Abstract
BACKGROUND:
Laparoscopic resection for colon cancer has not been associated with improvements in oncological outcomes in comparison to open resection. Robotic resections are associated with increased lymph node yield and radicality of mesenteric resection in patients with right-sided tumors. It is unclear whether lymph node yield is higher in robotic resections in other parts of the colon and whether higher lymph node yield is associated with improved survival.
OBJECTIVE:
To compare survival rates between robotic, laparoscopic, and open resections in a large cohort of patients with nonmetastatic colon cancer.
DESIGN:
This is a retrospective observational study.
SETTING:
This study was conducted at a single comprehensive cancer center.
PATIENTS:
Patients who underwent resection of nonmetastatic primary colon cancer between January 2006 and December 2018.
MAIN OUTCOME MEASURES:
Univariable and multivariable models were used to identify predictors of disease-free and overall survival. Lymph node yield and perioperative outcomes were compared between operative approaches.
RESULTS:
There were 2398 patients who met the inclusion criteria: 699 (29%) underwent open, 824 (34%) underwent laparoscopic, and 875 (36%) underwent robotic resection. Median follow-up was 3.8 years (45.4 months). Robotic surgery was associated with higher lymph node yield and radicality of mesenteric resection. On multivariable analysis, surgical approach was not associated with a difference in disease-free or overall survival. Minimally invasive colectomy was associated with fewer complications and shorter length of stay in comparison to open surgery. In a direct comparison between the two minimally invasive approaches, robotic colectomy was associated with fewer complications, shorter length of stay and lower conversion rate than laparoscopy.
LIMITATIONS:
This was a single-center retrospective study.
CONCLUSIONS:
Our data indicate that the three surgical approaches are similarly effective in treating primary resectable colon cancer and that differences in outcomes are seen primarily in the early postoperative period. See Video Abstract at http://links.lww.com/DCR/Bxxx.
Abstract
ANTECEDENTES:
La resección laparoscópica para el cáncer de colon no se ha asociado con mejoras en los resultados oncológicos en comparación con la resección abierta. Las resecciones robóticas se asocian con un mayor rendimiento de los ganglios linfáticos y la radicalidad de la resección mesentérica en pacientes con tumores del lado derecho. No está claro si el rendimiento de los ganglios linfáticos es mayor en las resecciones robóticas en otras partes del colon y si un mayor rendimiento de los ganglios linfáticos se asocia con una mejor supervivencia.
OBJETIVO:
Comparar las tasas de supervivencia entre resecciones robóticas, laparoscópicas y abiertas en una gran cohorte de pacientes con cáncer de colon no metastásico.
DISEÑO:
Este es un estudio observacional retrospectivo.
AJUSTE:
Este estudio se realizó en un único centro oncológico integral.
PACIENTES:
Pacientes que se sometieron a resección de cáncer de colon primario no metastásico entre enero de 2006 y diciembre de 2018.
PRINCIPALES MEDIDAS DE RESULTADO:
Se utilizaron modelos univariables y multivariables para identificar predictores de supervivencia libre de enfermedad y global. El rendimiento de los ganglios linfáticos y los resultados perioperatorios se compararon entre los enfoques operativos.
RESULTADOS:
Hubo 2398 pacientes que cumplieron con los criterios de inclusión: 699 (29%) se sometieron a cirugía abierta, 824 (34%) se sometieron a resección laparoscópica y 875 (36%) se sometieron a resección robótica. La mediana de seguimiento fue de 3,8 años (45,4 meses). La cirugía robótica se asoció con un mayor rendimiento de los ganglios linfáticos y la radicalidad de la resección mesentérica. En el análisis multivariable, el abordaje quirúrgico no se asoció con una diferencia en la supervivencia general o libre de enfermedad. La colectomía mínimamente invasiva se asoció con menos complicaciones y una estancia más corta en comparación con la cirugía abierta. En una comparación directa entre los dos enfoques mínimamente invasivos, la colectomía robótica se asoció con menos complicaciones, una estancia más corta y una tasa de conversión más baja que la laparoscopia.
LIMITACIONES:
Este fue un estudio retrospectivo de un solo centro.
CONCLUSIONES:
Nuestros datos indican que los tres enfoques quirúrgicos son igualmente efectivos en el tratamiento del cáncer de colon resecable primario y que las diferencias en los resultados se observan principalmente en el período posoperatorio temprano. Consulte Video Resumen en http://links.lww.com/DCR/Bxxx. (Pre-proofed version)
INTRODUCTION
Several large randomized control trials (RCTs) demonstrated that laparoscopic surgery for colon cancer is associated with better perioperative outcomes and similar long-term survival outcomes in comparison with open surgery.1–8 A small RCT recently did not find a meaningful difference in survival outcomes between robotic and laparoscopic colectomies,9 but it is unlikely that a large RCT will be conducted to compare long-term survival outcomes between open, laparoscopic, and robotic surgical approaches in colon cancer patients, because of the widespread adoption of minimally invasive surgery. Well annotated, large retrospective comparative studies can bridge this gap in our ability to compare the efficacies of different surgical approaches in the treatment of colon cancer. Such studies can provide the power, which is often hard to accrue in prospective trials, to detect small differences in survival and such studies can also provide the granularity (e.g. information on recurrence), which is often absent from big data studies, like those based on National Cancer Database (NCDB) data. Previous retrospective studies that compared survival outcomes between operative approaches obtained mixed results.10–14
A more radical mesenteric resection of stage I–III colon cancer may be associated with improved survival.15,16 Our group previously demonstrated that robotic resections of right-colon tumors are associated with more radical mesenteric resection and higher lymph node yield (LNY) in comparison with open and laparoscopic resections.17 It is unclear whether the robotic approach is associated with higher LNY and more radical mesenteric resection in all tumor locations and whether differences in LNY and radicality of mesenteric resection translate into longer survival.
The aim of this study was to compare long-term survival between robotic, laparoscopic, and open surgeries in a large and well annotated cohort of nonmetastatic colon cancer patients. Our hypothesis was that robotic resection is associated with a higher LNY and more radical mesenteric resection, and therefore longer survival. We evaluated the following outcomes: overall survival (OS), disease-free survival (DFS), LNY, and radicality of mesenteric resection. Short-term outcomes, such as 90-day postoperative complications, length of stay (LOS), and conversion rates of minimally invasive resections, were also evaluated.
MATERIALS AND METHODS
Patients
With a waiver of informed consent and approval from the institutional review board, prospectively maintained institutional databases were queried to identify patients with stage I–III primary colon adenocarcinoma who underwent curative resection at Memorial Sloan Kettering Cancer Center between January 1, 2006, and December 31, 2018. Patients with rectal cancer (≤12 cm from the anal verge) or metachronous colon cancer within 5 years prior to their colectomy were excluded. Patients with previous history of non-colorectal cancer in the previous 5 years were also excluded.
Resection
Available data on surgical procedures included operative approach, extent of resection, and conversion from minimally invasive surgery to open surgery. The determination of the extent of the resection and the choice of operative approach were made at each surgeon’s discretion. The method of anastomosis and extraction site depended on the surgeon’s preference. Operative approach was categorized according to intention to treat, and patients whose minimally invasive resection was converted to open surgery were categorized as having undergone minimally invasive surgery. Right, left, and transverse colectomies were considered segmental resections. Subtotal and total colectomies were considered extended resections.
Staging and Surveillance
Preoperative staging included colonoscopy and contrast-enhanced computed tomography of the chest, abdomen, and pelvis. Adjuvant chemotherapy was administered and surveillance was conducted according to the guidelines of the National Comprehensive Cancer Network. Recurrence was identified on the basis of radiologic, endoscopic or histological/biopsy evidence.
Outcomes
Data on LOS, 90-day postoperative complications, and rates of conversion to open surgery were calculated. Postoperative complications were classified using the Clavien-Dindo index.18 Lymphadenectomy data included LNY, number of positive nodes, and specimen length. The ratio of LNY to specimen length was calculated as an indicator of the radicality of the lymphadenectomy, as described previously.17 OS and DFS were calculated from the date of surgery. Events for DFS were local recurrence, distant recurrence, or death.
Statistical Analysis
Categorical variables are presented as frequencies and percentages, and continuous variables are presented as means and ranges. Groups were compared using the chi-square test of independence, Fisher exact test for categorical variables and the Kruskal-Wallis test for continuous variables. Survival statistics were estimated using the Kaplan-Meier method and compared using the log rank test. A Cox proportional-hazards model was used to evaluate the association between baseline characteristics and survival. Variables found to have a significant association with survival were included in a multivariable model to identify independent predictors. Statistical significance was defined as P < 0.05. All analyses were performed using R software version 3.6.2.
RESULTS
Surgical Approaches
Of the 2398 patients who met the inclusion criteria, 699 (29%) underwent open resection, 824 (34%) underwent laparoscopic resection, and 875 (36%) underwent robotic resection (Table 1). On average, patients in the open surgery group were older, had worse physical status and lower baseline albumin levels. They were also more likely to have had a history of cancer, to have an advanced T stage, to have a high baseline level of carcinoembryonic antigen, and to have undergone an extended resection. Median follow-up was 3.8 years (45.4 months) for the full cohort, 5.1 years (61.6 months) for the open surgery group, 5.0 years (60.5 months) for the laparoscopic surgery group, and 2.5 years (30 months) for the robotic surgery group. During the period examined, the relative frequency of each surgical approach changed substantially (Fig. 1).
TABLE 1.
Baseline, Procedure, and Tumor Characteristics
| Characteristic (N) | No. of Patients (%) | P a | ||
|---|---|---|---|---|
| Open, N = 699 | Laparoscopic, N = 824 | Robotic, N = 875 | ||
| Age (2398)b | 66 (26–99) yr | 64 (18–94) yr | 62 (23–100) yr | <0.001 |
| Sex (2398) | 0.088 | |||
| Female | 369/699 (53) | 391/824 (47) | 423/875 (48) | |
| Male | 330/699 (47) | 433/824 (53) | 452/875 (52) | |
| Race (2328) | 0.046 | |||
| Asian | 40/683 (5.9) | 47/811 (5.8) | 65/834 (7.8) | |
| Black | 61/683 (8.9) | 48/811 (5.9) | 47/834 (5.6) | |
| White | 572/683 (84) | 709/811 (87) | 709/834 (85) | |
| Other | 10/683 (1.5) | 7/811 (0.9) | 13/834 (1.6) | |
| Ethnicity (2164) | 0.093 | |||
| Hispanic or Latino | 39/576 (6.8) | 30/729 (4.1) | 51/859 (5.9) | |
| Not Hispanic | 537/576 (93) | 699/729 (96) | 808/859 (94) | |
| History of cancer (2398) | 124/699 (18) | 79/824 (9.6) | 76/875 (8.7) | <0.001 |
| ASA (2398) | <0.001 | |||
| 1 | 10/699 (1.4) | 14/824 (1.7) | 3/875 (0.3) | |
| 2 | 211/699 (30) | 298/824 (36) | 291/875 (33) | |
| 3 | 436/699 (62) | 481/824 (58) | 557/875 (64) | |
| 4 | 42/699 (6.0) | 31/824 (3.8) | 24/875 (2.7) | |
| BMI (2376)b | 28.5 (12.9–53.9) | 29.2 (15.8–62.5) | 28.7 (14.9–54.3) | 0.3 |
| CEA (2139) | <0.001 | |||
| <5 ng/mL | 387/615 (63) | 538/740 (73) | 572/784 (73) | |
| ≥5 ng/mL | 228/615 (37) | 202/740 (27) | 212/784 (27) | |
| Albumin (2353)b | 3.97 (1.70–5.20) g/dL | 4.18 (2.50–5.10) g/dL | 4.16 (2.70–5.20) g/dL | <0.001 |
| N category (2398) | 0.2 | |||
| 0 | 467/699 (67) | 565/824 (69) | 557/875 (64) | |
| 1 | 157/699 (22) | 187/824 (23) | 224/875 (26) | |
| 2 | 75/699 (11) | 72/824 (8.7) | 94/875 (11) | |
| T category (2398) | <0.001 | |||
| CIS or 1 | 98/699 (14) | 192/824 (23) | 192/875 (22) | |
| 2 | 93/699 (13) | 124/824 (15) | 136/875 (16) | |
| 3 | 387/699 (55) | 438/824 (53) | 464/875 (53) | |
| 4 | 121/699 (17) | 70/824 (8.5) | 83/875 (9.5) | |
| Tumor location (2398) | 0.004 | |||
| Left colon | 288/699 (41) | 319/824 (39) | 418/875 (48) | |
| Mid-transverse colon | 59/699 (8.4) | 71/824 (8.6) | 64/875 (7.3) | |
| Right colon | 352/699 (50) | 434/824 (53) | 393/875 (45) | |
| Colectomy (2398) | <0.001 | |||
| Extended | 67/699 (9.6) | 45/824 (5.5) | 25/875 (2.9) | |
| Segmental | 632/699 (90) | 779/824 (95) | 850/875 (97) | |
| Chemotherapy (915) | 268/699 (38) | 294/824 (36) | 353/875 (40) | 0.1 |
| Tumor grade (2318) | 0.6 | |||
| Low | 542/678 (80) | 641/788 (81) | 698/852 (82) | |
| High | 136/678 (20) | 147/788 (19) | 154/852 (18) | |
Kruskal-Wallis test, chi-square test of independence, or Fisher exact test.
Mean (minimum–maximum).
ASA = American Society of Anesthesiologists classification of physical status; BMI = body mass index; CEA = carcinoembryonic antigen; CIS = intramucosal carcinoma in situ.
Figure 1.
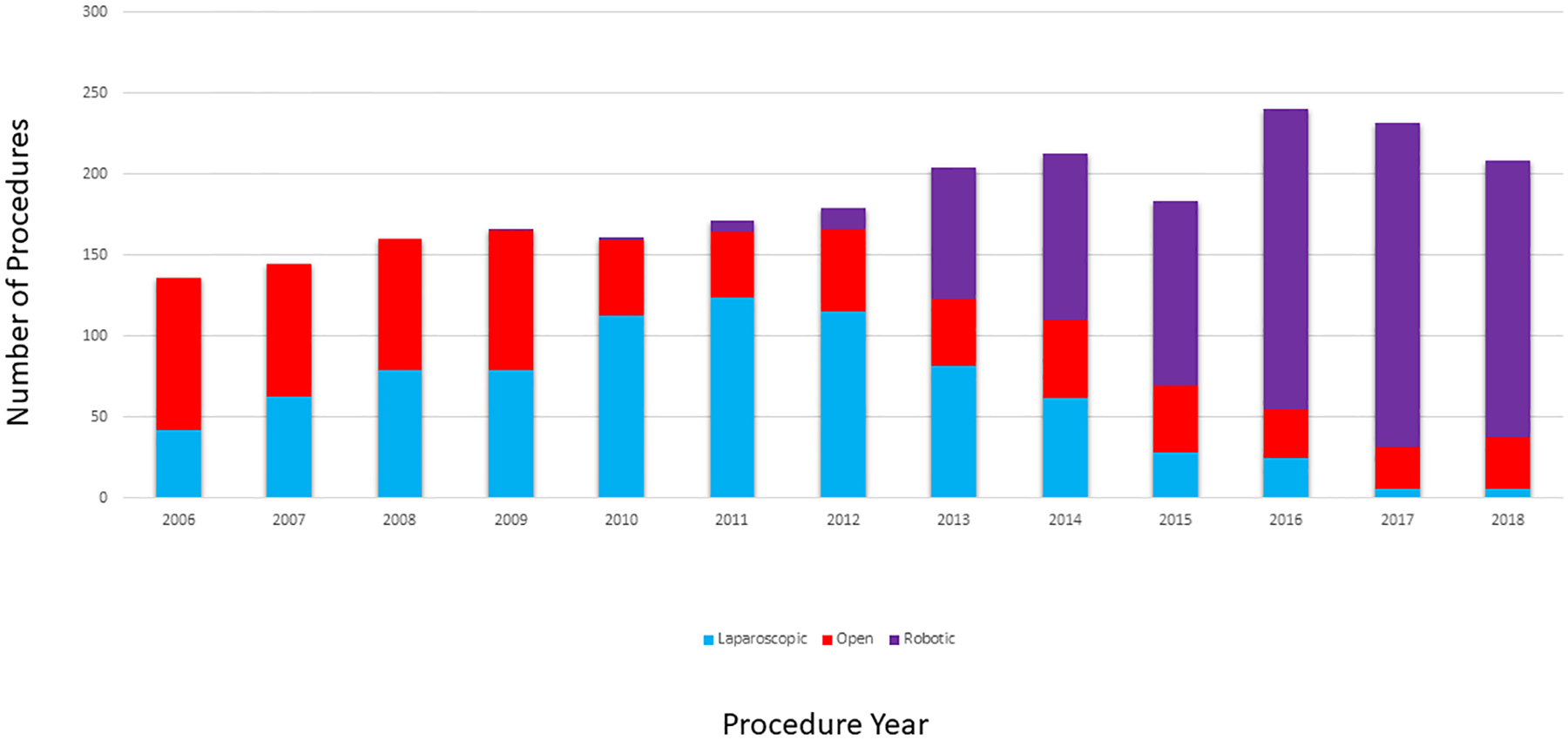
Open, laparoscopic, and robotic colectomies performed at Memorial Sloan Kettering in 2006–2018.
Short-term Outcomes
The 90-day rate of postoperative mortality for the full cohort was 0.63% (15 of 2398 patients). Open resections had a 90-day postoperative mortality rate of 1.1% (8 of 699 patients), laparoscopic resections had a rate of 0.12% (1 of 824 patients), and robotic resections had a rate of 0.69% (6 of 875 patients) (p = 0.40). Data on postoperative complications in the first 90 days were available for 2366 (99%) of 2398 patients. The 90-day rate of postoperative complications was 14.4% (341 of 2366 patients); the rate of severe complications (Clavien-Dindo grade 3 or higher) was 3.7% (88 of 2366 patients). The overall rate of complications of any grade and the rate of severe complications were significantly higher for open resections compared to laparoscopic or robotic resections (p < 0.001, Table 2).
TABLE 2.
Short-Term Outcomes
| Outcome (N) | No. of Patients (%) | P a | ||
|---|---|---|---|---|
| Open, N = 699 | Laparoscopic, N = 824 | Robotic, N = 875 | ||
| Complications (2366) | 147/690 (21) | 116/813 (14) | 78/863 (9.0) | <0.001 |
| Severe complications (2366)b | 45/690 (6.5) | 20/813 (2.5) | 23/863 (2.7) | <0.001 |
| Length of stay (2398) | 8.8 ± 6.8 days | 6.1 ± 3.4 days | 4.2 ± 3.9 days | <0.001 |
| Conversion (1699) | 115/824 (14) | 20/875 (2.3) | <0.001 | |
| Operative time (2096) | 2.9 ± 1.5 hours | 3.1 ± 1.2 hours | 3.6 ± 1.3 hours | 0.03 |
| Estimated Blood Loss (1929) | 167 ± 315 ml | 97 ± 158 ml | 60 ± 92 ml | <0.001 |
Kruskal-Wallis test, chi-square test of independence, or Fisher exact test.
Clavien-Dindo grade ≥3.
Detailed data on surgical site infections were available for 2067 (86%) of the 2398 patients. The rate of surgical site infections (SSI) was 6.1% for the full cohort (126 of 2067 patients with data available), 9.9% for open resections (53 of 533 patients), 7.0% for laparoscopic resections (51 of 726 patients), and 2.7% for robotic resections (22 of 808 patients) (p < 0.001). In November 2013 an SSI bundle was implemented at our institution.19 For patients undergoing surgery before the SSI bundle, the rate of surgical site infection was 9.0% for the full cohort (94 of 1039 patients with available data), 10.5% for open resections (38 of 363 patients), 8.1% for laparoscopic resections (49 of 603 patients), and 9.6% for robotic resections (7 of 73 patients) (P = 0.46). For patients undergoing surgery after the SSI bundle, the rate of surgical site infections was 3.1% for the full cohort (32 of 1028 patients with available data), 8.8% for open resections (15 of 170 patients), 1.6% for laparoscopic resections (2 of 123 patients), and 2.0% for robotic resections (15 of 735 patients) (p < 0.001).
Mean LOS was 6.2 ± 5.1 (range, 1–84) days for the full cohort, 8.8 ± 6.8 (range, 2–84) days for the open surgery group, 6.1 ± 3.4 (range, 2–39) days for the laparoscopic surgery group, and 4.2 ± 3.9 (range, 1–83) days for the robotic surgery group (p < 0.001). In January 2016 an enhanced recovery after surgery (ERAS) program was initiated at our institution.20 Before ERAS (1718 patients), mean LOS was 6.9 ± 5.3 (range, 2–84) days for the full cohort, 8.9 ± 6.8 (range, 2–84) days for the open surgery group, 6.1 ± 3.3 (range, 2–39) days for the laparoscopic surgery group, and 5.2 ± 5.1 (range, 2–83) days for the robotic surgery group (p < 0.001). After ERAS (680 patients), mean LOS was 4.3 ± 4.0 (range, 1–59) days for the full cohort, 8.1 ± 7.1 (range, 3–59) days for the open surgery group, 5.8 ± 4.2 (range, 2–21) days for the laparoscopic surgery group, and 3.6 ± 2.9 (range, 1–40) days for the robotic surgery group (p < 0.001).
Operative time was shortest in open resections (2.9 ± 1.5 hours), longer in laparoscopic resections (3.1 ± 1.2 hours) and longest in robotic resections (3.6 ± 1.3 hours) (p = 0.03; Table 2). Estimated blood loss was lowest in robotic resections (60 ± 92 ml), higher in laparoscopic resections (97 ± 158 ml) and highest in open resections (167 ± 315 ml) (p < 0.001; Table 2).
In a subgroup analysis comparing the two minimally invasive approaches, robotic resection was associated with a lower overall rate of complications (14% vs. 9.0%, p < 0.001), a lower rate of surgical site infections (7.0% vs. 2.7%, p < 0.001), shorter LOS (6.08 vs. 4.18 days, p < 0.001), and a lower conversion rate (14% vs. 2.3%, p < 0.001), but the two minimally invasive approaches had comparable 90-day postoperative mortality rates and comparable rates of severe complications.
Of the 1699 patients that underwent a minimally invasive colectomy, 135 (7.9%) underwent conversion to open surgery. The conversion rate was 14.0% (115 of 824 patients) for laparoscopic resections and 2.3% (20 of 875) for robotic resections (p < 0.001) (Table 2). The complication rate in patients who underwent a minimally invasive colectomy that was converted was 21%, and the complication rate in patients who did not undergo conversion was 11% (p < 0.001). The SSI rate in patients who underwent conversion was 10%, and the SSI rate in patients who did not undergo conversion was 4.3% (p = 0.002). Patients who underwent a conversion had a mean LOS of 7.3 ± 3.8 days, and patients that did not had a mean LOS of 4.9 ± 3.0 days (p < 0.001). There was no meaningful difference in severe complications between patients whose surgery was converted and those whose surgery was not (Supplemental Table at https://links.lww.com/DCR/CXX). The most common reasons for conversion in both laparoscopic and robotic resections were poor exposure (70% of laparoscopic and 55% of robotic conversions) and tumor involvement of surrounding structures (15% of laparoscopic and 55% of robotic conversions). Less common reasons included bleeding (6% of laparoscopic and 5% of robotic conversions), poor tolerance to insufflation (3.5% of laparoscopic and 5% of robotic conversions), inability to find the tumor (3.5% of laparoscopic and 0% of robotic conversions), trouble with the anastomosis (0.08% of laparoscopic and 5% of robotic conversions), and unclear causes (0.08% of laparoscopic and 5% of robotic conversions).
Robotic resections had the highest mean LNY and the highest ratio of LNY to specimen length (Table 3). There was no meaningful difference between operative approaches in the rate of LNY < 12 or positive margins.
TABLE 3.
Lymph Node Yield and Margin Status
| Outcome (N) | No. of Patients (%) | P a | ||
|---|---|---|---|---|
| Open, N = 699 | Laparoscopic, N = 824 | Robotic, N = 875 | ||
| LNY (2397) | 26 ± 15 nodes | 25 ± 12 nodes | 28 ± 15 nodes | <0.001 |
| LNY/specimen length (2396) | 0.79 ± 0.44 | 0.90 ± 0.47 | 1.06 ± 0.55 | <0.001 |
| LNY < 12 | 30/698 (4.3) | 39/824 (4.7) | 26/875 (3.0) | 0.200 |
| Positive Margins | 6/699 (0.9) | 2/824 (0.2) | 1/875 (0.1) | 0.066 |
Kruskal-Wallis test, chi-square test of independence, or Fisher exact test.
Clavien-Dindo grade ≥3.
LNY = lymph node yield.
Survival
There were 875 stage II patients, of whom 225 (25.7%) received adjuvant chemotherapy. Of these patients, 118 (52.4%) received multiagent treatment. There were 809 stage III patients, of whom 669 (82.7%) received adjuvant chemotherapy. Of these patients, 575 (85.9%) received multiagent treatment.
There were 499 (20.8%) patients that experienced a DFS event during follow-up. These events included 69 local recurrences, 225 distant recurrences and 205 deaths without recurrence. Estimated DFS for the full cohort was 84% at 3 years and 77% at 5 years. Estimated DFS in the open, laparoscopic, and robotic resection groups was 79%, 86%, and 89%, respectively, at 3 years and 70%, 80%, and 79%, respectively, at 5 years (Fig. 2). In univariable analysis, the open approach was associated with shorter DFS (hazard ratio, 1.34; 95% CI, 1.09–1.63; p = 0.004) in comparison with the laparoscopic approach, but the difference was not significant in multivariable analysis. No significant association with DFS was noted when comparing robotic and laparoscopic resections. Independent factors associated with shorter DFS were older age, history of other cancer, carcinoembryonic antigen ≥ 5 ng/mL, T4 tumor category, and increasing N stage (Table 4).
Figure 2.

Unadjusted disease-free survival in relation to surgical approach and disease stage (tumor-node staging of the National Comprehensive Cancer Network).
TABLE 4.
Univariable and Multivariable Analyses for Association with Disease-Free Survival
| Characteristic (N) | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (2398) | 1.04 (1.04–1.05) | <0.001 | 1.04 (1.03–1.05) | <0.001 |
| Sex (2398) | ||||
| Female | — | |||
| Male | 1.02 (0.86–1.22) | 0.8 | ||
| Race (2328) | ||||
| Asian | — | — | ||
| Black | 1.71 (1.03–2.84) | 0.038 | 1.20 (0.69–2.07) | 0.5 |
| White | 1.41 (0.93–2.14) | 0.11 | 1.08 (0.70–1.67) | 0.7 |
| Other | 0.61 (0.14–2.58) | 0.5 | 0.51 (0.12–2.21) | 0.4 |
| Ethnicity (2164) | ||||
| Hispanic or Latino | — | |||
| Not Hispanic | 1.52 (0.91–2.55) | 0.11 | ||
| History of cancer (2398) | ||||
| No | — | — | ||
| Yes | 1.55 (1.23–1.96) | <0.001 | 1.32 (1.01–1.72) | 0.039 |
| ASA (2398) | ||||
| 1 | — | — | ||
| 2 | 1.55 (0.49–4.88) | 0.5 | 0.67 (0.21–2.13) | 0.5 |
| 3 | 3.28 (1.05–10.2) | 0.041 | 1.01 (0.32–3.22) | >0.9 |
| 4 | 6.44 (1.99–20.9) | 0.002 | 1.42 (0.42–4.80) | 0.6 |
| BMI (2376) | 1.00 (0.99–1.02) | >0.9 | ||
| CEA level (2139) | ||||
| <5 | — | — | ||
| ≥5 | 1.67 (1.38–2.01) | <0.001 | 1.32 (1.07–1.62) | 0.009 |
| Albumin level (2353) | 0.56 (0.46–0.68) | <0.001 | 0.86 (0.67–1.10) | 0.2 |
| Tumor location (2398) | ||||
| Left colon | — | — | ||
| Mid-transverse colon | 1.02 (0.72–1.43) | >0.9 | 0.75 (0.51–1.12) | 0.2 |
| Right colon | 1.38 (1.14–1.66) | <0.001 | 1.05 (0.85–1.30) | 0.6 |
| Colectomy extent (2398) | ||||
| Extended | — | |||
| Segmental | 1.24 (0.85–1.82) | 0.3 | ||
| Tumor grade (2318) | ||||
| Low | — | — | ||
| High | 1.51 (1.24–1.86) | <0.001 | 1.11 (0.88–1.40) | 0.4 |
| N category (2398) | ||||
| 0 | — | — | ||
| 1 | 1.51 (1.23–1.85) | <0.001 | 1.62 (1.28–2.04) | <0.001 |
| 2 | 2.51 (1.98–3.18) | <0.001 | 2.66 (2.00–3.54) | <0.001 |
| T category (2398) | ||||
| CIS or 1 | — | — | ||
| 2 | 0.99 (0.68–1.46) | >0.9 | 1.02 (0.66–1.58) | >0.9 |
| 3 | 1.51 (1.13–2.01) | 0.005 | 1.23 (0.86–1.75) | 0.3 |
| 4 | 2.99 (2.16–4.13) | <0.001 | 1.95 (1.29–2.94) | 0.002 |
| Procedure yr (2398) | ||||
| 2006–2009 | — | |||
| 2010–2014 | 1.03 (0.83–1.27) | 0.8 | ||
| 2015–2018 | 1.16 (0.90–1.50) | 0.2 | ||
| Colectomy approach (2398) | ||||
| Laparoscopic | — | — | ||
| Open | 1.34 (1.09–1.63) | 0.004 | 1.05 (0.84–1.32) | 0.7 |
| Robotic | 0.94 (0.73–1.20) | 0.6 | 1.13 (0.86–1.48) | 0.4 |
HR = hazard ratio; CI = confidence interval; ASA = American Society of Anesthesiologists classification of physical status; BMI = body mass index; CEA = carcinoembryonic antigen; CIS = intramucosal carcinoma in situ.
There were 353 patients (14.7%) that died during follow-up. Estimated OS for the full cohort was 92% at 3 years and 85% at 5 years. Estimated OS in the open, laparoscopic, and robotic resection groups was 87%, 93%, and 96%, respectively, at 3 years and 78%, 87%, and 90%, respectively, at 5 years (Fig. 3). In univariable analysis, the open approach was associated with worse OS (hazard ratio, 1.32; 95% CI, 1.05–1.65; p = 0.02) and the robotic approach was associated with longer OS (hazard ratio, 0.63; 95% confidence interval, 0.44–0.89; p = 0.009) in comparison with the laparoscopic approach. However, the differences were not significant in multivariable analysis. Independent factors associated with shorter OS were older age, history of other cancer, and increasing N stage (Table 5).
Figure 3.
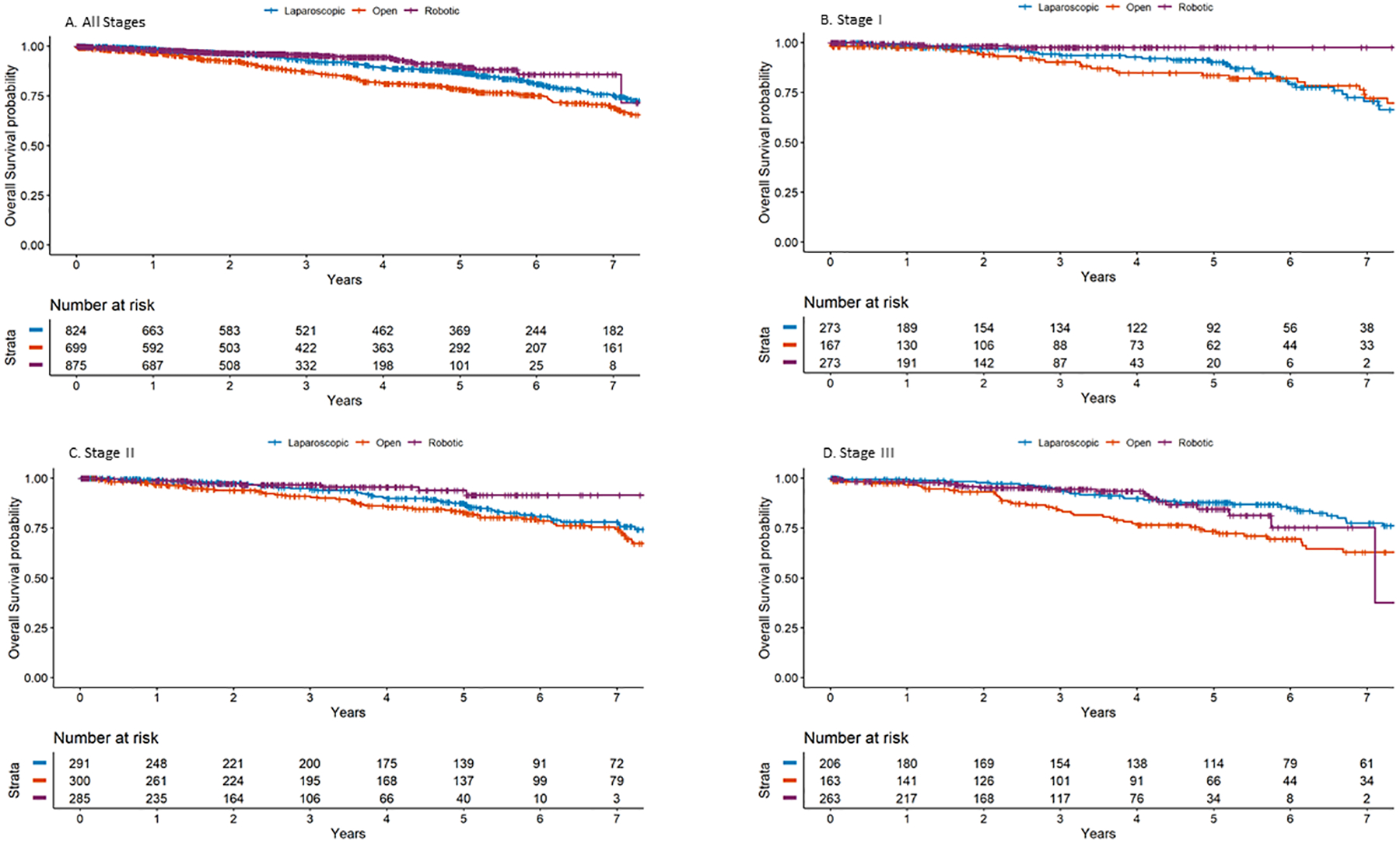
Unadjusted overall survival in relation to surgical approach and disease stage (tumor-node staging of the National Comprehensive Cancer Network).
TABLE 5.
Univariable and Multivariable Analyses for Association with Overall Survival
| Characteristic (N) | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (2398) | 1.08 (1.07–1.09) | <0.001 | 1.07 (1.06–1.09) | <0.001 |
| Sex (2398) | ||||
| Female | — | |||
| Male | 1.14 (0.92–1.40) | 0.2 | ||
| Race (2328) | ||||
| Asian | — | — | ||
| Black | 2.72 (1.23–6.00) | 0.013 | 1.66 (0.73–3.80) | 0.2 |
| White | 2.99 (1.48–6.03) | 0.002 | 1.84 (0.90–3.74) | 0.093 |
| Other | 2.22 (0.47–10.4) | 0.3 | 1.66 (0.34–8.00) | 0.5 |
| Ethnicity (2164) | ||||
| Hispanic or Latino | — | |||
| Not Hispanic | 1.69 (0.87–3.29) | 0.12 | ||
| History of cancer (2398) | ||||
| No | — | — | ||
| Yes | 1.88 1.45–2.45 | <0.001 | 1.35 (1.00–1.82) | 0.046 |
| ASA (2398) | ||||
| 1 | — | — | ||
| 2 | 1.25 (0.30–5.12) | 0.8 | 0.27 (0.06–1.13) | 0.073 |
| 3 | 4.65 (1.16–18.7) | 0.030 | 0.66 (0.16–2.73) | 0.6 |
| 4 | 12.0 (2.88–50.1) | <0.001 | 1.01 (0.23–4.45) | >0.9 |
| BMI (2376) | 0.99 (0.98–1.01) | 0.6 | ||
| CEA level (2139) | ||||
| <5 | — | — | ||
| ≥5 | 1.44 (1.15–1.80) | 0.001 | 1.16 (0.90–1.49) | 0.2 |
| Albumin level (2353) | 0.48 (0.38–0.60) | <0.001 | 0.87 (0.65–1.17) | 0.4 |
| Tumor location (2398) | ||||
| Left colon | — | — | ||
| Mid-transverse colon | 1.21 (0.81–1.81) | 0.3 | 0.70 (0.43–1.14) | 0.2 |
| Right colon | 1.64 (1.30–2.05) | <0.001 | 1.10 (0.84–1.43) | 0.5 |
| Colectomy extent (2398) | ||||
| Extended | — | |||
| Segmental | 1.22 (0.79–1.88) | 0.4 | ||
| Tumor grade (2318) | ||||
| Low grade | — | — | ||
| High grade | 1.55 (1.21–1.97) | <0.001 | 1.21 (0.91–1.59) | 0.2 |
| N category (2398) | ||||
| 0 | — | — | ||
| 1 | 1.18 (0.92–1.50) | 0.2 | 1.34 (1.01–1.78) | 0.043 |
| 2 | 1.73 (1.29–2.33) | <0.001 | 2.14 (1.48–3.11) | <0.001 |
| T category (2398) | ||||
| CIS or 1 | — | — | ||
| 2 | 1.05 (0.70–1.59) | 0.8 | 1.05 (0.65–1.69) | 0.9 |
| 3 | 1.04 (0.75–1.44) | 0.8 | 0.85 (0.56–1.27) | 0.4 |
| 4 | 2.11 (1.46–3.04) | <0.001 | 1.42 (0.88–2.29) | 0.15 |
| Procedure yr (2398) | ||||
| 2006–2009 | — | |||
| 2010–2014 | 1.02 (0.81–1.30) | 0.9 | ||
| 2015–2018 | 0.84 (0.59–1.20) | 0.3 | ||
| Colectomy approach (2398) | ||||
| Laparoscopic | — | — | ||
| Open | 1.32 (1.05–1.65) | 0.017 | 1.02 (0.78–1.32) | >0.9 |
| Robotic | 0.63 (0.44–0.89) | 0.009 | 0.79 (0.53–1.17) | 0.2 |
HR = hazard ratio; CI = confidence interval; ASA = American Society of Anesthesiologists classification of physical status; BMI = body mass index; CEA = carcinoembryonic antigen; CIS = intramucosal carcinoma in situ.
DISCUSSION
Our study found that robotic colectomy for stage I-III colon cancer is associated with a significant though small improvement in LNY and with a more radical mesenteric resection compared with open and laparoscopic colectomy, but no meaningful difference in DFS or OS was observed between the three groups. Minimally invasive procedures were associated with lower complication rates and shorter LOS. In a subgroup analysis, robotic resection was associated with lower complication rates, shorter LOS and fewer conversions than laparoscopic resections.
Although LNY was higher in the robotic surgery group, average LNY in both the open colectomy group and the laparoscopic colectomy group was above 24. According to NCDB data for more than a quarter of a million colon resections, LNY ≥ 24 is associated with longer OS, but further increases in LNY are not associated with an additional benefit.21 Because LNY can increase with increasing specimen length independently of the radicality of mesenteric resection, the modest differences in LNY may be of greater clinical importance when viewed in context of the ratio of LNY to specimen length (Table 3).
Similar survival rates for the three surgical approaches in our study are consistent with previous reports of similar survival in robotic and laparoscopic resections of colon cancer.9–12,14 This finding also confirms data from landmark RCTs1–8,22,23 that found no meaningful difference in survival between open and laparoscopic colectomies. The rates of DFS (84% and 77% at 3 and 5 years, respectively) and OS (92% and 85% at 3 and 5 years, respectively) in our cohort were considerably higher than the rates in the RCTs (DFS, 67%–76% at 3 years6,8 and 56%–73% at 5 years6,7,22,23; OS, 67%–84% at 3 years6,8 and 56%–78% at 5 years6,7,22,23). This difference is notable because, unlike the RCTs, our study included patients with advanced tumors and transverse colon tumors as well as morbidly obese patients. It likely reflects the multiple advances in colon cancer treatment of the past two decades,24 such as improvement in the accuracy of modern imaging,25 adoption of regional therapy for metastatic disease in select stage IV patients,26 incorporation of oxaliplatin chemotherapy,27 and increased use of chemotherapy for stage II disease.28
Our findings contrast with the results of two large retrospective analyses of NCDB data that found an association between operative approach and OS in patients with nonmetastatic colon cancer. These studies lacked recurrence information and the granularity of data available in our cohort. In a study that used propensity score matching for more than 15,000 patients, Mirkin et al.13 found an OS advantage in patients with stage II–III disease who underwent robotic resection compared with laparoscopic resection. Interestingly, the authors did not find a difference in LNY between the two approaches. Lee et al.29 used propensity score matching to compare 33,183 patients who underwent open resection of stage III colon cancer and 33,183 patients who underwent minimally invasive resection. Laparoscopic and robotic resections were associated with longer OS and a lower rate of delay to chemotherapy.
The above studies were subject to biases associated with large-database research, such as underreporting and unique confounding. Ofshteyn et al.30 demonstrated that NCDB data for patients who had undergone robotic resection for stage I–III rectal cancer were likely to come from white, privately insured men living in a metropolitan area, with at least a high school education. The patients were also likely to be treated at a high-volume academic center and to travel a considerable distance to the treating hospital. Underprivileged populations were less likely to have access to robotic surgery. This confounding may affect the association between operative approach and survival. Ofshteyn et al. concluded that the inherent bias in access to robotic surgery may skew NCDB data, preventing generalizability. Although our retrospective study was also subject to biases, the choice of operative approach was associated primarily with the year of the surgery and the surgeon’s clinical judgment. Surgical approach was not likely to be associated with socioeconomic status, given the relative homogeneity of the patient population at our institution.
Similar to the RCTs,1,2,4,5 our study found favorable short-term outcomes for minimally invasive colectomy, such as fewer complications, fewer SSI and shorter LOS. Our data indicate that robotic colectomy is associated with better short-term outcomes and a lower likelihood of conversion in comparison with laparoscopic colectomy, in agreement with previously reported findings in rectal cancer.31
The short-term benefits of robotic colectomy have financial implications that may offset the higher overhead cost.32 Conversion, which was rarer in the robotic surgery group (2%) than in the laparoscopic surgery group (14%), has been shown to significantly increase cost.33 Herein, conversion was associated with more complications, more SSI and longer LOS; all have been shown to increase cost.34–36 Financial benefits of robotic resection may extend beyond the index admission37: complications not only are expensive in terms of direct cost but also affect the likelihood and speed of returning to work,38 with nearly a third of patients failing to return to the workforce within 1 year following curative resection.39 The shorter LOS for robotic surgery can also potentially help decrease both direct inpatient cost and absenteeism from the workforce as seen with other diseases.40
The limitations of our study include potential selection bias inherent in retrospective design. Additionally, the homogeneity of the patient population from a single center may limit generalizability. The relative frequencies of the operative approaches changed during the 13-year study period, but during this time no meaningful changes in adjuvant chemotherapy guidelines were implemented. Given the robotic group’s shorter median follow-up, it is possible that survival for this group will decrease with additional follow-up. Although there is possible confounding from the adoption of an ERAS protocol and an SSI bundle which occurred alongside the adoption of the robotic platform, differences in LOS remained significant when analyzing the cohorts both before and after ERAS adoption. Differences in SSI rates remained meaningful between operative approaches only in the post-bundle period, so it is more difficult to dissect the effect of operative approach from the effect of the SSI bundle on the incidence of SSI, but the data suggest an additive value of bundle implementation and minimally invasive surgery. Short term outcomes were compared in an unadjusted analysis and demonstrate association and not causation. The strengths of the study include the large size, the standardization of surgery, surveillance, comprehensive histopathologic assessment, and the granularity of the clinical data, including recurrence information.
CONCLUSION
Survival rates for patients who undergo resection of nonmetastatic colon cancer at a specialized cancer center do not differ significantly between open, laparoscopic, and robotic resections, although robotic resections are associated with a small increase in LNY and a more radical mesenteric resection. Short-term outcomes are better for minimally invasive colectomy than for open colectomy and better for robotic colectomy than for laparoscopic colectomy. Additional studies are needed to investigate whether robotic colectomy is associated with economic advantages, if the costs of complications, conversion, and delay in return to work are taken into account.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Arthur Gelmis, BS, for editing the manuscript. J.B. Yuval acknowledges Yael Renert-Yuval for insightful conversations.
Funding/Support:
Research at Memorial Sloan Kettering is funded in part by grant P30 CA008748 from the National Cancer Institute. J. B. Yuval’s research fellowship at Memorial Sloan Kettering was funded in part by grant T32 CA009501 from the National Cancer Institute.
Footnotes
Financial Disclosures: Dr. Garcia-Aguilar has received honoraria from Intuitive Surgical, Medtronic and Johnson & Johnson.
REFERENCES
- 1.Lacy AM, García-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–2229. [DOI] [PubMed] [Google Scholar]
- 2.Nelson H, Sargent DJ, Wieand HS, et al. ; Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. [DOI] [PubMed] [Google Scholar]
- 3.Guillou PJ, Quirke P, Thorpe H, et al. ; MRC CLASICC trial group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. [DOI] [PubMed] [Google Scholar]
- 4.Veldkamp R, Kuhry E, Hop WC, et al. ; COlon cancer Laparoscopic or Open Resection Study Group (COLOR). Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–484. [DOI] [PubMed] [Google Scholar]
- 5.Hewett PJ, Allardyce RA, Bagshaw PF, et al. Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann Surg. 2008;248:728–738. [DOI] [PubMed] [Google Scholar]
- 6.Buunen M, Veldkamp R, Hop WC, et al. ; Colon Cancer Laparoscopic or Open Resection Study Group. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. [DOI] [PubMed] [Google Scholar]
- 7.Bagshaw PF, Allardyce RA, Frampton CM, et al. ; Australasian Laparoscopic Colon Cancer Study Group. Long-term outcomes of the australasian randomized clinical trial comparing laparoscopic and conventional open surgical treatments for colon cancer: the Australasian Laparoscopic Colon Cancer Study trial. Ann Surg. 2012;256:915–919. [DOI] [PubMed] [Google Scholar]
- 8.Jayne DG, Guillou PJ, Thorpe H, et al. ; UK MRC CLASICC Trial Group. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061–3068. [DOI] [PubMed] [Google Scholar]
- 9.Park JS, Kang H, Park SY, et al. Long-term oncologic after robotic versus laparoscopic right colectomy: a prospective randomized study. Surg Endosc. 2019;33:2975–2981. [DOI] [PubMed] [Google Scholar]
- 10.Xu M, Zhao Z, Jia B, Liu R, Liu H. Perioperative and long-term outcomes of robot-assisted versus laparoscopy-assisted hemicolectomy for left-sided colon cancers: a retrospective study. Updates Surg. 2021;73:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinar I, Fransgaard T, Thygesen LC, Gögenur I. Long-term outcomes of robot-assisted surgery in patients with colorectal cancer. Ann Surg Oncol. 2018;25:3906–3912. [DOI] [PubMed] [Google Scholar]
- 12.Spinoglio G, Bianchi PP, Marano A, et al. Robotic versus laparoscopic right colectomy with complete mesocolic excision for the treatment of colon cancer: perioperative outcomes and 5-year survival in a consecutive series of 202 patients. Ann Surg Oncol. 2018;25:3580–3586. [DOI] [PubMed] [Google Scholar]
- 13.Mirkin KA, Kulaylat AS, Hollenbeak CS, Messaris E. Robotic versus laparoscopic colectomy for stage I-III colon cancer: oncologic and long-term survival outcomes. Surg Endosc. 2018;32:2894–2901. [DOI] [PubMed] [Google Scholar]
- 14.Kang J, Park YA, Baik SH, Sohn SK, Lee KY. A comparison of open, laparoscopic, and robotic surgery in the treatment of right-sided colon cancer. Surg Laparosc Endosc Percutan Tech. 2016;26:497–502. [DOI] [PubMed] [Google Scholar]
- 15.Ow ZGW, Sim W, Nistala KRY, et al. Comparing complete mesocolic excision versus conventional colectomy for colon cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2021;47:732–737. [DOI] [PubMed] [Google Scholar]
- 16.Killeen S, Mannion M, Devaney A, Winter DC. Complete mesocolic resection and extended lymphadenectomy for colon cancer: a systematic review. Colorectal Dis. 2014;16:577–594. [DOI] [PubMed] [Google Scholar]
- 17.Widmar M, Keskin M, Strombom P, et al. Lymph node yield in right colectomy for cancer: a comparison of open, laparoscopic and robotic approaches. Colorectal Dis. 2017;19:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tufts LS, Jarnagin ED, Flynn JR, et al. A perioperative multidisciplinary care bundle reduces surgical site infections in patients undergoing synchronous colorectal and liver resection. HPB (Oxford). 2019;21:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei IH, Pappou EP, Smith JJ, et al. Monitoring an ongoing enhanced recovery after surgery (ERAS) program: adherence improves clinical outcomes in a comparison of three thousand colorectal cases. Clin Surg. 2020;5:5. [PMC free article] [PubMed] [Google Scholar]
- 21.Trepanier M, Erkan A, Kouyoumdjian A, et al. Examining the relationship between lymph node harvest and survival in patients undergoing colectomy for colon adenocarcinoma. Surgery. 2019;166:639–647. [DOI] [PubMed] [Google Scholar]
- 22.Fleshman J, Sargent DJ, Green E, et al. ; Clinical Outcomes of Surgical Therapy Study Group. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246:655–662. [DOI] [PubMed] [Google Scholar]
- 23.Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–1645. [DOI] [PubMed] [Google Scholar]
- 24.Konishi T, Shimada Y, Hsu M, et al. Contemporary validation of a nomogram predicting colon cancer recurrence, revealing all-stage improved outcomes. JNCI Cancer Spectr. 2019;3:pkz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahani DV, Kalva SP, Fischman AJ, et al. Detection of liver metastases from adenocarcinoma of the colon and pancreas: comparison of mangafodipir trisodium-enhanced liver MRI and whole-body FDG PET. AJR Am J Roentgenol. 2005;185:239–246. [DOI] [PubMed] [Google Scholar]
- 26.Adileh M, Yuval JB, Walch HS, et al. Primary tumor location and outcomes after cytoreductive surgery and intraperitoneal chemotherapy for peritoneal metastases of colorectal origin. Ann Surg Oncol. 2021;28:1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. [DOI] [PubMed] [Google Scholar]
- 28.Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ; Quasar Collaborative Group. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370:2020–2029. [DOI] [PubMed] [Google Scholar]
- 29.Lee L, Wong-Chong N, Kelly JJ, Nassif GJ, Albert MR, Monson JRT. Minimally invasive surgery for stage III colon adenocarcinoma is associated with less delay to initiation of adjuvant systemic therapy and improved survival. Surg Endosc. 2019;33:460–470. [DOI] [PubMed] [Google Scholar]
- 30.Ofshteyn A, Bingmer K, Towe CW, Steinhagen E, Stein SL. Robotic proctectomy for rectal cancer in the US: a skewed population. Surg Endosc. 2020;34:2651–2656. [DOI] [PubMed] [Google Scholar]
- 31.Crippa J, Grass F, Dozois EJ, et al. Robotic surgery for rectal cancer provides advantageous outcomes over laparoscopic approach: results from a large retrospective cohort. Ann Surg. 2021;274:e1218–e1222. [DOI] [PubMed] [Google Scholar]
- 32.Simianu VV, Gaertner WB, Kuntz K, et al. Cost-effectiveness evaluation of laparoscopic versus robotic minimally invasive colectomy. Ann Surg. 2020;272:334–341. [DOI] [PubMed] [Google Scholar]
- 33.Cleary RK, Mullard AJ, Ferraro J, Regenbogen SE. The cost of conversion in robotic and laparoscopic colorectal surgery. Surg Endosc. 2018;32:1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keenan JE, Speicher PJ, Thacker JK, Walter M, Kuchibhatla M, Mantyh CR. The preventive surgical site infection bundle in colorectal surgery: an effective approach to surgical site infection reduction and health care cost savings. JAMA Surg. 2014;149:1045–1052. [DOI] [PubMed] [Google Scholar]
- 35.Widmar M, Keskin M, Strombom P, et al. Evaluating the validity of the Clavien-Dindo classification in colectomy studies: a 90-day cost of care analysis. Dis Colon Rectum 2021;64:1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gantz O, Zagadailov P, Merchant AM. The cost of surgical site infections after colorectal surgery in the United States from 2001 to 2012: a longitudinal analysis. Am Surg. 2019;85:142–149. [PubMed] [Google Scholar]
- 37.Justiniano CF, Becerra AZ, Xu Z, et al. A population-based study of 90-day hospital cost and utilization associated with robotic surgery in colon and rectal cancer. J Surg Res. 2020;245:136–144. [DOI] [PubMed] [Google Scholar]
- 38.den Bakker CM, Anema JR, Huirne JAF, Twisk J, Bonjer HJ, Schaafsma FG. Predicting return to work among patients with colorectal cancer. Br J Surg. 2020;107:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhalla A, Williams JP, Hurst NG, et al. One-third of patients fail to return to work 1 year after surgery for colorectal cancer. Tech Coloproctol. 2014;18:1153–1159. [DOI] [PubMed] [Google Scholar]
- 40.Rørth R, Wong C, Kragholm K, et al. Return to the workforce after first hospitalization for heart failure: a Danish nationwide cohort study. Circulation. 2016;134:999–1009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


