Abstract
Aim
We aimed to assess the association between the use of Glucagon-like peptide-1 receptor agonists and the risk of 12 respiratory diseases in patients with type 2 diabetes, obesity, or overweight.
Method
The PubMed (MEDLINE), EMBASE, Cochrane Library, and ClinicalTrials.gov databases were searched from the establishment of the database to December 24, 2022. Dichotomous outcomes were analyzed using RR and 95% CI calculated from fixed-effects models.
Results
Twenty-eight RCTs were ultimately included for analysis, involving a total of 77,485 participants. Compared to controls, patients with GLP-1RAs have a 14% lower risk of respiratory disease (RR 0.86, 95% CI 0.81–0.93 p < 0.0001), with Semaglutid (RR 0.82, 95% CI 0.68–0.97, p = 0.02), Liraglutide (RR 0.86. 95% CI 0.75–0.98, p = 0.03), Dulaglutide (RR 0.82, 95% CI 0.70–0.96, p = 0.02), Albiglutide (RR 0.93,95% CI 0.79–1.10, p = 0.40), Exenatide (RR 0.93, 95% CI 0.74–1.18, p = 0.55), Lixisenatide (RR 0.83, 95% CI 0.62–1.12, p = 0.22), and Efpeglenatide (RR 0.76, 95% CI 0.46–1.24, p = 0.27). Semaglutide, Liraglutide and Dulaglutide reduce the risk of respiratory diseases by 18%, 14% and 18%, respectively.Trial duration, control type, and indication were not associated with the impact of GLP-1 receptor agonists on overall respiratory disease. Among secondary outcomes, the risk of Pulmonary edema (RR 0.66, 95% CI 0.44–0.98, p = 0.04), and Bronchitis (RR 0.86, 95% CI 0.74–1.00, p = 0.04) was reduced.
Conclusion
In conclusion, GLP-1RAs were linked to a lower risk of overall respiratory diseases, especially Pulmonary edema and Bronchitis. In the future, physicians should pay attention to the relationship between GLP-1 RA and the risk of respiratory diseases and evaluate the efficacy of GLP-1RAs in the primary and secondary prevention of respiratory diseases.
Trial registration CRD42023396138.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13098-023-01118-6.
Keywords: GLP-1RA; Diabetes mellitus, type 2; Respiratory illness; Randomized controlled trial; Meta-analysis
Introduction
Respiratory diseases are common disorders in life. Respiratory diseases are increasing in mortality and morbidity globally each year. It is a major contributor to the global health burden [1]. In addition, chronic respiratory disease is one of the top 10 causes of death worldwide [2]. Diabetes is a common comorbidity among patients with respiratory diseases. People with diabetes are at increased risk of developing sleep apnoea syndrome, chronic obstructive pulmonary disease (COPD), asthma, and lung injury. Hyperinsulinemia, hyperglycemia, and chronic pro-inflammatory states can lead to impaired lung function. There is evidence that GLP-1RAs may improve lung function through mechanisms such as reducing inflammation and tracheal hyperresponsiveness [3, 4].
GLP-1RAs are novel anti-hyperglycemic agents commonly used. In addition to cardiovascular benefits, emerging evidence suggests that such drugs may also be associated with respiratory benefits, for example in patients with COPD [5, 6].
Recently, a population-based cohort study (involving 56,243 participants) discovered that GLP-1 receptor agonists reduce the incidence of severe exacerbation of COPD [6]. Compared with sulfonylureas, GLP-1 receptor agonists were associated with a 30% decreased risk of severe exacerbation (3.5 v 5.0 events per 100 person years; HR 0.70, 95% CI 0.49–0.99) and moderate exacerbation (0.63, 0.43–0.94). Besides, in a previous retrospective cohort study, the use of GLP-1RA was found to result in fewer asthma attacks in adult asthmatics with type 2 diabetes [7]. GLP-1R agonists (reference) compared with SGLT-2 inhibitors (incidence rate ratio [IRR], 2.98; 95% confidence interval [CI], 1.30–6.80), DPP-4 inhibitors (IRR, 2.45; 95% CI 1.54–3.89), sulfonylureas (IRR, 1.83; 95% CI 1.20–2.77), and basal insulin (IRR, 2.58; 95% CI 1.72–3.88). Also, it allowed patients to potentially improve from obstructive sleep apnea syndrome and have less aggravation of the chronic lower respiratory disease [8, 9]. The adjusted incidence rate of first chronic lower respiratory disease admission during follow-up was 10.7 and 20.3 per 1000 person-years for GLP-1RA and DPP-4I users, respectively, resulting in an adjusted hazard ratio of 0.52 (95% CI 0.32–0.85). From the perspective of its possible underlying mechanism. The use of GLP-1RA was found to reduce inflammation and promote lung function in a mouse model of obstructive lung disease [10]. In another previous animal model, liraglutide pretreatment reduced acute pulmonary damage brought on by LPS in mice [11]. Furthermore, GLP-1RA has been shown to significantly attenuate LPS-stimulated eosinophil activation [12]. It may have immunomodulatory effects. Currently, the relationship between GLP-1RAs and respiratory diseases is unclear. To determine the link between GLP-1 agonists and the occurrence of respiratory diseases, we conducted a meta-analysis of all RCTs that satisfied the inclusion criteria. Meanwhile, since GLP-1RAs are clinically used for type 2 diabetes and weight loss, and diabetes and obesity are risk factors for the development of respiratory diseases, we included people with type 2 diabetes, obesity, or overweight for the study [5, 13].
Methods
Data sources and searches
We searched PubMed (MEDLINE), EMBASE, and Cochrane Library databases to find literature on GLP-1RA published from database creation to December 24, 2022, and the literature was not restricted by language. The following were the search keywords used: “Glucagon-like peptide 1 receptor agonist”, “liraglutide”, “albiglutide”, “lixisenatide”, “exenatide”, “dulaglutide”, “semaglutide”, “Diabetes Mellitus, Type 2”, “obesity”, “Overweight” and “randomized controlled trial”. Detailed search strategies can be found in the Additional file.
This meta-analysis follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines statement. The International Registry of Prospective Systematic Reviews has prospectively recorded the meta-procedure. (CRD42023396138).
Study selection
Inclusion criteria: (1) Adult people with type 2 diabetes mellitus, overweight or obese. (2) RCT comparing GLP-1 receptor agonist with placebo or positive control (Insulin or other oral hypoglycemic drugs). (3) The results reported at least one respiratory disease event of interest (all data from ClinicalTrials.gov on respiratory adverse events).
Exclusion criteria: (1) Pregnant women. (2) GLP-1RA in combination with other hypoglycemic agents (e.g. IDegLira). (3) Comparison between different GLP-1RAs (4) Multiple publications from the same RCT (using the most complete or most recently reported data), reviews, case reports, letters, and conference proceedings on GLP-1RA. (4) Studies with incomplete data or full text not available (Table 1).
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Participants | Adults(≥ 18 years old) with type 2 diabetes, obesity, or overweight | Pregnant women |
| Intervention | Use any GLP-1RA, GLP-1RA can also be used as add-on therapy to other interventions | Combination drugs of GLP-1RA or combination with other hypoglycemic drugs |
| Comparators | Placebo or active control (other antidiabetic drugs or insulin) | Comparing different categories of GLP-1RAs |
| Outcomes | Respiratory diseases | No relevant outcome data is available |
| Study design | RCTs | Post-hoc analysis, multiple articles from the same study |
Outcome measures
Primary outcome: overall respiratory disease prevalence.
Secondary outcomes: 12 subcategories of respiratory diseases: Pulmonary edema , pneumonia, Bronchitis, Pulmonary fibrosis, Dyspnoea, Acute respiratory failure, Pleural effusion, Asthma, COPD, Sleep apnoea syndrome, Pulmonary embolism, Pulmonary hypertension.
Data extraction and quality assessment
By reading the full text of the included literature, two investigators (MX and RX) independently extracted the following data: First author, year of study publication, clinical trial registration number, duration of follow-up, type of GLP-1 RA, control drug, and trial subject characteristics (age, sex, BMI, and glycosylated hemoglobin [HbA1c] levels). Any discrepancies arising from this process were resolved by consensus or by a third reviewer (PL). The Cochrane Risk of Bias tool was used to assess the quality of each RCT included [14].
Statistical analysis
We analyzed dichotomous outcomes using RR and 95% CI calculated from random or fixed effects models. Heterogeneity between studies was assessed using the χ2 test and the I2 statistic. If heterogeneity was low (P > 0 0.05; I2 ≤ 50%), analyses were performed using the Mantel–Haenszel method and fixed-effects models. Otherwise, a random-effects model using the DerSimonian-Laird method was used. We also assessed the association of specific GLP-1 RA drugs with respiratory disease. Also, we performed subgroup analyses according to control type, trial duration, and indication. Sensitivity analyses were performed by omitting eligible trials one by one. We used a funnel plot and the Egger test to check for publication bias. All of the analyses were conducted using RevMan 5.4 and Stata 14. We think it is statistically significant if the P-value is < 0.05.
Result
Study search and study characteristics
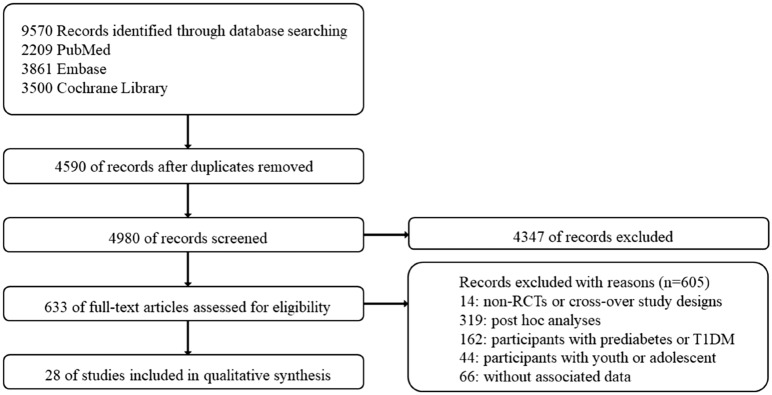
A search of the above databases based on keywords returned 9570 records in total. After excluding duplicates, and reading the titles and abstracts, a total of 633 documents were read in full. A total of 28 RCTs involving a total of 77485 participants were finally included for analysis [15–42]. Figure 1 shows the study screening flow chart. In the 28 included trials, the majority included subjects with type 2 diabetes (96.3%) with follow-up periods ranging from 24 weeks to 5.4 years. Among all participants, the average age of the study participants was between 46.0 and 74.2 years. The proportion of males ranged from 19.0 to 69.4%. The average BMI varied from 26.8 to 38.5 kg/m2. Mean glycosylated hemoglobin (HbA1c) ranged from 5.7 to 8.9%. (Table 2).
Fig. 1.
Process of trial selection
Table 2.
Baseline characteristics of included studies
| Study | NCT ID | Country/Region | Indication | Type of underlying diseases | Experimental | Control | Trial duration | Total | Average age(years) | Male (n,%) | BMI(kg/m2) | Mean HbA1c (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (Standard Deviation) | Mean (Standard deviation) | Mean (Standard deviation) | ||||||||||
| LEAD -2 (2009) | NCT00318461 | Europe, Oceania, Africa, Asia and South America | T2DM | T2DM | liraglutide 0.6 , 1.2, 1.8 mg once daily | placebo, glimepiride | 26 week | 1091 | 56.7 (9.5) | 58.20 | 31.0 (4.7) | 8.4 (0.9) |
| LIRA-RENAL(2016) | NCT01620489 | France, Poland, Russian Federation, Ukraine, United Kingdom, United States | T2DM | T2DM and moderate renal impairment | liraglutide 1.8 mg once daily | Placebo | 26 week | 279 | 67.2 (8.2) | 50.50 | 33.9 (5.4) | 8.0 (0.8) |
| HARMONY 3(2014) | NCT00838903 | Albania, Germany, Hong Kong, Mexico, Peru, Philippines, Russian Federation, South Africa, Spain, United Kingdom, United States | T2DM | T2DM | Albiglutide 30 mg once weekly | Placebo, Sitagliptin, Glimepiride | 104 week | 1012 | 54.5 (8.0) | 47.60 | 32.6 (5.5) | 8.1 (0.8) |
| Gallwitz et al. (2011) | NCT00434954 | Germany | T2DM | T2DM | Exenatide 10 μg twice daily | Premixed Insulin Aspart | 26 week | 494 | 57.1 (10.0) | 57.60 | 33.2 (4.3) | 7.88 (0.9) |
| GetGoal-O (2017) | NCT01798706 | Australia, Bulgaria, Canada, Denmark, Germany, Norway, Peru, Poland, South Africa, Spain, Sweden, United Kingdom, United States | T2DM | T2DM | Lixisenatide 20 μg once daily | Placebo | 24 week | 350 | 74.2 (3.9) | 52.00 | 30.0 (4.1) | 8.0 (0.7) |
| AWARD-CHN2 (2019) | NCT01648582 | China, Mexico, and Russian Federation | T2DM | T2DM | Dulaglutide 0.75, 1.5 mg once weekly | Insulin Glargine | 52 week | 774 | 55.0 (9.6) | 55.20 | 26.8 (3.7) | 8.4 (1.1) |
| AWARD-5 (2015) | NCT00734474 | United States, Canada, France, Germany,India, Korea, Mexico, Poland, Puerto Rico,Romania, Russian Federation, Spain, Taiwan | T2DM | T2DM | Dulaglutide 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 3.0 mg once weekly | sitagliptin | 104 week | 921 | 54.1 (9.9) | 46.50 | 31.3 (4.4) | 8.1 (1.1) |
| HARMONY 4 (2014) | NCT00838916 | United States, Russian Federation, South Africa, United Kingdom | T2DM | T2DM | Albiglutide 30 mg once weekly | insulin glargine | 52 week | 745 | 55.5 (9.5) | 56.10 | 33.1(5.5) | 8.3 (0.9) |
| Charbonnel et al. (2013) | NCT01296412 | Canada, Colombia, Czech Republic, Denmark, Finland, France, Germany, Hong Kong, Hungary, Israel, Italy, Lithuania, New Zealand, Poland, Puerto Rico, Slovakia, Slovenia, Spain, Sweden, United Kingdom, United States | T2DM | T2DM | liraglutide 1.2 mg once daily | sitagliptin | 26 week | 653 | 57.3 (10.4) | 54.80 | 32.7(6.0) | 8.2 (1.0) |
| SCALE Diabetes (2015) | NCT01272232 | Argentina, Brazil, France, Germany, India, Israel, Japan, Mexico, Romania, Russian Federation, South Africa, Turkey, Ukraine, United Kingdom, United States | Weight Loss | T2DM and overweight or obesity | Liraglutide 1.8, 3.0 mg once daily | Placebo | 56 week | 846 | 54.9 (10.5) | 50.20 | 37.1 (6.8) | 7.9 (0.8) |
| PIONEER 3 (2019) | NCT02607865 | France, Germany, India, Israel, Italy, Puerto Rico, South Africa, Spain, Sweden, Taiwan, Turkey, United Kingdom, United States | Cardiovascular Outcomes | T2DM | Oral Semaglutide 3, 7, 14 mg once daily | Sitagliptin | 78 week | 1864 | 58.0 (10.0) | 52.80 | 32.5(6.4) | 8.3 (0.9) |
| STEP 3 (2021) | NCT03611582 | United States | Weight Loss | Overweight or Obesity (without diabetes) | Semaglutide 2.4 mg once weekly | Placebo | 68 week | 611 | 46.0 (13.0) | 19.00 | 38.0(6.7) | 5.7 (0.3) |
| Harmony Outlets (2018) | NCT02465515 | Argentina, Belgium, Bulgaria, Canada, Czechia, Denmark, France, Germany, Greece, Hong Kong, Hungary, Italy, Korea, Republic of, Mexico, Netherlands, Norway, Peru, Philippines, Poland, Russian Federation, South Africa, Spain, Sweden, Taiwan, Thailand, Ukraine, United Kingdom, United States | Cardiovascular Outcomes | T2DM and cardiovascular disease | Albiglutide 30, 50 mg once weekly | Placebo | 1.6 Year | 9463 | 64.1 (8.7) | 69.40 | 32.3(5.9) | 8.7(1.5) |
| STEP 2 (2021) | NCT03552757 | Argentina, Canada, Germany, Greece, India, Japan, Puerto Rico, Russian Federation, South Africa, Spain, United Arab Emirates, United Kingdom, United States | Weight Loss | T2DM and overweight or obesity | Semaglutide 1.0, 2.4 mg once weekly | Placebo | 68 week | 1210 | 55.0 (11.0) | 49.10 | 35·7 (6·3) | 8·1 (0·8) |
| REWIND (2019) | NCT01394952 | Argentina, Australia, Brazil, Bulgaria, Canada, Chile, Colombia, Czechia, Germany, Hungary, Korea, Republic of, Latvia, Lithuania, Mexico, New Zealand, Poland, Puerto Rico, Romania, Russian Federation, South Africa, Spain, Sweden, Taiwan, United Kingdom, United States | Cardiovascular Outcomes | T2DM and cardiovascular disease( or high cardiovascular risk) | Dulaglutide 1.5 mg once daily | Placebo | 5.4 Year | 9901 | 66.2 (6.5) | 53.65 | 32.3 (5.7) | 7.4 (1.1) |
| AWARD-4 (2015) | NCT01191268 | Argentina, Australia, Belgium, Brazil, Canada, Denmark, Greece, Hungary, Mexico, Poland, Puerto Rico, Russian Federation, Spain, Sweden, Taiwan, United States | T2DM | T2DM | Dulaglutide 0.75, 1.5 mg once weekly | insulin glargine | 52 week | 884 | 59.4 (9.2) | 53.50 | 32.5 (5.2) | 8.5 (1.1) |
| EUREXA (2012) | NCT00359762 | Austria, Czech Republic, Finland, France, Germany, Hungary, Ireland, Israel, Italy, Mexico, Poland, Spain, Switzerland, United Kingdom | T2DM | T2DM | Exenatide 10 μg twice daily | glimepiride | 2.0 Year | 1029 | 56.4 (9.6) | 53.60 | 32.5 (4.1) | 7.4 (0.70) |
| LEAD-3 Mono (2009) | NCT00294723 | Mexico, Puerto Rico, United States | T2DM | T2DM | liraglutide 1.2 , 1.8 mg once daily | glimepiride | 104 week | 746 | 53.0 (10.9) | 49.70 | 33.1 (5.8) | 8.3 (1.1) |
| SUSTAIN 2 (2017) | NCT01930188 | Argentina, Bulgaria, Czechia, Hong Kong, Hungary, India, Japan, Mexico, Norway, Portugal, Romania, Russian Federation, South Africa, Spain, Sweden, Thailand, Turkey, Ukraine | T2DM | T2DM | Semaglutide 0.5, 1.0 mg once weekly | Sitagliptin | 56 week | 1225 | 55.1 (10.0) | 50.60 | 32.5(6.2) | 8.07 (0.9) |
| LEADER (2016) | NCT01179048 | Australia, Austria, Belgium, Brazil, Canada, China, Czechia, Denmark, Finland, France, Germany, Greece, India, Ireland, Israel, Italy, Korea, Republic of, Mexico, Netherlands, Norway, Poland, Puerto Rico, Romania, Russian Federation, Serbia, South Africa, Spain, Sweden, Taiwan, Turkey, United Arab Emirates, United Kingdom, United States | Cardiovascular Outcomes | T2DM and high cardiovascular risk | liraglutide 1.8 mg once daily | Placebo | 3.8 Year | 9340 | 64.3 (7.2) | 64.30 | 32.5 (6.3) | 8.7 (1.6) |
| PIONEER 6 (2019) | NCT02692716 | Algeria, Argentina, Brazil, Canada, Denmark, Germany, India, Israel, Italy, Malaysia, Mexico, Netherlands, Poland, Romania, South Africa, Spain, Taiwan, Thailand, Turkey, United Kingdom, United States | Cardiovascular Outcomes | T2DM and high cardiovascular risk | Oral Semaglutide 14 mg once daily | Placebo | 1.3 Year | 3183 | 66.0 (7.0) | 68.40 | 32.3 (6.5) | 8.2 (1.6) |
| ELIXA (2015) | NCT01147250 | Argentina, Australia, Austria, Belarus, Belgium, Brazil, Bulgaria, Canada, Chile, China, Colombia, Denmark, Ecuador, Egypt, Estonia, Finland, France, Georgia, Germany, Guatemala, India, Israel, Italy, Japan, Korea, Republic of, Latvia, Lithuania, Mexico, Netherlands, Norway, Panama, Peru, Philippines, Poland, Portugal, Romania, Russian Federation, Serbia, South Africa, Spain, Sweden, Switzerland, Taiwan, Tunisia, Turkey, Ukraine, United Arab Emirates, United Kingdom, United States | Cardiovascular Outcomes | T2DM and Acute Coronary Syndrome | Lixisenatide 20 μg once daily | Placebo | 2.1 Year | 6068 | 60.3 (9.7) | 69.30 | 30.2 (5.7) | 7.7 (1.3) |
| EXSCEL (2017) | NCT01144338 | Argentina, Australia, Austria, Belgium, Brazil, Bulgaria, Canada, Chile, China, Colombia, Czechia, Germany, Hong Kong, Hungary, Israel, Italy, Korea, Republic of, Latvia, Lithuania, Malaysia, Mexico, Netherlands, New Zealand, Philippines, Poland, Romania, Russian Federation, Slovakia, South Africa, Spain, Taiwan, Thailand, Ukraine, United Kingdom, United States | Cardiovascular Outcomes | T2DM | Exenatide 2 mg once weekly | Placebo | 3.2 Year | 14,752 | 61.9 (9.4) | 62.00 | 31.7 (5.9) | 8.0 (1.2) |
| Wilding et al. (2021) | NCT03548935 | Argentina, Belgium, Bulgaria, Canada, Denmark, Finland, France, Germany, India, Japan, Mexico, Poland, Puerto Rico, Russian Federation, Taiwan, United Kingdom, United States | Weight Loss | Overweight or Obesity (without diabetes) | Semaglutide 2.4 mg once weekly | Placebo | 68 week | 1961 | 46.0 (13) | 25.90 | 37.9 (6.6) | 5.7 (0.3) |
| SUSTAIN-6 (2016) | NCT01720446 | Algeria, Argentina, Australia, Brazil, Bulgaria, Canada, Denmark, Germany, India, Israel, Italy, Malaysia, Mexico, Poland, Russian Federation, Spain, Taiwan, Thailand, Turkey, United Kingdom, United States | Cardiovascular Outcomes | T2DM and cardiovascular disease | Semaglutide 0.5, 1.0 mg once weekly | Placebo | 2.1 Year | 3297 | 64.6 (7.4) | 60.70 | 32.8 (6.2) | 8.7 (1.5) |
| STEP 5 (2022) | NCT03693430 | Canada, Hungary, Italy, Spain, United States | Weight Loss | Overweight or Obesity (without diabetes) | Semaglutide 2.4 mg once weekly | Placebo | 104 week | 304 | 47.0 (11.0) | 22.40 | 38.5 (6.9) | 5.7 (0.4) |
| AMPLITUDE-O (2021) | NCT03496298 | Argentina, Bulgaria, Canada, Chile, Denmark, Estonia, Finland, Germany, Hungary, India, Italy, Korea, Republic of, Latvia, Lithuania, Mexico, Norway, Peru, Poland, Romania, Russian Federation, Serbia, Slovakia, South Africa, Spain, Sweden, Turkey, Ukraine, United States | Cardiovascular Outcomes | T2DM and cardiovascular disease or kidney disease | Efpeglenatide 4,, 6 mg once weekly | Placebo | 1.81 years | 4076 | 64.5 (8.2) | 67 | 32.7 (6.2) | 8.9(1.5) |
| AMPLITUDE-M(2022) | NCT03353350 | Germany, Poland, Ukraine, United Kingdom, United States | T2DM | T2DM | Efpeglenatide 2, 4, 6 mg once weekly | Placebo | 56 week | 406 | 58.5 (11.2) | 53.90 | 34.2 (6.8) | 8.1 (0.9) |
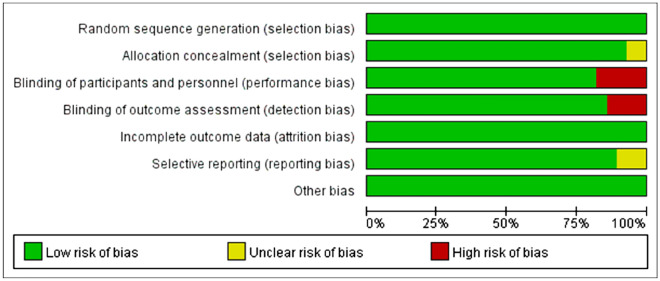
Risk of bias evaluation
The Cochrane Risk of Bias tool, which provides information on the risk of bias evaluation, was used to evaluate the quality of the included studies (Fig. 2).
Fig. 2.
Quality assessment (Cochrane risk of bias tool) for included RCTs
Main outcome
Incidence of respiratory diseases with all GLP-1 receptor agonists
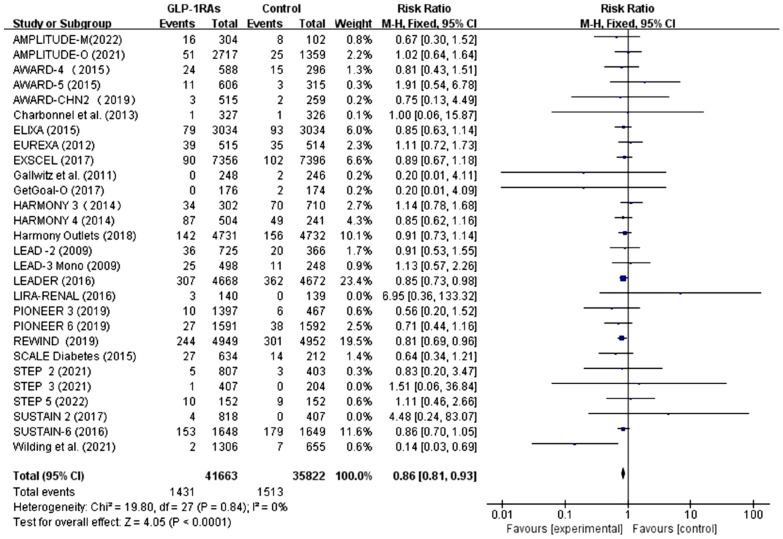
As shown in Fig. 3, this study included 41,663 participants using GLP-1RAs and 35,822 patients using placebo, oral hypoglycemic agents, or insulin. The GLP-1 RAs group had a lower event rate (3.43%) than the control group (4.22%). Patients with GLP-1RAs had a 14% lower risk of respiratory disease compared to controls (RR 0.86, 95% CI 0.81–0.93; p < 0.0001), with no significant heterogeneity between studies (I2 = 0). In the sensitivity analysis, the pooled results were not significantly altered after repeated omission of each trial. There was no obvious publication bias, according to the funnel plots of this analysis. (Additional file 1: Figure S1, Egger’s test P = 0.859 > 0.05).
Fig. 3.
Forest plot of GLP-1 receptor agonists versus comparators on the risk of overall Respiratory diseases
Incidence of respiratory diseases with different GLP-1 receptor agonists
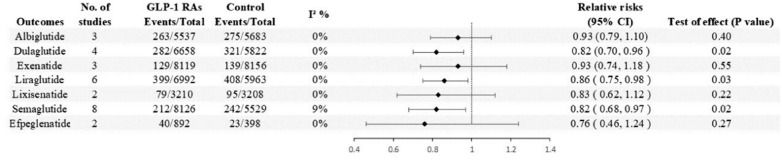
As shown in Fig. 4, eight trials totaling 13655 patients were included with Semaglutide as the experimental group. Semaglutide reduced the risk of developing respiratory diseases by 18% compared to placebo or other interventions (RR 0.82, 95% CI 0.68–0.97, p = 0.02). In addition, six trials totaling 12,955 patients were included with Liraglutide as the experimental group. Compared to the control group, Liraglutide reduced the risk of respiratory disease by 14% (RR 0.86, 95% CI 0.75–0.98, p = 0.03). Four RCT studies including 12,480 patients provided information on the use of Dulaglutide and the risk of respiratory disease. The results showed that Dulaglutide reduced the risk of respiratory disease by 18% compared to controls (RR 0.82, 95% CI 0.70–0.96, p = 0.02).
Fig. 4.
Forest plot of specific GLP-1 receptor agonists versus comparators on the risk of overall Respiratory diseases
However, other GLP-1 receptor agonists did not affect the overall respiratory disease. The RR for the occurrence of respiratory disease in patients receiving Albiglutide was 0.93 (95% CI 0.79–1.10, p = 0.40) compared to other interventions. The RR for the occurrence of respiratory disease in patients receiving Exenatide was 0.93 (95% CI 0.74–1.18, p = 0.55). The RR for the occurrence of respiratory disease in patients receiving Lixisenatide was 0.83 (95% CI 0.62–1.12, p = 0.22). The RR for the occurrence of respiratory disease in patients receiving Efpeglenatide was 0.76 (95% CI 0.46–1.24, p = 0.27). No inter-study heterogeneity was observed in all of the above analyses.
Subgroup analyses
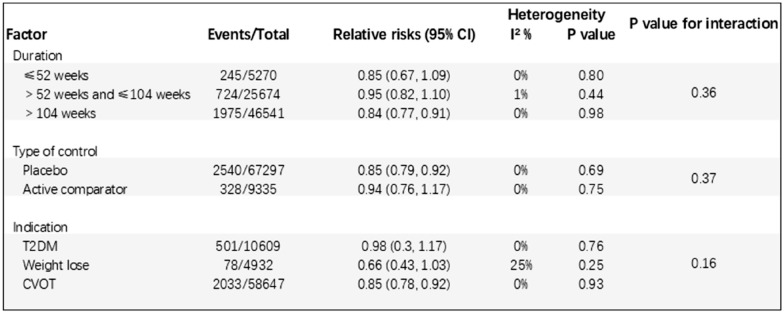
Based on subgroup analysis of trial duration, control type, and indication. The results showed that trial duration, control type, and indication had no significant effect on the effect of GLP-1 receptor agonists on overall respiratory disease (all P subgroups > 0.05; Fig. 5).
Fig. 5.
Subgroup analysis: GLP-1 receptor agonists and Respiratory disease. CVOT: Cardiovascular Outcomes Trial
Secondary outcome
Random assignment to GLP-1 RA treatment resulted in a reduced risk of Pulmonary edema (RR 0.66, 95% CI 0.44–0.98, p = 0.04; I2 = 11; Additional file 1: Fig S3), Bronchitis (RR 0.86, 95% CI 0.74–1.00, p = 0.04; I2 = 0; Additional file 1: Fig S4). Meanwhile, GLP-1RAs showed a trend towards a reduced risk for nine respiratory diseases, although not reaching statistical significance, including pneumonia (RR 0.89, 95% CI 0.78–1.00, p = 0.05; I2 = 0; Additional file 1: Fig S5), Pulmonary fibrosis (RR 0.39, 95% CI 0.13–1.16, p = 0.09; I2 = 0; Additional file 1: Fig. S6), Dyspnoea (RR 0.78, 95% CI 0.58–1.04, p = 0.09; I2 = 0; Additional file 1: Fig. S7), Acute respiratory failure (RR 0.81, 95% CI 0.57–1.13, p = 0.21; I2 = 0; Additional file 1: Fig. S8), Pleural effusion (RR 0.73, 95% CI 0.48–1.11, p = 0.15; I2 = 6; Additional file 1: Figure S9), Asthma (RR 0.85, 95% CI 0.59–1.24, p = 0.40; I2 = 0; Fig S10), and COPD (RR 0.93. 95% CI 0.76–1.13, p = 0.47; I2 = 0; Additional file 1: Fig S11), Sleep apnoea syndrome (RR 0.81, 95% CI 0.48–1.36, p = 0.43; I2 = 0; Additional file 1: Fig S12), Pulmonary embolism (RR 0.95, 95% CI 0.69–1.31, p = 0.76; I2 = 0; Additional file 1: Fig S13). GLP-1RAs did not affect Pulmonary hypertension (RR 1.02, 95% CI 0.51–2.05, p = 0.95; I2 = 5; Additional file 1: Fig S14). Heterogeneity was absent or low for all meta-analyses analyzed above.
Discussion
The relationship between GLP-1RA and respiratory disease has rarely been studied in clinical studies. In 2021, the results of a meta-analysis that included 7 RCT studies showed that GLP-RA uses resulted in a decreased risk of respiratory disease [43]. This meta-analysis included large RCTs with cardiovascular or renal outcomes as experimental endpoints in patients with type 2 diabetes. Our meta-analysis mainly focuses on people with type 2 diabetes, obesity, or overweight. We expand the crowd even further. Furthermore, it is to determine whether the risk may differ by GLP-1RA type, trial duration, control type, and indication. In this regard, we have further discussed and systematically elaborated.
This meta-analysis examined the connection between the injection of GLP-1 receptor agonists and the incidence of diverse respiratory diseases. Two main discoveries were discovered. Firstly, compared to placebo or other therapies, GLP-1 RAs greatly decreased the incidence of total respiratory illness by 14%, particularly with Semaglutid, Liraglutide, and Dulaglutide. In the forest plot, several studies including Harmony Outlets (2018), LEADER (2016), REWIND (2019), and SUSTAIN-6 (2016) appear to show a low risk of respiratory diseases. These studies are all Cardiovascular Outcomes Trials, which are large multi-country and multi-center RCTs. The weight of these studies is between 10.1 and 23.4%, and the total number of participants is between 3297 and 9901. Median follow-up time of these studies ranged from 1.6 to 5.4 years. Therefore, more participants and longer follow-up time may better reflect the relationship between the use of GLP-1RAs and the risk of respiratory disease. It is meaningful to further evaluate the relationship between long-term use of GLP-1RAs and the risk of respiratory diseases in the future. In the secondary outcome, the risk of pulmonary edema and bronchitis was reduced. On the other hand, although statistical significance was not reached, the use of GLP-1RAs showed a trend towards a reduced risk of nine respiratory diseases (pneumonia, pulmonary fibrosis, dyspnea, acute respiratory failure, pleural effusion, COPD, sleep apnea syndrome, and pulmonary embolism).
GLP-1RAs were highly expressed in the lung [4]. Among the extra-pancreatic organs, the lung had the highest level of Glp1r mRNA expression [44, 45]. Moreover, alveolar lining fluid had GLP-1 concentrations that were substantially higher than those in serum [46]. This provides a pathway for a potential effect of GLP1-RA beyond glucose regulation. So far, the mechanisms underlying the protective effects of GLP-1RA in respiratory disease are not fully understood. GLP-1 RA may reduce tracheal hyperresponsiveness by activating the cAMP-dependent protein kinase A pathway in the human airways [4]. It also mediates tracheal relaxation. In a study using female mice in an obstructive pulmonary disease model, the use of GLP-1RA was found to reduce inflammation and improve lung function [10]. Meanwhile, some studies on GLP-1RA's anti-inflammatory effects in T2D patients have been conducted. GLP-1RA inhibits oxidative stress and inflammatory mediator production in patients with type 2 diabetes [47]. GLP-1RA dramatically lowered the levels of eosinophil surface activation markers after LPS stimulation. Thus, it attenuated LPS-stimulated eosinophil activation and reduced the generation of IL-4, IL-8, and IL-13 [12]. This suggests that GLP-1 may have an immunomodulatory role in asthma. In addition, in a previous animal model, liraglutide pretreatment attenuated LPS-induced acute lung injury in mice. It was linked to a decrease in the activity of inflammatory cytokines and chemokine genes in the lung, and it significantly decreased lung injury scores, and lung apoptosis [11]. In a related study, liraglutide was shown to increase FVC, and carbon monoxide diffusion capacity, and significantly reduce serum surface active protein D (SP-D) levels (used as a biomarker to assess alveolar-capillary barrier integrity) in patients with type 2 diabetes [48, 49]. It is well known that pulmonary surface-active substances (PS) are composed of various lipids and proteins arranged on the alveolar surface. Its main function is to maintain lung compliance and lung fluid homeostasis. on the other hand, surfactant protein A is the most prevalent protein in PS. As well, it has been shown that liraglutide increases the expression of SP-A by activating the TTF-1 signaling pathway. Thus, it may reduce the occurrence of pulmonary edema [50].
In the results of a cohort study published in BMJ, new users of GLP-1 receptor agonists had a 30% lower risk of severe COPD exacerbation compared to new sulfonylurea users. There was also a reduced risk of moderate exacerbation [6]. In another retrospective study, it was shown that patients starting GLP-1RA in intensive glucose-lowering therapy for patients with combined asthma and type 2 diabetes had fewer asthma attacks than those using other alternatives. Asthma symptoms were also reduced [7]. Interestingly, insulin resistance may enhance the pro-asthmatic effects of obesity. GLP-1 RAs may reduce asthma through weight loss and improved insulin sensitivity [51–53]. In addition, in a study that included 16,690 patients, patients with GLP-1RA experienced less worsening of chronic lower respiratory disease compared to DPP-4I users [8]. Finally, GLP-1 receptor agonists may improve lung function by reducing body weight, as abdominal obesity decreases lung capacity by pushing the diaphragm into the chest cavity.
In our analysis, a trend towards a decreased risk of sleep apnea syndrome was found with the use of GLP-1RA (RR 0.86, 95% CI 0.50–1.45). GLP-1 RAs may benefit patients with this obstructive sleep apnea syndrome by weight loss, reducing systemic inflammation, improving metabolic dysregulation, and reversing endothelial dysfunction [9]. Glucagon-like peptide-1 agonists have been advocated in the literature as a one-stop shop for the obesity-diabetes-OSA triad [9]. Besides, our results suggest that GLP-1RAs may not affect Pulmonary hypertension. However, in previous studies, it was demonstrated that GLP-1RAs could diastole pulmonary arteries in a cAMP-dependent manner [54]. Meanwhile, Glucagon-like peptide 1 receptor agonists attenuate autophagy via Drp1/NOX- and Atg-5/Atg-7/Beclin-1/LC3β pathways to improve pulmonary hypertension [55]. The inconsistent results may be due to the low incidence and the incorporation of studies with limited sample sizes, statistical effectiveness was not greatly increased. And it may lead to small sample effect bias. In the study of several categories of GLP-1 receptor agonists, we discovered that Liraglutide, Semaglutid, and Dulaglutide were connected to the low incidence of respiratory disease. This may be related to the high amino acid homology of natural glucagon-like peptide-1, with Liraglutide reaching 97% [56]. In addition, the chemical structures of different GLP-1 RAs with different pharmacological characteristics from each other. And these different findings could also be explained by unbalanced sample sizes.
This article has several advantages. Firstly, this is the first meta-analysis to evaluate the relationship between GLP-1RAs and the risk of respiratory disease in greater detail. The overweight or obese population was also included and subgroup analyses were carried out. In addition, all included studies were randomized controlled trials. Finally, there was either no heterogeneity or very little heterogeneity in our meta-analysis. We admit several limitations of our research. Almost all included studies did not have respiratory diseases as a primary outcome, and these outcomes were derived from adverse event reports while not detecting changes in lung function. Second, the analysis included only trials reporting respiratory disease, resulting in an unknown publication bias. In addition, although 28 studies were incorporated into this analysis, the wide confidence intervals reduced the quality of our findings. Finally, the limited amount of events in the subgroups may have led to a lack of certainty in the subgroup analysis. The effect of using GLP-1RAs on respiratory disease is unknown. More large long-term RCTs with respiratory disease as the primary outcome are needed in the future to evaluate the link between the incidence of respiratory disease and GLP-1 receptor agonists.
Conclusion
In conclusion, using GLP-1RAs was linked to a lower risk of overall respiratory diseases, especially Pulmonary edema and Bronchitis. In the future, physicians should pay attention to the relationship between GLP-1 RA use and the risk of respiratory diseases and evaluate the efficacy of GLP-1RAs in the primary and secondary prevention of respiratory diseases.
Supplementary Information
Additional file 1: Figure S1. Risk of bias summary. Figure S2. Funnel plot and Egger’s test for the comparison of the incidence of overall Respiratory diseases with the use of GLP-1 receptor agonists versus placebo or other antidiabetic treatments. Figure S3. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Pulmonary edema. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S4. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Bronchitis. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S5. Forest plot of GLP-1 receptor agonists versus comparators on the risk of pneumonia. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S6. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Pulmonary fibrosis. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S7. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Dyspnoea. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S8. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Acute respiratory failure. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S9. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Pleural effusion. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S10. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Asthma. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S11. Forest plot of GLP-1 receptor agonists versus comparators on the risk of COPD. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S12. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Sleep apnoea syndrome. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S13. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Pulmonary embolism. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S14. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Pulmonary hypertension. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S15. Subgroup analyses (indication) of the effects of GLP-1 receptor agonists on the risk of overall respiratory disease. Figure S16. Subgroup analyses (control type) of the effects of GLP-1 receptor agonists on the risk of overall respiratory disease. Figure S17. Subgroup analyses (duration) of the effects of GLP-1 receptor agonists on the risk of overall respiratory disease. Figure S18. Forest plot of Albiglutide versus comparators on the risk of overall Respiratory diseases. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval. Figure S19. Forest plot of Dulaglutide versus comparators on the risk of overall Respiratory diseases. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval. Figure S20. Forest plot of Efpeglenatide versus comparators on the risk of overall Respiratory diseases. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval. Figure S21. Forest plot of Exenatide versus comparators on the risk of overall Respiratory diseases. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval. Figure S22. Forest plot of Liraglutide versus comparators on the risk of overall Respiratory diseases. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval. Figure S23. Forest plot of Lixisenatide versus comparators on the risk of overall Respiratory diseases. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval. Figure S24. Forest plot of Semaglutide versus comparators on the risk of overall Respiratory diseases. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval.
Acknowledgements
Not applicable
Abbreviations
- GLP-1 RAs
Glucagon-like peptide-1 receptor agonists
- T2DM
Type 2 diabetes mellitus
- COPD
Chronic obstructive pulmonary disease
- BMI
Body mass index
- HbA1c
Glycated hemoglobin
- RR
Relative risk
- CI
Confidence interval
- RCT
Randomized controlled trial
- CVOT
Cardiovascular outcome trial
- PRISMA
Preferred reporting items for systematic reviews and meta-analyses
Author contributions
MX contributed to the conception and design of the study. Literature retrieval and data extraction were performed by MX and RX. MX, RX, and PL analyze the data. JJ, HW, YW, and HL explain the results. LH, JH, and XF reviewed and revised this article. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Funding by Science and Technology Projects in Guangzhou (Grant Number: 202201020081).
Availability of data and materials
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
We have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Meixin Yu and Ruxin Wang have contributed equally to this work and share first authorship.
Contributor Information
Meixin Yu, Email: yumeixin2023@163.com.
Ruxin Wang, Email: wrxinxin@foxmail.com.
Ling Pei, Email: peiling@mail2.sysu.edu.cn.
Xiaofang Zhang, Email: 857154586@qq.com.
Jinjing Wei, Email: 2633100795@qq.com.
Yun Wen, Email: rhymewen@163.com.
Han Liu, Email: liuhan996@126.com.
Haowen Ye, Email: 849994689@qq.com.
Jinghao Wang, Email: wangjinghao@jnu.edu.cn.
Lihong Wang, Email: nd6688@163.com.
References
- 1.Zar HJ, Ferkol TW. The global burden of respiratory disease-impact on child health. Pediatr Pulmonol. 2014;49:430–434. doi: 10.1002/ppul.23030. [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visca D, Pignatti P, Spanevello A, Lucini E, La Rocca E. Relationship between diabetes and respiratory diseases-clinical and therapeutic aspects. Pharmacol Res. 2018;137:230–235. doi: 10.1016/j.phrs.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Rogliani P, Calzetta L, Capuani B, et al. Glucagon-like peptide 1 receptor: a novel pharmacological target for treating human bronchial hyperresponsiveness. Am J Respir Cell Mol Biol. 2016;55:804–814. doi: 10.1165/rcmb.2015-0311OC. [DOI] [PubMed] [Google Scholar]
- 5.ElSayed NA, Aleppo G, Aroda VR, et al. 9 Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46:S140–S157. doi: 10.2337/dc23-S009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pradhan R, Lu S, Yin H, et al. Novel antihyperglycaemic drugs and prevention of chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes: population based cohort study. BMJ. 2022;379:e071380. doi: 10.1136/bmj-2022-071380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foer D, Beeler PE, Cui J, Karlson EW, Bates DW, Cahill KN. Asthma exacerbations in patients with type 2 diabetes and asthma on glucagon-like peptide-1 receptor agonists. Am J Respir Crit Care Med. 2021;203:831–840. doi: 10.1164/rccm.202004-0993OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albogami Y, Cusi K, Daniels MJ, Wei YJ, Winterstein AG. Glucagon-like peptide 1 receptor agonists and chronic lower respiratory disease exacerbations among patients with type 2 diabetes. Diabetes Care. 2021;44:1344–1352. doi: 10.2337/dc20-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sultana R, Sissoho F, Kaushik VP, Raji MA. The case for early use of glucagon-like peptide-1 receptor agonists in obstructive sleep apnea patients with comorbid diabetes and metabolic syndrome. Life. 2022 doi: 10.3390/life12081222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viby NE, Isidor MS, Buggeskov KB, Poulsen SS, Hansen JB, Kissow H. Glucagon-like peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice. Endocrinology. 2013;154:4503–4511. doi: 10.1210/en.2013-1666. [DOI] [PubMed] [Google Scholar]
- 11.Zhou W, Shao W, Zhang Y, Liu D, Liu M, Jin T. Glucagon-like peptide-1 receptor mediates the beneficial effect of liraglutide in an acute lung injury mouse model involving the thioredoxin-interacting protein. Am J Physiol Endocrinol Metab. 2020;319:E568–E578. doi: 10.1152/ajpendo.00292.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell PD, Salter BM, Oliveria JP, et al. Glucagon-like peptide-1 receptor expression on human eosinophils and its regulation of eosinophil activation. Clin Exp Allergy. 2017;47:331–338. doi: 10.1111/cea.12860. [DOI] [PubMed] [Google Scholar]
- 13.Perdomo CM, Cohen RV, Sumithran P, Clément K, Frühbeck G. Contemporary medical, device, and surgical therapies for obesity in adults. Lancet. 2023 doi: 10.1016/S0140-6736(22)02403-5. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Altman DG, Gotzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahrén B, Johnson SL, Stewart M, et al. HARMONY 3: 104-week randomized, double-blind, placebo- and active-controlled trial assessing the efficacy and safety of albiglutide compared with placebo, sitagliptin, and glimepiride in patients with type 2 diabetes taking metformin. Diabetes Care. 2014;37:2141–2148. doi: 10.2337/dc14-0024. [DOI] [PubMed] [Google Scholar]
- 16.Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol. 2017;5:341–354. doi: 10.1016/S2213-8587(17)30092-X. [DOI] [PubMed] [Google Scholar]
- 17.Blonde L, Jendle J, Gross J, et al. Once-weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD-4): a randomised, open-label, phase 3, non-inferiority study. Lancet. 2015;385:2057–2066. doi: 10.1016/S0140-6736(15)60936-9. [DOI] [PubMed] [Google Scholar]
- 18.Charbonnel B, Steinberg H, Eymard E, et al. Efficacy and safety over 26 weeks of an oral treatment strategy including sitagliptin compared with an injectable treatment strategy with liraglutide in patients with type 2 diabetes mellitus inadequately controlled on metformin: a randomised clinical trial. Diabetologia. 2013;56:1503–1511. doi: 10.1007/s00125-013-2905-1. [DOI] [PubMed] [Google Scholar]
- 19.Davies MJ, Bain SC, Atkin SL, et al. Efficacy and safety of liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA-RENAL): a randomized clinical trial. Diabetes Care. 2016;39:222–230. doi: 10.2337/dc14-2883. [DOI] [PubMed] [Google Scholar]
- 20.Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314:687–699. doi: 10.1001/jama.2015.9676. [DOI] [PubMed] [Google Scholar]
- 21.Davies M, Færch L, Jeppesen OK, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397:971–984. doi: 10.1016/S0140-6736(21)00213-0. [DOI] [PubMed] [Google Scholar]
- 22.Frias JP, Choi J, Rosenstock J, et al. Efficacy and safety of once-weekly efpeglenatide monotherapy versus placebo in type 2 diabetes: the AMPLITUDE-M randomized controlled trial. Diabetes Care. 2022;45:1592–1600. doi: 10.2337/dc21-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallwitz B, Böhmer M, Segiet T, et al. Exenatide twice daily versus premixed insulin aspart 70/30 in metformin-treated patients with type 2 diabetes: a randomized 26-week study on glycemic control and hypoglycemia. Diabetes Care. 2011;34:604–606. doi: 10.2337/dc10-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallwitz B, Guzman J, Dotta F, et al. Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. Lancet. 2012;379:2270–2278. doi: 10.1016/S0140-6736(12)60479-6. [DOI] [PubMed] [Google Scholar]
- 25.Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473–481. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 26.Garvey WT, Batterham RL, Bhatta M, et al. Two-year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. 2022;28:2083–2091. doi: 10.1038/s41591-022-02026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 28.Gerstein HC, Sattar N, Rosenstock J, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385:896–907. doi: 10.1056/NEJMoa2108269. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (harmony outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 30.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 32.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 33.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meneilly GS, Roy-Duval C, Alawi H, et al. Lixisenatide therapy in older patients with type 2 diabetes inadequately controlled on their current antidiabetic treatment: the getgoal-o randomized trial. Diabetes Care. 2017;40:485–493. doi: 10.2337/dc16-2143. [DOI] [PubMed] [Google Scholar]
- 35.Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 37.Rosenstock J, Allison D, Birkenfeld AL, et al. Effect of additional oral semaglutide vs sitagliptin on glycated hemoglobin in adults with type 2 diabetes uncontrolled with metformin alone or with sulfonylurea: the PIONEER 3 randomized clinical trial. JAMA. 2019;321:1466–1480. doi: 10.1001/jama.2019.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325:1403–1413. doi: 10.1001/jama.2021.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang W, Nevárez L, Filippova E, et al. Efficacy and safety of once-weekly dulaglutide versus insulin glargine in mainly Asian patients with type 2 diabetes mellitus on metformin and/or a sulphonylurea: a 52-week open-label, randomized phase III trial. Diabetes Obes Metab. 2019;21:234–243. doi: 10.1111/dom.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinstock RS, Guerci B, Umpierrez G, Nauck MA, Skrivanek Z, Milicevic Z. Safety and efficacy of once-weekly dulaglutide versus sitagliptin after 2 years in metformin-treated patients with type 2 diabetes (AWARD-5): a randomized, phase III study. Diabetes Obes Metab. 2015;17:849–858. doi: 10.1111/dom.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weissman PN, Carr MC, Ye J, et al. HARMONY 4: randomised clinical trial comparing once-weekly albiglutide and insulin glargine in patients with type 2 diabetes inadequately controlled with metformin with or without sulfonylurea. Diabetologia. 2014;57:2475–2484. doi: 10.1007/s00125-014-3360-3. [DOI] [PubMed] [Google Scholar]
- 42.Wilding J, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 43.Wei JP, Yang CL, Leng WH, Ding LL, Zhao GH. Use of GLP1RAs and occurrence of respiratory disorders: a meta-analysis of large randomized trials of GLP1RAs. Clin Respir J. 2021;15:847–850. doi: 10.1111/crj.13372. [DOI] [PubMed] [Google Scholar]
- 44.Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology. 1996;137:2968–2978. doi: 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- 45.Campos RV, Lee YC, Drucker DJ. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology. 1994;134:2156–2164. doi: 10.1210/endo.134.5.8156917. [DOI] [PubMed] [Google Scholar]
- 46.Mendivil CO, Koziel H, Brain JD. Metabolic hormones, apolipoproteins, adipokines, and cytokines in the alveolar lining fluid of healthy adults: compartmentalization and physiological correlates. PLoS ONE. 2015;10:e0123344. doi: 10.1371/journal.pone.0123344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaudhuri A, Ghanim H, Vora M, et al. Exenatide exerts a potent antiinflammatory effect. J Clin Endocrinol Metab. 2012;97:198–207. doi: 10.1210/jc.2011-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altintas DA, Hilberg O, Hess S, Jensen TT, Bladbjerg EM, Juhl CB. Respiratory effects of treatment with a glucagon-like peptide-1 receptor agonist in patients suffering from obesity and chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2022;17:405–414. doi: 10.2147/COPD.S350133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lopez-Cano C, Ciudin A, Sanchez E, et al. Liraglutide improves forced vital capacity in individuals with type 2 diabetes: data from the randomized crossover LIRALUNG study. Diabetes. 2022;71:315–320. doi: 10.2337/db21-0688. [DOI] [PubMed] [Google Scholar]
- 50.Zhu T, Li C, Zhang X, et al. GLP-1 analogue liraglutide enhances SP-A expression in LPS-induced acute lung injury through the TTF-1 signaling pathway. Mediators Inflamm. 2018;2018:3601454. doi: 10.1155/2018/3601454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cardet JC, Ash S, Kusa T, Camargo CJ, Israel E. Insulin resistance modifies the association between obesity and current asthma in adults. Eur Respir J. 2016;48:403–410. doi: 10.1183/13993003.00246-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rodrigues T, Borges P, Mar L, et al. GLP-1 improves adipose tissue glyoxalase activity and capillarization improving insulin sensitivity in type 2 diabetes. Pharmacol Res. 2020;161:105198. doi: 10.1016/j.phrs.2020.105198. [DOI] [PubMed] [Google Scholar]
- 53.Rodrigues T, Matafome P, Sereno J, et al. Methylglyoxal-induced glycation changes adipose tissue vascular architecture, flow and expansion, leading to insulin resistance. Sci Rep. 2017;7:1698. doi: 10.1038/s41598-017-01730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richter G, Feddersen O, Wagner U, Barth P, Goke R, Goke B. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am J Physiol. 1993;265:L374–L381. doi: 10.1152/ajplung.1993.265.4.L374. [DOI] [PubMed] [Google Scholar]
- 55.Wu YC, Wang WT, Lee SS, et al. Glucagon-like peptide-1 receptor agonist attenuates autophagy to ameliorate pulmonary arterial hypertension through Drp1/NOX- and Atg-5/Atg-7/Beclin-1/LC3beta pathways. Int J Mol Sci. 2019 doi: 10.3390/ijms20143435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacobsen LV, Flint A, Olsen AK, Ingwersen SH. Liraglutide in type 2 diabetes mellitus: clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2016;55:657–672. doi: 10.1007/s40262-015-0343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Risk of bias summary. Figure S2. Funnel plot and Egger’s test for the comparison of the incidence of overall Respiratory diseases with the use of GLP-1 receptor agonists versus placebo or other antidiabetic treatments. Figure S3. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Pulmonary edema. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S4. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Bronchitis. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S5. Forest plot of GLP-1 receptor agonists versus comparators on the risk of pneumonia. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S6. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Pulmonary fibrosis. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S7. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Dyspnoea. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S8. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Acute respiratory failure. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S9. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Pleural effusion. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S10. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Asthma. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S11. Forest plot of GLP-1 receptor agonists versus comparators on the risk of COPD. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S12. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Sleep apnoea syndrome. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S13. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Pulmonary embolism. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S14. Forest plot of GLP-1 receptor agonists versus comparators on the risk of Pulmonary hypertension. GLP-1RAs: GLP-1 receptor agonists, RR: risk ratios, CI: confidence Interval. Figure S15. Subgroup analyses (indication) of the effects of GLP-1 receptor agonists on the risk of overall respiratory disease. Figure S16. Subgroup analyses (control type) of the effects of GLP-1 receptor agonists on the risk of overall respiratory disease. Figure S17. Subgroup analyses (duration) of the effects of GLP-1 receptor agonists on the risk of overall respiratory disease. Figure S18. Forest plot of Albiglutide versus comparators on the risk of overall Respiratory diseases. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval. Figure S19. Forest plot of Dulaglutide versus comparators on the risk of overall Respiratory diseases. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval. Figure S20. Forest plot of Efpeglenatide versus comparators on the risk of overall Respiratory diseases. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval. Figure S21. Forest plot of Exenatide versus comparators on the risk of overall Respiratory diseases. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval. Figure S22. Forest plot of Liraglutide versus comparators on the risk of overall Respiratory diseases. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval. Figure S23. Forest plot of Lixisenatide versus comparators on the risk of overall Respiratory diseases. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval. Figure S24. Forest plot of Semaglutide versus comparators on the risk of overall Respiratory diseases. GLP-1RAs, GLP-1 receptor agonists; RR, risk ratios; CI, confidence interval.
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its supplementary information files).