Abstract

Structural and functional modulation of three-dimensional artificial macromolecular systems is of immense importance. Designing supramolecular cages that can show stimuli mediated reversible switching between higher-order structures is quite challenging. We report here construction of a Pd6 trifacial barrel (1) by coordination self-assembly. Surprisingly, barrel 1 was found to exhibit guest-responsive behavior. In presence of fullerenes C60 and C70, 1 unprecedentedly transformed to its metastable higher homologue Pd8 tetrafacial barrel (2), forming stable host–guest complexes (C60)3⊂2 and (C70)2⊂2, respectively. Again, encapsulated fullerenes could be extracted from the cavity of 2 using 1,2-dichlorobenzene, leading to its facile conversion to the parent trifacial barrel 1. Such reversible structural interconversion between an adaptable molecular barrel and its guest stabilized higher homologue is an uncommon observation.
Keywords: Self-assembly, Molecular barrels, Fullerene encapsulation, Structural transformation, Coordination chemistry
Introduction
The flexible and adaptable nature of macromolecular biological receptors is responsible for their structural transformation upon substrate binding. Such flexible and adaptable behavior is mainly due to the noncovalent interactions that drive the biological processes. On the other hand, covalent architectures are generally less prone to structural reorganization to expand their cavity size significantly for better fitting of the substrates. Coordination-driven self-assembly is an efficient technique for designing adaptable large molecular architectures.1−9 The dynamic nature of the metal–ligand bonding allows for self-correction and conformational changes of the structures.10−15 The thermodynamically most stable architecture is formed from a given set of building blocks, whereas the other possible geometries remain inaccessible. However, external stimuli16−30 may induce structural changes to access metastable architectures that are not usually formed. Over the years, the internal cavities of such discrete architectures have been used for binding guest molecules for numerous applications such as catalysis,31−38 sensing,39−43 light-harvesting,44−49 biological applications,50−52 molecular separations,53−56 and modification of the dynamics and chemical reactivity of the trapped guests.57−59 Guest binding within the internal pockets is completely dictated by the size complementarity and favorable noncovalent interactions between the host and the guest. Thus, suitable hosts can be predesigned by incorporating specific functionalities within the building blocks for stabilizing a wide variety of guest molecules within their cavities. Among the various shapes, architectures resembling barrels are expected to be efficient for encapsulating guest molecules due to their large open windows and parallel orientation of the walls which ensures greater interactions with the incoming guest molecules.60,61
Fullerenes, due to their interesting physicochemical properties, find applications in several fields.62−67 The major drawback of working with fullerenes is their poor solubility in common organic solvents. Functionalization of fullerenes to generate soluble derivatives has been the common technique employed to overcome this issue. However, such functionalization usually needs multistep reactions and tedious separation steps.68 On the other hand, encapsulation of fullerenes within a soluble host is believed to be an easier solution to enhance the solubility of free fullerenes without their chemical modification. Designing suitable receptors that can bind fullerenes in solvents where fullerenes are insoluble has been the prime focus of supramolecular chemists.69−74 Synthesis of hosts that can encapsulate many molecules of fullerene is highly desirable to enhance their solubility. However, it is a challenging task to design such receptors with large, confined pockets for accommodating more than one fullerene molecule.
Herein, we report the formation of a [Pd6L3]12+ trifacial molecular barrel (1) upon coordination self-assembly (Scheme 1) of a tetratopic ligand L with cis-[(tmeda)Pd(ONO2)2] (A) as an acceptor in DMSO (tmeda = N,N,N′,N′-tetramethylethane-1,2-diamine; DMSO = dimethyl sulfoxide). Trifacial barrel (1) possesses a large internal cavity with open windows suitable for encapsulation of large guest molecules. Interestingly, upon treatment with fullerenes C60/C70, the trifacial barrel undergoes an uncommon structural transformation to its higher homologue [Pd8L4]16+ tetrafacial barrel (2) with the formation of fullerene encapsulated stable adducts (C60)3⊂2 and (C70)2⊂2, respectively. The labile and dynamic nature of the Pd–pyridine bond is ultimately responsible for the transformation of the trifacial barrel to its higher homologue in the presence of external guests to reach its thermodynamically most stable state.70,75−79 The addition of fullerene to barrel 1 induced reorganization of the building blocks to generate a metastable tetrafacial barrel (2) by effective host–guest interactions. Extraction of C60/C70 from the host–guest complexes led to facile conversion of 2 to its parent trifacial barrel 1 which indicates that the metastable barrel 2 could only be obtained in the presence of C60 or C70 as the guest. Such guest-induced reversible structural transformation between homologues of self-assembled molecular barrels and stabilization of a metastable homologue by multiple fullerene molecules are unusual observations. Further, these adducts [(C60)3⊂2 and (C70)2⊂2] were found to act as photosensitizers for singlet oxygen generation under visible light irradiation in acetonitrile where free C60/C70 is insoluble and shows no singlet oxygen generation.
Scheme 1. Schematic Representation of Synthesis of Trifacial Barrel 1 and Its Conversion to Metastable Higher Homologues in the Presence of C60/C70 Molecules.

Results and Discussion
The ligand L was synthesized by reacting 4,4′-(benzo[c][1,2,5]thiadiazole-4,7-diyl)dibenzaldehyde (P) with KOH, 4-acetylpyridine, and NH4OH in ethanol using a standard procedure for the synthesis of terpyridines.70,80,81 Precursor P was synthesized via Suzuki coupling between 4,7-dibromobenzo[c][1,2,5]thiadiazole and (4-formylphenyl)boronic acid (Scheme S1) following a reported procedure.82 Self-assembly of L and cis-[(tmeda)Pd(NO3)2] (A) was done in a 1:2 molar ratio in DMSO for 48 h at 70 °C which afforded a clear yellow solution (Scheme 1). 1 was isolated as a yellow solid in almost quantitative yield upon addition of excess ethyl acetate to the reaction mixture. 1H NMR of 1 was recorded in DMSO-d6 which showed downfield shifts of α-pyridyl protons and a 2-fold splitting of α-pyridyl (Ha and Ha’) and β-pyridyl (Hb and Hb’) protons of the ligand in comparison to the free L due to rigidification of asymmetric ligand L upon assembly formation (Figures 1a,b, S1, and S4). All the peaks in the 1H NMR spectrum were assigned with the help of 1H–1H COSY (Figure S6) and NOESY NMR (Figure S7). The same diffusion coefficient (D = 6.025 × 10–11 m2/s) for all the peaks in 1H-DOSY NMR confirmed the formation of a single assembly (Figure S5). 1 was converted to the PF6– analogue by treating the nitrate analogue with excess KPF6 for better ionization. ESI-MS analyses confirmed the formation of a Pd6L3 trifacial barrel 1 by the appearance of peaks at m/z = 1630.8743, 1186.9005, 920.5327, and 742.9542 which correspond to the charged fragments [1-3PF6]3+, [1-4PF6]4+, [1-5PF6]5+, and [1-6PF6]6+, respectively (Figures 1c,d and S9). The isotopic patterns corresponding to these fragments were in good agreement with the theoretically calculated patterns.
Figure 1.

(a) Stacked 1H NMR spectra of 1 and (b) L in DMSO-d6. (c) Experimental (blue) and (e) calculated (red) isotopic patterns of [1-4PF6]4+ fragment. (d) Experimental (blue) and (f) calculated (red) isotopic patterns of [1-5PF6]5+ fragment in ESI-MS analyses.
Several attempts to grow single crystals of 1 were fruitless. To gain structural insights, the geometry of 1 was optimized using the PM6 method. In the energy minimized structure of barrel 1, three tetrapyridyl ligands (L) are horizontally oriented in a trigonal disposition and the six corners are clipped by the Pd(II) acceptor A (Figure 2). There remains a possibility to obtain an isomeric trifacial tube in which all three ligands are vertically oriented and clipped to six Pd(II) acceptors. However, the formation of a trifacial tube (Figures S24) is energetically disfavored by about 132 kcal/mol compared to barrel 1 which can be attributed to the greater steric crowding among the vertical panels. From the optimized structure, it is observed that 1 possesses a large internal cavity of diameter 19.72 Å (Figure 2a), π-conjugated aromatic walls, and wide-open windows, suitable for encapsulation and stabilization of large guest molecules.
Figure 2.
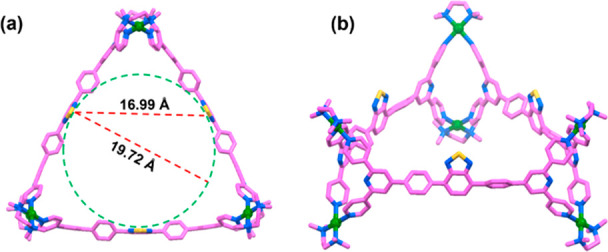
Structure of trifacial barrel 1 obtained by optimization: (a) top view and (b) side view, where ligands are horizontally oriented. Color codes: carbon (pink), nitrogen (blue), sulfur (yellow), and palladium (green). Hydrogen atoms are omitted for clarity.
Fullerenes (C60/C70), on the other hand, are electron-deficient large molecules with negligible solubility in a polar solvent like DMSO. This prompted us to carry out encapsulation of fullerenes C60 and C70 into barrel 1. We have recently shown structural conversion of a self-assembled trigonal cage to larger analogue upon binding of large guests like fullerenes.70 Four equivalents of C60 were added to a yellow solution of trifacial barrel 1 in DMSO and stirred at 70 °C for 12 h. The reaction mixture was centrifuged, and a clear brown solution was obtained. The product was isolated as brown solid by treating the solution with excess ethyl acetate. A new set of peaks was seen in the 1H NMR spectrum with downfield shifts of the α-pyridyl protons of the barrel, indicating the formation of a host–guest complex with C60 (Figures 3b and S10).
Figure 3.
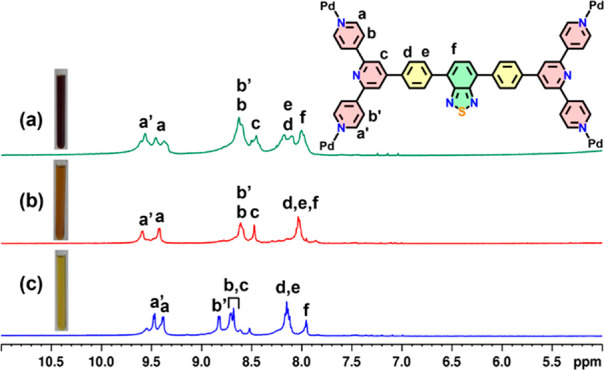
Stacked 1H NMR spectra of (a) (C70)2⊂2, (b) (C60)3⊂2, and (c) trifacial barrel 1 in DMSO-d6. Insets show the color of the solutions.
An encapsulation experiment with the ellipsoidal C70 was performed in a similar fashion. A deep-red solution was obtained, which upon treatment with excess ethyl acetate yielded a dark-red solid product. The 1H NMR spectrum of the product was broad due to the presence of unsymmetrical and ellipsoidal C70 (Figures 3a and S17). 13C NMR spectra of these complexes were recorded to detect the presence of encapsulated C60 or C70 molecules. Free fullerenes in DMSO do not show any peak in 13C NMR spectra due to poor solubility. Interestingly, two intense peaks of slightly different intensities were found at δ 141.39 and 140.80 ppm for the C60 complex (Figures 4b and S14). Spherically symmetrical C60 is expected to show a single intense peak in the 13C NMR spectrum. The presence of two peaks hinted toward the possibility of the presence of multiple C60 molecules encapsulated within the cavity of the barrel. 13C NMR spectrum of the C70 complex showed several intense peaks characteristic of C70 which also indicated the possible presence of multiple C70 molecules encapsulated within the barrel (Figures 4a and S21).
Figure 4.
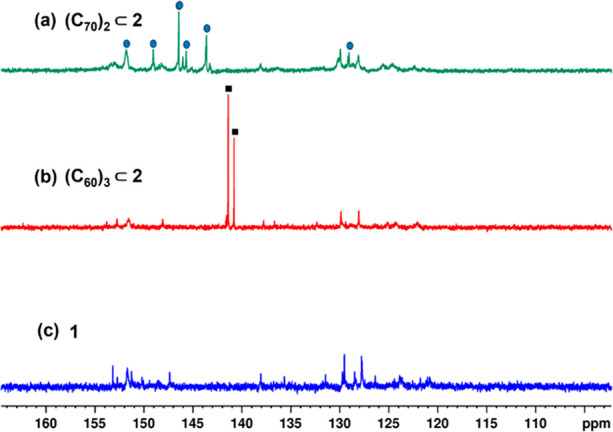
Stacked 13C NMR spectra of (a) (C70)2⊂2, (b) (C60)3⊂2, and (c) trifacial barrel 1 in DMSO-d6.
To get the exact composition of the host–guest complexes, the nitrate analogues were converted to hexafluorophosphates for easy ionization in ESI-MS experimental conditions. Surprisingly, a composition of three C60 molecules encapsulated in a homologous tetrafacial barrel 2 ([(C60)3⊂2]) was found for the C60 complex. Peaks were obtained at m/z = 2171.4499, 1708.1593, 1399.3139, 1178.6954, and 1013.2381 which correspond to the charged fragments [(C60)3⊂2-4PF6]4+, [(C60)3⊂2-5PF6]5+, [(C60)3⊂2-6PF6]6+, [(C60)3⊂2-7PF6]7+, and [(C60)3⊂2-8PF6]8+, respectively (Figures 5b,d, S15, and S16). The isotopic patterns were in good agreement with theoretically calculated patterns. On the other hand, a composition of two C70 molecules encapsulated in a similar tetrafacial barrel 2 ([(C70)2⊂2]) was obtained for the C70 complex from ESI-MS analyses. Peaks and the isotopic distribution patterns were obtained at m/z = 2051.1936, 1611.9548, 1319.1342, 1109.9847, and 953.1152 which correspond to the charged fragments [(C70)2⊂2-4PF6]4+, [(C70)2⊂2-5PF6]5+, [(C70)2⊂2-6PF6]6+, [(C70)2⊂2-7PF6]7+, and [(C70)2⊂2-8PF6]8+, respectively (Figures 5a,e, S22, and S23). Interestingly, neither the free trifacial barrel (1) nor the tetrafacial barrel (2) was detected from mass analyses. Therefore, trifacial barrel 1 undergoes a complete transformation to a homologous tetrafacial barrel 2 with encapsulated C60 or C70. While the free tetrafacial barrel was not formed during the reaction, it was isolated in the form of stable host–guest complexes with three C60 or two C70 molecules.
Figure 5.

Partial stacked ESI-MS spectra of (a) (C70)2⊂2, (b) (C60)3⊂2, and (c) 1. Experimental isotopic distribution patterns of (d) [(C60)3⊂2-5PF6]5+ and (e) [(C70)2⊂2-5PF6]5+ in ESI-MS.
1H-DOSY NMR confirmed the presence of a single species for both the complexes (C60)3⊂2 and (C70)2⊂2 (Figures S11 and S18). Further, a comparison of the diffusion coefficient values for the free trifacial barrel 1 (D = 6.025 × 10–11 m2/s) with host–guest complexes (C60)3⊂2 (D = 5.164 × 10–11 m2/s) and (C70)2⊂2 (D = 4.819 × 10–11 m2/s) revealed higher hydrodynamic radii for (C60)3⊂2 and (C70)2⊂2 than the free barrel 1, which also supports the transformation of 1 to the higher homologue (2).
Absorption spectra of 1, (C60)3⊂2, and (C70)2⊂2 were recorded (Figure 6) using 10 μM dimethyl sulfoxide solutions of the respective complexes. Trifacial barrel 1 displays two absorptions at 307 and 392 nm due to π–π* transitions. (C60)3⊂2 shows an absorption at 387 nm with a 5 nm blue shift compared to the peak at 392 nm for 1 and a hump at 324 nm (free C60 shows an absorption at 321 nm).83 (C70)2⊂2 shows peaks at 383 and 311 nm and a broad hump at 470 nm (characteristic of C70).84 These observations further confirmed the presence of encapsulated C60 and C70 in these complexes.
Figure 6.

Normalized absorption spectra of 1, (C60)3⊂2, and (C70)2⊂2 in DMSO (10 μM concentration).
The structures of 2, (C60)3⊂2, and (C70)2⊂2 were optimized by the PM6 method. The tetrafacial barrel 2 possesses a cavity large enough to accommodate three C60 or two C70 molecules (Figure S25). Inside the cavity of 2, the three C60 molecules are linearly oriented along one diagonal (Figure 7a). This explains the origin of two peaks corresponding to C60 in the 13C NMR spectrum of (C60)3⊂2. The two peripheral C60 molecules within the cavity of 2 are in a different chemical environment compared to the central C60 molecule, and thus, two time-averaged peaks of slightly different intensities are observed in the 13C NMR spectrum (Figure 4b). The two C70 molecules are diagonally oriented within the cavity (Figure 7b). Presumably, the π–π interactions between the cluster of aromatic rings of terpyridine-based building blocks of host 2 and ellipsoidal C70 provide the maximum stabilization in two corners of 2.
Figure 7.
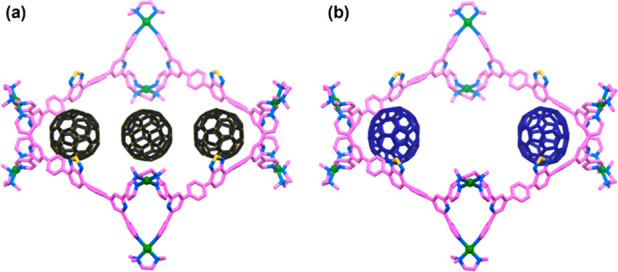
Optimized structures of (a) (C60)3⊂2 and (b) (C70)2⊂2. Color codes: carbons of host (pink), nitrogen (blue), sulfur (yellow), palladium (green), carbons of C60 guest (black), carbons of C70 guest (deep blue). Hydrogen atoms are omitted for clarity.
Next, competitive binding experiments were performed by treating 1 with excess C60 and C70 added simultaneously in a 1:1 ratio (4 equiv of each C60 and C70 was added to 1 equiv of 1 and mixture stirred at 70 °C for 12 h), and the results were monitored by ESI-MS. Interestingly, the peaks obtained correspond to C70 encapsulated barrel (C70)2⊂2 (Figure 8a). Again, when 4 equiv of C60 was added to the complex (C70)2⊂2, no change was observed in mass spectrum, confirming the high stability and selectivity of (C70)2⊂2 (Figure 8b). However, when 4 equiv of C70 was added to complex (C60)3⊂2, C70 could not displace C60 molecules from the cavity of 2 (Figure 8c). These results imply that the barrel-to-barrel transformation occurs in the presence of both C60 and C70. C70 has a slightly higher affinity toward the tetrafacial barrel (2); however, once three C60 molecules get encapsulated within the cavity of 2, it forms the very stable host–guest complex (C60)3⊂2, and C70 cannot replace the encapsulated C60 molecules from the cavity.
Figure 8.

Partial stacked ESI-MS spectra: (a) after treatment of 1 with excess C60 and C70 (1:1 ratio), (b) after treatment of (C70)2⊂2 with excess C60, (c) after treatment of (C60)3⊂2 with excess C70, (d) (C70)2⊂2, and (e) (C60)3⊂2 and (f) 1.
It was important to extract the fullerenes from the host–guest complexes to check the fate of the barrel. After screening with different solvents, 1,2-dichlorobenzene was found to be appropriate for the extraction experiments since barrel/host–guest complexes and fullerenes (C60/C70) have orthogonal solubilities in this solvent. C60 and C70 were successfully extracted from the complexes as was indicated by the pale purple/brown coloration of the 1,2-dichlorobenzene extract in the respective cases. Furthermore, ESI-MS analyses confirmed the existence of only trifacial barrel 1 and not tetrafacial barrel 2 after extraction in both cases (Figure 9a,c).
Figure 9.

Partial stacked ESI-MS spectra: (a) after extraction of C70 from (C70)2⊂2 using 1,2-dichlorobenzene, (b) (C70)2⊂2, (c) after extraction of C60 from (C60)3⊂2 using 1,2-dichlorobenzene, (d) (C60)3⊂2, and (e) 1.
This proves that the structural transformation from trifacial barrel to its higher homologue is feasible only in the presence of fullerenes. The removal of fullerenes from the host–guest complexes readily converted the tetrafacial barrel to the parent trifacial barrel in solution, which indicates that the tetrafacial barrel is a metastable form which can only exist in the presence of fullerenes (Scheme 2).
Scheme 2. Schematic Representation of Fullerene Induced Barrel to Barrel Transformation, Competitive Binding of C60 and C70, and Fullerene Extraction.

Singlet oxygen (1O2) generation is important for different uses. Photosensitizers upon light irradiation can transfer its absorbed energy to triplet oxygen to generate singlet oxygen. Fullerenes are well-known photosensitizers that can generate singlet oxygen, but their insolubility in common organic solvents limits their application. Good solubility of the PF6– analogues of (C60)3⊂2 and (C70)2⊂2 in acetonitrile prompted us to investigate the efficiencies of these complexes as photosensitizers for generating singlet oxygen using visible light. To the CD3CN solutions of anthracene, 2 mol % of PF6– analogues of these complexes were added separately and irradiated with visible light, and the generation of singlet oxygen was monitored by the formation of anthracene endoperoxide over time using 1H NMR. The complete conversion of anthracene to anthracene-endoperoxide was achieved in 4 and 5 h using (C70)2⊂2 and (C60)3⊂2, respectively (Figures S26 and S27). Although, (C70)2⊂2 has two molecules of fullerene, its slightly higher singlet oxygen generating ability compared to (C60)3⊂2 can be ascribed to its higher absorption in the visible region (Figure 6). The building block of free barrel 1, (C60)3⊂2, and (C70)2⊂2 contains a benzothiadiazole moiety which too is a photosensitizer. So, we also investigated the generation of singlet oxygen using 2 mol % of PF6– analogue of free barrel 1 as a photosensitizer in acetonitrile. Even after irradiation with visible light for 20 h, complete conversion of anthracene could not be achieved, thus showing the poor singlet oxygen generating ability of the free barrel 1 (Figures 10 and S28). Encapsulation of multiple fullerenes within the barrel enhanced singlet oxygen generation drastically (Figure 10). Notably, when an excess (20 mol %) of either C70 or C60 was used as a heterogeneous photocatalyst for conversion of anthracene to its endoperoxide in acetonitrile, no conversion was observed (Figures S29 and S30), thus highlighting the importance of solubilizing fullerenes C60/C70 in common organic solvents by encapsulation within a host for singlet oxygen generation.
Figure 10.
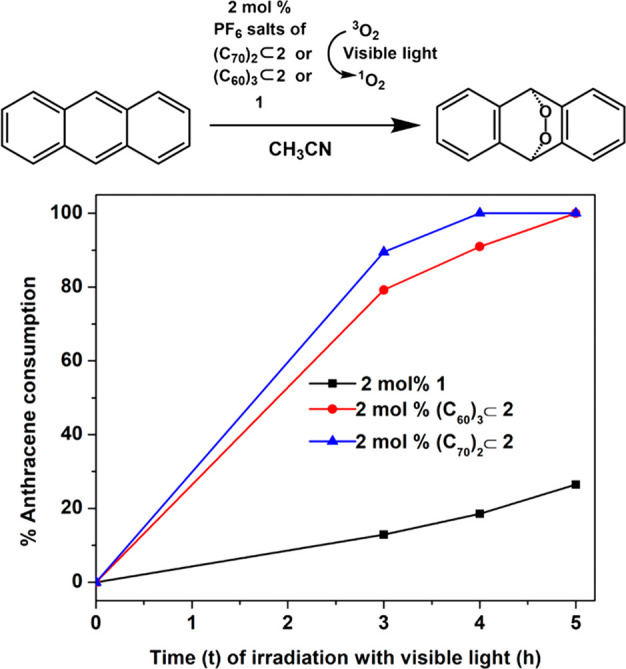
Comparison of singlet oxygen generating ability of (C70)2⊂2, (C60)3⊂2, and 1 obtained by monitoring anthracene consumption and formation of its endoperoxide over time upon visible light irradiation using 1H NMR.
Conclusions
In summary, we report here the synthesis of an adaptable trifacial molecular barrel (1) that undergoes structural change to a metastable higher homologue in the presence of suitable guests by efficient host–guest binding. The barrel (1) was obtained via self-assembly of a tetrapyridyl ligand L and a cis-blocked Pd(II) acceptor (A). The large cavity, open windows, and π-electron rich aromatic walls of 1 made it a potential candidate for electron deficient large guests like fullerenes C60 and C70. The trifacial barrel (1) showed an uncommon structural transformation70 to its metastable higher homologue tetrafacial barrel (2) in the presence of excess C60 and C70, stabilizing three C60 and two C70 molecules in its enlarged cavity, respectively. Competitive binding experiments with C60 and C70 confirmed that C70 has a higher affinity for tetrafacial barrel 2 possibly due to greater π-interactions with the walls of the molecular barrel because of the ellipsoidal shape of C70. The host–guest complexes (C60)3⊂2 and (C70)2⊂2 were thermodynamically stable and did not show guest exchange upon treatment with C70 and C60, respectively. Trifacial barrel 1 was thus found to be a guest-responsive system which undergoes a structural expansion to a larger homologous molecular container to stabilize multiple fullerene molecules within its cavity. Further, the encapsulated fullerenes were extracted by washing with an appropriate solvent which led to the facile conversion of the metastable tetrafacial barrel to parent trifacial barrel 1. Such a guest mediated reversible structural transformation between a 3D adaptable architecture and its higher homologue is an interesting observation. The PF6– analogues of (C60)3⊂2 and (C70)2⊂2 generated singlet oxygen under visible light irradiation in acetonitrile. The encapsulation of multiple fullerenes within host 2 was thus pivotal in solubilizing acetonitrile insoluble C60/C70 molecules for acting as visible light photosensitizers in acetonitrile.
Methods
All the chemicals were purchased from commercially available sources and used without further purification. NMR spectra were recorded on Bruker 400 and 500 MHz NMR spectrometers in DMSO-d6. Electrospray ionization mass spectra (ESI-MS) were recorded using an Agilent 6538 Ultra-High Definition (UHD) Accurate Mass Q-TOF spectrometer using standard spectroscopic grade solvents. Electronic absorption spectra were recorded on a LAMBDA 750 UV/vis spectrophotometer. White LED (45 W) was used for singlet oxygen generation experiments.
The Gaussian 09 package was used for the computational study.85 Trifacial barrel 1, trifacial tube, tetrafacial barrel 2, (C60)3⊂2, and (C70)2⊂2 were optimized using the PM6 semiempirical method. No symmetry constraints were used during optimization.
Acknowledgments
P.S.M. thanks the SERB (New Delhi) for the research grant. R.B. gratefully acknowledges PMRF (India) for the research fellowship and contingency grant. The authors thank Mr. Pallab Bhandari and Mrs. Arppitha Baby Sainaba for help in recording mass spectra.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.3c00224.
Experimental section, additional NMR spectra (1H, COSY, NOESY, DOSY), mass spectra, optimized structures (PDF)
Author Contributions
‡ R.B. and S.B. contributed equally. R.B. carried out the experimental works. S.B. carried out the optimization studies. R.B. and S.B. analyzed the experimental data. P.S.M. supervised the whole project. All authors contributed to writing this manuscript, and they have given approval to the final version of the manuscript. CRediT: Ranit Banerjee conceptualization, data curation, formal analysis, investigation, methodology, writing-original draft, writing-review & editing; Soumalya Bhattacharyya data curation, formal analysis, investigation, methodology, writing-original draft, writing-review & editing; Partha Sarathi Mukherjee conceptualization, funding acquisition, project administration, supervision, writing-original draft, writing-review & editing.
The authors declare no competing financial interest.
Supplementary Material
References
- Caulder D. L.; Brückner C.; Powers R. E.; König S.; Parac T. N.; Leary J. A.; Raymond K. N. Design, Formation and Properties of Tetrahedral M4L4 and M4L6 Supramolecular Clusters. J. Am. Chem. Soc. 2001, 123, 8923–8938. 10.1021/ja0104507. [DOI] [PubMed] [Google Scholar]
- Yoshizawa M.; Klosterman J. K. Molecular architectures of multi-anthracene assemblies. Chem. Soc. Rev. 2014, 43, 1885–1898. 10.1039/C3CS60315F. [DOI] [PubMed] [Google Scholar]
- Gao W.-X.; Feng H.-J.; Guo B.-B.; Lu Y.; Jin G.-X. Coordination-Directed Construction of Molecular Links. Chem. Rev. 2020, 120, 6288–6325. 10.1021/acs.chemrev.0c00321. [DOI] [PubMed] [Google Scholar]
- Hardy M.; Tessarolo J.; Holstein J. J.; Struch N.; Wagner N.; Weisbarth R.; Engeser M.; Beck J.; Horiuchi S.; Clever G. H.; Lützen A. A Family of Heterobimetallic Cubes Shows Spin-Crossover Behaviour Near Room Temperature. Angew. Chem., Int. Ed. 2021, 60, 22562–22569. 10.1002/anie.202108792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa S.; Meichsner S. L.; Holstein J. J.; Baksi A.; Kasanmascheff M.; Clever G. H. Long-Lived C60 Radical Anion Stabilized Inside an Electron-Deficient Coordination Cage. J. Am. Chem. Soc. 2021, 143, 9718–9723. 10.1021/jacs.1c02860. [DOI] [PubMed] [Google Scholar]
- Seidel S. R.; Stang P. J. High-Symmetry Coordination Cages via Self-Assembly. Acc. Chem. Res. 2002, 35, 972–983. 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]
- Fujita M.; Tominaga M.; Hori A.; Therrien B. Coordination Assemblies from a Pd(II)-Cornered Square Complex. Acc. Chem. Res. 2005, 38, 369–378. 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]
- Saha R.; Mondal B.; Mukherjee P. S. Molecular Cavity for Catalysis and Formation of Metal Nanoparticles for Use in Catalysis. Chem. Rev. 2022, 122, 12244–12307. 10.1021/acs.chemrev.1c00811. [DOI] [PubMed] [Google Scholar]
- Li Y.; Jiang Z.; Wang M.; Yuan J.; Liu D.; Yang X.; Chen M.; Yan J.; Li X.; Wang P. Giant, Hollow 2D Metalloarchitecture: Stepwise Self-Assembly of a Hexagonal Supramolecular. Nut. J. Am. Chem. Soc. 2016, 138, 10041–10046. 10.1021/jacs.6b06021. [DOI] [PubMed] [Google Scholar]
- Nitschke J. R. Construction, Substitution, and Sorting of Metallo-organic Structures via Subcomponent Self-Assembly. Acc. Chem. Res. 2007, 40, 103–112. 10.1021/ar068185n. [DOI] [PubMed] [Google Scholar]
- Debata N. B.; Tripathy D.; Chand D. K. Self-assembled coordination complexes from various palladium(II) components and bidentate or polydentate ligands. Coord. Chem. Rev. 2012, 256, 1831–1945. 10.1016/j.ccr.2012.04.001. [DOI] [Google Scholar]
- Bloch W. M.; Holstein J. J.; Dittrich B.; Hiller W.; Clever G. H. Hierarchical Assembly of an Interlocked M8L16 Container. Angew. Chem., Int. Ed. 2018, 57, 5534–5538. 10.1002/anie.201800490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G.; Yu S.; Saha M. L.; Zhou J.; Cook T. R.; Yung B. C.; Chen J.; Mao Z.; Zhang F.; Zhou Z.; Liu Y.; Shao L.; Wang S.; Gao C.; Huang F.; Stang P. J.; Chen X. A discrete organoplatinum (II) metallacage as a multimodality theranostic platform for cancer photochemotherapy. Nat. Commun. 2018, 9, 4335. 10.1038/s41467-018-06574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Wang K.; Xu Y.; Wang W.; Chen S.; Hart M.; Wojtas L.; Zhou L.-P.; Gan L.; Yan X.; Li Y.; Lee J.; Ke X.-S.; Wang X.-Q.; Zhang C.-W.; Zhou S.; Zhai T.; Yang H.-B.; Wang M.; He J.; Sun Q.-F.; Xu B.; Jiao Y.; Stang P. J.; Sessler J. L.; Li X. Hierarchical Self-Assembly of Nanowires on the Surface by Metallo-Supramolecular Truncated Cuboctahedra. J. Am. Chem. Soc. 2021, 143, 5826–5835. 10.1021/jacs.1c00625. [DOI] [PubMed] [Google Scholar]
- Stefankiewicz A. R.; Wałęsa-Chorab M.; Harrowfield J.; Kubicki M.; Hnatejko Z.; Korabik M.; Patroniak V. Self-assembly of transition metal ion complexes of a hybrid pyrazine-terpyridine ligand. Dalton Trans. 2013, 42, 1743–1751. 10.1039/C2DT31982A. [DOI] [PubMed] [Google Scholar]
- Kilbas B.; Mirtschin S.; Scopelliti R.; Severin K. A solvent-responsive coordination cage. Chem. Sci. 2012, 3, 701–704. 10.1039/C1SC00779C. [DOI] [Google Scholar]
- Henkelis J. J.; Fisher J.; Warriner S. L.; Hardie M. J. Solvent-Dependent Self-Assembly Behaviour and Speciation Control of Pd6L8 Metallo-supramolecular Cages. Chem. -Eur. J. 2014, 20, 4117–4125. 10.1002/chem.201304437. [DOI] [PubMed] [Google Scholar]
- Matsumoto K.; Kusaba S.; Tanaka Y.; Sei Y.; Akita M.; Aritani K.; Haga M.-a.; Yoshizawa M. A Peanut-Shaped Polyaromatic Capsule: Solvent-Dependent Transformation and Electronic Properties of a Non-Contacted Fullerene Dimer. Angew. Chem., Int. Ed. 2019, 58, 8463–8467. 10.1002/anie.201903117. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Banerjee R.; Zangrando E.; Mukherjee P. S. Solvent and Counteranion Assisted Dynamic Self-Assembly of Molecular Triangles and Tetrahedral Cages. Inorg. Chem. 2022, 61, 2368–2377. 10.1021/acs.inorgchem.1c03797. [DOI] [PubMed] [Google Scholar]
- Xie T.-Z.; Endres K. J.; Guo Z.; Ludlow J. M.; Moorefield C. N.; Saunders M. J.; Wesdemiotis C.; Newkome G. R. Controlled Interconversion of Superposed-Bistriangle, Octahedron, and Cuboctahedron Cages Constructed Using a Single, Terpyridinyl-Based Polyligand and Zn2+. J. Am. Chem. Soc. 2016, 138, 12344–12347. 10.1021/jacs.6b07969. [DOI] [PubMed] [Google Scholar]
- Davies J. A.; Ronson T. K.; Nitschke J. R. Twisted rectangular subunits self-assemble into a ferritin-like capsule. Chem. 2022, 8, 1099–1106. 10.1016/j.chempr.2022.01.003. [DOI] [Google Scholar]
- Cullen W.; Hunter C. A.; Ward M. D. An Interconverting Family of Coordination Cages and a meso-Helicate; Effects of Temperature, Concentration, and Solvent on the Product Distribution of a Self-Assembly Process. Inorg. Chem. 2015, 54, 2626–2637. 10.1021/ic502780b. [DOI] [PubMed] [Google Scholar]
- Shi W.-J.; Liu D.; Li X.; Bai S.; Wang Y.-Y.; Han Y.-F. Supramolecular Coordination Cages Based on N-Heterocyclic Carbene-Gold(I) Ligands and Their Precursors: Self-Assembly, Structural Transformation and Guest-Binding Properties. Chem. -Eur. J. 2021, 27, 7853–7861. 10.1002/chem.202100710. [DOI] [PubMed] [Google Scholar]
- Lewis J. E. M.; Gavey E. L.; Cameron S. A.; Crowley J. D. Stimuli-responsive Pd2L4 metallosupramolecular cages: towards targeted cisplatin drug delivery. Chem. Sci. 2012, 3, 778–784. 10.1039/C2SC00899H. [DOI] [Google Scholar]
- Lisboa L. S.; Findlay J. A.; Wright L. J.; Hartinger C. G.; Crowley J. D. A Reduced-Symmetry Heterobimetallic [PdPtL4]4+ Cage: Assembly, Guest Binding, and Stimulus-Induced Switching. Angew. Chem., Int. Ed. 2020, 59, 11101–11107. 10.1002/anie.202003220. [DOI] [PubMed] [Google Scholar]
- Scherer M.; Caulder D. L.; Johnson D. W.; Raymond K. N. Triple Helicate—Tetrahedral Cluster Interconversion Controlled by Host-Guest Interactions. Angew. Chem., Int. Ed. 1999, 38, 1587–1592. . [DOI] [PubMed] [Google Scholar]
- Wood D. M.; Meng W.; Ronson T. K.; Stefankiewicz A. R.; Sanders J. K. M.; Nitschke J. R. Guest-Induced Transformation of a Porphyrin-Edged FeII4L6 Capsule into a CuIFeII2L4 Fullerene Receptor. Angew. Chem., Int. Ed. 2015, 54, 3988–3992. 10.1002/anie.201411985. [DOI] [PubMed] [Google Scholar]
- Wang S.; Sawada T.; Fujita M. Capsule-bowl conversion triggered by a guest reaction. Chem. Commun. 2016, 52, 11653–11656. 10.1039/C6CC06551A. [DOI] [PubMed] [Google Scholar]
- Wang S.; Sawada T.; Ohara K.; Yamaguchi K.; Fujita M. Capsule-Capsule Conversion by Guest Encapsulation. Angew. Chem., Int. Ed. 2016, 55, 2063–2066. 10.1002/anie.201509278. [DOI] [PubMed] [Google Scholar]
- Yan D.-N.; Cai L.-X.; Cheng P.-M.; Hu S.-J.; Zhou L.-P.; Sun Q.-F. Photooxidase Mimicking with Adaptive Coordination Molecular Capsules. J. Am. Chem. Soc. 2021, 143, 16087–16094. 10.1021/jacs.1c06390. [DOI] [PubMed] [Google Scholar]
- Nishioka Y.; Yamaguchi T.; Yoshizawa M.; Fujita M. Unusual [2 + 4] and [2 + 2] Cycloadditions of Arenes in the Confined Cavity of Self-Assembled Cages. J. Am. Chem. Soc. 2007, 129, 7000–7001. 10.1021/ja071591x. [DOI] [PubMed] [Google Scholar]
- Chi X.; Tian J.; Luo D.; Gong H.-Y.; Huang F.; Sessler J. L. Texas-Sized Molecular Boxes: From Chemistry to Applications. Molecules 2021, 26, 2426. 10.3390/molecules26092426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings C. J.; Pluth M. D.; Bergman R. G.; Raymond K. N. Enzymelike Catalysis of the Nazarov Cyclization by Supramolecular Encapsulation. J. Am. Chem. Soc. 2010, 132, 6938–6940. 10.1021/ja102633e. [DOI] [PubMed] [Google Scholar]
- Murase T.; Horiuchi S.; Fujita M. Naphthalene Diels-Alder in a Self-Assembled Molecular Flask. J. Am. Chem. Soc. 2010, 132, 2866–2867. 10.1021/ja9107275. [DOI] [PubMed] [Google Scholar]
- Samanta D.; Mukherjee P. S. Multicomponent self-sorting of a Pd7 molecular boat and its use in catalytic Knoevenagel condensation. Chem. Commun. 2013, 49, 4307–4309. 10.1039/c2cc37377g. [DOI] [PubMed] [Google Scholar]
- Guo J.; Xu Y.-W.; Li K.; Xiao L.-M.; Chen S.; Wu K.; Chen X.-D.; Fan Y.-Z.; Liu J.-M.; Su C.-Y. Regio- and Enantioselective Photodimerization within the Confined Space of a Homochiral Ruthenium/Palladium Heterometallic Coordination Cage. Angew. Chem., Int. Ed. 2017, 56, 3852–3856. 10.1002/anie.201611875. [DOI] [PubMed] [Google Scholar]
- Das P.; Kumar A.; Howlader P.; Mukherjee P. S. A Self-Assembled Trigonal Prismatic Molecular Vessel for Catalytic Dehydration Reactions in Water. Chem. -Eur. J. 2017, 23, 12565–12574. 10.1002/chem.201702263. [DOI] [PubMed] [Google Scholar]
- Howlader P.; Mondal S.; Ahmed S.; Mukherjee P. S. Guest-Induced Enantioselective Self-Assembly of a Pd6 Homochiral Octahedral Cage with a C3-Symmetric Pyridyl Donor. J. Am. Chem. Soc. 2020, 142, 20968–20972. 10.1021/jacs.0c11011. [DOI] [PubMed] [Google Scholar]
- Shanmugaraju S.; Mukherjee P. S. Self-Assembled Discrete Molecules for Sensing Nitroaromatics. Chem. -Eur. J. 2015, 21, 6656–6666. 10.1002/chem.201406092. [DOI] [PubMed] [Google Scholar]
- Chowdhury A.; Howlader P.; Mukherjee P. S. Aggregation-Induced Emission of Platinum(II) Metallacycles and Their Ability to Detect Nitroaromatics. Chem. -Eur. J. 2016, 22, 7468–7478. 10.1002/chem.201600698. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Saha M. L.; Wang M.; Zhou Z.; Song B.; Lu C.; Yan X.; Li X.; Huang F.; Yin S.; Stang P. J. Multicomponent Platinum(II) Cages with Tunable Emission and Amino Acid Sensing. J. Am. Chem. Soc. 2017, 139, 5067–5074. 10.1021/jacs.6b12536. [DOI] [PubMed] [Google Scholar]
- Li Z.; Yan X.; Huang F.; Sepehrpour H.; Stang P. J. Near-Infrared Emissive Discrete Platinum(II) Metallacycles: Synthesis and Application in Ammonia Detection. Org. Lett. 2017, 19, 5728–5731. 10.1021/acs.orglett.7b02456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purba P. C.; Venkateswaralu M.; Bhattacharyya S.; Mukherjee P. S. Silver(I)-Carbene Bond-Directed Rigidification-Induced Emissive Metallacage for Picric Acid Detection. Inorg. Chem. 2022, 61, 713–722. 10.1021/acs.inorgchem.1c03527. [DOI] [PubMed] [Google Scholar]
- Chen S.; Li K.; Zhao F.; Zhang L.; Pan M.; Fan Y.-Z.; Guo J.; Shi J.; Su C.-Y. A metal-organic cage incorporating multiple light harvesting and catalytic centres for photochemical hydrogen production. Nat. Commun. 2016, 7, 13169. 10.1038/ncomms13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z.; Zhao Z.; Hou Y.; Wang H.; Li X.; He G.; Zhang M. Aqueous Platinum(II)-Cage-Based Light-Harvesting System for Photocatalytic Cross-Coupling Hydrogen Evolution Reaction. Angew. Chem., Int. Ed. 2019, 58, 8862–8866. 10.1002/anie.201904407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharyya K.; Bhattacharyya S.; Sepehrpour H.; Chakraborty S.; Lu S.; Shi B.; Li X.; Mukherjee P. S.; Stang P. J. Self-Assembled Fluorescent Pt(II) Metallacycles as Artificial Light-Harvesting Systems. J. Am. Chem. Soc. 2019, 141, 14565–14569. 10.1021/jacs.9b08403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia P.-P.; Xu L.; Hu Y.-X.; Li W.-J.; Wang X.-Q.; Ling Q.-H.; Shi X.; Yin G.-Q.; Li X.; Sun H.; Jiang Y.; Yang H.-B. Orthogonal Self-Assembly of a Two-Step Fluorescence-Resonance Energy Transfer System with Improved Photosensitization Efficiency and Photooxidation Activity. J. Am. Chem. Soc. 2021, 143, 399–408. 10.1021/jacs.0c11370. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Saha R.; Mukherjee P. S. Self-assembled metallasupramolecular cages towards light harvesting systems for oxidative cyclization. Chem. Sci. 2021, 12, 5319–5329. 10.1039/D1SC00097G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharyya K.; Bhattacharyya S.; Lu S.; Sun Y.; Mukherjee P. S.; Stang P. J. Emissive Platinum(II) Macrocycles as Tunable Cascade Energy Transfer Scaffolds. Angew. Chem., Int. Ed. 2022, 61, e202200715 10.1002/anie.202200715. [DOI] [PubMed] [Google Scholar]
- Sepehrpour H.; Fu W.; Sun Y.; Stang P. J. Biomedically Relevant Self-Assembled Metallacycles and Metallacages. J. Am. Chem. Soc. 2019, 141, 14005–14020. 10.1021/jacs.9b06222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S.; Venkateswarulu M.; Sahoo J.; Zangrando E.; De M.; Mukherjee P. S. Self-Assembled PtII8 Metallosupramolecular Tubular Cage as Dual Warhead Antibacterial Agent in Water. Inorg. Chem. 2020, 59, 12690–12699. 10.1021/acs.inorgchem.0c01777. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S.; Ali S. R.; Venkateswarulu M.; Howlader P.; Zangrando E.; De M.; Mukherjee P. S. Self-Assembled Pd12 Coordination Cage as Photoregulated Oxidase-Like Nanozyme. J. Am. Chem. Soc. 2020, 142, 18981–18989. 10.1021/jacs.0c09567. [DOI] [PubMed] [Google Scholar]
- Li G.; Yu W.; Ni J.; Liu T.; Liu Y.; Sheng E.; Cui Y. Self-Assembly of a Homochiral Nanoscale Metallacycle from a Metallosalen Complex for Enantioselective Separation. Angew. Chem., Int. Ed. 2008, 47, 1245–1249. 10.1002/anie.200704347. [DOI] [PubMed] [Google Scholar]
- Zhang W.-Y.; Lin Y.-J.; Han Y.-F.; Jin G.-X. Facile Separation of Regioisomeric Compounds by a Heteronuclear Organometallic Capsule. J. Am. Chem. Soc. 2016, 138, 10700–10707. 10.1021/jacs.6b06622. [DOI] [PubMed] [Google Scholar]
- Rajasekar P.; Pandey S.; Ferrara J. D.; Del Campo M.; Le Magueres P.; Boomishankar R. Chiral Separation of Styrene Oxides Supported by Enantiomeric Tetrahedral Neutral Pd(II) Cages. Inorg. Chem. 2019, 58, 15017–15020. 10.1021/acs.inorgchem.9b02389. [DOI] [PubMed] [Google Scholar]
- Sainaba A. B.; Venkateswarulu M.; Bhandari P.; Arachchige K. S. A.; Clegg J. K.; Mukherjee P. S. An Adaptable Water-Soluble Molecular Boat for Selective Separation of Phenanthrene from Isomeric Anthracene. J. Am. Chem. Soc. 2022, 144, 7504–7513. 10.1021/jacs.2c02540. [DOI] [PubMed] [Google Scholar]
- Samanta D.; Gemen J.; Chu Z.; Diskin-Posner Y.; Shimon L. J. W.; Klajn R. Reversible photoswitching of encapsulated azobenzenes in water. Proc. Natl. Acad. Sci. U.S.A. 2018, 115, 9379–9384. 10.1073/pnas.1712787115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader P.; Mondal B.; Purba P. C.; Zangrando E.; Mukherjee P. S. Self-Assembled Pd(II) Barrels as Containers for Transient Merocyanine Form and Reverse Thermochromism of Spiropyran. J. Am. Chem. Soc. 2018, 140, 7952–7960. 10.1021/jacs.8b03946. [DOI] [PubMed] [Google Scholar]
- Saha R.; Devaraj A.; Bhattacharyya S.; Das S.; Zangrando E.; Mukherjee P. S. Unusual Behavior of Donor-Acceptor Stenhouse Adducts in Confined Space of a Water-Soluble PdII8 Molecular Vessel. J. Am. Chem. Soc. 2019, 141, 8638–8645. 10.1021/jacs.9b03924. [DOI] [PubMed] [Google Scholar]
- Bhat I. A.; Jain R.; Siddiqui M. M.; Saini D. K.; Mukherjee P. S. Water-Soluble Pd8L4 Self-assembled Molecular Barrel as an Aqueous Carrier for Hydrophobic Curcumin. Inorg. Chem. 2017, 56, 5352–5360. 10.1021/acs.inorgchem.7b00449. [DOI] [PubMed] [Google Scholar]
- Bhandari P.; Modak R.; Bhattacharyya S.; Zangrando E.; Mukherjee P. S. Self-Assembly of Octanuclear PtII/PdII Coordination Barrels and Uncommon Structural Isomerization of a Photochromic Guest in Molecular Space. JACS Au 2021, 1, 2242–2248. 10.1021/jacsau.1c00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro E.; Murillo J.; Fernandez-Delgado O.; Echegoyen L. Progress in fullerene-based hybrid perovskite solar cells. J. Mater. Chem. C 2018, 6, 2635–2651. 10.1039/C7TC04302C. [DOI] [Google Scholar]
- Lucas S.; Leydecker T.; Samorì P.; Mena-Osteritz E.; Bäuerle P. Covalently linked donor-acceptor dyad for efficient single material organic solar cells. Chem. Commun. 2019, 55, 14202–14205. 10.1039/C9CC07179B. [DOI] [PubMed] [Google Scholar]
- Lee Y. W.; Yeop J.; Lim H.; Park W.-W.; Joung J. F.; Park S.; Kwon O.-H.; Kim J. Y.; Woo H. Y. Fullerene-Based Triads with Controlled Alkyl Spacer Length as Photoactive Materials for Single-Component Organic Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 43174–43185. 10.1021/acsami.1c14901. [DOI] [PubMed] [Google Scholar]
- Campidelli S.; Vázquez E.; Milic D.; Lenoble J.; Atienza Castellanos C.; Sarova G.; Guldi D. M.; Deschenaux R.; Prato M. Liquid-Crystalline Bisadducts of [60]Fullerene. J. Org. Chem. 2006, 71, 7603–7610. 10.1021/jo0609576. [DOI] [PubMed] [Google Scholar]
- Yamakoshi Y.; Umezawa N.; Ryu A.; Arakane K.; Miyata N.; Goda Y.; Masumizu T.; Nagano T. Active Oxygen Species Generated from Photoexcited Fullerene (C60) as Potential Medicines: O2–• versus 1O2. J. Am. Chem. Soc. 2003, 125, 12803–12809. 10.1021/ja0355574. [DOI] [PubMed] [Google Scholar]
- Castro E.; Garcia A. H.; Zavala G.; Echegoyen L. Fullerenes in biology and medicine. J. Mater. Chem. B 2017, 5, 6523–6535. 10.1039/C7TB00855D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A.; Lamparth I.; Karfunkel H. R. Fullerene Chemistry in Three Dimensions: Isolation of Seven Regioisomeric Bisadducts and Chiral Trisadducts of C60 and Di(ethoxycarbonyl)methylene. Angew. Chem., Int. Ed. Engl. 1994, 33, 437–438. 10.1002/anie.199404371. [DOI] [Google Scholar]
- García-Simón C.; Garcia-Borràs M.; Gómez L.; Parella T.; Osuna S.; Juanhuix J.; Imaz I.; Maspoch D.; Costas M.; Ribas X. Sponge-like molecular cage for purification of fullerenes. Nat. Commun. 2014, 5, 5557. 10.1038/ncomms6557. [DOI] [PubMed] [Google Scholar]
- Banerjee R.; Chakraborty D.; Jhang W.-T.; Chan Y.-T.; Mukherjee P. S. Structural Switching of a Distorted Trigonal Metal-Organic Cage to a Tetragonal Cage and Singlet Oxygen Mediated Oxidations. Angew. Chem., Int. Ed. 2023, e202305338 10.1002/anie.202305338. [DOI] [PubMed] [Google Scholar]
- Brenner W.; Ronson T. K.; Nitschke J. R. Separation and Selective Formation of Fullerene Adducts within an MII8L6 Cage. J. Am. Chem. Soc. 2017, 139, 75–78. 10.1021/jacs.6b11523. [DOI] [PubMed] [Google Scholar]
- Chen B.; Holstein J. J.; Horiuchi S.; Hiller W. G.; Clever G. H. Pd(II) Coordination Sphere Engineering: Pyridine Cages, Quinoline Bowls, and Heteroleptic Pills Binding One or Two Fullerenes. J. Am. Chem. Soc. 2019, 141, 8907–8913. 10.1021/jacs.9b02207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes-Espinosa C.; García-Simón C.; Pujals M.; Garcia-Borràs M.; Gómez L.; Parella T.; Juanhuix J.; Imaz I.; Maspoch D.; Costas M.; Ribas X. Supramolecular Fullerene Sponges as Catalytic Masks for Regioselective Functionalization of C60. Chem. 2020, 6, 169–186. 10.1016/j.chempr.2019.10.010. [DOI] [Google Scholar]
- Purba P. C.; Maity M.; Bhattacharyya S.; Mukherjee P. S. A Self-Assembled Palladium(II) Barrel for Binding of Fullerenes and Photosensitization Ability of the Fullerene-Encapsulated Barrel. Angew. Chem., Int. Ed. 2021, 60, 14109–14116. 10.1002/anie.202103822. [DOI] [PubMed] [Google Scholar]
- Cai L.-X.; Yan D.-N.; Cheng P.-M.; Xuan J.-J.; Li S.-C.; Zhou L.-P.; Tian C.-B.; Sun Q.-F. Controlled Self-Assembly and Multistimuli-Responsive Interconversions of Three Conjoined Twin-Cages. J. Am. Chem. Soc. 2021, 143, 2016–2024. 10.1021/jacs.0c12064. [DOI] [PubMed] [Google Scholar]
- Tsutsui T.; Catti L.; Yoza K.; Yoshizawa M. An atropisomeric M2L4 cage mixture displaying guest-induced convergence and strong guest emission in water. Chem. Sci. 2020, 11, 8145–8150. 10.1039/D0SC03223A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto F. J.; Nitschke J. R. Stereochemical plasticity modulates cooperative binding in a CoII12L6 cuboctahedron. Nat. Chem. 2017, 9, 903–908. 10.1038/nchem.2758. [DOI] [PubMed] [Google Scholar]
- Percástegui E. G. Guest-Induced Transformations in Metal-Organic Cages. Eur. J. Inorg. Chem. 2021, 2021, 4425–4438. 10.1002/ejic.202100657. [DOI] [Google Scholar]
- Yazaki K.; Akita M.; Prusty S.; Chand D. K.; Kikuchi T.; Sato H.; Yoshizawa M. Polyaromatic molecular peanuts. Nat. Commun. 2017, 8, 15914. 10.1038/ncomms15914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D.; Shao T.; Men J.; Chen X.; Gao G. Superaromatic terpyridines based on corannulene responsive to metal ions. Dalton Trans. 2014, 43, 1753–1761. 10.1039/C3DT52013G. [DOI] [PubMed] [Google Scholar]
- Gorczyński A.; Harrowfield J. M.; Patroniak V.; Stefankiewicz A. R. Quaterpyridines as Scaffolds for Functional Metallosupramolecular Materials. Chem. Rev. 2016, 116, 14620–14674. 10.1021/acs.chemrev.6b00377. [DOI] [PubMed] [Google Scholar]
- Dou C.; Chen D.; Iqbal J.; Yuan Y.; Zhang H.; Wang Y. Multistimuli-Responsive Benzothiadiazole-Cored Phenylene Vinylene Derivative with Nanoassembly Properties. Langmuir 2011, 27, 6323–6329. 10.1021/la200382b. [DOI] [PubMed] [Google Scholar]
- Saraswati T. E.; Setiawan U. H.; Ihsan M. R.; Isnaeni I.; Herbani Y. The Study of the Optical Properties of C60 Fullerene in Different Organic Solvents. Open Chem. 2019, 17, 1198–1212. 10.1515/chem-2019-0117. [DOI] [Google Scholar]
- Sachdeva S.; Singh D.; Tripathi S. K. Optical and electrical properties of fullerene C70 for solar cell applications. Opt. Mater. 2020, 101, 109717. 10.1016/j.optmat.2020.109717. [DOI] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell K. A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas D6.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09, Revision d.01; Gaussian. Inc.: Wallingford, CT, 2009.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


