Abstract
Introduction:
Timely colorectal cancer (CRC) screening has been shown to improve CRC-related morbidity and mortality rates. However, even with this preventative care tool, CRC screening rates remain below 70% among eligible United States (US) adults, with even lower rates among US immigrants. The aim of this scoping review is to describe the barriers to CRC screening faced by this unique and growing immigrant population and discuss possible interventions to improve screening.
Methods:
Four electronic databases were systematically searched for all original research articles related to CRC screening in US immigrants published after 2010. Following a full-text review of articles for inclusion in the final analysis, data extraction was conducted while coding descriptive themes. Thematic analysis led to the organization of this data into five themes.
Results:
Of the 4637 articles initially identified, 55 met inclusion criteria. Thematic analysis of the barriers to CRC screening identified five unique themes: access, knowledge, culture, trust, health perception, and beliefs. The most cited barriers were in access (financial burden and limited primary care access) and knowledge (CRC/screening knowledge).
Conclusions:
US immigrants face several barriers to the receipt of CRC screening. When designing interventions to increase screening uptake among immigrants, gaps in physician and screening education, access to care, and trust need to be addressed through culturally sensitive supports. These interventions should be tailored to the specific immigrant group, since a one-size-fits approach fails to consider the heterogeneity within this population.
Keywords: Colorectal cancer screening, Healthcare access, Immigrant healthcare, Immigrant, Screening barriers
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related death and the third most commonly diagnosed malignancy around the world.1,2 In the United States (US), localized CRC has 5-year survival rates of 90%, but only 38% of people present with this early stage of disease.3 As such, the overall 5-year CRC survival rate is 65% and it is projected that over 50,000 people will die of CRC this year.3,4 With improved screening rates, however, it is estimated that 68% of these deaths could be prevented.5
Despite the benefits of screening in reducing the incidence, morbidity, and mortality associated with CRC, screening rates remain below 70% among US adults aged 50–75 y old.6–9 These suboptimal rates of screening are especially prominent in immigrants who now make up almost 14% of the US population.10–13 By 2030, immigration is expected to become the primary driver of US population growth, and by 2060, over 17% of the US population will be foreign-born.14 Often, immigrant and nonimmigrant populations face similar barriers. However, these barriers are often exacerbated in immigrant populations. For example, although obtaining insurance to cover CRC screening can be a universal challenge, some immigrant populations are excluded from purchasing insurance plans from the Affordable Care Act altogether or have to wait 5 y before qualifying for Medicaid.15 Therefore, as efforts to increase rates of CRC screening become more widespread, it is critical that they address the unique barriers faced by US immigrants. This scoping review summarizes the literature evaluating the barriers to CRC screening among US immigrants. Through this analysis, the authors aim to guide future interventions specifically designed to support this vulnerable population.
Materials and Methods
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews framework.16
Data sources and search strategy
The initial literature search was conducted on January 15, 2020 with an updated, follow-up second search on January 14, 2022 by an information specialist using the following online databases: Ovid MEDLINE, EBSCOhost CINAHL, Elsevier Embase, and Clarivate Web of Science. Each search consisted of two concepts: CRC screening and immigrant populations. These concepts were defined in the search by both controlled vocabulary terms (such as Medical Subject Headings) and title and abstract keywords. The complete search strategies are available in Supplementary file 1. Citations were imported into EndNote 20 (Clarivate, Philadelphia, PA) and exported into an Excel spreadsheet once the duplicates were removed.
Study selection
All original research with a focus on CRC screening among immigrant populations in the US were eligible for inclusion. For the purposes of this review, an immigrant was defined as a person residing in the US who was born in a different country. Articles were excluded if they were not peer-reviewed, published before 2010, included study populations who had been diagnosed with early-onset CRC (before age 50), or if the study population had an identifiable inherited genetic predisposition to CRC.
Studies were selected using a two-stage process. In the first stage, each article’s study title and abstract was reviewed by two independent reviewers from the research team (A.V.P., A.L., S.D., L.H., C.E.R.). Articles that did not meet inclusion criteria were excluded. If there was any uncertainty or if the two independent reviewers disagreed in their assessment, the study was included for full-text review. In the second stage, two independent reviewers (A.V.P., L.H.) performed a full-text assessment to ensure the study met inclusion criteria. Studies were additionally excluded if they were only an abstract or report, did not study barriers to CRC screening, or did not clearly indicate an immigrant population was studied.
Data extraction
Data extraction was performed on papers included through the study selection process using a standardized chart consisting of 56 rows and 13 columns. Nine percent of the articles were reviewed by two authors (A.V.P., A.L.) to ensure reliability and consistency of data abstraction. The remaining articles were reviewed by a single author (A.V.P.).
Thematic analysis
Thematic analysis was modeled after the synthesis conducted by Thomas and Harden.17 Through the data extraction and thematic analysis processes, codes were generated for the qualitative data in the source texts and initial descriptive themes were identified by two reviewers (A.V.P., L.H.). In the final data review after extraction was completed, the themes were consolidated into the final set of five analytical themes presented in this paper (Access, Knowledge, Culture, Trust, Health Perception, and Beliefs).
Results
Evidence source
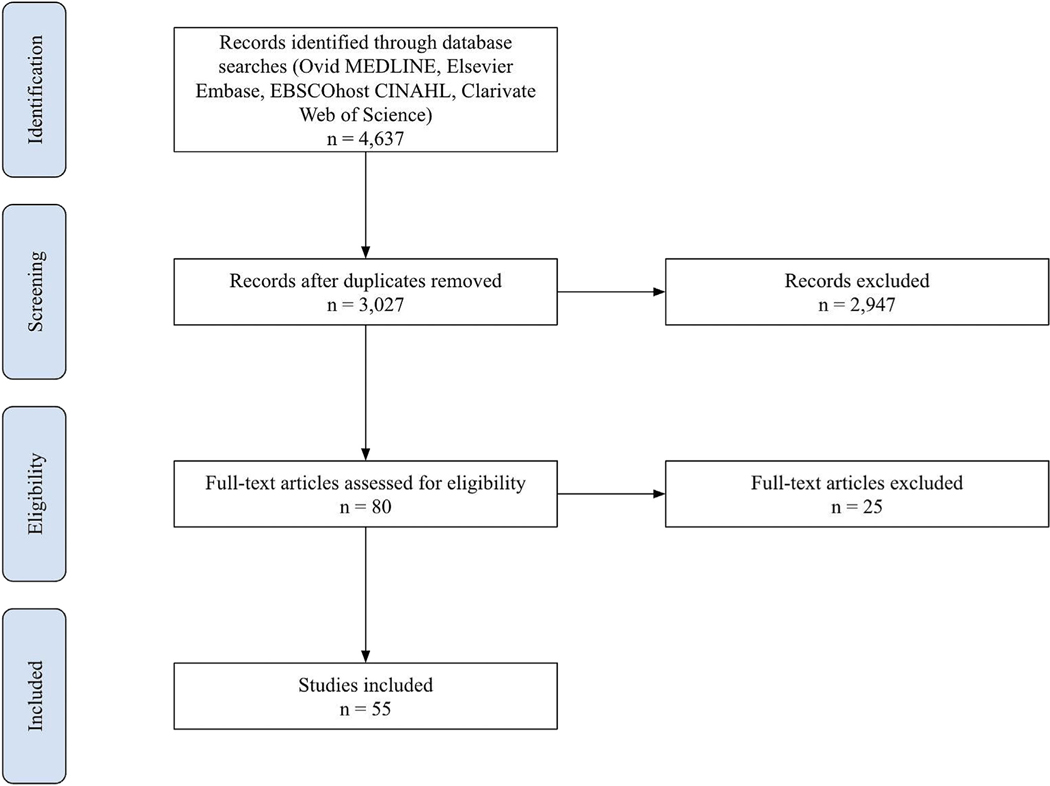
We identified 4637 articles using our database search. Of these papers, 250 met our inclusion criteria for full-text review and 55 of those were ultimately included in our study (Fig.). The study designs varied in both quantitative and qualitative analyses of aggregate patient data, coded interviews, and community interventions, as well as a few research reviews. Most papers implemented a cross-sectional approach to analyzing data. The most common CRC screening approach was Fecal Occult Blood Test (FOBT), but some studies evaluated the use of sigmoidoscopy and colonoscopy as well. Immigrant populations included South Asians, Filipinos, Vietnamese, Koreans, Bangladeshi, Hispanics, Latino(x), Somali, Jamaicans, Chinese, and many more. A majority of papers focused on immigrants from East and Southeast Asia. Commonly, studies were conducted in metro/city centers. A summary of study details can be found in Table 1.
Fig. –
Scoping review literature screening flowchart.
Table 1 –
Study characteristics.
| Title | Year | Study type | Participants | Location | Screening test |
|---|---|---|---|---|---|
|
| |||||
| Shahidi et al.10 | 2013 | Quantitative analysis of survey | 25,439 immigrants, 50 y or older | California | FOBT, sigmoidoscopy, colonoscopy |
| Rogers et al.11 | 2020 | Quantitative analysis of survey | 118 Lao immigrants, 50–75 y | Minnesota | FOBT, FIT, sigmoidoscopy, colonoscopy |
| Siddiq et al.18 | 2020 | Systematic review | - | - | - |
| Cofie et al.19 | 2020 | Quantitative analysis of surveys | 5529 immigrants, 50–75 y | United States | FOBT, sigmoidoscopy, colonoscopy |
| Maxwell et al.20 | 2000 | Quantitative analysis of surveys | 218 Filipino and 229 Korean female immigrants, 50 y or older | Los Angeles, CA | FOBT, sigmoidoscopy, colonoscopy |
| Ellison et al.21 | 2011 | Quantitative analysis of interviews | 225 Hispanic female immigrants, 50 y or older | Harlem, New York City, NY | FOBT, colonoscopy |
| Lee et al.22 | 2018 | Quantitative and qualitative analyses of surveys | 202 Korean immigrants, 50 y or older | Chicago Metropolitan Area, IL | FOBT |
| Lee et al.23 | 2018 | Quantitative and qualitative analyses of surveys | 210 Korean immigrants, 50 y or older | Chicago Metropolitan Area, IL | FOBT, sigmoidoscopy, colonoscopy |
| Tong et al.24 | 2017 | Randomized controlled trial of educational intervention | 329 Hmong immigrants, 50–75 y | Sacramento, CA | FOBT, sigmoidoscopy, colonoscopy |
| Ko et al.25 | 2016 | Quantitative analysis of survey | 193 Korean immigrants, 50–75 y | Seattle Metropolitan Area, WA | FOBT, FIT, colonoscopy |
| Ben Morrison et al.26 | 2012 | Quantitative patient chart review | 810 Somali immigrants, 18 y or older | Rochester, MN | FOBT, sigmoidoscopy, colonoscopy |
| Kim et al.27 | 2012 | Quantitative analysis of health intervention and survey | 113 Chinese immigrants, 50 y or older | Chicago, IL | FOBT |
| Shih et al.28 | 2008 | Quantitative analysis of survey | 1440 immigrants, 50 y or older | United States | FOBT, sigmoidoscopy, colonoscopy, proctoscopy |
| Breen et al.29 | 2010 | Quantitative analysis of survey | 5265 Mexican immigrants, 18 y or older | California | FOBT, endoscopy |
| Consedine et al.30 | 2011 | Quantitative analysis of survey | Jamaican immigrants, 45–75 y | Brooklyn, NY | - |
| Francois et al.31 | 2009 | Qualitative analysis of interviews | 45 Haitian immigrants, 40 y or older | New York City, NY | FOBT, colonoscopy |
| Siddiq et al.32 | 2020 | Qualitative analysis of interviews | 19 Afghan female immigrants, 50 y or older | San Diego, CA | Colonoscopy |
| Patel et al.33 | 2021 | Qualitative analysis of interviews and surveys | 51 Indian, Pakistani, Bangladeshi, and Nepali immigrants, 50–75 y | New York City, NY | FOBT, colonoscopy |
| Ly Van Manh et al.34 | 2020 | Quantitative analysis of survey | 701 African and 311 Asian immigrants, 50 y or older | New York City, NY | Colonoscopy |
| Samuel et al.35 | 2009 | Quantitative patient chart review; qualitative analysis of survey | 100 female Cambodian, Somali, and Vietnamese immigrants, 50–75 y | Maine | FOBT, sigmoidoscopy, colonoscopy |
| Ivey et al.36 | 2018 | Qualitative interviews | 22 Bangladeshi and 31 Indian immigrants, 50 y or older | California | FOBT, endoscopy |
| Oh et al.37 | 2019 | Qualitative analysis of interviews | 51 Korean immigrants, 40 y or older | Washington, DC | FOBT, sigmoidoscopy, colonoscopy |
| Fang and Ragin38 | 2020 | Research review | - | - | - |
| Ayash et al.39 | 2020 | Quantitative analysis of survey | 100 Arab immigrants, 50–75 y | New York City, NY | FOBT, FIT, colonoscopy |
| Juon et al.40 | 2018 | Quantitative analysis of survey | 274 Chinese, Korean, and Vietnamese immigrants, 50–75 y | Baltimore-Washington Metropolitan Area, MD | FOBT, colonoscopy |
| Kim et al.41 | 2015 | Qualitative and quantitative analyses of interviews | 237 Chinese, 69 Cambodian, and 168 Vietnamese immigrants, 50 y or older | Chicago, IL | Sigmoidoscopy, colonoscopy |
| Reyes and Miranda42 | 2015 | Quantitative analysis of surveys | 73,475 female immigrants, 40 y or older; 91,711 female immigrants, 21–65 y; and 80,811 male immigrants, 50–80 y | United States | FOBT, sigmoidoscopy, colonoscopy |
| Lee and Im43 | 2013 | Quantitative analysis of survey | 281 Korean immigrants, 50–88 y | New York Metropolitan Area | FOBT, sigmoidoscopy, colonoscopy |
| Gwede et al.44 | 2011 | Qualitative and quantitative analyses of interviews | 20 Caribbean and 20 Haitian immigrants, 50 y or older | Hillsborough County, FL | - |
| Maxwell et al.45 | 2008 | Quantitative analysis of survey | 487 Filipino immigrants, 50–75 y | Los Angeles, CA | FOBT, endoscopy |
| Yao et al.46 | 2022 | Quantitative analysis of survey | 3660 immigrants, 50 y or older | United States | FOBT, FIT, sigmoidoscopy, colonoscopy |
| Shapiro47 | 2021 | Qualitative analysis of interviews | 81 Russian, Ukrainian, Uzbekistani, Kazakhstani, Kyrgyzstani, Turkmenistani, and Tajikistani immigrants, 50–75 y | New York City, NY | Colonoscopy |
| Li et al.48 | 2021 | Quantitative analysis of surveys | 7220 immigrants | United States | FOBT, sigmoidoscopy, colonoscopy |
| Portillo et al.49 | 2020 | Health intervention | Hispanic and Latinx immigrants | El Paso, TX | - |
| Lee50 | 2011 | Survey/Health scale validation | 26 Korean immigrants, 50 y or older | Chicago Metropolitan Area, IL | FOBT |
| Rogers et al.51 | 2021 | Qualitative analysis of interview | 27 Somali males, 50–75 y | Minnesota | FOBT, FIT, sigmoidoscopy, colonoscopy |
| Manne et al.52 | 2018 | Behavioral intervention | 93 South Asians, 50–75 y | New Jersey | FOBT, sigmoidoscopy, colonoscopy |
| Coughlin et al.53 | 2015 | Research summary | Haitian immigrants | Hillsborough County, FL; Little Haiti, Miami-Dade County, FL; New York City, NY | Colonoscopy |
| Manne et al.54 | 2015 | Quantitative analysis of survey | 208 South Asian (Pakistan, India, Nepal, Bangladesh, Sri Lanka) immigrants, 50–75 y | New York/New Jersey Metropolitan Area | FOBT, sigmoidoscopy, colonoscopy |
| Gwede et al.55 | 2010 | Quantitative analysis of interview | 20 Caribbean and 20 Haitian immigrants, 50 y or older | Hillsborough County, FL | - |
| Walsh et al.56 | 2009 | Quantitative analyses of interviews | 808 Vietnamese immigrants, 50–79 y | Santa Clara, CA | FOBT, sigmoidoscopy, colonoscopy |
| Le et al.57 | 2014 | Quantitative analysis of survey | 238 Chinese, 217 Korean, and 199 Vietnamese immigrants, 50–75 y | Portland Metropolitan Area, OR | FOBT, sigmoidoscopy, colonoscopy |
| Nakajima et al.58 | 2021 | Quantitative analysis of educational intervention and surveys | 31 Somali male immigrants | Minneapolis, MN | - |
| Wang et al.59 | 2014 | Health education intervention | 14 Chinese immigrants, 50–68 y | San Francisco, CA | FOBT, colonoscopy |
| Lopez-Class et al.60 | 2012 | Quantitative patient chart review | 840 South/Central American immigrants, 50 y or older | District of Columbia, MD | FOBT, sigmoidoscopy, colonoscopy |
| Lee et al.61 | 2013 | Qualitative analysis of interviews | 26 Korean immigrants, 50 y or older | Chicago Metropolitan Area, IL | FOBT, endoscopy |
| Maxwell et al.62 | 2011 | Quantitative analysis of health intervention | 548 Filipino immigrants, 50–70 y | Southern California | FOBT, sigmoidoscopy, colonoscopy |
| Aragones et al.63 | 2010 | Randomized controlled trial of health intervention | 65 Latino immigrants, 50 y or older | New York City, NY | - |
| Miranda et al.64 | 2017 | Quantitative analyses of surveys | 14,968 female immigrants, 50 y or older (for CRC) | United States | FOBT, sigmoidoscopy, colonoscopy |
| Menon et al.65 | 2014 | Quantitative analysis of interview | 275 South Asian immigrants, 50 y or older | Chicago, IL | FOBT, sigmoidoscopy, colonoscopy |
| Morey et al.66 | 2021 | Quantitative analysis of survey | 400 Chinese and Korean immigrants, 50–75 y | Baltimore-Washington DC Metro Area | - |
| Alsayid et al.67 | 2019 | Qualitative analysis of interviews | 11 Arab male immigrants, 50–75 y | San Francisco Bay Area, CA; Worcester, MA | FIT, colonoscopy |
| Nakajima et al.68 | 2021 | Quantitative analysis of survey | 111 East African male immigrants, 45–75 y | Twin Cities Metro Area, MN | FOBT, sigmoidoscopy, colonoscopy |
| Li et al.69 | 2019 | Health intervention | Chinese immigrants, 56–75 y | Texas | FOBT |
| Consedine et al.70 | 2011 | Quantitative analysis of survey | 69 Jamaican immigrants, 45–70 y | Brooklyn, NY | - |
FOBT = fecal occult blood test; FIT = fecal immunochemical test.
Thematic categorization of barriers to colorectal cancer screening
Five common themes associated with barriers to CRC screening among US immigrant populations were identified: Access, Knowledge, Culture, Trust, Health Perception, and Beliefs. Table 2 shows the relevant studies under each theme.
Table 2 –
Data synthesis table.
| Theme | Barrier | Study reference |
|---|---|---|
|
| ||
| Access | Not having a PCP/usual source of care | 11,18–30 |
| No access to interpreter | 18,26,31–34 | |
| Difficulty with colonoscopy prepping | 33 | |
| Lacking transportation | 35 | |
| Difficulty navigating complexity of healthcare system | 36,37 | |
| Adherence to other screening tests | 21 | |
| Home-based screening | 38 | |
| Lack of insurance or ability to pay | 10,19,24,25,28,29,33,35–37,39–48 | |
| Unemployed | 22,41 | |
| Lack of citizenship | 46 | |
| Screening costs | 25,49,50 | |
| Ability to travel abroad for care | 25,37 | |
| Knowledge | Unfamiliarity with/lack of knowledge of CRC and CRC screening | 11,18,24,31–33,36,37,40,47,50–59 |
| Lack of physician recommendation | 18,21,22,30–33,36,37,39,50,55,57,60–63 | |
| Unaware of cancer risks and insurance coverage | 58 | |
| Physician did not follow screening guidelines | 44,53 | |
| Unaware of medications | 51 | |
| High school education only | 40,46 | |
| Sociocultural barriers | Length of US residency | 18–22,28,29,35,39,41,45,51,54,64,65 |
| Degree of westernization | 66 | |
| English language use | 24,33,34,36,42,66 | |
| Acculturation level | 25 | |
| Traditional care preference | 37,59 | |
| Trust | Health system mistrust | 36,51,65 |
| Lack of patient-provider rapport | 32,51,67 | |
| Patient-physician linguistic or gender concordance/preference | 31,32,35,37 | |
| Nonauthoritative patient-physician relationship | 47 | |
| Family encouragement | 32,33,61 | |
| Friends who screened | 57 | |
| Suspicion of pharmaceutical companies | 51 | |
| Health perception and beliefs | Health fatalism | 22,33,36,37,47,50 |
| Intervention averse for cancer diagnosis | 51 | |
| Faith influence over health | 33,39,51 | |
| Preventative health orientation (or lack thereof) | 22,36,37,47,50,61,68 | |
| Perception of CRC risk | 37 | |
| Perception of CRC severity | 37 | |
| Self-efficacy | 50,51,69 | |
| Fear, shame, perceived helplessness related to cancer diagnosis | 32,33,36,37,43,51 | |
| Fear of finding a health problem | 31 | |
| Concern of low-quality healthcare when uninsured | 31 | |
| Screening-related embarrassment | 30,35,37,47,70 | |
| Uncomfortable, risk, dehumanizing screening process | 32,35,37,47,67 | |
PCP = primary care provider.
Access
Barriers to access included a lack of healthcare resources and prohibitory financial burden. In regard to the lack of resources, one of the most commonly cited barriers was lacking access to a primary care physician or usual source of care.11,18–30 Beyond the point of initial care access, several studies highlighted language barriers, a lack of translated resources, and a critical gap in interpreter services which collectively limited CRC screening recommendation from the healthcare provider.18,26,31–34 Patel et al.33 found the difficulty in preparing for a colonoscopy to limit screening. Additionally, lack of transportation to healthcare facilities was suggested by Samuel et al.35 as a barrier. On a broader scale, some studies suggested that the complexity of the healthcare system led to problems in navigating and accessing CRC screening.36,37 Ellison et al.21 found that access and adherence to other testing, specifically mammography, increased CRC screening utilization. Access to home-based FOBT screening was found by Fang and Ragin38 to increase screening uptake.
Multiple studies also often reported cost-associated barriers, particularly pointing to a lack of insurance, ability to pay for healthcare (visits, routine medical tests, serious illness), and lower income.10,19,24,25,28,29,33,35–37,39–48 Additionally, unemployment was associated with limited access to CRC testing.22,41 Lack of citizenship status, crucially linked to health insurance attainment, was found by Yao et al.46 to restrict access as well. Given the costs, Portillo et al.49 found administering screening vouchers via community health workers (CHWs) to help with uptake. High costs associated with screening reduced CRC test utilization for both FOBT and colonoscopy. A couple of studies showed that lower test costs in South Korea prompted medical tourism, where immigrants traveled outside the US for more affordable CRC testing in South Korea.25,50 The ability to travel out of country for care was associated with higher screening rates.25,37
Knowledge
Many studies found low levels of CRC knowledge and screening tests as a barrier to timely CRC screening among immigrants, with many studies reporting that patients were generally unfamiliar with or had never heard of either the screening tests or CRC itself.11,18,24,31–33,36,37,40,47,50–59 The majority of papers underscored the importance of a physician recommendation in influencing people to get screened.18,21,22,30–33,36,37,39,50,55,57,60–63 The studies emphasized how a lack of coordination and communication among physicians, public health workers, CHWs, and others in disseminating healthcare information related to CRC limited access to screening tests. Nakajima et al.58 explored this further, finding the immigrants studied were reluctant to screen due to not knowing the risks of cancer and how insurance could be used to cover screening. Additionally, physicians did not always follow guidelines regarding age and risk-appropriate screening.44,53 Outside of physician recommendation and CRC screening test knowledge, immigrants’ lack of awareness regarding the availability of CRC treatment options has also been shown to limit receipt of testing.51 Finally, aside from knowledge particular to CRC, a couple of groups interestingly found that having more than a high school level of education correlated with a lower chance of screening.40,46 They hypothesized that those with more than a high school level education might not have time to screen.
Culture
Several studies found that hesitancy to screen was associated with low levels of acculturation to the US, which was measured based on time spent in the US, language proficiency, and attitudes about healthcare and screening. Specifically, many papers found that longer residencies in the US were associated with increased screening rates.18–21,28,29,35,39,41,45,51,54,64,65 In contrast, Lee and Lee22 found that a shorter residency facilitated willingness to undergo screening. This discrepancy was reasoned to be due to higher uptake of FOBT among new immigrants, a test covered by most US insurance companies unlike other more invasive methods of CRC screening. Morey etal.66 found the degree of westernization to influence whether family or providers could influence screening receipt. Aside from length of residency and acculturation, a lack of English proficiency posed an additional barrier for immigrants.24,33,34,36,42,66 South Korean immigrants who reported less acculturation to the US were less likely to have a usual source of care in the US and more likely to engage in medical tourism to access CRC screening.25 A couple of groups found that the immigrants they studied had preferred traditional approaches to care over screening.37,59
Trust
Many immigrant groups reported a general mistrust in the healthcare system that was associated with a reduced receipt of screening.36,51,65 Several elements of the physician-patient relationship acted as a barrier for immigrants such as low physician-patient rapport, lack of communication, and unclear screening explanations.32,51,67 Conversely, immigrants having linguistic or gender concordance with their physician helped facilitate screening.31,32,37 In another study, both male and female immigrants indicated a dislike in male physicians performing the screening.35 Shapiro47 interestingly found a nonauthoritative physician-patient relationship to dissuade screening uptake. Outside direct interactions with healthcare workers, a lack of family support to receive screening was associated with reduced CRC screening.32,33,61 Additionally, Le et al. found that immigrants with friends who had received screening were more likely to receive it themseleves.57 Rogers et al.51 found a suspicion of pharmaceutical companies to be a barrier for an immigrant community.
Health perception and beliefs
The decision to pursue screening was influenced by immigrants’ perceived control over their health. A perception of health fatalism (i.e., believing screening would not help with adverse health outcomes) was found by a few immigrant populations to lead to reduced screening.22,33,36,37,47,50 In this line, Rogers et al.51 found that some immigrants, due to religious beliefs, believed there should not be any intervention after a cancer diagnosis, limiting their likelihood to screen. Faith-based beliefs around health combined with the perception that cancer could not be treated, as it was in the hands of God, led some to not pursue screening.33,39,51 Additionally, many studies reported a lack of emphasis on preventative care among many immigrant populations as well as a tradition of only seeing a doctor when symptoms are present, both of which were barriers to CRC screening.36,37,47,50,61,68 Oh et al.37 found that some immigrants were reluctant to screen as they believed they had a lower than typical CRC risk compared to Americans. On the other hand, Lee and Lee22 found a preventive or future health temporal orientation, where people placed an importance on being healthy in the future and detecting adverse health outcomes, to be a facilitator for screening. When people knew about the severity of CRC they were found to have an increased openness to screen.37 An additional facilitator came from immigrants’ self-efficacy, where higher levels were related to an increased likelihood to screen.50,51,69
Another pattern common among immigrant populations was concern for a cancer diagnosis. For example, some groups discussed how the perceived seriousness, fear, shame, and accompanying sense of helplessness related to a cancer diagnosis stood as a barrier to getting screened.32,33,36,37,43,51 From a similar but more general perspective, Francois et al.31 found an association with the fear of finding a health problem as limiting CRC screening receipt. This group also reported immigrants’ concerns about the lack of quality healthcare for those without insurance. Particular to the screening process, a few studies found screening-related fecal/rectal embarrassment to result in a reluctance to screen.30,35,37,47,70 Other groups additionally reported the screening process to be too uncomfortable, risky, or even dehumanizing for some immigrant groups.32,35,37,47,67
Discussion
The results from this scoping review reveal a wide range of barriers to CRC screening for US immigrants. Frequently cited barriers to screening fell into five themes including access, knowledge, culture, trust, and health perception and beliefs. Commonly cited knowledge barriers included an unfamiliarity with CRC and screening, and a lack of physician recommendation—all of which restrict the ability of immigrants to navigate the healthcare system and learn about resources available to them. Access-related barriers included financial burden and lack of primary care. Collectively, these identified barriers help explain the low rates of CRC screening in immigrant population.
The barriers to CRC screening we identified in this study are similar to barriers immigrants face when seeking other forms of oncologic screening. For example, Ferdous et al.71 evaluated barriers to breast cancer screening faced by immigrant groups in Canada and highlighted a lack of physician recommendation and lack of education on screening to be major barriers, concordant with our findings. However, gender discordance between physicians and patients was cited as a barrier to seeking mammography screening, while this was not a commonly reported barrier with CRC screening—owing, as Ferdous et al.71 particularly points out, to the uncomfortable nature of having breast cancer screening conducted by a male physician. Separately, in a systematic review of cancer screening among African immigrants (breast, cervical, prostate, uterine, colorectal), limited cancer knowledge, fear of diagnosis, recency of immigration, shame and embarrassment related to testing, and difficulties in healthcare access were all cited as screening barriers—all of which were identified as barriers to CRC screening in this review.72 Interestingly, no studies in our review mentioned distrust in interpreters as a barrier to CRC screening which has been cited as a major barrier to cervical cancer screening among immigrant women in Europe.73 This is an important area of future study given the frequent use of interpreters in breaking down the patient-physician language barrier present during consultation.
The barriers we identified in this scoping review help explain the substantially lower rates of CRC screening among US immigrants—68% of people born in the US were up to date with screening compared to 26% of immigrants in the US who had been here fewer than 10 y.74 This discrepancy suggests that there are certainly barriers faced by both immigrant and nonimmigrant populations, but that many barriers may be exacerbated in immigrant populations. Interventions at the national and state level have been created to address general gaps in screening uptake. For example, at the national level, the Centers for Disease Control and Prevention and National Institutes of Health have created guidelines for CRC screening research and outreach goals while also funding primary care clinics to encourage screening education and community engagement.75,76 Although these programs have been shown to increase screening rates among US-born adults, there are still considerable challenges in translating similar benefits for immigrants. A summary review of studies evaluating the Centers for Disease Control and Prevention’s 2009–2015 Colorectal Cancer Control Program found challenges associated with high costs of program infrastructure, limited scope of program coverage, and lack of employing evidence-based interventions—reflecting considerable areas for improvement.77 These programs lacked a focus on health perception and belief-related barriers, with a lack of disaggregated population data to help better identify differences in CRC screening uptake between groups of people. Separately, Gupta et al.78 report on the difficulty in identifying unscreened individuals, as uninsured people cannot access healthcare and do not show up in insurance-based systems for identifying unscreened individuals. Considering the high rates of uninsured immigrant populations, this poses an additional challenge in identifying unscreened immigrants for screening recruitment programs. Furthermore, efforts in place from New York City and Delaware focus on colonoscopy uptake but do not address immigrants’ embarrassment with the screening process (i.e., need for anal insertion of an instrument) or inability to pay for colonoscopy, limiting uptake of this form of screening for some communities.78 When developing CRC screening uptake interventions, it is necessary to create culturally sensitive screening recommendations that consider screening costs and immigrant preferences for broader accessibility.
In evaluating interventions that would be most promising for targeting these barriers, we looked through current programs implemented at the national level. These focused on the impact of CHWs using a variety of interventions (group and one-on-one education, patient reminders, and appointment scheduling assistance), finding this increased screening demand and reduced out-of-pocket costs.79 This scoping review found education and access-related barriers to be significant in barring CRC receipt, which solidifies the importance of integrating CHWs into care teams—as indicated by the >1 benefit-cost ratio for urban public hospitals that utilized CHWs for education and appointment scheduling assistance.79 The incorporation of CHWs has many benefits in screening uptake, yet there remains a need to further research into the effectiveness of screening interventions and follow-up care. Interventions have varied from the use of language-concordant patient navigators to mitigate language barriers to mailing fecal immunochemical test kits for easier screening access.80,81 Research specific to the effect of culturally tailored interventions in facilitating screening within different immigrant groups can be helpful in accounting for differences in healthcare perceptions and beliefs. Supporting immigrants’ initial access to care is important, but research also needs to take a more longitudinal focus on the follow-up of care. The studies focused on in this review covered immigrant’s initial access to care with some discussion on meeting habitual screening guidelines, but research needs to also focus on how immigrants are supported in further care following test results. Interventions implemented for immigrants need to be culturally adaptable—available in multiple different languages relevant to immigrant communities served, with CHWs from these communities, interpreters in clinic, and immigrant recruitment into research/intervention development and implementation itself. Although there are no blanket solutions, progress could be made by leveraging preexisting interventions designed for nonimmigrants. For example, Frerichs et al.82 describe a culturally sensitive intervention that specifically considers the CRC screening beliefs of Native Americans. This intervention resulted in positive screening perceptions, self-efficacy, and increased intent to screen and could serve as an effective launch point in the design of interventions targeting immigrant populations. Liss et al. conducted a retrospective study on FOBT screening adherence among an uninsured Spanish-speaking population in an urban community health network. They concluded that systems-based interventions that increase adherence without requiring in-person clinic visits were essential.83 A couple of studies with low income, uninsured populations receiving care at 13 federal healthcare centers found that FOBT completion rates significantly improve when combined with enhanced care, literacy appropriate education tools, or education tools in addition to nurse support (P = 0.012).84,85 These studies emphasize the need for community-based screening programs paired with patient-centered health literacy, especially in under-resourced communities.
There are several limitations to this study. First, this work only included studies that were written in the English language. In doing so, perspectives from within immigrant communities, which could point to more culturally sensitive and accurate depictions of barriers faced, may have been missed. However, multiple studies included in this review centered data collection around direct interviews with immigrants—allowing for a more complete, holistic picture of their experiences. Second, many studies aggregated patient populations into general immigrant categories that lacked a distinction on immigrant group differences. To understand the distinct barriers specific populations of immigrants face, it is important to disaggregate demographic data as different groups vary in acculturation, immigration status and reason, and perceptions of healthcare. Third, we acknowledge the predominance of East Asians in the studied populations of the papers we reviewed. Given the majority of US immigrants are Hispanic, this can impact the generalizability of our results. However, prior work has shown substantial overlap in barriers faced by both populations.86,87 Finally, in our review methodology, we initially had two reviewers go through each paper in the set of 3027 papers (5 reviewers going through these in total) and two reviewers screen the full-text articles, but had one reviewer for data abstraction due to restricted personnel resources. To account for this, we compared our abstraction methodology with 10 different scoping reviews and consulted with an information specialist to create a consistent paper review process concordant with similarly structured scoping reviews. With one reviewer (A.V.P.) for most of the papers in the data extraction process, there is potential for bias with the barriers identified and chance of missing barriers through the review process. To try to mitigate this effect, we had 9% of the papers fully reviewed by another reviewer (A.L.) to ensure consistency in themes extracted.
Conclusions
This scoping review comprehensively summarizes the barriers to receipt of CRC in immigrant groups. Immigrants faced numerous barriers which fell into five themes: Access, Knowledge, Culture, Trust, Health Perception, and Beliefs. Unfortunately, there is a paucity of studies on interventions to address these barriers for immigrant populations. As the US immigrant population continues to grow, it will be critical that any intervention has at its core partnerships with immigrant communities in order to identify specific barriers, develop culturally sensitive interventions, and improve long-term adherence to proposed solutions.
Supplementary Material
Funding
Alisha Lussiez is supported by the National Cancer Institute (T32CA009672).
Footnotes
Disclosure
Dr. Kwakye is a member of the Editorial Board of the Journal of Surgical Research; as such, she was excluded from the entire peer-review and editorial process for this manuscript.
Supplementary Materials
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jss.2022.08.024.
Availability of Data
Data not publicly available but can be accessed upon request.
REFERENCES
- 1.Keum NN, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713–732. [DOI] [PubMed] [Google Scholar]
- 2.Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5:1749–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colorectal cancer: statistics. 2020. Available at: https://www.cancer.net/cancer-types/colorectal-cancer/statistics#:~:text=Forrectalcancer%2Ctheoverallyearsurvivalrateis71%25. Accessed April 20, 2021.
- 4.Cancer stat facts: colorectal cancer. National cancer Institute surveillance, epidemiology, and end results program. Available at: https://seer.cancer.gov/statfacts/html/colorect.html. Accessed August 12, 2021.
- 5.Sharma K, Grosse S, Maciosek M, et al. Preventing breast, cervical, and colorectal cancer deaths: assessing the impact of increased screening. Prev Chronic Dis. 2020;17:E123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colorectal (colon) cancer. 2021. Available at: https://www.cdc.gov/cancer/colorectal/statistics/index.htm. Accessed August 12, 2021.
- 7.Schoen R, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106–1114. [DOI] [PubMed] [Google Scholar]
- 9.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahidi NC, Homayoon B, Cheung WY. Factors associated with suboptimal colorectal cancer screening in us immigrants. Am J Clin Oncol. 2013;36:381–387. [DOI] [PubMed] [Google Scholar]
- 11.Rogers EA, Chanthanouvong S, Saengsudham C, et al. Factors associated with reported colorectal cancer screening among Lao-American immigrants in Minnesota. J Immigr Minor Health. 2020;22:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford J, Ahmad F, Beaton D, Bierman AS. Cancer screening behaviours among South Asian immigrants in the UK, US and Canada: a scoping study. Health Soc Care Community. 2016;24:123–153. [DOI] [PubMed] [Google Scholar]
- 13.Immigrants in the United States. 2020. Available at: https://www.americanimmigrationcouncil.org/research/immigrants-in-the-united-states. Accessed August 12, 2021.
- 14.Vespa J, Medina L, Armstrong D. Demographic turning points for the United States: population projections for 2020 to 2060. Curr Popul Rep. 2020;1144:1–15. [Google Scholar]
- 15.Fuentes L, Desai S, Dawson R. New Analyses on US Immigrant Health Care Access Underscore the Need to Eliminate Discriminatory Policies; 2022. Guttmacher Institute; 2022. Available at: https://www.guttmacher.org/report/new-analyses-us-immigrant-health-care-access-underscore-need-eliminate-discriminatory. Accessed August 12, 2021. [Google Scholar]
- 16.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–473. [DOI] [PubMed] [Google Scholar]
- 17.Thomas J, Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol. 2008;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siddiq H, Alemi Q, Mentes J, Pavlish C, Lee E. Preventive cancer screening among resettled refugee women from muslim-majority countries: a systematic review. J Immigr Minor Health. 2020;22:1067–1093. [DOI] [PubMed] [Google Scholar]
- 19.Cofie LE, Hirth JM, Cuevas AG, Farr D. A national study of gender and racial differences in colorectal cancer screening among foreign-born older adults living in the US. J Behav Med. 2020;43:460–467. [DOI] [PubMed] [Google Scholar]
- 20.Maxwell AE, Bastani R, Warda US. Demographic predictors of cancer screening among filipino and korean immigrants in the United States. Am J Prev Med. 2000;18:62–68. [DOI] [PubMed] [Google Scholar]
- 21.Ellison J, Jandorf L, Villagra C, Winkel G, DuHamel K. Screening adherence for colorectal cancer among immigrant hispanic women. J Natl Med Assoc. 2011;103:681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SL, Lee EE. Access to health care, beliefs, and behaviors about colorectal cancer screening among Korean Americans. Asian Pac J Cancer Prev. 2018;19:2021–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SY. Colorectal cancer screening among Korean Americans in Chicago: does it matter whether they had the screening in Korea or the US? Asian Pac J Cancer Prev. 2018;19:1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tong EK, Nguyen TT, Lo P, et al. Lay health educator education increases colorectal cancer screening among Hmong Americans: a clustered randomized controlled trial. Cancer. 2017;123:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko LK, Taylor VM, Yoon J, et al. The impact of medical tourism on colorectal screening among Korean Americans: a community-based cross-sectional study. BMC Cancer. 2016;16:931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ben Morrison T, Wieland ML, Cha SS, Rahman AS, Chaudhry R. Disparities in preventive health services among Somali immigrants and refugees. J Immigr Minor Health. 2012;14:968–974. [DOI] [PubMed] [Google Scholar]
- 27.Kim K, Chapman C, Vallina H. Colorectal cancer screening among Chinese American immigrants. J Immigr Minor Health. 2012;14:898–901. [DOI] [PubMed] [Google Scholar]
- 28.Shih YCT, Elting LS, Levin B. Disparities in colorectal screening between US-born and foreign-born populations: evidence from the 2000 national health interview survey. J Cancer Educ. 2008;23:18–25. [DOI] [PubMed] [Google Scholar]
- 29.Breen N, Rao SR, Meissner HI. Immigration, health care access, and recent cancer tests among Mexican-Americans in California. J Immigr Minor Health. 2010;12:433–444. [DOI] [PubMed] [Google Scholar]
- 30.Consedine NS, Ladwig I, Reddig MK, Broadbent EA. The many faeces of colorectal cancer screening embarrassment: preliminary psychometric development and links to screening outcome. Br J Health Psychol. 2011;16:559–579. [DOI] [PubMed] [Google Scholar]
- 31.Francois F, Elysée G, Shah S, Gany F. Colon cancer knowledge and attitudes in an immigrant Haitian community. J Immigr Minor Health. 2009;11:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siddiq H, Pavlish C, Alemi Q, Mentes J, Lee E. Beyond resettlement: sociocultural factors influencing breast and colorectal cancer screening among Afghan refugee women. J Cancer Educ. 2022;37:352–361. [DOI] [PubMed] [Google Scholar]
- 33.Patel S, Kranick J, Manne S, et al. A population health equity approach reveals persisting disparities in colorectal cancer screening in New York City South Asian communities. J Cancer Educ. 2021;36:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ly Van Manh A, Blondeau-Lecomte E, Makoni N, Jandorf L, Perumalswami P. Identifying factors associated with cancer screening in immigrant populations living in New York City. J Community Health. 2020;45:1027–1029. [DOI] [PubMed] [Google Scholar]
- 35.Samuel PS, Pringle JP, James Iv NW, Fielding SJ, Fairfield KM. Breast, cervical, and colorectal cancer screening rates amongst female Cambodian, Somali, and Vietnamese immigrants in the USA. Int J Equity Health. 2009;8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivey SL, Mukherjea A, Patel A, et al. Colorectal cancer screening among south Asians: focus group findings on attitudes, knowledge, barriers and facilitators. J Health Care Poor Underserved. 2018;29:1416–1437. [DOI] [PubMed] [Google Scholar]
- 37.Oh KM, Park B, Jacobsen KH. A qualitative analysis of barriers to colorectal cancer screening among Korean Americans. J Cancer Educ. 2019;36:261–270. [DOI] [PubMed] [Google Scholar]
- 38.Fang CY, Ragin CC. Addressing disparities in cancer screening amongU.S. Immigrants: progress and opportunities. Cancer Prev Res (Phila). 2020;13:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayash C, Badreddine D, Gatarny R, et al. Associations with the receipt of colon cancer screening among a diverse sample of Arab Americans in NYC. J Immigr Minor Health. 2020;22:503–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Juon HS, Guo J, Kim J, Lee S. Predictors of colorectal cancer knowledge and screening among Asian Americans aged 50–75 years old. J Racial Ethn Health Disparities. 2018;5:545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim K, Chandrasekar E, Lam H. Colorectal cancer screening among Chinese, Cambodian, and Vietnamese immigrants in Chicago. J Racial Ethn Health Disparities. 2015;2:473–480. [DOI] [PubMed] [Google Scholar]
- 42.Reyes AM, Miranda PY. Trends in cancer screening by citizenship and health insurance, 2000–2010. J Immigr Minor Health. 2015;17:644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HY, Im H. Colorectal cancer screening among Korean American immigrants: Unraveling the influence of culture. J Health Care Poor Underserved. 2013;24:579–598. [DOI] [PubMed] [Google Scholar]
- 44.Gwede CK, Jean-Francois E, Quinn GP, et al. Perceptions of colorectal cancer among three ethnic subgroups of US blacks: a qualitative study. J Natl Med Assoc. 2011;103:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maxwell AE, Danao LL, Crespi CM, Antonio C, Garcia GM, Bastani R. Disparities in receipt of FOBT versus endoscopy among Filipino American immigrants. Cancer Epidemiol Biomarkers Prev. 2008;17:1963–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yao JS, Paguio JA, Dee EC, Amen TB, Escota GV. Disparities in access to colorectal cancer screening among US immigrants. J Gen Intern Med. 2022;37:2126–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shapiro E. Barriers to colorectal cancer screening among Russian-speaking immigrants: the importance of culture and home country experiences. J Immigr Minor Health. 2022;24:1300–1308. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Toseef MU, Jensen GA, Ortiz K, González HM, Tarraf W. Gains in insurance coverage following the affordable care act and change in preventive services use among non-elderly US immigrants. Prev Med. 2021;148:106546. [DOI] [PubMed] [Google Scholar]
- 49.Portillo EM, Vasquez D, Brown LD. Policy, theory, and social issue promoting hispanic immigrant health via community health workers and motivational interviewing motivational interview. Int Q Community Health Educ. 2020;4:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SY. Validation of Health and Cultural Belief Scales About Colorectal Cancer Screening Among Korean Americans; 2011. Chicago, IL: University of Illinois at Chicago; 2011. Thesis. Available at: https://hdl.handle.net/10027/8920. Accessed August 12, 2021. [Google Scholar]
- 51.Rogers CR, Jessica Obidike O, Wallington SF, Hussein M, Mahamed ZA, Sampson J. A qualitative study of barriers and enablers associated with colorectal cancer screening among Somali men in Minnesota. Ethn Health. 2021;26:168–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manne SL, Islam N, Frederick S, Khan U, Gaur S, Khan A. Culturally-adapted behavioral intervention to improve colorectal cancer screening uptake among foreign-born South Asians in New Jersey: the Desi Sehat trial. Ethn Health. 2021;26:554–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coughlin SS, Lubetkin EI, Hay JL, Raphael R, Smith SA. Promoting colorectal cancer screening among Haitian Americans. J Ga Public Health Assoc. 2015;5:149–152. [PMC free article] [PubMed] [Google Scholar]
- 54.Manne S, Steinberg MB, Delnevo C, Ulpe R, Sorice K. Colorectal cancer screening among foreign-born South Asians in the metropolitan New York/New Jersey region. J Community Health. 2015;40:1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gwede CK, William CM, Thomas KB, et al. Exploring disparities and variability in perceptions and self-reported colorectal cancer screening among three ethnic subgroups of U.S. Blacks. Oncol Nurs Forum. 2010;37:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walsh J, Nguyen T, Nguyen L, Mcphee SJ, Pasick R. Healthy colon, healthy life (Ruôt Lành, Sống Khoe): patient and physician factors associated with colorectal cancer screening among Vietnamese Americans in a county medical care system. J Health Care Poor Underserved. 2009;20:74–89. [DOI] [PubMed] [Google Scholar]
- 57.Le D, Carney PA, Lee-Lin F, et al. Differences in knowledge, attitudes, beliefs, and perceived risks regarding colorectal cancer screening among Chinese, Korean, and Vietnamese sub-groups. J Community Health. 2014;39:248–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakajima M, Haji A, Mohamud S, Ahmed O, Hodges JS, Pratt R. A culturally adapted colorectal cancer education video for the Somali community in Minnesota: a pilot investigation. Am J Health Promot. 2021;0:1–4. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Burke A, Tsoh JY, et al. Exploring a culturally relevant model of cancer prevention involving traditional Chinese medicine providers in a Chinese American community. Eur J Integr Med. 2014;6:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lopez-Class M, Luta G, Noone AM, et al. Patient and provider factors associated with colorectal cancer screening in safety net clinics serving low-income, urban immigrant latinos. J Health Care Poor Underserved. 2012;23:1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee SY, Lee EE. Korean Americans’ beliefs about colorectal cancer screening. Asian Nurs Res (Korean Soc Nurs Sci). 2013;7:45–52. [DOI] [PubMed] [Google Scholar]
- 62.Maxwell AE, Crespi CM, Danao LL, Antonio C, Garcia GM, Bastani R. Alternative approaches to assessing intervention effectiveness in randomized trials: Application in a colorectal cancer screening study. Cancer Causes Control. 2011;22:1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aragones A, Schwartz MD, Shah NR, Gany FM. A randomized controlled trial of a multilevel intervention to increase colorectal cancer screening among Latino immigrants in a primary care facility. J Gen Intern Med. 2010;25:564–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miranda PY, Yao N, Snipes SA, BeLue R, Lengerich E, Hillemeier MM. Citizenship, length of stay, and screening for breast, cervical, and colorectal cancer in women, 2000e2010. Cancer Causes Control. 2017;28:589–598. [DOI] [PubMed] [Google Scholar]
- 65.Menon U, Szalacha L, Prabhughate A, Kue J. Correlates of colorectal cancer screening among South Asian immigrants in the United States. Cancer Nurs. 2014;37:E19–E27. [DOI] [PubMed] [Google Scholar]
- 66.Morey BN, Valencia C, Sunmin Lee. The influence of Asian subgroup and acculturation on colorectal cancer screening knowledge and attitudes among Chinese and Korean Americans. J Canc Educ. 2021:1e10. [e-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dimaano C, Alsayid M, Tlimat NM, Spigner C. Perceptions of colorectal cancer screening in the Arab American community: a pilot study. Prim Health Care Res Dev. 2019;20:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakajima M, Haji A, Sero A, et al. Psychosocial correlates of experience and intention to receive colorectal cancer screening: a cross-sectional study among East African men in the U.S. J Prim Prev. 2021;42:603–623. [DOI] [PubMed] [Google Scholar]
- 69.Li M, Yeh YL, Sun H, Chang B, Chen LS. Community-based participatory research: a family health history-based colorectal cancer prevention program among Chinese Americans. J Cancer Educ. 2019;35:485–492. [DOI] [PubMed] [Google Scholar]
- 70.Consedine NS, Reddig MK, Ladwig I, Broadbent EA. Gender and ethnic differences in colorectal cancer screening embarrassment and physician gender preferences. Oncol Nurs Forum. 2011;38:E409–E417. [DOI] [PubMed] [Google Scholar]
- 71.Ferdous M, Goopy S, Yang H, Rumana N, Abedin T, Turin TC. Barriers to breast cancer screening among immigrant populations in Canada. J Immigr Minor Health. 2020;22:410–420. [DOI] [PubMed] [Google Scholar]
- 72.Hurtado-de-Mendoza A, Song M, Kigen O, Jennings Y, Nwabukwu I, Sheppard VB. Addressing cancer control needs of African-born immigrants in the US: a systematic literature review. Prev Med. 2014;67:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marques P, Nunes M, Da Luz Antunes M, Heleno B, Dias S. Factors associated with cervical cancer screening participation among migrant women in Europe: a scoping review. Int J Equity Health. 2020;19:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.ACS. Colorectal cancer facts and figures 2020–2022. Am Cancer Soc. 2020;66:1–41. [Google Scholar]
- 75.Steinwachs D, Allen JD, Barlow WE, et al. NIH state-of-the-science conference statement: enhancing use and quality of colorectal cancer screening. NIH Consens State Sci Statements. 2010;27:1–31. [PubMed] [Google Scholar]
- 76.DeGroff A, Sharma K, Satsangi A, et al. Increasing colorectal cancer screening in health care systems using evidence-based interventions. Prev Chronic Dis. 2018;15:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Joseph DA, Degroff A. The CDC colorectal cancer control program, 2009e2015. Prev Chronic Dis. 2019;16:E159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gupta S, Sussman DA, Doubeni CA, et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014;106:dju032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Attipoe-Dorcoo S, Chattopadhyay SK, Verughese J, Ekwueme DU, Sabatino SA, Peng Y. Engaging community health workers to increase cancer screening: a community guide systematic economic review. Am J Prev Med. 2021;60:e189–e197. [DOI] [PubMed] [Google Scholar]
- 80.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved a randomized clinical trial. JAMA Intern Med. 2013;173:1725–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lasser KE, Murillo J, Lisboa S, et al. Colorectal cancer screening among ethnically diverse, low-income patients. Arch Intern Med. 2011;171:906–912. [DOI] [PubMed] [Google Scholar]
- 82.Frerichs L, Beasley C, Pevia K, et al. Testing a culturally adapted colorectal cancer screening decision Aid among American Indians: results from a pre–epost trial. Health Equity. 2020;4:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liss DT, Petit-Homme A, Feinglass J, Buchanan DR, Baker DW. Adherence to repeat fecal occult blood testing in an urban community health center network. J Community Health. 2013;38:829–833. [DOI] [PubMed] [Google Scholar]
- 84.Davis T, Arnold C, Rademaker A, et al. Improving colon cancer screening in community clinics. Cancer. 2013;119:3879–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davis TC, Arnold CL, Bennett CL, et al. Strategies to improve repeat fecal occult blood testing cancer screening. Cancer Epidemiol Biomarkers Prev. 2014;23:134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pérez-Escamilla R, Garcia J, Song D. HEALTH CARE ACCESS AMONG HISPANIC IMMIGRANTS: ¿ALGUIEN ESTÁ ESCUCHANDO? [IS ANYBODY LISTENING?]. NAPA Bull. 2010;34:47–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim W, Keefe RH. Barriers to healthcare among Asian Americans. Soc Work Public Health. 2010;25:286–295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data not publicly available but can be accessed upon request.