Abstract
DNA-mediated programming is emerging as an effective technology that enables controlled dynamic assembly/disassembly of inorganic nanocrystals (NC) with precise numbers and spatial locations for biomedical imaging applications. In this review, we will begin with a brief overview of the rules of NC dynamic assembly driven by DNA ligands, and the research progress on the relationship between NC assembly modes and their biomedical imaging performance. Then, we will give examples on how the driven program is designed by different interactions through the configuration switching of DNA-NC conjugates for biomedical applications. Finally, we will conclude with the current challenges and future perspectives of this emerging field. Hopefully, this review will deepen our knowledge on the DNA-guided precise assembly of NCs, which may further inspire the future development of smart chemical imaging devices and high-performance biomedical imaging probes.
Keywords: DNA nanotechnology, programmable materials, self-assembly, dynamic assembly, responsive materials, DNA probe, bioimaging, biomedical diagnosis
1. Introduction
Nano- and microstructures influence the physical properties of matter.1 In particular for inorganic nanocrystals (NCs),2,3 various unexpected properties have been explored, including in photonics,4,5 thermoelectrics,6,7 and magnetism.8−10 Surface ligands play a crucial role in regulating the assembly and disassembly of NCs, controlling their structures and functions at multiscales,11,12 and making them a strong candidate for the development of cutting-edge next-generation imaging probes. As a typical example, controlling the assembly structures of plasmonic NCs to modulate their plasmonic resonance properties has been widely exploited to construct functional nanoarchitectures for biosensors and optical analysis systems during the past decades,13−21 in which the precise assembly of NCs is the key to elaborate and verify plasmonic coupling effects.20−22
By tuning the various assembly/disassembly parameters, including the change of interparticle spacing, aggregation state, arrangement direction, and assembly composition, the properties of NCs can be regulated to suit a given bioimaging application.23−28 However, it is difficult to predict the organization and structure of nanoscale assembly due to the lack of interaction with sufficient directionality for driving NCs.29 In the past four decades, with the vigorous development of DNA nanotechnology, DNA-mediated self-assembly has been developed as an effective approach to prepare highly programmable nanostructures with controllable size and shape,30−34 which provides a promising methodology for driving the precise assembly of NCs via the directional base pairing.35 Specifically, Watson–Crick base-pairing interactions (A–T and G–C) define DNA directionality, and varying base amounts and sequences can program the geometry of DNA nanoassembly.36,37 Programmable DNAs are modified on the surface of NCs and drive their directional movement, hence manipulating the dynamic assembly of NCs.38−43 In addition to base complementarity, DNA possesses a variety of unique biological and chemical features. For example, the designed DNA sequences can develop a specific secondary structure and switch their configuration under the interaction of metal ions,44,45 small molecules,46,47 and proteins.47,48 Once stimulated, the switchable DNA structures can act as procedures to drive dynamic assembly/disassembly of NCs for changing the imaging performance of NCs, resulting in responsive signals. These elegant characteristics indicate that DNA is a reliable baseband for NCs to realize efficient biomedical imaging.49−52
In this review, the design principle and biomedical imaging applications of DNA-driven dynamic NC assemblies are summarized (Figure 1). First, we briefly discuss the fundamental mechanisms of DNA-mediated NC assemblies and their regulated imaging properties. Then, the biomedical imaging applications using DNA-driven dynamic NC assemblies are reviewed. Finally, the challenges and perspectives in the biomedical applications of DNA-mediated dynamic NC assemblies are discussed.
Figure 1.
Schematic illustration of DNA-driven dynamic assembly/disassembly of NCs for biomedical imaging.
2. Controllable Assembly of NCs
Controlling the placement of each component is absolutely a grand challenge to construct materials and tailor their properties for a given application. The interaction with sufficient directionality to accurately predict the final arrangement and orientation of components is highly important for controlled NC assembly.29 As a storage material for genetic information, DNA strictly follows the Watson–Crick hydrogen-bonding rules, which are able to control the hybridization of DNA strands with high selectivity and specificity by permuting the nucleobase sequence of DNA strands and assemble nanostructures via “bottom-up” strategies.53−55 Since Mirkin’s and Alivisatos’s groups demonstrated the powerful methodology of DNA-guided NC self-assembly in 1996,56,57 the field of material engineering based on the assembly of homogeneous and heterogeneous NCs’ crystal structures has been developed rapidly.58,59 The DNA-guided NC self-assembly methodology developed into two branches: “NCs template-assisted assembly” and “DNA template-assisted assembly”. In brief, the former strategy developed by the Mirkin group describes that DNA mainly acts as connectors, driving assembly and determining the spacing of NCs, in which the assembly orientation and arrangement pattern are dominated by the geometric characteristics of NCs.29 For the second strategy, a discrete number of DNA strands can guide the NCs’ directional assembly. Therefore, DNA not only cross-links the NCs, but also acts as a template to determine the arrangement direction and structural pattern of the NCs.60
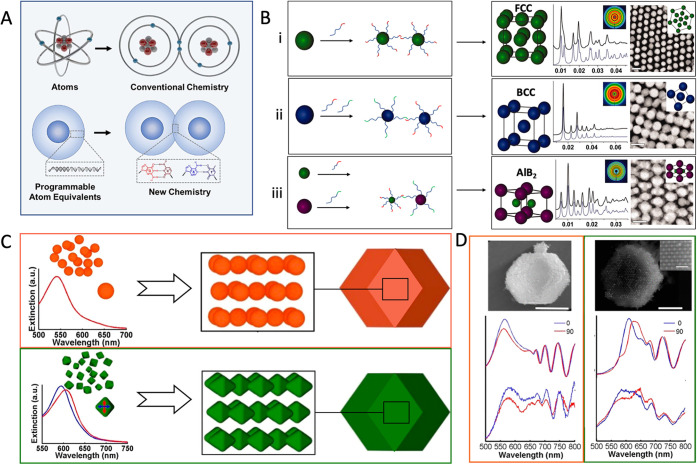
NCs as the template can be directionally assembled and programmed by the interaction forces of DNAs, analogous to atomic assembly manipulated by covalent bonds.29,61−64 The NCs modified with dense DNA ligands can act as programmable atom equivalents (PAEs), and DNA hybridization plays a role in controlling nanomodule construction as the “value” and “bond” (Figure 2A).65−67 Similar to the chemical bonds that control the arrangement of atoms in an ordered crystal structure, the DNA “bond” can also drive NCs to form various three-dimensional superlattices.40,68−70 By regulating the length or sequence of DNA and the size of PAEs, NCs can be precisely positioned into a variety of crystal structures.40 It is always the most stable state that maximizes the number of “bands” formed by DNA hybridization in a lattice arrangement, in which the surface-area contact between NCs bearing complementary linkers can be used as a proxy for counting the hybridization events.29,40 For instance, as shown in Figure 2B: (i) Sharing a single self-complementary DNA sequence that allows all PAEs to bond equivalently, face-centered cubic (FCC) crystal assembly maximizes the total number of closest neighbors for each NC within a lattice. (ii) Two different sequences as termini on DNA bonds can hybridize with each other (nonself-complementary) and assemble NCs with a body-centered cubic (BCC) crystal structure. (iii) Tuning the hydrodynamic radii of PAEs that are determined by NCs size and DNA length, NCs can be positioned within a given unit cell of lattice type to form a variety of crystallographic symmetry such as AlB2 crystals.
Figure 2.
(A) Schematic illustration of DNA-NC conjugates acting as programmable atom equivalents (PAEs) for bonding assembly in a manner analogous to a natural atom assembly system. Reproduced with permission from ref (65). Copyright (2021) American Chemical Society. (B) DNA-mediated FCC (i), BCC (ii), AlB2 (iii) superlattice assembly of NCs; each panel contains a model unit cell, 1D and 2D (inset) X-ray diffraction patterns, and a TEM image. Reproduced with permission from ref (40). Copyright (2011) The American Association for the Advancement of Science. (C) The schematic depiction and single nanoparticle extinction spectra of the superlattices made from either octahedral (green) or spherical (orange) NCs. (D) SEM image and backscattering spectra of (C). Reproduced with permission from ref (76). Copyright (2017) American Chemical Society.
It is crucial to note that the general method is advantageous for assembling various types of NCs, which examine the effect of the spatial arrangement of NCs with different chemical compositions,71,72 sizes,71 or shapes73,74 on their macro-properties.65 For example, by controlling the crystal habit of plasmonic NCs superlattices assembled by DNA sequence, Mirkin et al. illustrated how DNA-mediated NC assembly controls light confinement and far-field extinction in plasmonic assembly through the optical responsiveness, which is demonstrated by both experiments and electrodynamic simulations.75 Meanwhile, the three-dimensional lattice assembled from octahedral NCs exhibits a unique polarization-dependent optical responsiveness different from spherical NCs, as determined by finite-difference time-domain simulations and backscattering measurements (Figure 2C,D), which indicates that the incorporation of anisotropic NCs contributes to expanding the diversity of programmable NC assemblies for optical tools.76 Furthermore, Mirkin and Cruz’s group first discovered that the small DNA-NC conjugates with electronic-like properties can act as electronic equivalents (EEs) to stabilize the larger PAEs and diffuse within the lattice, analogous to the metallic bonding.77−80 Thus, the introduction of EEs provides an assembly method to break the high symmetry of NCs superlattice: the DNA as valence bond connected to NCs is not necessarily completely fixed in space and no longer restricts NC assembly on a single cell.77−80 Based on this concept, a series of crystal structures were successfully constructed, and the electron microscopy results and molecular dynamics simulation clearly demonstrated that the DNA-driven NC assembly can generate lower symmetrical crystal structures and even create unexpected lattice arrangements.77 Such a programmable assembly approach indicates that the diverse properties of nanomaterials can be regulated by both the length of DNA and the geometric configuration of NC, which facilitates the elucidation of the relationship between the assembly parameter and performance of NCs and will guide the development of biomedical imaging nanoplatforms.
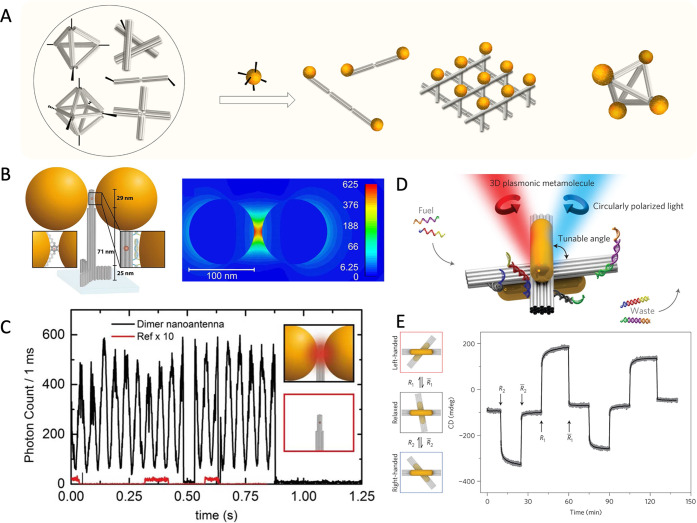
On the other hand, the hybridization of stands is able to create rigid structures that can be used as a template to precisely locate NCs. In the DNA template assembly strategy, DNA modified on the surface drives NC to be arranged at the predetermined position of the template, instead of cross-linking between NCs, achieving the nanoscale patterning with precise spatial location and number of NCs.81−86 Differing from the NCs template-assisted assembly, the arrangement of NC is determined by the geometry of the DNA pattern. Moreover, the development of advanced DNA nanostructures has given a new opportunity for DNA template-based NCs-assembly (such as DNA tiles87−89 and DNA origami32,90,91). As shown in Figure 3A, the rigidity of DNA structure provides a rational scheme for decorating NCs in arbitrary patterns or precisely fixing different NCs in the desired position. As a proof of concept, Alivisatos and colleagues constructed the pyramid assembly and chiral arrangement of NCs through the programming nucleic acids framework, demonstrating that the larger DNA nanostructures can be exploited as templates to integrate the monodisperse NCs into complex three-dimensional structures.60 Then, several groups developed the DNA origami-based NC assembly,92−96 which achieved great changes in properties at macro-scales through the precise and dynamic displacement of NCs.92−94 Concurrently, a series of improved functions based on the precise assembly of NCs have been found. For instance, Tinnefeld et al. assembled Au NCs dimer at a precise space distance through cylindrical DNA origami.97 The properties of dimers with various sizes have been systematically analyzed, which provides a simple solution for the investigation of the overlapping plasmonic fields in metal NC systems.97 Furthermore, it is predicted that the electric field intensity factor increased by more than 500 for the 100 nm-Au NC dimer with a gap of 12 nm at a dimer-parallel incident electric field polarization with a wavelength of 640 nm, which effectively improved the signals of single-molecule fluorescence transients (Figure 3B,C).98 Keyser et al. proposed the 40 nm2 DNA origami platforms as templates to assemble 40 nm Au NCs into dimer formation with a gap of 3.3 ± 1 nm. They observed a 7-fold enhancement in the coefficient for surface-enhanced Raman scattering (SERS).99 Meanwhile, Ke et al. developed a new type of modular DNA origami laden with magnetic NCs, which exhibit enhanced magnetic resonance imaging (MRI) contrast through the dynamic assembly of magnetic NCs.100
Figure 3.
(A) Schematic illustration of the programmable directional assembly of NCs in a DNA template. (B) Sketch of the DNA origami and two Au NCs to assemble the optical nanoantenna (left), and its numerical simulation of the electric field intensity (right). (C) Single-molecule fluorescence transient response for the dimer nanoantenna (black line) or the DNA origami without NCs (red line) at ten times excitation intensity. Reproduced with permission from ref (98). Copyright (2015) American Chemical Society. (D) Schematic diagram of the tunable angle Au nanorods hosted on switchable DNA origami template. (E) The circularly polarized light of plasmonic nanostructure modulated by switchable DNA templates. Reproduced with permission from ref (94). Copyright (2014) Springer Nature.
Furthermore, for the geometric features tuning of high-order anisotropic nanoscale building blocks, Liedl et al. developed a method for using DNA origami to guide the conformational changes of NRs assembly in order to modulate chirality, color, and intensity of nanodevice.94 By using strand displacement reactions (SDR), a switchable DNA origami with two bundles was used to perform dynamic spatial reconstruction driven by fuel DNA strands (Figure 3D), changing the relative angle of plasmonic Au NRs into desired states and causing strong circular dichroism (CD) changes (Figure 3E).94 Ke et al. constructed a 3D reconfigurable nanostructure by positioning three Au NRs on a reconfigurable DNA tripod. The inter-nanorod distance and angle were precisely regulated through toehold-mediated strand displacement while operating the origami tripod.101 Consequently, dynamic DNA origami nanotechnology has the potential to assemble high-order anisotropic building blocks, increase the complexity of coupling modes, and contribute to the development of signal conversion networks.94,102,103
3. Dynamic Assembly/Disassembly of NCs for Biomedical Imaging
Owing to the capability of structural conversion of DNA, well-designed DNA nanostructures allow for the dynamic assembly/disassembly of NCs, which can be applied in various biological stimulations104−106 to enhance biomedical imaging.107,108 This section focuses on DNA-driven dynamic assembly/disassembly of NCs with controllable optical and magnetic properties, and corresponding applications in biomedical imaging, in which the different interaction approaches of manipulation such as Watson–Crick base pairing interactions, noncanonical base pairing interactions, aptamer–target interactions, and enzymatic/DNAzymatic reaction mediated covalent interactions are summarized.
3.1. Watson–Crick Interaction
The Watson–Crick base-pairing interaction is crucial in both the control of nanoscaled DNA assembly and the regulation of dynamic DNA nanostructures. Most dynamic DNA systems are regulated by classical DNA-exchange reactions such as toehold-mediated SDR.109 The toehold enables the displacement of the original strand by the binding strand, resulting in a change in DNA configuration and dynamic assembly/disassembly of DNA-NC conjugates. For example, Gang and coauthors reprogrammed the DNA-mediated interactions with the NCs, selectively manipulating the transformations between NC superlattices, which showed that DNA-exchange reactions enable dynamic reconfiguration and switchable structures in NC assembly,69 providing multiple-stage activation and diverse functional capabilities.69,110,111
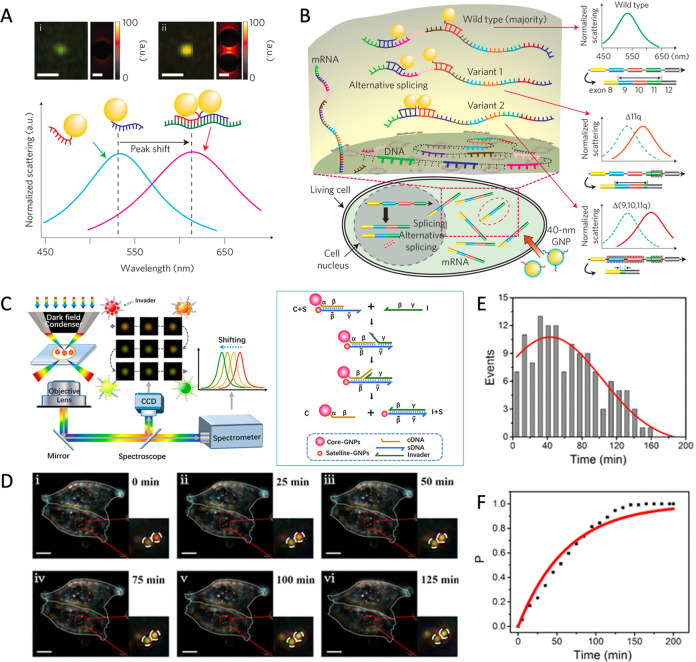
In the past decades, several new tools based on SDR have been developed for enhancing disease-related nucleic acid sensing, which demonstrates that DNA is capable of customizing dynamic NC assembly with controllable optical112 and magnetic113 properties, and expanding the applications of NCs in biomedical imaging.114,115 For instance, Ma et al. created a quantum dots (QD) computing system based on fluorescence resonance energy transfer (FRET) through the dynamic assembly of polychromatic QD with DNA, which can image disease-related nucleic acid molecules (e.g., microRNA and mRNA).116 Interacting with multiple nucleic acid targets, the imaging signals of QDs were generated, which improved the efficiency and accuracy of molecular diagnosis in vitro and in vivo.116 In addition, the change in the assembly state of superparamagnetic NCs has sensitive and reversible impacts on the relaxation rate of adjacent water molecules. Weissleder’s group developed a magnetic resonance (MR) switch for quantitative analysis of nucleic acids and identification of DNA sequences with single nucleotide mismatches.113,117 These nanoswitches for MRI imaging are nontoxic to mammals without the limitation of penetration depth, providing a promising option for in vivo nucleic acid sequencing.117 Moreover, Alivisatos et al. developed a DNA plasmonic ruler to measure the distance between NCs, utilizing the DNA double helix as the linear template to control the NCs distance.85 When plasmonic NCs approach one another, the scattering spectrum undergoes a spectral shift with the vibrant colors of NCs under a dark field microscope (DFM). This DNA plasmonic ruler offers a potential alternative to FRET for measuring nanoscale distances with a broader range and longer period, which is advantageous for sensing the interaction between biological macromolecules.85,118 Irudayaraj et al. combined hyperspectral imaging with plasmon rulers made of dynamically assembling Au NCs in cells to detect single-molecule mRNA with precision (Figure 4A).119 By measuring the position relationship of NCs which were captured by target sequence on mRNA, plasmon rulers monitored the spatial and temporal distribution of three selected splice variants of the breast cancer susceptibility gene BRCA1 in single copy resolution (Figure 4B).119 Chen et al. fabricated a plasmon ruler composed of Au NCs core–satellite structures, which were controlled by the toehold-mediated strand displacement of complementary micro-RNA (Figure 4C).120 The strand displacement induced by the target miRNA disassembles the plasmon ruler, resulting in synchronous changes in scattering intensity and wavelength in the DFM. Since miRNA gradually displaces, the color of the plasmon ruler changed from pink to yellow to green, allowing real-time detection of the dynamic biological distribution of miRNA 21 in HeLa cells at the single-molecule level (Figure 4D–F). The unique performance of plasmon rulers allows for single-molecule monitoring of biological processes in living cells.120 DNA-driven dynamic NC assembly has great potential to overcome the limitations of conventional probes, such as photobleaching and photoblinking.85,118−120 Furthermore, the described dynamic nanoassemblies are susceptible to “genetic” input, much like living matter is, potentially opening up new avenues for dynamic connections between biological and artificial systems. Undergoing the “genetic” modification to structural “evolution”, these dynamic inorganic nanocrystals have achieved a promising opportunity to mimic biological systems.69,70,101
Figure 4.
(A) Hyperspectral imaging and finite-difference time-domain simulation of monomeric and dimeric NCs. (B) The schematic diagram for single copy resolution imaging. Reproduced with permission from ref (119). Copyright (2014) Springer Nature. (C) Schematic illustration of the imaging model of core–satellite plasmon ruler (left) and the structure switching of plasmon ruler driven by toehold-mediated strand displacements (right). (D) The dark field microscope (DFM) imaging of plasmon ruler with time elapsing in living HeLa cells. Statistical analysis (E) of the disassembly events for the plasmon ruler, and cumulative probability (F) of the strand displacement in (D). Reproduced with permission from ref (120). Copyright (2018) American Chemical Society.
3.2. Noncanonical Base-Pairing Interaction
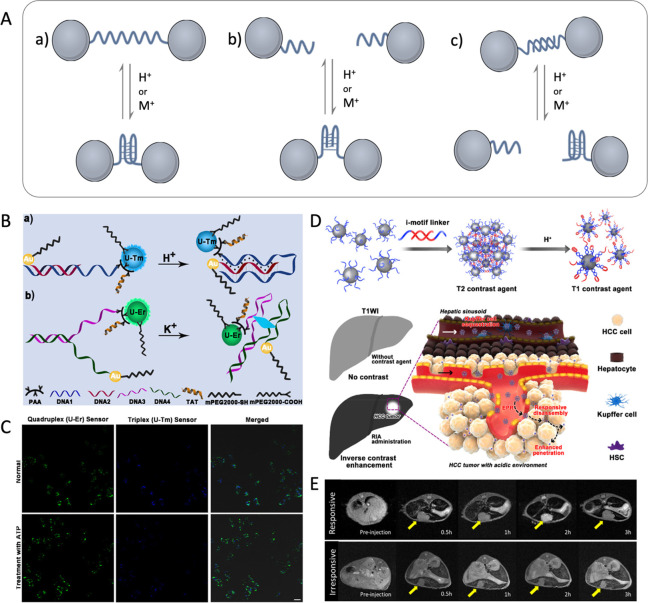
Noncanonical base pairing occurs when bases interact differently from the standard Watson–Crick rules, which enriches the diversity of DNA structure and facilitates the development of functional modules.121,122 Several DNA conformations function as natural gene regulators in specific pathological conditions, particularly the G-quadruplex121 and the i-motif,122 which form unique secondary structures through Hoogsteen base pairing or C:C+ base pairing. For instance, the low stability of G-quadruplex in K+-deprived environments is advantageous for gene transcription in invasive cancer cells containing the c-Myc protooncogene.123 The formation of the secondary structures of i-motif requires weak acidic conditions, similar to the acidic tumor microenvironment.124,125 Through conformation switching, the topological diversity and complexity of noncanonical DNA can provide a novel methodology for constructing nanoprobes.126 As shown in Figure 5A, there are three general strategies for fabricating nanoprobes through noncanonical DNA secondary structures, which can form in specific disease microenvironments and drive the assembly or disassembly of NCs: (a) the formation of noncanonical conformation accompanied by multiple folds with the geometric change alters the distance between conjugated NCs;127 (b) the assembly of NCs is driven by the intermolecular noncanonical conformation;127 (c) DNA conformation switching between intramolecular noncanonical and double helix dynamically regulates the assembly/disassembly of NCs.128
Figure 5.
(A) Scheme for the general strategies for the design of NC assembly/disassembly driven by noncanonical DNA. (B) Schematic illustration of the principle of nanosensors based on (a) DNA triplex and (b) G-quadruplex for correlating pH and K+ in lysosomes. (C) Confocal micrographs of HeLa cells with the signal change of nanosensors as stimulated by 100 μM ATP. Reproduced with permission from ref (129). Copyright (2021) Wiley-VCH GmbH, Weinheim. The (D) schematic diagram and (E) T1-weighted image of a pH MRI probe based on DNA i-motif applied for hepatocellular carcinoma imaging. Reproduced with permission from ref (130). Copyright (2018) American Chemical Society.
At present, a series of probes based on noncanonical DNA-driven NC assembly have been developed. Liu et al. established a nanoprobe based on DNA triplets and G-quadruplex for the subcellular monitoring of K+ and H+ in the lysosomal cavity.129 As the concentration of H+ or K+ increased, the DNA loading with UCNP and AuNP was folded into triplets or quadruplexes, resulting in a shorter distance between UCNP and Au NC (Figure 5B), which can dynamically respond in the pH range of 5.0–8.2 and 5–200 mM of [K+] conditions. Moreover, it is observed that the inflow of H+ is accompanied by the outflow of K+ during ATP-induced lysosomal acidification (Figure 5C).129 In addition, for the early diagnosis of tumors, Ling et al. designed an intelligent MRI probe based on the i-motif DNA functionalized ultrasmall superparamagnetic iron oxide nanocrystals (USIONs).130 The USIONs are assembled by the base pairing interaction of DNA, which can act as a responsive iron oxide nanocrystal assembly (RIA) with a high r2/r1 ratio (63.28), resulting in normal liver darkening. Subsequently, the tumor acidic microenvironment induces the RIA disassembly with i-motif DNA conformation, then switches into monodisperse USIONs with ideal T1-weighted imaging performance (r2/r1 = 7.12) (Figure 5D). Consequently, the normal tissues were darkened and the tumor tissues were brightened with a ∼4 times difference in signal intensity, which is highly valuable for the accurate imaging and early diagnosis of small hepatocellular carcinomas (Figure 5E).130
Besides the natural structures of G-quadruplex and i-motif, artificial noncanonical DNA structures have also been developed, such as metal base pairs131 that have been applied in the detection of heavy metal ions and water quality.132 While flexible base sequence programmability gives infinite possibilities to the DNA structures. We believe that more DNA interactions beyond the Watson–Crick rules could be discovered, which may further expand the applications of nanoprobes through the conformation switching of DNA structures.
3.3. Aptamer–Target Interaction
The DNA aptamer selected from the synthesis library has the ability to interact with certain bioactive molecules.133,134 The strategies have been developed for biomedical imaging through changes in the assembly state of DNA-NC conjugates by altering the interaction between the DNA aptamer and bioactive molecules. The interconversion between secondary structure conformations formed by the interaction of DNA with target molecules and the DNA double helix can facilitate the assembly and disassembly of DNA-NC conjugates. This section focuses on the typical applications of biomedical imaging utilizing aptamer–target interaction-mediated DNA-NC conformation switching.
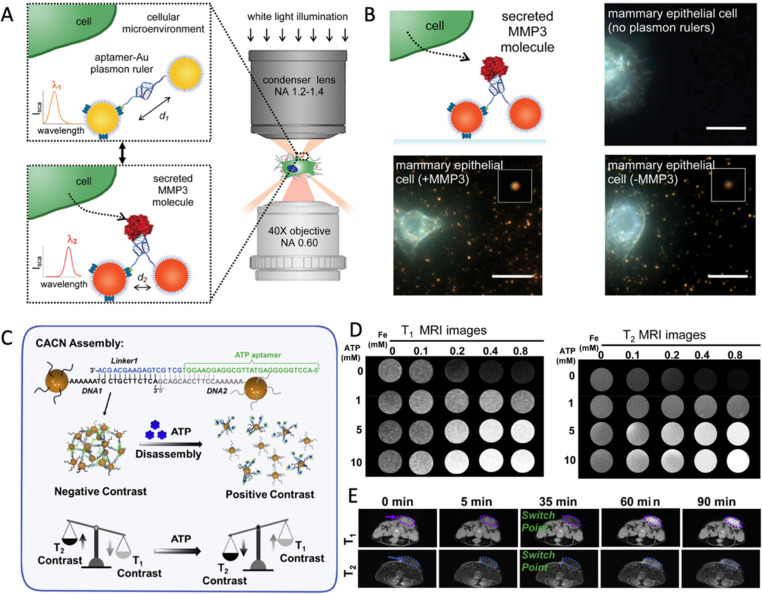
Bissell et al. developed reversible aptamer-Au plasmon rulers consisting of Au NCs coupled to a single aptamer strand.135 When the aptamer interacts with a single protein molecule with a high level of specificity, the spatial changes in DNA structures can result in the distance regulation of NCs conjugation at both ends of DNA (Figure 6A). The combination of the target secretory molecule MMP3 with plasmon rulers shows a 12 nm red shift with single molecule sensitivity, allowing for the visualization and quantification of MMP3 molecules secreted in the microenvironment of mammary epithelial cells (Figure 6B).135 The strategy showed great potential to characterize the spatiotemporal resolution of the secreted molecules’ form and evolution in the cell microenvironment. Meanwhile, Tan’s group created the first colorimetric technique for cancer cell imaging through the affinity between aptamer functionalized NCs and the target molecules.136 The well-designed aptamer is capable of recognizing the target protein on the surface of cancer cell membranes, and assembling the NCs to effectively enhance the interaction with light, allowing for the distinction of diverse types of cells based on the aptamer applied in the analysis. Then, they proved the universality of this targeted assembly methodology using a variety of NCs and aptamers to customize materials for fluorescent visualization of target cells.137−139 Moreover, the competition between Watson–Crick interaction and aptamer–target interaction provides an additional assembly/disassembly strategy with precise controllability. For example, Lu et al. described a protocol for disassembling purple Au NC assembly into red Au NCs through aptamer–target interaction, in which the aptamer sequence is more likely to bind with analytes and block the base pair interaction sites.140 Further utilized the interaction of ATP and related-aptamers as a model, they investigate the effect of the relative position of the non-base-pairing region between nucleotides and the Au NC surface.141 Song et al. constructed an MRI probe based on the ultrasmall magnetic NC assembly, which can change contrast performance according to the ATP concentrations in tumor microenvironments and achieve better tumor resolution.142 To enable ATP concentration-dependent disassembly, the probes were designed with a relative position between the pairing regions and the aptamers. As shown in Figure 6A, the linker 1-ATP aptamer strand connects DNA-NCs to act as ATP probes with the performance of T2 imaging in normal tissue. However, when probes detect an increase in ATP in the tumor microenvironment, the change in the aptamer conformation causes the probes to disassemble and switch the MRI from T2 to T1 contrast (Figure 6C), improving tumor resolution (Figure 6D,E).142
Figure 6.
(A) The switchable distance strategy of aptamer-Au plasmon rulers, and (B) darkfield scattering images to measure secreted protein molecules in the cellular microenvironment. Reproduced with permission from ref (135). Copyright (2015) American Chemical Society. (C) Scheme for ATP-triggered disassembly of MRI probes with the T1/T2 switchable. The T1-weighted and T2-weighted imaging of (D) in vitro and (E) in vivo triggered with various concentrations of ATP. Reproduced with permission from ref (142). Copyright (2022) Elsevier.
Moreover, owing to the technical advancements in the selection process and synthesis of aptamers, aptamer-based probes have favorable performance in advanced imaging technology, allowing for a wider range of biomarker analysis.143 This shows that the aptamer-based dynamic NC assembly has the potential to broaden the scope of biomedical imaging to include more diverse bioactive molecules.
3.4. Enzymatic/DNAzymatic Reaction Mediated Covalent Interaction
Protein enzyme and DNAzyme reactions have extended the boundaries of programs operated by DNA-functionalized materials via manipulating chemical bonds.144 Inspired by biological motors, the artificial DNA machines drive NCs to perform directional tasks via the conversion of chemical energy into controlled motions, which could be utilized as imaging probes for advanced imaging applications.145
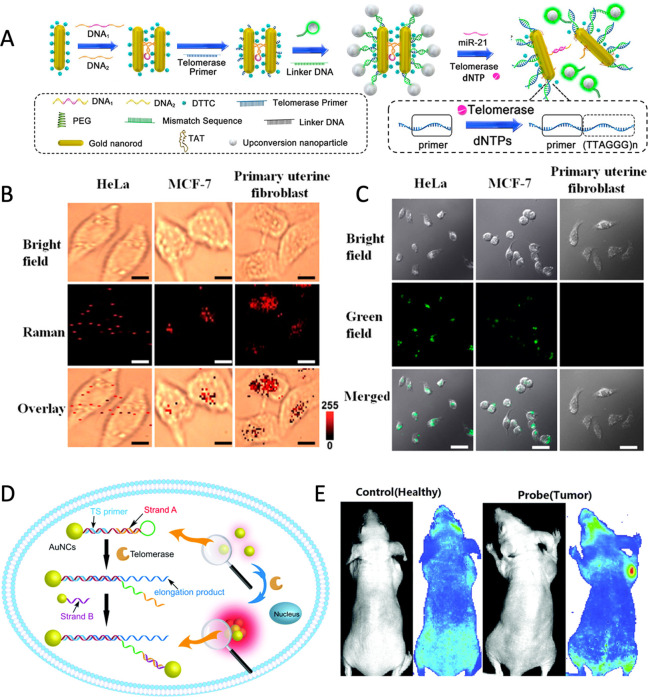
A series of enzymes have been created in nature to regulate DNA replication, transmission, error correction, and other life processes in order to effectively manage genetic information.144,146 It has been demonstrated that the DNA-related enzymes are equally active with DNA-NC conjugates,147−149 indicating that the natural enzyme toolkits could be employed to create a more advanced dynamic assembly/disassembly process, even expand the replication and repair functions of DNA-NC assembly.146 Kanaras et al. introduced the concept of protection and deprotection steps into NC assembly strategy using enzymes.147 Restriction enzymes such as EcoRI, StyI, and others are used to cleave DNA duplexes on NCs at specific recognition sites to generate cohesive ends. Then, T4 DNA ligase is used to covalently link the nucleic acid backbone at the hybrid cohesive ends, increasing the diversity of the assembly/disassembly of the DNA-NCs conjugation system.147,150 The protection/deprotection approach of NC assembly, similar to protecting-group chemistry, allows NCs to be programmed and manipulated to synthesize nanoassembled structures by selectively blocking and activating their functionality.151 The use of enzyme toolkits has increased the possibilities for dynamic assembly/disassembly of NCs, with the discovery of more than 3000 restrictive enzymes (hundreds of which are commercially available).147 In addition, enzyme-assisted reactions improve the traditional DNA motor mode driven by SDR.152,153 The free energy generated by the enzyme acting on DNA covalent bonds triggers the next step of SDR and drives DNA information transmission.144,154 Based on this fact, it opens up the possibility of developing a novel DNA motor with autonomous, unidirectional, and progressive linear translational motion.144 For instance, Salaita et al. developed a DNA rolling machine, which utilizes RNase H to selectively hydrolyze highly multivalent DNA-RNA duplexes on spherical particles as the driving force, resulting in an increase in movement by 3 orders of magnitude compared with the traditional DNA walkers.145 Wang et al. developed a 3D DNA rolling machine by integrating DNA walkers with Ag NCs to recognize the target and expose the toes for rolling, which provides the cascaded amplified signals.155 With an efficient chemical/mechanical energy conversion efficiency to amplify the signal of biomarkers, a DNA rolling machine driven by enzymes has achieved the accurate detection of p53 gene in blood samples,155 as well as the imaging of single nucleotide polymorphism (SNP) for simple equipment, allowing the possibility of imaging biomarkers with functional DNA such as aptamers. For in vivo imaging, the interactions between DNA and various enzymes can be designed to dynamically assemble NCs for the sensing of highly expressed enzymes in the disease microenvironment. Telomerase, which is highly expressed in most cancer cells, is essential for cancer diagnosis.156,157 DNA ligands can be designed to monitor telomerase activity in vivo by driving the assembly and disassembly of NCs. Wang et al. reported Au50@Au13 core–satellite nanostructures assembled by two sizes of Au NCs functionalized by DNA.158 Telomerase extends a repeat sequence (TTAGGG)n, the telomerase primer (TP), and then releases the complementary strands to drive Au NC assembly, which realizes the significant blue shift of the LSPR spectrum and in situ monitoring of the change of telomerase activity.158 Wei et al. used DNA programming to assemble Au NRs and UCNPs, including two Au NRs (forming a dimer structure) as a core and the UCNPs as satellites (Figure 7A).159 Then, Micro-RNA 21 triggered the disassembly of the Au NR dimer and telomerase released the UCNPs, generating two independent signals in living cells: a Raman signal from Au NR (Figure 7B) and a fluorescence signal from UCNP (Figure 7C).159 Qu et al. designed a hairpin-shaped TP DNA modified on Au NC to respond to telomerase.160 When internalized by cancer cells and recognized telomerase, the TP DNA could be extended and replaced to release a toehold domain of strand A, which subsequently interacted with strand B of another Au NC to generate the aggregation-induced emission (AIE) effect resulting from the assembly of Au NCs, allowing for the selective imaging of telomerase activity in living tumor cells in vitro and in vivo (Figure 7D,E).160 However, the limited number of enzymes with both disease markers and DNA conformation-switching properties restricts their application in biomedical imaging. Recently, chemical modification of DNA skeletons has been considered as a means of expanding the enzymatic toolkit to construct DNA nanoprobes for identifying highly expressed enzymes in diverse disease microenvironments. DNA probes containing molecular beacons at apurinic/pyramidic sites, for instance, can identify the human apurinic/pyramidic endonuclease 1 in the inflammatory microenvironment161 or tumor microenvironment.162 The capability of peptide-modified nucleic acid triblock copolymers to identify highly expressed proteases in the tumor microenvironment163 demonstrates the potential of using DNA chemical engineering approaches to customize the nanoprobes driven by enzymes.
Figure 7.
(A) Schematic illustration of the designed fabrication of the self-assembled satellite nanostructures of Au NR-dimer and UCNPs and their bioresponsive disassembly process. (B) Raman imaging and (C) confocal images of HeLa, MCF-7, and primary uterine fibroblast cells with core–satellite assemblies. Reproduced with permission from ref (159). Copyright (2017) American Chemical Society. (D) Schematic illustration of the telomerase-triggered DNA hybridization/substitution that can drive NC assembly. (E) The telomerase fluorescence imaging in healthy mice and tumor mice. Reproduced with permission from ref (160). Copyright (2021) Royal Society of Chemistry.
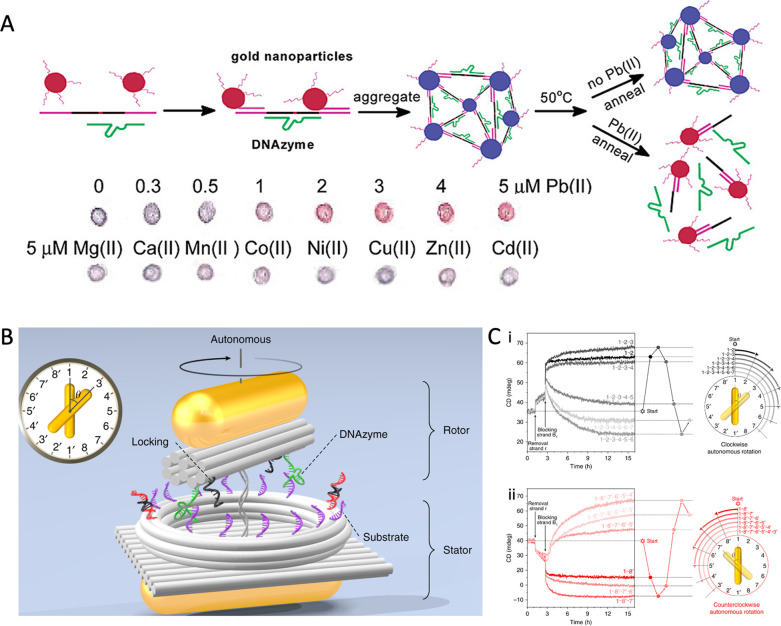
Additionally, since the discovery of catalytic nucleic acid (ribozyme), researchers have screened a series of DNAzymes with both programmability and catalytic activity, which is highly valuable for biosensor and medical diagnosis applications.164 DNAzyme has excellent affinity and selectivity for a certain biological target, allowing for the controlled capture and release of target DNA.164 Typically, the catalytic core of DNAzyme is maintained by the complexation of specific metal ions, which catalyzes the formation or breaking of phosphate diester bonds with target DNA or RNA.165 Therefore, DNAzyme is ideally suited for the construction of metal ion probes, which are capable of combining with ion specificity and catalyzing the substrate strand to drive the dynamic assembly of NCs.165,166 For example, Lu et al. reported a Pb2+ sensor based on a blue-colored aggregate composed of DNAzyme and AuNP-tagged substrate; in the presence of Pb2+, the DNAzyme cleaves the substrate strand, disassembling the AuNP aggregate and leading to a color change from blue to red (Figure 8A).167 DNAzymes can identify metal ions and drive the dynamic assembly/disassembly of Au NCs for a visual color change,167,168 which have acted as portable biosensors, such as transverse flow devices169,170 and paper sensors.171,172 In addition, driving magnetic NCs for dynamic assembly stimulated by metal ions, DNAzyme has also been proven to be useful for MRI detection.173,174 Furthermore, due to DNA’s physical and chemical properties, DNAzymes can be engineered to act as advanced molecular machines that can control the behavior of NCs to conduct complex mechanical tasks such as “walking”, “splitting”, and “rotating”.175−177 For instance, Choi and colleagues reported a DNA motor that can walk along the nanotube.176 DNAzyme converts the chemical energy from the substrate RNA on the nanotube into movement, driving the CdS NCs along the orbit in a programmatic manner. The movement of a single NC was observed in real-time with dual visible/near-infrared optical spectroscopy.176 Inspired by ATP synthetase, Liu et al. created a rotating plasma nanoclock made of DNA and Au NR.177 Two Au NRs are assembled and immobilized into stator and rotor by DNA, respectively. The rotor is able to rotate in a reversible, and omnidirectional manner, driven by DNAzyme-RNA interactions, which can be monitored in real-time using optical spectroscopy (Figure 8B).177 By leveraging the high energy conversion efficiency of DNAzyme and the exquisite programmability of DNA, it is anticipated that DNAzyme toolkits can enhance the capabilities of DNA and accelerate the speed of NC movement in programs with delicate signals of biological markers, making huge progress in the field of biomedical imaging.
Figure 8.
(A) Schematic illustration of DNAzyme-directed assembly of Au NCs and their sensing imaging with Pb2+ ions and interferent ions. Reproduced with permission from ref (167). Copyright (2003) American Chemical Society. (B) Schematic illustration of the rotary plasmonic nanoclock for autonomous rotation. (C) Time-course CD measurement for clockwise autonomous rotation (i) and counterclockwise autonomous rotation (ii). Reproduced with permission from ref (177). Copyright (2019) Springer Nature.
Overall, switching DNA configurations, through natural biological processes or synthetic chemical modifications, has broadened the scope of using dynamic assembly/disassembly of NCs for the specific imaging of various biomarkers. Furthermore, with the use of editable DNA, it is easy to alter the sequence of an interaction region to specifically respond to target molecules, creating a useful toolbox for developing universal and modular platforms for advanced biomedical imaging and clinical diagnoses.
4. Conclusion
The controllable assembly/disassembly of inorganic NCs remains a major challenge in nanotechnology. DNA nanotechnology provides a promising method tool to arrange NCs precisely into assemblies with the desired optical, electrical, or magnetic properties.93 Although several biomedical imaging strategies have been proposed based on DNA-driven dynamic assembly of NCs, the relevant research remains in its infancy, and there are still several challenges to be addressed. First, in order to enrich the library of structures for NC self-assembly, reasonable surface engineering is urgently needed to improve the modification efficiency of DNA with NCs, which enables more precise and programmable assembly with inorganic NCs, especially those without DNA affinity on surface sites. Second, DNA not only can manipulate the assembly state of NCs, but also has the potential to directly affect imaging-related parameters owing to their unique structures. For instance, when close to guanine-rich DNA sequences, the red fluorescence of DNA/Ag NCs is greatly amplified.178 The extensive and in-depth studies on the mechanisms of DNA that promote the imaging performance of NCs are helpful in expanding the applications of DNA-driven NC assembly in biomedical imaging. Third, the DNA-based dynamic NC assembly in response to a single endogenous stimulus (e.g., pH, redox, enzyme) could suffer from nonspecific excitation by the nontarget area in the complex microenvironments in vivo, resulting in false-positive imaging results. Notably, the design of DNA-driven NC assembly with responsiveness on both endogenous and exogenous stimuli (e.g., light, sound, magnetic) holds promise to improve the response accuracy and imaging specificity through precise spatial–temporal manipulation.
Taken together, DNA nanotechnology has revolutionized inorganic NC assembly with precise and programmable features, leading to the creation of functional materials with customized chemical and physical properties. Then, by utilizing stimuli-responsive DNA, dynamic NC assembly has been produced with the potential for sensing various biomarkers. Through “genetic” modification, NCs could undergo structural “evolution”, providing a methodology for the designed fabrication of advanced biomedical probes with outstanding imaging performance.
Acknowledgments
The authors acknowledge financial support by the National Key Research and Development Program of China (2022YFB3203801, 2022YFB3203804, 2022YFB3203800), the Leading Talent of “Ten Thousand Plan”-National High-Level Talents Special Support Plan, National Natural Science Foundation of China (32071374), Program of Shanghai Academic Research Leader under the Science and Technology Innovation Action Plan (21XD1422100), Program of Shanghai Science and Technology Development (22TS1400700), Zhejiang Provincial Natural Science Foundation of China (LR22C100001), Innovative Research Team of High-Level Local Universities in Shanghai (SHSMU-ZDCX20210900), and CAS Interdisciplinary Innovation Team (JCTD-2020-08).
Glossary
Vocabulary
- NC, inorganic nanocrystals
Inorganic nanocrystals are special particles that are composed of atoms in either a single- or poly crystalline arrangement, with sizes typically in the range of 1–100 nm
- PAEs, programmable atom equivalents
Programmable atom equivalents are oligonucleotide–nanoparticle conjugates that emulate the behavior of individual atoms, allowing for the precise assembly of larger structures
- SERS, surface-enhanced Raman scattering
Surface-enhanced Raman scattering is a spectroscopic technique that enhances the Raman scattering signal of molecules adsorbed on some nanostructures, leading to highly sensitive detection and analysis of trace amounts of molecules
- MRI, magnetic resonance imaging
Magnetic resonance imaging is a noninvasive medical imaging technique that creates two and three-dimensional detailed anatomical images of living subjects
- SDR, strand displacement reactions
Strand displacement reactions are biochemical processes in which a single-stranded nucleic acid molecule displaces a complementary strand from a double-stranded nucleic acid molecule.
Author Contributions
# S. Yang, Y. Wang, and Q. Wang contributed equally.
The authors declare no competing financial interest.
References
- Santos P. J.; Gabrys P. A.; Zornberg L. Z.; Lee M. S.; Macfarlane R. J. Macroscopic materials assembled from nanoparticle superlattices. Nature 2021, 591 (7851), 586–591. 10.1038/s41586-021-03355-z. [DOI] [PubMed] [Google Scholar]
- Udayabhaskararao T.; Altantzis T.; Houben L.; Coronado-Puchau M.; Langer J.; Popovitz-Biro R.; Liz-Marzan L. M.; Vukovic L.; Kral P.; Bals S.; Klajn R. Tunable porous nanoallotropes prepared by post-assembly etching of binary nanoparticle superlattices. Science 2017, 358, 514–518. 10.1126/science.aan6046. [DOI] [PubMed] [Google Scholar]
- Wang T.; Zhuang J.; Lynch J.; Chen O.; Wang Z.; Wang X.; LaMontagne D.; Wu H.; Wang Z.; CAO Y. C. Self-assembled colloidal superparticles from nanorods. Science 2012, 338 (6105), 358–363. 10.1126/science.1224221. [DOI] [PubMed] [Google Scholar]
- Li N.; Wu F.; Han Z.; Wang X.; Liu Y.; Yi C.; Ye S.; Lu G.; Yu L.; Nie Z.; Ding B. Shape complementarity modulated self-assembly of nanoring and nanosphere hetero nanostructures. J. Am. Chem. Soc. 2020, 142 (27), 11680–11684. 10.1021/jacs.0c04678. [DOI] [PubMed] [Google Scholar]
- Jeong H.; Park W.; Kim D. H.; Na K. Dynamic nanoassemblies of nanomaterials for cancer photomedicine. Adv. Drug Delivery Rev. 2021, 177, 113954. 10.1016/j.addr.2021.113954. [DOI] [PubMed] [Google Scholar]
- Ibanez M.; Luo Z.; Genc A.; Piveteau L.; Ortega S.; Cadavid D.; Dobrozhan O.; Liu Y.; Nachtegaal M.; Zebarjadi M.; Arbiol J.; Kovalenko M. V.; Cabot A. High-performance thermoelectric nanocomposites from nanocrystal building blocks. Nat. Commun. 2016, 7, 10766. 10.1038/ncomms10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X.; Sun R.; Yin L.; Chai Z.; Shi H.; Gao M. Light-Triggered Assembly of Gold Nanoparticles for photothermal therapy and photoacoustic imaging of tumors in vivo. Adv. Mater. 2017, 29 (6), 1604894. 10.1002/adma.201604894. [DOI] [PubMed] [Google Scholar]
- Lee H.; Sun E.; Ham D.; Weissleder R. Chip-NMR biosensor for detection and molecular analysis of cells. Nat. Med. 2008, 14 (8), 869–874. 10.1038/nm.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F.; Liang Z.; Liu J.; Sun J.; Hu X.; Zhao M.; Liu J.; Bai R.; Kim D.; Sun X.; Hyeon T.; Ling D. Dynamically reversible iron oxide nanoparticle assemblies for targeted amplification of T1-weighted magnetic resonance imaging of tumors. Nano Lett. 2019, 19 (7), 4213–4220. 10.1021/acs.nanolett.8b04411. [DOI] [PubMed] [Google Scholar]
- Park W.; Na K. Advances in the synthesis and application of nanoparticles for drug delivery.. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2015, 7 (4), 494–508. 10.1002/wnan.1325. [DOI] [PubMed] [Google Scholar]
- Begley M. R.; Gianola D. S.; Ray T. R. Bridging functional nanocomposites to robust macroscale devices. Science 2019, 364 (6447), eaav4299. 10.1126/science.aav4299. [DOI] [PubMed] [Google Scholar]
- Boles M. A.; Engel M.; Talapin D. V. Self-assembly of colloidal nanocrystals: from intricate structures to functional materials. Chem. Rev. 2016, 116 (18), 11220–11289. 10.1021/acs.chemrev.6b00196. [DOI] [PubMed] [Google Scholar]
- Oh E.; Hong M.-Y.; Lee D.; Nam S.-H.; Yoon H. C.; Kim H.-S. Inhibition Assay of biomolecules based on fluorescence resonance energy transfer (FRET) between quantum dots and gold nanoparticles. J. Am. Chem. Soc. 2005, 127, 3270–3271. 10.1021/ja0433323. [DOI] [PubMed] [Google Scholar]
- Maier S. A.; Kik P. G.; Atwater H. A.; Meltzer S.; Harel E.; Koel B. E.; Requicha A. A. Local detection of electromagnetic energy transport below the diffraction limit in metal nanoparticle plasmon waveguides. Nat. Mater. 2003, 2 (4), 229–232. 10.1038/nmat852. [DOI] [PubMed] [Google Scholar]
- Lee J.; Govorov A. O.; Kotov N. A. Nanoparticle assemblies with molecular springs: a nanoscale thermometer. Angew. Chem., Int. Ed. Engl. 2005, 44 (45), 7439–7442. 10.1002/anie.200501264. [DOI] [PubMed] [Google Scholar]
- Köker T.; Tang N.; Tian C.; Zhang W.; Wang X.; Martel R.; Pinaud F. Cellular imaging by targeted assembly of hot-spot SERS and photoacoustic nanoprobes using split-fluorescent protein scaffolds. Nat. Commun. 2018, 9, 607. 10.1038/s41467-018-03046-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Hernandez P.; Lee J.; Govorov A. O.; Kotov N. A. Exciton-plasmon interactions in molecular spring assemblies of nanowires and wavelength-based protein detection. Nat. Mater. 2007, 6 (4), 291–295. 10.1038/nmat1869. [DOI] [PubMed] [Google Scholar]
- Roller E. M.; Besteiro L. V.; Pupp C.; Khorashad L. K.; Govorov A. O.; Liedl T. Hot spot-mediated non-dissipative and ultrafast plasmon passage. Nat. Phys. 2017, 13 (8), 761–765. 10.1038/nphys4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra P. P.; Chikkaraddy R.; Tripathi R. P.; Dasgupta A.; Kumar G. V. Plasmofluidic single-molecule surface-enhanced Raman scattering from dynamic assembly of plasmonic nanoparticles. Nat. Commun. 2014, 5, 4357. 10.1038/ncomms5357. [DOI] [PubMed] [Google Scholar]
- Halas N. J.; Lal S.; Chang W.-S.; Link S.; Nordlander P. Plasmons in strongly coupled metallic nanostructures. Chem. Rev. 2011, 111 (6), 3913–3961. 10.1021/cr200061k. [DOI] [PubMed] [Google Scholar]
- Ghosh S. K.; Pal T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797–4862. 10.1021/cr0680282. [DOI] [PubMed] [Google Scholar]
- Prodan E.; Radloff C.; Halas N. J.; Nordlander P. A Hybridization Model for the Plasmon response of complex nanostructures. Science 2003, 302 (5644), 419–422. 10.1126/science.1089171. [DOI] [PubMed] [Google Scholar]
- Nie Z.; Petukhova A.; Kumacheva E. Properties and emerging applications of self-assembled structures made from inorganic nanoparticles. Nat. Nanotechnol. 2010, 5 (1), 15–25. 10.1038/nnano.2009.453. [DOI] [PubMed] [Google Scholar]
- Du H.; Wang Q.; Liang Z.; Li Q.; Li F.; Ling D. Designed fabrication of magnetic nanoprobes for ultra-high-field magnetic resonance imaging. Nanoscale 2022, 14 (47), 17483–17499. 10.1039/D2NR04979A. [DOI] [PubMed] [Google Scholar]
- Aubret A.; Martinet Q.; Palacci J. Metamachines of pluripotent colloids. Nat. Commun. 2021, 12 (1), 6398. 10.1038/s41467-021-26699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F.; Du Y.; Liu J.; Sun H.; Wang J.; Li R.; Kim D.; Hyeon T.; Ling D. Responsive assembly of upconversion nanoparticles for pH-activated and near-infrared-triggered photodynamic therapy of deep tumors. Adv. Mater. 2018, 30 (35), 1802808. 10.1002/adma.201802808. [DOI] [PubMed] [Google Scholar]
- Ling D. Dynamic nanoassembly-based drug delivery systems on the horizon. J. Controlled Release 2021, 339, 547–552. 10.1016/j.jconrel.2021.08.045. [DOI] [PubMed] [Google Scholar]
- Peng L.; Peng H.; Liu Y.; Wang X.; Hung C. T.; Zhao Z.; Chen G.; Li W.; Mai L.; Zhao D. Spiral self-assembly of lamellar micelles into multi-shelled hollow nanospheres with unique chiral architecture. Sci. Adv. 2021, 7, eabi7403. 10.1126/sciadv.abi7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. R.; Seeman N. C.; Mirkin C. A. Nanomaterials. Programmable materials and the nature of the DNA bond. Science 2015, 347 (6224), 1260901. 10.1126/science.1260901. [DOI] [PubMed] [Google Scholar]
- Andersen E. S.; Dong M.; Nielsen M. M.; Jahn K.; Subramani R.; Mamdouh W.; Golas M. M.; Sander B.; Stark H.; Oliveira C. L.; Pedersen J. S.; Birkedal V.; Besenbacher F.; Gothelf K. V.; Kjems J. Self-assembly of a nanoscale DNA box with a controllable lid. Nature 2009, 459 (7243), 73–76. 10.1038/nature07971. [DOI] [PubMed] [Google Scholar]
- Pinheiro A. V.; Han D.; Shih W. M.; Yan H. Challenges and opportunities for structural DNA nanotechnology. Nat. Nanotechnol. 2011, 6 (12), 763–772. 10.1038/nnano.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothemund P. W. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440 (7082), 297–302. 10.1038/nature04586. [DOI] [PubMed] [Google Scholar]
- Gerling T.; Wagenbauer K. F.; Neuner A. M.; Dietz H. Dynamic DNA devices and assemblies formed by shape-complementary, non–base pairing 3D components. Science 2015, 347 (6229), 1446–1452. 10.1126/science.aaa5372. [DOI] [PubMed] [Google Scholar]
- Benson E.; Mohammed A.; Gardell J.; Masich S.; Czeizler E.; Orponen P.; Hogberg B. DNA rendering of polyhedral meshes at the nanoscale. Nature 2015, 523 (7561), 441–444. 10.1038/nature14586. [DOI] [PubMed] [Google Scholar]
- Majewski P. W.; Michelson A.; Cordeiro L.; Tian C.; Ma C.; Kisslinger K.; Tian Y.; Liu W.; Stach E. A.; Yager K. G.; Gang O. Resilient three-dimensional ordered architectures assembled from nanoparticles by DNA. Sci. Adv. 2021, 7 (12), eabf0617 10.1126/sciadv.abf0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W.; Zhan Y.; Zhang Y.; Mao C.; Xie X.; Lin Y. The biological applications of DNA nanomaterials: current challenges and future directions. Signal Transduct. Target. Ther. 2021, 6 (1), 351. 10.1038/s41392-021-00727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y.; Yao C.; Zhu Y.; Yang L.; Luo D.; Yang D. DNA functional materials assembled from branched DNA: design, synthesis, and applications. Chem. Rev. 2020, 120 (17), 9420–9481. 10.1021/acs.chemrev.0c00294. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Lu F.; Yager K. G.; van der Lelie D.; Gang O. A general strategy for the DNA-mediated self-assembly of functional nanoparticles into heterogeneous systems. Nat. Nanotechnol. 2013, 8 (11), 865–872. 10.1038/nnano.2013.209. [DOI] [PubMed] [Google Scholar]
- Auyeung E.; Li T. I.; Senesi A. J.; Schmucker A. L.; Pals B. C.; de la Cruz M. O.; Mirkin C. A. DNA-mediated nanoparticle crystallization into Wulff polyhedra. Nature 2014, 505 (7481), 73–77. 10.1038/nature12739. [DOI] [PubMed] [Google Scholar]
- Macfarlane R. J.; Lee B.; Jones M. R.; Harris N.; Schatz G. C.; Mirkin C. A. Nanoparticle superlattice engineering with DNA. Science 2011, 334 (6053), 204–208. 10.1126/science.1210493. [DOI] [PubMed] [Google Scholar]
- Lin Q.-Y.; Mason J. A.; Li Z.; Zhou W.; O’Brien M. N.; Brown K. A.; Jones M. R.; Butun S.; Lee B.; Dravid V. P.; Aydin K.; Mirkin C. A. Building superlattices from individual nanoparticles via template-confined DNA-mediated assembly. Science 2018, 359 (6376), 669–672. 10.1126/science.aaq0591. [DOI] [PubMed] [Google Scholar]
- Kim Y.; Macfarlane R. J.; Jones M. R.; Mirkin C. A. Transmutable nanoparticles with reconfigurable surface ligands. Science 2016, 351 (6273), 579–582. 10.1126/science.aad2212. [DOI] [PubMed] [Google Scholar]
- Ross M. B.; Ku J. C.; Blaber M. G.; Mirkin C. A.; Schatz G. C. Defect tolerance and the effect of structural inhomogeneity in plasmonic DNA-nanoparticle superlattices. Proc. Natl. Acad. Sci. U.S.A. 2015, 112 (33), 10292–10297. 10.1073/pnas.1513058112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Nguyen K.; Spitale R. C.; Chaput J. C. A biologically stable DNAzyme that efficiently silences gene expression in cells. Nat. Chem. 2021, 13 (4), 319–326. 10.1038/s41557-021-00645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson I. A. P.; Zheng L.; Eisenstein M.; Soh H. T. Rational design of aptamer switches with programmable pH response. Nat. Commun. 2020, 11 (1), 2946. 10.1038/s41467-020-16808-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q. L.; Wang L. L.; Liu Y.; Lin J.; Xu L. A kinetically controlled platform for ligand-oligonucleotide transduction. Nat. Commun. 2021, 12 (1), 4654. 10.1038/s41467-021-24962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.; Wang Y.; Xu X.; Liu Y.; Lin B.; Zhang M.; Zhang J.; Wan S.; Yang C.; Tan W. Aptamer-based detection of circulating targets for precision medicine. Chem. Rev. 2021, 121 (19), 12035–12105. 10.1021/acs.chemrev.0c01140. [DOI] [PubMed] [Google Scholar]
- Zhao S.; Tian R.; Wu J.; Liu S.; Wang Y.; Wen M.; Shang Y.; Liu Q.; Li Y.; Guo Y.; Wang Z.; Wang T.; Zhao Y.; Zhao H.; Cao H.; Su Y.; Sun J.; Jiang Q.; Ding B. A DNA origami-based aptamer nanoarray for potent and reversible anticoagulation in hemodialysis. Nat. Commun. 2021, 12 (1), 358. 10.1038/s41467-020-20638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Shi L.; Kuang H.; Xu C. DNA-driven nanoparticle assemblies for biosensing and bioimaging. Top. Curr. Chem. 2020, 378 (1), 18. 10.1007/s41061-020-0282-z. [DOI] [PubMed] [Google Scholar]
- Samanta A.; Medintz I. L. Nanoparticles and DNA - a powerful and growing functional combination in bionanotechnology. Nanoscales 2016, 8 (17), 9037–9095. 10.1039/C5NR08465B. [DOI] [PubMed] [Google Scholar]
- Li L.; Xing H.; Zhang J.; Lu Y. Functional DNA molecules enable selective and stimuli-responsive nanoparticles for biomedical applications. Acc. Chem. Res. 2019, 52 (9), 2415–2426. 10.1021/acs.accounts.9b00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L.; Mu J.; Gang O.; Chen X. Rationally programming nanomaterials with dna for biomedical applications. Adv. Sci. 2021, 8 (8), 2003775. 10.1002/advs.202003775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathe M.; Rothemund P. W. K. DNA nanotechnology: a foundation for programmable nanoscale materials. MRS Bull. 2017, 42 (12), 882–888. 10.1557/mrs.2017.279. [DOI] [Google Scholar]
- Seeman N. C.; Sleiman H. F. DNA nanotechnology. Nat. Rev. Mater. 2018, 3 (1), 17068. 10.1038/natrevmats.2017.68. [DOI] [Google Scholar]
- Zhang F.; Jiang S.; Wu S.; Li Y.; Mao C.; Liu Y.; Yan H. Complex wireframe DNA origami nanostructures with multi-arm junction vertices. Nat. Nanotechnol. 2015, 10 (9), 779–784. 10.1038/nnano.2015.162. [DOI] [PubMed] [Google Scholar]
- Mirkin C. A.; Letsinger R. L.; Mucic C.; Storhoff J. J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996, 382, 607–609. 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- Alivisatos A. P.; Johnsson K. P.; Peng X.; Wilson T. E.; Loweth C. J.; Bruchez M. P. Jr; Schultz P. G. Organization of ’nanocrystal molecules’ using DNA. Nature 1996, 382 (15), 609–611. 10.1038/382609a0. [DOI] [PubMed] [Google Scholar]
- De Fazio A. F.; Misatziou D.; Baker Y. R.; Muskens O. L.; Brown T.; Kanaras A. G. Chemically modified nucleic acids and DNA intercalators as tools for nanoparticle assembly. Chem. Soc. Rev. 2021, 50 (23), 13410–13440. 10.1039/D1CS00632K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julin S.; Nummelin S.; Kostiainen M. A.; Linko V. DNA nanostructure-directed assembly of metal nanoparticle superlattices. J. Nanopart. Res. 2018, 20 (5), 119. 10.1007/s11051-018-4225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroianni A. J.; Claridge S. A.; Alivisatos A. P. Pyramidal and chiral groupings of gold nanocrystals assembled using DNA scaffolds. J. Am. Chem. Soc. 2009, 131 (24), 8455–8459. 10.1021/ja808570g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane R. J.; O’Brien M. N.; Petrosko S. H.; Mirkin C. A. Nucleic acid-modified nanostructures as programmable atom equivalents: forging a new ″table of elements″. Angew. Chem., Int. Ed. Engl. 2013, 52 (22), 5688–5698. 10.1002/anie.201209336. [DOI] [PubMed] [Google Scholar]
- Nykypanchuk D.; Maye M. M.; van der Lelie D.; Gang O. DNA-guided crystallization of colloidal nanoparticles. Nature 2008, 451 (7178), 549–552. 10.1038/nature06560. [DOI] [PubMed] [Google Scholar]
- Park S. Y.; Lytton-Jean A. K.; Lee B.; Weigand S.; Schatz G. C.; Mirkin C. A. DNA-programmable nanoparticle crystallization. Nature 2008, 451 (7178), 553–556. 10.1038/nature06508. [DOI] [PubMed] [Google Scholar]
- Senesi A. J.; Eichelsdoerfer D. J.; Macfarlane R. J.; Jones M. R.; Auyeung E.; Lee B.; Mirkin C. A. Stepwise evolution of DNA-programmable nanoparticle superlattices. Angew. Chem., Int. Ed. Engl. 2013, 52 (26), 6624–6628. 10.1002/anie.201301936. [DOI] [PubMed] [Google Scholar]
- Petrosko S. H.; Coleman B. D.; Drout R. J.; Schultz J. D.; Mirkin C. A. Spherical nucleic acids: integrating nanotechnology concepts into general chemistry curricula. J. Chem. Educ. 2021, 98 (10), 3090–3099. 10.1021/acs.jchemed.1c00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien M. N.; Girard M.; Lin H. X.; Millan J. A.; Olvera de la Cruz M.; Lee B.; Mirkin C. A. Exploring the zone of anisotropy and broken symmetries in DNA-mediated nanoparticle crystallization. Proc. Natl. Acad. Sci. U.S.A. 2016, 113 (38), 10485–10490. 10.1073/pnas.1611808113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S.; Yang S.; Xin H. L.; Nykypanchuk D.; Liu M.; Zhang H.; Gang O. Valence-programmable nanoparticle architectures. Nat. Commun. 2020, 11 (1), 2279. 10.1038/s41467-020-16157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. L.; Ross M. B.; Blaber M. G.; Rycenga M.; Jones M. R.; Zhang C.; Senesi A. J.; Lee B.; Schatz G. C.; Mirkin C. A. Using DNA to design plasmonic metamaterials with tunable optical properties. Adv. Mater. 2014, 26 (4), 653–659. 10.1002/adma.201302938. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Pal S.; Srinivasan B.; Vo T.; Kumar S.; Gang O. Selective transformations between nanoparticle superlattices via the reprogramming of DNA-mediated interactions. Nat. Mater. 2015, 14 (8), 840–847. 10.1038/nmat4296. [DOI] [PubMed] [Google Scholar]
- Maye M. M.; Kumara M. T.; Nykypanchuk D.; Sherman W. B.; Gang O. Switching binary states of nanoparticle superlattices and dimer clusters by DNA strands. Nat. Nanotechnol. 2010, 5 (2), 116–120. 10.1038/nnano.2009.378. [DOI] [PubMed] [Google Scholar]
- Sun D.; Tian Y.; Zhang Y.; Xu Z.; Sfeir M. Y.; Cotlet M.; Gang O. Light-harvesting nanoparticle core shell clusters with controllable optical output. ACS Nano 2015, 9 (6), 5657–5665. 10.1021/nn507331z. [DOI] [PubMed] [Google Scholar]
- Sun D.; Gang O. Binary heterogeneous superlattices assembled from quantum dots and gold nanoparticles with DNA. J. Am. Chem. Soc. 2011, 133 (14), 5252–5254. 10.1021/ja111542t. [DOI] [PubMed] [Google Scholar]
- Lin H.; Lee S.; Sun L.; Spellings M.; Engel M.; Glotzer S. C.; Mirkin C. A. Clathrate colloidal crystals. Science 2017, 355, 931–935. 10.1126/science.aal3919. [DOI] [PubMed] [Google Scholar]
- Lu F.; Yager K. G.; Zhang Y.; Xin H.; Gang O. Superlattices assembled through shape-induced directional binding. Nat. Commun. 2015, 6, 6912. 10.1038/ncomms7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross M. B.; Ku J. C.; Vaccarezza V. M.; Schatz G. C.; Mirkin C. A. Nanoscale form dictates mesoscale function in plasmonic DNA-nanoparticle superlattices. Nat. Nanotechnol. 2015, 10 (5), 453–458. 10.1038/nnano.2015.68. [DOI] [PubMed] [Google Scholar]
- Sun L.; Lin H.; Park D. J.; Bourgeois M. R.; Ross M. B.; Ku J. C.; Schatz G. C.; Mirkin C. A. Polarization-dependent optical response in anisotropic nanoparticle-DNA superlattices. Nano Lett. 2017, 17 (4), 2313–2318. 10.1021/acs.nanolett.6b05101. [DOI] [PubMed] [Google Scholar]
- Wang S.; Lee S.; Du J. S.; Partridge B. E.; Cheng H. F.; Zhou W.; Dravid V. P.; Lee B.; Glotzer S. C.; Mirkin C. A. The emergence of valency in colloidal crystals through electron equivalents. Nat. Mater. 2022, 21 (5), 580–587. 10.1038/s41563-021-01170-5. [DOI] [PubMed] [Google Scholar]
- Girard M.; Wang S.; Du J. S.; Das A.; Huang Z.; Dravid V. P.; Lee B.; Mirkin C. A.; Olvera de la Cruz M. Particle analogs of electrons in colloidal crystals. Science 2019, 364, 1174–1178. 10.1126/science.aaw8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H. F.; Wang S.; Mirkin C. A. Electron-equivalent valency through molecularly well-defined multivalent DNA. J. Am. Chem. Soc. 2021, 143 (4), 1752–1757. 10.1021/jacs.0c11843. [DOI] [PubMed] [Google Scholar]
- Wang S.; Du J. S.; Diercks N. J.; Zhou W.; Roth E. W.; Dravid V. P.; Mirkin C. A. Colloidal crystal ″alloys″. J. Am. Chem. Soc. 2019, 141 (51), 20443–20450. 10.1021/jacs.9b11109. [DOI] [PubMed] [Google Scholar]
- Loweth C. J.; Caldwell W. B.; Peng X.; Alivisatos A. P.; Schultz P. G. DNA-based assembly of gold nanocrystals. Angew. Chem., Int. Ed. Engl. 1999, 38 (12), 1808–1812. . [DOI] [PubMed] [Google Scholar]
- Fu A.; Micheel C. M.; Cha J.; Chang H.; Yang H.; Alivisatos A. P. Discrete Nanostructures of quantum dots/Au with DNA. J. Am. Chem. Soc. 2004, 126, 10832–10833. 10.1021/ja046747x. [DOI] [PubMed] [Google Scholar]
- Aldaye F. A.; Sleiman H. F. Dynamic DNA templates for discrete gold nanoparticle assemblies: control of geometry, modularity, write/erase and structural switching. J. Am. Chem. Soc. 2007, 129 (14), 4130–4131. 10.1021/ja070017i. [DOI] [PubMed] [Google Scholar]
- Mastroianni A. J.; Claridge S. A.; Alivisatos A. P. Pyramidal and chiral groupings of gold nanocrystals assembled using DNA Scaffolds. J. Am. Chem. Soc. 2009, 131 (24), 8455–8459. 10.1021/ja808570g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnichsen C.; Reinhard B. M.; Liphardt J.; Alivisatos A. P. A molecular ruler based on plasmon coupling of single gold and silver nanoparticles. Nat. Biotechnol. 2005, 23 (6), 741–745. 10.1038/nbt1100. [DOI] [PubMed] [Google Scholar]
- Zheng J.; Lukeman P. S.; Sherman W. B.; Micheel C.; Alivisatos A. P.; Constantinou P. E.; Seeman N. C. Metallic nanoparticles used to estimate the structural integrity of DNA motifs. Biophys. J. 2008, 95 (7), 3340–3348. 10.1529/biophysj.108.138479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B.; Leontis N. B.; Seeman N. C. Bulged 3-arm DNA branched junctions as components for nanoconstruction. Nanobiology 1994, 3, 177–188. [Google Scholar]
- He Y.; Ye T.; Su M.; Zhang C.; Ribbe A. E.; Jiang W.; Mao C. Hierarchical self-assembly of DNA into symmetric supramolecular polyhedra. Nature 2008, 452 (7184), 198–201. 10.1038/nature06597. [DOI] [PubMed] [Google Scholar]
- Wei B.; Dai M.; Yin P. Complex shapes self-assembled from single-stranded DNA tiles. Nature 2012, 485 (7400), 623–626. 10.1038/nature11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S.; Fan C.; Gothelf K. V.; Li J.; Lin C.; Liu L.; Liu N.; Nijenhuis M. A. D.; Saccà B.; Simmel F. C.; Yan H.; Zhan P. DNA origami. Nat. Rev. Methods Primers 2021, 1 (1), 13. 10.1038/s43586-020-00009-8. [DOI] [Google Scholar]
- Yao G.; Zhang F.; Wang F.; Peng T.; Liu H.; Poppleton E.; Sulc P.; Jiang S.; Liu L.; Gong C.; Jing X.; Liu X.; Wang L.; Liu Y.; Fan C.; Yan H. Meta-DNA structures. Nat. Chem. 2020, 12 (11), 1067–1075. 10.1038/s41557-020-0539-8. [DOI] [PubMed] [Google Scholar]
- Urban M. J.; Dutta P. K.; Wang P.; Duan X.; Shen X.; Ding B.; Ke Y.; Liu N. Plasmonic toroidal metamolecules assembled by DNA origami. J. Am. Chem. Soc. 2016, 138 (17), 5495–5498. 10.1021/jacs.6b00958. [DOI] [PubMed] [Google Scholar]
- Kuzyk A.; Schreiber R.; Fan Z.; Pardatscher G.; Roller E. M.; Hogele A.; Simmel F. C.; Govorov A. O.; Liedl T. DNA-based self-assembly of chiral plasmonic nanostructures with tailored optical response. Nature 2012, 483 (7389), 311–314. 10.1038/nature10889. [DOI] [PubMed] [Google Scholar]
- Kuzyk A.; Schreiber R.; Zhang H.; Govorov A. O.; Liedl T.; Liu N. Reconfigurable 3D plasmonic metamolecules. Nat. Mater. 2014, 13 (9), 862–866. 10.1038/nmat4031. [DOI] [PubMed] [Google Scholar]
- Tian Y.; Lhermitte J. R.; Bai L.; Vo T.; Xin H. L.; Li H.; Li R.; Fukuto M.; Yager K. G.; Kahn J. S.; Xiong Y.; Minevich B.; Kumar S. K.; Gang O. Ordered three-dimensional nanomaterials using DNA-prescribed and valence-controlled material voxels. Nat. Mater. 2020, 19 (7), 789–796. 10.1038/s41563-019-0550-x. [DOI] [PubMed] [Google Scholar]
- Wang M.; Dai L.; Duan J.; Ding Z.; Wang P.; Li Z.; Xing H.; Tian Y. Programmable assembly of nano-architectures through designing anisotropic DNA origami Patches. Angew. Chem., Int. Ed. Engl. 2020, 59 (16), 6389–6396. 10.1002/anie.201913958. [DOI] [PubMed] [Google Scholar]
- Acuna G. P.; Moller F. M.; Holzmeister P.; Beater S.; Lalkens B.; Tinnefeld P. Fluorescence enhancement at docking sites of dna-directed self-assembled nanoantennas. Science 2012, 338 (6106), 506–510. 10.1126/science.1228638. [DOI] [PubMed] [Google Scholar]
- Puchkova A.; Vietz C.; Pibiri E.; Wunsch B.; Sanz Paz M.; Acuna G. P.; Tinnefeld P. DNA origami nanoantennas with over 5000-fold fluorescence enhancement and single-molecule detection at 25 μM. Nano Lett. 2015, 15 (12), 8354–9359. 10.1021/acs.nanolett.5b04045. [DOI] [PubMed] [Google Scholar]
- Thacker V. V.; Herrmann L. O.; Sigle D. O.; Zhang T.; Liedl T.; Baumberg J. J.; Keyser U. F. DNA origami based assembly of gold nanoparticle dimers for surface-enhanced Raman scattering. Nat. Commun. 2014, 5, 3448. 10.1038/ncomms4448. [DOI] [PubMed] [Google Scholar]
- Meyer T. A.; Zhang C.; Bao G.; Ke Y. Programmable assembly of iron oxide nanoparticles using DNA origami. Nano Lett. 2020, 20 (4), 2799–2805. 10.1021/acs.nanolett.0c00484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan P.; Dutta P. K.; Wang P.; Song G.; Dai M.; Zhao S. X.; Wang Z. G.; Yin P.; Zhang W.; Ding B.; Ke Y. Reconfigurable three-dimensional gold nanorod plasmonic nanostructures organized on DNA origami tripod. ACS Nano 2017, 11 (2), 1172–1179. 10.1021/acsnano.6b06861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Nguyen M. K.; Natarajan A. K.; Nguyen V. H.; Kuzyk A. A DNA origami-based chiral plasmonic sensing device. ACS Appl. Mater. Interfaces 2018, 10 (51), 44221–44225. 10.1021/acsami.8b19153. [DOI] [PubMed] [Google Scholar]
- Funck T.; Nicoli F.; Kuzyk A.; Liedl T. Sensing picomolar concentrations of RNA using switchable plasmonic chirality. Angew. Chem., Int. Ed. Engl. 2018, 57 (41), 13495–13498. 10.1002/anie.201807029. [DOI] [PubMed] [Google Scholar]
- Nummelin S.; Shen B.; Piskunen P.; Liu Q.; Kostiainen M. A.; Linko V. Robotic DNA nanostructures. ACS Synth. Biol. 2020, 9 (8), 1923–1940. 10.1021/acssynbio.0c00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.; Jiang Q.; Wang Y.; Ding B. Biomedical Applications of DNA-Based Molecular Devices. Adv. Healthc. Mater. 2019, 8 (10), e1801658 10.1002/adhm.201801658. [DOI] [PubMed] [Google Scholar]
- Augspurger E. E.; Rana M.; Yigit M. V. Chemical and biological sensing using hybridization chain reaction. ACS Sens. 2018, 3 (5), 878–902. 10.1021/acssensors.8b00208. [DOI] [PubMed] [Google Scholar]
- Liu N.; Liedl T. DNA-assembled advanced plasmonic architectures. Chem. Rev. 2018, 118 (6), 3032–3053. 10.1021/acs.chemrev.7b00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F.; Mao X.; Li M.; Zuo X. Stimuli-responsive DNA-switchable biointerfaces. Langmuir 2018, 34 (49), 15055–15068. 10.1021/acs.langmuir.8b02185. [DOI] [PubMed] [Google Scholar]
- Del Grosso E.; Irmisch P.; Gentile S.; Prins L. J.; Seidel R.; Ricci F. Dissipative control over the toehold-mediated DNA strand displacement reaction. Angew. Chem., Int. Ed. Engl. 2022, 61 (23), e202201929 10.1002/anie.202201929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Increasingly complex DNA assemblies. Nat. Mater. 2020, 19 ( (7), ), 689. 10.1038/s41563-020-0727-3. [DOI] [PubMed] [Google Scholar]
- Eiser E. DNA-nanoparticle crystals: Flip-flop lattices. Nat. Mater. 2015, 14 (8), 751–752. 10.1038/nmat4370. [DOI] [PubMed] [Google Scholar]
- Tikhomirov G.; Hoogland S.; Lee P. E.; Fischer A.; Sargent E. H.; Kelley S. O. DNA-based programming of quantum dot valency, self-assembly and luminescence. Nat. Nanotechnol. 2011, 6 (8), 485–490. 10.1038/nnano.2011.100. [DOI] [PubMed] [Google Scholar]
- Perez J. M.; Josephson L.; O’Loughlin T.; Hogemann D.; Weissleder R. Magnetic relaxation switches capable of sensing molecular interactions. Nat. Biotechnol. 2002, 20 (8), 816–820. 10.1038/nbt720. [DOI] [PubMed] [Google Scholar]
- Li L.; Xing H.; Zhang J.; Lu Y. Functional DNA molecules enable selective and stimuli-responsive nanoparticles for biomedical applications. Acc. Chem. Res. 2019, 52 (9), 2415–2426. 10.1021/acs.accounts.9b00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L. H.; Xing H.; Lu Y. DNA as a powerful tool for morphology control, spatial positioning, and dynamic assembly of nanoparticles. Acc. Chem. Res. 2014, 47 (6), 1881–1890. 10.1021/ar500081k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X.; Li Z.; Chen M.; Ma N. DNA-programmed dynamic assembly of quantum dots for molecular computation. Angew. Chem., Int. Ed. Engl. 2014, 53 (52), 14447–14450. 10.1002/anie.201408479. [DOI] [PubMed] [Google Scholar]
- Josephson L.; Perez J. M.; Weissleder R. Magnetic nanosensors for the detection of oligonucleotide sequences. Angew. Chem., Int. Ed. Engl. 2001, 40 (17), 3204–3206. . [DOI] [PubMed] [Google Scholar]
- Thaxton C. S.; Mirkin C. A. Plasmon coupling measures up. Nat. Biotechnol. 2005, 23 (6), 681–682. 10.1038/nbt0605-681. [DOI] [PubMed] [Google Scholar]
- Lee K.; Cui Y.; Lee L. P.; Irudayaraj J. Quantitative imaging of single mRNA splice variants in living cells. Nat. Nanotechnol. 2014, 9 (6), 474–480. 10.1038/nnano.2014.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. X.; Xu C. H.; Zhang N.; Qian G. S.; Zhao W.; Xu J. J.; Chen H. Y. Exploration of the kinetics of toehold-mediated strand displacement via plasmon rulers. ACS Nano 2018, 12 (4), 3341–3350. 10.1021/acsnano.7b08673. [DOI] [PubMed] [Google Scholar]
- Teng X.; Dai Y.; Li J. Methodological advances of bioanalysis and biochemical targeting of intracellular G-quadruplexes. Exploration 2022, 2 (2), 20210214. 10.1002/EXP.20210214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou Assi H.; Garavis M.; Gonzalez C.; Damha M. J i-Motif DNA: structural features and significance to cell biology. Nucleic Acids Res. 2018, 46 (16), 8038–8056. 10.1093/nar/gky735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi-Karimata H.; Kawauchi K.; Sugimoto N. Destabilization of DNA G-Quadruplexes by chemical environment changes during tumor progression facilitates transcription. J. Am. Chem. Soc. 2018, 140 (2), 642–651. 10.1021/jacs.7b09449. [DOI] [PubMed] [Google Scholar]
- Idili A.; Vallee-Belisle A.; Ricci F. Programmable pH-triggered DNA nanoswitches. J. Am. Chem. Soc. 2014, 136 (16), 5836–5839. 10.1021/ja500619w. [DOI] [PubMed] [Google Scholar]
- Choi J.; Kim S.; Tachikawa T.; Fujitsuka M.; Majima T. pH-induced intramolecular folding dynamics of i-motif DNA. J. Am. Chem. Soc. 2011, 133 (40), 16146–16153. 10.1021/ja2061984. [DOI] [PubMed] [Google Scholar]
- He S.; Ge Z.; Zuo X.; Fan C.; Mao X. Dynamic regulation of DNA nanostructures by noncanonical nucleic acids. NPG Asia Mater. 2021, 13 (1), 14. 10.1038/s41427-021-00309-9. [DOI] [Google Scholar]
- Zhu J.; Kim Y.; Lin H.; Wang S.; Mirkin C. A. pH-responsive nanoparticle superlattices with tunable DNA bonds. J. Am. Chem. Soc. 2018, 140 (15), 5061–5064. 10.1021/jacs.8b02793. [DOI] [PubMed] [Google Scholar]
- Han M. S.; Lytton-Jean A. K. R.; Mirkin C. A. A gold nanoparticle based approach for screening triplex DNA binders. J. Am. Chem. Soc. 2006, 128 (15), 4954–4955. 10.1021/ja0606475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F.; Lu Q.; Huang L.; Liu B.; Liu M.; Zhang Y.; Liu J. DNA triplex and quadruplex assembled nanosensors for correlating K+ and pH in lysosomes. Angew. Chem., Int. Ed. Engl. 2021, 60 (10), 5453–5458. 10.1002/anie.202013302. [DOI] [PubMed] [Google Scholar]
- Lu J.; Sun J.; Li F.; Wang J.; Liu J.; Kim D.; Fan C.; Hyeon T.; Ling D. Highly sensitive diagnosis of small hepatocellular carcinoma using pH-responsive iron oxide nanocluster assemblies. J. Am. Chem. Soc. 2018, 140 (32), 10071–10074. 10.1021/jacs.8b04169. [DOI] [PubMed] [Google Scholar]
- Clever G. H.; Kaul C.; Carell T. DNA–metal base pairs. Angew. Chem., Int. Ed. Engl. 2007, 46 (33), 6226–6236. 10.1002/anie.200701185. [DOI] [PubMed] [Google Scholar]
- Huang D.; Niu C.; Wang X.; Lv X.; Zeng G. Turn-On” fluorescent sensor for Hg2+ based on single-stranded DNA functionalized Mn: CDs/ZnS quantum dots and gold nanoparticles by time-gated mode.. Anal. Chem. 2013, 85 (2), 1164–1170. 10.1021/ac303084d. [DOI] [PubMed] [Google Scholar]
- Iliuk A. B.; Hu L.; Tao W. A. Aptamer in bioanalytical applications. Anal. Chem. 2011, 83 (12), 4440–4452. 10.1021/ac201057w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S.; Wang L.; Li J.; Fan C.; Zhao J. Aptamer-based biosensors. Trends Analyt. Chem. 2008, 27 (2), 108–117. 10.1016/j.trac.2007.12.004. [DOI] [Google Scholar]
- Lee S. E.; Chen Q.; Bhat R.; Petkiewicz S.; Smith J. M.; Ferry V. E.; Correia A. L.; Alivisatos A. P.; Bissell M. J. Reversible aptamer-Au plasmon rulers for secreted single molecules. Nano Lett. 2015, 15 (7), 4564–4570. 10.1021/acs.nanolett.5b01161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medley C. D.; Smith J. E.; Tang Z.; Wu Y.; Bamrungsap S.; Tan W. Gold nanoparticle-based colorimetric assay for the direct detection of cancerous Cells. Anal. Chem. 2008, 80 (4), 1067–1072. 10.1021/ac702037y. [DOI] [PubMed] [Google Scholar]
- Huang Y. F.; Sefah K.; Bamrungsap S.; Chang H. T.; Tan W. Selective photothermal therapy for mixed cancer cells using aptamer-conjugated nanorods. Anal. Chem. 2008, 24 (20), 11860–11865. 10.1021/la801969c. [DOI] [PubMed] [Google Scholar]
- Smith J. E.; Medley C. D.; Tang Z.; Shangguan D.; Lofton C.; Tan W. Aptamer-conjugated nanoparticles for the collection and detection of multiple cancer cells. Anal. Chem. 2007, 79 (8), 3075–3082. 10.1021/ac062151b. [DOI] [PubMed] [Google Scholar]
- Herr J.; Smith J. E.; Medley C. D.; Shangguan D.; Tan W. Aptamer-conjugated nanoparticles for selective collection and detection of cancer cells. Anal. Chem. 2006, 78 (9), 2918–2924. 10.1021/ac052015r. [DOI] [PubMed] [Google Scholar]
- Liu J.; Lu Y. Preparation of aptamer-linked gold nanoparticle purple aggregates for colorimetric sensing of analytes. Nat. Protoc. 2006, 1 (1), 246–252. 10.1038/nprot.2006.38. [DOI] [PubMed] [Google Scholar]
- Liu J.; Lu Y. Non-base pairing DNA provides a new dimension for controlling aptamer-linked nanoparticles and sensors. J. Am. Chem. Soc. 2007, 129 (27), 8634–8643. 10.1021/ja072075+. [DOI] [PubMed] [Google Scholar]
- Yue R.; Zhang C.; Xu L.; Wang Y.; Guan G.; Lei L.; Zhang X.; Song G. Dual key co-activated nanoplatform for switchable MRI monitoring accurate ferroptosis-based synergistic therapy. Chem. 2022, 8 (7), 1956–1981. 10.1016/j.chempr.2022.03.009. [DOI] [Google Scholar]
- Seok Kim Y.; Ahmad Raston N. H.; Bock Gu M. Aptamer-based nanobiosensors. Biosens. Bioelectron. 2016, 76, 2–19. 10.1016/j.bios.2015.06.040. [DOI] [PubMed] [Google Scholar]
- Chen J.; Luo Z.; Sun C.; Huang Z.; Zhou C.; Yin S.; Duan Y.; Li Y. Research progress of DNA walker and its recent applications in biosensor. Trends Analyt. Chem. 2019, 120, 115626. 10.1016/j.trac.2019.115626. [DOI] [Google Scholar]
- Yehl K.; Mugler A.; Vivek S.; Liu Y.; Zhang Y.; Fan M.; Weeks E. R.; Salaita K. High-speed DNA-based rolling motors powered by RNase H. Nat. Nanotechnol. 2016, 11 (2), 184–190. 10.1038/nnano.2015.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claridge S. A.; Mastroianni A. J.; Au Y. B.; Liang H. W.; Micheel C. M.; Fréchet J. J. M.; Alivisatos A. P. Enzymatic ligation creates discrete multinanoparticle building blocks for self-assembly. J. Am. Chem. Soc. 2008, 130 (29), 9598–9605. 10.1021/ja8026746. [DOI] [PubMed] [Google Scholar]
- Kanaras A. G.; Wang Z.; Bates A. D.; Cosstick R.; Brust M. Towards multistep nanostructure synthesis: programmed enzymatic self-assembly of DNA/Gold systems. Angew. Chem., Int. Ed. Engl. 2003, 42 (2), 191–194. 10.1002/anie.200390075. [DOI] [PubMed] [Google Scholar]
- Nicewarner Peña S. R.; Raina S.; Goodrich G. P.; Fedoroff N. V.; Keating C. D. Hybridization and Enzymatic Extension of Au Nanoparticle-Bound Oligonucleotides. J. Am. Chem. Soc. 2002, 124 (25), 7314–7323. 10.1021/ja0177915. [DOI] [PubMed] [Google Scholar]
- Andreadis J. D.; Chrisey L. A. Use of immobilized PCR primers to generate covalently immobilized DNAs for in vitro transcription/translation reactions. Nucleic Acids Res. 2000, 28 (2), e5. 10.1093/nar/28.2.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaras A. G.; Wang Z.; Brust M.; Cosstick R.; Bates A. D. Enzymatic disassembly of DNA–gold nanostructures. Small 2007, 3 (4), 590–594. 10.1002/smll.200600494. [DOI] [PubMed] [Google Scholar]
- Kanaras A. G.; Wang Z.; Hussain I.; Brust M.; Cosstick R.; Bates A. D. Site-specific ligation of DNA-modified gold nanoparticles activated by the restriction enzyme StyI. Small 2007, 3 (1), 67–70. 10.1002/smll.200600464. [DOI] [PubMed] [Google Scholar]
- Yin P.; Yan H.; Daniell X. G.; Turberfield A. G.; Reif J. H. A unidirectional DNA walker that moves autonomously along a track. Angew. Chem., Int. Ed. Engl. 2004, 116 (37), 5014–5019. 10.1002/ange.200460522. [DOI] [PubMed] [Google Scholar]
- Bath J.; Green S. J.; Turberfield A. J. A free-running DNA motor powered by a nicking enzyme. Angew. Chem., Int. Ed. Engl. 2005, 117 (28), 4432–4435. 10.1002/ange.200501262. [DOI] [PubMed] [Google Scholar]
- Xu M.; Tang D. Recent advances in DNA walker machines and their applications coupled with signal amplification strategies: A critical review. Anal. Chim. Acta 2021, 1171, 338523. 10.1016/j.aca.2021.338523. [DOI] [PubMed] [Google Scholar]
- Wu N.; Wang K.; Wang Y. T.; Chen M. L.; Chen X. W.; Yang T.; Wang J. H. Three-dimensional DNA nanomachine biosensor by integrating DNA walker and rolling machine cascade amplification for ultrasensitive detection of cancer-related gene. Anal. Chem. 2020, 92 (16), 11111–11118. 10.1021/acs.analchem.0c01074. [DOI] [PubMed] [Google Scholar]
- Arndt G. M.; MacKenzie K. L. New prospects for targeting telomerase beyond the telomere. Nat. Rev. Cancer 2016, 16 (8), 508–524. 10.1038/nrc.2016.55. [DOI] [PubMed] [Google Scholar]
- Roake C. M.; Artandi S. E. Regulation of human telomerase in homeostasis and disease. Nat. Rev. Mol. Cell Biol. 2020, 21 (7), 384–397. 10.1038/s41580-020-0234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.; Shangguan L.; Liu Y.; Jiang L.; Zhang F.; Wei Y.; Zhang Y.; Qi Z.; Wang K.; Liu S. In situ detection and imaging of telomerase activity in cancer cell lines via disassembly of plasmonic core-satellites nanostructured probe. Anal. Chem. 2017, 89 (13), 7262–7268. 10.1021/acs.analchem.7b01882. [DOI] [PubMed] [Google Scholar]
- Ma W.; Fu P.; Sun M.; Xu L.; Kuang H.; Xu C. Dual quantification of micrornas and telomerase in living cells. J. Am. Chem. Soc. 2017, 139 (34), 11752–11759. 10.1021/jacs.7b03617. [DOI] [PubMed] [Google Scholar]
- Ran X.; Wang Z.; Pu F.; Ju E.; Ren J.; Qu X. Nucleic acid-driven aggregation-induced emission of Au nanoclusters for visualizing telomerase activity in living cells and in vivo. Mater. Horiz. 2021, 8 (6), 1769–1775. 10.1039/D0MH01875A. [DOI] [PubMed] [Google Scholar]
- Sheng C.; Zhao J.; Di Z.; Huang Y.; Zhao Y.; Li L. Spatially resolved in vivo imaging of inflammation-associated mRNA via enzymatic fluorescence amplification in a molecular beacon. Nat. Biomed. Eng. 2022, 6 (9), 1074–1084. 10.1038/s41551-022-00932-z. [DOI] [PubMed] [Google Scholar]
- Shao Y.; Zhao J.; Yuan J.; Zhao Y.; Li L. Organelle-specific photoactivation of DNA nanosensors for precise profiling of subcellular enzymatic activity. Angew. Chem., Int. Ed. Engl. 2021, 60 (16), 8923–8931. 10.1002/anie.202016738. [DOI] [PubMed] [Google Scholar]
- Xiang Z.; Zhao J.; Yi D.; Di Z.; Li L. Peptide nucleic acid (PNA)-guided peptide engineering of an aptamer sensor for protease-triggered molecular imaging. Angew. Chem., Int. Ed. Engl. 2021, 60 (42), 22659–22663. 10.1002/anie.202106639. [DOI] [PubMed] [Google Scholar]
- McConnell E. M.; Cozma I.; Mou Q.; Brennan J. D.; Lu Y.; Li Y. Biosensing with DNAzymes. Chem. Soc. Rev. 2021, 50 (16), 8954–8994. 10.1039/D1CS00240F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner I.; Shlyahovsky B.; Zayats M.; Willner B. DNAzymes for sensing, nanobiotechnology and logic gate applications. Chem. Soc. Rev. 2008, 37 (6), 1153–1165. 10.1039/b718428j. [DOI] [PubMed] [Google Scholar]
- Liu J.; Wernette D. P.; Lu Y. Proofreading and error removal in a nanomaterial assembly. Angew. Chem., Int. Ed. Engl. 2005, 44 (44), 7290–7293. 10.1002/anie.200501815. [DOI] [PubMed] [Google Scholar]
- Liu J.; Lu Y. A colorimetric lead biosensor using DNAzyme-directed assembly of gold nanoparticles. J. Am. Chem. Soc. 2003, 125 (22), 6642–6643. 10.1021/ja034775u. [DOI] [PubMed] [Google Scholar]
- Chen J.; Pan J.; Chen S. A naked-eye colorimetric sensor for Hg (2+) monitoring with cascade signal amplification based on target-induced conjunction of split DNAzyme fragments. Chem. Commun. 2017, 53 (73), 10224–10227. 10.1039/C7CC05445A. [DOI] [PubMed] [Google Scholar]
- Mazumdar D.; Liu J.; Lu G.; Zhou J.; Lu Y. Easy-to-use dipstick tests for detection of lead in paints using non-cross-linked gold nanoparticle-DNAzyme conjugates. Chem. Commun. 2010, 46 (9), 1416–1418. 10.1039/b917772h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z.; Huang J.; Lie P.; Xiao Z.; Ouyang C.; Wu Q.; Wu Y.; Liu G.; Zeng L. Lateral flow nucleic acid biosensor for Cu2+ detection in aqueous solution with high sensitivity and selectivity. Chem. Commun. 2010, 46 (47), 9043–9045. 10.1039/c0cc02782k. [DOI] [PubMed] [Google Scholar]
- Zhang Y.; Xu J.; Zhou S.; Zhu L.; Lv X.; Zhang J.; Zhang L.; Zhu P.; Yu J. DNAzyme-triggered visual and ratiometric electrochemiluminescence dual-readout assay for Pb(II) based on an assembled paper device. Anal. Chem. 2020, 92 (5), 3874–3881. 10.1021/acs.analchem.9b05343. [DOI] [PubMed] [Google Scholar]
- Xu J.; Zhang Y.; Li L.; Kong Q.; Zhang L.; Ge S.; Yu J. Colorimetric and electrochemiluminescence dual-mode sensing of lead ion based on integrated lab-on-paper device. ACS Appl. Mater. Interfaces 2018, 10 (4), 3431–3440. 10.1021/acsami.7b18542. [DOI] [PubMed] [Google Scholar]
- Xu L.; Yin H.; Ma W.; Wang L.; Kuang H.; Xu C. MRI biosensor for lead detection based on the DNAzyme-induced catalytic reaction. J. Phys. Chem. B 2013, 117 (46), 14367–14371. 10.1021/jp4087656. [DOI] [PubMed] [Google Scholar]
- Yin H.; Kuang H.; Liu L.; Xu L.; Ma W.; Wang L.; Xu C. A ligation DNAzyme-induced magnetic nanoparticles assembly for ultrasensitive detection of copper ions. ACS Appl. Mater. Interfaces 2014, 6 (7), 4752–4757. 10.1021/am405482a. [DOI] [PubMed] [Google Scholar]
- Ma L.; Liu J. Catalytic Nucleic Acids: Biochemistry, Chemical Biology, Biosensors, and Nanotechnology. iScience 2020, 23 (1), 100815. 10.1016/j.isci.2019.100815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha T. G.; Pan J.; Chen H.; Salgado J.; Li X.; Mao C.; Choi J. H. A synthetic DNA motor that transports nanoparticles along carbon nanotubes. Nat. Nanotechnol. 2014, 9 (1), 39–43. 10.1038/nnano.2013.257. [DOI] [PubMed] [Google Scholar]
- Xin L.; Zhou C.; Duan X.; Liu N. A rotary plasmonic nanoclock. Nat. Commun. 2019, 10 (1), 5394. 10.1038/s41467-019-13444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh H. C.; Sharma J.; Han J. J.; Martinez J. S.; Werner J. H. A DNA–silver nanocluster probe that fluoresces upon hybridization. Nano Lett. 2010, 10 (8), 3106–3110. 10.1021/nl101773c. [DOI] [PubMed] [Google Scholar]