Abstract
Aims:
Alzheimer’s disease (AD) is a complex neurodegenerative disorder characterized by cerebral amyloid β (Aβ) deposition and tau pathology. The AD-mediated degeneration of the brain neuro-signaling pathways, together with a potential peripheral amyloid accumulation, may also result in the derangement of the peripheral nervous system, culminating in detrimental effects on other organs, including the heart. However, whether and how AD pathology modulates cardiac function, neurotrophins, innervation, and amyloidosis is still unknown. Here, we report for the first time that cardiac remodeling, amyloid deposition, and neuro-signaling dysregulation occur in the heart of Tg2576 mice, a widely used model of AD and cerebral amyloidosis.
Methods ad Results:
Echocardiographic analysis showed significant deterioration of left ventricle function, evidenced by a decline of both ejection fraction and fraction shortening percentage in 12-month-old Tg2576 mice compared to age-matched WT littermates. Tg2576 mice hearts exhibited an accumulation of amyloid aggregates, including Aβ, an increase in interstitial fibrosis and severe cardiac nervous system dysfunction. The transgenic mice also showed a significant decrease in cardiac nerve fiber density, including both adrenergic and regenerating nerve endings. This myocardial denervation was accompanied by a robust reduction in NGF and BDNF protein expression as well as GAP-43 expression (regenerating fibers) in both the brain and heart of Tg2576 mice. Accordingly, cardiomyocytes and neuronal cells challenged with Aβ oligomers showed significant downregulation of BDNF and GAP-43, indicating a causal effect of Aβ on the loss of cardiac neurotrophic function.
Conclusions:
Overall, this study uncovers possible harmful effects of AD on the heart, revealing cardiac degeneration induced by Aβ through fibrosis and neuro-signaling pathway deregulation for the first time in Tg2576 mice. Our data suggest that AD pathology can cause deleterious effects on the heart, and the peripheral neurotrophic pathway may represent a potential therapeutic target to limit these effects.
Keywords: Amyloid-β (Aβ), Alzheimer’s disease, cardiac dysfunction, neurotrophic pathway, peripheral nervous system, brain-heart axis
1. Introduction
Alzheimer’s disease (AD) is the most common form of dementia, with a significantly growing incidence that is expected to triple by 2050, especially due to the increasing population age in developed countries[1]. This complex neurodegenerative disorder is characterized by the progressive accumulation of insoluble amyloid-β (Aβ) plaques in the brain parenchyma and vasculature, together with neurofibrillary tangles of hyperphosphorylated tau, resulting in a widespread cerebral derangement[2]. This culminates in severe neuronal loss, neuroinflammation, and vascular dysfunction with increased blood-brain barrier (BBB) permeability and progressive cognitive decline[3–5]. Albeit the main symptoms of the disease predominantly concern the central nervous system (CNS), recent evidence described extracerebral effects of AD that involve the heart, among other peripherical organs and tissues[6]. Indeed, several studies have demonstrated a connection between AD and cardiac disorders[7–9]. Particularly, beyond the neuronal areas, Aβ accumulation was found in the cardiac tissue of AD patients[10] similar to that detected in patients with idiopathic dilated cardiomyopathy[11, 12]. In parallel to the described neuronal and vascular dysfunction, AD is accompanied by a dysregulation in the brain neurotrophic signaling, with a progressive loss of the two main neuromodulators, nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF)[13]. Specifically, Aβ significantly affects the expression of these neurotrophins, and the maladaptive remodeling of the neuro-signaling pathway is associated with cognitive decline[14]. Therefore, it is plausible to hypothesize that the progressive neurotrophic signaling degeneration observed in the brain of AD patients may trigger a gradual decline in peripheral levels of neuromodulators. The latter, in turn, may result in a severe derangement of other peripheral organs, including the cardiac nervous system, potentially culminating in lethal heart dysfunction. However, whether and how AD pathology modulates neurotrophic factors (NTFs) levels, innervation, and amyloidosis in the cardiac tissue remains to be explored. Previous studies have described the accumulation of both Aβ peptides, Aβ42 and Aβ40, in the myocardial parenchyma of AD patients[10], associated with progressive cardiac dysfunction, supporting the hypothesis that AD is a multi-organ disease. Yet, the mechanisms involved in cardiac dysfunction and peripheral autonomic nervous system derangement in AD remain elusive, and whether Aβ aggregates reside outside the central nervous system colonizing the myocardial parenchyma and fueling cardiac impairment is still under debate. Moreover, early reports have attested to the detrimental effects mediated by Aβ oligomers on the neurotrophic signaling pathway in the brain, with a progressive reduction in both production and release of neurotrophins, associated with severe cerebral degeneration[15, 16]. However, there are no studies about the potential impacts of AD pathology on the modulation of peripheral nervous system activity and cardiac availability of neurotrophins. Of note, it is known that a decline in circulating BDNF levels promotes the progression of cardiac disease, especially that of ischemic etiology[17–19]. Nevertheless, to the best of our knowledge, no studies thus far analyzed whether AD pathology and more specifically cerebral amyloidosis have an impact on cardiac functional homeostasis. This study, moving on from clinical studies that show diastolic dysfunction in AD patients, directly demonstrates the effect of Aβ pathology on cardiac dysfunction mechanisms. Moreover, we investigated whether the accumulation of cardiac Aβ aggregates is associated with alterations of peripheral innervation and neurotrophins, such as BDNF and NGF, in the heart. Overall, we determined for the first time the presence of cardiac functional impairments, cardiac parenchymal fibrosis and amyloidosis, neurotrophic signaling pathway deficiency, and nerve fiber loss in the Tg2576 mouse model of AD.
2. Methods
2.1. Animals
Male and female Tg2576 mice, a model of cerebral amyloidosis bearing the Swedish mutation (KM670/671NL), and age-matched WT littermates were bred internally. Mice were maintained under controlled conditions (~22°C, and in a 12-hour light-dark cycle, lights on 7 am to 7 pm) with unrestricted access to food and water. The generation of B6;SJL-Tg(APPSWE)2576Kha mice (Tg2576) on a B6;SJL Mixed Background was as described[20].Tg-2576 mice develop numerous parenchymal amyloid beta plaques at 11–13 months and cerebral amyloid angiopathy (CAA) at 10–11 months of age[21]. Tg2576 and age-matched WT littermates (males and females) were evaluated at 4- and 12-month-old age. All experiments and animal protocols were performed according to protocols approved by the Institutional Animal Care and Use Committee of Temple University School of Medicine and conformed to the National Research Council Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (2011, eighth edition).
2.2. Assessment of cardiac function by transthoracic echocardiography
Transthoracic bi-dimensional M-mode echocardiographic analysis was accomplished in 4 and 12 months-old mice under anesthesia, before and after the development of Aβ pathology, using a VisualSonics Vevo 2100 system (VisualSonics, Toronto, Canada). Cardiac function and structure were evaluated by measuring left ventricle (LV) wall thickness, end-diastolic diameter (LVEDD), end-systolic diameter (LVESD), end-diastolic volume (LVEDV), end-systolic volume (LVESV), ejection fraction (EF%), and fraction shortening (FS%) percentage[22]. Animals were euthanized at 12 months for organ explant and biomolecular and histological analysis.
2.3. Tissue processing and morphological evaluations
Cardiac specimens were fixed in Zamboni’s solution (2% paraformaldehyde and picric acid) overnight at 4°C, then cryoprotected with 20% sucrose in PBS for 24 hours at 4°C and cut in 5-μm-consecutive thick longitudinal sections using a freezing sliding microtome (Leica 2000R, Germany). Interstitial fibrosis was assessed via Picro Sirius Red Stain Kit (Abcam, ab150681), and images were acquired using a Nikon Eclipse Ti fluorescence microscope with an 20x objective[23]. Cardiac fibrosis density was measured using ImageJ software (NIH, version 1.30) and expressed as a percentage of fibrotic area over the total area. A single operator blindly executed all the quantifications.
2.4. Immunostaining
The cardiac sections were processed via the indirect immunofluorescence method using rabbit polyclonal antibodies against tyrosine hydroxylase (TH, Millipore, #AB152; 1:100) to stain adrenergic nerve fibers and growth association protein 43 (GAP-43, Millipore, #AB5220;1:100) for regenerating nerve terminals. Cardiac nerve fibers showed a regular pattern along cardiomyocytes[24, 25]. Heart nerve endings density was quantified using a square grid with 24 probes (area: 1 cm2 each) overlapped to the digital images, which were acquired using a Nikon Eclipse Ti fluorescence microscope with an 20x objective. Five areas were randomly chosen for quantification from each of the three sections of each sample. The total number of intercepts between nerve fibers and probes for each area was assessed, and cardiac nerve fiber density was measured and expressed as the mean number of intercepts per area (fibers/mm2). A single operator assessed all the measurements in a blinded manner. Next, to evaluate amyloid-β accumulation, 5- μm-thick cardiac sections from each experimental group were marked with 6E10 anti-Aβ antibody (Covance, SIG-39320, 1:500) and co-stained with cardiac troponin T (cTnT, Thermo Scientific, #MA5-12960; 1:250), collagen type I (Rockland Immunochemicals, #600-401-103-0.1; 1:100) or cleaved caspase-3 (Asp175) (Cl-Casp-3, Cell Signaling, #94530; 1:200) antibodies. Nuclei were labeled with 4’, 6-diamidino-2-phenylindole (DAPI, Sigma Aldrich, D9542; 1:5000) and sections were mounted using an aqueous mounting medium. Digital images were captured and analyzed by a Nikon Eclipse Ti fluorescence microscope with 40x and 60x objectives. To evaluate vascular components, after fixation, cardiac specimens were cut into 5-μm-thick longitudinal sections using a freezing sliding microtome (Leica 2000R, Germany) (15723985). Next, tissue sections were incubated for 30’ minutes at 4°C in a blocking solution (10% donkey serum supplemented with 0.3% Triton X-100 in phosphate-buffered saline). Via indirect immunofluorescence procedure, sections were analyzed using rabbit polyclonal antibodies against CD31 (AF3628- R&D Systems, 1:30) and α-smooth muscle actin (α-SMA, ab32575- Abcam, 1:500) at 4°C overnight, followed by specific secondary antibodies conjugated with cyanine 3 or cyanin 5 respectively, to stain vascular network. For serum amyloid A3 immunostaining (SAA), fixed 5-μm-thick cardiac sections were retrieved with formic acid 70% solution (Thermo Scientific, #270480010) for twenty minutes at room temperature, next they were processed using rat monoclonal antibody against SAA (Abcam, #ab231680; 1:50) and co-stained with rabbit polyclonal antibody against fibronectin (Abcam, #ab2413; 1:100) for two hours at 37°C, followed by specific secondary antibodies combined with Alexa Fluor 488-conjugated anti-rat IgG and Alexa Fluor 555-conjugated anti-rabbit IgG, respectively. DAPI was used to counterstain nuclei, and cardiac sections were mounted with a Vectashield vibrance antifade mounting medium. Images were acquired and examined using a Nikon Eclipse Ti2 fluorescence microscope with 20x and 40x objectives. A single operator blindly performed all the procedures.
2.5. Preparation of cell and tissue lysates and immunoblotting analysis
Cellular and cardiac samples were homogenized using RIPA cell lysis buffer (Thermo Scientific) enriched with phosphatases and protease inhibitors (Thermo Scientific). Then, the lysates underwent sonication and were centrifuged for 10 min at 4°C at 13,000 rpm to discard the insoluble debris. Next, total protein amounts were quantified via a dye-binding Pierce BCA protein assay kit (Thermo Scientific) and detected using a spectrophotometer reader (SpectraMax i3x Multi-mode Microplate Reader, Molecular Devices) at a wavelength of 512 nm. Equal yields of protein (20–40 μg) were separated through SDS-PAGE and identified by western blot analysis. Total lysates were used to evaluate the protein levels of NGF (AN-240; Alomone labs; 1:1000), BDNF (ANT-010; Alomone labs; 1:1000), GAP-43 (Millipore AB552; 1:1000), Cleaved Caspase-3- Asp175 (Cl-Casp-3- Cell Signaling- #94530; 1:200), dopamine β hydroxylase (DβH; AB1536; Millipore), human Aβ [mouse monoclonal anti-Aβ antibodies mixture composed of 4G8 epitope (residues Aβ18–22; SIG-39320; Covance) and 6E10 epitope (residues Aβ3–8; SIG-39220; Covance)], and GAPDH (sc-32233,6C5; Santa Cruz Biotechnology; 1: 2000), the latter which was used as the loading control. Protein bands were detected by using Odyssey® CLx Imaging System according to the manufacturer’s instructions and quantified with Image Studio™ Lite Software[26].
2.6. ELISA
Aβ40 and Aβ42 peptides levels were detected using a commercial ELISA kit (Invitrogen KHB3481 and KHB3441, respectively). Briefly, cardiac specimens for each mouse were collected at the end of the experimental study and homogenized with RIPA cell lysis buffer (Thermo Scientific), supplemented with phosphatases and protease inhibitors (Thermo Scientific, Waltham, MA). Then, ELISA was performed using an equal amount of total cardiac protein following the company’s specifications, and the plate was evaluated at 450 nm with a spectrophotometer reader (SpectraMax i3x Multi-mode Microplate Reader, Molecular Devices).
2.7. Cell culture and treatments
Human cardiomyocytes (AC16, purchased from Millipore, #SCC109) were expanded in 10 cm2 dishes in a humidified environment at 37°C with 5% CO2 in DMEM/F12 medium (Lonza Ltd. Basel, Switzerland) supplemented with 12.5% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S, Lonza). Human SH-SY5Y neuroblastoma cells (ATCC, CRL-2266, a neuronal cell line used widely in experimental neurological studies), were grown in 10 cm2 dishes in a humidified environment at 37°C with 5% CO2 in DMEM/F12 medium (Lonza Ltd. Basel, Switzerland) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (P/S, Lonza). For both cell types, SH-SY5Y and AC16, an equal number of cells were seeded in 6 well plates and, after 24h, cells were challenged with human Aβ40 or Aβ42 oligomers for 16 hours.
2.8. Aβ peptides
Pre-aggregated Aβ40 and Aβ42 oligomers were generated from HFIP-treated and lyophilized peptides as previously described[27, 28]. Briefly, oligomer enriched Aβ40 and Aβ42 preparations were obtained after a dilution of the peptide in DMSO at a concentration of 5 mM, then diluted to 100 μM in ice-cold DMEM/F12 media and incubated at 4°C for 16 hours. Next, Aβ40 and Aβ42 oligomers were resuspended in DMEM/F12 medium to 10 μM as a final concentration for the cell treatments.
2.9. Statistical methods and analysis
All in vitro and in vivo experiments during the study period were performed blindly. Data are expressed as means ± SEM. Statistical significance between 2 groups was determined by two-tailed Student’s t-test. When comparing multiple groups, data were analyzed using a one-way analysis of variance (ANOVA) test, followed by Tukey’s post hoc test. Differences between groups were considered statistically significant when p≤0.05. All data were analyzed and graphically represented using GraphPad Prism software version 9 (GraphPad, La Jolla, CA).
3. Results
3.1. AD amyloid pathology impairs cardiac function and promotes myocardial interstitial fibrosis.
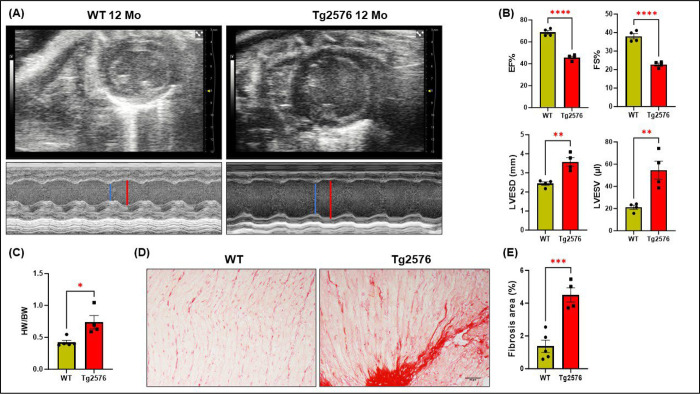
To evaluate the effects of AD amyloid pathology on cardiac tissue functionality and systolic activity, LV function and cardiac chamber dimensions were determined by echocardiographic analysis before or after the development of cerebral Aβ pathology (4- or 12-months) in Tg2576 transgenic mice, a widely used model of cerebral amyloidosis, and age-matched WT littermates (Study design in supplementary Fig.1). Bi-dimensional M-mode tracings revealed a significant decrease in both, ejection fraction (EF%) and fractional shortening (FS%) percentage of 12-month-old Tg2576 mice compared to WT littermates (Fig. 1A–B). No significant changes were found in systolic function in young (4month-old) Tg2576 mice (Supplementary Fig.1). Additionally, 12-month-old transgenic mice showed a remarkable left ventricle enlargement, as evidenced by the increased left ventricular end-systolic diameter (LVESD) (Fig.1A–B). The LV chamber dilation resulted in a relevant increase in left ventricle volumes, expressed as left ventricular end-systolic volume (LVESV) (Fig.1B). Differences in gravimetric parameters were confirmed by measuring the heart weight/body weight ratio, which significantly increased in Tg2576 mice compared to the WT group (Fig.1C). This severe deterioration of systolic function and increased cardiac mass in the 12-month-old Tg2576 mouse was associated with a prominent increase in myocardial interstitial fibrosis, as detected by Picrosirius red staining (Fig.1D–E). Overall, these findings suggest that AD pathology causes detrimental effects on systolic function and parenchymal tissue structure of the heart.
Figure 1. Alzheimer’s disease severely impairs cardiac tissue.
(A) M-mode representative images of echocardiographic analysis in 12-month-old WT and Tg2576 mice. Red lines point to the left ventricular diameter at end-diastole while blue lines show the left ventricular diameter at end-systole on M-mode images. (B) Left ventricle (LV) systolic function is impaired in the AD model, as evidenced by a significant decline both in ejection fraction (EF%) and fraction shortening percentage (FS%), accompanied by a significant cardiac chamber dilation, as assessed by measuring left ventricular dimension (LVESD) and left ventricular volume (LVESV) in the Tg2576 group, compared to age-matched WT mice (12-month-old). (C) The heart weight/body weight ratio shows a significant increase in Tg2576 mice compared to the WT group. (D-E) Representative images (left panels) and quantitative data (right panels) showing the percentage (%) of cardiac fibrosis in heart sections from WT and Tg2576 mice, assessed via PicroSirius red staining (scale bar 50μm). n=4– 5 mice/group. Data are presented as a mean±SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 and vs WT. Student t-tests have been performed between the two groups.
3.2. AD increases Aβ accumulation within the myocardial parenchyma in association with apoptosis.
Recently, a clinical report demonstrated the presence of Aβ deposits in the myocardial tissue of patients affected by AD, resulting in a progressive diastolic impairment[10]. In line with this, our data revealed a degradation of the extracellular matrix with increased collagen deposition and Aβ40 accumulation, the latter quantified by ELISA, in 12-month-old Tg2576 cardiac tissue compared to the WT group (Fig. 2A). Intriguingly, no changes in Aβ42 levels were observed between the WT and Tg2576 groups (Fig.2B). These results are consistent with the age-dependent production of Aβ peptides in the Tg2576 mouse model, which exhibits higher abundance and a progressive increase in cerebral Aβ40 levels compared to Aβ42[29]. The cardiac Aβ40 deposition evidenced by ELISA was validated by western blot analysis, which confirmed a significant increase of Aβ dimers and oligomers in total heart lysates of Tg2576 as opposed to WT littermates (Fig. 2C–D). Interestingly, the immunofluorescence staining revealed that amyloid aggregates accumulate in the interstitial spaces between cardiomyocytes, together with collagen type I fibers (Fig. 2F–G, top panels), thus corroborating the myocardial interstitial fibrosis evidenced by Picro-sirius red staining. Aβ accumulation is known to cause increase in apoptosis in vascular and neuronal cells and in AD brains, as well as in cardiac cells in culture [30–34]. Indeed, analysis of the cardiac tissue by WB and IHC revealed upregulation of cleaved-caspase 3 levels in Tg2576 hearts (Fig.2C–E, and F–G). In summary, these data suggest that the accumulation of Aβ in the myocardial interstitium results in cardiac cell apoptosis and dysfunction.
Figure 2: Aβ aggregated species accumulate in the myocardial parenchyma in association with cardiac cell apoptosis.
(A-B): Plots show Aβ40 (A) Aβ42 (B) levels (pg/mL), assessed by ELISA assay in heart lysates from 12 months old WT and Tg2576 mice. (C-E): Representative immunoblots (left panels) and densitometric analysis (right panels) showing levels of Aβ dimers, oligomers (D) and Cleaved-Caspase3 (Cl-Casp3, E) in total heart lysates of 12-month-old WT and Tg2576 mice. GAPDH levels are used as a loading control. (F-G): Representative digital images (top panels, scale bar 100μm) showing collagen type I fibers (COL1A1, in green) and Aβ aggregates (in red) and, representative digital images (bottom panels; scale bar 100μm) showing Cleaved-Caspase3 (Cl-Casp3, in green) and Aβ aggregates (in red) in cardiac sections from WT (left panels) and Tg2576 (right panels) mice. n=3– 5 mice/group. Data are presented as a mean±SEM. *P<0.05, **P<0.01, ***P<0.001 and vs WT. Student t-tests have been performed between the 2 groups.
3.3. Amyloid-β pathology induces misfolding of serum amyloid A3 and its deposition in the cardiac vessel walls.
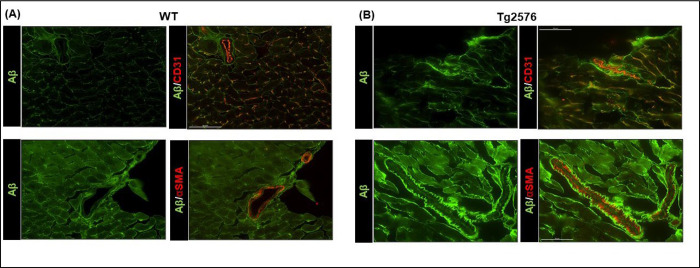
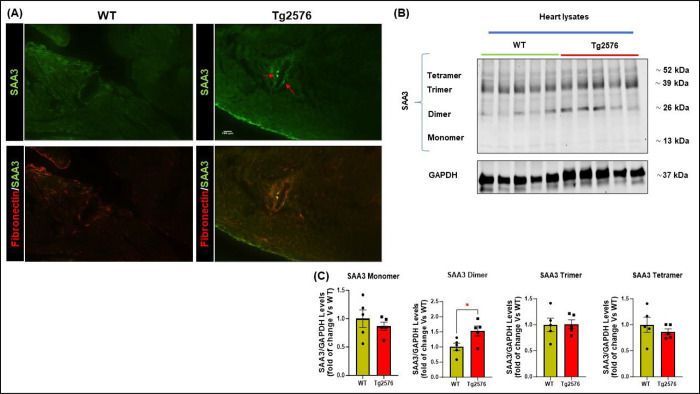
Protein misfolding diseases, including AD, are characterized by a progressive derangement in protein organization, during which seeding processes may be triggered by different forms of amyloid interacting with each other[35]. It is therefore conceivable that the progressive accumulation of misfolded Aβ oligomers in the heart tissue may result in both, a significant increase in fibrosis and the seeding of other amyloid peptides such as serum amyloid A (SAA) proteins. SAAs belong to the apolipoproteins category, associated with inflammatory stimuli and activation of enzymes involved in extracellular matrix deterioration[36]. High levels of SAAs proteins usually do not lead to fibrillar deposits. However, a chronic inflammatory state, or a seeding process (such as that promoted by Aβ), may induce these SAAs peptides to accumulate in peripheral tissues. Indeed, we found accumulation of Aβ aggregates around cardiac vessels in the Tg2576 mice, with infiltration of the vascular basal lamina (Fig.3 and Supplementary Fig.2D), which was accompanied by an increase of serum amyloid A3 (SAA3) vascular deposits (Fig 4A). As confirmed by immunoblot analysis, SAA3 dimers were increased in the cardiac tissue of Tg mice (Fig.4B). This accumulation and promoted aggregation of serum amyloid proteins may further contribute to the impairment of cardiac function and to the fibrotic phenotype.
Figure 3: Aβ aggregates surround cardiac vessels and infiltrate the vascular basal lamina.
Top panels: Representative digital images (scale bar 50μm) showing endothelial cells (stained with CD31, in orange) and Aβ (in green). Bottom panels: representative digital images (scale bar 50μm) showing smooth muscle cells by α-Smooth muscle alpha-actin staining (αSMA, in red) and Aβ (in green) in cardiac sections from WT and Tg2576 mice. n=3– 4 mice/group. WT mice are represented in the left panels and Tg2576 mice in the right panels.
Figure 4: Serum amyloid A3 (SAA3) perivascular deposition in the heart of Tg2576 mice.
(A): Representative images of 12-month-old WT and Tg2576 cardiac sections stained with serum amyloid A3 (SAA3, in green) and fibronectin fibers (in red, scale bar 100μm). (B-C): representative immunoblots (top panels) and densitometric quantitative analysis (bottom panels) showing the levels of different aggregates of SAA3 in total cardiac lysates from WT and Tg2576 mice. GAPDH levels were used as a loading control. n= 5 mice/group. Data are presented as a mean±SEM. *P<0.05 and vs WT. Student t-tests have been performed between the 2 groups.
3.4. Aβ pathology severely impacts neurotrophic signaling in the brain and heart.
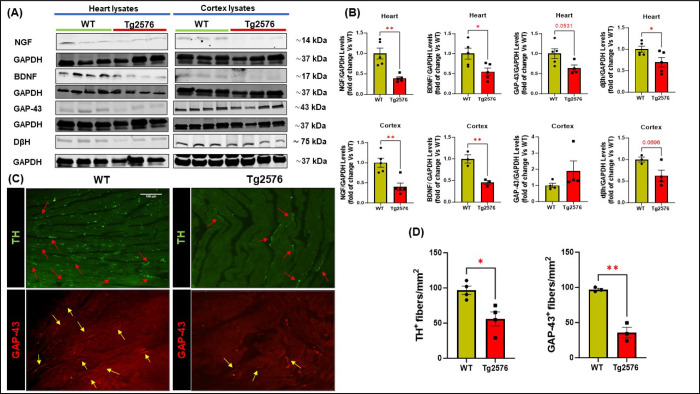
AD pathology and Aβ deposition significantly impair the production of neurotrophins in the brain, leading to a progressive loss of the two major neuromodulators, NGF and BDNF, thus contributing to cognitive impairment and dementia[37, 38]. However, whether AD pathology can modulate peripheral neurotrophic activity and cardiac innervation remains to be elucidated. Therefore, we explored the regulation of neuro-signaling modulators and innervation factors both in the cerebral cortex and cardiac lysates of 12-month-old Tg2576 mice. Immunoblotting analysis showed a robust decline in both cerebral and cardiac levels of NGF and BDNF expression in the Tg2576 group compared to WT mice (Fig.5 A–B). This decrease in the levels of neurotrophins exhibited by transgenic mice was associated with a relevant reduction in the adrenergic marker (DβH) and in the neuronal fibers’ regeneration marker, GAP-43 (Fig.4A–B). Interestingly, IHC analysis confirmed an evident loss in cardiac nerve fibers density in Tg2576 mice compared to WT animals, including both sympathetic and regenerating nerve endings, labeled with TH and GAP-43 markers, respectively (Fig.5 C–D). Taken together, these results indicate that AD amyloid pathology also results in harmful effects on peripheral neurotrophic pathways, hence impacting the peripheral nervous system and cardiac innervation.
Figure 5: The neuro-signaling pathway is compromised in brain and heart of Tg2576 AD mice.
(A-B): Representative immunoblots (A) and densitometric quantitative analysis (B) showing levels of NGF, BDNF, GAP-43, and DβH, in total cardiac (top panels) and cerebral cortex (bottom panels) lysates from WT and Tg2576 mice. (C-D): digital images (C, scale bar 100μm) and quantifications (D) showing cardiac adrenergic nerve fibers, labeled with anti-tyrosine-hydroxylase (TH, in green), and cardiac regenerating nerve endings, labeled with anti-neuronal regeneration marker (GAP-43, in red) in heart sections from WT and Tg2576 mice. n=3– 5 mice/group. Data are presented as a mean±SEM. *P<0.05, **P<0.01 and vs WT. Student t-tests have been performed between the 2 groups.
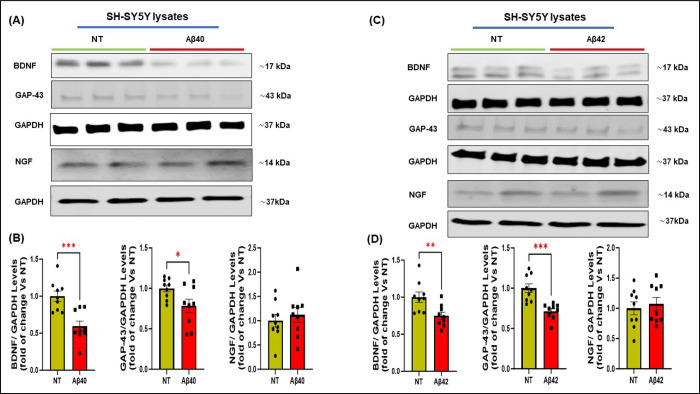
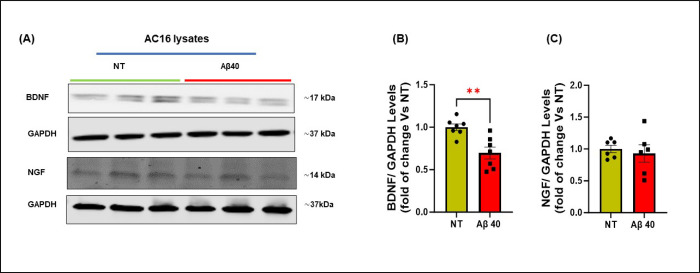
3.5. Aβ oligomers impair neurotrophins production in cardiomyocytes and neuroblastoma cells.
Our in vivo studies in Tg2576 mice, a widely used AD model, demonstrated a remarkable reduction of neurotrophic markers and nerve fibers within the cardiac tissue. To demonstrate that this loss of NTFs is caused by Aβ aggregates that accumulate in the heart, we assessed the effects of Aβ40 oligomers, known as the most toxic aggregation species, on the production of neurotrophins and neuronal regeneration markers in human cardiomyocytes and neuronal SH-SY5Y cells (Fig.6– 7). In addition to Aβ40, Aβ42 effects were also tested in SH-SY5Y cells, since neurons are typically exposed to both peptides. Incubation of the cells for 16 hours with Aβ peptides significantly reduced protein levels of GAP-43 and BDNF in neuronal cells compared with the non-treated group, with no significant changes in NGF expression (Fig.6), and significantly reduced the expression of BDNF in cardiomyocytes (Fig. 7). These data support the hypothesis that amyloid aggregates are, at least in part, responsible for the impairment of the cardiac neuro-signaling pathway and neuronal regeneration, particularly by reducing the production of BDNF in both neurons and cardiomyocytes and the levels of GAP-43 in neurons.
Figure 6: Effects of human Aβ40 or Aβ42 oligomers on the expression of neurotrophic factors and neuronal regeneration markers in SH-SY5Y cells.
Representative immunoblots (A and C) and densitometric quantitative analysis (B and D) showing protein levels of BDNF, NGF, and GAP-43 (B-D) in total protein lysates from human neuroblastoma cells (SH-SY5Y) stimulated with human Aβ40 (left) or Aβ42 (right) oligomers for 16 h. GAPDH levels were used as a loading control. n=9 biological replicates. Data are presented as a mean±SEM. *P<0.05, **P<0.01, ***P<0.001 and vs WT. Student t-tests have been performed between the 2 groups. (NT= Not Treated).
Figure 7: Effects of human Aβ40 oligomers on the expression of neurotrophic factors in AC16 Human Cardiomyocytes.
(A-C): Representative immunoblots (A) and densitometric quantitative analysis (B-C) showing protein levels of BDNF (B), and NGF (C) in total protein lysates from human cardiomyocytes (AC16) stimulated with human Aβ-40 oligomers for 16 h. GAPDH levels were used as a loading control. n= 6–7 biological replicates. Data are presented as a mean±SEM. **P<0.01 and vs WT. Student t-tests have been performed between the groups. (NT= Not Treated).
4. Discussion
This study demonstrates for the first time a severe cardiac functional, morphological, and neurotrophic impairment in the Tg2576 mice, a widely used animal model of cerebral amyloidosis and AD. These alterations include dramatically reduced EF% and FS%, increased LV volume and heart weight. Our data suggests that cardiac amyloidosis (including Aβ and SAA3 deposition) promotes interstitial fibrosis and a loss of cardiac innervation, neurotrophins and neuro-signaling pathway impairment, which appear to be essential mediators of the observed cardiac dysfunction. Importantly, these findings are consistent with recent clinical studies that showed cardiac defects and Aβ accumulation in the hearts of AD patients [10, 39].
The heart-brain axis constitutes a complex and highly regulated bi-directional interconnection between the central and peripheral nervous systems[40, 41]. Neurodegenerative pathologies, such as AD, through the modulation of both the central and the peripheral nervous system, may have serious and debilitating effects on multiple peripheral organs, including the heart. However, the effects of AD pathology and Aβ accumulation in the heart of AD patients and animal models have not been clearly elucidated. Age-related heart-brain axis remodeling increases the susceptibility to both neurodegenerative and chronic cardiac disorders, thus leading to a raise in morbidities and mortality risk[42–44]. An increasing number of studies are starting to highlight the involvement of the cardiac tissue in neurodegenerative diseases, revealing a compelling interplay between AD and cardiac disorders, such as atrial fibrillation[45], heart failure[46–48], and coronary artery disease[49, 50]. It has been recently shown that AD patients exhibit myocardial dysfunction following the deposition of Aβ aggregates in the cardiac tissue[10]. In line with this, Sanna and coworkers described the presence of structural and functional abnormalities (diastolic impairment, ECG alterations, and interventricular septum thickness enhancement) in the cardiac tissue of a small cohort of AD subjects[6]. Interestingly, short N-terminal cleavage products of APP were found in the myocardium. Their frequency correlated with age, degree of myocardial fibrosis in individuals with hypertension and with the severity of cerebral amyloid angiopathy (CAA), suggesting a link between cerebrovascular and cardiac APP-related amyloidosis[39]. AD pathology has been shown to severely impact the production and release of the main neurotrophic factors, NGF and BDNF, in the brain[37, 51, 52]. These two neurotrophins are mainly involved in the maturation and differentiation of neurons, ensuring neurogenesis and synaptogenesis[53, 54]. The progressive decline in neurotrophic factor expression observed in aging and exacerbated by AD pathology may lead to an adverse remodeling of the neuro-signaling pathway, driving, together with other AD pathological factors, a possible impairment of the peripheral innervation[38].
This study is the first to directly demonstrate, in a transgenic model of AD with CAA, the effect of Aβ pathology on cardiac physiology, heart tissue dysfunction and cardiac cell death, showing their association with peripheral innervation impairment and loss of neurotrophins, such as BDNF and NGF. Since both cerebral parenchymal amyloid pathology and CAA have been well described in the Tg2576 mice (e.g., progressive age-dependent accumulation of Aβ aggregates in the brain parenchyma as well as in the brain vasculature), we decided to assess whether a progressive impairment of cardiac morphology and function was evident in this AD transgenic murine model[20, 55, 56]. We found that Tg2576 mice show an age- dependent deterioration in cardiac systolic function and a gradual replacement of viable myocardium with collagen fibers, together with a progressive alteration of the extracellular matrix resulting in severe interstitial fibrosis (Fig. 1). This cardiac maladaptive remodeling is accompanied by a significant accumulation of Aβ, particularly Aβ40, colocalized in perivascular and interstitial spaces with collagen deposits, as well as caspase 3 activation in cardiomyocytes, suggesting that Aβ deposition in the heart tissue contributes to the development of interstitial fibrosis and apoptotic cardiac cell death (Fig. 2). The preferential accumulation of Aβ40, rather than Aβ42, in Tg2576 mice hearts, is in line with previous studies describing the progressive increase mostly of Aβ40 levels in both serum and brain parenchyma of Tg2576 mice during aging[29]. In addition, the Aβ40 peptide is preferentially associated with vascular deposits and CAA in the human brain, resulting in degenerative phenotypes in cerebral endothelial cells[31], which culminate in vessel wall dysfunction[57, 58], contributing to BBB permeability and microhemorrhages[59, 60]. This BBB dysfunction in AD brains may enhance the systemic propagation of Aβ (including aggregated forms such as oligomers) through the blood and its accumulation in peripheral organs, including the heart. Aβ40 is the most abundant peptide in the cerebrospinal fluid and blood of AD patients as well as in this mouse model and multiple biomarker studies have shown that this peptide does not decrease in peripheral organs and tissues with AD progression[61]. Conversely, as Aβ42 fibrils deposit in the brain parenchyma, Aβ42 levels decrease in the cerebrospinal fluid and in the peripheral blood[62].
Aβ oligomers may also act as “seeds”[63], to induce aggregation of other amyloids, such as SAA3 (as shown by our data), thus leading to extracellular matrix protein accumulation in the heart and progressive cardiac impairment. At the molecular level, dysregulation and misfolding of amyloid peptides induce a progressive rearranging of protein structure, with seeding of other amyloids, gradually precipitating in the tissue parenchyma[64]. This process characterizes a wide array of human diseases, including AD and CAA[35]. Some of these aggregation species fuel cell stress and inflammation, resulting in degenerative processes in multiple cell types, including endothelial and cardiac cells[30, 57, 58, 65–67]. We propose that this amyloid cross-seeding process progressively induces cardiac SAA3 apolipoproteins deposition in Tg2576 mice, culminating in significant extracellular matrix deterioration and fibrosis[36]. Notably, SAA3 aggregates, together with Aβ deposits, accumulated primarily around cardiac vessels (Fig. 3– 4), suggesting that misfolded Aβ peptides or oligomers may diffuse through the vascular system, with consequential seeding of other amyloids around the myocardial vessels. This evidence suggests that the progressive deposition of amyloids in the cardiac tissue may initiate the observed cardiac cell death, extracellular matrix alterations, heart fibrosis, cardiac dysfunction, and the rarefaction of cardiac nerve fibers.
It is conceivable that, in this model, contractile dysfunction due to amyloid-mediated cardiomyocytes cell death and neuronal dysregulation may proceed simultaneously or that one may precede the other. As we have shown in cardiomyocytes and coronary endothelial cells, as well as in cerebral endothelial cells, Aβ challenge plays a causal role in inducing mitochondrial dysfunction and increased ROS production [27, 30], through diminished energy metabolism, resulting in cardiac and endothelial cell dysfunction and death. This cascade of events is likely to lead to the loss of cardiac cells, hypertrophy, and deteriorated peripheral neurotrophic pathways in the heart. Specifically, we observed a marked depletion in both adrenergic nerve endings and regenerated nerve terminals in transgenic mouse hearts (Fig.5). This myocardial denervation was associated with a robust reduction of both neurotrophic factors, NGF and BDNF, at the cerebral and cardiac level, accompanied by significant impairment in the neuronal sprouting marker GAP-43 (Fig. 5). In AD patients, the decline of neuromodulators in the brain accelerates the age-related synaptic loss, resulting in cognitive impairment[48, 68]. This process starts in the early stages of AD pathology, worsening due to cellular and metabolic modifications, pinnacling in a dramatic loss of neurons, synaptic rarefaction, and brain atrophy[69–71]. NTFs are powerful biomolecules with significant pleiotropic effects involved in cellular growth and survival pathways, both in the brain and on cells/tissues outside the brain, including endothelial cells, muscle cells, and the cardiovascular system[17, 72–74]. Here, we demonstrated that Tg2576 mice exhibit a marked downregulation of the neuro signaling pathway in both the brain and the heart. Thus, akin to elderly individuals and heart failure subjects, AD patients may also be more exposed to cardiac impairment because of neuronal signaling dysregulation, which may be both a consequence of myocardial amyloid deposition, as well as of neurotrophic signaling impairment in neuronal and cardiac cells. Notably, we observed that after treatment with Aβ oligomers, BDNF and GAP-43 expression in human neuroblastoma SH-SY5Y cells and cardiomyocytes (Fig. 6– 7) were considerably reduced. These findings suggest a direct effect of Aβ on the reduction of NTFs in neurons, including those innervating the heart, and in cardiac cells (Fig.6) and represents the first report of an Aβ-induced reduction in neurotrophic factors in cardiomyocytes.
Since neurotrophic factors are also involved in angiogenesis and relaxation/contraction coupling processes, their deficit sharply impairs cardiac function[75, 76]. In this regard, the observed loss of BDNF and NGF is likely to be a key contributor to the cardiac pathological state observed in our AD model, providing additional clarification on the reasons why AD patients may be more prone to develop cardiovascular disorders of different etiology[77, 78]. The decline in NGF and BDNF in the Tg2576 animals was associated with a downregulation of TH and GAP-43, confirming the rarefaction of cardiac nerve fibers, and the impairment of their neuronal regeneration capability.
Aβ challenge may also have additional toxic effects on cardiac cells. As we have recently demonstrated, Aβ reduced cell survival and increased mitochondrial swelling and ROS production on H9c2 cardio-myoblasts and human coronary artery endothelial cells[30]. This is in line with the current findings showing an increased activation of caspase-3 in cardiac cells of Tg2576 mice.
Collectively, our novel findings suggest that AD pathology could be responsible for structural and functional alterations in the heart, including deficits in the cardiac nervous system, as previously shown for aging and heart failure[15, 17, 25].
4.1. Limitations.
The present study is not free of limitations. First, larger longitudinal studies involving heart IHC and biochemistry at many stages of AD pathology in mouse models and humans will be needed to demonstrate whether amyloidosis and loss of cardiac innervation are simultaneous, or if one precedes the other. Also, we did not investigate LV diastolic function in our in ²vivo model. Previous investigations have demonstrated diastolic impairment correlated with cardiac electric abnormalities in a small group of AD patients. Here, we confirmed cardiac dysfunction through systolic evaluations. Our investigation of the neuro-signaling pathway may miss other neurotrophins involved in cardiac function such as NT3 and NT4. In this study, we focused on the two main neuromodulators (NGF and BDNF), which are well-characterized and accountable for regulating and preserving the brain/heart axis equilibrium. Additionally, it cannot be excluded that this transgenic model may involve the production of Aβ by peripheral neurons. However, this reflects the case of human aging and AD patients, in which the production of Aβ may be expected also in peripheral neuronal cells, and overall clearance mechanisms are compromised by aging. In fact, the validity and clinical relevance of our findings is confirmed by the presence of Aβ deposits in the heart of AD patients[10]. Lastly, future investigations are needed to better elucidate the mechanisms responsible for the cardiac neuro-signaling impairment and loss of nerve terminals that occurs in Alzheimer’s pathology. Our in vitro data suggests that Aβ is, at least in part, causal for these effects.
4.2. Conclusions.
Overall, our findings reveal a maladaptive remodeling induced by AD amyloid pathology on the heart, with Aβ and SAA3 amyloid deposition, fibrosis, and neurotrophic signaling pathway dysregulation, resulting in an important impairment of cardiac function. This progressive brain/heart axis deterioration is very likely to increase the risk of developing cardiovascular disorders in AD patients. The discovery of these previously uninvestigated AD-mediated myocardial pathological mechanisms, in addition to improving our knowledge on the peripheral implications of AD, provides novel appealing therapeutic targets, such as the neurotrophic signaling pathway, to delay the harmful changes induced by AD and amyloid pathology on the cardiovascular system.
Supplementary Material
5. Funding
This work was supported by NIH R01NS104127 and R01AG062572 grants, the Alzheimer’s Association (AARG-20-685663), the Pennsylvania Department of Heath Collaborative Research on Alzheimer’s Disease (PA Cure) Grant, awarded to SF, by the NIH SC1GM128210 grant awarded to SJ, and by the Karen Toffler Charitable Trust, and the Lemole Center for Integrated Lymphatics research.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
8. Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
9. References
- 1.Hebert L.E., et al. , Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 2013. 80(19): p. 1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masters C.L., et al. , Alzheimer’s disease. Nat Rev Dis Primers, 2015. 1: p. 15056. [DOI] [PubMed] [Google Scholar]
- 3.Castellani R.J., Rolston R.K., and Smith M.A., Alzheimer disease. Dis Mon, 2010. 56(9): p. 484–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fratiglioni L., De Ronchi D., and Aguero-Torres H., Worldwide prevalence and incidence of dementia. Drugs Aging, 1999. 15(5): p. 365–75. [DOI] [PubMed] [Google Scholar]
- 5.Small G.W., et al. , Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA, 1997. 278(16): p. 1363–71. [PubMed] [Google Scholar]
- 6.Sanna G.D., et al. , Cardiac Abnormalities in Alzheimer Disease: Clinical Relevance Beyond Pathophysiological Rationale and Instrumental Findings? JACC Heart Fail, 2019. 7(2): p. 121–128. [DOI] [PubMed] [Google Scholar]
- 7.Paciaroni M. and Bogousslavsky J., Connecting cardiovascular disease and dementia: further evidence. J Am Heart Assoc, 2013. 2(6): p. e000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tini G., et al. , Alzheimer’s Disease and Cardiovascular Disease: A Particular Association. Cardiol Res Pract, 2020. 2020: p. 2617970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang M., et al. , Interrelationship between Alzheimer’s disease and cardiac dysfunction: the brain-heart continuum? Acta Biochim Biophys Sin (Shanghai), 2020. 52(1): p. 1–8. [DOI] [PubMed] [Google Scholar]
- 10.Troncone L., et al. , Abeta Amyloid Pathology Affects the Hearts of Patients With Alzheimer’s Disease: Mind the Heart. J Am Coll Cardiol, 2016. 68(22): p. 2395–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianni D., et al. , Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation, 2010. 121(10): p. 1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li D., et al. , Mutations of presenilin genes in dilated cardiomyopathy and heart failure. Am J Hum Genet, 2006. 79(6): p. 1030–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen S.J., Watson J.J., and Dawbarn D., The neurotrophins and their role in Alzheimer’s disease. Curr Neuropharmacol, 2011. 9(4): p. 559–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garzon D., Yu G., and Fahnestock M., A new brain-derived neurotrophic factor transcript and decrease in brain-derived neurotrophic factor transcripts 1, 2 and 3 in Alzheimer’s disease parietal cortex. J Neurochem, 2002. 82(5): p. 1058–64. [DOI] [PubMed] [Google Scholar]
- 15.Poon W.W., et al. , beta-Amyloid (Abeta) oligomers impair brain-derived neurotrophic factor retrograde trafficking by down-regulating ubiquitin C-terminal hydrolase, UCH-L1. J Biol Chem, 2013. 288(23): p. 16937–16948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Z., Sabirzhanov B., and Keifer J., Oligomeric amyloid-beta inhibits the proteolytic conversion of brain-derived neurotrophic factor (BDNF), AMPA receptor trafficking, and classical conditioning. J Biol Chem, 2010. 285(45): p. 34708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elia A., et al. , Aging is associated with cardiac autonomic nerve fiber depletion and reduced cardiac and circulating BDNF levels. J Geriatr Cardiol, 2021. 18(7): p. 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halade G.V., et al. , Reduced BDNF attenuates inflammation and angiogenesis to improve survival and cardiac function following myocardial infarction in mice. Am J Physiol Heart Circ Physiol, 2013. 305(12): p. H1830–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiltunen J.O., et al. , Nerve growth factor and brain-derived neurotrophic factor mRNAs are regulated in distinct cell populations of rat heart after ischaemia and reperfusion. J Pathol, 2001. 194(2): p. 247–53. [DOI] [PubMed] [Google Scholar]
- 20.Hsiao K., et al. , Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science, 1996. 274(5284): p. 99–102. [DOI] [PubMed] [Google Scholar]
- 21.Robbins E.M., et al. , Kinetics of cerebral amyloid angiopathy progression in a transgenic mouse model of Alzheimer disease. J Neurosci, 2006. 26(2): p. 365–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rigaud V.O., et al. , UCP2 modulates cardiomyocyte cell cycle activity, acetyl-CoA, and histone acetylation in response to moderate hypoxia. JCI Insight, 2022. 7(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cannavo A., et al. , Aldosterone Jeopardizes Myocardial Insulin and beta-Adrenergic Receptor Signaling via G Protein-Coupled Receptor Kinase 2. Front Pharmacol, 2019. 10: p. 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Lucia C., et al. , Long-Term Caloric Restriction Improves Cardiac Function, Remodeling, Adrenergic Responsiveness, and Sympathetic Innervation in a Model of Postischemic Heart Failure. Circ Heart Fail, 2018. 11(3): p. e004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parisi V., et al. , Increased Epicardial Adipose Tissue Volume Correlates With Cardiac Sympathetic Denervation in Patients With Heart Failure. Circ Res, 2016. 118(8): p. 1244–53. [DOI] [PubMed] [Google Scholar]
- 26.Marzano F., et al. , Genetic Catalytic Inactivation of GRK5 Impairs Cardiac Function in Mice Via Dysregulated P53 Levels. JACC Basic Transl Sci, 2022. 7(4): p. 366–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fossati S., et al. , The carbonic anhydrase inhibitor methazolamide prevents amyloid beta-induced mitochondrial dysfunction and caspase activation protecting neuronal and glial cells in vitro and in the mouse brain. Neurobiol Dis, 2016. 86: p. 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stine W.B. Jr., et al. , In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J Biol Chem, 2003. 278(13): p. 11612–22. [DOI] [PubMed] [Google Scholar]
- 29.Kawarabayashi T., et al. , Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci, 2001. 21(2): p. 372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang S., et al. , Beta-Amyloid Instigates Dysfunction of Mitochondria in Cardiac Cells. Cells, 2022. 11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parodi-Rullan R., et al. , Alzheimer’s amyloid beta heterogeneous species differentially affect brain endothelial cell viability, blood-brain barrier integrity, and angiogenesis. Aging Cell, 2020. 19(11): p. e13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J., et al. , Beta-amyloid oligomers activate apoptotic BAK pore for cytochrome c release. Biophys J, 2014. 107(7): p. 1601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fossati S., Ghiso J., and Rostagno A., TRAIL death receptors DR4 and DR5 mediate cerebral microvascular endothelial cell apoptosis induced by oligomeric Alzheimer’s Abeta. Cell Death Dis, 2012. 3: p. e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eckert A., et al. , Increased apoptotic cell death in sporadic and genetic Alzheimer’s disease. Ann N Y Acad Sci, 2003. 1010: p. 604–9. [DOI] [PubMed] [Google Scholar]
- 35.Dobson C.M., Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol, 2004. 15(1): p. 3–16. [DOI] [PubMed] [Google Scholar]
- 36.Uhlar C.M. and Whitehead A.S., Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem, 1999. 265(2): p. 501–23. [DOI] [PubMed] [Google Scholar]
- 37.Arancio O. and Chao M.V., Neurotrophins, synaptic plasticity and dementia. Curr Opin Neurobiol, 2007. 17(3): p. 325–30. [DOI] [PubMed] [Google Scholar]
- 38.Nasrolahi A., et al. , Therapeutic potential of neurotrophic factors in Alzheimer’s Disease. Mol Biol Rep, 2022. 49(3): p. 2345–2357. [DOI] [PubMed] [Google Scholar]
- 39.Kramer L.M., et al. , Amyloid precursor protein-fragments-containing inclusions in cardiomyocytes with basophilic degeneration and its association with cerebral amyloid angiopathy and myocardial fibrosis. Sci Rep, 2018. 8(1): p. 16594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samuels M.A., The brain-heart connection. Circulation, 2007. 116(1): p. 77–84. [DOI] [PubMed] [Google Scholar]
- 41.Tahsili-Fahadan P. and Geocadin R.G., Heart-Brain Axis: Effects of Neurologic Injury on Cardiovascular Function. Circ Res, 2017. 120(3): p. 559–572. [DOI] [PubMed] [Google Scholar]
- 42.Daniele G., et al. , Heart and Brain: Complex Relationships for Left Ventricular Dysfunction. Curr Cardiol Rep, 2020. 22(8): p. 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hooghiemstra A.M., et al. , Frequent Cognitive Impairment in Patients With Disorders Along the Heart-Brain Axis. Stroke, 2019. 50(12): p. 3369–3375. [DOI] [PubMed] [Google Scholar]
- 44.Veugen M.G.J., et al. , Cross-Sectional Associations Between Cardiac Biomarkers, Cognitive Performance, and Structural Brain Changes Are Modified by Age. Arterioscler Thromb Vasc Biol, 2018. 38(8): p. 1948–1958. [DOI] [PubMed] [Google Scholar]
- 45.Ihara M. and Washida K., Linking Atrial Fibrillation with Alzheimer’s Disease: Epidemiological, Pathological, and Mechanistic Evidence. J Alzheimers Dis, 2018. 62(1): p. 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cermakova P., et al. , Heart failure and Alzheimer’s disease. J Intern Med, 2015. 277(4): p. 406–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen M.B. and Mather P.J., A review of the association between congestive heart failure and cognitive impairment. Am J Geriatr Cardiol, 2007. 16(3): p. 171–4. [DOI] [PubMed] [Google Scholar]
- 48.Hoth K.F., et al. , Cardiac dysfunction and cognition in older adults with heart failure. Cogn Behav Neurol, 2008. 21(2): p. 65–72. [DOI] [PubMed] [Google Scholar]
- 49.Roger V.L., Epidemiology of heart failure. Circ Res, 2013. 113(6): p. 646–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tublin J.M., et al. , Getting to the Heart of Alzheimer Disease. Circ Res, 2019. 124(1): p. 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cade S., Zhou X.F., and Bobrovskaya L., The role of brain-derived neurotrophic factor and the neurotrophin receptor p75NTR in age-related brain atrophy and the transition to Alzheimer’s disease. Rev Neurosci, 2022. 33(5): p. 515–529. [DOI] [PubMed] [Google Scholar]
- 52.Gao L., et al. , Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl Neurodegener, 2022. 11(1): p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vitaliano G.D., et al. , Clathrin-nanoparticles deliver BDNF to hippocampus and enhance neurogenesis, synaptogenesis and cognition in HIV/neuroAIDS mouse model. Commun Biol, 2022. 5(1): p. 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C.S., Kavalali E.T., and Monteggia L.M., BDNF signaling in context: From synaptic regulation to psychiatric disorders. Cell, 2022. 185(1): p. 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Irizarry M.C., et al. , APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol, 1997. 56(9): p. 965–73. [DOI] [PubMed] [Google Scholar]
- 56.Jung J.H., et al. , Pathway-specific alteration of synaptic plasticity in Tg2576 mice. Mol Cells, 2011. 32(2): p. 197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fossati S., et al. , Differential activation of mitochondrial apoptotic pathways by vasculotropic amyloid-beta variants in cells composing the cerebral vessel walls. FASEB J, 2010. 24(1): p. 229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fossati S., Ghiso J., and Rostagno A., TRAIL death receptors DR4 and DR5 mediate cerebral microvascular endothelial cell apoptosis induced by oligomeric Alzheimer’s Abeta. Cell Death Dis, 2012. 3(6): p. e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghiso J., Fossati S., and Rostagno A., Amyloidosis associated with cerebral amyloid angiopathy: cell signaling pathways elicited in cerebral endothelial cells. J Alzheimers Dis, 2014. 42 Suppl 3(0 3): p. S167–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lemon N., et al. , Carbonic Anhydrases as Potential Targets Against Neurovascular Unit Dysfunction in Alzheimer’s Disease and Stroke. Front Aging Neurosci, 2021. 13: p. 772278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanon O., et al. , Plasma amyloid beta predicts conversion to dementia in subjects with mild cognitive impairment: The BALTAZAR study. Alzheimers Dement, 2022. [DOI] [PubMed] [Google Scholar]
- 62.Zicha S., et al. , Comparative analytical performance of multiple plasma Abeta42 and Abeta40 assays and their ability to predict positron emission tomography amyloid positivity. Alzheimers Dement, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ulm B.S., Borchelt D.R., and Moore B.D., Remodeling Alzheimer-amyloidosis models by seeding. Mol Neurodegener, 2021. 16(1): p. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lyubchenko Y.L., Amyloid misfolding, aggregation, and the early onset of protein deposition diseases: insights from AFM experiments and computational analyses. AIMS Mol Sci, 2015. 2(3): p. 190–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fossati S., Ghiso J., and Rostagno A., Insights into caspase-mediated apoptotic pathways induced by amyloid-beta in cerebral microvascular endothelial cells. Neurodegener Dis, 2012. 10(1–4): p. 324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parodi-Rullan R., Sone J.Y., and Fossati S., Endothelial Mitochondrial Dysfunction in Cerebral Amyloid Angiopathy and Alzheimer’s Disease. J Alzheimers Dis, 2019. 72(4): p. 1019–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parodi-Rullan R.M., Javadov S., and Fossati S., Dissecting the Crosstalk between Endothelial Mitochondrial Damage, Vascular Inflammation, and Neurodegeneration in Cerebral Amyloid Angiopathy and Alzheimer’s Disease. Cells, 2021. 10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Numakawa T. and Odaka H., The Role of Neurotrophin Signaling in Age-Related Cognitive Decline and Cognitive Diseases. Int J Mol Sci, 2022. 23(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Leary T.P. and Brown R.E., Visuo-spatial learning and memory impairments in the 5xFAD mouse model of Alzheimer’s disease: Effects of age, sex, albinism, and motor impairments. Genes Brain Behav, 2022. 21(4): p. e12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paasila P.J., et al. , Synapses, Microglia, and Lipids in Alzheimer’s Disease. Front Neurosci, 2021. 15: p. 778822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Urayama A., et al. , Preventive and therapeutic reduction of amyloid deposition and behavioral impairments in a model of Alzheimer’s disease by whole blood exchange. Mol Psychiatry, 2022. 27(10): p. 4285–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daquinag A.C., et al. , Endothelial TrkA coordinates vascularization and innervation in thermogenic adipose tissue and can be targeted to control metabolism. Mol Metab, 2022. 63: p. 101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fang C.N., et al. , NGF/TrkA promotes the vitality, migration and adhesion of bone marrow stromal cells in hypoxia by regulating the Nrf2 pathway. Metab Brain Dis, 2022. 37(6): p. 2017–2026. [DOI] [PubMed] [Google Scholar]
- 74.Pius-Sadowska E. and Machalinski B., BDNF - A key player in cardiovascular system. J Mol Cell Cardiol, 2017. 110: p. 54–60. [DOI] [PubMed] [Google Scholar]
- 75.Abcejo A.J., et al. , Brain-derived neurotrophic factor enhances calcium regulatory mechanisms in human airway smooth muscle. PLoS One, 2012. 7(8): p. e44343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cannavo A., et al. , beta3AR-Dependent Brain-Derived Neurotrophic Factor (BDNF) Generation Limits Chronic Postischemic Heart Failure. Circ Res, 2023. 132(7): p. 867–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bahls M., et al. , Brain-derived neurotrophic factor is related with adverse cardiac remodeling and high NTproBNP. Sci Rep, 2019. 9(1): p. 15421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takashio S., et al. , Significance of low plasma levels of brain-derived neurotrophic factor in patients with heart failure. Am J Cardiol, 2015. 116(2): p. 243–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.