Abstract
Indigo carmine (E 312) was re‐evaluated in 2014 by the EFSA Panel on Food Additives and Nutrient sources added to Food (ANS). The ANS Panel confirmed the acceptable daily intake (ADI) of 5 mg/kg body weight (bw) per day for indigo carmine allocated by JECFA (1975). The ANS Panel indicated that the ADI was applicable to a material with a purity of 93% pure colouring and manufactured using processes resulting in comparable residuals as material used in the Borzelleca et al. studies (1985, 1986) and Borzelleca and Hogan (1985) which were the basis for deriving the ADI. The ANS Panel considered that any extension of the ADI to indigo carmine of lower purity and/or manufactured using a different process would require new data to address the adverse effects on the testes observed in the Dixit and Goyal (2013) study. Following a European Commission call for data to submit data to fill the data gaps, an IBO submitted technical and toxicological data. Considering the technical data, the EFSA Panel on Food Additives and Flavourings (FAF Panel) recommended some modifications of the existing EU specifications for E 132, mainly to lower the limits for toxic elements. Considering the toxicological data, an IBO has submitted a 56‐day dietary study to address the adverse effects on testes using a material with 88% purity. The results of this study submitted did not confirm the severe adverse effects observed in the Dixit and Goyal study. Considering all the available information, the Panel confirmed the ADI of 5 mg/kg bw per day for indigo carmine (E 132) disodium salts, meeting the proposed revisions of the specifications (85% minimum for the colouring matter). The Panel concluded that there is no safety concern for the use of indigo carmine (E 132) disodium salts at the reported use levels and submitted analytical data.
Keywords: Indigo Carmine; Indigotine; E 132; disodium 3,3‐dioxo‐2,2‐bi‐indolylidene‐5,5‐disulfonate; aniline; food additive; EINECS 212‐728‐8; FD&C Blue No. 2
Summary
The re‐evaluation of indigo carmine (E 132) was completed by the EFSA ANS Panel in 2014 (EFSA ANS Panel, 2014). The ANS Panel confirmed the acceptable daily intake (ADI) of 5 mg/kg bw per day for indigo carmine allocated by JECFA in 1975 and endorsed by Scientific Committee on Food (SCF) in 1975. The ANS Panel indicated that the ADI was applicable to a material with a purity of 93% pure colouring and manufactured using processes resulting in comparable residuals as material used in the Borzelleca et al. studies (1985, 1986) and Borzelleca and Hogan (1985) which were the basis for deriving the ADI. The ANS Panel considered that any extension of the ADI to indigo carmine of lower purity and/or manufactured using a different process would require new data which would need to address the adverse effects on testes observed in the Dixit and Goyal (2013) study. The chemical identity of the tested food additive, including the presence of possible impurities and/or contaminants, should be investigated and reported.
The data gaps and uncertainties identified by the ANS Panel required a follow‐up by the European Commission by means of a subsequent call for additional data. The present opinion deals with the assessment of the data provided by interested business operators (IBOs) in response to this call.
For this assessment, dietary exposure to indigo carmine (E 132) was newly estimated because food consumption data gathered by EFSA have substantially changed since the time of the re‐evaluation of E 132 in 2014 and new analytical data are available. Based on the current authorisation of E 132 as a food colour, the Panel considered the brand‐loyal scenario as the most relevant exposure scenario for the safety evaluation of indigo carmine (E 132).
New data on toxic elements (lead, mercury, cadmium and arsenic) in samples of indigo carmine (E 132) were submitted by an IBO. The Panel considered the potential presence of these toxic elements in E 132 at (i) the existing limit in EU specifications; (ii) the rounded highest measured value of the level of toxic element. The potential exposure to these impurities from the use of E 132 was compared against the available health‐based guidance values (HBGV) and reference points (RP). The resulting figures show that for As, in both scenarios (i and ii), the lower end of the range of calculated margin of exposure (MOE) values was insufficient. For the other toxic elements (lead, mercury and cadmium), exposure to them due to the use of the food additive does not give rise to safety concerns. Considering the results of the estimation calculated by the Panel and the fact that the food additive is not the only potential dietary source of toxic elements, the Panel recommended that the maximum limits be lowered.
Data on the identity of the unsulfonated aromatic amines present in the food additive E 132, as well as data on their individual lowest achievable limits were requested in the European Commission call for data. Only aniline was identified and analysed at the range of 2.1–69 mg/kg indigo carmine, while no information was provided on the identity of the additional peaks detected in the analysis of unsulfonated primary aromatic amines. The risk assessment for unsulfonated primary aromatic amines was conducted assuming that these unidentified components have a toxicity similar to aniline a probable carcinogen according to IARC (Group 2A).
The Panel calculated the MOEs that would result if (i) aniline was present in E 132 at the rounded highest measured value of aniline (70 mg/kg) and (ii) unsulfonated primary aromatic amines were present at the existing limit (0.01%) in the EU specifications, assuming that they have the same toxicity as aniline. This results in MOEs for both measured aniline and assumed total unsulfonated primary aromatic amines above the target value of 25,000, which resulted from the value available for T25. The MOE for unsulfonated primary aromatic amines would still be larger than the target value even though some aromatic amines are more potent than aniline, but not by much more than factor 10 (Crabtree et al., 1991). Therefore, the Panel considered that the presence of these unsulfonated primary aromatic amines, calculated as aniline, at the specifications limit of 0.01% would not raise a concern.
Information on the solubility of indigo carmine (E 132) (disodium salts) was provided. The Panel took into account the solubility of indigo carmine which is ca. 1.6% (16 g/kg or L) in water at 20°C and noted that this is 32‐times higher than the maximum use level of 500 mg/kg food permitted in the regulation. Taking into account the reported uses and use levels and the MPLs along with the reported solubility, the Panel considered that full dissolution of indigo carmine E 132 (disodium salts) is to be expected in the food and/or in the gastrointestinal tract and that ingested particles (if any) would not persist. Therefore, the Panel concluded there is no concern with regards to the potential presence of small particles, including nanoparticles, in indigo carmine (E 132) (disodium salts) when used as a food additive and considered that a conventional risk assessment of the disodium salts of indigo carmine (E 132) can be performed according to the EFSA Guidance for submission for food additive evaluations (EFSA ANS Panel, 2012). Relevant information according to EFSA Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles (2021) would be needed to confirm the adequacy of a conventional risk assessment also for alternative forms of indigo carmine (i.e. calcium and potassium salts and the aluminium lakes).
As a result of the European Commission call for data, an IBO has also submitted a 56‐day dietary study with the aim to evaluate the effect on the reproductive performance in male mice. The Panel noted that the test item was described with a purity of 88% in terms of colouring matter (disodium salts). No information was provided on the manufacturing process and on the presence of unsulfonated aromatic amines. The Panel noted that no test item‐related adverse effects were observed, in particular on parameters of male reproduction. From this study, the Panel identified a no observed adverse effect level (NOAEL) of 548 mg indigo carmine/kg body weight (bw) per day, the highest dose tested.
The results of this study did not confirm the severe adverse effects observed in the testes in a 45‐day oral toxicity study with adult male mice (Dixit and Goyal, 2013) noted at the time of the re‐evaluation. Further, the results of the 56‐day study support the considerations of the ANS Panel that the adverse effects in the testes reported by Dixit and Goyal (2013) may be ascribed to impurities or contaminants present in the additive tested and not to indigo carmine itself. The Panel noted that the purity of E 132 tested in the 56‐day study was 88%, hence the Panel considered that the current specification of 85% minimum for the colouring matter is still appropriate.
Based on the available database evaluated during the re‐evaluation of indigo carmine (E 132) and the results of the study report submitted as a follow‐up of the re‐evaluation, the Panel confirmed the ADI of 5 mg/kg bw per day for indigo carmine (E 132) disodium salts, meeting the proposed revisions of the specifications.
The exposure estimates at the 95th percentile of indigo carmine (E 132) for infants, toddlers and children would exceed the ADI at the maximum permitted level of use. However, using the available use levels and analytical data, the exposure estimates to indigo carmine (E 132) in the refined brand‐loyal exposure scenario for all population groups were below the ADI.
The Panel concluded that there was no safety concern for the use of indigo carmine (E 132) disodium salts, meeting the proposed revision of the specification, at the reported use levels and submitted analytical data.
1. Introduction
The re‐evaluation of the safety of indigo carmine (E 132) as a food additive under Regulation (EU) No 257/2010 was completed by EFSA in 2014 (EFSA ANS Panel, 2014). The EFSA ANS Panel confirmed the ADI of 5 mg/kg body weight (bw) per day for indigo carmine (E 132) established by JECFA in 1975 (JECFA, 1975) and endorsed by the SCF in the same year (SCF, 1975). In its opinion, the ANS Panel indicated that the ADI was applicable to a material with a purity of 93% pure colouring and manufactured using processes resulting in comparable residuals as material used in the Borzelleca et al. studies (1985, 1986) and Borzelleca and Hogan (1985). In addition, the ANS Panel considered that the EU specifications should be revised in order to restrict the indigo carmine material permitted as a food additive (E 132) to the material for which the ADI is applicable.
The data gaps and uncertainties identified by the ANS Panel required to be followed‐up by the European Commission by means of a subsequent call for additional data. The present opinion deals with the assessment of the data provided by IBOs in response to the European Commission call for scientific and technical data on the permitted food additive indigotine, indigo carmine (E 132).
The Panel noted that in Regulation (EC) No 1333/2008 and Commission Regulation (EU) No 231/2012 the food additive E 132 is always referred to as ‘Indigotine, indigo carmine’. However, for the sake of simplicity only the term indigo carmine (E 132) is used in the present opinion.
1.1. Background and Terms of Reference
1.1.1. Background
The use of food additives is regulated under the European Parliament and Council Regulation (EC) No 1333/2008 on food additives. 1 Only food additives that are included in the Union list, in particular in Annex II to that Regulation, may be placed on the market and used in foods under the conditions of use specified therein. Moreover, food additives shall comply with the specifications as referred to in Article 14 of that Regulation and laid down in Commission Regulation (EU) No 231/2012.
Indigotine, indigo carmine (E 132) is authorised for use as food additives in the Union. Since indigotine, indigo carmine (E 132), was permitted in the Union before 20 January 2009, it belongs to the group of food additives which are subject to a re‐evaluation by the European Food Safety Authority (EFSA), according to Commission Regulation (EU) No 257/2010 2 , and in line with the provisions of Regulation (EC) No 1333/2008.
EFSA completed the re‐evaluation of indigotine, indigo carmine (E 132), as a food additive and published a scientific opinion on 25 July 2014 (EFSA ANS Panel, 2014). In that opinion, EFSA concluded that the ADI of 5 mg/kg bw per day for indigotine, indigo carmine (E 132), is only applicable for a material of at least 93% purity, manufactured using the same or equivalent manufacturing process resulting in the material tested in Borzelleca et al. studies (Borzelleca et al., 1985, 1986) and Borzelleca and Hogan (1985). Consequently, for an extension of this ADI to indigotine, indigo carmine, complying with the current EU specifications for indigotine, indigo carmine (E 132), (which allow for a lower purity of “not less than 85% total colouring matters, calculated as the sodium salt”), new data addressing the adverse effects on testis observed in the Dixit and Goyal (2013) study should be generated. The chemical identity (identity and percentage of colouring matters and non‐colouring components) of the tested food additive, including the presence of possible impurities and/or contaminants, should be investigated and reported. In addition, EFSA expressed concerns with respect to the current limits for unsulphonated aromatic amines in the specifications for E 132.
Therefore, the European Commission published on 9 March 2018 a call for data addressing the recommendations made by EFSA in the scientific opinion on the re‐evaluation of indigotine, indigo carmine (E 132), as a food additive, which led to the submission by the interested business operator (IBO) International Association of Colour Manufacturers (IACM) of new technical and toxicological data on indigotine, indigo carmine (E 132) in November 2020.
Consequently, the European Commission has decided to consult EFSA of this matter.
1.1.2. Terms of Reference
In accordance with Article 29(1)(a) of Regulation (EC) No 178/2002 3 , the European Commission requests the European Food Safety Authority (EFSA) to provide a scientific opinion as regards the safety of the food additive indigotine, indigo carmine (E 132).
In particular, EFSA is requested to:
confirm that the new technical data provided by IBOs adequately support the proposed amendment of the specifications of the food additive indigotine, indigo carmine (E 132).
assess the new toxicity data on the safety of indigotine, indigo carmine (E 132) containing not less than 85% total colouring matters, calculated as the sodium salt, as a food additive.
1.2. Background information
Indigo carmine (E 132) was re‐evaluated by the ANS Panel in 2014 and confirmed an ADI of 5 mg/kg bw per day for indigo carmine, established by JECFA (1975) and endorsed by SCF in 1975.
In its re‐evaluation opinion, the ANS Panel reported that JECFA had established a temporary ADI of 0–2.5 mg/kg bw per day in 1969 that was increased to a full ADI of 0–5 mg/kg bw per day in 1975 (JECFA, 1969, 1975). These ADIs were probably based on the long‐term rat study by Hansen et al. (1966) in which a significantly inhibited growth of male rats was observed at dietary doses of 1000 and 2500 mg/kg bw per day. In this study, the NOAEL was determined to be equivalent to 500 mg/kg bw per day. JEFCA established a temporary ADI in 1969 probably by applying an uncertainty factor of 200 to the above NOAEL. In 1975, JECFA applied an uncertainty factor of 100 to the NOAEL of 500 mg/kg bw per day to derive an ADI of 0–5 mg/kg bw per day for indigo carmine. In 1975 the SCF endorsed an ADI of 0–5 mg/kg bw per day established by JECFA. In the latest evaluation of 1984, taking into consideration more recent studies, the SCF agreed to retain this ADI (SCF, 1984).
The ANS Panel considered that the ADI was applicable to a material with a purity of 93% pure colouring and manufactured using processes resulting in comparable residuals as material used in the Borzelleca et al. studies (1985, 1986) and Borzelleca and Hogan (1985). Given the uncertainties in the database, the ANS Panel was not able to conclude whether the ADI should apply to indigo carmine with lower purity manufactured using these same processes or material manufactured using a different but not equivalent process. The ANS Panel considered that any extension of the ADI to indigo carmine of lower purity and/or manufactured using a different process would require new data which would need to address the adverse effects on testis observed in the Dixit and Goyal (2013) study. The chemical identity of the tested food additive, including the presence of possible impurities and/or contaminants, should be investigated and reported. The ANS Panel acknowledged that the EFSA Guidance for submission for food additives evaluation (EFSA ANS Panel, 2012) requires information on the manufacturing process to identify hazards which may need to be controlled in the specifications.
Furthermore, the ANS Panel considered that the current specifications should be revised in order to restrict the indigo carmine material permitted as a food additive (E 132) to the material for which the ADI is applicable.
In 2015, the EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP Panel) adopted a scientific opinion on the re‐evaluation on the safety and efficacy of the feed additive of indigo carmine for cats and dogs and ornamental fish (EFSA FEEDAP Panel, 2015). In 2017 EFSA received a request from the EC to update the opinion adopted by the FEEDAP Panel in 2015, considering the new data submitted by the applicant. 4
2. Data and methodologies
2.1. Data
The Panel based its assessment on:
Information submitted in response to the public call for data issued by the European Commission (Documentation provided to EFSA 1,2) and additional information submitted during the assessment process by IBOs in response to follow‐up requests from EFSA (Documentation provided to EFSA 3,4,5,6);
Food consumption data from the EFSA Comprehensive European Food Consumption Database (Comprehensive Database), which were used to estimate the dietary exposure to indigo carmine (E 132);
Use levels and analytical data to estimate the dietary exposure to indigo carmine (E 132);
Information from Mintel's Global New Products Database (GNPD) to identify the use of indigo carmine (E 132) in food and beverage products and food supplements. Mintel's GNPD is an online database that contains the compulsory ingredient information present on the label of numerous products.
Following the request for additional data sent by EFSA on 22 December 2021, an IBO requested a clarification teleconference, which was held on 1 March 2022.
2.2. Methodologies
This opinion was formulated following the principles described in the EFSA Guidance on transparency with regard to scientific aspects of risk assessment (EFSA Scientific Committee, 2009) and following the relevant existing guidance documents from the EFSA Scientific Committee.
The FAF Panel assessed the safety of indigo carmine (E 132) as food additives in line with the Guidance for submission for food additive evaluations in 2012 (EFSA ANS Panel, 2012).
In the animal study considered in this assessment, the test substance was administered in feed and the dose in mg/kg bw per day was calculated by the authors of the study report based on these reported concentrations and on reported consumption data for feed, the dose was expressed as ‘equal to mg/kg bw per day’.
3. Assessment
3.1. Identity and specifications
According to Commission Regulation (EU) No 231/2012, indigo carmine (E 132), also named as indigotine, consists essentially of a mixture of disodium 3,3′‐dioxo‐2,2′‐bi‐indo‐lylidene‐5,5′‐disulfonate, its positional isomer disodium 3,3′‐dioxo‐2,2′‐bi‐indolylidene‐5,7′‐disulfonate and subsidiary colouring matters together with sodium chloride and/or sodium sulfate as the principal uncoloured components. The structural formula of the main constituent disodium 3,3′‐dioxo‐2,2′‐bi‐indo‐lylidene‐5,5′‐disulfonate (CAS No 860‐22‐0) is given in Figure 1.
Figure 1.
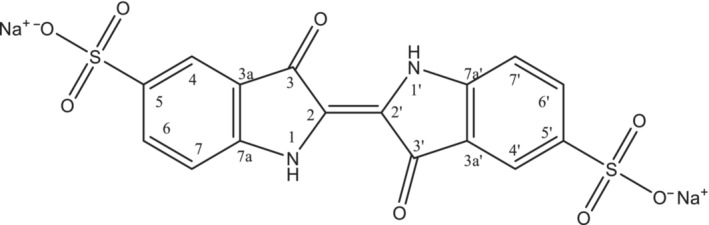
Structural formula of disodium 3,3′‐dioxo‐2,2′‐bi‐indo‐lylidene‐5,5′‐disulfonate
Specifications for indigo carmine (E 132) have been defined in Commission Regulation (EU) No 231/2012 5 and by JECFA (JECFA, 2018) (Table 1).
Table 1.
Specifications for indigo carmine according to Commission Regulation (EU) No 231/2012 and JECFA (2018)
| Purity | Commission Regulation (EU) No 231/2012 | JECFA (2018) |
|---|---|---|
| Synonyms | CI Food Blue 1 | NS No. 132, CI Food Blue 1, CI (1975) No. 73015, indigo carmine, Food Blue No. 2, FD&C Blue No. 2 |
| Definition | Indigotine consists essentially of a mixture of disodium 3,3'dioxo‐ 2,2′‐bi‐indolylidene‐5,5′‐disulfonate, and disodium 3,3′‐dioxo‐2,2′‐bi‐indolylidene‐5,7′‐disulfonate and subsidiary colouring matters together with sodium chloride and/or sodium sulfate as the principal uncoloured components. Indigotine is described as the sodium salt. The calcium and the potassium salt are also permitted. indigo carmine is obtained by sulfonation of indigo. This is accomplished by heating indigo (or indigo paste) in the presence of sulfuric acid. The dye is isolated and subjected to purification procedures |
Indigotine consists of a mixture of disodium 3,3′‐dioxo‐[delta2,2′‐biindoline]‐5,5′‐disulfonate and disodium 3,3′‐dioxo‐[delta2,2′‐biindoline]‐5,7′‐disulfonate and subsidiary colouring matters. Sodium chloride and/or sodium sulfate are the principal uncoloured components. Indigotine is manufactured by heating indigo in the presence of sulfuric acid. The indigo (or indigo paste) is manufactured by the fusion of N‐phenylglycine (prepared from aniline and formaldehyde) in a molten mixture of sodamide and sodium and potassium hydroxides under ammonia pressure. It is isolated and subjected to purification procedures prior to sulfonation Indigotine may be converted to the corresponding aluminium lake in which case only the requirements in the General Specifications for Aluminium Lakes of Colouring Matters apply |
| Colour Index No | 73015 | |
| Einecs | 212‐728‐8 |
860‐22‐0 (5,5′ isomer) |
| CAS number | ||
| Chemical name | Disodium 3,3′‐dioxo‐2,2′‐bi‐indolylidene‐5,5′‐disulfonate |
Disodium 3,3′‐dioxo‐[delta2,2′‐biindoline]‐5,5′‐disulfonate Disodium (2E)‐3‐oxo‐2‐(3‐oxo‐5‐sulfonato‐2,3‐dihydro‐1H‐indol‐2‐ylidene)‐2,3‐dihydro‐1H‐indole‐5‐sulfonate Disodium;(2E)‐3‐oxo‐2‐(3‐oxo‐5‐sulfonato‐1H‐indol‐2‐ylidene)‐1H‐indole‐5‐sulfonate |
| Chemical formula | C16H8N2Na2O8S2 | C16H8N2Na2O8S2 |
| Molecular weight | 466,36 | 466,36 |
| Assay |
≥ 85% total colouring matters (calculated as sodium salt) ≤ 18% disodium 3,3′‐dioxo‐2,2′‐bi‐indolylidene‐5,7′‐disulfonate E1% 1cm 480 at ca. 610 nm in aqueous solution |
≥ 85% total colouring matters. ≤ 18% of disodium 3,3′ dioxo‐[delta‐2,2′‐biindoline]‐5,7′‐disulfonate |
|
Description Appearance of the aqueous solution |
Dark‐blue powder or granules Blue |
Blue powder or granules |
| Identification | ||
| Spectroscopy | Maximum in water at ca. 610 nm |
Maximum in water at ca. 610 nm Determine the UV–visible absorption spectrum of the sample dissolved in water. |
| Solubility | Soluble in water, sparingly soluble in ethanol | |
| Purity | ||
| Loss on drying, chloride and sulfate as sodium salts |
Not more than 15% Determine chloride as sodium chloride, sulfate as sodium sulfate and loss on drying (135°, 6 h) |
|
| Water insoluble matter | ≤ 0.2% | ≤ 0.2% |
| Subsidiary colouring matters | ≤ 1.0% (excluding disodium 3,3′‐dioxo‐2,2′‐bi‐indolylidene‐5,7′‐disulfonate) |
Not more than 18% disodium 3,3′‐dioxo‐[delta2,2′‐biindoline]‐5,7′‐disulfonate (isomeric subsidiary colouring matter) ≤ 1.0% other subsidiary colouring matters |
|
Organic compounds other than colouring matters |
||
|
– Isatin‐5‐sulfonic acid – 5‐sulfoanthranilic acid – Anthranilic acid |
≤ 0.5% |
≤ 0.5% |
| Unsulfonated primary aromatic amines | ≤ 0.01% (calculated as aniline) | ≤ 0.01% (calculated as aniline) |
| Ether extractable matter | ≤ 0.2% (under neutral conditions) | ≤ 0.2% |
| Arsenic | ≤ 3 mg/kg | – |
| Lead | ≤ 2 mg/kg | ≤ 2 mg/kg |
| Mercury | ≤ 1 mg/kg | – |
| Cadmium | ≤ 1 mg/kg | – |
The ANS Panel noted that the specifications for the purity of indigo carmine would permit concentrations of unsulfonated aromatic amines to be present in concentrations of up to 100 mg/kg indigo carmine (calculated as aniline) (EFSA ANS Panel, 2014).
The Panel noted that EINECS/EC number, name, chemical formula and molecular weight provided in the EU specifications for E 132 (Commission Regulation (EU) No 231/2012) correspond to the disodium salt of 3,3′‐dioxo‐2,2′‐bi‐indolylidene‐5,5′‐disulfonate despite according to the definition of the food additive, calcium and the potassium salts are also permitted. The Panel also noted that aluminium lakes 6 of E 132 are authorised to be used as food additive.
The Panel noted that no CAS number is included in the EU specifications and CAS No 860‐22‐0 corresponds to the EINECS/EC No 212‐728‐8.
According to the IBO, the samples used in the different analyses/studies for which data have been submitted were principally the disodium salt of 2‐(1,3‐dihydro‐3‐oxo‐5‐sulfo‐2H‐indol‐2‐ylidene)‐2,3‐dihydro‐3‐oxo‐1H‐indole‐5‐sulfonic acid (CAS No. 860‐22‐0) with smaller amounts of the disodium salt of 2‐(1,3‐dihydro‐3‐oxo‐7‐sulfo‐2H‐indol‐2‐ylidene)‐2,3‐dihydro‐3‐oxo‐1H‐indole‐5‐sulfonic acid (CAS No. 54947‐75‐0) and the sodium salt of 2‐(1,3‐dihydro‐3‐oxo‐2H‐indol‐2‐ylidene)‐2,3‐dihydro‐3‐oxo‐1H‐indole‐5‐sulfonic acid (indigo sulfonic acid, CAS No. 605‐18‐5) (Documentation provided to EFSA No 5). Figure 2 shows the structures of disodium salt of 2‐(1,3‐dihydro‐3‐oxo‐7‐sulfo‐2H‐indol‐2‐ylidene)‐2,3‐dihydro‐3‐oxo‐1H‐indole‐5‐sulfonic acid and indigo sulfonic acid.
Figure 2.
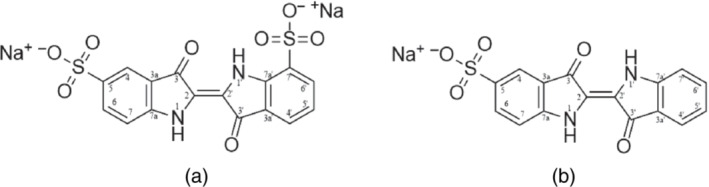
Structural formula of (a) disodium salt of 2‐(1,3‐dihydro‐3‐oxo‐7‐sulfo‐2H‐indol‐2‐ylidene)‐2,3‐dihydro‐3‐oxo‐1H‐indole‐5‐sulfonic acid and (b) indigo sulfonic acid
The Panel noted that indigo sulfonic acid, an intermediate in one of the routes of the manufacturing process (see Section 3.2.1), is not explicitly listed as an impurity in the EU specifications for E 132 but it can be considered to be part of the ‘subsidiary colouring matters’ mentioned in the definition of the food additive.
3.2. Technical data submitted
The following was requested in the European Commission call for data 7 :
Data on the lowest achievable limits for the impurities of toxic elements (arsenic, lead, mercury and cadmium) in E 132: The current maximum limits for those impurities set in the EU specifications for indigo carmine (E 132) in Commission Regulation (EU) No 231/20126 are too high and therefore should be revised to ensure that food additives will not be a significant source of exposure to those toxic elements in food. Therefore, data on the lowest achievable limits for arsenic, lead, mercury and cadmium in E 132 are requested.
Data on the identity of unsulfonated aromatic amines and their lowest achievable limits in E 132: The current EU specifications for indigo carmine (E 132) allow for the presence of not more than 0.01% unsulfonated aromatic amines (calculated as aniline). Data are requested on the identity of the unsulfonated aromatic amines present in the food additive E 132, as well as data on their individual lowest achievable limits.
3.2.1. Information on manufacturing process
According to IBO (Documentation provided to EFSA No 3), three manufacturing processes are used for the synthesis of indigo carmine.
N‐Phenylglycine is self‐coupled in the presence of sodium amide (NaNH2) and a strong hydroxide base solution (NaOH/KOH) under an ammonia atmosphere to form indigo that is subsequently used for the production of indigo carmine as described below.
Indole is oxidatively coupled using cumene hydroperoxide in the presence of a molybdenum hexacarbonyl catalyst to produce indigo, which is subsequently disulfonated with sulfuric acid to produce indigo sulfonic acid. This is then converted to indigo carmine by treatment with sodium hydroxide.
Synthetic indigo is directly sulfonated to produce 5,5′‐indigosulfonic acid, which is subsequently reduced to leuco‐5,5′‐indigosulfonic acid. The leuco‐5,5′‐indigosulfonic acid is oxidised to disodium‐5,5′‐indigosulfonate.
It has been stated that natural indigo is uncommonly used for the production of indigo carmine.
3.2.2. Impurities
3.2.2.1. Toxic elements
An IBO has submitted data on concentration levels of toxic elements (Pb, Hg, Cd and As) on behalf of three manufacturers (Documentation provided to EFSA No 1 and 3). One of the manufacturers reported values as being below their reporting limits (i.e. 1, 2, 1 and 1 mg/kg for As, Pb, Hg and Cd, respectively) without indicating the corresponding limit of quantifications (LOQs) which therefore cannot be used for the risk assessment. Summary of the information submitted by the other two IBOs is presented in Table 2.
Table 2.
Summary information submitted by IBOs (Documentation provided to EFSA No 1 and 3, call and Nov 2021)
| Manufacturer | No Lots | No of lots below LOQ | Min‐max quantified level (mg/kg) reported | |
|---|---|---|---|---|
| Pb (a) | A | 43 | 39 (d) | 0.11–0.2 |
| B | 29 | 0 | 0.97–1.48 | |
| Hg (b) | A | 43 | 37 (d) | 0.2–0.6 |
| B | 29 | 2 (e) | 0.10–0.35 | |
| Cd (c) | A | 43 | 43 (d) | |
| B | 29 | 5 (e) | 0.03–0.09 | |
| As (a) | A | 43 | 43 (d) | |
| B | 29 | 2 (e) | 0.36–0.93 |
Method 7.01 established by the US Food and Drug Administration, ‘Determination of lead and arsenic in Certifiable Color Additives and Color Additive Lakes by X‐ray Fluorescence Spectrometry’ (Documentation provided to EFSA No 3).
Method 7.05 established by the US Food and Drug Administration, ‘Determination of mercury in Certifiable Color Additives and Color Additive Lakes by Automated Microwave Digestion and Dedicated Mercury Analyzer’ (Documentation provided to EFSA No 3).
Method to measure cadmium vary from manufacturer to manufacturer (Documentation provided to EFSA No 3).
Limit of quantification (LOQ) of 0.1 mg/kg (Documentation provided to EFSA No 3).
Unknow LOQ; reported as ‘not detected’.
The IBO proposed to maintain the current limits in the EU specifications for toxic elements (Documentation provided to EFSA No 1).
3.2.2.2. Unsulfonated primary aromatic amines
The IBO submitted information on six batches of indigo carmine analysed for the levels and identification of unsulfonated primary aromatic amines that may be present as impurities (Documentation provided to EFSA No 1 and 3). The samples were analysed using a method based on solvent extraction followed by gas chromatography coupled to a nitrogen/phosphorous‐specific detector, a sulfur‐specific (chemiluminescence) detector or to mass spectrometry, respectively (GC–NPD, GC–SPD and GC–MS). No derivatisation was applied and therefore the analysis was capable only for the detection of substances that would be volatile under the GC conditions used. The samples were not analysed in parallel, using for example the colorimetric method for measurement of total unsulfonated primary aromatic amines that is referred to in the JECFA specifications for indigo carmine, which is a more sensitive and a more comprehensive method, albeit non‐specific. Such an analysis would have provided an indication of the total content of unsulfonated primary aromatic amines that the GC‐based approach could then aim to account for. The sample preparation steps used before the GC‐based analysis also had several weaknesses and the reported data are therefore considered by the Panel to be incomplete with respect to the detection and identification of unsulfonated primary aromatic amines possibly present as impurities and to be semi‐quantitative only.
The six batches were reported to contain aniline, based on retention time by GC‐NPD, at 2.1, 2.5, 2.9, 8.9, 50 and 69 mg/kg indigo carmine. The sample containing the highest aniline concentration was re‐analysed by GC–MS and aniline was confirmed as present.
In addition to aniline that was in all six samples of indigo carmine, the GC‐NPD analysis detected a further nine nitrogen‐containing peaks/substances in two of the six samples, corresponding to the two samples in which the highest content of aniline was measured. Based on retention times, six peaks/substances were common to both of the samples while a further three peaks/substances were unique to each of the two samples in turn. Therefore, in total, these two samples contained 12 nitrogen‐containing peaks/substances in addition to aniline. One peak/substance that was common to both samples, eluted earlier than aniline whereas all of the other 11 nitrogen‐containing peaks eluted later than aniline and therefore are likely to have higher molecular weight (based on the lower volatility). For the sample showing the highest abundance, the nine nitrogen‐containing peaks/substances were individually in the range of 1.1. to 24.2 mg/kg and the nine totalled 42 mg/kg, when assuming they each had the same GC‐NPD response factor as aniline. For the other sample, the nine nitrogen‐containing peaks/substances were individually in the range of 0.8 to 4.1 mg/kg and the nine totalled 17 mg/kg when making the same assumption on response factors. None of these 12 nitrogen‐containing peaks/substances were detected in the other four batches of indigo carmine that were analysed.
The Panel noted that these data are subject to considerable uncertainty. Apart from aniline which was identified and quantified by comparison to an analytical standard, there was no information provided on the identity of the additional peaks detected. These peaks/substances are likely to contain nitrogen (being detected by the NPD) but the failure of the GC–MS analysis that was attempted prevents any conclusion on whether they may be unsulfonated primary aromatic amines or some other nitrogen‐containing impurities.
3.2.3. Solubility
Three batches of indigo carmine (disodium salts) were tested for solubility in water at 20°C using the OECD test method 105 (27 July 1995) followed by centrifugation and quantification of dissolved indigo carmine using an in‐house validated high‐pressure liquid chromatography (HPLC) method (Documentation provided to EFSA No 4). The Panel noted that the performed solubility test was not fully in line with the EFSA Guidance on Technical Requirements (EFSA Scientific Committee, 2021) as the recommended ultrafiltration step was not applied; the samples were simply centrifuged rather than ultra‐centrifuged which is an acceptable alternative to the ultrafiltration step. There was some scatter in the data reported for the three batches tested with three different equilibration times (1, 2 and 3 days). The solubility results were in the range of 1.25 to 1.90% and the average of the nine results was 1.59% (15.9 g/kg water at 20°C). This result is consistent with the solubility of indigo carmine that was reported to be 1.6% at 20°C at the time of the re‐evaluation (EFSA ANS Panel, 2014).
Tests were conducted for the rate of dissolution of three batches of indigo carmine (disodium salts) in water containing 40 mM NaCl and 85 mM NaHCO3 in a stirred system at 30°C (Documentation provided to EFSA No 4). Portions were withdrawn at intervals and subjected to an HPLC analysis for indigo carmine both with‐ and without prior filtration through a glass microfibre (GMF) filter with 1.2 μm nominal particle retention and a dispersed oil penetration (DOP) 0.3 μm value of < 0.001%. For the filtered samples, the results were inconsistent, there was material retained on the GMF filter at every sampling point and the filter would not be capable of retaining particles at the nanoscale. For the unfiltered samples, it cannot be excluded that suspended particulates were present and subsequent dissolved after removal from the stirred system, because the withdrawn aliquots (nominally 20,000 mg/L) were diluted 200‐fold to 100 mg/L for the subsequent HPLC analysis. The Panel concluded that these results cannot be used to conclude on the dissolution rate of indigo carmine because of these limitations.
3.3. Exposure assessment
A new exposure assessment to indigo carmine (E 132) has been performed because food consumption data gathered by EFSA have substantially changed since the time of the re‐evaluation of E 132 in 2014 and new analytical data are available.
3.3.1. Authorised uses and use levels
Maximum levels of indigo carmine (E 132) have been defined in Annex II to Regulation (EC) No 1333/2008 on food additives, as amended. In this document, these levels are named maximum permitted levels (MPLs).
Currently, indigo carmine (E 132) belongs to Group III food colours with combined maximum limit and as such is authorised in 37 food categories. Annex A lists the 37 food categories in which indigo carmine (E 132) is authorised.
3.3.2. Exposure data
Indigo carmine (E 132) is authorised at MPLs in all food categories (see Annex A). To assess the actual dietary exposure to this food additive, concentration data (use levels and/or analytical data) are required.
3.3.2.1. Reported use levels of indigo carmine (E 132)
Data on the occurrence of indigo carmine (E 132) in food were collected at the time of its the re‐evaluation by the ANS Panel by means of a call for data launched in 2013. In response to this call, seven use levels were submitted to EFSA by industry (EFSA ANS Panel, 2014). 8
3.3.2.2. Summarised data on analytical results of indigo carmine (E 132) provided by Member States
In total, 6281 analytical results were reported to EFSA between 2013 and 2022.
Data coming from UK (n = 171), from non‐accredited laboratories (n = 112) and qualitative results (n = 1,591) were discarded.
A total of 4,407 data remained coming from 14 countries: Austria (n = 478), Belgium (n = 604), Germany (n = 2,988), Denmark (n = 35), Spain (n = 269), France (n = 120), Greece (n = 83), Croatia (n = 89), Hungary (n = 1,065), Italy (n = 142), Lithuania (n = 12), Luxembourg (n = 95), Montenegro (n = 17), Slovakia (n = 113). These data were mainly for confectionary (FC 05.2), edible ice (FC 03), fine bakery wares (FC 07.2) and flavoured drinks (FC 14.1.4). Foods were sampled between 2013 and 2021. The majority of analytical results on indigo carmine (E 132) were either not quantified (< LOQ) in 1,719 samples or not detected (< LOD) in 2,619 samples. 69 samples had quantified values ranging from 0.04 to 213.4 mg/kg.
Annex B shows the analytical results of indigo carmine (E 132) in foods as reported by Member States.
3.3.2.3. Summarised data extracted from the Mintel's Global New Products Database
Mintel's GNPD is an online database which monitors new introductions of packaged goods in the market worldwide. It contains information of over 4 million food and beverage products of which more than 1,290,000 are or have been available on the European food market. Mintel started covering EU's food markets in 1996, currently having 24 out of its 27 member countries, Norway and UK presented in the Mintel GNPD. 9
For the purpose of this Scientific Opinion, Mintel's GNPD was used for checking the labelling of food and beverage products and food supplements for indigo carmine (E 132) within the EU's food market as the database contains the compulsory ingredient information on the label.
According to Mintel's GNPD, indigo carmine (E 132) was labelled on a few products (n = 481) of confectionary, vitamins and dietary supplements and chocolates products.
Annex C lists the percentage of the food products labelled with indigo carmine (E 132) out of the total number of food products per food subcategory according to Mintel's GNPD food classification. The percentages ranged from less than 0.1% in many food subcategories to 6.7% in Mintel's GNPD food subcategory ‘Mixed Assortments’. The average percentage of foods labelled to contain indigo carmine (E 132) was 0.3%.
3.3.2.4. Food consumption data used for the exposure assessment
EFSA Comprehensive European Food Consumption Database
Since 2010, the EFSA Comprehensive European Food Consumption Database (Comprehensive Database) has been populated with national data on food consumption at a detailed level. Competent authorities in the European countries provide EFSA with data on the level of food consumption by the individual consumer from the most recent national dietary survey in their country (cf. Guidance of EFSA on the ‘Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment’) (EFSA, 2011). The version of the Comprehensive database taken into account in the exposure assessment was published in December 2022. 10 Data from EU Member States were considered for the estimations.
The food consumption data gathered by EFSA were collected by different methodologies and thus direct country‐to‐country comparisons may not be appropriate. Depending on the food category and the level of detail used for exposure calculations, uncertainties could be introduced owing to possible subjects' underreporting and/or misreporting of the consumption amounts. Nevertheless, the EFSA Comprehensive Database includes the currently best available food consumption data across Europe.
Food consumption data from the following population groups were used for the exposure assessment: infants, toddlers, children, adolescents, adults and the elderly. For the present assessment, food consumption data were available from 41 different dietary surveys carried out in 23 EU Member States (Table 4). Since more dietary surveys are available in the EFSA comprehensive database compared to 2016, more countries are now considered in each population group. As the 95th percentile of exposure was only calculated for those population groups (Table 3) with a sufficiently large sample size (EFSA, 2011), in the present assessment, it was not estimated for infants from Italy and France, for toddlers from Belgium and Italy and for adolescents from Estonia.
Table 4.
Summary of dietary exposure to indigo carmine (E 132) from its use as a food additive in the regulatory maximum level exposure assessment scenario, and refined exposure assessment scenario in six population groups (minimum – maximum across the dietary surveys in mg/kg bw per day)
| Infants (12 weeks‐11 months) | Toddlers (12–35 months) | Children (3–9 years) | Adolescents (10–17 years) | Adults (18–64 years) | The elderly (≥ 65 years) | |
|---|---|---|---|---|---|---|
| Regulatory maximum level exposure assessment scenario | ||||||
|
0.05–1.3 0.2–6.2 |
0.6–3.9 1.7–9.6 |
0.6–3.7 1.5–7.4 |
0.4–1.9 1.1–4.1 |
0.2–1.2 0.6–3.2 |
0.1–0.7 0.4–1.6 |
| Refined exposure assessment scenario | ||||||
| Brand‐loyal refined scenario | ||||||
|
0.001–0.05 0.01–0.31 |
0.02–0.44 0.06–1.26 |
0.05–0.41 0.14–0.91 |
0.03–0.22 0.11–0.53 |
0.02–0.12 0.06–0.35 |
0.01–0.04 0.03–0.14 |
| Non‐brand‐loyal refined scenario | ||||||
|
< 0.001–0.01 0.001–0.09 |
0.002–0.05 0.01–0.18 |
0.007–0.05 0.03–0.16 |
0.004–0.02 0.01–0.09 |
0.002–0.02 0.01–0.06 |
0.001–0.01 0.003–0.05 |
bw: body weight.
Table 3.
Population groups considered for the exposure estimates of indigo carmine (E 132)
| Population | Age range | Countries with food consumption surveys covering more than 1 day |
|---|---|---|
| Infants | From more than 12 weeks up to and including 11 months of age | Bulgaria, Cyprus, Denmark, Estonia, Finland, France, Germany, Italy, Latvia, Portugal, Slovenia |
| Toddlers (a) | From 12 months up to and including 35 months of age | Belgium, Bulgaria, Cyprus, Denmark, Estonia, Finland, France, Germany, Hungary, Italy, Latvia, Netherlands, Portugal, Slovenia, Spain |
| Children (b) | From 36 months up to and including 9 years of age | Austria, Belgium, Bulgaria, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Latvia, Netherlands, Portugal, Spain, Sweden |
| Adolescents | From 10 years up to and including 17 years of age | Austria, Belgium, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Latvia, Netherlands, Portugal, Romania, Slovenia, Spain, Sweden |
| Adults | From 18 years up to and including 64 years of age | Austria, Belgium, Croatia, Cyprus, Czechia, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Netherlands, Portugal, Romania, Slovenia, Spain, Sweden |
| The elderly (b) | From 65 years of age and older | Austria, Belgium, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Netherlands, Portugal, Romania, Slovenia, Spain, Sweden |
The term ‘toddlers’ in the Comprehensive Database (EFSA, 2011) corresponds to ‘young children’ in Regulations (EC) No 1333/2008 and (EU) No 609/2013.
The terms ‘children’ and ‘the elderly’ correspond, respectively, to ‘other children’ and the merge of ‘elderly’ and ‘very elderly’ in Comprehensive Database (EFSA, 2011).
Consumption records were codified according to the FoodEx2 classification system (EFSA, 2015). Nomenclature from the FoodEx classification system was linked to the food categorisation system (FCS) as presented in Annex II of Regulation (EC) No 1333/2008, part D, to perform the exposure assessments. In practice, the FoodEx2 food codes were matched to the FCS food categories.
3.3.2.5. Food categories considered for the exposure assessment of indigo carmine (E 132)
The food categories for which MPLs are set and/or, use levels and analytical data of indigo carmine (E 132) were provided were selected from the nomenclature of the Gundert?Remy, U. (FoodEx classification system), at the most detailed level possible (up to FoodEx Level 4) (EFSA, 2011).
Few food categories or their restrictions/exceptions are not referenced in the EFSA Comprehensive Database and could therefore not be considered in the present assessment. This was the case for four food categories (Annex D) and may have resulted in an underestimation of the exposure. The food categories which were not taken into account are listed below:
-
–
01.7.1 Edible cheese rind.
-
–
04.2.4.1 Fruit and vegetable preparations excluding compote, only mostarda di frutta.
-
–
06.6 Batters, only batters for coating.
-
–
08.3.3 Casings and coatings and decorations for meat, only decorations and coatings except edible external coating of pasturmas and only edible casings.
Annex D indicates the concentration levels of indigo carmine (E 132) used in the exposure assessment scenarios.
3.3.3. Exposure estimates to indigo carmine (E 132)
The Panel estimated the chronic dietary exposure to indigo carmine (E 132) for the following population groups: infants, toddlers, children, adolescents, adults and the elderly. The methodology to estimate dietary exposure to indigo carmine (E 132) for the different scenarios – regulatory maximum level exposure assessment scenario and refined exposure assessment scenarios (brand‐loyal and non‐brand‐loyal) – presented in the current assessment are described in the approach for the refined exposure assessment of food additives under re‐evaluation (EFSA ANS Panel, 2017).
Table 4 summarises the estimated exposure to indigo carmine (E 132) from its use as a food additive in six population groups according to the different exposure scenarios. Detailed results per population group and survey are presented in Annex E.
In the regulatory maximum level exposure assessment scenario, mean exposure to indigo carmine (E 132) from its use as a food additive ranged from 0.05 mg/kg bw per day in infants to 3.9 mg/kg bw per day in toddlers. At the 95th percentile, exposure to indigo carmine (E 132) ranged from 0.2 mg/kg bw per day in infants to 9.6 mg/kg bw per day for toddlers.
In the refined estimated exposure scenario, in the brand‐loyal scenario, mean exposure to indigo carmine (E 132) from its use as a food additive ranged from 0.001 mg/kg bw per day in infants to 0.44 mg/kg bw per day in toddlers. At the 95th percentile, exposure to indigo carmine (E 132) ranged from 0.01 mg/kg bw per day in infants to 1.26 mg/kg bw per day in toddlers. In the non‐brand‐loyal scenario, mean exposure to indigo carmine (E 132) from its use as a food additive ranged from 0.001 mg/kg bw per day in infants to 0.05 mg/kg bw per day in toddlers and children. At the 95th percentile, exposure to indigo carmine (E 132) ranged from 0.001 mg/kg bw per day in infants to 0.18 mg/kg bw per day in toddlers.
Main contributing food categories
In the regulatory scenario, for infants, toddlers and children flavoured fermented milk products including heat‐treated products is the main food category contributing to the mean exposure. For all population groups, flavoured drinks and fine bakery wares contributes mainly to the mean exposure to indigo carmine (E 132).
In the refined brand‐loyal scenario, main contributing food categories are:
-
–
flavoured drinks for all population groups;
-
–
edible ices for infants, toddlers, children and the elderly;
-
–
fine bakery wares for infants and the elderly.
In the refined non‐brand‐loyal scenario, main contributing food categories are:
-
–
desserts for all population groups;
-
–
flavoured drinks and fine bakery wares;
-
–
flavoured fermented milk products including heat‐treated products for infants and toddlers;
-
–
Potato‐, cereal‐, flour‐ or starch‐based snacks for toddlers, children, adolescents and adults.
See Annex F for the detailed contributing food categories.
In accordance with the guidance provided in the EFSA opinion related to uncertainties in dietary exposure assessment (EFSA, 2007), potential sources of uncertainties in the exposure assessment of indigo carmine (E 132) have been considered and are summarised in Annex G.
Indigo carmine (E 132) is authorised in 37 food categories (mostly with numerical MPL and at quantum satis for two food categories). Information from the Mintel GNPD (Annex C) indicated that indigo carmine (E 132) is used in 17 food categories, on average in 0.3% of the foods belonging to these food categories (maximum is 6.8% of foods belonging to Mintel sub‐category ‘Mixed Assortments’ i.e. confectionary). Most of the Mintel sub‐category in which indigo carmine (E 132) is labelled are included in the current exposure assessment, considering 100% of the foods belonging to an authorised food category contained the additive.
Given these observations, the Panel considered overall that the uncertainties identified resulted in an overestimation of the exposure to Indigo carmine (E 132) from its use as a food additive according to Annex II for the regulatory maximum level exposure scenario. Based on the assumption that the food additive is not used in those food categories in which it is permitted but for which no use data were provided by the stakeholders, also the refined scenario would in general result in an overestimation of exposure.
3.4. Proposed revision to existing EU specifications
The potential exposure to impurities from the use of indigo carmine (E 132) can be calculated by assuming that the impurity is present in the food additive up to a limit value, and then by calculation pro‐rata to the estimates of exposure to the food additive itself.
For the current assessment, considering the refined brand‐loyal exposure scenario as the most representative, the highest exposure levels for the mean and 95th percentile among the different population groups were considered, i.e. 0.44 and 1.26 mg/kg bw per day, for toddlers, respectively (Table 4, Section 3.3).
The level of impurities in the food additive combined with the estimated intakes of E 132, presented in Table 4, could result in an exposure which can be compared with the following reference points (RP) or HBGV (Table 5) for the undesirable impurities.
Table 5.
Reference points and HBGV for impurities potentially present in indigo carmine (E 132)
| Impurity HBGV/RP | Basis/Reference |
|---|---|
| Lead (Pb)/0.5 μg/kg bw per day (BMDL01) |
The reference point is based on a study demonstrating perturbation of intellectual development in children with the critical response size of 1 point reduction in IQ. The EFSA CONTAM Panel mentioned that a 1 point reduction in IQ is related to a 4.5% increase in the risk of failure to graduate from high school and that a 1 point reduction in IQ in children can be associated with a decrease of later productivity of about 2%. A risk cannot be excluded if the exposure exceeds the BMDL01 (MOE lower than 1). EFSA CONTAM Panel (2010) |
| Mercury (Hg)/4 μg/kg bw per week (TWI) |
The HBGV was set using kidney weight changes in male rats as the pivotal effect. Based on the BMDL10 of 0.06 mg/kg bw per day, expressed as mercury, and an uncertainty factor of 100 to account for inter and intra species differences, with conversion to a weekly basis and rounding to one significant figure, a TWI for inorganic mercury of 4 μg/kg bw per week, expressed as mercury was established. EFSA CONTAM Panel (2012) |
| Cadmium (Cd)/2.5 μg/kg bw per week (TWI) |
The derivation of the reference point is based on a meta‐analysis to evaluate the dose–response relationship between selected urinary cadmium and urinary beta‐2‐microglobulin (B2M) as the biomarker of tubular damage recognised as the most useful biomarker in relation to tubular effects. A group‐based BMDL5 of 4 μg Cd/g creatinine for humans was derived. A chemical specific adjustment factor of 3.9 was applied to account for human variability in urinary cadmium within each dose‐subgroup in the analysis resulting in a reference point of 1.0 μg Cd per g creatinine. In order to remain below 1 μg Cd/g creatinine in urine in 95% of the population by age 50, the average daily dietary cadmium intake should not exceed 0.36 μg Cd/kg bw, corresponding to a weekly dietary intake of 2.5 μg Cd/kg bw. EFSA CONTAM Panel (2009a) |
| Arsenic (As)/0.3–8 μg/kg bw per day (BMDL01) |
The reference point is based on a range of benchmark dose lower confidence limit (BMDL01) values between 0.3 and 8 μg/kg bw per day identified for cancers of the lung, skin and bladder, as well as skin lesions. In general, the MOE should be at least 10,000 if the reference point is based on carcinogenicity in animal studies. However, as the BMDL for As is derived from human studies, an interspecies extrapolation factor (i.e. 10) is not needed, i.e. a MOE of 1,000 would be sufficient. |
| Aniline 46,000 μg/kg bw per day (T25) |
From the rat carcinogenicity data a T25 of 46 mg/kg bw per day was obtained (72 mg/kg bw per day 25%/39%, no correction for spontaneous incidence or duration of experiment necessary). The MOE should be at least 25,000 since the reference point is based on carcinogenicity in animal studies taking into account that the reference point was T25. European Chemical Bureau (2004) |
HBGV: health based guidance value; RP: Reference point; BMDL01: benchmark dose (lower confidence limit); bw: body weight; TWI: tolerable weekly intake; MOE: margin of exposure; T25: the chronic dose rate, which will give 25% of the animal tumours at a specific tissue site, after specific correction for the spontaneous incidence within the standard lifetime of that species.
The risk assessment of the impurities helps determine whether there could be a possible health concern if it would be present at the limit value in the food additive. The assessment is performed by calculating the MOE by dividing the reference point (e.g. BMDL Table 5) by the exposure estimate (Table 4).
3.4.1. Toxic elements
Data provided as reporting limits for one of the manufacturers could not be used for the risk assessment. The Panel noted that the values reported for toxic elements by the other two manufacturers are different. While for As, Pb and Cd the values reported for one of the manufacturers are very low (median value below LOQ of 0.1 mg/kg for the three elements) compared to the other manufacturer, the maximum level of Hg is higher. No information on the route of manufacturing (Section 3.2.1) used for each manufacturer was provided.
The Panel noted that the occurrence data submitted by the IBOs on cadmium and arsenic are substantially lower than the current limits in the EU specifications (Documentation provided to EFSA No 1 and 3). The Panel considered that the maximum limits in the EU specifications for toxic elements should be established based on actual levels measured in the commercial food additive. If the European Commission decides to revise the current limits in the EU specifications, the estimates of toxic elements intake as described below could be considered.
The Panel performed the risk assessment that would result if the toxic elements were present in E 132 at: (i) the existing limit in EU specifications; (ii) the rounded highest measured value of the level of toxic element, that is the same as the maximum current limit for Pb and Hg, and therefore only calculated for Cd and As (Table 6).
Table 6.
Different scenarios for the potential exposure to toxic elements from the use of E 132
| Lead | Mercury | Cadmium | Arsenic | |
|---|---|---|---|---|
| Current limits in the EU specifications (mg/kg) | 2 | 1 | 1 | 3 |
| Rounded highest measured value (mg/kg) | 2 | 1 | 0.1 | 1 |
The potential exposure to these impurities from the use of E 132 was compared with the available HBGV and reference points (RP) (Table 7).
Table 7.
Risk assessment for toxic elements
| (i) Considering the presence of toxic elements at the current limits of the EU specifications for E 132 (Commission Regulation (EU) No 231/2012) | ||||
|---|---|---|---|---|
| Exposure to E 132 (mg/kg bw per day) | MOE for Pb at 2 mg/kg | % of the TWI for Hg at 1 mg/kg | % of the TWI for Cd at 1 mg/kg | MOE for As at 3 mg/kg |
| 0.44 (a) | 568 | 0.1 | 0.1 | 277–6060 |
| 1.26 (b) | 198 | 0.2 | 0.4 | 79–2116 |
| (ii) Considering the presence of toxic elements at the rounded highest measured value | ||||
| Exposure to E 132 (mg/kg bw per day) | % of the TWI for Cd at 0.1 mg/kg | MOE for As at 1 mg/kg | ||
| 0.44 (a) | 0.01 | 681–18181 | ||
| 1.26 (b) | 0.04 | 238–6350 | ||
bw: body weight; TWI: tolerable weekly intake; MOE: margin of exposure.
Highest exposure level among the different population groups (refined brand‐loyal scenario – toddlers – mean) (Table 4).
Highest exposure level among the different population groups (refined brand‐loyal scenario – toddlers – 95th percentile) (Table 4).
The potential exposure to these impurities from the use of E 132 was compared with the available HBGV and RP (Table 7). For As, in both scenarios, i.e. (i) the maximum current limit in the EU specification and (ii) the rounded highest measured value, the lower end of the range of calculated MOE values was insufficient, i.e. below the target value of 1,000. For the other toxic elements (lead, mercury and cadmium), exposure to them due to the use of the food additive does not give rise to safety concerns (Table 7).
The Panel considered that the maximum limits in the EU specifications for toxic elements should be established based on actual levels in the commercial food additive. Taking into account the results of the estimation calculated by the Panel (Table 7) and the fact that the food additive is not the only potential dietary source of toxic elements, the Panel recommended the maximum limits to be lowered on the basis of the information provided by the IBOs and on the considerations of the Panel (see Table 9). If the European Commission decides to revise the current limits in the EU specifications, the estimates of toxic elements intake as above could be considered.
Table 9.
Proposal for a revised version of the existing EU Specifications for indigo carmine (E 132)
| Purity | Commission Regulation (EU) No 231/2012 | Comment/justification for revision |
|---|---|---|
| Name | Indigotine, Indigo carmine | To harmonise the name in the definition to only indigo carmine or indigotine and insert the other as a synonym |
| Synonyms | CI Food Blue 1 | Please see above |
| Definition | Indigotine consists essentially of a mixture of disodium 3,3'dioxo‐ 2,2′‐bi‐indolylidene‐5,5′‐disulfonate, and disodium 3,3′‐dioxo‐2,2′‐bi‐indolylidene‐5,7′‐disulfonate and subsidiary colouring matters together with sodium chloride and/or sodium sulfate as the principal uncoloured components. Indigotine is described as the sodium salt. The calcium and the potassium salt are also permitted. Indigo carmine is obtained by sulfonation of indigo. This is accomplished by heating indigo (or indigo paste) in the presence of sulfuric acid. The dye is isolated and subjected to purification procedures | See consideration of the Panel regarding the calcium and potassium salts and the aluminium lakes |
| Colour Index No | 73015 | |
| Einecs | 212‐728‐8 | |
| CAS number | 860‐22‐0 (5,5′ isomer) | |
| Chemical name | Disodium 3,3′‐dioxo‐2,2′‐bi‐indolylidene‐5,5′‐disulfonate | Unchanged |
| Chemical formula | C16H8N2Na2O8S2 | Unchanged |
| Molecular weight | 466,36 | Unchanged |
| Assay |
≥ 85% total colouring matters (calculated as sodium salt) ≤ 18% disodium 3,3′‐dioxo‐2,2′‐bi‐indolylidene‐5,7′‐disulfonate E1% 1cm 480 at ca. 610 nm in aqueous solution |
Unchanged |
|
Description Appearance of the aqueous solution |
Dark‐blue powder or granules Blue |
Unchanged |
|
Identification Spectroscopy |
Maximum in water at ca. 610 nm |
Maximum absorbance* in water at ca. 610 nm |
| Purity | ||
| Water insoluble matter | ≤ 0.2% | Unchanged |
| Subsidiary colouring matters | ≤ 1.0% (excluding disodium 3,3′‐dioxo‐2,2′‐bi‐indolylidene‐5,7′‐disulfonate) | Unchanged |
|
Organic compounds other than colouring matters |
Unchanged | |
|
– Isatin‐5‐sulfonic acid – 5‐sulfoanthranilic acid – Anthranilic acid |
≤ 0.5% |
|
| Unsulfonated primary aromatic amines | ≤ 0.01% (calculated as aniline) | Unchanged** |
| Ether extractable matter | ≤ 0.2% (under neutral conditions) | Unchanged |
| Arsenic | ≤ 3 mg/kg | Maximum limit to be lowered on the basis of the information provided by the IBO and on the considerations of the Panel |
| Lead | ≤ 2 mg/kg | Unchanged |
| Mercury | ≤ 1 mg/kg | Unchanged |
| Cadmium | ≤ 1 mg/kg | Maximum limit to be lowered on the basis of the information provided by the IBO and on the considerations of the Panel |
For clarity.
Based on the limited data submitted, the identity of any unsulfonated aromatic amines, apart from aniline, if present in E 132, remain uncertain.
3.4.2. Unsulfonated primary aromatic amines
The IBO did not provide data on the identities of any unsulfonated primary aromatic amines apart from aniline. Although several peaks were detected using GC–NPD, GC–MS was not successful, and those peaks remain unidentified.
The IBO did not use a colorimetric method capable of quantifying total unsulfonated primary aromatic amines expressed as aniline equivalents as indicated in the JECFA specifications.
The Panel noted that according to JECFA specifications (JECFA, 2018), N‐phenylglycine, that can be used for manufacturing indigo (or indigo paste), is prepared from aniline (see Table 1).
The IBO also did not propose lowest achievable limits for unsulfonated primary aromatic amines in E 132 as requested in the European Commission call for data.
As indicated in Section 3.2.2.2, aniline was reported in the six analysed batches at the range of 2.1–69 mg/kg indigo carmine. The Panel performed the risk assessment that would result if (i) aniline was present in E 132 at the rounded highest measured value of aniline (70 mg/kg) and (ii) unsulfonated primary aromatic amines were present at the existing limit in the EU specifications (0.01%), assuming that they have the same toxicity as aniline (Table 8).
Table 8.
Risk assessment for aniline and unsulfonated primary aromatic amines, assuming that all unsulfonated primary aromatic amines are aniline
| MOE | |
|---|---|
| Exposure to E 132 (mg/kg bw per day) | (i) Considering the presence of aniline at the rounded highest measured value (70 mg/kg) |
| 0.44 (a) | 1,490,000 |
| 1.26 (b) | 520,000 |
| (ii) Considering the presence of unsulfonated primary aromatic amines at the current limit in the EU specifications for E 132 of 0.01% (100 mg/kg) assuming that the whole group is aniline (Commission Regulation (EU) No 231/2012) | |
| 0.44 (a) | 1,050,000 (c) |
| 1.26 (b) | 365,000 (c) |
bw: body weight; MOE: margin of exposure.
Highest exposure level among the different population groups (refined brand‐loyal scenario – toddlers – mean) (Table 2).
Highest exposure level among the different population groups (refined brand‐loyal scenario – toddlers – 95th percentile) (Table 2).
MOE calculated assuming that all unsulfonated primary aromatic amines have the same toxicity as aniline.
The risk assessment of aniline (scenario (i) Table 8) showed a MOE above the target value of 25,000 at the highest exposure of E 132.
Apart from aniline which was identified and quantified by comparison to an analytical standard, there was no information provided on the identities of the additional peaks detected in the analysis of unsulfonated primary aromatic amines (scenario (ii) Table 8). The Panel noted that these peaks/substances are likely to contain nitrogen (being detected by the NPD) but the failure of the GC–MS analysis to identify the nature of those components prevents any conclusion. However, assuming that these unidentified components have a toxicity similar to aniline, and assuming that the sample may contain unsulfonated primary aromatic amine up to the specifications limit of 0.01%, a large MOE (more than 10‐fold higher than the target value of 25,000) was estimated. The MOE for unsulfonated primary aromatic amines would still be larger than the target value even though some aromatic amines are more potent than aniline, but not by much more than factor 10 (Crabtree et al., 1991). The Panel considered that the presence of these unsulfonated primary aromatic amines, calculated as aniline, at the specifications limit of 0.01% would not raise a concern.
3.4.3. Summary of the proposed revision to the EU specifications
The Panel noted that no information for the calcium and potassium salts and the aluminium lakes, that according to the Commission Regulation (EU) No 231/2012 are also authorised to be used as E 132, was submitted. According to the IBO, the main form of E 132 used globally is the disodium salts and all the samples tested analytically and toxicologically were the disodium salts (Documentation provided to EFSA No 5, 6). However, as also stated by the IBO, in the European Union, manufacturers of E 132 may use the calcium and potassium salts (Documentation provided to EFSA No 5). The Panel has no information if the aluminium lakes are actually used as E 132. The Panel cannot exclude that the content of toxic elements and unsulfonated aromatic amines of these alternative forms (calcium and potassium salts and the aluminium lakes) is different from the disodium salts but notes that the proposed changes to the specifications are applicable for indigo carmine in all forms.
Overall, based on the information provided by the IBOs (Documentation provided to EFSA No 1,3,4,5 and 6) and the above considerations, the Panel recommends the following revisions of the existing EU specifications for indigo carmine (E 132) as listed in Table 9. The Panel noted that the choice of maximum limits for impurities in the EU specifications is in the remit of risk management.
The Panel noted that the JECFA specifications (2018) request not more than 15% for the ‘loss on drying, chloride and sulfate as sodium salts’. At the time of the re‐evaluation, the ANS Panel also noted that ‘if the EU specifications were extended to include < 15% of sodium chloride and/or sodium sulphate as the principal uncoloured components, most of the material would be accounted for’ since the assay requests at least 85% of the colouring matter. No information on this aspect was requested in the European Commission call for data 7 and there are no data to support an amendment of the EU Specifications for E 132 on this parameter.
3.5. Toxicological data
3.5.1. Summary of the biological and toxicological data considered during the re‐evaluation of E 132 (EFSA ANS Panel, 2014)
A summary of the main conclusions for the biological and toxicological data from the assessment of the ANS Panel during the re‐evaluation of E 132 (EFSA ANS Panel, 2014) is presented below.
“In vivo studies demonstrated that radioactivity from 35 S‐Indigo Carmine was poorly detected in urine (1.6% to 2.1% of the radioactivity) after oral administration in rats. Moreover, incubation of 35 S‐Indigo Carmine with intestinal contents of rats for 48 hours suggested that isatin‐5‐sulphonic acid and 5‐sulphoanthranilic acid might be metabolites formed by intestinal bacteria. By comparison to the data obtained after intravenous administration, the Panel considered that the data available on the absorption, metabolism and excretion indicated that Indigo Carmine or its metabolites were poorly absorbed. However, the Panel noted that both identification of faecal metabolites and tissue distribution of radioactivity were not investigated in these studies.
The Panel considered that based on the available data, including a newly performed in vivo study on micronuclei induction, Indigo Carmine does not raise concern for genotoxicity.
In a subacute toxicity study (45‐day) performed on adult male Swiss albino mice of B‐6 strain (5 animals/group) at oral doses of 0, 17 and 39 mg Indigo Carmine/kg bw/day, statistically significant severe adverse effects on the testis were described (Dixit and Goyal, 2013 ). The Panel noted that no NOAEL could be identified in this study and the lowest observed adverse effect level (LOAEL) was 17 mg/kg bw/day, the lowest dose level tested. The Panel noted limitations in the design of the study and lack of information on the specifications of the test material used in the study (E 132; FD&C Blue # 2; CI 73015) was procured from the local market in India.
Several chronic toxicity studies were available when JECFA allocated the ADI (1975). Two chronic and carcinogenicity studies, one in rats and one in mice were published since the latest evaluation of Indigo Carmine by JECFA in 1975.
In the mouse study, microscopic evaluation of selected tissues revealed a variety of randomly distributed neoplastic, degenerative, hyperplastic and inflammatory changes usually encountered in ageing mice (Borzelleca and Hogan, 1985 ). These changes occurred without relationship to dose and are considered to be unrelated to the dietary administration of Indigo Carmine. It was therefore concluded that this lifetime exposure of mice to Indigo Carmine did not demonstrate carcinogenic or toxic effects. The Panel identified a NOAEL in this study equal to 5% in the diet providing an average intake of 8259 mg/kg bw per day for males and 9456 mg/kg bw per day for females.
In a chronic/carcinogenicity study, a statistically significant increased incidence of gliomas and malignant mammary gland tumours was seen in the male rats at the highest dose level of 1282 mg Indigo Carmine/kg bw/day. Overall, the Panel identified a NOAEL in this study of 632 mg/kg bw per day for male rats, the mid‐dose, based on these carcinogenic effects. In the absence of any genotoxic activity, the Panel considered that Indigo carmine was acting as a non‐genotoxic carcinogen and therefore, that there would be a threshold for this effect.
In a multigeneration reproductive toxicity study no test substance related effects were observed up to doses of 250 mg/kg bw/day. No adverse effects have been detected in developmental studies in rats and rabbits at tested doses (0, 25, 75 or 250 mg/kg bw/day). The Panel noted that the concentrations tested in the developmental toxicity studies were lower than the dose levels up to 2000 mg/kg bw per day used in several subacute or long‐term toxicity studies but close to the NOAEL of 500 mg/kg bw/day on which JECFA based its ADI.
No cases of Indigo Carmine intolerance or allergy have been reported after ingestion.
No adverse effects in subacute, chronic, reproduction and developmental toxicity studies, and no modifications of haematological and biological parameters in chronic toxicity studies have been identified at doses less than or equal to 500 mg/kg bw/day. The only report of an adverse effect was in testis with a LOAEL of 17 mg/kg bw/day by Dixit and Goyal ( 2013 ). No such adverse effects have been described on testis in long‐term toxicity studies in mice and rats (weight of testis, histopathology examination) at higher doses. Age‐related testicular atrophy of the seminiferous tubules, accompanied by oligospermia or aspermia, was present in male rats of all groups in the 2‐year chronic toxicity/carcinogenicity study (Borzelleca et al., 1985 ) but these lesions were randomly distributed in treated and control animals and were not dose‐related. The Panel noted also that fertility indices were reduced in F2b and F2c group of male rats, but these changes were not considered to be compound‐related because they were not dose‐related in the 3‐generation reproductive toxicity study (Borzelleca et al., 1986 ). Overall, the Panel noted that no effects on the testes or reproductive function were observed in chronic toxicity studies and in a 3‐generation reproduction toxicity study undertaken with Indigo Carmine (FD&C Blue No 2) containing approximately 93% pure colouring and 7% volatile matter (Borzelleca et al., 1985 , 1986 ; Borzelleca and Hogan, 1985 ). The Panel considered that the Dixit and Goyal ( 2013 ) study has shortcomings since it is not clear to the Panel whether the adverse effects observed were due to the food additive itself or to impurities and/or contaminants present in the material tested and/or to the conduct of the study.
The Panel considered that the current ADI of 5 mg/kg bw/day was applicable to a material with a purity of 93% pure colouring matter and manufactured using processes resulting in comparable residuals as material used in the Borzelleca et al. studies ( 1985 , 1986 ) and Borzelleca and Hogan ( 1985 ).”
3.5.2. Toxicological data submitted
The following was requested in the European Commission call for data:
According to EFSA the ADI of 5 mg/kg bw per day for indigo carmine is only applicable for indigo carmine of at least 93% purity, manufactured using the same or equivalent manufacturing process resulting in the material tested in Borzelleca et al. studies. For an extension of this ADI to indigo carmine complying with the current EU specifications for E 132 indigo carmine (which allow for a lower purity of ‘not less than 85% total colouring matters, calculated as the sodium salt’) new data addressing the adverse effects on testis observed in the Dixit and Goyal (2013) study should be generated. The chemical identity (identity and percentage of colouring matters and non‐colouring components) of the tested food additive, including the presence of possible impurities and/or contaminants, should be investigated and reported. The manufacturing process of the tested food additive should also be described. In the absence of a commitment for the submission of the requested data, the specifications of E 132 indigo carmine will be amended, in line with EFSA's conclusions on the characteristics of the material to which the established ADI should apply.
As a result of the European Commission call for data, an IBO submitted a study with the aim to evaluate the effect on the reproductive performance in male mice (Documentation provided to EFSA No 2). Details are provided in Table A.1 of the Appendix A.
Indigo carmine was tested in male mice (10/group) at concentrations of 0, 834, 1,667 and 3,334 mg indigo carmine/kg diet (equal to 135, 277 and 548 mg/kg per day) for 56 days. Apart from the duration and the use of one sex, the study was in accordance with OECD TG 408 and compliant with GLP. The aim of the study was to evaluate the effect of indigo carmine (E 132) on the reproductive function in male mice. Therefore, the study was performed in males only and the duration is considered sufficient since it covers the complete spermatogenetic cycle. The Panel noted that the test item was described with a purity of 88% in terms of colouring matter and at 12% of ‘loss of drying (water) and chloride and sulphates expressed as sodium salt’; isatin‐5‐sulfonic acid < 0.5%; subsidiary dye < 1%. No information was provided on the manufacturing process and on the presence of unsulfonated aromatic amines. The Panel considered the study valid although the full characterisation of the test item was not provided but claimed by the IBO to be used as E 132, hence being in accordance with the EU specifications. There were no test item‐related adverse effects observed, in particular on parameters of male reproduction, within the dose range tested (135, 277 and 548 mg/kg bw per day). The Panel identified the highest dose tested of 548 mg indigo carmine/kg bw per day as the NOAEL of this study.
4. Discussion
The re‐evaluation of indigo carmine (E 132) was completed by the EFSA ANS Panel in 2014 (EFSA ANS Panel, 2014). The ANS Panel confirmed the ADI of 5 mg/kg bw per day for indigo carmine allocated by JECFA in 1975 and endorsed by SCF in 1975. The ANS Panel indicated that the ADI was applicable to a material with a purity of 93% pure colouring and manufactured using processes resulting in comparable residuals as material used in the Borzelleca et al. studies (1985, 1986) and Borzelleca and Hogan (1985). Given the uncertainties in the database, the ANS Panel was not able to conclude whether the ADI should apply to indigo carmine with lower purity manufactured using these same processes or material manufactured using a different but not equivalent process. The ANS Panel considered that any extension of the ADI to indigo carmine of lower purity and/or manufactured using a different process would require new data which would need to address the adverse effects on testis observed in the Dixit and Goyal (2013) study. The chemical identity of the tested food additive, including the presence of possible impurities and/or contaminants, should be investigated and reported.
The data gaps and uncertainties identified by the ANS Panel required a follow‐up by the European Commission by means of a subsequent call for additional data. The present opinion deals with the assessment of the data provided by IBOs in response to this call.
For this assessment, dietary exposure to indigo carmine (E 132) was newly estimated because food consumption data gathered by EFSA have substantially changed since the time of the re‐evaluation of E 132 in 2014 and new analytical data are available. For the current exposure estimate, data provided through the call for data in 2013 and considered in the re‐evaluation of the food additive (EFSA ANS Panel, 2014) have been used together with the available analytical data. Based on the current authorisation of E 132 as a food colour, the Panel considered that brand‐loyalty could be expected. Therefore, the Panel considered the brand‐loyal scenario as the most relevant exposure scenario for the safety evaluation of indigo carmine (E 132). In that scenario, mean exposure to indigo carmine (E 132) from its use as a food additive ranged from 0.001 mg/kg bw per day in infants to 0.44 mg/kg bw per day in toddlers. At the 95th percentile, exposure to indigo carmine (E 132) ranged from 0.01 mg/kg bw per day in infants to 1.26 mg/kg bw per day in toddlers (Table 4).
An IBO has submitted data on concentration levels of toxic elements (Pb, Hg, Cd and As) on behalf of three manufacturers. Analytical results were reported by one of the manufacturers as being below their reporting limits (i.e. 1, 2, 1 and 1 mg/kg for As, Pb, Hg and Cd, respectively) without indicating the corresponding LOQs, therefore they could not be used for the risk assessment. The Panel noted that the occurrence data submitted by the IBOs on cadmium and arsenic are substantially lower than the current limits in the EU specifications. Nevertheless, the IBO proposed to maintain the current limits in the EU specifications for toxic elements.
The Panel considered the potential presence of Pb, Cd, Hg and As in E 132 at (i) the existing limit in EU specifications; (ii) the rounded highest measured value of the level of toxic element, that is the same as the maximum current limit for Pb and Hg, and therefore only calculated for Cd and As.
The Panel considered the brand‐loyal exposure scenario to calculate the exposure to the toxic elements from the use of E 132. The highest exposure levels for the mean and 95th percentile among the different population groups were considered, i.e., 0.44 and 1.26 mg/kg bw per day, respectively, for toddlers.
The potential exposure to these impurities from the use of E 132 was compared against the available HBGV and RP. The resulting figures (see Table 7) show that for As, in both scenarios (i and ii), the lower end of the range of calculated MOE values was insufficient, i.e. below the target value of 1,000. For the other toxic elements (lead, mercury and cadmium), exposure to them due to the use of the food additive does not give rise to safety concerns.
The Panel considered that the maximum limits in the EU specifications for toxic elements should be established based on actual levels in the commercial food additive. Taking into account the results of the estimation calculated by the Panel (Table 7) and the fact that the food additive is not the only potential dietary source of toxic elements, the Panel recommended the maximum limits to be lowered on the basis of the information provided by the IBOs and on the considerations of the Panel (see Table 9).
Data on the identity of the unsulfonated aromatic amines present in the food additive E 132, as well as data on their individual lowest achievable limits were requested in the European Commission call for data. Only aniline was identified and reported in the six analysed batches at the range of 2.1–69 mg/kg indigo carmine. Apart from aniline which was identified and quantified by comparison to an analytical standard, there was no information provided on the identity of the additional peaks detected in the analysis of unsulfonated primary aromatic amines. The Panel noted that these peaks/substances are likely to contain nitrogen (being detected by the NPD) but the GC–MS analysis failed to identify these components. The risk assessment for unsulfonated primary aromatic amines was conducted assuming that these unidentified components have a toxicity similar to aniline a probable carcinogen according to IARC (2A).
The Panel calculated the MOEs that would result if (i) aniline was present in E 132 at the rounded highest measured value of aniline (70 mg/kg) and (ii) unsulfonated primary aromatic amines were present at the existing limit (0.01%) in the EU specifications, assuming that they have the same toxicity as aniline. This results in MOEs for both measured aniline and assumed total unsulfonated primary aromatic amines above the target value of 25,000.
The MOE for unsulfonated primary aromatic amines would still be larger than the target value even though some aromatic amines are more potent than aniline, but not by much more than factor 10 (Crabtree et al., 1991). Therefore, the Panel considered that the presence of these unsulfonated primary aromatic amines, calculated as aniline, at the specifications limit of 0.01% would not raise a concern.
According to the IBO, the main form of E 132 used globally is the disodium salts and all the samples tested analytically and toxicologically were the disodium salts. However, as also stated by the IBO, in the European Union, manufacturers of E 132 may use the calcium and potassium salts. The Panel noted that the proposed changes to the specifications are applicable for indigo carmine in all forms of the food additive (E 132).
Information on the solubility of indigo carmine (E 132) (disodium salts) was provided (see Section 3.2.3). The Panel took into account the solubility of indigo carmine which is ca. 1.6% (16 g/kg or L) in water at 20°C and noted that this is 32‐times higher than the maximum use level of 500 mg/kg food permitted in the regulation. Taking into account the reported uses and use levels and the MPLs along with the reported solubility, the Panel considered that full dissolution of indigo carmine E 132 (disodium salts) is to be expected in the food and/or in the gastrointestinal tract and that ingested particles (if any) would not persist. Therefore, the Panel concluded there is no concern with regards to the potential presence of small particles, including nanoparticles, in indigo carmine (E 132) (disodium salts) when used as a food additive and considered that a conventional risk assessment of the disodium salts of indigo carmine (E 132) can be performed according to the EFSA Guidance for submission for food additive evaluations (EFSA ANS Panel, 2012). Relevant information according to EFSA guidance TG would be needed to confirm the adequacy of a conventional risk assessment also for alternative forms of indigo carmine (i.e. calcium and potassium salts and the aluminium lakes). The Panel noted that no such information for the alternative forms of indigo carmine was provided in response to the follow‐up call issue by the European Commission. Therefore, the present assessment is applicable only to the indigo carmine (E 132) disodium salts.
As a result of the European Commission call for data, an IBO has also submitted a 56‐day dietary study with the aim to evaluate the effect on the reproductive performance in male mice. The Panel noted that the test item was described with a purity of 88% in terms of colouring matter (disodium salts) and at 12% of loss of drying (Water) and chloride sulfates expressed as sodium salt; Isatin‐5‐sulfonic acid, < 0.500%; Subsidiary dye, < 1.000%. No information was provided on the manufacturing process and on the presence of unsulfonated aromatic amines. The Panel noted that no test item‐related adverse effects were observed, in particular on parameters of male reproduction, within the dose range tested (135, 277 and 548 mg/kg per day). From this study, the Panel identified a NOAEL of 548 mg indigo carmine/kg bw per day, the highest dose tested.
The results of this study did not confirm the severe adverse effects observed in the testis in a 45‐day oral toxicity study with adult male mice tested at 17 mg/kg bw per day and 39 mg/kg bw per day (Dixit and Goyal, 2013) noted at the time of the re‐evaluation (EFSA ANS Panel, 2014). The results of 56‐day study are in accordance with the observations in chronic toxicity studies (Oettel et al., 1965; Borzelleca et al., 1985) and in a three‐generation reproduction toxicity study with indigo carmine (Borzelleca et al., 1986), where no effects on testes or reproductive function were observed. Further, the results of the 56‐day study support the considerations of the ANS Panel that the adverse effects in the testes reported by Dixit and Goyal (2013) may be ascribed to impurities or contaminants present in the additive tested and not to indigo carmine itself. Therefore, the results of the Dixit and Goyal (2013) study are not considered for the risk assessment of E 132.
The Panel noted that the purity of E 132 tested in the 56‐day study was 88%; the Panel considered that the current specification of 85% minimum for the colouring matter is still appropriate.
Based on the available database evaluated during the re‐evaluation of indigo carmine (E 132) and the results of the study report submitted as a follow‐up of the re‐evaluation, the Panel confirmed the ADI of 5 mg/kg bw per day for indigo carmine (E 132) disodium salts, meeting the proposed revisions of the specifications as presented in Table 9.
The Panel noted that at the maximum permitted level of use, exposure estimates of indigo carmine (E 132) would exceed the ADI of 5 mg/kg bw per day for infant, toddlers and children at the 95th percentile of exposure. The Panel also noted the exposure estimates to indigo carmine (E 132), using the available usage and analytical data, for the different population groups of the refined brand‐loyal exposure scenario, did not exceed the ADI.
5. Conclusions
The Panel concluded that the technical data provided by the interested business operator support an amendment of the specifications for indigo carmine (E 132) laid down in Commission Regulation (EU) No 231/2012, as presented by the recommendations made in Table 9.
Based on the available database, the Panel confirmed the ADI of 5 mg/kg bw per day for indigo carmine (E 132) disodium salts, meeting the proposed revisions of the specifications.
The Panel concluded that at the maximum permitted level of use, the exposure estimates at the 95th percentile of indigo carmine (E 132) for infants, toddlers and children would exceed the ADI. However, using the available use levels and analytical data, the exposure estimates to indigo carmine (E 132) in the refined brand‐loyal exposure scenario for all population groups were below the ADI.
The Panel concluded that there is no safety concern for the use of indigo carmine (E 132) disodium salts, meeting the proposed revision of the specification, at the reported use levels and submitted analytical data.
Documentation as provided to EFSA
Submission of data response to the European Commission call for technical data on the permitted food indigo carmine (E 132). Submitted by IACM to the European Commission. https://dms.efsa.europa.eu/otcs/cs.exe/link/28603094
Indigo Carmine: A 56‐day dietary study in mice. Product Safety Labs, 2020. Study Number 511821. Submitted by IACM to the European Commission https://dms.efsa.europa.eu/otcs/cs.exe/link/24781393
Additional information submitted in response to a request from EFSA. Submitted by IACM November 2021 https://dms.efsa.europa.eu/otcs/cs.exe/link/25426059
Additional information submitted in response to a request from EFSA. Submitted by IACM October 2022 https://dms.efsa.europa.eu/otcs/cs.exe/link/27245106
Additional information submitted in response to a request from EFSA. Submitted by IACM April 2023 https://dms.efsa.europa.eu/otcs/cs.exe/link/29059721
Additional information submitted in response to a request from EFSA. Submitted by ROHA April 2023 https://dms.efsa.europa.eu/otcs/cs.exe/link/29038651
Abbreviations
- ADI
acceptable daily intake
- AFC
Panel on Food additives, Flavourings, Processing Aids and Materials in contact with Food
- ANS
Panel on Food Additives and Nutrient Sources added to Food
- BMDL
Bench Mark Dose (lower confidence interval)
- bw
body weight
- CEF
Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CONTAM
Panel on Contaminants in the Food Chain
- ECHA
European Chemicals Agency
- FAIM
Food Additives Intake Model
- FC
Food category
- FCS
Food categorisation system
- FEEDAP
Panel on Additives and Products or Substances used in Animal Feed
- GC
gas chromatography
- HBGV
health‐based guidance value
- HPLC
high‐pressure liquid chromatography
- HS‐GC
headspace gas chromatography
- IBO
interested business operator
- ICP
inductively coupled plasma
- IQ
Intelligence quotient
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LC–HRMS
liquid chromatography– high‐resolution mass spectrometry
- LOAEL
lowest observed adverse effect level
- LOQs
limit of quantifications
- MOE
margin of exposure
- MS
mass spectrometry
- NOAEL
no observed adverse effect level
- OECD
Organisation for Economic Co‐operation and Development
- RH
relative humidity
- RP
reference point
- T25
T25: the chronic dose rate, which will give 25% of the animal tumours at a specific tissue site, after specific correction for the spontaneous incidence within the standard lifetime of that species.
- SCF
Scientific Committee on Food
- TG
Test Guideline
- TWI
Tolerable weekly intake
Appendix A – Summary of the 56‐day dietary study in mice submitted in the call for data
Table A.1.
Summary of the 56‐day dietary study in mice submitted in the call for data
| Study ID | |
| Reference | INDIGO CARMINE: A 56‐DAY DIETARY STUDY IN MICE, (Documentation provided to EFSA No 2) |
| Source (published/unpublished) | Unpublished |
| Overview of the study and guideline | |
| Good laboratory practice (yes/no) | Yes |
| Guideline studies (if yes, specify) | Claimed to be 407, but based on 408 |
| Animal model | |
| Species and strain | CD‐1 Mouse, albino |
| Disease models (e.g. diabetes, allergy, obesity) | No |
| Housing conditions | |
| Housing condition | Individually |
| Diet name and source (if reported) | 2016 Certified Envigo Teklad Global Rodent Diet® ad libitum, water ad libitum |
| Treatment | |
| Test material | Indigo Carmine (also referred to as FD&C Blue No.2), batch number Mix no. 153. No information on the manufacturing process was provided. |
| Provider | IACM |
| Compound purity | 87.87% |
| Vehicle used | Feed |
| Dose regimen (dose level or concentration per group and frequency) and achieved doses if available | 0, 834, 1,667 and 3,334 mg indigo carmine/kg diet, equal to 135, 277 and 548 mg/kg per day |
| Route of administration (diet, drinking water, gavage) | Diet |
| Period of exposure (pre‐mating, mating, gestation, lactation, adult) | Adult |
| Duration of the exposure | 56 days |
| Study design | |
| Sex and age at the start of the treatment | Male, 5 weeks of age |
| Number animals/sex/group | 10/male/group |
| Measured endpoints | According to guidelines |
| Time of measurement/observation period | According to guidelines |
| Methods to measure the endpoints | Sperm analysis: CASA; testis fixative: modified Davidsons. |
| Statistical analysis | |
| Statistical methods |
Two‐way or one‐way analysis of variance (ANOVA) Kruskal–Wallis non‐parametric ANOVA test (SAS/STAT User's Guide, 1989). If a significant difference occurs (p < 0.05), the Mann–Whitney U test |
| Results | |
| Findings reported by the study author/s | There were no adverse histopathological changes in testes and no test item‐related changes in sperm motility, sperm count or sperm morphology. |
| No observed adverse effect level, lowest observed adverse effect level, benchmark dose/benchmark dose lower bound | The Panel noted that no adverse findings were observed within the dose range tested and identified a NOAEL of 548 mg/kg bw per day, the highest dose tested. |
| Further information | |
|
The Panel noted some discrepancies in the Summary compared to the Report. The Summary refers to 5 mice/sex/group but the Methods specify 10 male mice per group. The Conclusion of the report refers to rats instead of mice. The results of the study are nonetheless considered valid as it was clear from the report (text, summary tables and individual data), that 10 male mice were used. The Panel noted that the large variation in some organ weights (e.g. adrenals) suggests that the collection of the small organs was not very well performed. This large weight variation did not influence the most important aim of this study to investigate a possible effect of indigo carmine on reproductive function. | |
Annex A – MPLs of indigo carmine (E 132) in foods according to the Annex II to Regulation (EC) No 1333/2008
Annex B – Summary of analytical results (mg/kg or mg/L as appropriate) of indigo carmine (E 132) provided by Member States
Annex C – Number and percentage of food products labelled with indigo carmine (E 132) out of the total number of food products present in the Mintel GNPD per food subcategory between 2018 and 2023
Annex D – Concentration levels of indigo carmine (E 132) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Annex E – Summary of total estimated exposure of indigo carmine (E 132) from its use as a food additive for the regulatory maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Annex F – Main food categories contributing to exposure to indigo carmine (E 132) using the regulatory maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Annex G – Table of uncertainties
1.
Annexes Annex A – MPLs of indigo carmine (E 132) in foods according to the Annex II to Regulation (EC) No 1333/2008, Annex B – Summary of analytical results (mg/kg or mg/L as appropriate) of indigo carmine (E 132) provided by Member States, Annex C – Number and percentage of food products labelled with indigo carmine (E 132) out of the total number of food products present in the Mintel GNPD per food subcategory between 2018 and 2023, Annex D – Concentration levels of indigo carmine (E 132) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate), Annex E – Summary of total estimated exposure of indigo carmine (E 132) from its use as a food additive for the regulatory maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day), Annex F – Main food categories contributing to exposure to indigo carmine (E 132) using the regulatory maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)–G can be found in the online version of this output (in the ‘Supporting information’ section).
Supporting information
MPLs of indigo carmine (E 132) in foods according to the Annex II to Regulation (EC) No 1333/2008
Summary of analytical results (mg/kg or mg/L as appropriate) of indigo carmine (E 132) provided by Member States
Number and percentage of food products labelled with indigo carmine (E 132) out of the total number of food products present in the Mintel GNPD per food subcategory between 2018 and 2023
Concentration levels of indigo carmine (E 132) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of indigo carmine (E 132) from its use as a food additive for the regulatory maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to indigo carmine (E 132) using the regulatory maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Table of uncertainties
Suggested citation: EFSA FAF Panel (EFSA Panel on Food Additives and Flavourings) , Younes, M ., Aquilina, G ., Degen, G ., Engel, K.‐H ., Fowler, P ., Frutos Fernandez, M . J., Fürst, P ., Gürtler, R ., Husøy, T ., Manco, M ., Mennes, W ., Passamonti, S ., Moldeus, P ., Shah, R ., Waalkens‐Berendsen, I ., Wright, M ., Cheyns, K ., FitzGerald, R ., … Gundert‐Remy, U . (2023). Follow‐up of the re‐evaluation of indigo carmine (E 132) as a food additive. EFSA Journal, 21(7), 1–31. 10.2903/j.efsa.2023.8103
Requestor European Commission
Question number EFSA‐Q‐2021‐00180
Panel members Gabriele Aquilina, Polly Boon, Gisela Degen, Karl‐Heinz Engel, Paul Fowler, Maria Jose Frutos Fernandez, Peter Fürst, Ursula Gundert‐Remy, Rainer Gürtler, Trine Husøy, Melania Manco, Wim Mennes, Sabina Passamonti, Peter Moldeus, Romina Shah, Ine Waalkens‐Berendsen, Matthew Wright and Maged Younes.
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements The Panel wishes to thank the following for the support provided to this scientific output: Vasantha Palaniappan.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Adopted: 15 June 2023
Annexes A–G are available under the Supporting Information section .
Notes
OJ L 354, 31.12.2008, p. 16.
OJ L 80, 26.3.2010, p. 19.
OJ L 31, 1.2.2002, p. 1.
Commission Regulation (EU) No 231/2012 of 9 March 2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council. OJ L 83, 22.3.2012.
Aluminium lakes are prepared by reacting colours complying with the purity criteria set out in the appropriate specification monograph with alumina under aqueous conditions. The alumina is usually freshly prepared undried material made by reacting aluminium sulfate or chloride with sodium or calcium carbonate or bicarbonate or ammonia. Following lake formation, the product is filtered, washed with water and dried. Unreacted alumina may also be present in the finished product (Commission Regulation 231/2012).
Missing Cyprus, Luxembourg and Malta.
References
- Borzelleca JF and Hogan GK, 1985. Chronic toxicity/carcinogenicity study of FD&C Blue No. 2 in mice. Food and Chemical Toxicology, 23, 719–722. 10.1016/0278-6915(85)90264-9 [DOI] [PubMed] [Google Scholar]
- Borzelleca JF, Hogan GK and Koestner A, 1985. Chronic toxicity/carcinogenicity study of FD&C Blue No. 2 in rats. Food and Chemical Toxicology, 23, 551–558. 10.1016/0278-6915(85)90178-4 [DOI] [PubMed] [Google Scholar]
- Borzelleca JF, Goldenthal EI and Wazeter FX, 1986. Multigeneration study of FD&C Blue No. 2 in rats. Food and Chemical Toxicology, 24, 159–163. 10.1016/0278-6915(86)90351-0 [DOI] [PubMed] [Google Scholar]
- Crabtree HC, Hart D, Thomas MC, Witham BH, McKenzie IG and Smith CP, 1991. Carcinogenic ranking of aromatic amines and nitro compounds. Mutation Research, 264, 155–162. 10.1016/0165-7992(91)90071-b [DOI] [PubMed] [Google Scholar]
- Dixit A and Goyal RP, 2013. Evaluation of Reproductive toxicity caused by Indigo carmine on male swiss albino mice. Pharmacology Online, 1, 218–224. Available online: https://www.researchgate.net/publication/287328182_Evaluation_of_Reproductive_toxicity_caused_by_indigo_carmine_on_male_swiss_albino_mice [Google Scholar]
- EFSA (European Food Safety Authority) , 2007. Opinion of the Scientific Committee related to Uncertainties inDietary Exposure Assessment. EFSA Journal 2007;5(1):438, 54 pp. 10.2903/j.efsa.2007.438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2011. Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA Journal 2011;9(3):2097, 34 pp. 10.2903/j.efsa.2011.2097 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2015. The food classification and description system FoodEx2 (revision 2). EFSA Supporting Publication 2015;12(5):EN‐804, 90 pp. 10.2903/sp.efsa.2015.EN-804 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources added to Food) , 2017. Statement on approach followed for the refined exposure assessment as part of the safety assessment of food additives under re‐evaluation. EFSA Journal 2017;15(10):5042, 9 pp. 10.2903/j.efsa.2017.5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources Added to Food) , 2012. Guidance for submission of food additives evaluations. EFSA Journal 2012;10(7):2760, 60 pp. 10.2903/j.efsa.2012.2760 [DOI] [Google Scholar]
- EFSA ANS Panel (EFSA Panel on Food Additives and Nutrient Sources Added to Food) , 2014. Scientific Opinion on the re‐evaluation of Indigo Carmine (E 132) as a food additive. EFSA Journal 2014;12(7):3768, 51 pp. 10.2903/j.efsa.2014.3768 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , 2009a. Scientific Opinion on arsenic in food. EFSA Journal 2009;7(10):1351, 199 pp. 10.2903/j.efsa.2009.1351 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , 2009b. Scientific opinion on cadmium in food. EFSA Journal 2009;7(10):980, 139 pp. 10.2903/j.efsa.2009.98 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , 2010. Scientific Opinion on lead in food. EFSA Journal 2010;8(4):1570, 151 pp. 10.2903/j.efsa.2010.1570 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , 2012. Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA Journal 2012;10(12):2985, 241 pp. 10.2903/j.efsa.2012.2985 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) , 2015. Scientific Opinion on the safety and efficacy of indigo carmine (E 132) for cats and dogs and ornamental fish. EFSA Journal 2015;13(5):4108, 15 pp. 10.2903/j.efsa.2015.4108 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2009. Guidance of the Scientific Committee on transparency in the scientific aspects of risk assessments carried out by EFSA. Part 2: general principles. EFSA Journal 2009;7(7):1051, 22 pp. 10.2903/j.efsa.2009.1051 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2012. Scientific Opinion on the applicability of the Margin of Exposure approach for the safety assessment of impurities which are both genotoxic and carcinogenic in substances added to food/feed. EFSA Journal 2012; 10(3):2578, 5 pp. 10.2903/j.efsa.2012.2578 [DOI] [Google Scholar]
- EFSA Scientific Committee , 2021. Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA Journal 2021;19(8):6769, 48 pp. 10.2903/j.efsa.2021.6769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Chemical Bureau, Institute for Health and Consumer Product , 2004. European Union Risk Assessment Report Aniline. First priority list. Volume 50. Available online: https://echa.europa.eu/documents/10162/0abd36ad-53de-4b0f-b258-10cf90f90493
- Hansen WH, Fitzhugh OG, Nelson AA and Davis KJ, 1966. Chronic toxicity of two food colors, brilliant blue FCF and indigotine. Toxicology and Applied Pharmacology, 8, 29–36. 10.1016/0041-008X(66)90097-4 [DOI] [PubMed] [Google Scholar]
- JECFA (Joint FAO, WHO Expert Committee on Food Additives) , 2018. Indigotine. In: Compendium of Food Additive Specifications, 86th meeting 2018. FAO JECFA Monographs 22, 2018. Available online: https://www.fao.org/3/ca3738en/ca3738en.pdf
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) , 1969. Toxicological evaluation of some food colours, emulsifiers, stabilizers, anti‐caking agents and certain other substances. FAO Nutrition Meeting Report Serie 46a WHO/FOOD ADD/70.36. Available online: http://www.inchem.org/documents/jecfa/jecmono/v46aje14.htm [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives) , 1975. Toxicological evaluation of some food colours, enzymes flavour enhancers, thickening agents, and certain other food additives. WHO Food Additives Series 6, 18th report of the Joint FAO/WHO Expert Committee on Food Additives. Available online: http://www.inchem.org/documents/jecfa/jecmono/v06je22.htm [PubMed]
- Oettel H, Frohberg H, Nothdurft H and Wilhelm G, 1965. Die Prüfung einiger synthetischer Farbstoffe auf ihre Eignung zur Lebensmittelfärbung. Archiv für Toxikologie, 21, 9–29. 10.1007/BF00578966 [DOI] [PubMed] [Google Scholar]
- SCF (Scientific Committee for Food) , 1975. Reports of the Scientific Committee for Food (1st series), opinion expressed 31 December 1975. Available online: http://aei.pitt.edu/40814/1/food_1st.pdf
- SCF (Scientific Committee for Food) , 1984. Reports of the Scientific Committee for Food (14thseries), ISBN 92–825–3893‐1, Brussels‐Luxembourg, 1983. Available online: http://aei.pitt.edu/40823/1/14th_food.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MPLs of indigo carmine (E 132) in foods according to the Annex II to Regulation (EC) No 1333/2008
Summary of analytical results (mg/kg or mg/L as appropriate) of indigo carmine (E 132) provided by Member States
Number and percentage of food products labelled with indigo carmine (E 132) out of the total number of food products present in the Mintel GNPD per food subcategory between 2018 and 2023
Concentration levels of indigo carmine (E 132) used in the exposure assessment scenarios (mg/kg or mL/kg as appropriate)
Summary of total estimated exposure of indigo carmine (E 132) from its use as a food additive for the regulatory maximum level exposure scenario and the refined exposure assessment scenarios per population group and survey: mean and 95th percentile (mg/kg bw per day)
Main food categories contributing to exposure to indigo carmine (E 132) using the regulatory maximum level exposure assessment scenario and the refined exposure assessment scenarios (> 5% to the total mean exposure)
Table of uncertainties



