Abstract
Background
The transfemoral (TF) approach drives most of the advantages of transcatheter aortic valve implantation (TAVI) over surgical aortic valve replacement. Alternative accesses for TAVI are associated with higher complication rates, but are still considered in ∼5% of cases due to peripheral arterial disease (PAD). Percutaneous transluminal angioplasty can still allow TF-TAVI in selected cases with severe calcific PAD; however, ancillary techniques for calcium management are often needed.
Case Summary
Orbital atherectomy was selected to facilitate TF-TAVI in two patients with different degrees and aspects of calcific PAD. Pre-procedural computed tomography analysis was key to choose the most appropriate technique for calcium management. We describe our experience with a step-by-step procedural approach to orbital atherectomy-assisted TF-TAVI.
Discussion
PAD is not uncommon in patients affected by severe symptomatic aortic valve stenosis. Orbital atherectomy can still allow TF-TAVI in selected cases with severe calcific PAD. A meticulous patient selection and a standardized, step-wise procedural execution are mandatory to optimize outcomes.
Keywords: Transcatheter aortic valve implantation, Transfemoral, Peripheral arterial disease, Calcium modification, Orbital atherectomy, Case report
Learning points.
Use of the transfemoral route to perform transcatheter aortic valve implantation (TAVI) should be the preferred approach and is the main driver for lower morbidity and mortality in trials comparing transcatheter with surgical aortic valve replacement (SAVR).
Calcific iliofemoral disease can be treated to make the iliofemoral route suitable for TAVI—hereby, choosing the most appropriate calcium modification technique is paramount.
Orbital atherectomy can be applied for treatment of both concentric and eccentric calcified lesions that require calcium debulking and can as such expand indications for transfemoral TAVI.
Introduction
Transfemoral (TF) access for transcatheter aortic valve implantation (TAVI) is the preferred approach for the treatment of severe aortic stenosis (AS) and is the main driver for lower morbidity and mortality in randomized clinical trials comparing transcatheter with surgical aortic valve replacement (SAVR).1 TAVI by alternative access is associated with increased peri-procedural complications and worse clinical outcomes, which negate the benefits of TAVI over SAVR, and is also discouraged in current guidelines.2–4
However, not infrequently patients with AS may have extensive peripheral arterial disease (PAD), with 5% to 8% considered ineligible for safe TF-TAVI due to severe arterial calcification and a narrowed luminal diameter.5 To render these patients suitable for TF approach, treating the diseased iliofemoral segments with percutaneous transluminal balloon angioplasty (PTA) alone is often inadequate and not safe (risk of vessel rupture, spiral dissection), particularly in the presence of severe arterial calcification. Hence, ancillary techniques for calcium management are required.
Intravascular lithotripsy (IVL) has been reported to be a safe and effective method to treat concentric calcifications in this context.6 The generation of micro-fractures within the calcified segment increases vessel compliance and facilitates luminal expansion by PTA and subsequent safe TAVI delivery system advancement. In the case of presence of eccentric and/or intraluminal calcific disease, the fracturing effect of acoustic waves can be reduced and a debulking procedure by means of atherectomy may be required.7,8
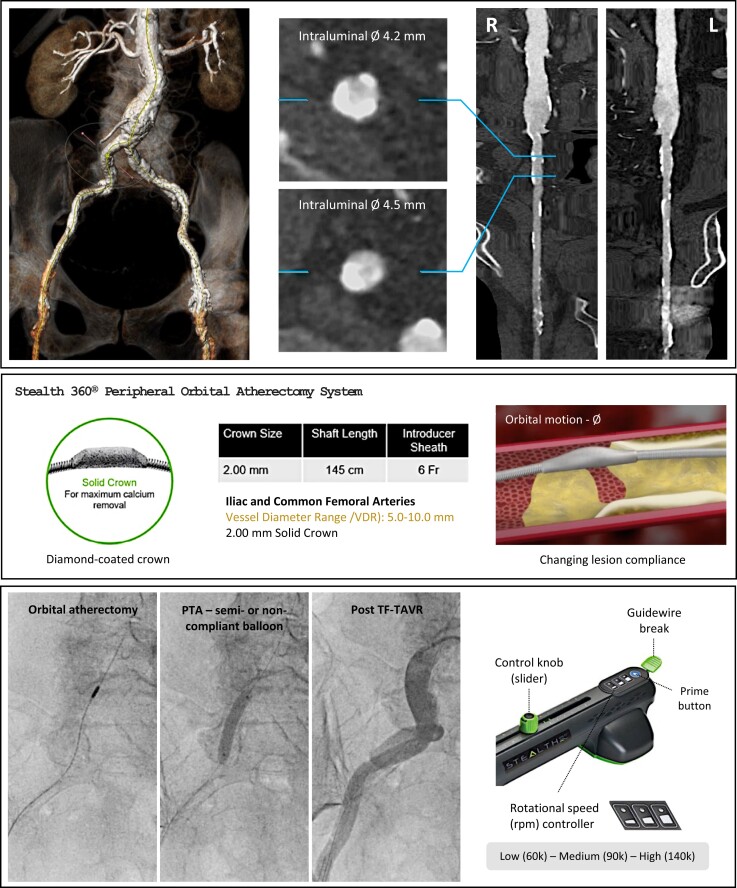
We report on the first European experience with the Stealth 360® peripheral Orbital Atherectomy System (OAS; Cardiovascular Systems CSI, USA) utilized to facilitate TF-TAVI in two patients with severely calcified iliofemoral arteries, sharing a detailed procedural plan including tips and tricks to enhance the safety and efficacy of this procedure. The Stealth 360® peripheral OAS with a diamond-coated solid crown uses an off-axis centrifugal force to achieve 360°contact with the vessel wall and enables the differential sanding of calcium to facilitate debulking (Figure 1). The crown of OA presents diamond chips on front and back, allowing calcium ablation during both antegrade and retrograde motion and making crown entrapment or lodging unlikely. This risk may be considered higher in case of rotablation.9
Figure 1.
Timeline. Patient selection and execution of orbital atherectomy-assisted TF-TAVI. Upper panel: Preliminary computed tomography imaging showing severe bilateral iliofemoral calcific disease. Middle panel: Stealth 360® peripheral Orbital Atherectomy System (OAS; Cardiovascular Systems CSI, USA). Lower panel: Intraprocedural sequence of an OAS-assisted TF-TAVI case. Left to right: orbital atherectomy, balloon angioplasty, final result after TF-TAVI. PTA, percutaneous transluminal angioplasty; TF-TAVI, transfemoral transcatheter aortic valve implantation.
Case 1
A 75-year-old patient with severe, symptomatic AS was referred for TAVI. A contrast-enhanced multi-slice computed tomography (MSCT) of the aorta and iliofemoral arteries showed severe bilateral iliofemoral calcific disease. The right iliofemoral artery was the least diseased, but showed eccentric calcifications at the level of the right common iliac artery and a residual lumen of 4.2–4.5 mm (Figure 1). The puncture site at the level of the common femoral artery (CFA) was relatively calcium-free. Orbital atherectomy with a Stealth 360® OAS 2.0 mm solid crown was performed. A detailed description of all procedural steps can be found in Figure 2. Several passages were required to cross the narrowest calcific arterial stenoses, with speeds of 90 000 rpm ultimately achieving satisfactory calcium debulking. Following orbital atherectomy, the TAVI access vessel was protected by means of a contralateral 0.018 in safety wire. The diseased segment was further dilated using a 7 mm Z-MED® balloon (NuMED, USA). The ViperWire was finally exchanged with a 0.035 in stiff guidewire, on which a 14F expandable TAVI introducer sheath was advanced without difficulty. TF-TAVI was successfully performed with a Navitor 25 mm transcatheter aortic valve (Abbott, IL, USA). The large-bore access site was closed utilizing the ProStyleTM (Abbott, USA) closure system (Supplementary material, Video 1).
Figure 2.
OAS-assisted TF-TAVI. Procedural tips and tricks to enhance the safety and efficacy of Stealth 360-assisted PTA and facilitate large-bore access in calcified iliofemoral arteries. OAS, orbital atherectomy system; PTA, percutaneous transluminal angioplasty; TF-TAVI, transfemoral transcatheter aortic valve implantation.
Case 2
A 79-year-old patient with severe AS was referred for TAVI. An MSCT scan demonstrated bilateral iliofemoral PAD with concentric vessel calcifications and a minimal residual luminal diameter of 4.5 mm, considered suitable for OAS-assisted TF-TAVI. The CFA puncture site was free from calcium. Stealth 360® OAS was performed and calcium debulking was achieved following multiple passages of a 2.0 mm solid crown at a maximal speed of 90 000 rpm. Subsequent to this, a safety wire was positioned through the contralateral access and further intraluminal expansion was obtained with a 7 mm Z-MED® balloon. This allowed the introduction of a 14F expandable sheath and permitted successful TF-TAVI with a Navitor 27 mm without vascular complications.
Both patients were mobilized and discharged homewards the following day. No vascular complications were reported after discharge.
Discussion
TAVI is best performed via the TF route for a multitude of reasons. First of all, clinical outcomes including mortality and major morbidities such as stroke, vascular complications and major bleeding are demonstrably lower in case of TF-TAVI as compared to non-TF-TAVI approaches.1,2 Secondly, most TAVI are performed in conventional catheterization laboratories rather than hybrid rooms, where surgical assistance to support alternative access routes can be difficult. Thirdly, transaxillary or transcarotid access increases radiation exposure for the structural interventionalist and its team. Finally, the ability to perform TF-TAVI using a minimalist approach with local anesthesia or mild conscious sedation also mitigates the risk of post-procedural delirium. Efforts should therefore be made to pursue a TF-TAVI option whenever possible, and this without jeopardizing procedural safety.
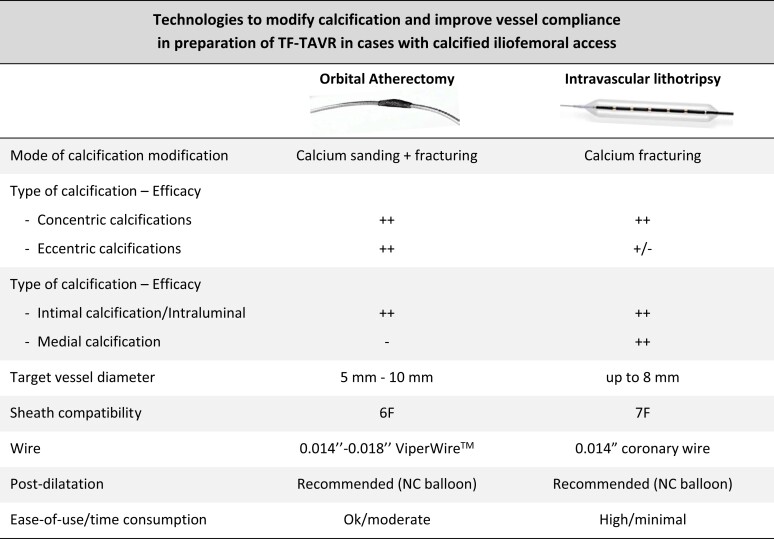
Converting a hostile iliofemoral arterial access to one that allows the safe delivery of a transcatheter heart valve is an integral part of this strategy. To achieve this, adjunctive therapies to PTA are required in case of the presence of severe arterial calcification. While IVL has been used successfully to facilitate TF-TAVI in predominantly concentric calcified lesions, orbital atherectomy can be applied for treatment of both concentric and eccentric calcifications that require calcium debulking10 (Figure 3).
Figure 3.
Technologies to modify calcification and improve vessel compliance in preparation of TF-TAVI in cases with calcified iliofemoral access. Comparison of orbital atherectomy and intravascular lithotripsy. NC, non-compliant; TF-TAVI, transfemoral transcatheter aortic valve implantation.
When engaging in these more challenging TF-TAVI cases, it is strongly recommended to use a 0.018 in safety wire—either introduced from a contralateral or a lower ipsilateral arterial access. A safety wire can be useful to treat complications at the level of the IVL- or orbital atherectomy-treated segments and to bail-out vascular closure failures. Finally, a control contrast angiography at the end of the procedure should also be encouraged in order to exclude important vascular injury at the iliac and/or femoral artery.
Importantly, a limitation of both IVL- and orbital atherectomy-assisted TF-TAVI is that the puncture site cannot be treated with either technique. Proceeding with TF-TAVI in case the puncture site is calcified or significantly diseased still carries a higher risk of vascular closure device failure.
Conclusions
Orbital atherectomy is a new tool in the arsenal to facilitate TF-TAVI in selected cases with calcific iliofemoral disease. A meticulous stepwise approach to this technique is paramount to its success.
Supplementary Material
Contributor Information
Angelo Quagliana, The Heart Center, Rigshospitalet, Copenhagen University Hospital, Section 9441, Blegdamsvej 9, DK-2100 Copenhagen, Denmark; Istituto Cardiocentro Ticino, Università della Svizzera Italiana, Lugano, Switzerland.
Nicholas J Montarello, The Heart Center, Rigshospitalet, Copenhagen University Hospital, Section 9441, Blegdamsvej 9, DK-2100 Copenhagen, Denmark.
Maarten Vanhaverbeke, The Heart Center, Rigshospitalet, Copenhagen University Hospital, Section 9441, Blegdamsvej 9, DK-2100 Copenhagen, Denmark.
Yannick Willemen, The Heart Center, Rigshospitalet, Copenhagen University Hospital, Section 9441, Blegdamsvej 9, DK-2100 Copenhagen, Denmark.
Laurence Campens, The Heart Center, Rigshospitalet, Copenhagen University Hospital, Section 9441, Blegdamsvej 9, DK-2100 Copenhagen, Denmark.
Lars Sondergaard, The Heart Center, Rigshospitalet, Copenhagen University Hospital, Section 9441, Blegdamsvej 9, DK-2100 Copenhagen, Denmark.
Ole De Backer, The Heart Center, Rigshospitalet, Copenhagen University Hospital, Section 9441, Blegdamsvej 9, DK-2100 Copenhagen, Denmark.
Lead author biography
 Interventional Cardiologist and Researcher at Cardiocentro Ticino Institute, Università della Svizzera Italiana—Lugano (CH). Fellow in Structural Heart Interventions at Rigshospitalet, Copenhagen (DK).
Interventional Cardiologist and Researcher at Cardiocentro Ticino Institute, Università della Svizzera Italiana—Lugano (CH). Fellow in Structural Heart Interventions at Rigshospitalet, Copenhagen (DK).
Supplementary material
Supplementary material is available at European Heart Journal – Case Reports.
Consent: Publication consent has been obtained by all subjects involved in the case series, according to COPE guidelines.
Funding: Y.W. received funding from the European Association of Percutaneous Cardiovascular Interventions (EAPCI Education & Training Grant App000092924).
Data availability
The data underlying this article are available in the article and in its online Supplementary material.
References
- 1. Siontis GC, Overtchouk P, Cahill TJ, Modine T, Prendergast B, Praz F, et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis. Eur Heart J 2019;40:3143–3153. [DOI] [PubMed] [Google Scholar]
- 2. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2022;43:561–632.34453165 [Google Scholar]
- 3. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2021;77:450–500. [DOI] [PubMed] [Google Scholar]
- 4. De Backer O, Quagliana A, Vanhaverbeke M, Nuyens P, Søndergaard L. Alternative access options for transcatheter aortic valve replacement. JACC Cardiovasc Interv 2022;15:1683–1685. [DOI] [PubMed] [Google Scholar]
- 5. Costa G, Bieliauskas G, Fukutomi M, Ihlemann N, Søndergaard L, De Backer O. Feasibility and safety of a fully percutaneous transcatheter aortic valve replacement program. Catheter Cardiovasc Interv 2021;97:E418–E424. [DOI] [PubMed] [Google Scholar]
- 6. Sawaya FJ, Bajoras V, Vanhaverbeke M, Wang C, Bieliauskas G, Søndergaard L, et al. Intravascular lithotripsy-assisted transfemoral TAVI: the Copenhagen experience and literature review. Front Cardiovasc Med 2021;8:739750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mattesini A, Nardi G, Martellini A, Sorini Dini C, Hamiti B, Stolcova M, et al. Intravascular imaging to guide lithotripsy in concentric and eccentric calcific coronary lesions. Cardiovasc Revasc Med 2020;21:1099–1105. [DOI] [PubMed] [Google Scholar]
- 8. Kaluski E, Khan SU, Singh M, Reitknecht F, Sattur S, Rogers Get al. Iliofemoral peripheral orbital atherectomy for optimizing TAVR access: an innovative strategy in the absence of alternative access options. Cardiovasc Revasc Med. 2018; 19(8S):71–76. [DOI] [PubMed] [Google Scholar]
- 9. De Maria GL, Scarsini R, Banning AP. Management of calcific coronary artery lesions: is it time to change our interventional therapeutic approach? JACC Cardiovasc Interv 2019;12:1465–1478. [DOI] [PubMed] [Google Scholar]
- 10. Lee MS, Martinsen BJ, Hollowed J, Heikali D, Mustapha J, Adams G, et al. Acute procedural outcomes of orbital atherectomy for the treatment of iliac artery disease: sub-analysis of the CONFIRM registries. Cardiovasc Revasc Med 2018;19:503–505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary material.