Abstract
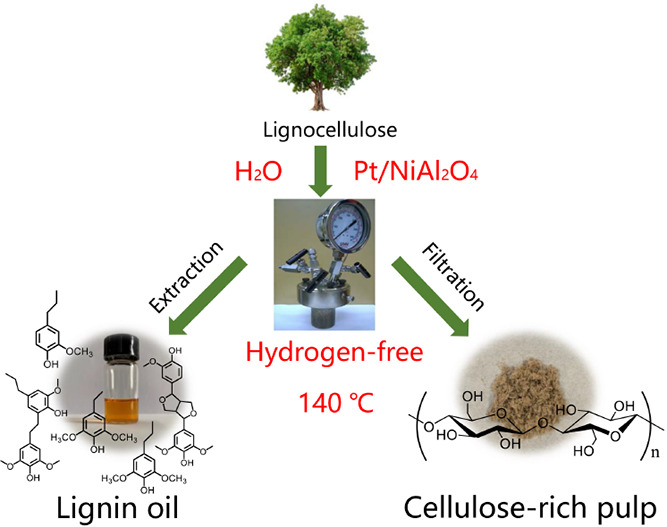
Lignocellulosic biomass is one of the most well-studied and promising green carbon sources. The fullest utilization of lignocellulosic biomass in hydrogen-free and mild conditions to produce phenolic monomers while preserving cellulose-rich pulps is challenging and has far-reaching significance. Here, we report an innovative strategy to convert lignocellulosic biomass into lignin oils and cellulose-rich pulps without exogenous hydrogen under mild conditions over a Pt/NiAl2O4 catalyst. In this process, the structural hydrogens in hemicellulose acted as a hydrogen source to realize the fractionation and depolymerization of lignin into phenolic monomers while keeping the cellulose intact, which is named self-hydrogen supplied catalytic fractionation (SCF). By using water as a solvent, the theoretical yield of phenolic monomers (46.6 wt %, with propyl(ethyl) end-chained syringol and guaiacol as main products) is achieved at 140 °C for 24 h, with 90% cellulose intact in birch sawdust. This H2-free process can be extended to other biomass (hardwood, softwood, and grass) and can be scaled up. The Pt/NiAl2O4 catalyst also shows good stability in recycling as well as regeneration treatment. This work provides a new strategy to achieve high utilization of lignocellulosic biomass for sustainable biorefinery by using water as a solvent without exogenous hydrogen under mild conditions.
Keywords: heterogeneous catalysis, biomass fractionation, self-hydrogen supplied, lignin depolymerization, cellulose-rich pulps
1. Introduction
In the context of carbon neutrality, there has been an interest in the use of clean and sustainable sources due to the growing global concern about energy and environmental problems caused by over-reliance on fossil resources. Lignocellulosic biomass, which comprises cellulose (30–50%), hemicellulose (20–35%), and lignin (15–30%), is the most abundant and sustainable green carbon resource on Earth.1−5 Utilizing lignocellulosic biomass as a renewable resource is a feasible way to achieve the target of carbon neutrality, and the fractionation of lignocellulose into (hemi)cellulose and lignin is normally the first step to obtain materials and high value-added chemicals.6−13 Conventionally, the lignocellulose fractionation strategy focuses on (hemi)cellulose utilization, which is usually called the carbohydrate-first process, e.g., the kraft, sulfite, or soda process, whereas lignin is irreversibly degraded or converted to a highly condensed biopolymer or sulfonated lignin and burned as energy. In addition, other methods, such as organosolv and hydrolytic fractionation, have also been widely used to fractionate lignocellulosic biomass, but organic solvents or acids are still necessary, and the resulting lignin requires further upgrading to obtain lignin phenolic monomers.14,15
Recently, reductive catalytic fractionation (RCF) of lignocellulosic biomass is regarded as one of the most promising strategies, which can strip and depolymerize lignin into lignin oils with the cellulose intact under a hydrogen atmosphere; this process is also called the lignin-first strategy.16,17 Various catalysts with different reaction conditions have been developed for RCF.18−35 However, H2 is generally used, and the operating temperature of most of the process is above 200 °C. This would be unfavorable for the industrialization of RCF considering the explosive property of H2 and the relatively high temperature. Therefore, H2-free fractionation of raw biomass is pursued with high demand. Samec et al. have achieved fractionation of lignocellulosic biomass in an ethanol/water solvent without H2 in which partial hemicellulose was consumed to supply some H2. However, they also demonstrated that ethanol was consumed via hydrogen transfer to be involved in the fractionation.36,37 Wang and co-workers described the hydrogenolysis of native lignin in water under H2-free conditions, and about 40 wt % yields of lignin monomers can be obtained from poplar lignin at 180 °C. However, cellulose is inevitably degraded to formic acid in the process.38
It is well-known that lignocellulosic biomass is rich in −C–OH and −C–OCH3 groups and can generate H2 through aqueous-phase reforming (APR). For example, Lu and co-workers realized H2 production over a nickel-molybdenum catalyst by using raw biomass as substrates, and 58.6 mmol H2 per gram wood with a H2 selectivity of 79.4% can be reached at 310 °C.39 Chen et al. reported a modified Pt-supported carbon nitride photocatalyst, which exhibited a hydrogen production rate of 3.39 mmol per gram per hour from glucose solution under an LED light source.40 In our laboratory, using structural H in lignin (hydroxypropyl and −OCH3 groups), the transformation of lignin into 4-alkylphenol was realized over a Pt/NiAl2O4 catalyst without additional hydrogen sources.41
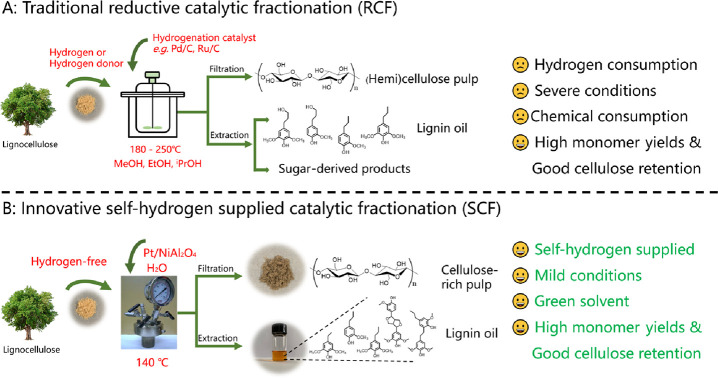
Inspired and encouraged by these interesting works, we herein develop an innovative strategy for lignocellulose fractionation by using structural H as a hydrogen source, which is named self-hydrogen supplied catalytic fractionation (SCF). Through this process, various lignocellulosic biomass can be efficiently converted into lignin oils and cellulose-rich pulps without exogenous hydrogen sources (H2 or alcohols) under mild conditions over a Pt/NiAl2O4 catalyst with hemicellulose acting as a hydrogen source. This strategy exhibited high efficiency in the fractionation of birch sawdust and achieved nearly theoretical maximum yields of phenolic monomers (mainly propyl(ethyl) end-chained syringol and guaiacol) and 90% cellulose retention at 140 °C for 24 h. The comparison of traditional reductive catalytic fractionation (RCF) with the newly developed self-hydrogen supplied catalytic fractionation (SCF) process is drawn in Scheme 1. This catalyst has good stability in recycling as well as regeneration treatment. This study provides an efficient, self-hydrogen supplied catalytic fractionation (SCF) for lignocellulosic biomass utilization.
Scheme 1. Comparison of Traditional Reductive Catalytic Fractionation (RCF) and Newly Developed Self-Hydrogen Supplied Catalytic Fractionation (SCF).
2. Experimental Methods
2.1. Catalyst Preparation
NiAl2O4 spinel was synthesized by a coprecipitation method at a Ni/Al mole ratio of 1:2 according to our previous works.41,42 A typical procedure was as follows: 40 mmol of nickel nitrate hexahydrate and 80 mmol of aluminum nitrate nonahydrate were dissolved in 200 mL of ultrapure water under vigorous stirring at room temperature. Aqueous ammonia (25–28 wt %) was added dropwise to the above solution until the pH reached 8 to obtained precipitates. Then, the formed precipitates were filtered and washed with deionized water until the pH of the filtrate reached 7. Finally, the filter cake was dried at 100 °C for 24 h followed by calcination at 800 °C for 8 h under an Ar atmosphere. Pt/NiAl2O4 was prepared by the incipient wetness impregnation method with appropriate amounts of aqueous solution of chloroplatinic acid (H2PtCl6). The obtained samples were dried at 100 °C for 12 h and then reduced in a 10% H2/Ar flow at 300 °C for 3 h. The Pt loading in the catalyst was 2 wt %.
2.2. Catalyst Characterizations
The powder X-ray diffraction (XRD) patterns were recorded on a Rigaku D/max-2550VB/PC diffractometer by using Cu Kα radiation (λ = 0.15406 nm). Nitrogen adsorption/desorption isotherms of the catalysts were measured on a Micromeritics ASAP 2020M sorption analyzer at 77 K. The Brunauer–Emmett–Teller (BET) method was used to calculate the surface area. Scanning transmission electron microscopy (STEM) characterization was performed using a Thermo Fisher Talos F200X microscope. High-angle annular dark-field (HAADF)-STEM images were recorded using a convergence semiangle of 11 mrad and inner and outer collection angles of 59 and 200 mrad, respectively. Chemical analysis of the samples was performed by using inductively coupled plasma-atomic emission spectrometry (ICP-AES).
H2 temperature-programmed reduction tests were carried out on a Huasi DAS-7200 automatic chemisorption instrument. In a typical run, 0.1 g of the catalyst was put into the quartz tube. Before reduction, the catalyst was degassed and dehydrated at 150 °C in Ar. The temperature was controlled from 20 to 800 °C at a rate of 10 °C min–1.
X-ray photoelectron (XPS) spectra were recorded on a Thermo Scientific Escalab 250 Xi system with monochromatic Al Kα radiation, and all results were calibrated using the C 1s peak at 284.8 eV.
2.3. Catalytic Reactions
In a typical SCF reaction, 0.5 g of birch sawdust (2–5 mm), 0.1 g of the 2%Pt/NiAl2O4 catalyst, and 10 mL of H2O were put into the 50 mL stainless-steel autoclave reactor. The reactor was sealed and purged with N2 at room temperature. The reaction mixture was heated to different temperatures and kept for a certain time at a magnetic stirring speed of 700 rpm. At the end of the reaction, it was cooled to room temperature in ice water. The soluble fraction and residue were subsequently separated by centrifugation.
2.4. Lignin-Derived Product Analyses
After the SCF reaction, the liquid phase was extracted by ethyl acetate and analyzed by GC–MS (Agilent 7890A) and quantitatively analyzed by GC (Agilent 7890B) with a flame ionization detector, both quipped with HP-5 capillary columns. Tridecane was used as an internal standard for the quantification of the liquid products. All experiments were performed at least three times. The phenolic monomer yields were calculated based on the Klason lignin weight, with the calculation formula as shown below:
The mass of lignin in biomass was measured by the Klason method, and the mole of monomers in lignin was measured by the NBO method.43
The retentions of cellulose and hemicellulose in pulps were calculated based on the biomass compositional analyses of solid pulps, with the calculation formula as shown below:
The gas products were collected in a gas bag and qualitatively analyzed on an online gas chromatographer equipped with methanator, FID, and TCD detectors. The H2 production was calculated based on the mass of substrates, with the calculation formula as shown below:
The H2 selectivity was determined based on the following formula:
where RR is the reforming ratio of H2/CO2. The carbon yield of products was calculated on the basis of each mole number in products and substrates. The calculation formula is shown below:
2.5. Determination of Structural Carbohydrates and Lignin
The structural carbohydrates and lignin in untreated biomass and the solid residue after treatment were determined according to a technical report NREL/TP-510-42618.44 Saccharides and degradation products in liquid extract were determined according to a technical report NREL/TP-510-42623.45 Saccharides were determined using an HPLC system (Agilent 1260 series) equipped with a refractive index dectector (Agilent G1362A) and using a Biorad Aminex HPX-87H sugar column at 55 °C. The mobile phase was 0.004 M H2SO4 with flow rate of 0.8 mL min–1. The quantification of products was performed using an external standard method. The total lignin content in biomass was determined as the sum of acid-insoluble lignin and acid-soluble lignin. The equation 100 – 100 × Lsr × Ysr/Lub was used for the delignification value, where Lsr is the lignin content in the solid residue, Ysr is the yield of the solid residue after treatment, and Lub is the lignin content in untreated biomass.
3. Results and Discussion
3.1. Performance of Pt/NiAl2O4 in the Self-Hydrogen Supplied Catalytic Fractionation (SCF) of Raw Biomass
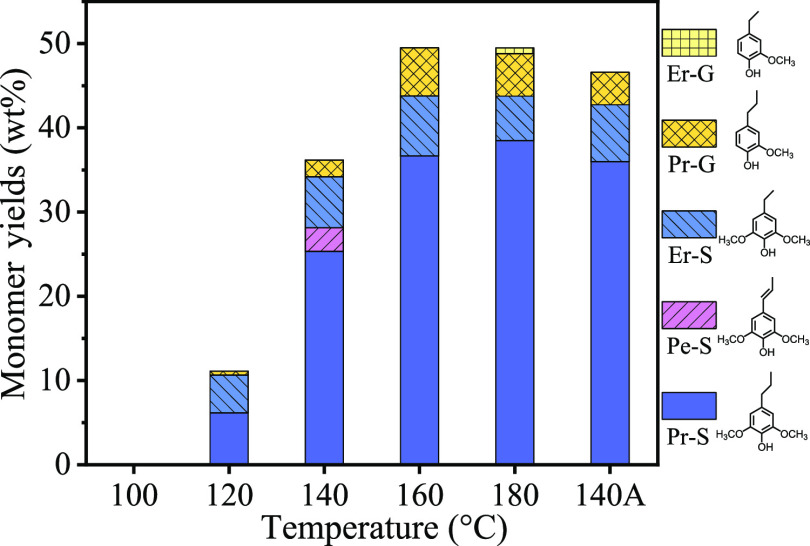
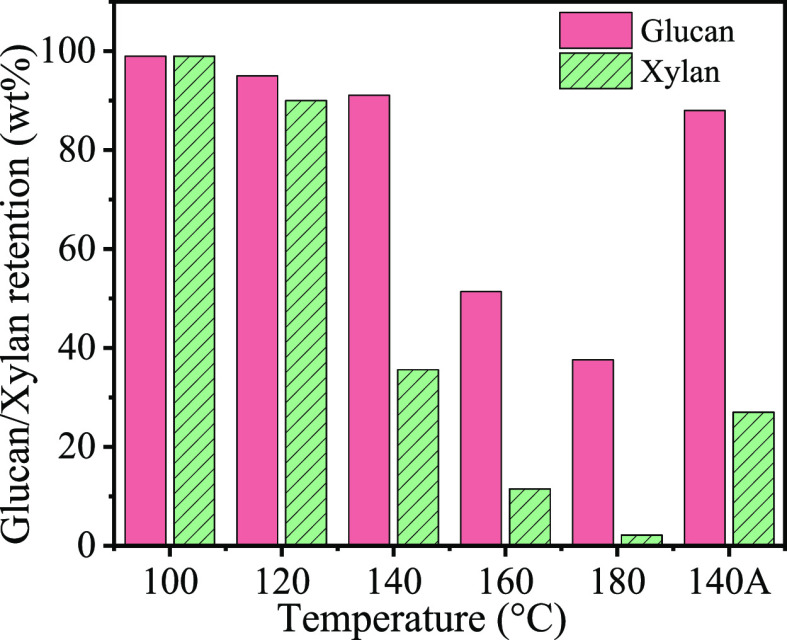
Here, Pt/NiAl2O4 was selected as the catalyst in the self-hydrogen supplied catalytic fractionation (SCF) of raw biomass because it is active and has excellent stability in the APR process.42 The actual Pt loading of Pt/NiAl2O4 was 1.7 wt % as determined by ICP-OES, other characterizations including XRD, STEM, H2-TPR, XPS, and N2 sorption are supplied in the Supporting Information (Figures S1–S4 and Table S1). Birch wood was taken as a benchmark hardwood substrate, and its composition was analyzed and is provided in Table S2. We first evaluated the performance of Pt/NiAl2O4 at different temperatures by detecting the yields of lignin monomers and the retention of cellulose, and the results are present in Figure 1. It is shown that with the increase of reaction temperature from 100 to 180 °C, the phenolic monomer yield increased from 0 to 49.5 wt %, confirming that the Pt/NiAl2O4 catalyst has an excellent ability to depolymerize lignin. However, the glucan and xylan retention sharply decreased when the temperature reached 160 °C (Figure 2), which is unfavorable for sustainable biorefinery. Pleasantly, we found that glucan showed good retention (91 wt %) at 140 °C, while the yield of phenolic monomers was kept at a high value of 36.2 wt %. When the reaction time was prolonged to 24 h at 140 °C, the phenolic monomer yield further increased to 46.6 wt %, approaching the theoretical maximum yields of phenolic monomers,16 and the glucan retention only had a slight decrease. Among the monomers, 4-propyl syringol (Pr-S, 36 wt %), 4-ethyl syringol (Er-S, 6.8 wt %), and 4-propyl guaiacol (Pr-G, 3.9 wt %) were identified as three major products; the intermediate of 4-propenyl syringol (Pe-S) was absent after the reaction for 24 h, whereas it was detected at 12 h (Figure 1 and Figure S5). This result indicated that the SCF strategy is indeed an excellent process to fractionate cellulose and lignin. The high selectivity to propyl(ethyl) end-chained syringol and guaiacol allowed them to be readily purified.33,46 In order to analyze the type of dimer, the obtained lignin oil was silylated and characterized with GC–MS, and seven dimers were identified, which all had C–C linkages, including β-β and β-5 linkages (Figure S6).
Figure 1.
Monomer distribution and yields of SCF over the Pt/NiAl2O4 catalyst at different temperatures. Reaction conditions: birch sawdust (0.5 g), Pt/NiAl2O4 (0.1 g), H2O (10 mL), N2 at 1 atm, 12 h (note: the label “140A” represents that the reaction time was prolonged to 24 h).
Figure 2.
Glucan/xylan retention of SCF over the Pt/NiAl2O4 catalyst at different temperatures. Reaction conditions: birch sawdust (0.5 g), Pt/NiAl2O4 (0.1 g), H2O (10 mL), N2 at 1 atm, 12 h (note: the label “140A” represents that the reaction time was prolonged to 24 h).
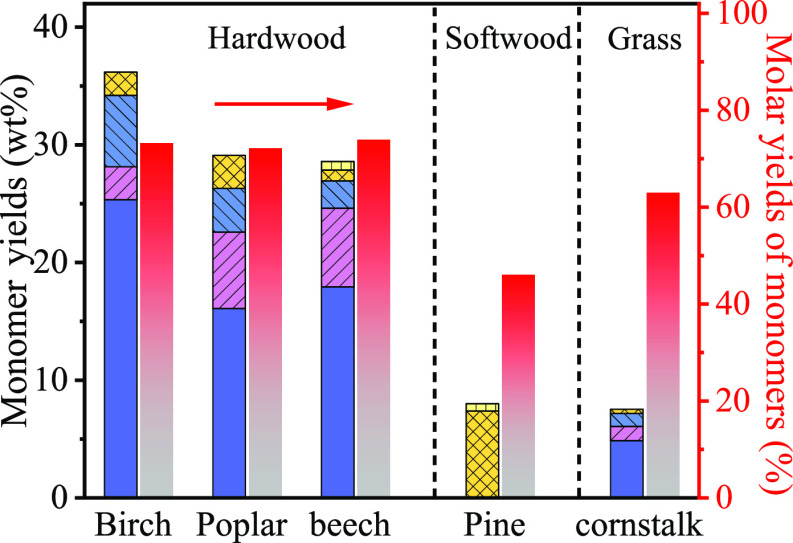
We further evaluated the SCF performance of other types of wood including softwood and grass over the Pt/NiAl2O4 catalyst. As for the substrate expansion, the yields of phenolic monomers from hardwood (birch, poplar, and beech) were between 28.6 and 36.2 wt % with Pr-S, Pe-S, Er-S, and Pr-G as the dominant products for 12 h (Figure 3). Additionally, the total molar yield of phenolic monomers approached 72.1 to 73.9% from corresponding lignin (see the monomer concentrations in Table S3). In contrast to hardwood, lignin in softwood (pine), mainly composed of G-units with less cleavable β-O-4 bonds,6 afforded 7.9 wt % phenolic monomers (46% molar yield of phenolic monomers). As expected, only G-units including Pr-G and Er-G were detected. Cornstalk, a straw grass, provided 7.5 wt % yield of the phenolic monomers (63% molar yield of phenolic monomers from cornstalk lignin).
Figure 3.
SCF of various biomass sources. Reaction conditions: biomass sawdust (0.5 g), Pt/NiAl2O4 (0.1 g), H2O (10 mL), 140 °C, N2 at 1 atm, 12 h.
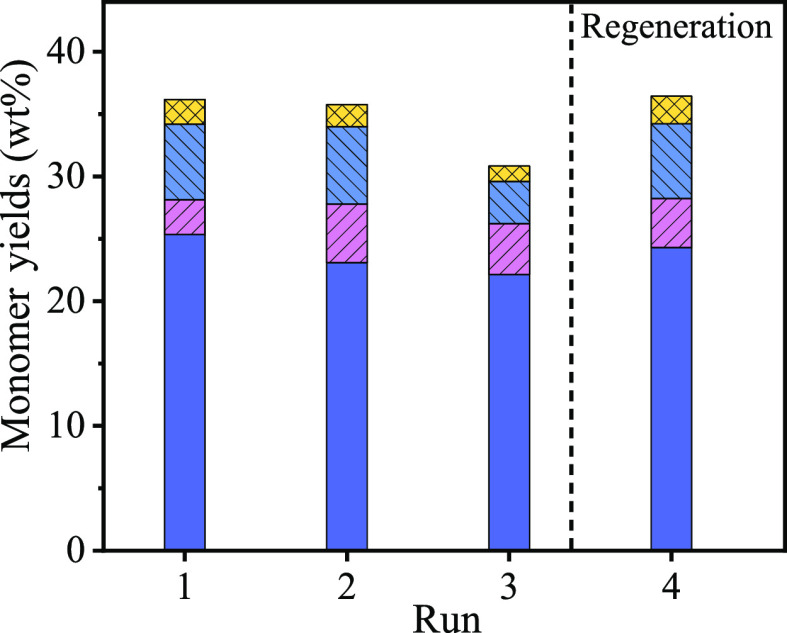
To evaluate the recyclability of Pt/NiAl2O4, the spent catalyst separated from the cellulose-rich pulps by stripping and sieving was directly used for the next run (Figure 4 and Figure S7). In the third run, a decreased catalytic performance with 30.8 wt % yields of phenolic monomers was observed. Considering that the degradation products or byproducts may be adsorbed on the catalyst, further affecting the catalyst activity, the catalyst was regenerated (by calcining at 500 °C and reducing) and used for the fourth run; the phenolic monomer yield of 36.3 wt % was achieved, suggesting almost complete recovery of catalytic performance. In addition, we carried out the scale-up experiment over Pt/NiAl2O4, and the results are shown in Table S4. The total phenolic monomer yield reached 41.2 wt %, a slight decrease compared to the result of 46.6 wt % at 24 h (Figure 1). These results imply that the catalyst has good recyclability and the process can be scaled up.
Figure 4.
Recyclability test of the Pt/NiAl2O4 catalyst. Reaction conditions: birch sawdust (0.5 g), Pt/NiAl2O4 (0.1 g), H2O (10 mL), 140 °C, N2 at 1 atm, 12 h.
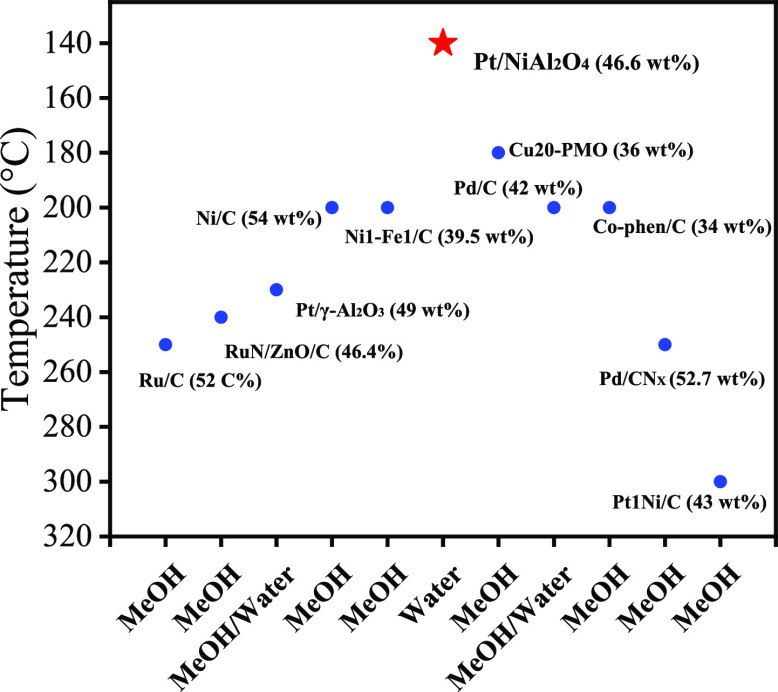
The performance of the here-developed SCF and the conventional RCF process was compared (Figure 5 and Table S5). Traditional RCF mostly requires high hydrogen pressure (Ru/C for 3 MPa18 and Pd/C24 for 2 MPa), while SCF could proceed without exogenous hydrogen. The depolymerization of lignocellulose can be completed at only 140 °C of the SCF process, but most of RCF requires above 200 °C (Table S5). What is more, RCF mostly uses alcohols as solvents (such as MeOH and EtOH), while SCF uses a green solvent (H2O) as a solvent. The limitation of SCF at present is that it takes a long time (24 h) to achieve the high yield; therefore, a catalyst with high activity is required for the depolymerization and upgrading of lignocellulosic biomass. From the above analysis, the greatest advantage of SCF is that it could rationally utilize the structural hydrogen to depolymerize lignocellulosic biomass into phenolic monomers with the cellulose part intact without consumption of exogenous hydrogen. Meanwhile, it utilizes mild reaction conditions and a green solvent (H2O) compared with the other RCF process with good to excellent phenolic monomer yields.
Figure 5.
Reported reductive catalytic fractionation conditions and results over different catalysts.
3.2. Investigation of the Origin of the Hydrogen Source
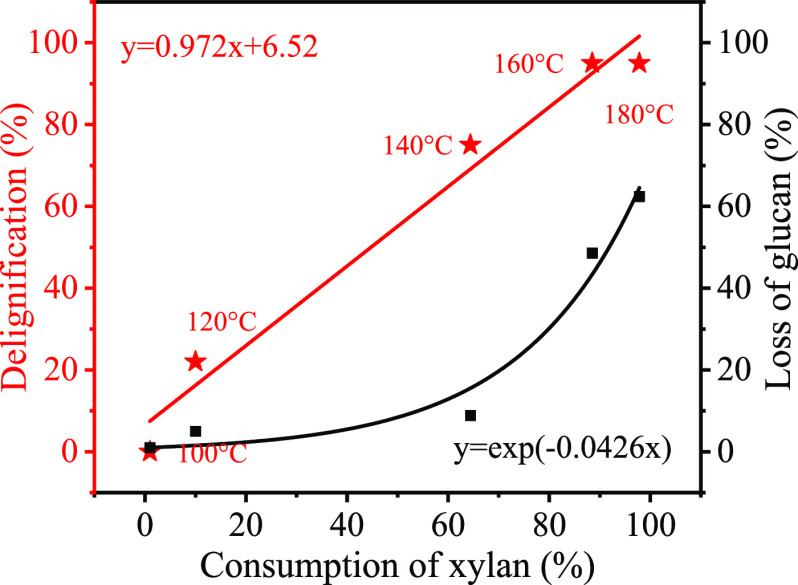
As known that hydrogen is necessary in the fractionation and depolymerization of lignin into phenolic monomers,25 so the origination of hydrogen in this process needs investigation. According to the results in Figures 1 and 2, we correlated the consumption of xylan, loss of glucan, and delignification at different temperatures (Figure 6). It showed that the consumption of xylan is basically linearly corrected to delignification, implying perhaps that there is relevance between lignin depolymerization and hemicellulose consumption. Meanwhile, the loss of glucan was less than 10% below 140 °C. Therefore, we speculated that hemicellulose played the role of a hydrogen source.
Figure 6.
Correlation between consumption of xylan, loss of glucan, and delignification at different temperatures.
To further confirm it, xylan and cellulose were taken as substrates to investigate the capacity of supplying hydrogen. As shown in Figure S8, the hydrogen production reached up to 4668 μmol g–1 with a hydrogen selectivity of 76.5% by using xylan as the substrate at 140 °C for 12 h, whereas it was only 227 μmol g–1 by using cellulose as the substrate. The high solid retention of 90 wt % in SCF of cellulose (Table S6) also confirmed the reactive inertness of cellulose. In order to explore the hydrogen production capacity of lignin at such mild conditions, we conducted the experiment by using lignin as the substrate, and a small quantity of monomers (1.7 wt %) was detected, whereas the yields of monomers increase to 7.9 wt % by adding xylan and to 19.5 wt % by adding xylose (Table S7). In addition, we also conducted a control experiment without any catalyst to see whether the hydrolysis of hemicellulose happens under reaction conditions; the results (Figure S9) showed that xylose was the main product (48.6%, based on a hemicellulose content of 18.4% in birch sawdust) in the liquid phase after the reaction. Thus, we propose that during the SCF process, hemicellulose is first hydrolyzed to xylose followed by dehydrogenation, C–C cleavage, or C–O cleavage to form CO intermediates and finally produce H2 and CO2 through the water gas shift reaction over the Pt/NiAl2O4 catalyst (Scheme S1). All the above results confirmed that hemicellulose in lignocellulosic biomass provided the hydrogen to drive the catalytic “reductive” fractionation.
3.3. Characterization of Cellulose in Carbohydrate Pulps
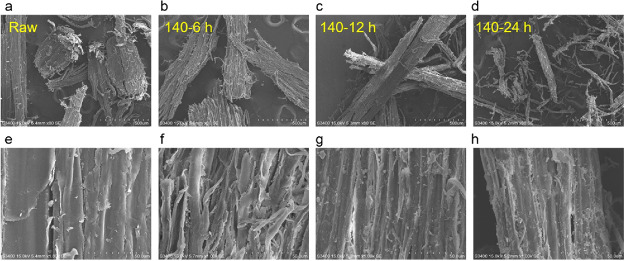
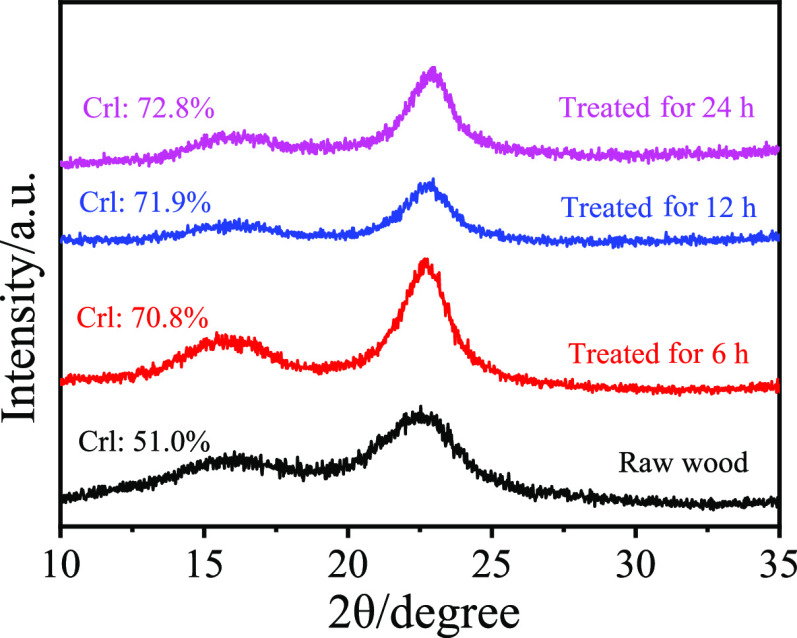
Scanning electron microscopy (SEM) was used to compare the microstructure and micromorphology of the cellulose-rich pulps obtained from SCF. As shown in Figure 7 at two different magnifications (80× and 1000×), the fibrous structure of the pulps was well-retained at different reaction times (6, 12, and 24 h), compared to that of the raw birch sawdust. It is worth noting that some small particles appeared on the surface of the pulps after reacting for 24 h (Figure 7h), which may be some wood fragments or pseudo-lignin.47 In addition, the pulps were also characterized by XRD, and the crystallinity index (CrI) was determined according to an empirical method developed by Segal et al.48 As shown in Figure 8, the CrI increased with prolonging of the reaction time because of gradual removal of the amorphous lignin and hemicellulose. Meanwhile, further prolonging the reaction time seems to not affect the crystallinity of the cellulose-rich pulps. The above results confirmed that the cellulose could be well-preserved in the SCF.
Figure 7.
Scanning electron microscopy images of the pulps obtained from raw birch wood (a,e), treated for 6 h (b,f), treated for 12 h (c,g), and treated for 24 h (d,h); a–d and e–h represent 80× and 1000× magnification, respectively.
Figure 8.
Crystallinity index (CrI) measured by X-ray diffraction at different reaction times.
4. Conclusions
In summary, we developed an innovative self-hydrogen supplied catalytic fraction (SCF) strategy by using structural hydrogens in hemicellulose as a hydrogen source to realize the fractionation and depolymerization of lignin to phenolic monomers while keeping the cellulose intact over the Pt/NiAl2O4 catalyst. This strategy exhibited high efficiency in the fractionation of birch sawdust and achieved nearly theoretical maximum yields of phenolic monomers with high selectivity for propyl(ethyl) end-chained syringol and guaiacol at 140 °C for 24 h, with 90% cellulose intact in birch sawdust. Meanwhile, good recyclability and regeneration could be achieved in this catalyst over operating conditions. This work has made significant progress in the development of high-performance, environment-friendly, and stable catalysts for lignin depolymerization as well as valorization of lignocellulosic biomass for sustainable biorefinery without consumption of exogenous hydrogen.
Acknowledgments
The authors acknowledge financial support from the National Key Research and Development Program of China (2022YFA1504903) and the National Natural Science Foundation of China (21832002 and 22102056).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacsau.3c00154.
Additional experimental results of the studied samples (Figures S1–S8 and Tables S1–S7), including XRD, HAADF-STEM, H2-TPR, XPS spectra, GC spectra, and GC–MS spectra (PDF)
Author Contributions
CRediT: Hao Zhou data curation, formal analysis, investigation, writing-original draft; Xiaohui Liu methodology, validation, writing-original draft; Yong Guo investigation, methodology, resources, writing-review & editing; Yanqin Wang conceptualization, resources, supervision, visualization, writing-review & editing.
The authors declare no competing financial interest.
Supplementary Material
References
- Tuck C. O.; Pérez E.; Horváth I. T.; Sheldon R. A.; Poliakoff M. Valorization of biomass: deriving more value from waste. Science 2012, 337, 695–699. 10.1126/science.1218930. [DOI] [PubMed] [Google Scholar]
- Alonso D. M.; Hakim S. H.; Zhou S.; Won W.; Hosseinaei O.; Tao J.; Garcia-Negron V.; Motagamwala A. H.; Mellmer M. A.; Huang K.; et al. Increasing the revenue from lignocellulosic biomass: Maximizing feedstock utilization. Sci. Adv. 2017, 3, e1603301 10.1126/sciadv.1603301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corma A.; Iborra S.; Velty A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. 10.1021/cr050989d. [DOI] [PubMed] [Google Scholar]
- Huber G. W.; Iborra S.; Corma A. Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. 10.1021/cr068360d. [DOI] [PubMed] [Google Scholar]
- Schutyser W.; Renders T.; Van den Bosch S.; Koelewijn S.-F.; Beckham G. T.; Sels B. F. Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. 10.1039/c7cs00566k. [DOI] [PubMed] [Google Scholar]
- Rinaldi R.; Jastrzebski R.; Clough M. T.; Ralph J.; Kennema M.; Bruijnincx P. C.; Weckhuysen B. M. Paving the way for lignin valorisation: recent advances in bioengineering, biorefining and catalysis. Angew. Chem., Int. Ed. 2016, 55, 8164–8215. 10.1002/anie.201510351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z.; Fridrich B.; De Santi A.; Elangovan S.; Barta K. Bright side of lignin depolymerization: toward new platform chemicals. Chem. Rev. 2018, 118, 614–678. 10.1021/acs.chemrev.7b00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakzeski J.; Bruijnincx P. C.; Jongerius A. L.; Weckhuysen B. M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. 10.1021/cr900354u. [DOI] [PubMed] [Google Scholar]
- Li C.; Zhao X.; Wang A.; Huber G. W.; Zhang T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. 10.1021/acs.chemrev.5b00155. [DOI] [PubMed] [Google Scholar]
- Park J.; Mushtaq U.; Sugiarto J. R.; Verma D.; Kim J. Total chemocatalytic cascade conversion of lignocellulosic biomass into biochemicals. Appl. Catal., B 2022, 310, 121280 10.1016/j.apcatb.2022.121280. [DOI] [Google Scholar]
- Ma Z.; Kasipandi S.; Wen Z.; Yu L.; Cui K.; Chen H.; Li Y. Highly efficient fractionation of corn stover into lignin monomers and cellulose-rich pulp over H2WO4. Appl. Catal., B 2021, 284, 119731 10.1016/j.apcatb.2020.119731. [DOI] [Google Scholar]
- Chou W.; Liu D.; Li W.; Chou X.; Liu H.; Wu C.; Wu P.; Men Z.; Li Z. Full utilization of lignocellulose through one-pot in-situ hydro-liquefaction with versatile Pt/CeCrO2–x catalyst. Appl. Catal., B 2022, 316, 121625 10.1016/j.apcatb.2022.121625. [DOI] [Google Scholar]
- Adler A.; Kumaniaev I.; Karacic A.; Baddigam K. R.; Hanes R. J.; Subbotina E.; Bartling A. W.; Huertas-Alonso A. J.; Moreno A.; Håkansson H.; et al. Lignin-first biorefining of Nordic poplar to produce cellulose fibers could displace cotton production on agricultural lands. Joule 2022, 6, 1845–1858. 10.1016/j.joule.2022.06.021. [DOI] [Google Scholar]
- Shui T.; Feng S.; Yuan Z.; Kuboki T.; Xu C. C. Highly efficient organosolv fractionation of cornstalk into cellulose and lignin in organic acids. Bioresour. Technol. 2016, 218, 953–961. 10.1016/j.biortech.2016.07.054. [DOI] [PubMed] [Google Scholar]
- Mahmood N.; Yuan Z.; Schmidt J.; Xu C. C. Hydrolytic depolymerization of hydrolysis lignin: Effects of catalysts and solvents. Bioresour. Technol. 2015, 190, 416–419. 10.1016/j.biortech.2015.04.074. [DOI] [PubMed] [Google Scholar]
- Abu-Omar M. M.; Barta K.; Beckham G. T.; Luterbacher J. S.; Ralph J.; Rinaldi R.; Román-Leshkov Y.; Samec J. S. M.; Sels B. F.; Wang F. Guidelines for performing lignin-first biorefining. Energy Environ. Sci. 2021, 14, 262–292. 10.1039/d0ee02870c. [DOI] [Google Scholar]
- Renders T.; Van den Bosch S.; Koelewijn S.-F.; Schutyser W.; Sels B. F. Lignin-first biomass fractionation: the advent of active stabilisation strategies. Energy Environ. Sci. 2017, 10, 1551–1557. 10.1039/c7ee01298e. [DOI] [Google Scholar]
- Van den Bosch S.; Schutyser W.; Vanholme R.; Driessen T.; Koelewijn S. F.; Renders T.; De Meester B.; Huijgen W. J. J.; Dehaen W.; Courtin C. M.; et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748–1763. 10.1039/c5ee00204d. [DOI] [Google Scholar]
- Brienza F.; Van Aelst K.; Devred F.; Magnin D.; Sels B. F.; Gerin P. A.; Cybulska I.; Debecker D. P. Reductive Catalytic Fractionation of Wheat Straw Biomass. ACS Sustainable Chem. Eng. 2022, 10, 11130–11142. 10.1021/acssuschemeng.2c02012. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Li H.; Su S.; Chen J.; Liu C.; Wang S.; Xu X.; Song G. Integration of Ru/C and base for reductive catalytic fractionation of triploid poplar. Chin. J. Catal. 2022, 43, 802–810. 10.1016/s1872-2067(21)63881-0. [DOI] [Google Scholar]
- O’Dea R. M.; Pranda P. A.; Luo Y.; Amitrano A.; Ebikade E. O.; Gottlieb E. R.; Ajao O.; Benali M.; Vlachos D. G.; Ierapetritou M.; et al. Ambient-pressure lignin valorization to high-performance polymers by intensified reductive catalytic deconstruction. Sci. Adv. 2022, 8, eabj7523 10.1126/sciadv.abj7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. M.; Katahira R.; Reed M.; Resch M. G.; Karp E. M.; Beckham G. T.; Román-Leshkov Y. Reductive Catalytic Fractionation of Corn Stover Lignin. ACS Sustainable Chem. Eng. 2016, 4, 6940–6950. 10.1021/acssuschemeng.6b01858. [DOI] [Google Scholar]
- Van Aelst K.; Van Sinay E.; Vangeel T.; Cooreman E.; Van den Bossche G.; Renders T.; Van Aelst J.; Van den Bosch S.; Sels B. F. Reductive catalytic fractionation of pine wood: elucidating and quantifying the molecular structures in the lignin oil. Chem. Sci. 2020, 11, 11498–11508. 10.1039/d0sc04182c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renders T.; Schutyser W.; Van den Bosch S.; Koelewijn S.-F.; Vangeel T.; Courtin C. M.; Sels B. F. Influence of Acidic (H3PO4) and Alkaline (NaOH) Additives on the Catalytic Reductive Fractionation of Lignocellulose. ACS Catal. 2016, 6, 2055–2066. 10.1021/acscatal.5b02906. [DOI] [Google Scholar]
- Ouyang X.; Huang X.; Zhu J.; Boot M. D.; Hensen E. J. M. Catalytic Conversion of Lignin in Woody Biomass into Phenolic Monomers in Methanol/Water Mixtures without External Hydrogen. ACS Sustainable Chem. Eng. 2019, 7, 13764–13773. 10.1021/acssuschemeng.9b01497. [DOI] [Google Scholar]
- Yan N.; Zhao C.; Dyson P. J.; Wang C.; Liu L. T.; Kou Y. Selective degradation of wood lignin over noble-metal catalysts in a two-step process. ChemSusChem 2008, 1, 626–629. 10.1002/cssc.200800080. [DOI] [PubMed] [Google Scholar]
- Song Q.; Wang F.; Cai J.; Wang Y.; Zhang J.; Yu W.; Xu J. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ. Sci. 2013, 6, 994–1007. 10.1039/c2ee23741e. [DOI] [Google Scholar]
- Li C.; Zheng M.; Wang A.; Zhang T. One-pot catalytic hydrocracking of raw woody biomass into chemicals over supported carbide catalysts: simultaneous conversion of cellulose, hemicellulose and lignin. Energy Environ. Sci. 2012, 5, 6383–6390. 10.1039/c1ee02684d. [DOI] [Google Scholar]
- Chen J.; Lu F.; Si X.; Nie X.; Chen J.; Lu R.; Xu J. High Yield Production of Natural Phenolic Alcohols from Woody Biomass Using a Nickel-Based Catalyst. ChemSusChem 2016, 9, 3353–3360. 10.1002/cssc.201601273. [DOI] [PubMed] [Google Scholar]
- Zhai Y.; Li C.; Xu G.; Ma Y.; Liu X.; Zhang Y. Depolymerization of lignin via a non-precious Ni–Fe alloy catalyst supported on activated carbon. Green Chem. 2017, 19, 1895–1903. 10.1039/c7gc00149e. [DOI] [Google Scholar]
- Rautiainen S.; Di Francesco D.; Katea S. N.; Westin G.; Tungasmita D. N.; Samec J. S. M. Lignin Valorization by Cobalt-Catalyzed Fractionation of Lignocellulose to Yield Monophenolic Compounds. ChemSusChem 2019, 12, 404–408. 10.1002/cssc.201802497. [DOI] [PubMed] [Google Scholar]
- Park J.; Cahyadi H. S.; Mushtaq U.; Verma D.; Han D.; Nam K.-W.; Kwak S. K.; Kim J. Highly Efficient Reductive Catalytic Fractionation of Lignocellulosic Biomass over Extremely Low-Loaded Pd Catalysts. ACS Catal. 2020, 10, 12487–12506. 10.1021/acscatal.0c03393. [DOI] [Google Scholar]
- Liu Z.; Li H.; Gao X.; Guo X.; Wang S.; Fang Y.; Song G. Rational highly dispersed ruthenium for reductive catalytic fractionation of lignocellulose. Nat. Commun. 2022, 13, 4716. 10.1038/s41467-022-32451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.; Pan L.; van Muyden A. P.; Bai L.; Li J.; Tong Y.; Fei Z.; Hagfeldt A.; Laurenczy G.; Dyson P. J. Anchoring single platinum atoms onto nickel nanoparticles affords highly selective catalysts for lignin conversion. Cell Rep. Phys. Sci. 2021, 2, 100567 10.1016/j.xcrp.2021.100567. [DOI] [Google Scholar]
- Wang S.; Zhang K.; Li H.; Xiao L. P.; Song G. Selective hydrogenolysis of catechyl lignin into propenylcatechol over an atomically dispersed ruthenium catalyst. Nat. Commun. 2021, 12, 416. 10.1038/s41467-020-20684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkin M. V.; Samec J. S. Selective route to 2-propenyl aryls directly from wood by a tandem organosolv and palladium-catalysed transfer hydrogenolysis. ChemSusChem 2014, 7, 2154–2158. 10.1002/cssc.201402017. [DOI] [PubMed] [Google Scholar]
- Galkin M. V.; Smit A. T.; Subbotina E.; Artemenko K. A.; Bergquist J.; Huijgen W. J.; Samec J. S. Hydrogen-free catalytic fractionation of woody biomass. ChemSusChem 2016, 9, 3280–3287. 10.1002/cssc.201600648. [DOI] [PubMed] [Google Scholar]
- Dou Z. L.; Zhang Z.; Wang M. Self-hydrogen transfer hydrogenolysis of native lignin over Pd-PdO/TiO2. Appl. Catal., B 2022, 301, 120767 10.1016/j.apcatb.2021.120767. [DOI] [Google Scholar]
- Si X.; Zhao Z.; Chen J.; Lu R.; Lu F. Low-Temperature Efficient Hydrogen Production from Raw Biomass on the Ni–Mo Catalyst. ACS Catal. 2022, 12, 10629–10637. 10.1021/acscatal.2c02706. [DOI] [Google Scholar]
- Wang E.; Mahmood A.; Chen S.-G.; Sun W.; Muhmood T.; Yang X.; Chen Z. Solar-Driven Photocatalytic Reforming of Lignocellulose into H2 and Value-Added Biochemicals. ACS Catal. 2022, 12, 11206–11215. 10.1021/acscatal.2c02624. [DOI] [Google Scholar]
- Li L.; Dong L.; Li D.; Guo Y.; Liu X.; Wang Y. Hydrogen-Free Production of 4-Alkylphenols from Lignin via Self-Reforming-Driven Depolymerization and Hydrogenolysis. ACS Catal. 2020, 10, 15197–15206. 10.1021/acscatal.0c03170. [DOI] [Google Scholar]
- Li D.; Li Y.; Liu X.; Guo Y.; Pao C.-W.; Chen J.-L.; Hu Y.; Wang Y. NiAl2O4 Spinel Supported Pt Catalyst: High Performance and Origin in Aqueous-Phase Reforming of Methanol. ACS Catal. 2019, 9, 9671–9682. 10.1021/acscatal.9b02243. [DOI] [Google Scholar]
- Shuai L.; Amiri M. T.; Questell-Santiago Y. M.; Héroguel F.; Li Y.; Kim H.; Meilan R.; Chapple C.; Ralph J.; Luterbacher J. S. Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization. Science 2016, 354, 329–333. 10.1126/science.aaf7810. [DOI] [PubMed] [Google Scholar]
- Sluiter A.; Hames B.; Ruiz R.; Scarlata C.; Sluiter J.; Templaton D.; Crocker D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1–15. [Google Scholar]
- Sluiter A.; Hames B.; Ruiz R.; Scarlata C.; Sluiter J.; Templaton D. Determination of sugars, byproducts, and degradation products in liquid fraction process samples. Golden: Natl. Renewable Energy Lab. 2006, 65–71. [Google Scholar]
- Ren T.; Zhang Z.; You S.; Qi W.; Su R.; He Z. Isolation and purification of 4-propylguaiacol and 4-propylsyringol by extraction and crystallization from the products of reductive catalytic fractionation processes. Green Chem. 2022, 24, 7355–7361. 10.1039/d2gc01863b. [DOI] [Google Scholar]
- Zhuang J.; Wang X.; Xu J.; Wang Z.; Qin M. Formation and deposition of pseudo-lignin on liquid-hot-water-treated wood during cooling process. Wood Sci. Technol. 2017, 51, 165–174. 10.1007/s00226-016-0872-7. [DOI] [Google Scholar]
- Segal L.; Creely J. J.; Martin A. E.; Conrad C. M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. 10.1177/004051755902901003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.