Key Points
Question
What percentage of birthing adults in the US with prepregnancy cardiovascular disease risk factors or adverse pregnancy outcomes received cardiovascular health counseling at the postpartum visit from 2016 through 2020?
Findings
In a serial cross-sectional analysis of nationally representative data from the Pregnancy Risk Assessment Monitoring System, prevalence of self-reported postpartum counseling for healthy eating, exercise, and losing weight gained during pregnancy was approximately 60% and experienced small declines from 2016 through 2020 among individuals with prepregnancy cardiovascular disease risk factors or adverse pregnancy outcomes.
Meaning
At-risk individuals may not be receiving cardiovascular health counseling at their postpartum visit.
Abstract
Importance
Poor prepregnancy cardiovascular health (CVH) and adverse pregnancy outcomes (APOs) are key risk factors for subsequent cardiovascular disease (CVD) in birthing adults. The postpartum visit offers an opportunity to promote CVH among at-risk individuals.
Objective
To determine prevalence, predictors, and trends in self-reported CVH counseling during the postpartum visit.
Design, Setting, and Participants
Serial, cross-sectional analysis of data from 2016-2020 from the Pregnancy Risk Assessment Monitoring System (PRAMS), a nationally representative, population-based survey. The primary analysis included individuals who attended a postpartum visit 4 to 6 weeks after delivery with available data on receipt of CVH counseling, self-reported prepregnancy CVD risk factors (obesity, diabetes, and hypertension), and APOs (gestational diabetes, hypertensive disorders of pregnancy, and preterm birth) (N = 167 705 [weighted N = 8 714 459]).
Exposures
Total number of CVD risk factors (0, 1, or ≥2 prepregnancy risk factors or APOs).
Main Outcomes and Measures
Annual, age-adjusted prevalence of self-reported postpartum CVH counseling per 100 individuals, defined as receipt of counseling for healthy eating, exercise, and losing weight gained during pregnancy, was calculated overall and by number of CVD risk factors. Average annual percent change (APC) assessed trends in CVH counseling from 2016 through 2020. Data were pooled to calculate rate ratios (RRs) for counseling that compared individuals with and without CVD risk factors after adjustment for age, education, postpartum insurance, and delivery year.
Results
From 2016 through 2020, prevalence of self-reported postpartum CVH counseling declined from 56.2 to 52.8 per 100 individuals among those with no CVD risk factors (APC, −1.4% [95% CI, −1.8% to −1.0%/y]), from 58.5 to 57.3 per 100 individuals among those with 1 risk factor (APC, −0.7% [95% CI, −1.3% to −0.1%/y]), and from 61.9 to 59.8 per 100 individuals among those with 2 or more risk factors (APC, −0.8% [95% CI, −1.3% to −0.3%/y]). Reporting receipt of counseling was modestly higher among individuals with 1 risk factor (RR, 1.05 [95% CI, 1.04 to 1.07]) and with 2 or more risk factors (RR, 1.11 [95% CI, 1.09 to 1.13]) compared with those who had no risk factors.
Conclusions and Relevance
Approximately 60% of individuals with CVD risk factors or APOs reported receiving CVH counseling at their postpartum visit. Prevalence of reporting CVH counseling decreased modestly over 5 years.
Using data from the Pregnancy Risk Assessment Monitoring System, this study evaluates trends (2016-2020) in prevalence of postpartum counseling for healthy eating, exercise, and losing weight gained during pregnancy among individuals with prepregnancy cardiovascular disease risk factors or adverse pregnancy outcomes.
Introduction
Cardiovascular disease (CVD) is the leading cause of mortality among women in the US, and it has contributed to an increasing number of deaths since 2010.1 Risk of CVD morbidity and mortality during the peripartum period is greater among individuals who have poor prepregnancy cardiovascular health (CVH), those who experience adverse pregnancy outcomes (APOs), or both. However, over the past decade, CVH prior to pregnancy has declined such that in 2019, less than half of individuals entered pregnancy without obesity, diabetes, or hypertension.2,3,4,5 Concurrently, incidence of APOs, including hypertensive disorders of pregnancy and gestational diabetes, has increased in the US. Disparities in poor prepregnancy CVH and APOs by race and ethnicity have persisted, with the highest burden observed among individuals who self-identified as non-Hispanic Black or American Indian/Alaska Native.5,6,7
Beyond the peripartum period, APOs are recognized as important risk factors for CVD across the life course and are now designated as risk-enhancing factors for CVD by the American Heart Association/American College of Cardiology Prevention Guidelines.8 In addition, the American College of Obstetricians and Gynecologists recommends that individuals with APOs receive counseling for postpartum weight loss.9 The postpartum period is an opportune time when there is contact with the health care system, and counseling for CVH could potentially improve CVD-related outcomes during subsequent pregnancies and throughout the life course because individuals with poor CVH are more likely to experience subsequent APOs and CVD later in life.10,11,12 However, in 2016-2017, only 40% of individuals in the US who attended a 4- to 6-week postpartum visit reported receiving CVD prevention messages, and receipt of these messages was only slightly higher among those with CVD risk factors or APOs.13 Since then, the American Heart Association has released several consensus statements describing opportunities to improve CVH during the peripartum period.14,15,16
The objectives of the current analysis have been to determine current prevalence, predictors, and trends in self-reported CVH counseling at the 4- to 6-week postpartum visit, using a nationally representative sample of individuals with a recent live birth in the US from 2016 through 2020.
Methods
This was a cross-sectional analysis using data obtained from the Centers for Disease Control and Prevention (CDC) Pregnancy Risk Assessment Monitoring System (PRAMS), a national population-based survey conducted annually of individuals at 2 to 6 months after having a live birth. Surveys were completed via mail or telephone to collect data regarding sociodemographic and health-related factors and behaviors before, during, and after delivery. States stratified samples to allow oversampling of subpopulations of public health interest including individuals living in high-risk areas or belonging to historically underrepresented racial and ethnic groups.17 PRAMS methods and protocols were approved by the CDC institutional review board.
First, we determined the percentage of individuals who attended a postpartum visit 4 to 6 weeks after delivery overall and by sociodemographic characteristics. For the primary analysis (receipt of postpartum CVH counseling), we excluded individuals who did not attend a postpartum visit or were missing data on self-reported CVH counseling, prepregnancy CVD risk factors (obesity, diabetes, hypertension, or all 3), and APOs (gestational diabetes, hypertensive disorders of pregnancy, preterm birth, or all 3). Gestational diabetes was defined based on self-report of new diabetes during pregnancy, and hypertensive disorders of pregnancy were defined as self-report of new-onset hypertension during pregnancy (ie, gestational hypertension, preeclampsia or eclampsia [excluding individuals with prepregnancy hypertension]). Preterm birth was defined as birth prior to 37 weeks.
Race and ethnicity were determined via self-report using close-ended questions from birth certificate data linked to the PRAMS database. Birthing individuals were asked to check 1 or more racial or ethnic groups to indicate “what they consider themselves to be” by checking 1 or more of the following boxes: American Indian or Alaska Native, Asian Indian, Black or African American, Chinese, Filipino, Guamanian or Chamorro, Japanese, Korean, Native Hawaiian, Other Asian, Other Pacific Islander, Samoan, Vietnamese, White, or other. Those who checked more than 1 box grouped into the category of Multiracial or other. These individuals were separately asked to specify whether they considered themselves to be Spanish, Hispanic, or Latina. Race and ethnicity were reported in this article as a social determinant of health by which there are known disparities in prepregnancy CVH and APOs. The outcome of interest was self-reported CVH counseling at the postpartum visit, defined as self-reported counseling regarding healthy eating, exercise, and losing weight gained during pregnancy, as provided by a physician, nurse, or other health care professional.
Statistical Analysis
We calculated the prevalence of self-reported CVH counseling annually from 2016 through 2020, age-standardized to the 2016 population per 100 individuals, overall and by the total number of CVD risk factors (0, 1, or ≥2 prepregnancy risk factors and APOs) present. We secondarily determined the annual prevalence of CVH counseling by each prepregnancy risk factor (diabetes, hypertension, obesity) and APO (gestational diabetes, hypertensive disorders of pregnancy, preterm birth). We then quantified the annual average percent change (APC) from 2016 through 2020 using joinpoint software, overall and in each subgroup.18 Joinpoint calculated the APC by fitting a log-linear model based on data we supplied regarding the annual age-standardized prevalence of CVH counseling and standard errors. Given the potential impact from the COVID-19 pandemic on postpartum health care in 2020, we conducted a sensitivity analysis of APCs from 2016 through 2019 prior to the onset of the pandemic and compared the APC excluding 2020 with the full sample. Lastly, we pooled data from 2016 through 2020 and calculated rate ratios (RRs) of CVH counseling receipt, comparing individuals with and without prepregnancy CVD risk factors or APOs. Multivariable logistic regression models adjusted for age, education (≤high school or >high school), health insurance status at 2 to 6 months postpartum (any private insurance or no private insurance), and year of delivery (2016-2020). All analyses were repeated in each 5-year age group, by self-identified race or ethnicity (Hispanic, non-Hispanic American Indian/Alaska Native, non-Hispanic Asian/Pacific Islander, non-Hispanic Black, or non-Hispanic White), educational status, and insurance status. To account for the PRAMS complex sampling design, a total analysis weight was assigned as recommended by the PRAMS analysis guide and consistent with prior publications.17,19,20 PRAMS supplies a sampling weight to account for selection probability, a nonresponse weight to account for survey completion failure, and a noncoverage weight to account for individuals who were not sampled. The total analytic weight was calculated as the product of these analytic weights per PRAMS recommendations.17,19,20 Analyses were completed using Stata 14.0.21
Results
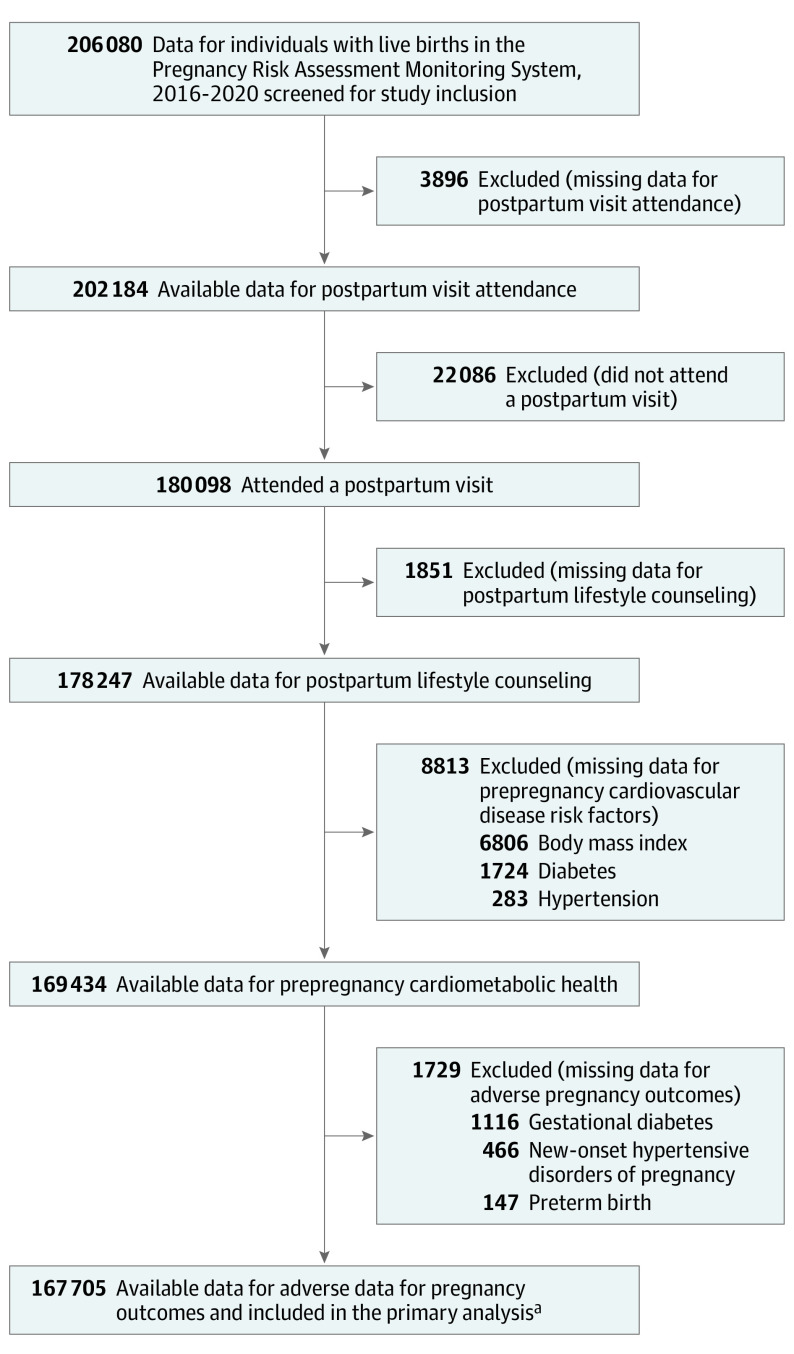
Of 206 080 individuals (weighted N=10 630 861) in PRAMS with a live birth in the US from 2016 through 2020, the weighted percent of individuals who attended a postpartum visit was 88.2% (95% CI, 88.0% to 88.5%). Postpartum follow-up was lowest among individuals who were younger than 20 years old (80.2% [95% CI, 78.8%-81.6%]), who identified as American Indian/Alaska Native (77.4% [95% CI, 75.7% to 79.1%%]), had a high school education or less (81.0% [95% CI, 80.5% to 81.4%]), and were enrolled in Medicaid (82.3% [95% CI, 81.8% to 82.8%]) (Table 1). In the primary analysis examining receipt of postpartum CVH counseling, 18.6% of individuals were excluded: 1.9% (3896) with missing postpartum visit data, 10.7% (22 086)who did not attend a postpartum visit, and 6.0% (12 393) with missing data for CVH counseling or CVD risk factors (Figure 1). Individuals who were excluded were more likely to be younger, identify as Hispanic or non-Hispanic Black, complete the survey in Spanish, have a high school education or less, be enrolled in Medicaid, and have any prepregnancy cardiovascular risk factor or APO (eTables 1 and 2 in Supplement 1).
Table 1. Individuals Who Attended a Postpartum Visit Among All With a Live Birth in the Pregnancy Risk Assessment Monitoring System, Overall and by Sociodemographic Characteristics, 2016-2020a.
| Sociodemographic factor | Attended a postpartum visit, % (95% CI) |
|---|---|
| No. with a live birth | 206 080 |
| Weighted No. | 10 630 861 |
| Overall | 88.2 (88.0-88.5) |
| Age group, y | |
| <20 | 80.2 (78.8-81.6) |
| 20-24 | 83.8 (83.2-84.4) |
| 25-29 | 88.0 (87.5-88.4) |
| 30-34 | 91.1 (90.8-91.5) |
| 35-39 | 90.8 (90.3-91.3) |
| ≥40 | 88.3 (87.0-89.4) |
| Race and ethnicity | |
| American Indian or Alaska Native | 77.4 (75.7-79.1) |
| Asian or Pacific Islander | 89.2 (88.4-90.0) |
| Hispanic | 83.2 (82.5-83.8) |
| Non-Hispanic Black | 83.6 (83.0-84.3) |
| Non-Hispanic White | 91.5 (91.2-91.7) |
| Other or multiracialb | 85.3 (83.8-86.6) |
| Language of survey | |
| English | 89.1 (88.8-89.3) |
| Spanish | 79.7 (78.7-80.7) |
| Chinese | 94.8 (91.2-97.0) |
| Education | |
| High school or less | 81.0 (80.5-81.4) |
| Greater than high school | 92.6 (92.4-92.8) |
| Postpartum insurancec | |
| Any private insurance | 93.9 (93.6-94.1) |
| Medicaid | 82.3 (81.8-82.8) |
| Other government insurance | 87.3 (85.9-88.6) |
| Other insurance | 82.5 (80.8-84.1) |
| No insurance | 78.9 (77.9-79.9) |
Weighted to account for the Pregnancy Risk Assessment Monitoring System sampling design.
The category of other includes individuals who did not identify as any of the races or ethnicities listed in this table.
Indicates insurance at survey completion (2-6 months postpartum).
Figure 1. Individuals Included From the Pregnancy Risk Assessment Monitoring System, 2016-2020.
Of 206 080 individuals with a live birth from 2016 through 2020, 18.6% were excluded from the primary analysis examining receipt of postpartum cardiovascular health counseling (1.9% with missing postpartum visit data; 10.7% who did not attend a postpartum visit; and 6.0% with missing data for postpartum lifestyle counseling, prepregnancy cardiovascular disease risk factors, and adverse pregnancy outcomes).
aWeighted analytical N = 8 714 459.
Of the included 167 705 individuals (weighted n = 8 714 459), 77.2% were 20 to 34 years old, 67.4% had more than a high school education, and 26.7% were enrolled in Medicaid at 2 to 6 months postpartum. The majority identified as Hispanic (16.1%), non-Hispanic Black (14.2%), and non-Hispanic White (60.2%). Individuals with a live birth in 2020 were more likely to be 35 to 39 years old, identify as non-Hispanic White, have greater than a high school education, and be enrolled in Medicaid (Table 2). From 2016 through 2020, the percent of individuals reporting the following risk factors increased: prepregnancy obesity (from 23.3% to 27.5%), gestational diabetes (from 8.0% to 10.1%), and hypertensive disorders of pregnancy (from 9.7% to 12.3%).
Table 2. Characteristics of Individuals With a Live Birth in the Pregnancy Risk Assessment Monitoring System, 2016-2020.
| No. of participants (weighted %) by delivery yeara | |||||
|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | |
| No. included in the primary analysis | 29 346 | 31 276 | 36 476 | 37 347 | 33 260 |
| Weighted No.a | 1 655 515 | 1 604 942 | 1 829 709 | 1 922 077 | 1 702 215 |
| Age group, y | n = 29 346 | n = 31 276 | n = 36 476 | n = 37 343 | n = 33 258 |
| <20 | 1316 (4.3) | 1371 (3.9) | 1558 (3.8) | 1364 (3.7) | 1221 (3.5) |
| 20-24 | 5189 (17.1) | 5546 (17.7) | 6422 (18.1) | 6141 (17.3) | 5604 (16.8) |
| 25-29 | 8618 (29.5) | 9318 (29.2) | 10 584 (29.1) | 10 678 (28.8) | 9322 (28.2) |
| 30-34 | 8835 (31.2) | 9368 (30.7) | 11 081 (30.1) | 11 637 (30.6) | 10 427 (31.7) |
| 35-39 | 4409 (14.7) | 4668 (15.2) | 5624 (15.7) | 6177 (16.2) | 5465 (16.3) |
| ≥40 | 979 (3.2) | 1005 (3.3) | 1207 (3.3) | 1346 (3.3) | 1219 (3.5) |
| Race and ethnicity | n = 29 121 | n = 31 071 | n = 36 278 | n = 37 131 | n = 33 073 |
| American Indian or Alaskan Native | 890 (0.7) | 1182 (0.8) | 1294 (0.8) | 1143 (0.5) | 1290 (0.7) |
| Asian or Pacific Islander | 2261 (6.2) | 1745 (5.2) | 2315 (5.0) | 2606 (4.8) | 2511 (5.2) |
| Hispanic | 5073 (20.2) | 5142 (14.1) | 6052 (13.8) | 6421 (15.9) | 5607 (17.0) |
| Non-Hispanic Black | 4623 (12.2) | 5482 (14.2) | 6492 (14.2) | 6153 (15.8) | 5565 (14.6) |
| Non-Hispanic White | 14 703 (57.9) | 16 087 (62.7) | 18 123 (63.2) | 18 747 (59.9) | 16 055 (59.4) |
| Other or multiracialb | 1571 (2.9) | 1433 (2.9) | 2002 (3.0) | 2061 (3.0) | 2045 (3.2) |
| Language of survey | n = 29 346 | n = 31 276 | n = 36 476 | n = 37 347 | n = 33 260 |
| English | 27 641 (92.8) | 28 958 (93.8) | 33 972 (94.1) | 34 653 (93.6) | 31 181 (94.0) |
| Spanish | 1670 (7.0) | 2277 (6.0) | 2473 (5.8) | 2646 (6.1) | 2055 (5.9) |
| Chinese | 35 (0.2) | 41 (0.2) | 31 (0.1) | 48 (0.2) | 24 (0.1) |
| Education | n = 29 031 | n = 31 040 | n = 36 185 | n = 37 103 | n = 33 055 |
| High school or less | 9512 (32.3) | 10 150 (31.7) | 11 911 (32.6) | 11 236 (32.1) | 10 399 (31.9) |
| Greater than high school | 19 519 (67.7) | 20 890 (68.3) | 24 274 (67.4) | 25 867 (67.9) | 22 656 (68.1) |
| Postpartum insurancec | n = 29 012 | n = 30 873 | n = 36 098 | n = 36 867 | n = 32 914 |
| Any private insurance | 16 406 (58.8) | 17 072 (59.0) | 20 015 (58.3) | 21 222 (58.7) | 18 243 (57.7) |
| Medicaid | 8185 (22.6) | 9397 (26.8) | 10 807 (26.5) | 10 761 (26.8) | 11 455 (32.2) |
| Other government insurance | 1232 (4.2) | 1177 (3.6) | 1445 (3.9) | 1231 (3.2) | 1154 (3.2) |
| Other insurance | 766 (3.3) | 790 (2.4) | 892 (2.6) | 809 (2.2) | 634 (2.1) |
| No insurance | 2423 (11.3) | 2437 (8.1) | 2939 (8.7) | 2844 (9.0) | 1428 (4.8) |
| Prepregnancy clinical cardiovascular risk factors | n = 29 346 | n = 31 276 | n = 36 476 | n = 37 347 | n = 33 260 |
| Any cardiovascular risk factord | 8461 (27.2) | 9658 (28.7) | 11 309 (29.7) | 11 562 (30.0) | 10 821 (30.7) |
| Prepregnancy diabetes | 1116 (3.8) | 1162 (3.0) | 1187 (3.5) | 1099 (2.9) | 1077 (2.6) |
| Prepregnancy hypertension | 1764 (5.3) | 1964 (5.0) | 2167 (5.6) | 2082 (4.7) | 2073 (5.1) |
| Prepregnancy obesity | 7225 (23.3) | 8360 (25.3) | 9957 (26.0) | 10 266 (26.9) | 9532 (27.5) |
| Adverse pregnancy outcomes | n = 29 346 | n = 31 276 | n = 36 476 | n = 37 347 | n = 33 260 |
| Any adverse pregnancy outcomee | 9246 (22.3) | 9547 (23.0) | 11 303 (23.5) | 12 294 (25.4) | 11 360 (26.1) |
| Gestational diabetes | 2483 (8.0) | 2824 (8.2) | 3285 (8.2) | 3600 (9.0) | 3529 (10.1) |
| Hypertensive disorder of pregnancy | 3528 (9.7) | 3918 (10.5) | 4655 (11.2) | 5218 (12.4) | 4879 (12.3) |
| Preterm birth | 5474 (8.7) | 5170 (8.6) | 6177 (8.6) | 6550 (9.0) | 5909 (8.9) |
| No. of total cardiovascular risk factorsf | n = 29 346 | n = 31 276 | n = 36 476 | n = 37 347 | n = 33 260 |
| No risk factors | 15 081 (59.1) | 15 882 (57.2) | 18 460 (56.5) | 18 396 (55.1) | 15 961 (54.8) |
| 1 Risk factor | 8749 (26.7) | 9370 (28.7) | 10 947 (28.0) | 11 546 (29.3) | 10 072 (28.5) |
| ≥2 Risk factors | 5516 (14.2) | 6024 (14.1) | 7069 (15.4) | 7405 (15.7) | 7227 (16.7) |
Weighted to account for Pregnancy Risk Assessment Monitoring System sampling design.
The category of other includes individuals who did not identify as any of the races or ethnicities listed in this table.
Indicates insurance at survey completion (2-6 months postpartum).
Category is defined as the presence of prepregnancy diabetes, prepregnancy hypertension, and/or prepregnancy obesity.
Category is defined as the presence of gestational diabetes, hypertensive disorders of pregnancy, and/or preterm birth.
Indicates the sum of the following risk factors: prepregnancy diabetes, prepregnancy hypertension, prepregnancy obesity, gestational diabetes, hypertensive disorders of pregnancy, and preterm birth.
Prevalence and Trends in Self-Reported Postpartum CVH Counseling
From 2016 through 2020, prevalence of self-reported CVH counseling at the 4- to 6-week postpartum visit decreased from 56.2% (95% CI, 55.0% to 57.4%) to 52.8% (95% CI, 51.7% to 54.0%) (APC, −1.4% [95% CI, −1.8% to −1.0%/y]) among individuals with no CVD risk factors (prepregnancy risk factors or APOs), from 58.5% (95% CI, 56.8% to 60.3%) to 57.3% (95% CI, 55.7% to 58.8%) (APC, −0.7% [95% CI, −0.7% [−1.3% to −0.1%/y]) among those with 1 risk factor, and from 61.9% (95% CI, 59.6% to 64.2%) to 59.8% (95% CI, 57.8% to 61.7%) (APC, −0.8% [95% CI, −1.3% to −0.3%/y]) among those with 2 or more risk factors (Table 3). In sensitivity analyses, trends did not differ significantly from 2016 through 2019 (prepandemic) compared with the full study period from 2016 through 2020 (eTable 3 in Supplement 1).
Table 3. Trends in Annual Self-Reported Postpartum Cardiovascular Health Counseling by Number of Cardiovascular Risk Factors, 2016-2020a.
| Year | No. of cardiovascular disease risk factors, rate/100 individualsb | Cardiovascular health counseling, adjusted rate ratio (95% CI)c | ||||
|---|---|---|---|---|---|---|
| Overall | 0 | 1 | ≥2 | 0 vs 1 risk factor | 0 vs ≥2 risk factors | |
| Overall, 2016-2020 | 56.4 (56.0 to 56.8) | 54.5 (54.0 to 55.0) | 58.0 (57.2 to 58.7) | 60.7 (59.8 to 61.6) | 1.05 (1.04 to 1.07) | 1.11 (1.09 to 1.13) |
| 2016 | 57.6 (56.7 to 58.5) | 56.2 (55.0 to 57.4) | 58.5 (56.8 to 60.3) | 61.9 (59.6 to 64.2) | 1.03 (1.00 to 1.07) | 1.09 (1.05 to 1.14) |
| 2017 | 57.0 (56.1 to 57.8) | 54.9 (53.8 to 56.0) | 58.9 (57.4 to 60.4) | 61.2 (59.1 to 63.3) | 1.06 (1.02 to 1.10) | 1.10 (1.06 to 1.15) |
| 2018 | 56.6 (55.8 57.3) | 54.5 (53.5 to 55.6) | 58.1 (56.7 to 59.6) | 61.2 (59.3 to 63.1) | 1.05 (1.02 to 1.08) | 1.11 (1.07 to 1.15) |
| 2019 | 55.9 (55.1 to 56.7) | 53.8 (52.7 to 55.0) | 57.4 (55.9 to 58.9) | 60.7 (58.7 to 62.8) | 1.05 (1.02 to 1.09) | 1.12 (1.07 to 1.16) |
| 2020 | 55.1 (54.3 to 56.0) | 52.8 (51.7 to 54.0) | 57.3 (55.7 to 58.8) | 59.8 (57.8 to 61.7) | 1.07 (1.04 to 1.11) | 1.12 (1.08 to 1.17) |
| Absolute % difference, 2016-2020 | −2.5 | −3.4 | −1.2 | −2.1 | ||
| Average annual percent change 2016-2020 (95% CI) | −1.1 (−1.3 to −0.8) | −1.4 (−1.8 to −1.0) | −0.7 (−1.3 to −0.1) | −0.8 (−1.3 to −0.3) | ||
Cardiovascular risk factors: prepregnancy obesity, prepregnancy diabetes, prepregnancy hypertension, hypertensive disorders of pregnancy, gestational diabetes, and preterm birth.
Percent (95% CI) values for No. of cardiovascular disease risk factors were age standardized, and they were weighted to account for the Pregnancy Risk Assessment Monitoring System sampling design.
Adjusted for maternal age, education (≤high school or > high school), and postpartum insurance type (any private insurance or no private insurance). The overall rate ratio was additionally adjusted for year of delivery.
Prevalence and trends were similar among individuals with any prepregnancy CVD risk factor and any APO (eTable 4 in Supplement 1). Among individuals with prepregnancy CVD risk factors, prevalence of self-reported counseling was similar for each risk factor in each year. For individuals with APOs, point estimates for the prevalence of counseling were highest among those with gestational diabetes compared with hypertensive disorders of pregnancy or preterm birth. In 2020, 62.3% (95% CI, 59.5% to 65.1%) of individuals with gestational diabetes reported postpartum CVH counseling compared with 55.1% (95% CI, 52.7% to 57.4%) of those with hypertensive disorders of pregnancy and 57.2% (95% CI, 54.9% to 59.4%) of those with preterm birth.
RRs for Self-Reported Postpartum CVH Counseling Among Individuals With Cardiovascular Risk Factors
Individuals with prepregnancy CVD risk factors or APOs were modestly more likely to report postpartum CVH counseling than those without risk factors in each year (Table 3). After pooling data from 2016 through 2020, the adjusted RR was 1.05 (95% CI, 1.04 to 1.07) times higher among individuals with 1 risk factor and 1.11 (95% CI, 1.09 to 1.13) times higher among those with ≥2 or more risk factors (prepregnancy risk factors or APOs) compared with individuals without any risk factors. In subgroup analyses, the prevalence of self-reported CVH counseling was higher among individuals with each prepregnancy CVD risk factor and APO compared with those without risk factors, with the adjusted RR ranging from 1.02 (95% CI, 1.0 to 1.05) among those with hypertensive disorders of pregnancy, from 1.02 (95% CI, 1.0 to 1.04) among those with preterm birth, to 1.11 (95% CI, 1.08 –1.13) among those with gestational diabetes (eTable 5 in Supplement 1).
Prevalence of Self-Reported Postpartum CVH Counseling by Sociodemographic Subgroups
Prevalence of self-reported CVH counseling was stable or declined slightly from 2016 through 2020 in each age, race and ethnicity, education, and insurance subgroup (eTable 6 in Supplement 1). In 2016 through 2020, prevalence of reported postpartum CVH counseling was highest among individuals younger than 20 years old (63.8% [95% CI, 61.8% to 65.7%]), who identified as non-Hispanic Black (69.2% [95% CI 68.3% to 70.1%]), who had at most a high school education (60.9% [95% CI, 60.2% to 61.6%]), and who did not have private insurance (61.1% [95% CI, 60.5% to 61.7%]) (Figure 2). Individuals with 2 or more CVD risk factors were more likely to report CVH counseling than those without risk factors with similar odds in each sociodemographic subgroup (eTable 7 in Supplement 1).
Figure 2. Prevalence of Self-Reported Cardiovascular Health Counseling at the Postpartum Visit by Number of Cardiovascular Disease Risk Factors and Sociodemographic Characteristics.
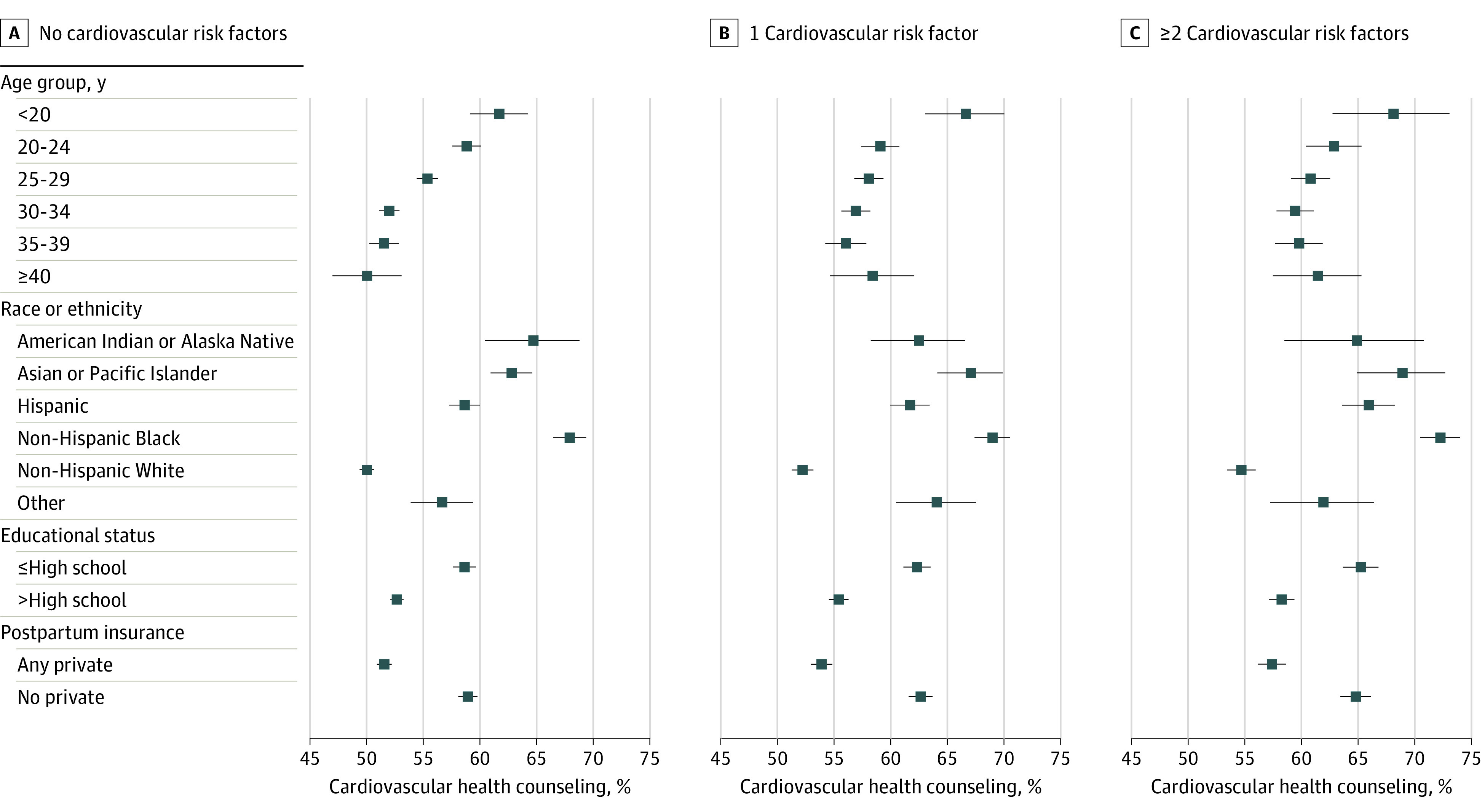
Prevalence of reported postpartum cardiovascular health counseling was highest among individuals younger than 20 years old, who identified as non-Hispanic Black, had at most a high school education, and did not have private insurance. Cardiovascular risk factors: prepregnancy obesity, prepregnancy diabetes, prepregnancy hypertension, hypertensive disorders of pregnancy, gestational diabetes, and preterm birth. Individuals categorized by race or ethnicity as other self-reported as any race or ethnicity that is not shown in this figure, or they self-reported as multiracial. Race and ethnicity categorization is based on available data in the Pregnancy Risk Assessment Monitoring System.
Discussion
Among a national sample of postpartum individuals in the US, approximately 6 in 10 self-reported CVH counseling about healthy eating, exercise, and postpartum weight loss at the 4- to 6-week postpartum visit. While the prevalence of CVD risk factors increased from 2016 through 2020, receipt of self-reported CVH counseling was stable or experienced small declines during the study period. Individuals with 2 or more prepregnancy CVD risk factors or APOs were slightly more likely to report CVH counseling at their postpartum visit than those without risk factors; however, up to 40% of individuals with these risk factors did not report receiving CVH counseling. Prevalence of self-reported CVH counseling differed based on social determinants of health and was highest among individuals who identified as non-Hispanic Black, American Indian/Alaska Native, had a high school education or less, or did not have private insurance during the postpartum period.
This study builds upon recent data from PRAMS demonstrating that approximately 57% of all individuals with a recent live birth in the US received diet and exercise counseling at the 4- to 6-week postpartum visit from 2016 through 2017.13 Our study newly demonstrates no meaningful differences or changes in the prevalence of CVH counseling among individuals with CVD risk factors from 2016 through 2020 despite increasing awareness of the importance of CVH promotion. Potential reasons for relatively low counseling rates include clinicians having competing priorities and time constraints at the postpartum visit and patients’ recall bias. Although this study spanned the COVID-19 pandemic during which there were dramatic changes in health care delivery and practices, prevalence of self-reported CVH counseling at the postpartum visit did not change significantly from 2019 to 2020. In addition, the APC in self-reported CVH counseling from 2016 through 2019 (prepandemic) and from 2016 through 2020 were similar, suggesting that trends in counseling were not substantially affected by the COVID-19 pandemic in this sample.
Prevalence of self-reported CVH counseling in 2020 was lowest among individuals with hypertensive disorders of pregnancy (55%), which is an APO that is a recognized risk-enhancing factor for CVD and associated with a 2-fold higher lifetime risk of heart failure and ischemic heart disease.22 Although previous data regarding CVH counseling after APOs are limited, one small study of 350 individuals with hypertensive disorders of pregnancy at a single institution revealed that only 6% had counseling on CVD risk documented in the medical record at the postpartum visit.23 It is possible that CVD counseling may be underdocumented in the medical record and prevalence of CVH counseling in the present analysis may have been higher due to use of self-report. However, awareness of CVD risk by birthing individuals and by clinicians after an APO remains low, suggesting missed opportunities for CVH counseling during postpartum care, especially among individuals with prepregnancy CVD risk factors or APOs.24
Postpartum follow-up and self-reported CVH counseling differed significantly by social determinants of health. Individuals who did not attend a postpartum visit or who did not respond to survey questions regarding CVH counseling and risk factors were more likely to identify as Hispanic or non-Hispanic Black, to have a high school education or less, and to lack private health insurance. Conversely, among individuals who attended a postpartum visit, prevalence of self-reported postpartum counseling was modestly higher among individuals from these groups, regardless of the number of CVD risk factors present. Potential reasons for higher rates of counseling among individuals from racial and ethnic minority groups and among those with a lower socioeconomic status may be related to appropriate awareness by clinicians of the role of adverse social determinants of health on CVD risk and barriers to care. In addition to lower rates of postpartum visit attendance demonstrated in the present study, previous work has shown that individuals who identify as non-Hispanic Black or who have less than a high school education initiate prenatal care 1 to 4 weeks later in pregnancy than those who identify as non-Hispanic White or have greater than a high school education.25 With less opportunity for CVH counseling during pregnancy, clinicians may prioritize CVH counseling at the postpartum visit, although more data are needed to better understand these associations. Lastly, it is possible that individuals who attended their postpartum visit were more likely to prioritize and recall CVH counseling than those who missed the appointment, leading to overestimation of self-reported CVH counseling among those from racial or ethnic minority groups and those with lower socioeconomic status.
However, up to 40% of individuals did not self-report counseling in this study, and substantial disparities in postpartum weight retention, diet, and long-term CVD risk persist.15,26,27 Therefore, while CVH counseling at the postpartum visit is an important first step, multilevel interventions are needed to equitably promote CVH during the peripartum period and beyond. First, public health and hospital policies must address barriers that drive inequities in timely postpartum care including challenges with transportation, childcare, and insurance coverage.28 Second, standardized protocols for CVH counseling, enhanced CVD screening, and improved surveillance among individuals with CVD risk factors during the peripartum period need to be developed and implemented as recommended by the American College of Obstetricians and Gynecologists and the American Heart Association.14,15,29 One opportunity may be to leverage the American Heart Association’s Life’s Essential 8 framework, which highlights and promotes a positive construct of health with a score from 0 to 100 to prioritize primordial and primary prevention.30 Third, given that only approximately 60% of individuals self-report CVH counseling at their postpartum visit, improving transitions to long-term preventive care for continued CVH counseling and monitoring is essential. The postpartum visit can serve as an important opportunity to screen for cardiovascular risk factors, assess barriers to achieving ideal CVH, and refer to services and clinicians (ie, primary care clinicians, obstetricians, or cardiologists) that best meet a patient’s needs. Several gaps in the transition to long-term preventive care exist, however, and include difficulty identifying in-network clinicians, fragmented medical systems, challenges accessing transportation and finding affordable childcare, and experiencing discrimination within the health care system.31,32,33 In addition to addressing these patient- and systems-level barriers, care transitions should be coupled with physician education regarding the associations between APOs and CVD risk. Education is particularly important in care transitions to primary care clinicians, who are less likely to screen for a pregnancy history but are well trained in CVD risk factor assessment and management.34,35 Utilization of the electronic medical record to promote physician communication, document APOs, and design clinical decision support tools may help promote successful care transitions.36
Referral to a postpartum diet and physical activity modification programs has shown some promise in improving knowledge about CVD risk factors, promoting weight loss, and reducing markers of metabolic syndrome among individuals with a history of hypertensive disorders of pregnancy and/or gestational diabetes, but data on generalizability and sustainability of these approaches are lacking.29 Similar to successful interventions implemented in nonpregnant populations, these programs typically involve intensive lifestyle modification that occurs during regularly scheduled visits over the course of several months and utilize lifestyle coaches, dieticians and/or diet and physical activity tracking.29,37,38 Ongoing national programs such as the Early Intervention to Promote Cardiovascular Health of Mothers and Children network aim to reduce CVH disparities and address barriers to postpartum CVH promotion among individuals from low-income, underserved, and high-risk communities utilizing prenatal and postpartum home visiting programs.39
Limitations
This study was strengthened by use of PRAMS, which is a large, nationally representative data set of individuals with a recent live birth in the US. Limitations include exclusion of individuals without live births (ie, intrauterine fetal demise, abortions) who may be at higher risk of CVD later in life and the potential for nonresponse bias. PRAMS data are weighted to account for nonresponse bias and are oversampled to recruit individuals from high-risk populations. Although these analytic weights cannot fully account for individuals excluded from our main analysis (ie, those who did not attend a postpartum visit), we present data regarding the sociodemographic characteristics of these excluded individuals so that results can be interpreted in this context.
Second, use of self-report data may lead to misclassification. Therefore, it is possible that individuals may have received counseling at the postpartum visit but did not remember the counseling received. However, self-reported counseling is an important public health metric given that behavior change is unlikely to occur if counseling and its content are not retained. While sensitivity of self-reported overweight/obesity and APO data are low, specificity is high. Therefore, we may have underestimated the prevalence of risk factors in the population.40,41,42 This may have reduced differences in CVH counseling among individuals with and without risk factors; however, counseling was still low in all groups, emphasizing the need to improve postpartum CVH counseling overall.
Third, data were only described for counseling at a single postpartum visit, so it is possible that we missed CVH counseling that might have occurred during antenatal visits, the delivery hospitalization, and/or subsequent postpartum visits.
Fourth, PRAMS lacks data regarding postpartum smoking cessation counseling and therefore could not be assessed in this analysis.
Conclusions
Approximately 60% of individuals with prepregnancy CVD risk factors or APOs reported receiving CVH counseling at their postpartum visit in the US. Prevalence of self-reported counseling was stable or declined slightly from 2016 through 2020, despite increasing prevalence of prepregnancy cardiovascular risk factors and APOs, identifying a key opportunity to improve CVH counseling during the postpartum period with effective and equitable interventions.
eTable 1. Sociodemographic Characteristics of All Individuals With a Live Birth, Those Excluded From the Analysis and the Final Analytic Sample, 2016-2020
eTable 2. Prevalence of Prepregnancy and Pregnancy-Related Cardiovascular Disease Risk Factors Among All Individuals With a Live Birth, Those Excluded From the Analysis, and the Final Analytic Sample, 2016-2020
eTable 3. Average Annual Percent Change (APC) in Self-Reported Cardiovascular Health Counseling From 2016-2019 (Pre-COVID) and 2016-2020 Overall, by Sociodemographic Characteristics, and Peripartum Cardiovascular Disease Risk Factors
eTable 4. Age-Standardized, Weighteda Percent (95% CI) and Average Annual Percent Change (%/y) (95% CI) in Self-Reported Postpartum Cardiovascular Health Counseling by Prepregnancy Cardiovascular Risk Factor and Adverse Pregnancy Outcome Status From 2016-2020
eTable 5. Weighteda Percent (95% CI) and Adjustedb Odds Ratios of Self-Reported, Postpartum Cardiovascular Health Counseling Among Individuals With Cardiovascular Risk Factors and Adverse Pregnancy Outcomes Compared With Those Without Risk Factors, 2016-2020
eTable 6. Weighteda Percent (95% CI) and Average Annual Percent Change (%/y) (95% CI) in Postpartum Cardiovascular Health Counseling by Age, Educational Status, and Postpartum Insurance
eTable 7. Unadjusted Risk Ratios of Self-Reported Postpartum Cardiovascular Health Counseling Among Those With Cardiovascular Disease Risk Factorsa Compared With Those Without Risk Factors by Age, Educational Status, and Postpartum Insurance
Data Sharing Statement
References
- 1.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2022 update. Circulation. Published online January 26, 2022. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 2.Gregory CWE, Danielle ME. Trends and Characteristics in Gestational Diabetes: United States, 2016–2020. National Center for Health Statistics; 2022, doi: 10.15620/cdc:118018. [DOI] [PubMed] [Google Scholar]
- 3.Freaney PM, Harrington K, Molsberry R, et al. Temporal trends in adverse pregnancy outcomes in birthing individuals aged 15 to 44 years in the United States, 2007 to 2019. J Am Heart Assoc. Published online May 18, 2022. doi: 10.1161/JAHA.121.025050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah NS, Wang MC, Freaney PM, et al. Trends in gestational diabetes at first live birth by race and ethnicity in the US, 2011-2019. JAMA. 2021;326(7):660-669. doi: 10.1001/jama.2021.7217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang MC, Freaney PM, Perak AM, et al. Trends in prepregnancy cardiovascular health in the United States, 2011-2019. Am J Prev Cardiol. 2021;7:100229. doi: 10.1016/j.ajpc.2021.100229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron NA, Everitt I, Seegmiller LE, Yee LM, Grobman WA, Khan SS. Trends in the incidence of new-onset hypertensive disorders of pregnancy among rural and urban areas in the United States, 2007 to 2019. J Am Heart Assoc. 2022;11(2):e023791. doi: 10.1161/JAHA.121.023791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah LM, Varma B, Nasir K, et al. Reducing disparities in adverse pregnancy outcomes in the United States. Am Heart J. 2021;242:92-102. doi: 10.1016/j.ahj.2021.08.019 [DOI] [PubMed] [Google Scholar]
- 8.Lloyd-Jones DM, Braun LT, Ndumele CE, et al. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease. Circulation. 2019;139(25):e1162-e1177. doi: 10.1161/CIR.0000000000000638 [DOI] [PubMed] [Google Scholar]
- 9.ACOG committee opinion No. 736: optimizing postpartum care. Obstet Gynecol. 2018;131(5):e140-e150. doi: 10.1097/AOG.0000000000002633 [DOI] [PubMed] [Google Scholar]
- 10.Perak AM, Ning H, Khan SS, et al. Associations of late adolescent or young adult cardiovascular health with premature cardiovascular disease and mortality. J Am Coll Cardiol. 2020;76(23):2695-2707. doi: 10.1016/j.jacc.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang MC, Freaney PM, Perak AM, et al. Trends in prepregnancy obesity and association with adverse pregnancy outcomes in the United States, 2013 to 2018. J Am Heart Assoc. 2021;10(17):e020717. doi: 10.1161/JAHA.120.020717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang MC, Freaney PM, Perak AM, et al. Association of pre-pregnancy cardiovascular risk factor burden with adverse maternal and offspring outcomes. Eur J Prev Cardiol. Published online July 20, 2021. doi: 10.1093/eurjpc/zwab121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanhope KK, Kramer MR. Variation in the content of postpartum visits by maternal race/ethnicity, preconception, and pregnancy-related cardiovascular disease risk, PRAMS, 2016-2017. Public Health Rep. 2022;137(3):516-524. doi: 10.1177/00333549211005814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown HL, Warner JJ, Gianos E, et al. ; American Heart Association and the American College of Obstetricians and Gynecologists . Promoting risk identification and reduction of cardiovascular disease in women through collaboration with obstetricians and gynecologists. Circulation. 2018;137(24):e843-e852. doi: 10.1161/CIR.0000000000000582 [DOI] [PubMed] [Google Scholar]
- 15.Mehta LS, Sharma G, Creanga AA, et al. ; American Heart Association Advocacy Coordinating Committee . Call to action: maternal health and saving mothers. Circulation. 2021;144(15):e251-e269. doi: 10.1161/CIR.0000000000001000 [DOI] [PubMed] [Google Scholar]
- 16.Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women. Circulation. 2021;143(18):e902-e916. doi: 10.1161/CIR.0000000000000961 [DOI] [PubMed] [Google Scholar]
- 17.Shulman HB, D’Angelo DV, Harrison L, Smith RA, Warner L. The Pregnancy Risk Assessment Monitoring System (PRAMS). Am J Public Health. 2018;108(10):1305-1313. doi: 10.2105/AJPH.2018.304563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute . Joinpoint regression program. Accessed May, 25, 2023. https://surveillance.cancer.gov/joinpoint/
- 19.Interrante JD, Admon LK, Carroll C, Henning-Smith C, Chastain P, Kozhimannil KB. Association of health insurance, geography, and race and ethnicity with disparities in receipt of recommended postpartum care in the US. JAMA Health Forum. 2022;3(10):e223292-e223292. doi: 10.1001/jamahealthforum.2022.3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention . Pregnancy Risk Assessment Monitoring System Methodology. Published March 28, 2023. Accessed May 28, 2023. https://www.cdc.gov/prams/methodology.htm
- 21.Stata Corp LP . Stata Statistical Software version 14. Accessed July 26, 2021. https://www.stata.com/stata14/
- 22.Khosla K, Heimberger S, Nieman KM, et al. Long-term cardiovascular disease risk in women after hypertensive disorders of pregnancy. Hypertension. 2021;78(4):927-935. doi: 10.1161/HYPERTENSIONAHA.121.16506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Triebwasser JE, Janssen MK, Sehdev HM. Postpartum counseling in women with hypertensive disorders of pregnancy. Am J Obstet Gynecol MFM. 2021;3(1):100285. doi: 10.1016/j.ajogmf.2020.100285 [DOI] [PubMed] [Google Scholar]
- 24.Beussink-Nelson L, Baldridge AS, Hibler E, et al. Knowledge and perception of cardiovascular disease risk in women of reproductive age. Am J Prev Cardiol. 2022;11:100364. doi: 10.1016/j.ajpc.2022.100364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krukowski RA, Jacobson LT, John J, et al. Correlates of early prenatal care access among US women: data from the Pregnancy Risk Assessment Monitoring System (PRAMS). Matern Child Health J. 2022;26(2):328-341. doi: 10.1007/s10995-021-03232-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boardley DJ, Sargent RG, Coker AL, Hussey JR, Sharpe PA. The relationship between diet, activity, and other factors, and postpartum weight change by race. Obstet Gynecol. 1995;86(5):834-838. doi: 10.1016/0029-7844(95)00283-W [DOI] [PubMed] [Google Scholar]
- 27.Gunderson EP. Childbearing and obesity in women. Obstet Gynecol Clin North Am. 2009;36(2):317-332, ix. doi: 10.1016/j.ogc.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson V, Stumbras K, Caskey R, Haider S, Rankin K, Handler A. Understanding factors associated with postpartum visit attendance and contraception choices: listening to low-income postpartum women and health care providers. Matern Child Health J. 2016;20(suppl 1):132-143. doi: 10.1007/s10995-016-2044-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jowell AR, Sarma AA, Gulati M, et al. Interventions to mitigate risk of cardiovascular disease after adverse pregnancy outcomes: a review. JAMA Cardiol. 2022;7(3):346-355. doi: 10.1001/jamacardio.2021.4391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lloyd-Jones DM, Allen NB, Anderson CAM, et al. Life’s essential 8: updating and enhancing the American Heart Association’s construct of cardiovascular health. Circulation. Published online June 29, 2022. doi: 10.1161/CIR.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellerose M, Rodriguez M, Vivier PM. A systematic review of the qualitative literature on barriers to high-quality prenatal and postpartum care among low-income women. Health Serv Res. 2022;57(4):775-785. doi: 10.1111/1475-6773.14008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Filicko A, Huennekens K, Davis K, et al. Primary care clinician perspectives on patient navigation to improve postpartum care for patients with low income. Womens Health Rep (New Rochelle). 2022;3(1):1006-1015. doi: 10.1089/whr.2022.0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruderman RS, Dahl EC, Williams BR, et al. Provider perspectives on barriers and facilitators to postpartum care for low-income individuals. Womens Health Rep (New Rochelle). 2021;2(1):254-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkins-Haug L, Celi A, Thomas A, Frolkis J, Seely EW. Recognition by women’s health care providers of long-term cardiovascular disease risk after preeclampsia. Obstet Gynecol. 2015;125(6):1287-1292. doi: 10.1097/AOG.0000000000000856 [DOI] [PubMed] [Google Scholar]
- 35.Gogineni VSM, Manfrini D, Aroda SH, et al. Variations in awareness of association between adverse pregnancy outcomes and cardiovascular risk by specialty. Cardiol Ther. 2021;10(2):577-592. doi: 10.1007/s40119-021-00220-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atasoy H, Greenwood BN, McCullough JS. The digitization of patient care: a review of the effects of electronic health records on health care quality and utilization. Annu Rev Public Health. 2019;40:487-500. doi: 10.1146/annurev-publhealth-040218-044206 [DOI] [PubMed] [Google Scholar]
- 37.Nicklas JM, Zera CA, England LJ, et al. A web-based lifestyle intervention for women with recent gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol. 2014;124(3):563-570. doi: 10.1097/AOG.0000000000000420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krist AH, Davidson KW, Mangione CM, et al. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors. JAMA. 2020;324(20):2069-2075. doi: 10.1001/jama.2020.21749 [DOI] [PubMed] [Google Scholar]
- 39.National Institutes of Health; Health Resources and Services Administration; Administration for Children and Families . RFA-HL-22-008: Early Intervention to Promote Cardiovascular Health of Mothers and Children (ENRICH) Multisite Resource and Coordinating Center (U24 Clinical Trial Required). Accessed April 21, 2022. https://grants.nih.gov/grants/guide/rfa-files/RFA-HL-22-008.html
- 40.Dietz P, Bombard J, Mulready-Ward C, et al. Validation of self-reported maternal and infant health indicators in the Pregnancy Risk Assessment Monitoring System. Matern Child Health J. 2014;18(10):2489-2498. doi: 10.1007/s10995-014-1487-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dietz P, Bombard J, Mulready-Ward C, et al. Validation of selected items on the 2003 US standard certificate of live birth: New York City and Vermont. Public Health Rep. 2015;130(1):60-70. doi: 10.1177/003335491513000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deputy NP, Sharma AJ, Bombard JM, et al. Quality of maternal height and weight data from the revised birth certificate and pregnancy risk assessment monitoring system. Epidemiology. 2019;30(1):154-159. doi: 10.1097/EDE.0000000000000936 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Sociodemographic Characteristics of All Individuals With a Live Birth, Those Excluded From the Analysis and the Final Analytic Sample, 2016-2020
eTable 2. Prevalence of Prepregnancy and Pregnancy-Related Cardiovascular Disease Risk Factors Among All Individuals With a Live Birth, Those Excluded From the Analysis, and the Final Analytic Sample, 2016-2020
eTable 3. Average Annual Percent Change (APC) in Self-Reported Cardiovascular Health Counseling From 2016-2019 (Pre-COVID) and 2016-2020 Overall, by Sociodemographic Characteristics, and Peripartum Cardiovascular Disease Risk Factors
eTable 4. Age-Standardized, Weighteda Percent (95% CI) and Average Annual Percent Change (%/y) (95% CI) in Self-Reported Postpartum Cardiovascular Health Counseling by Prepregnancy Cardiovascular Risk Factor and Adverse Pregnancy Outcome Status From 2016-2020
eTable 5. Weighteda Percent (95% CI) and Adjustedb Odds Ratios of Self-Reported, Postpartum Cardiovascular Health Counseling Among Individuals With Cardiovascular Risk Factors and Adverse Pregnancy Outcomes Compared With Those Without Risk Factors, 2016-2020
eTable 6. Weighteda Percent (95% CI) and Average Annual Percent Change (%/y) (95% CI) in Postpartum Cardiovascular Health Counseling by Age, Educational Status, and Postpartum Insurance
eTable 7. Unadjusted Risk Ratios of Self-Reported Postpartum Cardiovascular Health Counseling Among Those With Cardiovascular Disease Risk Factorsa Compared With Those Without Risk Factors by Age, Educational Status, and Postpartum Insurance
Data Sharing Statement