Abstract
Background
Hydatigera (Cestoda: Taeniidae) is a recently resurrected genus with the description of a new species, Hydatigera kamiyai, a cryptic entity within the Hydatigera taeniaeformis species complex. Rodents are intermediate hosts and correct taxonomic identification of H. taeniaeformis sensu lato (s.l.) species is difficult without the use of molecular methods. The aim of this study was to identify and explore the genetic diversity of Hydatigera and other taeniid species.
Methods
Ten different small mammals species (856 individuals) (Rattus rattus, three Apodemus, three Arvicolinae and three Soricidae species) were examined from 2013 to 2023. Captured animals were visually examined for cysts and visible lesions. Two markers were used for amplification and sequencing: cox1 and 12S rDNA.
Results
Molecular analysis of cysts and visible lesions revealed four taeniid species: Hydatigera kamiyai, H. taeniaeformis sensu stricto (s.s.), Taenia martis and T. crassiceps. Hydatigera kamiyai was found in Apodemus flavicollis, A. agrarius, Microtus arvalis and Crocidrua leucodon, while H. taeniaeformis s.s. is registered in R. rattus. Hydatigera kamiyai cox1 sequences clustered with European populations and showed at least 25 nucleotid differences compared to Asian, African, Australian and one of our isolates of H. taeniaeformis s.s acquired from a rat, followed by large sequence distances (9.4% to 12.9%), indicating clear molecular distinction of two species.
Conclusions
This is one of the few mitochondrial gene-based studies performed after the description of cryptic entities within the Hydatigera taeniaeformis s.l. complex and represents a valuable contribution to understanding of genetic diversity, host suitability and geographic distribution of these tapeworm species. Also, our study provides an important basis of molecular data from this part of Europe for further studies. We emphasize the importance of additional studies of intermediate hosts, especially rats from Europe and Apodemus spp. and voles from Asia and Africa.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-023-05879-x.
Keywords: Parasite, Taenia, Rodents, cox1, 12S rDNA, Phylogenetics
Background
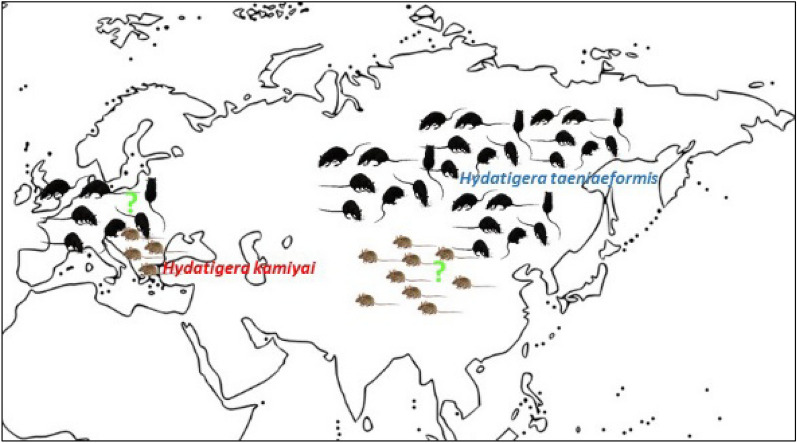
The cyclophyllidean family, Taeniidae, consists of four genera: Taenia Linnaeus, 1758; Echinococcus Rudolphi, 1801, Hydatigera Lamarck, 1816; and Versteria. Until recently, only two genera were recognized. Nakao et al. [1] proposed the creation of a new genus, Versteria, for Taenia mustelae and the resurrection of Hydatigera for Taenia taeniaeformis, Taenia parva and Taenia krepkogorski. There have also been other recent changes, with the description of a new species, Hydatigera kamiyai, a cryptic entity within the Hydatigera taeniaeformis species complex. Finally, Hydatigera genus consists of four valid species: H. kamiyai, H. taeniaeformis s.s., H. krepkogorski and H. parva [1, 2]. Hydatigera kamiyai is distributed across Europe to western Siberia, with an unexplained isolated case in Japan, while H. taeniaeformis s.s. probably originated in Asia but has spread worldwide [2]. In addition, there is a third cryptic lineage within the H. taeniaeformis complex that is restricted to the Mediterranean region, but its taxonomic status is still unclear, and it is currently referred to as Hydatigera sp. [2, 3].
The life cycle of taeniid cestodes includes two obligate mammalian hosts. Carnivorous or omnivorous animals are definitive hosts, while other mammals, particularly rodents, play an important role as intermediate hosts. Some species have zoonotic potential due to human infection by the larval stage [4]. In addition to detecting infection in the final host, knowledge of the role of rodents in the parasite life cycle and identification of parasite species is also important for determining potential transmission and management of zoonoses [5, 6]. Several species of Arvicolinae are considered the most important intermediate host for Echinococcus multilocularis [5, 7], a parasite that causes one of the most severe parasitic infections in humans. Infection with H. taeniaeformis s.l. is widespread in wild rodents (rats, voles and mice) and cats as the main definitive host [2, 3, 8]. Cysticercosis of H. taeniaeformis, Taenia crassiceps, T. martis and Versteria sp. develops naturally in rodents and accidentally in humans. The definitive hosts for T. crassiceps are various Carnivora species, while the carriers of T. martis and Versteria sp. are mainly mustelids [4]. In some tapeworm genera, differences between species (in adults and larvae stage) are subtle because of high morphological plasticity. Identification could be difficult without the use of molecular methods to ensure correct taxonomic identification, especially in the not yet fully developed larval stage [7, 9–12]. Hydatigera taeniaeformis was recently confirmed as a complex of three cryptic entities that differ very little morphologically (practically only in the dimensions of the rostellar hooks) despite extraordinary genetic divergence [2], highlighting the need of molecular identification. Since then, several studies have been conducted on the H. taeniaeformis s.l. complex, mainly based on analyses of the mitochondrial marker cytochrome c oxidase subunit 1 (cox1) [8, 13–18]. However, due to the small number of studies, geographic distribution and host susceptibility of this group of parasites remains poorly undertood. In this context, aims of the present study were: (i) to identify presence of Hydatigera and other taeniid species in various small mammals using molecular methods; (ii) to investigate genetic diversity and provide molecular data for further studies on intraspecific variation within the H. taeniaeformis s.l. complex. Considering the geographical location of the studied area and the targeted intermediate hosts, we expected the presence of H. kamiyai in our sample. Moreover, this is the first molecular and genetic study of larval taeniid infections in small mammals in Serbia.
Methods
Trapping of small mammals and necropsy
A total of 856 small mammals from 45 different locations (Additional file 1: Figure S1) in Serbia were captured from 2013 to 2023 (March-October). Ten different species of small mammals were identified morphologicaly: Apodemus flavicollis (n = 520), A. agrarius (n = 152), A. sylvaticus (n = 34), Microtus arvalis (n = 48), M. subterraneus (n = 17), Myodes glareolus (n = 52), Rattus rattus (n = 10), Crocidura suaveolens (n = 13), C. leucodon (n = 7) and Sorex araneus (n = 3). The animals were trapped using Longworth live traps containing dry hay and wheat grains in the nest box and baited with a mixture of oatmeal and sardines. Traps set in the afternoon were checked in the early morning, and captured animals were transported to the laboratory in suitable cages. Captured animals were killed and visually examined during necropsy for cysts and visible lesions in organs, abdominal and thoracic cavities. All tissue lesions were stored at −20 ℃ for further molecular studies.
DNA extraction, amplification and sequencing
Genomic DNA from parasite specimens (small pieces of host’s infected tissue) was extracted using the Quick-DNA MicroPrep Kit (Zymo Research, USA) according to the manufacturer's instructions, preceded by an overnight digestion with proteinase K. Two markers were used for amplification and sequencing: a fragment of approximately 400 bp of the cytochrome c oxidase subunit 1 (cox1) was amplified using the primers JB3 and JB45 [19] and a fragment of approximately 350 bp of mitochondrial (mt) 12S rDNA was amplified using the primers P60 for and P375 rev [20]). Primer sequences and PCR details and conditions are given in Additional file 1: Tables S1 and S2. The amplification products were separated by agarose gel electrophoresis, stained with Midori Green Direct (Nippon Genetics Europe), visualised on a Bio-Rad Gel Doc 1000 (Bio-Rad Laboratories, Hercules, California, USA) and subsequently sent for commercial sequencing in both directions.
Data analysis
Molecular and phylogenetic analyses
Phylogenetic analyses were conducted separately for each molecular marker. Sequences were aligned and visually inspected using Clustal W in MEGA (v.11) software. Sequences were trimmed to a uniform length of 318 nucleotides for cox1 gene and 238 nucleotides for 12S rDNA and compared with sequences deposited in the GenBank database. Sequences deposited from 2010 to the present were used to correspond to the period of collection of our samples. A Neighbour-joining tree was constructed with MEGA (v.11) using the Tamura-Nei model, with 10.000 bootstrap replicates.
Population genetic analysis
Species’ identity was established by matching the obtained sequences with ones in GenBank using the BLAST tool. The genetic diversity (number of haplotypes, haplotype diversity and nucleotide diversity) and neutrality indices (Fu’s Fs and Tajima’s D) were calculated using DnaSP 6.12.03 [21]. A median-joining network [22] was constructed with 13 cox1 nucleotide sequences from Serbia (present study) and 28 from the GenBank database, using PopART 1.7. Pairwise nucleotide sequence divergences were calculated using the Kimura 2-parameter (K2P) model [23] with a gamma setting of 0.5 in MEGA (V.11) software.
Results
Visible lesions and cysts were detected in 57 animals (6.7%). The cysticercus form of the parasite was predominantly found in the liver (75.4%), making it the most common site of infestation. Following the liver, the thoracic cavity accounted for the next highest occurrence (19.3%). Additionally, a smaller proportion of cases involved the mesentery and abdominal wall (5.3%). All observed pathological changes exhibited the characteristic morphology of classical metacestodes, representing the typical form of cysticercus. However, one exceptional case was identified, characterized by a unique phenomenon known as the budding of cysticerci. This rare occurrence resulted in a significant and distinctive infection (Additional file 1: Figure S2).
Of the total visible lesions and cysts observed in 57 animals, successful amplification of cox1 and 12S rDNA fragments was achieved in 16 and 13 larval samples, respectively. Amplification of 12S rDNA gene was performed only when cox1 was negative. Hydatigera kamiyai was found in 17 A. flavicollis, one A. agrarius, two M. arvalis and one C. leucodon. Hydatigera taeniaeformis s.s. was registred in one rat (R. rattus). Taenia martis was found in four A. flavicollis, one A. sylvaticus and one M. glareolus, while T. crassiceps was detected in only one animal (M. arvalis) (Table 1). Neither a cyst nor visible lesions were detected in M. subterraneus, C. suaveolens and S. araneus. All 13 cox1 sequences and eight 12S rDNA sequences of H. kamiyai had 99.37–100% identity with NC037071 sequence from GenBank. One cox1 sequence of H. taeniaeformis s.s had 100% identity with KT693060 and MH938573 previously published sequences. Two larval samples were identified as T. martis, based on 100% identity of cox1 marker with isolated origin from a human brain from France (KP198618). Four 12S rDNA sequences of T. martis had 100% identity with three sequences from the GenBank database (LT837855, KT943415, AB731758). Comparison between our 12S rDNA sequence of T. crassiceps and the sequences from the GenBank database (AF216699 and MN505206) showed 100% identity.
Table 1.
Host data and GenBank accession number for all sequences
| Genus | ID | Host species, gender | Localities, year | Parasite species | GenBank ID | |
|---|---|---|---|---|---|---|
| 12S rDNA | COX1 | |||||
| Rattus | 3/23 | R. rattus, f | Belgrade-New Belgrade, 2023 | H.taeniaeformis s.s | / | OQ832778 |
| Apodemus | 3669 | A. flavicollis, m | Belgrade-Košutnjak, 2013 | H. kamiyai | OQ834423 | / |
| 3754 | A. flavicollis, f | Petnica 2014 | H. kamiyai | OQ834422 | / | |
| 3922 | A. flavicollis, m | Petnica, 2014 | H. kamiyai | OQ834421 | / | |
| 4440 | A. flavicollis, f | Belgrade-Ada Ciganlija, 2021 | H. kamiyai | OQ834420 | / | |
| 4471 | A. flavicollis, m | Stara planina -Senokos, 2021 | H. kamiyai | OQ834419 | / | |
| 4521 | A. flavicollis, f | Ada Ciganlija, 2021 | H. kamiyai | OQ834418 | / | |
| 3702 | A. flavicollis, f | Belgrade-Košutnjak, 2014 | T. martis | OQ834427 | / | |
| 3712 | A. flavicollis, f | Petnica, 2014 | T. martis | OQ834428 | / | |
| 3853 | A. flavicollis, f | Misača, 2014 | T. martis | / | OQ592885 | |
| 4155 | A. flavicollis, f | Petnica, 2017 | T. martis | / | OQ592884 | |
| 3708 | A. flavicollis, f | Petnica, 2014 | H. kamiyai | / | OQ569719 | |
| 3823 | A. flavicollis, m | Misača, 2014 | H. kamiyai | / | OQ569721 | |
| 3961 | A. flavicollis, m | Belgrade-Košutnjak, 2015 | H. kamiyai | / | OQ569723 | |
| 4158 | A. flavicollis, m | Petnica, 2017 | H. kamiyai | / | OQ569724 | |
| 4587 | A. flavicollis, f | Belgrade-Autoput, Kvantaš, 2022 | H. kamiyai | / | OQ569725 | |
| 4591 | A. flavicollis, f | Belgrade-Autoput, Kvantaš, 2022 | H. kamiyai | / | OQ569726 | |
| 3711 | A. flavicollis, f | Petnica, 2014 | H. kamiyai | / | OQ569728 | |
| 3758 | A. flavicollis, f | Ruski Krstur, 2014 | H. kamiyai | / | OQ569729 | |
| 3895 | A. flavicollis, m | Ruski Krstur, 2014 | H. kamiyai | / | OQ569730 | |
| 3954 | A. flavicollis, m | Belgrade-Košutnjak, 2015 | H. kamiyai | / | OQ569731 | |
| 4627 | A. flavicollis, m | Belgrade-Autoput, Kvantaš, 2022 | H. kamiyai | / | OQ569727 | |
| 3873 | A. agrarius, m | Misača, 2014 | H. kamiyai | / | OQ569722 | |
| 4152 | A. sylvaticus f | Petnica, 2017 | T. martis | OQ834429 | / | |
| Microtus | 3761 | M. arvalis f | Ruski Krstur, 2014 | H. kamiyai | OQ834424 | / |
| 4449 | M. arvalis, f | Belgrade-Ada ciganlija, 2021 | H. kamiyai | OQ834425 | / | |
| 4514 | M. arvalis, f | Belgrade -Ada Ciganlija, 2021 | T. crassiceps | OQ834430 | / | |
| Myodes | 4161 | M. glareolus, f | Petnica, 2017 | T. martis | OQ834426 | / |
| Crocidura | 3989 | C. leucodon, m | Cer, 2015 | H. kamiyai | / | OQ569720 |
ID protocol number, m male, f female
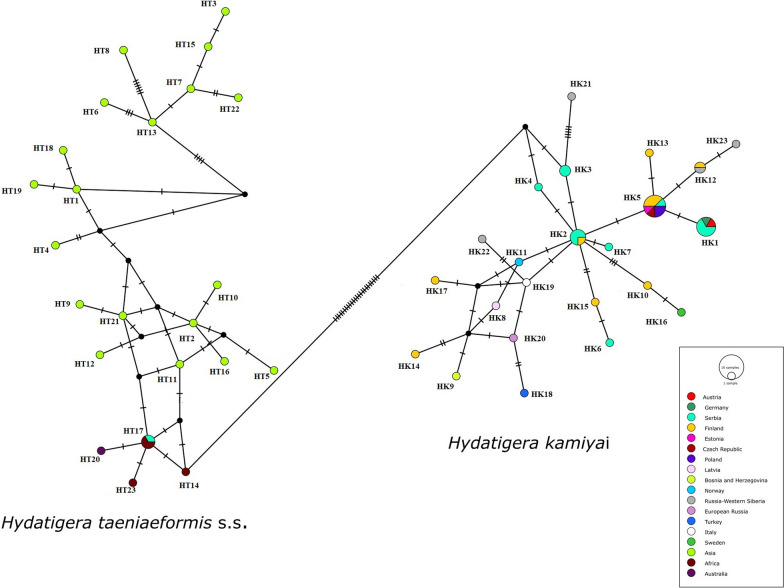
In this study, we recorded seven haplotypes of cox1 gene, out which four were reported for the first time (HK3, HK4, HK6 and HK7) and submitted to GenBank. Thirteen H. kamiyai cox1 gene partial sequences from this study and 28 from GenBank database corresponded to 23 haplotypes (HK1-HK23), whereas 19 were already known from deposited sequences (Table 2). Lavikainen et al. [2] described 22 haplotypes (named B1-B22 in original study) using 396 bp of the gene that had to be truncated in this study to the 318 bp length, resulting in a reduction of the number of haplotypes to 19. New haplotypes HK4, HK6 and HK7 were represented with a single sequence each, and haplotype HK3 was represented with two sequences. Eight sequences from our study clustered with previously recorded B16 (HK1), B7 (HK2) and B3/B12 (HK5) (Table 2). According to the median-joining network, all the 13 H. kamiyai cox1 sequences from this study clustered with populations from Europe. Two main haplotypes (HK1 and HK5) were observed, representing 34.1% of the total sample of sequences. A high presence of haplotypes HK1 and HK2 is registered in our samples. HK2 takes a central position in the haplotype network, with no more than 1–6 nucleotide differences from other haplotypes. European haplotypes had at least 25 mutation steps from Asian, African and Australian isolates (H. taeniaeformis s.s.). One isolate of H. taeniaeformis s.s. from this study derived from rat shared same haplotype with samples from Africa and clustered with African populations (Fig. 1). These results confirm and highlight the differential geographic distribution and molecular distinctiveness in H. taeniaeformis s.l. complex.
Table 2.
Haplotypes (cox1) of Hydatigera kamiyai found in Serbia and other European countries
| cox 1 haplotypes (HK1-HK23) | Cox1 haplotypes original name and GenBank ID | Country | Hosts | References |
|---|---|---|---|---|
| HK1 |
B16 (JQ663994) |
Germany Austria Serbia Serbia Serbia Serbia |
Felis silvestris catus, Microtus arvalis Apodemus flavicollis Crocidura leucodon Apodemus flavicollis Apodemus flavicollis |
[25] [2] |
| HK2 |
B7 (KT693082) |
Finland Serbia Serbia Serbia |
Felis silvestris catus Apodemus agrarius Apodemus flavicollis Apodemus flavicollis |
[2] |
| HK3 | SRB1 (OQ569724)* SRB1 (OQ569730)* |
Serbia Serbia |
Apodemus flavicollis Apodemus flavicollis |
|
| HK4 | SRB2 (OQ569725)* | Serbia | Apodemus flavicollis | |
| HK5 |
B3 (EU861478) B12 (KT693087) |
Finland Finland Finland Estonia Czech Republic Poland Poland Serbia |
Felis catus, Felis silvestris catus Felis catus Felis catus Falco tinnunculus Microtus arvalis Myodes glareolus Apodemus flavicollis |
[15] [2] |
| HK6 | SRB3 (OQ569728)* | Serbia | Apodemus flavicollis | |
| HK7 | SRB4 (OQ569731)* | Serbia | Apodemus flavicollis | |
| HK8 | B1 (KT693076) | Latvia | Apodemus flavicollis | [2] |
| HK9 | B2 (KT693077) | Bosnia and Herzegovina | Apodemus flavicollis | [2] |
| HK10 | B4 (KT693079) | Finland | Microtus agrestis | [2] |
| HK11 | B5 (KT693080) | Norway | Microtus agrestis | [2] |
| HK12 |
B6 (KT693081) B19 (KT693091) |
Finland, Russia-Western Siberia |
Felis silvestris catus Microtus agrestis |
[2] |
| HK13 | B8 (KT693083) | Finland | Felis silvestris catus | [2] |
| HK14 | B9 (KT693084) | Finland | Felis silvestris catus | [2] |
| HK15 | B10 (KT693085) | Finland | Felis silvestris catus | [2] |
| HK16 | B11 (KT693086) | Sweden | Arvicola amphibius | [2] |
| HK17 | B13 (KT693088) | Finland | Felis silvestris catus | [2] |
| HK18 | B14 (EU544596) | Turkey | Apodemus sylvaticus | [2] |
| HK19 | B15 (FN547850) | Italy | Felis silvestris silvestris | [2] |
| HK20 |
B17(KT693089) B18 (KT693090) |
European Russia European Russia |
Apodemus uralensis Apodemus uralensis |
[2] |
| HK21 | B20 (KT693092) | Russia-Western Siberia | Alticola strelzowi | [2] |
| HK22 | B21 (KT693093) | Russia-Western Siberia | Mus musculus | [2] |
| HK23 | B22 (KT693094) | Russia-Western Siberia | Myodes rutilus | [2] |
Bold-samples/sequences from this study
Fig. 1.
Median-joining network of Hydatigera kamiyai isolates from Serbian small mammals and other different hosts from Europe and Western Siberia based on cox1 gene sequences (as given in Table 2). Haplotype network also shows relations between European and West Siberian H. kamiyai isolates and Asian, Australian, African and one of our isolates of Hydatigera taeniaeformis s.s. (19 sequences [2]; 1 sequence [27]; 1 sequence [46]; 1 sequence [47]; 1 sequence [18])
Sequences of cox1 gene from this study differed by 0–2.7% from H. kamiyai isolates from Europe and western Siberia. On the other hand, pairwise divergences with Asian, African, Australian and a single rat-derived isolate of H. taeniaeformis s.s from Serbia were high, ranging from 9.4 to 12.9%. In addition, 12S rDNA sequences from this study differed up to 0.42% from the rest of European isolates and by 7.46–8.07% from Asian isolates. In the phylogenetic analyses results the Serbian samples were always clustered with the European ones and were distant from the Asian and African isolates (Figs. 2, 3).
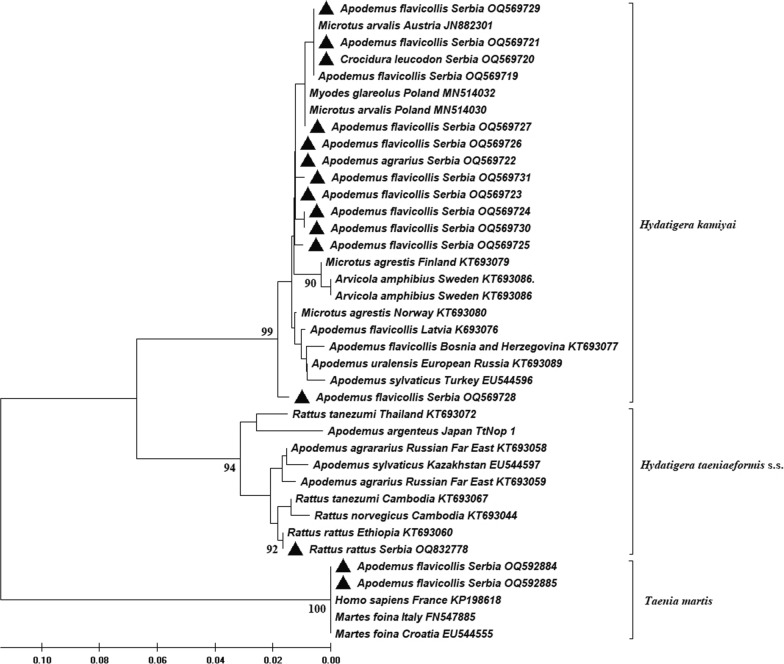
Fig. 2.
Phylogenetic tree of the Hydatigera taeniaeformis species complex based on 318 bp cox1 gene sequences. The tree was inferred using neighbour-joining method constructed with MEGA (v.11) using the Tamura-Nei model, with 10,000 bootstrap replicates. Values > 90% are shown. The sequences of Taenia martis from this and other studies are shown in the tree. Black triangles indicate our isolates
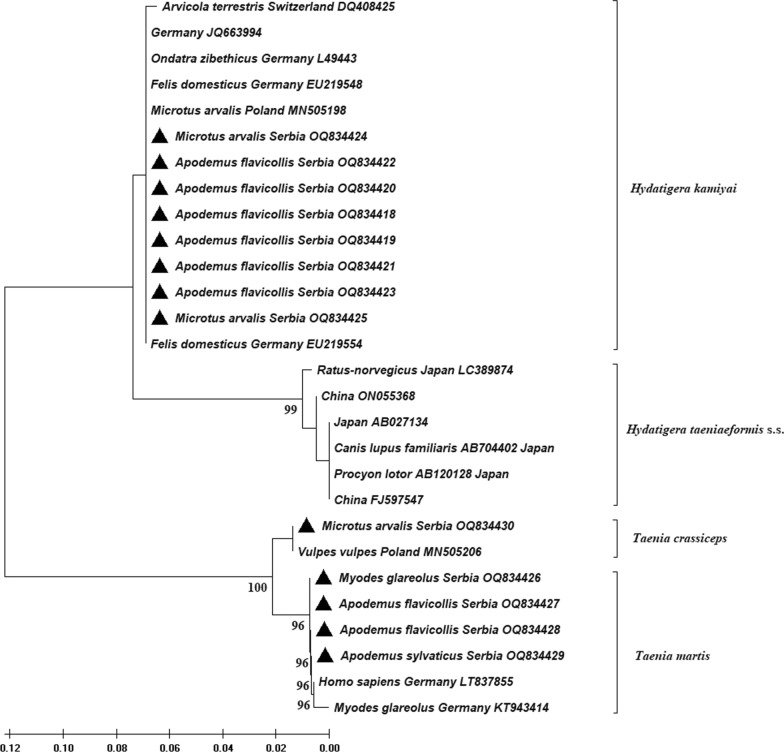
Fig. 3.
Phylogenetic tree of the Hydatigera taeniaeformis species complex based on 238 bp 12S rDNA gene sequences. The tree was inferred using neighbour-joining method constructed with MEGA (v.11) using the Tamura-Nei model, with 10,000 bootstrap replicates. Values > 90% are shown. The sequences of Taenia martis and T. crassiceps from this and other study are shown in the tree. Black triangles indicate our isolates
Polymorphism analysis of 13 H. kamiyai cox1 sequences showed eight polymorphic sites, three parsimony informative sites and five singleton variable sites, resulting in seven haplotypes. Haplotype diverstiy (Hd) and nucleotide diversity (π) of 0.872 and 0.00637, respectively, were observed, with no significant negative Tajima’s D (0.82982) and Fu’s F (−2.255) values (Table 3). All 12S rDNA sequences (238 bp) of H. kamiyai from this study corresponded to the same haplotype, revealing no genetic diversity.
Table 3.
Summary statistics of DNA polymorphism and neutrality tests based on sequences of the cox1 gene from this study
| Indices | Cox1 (318 bp) |
|---|---|
| No. of sequences | 13 |
| Variable (polymorphic) sites | 8 |
| Parsimony informative sites | 3 |
| Singleton variable site | 5 |
| No. of haplotypes | 7 |
| Haplotype diversity (Hd) | 0.872 |
| Nucleotide diversity (π) | 0.00637 |
| Nucleotide differences (k)* | 2.026 |
| Tajima’s D | -0.82982 (NS) |
| Fu's Fs | −2.255 (NS) |
*Average number of pairwise nucleotide differences (k)
NS = not significant
Discussion
In the present study, ten different species of small mammals (R. ratus, three Apodemus, three Arvicolinae and three Soricidae species) were examined for the presence of taeniid metacestodes. Despite targeting intermediate host, E. multilocullaris was not recorded. This can be explained by the absence of samples collected in a hot-spot area of alveolar echinococcosis in the northern part of Serbia (Vojvodina, Srem) [11, 24]. This result may also indicate that the disease is still present only in the registered focal area and has not spread south of the Sava and Danube Rivers. Molecular analysis of cysts and visible lesions revealed four taeniid species: H. kamiyai, H. taeniaeformis s.s., T. martis and T. crassiceps in voles, rats and Apodemus spp. These wild rodents are suitable intermediate hosts for registered parasite species, which is consistent with other studies [2, 7, 15, 25, 26]. Shrews (family Soricidae) are not considered intermediate hosts for Hydatigera species, but we found H. kamiyai at larval stage in one C. leucodon. To the best of our knowledge, this is the first record of H. kamiyai in a small insectivorous mammal. Similarly, Al-Sabi et al. [6] found H. taeniaeformis s.s. infection in only one S. araneus in Denmark and noted that this does not necessarily mean that cysts can successfully develop in these hosts, making them a reservoir for the parasite, and implicating their exposure to infectious eggs. We did not recorded any cysts or lesions in Sorex shrews of our sample. Catalano et al. [13] did not detect H. taeniaeformis s.s. in 28 shrews (Crocidura) examined in Senegal.
Of the other animals infected with H. kamiyai in our study, 18 belong to Apodemus (predominantly A. flavicollis) and two belong to M. arvalis (Arvicolinae). One R. rattus from our study was infected with H. taeniaeformis s.s. These results are in accordance with the fact that H. kamiyai mainly uses Arvicolinae and mice of the genus Apodemus as intermediate hosts, whereas H. taeniaeformis s.s. is restricted to murine rodents (mainly rats) [2]. The presence of H. kamiyai was also recently confirmed by molecular analyses in the same group of intermediate hosts in Europe such as M. arvalis and M. glareolus in Poland [15] and Ondatra zibethicus in Luxembourg [8]. Considering that we conducted the study on a large sample and a wide range of intermediate host species from Serbia (Southeastern Europe) and found H. kamiyai in members of Arvicolinae and Apodemus spp., our results are concordant with the current biogeography and host range of this recently described cryptic species and contribute to its clarification. On the other hand, it should be noted that we provided the sequence of H. taeniaeformis s.s from the black rat, but molecular studies of Hydatigera taeniaeformis s.l. in Europe are limited to Arvicolinae and Apodemus hosts [2, 8, 15]. Similarly, molecular studies of Hydatigera taeniaeformis s.l. from areas in Asia and Africa have been conducted mainly on intermediate hosts from the family Muridae [2, 13, 16, 17] and several individuals of Apodemus spp. that were found to be infected with H. taeniaeformis s.s. [2, 27], a parasite species for which members of the family Muridae (mainly rats) are considered suitable hosts. The previous discovery of H. taeniaeformis s.s. in the genus Apodemus in Asia (Fig. 2) and the lack of molecular studies of Hydatigera taeniaeformis s.l. in rats in Europe impose the need for additional research to obtain a more accurate view of the intermediate host spectrum from the different species of the genus. In a molecular study from Brazil, H. taeniaeformis s.s. was detected for the first time in the non-murine rodent Ingram's squirrel (Guerlinguetus ingrami), and the authors also point out that the purported host specificity of cryptic Hydatigera species is not strict [14]. Further testing on different host species from different areas is needed (especially rats from Europe and Arvicoline and Apodemus from Asia), and the possibility of infection of other small mammal species, e.g., shrews, should be considered. According to the median-joining haplotype network in the present study, H. kamiyai haplotypes from this study were clustered with other haplotypes from Europe and had at least 25 mutation steps from Asian, African and Australian haplotypes of H. taeniaeformis s.s., indicating clear molecular distinction of two species. Alvi et al. [17] also detected 20 mutation steps from the Pakistani H. taeniaeformis s.s. sequences compared to the European isolates (H. kamiyai). Pairwise divergences of our samples (H. kamiyai) with Asian, African and Australian isolates (H. taeniaeformis s.s.) from GenBank were high, ranging from 9.4% to 12.9%, supporting existence of two distinct taxa. These results are supported by the variation in the partial sequence of the mitochondrial cox1 gene between two clades of H. taeniaeformis s.l., reaching up to 13.3% [2]. The metacestode in Ingram's squirrel (G. ingrami) from Brazil found by Mello et al. [14] was identified as a H. taeniaeformis s.s and differed from H. kamiyai by 11.3–11.8%. A comparison of the complete mitochondrial (mt) genome between some European and Asian H. taeniaeformis s.l. species performed in two studies revealed a difference of 11.8–12.1%, and the presence of two distinct clades referred to as sister taxa, leading to the conclusion that H. taeniaeformis represents a species complex [18, 28]. All this recent molecular research and our results support the earlier discovery from the 1990s, when scientists noticed the existence of differences based on four rare laboratory isolates (KRN from the gray rat Rattus norvegicus from Malaysia; ACR from the gray red-backed vole Myodes rufocanus from Japan; SRN from the gray rat R. norvegicus from Japan; BMM from the house mouse Mus musculus from Belgium) and several wild isolates of H. taeniaeformis [Japan: TtMar, TtTom, TtKaRN (R. norvegicus); TtKaAA, TtNop (A. argenteus) and China: TtChi (M. musculus)]. They noted that the ACR isolate (now known as H. kamiyai) differed from the others in terms of infectivity, morphology and genetics (intraspecific variation of isoenzymes, nucleotide variations in the cox1 gene and protein composition of metacestodes) and concluded that it might be a different strain of H. taeniaeformis or even a new species [27, 29–31]. Based on cox 1 sequence analysis of the 13 H. kamiyai isolates, we found 7 haplotypes (Hd: 0.872), which is similar to the diversity level of the H. kamiyai specimens from Luxembourg based on 26 cox 1 sequences and 9 haplotypes defined, with Hd = 0.883 [8]. The authors suggested that H. kamiyai in Europe has a heterogeneous genetic structure and the different haplotypes are widely distributed across the continent, which could indicate a long and undisturbed presence of this ubiquitous parasite. The haplotype diversity of H. taeniaeformis s.s. from Pakistan composed of 38 cox1 sequences was 0.757, with 10 haplotypes [17], while the greatest diversity was found in Chinese isolates, where 10 haplotypes were recorded from 13 cox1 sequences [16].
In this study, two additional parasite species were also detected. Taenia martis was identified in A. flavicollis, A. sylvaticus and M. glareouls using both 12S rDNA and cox1 gene, and these are the first obtained sequences of this tapeworm from Serbia, providing new insights into its genetic diversity. Due to small sample size, of only six sequences in total (for both genes), no relevant population genetic analyses could be performed. Based on morphological identification, T. martis was found to infect 4.1% of 588 examined bank voles on Fruška gora mountain in Serbia [32]. Cysticercosis of T. martis in rodents appears to occur throughout Europe with variable prevalence, while adult forms infect wild carnivores, mainly martens [4]. Cases of the larval stage have also been detected in humans [33–36] and non-human primates [37, 38]. In 10 years of our research and 856 examined animals, only one small mammal (M. arvalis) captured in 2021 was found to be infected with T. crassiceps. A low prevalence (0.22%–2.9%) of metacestodes in rodent hosts has also been found in other European countries [5, 7, 39]. In several studies that included definitive and intermediate hosts from Europe, the presence of T. crassiceps was not recorded [24, 40–42]. Although this infection is rare, it can be very severe and lead to death or serious pathological changes in intermediate and paratenic hosts [4]. This massive and specific-looking infection, which affected the subcutaneous and pleural cavities in our sample (Additional file 1: Figure S2), was detected and confirmed by PCR analyses (12S rDNA gene). In natural intermediate hosts such as rodents, T. crassiceps metacestodes reproduce by asexual reproduction, particularly by exogenous budding, which is unique to this species and results in the formation of multiple infective scolices [43–45]. A number of well-documented cases of cysticercosis in humans have been published, most of them from Central Europe (Switzerland, France and Germany) [4]. It appears that this species has serious zoonotic potential and should be monitored in animals in the future.
Conclusions
Further studies are needed to better understand the specificity of two Hydatigera species (H. kamiyai and H. taeniaeformis s.s.) towards the intermediate host and their geographic distribution, particularly in rats from Europe and Apodemus spp. and voles from Asia and Africa. The possibility of infection of other small mammalian species, e.g., shrews, should also be considered. This is one of the few mitochondrial gene-based studies performed after the description of cryptic entities within the H. taeniaeformis s.l. complex and represents a valuable contribution to understanding of genetic diversity, host suitability and geographic distribution of these tapeworm species. Also, our study provides an important basis of molecular data from this part of Europe for further studies.
Supplementary Information
Additional file 1: Table S1. Primer sequences used for PCR analysis; Table S2. PCR details and conditions for two molecular markers used in this study; Fig. S1. Map of sampling sites in Serbia. The circles on the map show places where small mammals were collected. The red circles indicate the places where the animals were infected with some of the taeniids larval stages; Fig. S2. Cysticercosis caused by larval Taenia crassiceps tapeworm in common vole (Microtus arvalis).
Author contributions
MM and JB designed the study; MM, MR, BB, OBČ, IB and JB collected the samples; MM, GU, MR and JB performed experiments; MM and JB analyzed the data; MM wrote the manuscript; MR, GU, BB, IB, OBČ and JB revised the text. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia, Contract No. 451–03-47/2023–01/ 200007.
Availability of data and materials
Nucleotide sequences of cox1 and 12S rDNA genes from the present study have been deposited in the GenBank database under the accession numbers OQ569719-OQ569731; OQ834418-OQ834430; OQ832778.
Declarations
Ethics approval and consent to participate
The animals were treated in accordance with the legal and ethical guidelines as laid down in the Directive 2010/63/ EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. The animal study protocol was approved by the Ethics Committee of Institute for Biological Research “Siniša Stanković”, National Institute of Republic of Serbia (protocol code 01–1514/1 and date of approval: 23 September 2020).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Milan Miljević, Email: milan.miljevic@ibiss.bg.ac.rs.
Marija Rajičić, Email: marija.rajicic@ibiss.bg.ac.rs.
Gérald Umhang, Email: Gerald.UMHANG@anses.fr.
Branka Bajić, Email: branka.pejic@ibiss.bg.ac.rs.
Olivera Bjelić Čabrilo, Email: olivera.bjelic-cabrilo@dbe.uns.ac.rs.
Ivana Budinski, Email: ivana.budinski@ibiss.bg.ac.rs.
Jelena Blagojević, Email: jelena.blagojevic@ibiss.bg.ac.rs.
References
- 1.Nakao M, Lavikainen A, Iwaki T, Haukisalmi V, Konyaev S, Oku Y, et al. Molecular phylogeny of the genus Taenia (Cestoda: Taeniidae): Proposals for the resurrection of Hydatigera Lamarck, 1816 and the creation of a new genus Versteria. Int J Parasitol. 2013;43:427–437. doi: 10.1016/j.ijpara.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Lavikainen A, Iwaki T, Haukisalmi V, Konyaev SV, Casiraghi M, Dokuchaev NE, et al. Reappraisal of Hydatigera taeniaeformis (Batsch, 1786) (Cestoda: Taeniidae) sensu lato with description of Hydatigera kamiyai n. sp. Int J Parasitol. 2016;46:361–374. doi: 10.1016/j.ijpara.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Galimberti A, Romano DF, Genchi M, Paoloni D, Vercillo F, Bizzarri L, et al. Integrative taxonomy at work: DNA barcoding of taeniids harboured by wild and domestic cats. Mol Ecol Resour. 2012;12:403–413. doi: 10.1111/j.1755-0998.2011.03110.x. [DOI] [PubMed] [Google Scholar]
- 4.Deplazes P, Eichenberger RM, Grimm F. Wildlife-transmitted Taenia and Versteria cysticercosis and coenurosis in humans and other primates. Int J Parasitol Parasites Wildl. 2019;9:342–358. doi: 10.1016/j.ijppaw.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burlet P, Deplazes P, Hegglin D. Age, season and spatio-temporal factors affecting the prevalence of Echinococcus multilocularis and Taenia taeniaeformis in Arvicola terrestris. Parasit Vectors. 2011;4:1–9. doi: 10.1186/1756-3305-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Sabi MNS, Jensen PM, Christensen MU, Kapel CMO. Morphological and molecular analyses of larval taeniid species in small mammals from contrasting habitats in Denmark. J Helminthol. 2015;89:112–117. doi: 10.1017/S0022149X13000680. [DOI] [PubMed] [Google Scholar]
- 7.Reperant LA, Hegglin D, Tanner I, Fischer C, Deplazes P. Rodents as shared indicators for zoonotic parasites of carnivores in urban environments. Parasitology. 2009;136:329–337. doi: 10.1017/S0031182008005428. [DOI] [PubMed] [Google Scholar]
- 8.Martini M, Dumendiak S, Gagliardo A, Ragazzini F, La Rosa L, Giunchi D, et al. Echinococcus multilocularis and other taeniid metacestodes of muskrats in Luxembourg: Prevalence, risk factors, parasite reproduction, and genetic diversity. Pathogens. 2022;11:1414. doi: 10.3390/pathogens11121414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosbie PR, Nadler SA, Platzer EG, Kerner C, Mariaux J, Boyce WM. Molecular Systematics of Mesocesotides spp. (Cestoda: Mesocestoididae) from domestic dog (Canis familiaris ) and coyotes (Canis latrans) J Parasitol. 2000;86:350–357. doi: 10.1645/0022-3395(2000)086[0350:MSOMSC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Padgett KA, Nadler SA, Munson L, Sacks B, Boyce WM. Systematics of Mesocestoides (Cestoda: Mesocestoididae): evaluation of molecular and morphological variation among isolates. J Parasitol. 2005;91:1435–1443. doi: 10.1645/GE-3461.1. [DOI] [PubMed] [Google Scholar]
- 11.Miljević M, Lalošević D, Simin V, Blagojević J, Čabrilo B, Čabrilo OB. Intestinal helminth infections in the golden jackal (Canis aureus L.) from Vojvodina: Hotspot area of multilocular echinococcosis in Serbia. Acta Vet Hung. 2021;69:274–281. doi: 10.1556/004.2021.00030. [DOI] [PubMed] [Google Scholar]
- 12.Tran Thi G, Azzena I, Scarpa F, Cossu P, Le Danh C, Ton Nu PA, et al. Molecular identification and appraisal of the genetic variation of Taenia saginata in central regions of Vietnam. Life. 2022;12:70. doi: 10.3390/life12010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catalano S, Bâ K, Diouf ND, Léger E, Verocai GG, Webster JP. Rodents of Senegal and their role as intermediate hosts of Hydatigera spp. (Cestoda: Taeniidae) Parasitology. 2019;146:299–304. doi: 10.1017/S0031182018001427. [DOI] [PubMed] [Google Scholar]
- 14.Mello ÉM, Furtado LFV, Rabelo ÉML, Pinto HA. DNA barcoding of metacestodes found in the Guerlinguetus ingrami (Rodentia: Sciuridae) reveals the occurrence of Hydatigera taeniaeformis sensu stricto (Cyclophyllidea: Taeniidae) in the Americas. Parasitol Int. 2018;67:115–118. doi: 10.1016/j.parint.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Bajer A, Alsarraf M, Dwużnik D, Mierzejewska EJ, Kołodziej-Sobocińska M, Behnke-Borowczyk J, et al. Rodents as intermediate hosts of cestode parasites of mammalian carnivores and birds of prey in Poland, with the first data on the life-cycle of Mesocestoides melesi. Parasit Vectors. 2020;13:1–10. doi: 10.1186/s13071-020-3961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao F, Zhou Y, Wu Y, Zhou K, Liu A, Yang F, et al. Prevalence and genetic characterization of two mitochondrial gene sequences of Strobilocercus fasciolaris in the livers of brown rats (Rattus Norvegicus) in Heilongjiang province in Northeastern China. Front Cell Infect Microbiol. 2020;10:588107. doi: 10.3389/fcimb.2020.588107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alvi MA, Li L, Ohiolei JA, Qamar W, Saqib M, Tayyab MH, et al. Hydatigera taeniaeformis in urban rats (Rattus rattus) in Faisalabad. Pakistan Infect Genet Evolut. 2021;92:104873. doi: 10.1016/j.meegid.2021.104873. [DOI] [PubMed] [Google Scholar]
- 18.Wang H-M, Li R, Deng Y-P, Liu G-H, Fu Y-T. Comparative mitochondrial genomic analysis robustly supported that cat tapeworm Hydatigera taeniaeformis (Platyhelminthes: Cestoda) represents a species complex. Front Vet Sci. 2022;9:931137. doi: 10.3389/fvets.2022.931137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowles J, Blair D, Mcmanus D. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasit. 1992;54:165–173. doi: 10.1016/0166-6851(92)90109-W. [DOI] [PubMed] [Google Scholar]
- 20.von Nickisch-Rosenegk M, Silva-Gonzalez R, Lucius R. Modification of universal 12S rDNA primers for specific amplification of contaminated Taenia spp. (Cestoda) gDNA enabling phylogenetic studies. Parasitol Res. 1999;85:819–825. doi: 10.1007/s004360050638. [DOI] [PubMed] [Google Scholar]
- 21.Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 2017;34:3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 22.Bandelt HJ, Forster P, Rohl A. Median-joining networks for inferring intraspecific phylogenies. Mol Biol Evol. 1999;16:37–48. doi: 10.1093/oxfordjournals.molbev.a026036. [DOI] [PubMed] [Google Scholar]
- 23.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 24.Miljević M, Bjelić Čabrilo O, Simin V, Čabrilo B, Miljević JB, Lalošević D. Significance of the red fox as a natural reservoir of intestinal zoonoses in Vojvodina. Serbia Acta Vet Hung. 2019;67:561–571. doi: 10.1556/004.2019.055. [DOI] [PubMed] [Google Scholar]
- 25.Fuehrer H, Siehs C, Schneider R, Auer H. Morphometrical analysis of Taenia taeniaeformis and Taenia crassiceps in the common vole (Microtus arvalis) and the water vole (Arvicola terrestris) in Vorarlberg. Austria Helminthologia. 2012;49:169–173. doi: 10.2478/s11687-012-0034-x. [DOI] [Google Scholar]
- 26.Loxton KC, Lawton C, Stafford P, Holland CV. Reduced helminth parasitism in the introduced bank vole (Myodes glareolus): more parasites lost than gained. Int J Parasitol Parasites Wildl. 2016;5:175–183. doi: 10.1016/j.ijppaw.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamoto M, Bessho Y, Kamiya M, Kurosawa T, Horri T. Phylogenetic relationships within Taenia taeniaeformis variants and other taeniid cestodes inferred from the nucleotide sequence of the cytochrome c oxidase subunit I gene. Parasitol Res. 1995;81:451–458. doi: 10.1007/BF00931785. [DOI] [PubMed] [Google Scholar]
- 28.Jia W, Yan H, Lou Z, Ni X, Dyachenko V, Li H, et al. Mitochondrial genes and genomes support a cryptic species of tapeworm within Taenia taeniaeformis. Acta Trop. 2012;123:154–163. doi: 10.1016/j.actatropica.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Iwaki T, Nonaka N, Okamoto M, Oku Y, Kamiya M. Developmental and morphological characteristics of Taenia taeniaeformis (Batsch, 1786) in Clethrionomys rufocanus bedfordiae and Rattus norvegicus from different geographical locations. J Parasitol. 1994;80:461. doi: 10.2307/3283418. [DOI] [PubMed] [Google Scholar]
- 30.Okamoto M, Ito A, Kurosawa T, Kamiya M, Agatsuma T. Intraspecific variation of isoenzymes in Taenia taeniaeformis. Int J Parasitol. 1995;25:221–228. doi: 10.1016/0020-7519(94)00099-A. [DOI] [PubMed] [Google Scholar]
- 31.Azuma H, Okamoto M, Oku Y, Kamiya M, Azuma H, Okamoto M, Oku Y. Kamiya M (1995) Intraspecific variation of Taenia taeniaeformis as determined by various criteria. Parasitol Res. 1995;81:103–108. doi: 10.1007/BF00931613. [DOI] [PubMed] [Google Scholar]
- 32.Bjelić Čabrilo O, Kostić D, Popović E, Ćirković M, Aleksić N, Lujić J. Helminth fauna of the bank vole Myodes glareolus (Rodentia, Arvicolinae) on the territory of Fruska Gora Mountain (Serbia)—a potential source of zoonoses. Bulg J Agric Sci. 2011;17:829–836. [Google Scholar]
- 33.Eberwein P, Haeupler A, Kuepper F, Wagner D, Kern WV, Muntau B, et al. Human infection with marten tapeworm. Emerg Infect Dis. 2013;19:1152–1154. doi: 10.3201/eid1907.121114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunet J, Benoilid A, Kremer S, Dalvit C, Lefebvre N, Hansmann Y, et al. First case of human cerebral Taenia martis cysticercosis. J Clin Microbiol. 2015;53:2756–2759. doi: 10.1128/JCM.01033-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koch T, Schoen C, Addo M, Tappe D, Muntau B, Wiechens B, et al. Molecular diagnosis of human Taenia martis eye infection. Am J Trop Med Hyg. 2016;94:1055–1057. doi: 10.4269/ajtmh.15-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudelius M, Brehm K, Poelcher M, Spinner C, Rosenwald A, Costa CP. First case of human peritoneal cysticercosis mimicking peritoneal carcinosis: necessity of laparoscopy and histologic assessment for the correct diagnosis. JMM Case Rep. 2017 doi: 10.1099/jmmcr.0.005097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunet J, Pesson B, Chermette R, Regnard P, Grimm F, Deplazes P, et al. First case of peritoneal cysticercosis in a non-human primate host (Macaca tonkeana) due to Taenia martis. Parasit Vectors. 2014;7:422. doi: 10.1186/1756-3305-7-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Liberato C, Berrilli F, Meoli R, Friedrich KG, Di Cerbo P, Cocumelli C, et al. Fatal infection with Taenia martis metacestodes in a ring-tailed lemur (Lemur catta) living in an Italian zoological garden. Parasitol Int. 2014;63:695–697. doi: 10.1016/j.parint.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 39.Schaerer O. Die Metacestoden der Kleinsauger (Insectivora und Rodentia) und ihre Wirtsarten, Verbreitung und Haufigkeit im Kanton Thurgau (Schweiz). [Zurich, Switzerland]: University of Zurich; 1987.
- 40.Miller AL, Olsson GE, Walburg MR, Sollenberg S, Skarin M, Ley C, et al. First identification of Echinococcus multilocularis in rodent intermediate hosts in Sweden. Int J Parasitol Parasites Wildl. 2016;5:56–63. doi: 10.1016/j.ijppaw.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krücken J, Blümke J, Maaz D, Demeler J, Ramünke S, Antolová D, et al. Small rodents as paratenic or intermediate hosts of carnivore parasites in Berlin, Germany. PLoS ONE. 2017;12:e0172829. doi: 10.1371/journal.pone.0172829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loxton KC, Lawton C, Stafford P, Holland CV. Parasite dynamics in an invaded ecosystem: helminth communities of native wood mice are impacted by the invasive bank vole. Parasitology. 2017;144:1476–1489. doi: 10.1017/S0031182017000981. [DOI] [PubMed] [Google Scholar]
- 43.Freeman RS. Studies on the biology of Taenia crassiceps (Zeder, 1800) Rudolphi, 1810 (Cestoda) Can J Zool. 1962;40:969–990. doi: 10.1139/z62-086. [DOI] [Google Scholar]
- 44.Ntoukas V, Tappe D, Pfütze D, Simon M, Holzmann T. Cerebellar cysticercosis caused by larval Taenia crassiceps tapeworm in immunocompetent woman, Germany. Emerg Infect Dis. 2013;19:2008–2011. doi: 10.3201/eid1912.130284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowman DD. Georgis parasitology for veterinarians. 11. Philadelphia: Elsevier, Inc; 2020. [Google Scholar]
- 46.Lavikainen A, Haukisalmi V, Lehtinen MJ, Henttonen H, Oksanen A, Meri S. A phylogeny of members of the family Taeniidae based on the mitochondrial cox1 and nad1 gene data. Parasitology. 2008;135:1457–1467. doi: 10.1017/S003118200800499X. [DOI] [PubMed] [Google Scholar]
- 47.Liu G-H, Lin R-Q, Li M-W, Liu W, Liu Y, Yuan Z-G, et al. The complete mitochondrial genomes of three cestode species of Taenia infecting animals and humans. Mol Biol Rep. 2011;38:2249–2256. doi: 10.1007/s11033-010-0355-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Primer sequences used for PCR analysis; Table S2. PCR details and conditions for two molecular markers used in this study; Fig. S1. Map of sampling sites in Serbia. The circles on the map show places where small mammals were collected. The red circles indicate the places where the animals were infected with some of the taeniids larval stages; Fig. S2. Cysticercosis caused by larval Taenia crassiceps tapeworm in common vole (Microtus arvalis).
Data Availability Statement
Nucleotide sequences of cox1 and 12S rDNA genes from the present study have been deposited in the GenBank database under the accession numbers OQ569719-OQ569731; OQ834418-OQ834430; OQ832778.