Abstract
We studied 83 cardiac-surgery patients with nasal S. aureus carriage who received 4 intranasal administrations of XF-73 nasal gel or placebo <24 hours before surgery. One hour before surgery, patients exhibited a S. aureus nasal carriage reduction of 2.5 log10 with XF-73 compared to 0.4 log10 CFU/mL for those who received placebo (95% CI, −2.7 to −1.5; P < .0001).
Methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) are the most common causes of healthcare-associated infections in hospitals worldwide. 1 Between 15% and 30% of healthy adults are nasally colonized with MSSA and 1%–3% are colonized with MRSA. 2 S. aureus carriers are at a higher risk of staphylococcal infections after surgery than noncarriers and are up to 9 times more likely to develop surgical-site infections (SSIs). 3,4
In a large, randomized, controlled study comparing topical, nasal mupirocin versus placebo for a variety of surgical procedures, mupirocin prophylaxis decreased the rate of S. aureus SSIs among S. aureus carriers: 3.7% in mupirocin group versus 5.9% in controls. 5 A consensus of the Surgical Infection Society (SIS), Infectious Disease Society of America (IDSA), and the Society for Healthcare Epidemiology for America (SHEA) recommends nasal decolonization in those with documented S. aureus colonization for high-risk cardiac and orthopedic surgeries. 6
Although not a formal indication, mupirocin is used as a topical antibiotic prior to surgery for nasal decolonization of MSSA and MRSA, worldwide. 7 Implementation is affected by challenges with screening, ordering and administration, course duration, mupirocin resistance, and issues with adherence because it requires a multidisciplinary approach coupled with patient education. 4,7
Exeporfinium chloride (XF-73) is a dicationic porphyrin derivative with rapid potent bactericidal properties and a low propensity for engendering bacterial resistance. 8,9 It is being developed as a gel for nasal S. aureus decolonization to prevent postoperative staphylococcal SSIs. This phase 2 study was designed to assess microbiological efficacy and safety of XF-73 in reducing nasal S. aureus prior to open-chest cardiac surgery.
Methods
In this multicenter, randomized, placebo-controlled, phase 2 study, we assessed the effect of nasal XF-73 versus placebo on S. aureus nasal burden in patients undergoing cardiac surgery (Clinical trial identifier: NCT03915470). The study was conducted between August 29, 2019, and March 29, 2021, under good clinical practice guidelines (ICH E6 GCP) with all regulatory and ethical approvals in place. We included male and female patients, aged 18–75 years, who were due to undergo cardiac surgery. Patients were initially screened using a PCR assay (Cepheid Xpert S. aureus Nasal Complete Assay, Cepheid, Sunnyvale, CA) to identify nasal S. aureus carriage to be eligible to participate.
Patients were randomized (1:1) to receive 0.2% (w/w) XF-73 or color-matched placebo as 3 doses (ie, the primary end point), 1 dose immediately before cardiac surgery, and a fifth dose after surgery (ie, 5 doses in total). Of the 5 doses, 4 were administered <24 hours prior to surgery. Destiny staff trained research staff in nasal swab collection and dose administration; nasal swab cultures were obtained just prior to the next application of XF-73 or placebo by research staff. All nasal swabs were analyzed in a central laboratory within each country and the same techniques were followed in all laboratories. Nasal swabs were plated onto BBL ChromAgar plates. S. aureus colonies were converted to log10 CFU/mL to measure nasal burden, at baseline and immediately before and after surgery. Perioperative antibiotic use and preoperative antiseptic skin decolonization were left up to each center’s standard of care and were recorded. Antiseptic skin decolonization refers to “full-body skin decolonization” implemented preoperatively (eg, at home or on the inpatient unit prior to arriving to the operating room), with at least 1 application.
The primary end point of interest in this study was change in S. aureus log10 CFU/mL from baseline to 1 hour before surgery (after 3 doses), in the micro-ITT set (ie, patients with confirmed S. aureus nasal carriage at baseline). Safety measures included adverse events; ear, nose, and throat (ENT) examinations; and a brief smell-identification test (B-SIT, Sensonics International, NJ). Primary analyses of change in log10 CFU/mL from baseline to 1 hour pre-surgery were performed using an analysis of covariance (ANCOVA) model including baseline log10 CFU/mL as a covariate.
Results
Patients were recruited in the countries of Georgia (89.2%, 9 centers), Serbia (7.2%, 2 centers), and the United State (3.6%, 2 centers). Most of these patients were male (75.9%). The most common surgeries were coronary artery bypass graft (63.8%), mitral valve replacement and/or repair (16.9%), and aortic valve replacement (16.9%). A foreign implant was placed in 39.8% of patients in the micro-ITT primary efficacy set; 100% of patients received all 5 doses. MSSA colonization occurred in 96.8% of patients, and 3.2% had MRSA.
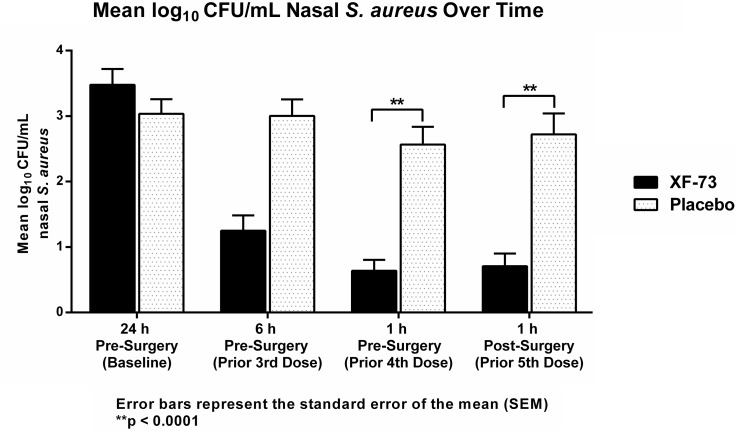
The baseline nasal S. aureus log10 CFU/mL were similar in both groups (Fig. 1). After 3 applications of XF-73 nasal gel over <24 hours (1 hour prior to surgery, the primary end point), we detected a greater decrease in nasal S. aureus observed in the XF-73 arm (−2.842 log10 CFU/mL) versus placebo (−0.469 log10 CFU/mL). The adjusted least-squares mean difference between the 2 groups was −2.1 log10 CFU/mL and was statistically significant (95% CI, −2.7 to −1.5; P < .0001).
Fig. 1.
Change in burden of nasal S. aureus before and after surgery. Note. CFU, colony-forming units; h, hour.
Within 1 hour of incision closure, the decrease in S. aureus was also significantly greater in the XF-73 cohort compared to the placebo group, with a least-squares mean difference of −2.2 log10 CFU/mL (95% CI, −2.7 to −1.6; P < .0001) (Fig. 1, postsurgery).
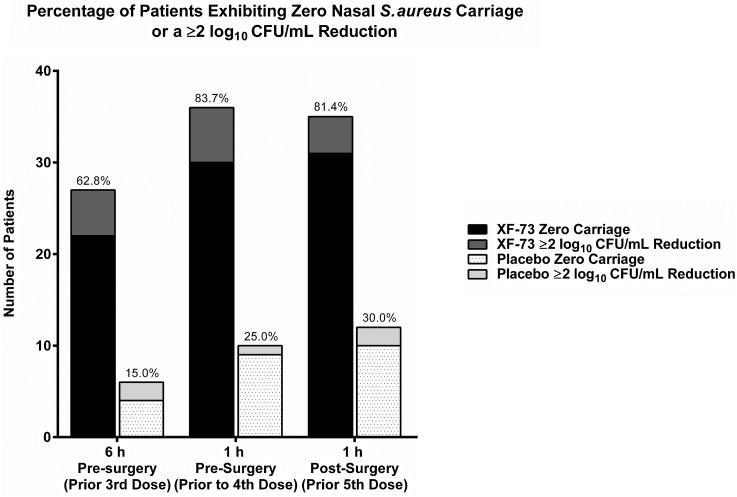
When assessing the percentage of patients exhibiting zero nasal S. aureus carriage, (decolonization), or a ≥2 log10 CFU/ml reduction, 83.7% of patients treated with XF-73 met this metric within 1 hour prior to surgery (Fig. 2). For antiseptic skin decolonization, 12 of 13 centers used chlorhexidine gluconate, benzalkonium chloride, or “other” as their standard of care. One site recorded no skin decolonization.
Fig. 2.
Percentage of patients with zero nasal S. aureus carriage or ≥2 log10 CFU/mL reduction. Note. CFU, colony-forming units; h, hour.
No SSIs were identified during the study or follow-up periods of 1 month or 3 months (for those with a foreign implant). Overall, 95.2% of patients received prophylactic skin decolonization preoperatively.
No treatment-emergent adverse events (TEAEs), laboratory or other safety findings were considered related to either arm in the 124 patients evaluated as the safety population. In the XF-73 and placebo groups, respectively, the most frequently reported TEAEs were pleural effusion (16 and 14 patients), anemia (5 and 6 patients), pericardial effusion (5 and 5 patients), and atrial fibrillation (5 and 4 patients).
No local reactions at the application site (ie, anterior nares) occurred according to an ENT specialist and there were no significant changes to sense of smell (B-SIT) during the study.
Discussion
Administration of XF-73 nasal gel <24 hours prior to surgery rapidly and significantly reduced nasal S. aureus burden preoperatively. The primary end point of this phase 2 study was met: XF-73 nasal gel reduced nasal S. aureus burden from baseline to 1 hour prior to surgery, with a highly statistically significant reduction of 2.1 log10 CFU/mL more than placebo. No safety or tolerability issues were identified.
The most widespread topical antibiotic recommended within SSI prevention guidelines for nasal S. aureus decolonization is mupirocin. However, guidelines also carry a warning to limit widespread use of mupirocin, due to concerns of generating mupirocin-resistant strains of S. aureus, which could dominate. 7 Global reports indicate that mupirocin-resistant S. aureus prevalence has increased to 7.6% and mupirocin-resistant MRSAs have significantly increased to 13.8%. 10 In this study, XF-73 nasal gel had a favorable safety profile in this patient population without any TEAE’s related to the study drug nor placebo. No postoperative SSIs were observed in either study arm.
This study had several limitations. Infection prevention training was not standardized across countries; a large proportion of the recruitment population was from Georgia; and only those patients with intravascular implants were followed for 90 days.
The advantages of XF-73 nasal gel include (1) a short presurgical dosing period required, (2) rapidity of S. aureus decolonization, and (3) previously demonstrated remote likelihood of generating staphylococcal strains that are resistant to XF-73. 9
Acknowledgments
Medical writing support was provided by Scinopsis Ltd (Brighton, UK).
Financial support
The study was funded by Destiny Pharma (Brighton, UK).
Conflicts of interest
J.P.L., W.G.L., and W.R.W. are employed by Destiny Pharma. J.G.M. was formerly an employee of Destiny Pharma and was funded to attend the ECCMID 2021 conference. W.G.L. has stock and/or stock options in Destiny Pharma, and J.G.M. formerly held stock while he was an employee. A.D. received consultancy fees from Destiny Pharma for statistical consultancy and support for the present study. J.E.M. and M.S.F. received funding from the Sponsor to serve on the data monitoring committee for the present study in 2020 and 2021. R.K.C.M. has leadership roles on the Thoracic Surgery Directors Association Executive Committee and the STS Workforce on E-Learning.
References
- 1. Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG, Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015;28:603–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Septimus EJ. Nasal decolonization: what antimicrobials are most effective prior to surgery? Am J Infect Control 2019;47 suppl 1:A53–A57. [DOI] [PubMed] [Google Scholar]
- 3. Kalmeijer MD, van Nieuwland-Bollen E, Bogaers-Hofman D, de Baere GA. Nasal carriage of Staphylococcus aureus is a major risk factor for surgical-site infections in orthopedic surgery. Infect Control Hosp Epidemiol 2000;21:319–323. [DOI] [PubMed] [Google Scholar]
- 4. Humphreys H, Becker K, Dohmen PM, et al. Staphylococcus aureus and surgical site infections: benefits of screening and decolonization before surgery. J Hosp Infect 2016;94:295–304. [DOI] [PubMed] [Google Scholar]
- 5. Perl TM, Cullen JJ, Wenzel RP et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med 2002;346:1871–1877. [DOI] [PubMed] [Google Scholar]
- 6. Bratzler DW, Dellinger EP, Olsen KM, et al. American Society of Health-System Pharmacists (ASHP), Infectious Diseases Society of America (IDSA), Surgical Infection Society (SIS), & Society for Healthcare Epidemiology of America (SHEA). Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect 2013;14:73–156. [DOI] [PubMed] [Google Scholar]
- 7. Global Guidelines for the Prevention of Surgical Site Infection, Second Edition. Geneva: World Health Organization; 2019.
- 8. Ooi N, Miller K, Hobbs J, et al. XF-73, a novel antistaphylococcal membrane-active agent with rapid bactericidal activity. J Antimicrob Chemother 2009;64:735–740. [DOI] [PubMed] [Google Scholar]
- 9. Farrell DJ, Robbins M, Rhys Williams W, Love WG. Investigation of the potential for mutational resistance to XF-73, retapamulin, mupirocin, fusidic acid, daptomycin, and vancomycin in methicillin-resistant Staphylococcus aureus isolates during a 55-passage study. Antimicrob Agents Chemother 2011;55:1177–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dadashi M, Hajikhani B, Darban-Sarokhalil D, van Belkum A, Goudarzi M. Mupirocin resistance in Staphylococcus aureus: a systematic review and meta-analysis. J Glob Antimicrob Resist 2020;20:238–247. [DOI] [PubMed] [Google Scholar]