Abstract
The conclusions of EFSA following the peer review of the initial risk assessments carried out by the Assessment Group on Glyphosate (AGG), consisting of the competent authorities of France, the Netherlands, Sweden and Hungary, acting jointly as rapporteur Member State for the pesticide active substance glyphosate are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012. The conclusions were reached on the basis of the evaluation of the representative uses of glyphosate as a herbicide as proposed by the applicants, covering uses pre‐sowing, pre‐planting and pre‐emergence plus post‐harvest in vegetables and sugar beet; post‐emergence of weeds in orchards, vineyards, row vegetables, railway tracks against emerged annual, biennial and perennial weeds. Moreover, uses as spot treatment against invasive species in agricultural and non‐agricultural areas, and in vegetables and sugar beet against couch grass are also included. The reliable endpoints, appropriate for use in regulatory risk assessment, are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are reported where identified.
Keywords: glyphosate, peer review, risk assessment, pesticide, herbicide
Summary
Commission Implementing Regulation (EU) No 844/2012 lays down the procedure applicable for the renewal of the approval of glyphosate submitted under Article 14 of Regulation (EC) No 1107/2009. Glyphosate is covered under the fifth stage of the renewal work programme (AIR V). By Commission Implementing Regulation (EU) 2019/724 amending Commission Implementing Regulation (EU) No 686/2012, on 10 May 2019, four Member States (France, Hungary, the Netherlands and Sweden) were appointed to act jointly as rapporteurs for the assessment of the application for renewal of the approval for glyphosate. The four Member States formed the Assessment Group on Glyphosate (AGG) and jointly assumed the role of the rapporteur Member State (RMS).
In accordance with Article 1 of Regulation (EU) No 844/2012, an application for the renewal of the approval for glyphosate was submitted by the deadline of 15 December 2019 by a consortium of 8 companies 1 – the Glyphosate Renewal Group (GRG).
An initial evaluation of the dossier on glyphosate was provided by the four RMSs of the AGG in the renewal assessment report (RAR) and subsequently, a peer review of the pesticide risk assessment on the RMS evaluation was conducted by EFSA in accordance with Article 13 of Commission Implementing Regulation (EU) No 844/2012.
For glyphosate, the formal assessment of the proposal for harmonised classification and labelling in accordance with Regulation (EC) No 1272/2008 has been conducted by the European Chemicals Agency (ECHA) in parallel to the EFSA peer review. When carrying out the risk assessment in the framework of the peer review, EFSA adopted ECHA's hazard assessment and the conclusions of the ECHA Committee for Risk Assessment (RAC) on harmonised classification and labelling delivered in their Opinion on 30 May 2022 (ECHA, 2022).
The following overall conclusions were derived by the peer review.
The representative uses of glyphosate proposed at EU level were a herbicide applied as a foliar spray to target weeds when growing vegetables, sugar beet, in orchards, in vineyards, on railway tracks, on fallow agricultural and non‐agricultural land. These uses result in a sufficient herbicidal efficacy against the target emerged annual weeds, emerged perennial and biennial weeds, giant hogweed and Japanese knotweed, and couch grass.
The assessment of the data package revealed no issues that could not be finalised or that needed to be included as critical areas of concern with respect to identity, physical–chemical and technical properties of the active substance and the formulation for representative uses, and analytical methods.
In the area of mammalian toxicology and non‐dietary exposure, no critical areas of concern were identified. The assessment of the reference specification could not be finalised since one of the impurities showed a potential for clastogenicity in an in vitro chromosome aberration test that was not appropriately followed up in vivo. This impurity was present in some of the batches used in toxicity studies at levels representative of the proposed reference specification, however a maximum level for this impurity cannot be established while this issue is not clarified. There were no indications of acute toxicity or genotoxicity in studies performed with the formulation for representative uses ‘MON 52276’. Toxicological studies were available for all co‐formulants but one (present in significant amount in the final formulation), for which repeated‐dose toxicity information over short‐ and long term was not available. In order to reach a final conclusion on the risk assessment of ‘MON 52276’, repeated‐dose toxicity data for this component should be assessed.
In the area of residues, the consumer risk assessment could not be finalised. Although preliminary results indicated residues in rotational crops above the limit of quantification, the number of rotational crop field trials was insufficient to address all relevant scenarios. Therefore, a higher consumer exposure to residues of glyphosate than the one considered in the current risk assessment cannot be excluded. However, it is not expected that this might lead to an exceedance of the toxicological reference values. Therefore, no critical concern was identified.
The data available on environmental fate and behaviour were sufficient to carry out the required environmental exposure assessments at EU level for the representative uses. In some small hydrological catchments and some larger river systems, the route of groundwater exposure via bank infiltration and the connectivity of surface water bodies to groundwater aquifers may be relevant. Therefore, further information would be useful for assessors in national regulatory competent authorities to assess groundwater concentrations that may result from this exposure pathway. However, the groundwater exposure assessment was finalised for most typical small hydrological catchments and most typical larger river systems, where the connectivity of surface water bodies to groundwater aquifers is limited.
The assessment of the data package revealed no issues that could not be finalised or that needed to be included as critical areas of concern with respect to ecotoxicology for the representative uses assessed. A high long‐term risk to mammals was concluded for 12 of the 23 representative uses based on tier 1 assumptions. Suitable data to refine the risk assessment were not available. The assessment for aquatic macrophytes, when contact exposure via spray drift occurs, could not be finalised. Insufficient information was provided to draw a firm conclusion on the impact to biodiversity via indirect effects and trophic interactions for the representative uses. In addition, the experts acknowledged the lack of harmonised methodologies and agreed specific protection goals, and that the risks for biodiversity are complex and depend on multiple factors.
Studies reporting effects on microbiome were considered and taken into account for the risk assessment in the areas of mammalian toxicology and ecotoxicology. Currently, no internationally agreed guidelines for the risk assessment of microbiome are in place in the pesticide area. Further research in the field of microbiome is needed to understand its relevance for risk assessment and to develop dedicated strategies and methodologies accordingly.
Following the assessment based on the available evidence, glyphosate does not meet the criteria for endocrine disruption as laid down in points 3.6.5 and 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) No 2018/605.
Background
Commission Implementing Regulation (EU) No 844/2012 2 (hereinafter referred to as ‘the Regulation’), lays down the provisions for the procedure of the renewal of the approval of active substances, submitted under Article 14 of Regulation (EC) No 1107/2009 3 . This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States (MSs), the applicant(s) and the public on the initial evaluation provided by the rapporteur Member State (RMS) and/or co‐rapporteur Member State (co‐RMS) in the renewal assessment report (RAR), and the organisation of an expert consultation where appropriate.
In accordance with Article 13 of the Regulation, unless formally informed by the European Commission that a conclusion is not necessary, EFSA is required to adopt a conclusion on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 within 5 months from the end of the period provided for the submission of written comments, subject to an extension of an additional 3 months where additional information is required to be submitted by the applicant(s) in accordance with Article 13(3).
Glyphosate is covered under the fifth stage of the renewal work programme (AIR V). By Commission Implementing Regulation (EU) 2019/724 4 amending Commission Implementing Regulation (EU) No 686/2012 5 , on 10 May 2019, four MSs (France, Hungary, the Netherlands and Sweden) were appointed to act jointly as rapporteurs for the assessment of the application for renewal of the approval for glyphosate. The four MSs formed the Assessment Group on Glyphosate (AGG) and jointly assumed the role of the RMS.
In accordance with Article 1 of the Regulation, an application for the renewal of the approval for glyphosate has been submitted by the deadline of 15 December 2019 by a consortium of 8 companies 1 – the Glyphosate Renewal Group (GRG).
On 8 June 2020, a supplementary dossier for renewal of the approval for glyphosate had been submitted by the GRG to the four RMSs of the AGG. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and on 18 August 2020 informed the applicants (GRG), the European Commission and EFSA about the admissibility.
The RMS provided its initial evaluation of the dossier on glyphosate in the RAR, which was received by EFSA on 15 June 2021 (AGG, 2021).
In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the MSs and the applicants, the GRG, for consultation and comments on 23 September 2021. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 24 November 2021. At the same time, the collated comments were forwarded to the RMS for compilation and evaluation in the format of reporting tables. In addition, the applicants were invited to respond to the comments received. The comments and the applicants' response were evaluated by the RMS in column 3 of the reporting tables.
The need for expert consultation and the necessity for additional information to be submitted by the applicants in accordance with Article 13(3) of the Regulation were considered in a telephone conference between EFSA, the RMS and the European Commission on 9 February 2022. On the basis of the comments received, the applicants' response to the comments and the RMS' evaluation thereof, it was concluded that additional information should be requested from the applicants, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology, residues, environmental fate and behaviour and ecotoxicology.
The outcome of the telephone conference, together with EFSA's further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting tables. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an experts' consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation tables, together with the outcome of the experts' consultation and the written consultation on the assessment of additional information, were reported in the final column of the evaluation tables.
A final consultation on the conclusions arising from the peer review of the risk assessment took place with MSs via a written procedure in May 2023.
This conclusion report summarises the outcome of the peer review of the risk assessment of the active substance and the formulation for representative uses, evaluated on the basis of the representative uses of glyphosate as a herbicide as proposed by the applicants, covering uses as pre‐sowing, pre‐planting and pre‐emergence plus post‐harvest in vegetables and sugar beet; post‐emergence of weeds in orchards, vineyards, row vegetables, railway tracks against emerged annual, biennial and perennial weeds. Moreover, uses as spot treatment against invasive species in agricultural and non‐agricultural areas, and in vegetables and sugar beet against couch grass are also included in the EU peer review.
In accordance with Article 12(2) of Regulation (EC) No 1107/2009, risk mitigation options identified in the RAR and considered during the peer review, if any, are presented in the conclusion.
A list of the relevant end points for the active substance and the formulation for representative uses is provided in Appendix B. In addition, the considerations as regards the cut‐off criteria for glyphosate according to Annex II of Regulation (EC) No 1107/2009 are summarised in Appendix A.
A key supporting document to this conclusion is the Peer Review Report (EFSA, 2023a), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the RAR;
the reporting tables (17 February 2022);
the evaluation tables (July 2023);
the reports of the scientific consultation with MS experts, including their Annexes where relevant;
the comments received on the assessment of the additional information;
the comments received on the draft EFSA conclusion.
Given the importance of the RAR, including its revisions (AGG, 2023), and the Peer Review Report, both documents are considered as background documents to this conclusion and thus are made publicly available. In addition, the list of newly available publications on glyphosate brought to EFSA's attention after the public consultation phase until the time point of drafting the EFSA conclusion, and screened for potential impact on the risk assessment, is also made publicly available as part of the background documentation to the conclusion (EFSA, 2023b).
It is recommended that this conclusion and its background documents would not be accepted to support any registration outside the EU for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulation for representative uses
Glyphosate is the ISO common name for N‐(phosphonomethyl)glycine (IUPAC).
The formulation for representative uses for the evaluation was ‘MON 52276’, a soluble concentrate (SL) containing 360 g/L of glyphosate as isopropylammonium salt (IUPAC name isopropylammonium N‐(phosphonomethyl)glycinate) (486 g/L), plus co‐formulants.
The representative uses evaluated are:
pre‐sowing, pre‐planting and pre‐emergence applications by tractor‐mounted broadcast spraying in vegetables (root, tuberous, bulb, fruit‐vegetable, Brassica, leaf and stem) and sugar beet against emerged annual, biennial and perennial weeds;
post‐harvest, pre‐sowing and pre‐planting applications by tractor‐mounted broadcast spraying in vegetables (root, tuberous, bulb, fruit‐vegetable, Brassica, leaf and stem) and sugar beet against emerged annual, biennial and perennial weeds and cereal volunteers;
post‐emergence of weeds inter‐row application by ground‐directed, fully shielded (hooded) spraying in vegetables (root, tuberous, bulb, fruit‐vegetable, legume and leaf vegetables) against emerged annual, biennial and perennial weeds;
post‐emergence of weeds in‐row band application by ground‐directed, fully shielded (hooded) spraying in orchards (citrus, stone and pome fruits, kiwi, nut, banana and table olives) and vines (table and wine grape, leaves not intended for human consumption) against emerged annual, biennial and perennial weeds;
train spray applications directed on railway tracks against emerged annual, biennial and perennial weeds;
post‐emergence‐shielded spot treatment spray applications against invasive species (giant hogweed and Japanese knotweed) in agricultural and non‐agricultural areas, and against couch grass in vegetables (root, tuberous, bulb, fruit‐vegetable, Brassica, leaf and stem vegetable) and sugar beet for post‐harvest, pre‐sowing and pre‐planting applications.
Full details of the Good Agricultural Practices (GAPs) can be found in the list of end points in Appendix B.
Data were submitted to conclude that the representative uses of glyphosate proposed at EU level result in a sufficient herbicidal effect following the guidance document SANCO/2012/11251‐rev. 4 (European Commission, 2014b).
The information on the active substance, co‐formulants and isopropylammonium counter ion declared in the formulation for representative uses has all been considered for the assessments during the peer review.
As regards the literature search carried out by the applicants, there is evidence that the exclusion criteria for relevance of literature used by the applicants at the rapid screening were not properly applied, as also noted by the RMS. Reasons for having excluded several of the ecotoxicology‐related publications identified by the literature search at the rapid screening step seemed not pertinent after reading the title and/or abstract. However, where subsequently identified as potentially relevant, these publications were added to the RAR and further assessed. Overall, considering that the public consultation also resulted in available scientific literature being assessed also from a broader time frame than that required by the regulatory framework, EFSA concludes that it is unlikely that relevant evidence from the peer‐reviewed scientific literature has been missed by the peer review.
Conclusions of the evaluation
1. Identity, physical/chemical/technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion: European Commission (2000b, 2010, 2012).
An updated common EU reference specification was proposed by the RMS and the GRG comprising of eight applicants. The proposed common reference specification was based on batch data from industrial plant productions. The proposed minimum purity of the active substance as manufactured is 950 g/kg (the minimum purity for individual sources ranged from 950 to 990 g/kg). The technical grade active ingredient was manufactured in the majority of cases as a technical material (TC), but also as a technical concentrate (TK). Based on the data submitted in support of the renewal of approval process, an update of the common EU reference specification is proposed (i.e. two additional relevant impurities were identified: triethylamine and formic acid, and some of the significant impurities were deleted from the specification). N‐nitroso‐glyphosate (NNG), formaldehyde, triethylamine and formic acid were considered relevant impurities at levels of < 1 mg/kg, < 1 g/kg, ≤ 2 g/kg and ≤ 4 g/kg, respectively (see Section 2). It is noted that the toxicological relevance of one impurity is inconclusive (see Section 2); hence additional data consisting of spectral data, content of the impurity before and after storage of the formulation and method for its analysis in the formulation might be required. The current and the proposed common reference specifications cannot be concluded as sufficiently supported by the toxicological information available, whilst the genotoxicity profile of one impurity needs clarification (see Section 2). The proposed reference specification is supported by the batches used in the ecotoxicological studies (see Section 5).
The proposed minimum purity of 950 g/kg met the requirements of the FAO specification 284/TC (2016), covering glyphosate technical materials of Monsanto, Cheminova, Syngenta and Helm. It should be noted that the FAO specification contains only NNG and formaldehyde as relevant impurities, with a higher specification level of 1.3 g/kg for formaldehyde.
For each source, an individual technical specification was derived based on the batch data submitted for the renewal. The RMS compared each individual source specification to the newly proposed EU reference specification according to the criteria given in the guidance document SANCO/10597/2003 rev. 10.1 (European Commission, 2012) and concluded that they were equivalent except from some sources, however EFSA notes that this equivalence check should be considered as provisional for all sources due to the inconclusive toxicological relevance of an impurity (see Section 2). Batch data were not submitted by applicant Ciech Sarzyna, therefore no further consideration could be made.
Some data gaps relevant to the specifications and batch analysis were set (see Section 10).
The main data regarding the identity of glyphosate and its physical and chemical properties are given in Appendix B. A data gap for n‐octanol/water partition coefficient for the metabolite N‐acetyl AMPA was identified. A data gap was also set for determination of the content of the relevant impurities: formic acid and triethylamine before and after 2‐year storage at ambient temperature of the formulation for representative uses (see Section 10).
In general, adequate methods are available for the generation of data required for the risk assessment, except for specific plant residue studies for which EFSA considers that the efficiency for the extraction procedure used was not addressed according to SANTE/2017/10632 (European Commission, 2022). 6 The RMS disagrees. In addition, a data gap for validation data for the method used in a toxicological study was identified (see Sections 2 and 10).
Appropriate methods of analysis are available for the determination of the active substance and impurities in the technical material, and for the determination of the active substance and the relevant impurities formaldehyde, NNG, triethylamine and formic acid in the formulation for representative uses. Pending on the outcome of the data gap on toxicological data on a component of a co‐formulant (see Sections 2 and 10), a method for its determination in the formulation might be required at MS level.
Appropriate liquid chromatography with tandem mass spectrometry (LC–MS/MS) methods are available for monitoring the components of the residue definition for food and feed of plant origin, with limits of quantification (LOQs) of 0.025 mg/kg for glyphosate, (aminomethyl)phosphonic acid (AMPA) and N‐acetyl glyphosate in all representative commodity groups. It should be noted that different options for the residue definition for enforcement for plant matrices are proposed to risk managers for consideration (see Section 3).
Residues of glyphosate and N‐acetyl glyphosate can be monitored in food of animal origin by the LC–MS/MS method with LOQs of 0.025 mg/kg in meat, milk, egg, liver, kidney and fat, respectively. Residues of glyphosate and AMPA in honey can be determined by the LC–MS/MS method with a LOQ of 0.025 mg/kg for each analyte. However, N‐acetyl glyphosate was also included in the residue definition for monitoring in honey; therefore, a validated monitoring method for N‐acetyl glyphosate residues in honey is needed (data gap, see Section 10). It is noted that different options for the residue definition for enforcement in honey are proposed to risk managers for consideration (see Section 3).
The residue definition for monitoring in soil was defined as glyphosate and AMPA. The compounds of the residue definition in soil can be monitored by LC–MS/MS, with LOQs of 0.05 mg/kg for both compounds. An appropriate LC–MS/MS method is available for monitoring residues of glyphosate and AMPA in groundwater, drinking water and surface water with LOQs of 0.03 μg/L for both substances. Residues of glyphosate in air can be monitored by gas chromatography–mass spectrometry (GC–MS) with a LOQ of 5 μg/m3.
Residues of glyphosate and AMPA in body fluids can be monitored by LC–MS/MS with LOQs of 0.01 mg/L, while residues of glyphosate and AMPA in tissues can be determined by the LC–MS/MS method with LOQs of 0.025 mg/kg for each analyte.
2. Mammalian toxicity
The toxicological profile of glyphosate and its metabolites was discussed at the Pesticides Peer Review Experts' Teleconference (TC) 80 in November–December 2022. The following guidance documents were followed in the production of this conclusion: European Commission (2003, 2012), EFSA (2014b), EFSA PPR Panel (2017), EFSA (2022) and ECHA (2017).
The assessment relies on studies submitted by the applicants and carried out according to internationally agreed guidelines and quality standards, as well as on relevant studies from peer reviewed scientific literature. Studies using formulated products other than the one for the representative uses as test material were considered for their reliability and relevance, and discussed as part of the weight of evidence (WoE) in the risk assessment for the active substance and the formulation for representative uses.
Regarding the proposed reference specification, the impurities N‐nitroso‐glyphosate (NNG), formaldehyde, triethylamine and formic acid are identified as relevant (see Section 1) based on their hazard properties, as classified according to Annex VI of Regulation (EC) No 1272/2008 7 (CLP Regulation). Regarding the other impurities occurring in batches from the different manufacturing sources, none were found to be relevant, except for one impurity, which showed a potential for clastogenicity in an in vitro chromosome aberration test that was not appropriately followed up in vivo. Therefore, the toxicological relevance for this impurity is inconclusive (data gap, see Section 9.1). This impurity was present in some of the batches used in toxicity studies at levels representative of the proposed reference specification, however its maximum level in any of the specifications cannot be established while its genotoxicity profile has not been clarified. Accordingly, the assessment of any reference specification cannot be finalised (see Section 9.1). 8 The RMS disagrees with this conclusion and considers the genotoxic potential not to be of toxicological concern at the level of the proposed reference specification, since the impurity was present at a 7‐fold higher level than that proposed for the reference specification in one in vivo micronucleus test performed with glyphosate. It is noted that the relevance assessment of the impurities was based on toxicological studies and quantitative structure–activity relationship (QSAR) analysis; a detailed summary of the QSAR assessment has not been provided by the applicants and was identified as a data gap (see Section 10). Another data gap was identified for clarification on the composition of some of the batches used in the toxicological studies (see Section 10).
The analytical methods used in feed, body fluids and tissues, air and any additional matrices in support of the critical toxicity studies used to set reference values are overall considered fit‐for‐purpose (see Section 1). A data gap was identified due to the lack of the analytical report including information on the analytical method validation in a toxicological study (see Sections 1 and 10).
The oral absorption of glyphosate is estimated to account for 20% of the administered doses (in the range between 1 and 10 mg/kg body weight (bw)). Excretion occurs predominantly through faeces and to a lesser extent in urine and it is almost completed within 48 h; biliary and pulmonary routes of elimination are negligible. In rats, glyphosate is rapidly distributed, with the highest levels being reached in bones, kidneys and liver; the evidence does not suggest bioaccumulation in mammals. 9 The metabolism of glyphosate is limited; less than 1% of the parent compound is eliminated as AMPA and major rat metabolites were not detected in the available studies. Based on comparative in vitro metabolism, major metabolic interspecies (mouse, rat, rabbit, dog) differences were not observed and unique human metabolites were not identified.
The residue definition for body fluids and tissues consists of glyphosate and AMPA.
Glyphosate has low acute toxicity by the oral, dermal and inhalation exposure routes. Clinical signs including diarrhoea, reduced activity, ataxia, piloerection, convulsions, and hunched posture were observed in rats and mice only following acute oral exposure to > 2,000 mg/kg bw. Glyphosate does not have skin irritating or sensitising properties. It is a severe eye irritant (ECHA, 2022). Testing for phototoxicity is not required for glyphosate in accordance with data requirement provisions stipulated in Commission Regulation (EU) No 283/2013. The currently available data do not give rise to any concern between glyphosate exposure and respiratory health effects (i.e. irritation and sensitisation). 10 The ECHA Committee for Risk Assessment (RAC) (ECHA, 2022) concluded that there were no clear human data to support classification for respiratory tract irritation and no specific data which clearly indicated respiratory tract irritation in studies with animals. For respiratory sensitisation, RAC considered that no classification is warranted based on insufficient data.
Many short‐term oral toxicity studies were provided for rats, mice and dogs. The dog and the rat were the most sensitive species, followed by the mouse. Common target organs/critical effects for toxicity included the gastrointestinal tract, decreased body weight gain and reduced food consumption, and changes in clinical chemistry including increased alanine aminotransferase (ALT) and alkaline phosphatase (ALP) in plasma, possibly indicative of altered liver metabolism. Effects in salivary glands, consisting of cellular alterations in the parotid gland (basophilic staining of the cytoplasm and hypertrophy not associated with degeneration/necrosis inflammatory conditions and not progressing to preneoplastic lesions in long‐term studies), were observed in rodents. They were considered as a local effect of unclear adversity based on the nature of the histopathological characteristics with lack of clinical correlates in rodents and of unclear human relevance. 11 The relevant short‐term no observed adverse effect level (NOAEL) in dog is 53 mg/kg bw per day based on decreased food consumption, increased gamma‐glutamyl transferase (GGT), increased ALP and bilirubin at the lowest observable adverse effect level (LOAEL) of 252 mg/kg bw per day in a 90‐day repeated dose toxicity study. In rats, the relevant short‐term oral NOAEL is 79 mg/kg bw per day, based on effects on the caecum (i.e., mucosal atrophy) and increased ALP reported at the LOAEL of 730 mg/kg bw per day in a 90‐day repeated dose toxicity study. The relevant short‐term oral NOAEL in mice is 1,221 mg/kg bw per day derived from a 90‐day repeat dose toxicity study, based on decreased food consumption, liver effects (increased ALP), caecum (distension not accompanied by histopathological changes) and increased incidence of cystitis in the urinary bladder, reported at the LOAEL of 6,295 mg/kg bw per day.
Glyphosate is unlikely to be genotoxic based on a WoE approach 12 ; this is in line with ECHA RAC assessment (ECHA, 2022).
After long‐term exposure, target organs/critical effects regarding toxicity included the gastro‐intestinal tract, salivary glands (local effects), eyes, liver and lungs in rats; and reduced body weight gain and urinary bladder in mice, with higher dose levels producing liver and kidney lesions, stomach cysts and increased mortality in mice. 13 The relevant long‐term NOAEL is 59.4 mg/kg bw per day based on increased incidences of liver (small livers, focal haemorrhage small cyst, and pale and mottled appearance) and lung (emphysema, collapse, petechiae and ecchymoses) lesions, increased ALP and cataracts observed at the LOAEL of 595.2 mg/kg bw per day in a 2‐year study in rats. 14 Lower LOAELs were identified ranging from 300 to 362 mg/kg bw per day in other long‐term studies for stomach mucosal irritation, 15 increased caecum weight, clinical chemistry (increase ALP) and decreased adrenal weight. Glyphosate may induce oxidative stress as shown in some in vitro and in vivo studies, but increased oxidative stress was not consistently demonstrated in the available studies. Regarding epidemiological studies investigating oxidative stress endpoints, a conclusion could not be drawn on the possible relationship between glyphosate exposure and changes in oxidative stress parameters based on the limited database and outcome from available human observational studies. 16 Based on all the available evidence, it was agreed that glyphosate is not carcinogenic in rats up to the highest dose level tested of 1,214 mg/kg bw per day in males and 1,498 mg/kg bw per day in females. In the mouse studies, no carcinogenic effects were seen up to 988 mg/kg bw per day in males and 1,081 mg/kg bw per day in females. 17 The currently available human epidemiological studies do not provide conclusive evidence that glyphosate exposure is associated with any cancer‐related health effect. 18 ECHA RAC concluded that glyphosate is unlikely to be carcinogenic for humans (ECHA, 2022).
With regard to reproductive toxicity studies, the relevant reproductive toxicity NOAEL is 351 mg/kg bw per day, based on decrease in homogenised resistant spermatid count in F0 males observed at the limit dose of 1,063 mg/kg bw per day in a two‐generation reproductive toxicity study in rats. For offspring toxicity, the relevant NOAEL is 293 mg/kg bw per day, based on reduced body weight observed at the LOAEL of 985 mg/kg bw per day in another two‐generation toxicity study in rats. For parental toxicity, the relevant NOAEL is 417 mg/kg bw per day, based on increased liver and kidney weights observed at the LOAEL of 2,151 mg/kg bw per day in a further two‐generation toxicity study in rats. 19 , 20 From the assessment of currently available human epidemiological studies, no conclusions could be drawn on a causal association between glyphosate exposure and effects on reproductive endpoints. 21
With regard to developmental toxicity, the relevant maternal toxicity NOAEL is 300 mg/kg bw per day, based on findings observed at 1,000 mg/kg bw per day in two rat developmental toxicity studies, including clinical signs (in both studies); the relevant developmental toxicity NOAEL is 300 mg/kg bw per day, based on reduced ossification and skeletal variations in foetuses observed in a rat developmental toxicity study at 1,000 mg/kg bw per day.
With regard to fetal development in rabbits, no teratogenic effect was observed. The relevant NOAELs for developmental and maternal toxicity were identified in a rabbit developmental toxicity study. For developmental toxicity, a NOAEL of 150 mg/kg per day was identified, based on increased incidence of post‐implantation loss at 450 mg/kg bw per day and reduced fetal weight at 300 mg/kg bw per day; the relevant maternal toxicity NOAEL is 50 mg/kg bw per day based on reduced body weight gain between gestation days 11 to 29. 22
In 2022, the ECHA RAC Committee (ECHA, 2022) concluded that no classification is warranted for adverse effects on reproduction and development.
There is no indication of neurotoxicity potential of glyphosate from one acute and two subchronic toxicity studies in rats and one delayed neurotoxicity study in domestic hens. The overall NOAEL is 1,000 mg/kg bw for acute systemic toxicity and 2,000 mg/kg bw (highest tested dose) for acute neurotoxicity; the NOAEL for subchronic systemic toxicity is 395 mg/kg bw per day based on reduced body weight gain and food consumption, while in the absence of neurotoxicity findings in the 90‐day neurotoxicity study in rats, the NOAEL for subchronic neurotoxicity is 1,499 mg/kg bw per day (highest tested dose).
There is insufficient evidence of an effect of glyphosate active substance and glyphosate‐based herbicides (GBHs) on neurotransmitters. 23 The integration of human observational studies with the limited experimental evidence from in vitro and in vivo studies does not trigger a concern for parkinsonism. 23 From the epidemiological studies, insufficient evidence on the possible association between glyphosate exposure and autism spectrum disorder (ASD) or amyotrophic lateral sclerosis (ALS) was concluded. 23 A developmental neurotoxicity study (DNT) with glyphosate is not present in the dossier and considered not needed based on the lack of neurotoxicity effects in the regulatory dataset on glyphosate active substance. New evidence on glyphosate was highlighted during the experts' meeting discussion 24 : an in vivo study in rats where DNT‐related endpoints were assessed and considered as not affected by the high doses administered to dams (2.16 and 4.65 g/kg bw per day during gestation and lactation period, respectively), and ToxCast/Tox 21 data, where glyphosate was not showing any activity in all tested in vitro assays, except for one parameter at high concentrations (i.e. AC50 31.7 μM). Additional data, including public literature studies on GBHs and studies on other glyphosate salts (including glyphosate‐trimesium), showing some DNT effects, were also assessed by the peer review. 23 Considering the overall body of evidence, a pattern of effects suggesting DNT liabilities was not clearly identified for glyphosate and the current toxicological reference values were considered protective. However, a data gap is identified for the applicants to clarify the cause of the DNT effects seen in the public literature studies with GBHs and in the study with glyphosate‐trimesium (see Section 10).
There are no indications of immunotoxicity potential for glyphosate in the available 28‐day toxicity study in female mice; a NOAEL of 1,448 mg/kg bw per day (highest tested dose) has been derived. 25
Several studies from the published literature investigated the potential effects of glyphosate on the human and animal gut microbiome, and possible consequent effects on health. Based on the current state of knowledge, considering that standardised regulatory guidance and/or established harmonised criteria are currently not available for the assessment of microbiome, no definitive conclusions can be drawn from these studies. However, the available mammalian toxicity dataset supports a sufficiently protective assessment for any health impact possibly mediated by the microbiome on humans, livestock and pet animals. Consistently, the previous conclusions on the lack of impact of glyphosate on animal gut microbiome and health (EFSA, 2018a) remain valid. Further developments are needed to understand the importance of the microbiome in risk assessment and identify dedicated strategies and methodologies accordingly (Merten et al., 2020). 26
The impact of glyphosate on the microbiome was also discussed at the Pesticides Peer Review Experts' TC 82 on ecotoxicology and similar conclusions were reached.
Toxicological reference values (TRVs) have been derived for glyphosate 27 as follows. The acceptable daily intake (ADI) is 0.5 mg/kg bw per day, based on a NOAEL of 53 mg/kg bw per day from a 90‐day study in dogs. The ADI is supported by the NOAEL of 59.4 mg/kg bw per day from a 2‐year rat study and covering the NOAEL of 50 mg/kg bw per day for maternal toxicity identified in a rabbit developmental toxicity study. The standard uncertainty factor (UF) of 100 was applied. Glyphosate‐induced effects on the salivary glands in rodents are likely to be a local effect of unclear adversity and human relevance, that were considered as not relevant for the derivation of TRVs. 28 The acute reference dose (ARfD) is 1.5 mg/kg bw, based on a NOAEL for developmental effects of 150 mg/kg bw per day identified in a rabbit developmental toxicity study. The standard UF of 100 was applied. During the previous peer review of glyphosate (EFSA, 2015), maternal and developmental NOAELs from a rabbit developmental toxicity study were selected for the derivation of the previous ADI (0.5 mg/kg bw per day) and ARfD (0.5 mg/kg bw), respectively. In the current peer review process, the reliability of this rabbit developmental toxicity study was re‐considered; another study, as reported above, was deemed as more appropriate to derive TRVs. 22
The acceptable operator exposure level (AOEL) is 0.1 mg/kg bw per day, based on the same considerations as for the ADI, applying a correction factor for limited oral absorption of 20%. This value is the same as previously established by the peer review (EFSA, 2015).
The acute AOEL (AAOEL) is 0.3 mg/kg bw, based on the same point of departure as for setting the ARfD, applying a correction factor for limited oral absorption of 20%.
Regarding glyphosate metabolites, an overview of their toxicological profile can be found in Table 1 and in Table 3 in Section 7.
Table 1.
Overview table of the toxicological profile of metabolites found as residues in livestock and/or crops
| Metabolite | Genotoxicity | General toxicity Toxicological reference values (TRVs) | Additional source of human exposure (a) (e.g. groundwater) |
|---|---|---|---|
| AMPA | Unlikely to be genotoxic | TRVs of glyphosate apply | No |
| N‐acetyl AMPA | Unlikely to be genotoxic | TRVs of glyphosate apply | No |
| N‐acetyl glyphosate | Negative for both mutagenicity and clastogenicity; aneugenicity not sufficiently investigated (data gap) | TRVs of glyphosate apply | No |
| N‐methyl AMPA | Unlikely to be genotoxic | No data, not needed for consumer risk assessment | No |
| N‐glyceryl AMPA | Negative for mutagenicity. Clastogenicity and aneugenicity not sufficiently investigated(data gap) | No data, not needed for consumer risk assessment | No |
| N‐malonyl AMPA | Negative for mutagenicity. Clastogenicity and aneugenicity not sufficiently investigated(data gap) | No data, not needed for consumer risk assessment | No |
As a groundwater metabolite please refer to the assessment summarised under Section 7.
Table 3.
Groundwater (a)
| Compound (name and/or code) | > 0.1 μg/L at 1 m depth for the representative uses (b) Step 2 | Biological (pesticidal) activity/relevance Step 3a. | Hazard identified Steps 3b. and 3c. | Consumer RA triggered Steps 4 and 5 | Human health relevance |
|---|---|---|---|---|---|
| Glyphosate | No | Yes | – | – | Yes |
| AMPA | No | No, though assessment not triggered | No Unlikely to be genotoxic; Same TRVs as glyphosate apply | No | Assessment not triggered |
Assessment according to European Commission guidance of the relevance of groundwater metabolites (2003).
FOCUS scenarios or a relevant lysimeter.
The metabolites AMPA, N‐methyl AMPA and N‐acetyl AMPA were concluded as unlikely to be genotoxic, based on the available data. For the minor metabolites in genetically modified (GM)‐tolerant crops N‐glyceryl AMPA and N‐malonyl AMPA, the submitted QSAR analysis did not suggest any specific concern for genotoxicity. Nonetheless, the analysis itself was not sufficiently reliable to cover clastogenicity and aneugenicity potential (data gap, see Section 10).
As regards general toxicity, AMPA and N‐acetyl AMPA displayed a similar qualitative and quantitative toxicological profile to glyphosate and the TRVs of glyphosate were concluded as applicable. For N‐acetyl glyphosate, general toxicity was sufficiently investigated, while the aneugenic potential was not addressed (data gap, see Section 10). Since aneugenicity has a threshold‐based mechanism and this metabolite is of no greater toxicity than glyphosate (similar toxicological profile), the same TRVs were concluded as applicable.
Based on an in vitro study with human skin conducted with the formulation for representative uses, ‘MON 52276’, the dermal absorption values are 0.096% for the concentrate (360 g/L) and 0.23% and 0.68% for the two in‐use dilutions (28.8 g/L and 2.4 g/L, respectively). Appropriate pro‐rata corrections were applied when necessary for the representative uses under consideration. 29
Based on the EFSA model predictions for tractor‐mounted and hand‐held application techniques, the operator exposure estimates are below the (A)AOEL for all representative uses, for an operator wearing workwear and no further personal protective equipment (PPE). Similarly, the predicted exposure levels for residents and bystanders (both adults and children) are lower than the (A)AOEL, without specific risk mitigation measure (considering the default buffer zone of 2–3 m), and the estimates for recreational exposure (in non‐agricultural areas) are also below the AOEL. Different scenarios were considered for workers, 29 for which no re‐entry is expected shortly after application for applications on bare‐soil (pre‐planting) or on railway tracks. For the uses on vegetables, the predicted worker exposure is below the AOEL for both tasks of inspection (2 or 8 h) and reaching/picking. For the uses in orchards crops and vines, considering the downward application of the herbicide, only re‐entry for inspection (8 h) is considering relevant, also triggering exposure estimates below the AOEL (without the need of gloves). The same outcome applies to the uses on invasive species.
Based on the available biomonitoring studies, the estimated systemic exposure levels to glyphosate are all below the AOEL/AAOEL or ADI/ARfD for the EU population. 30 It is noted that existing uncertainties due to limited relevance and reliability of some data were addressed by using the P95/max concentrations when available. 31
With regard to the toxicological information available for the formulation for representative uses ‘MON 52276’, studies were performed on acute toxicity and genotoxicity endpoints. With regard to the co‐formulants contained in ‘MON 52276’, toxicological studies were available for all components but one (present in significant amount in the final formulation). This component is exempted from REACH requirements because of its chemical nature. MS experts and the RMS considered that the available toxicological information is sufficient to conclude on the safety of ‘MON 52276’. However, EFSA concludes that repeated‐dose toxicity data for this component should be assessed to reach a final conclusion on the risk assessment of ‘MON 52276’ (a data gap has been identified by EFSA post‐experts' meeting, see Section 10).
3. Residues
The assessment in the residue section is based on the following guidance documents: OECD (2009, 2011), European Commission (2011) and JMPR (2004, 2007). All data assessed as reliable that inform on the defined data requirements, approval criteria 32 or criteria for product authorisation, 33 whether unpublished regulatory studies provided by the applicants or published peer reviewed scientific literature, have been used for the assessment of residues in plant and animal commodities. Where the test material used in an investigation was a formulated product, this information was assessed if relevant for the assessment of the active substance or the formulation for representative uses.
Glyphosate was discussed at the Pesticides Peer Review Experts' TC 83 on residues in November–December 2022.
The metabolism of glyphosate in primary crops was investigated in several crops, including genetically modified plants containing the CP4‐EPSPS, 34 GOX 35 or GAT 36 modifications.
In conventional crops (non‐tolerant), 37 acceptable metabolism studies were available for the categories fruit (lemon and grapes), cereal/grass crops (wheat) and pulses and oil seeds (soya bean, coffee). In addition, several other metabolism studies in fruit, root crops, cereals, pulses and sugar cane were considered supportive. The acceptable studies investigated the metabolism of 14C‐glyphosate when applied as soil (citrus, grapes, soya bean and coffee) and foliar treatment (grapes, wheat and coffee). Soil applied coffee experiments also investigated the metabolite 14C‐AMPA. Most studies were conducted with 14C‐glyphosate‐trimesium. Evidence provided from the peer reviewed scientific literature (Jianmei et al., 2005; Satchivi et al., 2000) showed that no differences ‐ neither in the rate nor the amount of glyphosate absorbed – were observed when compared with diammonium and isopropylammonium salt formulations. Therefore, all studies, regardless of the salt formulation, can be used to assess the metabolism of glyphosate in plants. Following soil application, the uptake of glyphosate was very low in comparison to when application was to foliage. Limited translocation was also observed after local foliar application. Unchanged glyphosate was observed as the major component with low amounts of AMPA (up to 6.4% TRR in soya bean straw). N‐methyl AMPA, N‐methyl glyphosate and methylphosphonic acid were only found in hydroponic experiments classified as supportive and were considered not needing further consideration with respect to the residues assessment.
Several studies with glyphosate‐tolerant crops with CP4‐EPSPS, with GOX and with GAT modifications were available. It is noted that the representative uses evaluated in the current renewal process do not include tolerant crops. Therefore, the studies were considered solely to complete the scientific assessment. Especially the studies with EPSPS and with GOX modifications confirm the metabolic picture found in the conventional crops. Some minor metabolites found in these modified crops (N‐glyceryl AMPA, N‐malonyl AMPA and N‐methyl AMPA) were not considered relevant to conventional crops, but require screening for genotoxic potential to address the safety of glyphosate residues in tolerant crops (outstanding data gaps for N‐glyceryl AMPA and N‐malonyl AMPA; for N‐methyl AMPA it was concluded that it is unlikely to be genotoxic; see Sections 2 and 10). In GAT modified crops, the specific metabolites N‐acetyl glyphosate and N‐acetyl AMPA were found. 38 It is noted that the aneugenic potential of N‐acetyl glyphosate has not been addressed (data gap, see Sections 2 and 10).
Acceptable confined rotational crop studies dosed with radiolabelled glyphosate or glyphosate‐trimesium in conventional crops are available for leafy crops (lettuce), root crops (radish and carrot) and cereals (wheat and barley). Several non‐fully guideline compliant studies were supporting these results. The main residue component found in food and feed parts of the investigated conventional crops is the metabolite AMPA.
The data selected as reliable are considered sufficient to elucidate the metabolic pathway and the nature of residues in plants (including those derived from soil residue uptake in crops planted in rotation) to cover all crop categories. Based on this evidence, separate plant residue definitions for risk assessment can be proposed for conventional crops: Sum of glyphosate and AMPA, expressed as glyphosate; and for glyphosate‐tolerant crops: Sum of glyphosate, AMPA, N‐acetyl glyphosate and N‐acetyl AMPA, expressed as glyphosate. For enforcement purposes, two options are proposed for risk managers to consider. Both options address crops with glyphosate‐tolerant modifications that were identified as being on the market in 2019 in the context of the Article 12 MRL review (EFSA, 2019) and consider specific metabolites that prevail in the crops. Option 1 is according to Codex (FAO‐WHO, 2019) 39 and relevant for soya bean, oilseed rape (OSR), maize (including sweet corn) 40 : Sum of glyphosate and N‐acetyl glyphosate, expressed as glyphosate; and for all other crops: Glyphosate only. Option 2 is according to the proposal in the EFSA MRL Art.12 Reasoned Opinion (EFSA, 2019) and relevant for soya bean, OSR, cotton, maize (including sweet corn), sugar beet 40 : sum of glyphosate, AMPA and N‐acetyl glyphosate, expressed as glyphosate; for all other crops: glyphosate only.
Public peer reviewed studies 41 did not confirm the transfer of AMPA in relevant amounts to crops from sources other than the use of glyphosate based herbicide products, suggesting AMPA as a specific marker for glyphosate use. AMPA was found in rotational field trials (see below). This is in line with the assessment that AMPA is a good environmental marker for glyphosate (see Section 4). As further information on additional residue trials for the representative uses and on the magnitude of residues in rotational crops is required (data gap, see below), and depending on the outcome of these trials, AMPA might be a better marker compound than glyphosate and risk managers may further consider the need to include AMPA in the enforcement residue definition for plants.
A large number of residue trials in conventionally grown crops were submitted, in most of them samples were analysed for glyphosate and AMPA. Many of these residue trials deviated from guidance and/or the critical GAPs (cGAPs). Those residue trials that can be considered reliable, i.e. cGAP compliant and analysing for glyphosate and AMPA with a valid analytical method and supported by sufficient storage stability data, are given in the list of endpoints in Appendix B (for further information on the validity assessment see Appendix D of this conclusion) and the data gaps identified in line with the current guideline SANTE/2019/12752 (European Commission, 2019) are detailed in Section 10.
It is noted that the RMS and the MS experts present at the experts' meeting do not agree with the data gaps set by EFSA to provide a sufficient number of GAP compliant residue field trials that are supported by storage stability data and a validated analytical method for some individual crop groups, except for residue trials for table olives in Northern EU (NEU). Instead, they suggested a wider extrapolation from the existing data to all crop groups (except table olives) and also to address pre‐sowing, pre‐planting, pre‐emergence and inter‐row uses based on the argument that residues for glyphosate and AMPA were below the LOQ in all cases except that of table olives. 42 In EFSA's view, the data need to be provided for completeness even if, taking into account the large amount of data available, their current absence does not raise an area of concern. In addition, the different views between EFSA and RMS on the validity of several trials with respect to the interpretation of the application of the agreed demonstrated storage stability and the analytical method for AMPA have been reflected and transparently reported in Appendix D.
Processing studies were submitted demonstrating the stability of glyphosate and AMPA under standard conditions simulating food processing operations, and processing factors were proposed for several crop commodities (see Appendix B).
Confined rotational crop studies for glyphosate‐tolerant rotational crops are not available and would be needed in case glyphosate‐tolerant crops were ever authorised in the EU. For the uses in conventional crops, an interim report of a study on the magnitude of residues in rotational crops in lettuce, carrot and wheat (results for only maximum two plant back intervals) indicated that residues of AMPA were present in rotational crops at levels above the LOQ (LOQ = 0.025 mg/kg), and therefore the study should be completed to enable the full assessment of rotational crop residues (data gap, see Section 9.1). In addition, a data gap has been identified for sufficient studies investigating the magnitude of residues in rotational crops (i.e. carrot, lettuce, wheat), as well as in additional crops, as appropriate. Given the limited data available, these data are considered necessary to finalise the consumer risk assessment (see Section 9.1).
Taking into account the residues from primary crops and the limited results from rotational crops, animal studies for all groups of livestock are triggered. Metabolism in ruminants and poultry was addressed in several studies administering radiolabelled forms of glyphosate alone (as such or as trimesium salt), as a mixture of glyphosate with AMPA (9:1) or as N‐acetyl glyphosate. Despite some shortcomings, all studies were considered acceptable except those dosed with glyphosate (acid form) that deviated from the guideline. Overall, glyphosate is the main component of the residue and only one metabolite (AMPA), major in several matrices, has been identified in these studies. On this basis and considering only the representative non‐ GM plant uses, the residue definition for risk assessment in animal commodities is proposed as sum of glyphosate and AMPA, expressed as glyphosate. In view of future MRL‐setting procedures and assuming that conventional and glyphosate‐tolerant crops could be included in the animal diet, the residue definition should be extended as follows: sum of glyphosate, AMPA, N‐acetyl glyphosate and N‐acetyl AMPA, expressed as glyphosate. It is noted, that the aneugenic potential of N‐acetyl glyphosate has not been addressed (data gap, see Sections 2 and 10). Given that the main compounds are good markers and considering that it cannot be excluded that livestock are fed with genetically GAT‐modified crops imported from third countries, the residue definition for enforcement purposes in animal commodities is confirmed as sum of glyphosate and N‐acetyl glyphosate, expressed as glyphosate, with the view of future MRL‐setting procedures. Several feeding studies conducted on dairy cows and laying hens fed with the same substances as in the metabolism studies were submitted. A feeding study on pig using the glyphosate/AMPA mixture was also provided. The studies employing glyphosate‐trimesium were not considered acceptable due to a non‐valid analytical method and lack of scientific evidence addressing its comparable absorption with respect to glyphosate. The studies with the mixture of glyphosate and AMPA are valid and sufficient to exclude residues above the LOQ in animal commodities with regard to the representative uses. Based on the latter studies and the preliminary estimated residue intakes by livestock, MRLs were proposed for animal commodities. However, these proposals are based on the representative uses limited to conventional crops only and MRL proposals might be significantly changed if the nature and level of residues present in feed commodities from glyphosate‐tolerant GM crops are taken into account.
According to the SANCO Technical guidelines for MRL setting in honey (European Commission, 2016), the same residue definitions as for plant commodities should be applicable. It is noted that a validated analytical method for monitoring of residues of N‐acetyl glyphosate in honey (not originating from the representative use but that has the potential to be present in imported honey) is not available (data gap, see Sections 1 and 10). Recent valid field studies analysing glyphosate and AMPA in honey were presented and indicate the need to increase the current MRL of 0.05–15 mg/kg.
The consumer risk assessment limited to the representative uses was performed using the EFSA PRIMo version 3.1 and using the supervised trials median residue (STMR) and highest residue (HR) values derived for plants grown as primary and rotational crops and animal commodities. The maximum chronic intake was calculated to be 3% of the ADI (NL toddler) and the highest acute intake is 2% of the ARfD for honey and other apicultural products. These assessment results are provisional, and a finalisation is still pending the data gaps identified on rotational crops and consequently the update of the animal dietary burden calculation.
4. Environmental fate and behaviour
Glyphosate was discussed at the Pesticides Peer Review Experts' TC 81 in November 2022. All data assessed as reliable that inform on the defined data requirements, approval criteria or criteria for product authorisation, whether unpublished regulatory studies provided by the applicants or published peer reviewed scientific literature, have been used for the assessment of environmental fate and behaviour. Where the test material used in an investigation was a formulated product, this information coming from different formulations was assessed equally, independently of whether the material was ‘MON 52276’ or another formulation.
The rates of dissipation and degradation in the environmental matrices investigated were estimated using the FOCUS (2006) kinetics guidance. In soil laboratory incubations under aerobic conditions in the dark, glyphosate exhibited low to high persistence, forming the major (> 10% applied radioactivity (AR)) metabolite AMPA (max. 42% AR), which exhibited moderate to very high persistence. Mineralisation of the phosphonomethyl 14C radiolabel to carbon dioxide accounted for 17–71% AR after 70–364 days. The formation of unextractable residues (not extracted by aqueous ammonium hydroxide) for this radiolabel accounted for 2.5–22% AR after 14–364 days. In anaerobic soil incubations glyphosate was stable compared to aerobic incubation conditions. Under the conditions of a laboratory soil photolysis study the only metabolite reaching levels triggering assessment was AMPA. Glyphosate and AMPA both exhibited characteristics between having low mobility and being immobile in soil. It was concluded that the adsorption of both glyphosate and AMPA was not pH dependent. In satisfactory field dissipation studies carried out at two sites in Germany, one in Switzerland, one in Ontario (Canada) and two in California (USA) (spray applications to the soil surface on bare soil plots) glyphosate exhibited low to moderate persistence. Sample analyses were carried out for AMPA in addition to glyphosate. This confirmed that AMPA was a major soil metabolite also under field conditions (max. 49% as parent equivalents). However, reliable AMPA dissipation rates could not be estimated from the available field studies leading to the identification of a data gap (see Section 10). Consequently, the exposure assessment for the representative uses being assessed was completed with the available laboratory AMPA kinetic endpoints. Field study DegT50 values for glyphosate were derived following normalisation to FOCUS reference conditions (20°C and pF2 soil moisture) in line with the EFSA (2014a) DegT50 guidance for one of the German and both California USA trial sites. The glyphosate field data endpoints were combined with laboratory values to derive modelling endpoints in line with the DegT50 guidance. The peer review confirmed the RMS assessment that soil degradation of glyphosate was best described by biphasic kinetics (except for an incubation in one soil) and that both glyphosate and AMPA degradation was pH dependent, with both compounds degrading more slowly under acidic soil conditions than when soil pH was in the neutral to alkaline range. The experts at the Pesticides Peer Review Experts' TC 81 agreed the use of the kinetic endpoints from the experiments that represented the slowest degradation (and fastest degradation for glyphosate when AMPA is kinetically generated from its glyphosate precursor), be used for exposure modelling for assessing the representative uses at EU level. This approach ensures that assessments covered use situations in acidic soils where degradation was slower, but also neutral/ alkaline conditions where the formation of AMPA might be greater. However, they agreed that if refinement would be needed for other uses in future exposure assessments, geomean soil DegT50 values should be used, splitting the dataset of reliable kinetic endpoints using the geomean value below pH(water) 6.5 to cover fields/areas with acidic soil conditions, and those above this value for alkaline fields/areas. The geomean endpoints that result from this approach have been included in Appendix B. It was agreed to use the arithmetic mean kinetic formation fraction for AMPA from glyphosate from all reliable soils in exposure modelling, independent of the pH of the soil incubation.
In laboratory incubations in dark aerobic natural sediment water systems, glyphosate exhibited moderate to high persistence, forming the major metabolites AMPA (max. 16% AR in water and 19% AR in sediment) and HMPA (max. 10% AR in water). Like glyphosate, these two metabolites also exhibited moderate to high persistence. The unextractable sediment fraction (not extracted by aqueous monopotassium phosphate or aqueous sodium hydroxide) was a sink for the phosphonomethyl 14C radiolabel, accounting for 14–22% AR at study end (100 days). Mineralisation of this radiolabel accounted for 6–48% AR at the end of the studies. In incubations where AMPA was applied as test substance, two further unidentified sediment metabolites were elucidated and ascribed the identifiers P1a and M3.3; they were estimated (estimates agreed in the Pesticides Peer Review Experts' TC 81) to have the potential to be formed at levels triggering exposure assessment at 14% and 6% of glyphosate respectively (as glyphosate molecular weight equivalents). The rate of decline of glyphosate in laboratory sterile aqueous photolysis experiments was enhanced compared to that in dark controls, with AMPA and methanediol being formed at up to 20% and 52% respectively. According to EFSA PPR Panel (2013) guidance on aquatic risk assessment and to European Commission (2003) guidance on the relevance of groundwater metabolites, the simple chemical structure of methanediol means it is considered to be not (eco)toxicologically relevant, and therefore of low risk or non‐relevant. The necessary surface water and sediment exposure assessments (predicted environmental concentration (PEC) calculations) were carried out for the metabolites AMPA, HMPA, P1a and M3.3 as well as for glyphosate, using the FOCUS (2001) step 1 and step 2 approach (version 3.2 of the Steps 1–2 in FOCUS calculator). In addition for glyphosate, appropriate step 3 (FOCUS, 2001) results were available. 43 For the representative use on railways, PEC were also available using the model and scenario parameterised in HardSpec 44 that represents UK civil engineering and climatic conditions.
The necessary groundwater exposure assessments were appropriately carried out using FOCUS (European Commission, 2014a) scenarios and the models PEARL 4.4.4, PELMO 5.5.3 and MACRO 5.5.4. 43 For the representative use on railways, PEC were also available using the model and scenario parameterised in HardSpec that represents UK civil engineering and climatic conditions. The potential for groundwater exposure from the representative uses by glyphosate and AMPA above the parametric drinking water limit of 0.1 μg/L was concluded to be low in geoclimatic situations that are represented by all 9 FOCUS groundwater scenarios and the HardSpec groundwater scenario. In a targeted monitoring study conducted in Sweden and peer reviewed in a scientific literature article (Cederlund, 2022), groundwater sampling wells (3 to 6 per site) were installed at 12 sites associated with railways; a total of 603 groundwater samples were collected in two different periods (2007–2010 and 2015–2019) and analysed for glyphosate and AMPA. Useful results were derived for wells adjacent to the railway down gradient regarding groundwater flow direction (i.e. those that have not been over sprayed so not below the rail track which were potentially influenced by preferential flow pathways). It was concluded that this information supported the exposure assessment for the single use pattern set out in the good agricultural practice table (1 × 1.8 kg a.s./ha) regarding the representative use on railways and the Swedish conditions in these periods. The results provide reassurance that groundwater exposure to glyphosate and AMPA above the parametric drinking water value of 0.1 μg/L generally did not occur in the monitored situations.
The applicants provided appropriate information to address the effect of water treatment processes on the nature of the residues that are present in surface water, when surface water is abstracted for the production of drinking water. The conclusion of this consideration was that consequent to oxidation at the disinfection stage of usual water treatment processes, glyphosate and its degradation products that trigger assessment (AMPA and HMPA) produce low molecular weight compounds with simple structures common to the degradation of naturally occurring substances in raw water, such as amino acids. The compounds identified were concluded as not being of toxicological concern.
A comprehensive review of environmental monitoring data, including collection of public monitoring data (raw data and aggregated data from national authorities and any regional/national agencies or research institutes) as well as open literature data was available. The monitoring reports and the published peer‐reviewed papers covered the monitoring of glyphosate and its main metabolite AMPA in soil, groundwater, surface water, transitional/tidal water, sediment, drinking water and air across several European countries and different temporal scales, ranging from a single sampling occasion to multi‐monthly and annual sampling schemes. The data from public monitoring have been collated and analysed by the applicants with regard to compliance with regulatory triggers (i.e. Regulatory Acceptable Concentrations (RAC) or the Drinking Water Directive 45 thresholds or Acceptable Daily Intake), considering that the whole EU data set was large enough to capture a range of agronomic, geographical, pedoclimatic and hydrogeological situations, as well as providing a good temporal coverage allowing assessment of the state of a compartment in different seasons and hydrological regimes. The applicants' approach to assess the environmental monitoring data and the reported conclusions were discussed at the Pesticides Peer Review Experts' TC 81. 46 Overall, the experts agreed that the monitoring datasets available for all the environmental compartments for glyphosate and AMPA were insufficient to use for exposure assessments in the EU regulatory framework and be assessed against a regulatory exposure assessment goal without additional information being provided (e.g. aspects such as agricultural context, including farmer usage of plant protection products, or site characterisation such as hydrogeological information). Because they are not aimed at fulfilling any higher tier assessment requirements, the results need to be taken with caution. In particular, the peer review agreed that the available groundwater monitoring data for glyphosate and AMPA cannot be used to overrule the available FOCUS PECgw values in the regulatory risk assessment of pesticides. Likewise, the measured concentrations of glyphosate and AMPA from public monitoring programmes or literature articles for the soil compartment are only valid for the time and place they represent and are not equivalent to the PECsoil calculated for risk assessment purposes. The experts at the Pesticides Peer Review Experts' TC 81 also acknowledged that the large proportion of land treated with glyphosate may make the route of groundwater exposure via bank infiltration and the connectivity of surface water bodies to groundwater aquifers more important issues than for other active substances. As information to address this exposure route was not available, a data gap was identified. However, as there are small hydrological catchments and river catchments where hydrology means groundwater would not be significantly connected with ponds, ditches, streams and rivers, this is not always a consideration (see Section 10). Consequently, an assessment not finalised has not been identified. The monitoring data for surface waters indicated concentrations below the RAC values for glyphosate and AMPA (those described in Section 5) in a very high proportion of the samples in the dataset (about 99%). In the few cases where glyphosate concentrations were above the RAC, the sites had mostly been sampled only once. Only two sites had exceedances in consecutive samples. Overall, the peer review concluded that, for regulatory purposes, the available surface water monitoring data can only be considered as supportive. For the sediment monitoring, the limited dataset provided is not representative of the EU and a comparison of sediment concentrations with the RAC value is of limited use. Since transitional/tidal water is usually not accounted for in the regulatory assessment for active substance approval, the monitoring data related to this environmental compartment were considered as supportive only. The available data from individual drinking water samples were of limited value for assessment for the whole EU as unaggregated values only originated from a few countries. For the air compartment, a limited monitoring dataset for glyphosate and AMPA was available. Despite the limited monitoring information available, also considering the intrinsic properties of glyphosate defined according to FOCUS Guidance Air (FOCUS, 2008), particulate‐bound concentration as a result of wind‐eroded particle transport at the short and medium range, and medium range transport during periods of spraying due to the formation of aerosols are expected to occur. Long range atmospheric transport of glyphosate in the upper atmosphere is not expected to occur due to the atmospheric half‐life estimated being below 2 days (regarding photochemical oxidative degradation in air, resulting from reaction with hydroxyl radicals present in the upper atmosphere). As for the other monitoring results, the monitored results from air samplers were considered difficult to equate directly to the representative uses being assessed.
The PEC in soil, surface water, sediment and groundwater covering the representative uses assessed can be found in Appendix B of this conclusion. A key to the wording used to describe the persistence and mobility of the compounds assessed can be found in Appendix C of this conclusion.
5. Ecotoxicology
The risk assessment was based on the following guidance documents: European Commission (2002)), SETAC (2001), EFSA (2009, 2013) and EFSA PPR Panel (2013).
Several aspects were discussed at the Pesticides Peer Review Experts' TC 82 in November – December 2022. The batches used in the regulatory dossier ecotoxicity studies were demonstrated to be in compliance with the proposed technical specification.
All data assessed as reliable and relevant for informing on the defined data requirements, approval criteria or criteria for product authorisation, whether unpublished regulatory dossier studies submitted by the applicants or published peer‐reviewed scientific literature, have been used for the assessment of ecotoxicology and environmental risk. Where a formulated product was used in literature studies, it was necessary to understand the relevance of the tested formulation relative to the formulation for representative uses, ‘MON 52276’. Therefore, the applicants were requested to provide the composition of formulations used in the literature studies together with a consideration of whether the tested formulation is comparable to the formulation for representative uses, ‘MON 52276’. This was addressed for only a number of the tested formulations and an explanation was not provided to justify why it was not possible for other formulations. The lack of this information may represent a source of uncertainty regarding the selection of the endpoints for risk assessment. A data gap was identified (see Section 10).
The criteria followed by the RMS for the assessment of the relevance of the tested material 47 and for the relevance and reliability of the endpoints 48 were discussed in detail during the Pesticides Peer Review Experts' TC 82. As a result of the discussions, the RMS was requested to update their evaluations following the agreed criteria.
Pending on the outcome on the data gap identified in Section 2 for one of the components in the formulation for representative uses, further consideration to non‐target organisms may be necessary.
For the risk assessment for birds, suitable acute and reproductive toxicity data were available with glyphosate. The reliability of the endpoints from the reproduction studies was discussed and agreed at the Pesticides Peer Review Experts' TC 82. 49 In addition, three scientific peer reviewed open literature studies providing sublethal endpoints were available and evaluated in the RAR. These studies were also discussed at the Pesticides Peer Review Experts' TC 82 50 but were not considered to provide endpoints for the risk assessment.
For the risk assessment for wild mammals, multiple acute toxicity studies with mammals were available and the appropriate acute endpoint for the risk assessment was discussed at the experts' meeting 51 where the experts agreed with the acute endpoint selected by the RMS. The experts also discussed and agreed on the appropriate endpoint to be used in the long‐term risk assessment for wild mammals. 52 Acute toxicity data for mammals were available for the formulation for representative uses, ‘MON 52276’.
The risk assessment for birds and mammals was conducted in line with EFSA (2009), however, several representative uses of ‘MON 52276’ are not explicitly covered by the guidance. Consequently, for some uses, the exposure assessment for birds and mammals was performed using surrogate scenarios. The available risk assessment demonstrated a low acute (screening‐level) and long‐term (screening or tier 1) risk to birds from dietary exposure to glyphosate for all representative uses. The acute risk to mammals, from dietary exposure, was also demonstrated to be low for all representative uses. The screening‐level long‐term risk assessment for mammals indicated a low risk for uses at 1 × 0.54 kg a.s./ha 53 and at 1 × 0.72 kg a.s./ha. 54 For all other representative uses the screening‐level long‐term assessment did not exclude a risk to wild mammals. The tier 1 risk assessment resulted in a high long‐term risk only to small herbivorous mammals for all uses assessed.
The refined long‐term risk assessment for small herbivorous mammals considered several options.
For the representative uses to railway tracks and for the spot applications to invasive species, various exposure refinements were agreed during the Pesticides Peer Review Experts' TC 82, 55 which resulted in a low long‐term risk to mammals.
For the remaining representative uses, the applicants proposed two types of refinement (i.e. degradation of glyphosate on plant material and population modelling). These refinements were discussed at the Pesticides Peer Review Experts' TC 82. 56 Regarding the degradation value (DT50) used in the exposure assessment, the experts agreed that there were insufficient reliable data to use the applicants' proposed value in a refined assessment. Nevertheless, the experts acknowledged that the data suggested that the degradation of glyphosate may be faster than assumed for a tier 1 exposure assessment. Regarding the population modelling, it was performed for the common vole (representing small herbivorous mammals) in orchards. The RMS provided an in‐depth assessment of the modelling according to EFSA PPR Panel (2014). The experts agreed with the RMS that, while the model showed potential for being useful, the landscape assumptions and parametrisation of the modelling were not considered appropriate. As a result, the modelling was not used for the refined assessment. Overall, there were no reliable higher tier data deemed suitable for refining the long‐term risk assessment to small herbivorous mammals. Considering the diversity and complexity of the list of representative uses for glyphosate, the experts reconsidered the problem formulation by discussing which scenarios within the representative uses lead to exposure, hence risk, of small herbivorous mammals. The experts agreed that a small herbivorous mammal is likely to be exposed for the majority of the representative uses. The exceptions were for field crops 57 (i) where the product is applied pre‐emergent of the crop (but post sowing/planting) and (ii) post‐emergent but when the application is made before growth stage BBCH 20. For these two scenarios, a low long‐term risk to mammals was concluded. For the remaining representative uses, a high long‐term risk to mammals was concluded. For a complete overview of the outcome of the risk assessment for mammals, please see Section 9.3.
The experts at the meeting agreed with the RMS that the risk to birds and mammals from the formulation for representative uses (‘MON 52267’) was sufficiently addressed by the risk assessment carried out for the active substance given that the available acute toxicity data for mammals did not indicate increased toxicity. 58
From plant metabolism studies, only metabolite AMPA was identified to require further risk assessment for birds and mammals (i.e. occurring at > 10% total radioactive residues (TRR)). The available risk assessment indicated a low risk for birds and mammals for all representative uses. 59 A low risk to birds and mammals via secondary poisoning was concluded since glyphosate and metabolites AMPA and HMPA have a log Kow < 3, meaning that a quantitative risk assessment was not required. A low risk to birds and wild mammals from ingestion of contaminated water was concluded for all representative uses.
According to Commission Regulation (EU) No 283/2013 60 , available and relevant data for terrestrial vertebrates, including amphibians and reptiles, if any, should be provided and taken into account in the risk assessment. Several scientific peer‐reviewed open literature studies were available which investigated the effects of glyphosate formulations on reptiles and terrestrial phases of amphibians. As mentioned above, the criteria for assessing the relevance and reliability of the studies were discussed and agreed at the experts' meeting. 61 Few studies were considered to provide endpoints which are potentially relevant to populations. However, when considering the available information, adverse and biologically relevant endpoints were not obtained.
The available data package for assessing the effects of glyphosate as active substance or in formulations on aquatic organisms was notably large in size and diversity. Overall, more than 600 endpoints were available for about a hundred species.
Unpublished regulatory dossier studies provided by the applicants were available to address the effects of exposure via surface water to glyphosate and the formulation for representative uses ‘MON 52276’ to fish, aquatic invertebrates, algae and macrophytes. The formulation for representative uses ‘MON 52276’ was shown to be less toxic than glyphosate. 62 Therefore, the current risk assessment covers the formulation for representative uses. Chronically exposed fish were the aquatic organisms showing adverse effects at the lowest glyphosate concentration. The chronic fish endpoint to be used for risk assessment was discussed at the Pesticides Peer Review Experts' TC 82. 63 Despite many studies were retrieved from the scientific peer‐reviewed open literature, none of those considered sufficiently reliable and relevant provided an endpoint lower than the one selected from unpublished regulatory dossier studies provided by the applicants. 64 Thus, the surface water Ecological Threshold Option Regulatory Acceptable Concentration (ETO‐RAC = 0.1 mg a.e./L) was derived from the selected chronic fish endpoint (NOEC = 1 mg a.e./L). Based on this RAC and on the estimated predicted environmental concentrations (PECsw), a low risk due to exposure via surface water to fish, aquatic invertebrates, algae and macrophytes could be concluded for all the representative uses of glyphosate.
The effects of glyphosate (either as active substance or formulated) to the aquatic stage of amphibians were investigated in several studies retrieved from the open literature. A comparison of the hazard data with fish was carried out and discussed at the Pesticides Peer Review Experts' TC 82. 65 For acute, lethal effects, due to exposure to glyphosate, the lowest fish endpoint was agreed to be protective for amphibians. For chronic exposure to glyphosate, a proper comparison between fish and amphibians could not be carried out, since relevant and reliable chronic endpoints for amphibians were not available. A full comparability between fish and aquatic stages of amphibians would anyway be hampered by the different response types being measured for the two groups.
Unpublished regulatory dossier studies provided by the applicants were also available to address the toxicity of glyphosate due to exposure via contaminated sediment to sediment‐dwelling organisms. Based on this information and on the estimated PECsed, a low risk due to sediment‐borne exposure was concluded for all the representative uses of glyphosate.
One study from the public literature (Sesin et al., 2021) 66 investigated how a single glyphosate formulation would result in different levels of effect to aquatic macrophytes, depending on the route of exposure. In particular, the study highlighted that overspraying the emerged parts of the plants resulted in larger effects when compared to other routes of exposure, including the standard exposure via contaminated surface water normally considered in the risk assessment. The study was considered reliable and its general findings plausible, also in light of the mode of action (MoA) of glyphosate; however, a regulatory endpoint was not derived since the study is not relevant due to the test material (experiment was carried out with a formulation containing the surfactant polyethoxylated tallow amine); in addition, two non‐standard species, whose general level of sensitivity is not known, were tested. Nonetheless, direct contact of the emerged parts of macrophytes via spray drift is likely to occur in the field for the representative uses, and the standard hazard assessment is not considered suitable to address this route of exposure for glyphosate. Considering the lack of further data, a data gap for addressing the risk to aquatic macrophytes due to contact exposure via spray drift of glyphosate was identified and this resulted in an assessment not finalised (see Section 9.1).
The available information was sufficient to conclude a low risk for metabolites AMPA and HMPA for all the representative uses of glyphosate. Data were not available for metabolites P1a and M3.3 which are expected to form in the sediment (see Section 4). Nonetheless, assuming as a worst‐case M3.3/P1a as 10 times more toxic than the parent compound AMPA, and thus using the AMPA endpoint for sediment‐dwelling organisms divided by a factor of 10 in a screening risk assessment, a low risk for all the representative uses of glyphosate was concluded.
A number of regulatory dossier toxicity studies provided by the applicants were available to address the effects of glyphosate and of the formulation for representative uses ‘MON 52276’ to honey bees (Apis mellifera). The available data package included all the required study types (i.e. acute oral and contact, chronic and larval toxicity studies). Since the necessary acute tests were available using the formulation ‘MON 52276’, the current risk assessment covers the formulation for representative uses. In addition, acute studies (oral and contact) were available to Bombus terrestris and an acute contact test was available to Osmia bicornis for glyphosate (test material glyphosate‐isopropylammonium). Reliable and relevant information for lethal effects from scientific peer‐reviewed open literature evaluated in the revised RAR did not indicate higher toxicity when compared to the regulatory studies.
The acute risk to honey bees in accordance with European Commission (2002) was concluded to be low for all the representative uses. Similarly, the risk from acute exposure was predicted to be low for all the representative uses when assessed in accordance with EFSA (2013) for honey bees and considering the available endpoints for the non‐Apis bees. By using EFSA (2013), low chronic risk to adult and larvae honey bees was concluded for all the representative uses (at screening level risk assessment or Tier 1). Risk assessments for chronic exposure (adult and larvae) for non‐Apis bees were not available.
A number of laboratory studies from scientific peer‐reviewed open literature investigating different types of sublethal effects were available. 67 Furthermore, a colony feeder study, which included an assessment of sublethal effects, was available. However, with a lack of a quantified link between the observed effects and the consequences for the colony, the endpoints derived from these studies can be used to inform the overall assessment, but they could not be used for a quantitative risk assessment.
An assessment of the accumulative effects was not available. For the relevant plant metabolite AMPA, data gaps were identified in Section 3. Therefore, the potential occurrence of AMPA in pollen and nectar could not be estimated for a risk assessment to bees (data gap, see Section 10).
To address the risk for non‐target arthropods other than bees, extended laboratory studies with the formulation for representative uses ‘MON 52276’ were available with the standard species, Aphidius rhopalosiphi and Typhlodromus pyri, as well as with the ground beetle Poecilus cupreus and the spider Pardosa sp. 68 Therefore, the current risk assessment covers the formulation for representative uses. Tier 1 (glass plate) studies with the standard species were only considered supporting; however, all experts agreed that the data set available was sufficient to perform the risk assessment according to European Commission (2002). 69 Relevant scientific peer‐reviewed publications evaluating direct effects of glyphosate on non‐target arthropods were not identified in the open literature in accordance with the criteria agreed at the Pesticides Peer Review Experts' TC 82. Based on the available data and risk assessment, low in‐ and off‐field risk to non‐target arthropods other than bees was concluded for all the representative uses of glyphosate.
Chronic toxicity studies were conducted with earthworms (Eisenia fetida), and soil meso‐ and macrofauna (the collembola Folsomia candida and the predatory mite Hypoaspis aculeifer) for the active substance and the formulation for representative uses ‘MON 52276’. Furthermore, a chronic toxicity study was provided with the only pertinent soil metabolite of glyphosate (i.e. AMPA). The formulation for representative uses ‘MON 52276’ was not shown to be of higher toxicity than glyphosate. Therefore, the current risk assessment covers the formulation for representative uses. The endpoints used for risk assessment for soil organisms were agreed by the experts. 70 Relevant and reliable peer‐reviewed publications evaluating direct effects of glyphosate on soil organisms were not identified in the open literature in accordance with the criteria agreed at the Pesticides Peer Review Experts' TC 82. Low chronic risk to earthworms and soil meso‐ and macrofauna (other than earthworms) was concluded for glyphosate and the metabolite AMPA for all representative uses. Studies on the effects of glyphosate, the formulation for representative uses and the metabolite AMPA on soil microorganisms were available. Based on the available data, which included a relevant peer‐reviewed publication identified from the open literature, the risk to soil microorganisms from exposure to glyphosate, the formulation for representative uses and the metabolite AMPA was considered low for all representative uses.
Appropriate data for risk assessments for non‐target terrestrial plants were available (i.e. vegetative vigour and seedling emergence tests with the formulation for representative uses ‘MON 52276’). In accordance with the criteria as agreed at the Pesticides Peer Review Experts' TC 82, 71 relevant and reliable data from the scientific peer‐reviewed open literature studies were not identified for non‐target terrestrial plants.
The deterministic risk assessment considering the lowest available endpoint as agreed by the experts 71 indicated a high risk for all representative uses. However, a low risk for non‐target terrestrial plants was identified for all representative uses by implementing appropriate risk mitigation measures. The required risk mitigation measures (5 to 10 m in‐field non‐sprayed buffer strip without or with combination of other drift reducing technology) vary with the different representative uses (see Section 8.1).
An assessment of risk to biodiversity via indirect effects and trophic interactions was submitted for the representative uses of glyphosate due to the specific condition related to effects on biodiversity laid down in Commission Implementing Regulation (EU) 2017/2324 72 . Such assessment considered the different environmental compartments and taxa (i.e. terrestrial vertebrates, aquatic organisms, bees, non‐target arthropods, soil organisms and non‐target terrestrial plants). It considered also risk mitigation and biodiversity conservation measures.
The assessment was extensively discussed at the Pesticides Peer Review Experts' TC 82. 73 In general, the current lack of a harmonised approach to assess biodiversity within the prospective risk assessment was recognised by the experts. EFSA suggested that this is a general issue that could be addressed during the development and agreement on specific protection goals for non‐target organisms. 74 The MS experts agreed with this suggestion, and they also agreed that the standard risk assessments address direct effects only, with the partial exception of aquatic organisms (see below for more details). In relation to the specific peer review of the submitted assessment, the discussion considered (i) the adequacy of the data collection, (ii) the criteria for the assessment of relevance and reliability of the available data, and (iii) the proposed mitigation measures.
Regarding the data collection, it was noted that a specific systematic literature search was not available, although requested to the applicants during the peer review process following the public consultation. Therefore, the assessment provided is lacking an appropriate problem formulation, search strategy and methodology. Overall, the experts considered the data presented of questionable scientific quality and of limited use to address the topic.
Regarding the assessment of relevance of the studies in relation to the representative uses, the experts agreed that the criteria are determined by the test material and the test conditions; therefore, it was agreed to re‐evaluate the studies according to those criteria and consider them appropriately in the overall WoE. 75 It is noted that the relevance of the test material was not considered applicable for studies reporting indirect effects due to plant removal.
Regarding the risk mitigation measures, the applicants proposed the implementation of a multi‐functional field margin (MFFM) in areas where more than 15 hectares are treated (for GAPs where 100% of the area is treated). Although this mitigation was considered as potentially useful, the experts noted that its effectiveness will be context and landscape dependent. The adequacy of the size limitation of the field (> 15 ha) and threshold for 100% of the treated area was not scientifically supported. The quality of the MFFM, in terms of structure and composition, was not specified and the extent to which the MFFM, and its quality, could mitigate effects on biodiversity was not quantified.
Based on the evaluation presented in the revised RAR by the RMS for the different groups of non‐target organisms, most of the studies were considered to be of low relevance for the representative uses.
For terrestrial vertebrates, the studies reported evidence of negative indirect effects of glyphosate, but generally reversible (i.e. recovery occurred within a few years post‐application). However, the dataset was considered too limited to reach a firm conclusion.
For aquatic organisms, the experts agreed that in principle the ETO‐RAC is suitable to cover both direct and indirect effects including trophic interaction among the aquatic food chain, as indicated in the EFSA PPR Panel (2013). However, the experts noted that some specific issues (e.g. disruption of the biofilm, community shifts in microbes, effects via contact on emergent macrophytes via spray drift, indirect effects driven by direct effects occurring outside of the water system) are not currently covered in the EFSA PPR Panel (2013). Overall, for aquatic organisms, the dataset was also considered too limited to reach a conclusion for indirect effects not covered by the direct effects.
For bees, relevant studies investigating impact on indirect effects due to removal of weeds and the reduction of floral resources were not provided.
For terrestrial non‐target plants, it was highlighted during the Pesticides Peer Review Experts' TC 82 that it would be necessary to ensure that the function of any MFFM would be effective. This would require measures to effectively limit the spray‐drift reaching the MFFM.
Overall, the experts recognised that the risks associated with the representative uses of glyphosate for biodiversity are complex and depend on multiple factors. Furthermore, it was reflected that indirect effects as a result of removal of the target weeds are likely to be similar for any broad‐spectrum herbicide used in the same manner. The experts also recognised that indirect effects on non‐target organisms may not be addressed by the assessment of direct toxicity effects, i.e. a low risk based on standard toxicity effects cannot be used to exclude indirect effects. However, a quantification of direct toxic effects could be useful to understand the resulting effects on higher trophic levels. Overall, on the basis of the information provided, the experts agreed that a conclusion cannot be reached to exclude possible negative impacts on non‐target species, habitats and ecosystems due to indirect effects via trophic interactions for all the representative uses of glyphosate, including uses where less than 50% of the surface is treated (i.e. band and spot applications) and railway uses. The experts also recognised that risk mitigation measures for the off‐field (i.e. the use of the proposed 75% drift reduction nozzles) as well as the implementation of a MFFM could be beneficial. A general data gap was identified to address several aspects (see Section 10). When addressing this general data gap, the issue should be considered more specifically for the different groups of non‐target organisms, including a consideration of the effectiveness of possible risk mitigation measures at landscape level, for all the uses being assessed.
For the current assessment, studies were identified (both via literature search and submitted during the consultation phase) on the potential effects of glyphosate and formulations on the microbiome of non‐target organisms. The information was assessed for relevance and reliability using criteria agreed during the Pesticides Peer Review Experts' TC 82. 76 The impact of glyphosate on the microbiome was discussed at the Pesticides Peer Review Experts' TC 82 77 and also at the Pesticides Peer Review Experts' TC 80 78 on mammalian toxicology. Only for bees, the studies identified were evaluated as relevant and reliable and responses due to glyphosate exposure on bees' gut microbiota identified, such as changes in the abundance of core microbial species. In particular, a decrease in abundance and growth of bee gut bacterium Snodgrasella alvi was observed. Generally, it was acknowledged that the relevance of these effects at the population level is unknown.
6. Endocrine disruption properties
The assessment of the endocrine disruption (ED) potential of glyphosate was discussed at the Pesticides Peer Review Experts' TC 84 on Mammalian Toxicology and Ecotoxicology joint session on endocrine disruption (December 2022) for both humans and non‐target organisms.
In the context of the peer review, considering the extensive amount of data available in the RAR, both regulatory studies and studies retrieved through a systematic literature search, EFSA with the support of the EFSA Working Group on Endocrine Disruptors (EFSA ED WG) conducted an ED assessment in line with the ECHA/EFSA (2018) guidance using a structured approach. A number of studies were also available with formulated products other than the one for the representative uses. However, the criteria for the identification of endocrine disruptors, as laid down in Commission Regulation (EU) No 2018/605 79 , do not apply to formulations. Therefore, studies with formulated products were only considered by the EFSA ED WG to understand their possible impact on the ED assessment of glyphosate active substance.
The approach used by EFSA and the RMS was not fully congruent in terms of data included in the ED assessment, 80 partially owing to slight difference in the assessment of relevance and reliability of the available data. Nevertheless, the overall outcomes reached individually by EFSA and the RMS were aligned and agreed by the experts at the Pesticides Peer Review Experts' TC 84. 81
With regard to the assessment of the ED potential of glyphosate for both humans and non‐target organisms according to the ECHA/EFSA (2018) guidance, in determining whether glyphosate interacts with the oestrogen, androgen, steroidogenesis (EAS) and thyroid (T) mediated pathways, the number and type of effects induced, and the magnitude and pattern of responses observed across the available information were considered. Additionally, the conditions under which effects occur were considered, in particular, whether or not endocrine‐related responses occurred at dose(s) that also resulted in overt toxicity. The assessment is therefore providing a WoE analysis of the potential interaction of glyphosate with the EAS and T signalling pathways, using the available evidence in the dataset.
For humans, with regard to the T‐modality, the data set was considered complete and a pattern of T‐mediated adversity was not identified. With regard to the EAS‐modalities, the dataset was also considered complete and a pattern of EAS‐mediated adversity was not observed.
In conclusion, based on the available information 82 and according to the ECHA/EFSA (2018) guidance, the ED criteria according to point 3.6.5 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605, are not met for the EAS‐ and T‐modalities for the active substance glyphosate. 83
For mammals as non‐target organisms, the same conclusion drawn for humans was reached.
Regarding the non‐mammalian species, several species and taxa were tested with glyphosate active substance including fish, birds, amphibians, reptiles, aquatic and terrestrial invertebrates, and many in vivo mechanistic, EATS‐mediated and ‘sensitive to but not diagnostic’ parameters were investigated. Therefore, the dataset was considered, overall, complete for the investigation of both EATS‐adversity and endocrine activity.
The overall WoE did not show any convincing pattern of EATS‐mediated adversity and/or endocrine activity.
Although invertebrate non‐target organisms are currently not fully addressed by the ECHA/EFSA (2018) guidance due to the lack of knowledge and test guidelines, especially at the mechanistic level, the guidance recommends evaluating the data with invertebrates when available by applying the general principles of the guidance. Although a clear pattern of adversity attributable to an ED MoA was not observed, in general, a clear conclusion on the ED potential of glyphosate on invertebrates could not be drawn as, in the vast majority of the studies, there was no clear dose‐ response and several drawbacks were noted in the studies impacting their reliability.
Overall, based on the available evidence and assessment, glyphosate does not meet the criteria for the EATS‐modalities as laid down in point 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605.
7. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 2, 3, 4–5)
Table 2.
Soil
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Glyphosate | Low risk to soil organisms |
| AMPA | Low risk to soil organisms |
Table 4.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Glyphosate |
Low risk to aquatic and sediment‐dwelling organisms via surface water and sediment Data gap for the risk to aquatic macrophytes due to contact exposure via spray drift |
| AMPA | Low risk to aquatic and sediment‐dwelling organisms |
| HMPA | Low risk to aquatic and sediment‐dwelling organisms |
| P1a (sediment only) | Low risk to sediment‐dwelling organisms |
| M3.3 (sediment only) | Low risk to sediment‐dwelling organisms |
Table 5.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
| Glyphosate | > 5 mg/L air/4 h (nose‐only) |
8. Particular conditions proposed to be taken into account by risk managers
Risk mitigation measures (RMMs) identified following consideration of MS and/or applicant's proposal(s) during the peer review, if any, are presented in this section (see Table 6). These measures applicable for human health and/or the environment leading to a reduction of exposure levels of operators, workers, bystanders/residents, environmental compartments and/or non‐target organisms for the representative uses are listed below. The list may also cover any RMMs as appropriate, leading to an acceptable level of risks for the respective non‐target organisms.
Table 6.
Risk mitigation measures (RMMs) proposed for the representative uses assessed in addition to those already specified in the GAP table as part of the representative uses applied for (a)
| Representative use | PRE‐SOWING, PRE‐PLANTING, PRE‐EMERGENCE | POST‐HARVEST, PRE‐SOWING, PRE‐PLANTING | ||||||
|---|---|---|---|---|---|---|---|---|
| Root vegetable plants & tuberous plants, bulb plants, fruit‐vegetable plants, Brassica, leaf and stem vegetable plants, Sugar beet | Root vegetable plants & tuberous plants, bulb plants, fruit‐vegetable plants, Brassica, leaf and stem vegetable plants, Sugar beet | |||||||
| Tractor‐mounted broadcast spray | Tractor‐mounted broadcast spray | |||||||
| 1× 1.44 kg a.s./ha | 1× 1.08 kg a.s./ha | 1× 0.72 kg a.s./ha | 1–2 × 1.08/1.44 kg a.s./ha | 1–3 × 0.72/1.08 kg a.s./ha | 1–3 × 0.72 kg a.s./ha |
Cereal volunteers 1 × 0.54 kg a.s./ha |
Cereal volunteers 1 × 0.54 kg a.s./ha once every 3 years |
|
| Max appl. rate of 1.44 kg a.s./ha in any 12 months period | Max appl. rate of 1.08 kg a.s./ha in any 12 months period | Max appl. rate of 0.72 kg a.s./ha in any 12 months period | Max appl. rate of 2.16 kg a.s./ha in any 12 months period | Max appl. rate of 2.16 kg a.s./ha in any 12 months period | Max appl. rate of 2.16 kg a.s./ha in any 12 months period | Max appl. rate of 0.54 kg a.s./ha in any 12 months period | Max appl. rate of 0.54 kg a.s./ha in any 36 months period | |
| Use No 1a | Use No 1b | Use No 1c | Use No 2a | Use No 2b | Use No 2c | Use No 3a | Use No 3b | |
| Risk to non‐target terrestrial plants | RMMs already specified in the GAP table are not sufficient. More efficacious measures are needed. (b) | RMMs already specified in the GAP table are not sufficient. More efficacious measures are needed. (b) | No additional measures needed. | RMMs already specified in the GAP table are not sufficient. More efficacious measures are needed. (b) | RMMs already specified in the GAP table are not sufficient. More efficacious measures are needed. (b) | No additional measures needed. | ||
Use of at least 75% drift reducing nozzles is specified in the GAP table.
RMMs such as 10‐m no‐spray buffer zone or 5 m no‐spray buffer zone in combination with an application of 50% drift‐reducing nozzles or application of 90% drift‐reducing nozzle.
It is noted that final decisions on the need of RMMs to ensure the safe use of the plant protection product containing the concerned active substance will be taken by risk managers during the decision‐making phase. Consideration of the validity and appropriateness of the RMMs remains the responsibility of MSs at product authorisation, taking into account their specific agricultural, plant health and environmental conditions at national level.
8.1. Particular conditions proposed for the representative uses evaluated
| Representative use | POST‐EMERGENCE OF WEEDS | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orchard crops: citrus, stone and pome fruits, kiwi, nut crops, banana, and table olives | Vines (table and wine grape, leaves not intended for human consumption) | Vegetables (Root vegetable plants and tuberous plants, bulb plants, fruit‐vegetable plants, Legume vegetables, Leafy vegetables | Railway tracks | Invasive species in agricultural and non‐agricultural areas | Root vegetable plants and tuberous plants, bulb plants, fruit‐vegetable plants, Brassica, leaf and stem vegetable plants, Sugar beet | ||||||||||
| Ground directed, fully‐shielded (hooded) spray, band application (Band application in the rows below the trees or as spot treatments. The treated area represents not more than 50% of the total orchard area) | Ground directed, fully‐shielded (hooded) spray, band application (Band application in the rows below the vine stock or as spot treatments. The treated area represents not more than 50% of the total vineyard area) | Inter‐row application: ground directed, fully‐shielded (hooded) spray (Applications are made between the crop rows. The rate refers to the treated area only, which represents not more than 50% of the total area) | Ground directed, spray | Spot treatment (shielded) | Spot treatment (shielded), cut stem: spray appl. |
Spot treatment (shielded) Post‐harvest, pre‐sowing, pre‐planting Application to existing row cropland after harvest for removal of couch grass The treated area represents not more than 20% of the cropland |
|||||||||
| 1–2 × 1.08/1.44 kg a.s./ha | 1–3 × 0.72/1.08 kg a.s./ha | 1–3 × 0.72 kg a.s./ha | 1–2 × 1.08/1.44 kg a.s./ha | 1–3 × 0.72/1.08 kg a.s./ha | 1–3 × 0.72 kg a.s./ha | 1 × 1.08 kg a.s./ha | 1 × 0.72 kg a.s./ha | 2 × 1.8 kg a.s./ha | 1 × 1.8 kg a.s./ha | 1 × 1.8 kg a.s./ha | 1 × 1.8 kg a.s./ha | 1 × 1.08 kg a.s./ha | 1 × 0.72 kg a.s./ha | 1 × 0.72 kg a.s./ha once every three years | |
| Max appl. rate of 2.88 kg a.s./ha treated area in any 12 months period | Max appl. rate of 2.16 kg a.s./ha in any 12 months period | Max appl. rate of 2.88 kg a.s./ha treated area in any 12 months period | Max appl. rate of 2.16 kg a.s./ha treated area in any 12 months period | Max appl. rate of 1.08 kg a.s./ha in any 12 months period | Max appl. rate of 0.72 kg a.s./ha in any 12 months period | Max appl. rate of 3.6 kg a.s./ha in any 12 months period | Maximum application rate of 1.8 kg a.s./ha in any 12 months period | Max appl. rate of 1.08 kg a.s./ha in any 12 months period | Max appl. rate of 0.72 kg a.s./ha in any 12 months period | Max appl. rate of 0.72 kg a.s./ha in any 36 months period | |||||
| Use No 4a | Use No 4b | Use No 4c | Use No 5a | Use No 5b | Use No 5c | Use No 6a | Use No 6b | Use No 7a | Use No 7b | Use No 8 | Use No 9 | Use No 10a | Use No 10b | Use No 10c | |
| Risk to non‐target terrestrial plants | RMMs already specified in the GAP table are not sufficient. More efficacious measures are needed. (b) | No additional measures needed. | RMMs already specified in the GAP table are not sufficient. More efficacious measures are needed. (b) | No additional measures needed. | RMMs already specified in the GAP table are not sufficient. More efficacious measures are needed (b) | No additional measures needed. | RMMs already specified in the GAP table are not sufficient. More efficacious measures such as 90% drift‐reducing nozzles are needed. | RMMs already specified in the GAP table are not sufficient. More efficacious measures are needed. (b) | No additional measures needed. | ||||||
Use of at least 75% drift reducing nozzles is specified in the GAP table.
RMMs such as 10 m no‐spray buffer zone or 5 m no‐spray buffer zone in combination with an application of 50% drift‐reducing nozzles or application of 90% drift‐reducing nozzles.
9. Concerns and related data gaps
9.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for one or more of the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011 33 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following issues or assessments that could not be finalised have been identified, together with the reasons including the associated data gaps where relevant, which are reported directly under the specific issue to which they are related:
- The assessment of the reference specification cannot be finalised since one of the impurities showed a potential for clastogenicity in an in vitro chromosome aberration test that was not appropriately followed up in vivo. Although some batches used in the toxicological studies contained this impurity at levels representative of the proposed reference specification, a conclusion on the maximum level of this impurity in any reference specification cannot be drawn without a clarification on its clastogenic potential (see Section 2).
- Clarification on the clastogenic potential of one impurity present in the reference specification, following up on the positive results obtained in an in vitro chromosome aberration test needs to be provided (relevant for all applicants/sources of glyphosate, see Section 2).
- The consumer dietary risk assessment could not be finalised since the data set on the magnitude of residues in rotational crops is not complete (see Section 3).
- Final report of the magnitude of the residues in rotational crops study in carrot, lettuce and wheat is required (relevant for the representative uses in all crops which are grown in rotation; see Section 3).
- Sufficient studies investigating the magnitude of residues in rotational crops (i.e. carrot, lettuce, wheat) including additional crops (as appropriate) are required (relevant for the representative uses in all crops which are grown in rotation; see Section 3).
- The risk assessment for aquatic macrophytes due to contact exposure via spray drift could not be finalised (see Section 5).
- Further information to investigate the risk for aquatic macrophytes due to contact exposure via spray drift is needed, including an assessment of the toxicity of the active substance and the formulation to standard macrophytes species via this route of exposure (relevant for all representative uses, see Section 5).
9.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 regarding the hazard cut‐off criteria outlined in Appendix A.
The following critical areas of concern are identified, together with any associated data gaps, where relevant, which are reported directly under the specific critical area of concern to which they are related:
Critical areas of concern were not identified.
9.3. Overview of the concerns identified for each representative use considered (Table 7)
Table 7.
Overview of concerns reflecting the issues not finalised, critical areas of concerns and the risks identified that may be applicable for some but not for all representative uses or risk assessment scenarios
| Representative use | PRE‐SOWING, PRE‐PLANTING, PRE‐EMERGENCE | POST‐HARVEST, PRE‐SOWING, PRE‐PLANTING | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Root vegetable plants & tuberous plants, bulb plants, fruit‐vegetable plants, Brassica, leaf and stem vegetable plants, Sugar beet | Root vegetable plants & tuberous plants, bulb plants, fruit‐vegetable plants, Brassica, leaf and stem vegetable plants, Sugar beet | ||||||||
| Tractor‐mounted broadcast spray | Tractor‐mounted broadcast spray | ||||||||
| 1× 1.44 kg a.s./ha | 1× 1.08 kg a.s./ha | 1 × 0.72 kg a.s./ha | 1–2 × 1.08/1.44 kg a.s./ha | 1–3 × 0.72/1.08 kg a.s./ha | 1–3 × 0.72 kg a.s./ha |
Cereal volunteers 1 × 0.54 kg a.s./ha |
Cereal volunteers 1 × 0.54 kg a.s./ha once every 3 years |
||
| Max appl. rate of 1.44 kg a.s./ha in any 12 months period | Max appl. rate of 1.08 kg a.s./ha in any 12 months period | Max appl. rate of 0.72 kg a.s./ha in any 12 months period | Max appl. rate of 2.16 kg a.s./ha in any 12 months period | Max appl. rate of 2.16 kg a.s./ha in any 12 months period | Max appl. rate of 2.16 kg a.s./ha in any 12 months period | Max appl. rate of 0.54 kg a.s./ha in any 12 months period | Max appl. rate of 0.54 kg a.s./ha in any 36 months period | ||
| Use No 1a | Use No 1b | Use No 1c | Use No 2a | Use No 2b | Use No 2c | Use No 3a | Use No 3b | ||
| Operator risk | Risk identified | ||||||||
| Assessment not finalised | |||||||||
| Worker risk | Risk identified | ||||||||
| Assessment not finalised | |||||||||
| Resident/bystander risk | Risk identified | ||||||||
| Assessment not finalised | |||||||||
| Consumer risk | Risk identified | ||||||||
| Assessment not finalised | X2 (f) | X2 (f) | X2 (f) | X2 (f) | X2 (f) | X2 (f) | X2 (f) | X2 (f) | |
| Risk to wild non‐target terrestrial vertebrates | Risk identified | X (c) | X (c) | X (e) | X (d) | X (d) | |||
| Assessment not finalised | |||||||||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | ||||||||
| Assessment not finalised | |||||||||
| Risk to aquatic organisms | Risk identified | ||||||||
| Assessment not finalised | X3 (g) | X3 (g) | X3 (g) | X3 (g) | X3 (g) | X3 (g) | X3 (g) | X3 (g) | |
| Groundwater exposure to active substance | Legal parametric value breached | ||||||||
| Assessment not finalised | |||||||||
| Groundwater exposure to metabolites | Legal parametric value breached (a) | ||||||||
| Parametric value of 10 μg/L (b) breached | |||||||||
| Assessment not finalised | |||||||||
The superscript numbers relate to the numbered points indicated in Section 9.1. Where there is no superscript number, see Section 5 for further information.
It should be noted that the classification proposed in the context of this evaluation procedure under Regulation (EC) No 1107/2009 concurs with the harmonised classification and labelling in accordance with Regulation (EC) No 1272/2008.
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission (2003).
High long‐term risk to mammals (identified at tier 1) for pre‐sowing and pre‐planting uses. Low risk to mammals for the pre‐emergent uses (but after the sowing/planting).
High long‐term risk to mammals (identified at tier 1) for 2 or 3 applications of 0.72 kg a.s/ha and for 1, 2 or 3 applications of 1.08 kg a.s/ha. Low risk to mammals for a single application of 0.72 kg a.s./ha.
High long‐term risk to mammals (identified at tier 1) for all scenarios within this representative use.
Rotational crop field trials are required regarding uses on agricultural/cropped land for all crop groups that can be grown in rotation (i.e. not for orchard crops such as kiwi, olives, grapes, citrus, stone, pome and tree fruit), to finalise the livestock dietary burden calculation and the consumer risk assessment.
Assessment not finalised for aquatic macrophytes, only for contact exposure via spray drift.
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 7.)
POST‐EMERGENCE OF WEEDS
| Representative use | POST‐EMERGENCE OF WEEDS | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orchard crops: citrus, stone and pome fruits, kiwi, nut crops, banana, and table olives | Vines (table and wine grape, leaves not intended for human consumption) | Vegetables (Root vegetable plants & tuberous plants, bulb plants, fruit‐vegetable plants, Legume vegetables, Leafy vegetables | Railway tracks | Invasive species in agricultural and non‐agricultural areas | Root vegetable plants & tuberous plants, bulb plants, fruit‐vegetable plants, Brassica, leaf and stem vegetable plants, Sugar beet | |||||||||||
| Ground‐directed, fullyshielded (hooded) spray, band application (Band application in the rows below the trees or as spot treatments. The treated area represents not more than 50% of the total orchard area) | Ground‐directed, fully shielded (hooded) spray, band application (Band application in the rows below the vine stock or as spot treatments. The treated area represents not more than 50% of the total vineyard area) | Inter‐row application: ground‐directed, fully shielded (hooded) spray (Applications are made between the crop rows. The rate refers to the treated area only, which represents not more than 50% of the total area) Crop BBCH < 20 | Ground‐directed, spray | Spot treatment (shielded) | Spot treatment (shielded), cut stem: spray appl. |
Spot treatment (shielded) Post‐harvest, pre‐sowing, pre‐planting Application to existing row cropland after harvest for removal of couch grass The treated area represents not more than 20% of the cropland |
||||||||||
| 1–2 × 1.08/1.44 kg a.s./ha | 1–3 × 0.72/1.08 kg a.s./ha | 1–3 × 0.72 kg a.s./ha | 1–2 × 1.08/1.44 kg a.s./ha | 1–3 × 0.72/1.08 kg a.s./ha | 1–3 × 0.72 kg a.s./ha | 1 × 1.08 kg a.s./ha | 1 × 0.72 kg a.s./ha | 2 × 1.8 kg a.s./ha | 1 × 1.8 kg a.s./ha | 1 × 1.8 kg a.s./ha | 1 × 1.8 kg a.s./ha |
1 × 1.08 kg a.s./ha |
1 × 0.72 kg a.s./ha | 1 × 0.72 kg a.s./ha once every three years | ||
| Max appl. rate of 2.88 kg a.s./ha treated area in any 12 months period | Max appl. rate of 2.16 kg a.s./ha in any 12 months period | Max appl. rate of 2.88 kg a.s./ha treated area in any 12 months period | Max appl. rate of 2.16 kg a.s./ha treated area in any 12 months period | Max appl. rate of 1.08 kg a.s./ha in any 12 months period | Max appl. rate of 0.72 kg a.s./ha in any 12 months period | Max appl. rate of 3.6 kg a.s./ha in any 12 months period | Maximum application rate of 1.8 kg a.s./ha in any 12 months period | Max appl. rate of 1.08 kg a.s./ha in any 12 months period | Max appl. rate of 0.72 kg a.s./ha in any 12 months period | Max appl. rate of 0.72 kg a.s./ha in any 36 months period | ||||||
| Use No 4a | Use No 4b | Use No 4c | Use No 5a | Use No 5b | Use No 5c | Use No 6a | Use No 6b | Use No 7a | Use No 7b | Use No 8 | Use No 9 | Use No 10a | Use No 10b | Use No 10c | ||
| Operator risk | Risk identified | |||||||||||||||
| Assessment not finalised | ||||||||||||||||
| Worker risk | Risk identified | |||||||||||||||
| Assessment not finalised | ||||||||||||||||
| Resident/bystander risk | Risk identified | |||||||||||||||
| Assessment not finalised | ||||||||||||||||
| Consumer risk | Risk identified | |||||||||||||||
| Assessment not finalised | X2 (e) | X2 (e) | X2 (e) | X2 (e) | X2 (e) | X2 (e) | X2 (e) | |||||||||
| Risk to wild non‐target terrestrial vertebrates | Risk identified | X (d) | X (c) | X (c) | X (d) | X (c) | X (c) | X (d) | ||||||||
| Assessment not finalised | ||||||||||||||||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | |||||||||||||||
| Assessment not finalised | ||||||||||||||||
| Risk to aquatic organisms | Risk identified | |||||||||||||||
| Assessment not finalised | X3 (f) | X3 (f) | X3 (f) | X3 (f) | X3 (f) | X3 (f) | X3 (f) | X3 (f) | X3 (f) | X3 (f) | X3 (f) | X3 (f) | X3 (f) | X3 (f) | X3 (f) | |
| Groundwater exposure to active substance | Legal parametric value breached | |||||||||||||||
| Assessment not finalised | ||||||||||||||||
| Groundwater exposure to metabolites | Legal parametric value breached (a) | |||||||||||||||
| Parametric value of 10 μg/L (b) breached | ||||||||||||||||
| Assessment not finalised | ||||||||||||||||
The superscript numbers, if any, relate to the numbered points indicated in Section 9.1. Where there is no superscript number, see Section 5 for further information.
It should be noted that the classification proposed in the context of this evaluation procedure under Regulation (EC) No 1107/2009 concurs with the harmonised classification and labelling in accordance with Regulation (EC) No 1272/2008.
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission (2003).
High long‐term risk to mammals (identified at tier 1) for 2 or 3 applications of 0.72 kg a.s/ha and for 1, 2 or 3 applications of 1.08 kg a.s/ha. Low risk to mammals for a single application of 0.72 kg a.s./ha.
High long‐term risk to mammals (identified at tier 1) for all scenarios within this representative use.
Rotational crop field trials are required regarding uses on agricultural/cropped land for all crop groups that can be grown in rotation (i.e. not for orchard crops such as kiwi, olives, grapes, citrus, stone, pome and tree fruit), to finalise the livestock dietary burden calculation and the consumer risk assessment.
Assessment not finalised for aquatic macrophytes, only for contact exposure via spray drift.
10. List of other outstanding issues
Remaining data gaps not leading to critical areas of concern or issues not finalised but considered necessary to comply with the data requirements, and which are relevant for some or all of the representative uses assessed at EU level (unless stated otherwise). Although not critical, these data gaps may lead to uncertainties in the assessment and are considered relevant.
These data gaps refer to the representative uses assessed (unless stated otherwise) and are listed in the order of the sections:
n‐octanol/water partition coefficient for metabolite N‐acetyl AMPA (relevant for all representative uses evaluated except that on railway tracks; see Section 1).
Determination of the content of the relevant impurities: formic acid and triethylamine before and after storage of the formulation for representative uses, for 2 years at ambient temperature (relevant for all representative uses evaluated; see Section 1).
Additional validation data for the determination of repeatability and recovery for formaldehyde, and for repeatability for NNG of the method for the determination of impurities in the technical active substance of one source (relevant for Industrias Afrasa and all representative uses evaluated; see Section 1).
Determination of the precision of the method used for the analysis of two non‐relevant impurities in a batch (relevant for Industrias Afrasa and all representative uses evaluated; see Section 1).
Validation data to demonstrate a sufficiently validated LOQ of at least 0.8 g/kg for formaldehyde and 0.8 mg/kg for NNG of the methods for the determination of the impurity in the technical material (relevant for Industrias Afrasa and all representative uses evaluated; see Section 1).
Validation data of the method used for determination of triethylamine in the submitted quality control data for one source (relevant for Industrias Afrasa and all representative uses evaluated; see Section 1).
Validation data to demonstrate a sufficiently validated LOQ of at least 3.2 g/kg for formic acid of the method for the determination of the impurity in the technical active substance (relevant for Albaugh and all representative uses evaluated; see Section 1).
Analysis of formic acid, with a validated method, in 5 representative and recent (within the last 5 years of manufacture) batches according to Good Laboratory Practice (GLP) (relevant for Industrias Afrasa and all representative uses evaluated; see Section 1).
Analysis of formic acid in five representative and recent (within the last 5 years of manufacture) batches according to GLP (relevant to Sinon, and all representative uses evaluated; see Section 1).
Validated analytical method for monitoring of N‐acetyl glyphosate residues in honey (not relevant for the representative uses assessed, but relevant when there is a need to enforce MRLs in imported honey; see Sections 1 and 3).
Analytical report including information on the validation of the analytical method used in a toxicological study (study CA 5.4.2/0.15, report no. 14613.402.078.14) (relevant for all representative uses evaluated; see Sections 1 and 2 and open point in the evaluation table set under experts' consultation point 2.3 (EFSA, 2023a)).
Detailed summary of the QSAR analysis provided to assess the toxicological relevance of the impurities present in the reference specification according to the recommendations given in the ‘Outcome of the pesticides peer review meeting on general recurring issues in mammalian toxicology’ (EFSA, 2018b) (relevant for all representative uses evaluated; see Section 2).
Applicant to clarify the identity (CAS, name, structure) of some of the impurities listed in the composition of the batches used in toxicological studies and to clarify whether these impurities are the ones in the reference specification (relevant for all representative uses evaluated; see Section 2).
A data gap is set to identify whether the DNT findings reported in the studies with glyphosate‐trimesium and with GBHs are due to glyphosate (relevant for all representative uses evaluated; see Section 2).
The aneugenic potential of the metabolite N‐acetyl glyphosate has not been addressed (not relevant for the representative uses assessed, but relevant for future uses on modified crops or consumer risk assessment for animal products as a result of the import of feed that is modified crops or imported honey; see Sections 2 and 3).
The aneugenicity and clastogenicity of metabolites N‐glyceryl AMPA and N‐malonyl AMPA have not been sufficiently investigated. An in vitro micronucleus test will be needed to address the metabolites' clastogenic/aneugenic potential (not relevant for the representative uses assessed, but relevant for future uses on modified crops to ensure safety with respect to minor metabolites; see Sections 2 and 3).
For one of the components of the formulation for representative uses ‘MON 52276’, repeated‐dose toxicity information over short‐ and long term was not available (see Section 2); therefore, in order to allow a final conclusion on the risk assessment of ‘MON 52276’, repeated dose toxicity data for this component (short‐ and long term) should be assessed (relevant for all representative uses evaluated; see Section 2).
Additional field residue trials are needed to complete the data package for the proposed representative uses assessed, as detailed below. For detailed explanation regarding the possibility for extrapolation between crop groups, please refer to the list of endpoints in Appendix B. It should be noted that the number of trials reflect the minimum requirements and more trials than indicated below may be needed if residues > LOQ are obtained either for glyphosate or AMPA (for relevant uses see below and Section 3):
For post‐emergence uses, supervised residue field trials with an acceptable method of analysis validated for glyphosate and AMPA, and analysed within the demonstrated storage stability of residues period for glyphosate and AMPA, are requested for:
Banana: At least three Southern EU (SEU) additional residue trials with AMPA/glyphosate analysed within the demonstrated storage stability period or studies demonstrating a longer storage stability of AMPA in banana are needed (relevant for the representative uses in banana; see Section 3).
Grapes: Six Northern EU (NEU) and seven SEU additional residue trials with AMPA/glyphosate analysed within the demonstrated storage stability period or studies demonstrating a longer storage stability of AMPA are needed. The results from these trials can cover the uses in kiwi, pome fruit, citrus fruit, stone fruit and tree nuts (relevant for the representative uses in grapes, kiwi, pome fruit, citrus fruit, stone fruit and tree nuts; see Section 3).
Table olives: At least four NEU residue trials with analysis of glyphosate and AMPA and at least four SEU residue trials with AMPA/glyphosate analysed within the demonstrated storage stability period are needed (relevant for the representative uses in table olives; see Section 3).
For post‐harvest, pre‐sowing, pre‐planting, pre‐emergence uses, supervised residue field trials with an acceptable method of analysis validated for glyphosate and AMPA, and analysed within the demonstrated storage stability of residues period for glyphosate and AMPA, are requested for:
- Root and tuber vegetables:
-
–carrot: at least two NEU and two SEU additional trials;
-
–potato: at least two NEU and two SEU additional trials;
-
–sugar beet: at least four NEU and two SEU additional trials.
-
–
- Bulb vegetables:
-
–bulb onion: at least two NEU and two SEU additional trials;
-
–spring onion (possible extrapolation from leek, see data gap for leek);
-
–bulb vegetables (other than onion and spring onion): at least three NEU and three SEU additional trials for each.
-
–
- Fruiting vegetables:
-
–cucumber or courgette: at least three NEU and two SEU additional trials;
-
–tomato: at least two NEU and four SEU additional trials.
-
–
- Brassica vegetables:
-
–cauliflower or broccoli: at least two NEU and three SEU additional trials;
-
–head cabbage: at least two NEU and two SEU additional trials;
-
–kale (leafy Brassica): at least three NEU and three SEU additional trials or extrapolation from pre‐emergence trials in lettuce may be considered;
-
–Kohlrabies: at least three NEU and three SEU additional trials.
-
–
- Leaf vegetables:
-
–lettuce (pre‐emergence): at least two NEU and two SEU additional trials;
-
–open leaf lettuce: at least one NEU and three SEU additional trials;
-
–vine leaves: at least three NEU and three SEU additional trials;
-
–witloof: at least three NEU and three SEU additional trials.
-
–
- Stem vegetables:
-
–leek: at least two NEU and one SEU additional trials (extrapolation possible to spring onion);
-
–stem vegetables (other than leek): at least three NEU and three SEU additional trials for each.
-
–
For inter‐row uses, supervised residue field trials with an acceptable method of analysis validated for glyphosate and AMPA, and analysed within the demonstrated storage stability of residues period for glyphosate and AMPA, are requested for:
- Root and tuber vegetables:
-
–carrot: at least four NEU additional trials;
-
–potato: at least four NEU and four SEU additional trials.
-
–
- Bulb vegetables:
-
–bulb onion: at least two NEU and one SEU additional trials;
-
–bulb vegetables (other than onion): at least three NEU and three SEU additional trials for each.
-
–
- Fruiting vegetables:
-
–tomato: at least four NEU additional trials;
-
–cucumber or courgette: at least one NEU additional trials.
-
–
- Leaf vegetables:
-
–open leaf lettuce: at least four NEU and four SEU additional trials;
-
–vine leaves: at least three NEU and three SEU additional trials;
-
–witloof: at least three NEU and three SEU additional trials.
-
–
- Legume vegetables (fresh):
-
–beans (with pods): at least one SEU additional trial.
-
–
Reliable AMPA soil DegT50 endpoints from at least three field trial sites were not available (relevant for all representative uses evaluated; see Section 4).
Further information to address the route of groundwater exposure via bank infiltration and the connectivity of surface water bodies to groundwater aquifers, which can be relevant in some small hydrological catchments and some larger river systems (relevant for all representative uses evaluated; see Section 4).
Although available for some tested formulations, a complete consideration of the composition of formulations used in the literature studies used for the ecotoxicological assessment together with a consideration of whether the tested formulation is comparable to the formulation for representative uses, ‘MON 52276’, was missing (see Section 5).
The potential occurrence of metabolite AMPA in pollen and nectar needs to be further investigated (relevant for all representative uses, see Section 5).
Although the studies were fully considered in the assessments described in Section 5, sufficiently detailed summaries were not provided for several of the literature studies 84 (relevant for all representative uses, see Section 5).
For further addressing the risk to biodiversity via indirect effects and trophic interactions it was considered needed (1) to perform a systematic literature search for data collection; (2) to quantify, in a spatial and temporal context, the direct effects on the weeds (including the impact on the seed bank), non‐target plants, non‐target arthropods and bees in order to inform the extent of potential indirect effects via trophic interactions; (3) to demonstrate how both specific and general mitigation measures may address the impact due to indirect effects (see Section 5).
Abbreviations
- AAOEL
acute acceptable operator exposure level
- AMPA
(aminomethyl)phosphonic acid
- a.s.
active substance
- ADI
acceptable daily intake
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- ALS
amyotrophic lateral sclerosis
- AOEL
acceptable operator exposure level
- AR
applied radioactivity
- ARfD
acute reference dose
- ASD
autism spectrum disorder
- bw
body weight
- CAS
Chemical Abstracts Service
- DNT
developmental neurotoxicity study
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- EAS
oestrogen, androgen and steroidogenesis modalities
- ECHA
European Chemicals Agency
- EEC
European Economic Community
- EPSPS
5‐enolpyruvylshikimate‐3‐phosphate synthase
- ETO
ecological threshold option
- FAO
Food and Agriculture Organization of the United Nations
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
Good Agricultural Practice
- GBH
glyphosate‐based herbicides
- GAT
glyphosate N‐acetyltransferase
- GC–MS
gas chromatography–mass spectrometry
- GGT
gamma glutamyl transferase
- GLP
Good Laboratory Practice
- GOX
glyphosate oxidoreductase
- HR
hazard rate
- InChiKey
International Chemical Identifier Key.
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- JMPR
Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Expert Group on Pesticide Residues (Joint Meeting on Pesticide Residues)
- Kdoc
organic carbon linear adsorption coefficient
- KFoc
Freundlich organic carbon adsorption coefficient
- Koc
normalised organic carbon to water partition coefficient
- LC–MS/MS
liquid chromatography with tandem mass spectrometry
- LOAEL
lowest observable adverse effect level
- LOQ
limit of quantification
- MFFM
multi‐functional field margin
- MOA
mode of action
- MRL
maximum residue level
- MS
Member State
- NNG
N‐Nitroso‐glyphosate
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- OSR
oilseed rape
- PEC
predicted environmental concentration
- PECair
predicted environmental concentration in air
- PECgw
predicted environmental concentration in groundwater
- PECsed
predicted environmental concentration in sediment
- PECsoil
predicted environmental concentration in soil
- PECsw
predicted environmental concentration in surface water
- PPE
personal protective equipment
- QSAR
quantitative structure–activity relationship
- RAC
regulatory acceptable concentration
- RAR
Renewal Assessment Report
- REACH
Registration, Evaluation, Authorisation of Chemicals Regulation
- SFO
single first‐order
- SL
soluble concentrate
- SMILES
simplified molecular‐input line‐entry system
- STMR
supervised trials median residue
- TK
technical concentrate
- TRR
total radioactive residue
- UF
uncertainty factor
- WHO
World Health Organization
Appendix A – Consideration of cut‐off criteria for glyphosate according to Annex II of Regulation (EC) No 1107/2009 of the European Parliament and of the Council
1.
| Properties | Conclusion (a) | |
|---|---|---|
| CMR | Carcinogenicity (C) | Glyphosate is not classified as carcinogen category 1A or 1B from: Harmonised classification according to Regulation (EC) No 1272/2008 and its Adaptations to Technical Process (Table 3.1 of Annex VI of Regulation (EC) No 1272/2008 as amended): CLP00, and proposed classification according to ECHA RAC opinion (May 2022). |
| Mutagenicity (M) | Glyphosate is not classified as mutagen category 1A or 1B from: Harmonised classification according to Regulation (EC) No 1272/2008 and its Adaptations to Technical Process [Table 3.1 of Annex VI of Regulation (EC) No 1272/2008 as amended]: CLP00, and proposed classification according to ECHA RAC opinion (May 2022). | |
| Toxic for Reproduction (R) | Glyphosate is not classified as toxic for reproduction category 1A or 1B from: Harmonised classification according to Regulation (EC) No 1272/2008 and its Adaptations to Technical Process (Table 3.1 of Annex VI of Regulation (EC) No 1272/2008 as amended): CLP00, and proposed classification according to ECHA RAC opinion (May 2022). | |
| Endocrine disrupting properties | Glyphosate is not considered to meet the criteria for endocrine disruption for humans and non‐target organisms according to points 3.6.5 and 3.8.2 of Annex II of Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605. | |
| POP | Persistence | Glyphosate is not considered to be a persistent organic pollutant (POP) according to point 3.7.1 of Annex II of Regulation (EC) No 1107/2009. |
| Bioaccumulation | ||
| Long‐range transport | ||
| PBT | Persistence | Glyphosate is not considered to be a persistent, bioaccumulative and toxic (PBT) substance according to point 3.7.2 of Annex II of Regulation (EC) No 1107/2009. |
| Bioaccumulation | ||
| Toxicity | ||
| vPvB | Persistence | Glyphosate is not considered to be a very persistent, very bioaccumulative substance according to point 3.7.3 of Annex II of Regulation (EC) No 1107/2009. |
| Bioaccumulation | ||
Origin of data to be included where applicable (e.g. EFSA, ECHA RAC, Regulation).
Appendix B – List of end points for the active substance and the formulation for representative uses
1.
Appendix B can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2023.8164
Appendix C – Wording EFSA used in Section 4 of this conclusion, in relation to DT and Koc ‘classes’ exhibited by each compound assessed
1.
| Wording | DT50 normalised to 20°C for laboratory incubations 85 or not normalised DT50 for field studies (SFO equivalent, when biphasic, the DT90 was divided by 3.32 to estimate the DT50 when deciding on the wording to use) |
|---|---|
| Very low persistence | < 1 day |
| Low persistence | 1 to < 10 days |
| Moderate persistence | 10 to < 60 days |
| Medium persistence | 60 to < 100 days |
| High persistence | 100 days to < 1 year |
| Very high persistence | A year or more |
Note these classes and descriptions are unrelated to any persistence class associated with the active substance cut‐off criteria in Annex II of Regulation (EC) No 1107/2009. For consideration made in relation to Annex II, see Appendix A.
| Wording | Koc (either KFoc or Kdoc) mL/g |
|---|---|
| Very high mobility | 0–50 |
| High mobility | 51–150 |
| Medium mobility | 151–500 |
| Low mobility | 501–2,000 |
| Slight mobility | 2,001–5,000 |
| Immobile | > 5,000 |
Based on McCall et al. (1980).
Appendix D – EFSA assessment of residue field trials – primary crops
1.
Appendix D can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2023.8164
Appendix E – Used compound codes
1.
| Code/trivial name (a) | IUPAC name/SMILES notation/InChiKey (b) | Structural formula (c) |
|---|---|---|
| glyphosate |
N‐(phosphonomethyl)glycine C(C(=O)O)NCP(=O)(O)O XDDAORKBJWWYJS‐UHFFFAOYSA‐N |
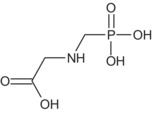
|
| glyphosate‐isopropylammonium |
N‐(phosphonomethyl)glycine ‐ isopropylamine (1:1) or isopropylammonium N‐(phosphonomethyl)glycinate O=C([O‐])CNCP(=O)(O)O.[NH3+]C(C)C ZEKANFGSDXODPD‐UHFFFAOYSA‐N |
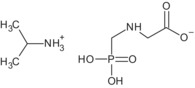
|
| glyphosate‐trimesium |
trimethylsulfonium N‐[(hydroxyphosphinato)methyl]glycine [O‐]P(=O)(O)CNCC(O)=O.C[S+](C)C RUCAXVJJQQJZGU‐UHFFFAOYSA‐M |
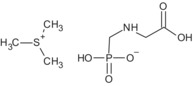
|
| glyphosate‐diammonium |
diammonium N‐(phosphonatomethyl)glycine [NH4+].[NH4+].[O‐]P([O‐])(=O)CNCC(O)=O CPHCYTUHSKEDOI‐UHFFFAOYSA‐N |
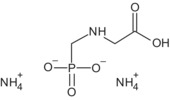
|
| AMPA ((aminomethyl)phosphonic acid) (M02) |
(aminomethyl)phosphonic acid NCP(=O)(O)O MGRVRXRGTBOSHW‐UHFFFAOYSA‐N |
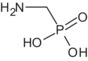
|
| HMPA |
(hydroxymethyl)phosphonic acid OCP(=O)(O)O GTTBQSNGUYHPNK‐UHFFFAOYSA‐N |
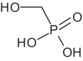
|
| methanediol |
Methanediol OCO CKFGINPQOCXMAZ‐UHFFFAOYSA‐N |
|
| N‐acetyl glyphosate (M04) |
N‐acetyl‐N‐(phosphonomethyl)glycine OC(=O)CN(CP(=O)(O)O)C(C) = O BFECXRMSKVFCNB‐UHFFFAOYSA‐N |
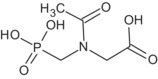
|
| N‐acetyl AMPA (M05) |
(acetamidomethyl)phosphonic acid CC(=O)NCP(=O)(O)O FDNUAHPLMXZWLS‐UHFFFAOYSA‐N |
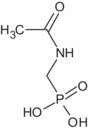
|
| N‐methyl AMPA (M03) |
[(methylamino)methyl]phosphonic acid CNCP(=O)(O)O HSMRCPIZVMDSHN‐UHFFFAOYSA‐N |
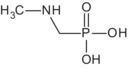
|
| N‐methyl glyphosate (M09) |
N‐methyl‐N‐(phosphonomethyl)glycine CN(CC(=O)O)CP(=O)(O)O SGVDYFNFBJGOHB‐UHFFFAOYSA‐N |
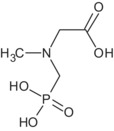
|
| N‐glyceryl AMPA (M06) |
[(2RS)‐(2,3‐dihydroxypropanamido)methyl]phosphonic acid O=C(NCP(=O)(O)O)C(O)CO LFMJDSWPGBSPFL‐UHFFFAOYSA‐N |
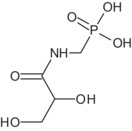
|
| N‐malonyl AMPA (M07) |
3‐oxo‐3‐[(phosphonomethyl)amino]propanoic acid O=C(CC(=O)O)NCP(=O)(O)O XVCISTXLOJFXDA‐UHFFFAOYSA‐N |
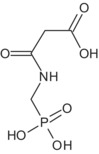
|
| methylphosphonic acid (M08) |
methylphosphonic acid CP(=O)(O)O YACKEPLHDIMKIO‐UHFFFAOYSA‐N |
|
| N‐nitroso‐glyphosate (NNG) |
[nitroso(phosphonomethyl)amino]acetic acid O=NN(CC(=O)O)CP(=O)(O)O BJYYBQPCMQGLLZ‐UHFFFAOYSA‐N |
|
| triethylamine |
N,N‐diethylethanamine CCN(CC)CC ZMANZCXQSJIPKH‐UHFFFAOYSA‐N |
|
| formic acid |
formic acid O=CO BDAGIHXWWSANSR‐UHFFFAOYSA‐N |
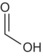
|
| formaldehyde |
Formaldehyde C=O WSFSSNUMVMOOMR‐UHFFFAOYSA‐N |
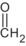
|
The name in bold is the name used in the conclusion.
ACD/Name 2021.1.3 ACD/Labs 2021.1.3 (File Version N15E41, Build 123232, 7 July 2021).
ACD/ChemSketch 2021.1.3 ACD/Labs 2021.1.3 (File Version C25H41, Build 123835, 28 August 2021).
Supporting information
List of end points for the active substance and the formulation for representative uses
EFSA assessment of residue field trials – primary crops
Suggested citation: Álvarez, F. , Arena, M. , Auteri, D. , Binaglia, M. , Castoldi, A. F. , Chiusolo, A. , Crivellente, F. , Egsmose, M. , Fait, G. , Ferilli, F. , Gouliarmou, V. , Nogareda, L. H. , Ippolito, A. , Istace, F. , Jarrah, S. , Kardassi, D. , Kienzler, A. , Lanzoni, A. , … Villamar‐Bouza, L . (2023). Peer review of the pesticide risk assessment of the active substance glyphosate. EFSA Journal, 21(7), 1–52. http://doi.org.10.2903/j.efsa.2023.8164
Requestor European Commission
Question number EFSA‐Q‐2020‐00140
Note/Update This scientific output, approved on 6 July 2023, supersedes the previous output published on 12 November 2015 (EFSA, 2015).
Declaration of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements EFSA wishes to thank the Assessment Group on Glyphosate (AGG), consisting of FR, NL, SE, HU acting jointly as rapporteur Member State, for the preparatory work on this scientific output. In addition, EFSA wishes to acknowledge the experts of the EFSA Working Group on Glyphosate Renewal for their support during the peer review and their contribution to the production of this scientific output, as well the support of the external chairpersons of the Pesticides Peer Review Experts' Teleconferences (Bernadette Ossendorp and Susanne Hougaard Bennekou: TC 80 – Mammalian toxicology; Thomas Poiger: TC 81 – Environmental fate and behaviour; Simon More: TC 82 – Ecotoxicology; Martin Wilks: TC 84 – Mammalian toxicology/ecotoxicology joint session on endocrine disruption).
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Approved: 6 July 2023
Appendix B(List of endpoint)and Appendix D (Appendix D ‐ EFSA assessment of residue field trials primary crops) are available under the supporting information section .
Notes
It is noted that at the time of application (December 2019) there were 9 companies in the consortium of GRG. By the time of dossier submission (June 2020) and thereafter, 8 companies remained in GRG supporting the renewal of approval of glyphosate.
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, p. 26–32.
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Implementing Regulation (EU) 2019/724 of 10 May 2019 amending Implementing Regulation (EU) No 686/2012 as regards the nomination of rapporteur Member States and co‐rapporteur Member States for the active substances glyphosate, lambda‐cyhalothrin, imazamox and pendimethalin and amending Implementing Regulation (EU) No 844/2012 as regards the possibility that a group of Member States assumes jointly the role of the rapporteur Member State. OJ L 124, 13.5.2019, p. 32–35.
Commission Implementing Regulation (EU) No 686/2012 of 26 July 2012 allocating to Member States, for the purposes of the renewal procedure, the evaluation of the active substances whose approval expires by 31 December 2018 at the latest. OJ L 200, 27.7.2012, p. 5–10.
See Evaluation Table, section 1, open point 1.31 (EFSA, 2023a).
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, p. 1–1355.
See experts' consultation point 2.36 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.1 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.32 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation points 2.2 and 2.15 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation points 2.1, 2.2, 2.3, 2.4 (identified following comments by public) and experts' consultation 2.17 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.16 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.14 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.18 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.17 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.5 (identified following comments by the public) at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.19 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.20 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.22 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.7 (identified following comments by the public) at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.21 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.27 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.27 and Annex 8 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.28 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.30 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a). See also Annex 9 to experts' consultation point 2.30.
See experts' consultation point 2.34 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.15 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.35 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See Annex 10 to experts' consultation point 2.33 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
See experts' consultation point 2.33 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
Provided for in Article 4 of Regulation (EC) No 1107/2009.
Set out in Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
CP4‐EPSPS: Tolerance to glyphosate is obtained by the introduction of a gene that codes for the expression of a modified EPSPS (5‐enolpyruvylshikimate‐3‐phosphate synthase) enzyme, making the plant insensitive towards glyphosate EPSPS inhibition. This modification is considered not to have an effect on the nature of the residues of glyphosate upon metabolisation by the plant.
GOX: Glyphosate oxidoreductase, protein obtained by the introduction of a gene from Ochrobactrum anthrop acting by breaking down glyphosate to AMPA and glyoxylate, which have no herbicidal activity. This modification is considered not to have an effect on the nature of the residues of glyphosate upon metabolisation by the plant.
GAT: Glyphosate N‐acetyltransferase, protein obtained by the introduction of a gene from Bacillus licheniformis, giving rise to N‐acetyl glyphosate which denotes no herbicidal activity. This modification is considered to affect the nature of the residues of glyphosate upon metabolisation by the plant by forming N‐acetyl metabolites.
Traditionally bred variety that does not exhibit resistance to glyphosate.
Further information is given in the report of the Pesticides Peer Review Experts' TC 83 under experts' consultation point 3.4 (EFSA, 2023a) and in the EFSA Reasoned Opinion on the review of the existing maximum residue levels (MRLs) for glyphosate according to Article 12 of Regulation (EC) No 396/2005 (EFSA, 2019).
FAO and WHO (2019)). Pesticide residues in food 2019 – Extra Joint FAO/WHO Meeting on Pesticide Residues Evaluation Part I: Residues. Rome. https://www.fao.org/publications/card/en/c/CA6010EN/
Due to the potential presence of glyphosate tolerant sources in the market (e.g. imported products), risk managers should consider to apply the proposed residue definition to all the monitored samples from these crops.
Further information is given in the report of the Pesticides Peer Review Experts' TC 83 under experts' consultation point 3.6 (EFSA, 2023a).
Simulations utilised the agreed Q10 of 2.58 (following EFSA, 2008) and Walker equation coefficient of 0.7.
Hollis, Ramwell, Holman and Whelan, HardSPEC A First‐tier Model for Estimating Surface‐ and Ground‐Water Exposure resulting from Herbicides applied to Hard Surfaces. Updated Technical Guidance on Model Principles and Application for version 1.4.3.2. Version 2.1 April 2017. https://www.hse.gov.uk/pesticides/pesticides-registration/data-requirements-handbook/fate/hardspec/HardSPEC_Guidance.pdf
Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. OJ L 330, 5.12.1998, p. 32–54.
See experts' consultation points 4.3, 4.4, 4.5, 4.6 and 4.7 at the Pesticides Peer Review Experts' TC 81 (EFSA, 2023a).
See experts' consultation point 5.10 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See experts' consultation points 5.12, 5.4 and 5.23 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See experts' consultation point 5.2 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See experts' consultation point 5.4 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See experts' consultation point 5.3 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See experts' consultation point 5.1 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
Root vegetable plants & tuberous plants, bulb plants, fruit‐vegetable plants, Brassica, leaf and stem vegetable plants, sugar beet.
Citrus orchards, stone fruit orchards and pome fruit orchards, kiwi, nut crops, banana, table olives, vines, root vegetable plants & tuberous plants, bulb plants, fruit‐vegetable plants, Brassica, leaf and stem vegetable plants, sugar beet, legume vegetables.
See experts' consultation point 5.7 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See experts' consultation point 5.6 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
Field crops: Root vegetable plants & tuberous plants, bulb plants, fruit‐vegetable plants, Brassica, leaf and stem vegetable plants, sugar beet.
See experts' consultation points 5.3 and 5.4 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See experts' consultation point 5.5 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 93, 3.4.2013, p. 1–84.
See experts' consultation point 5.23 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
The only exception to this pattern was for macrophytes: in that case ‘MON 52276’ presented a slightly lower endpoint, which was retained for the risk assessment of glyphosate as well.
See experts' consultation point 5.15 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See experts' consultation point 5.13 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See experts' consultation point 5.11 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
The reliability of this study and its impact on the risk assessment were discussed at the experts' meeting; see experts' consultation point 5.14 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See experts' consultation point 5.16 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
The reliability of the available toxicity studies as well as the endpoints derived from some of the studies were discussed at the Pesticides Peer Review Experts' TC 82. See experts' consultation points 5.18, 5.19 and 5.20 for further details (EFSA, 2023a).
See experts' consultation point 5.17 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See experts' consultation point 5.21 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See experts' consultation point 5.22 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
Commission Implementing Regulation (EU) 2017/2324 of 12 December 2017 renewing the approval of the active substance glyphosate in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market, and amending the Annex to Commission Implementing Regulation (EU) No 540/2011. OJ L 333, 15.12.2017, p. 10–16.
See experts' consultation point 5.25 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See experts' Annex ‘Glyphosate: biodiversity assessment within the context of Regulation (EC) No 1107/2009: A discussion paper from the EFSA WG on Glyphosate (sub‐group biodiversity)’ to the report of the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See expert consultation point 5.10 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a) for the general discussion on the relevance of the formulations.
See experts' consultation points 5.10 and 5.12 at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See experts' consultation point 5.1 (identified following comments by public) at the Pesticides Peer Review Experts' TC 82 (EFSA, 2023a).
See experts' consultation point 2.30 at the Pesticides Peer Review Experts' TC 80 (EFSA, 2023a).
Commission Regulation (EU) 2018/605 of 19 April 2018 amending Annex II to Regulation (EC) No 1107/2009 by setting out scientific criteria for the determination of endocrine disrupting properties. OJ L 101, 20.4.2018, p. 33–36.
The RMS did not consider in their assessment studies conducted with formulated products.
See experts' consultation point 5.24 at the Pesticides Peer Review Experts' TC 84 (EFSA, 2023a) including its Annexes with the EFSA ED WG report.
See experts' consultation point 2.29 at the Pesticides Peer Review Experts' TC 84 (EFSA, 2023a).
See experts' consultation point 2.23 at the Pesticides Peer Review Experts' TC 84 (EFSA, 2023a).
Refer to Evaluation table section 5, data requirements 5.66, 5.71, 5.72, 5.81 and 5.83 (EFSA, 2023a).
For laboratory soil incubations normalisation was also to field capacity soil moisture (pF2/10 kPa). For laboratory sediment water system incubations, the whole system DT values were used.
References
- AGG (Assessment Group on Glyphosate) , 2021. Renewal Assessment Report (RAR) on the active substance glyphosate prepared by the four rapporteur Member States of AGG, consisting of FR, HU, NL and SE, in the framework of Commission Implementing Regulation (EU) No 844/2012, June 2021. Available online: www.efsa.europa.eu
- AGG (Assessment Group on Glyphosate) , 2023. Revised Renewal Assessment Report (RAR) on glyphosate prepared by the four rapporteur Member States of AGG, consisting of FR, HU, NL and SE, in the framework of Commission Implementing Regulation (EU) No 844/2012, May 2023. Available online: www.efsa.europa.eu
- Cederlund H, 2022. Environmental fate of glyphosate used on Swedish railways — Results from environmental monitoring conducted between 2007–2010 and 2015–2019. Science of The Total Environment, 811, 152361. 10.1016/j.scitotenv.2021.152361 [DOI] [PubMed] [Google Scholar]
- Eaton JL, Cathey AL, Fernandez JA, Watkins DJ, Silver MK, Milne GL, Velez‐Vega C, Rosario Z, Cordero J, Alshawabkeh A and Meeker JD, 2022. The association between urinary glyphosate and aminomethyl phosphonic acid with biomarkers of oxidative stress among pregnant women in the PROTECT birth cohort study. Ecotoxicology and Environmental Safety, 233, 113300. 10.1016/j.ecoenv.2022.113300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHA (European Chemicals Agency) , 2017. Guidance on the Application of the CLP Criteria; Guidance to Regulation (EC) No 1272/2008 on classification, labelling and packaging (CLP) of substances and mixtures. Version 5.0 July 2017. Reference: ECHA‐17‐G‐21‐EN; ISBN: 978–92–9020‐050‐5. Available online: https://echa.europa.eu/guidance-documents/guidance-on-clp
- ECHA (European Chemicals Agency) , 2022. Committee for Risk Assessment (RAC) Opinion proposing harmonised classification and labelling at EU level of glyphosate (ISO); N‐(phosphonomethyl)glycine. CLH‐O‐0000007122‐85‐01/F. Available online: www.echa.europa.eu [Accessed: 30 May 2022].
- ECHA and EFSA (European Chemicals Agency and European Food Safety Authority with the technical support of the Joint Research Centre (JRC)) , Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311, 135 pp. 10.2903/j.efsa.2018.5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2008. Opinion on a request from EFSA related to the default Q10 value used to describe the temperature effect on transformation rates of pesticides in soil. EFSA Journal 2008;6(1):622, 32 pp. 10.2903/j.efsa.2008.622 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2009. Guidance on Risk Assessment for Birds and Mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2013. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014a. EFSA Guidance Document for evaluating laboratory and field dissipation studies to obtain DegT50 values of active substances of plant protection products and transformation products of these active substances in soil. EFSA Journal 2014;12(5):3662, 37 pp. 10.2903/j.efsa.2014.3662 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014b. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products. EFSA Journal 2014;12(10):3874, 55 pp. 10.2903/j.efsa.2014.3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2015. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA Journal 2015;13(11):4302, 107 pp. 10.2903/j.efsa.2015.4302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2018a. Scientific Report on evaluation of the impact of glyphosate and its residues in feed on animal health. EFSA Journal 2018;16(5):5283, 22 pp. 10.2903/j.efsa.2018.5283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2018b. Technical report on the outcome of the pesticides peer review meeting on general recurring issues in mammalian toxicology. EFSA supporting publication 2018;15(9):EN‐1485, 11 pp. 10.2903/sp.efsa.2018.EN-1485 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2019. Review of the existing maximum residue levels for glyphosate according to Article 12 of Regulation (EC) No 396/2005 – revised version to take into account omitted data. EFSA Journal 2019;17(10):5862, 211 pp. 10.2903/j.efsa.2019.5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2023a. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance glyphosate. Available online: www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- EFSA (European Food Safety Authority) , 2023b. Consolidated list of newly available publications on glyphosate raised to EFSA's attention after the public consultation phase until the time point of drafting the EFSA conclusion. Available online: www.efsa.europa.eu
- EFSA (European Food Safety Authority) , Charistou A, Coja T, Craig P, Hamey P, Martin S, Sanvido O, Chiusolo A, Colas M and Istace F, 2022. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment of plant protection products. EFSA Journal 2022;20(1):7032, 134 pp. 10.2903/j.efsa.2022.7032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , 2013. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(7):3290, 186 pp. 10.2903/j.efsa.2013.3290 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , 2014. Scientific Opinion on good modelling practice in the context of mechanistic effect models for risk assessment of plant protection products. EFSA Journal 2014;12(3):3589, 92 pp. 10.2903/j.efsa.2014.3589 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , 2017. Guidance on dermal absorption. EFSA Journal 2017;15(6):4873, 60 pp. 10.2903/j.efsa.2017.4873 [DOI] [Google Scholar]
- European Commission , 2000a. Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000.
- European Commission , 2000b. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000.
- European Commission , 2002. Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. SANCO/10329/2002‐rev. 2 final, 17 October 2002.
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003.
- European Commission , 2010. Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2011. Guidelines on comparability, extrapolation, group tolerances and data requirements for setting MRLs. SANCO 7525/VI/95‐rev. 9 March 2011. pp. 1–46.
- European Commission , 2012. Guidance document on the assessment of the equivalence of technical materials of substances regulated under Regulation (EC) No 1107/2009. SANCO/10597/2003‐rev. 10.1, 13 July 2012.
- European Commission , 2014a. Assessing potential for movement of active substances and their metabolites to ground water in the EU. Report of the FOCUS Workgroup. EC Document Reference SANCO/13144/2010‐v. 3, 613 pp, as outlined in Generic guidance for tier 1 FOCUS groundwater assessment, v. 2.2 May 2014.
- European Commission , 2014b. Guidance document on the renewal of approval of active substances to be assessed in compliance with Regulation (EU) No 844/2012. SANCO/2012/11251‐rev. 4, 12 December 2014.
- European Commission , 2016. Technical guidelines for determining the magnitude of pesticide residues in honey and setting Maximum Residue Levels in honey. SANTE/11956/2016 rev. 9, 14 September 2018.
- European Commission , 2019. Technical guideline on data requirements for setting maximum residue levels, comparability of residue trials and extrapolation of residue data on products from plant and animal origin. SANTE/2019/12752.
- European Commission , 2022. Technical guideline on the evaluation of extraction efficiency of residue analytical methods. SANTE/2017/10632 Rev. 4, 23 February 2022.
- FAO and WHO , 2019. Pesticide residues in food 2019 – Extra Joint FAO/WHO Meeting on Pesticide Residues Evaluation Part I: Residues. Rome. Available online: https://www.fao.org/publications/card/en/c/CA6010EN/
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2001. FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.4 May 2015.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2006. Guidance document on estimating persistence and degradation kinetics from environmental fate studies on pesticides in EU Registration Report of the FOCUS Work Group on Degradation Kinetics. EC Document Reference SANCO/10058/2005‐v. 2.0, 434 pp., as updated by the Generic guidance for Estimating Persistence and Degradation Kinetics from Environmental Fate Studies on Pesticides in EU Registration, v. 1.1 December 2014.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2008. Pesticides in air: considerations for exposure assessment. Report of the FOCUS Working Group on Pesticides in Air. EC Document Reference SANCO/10553/2006‐rev. 2, June 2008.
- Grandcoin A, Piel S and Baurès E, 2017. AminoMethylPhosphonic acid (AMPA) in natural waters: its sources, behavior and environmental fate. Water Research, 117, 187–197. 10.1016/j.watres.2017.03.055 [DOI] [PubMed] [Google Scholar]
- JMPR (Joint Meeting on Pesticide Residues) , 2004. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Rome, Italy, 20–29 September 2004, 383 pp.
- JMPR (Joint Meeting on Pesticide Residues) , 2007. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Geneva, Switzerland, 18–27 September 2007, 164 pp.
- Jianmei L, Smeda RJ, Sellers BA and Johnson WG, 2005. Influence of formulation and glyphosate salt on absorption and translocation in three annual weeds. Weed Science, 53, 153–159. 10.1614/WS-03-075R1 [DOI] [Google Scholar]
- McCall PJ, Laskowski DA, Swann RL and Dishburger HJ, 1980. Measurements of sorption coefficients of organic chemicals and their use in environmental fate analysis. In: Test Protocols for Environmental Fate and Movement of Toxicants. In: Proceedings of the 94th Annual Meeting of the American Association of Official Analytical Chemists (AOAC). October 21–22, Washington, DC. pp. 89–109.
- Merten C, Schoonjans R, Di Gioia D, Peláez C, Sanz Y, Maurici D and Robinson T, 2020. Editorial: exploring the need to include microbiomes into EFSA's scientific assessments. EFSA Journal 2020;18(6):e18061, 7 pp. 10.2903/j.efsa.2020.e18061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 2009. Guidance document on overview of residue chemistry studies. ENV/JM/MONO(2009)31, 28 July 2009.
- OECD (Organisation for Economic Co‐operation and Development) , 2011. OECD MRL calculator: spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. Available online: www.oecd.org
- Satchivi NM, Wax LV, Stoller EW and Briskin DP, 2000. Absorption and translocation of glyphosate isopropylamine and trimethylsulfonium in Abutilon theophrasti and Setaria faberi. Weed Science, 48, 675–679. [Google Scholar]
- Sesin V, Davy CM, Stevens KJ, Hamp R and Freeland JR, 2021. Glyphosate toxicity to native nontarget macrophytes following three different routes of incidental exposure. Integrated Environmental Assessment Management, 17, 597–613. 10.1002/ieam.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETAC (Society of Environmental Toxicology and Chemistry) , Candolfi MP, Barrett KL, Campbell PJ, Forster R, Grandy N, Huet MC, Lewis G, Oomen PA, Schmuck R and Vogt H, 2001. Guidance document on regulatory testing and risk assessment procedures for plant protection products with non‐target arthropods. ESCORT 2 workshop.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the formulation for representative uses
EFSA assessment of residue field trials – primary crops


