Abstract
Background
The surge in malaria cases and deaths in recent years, particularly in Africa, despite the widespread implementation of malaria-control measures could be due to inefficiencies in malaria control and prevention measures in malaria-endemic communities. In this context, this study provides the malaria situation report among children in three Municipalities in Northern Ghana, where Seasonal Malaria Chemotherapy (SMC) is implemented by Ghana Health Service (GHS).
Methods
A cross-sectional household survey was carried out to assess the malaria knowledge, attitudes, and practices (KAP) and malaria prevalence in 394 households in 13 rural communities in the Kumbugu, Nanton and Tolon Municipalities, Northern Region, Ghana. This was followed by screening for P. falciparum infection with anti-HRP2 RDT and PCR among children 1–17 years in the households. Plasma levels of IgG specific for crude P. falciparum antigen (3D7) and four recombinant malaria antigens (CSP, GLURP, MSP3, and Pfs230) were assessed by ELISA. The malaria and parasitaemia data were converted into frequency and subgroup proportions and disaggregated by study sites and demographic information of the participants. The ELISA data was converted to arbitrary units (AU) and similarly compared across study sites and demographic information.
Results
The P. falciparum infection rate and frequency of malaria were high in the study areas with significant age-dependent and inter-community differences, which were reflected by differences in plasma levels of P. falciparum-specific IgG. Over 60% of households reported the use of bed nets and indoor insecticide sprays/coils, and 14% mentioned bush clearing around homes (14%) as malaria preventive measures. Community health centres were the preferred place for households (88%) to seek malaria treatment but over-the-counter drug stores were the major source (66%) of their antimalarials. Overall, malaria preventive and treatment practices were sub-optimal.
Conclusions
P. falciparum infection and malaria are still high in the studied communities, indicating that preventive and control measures against the disease in the region remain inadequate. Efforts to ensure high SMC compliance and to improve preventative and treatment practices thus seem cost-beneficial “low-hanging fruits” in the fight against malaria in the Northern Region of Ghana.
Keywords: Seasonal malaria chemotherapy (SMC), Plasmodium falciparum, Malaria, Recombinant malaria antigens, Naturally acquired immunity, Crude antigens, Northern Ghana
1. Introduction
Malaria remains a major public health concern, with a disproportionately high morbidity among children under the age of 5 years and pregnant women, especially in Sub-Saharan Africa (WHO, 2021). An estimated 228 million malaria cases, and 602,000 deaths were recorded in Africa in 2020 (WHO, 2021). A combination of several malaria-control strategies, including the use of long-lasting insecticide-treated bed nets (ITNs), indoor residual spraying (IRS), enhanced diagnosis and treatment with artemisinin-based combination treatment (ACT), intermittent preventive treatment (IPT) in pregnancy (IPTp) and in infancy (IPTi), are widely employed in endemic communities in Africa to reduce this burden (Ansah et al., 2021; WHO, 2012; WHO, 2016).
In addition to the above, the WHO recommends since 2012 the use of seasonal malaria chemoprevention (SMC) as part of a paradigm shift from “one size fits all” to targeting malaria control strategies to specific populations and locations, (WHO, 2012; WHO, 2013). This strategy has proven effective, cost-effective, safe, and feasible for the prevention of malaria among children younger than 5 years of age in areas with highly seasonal P. falciparum transmission (Ansah et al., 2021; ACCESS-SMC Partnership, 2020; Meremikwu et al., 2012). SMC is therefore now widely adopted in several countries, especially in the Sahel and sub-Sahel regions of West Africa (Coldiron et al., 2017; Ashley and Yeka, 2020; Ambe et al., 2020), where the WHO recommends sulfadoxine-pyrimethamine plus amodiaquine as the drugs of choice (WHO, 2012; WHO, 2013).
The P. falciparum prevalence range between 16% and 28% in Southern Ghana, but can be as high as 51% in Northern Ghana, reflecting a higher transmission intensity (Dieng et al., 2019; Diallo et al., 2017; Amoah et al., 2019). Ghana adopted SMC in 2014, and following pilot studies in 2015 (Ansah et al., 2021) that showed that SMC provided subtantial protection against malaria and parasite carriage it is now implemented in Northern Ghana (Id et al., 2019; Adjei et al., 2022).
Despite increased coverage of the aforementioned malaria-control strategies globally, the steady decline in global malaria cases and deaths since 2000 has been dwindling in the last decade. The most recent evidence even suggests that the trend has reversed (WHO, 2021). While the disruptions to malaria control and prevention programs during the COVID-19 pandemic might be partially responsible, increasing numbers of malaria cases have been observed prior to the pandemic. Thus, an estimated 500,000 new malaria cases were reported in Ghana 2017–2018 (WHO, 2019), prompting the introduction of the high burden, high impact approach in 2019 (USAID, CDC, U.S, 2020).
Besides changes in demography, mosquito behaviour, and parasite biology (De Silva and Marshall, 2012), changes in the malaria knowledge, attitudes, and practices (KAP) could result in inefficient malaria treatment, control, and prevention seeking behaviour and promote malaria transmission (Rek et al., 2020). Although several studies have been conducted to assess the malaria KAP in Ghana (Diema Konlan et al., 2019; Attu and Adjei, 2018; Owusu et al., 2018; Tetteh-Quarcoo et al., 2021; Ayanore et al., 2019; Assan et al., 2017) and elsewhere (Rek et al., 2020; Ayanore et al., 2019; Nejati et al., 2018; Hlongwana et al., 2009), they did not explore the relationship between changes in malaria preventive control practices and the recent increases in malaria cases.
We therefore assessed malaria preventive control practices and treatment-seeking behaviours of rural households in three municipalities in the Northern Region of Ghana (Kumbugu, Nanton and Tolon), where SMC was already implemented by the Ghana Health Service. We compared the results with data on malaria prevalence, parasite carriage, and exposure-risk among children in the same area.
2. Materials and methods
2.1. Ethical statement
This study was approved by the Ethics Review Committee of the Ghana Health Service (GHS) (GHS-ERC 008/07/19). Declarations of free willingness to participate in the study and informed consent were obtained in writing from all study participants or guardians prior to enrolment.
2.2. Study design, site, and participants
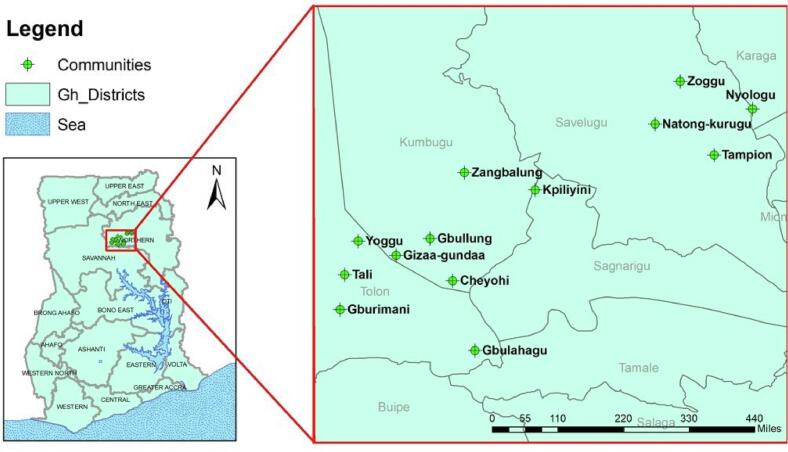
Two independent cross-sectional studies were conducted June–September 2020. The study was conducted in 13 rural communities in the Kumbugu, Nanton, and Tolon municipalities of Northern Region, Ghana, where GHS had already implemented SMC for children <5 years of age. The GIS co-ordinates of the thirteen communities were used to construct a graphical plot for visualization of the study communities using the ArcMap tool employed in ArcGIS v10.1. (ESRI, Redlands, California, USA) (Fig. 1).
Fig. 1.
Location of study sites.
Ghana it is shown on the left with black boundary lines. The location of Northern Region it is highlighted with the red-square. Study communities are indicated with green-coloured labels. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The first cross-sectional household survey was conducted June–July, during the second round of the SMC campaign in the communities. The survey was conducted to assess the malaria preventive control practices, and treatment-seeking behaviours, as well as the prevalence of malaria among children aged 1–14 years, assessed by verbal reports from the adult household members using a structured questionnaire. A total of 394 households including 543 children, were randomly sampled across the 13 communities. In recruiting the participants, 394 households were randomly selected and a maximum of two children were randomly recruited in a household with more than three children and a child in those with less than two children.
The second cross-sectional study was conducted at least two weeks after the completion of the final round/third round of the SMC campaign in the communities, between August–September. In this study, we directly assayed the degree of anaemia and the risk of P. falciparum infection and malaria exposure in children. A total of 1002 healthy children aged 1–17 years were enrolled, at least two weeks after the SMC was administered in each community.
2.3. Data and blood sample collection
In the household survey, interviews with adult household members were conducted using a structured questionnaire (supplementary material Q1), to obtain data on socio-demographic information and knowledge of malaria treatment, control, prevention, and treatment-seeking attitudes, especially for the children in their households. Socio-demographic information about the study children was also collected. Finally, body weight and temperature were recorded, using a digital scale and a contactless infrared thermometer, respectively. Data were recorded in the mWater Surveyor app (mWater, USA).
In the second study, finger-prick whole blood samples were collected for measurements of haemoglobin (Hb) levels by a URIT-12 haemoglobin meter (URIT Medical Electronic Ltd., China) and to test for P. falciparum infection using RDT. A drop of blood was also used to prepare dried blood spots (DBS) on filter paper (Whatman, USA) for retrospective confirmation of RDT results by PCR. Venous blood samples (2–5 mL) was collected into heparin tubes and plasma stored at −30 °C for later determination of malaria-specific IgG levels.
2.4. Diagnosis of malaria with anti-HRP2 rapid diagnosis test and by PCR
The First Response Malaria Antigen-HRP2 Card Test (Premier Medical Corporation, Gujarat, India) was used for on-site diagnosis of P. falciparum infection, following the manufacturer's instructions. DNA was extracted from DBS using the Chelex method (Baidjoe et al., 2013), and P. falciparum infection was confirmed by PCR, using the primers (forward-5’-AACAGACGGGTAGTCATGATTGAG-3′, reverse-5’-GTATCTGATCGTCTTCACTCCC-3′) against the 18 s rRNA gene, as previously reported (Padley et al., 2003).
2.5. Recombinant proteins
Recombinant glutamate-rich protein (GLURP) domain R0 was produced in E. coli (Theisen et al., 1995), while the merozoite surface protein 3 (PfMSP3) (Amoah et al., 2017) and a 230-kDa sexual stage protein (Pfs230) (Acquah et al., 2017) were produced in Lactococcus lactis as described previously. A crude lysate of asexual infected erythrocytes was prepared from P. falciparum 3D7 (Moreno et al., 2019). The circumsporozoite protein (PfCSP) was bought from IPT peptides (GeneScript, Germany).
2.6. P. falciparum-specific IgG levels
Plasma IgG levels against four recombinant P. falciparum proteins and a crude parasite lysate were measured using indirect ELISA, as previously described (Lopez-Perez et al., 2021). Briefly, 96-well flat-bottom microtitre plates (Nunc MaxiSorp; Thermo Fisher Scientific) were coated with 50 μL of recombinant protein (0.5 μg/mL) or crude antigen lysate (5 × 105 IEs/mL) in PBS and incubated overnight at 4 °C. After blocking with 1% BSA/PBS, plasma samples (1:300) were added in duplicate, followed by horseradish peroxidase-conjugated rabbit anti-human IgG (1:3000; Dako, Denmark). Bound plasma antibodies were detected by adding TMB (abcam, UK), stopping the reaction with 0.2 M H2SO4. The optical density (OD) was read at 450 nm (Varioskan LUX; Thermo Fisher, USA). The specific antibody levels were calculated in arbitrary units (AU), using the equation [(ODSAMPLE - ODBLANK)/ (ODPOSITIVE CONTROL - ODBLANK)] × 100 (Guitard et al., 2008). Plasma samples from malaria-unexposed Danish individuals and malaria-exposed Ghanaian children were included as negative and positive controls, respectively.
2.7. Data analysis and statistical considerations
Data from both surveys were independently analysed using R v. 3.6.3 (R Core Team, 2020) in Rstudio and GraphPad Prism version 9.3.1 (GraphPad Software, San Diego, CA, USA). The data were converted into frequency and subgroup proportions tables using the R package (Thomas et al., 2020). The proportions between different categories were compared using Chi-square or Fisher's exact tests. The “glm” function in R package was used to perform a multivariable analysis using a logistic regression model where the main outcome was reported malaria cases in households, and parasite carriage/parasite infection. In both models, the main exposure variables were age, gender and the municipalities. Municipalities and age, were cofounders and as such were controlled in both models. The forest plots for the models were generated with the “sjplot” package in R (Lüdecke, 2021). All p-values <0.05 at 95% confidence interval (95% CI) were deemed statistically significant.
3. Results
3.1. Study participant information
One adult household member from each of the 394 households was interviewed during the household survey and their information is presented in Table 1. The information collected from 543 children during the household survey and from the 1002 children included in the second study is summarized in Table 2. Most of the children were males and were younger than 7 years old in all the municipalities. Mean haemoglobin levels and bodyweight differed significantly across the municipalities (Table 2).
Table 1.
Demographics characteristics of adult household members.
| Municipalities |
||||||
|---|---|---|---|---|---|---|
| Variable | Level | Kumbugu (n = 137) | Nanton (n = 130) | Tolon (n = 127) | Total (n = 394) | p-valuea |
| Age | mean (SD) | 33.4 (5.4) | 33.9 (5.8) | 34.1 (5.6) | 33.8 (5.6) | 0.57 |
| Gender | Female | 126 (92.0) | 107 (82.3) | 107 (84.3) | 340 (86.3) | |
| Male | 11 (8.0) | 23 (17.7) | 20 (15.7) | 54 (13.7) | 0.052 | |
| Religion | Moslem | 129 (94.2) | 123 (94.6) | 127 (100.0) | 379 (96.2) | |
| Christian | 8 (5.8) | 7 (5.4) | 0 (0.0) | 15 (3.8) | 0.024 | |
| Education | No education | 113 (82.5) | 106 (81.5) | 111 (87.4) | 330 (83.8) | |
| Basic education | 13 (9.5) | 13 (10.0) | 10 (7.9) | 36 (9.1) | ||
| JHS education | 7 (5.1) | 6 (4.6) | 6 (4.7) | 19 (4.8) | ||
| SHS Education | 3 (2.2) | 1 (0.8) | 0 (0.0) | 4 (1.0) | ||
| Tertiary education | 1 (0.7) | 4 (3.1) | 0 (0.0) | 5 (1.3) | 0.33 | |
p-value using Pearson chi-square test or Fisher's exact test. SHS – Senior High School, JHS – Junior High School.
Table 2.
Information of children in the first and second study.
| 1st study |
Municipalities |
|||||
|---|---|---|---|---|---|---|
| Variable | Level | Kumbugu (n = 184) | Nanton (n = 130) | Tolon (n = 229) | Total (n = 543) | p-valuea |
| Gender | Female | 82 (44.6) | 69 (53.1) | 106 (46.3) | 257 (47.3) | |
| Male | 102 (55.4) | 61 (46.9) | 123 (53.7) | 286 (52.7) | 0.30 | |
| Age | mean(SD) | 5.4 (3.3) | 5.6 (3.4) | 5.3 (3.2) | 5.4 (3.3) | 0.65 |
| Age groups | < 5 | 85 (46.2) | 56 (43.1) | 109 (47.6) | 250 (46.0) | |
| 5–7 | 50 (27.2) | 37 (28.5) | 61 (26.6) | 148 (27.3) | ||
| 8–10 | 29 (15.8) | 23 (17.7) | 37 (16.2) | 89 (16.4) | ||
| 11+ | 20 (10.9) | 14 (10.8) | 22 (9.6) | 56 (10.3) | 0.99 | |
| 2nd study |
||||||
|---|---|---|---|---|---|---|
| Variable | Level | Kumbugu (n = 256) | Nanton (n = 280) | Tolon (n = 466) | Total (n = 1002) | p-valuea |
| Gender | Female | 102 (39.8) | 117 (41.8) | 175 (37.6) | 394 (39.3) | |
| Male | 154 (60.2) | 163 (58.2) | 291 (62.4) | 608 (60.7) | 0.51 | |
| Age | mean(SD) | 7.4 (3.3) | 6.8 (3.5) | 7.4 (3.4) | 7.2 (3.4) | 0.05 |
| Age groups | < 5 | 51 (19.9) | 82 (29.3) | 113 (24.2) | 246 (24.6) | |
| 5–7 | 100 (39.1) | 94 (33.6) | 156 (33.5) | 350 (34.9) | ||
| 8–10 | 58 (22.7) | 60 (21.4) | 101 (21.7) | 219 (21.9) | ||
| 11+ | 47 (18.4) | 44 (15.7) | 96 (20.6) | 187 (18.7) | 0.18 | |
| Hb level (g/dL) | mean(SD) | 11 (1.3) | 10.6 (1.3) | 10.9 (1.3) | 10.8 (1.3) | 0.014 |
| Bodyweight (kg) | mean(SD) | 22.6 (8.3) | 21.4 (8.5) | 23.5 (9) | 22.7 (8.7) | 0.008 |
| Body temperature | mean(SD) | 36.6 (0.4) | 36.6 (0.3) | 36.6 (0.4) | 36.6 (0.4) | 0.91 |
p-value using Pearson chi-square test.
3.2. Malaria control and prevention practices and treatment-seeking behaviour among households
Adult household members were interviewed about their malaria control and preventive practices. Use of bed nets (65%) and indoor insecticide sprays/coils (59%) were commonly and similarly used in all the communities. In contrast, only a minority mentioned clearing of bush/weeds around homes (14%) and avoiding sitting outside in the night (5.8%) as a malaria control and preventive measures (Supplementary Table 1). The average bed net ownership/coverage was relatively high (79%), although only 49% of the households were actually using their bed nets, and in some cases the use was not adequate (Supplementary Fig. S1).
The majority of households (87%) indicated that they sought malaria treatment at community health centres (CHC) when a member of their household was suspected of having malaria. About 12% reported self-medicating at home, and 1.5% reported going to over-the-counter drug stores (OCDS) to seek treatment. Despite the high preference to seek malaria treatment at the CHC, the majority of the households (70%) reported getting their anti-malaria medications from OCDS. Indeed, 59% reported having self-medicated at least one household member within the month prior to the interview (Supplementary table 2), mainly with artemether-lumefantrine combination drugs, but also with quinine-based syrups, sulphadoxine-pyrimethamine combination drugs, and antibiotics.
3.3. Prevalence of malaria among children based on the household survey
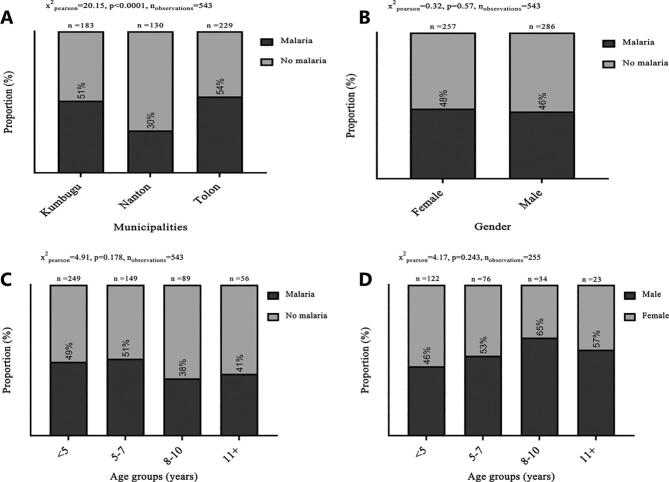
In the household survey, each informant was asked if any of the household children had been sick within the past two weeks prior to the interview. Those who answered in the affirmative were further asked to specify what was wrong with the child. The responses of those who specifically mentioned malaria were assumed to have had malaria although that might not necessarily correlate with clinical malaria among the children. Based on their responses over 50% of the children in Kumbugu and Tolon reportedly had malaria prior to the survey, compared to 30% in Nanton (Fig. 2A). The self-reported malaria cases were similar in males and females (Fig. 2B). Disaggregating the data by age, slightly more malaria cases were reported among those ≤7 years old, although the difference across age groups was not statistically significant (Fig. 2C).
Fig. 2.
Prevalence of malaria across municipalities.
Prevalence of reported malaria cases by municipalities (A), by gender (B), and by age groups (C). Percentage of those who reportedly had malaria (darker grey) and those without (lighter grey) are shown. (D) Gender of those who had malaria across age groups. Sample size and p-values using Chi-square test are also shown.
A multivariable analysis performed to determine the risk of malaria among the children), revealed that children in Nanton were about 67% less likely to develop malaria than those in Kumbugu, and this difference was highly significant. Younger children were slightly more likely to be reported as having had malaria than older children, but the difference was not statistically significant (Fig. 3).
Fig. 3.
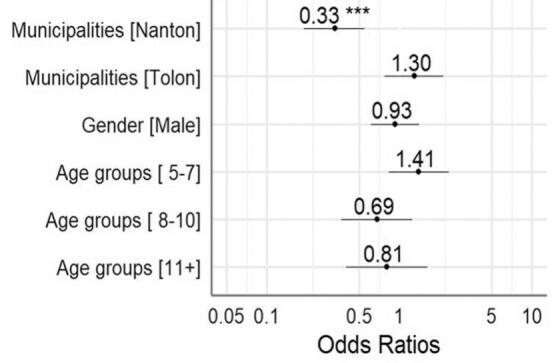
Multivariable analysis of malaria risk of children.
The y-axis labels are characteristics of the participants and x-axis labels are odds ratios (OR). The OR for each y-axis label is the aligned value in the plot. The black-dots represents the OR values and its whiskers indicates 95% confidence interval ranges. ** p-value <0.01, *** p-value <0.001.
3.4. Plasmodium falciparum infection and parasite carriage
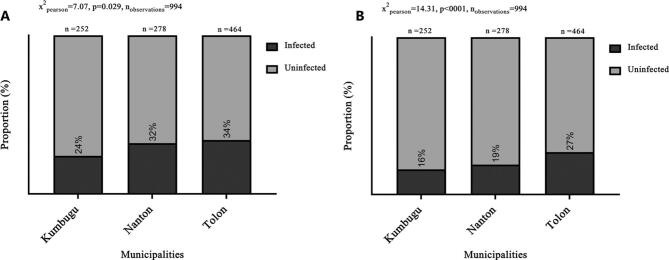
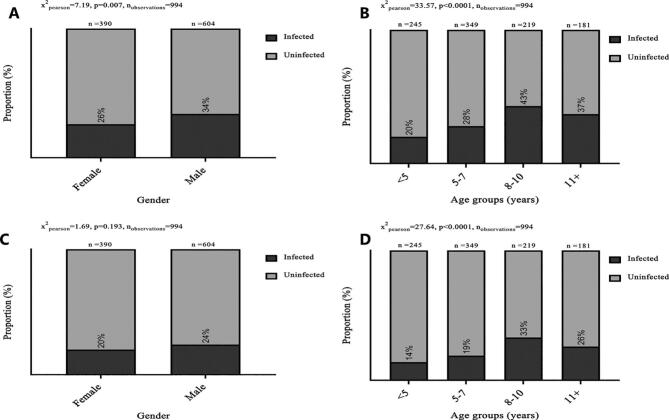
In the second survey, the P. falciparum infection rates were estimated to be 24–34% by RDT and 16–27% by PCR, and differed significantly among the three communities, being highest in Tolon (Fig. 4). When the data were disaggregated by gender, the P. falciparum infection rate was slightly higher in males, although the difference was only significant for the RDT data (Fig. 5A). Older children were more likely to carry parasites than younger children, both by RDT (Fig. 5B) and by PCR (Fig. 5D).
Fig. 4.
Prevalence of P. falciparum infections by RDT (A) and parasite-carriage rate by PCR (B).
Positive (darker grey) and negative (lighter grey) for P. falciparum are shown. Sample size and p-values using Chi-square test are also shown.
Fig. 5.
Prevalence of P. falciparum infections and parasite-carriage by gender and age.
Prevalence of P. falciparum infections by gender, using RDT (A) or PCR (C) and by age groups, using RDT (B) or PCR (D). Positive (darker grey) and negative (lighter grey) for P. falciparum are shown. Sample size and p-values using Chi-square test are also shown.
Consistent with the parasite-carriage data across municipalities (Fig. 4), a multivariate analysis of P. falciparum infection risk showed that children from Tolon were at the highest risk of being infected (Fig. 6). The risk of infection peaked in the 8–10 years age group and was lowest among those <5 years old (Supplementary Fig. S3). As expected, infected children were more anaemic than uninfected children (Fig. 6).
Fig. 6.
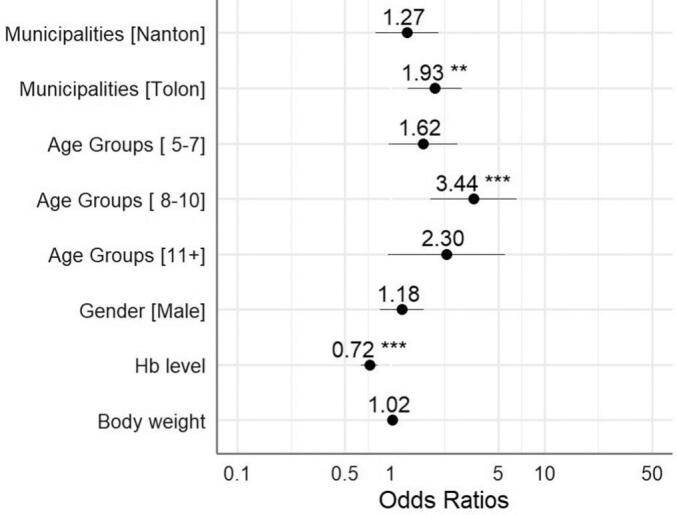
Multivariate analysis of P. falciparum infection risk based on participant's characteristics.
The y-axis labels are characteristics of the participants and x-axis labels are odds ratios (OR). The OR for each y-axis label is the aligned value in the plot. The black dots represents the OR values and its whiskers indicates 95% confidence interval ranges. ** p-value <0.01, *** p-value <0.001.
3.5. P. falciparum-specific IgG levels
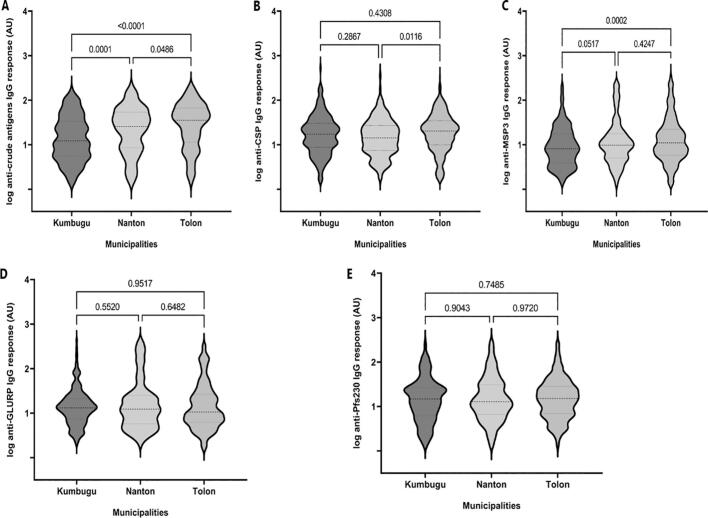
Consistent with the reported risk of malaria and infection risk data, levels of IgG specific for a crude P. falciparum lysate were lowest in Kumbugu and highest in Tolon (Fig. 7). A similar difference was observed for MSP3, but not for the other recombinant antigens. IgG levels correlated positively with age for all antigens and in all communities (Supplementary Fig. S4). Although these correlations were weak, they were highly significant in almost all cases.
Fig. 7.
Total IgG response to P. falciparum antigens according to the municipalities.
Antibody levels against (A) PfCSP (n = 852), (B) GLURP (n = 870), (C), MSP3 (n = 864), (D) crude antigen (n = 880), and (E) Pfs230 (n = 837) were determined by an ELISA. The number of samples against PfCSP, GLURP, MSP3, Crude antigen were respectively 244, 244, 245, 246 and 219 in Kumbugu, 179, 177, 178, 185 and 172 in Naton, and 429, 449, 441, 449 and 446 in Tolon. Values are expressed in log arbitrary units (AU). P-values were determined using Kruskal-Wallis test followed by Dunnett's Multiple Comparison.
4. Discussion
Ghana adopted SMC in 2014, and is in place in Northern Ghana (Id et al., 2019; Adjei et al., 2022). The intervention targets children younger than 5 years of age and has previously been shown to reduce parasite carriage and malaria morbidity among SMC recipients (Ansah et al., 2021; Ambe et al., 2020; Druetz, 2018; Yaro et al., 2011; Dicko et al., 2011). The present study supports these earlier findings, as the lowest risk of parasitemia and perceived malaria was found among the youngest study participants (Fig. 3, Fig. 5, and Fig. 6). Nevertheless, based on our questionnaire data and biological assays, P. falciparum parasitemia remains prevalent among children in Northern Ghana, including those in the SMC intervention age bracket (Fig. 3, Fig. 3, Fig. 4, Fig. 5). Furthermore, it varied substantially among the three study communities.
These findings were supported by the high and age-dependent levels of P. falciparum-specific IgG antibodies measured. Taking into consideration of the high SMC retention rates reported in a pilot study conducted seven years ago in the region (Ansah et al., 2021), it will be necessary to also look at the other intervention tools both generally and at the community level. This concern is supported by the high parasite carriage rates in older children reported here, which indicate continued substantial transmission of P. falciparum in Northern Ghana. It thus appears that more efficient interventions are needed to achieve the malaria reduction ambitions of national and international authorities. Furthermore, our data indicate that even when interventions that are known to be efficient, such as insecticide-treated bed nets (Lengeler, 2004) and clearing of shrubs and weeds around houses (Opiyo et al., 2007), are available, they may either not be used or used only inappropriately (Teye and Awetoriyaro, 2013) (e.g., Supplementary Fig. S1). Conversely, practices that are quite inefficient, such as mosquito repellent coils (Hogarh et al., 2018), are popular in our study area and could instil in the users an unjustified sense of having achieved protection against malaria (Hogarh et al., 2016).
Finally, although a high proportion of study participants reported to seek malaria treatment at nearby community health centres, in agreement with previous findings (Dalaba et al., 2018), the majority of the household informants reported getting their anti-malarial medication from over-the-counter drug stores, implying a limited or unreliable access to antimalarial medications at the health centres. This is obviously of concern, as it suggests widespread reliance on other sources which might be substandard or counterfeit drugs of questionable provenance, as observed elsewhere (El-Duah and Ofori-Kwakye, 2012; Osei-Safo et al., 2014; Ofori-Kwakye et al., 2008) although there has not been any reported substandard drugs in the region. Apart from the immediate inability of substandard drugs to achieve cure, they may also accelerate the development of resistance to bona fide anti-malarial drugs (Newton et al., 2016). A related worry is the likely over-treatment occurring as a result of poor quality of the malaria (often self-) diagnosis, which often does not involve any attempt to detect presence of parasites (Prah et al., 2019).
Malaria naturally acquired immunity develops after repeated exposure (Hviid, 2005; Fowkes et al., 2016) and sustained by premunition (Obi et al., 2010). Acquisition of protection may thus be compromised by SMC, as it limits exposure by ensuring early clearance of infections (Moustapha et al., 2021; Ndiaye et al., 2015; Mahamar et al., 2017), although the evidence regarding the clinical and epidemiological consequences is equivocal (Ceesay et al., 2008; Brasseur et al., 2011; Chen et al., 2016; Greenwood and David, 1995).
Overall, our study suggests that efforts to ensure high SMC compliance and to improve preventative and treatment practices are cost-beneficial “low-hanging fruits” in the fight against malaria.
4.1. Limitations of the study
The first cross-sectional study was an interviewed-based survey. So, the conclusions are based on the response of the households, which may be affected by recall-bias and/or disinformation from some of the respondents. However, we do not believe that the results is appreciably compromised by these limitations, as the findings are consistent with literature.
Authors' contributions
ZS, HL, MLP, LH, MFO and GOA conceived and designed the study. ZS, HL, PANA, NOWand EKB collected data. ZS, PANA and HL analysed the data. LH, MFO, GOA acquired the funding and supervised the work. ZS wrote the first draft. All authors reviewed and approved the final manuscript.
Funding
This work was funded by Danida (grant 17–02-KU) and by the Independent Research Fund Denmark (grant 0134-00123B). ZS was supported by a PhD scholarship from the Danida-sponsored Building Stronger Universities initiative (grant BSU3-UG). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgements
We are grateful to all the Ghanaian families participating in this study, and to the members of the field team, especially Naporo Mathias and Alex Kofi-Denso. We thank Michael Theisen (University of Copenhagen, Denmark), Asamoah Kusi, and Linda Eva Amoah (University of Ghana) for providing the GLURP, CSP, MSP-3 and PfS230 recombinant proteins, respectively. Finally, we thank all the staff of GHS in the study communities for facilitating the fieldwork.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.parepi.2023.e00317.
Appendix A. Supplementary data
Supplementary material
References
- ACCESS-SMC Partnership Effectiveness of seasonal malaria chemoprevention at scale in west and Central Africa: an observational study. Lancet. 2020;396:1829–1840. doi: 10.1016/S0140-6736(20)32227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acquah F.K., Obboh E.K., Asare K., Boampong J.N., Nuvor S.V., Singh S.K., et al. Antibody responses to two new Lactococcus lactis-produced recombinant Pfs48/45 and Pfs230 proteins increase with age in malaria patients living in the central region of Ghana. Malar. J. 2017;16:306. doi: 10.1186/s12936-017-1955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjei M.R., Kubio C., Buamah M., Sarfo A., Suuri T., Ibra S., et al. Effectiveness of seasonal malaria chemoprevention in reducing under-five malaria morbidity and mortality in the Savannah Region, Ghana. Ghana Med. J. 2022;56:64–70. doi: 10.4314/gmj.v56i2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambe J.P., Balogun S.T., Waziri M.B., Nglass I.N., Saddiq A. Impacts of seasonal malaria chemoprevention on malaria burden among under five-year-old children in Borno state, Nigeria. J. Trop. Dis. 2020;2020 doi: 10.1155/2020/9372457. Article ID 9372457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoah L.E., Nuvor S.V., Obboh E.K., Acquah F.K., Asare K., Singh S.K., et al. Natural antibody responses to plasmodium falciparum MSP3 and GLURP(R0) antigens are associated with low parasite densities in malaria patients living in the central region of Ghana. Parasit. Vectors. 2017;10:395. doi: 10.1186/s13071-017-2338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoah L.E., Dickson D., Abuaku B., Ahorlu C., Arhinful D., Afari E., et al. Probing the composition of plasmodium species contained in malaria infections in the eastern region of Ghana. BMC Public Health. 2019;19:1617. doi: 10.1186/s12889-019-7989-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansah P.O., Ansah N.A., Malm K., Awuni D., Peprah N., Dassah S., et al. Evaluation of pilot implementation of seasonal malaria chemoprevention on morbidity in young children in northern Sahelian Ghana. Malar. J. 2021;20:440. doi: 10.1186/s12936-021-03974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley E.A., Yeka A. Comment seasonal malaria chemoprevention : closing the know – do gap. Lancet. 2020;396:1778–1779. doi: 10.1016/S0140-6736(20)32525-3. [DOI] [PubMed] [Google Scholar]
- Assan A., Takian A., Hanafi-Bojd A.A., Rahimiforoushani A., Nematolahi S. Knowledge, attitude, and practice about malaria: socio-demographic implications for malaria control in rural Ghana. J. Public Health Policy. 2017;38:445–463. doi: 10.1057/s41271-017-0088-6. [DOI] [PubMed] [Google Scholar]
- Attu H., Adjei J.K. Local knowledge and practices towards malaria in an irrigated farming community in Ghana. Malar. J. 2018;17:150. doi: 10.1186/s12936-018-2291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayanore M.A., Tetteh J., Ameko A., Axame W.K., Alhassan R.K., Adoliba Ayanore A., et al. Reproductive-age women’s knowledge and care seeking for malaria prevention and control in Ghana: analysis of the 2016 malaria indicator survey. J. Trop. Med. 2019;2019 doi: 10.1155/2019/2316375. Article ID 2316375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baidjoe A., Stone W., Ploemen I., Shagari S., Grignard L., Osoti V., et al. Combined DNA extraction and antibody elution from filter papers for the assessment of malaria transmission intensity in epidemiological studies. Malar. J. 2013;12:272. doi: 10.1186/1475-2875-12-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasseur P., Badiane M., Cisse M., Agnamey P., Vaillant M.T., Olliaro P.L. Changing patterns of malaria during 1996-2010 in an area of moderate transmission in southern Senegal. Malar. J. 2011;10:203. doi: 10.1186/1475-2875-10-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceesay S.J., Casals-Pascual C., Erskine J., Anya S.E., Duah N.O., Fulford A.J., et al. Changes in malaria indices between 1999 and 2007 in the Gambia: a retrospective analysis. Lancet. 2008;372:1545–1554. doi: 10.1016/S0140-6736(08)61654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen I., Clarke S.E., Gosling R., Hamainza B., Killeen G., Magill A., et al. “Asymptomatic” malaria: a chronic and debilitating infection that should be treated. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coldiron M.E., Von Seidlein L., Grais R.F. Seasonal malaria chemoprevention : successes and missed opportunities. Malar. J. 2017;16:481. doi: 10.1186/s12936-017-2132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalaba M.A., Welaga P., Oduro A., Danchaka L.L., Matsubara C. Cost of malaria treatment and health seeking behaviour of children under-five years in the upper west region of Ghana. PLoS One. 2018;13 doi: 10.1371/journal.pone.0195533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva P.M., Marshall J.M. Factors contributing to urban malaria transmission in sub-saharan Africa: a systematic review. J. Trop. Med. 2012;2012 doi: 10.1155/2012/819563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo N., Akweongo P., Maya E., Aikins M., Sarfo B. Burden of malaria in mobile populations in the Greater Accra region, Ghana: a cross- sectional study. Malar. J. 2017;16:109. doi: 10.1186/s12936-017-1751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicko A., Diallo A.I., Tembine I., Dicko Y., Dara N., Santara G., et al. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Mali: A randomised, double-blind, placebo- controlled trial. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diema Konlan K., Amu H., Konlan K.D., Japiong M. Awareness and malaria prevention practices in a rural community in the ho municipality, Ghana. Interdiscip. Perspect. Infect. Dis. 2019;2019:9365823. doi: 10.1155/2019/9365823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieng C.C., Gonzalez L., Pestana K., Dhikrullahi S.B., Amoah L.E., Afrane Y.A., et al. Contrasting asymptomatic and drug resistance gene prevalence of plasmodium falciparum in Ghana: implications on seasonal malaria chemoprevention. Genes (Basel) 2019;10:538. doi: 10.3390/genes10070538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druetz T. Evaluation of direct and indirect effects of seasonal malaria chemoprevention in Mali. Sci. Rep. 2018;8:8104. doi: 10.1038/s41598-018-26474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Duah M., Ofori-Kwakye K. Substandard artemisinin-based antimalarial medicines in licensed retail pharmaceutical outlets in Ghana. J. Vector Borne Dis. 2012;49:131–139. [PubMed] [Google Scholar]
- Fowkes F.J.I., Boeuf P., Beeson J.G. Immunity to malaria in an era of declining malaria transmission. Parasitology. 2016;143:139–153. doi: 10.1017/S0031182015001249. [DOI] [PubMed] [Google Scholar]
- Greenwood B.M., David P.H. Mortality and morbidity from malaria after stopping malaria chemoprophylaxis. Trans. R. Soc. Trop. Med. Hyg. 1995;89:629–633. doi: 10.1016/0035-9203(95)90419-0. [DOI] [PubMed] [Google Scholar]
- Guitard J., Cottrell G., Magnouha N.M., Salanti A., Li T., Sow S., et al. Differential evolution of anti-VAR2CSA- IgG3 in primigravidae and multigravidae pregnant women infected by plasmodium falciparum. Malar. J. 2008;7:10. doi: 10.1186/1475-2875-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlongwana K.W., Mabaso M.L.H., Kunene S., Govender D., Maharaj R. Community knowledge, attitudes and practices (KAP) on malaria in Swaziland: a country earmarked for malaria elimination. Malar. J. 2009;8:29. doi: 10.1186/1475-2875-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarh J.N., Antwi-Agyei P., Obiri-Danso K. Application of mosquito repellent coils and associated self-reported health issues in Ghana. Malar. J. 2016;15:61. doi: 10.1186/s12936-016-1126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarh J.N., Agyekum T.P., Bempah C.K., Owusu-Ansah E.D.J., Avicor S.W., Awandare G.A., et al. Environmental health risks and benefits of the use of mosquito coils as malaria prevention and control strategy. Malar. J. 2018;17:265. doi: 10.1186/s12936-018-2412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hviid L. Naturally acquired immunity to plasmodium falciparum malaria in Africa. Acta Trop. 2005;95:270–275. doi: 10.1016/j.actatropica.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Id S.C., Ansah N.A., Awuni D.A., Oduro A., Ansah O. Community acceptability of seasonal malaria chemoprevention of morbidity and mortality in young children : a qualitative study in the upper west region of Ghana. PLoS One. 2019;14 doi: 10.1371/journal.pone.0216486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria (review) Cochrane Database Syst. Rev. 2004;2004 doi: 10.1002/14651858.cd000363.pub2. Art. No.: CD000363. [DOI] [PubMed] [Google Scholar]
- Lopez-Perez M., Viwami F., Seidu Z., Jensen A.T.R., Doritchamou J., Ndam N.T., et al. PfEMP1-specific immunoglobulin G reactivity among Beninese pregnant women with sickle cell trait, open forum. Infect. Dis. Ther. 2021;8:ofab527. doi: 10.1093/ofid/ofab527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdecke D. 2021. sjPlot: Data Visualization for Statistics in Social Science. [Google Scholar]
- Mahamar A., Issiaka D., Barry A., Attaher O., Dembele A.B., Traore T., et al. Effect of seasonal malaria chemoprevention on the acquisition of antibodies to Plasmodium falciparum antigens in Ouelessebougou, Mali. Malar. J. 2017;16:289. doi: 10.1186/1475-2875-10-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meremikwu M., Donegan S., Sinclair D., Esu E., Oringanje C., Mm M., et al. Intermittent preventive treatment for malaria in children living in areas with seasonal transmission (review) Cochrane Database Syst. Rev. 2012 doi: 10.1002/14651858. http://www.cochranelibrary.com Art. No.: CD003756. CD003756.pub4. Art. No.: CD003756. CD003756.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno Y.R., Donato S.T., Nogueira F., Silva M.S. Comparative analysis of the serological reactivity of individuals with clinical history of malaria using two different ELISA tests. Diagnostics. 2019;9:168. doi: 10.3390/diagnostics9040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustapha L.M., Adamou R., Ibrahim M.L., Padounou M. Abdoulaye Louis, Diallo A., Courtin D., et al. Evidence that seasonal malaria chemoprevention with SPAQ influences blood and pre-erythrocytic stage antibody responses of Plasmodium falciparum infections in Niger. Malar. J. 2021;20:1. doi: 10.1186/s12936-020-03550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiaye M., Sylla K., Sow D., Tine R., Faye B., Ndiaye J.L., et al. Potential impact of seasonal malaria chemoprevention on the acquisition of antibodies against glutamate-rich protein and apical membrane antigen 1 in children living in southern Senegal. Am. J. Trop. Med. Hyg. 2015;93:798–800. doi: 10.4269/ajtmh.14-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejati J., Moosa-Kazemi S.H., Saghafipour A., Soofi K. Knowledge, attitude and practice (KAP) on malaria, from high malaria burden rural communities, southeastern Iran. J. Parasit. Dis. 2018;42:62–67. doi: 10.1007/s12639-017-0965-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton P.N., Caillet C., Guerin P.J. A link between poor quality antimalarials and malaria drug resistance? Expert Rev. Anti-Infect. Ther. 2016;14:531–533. doi: 10.1080/14787210.2016.1187560. [DOI] [PubMed] [Google Scholar]
- Obi R.K., Okangba C.C., Nwanebu F.C., Ndubuisi U.U., Orji N.M. Premunition in Plasmodium falciparum malaria, African. J. Biotechnol. 2010;9:1397–1401. doi: 10.5897/ajbx09.034. [DOI] [Google Scholar]
- Ofori-Kwakye K., Asantewaa Y., Gaye O. Quality of artesunate tablets sold in pharmacies in Kumasi, Ghana. Trop. J. Pharm. Res. 2008;7:1179–1184. doi: 10.4314/tjpr.v7i4.14704. [DOI] [Google Scholar]
- Opiyo P., Mukabana W.R., Kiche I., Mathenge E., Killeen G.F., Fillinger U. An exploratory study of community factors relevant for participatory malaria control on Rusinga Island, western Kenya. Malar. J. 2007;6:48. doi: 10.1186/1475-2875-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei-Safo D., Agbonon A., Konadu D.Y., Harrison J.J.E.K., Edoh M., Gordon A., et al. Evaluation of the quality of artemisinin-based antimalarial medicines distributed in Ghana and Togo. Malar. Res. Treat. 2014;2014 doi: 10.1155/2014/806416. Article ID 806416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu E.D.A., Cremers A.L., Brown C.A., Mens P.F., Grobusch M.P. Knowledge, attitudes and practices regarding malaria in people living with HIV in rural and urban Ghana. Acta Trop. 2018;181:16–20. doi: 10.1016/j.actatropica.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Padley D., Moody A., Chiodini P., Saldanha J. Use of a rapid, single-round, multiplex PCR to detect malarial parasites and identify the species present. Ann. Trop. Med. Parasitol. 2003;97:131–137. doi: 10.1179/000349803125002977. [DOI] [PubMed] [Google Scholar]
- Prah J.K., Yeboah-Sarpong A., Pinkrah R., Ewudzi-Acquah E. Assessment of the knowledge, attitude and practices of prescribers regarding malaria diagnosis: a cross sectional study among Ghanaian prescribers. Pan Afr. Med. J. 2019;34:207. doi: 10.11604/pamj.2019.34.207.19940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rek J., Musiime A., Zedi M., Otto G., Kyagamba P., Rwatooro J.A., et al. Non-adherence to long-lasting insecticide treated bednet use following successful malaria control in Tororo, Uganda. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetteh-Quarcoo P.B., Dayie N.T.K.D., Adutwum-Ofosu K.K., Ahenkorah J., Afutu E., Amponsah S.K., et al. Unravelling the perspectives of day and night traders in selected markets within a sub-saharan african city with a malaria knowledge, attitude and practice survey. Int. J. Environ. Res. Public Health. 2021;18:3468. doi: 10.3390/ijerph18073468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teye J.K., Awetoriyaro J. Malaria control strategies in the Kassena-Nankana east and west districts of Ghana, Ghana. J. Geogr. 2013;5:102–120. https://www.ajol.info/index.php/gjg/article/view/109454 [Google Scholar]
- Theisen M., Vuust J., Gottschau A., Jepsen S., Hogh B. Antigenicity and immunogenicity of recombinant glutamate-rich protein of Plasmodium falciparum expressed in Escherichia coli. Clin. Diagn. Lab. Immunol. 1995;2:30–34. doi: 10.1128/cdli.2.1.30-34.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A.G.A., Torp-pedersen C., Holst K.K., Ozenne B. 2020. Publish: Format Output of Various Routines in a Suitable Way for Reports and Publication. R Package Version. 12.23. 2021. [Google Scholar]
- USAID, CDC, U.S . 2020. President ’ s Malaria Initiative Ghana: Malaria Operational Plan FY 2020. [Google Scholar]
- WHO . 2012. WHO policy Recommendation : Seasonal Malaria Chemoprevention (SMC) for Plasmodium Falciparum Malaria Control in Highly Seasonal Transmission Areas of the Sahel Sub - Region in Africa March 2012, Geneva, Switzerland. [Google Scholar]
- WHO . 2013. Seasonal Malaria Chemoprevention with Sulfadoxine– Pyrimethamine plus Amodiaquine in Children- a Field Guide, Geneva, Switzerland. [Google Scholar]
- WHO . 2016. Global Technical Strategy for Malaria 2016–2030, Geneva, Switzerland. [Google Scholar]
- WHO . 2019. World Malaria Report 2019, Geneva, Switzerland. [Google Scholar]
- WHO . 2021. World Malaria Report 2021, Geneva, Switzerland. [Google Scholar]
- Yaro J.B., Oue A.Z., Kabore Y., Oue A., Chandramohan D., Cousens S., et al. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated Bednet in Burkina Faso : A randomised, double-blind, placebo- controlled trial. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material