Abstract
The use of senolytic agents to remove senescent cells from atherosclerotic lesions is controversial. A common limitation of previous studies is the failure to rigorously define the effects of senolytic agent ABT-263 (Navitoclax) on smooth muscle cells (SMC) despite studies claiming that they are the major source of senescent cells. Moreover, there are no studies of the effect of ABT-263 on endothelial cells (EC), which along with SMC comprise 90% of α-SMA+ myofibroblast-like cells in the protective fibrous cap. Here we tested the hypothesis that treatment of advanced atherosclerotic mice with the ABT-263 will reduce lesion size and increase plaque stability. SMC (Myh11-CreERT2-eYFP) and EC (Cdh5-CreERT2-eYFP) lineage tracing Apoe−/− mice were fed a WD for 18 weeks, followed by ABT-263 100mg/kg/bw for six weeks or 50mg/kg/bw for nine weeks. ABT-263 treatment did not change lesion size or lumen area of the brachiocephalic artery (BCA). However, ABT-263 treatment reduced SMC by 90% and increased EC-contributions to lesions via EC-to-mesenchymal transition (EndoMT) by 60%. ABT-263 treatment also reduced α-SMA+ fibrous cap thickness by 60% and increased mortality by >50%. Contrary to expectations, treatment of WD-fed Apoe−/− mice with the senolytic agent ABT-263 resulted in multiple detrimental changes including reduced indices of stability, and increased mortality.
Keywords: Atherosclerosis, senescence, senolytics, ABT-263, Klf4, smooth muscle cells, endothelial cells
Graphical abstract

Introduction:
The rupture or erosion of advanced atherosclerotic plaques resulting in ischemic heart disease or stroke are the leading causes of death worldwide1. Human histopathological studies have clearly established that lesions containing a thick extracellular matrix (ECM)-rich fibrous cap and an abundance of α-SMA+ versus CD68+ cells are less likely to rupture2. Most investigators in the field have assumed that α-SMA+ lesion cells are derived from SMC and have a beneficial role in lesion pathogenesis by being the exclusive source of ECM-producing fibrous cap cells3. In addition, they assumed that CD68+ cells are macrophages that exacerbate lesion pathogenesis by increasing inflammation and giving rise to foam cells. However, subsequent studies by our lab4 and many others5,6,7,8 have either refuted several key aspects of these assumptions or shown that they are overly simplistic. This includes the following. First, α-SMA+ staining is highly unreliable for identifying SMC-derived cells in lesions given that nearly 80% of SMC-derived lesion cells lack detectable expression of this marker and activate markers of other cell types including macrophages4. In addition, we recently showed that 40% of α-SMA+ fibrous cap cells are derived from a non-SMC including EC that have undergone EC to mesenchymal transition (EndoMT) and macrophages undergoing macrophage to myofibroblast transition (MMT) in advanced BCA lesions from Apoe−/− and Ldlr deficient mice fed a WD for 18 weeks9. Second, there are qualitative differences in plaque stability depending on the source of α-SMA+ fibrous cap cells with increased EndoMT and MMT only being able to transiently compensate for the loss of SMC investment into lesions induced by SMC knockout (KO) of Pdgfβr or lethal irradiation9. Third, there is compelling evidence showing that each of the major cell types involved in atherosclerosis including macrophages10, T-Cells11, B-cells12, neutrophils13, endothelial cells9, and SMC4,14,15,16 can have beneficial or detrimental effects on lesion development or late-stage pathogenesis depending on the nature of their phenotypic transitions17. For example, using a combination of SMC lineage tracing, SMC-specific KO of Klf4 or Oct4, and scRNAseq analysis of lesions, we showed that SMC can have a beneficial or detrimental role in lesion pathogenesis15. Results with SMC KO of Klf4 were particularly intriguing since this resulted in what would be the ideal therapeutic outcome in patients at risk for development of atherosclerosis. Specifically, SMC-Klf4 KO lesions were 50% smaller and exhibited features associated with increased plaque stability including a two-fold increase in the thickness of the extracellular matrix (ECM) rich α-SMA+ fibrous cap4. Klf4 was subsequently identified as a coronary artery disease GWAS variant18. Moreover, our lab recently showed that loss of Klf4 in SMCs resulted in a marked reduction of mRNA transcripts associated with cellular senescence and inflammation including >40 putative KLF4 target genes previously identified as coronary artery disease GWAS variants. Taken together, results suggest that the beneficial effects of SMC Klf4 KO in mouse models, and for Klf4 being a CAD GWAS variant are due in part to its role in enhancing cell senescence and inflammation.
Senescence is a term used to define a state of irreversible growth arrest and includes replicative senescence, or stress-induced premature senescence (SIPS)19. Indeed, previous studies have shown that the atherosclerotic lesions in humans and mice contain relatively large numbers of senescent cells20,21,22, and claim that these senescent cells promote atherosclerosis and contribute to destabilization of plaques23. However, the origins and functional properties of these senescent cells are uncertain since they relied on methods that eliminated “senescent cells” based on expression of a single marker gene which is not specific for senescent cells, and/or relied on one or two traditional, albeit unreliable, markers to identify SMC-, EC- and macrophage-derived cells within lesions23,24. For example, when Childs et al.25 reduced senescent cells using a ganciclovir (GCV) dependent-p16 driven thymidine kinase genetic approach to induce death of p16Ink4a+ cells they discovered reduced Sudan-IV+ plaque burden and increased fibrous cap thickness of atherosclerotic lesions within the descending aorta of Ldl2−/− female mice. This same study showed that treatment of Ldlr−/− female mice at a dosage of 100 milligram/kilogram/body weight (mg/kg/bw) of the senolytic drug ABT-263 (Navitoclax) throughout 88 days of high-fat diet feeding reduced the Sudan-IV+ area of the aorta25. In addition, a more recent study by Childs et al.26 showed that aortic arch lesions of Myh11-CreERT2 TdTomato Ldlr−/− male mice fed a WD for 14 weeks followed by 9 weeks of a low-fat diet and treatment with ABT-263 had a reduced proportion of tdTomato+ cells that expressed the osteochondrogenic marker Runx1 as compared to vehicle treated mice26. ABT-263 inhibits interactions between the anti-apoptotic protein BCL-XL and the pro-apoptotic protein BAX thus enhancing clearance of senescent cells by reducing their capacity to evade efferocytotic clearance27. ABT-263 disrupts Bcl-2/Bcl-xL interactions with pro-death proteins (e.g., Bim), leading to the initiation of apoptosis39. Thus, Childs et al.,25,26 interpreted their results as evidence that the beneficial effects on atherosclerosis observed with ABT-263 treatment were the direct result of enhanced clearance of senescent cells from lesions. In recent years these results have been challenged by results of studies by Martin Bennett and co-workers who showed the following28. First, they found that “presumed” selective clearance of senescent cells using a p16 driven thymidine kinase genetic approach similar to Childs et al.25 did not reduce Oil red O+ plaque burden, aortic root lesion size, fibrous cap thickness, or necrotic core area in Apoe−/− mice (males and females combined), but instead increased apoptotic cells and induced inflammation. Second, treatment of Apoe−/− male and female mice with established lesions with 50 mg/kg/bw of ABT-263 did reduce aortic root lesion size but did not increase cap thickness. Third, ABT-263 treatment of Apoe−/− mice did not reduce relative mRNA expression of p16 and senescence-associated secretory phenotypes, including IL-6, IL-1α, Tnf-α, IL-18 and Mmp-12 in aortic arch tissue, but increased thrombocytopenia and reduced monocyte and leukocyte populations. Another independent study from James Kirkland’s lab showed that treatment of Apoe−/− mice with the senolytic agent Dasatinib plus Quercetin did not reduce lesion size, but reduced plaque calcification29. The reasons for the disparate results of the preceding studies are unclear but may be related to male versus female sex, Apoe−/− versus Ldlr−/− mice, and differences in the senolytic drugs tested and/or dosing regimens. However, a common limitation of these previous studies was the failure to rigorously define the effects of ABT-263 on SMC despite each study claiming this is the major source of senescent cells. In addition, there have been no studies of the effects of ABT-263 on endothelial cells (EC) which along with SMC comprise 90% of α-SMA+ myofibroblast-like cells in the protective fibrous cap. These studies also include minimal assessment of indices of plaque stability and were largely focused on assessment of aortic root lesions, which poorly replicate the histology of human lesions. As such, there are still major uncertainties regarding the effects of senolytic therapies on the cellular composition of advanced atherosclerotic lesions, the phenotypes of the lesion cells, and overall indices of plaque stability that need to be clarified before determining their usefulness in treating patients with advanced atherosclerotic disease.
Studies herein test two hypotheses. First, we hypothesized that the beneficial effects of SMC KO of Klf4 are due in part to reduced cell senescence and an associated reduction in inflammation. Second, we hypothesized that treatment of Western Diet (WD) fed SMC- and EC- lineage tracing Apoe−/− mice with the senolytic agent ABT-263 will result in loss of senescent cells from atherosclerotic lesions thereby reducing lesion inflammation and size, as well as inducing changes consistent with increased plaque stability including a thicker SMC- and ECM-rich α-SMA+ protective fibrous cap. Consistent with our first hypothesis, KO of Klf4 in SMC in WD fed Apoe−/− mice was associated with marked reductions in plaque size, lipid deposition, and the overall prevalence of senescent cells. However, contrary to expectations, the results of studies testing our second hypothesis showed that treatment of WD-fed Apoe−/− mice with ABT-263 had multiple detrimental effects including inducing marked reductions in Myh11-eYFP+ SMC-derived cells within lesions and the fibrous cap, reduced α-SMA+ fibrous cap thickness, increased EndoMT, and increased mortality.
Methods:
Mice:
The University of Virginia Animal Care and Use Committee approved animal protocols (Protocol 2400). The Myh11-CreERT2-eYFP and Cdh5-CreERT2-eYFP Apoe−/− mice used in the intervention study have been described in our previous studies4,9. In addition, Myh11-CreERT2-eYFP Klf4 WT vs KO Apoe−/− mice were used for the studies related to loss of Klf4 in SMC experiments as previously reported4.
Diet and treatment: ABT-263 intervention studies on advanced atherosclerotic mice:
In Myh11-CreERT2-eYFP and Cdh5-CreERT2-eYFP Apoe−/− mice, Cre recombinase was activated with a series of ten tamoxifen injections (1mg/day/mouse; Sigma Aldrich, T-5648) over a 2-week period. One week after the tamoxifen treatment, mice were switched from a normal chow diet (Harlan Teklad TD.7012) to a high fat Western-type diet (WD), containing 21% milk fat and 0.15% cholesterol (Harlan Teklad; TD.88137) for 18 weeks followed by 100mg/kg/bw ABT-263 treatment for 6 (2 cycles- 5 days ON and 14 days OFF) weeks or 50mg/kg/bw ABT-263 treatment for 9 (3 cycles- 5 days ON and 14 days OFF) weeks. ABT-263 (S1001) was obtained from Selleck (Houston, TX 77014, USA), formulated in Vehicle (PBS with 15% dimethylsulfoxide and 7% Tween-20) and injected intraperitoneally (IP) at a dose of 100mg/kg/bw or 50mg/kg/bw as shown in the figures, figure legends, and results sections.
Atherosclerotic plaque morphometry:
Paraformaldehyde-fixed paraffin-embedded BCAs were serially cut into 10 μm thick sections from the aortic arch to the bifurcation of the right subclavian artery. For morphometric analysis, we performed modified Russell-Movat staining on three locations along the BCA at 150 μm, 450 μm, and 750 μm, from the aortic arch as previously described9. The lesion, lumen, external elastic lamina (outward remodeling), and necrotic core areas as well as the internal elastic lamina area were measured on digitized images of the Movat staining using Fiji (Image J) software. Picrosirius Red staining was performed to assess collagen content and digitized images of the picrosirius staining was measured using Fiji software. Masson trichrome staining was performed to assess the fibrous tissue in the liver sections.
Immunofluorescent staining:
BCA sections were de-paraffinized and rehydrated in xylene and ethanol series. After antigen retrieval (H-3300–250, Vector Laboratories, Newark, CA 94560, USA), sections were blocked with fish skin gelatin–PBS (6 g/L) containing 10% horse serum for 1 hour at room temperature. Slides were incubated with the following antibodies: mouse monoclonal smooth muscle α-actin-Cy3 (α-SMA) (4.4 μg/mL, clone 1A4, C6198, Sigma Aldrich, Rockville, MD 20850, USA), goat polyclonal anti-GFP (4 μg/mL, ab6673, Abcam, Waltham, MA 02453, USA) for detection of eYFP, rabbit polyclonal p21 (18 μg/mL, 10355-1-AP, Proteintech, Rosemont, IL 60018, USA), TUNEL (Cat 30074 Bioutium, Fremont, CA, USA). The secondary antibodies donkey anti-goat conjugated to Alexa 488 (5 μg/mL), donkey anti-rabbit conjugated to Alexa 647 (A31573 5 μg/mL), DAPI (0.05 mg/mL, D3571,) was used as a nuclear counterstain and slides were mounted using Prolong Gold Antifade (P36930) were purchased from Thermo Fisher Scientific, Waltham, MA 02451, USA.
Imaging:
Movat and picrosirius red staining of brachiocephalic arteries and masson trichrome staining of liver sections were imaged by using a Leica thunder imager microscope. Image acquisition was performed with Leica software. Digitized images were analyzed with Fijisoftware. Immunofluorescent staining was imaged using a Zeiss LSM880 airy scan confocal microscope to acquire a series of z-stack images at 1-μm intervals. Zen 2009 Light Edition Software (Zeiss) was used for the analysis of each z-stack image and single-cell counting was performed for phenotyping and quantifying the cell population comprised within the 30μm thick layer proximal to the lumen (i.e., fibrous cap area). Assessment of α-SMA+ cap thickness (normalized to lesion) was performed using Zen 2009 Light Edition Software. Maximal intensity projection of representative images wereused to generate the representative images included in the figures.
Western blotting:
The method has been described previously30. The primary antibodies used for p16 were from Abcam, 2μg/mL, Ab189034, Waltham, MA 02453, USA ), Klf4 (1 μg/mL, CST4038T) and β-actin (1 μg/mL, 4967S) from Cell Signaling Technology, Danvers, MA 01923, USA.The anti-Rabbit IgG (A4914) HRP-conjugated secondary antibody was purchased from Sigma Aldrich, Rockville, MD 20850, USA.
Senescence-Associated β–Galactosidase (SAβG) Staining:
SaβG staining of aortas for senescent cells was examined using a Senescence detection assay kit (Millipore-Sigma Cat No.KAA002, Rockville, MD 20850, USA)30. Freshly isolated aortas were kept on ice in a 12 well plate containing PBS until all the aortas were harvested. Aortas were washed twice with the PBS, and then fixed with the diluted fixation solution provided in the kit for 10 minutes as per the manufacturer’s instructions. After the fixation, aortas were washed thrice with PBS and then 1mL of freshly prepared SAβG solution was added to a well of 12 well plates containing one aorta per well. Then, plates were wrapped with aluminum foil to avoid light exposure and placed in a 37°C incubator for 24h. After that, aortas were washed thrice with PBS, and photographs were taken with a mobile phone camera.
Statistics:
Statistics were performed using GraphPad prism 9. Data are presented as mean ± SEM. Animal numbers and type of statistical analysis done are reflected within figures and figure legends. A p-value ≤ 0.05 was considered statistically significant.
Results
Klf4 knockout in SMC resulted in reduced overall lesion senescence.
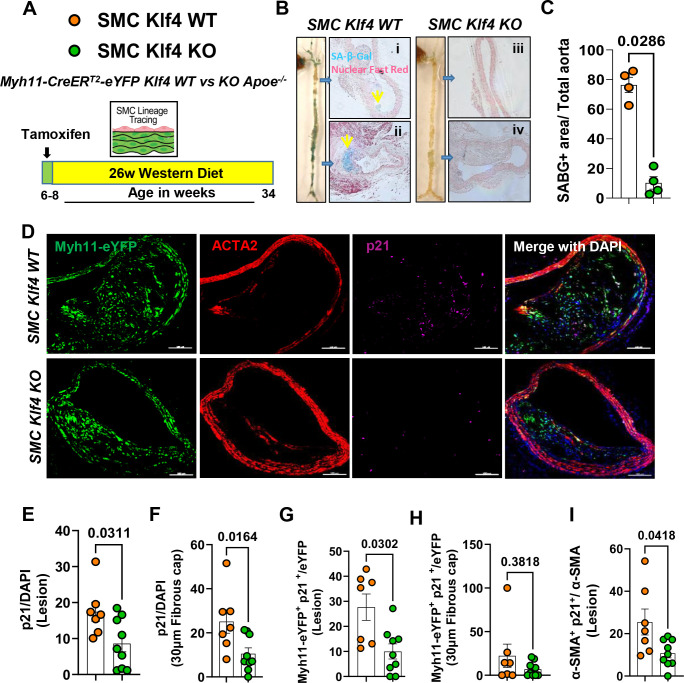
As an initial test of our hypothesis that the multiple beneficial effects of SMC KO of Klf4 are due in part to reduced cell senescence, male SMC Klf4 WT and KO Apoe−/− mice were fed a WD for 18 (Figure I in the data supplement) or 26 weeks (Fig.1A) and aortas were then stained for senescence-associated-β-gal (SAβG). Interestingly, SMC Klf4 WT mice aortas showed highly prevalent SAβG+ senescent cells in the brachiocephalic artery (BCA), aortic root, aortic arch, and abdominal aorta (Fig.1B). In contrast, there was almost no SAβG+ staining in SMC Klf4 KO mice (Fig.1B) as seen in whole aortic preparations and in cross sections of the thoracic and abdominal aorta counter stained with nuclear fast red (Fig.1Bi–iv). Similar results were found in mice fed WD for 18 weeks (Figure IB-IC in the data supplement). Loss of Klf4 in SMC was also associated with an increase in the Telomere Reverse Transcriptase (Tert+) anti-senescence marker, but a decrease in p16+ (pro-senescence marker) clusters, as assessed from uniform manifold approximation and projections (UMAPs) of scRNAseq data sets of BCA lesions from SMC Klf4 WT and KO mice15 (Figure ID-IE in the data supplement). KO of Klf4 in SMC preserved the Tert+ cells in most of the clusters of lesion cells as shown by UMAP analysis (Figure ID in the data supplement). In contrast, KO of Klf4 in SMC reduced the number of SMC-derived cells positive for the pro-senescence marker p16 (Cdkn2a) (Figure IE in the data supplement). Indeed, our previous study showed that Klf4 binds to Telomeric RNA (TERC) in SMCs of advanced atherosclerotic lesions based on chromatin immunoprecipitation (CHIP) assays4. Next, cultured murine aortic SMC were transfected with siKlf4 to determine if knock down (KD) of Klf4 in SMC reduces the pro-senescent marker p16 directly or through crosstalk with other cell types in the lesion. Results showed that KD of Klf4 reduced p16 expression in SMC at both the mRNA and protein levels (Figure IF-IG in the data supplement).
Figure 1. SMC-Klf4 KO resulted in a marked reduction in overall lesion senescence including reduced expression of the pro-senescence markers p21, p16, and SAβG, but preserved anti-senescence marker telomerase reverse transcriptase positive cells.
(A) Experimental design, SMC ((Myh11-CreERT2-eYFP)) lineage tracing Klf4 WT vs KO Apoe−/− mice were injected with tamoxifen at 6 to 8 weeks of age and subsequently placed on Western diet (WD) for 26 weeks to induce advanced atherosclerosis. (B) SAβG staining was performed as described in the methods section on freshly isolated aortas. (Bi-iv) thoracic and abdominal aortic sections from SAβG stained aortas from B and counter stained with nuclear fast red. (C) Quantification of SAβG+ area of the aorta. (D) Representative images of co-staining for eYFP (for detecting SMC), α-SMA, p21 (a marker of senescence) and DAPI (nucleus) in advanced BCA lesions from SMC Klf4 WT and KO animals fed a WD for 26 weeks. The confocal images show a maximum intensity projection ×20 zoom with a scale bar of 100μm. Quantification of the frequency of p21+ (p21+/DAPI) senescent cells in the lesion (E) and fibrous cap (F) as a percent of total cells in the lesions. Quantification of SMC derived p21+ (eYFP+ p21+/eYFP) senescent cells in the lesion (G) and the fibrous cap (H) as a percent of total SMC. Quantification of α-SMA+ senescent (α-SMA+ p21+/ α-SMA) cells in the lesion (I) as a percentage of total α-SMA+ cells. Mann-Whitney U-tests were used in C, and E-I. The error bars show the standard error of the mean (SEM). Independent animals are indicated as individual dots (WT, n=7, and KO, n=9). The p-values are indicated on the respective graphs.
A previous study from our lab showed that Klf4 is a suppressor of SMC differentiation markers and an inhibitor of SMC proliferation by inducing p21WAF1/Cip1 expression in concert with p5331. Both p53 and p21 are widely accepted pro-senescence markers32. Therefore, we stained BCA sections of SMC Klf4 WT and KO mice fed a WD for 18 weeks with a p21 antibody to determine if SMC Klf4 KO would reduce the frequency of p21+ senescent cells in atherosclerotic lesions (Fig.1D). As predicted, there was a reduction in p21+ senescent cells (p21/DAPI) in the BCA lesions and fibrous cap of SMC Klf4 KO versus WT control mice (Fig.1E and 1F). The fraction of SMC-derived p21+ cells (Myh11-eYFP+ p21+/eYFP) was also reduced in the lesions but not in the fibrous cap of these same mice (Fig.1G–1H). Furthermore, the fraction of α-SMA+ cells which expressed the senescence marker p21 was also reduced in the lesions of SMC Klf4 KO versus WT control mice (Fig.1I). Results show that KO of Klf4 in SMC was associated with a marked reduction in the prevalence of senescent cells within the aorta and BCA lesions of Apoe−/− mice fed WD for 18 or 26 weeks.
Taken together, the preceding results extend results of previous studies from our lab4,15 showing that SMC Klf4 KO has multiple beneficial effects including resulting in smaller lesions with a thicker α-SMA+ fibrous cap4 containing reduced numbers of senescent cells (Figure 1 and supplemental figure I from present study). However, it is not clear if the reduction in senescent cells contributes causally to the beneficial effects on lesions or if the reduced number of senescent cells is secondary to loss of Klf4 in SMC acting through other mechanisms. To distinguish these possibilities, we sought alternative approaches to reduce the prevalence of senescent cells within advanced lesions.
Treatment of Apoe−/− mice with advanced atherosclerotic lesions with the senolytic drug ABT-263 reduced the number of SMC within BCA lesions and was associated with increased EndoMT derived lesion cells and mortality.
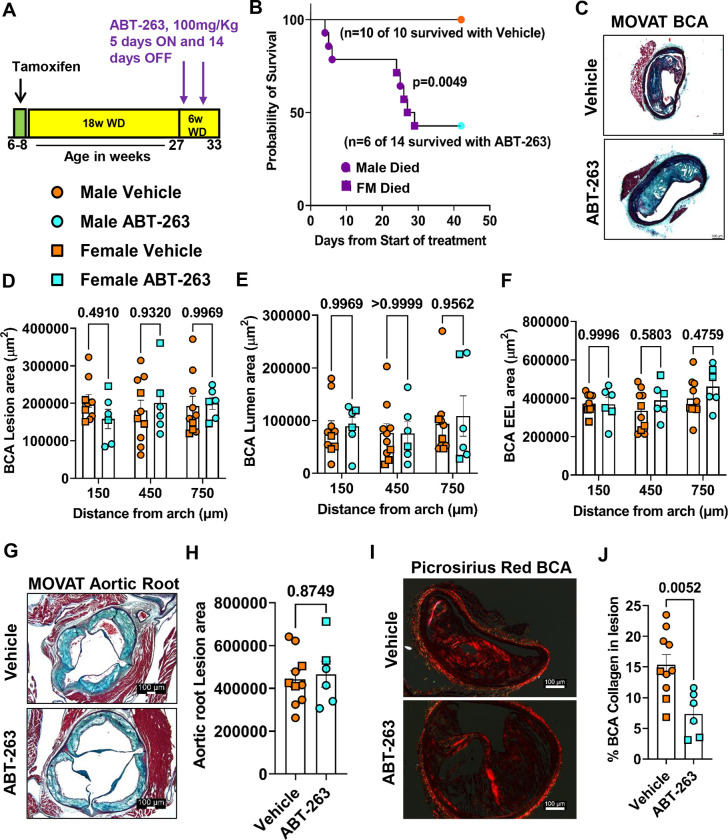
To determine if increased clearance of senescent cells could induce beneficial changes in late stage complex atherosclerotic lesions, SMC- and EC-lineage tracing Apoe−/− mice were fed a WD for 18 weeks and then treated with 3 cycles (Fig.2A) of 100mg/kg/bw ABT-263 as used previously by Childs et al.25. We elected to do an intervention rather than a prevention study to better model clinical scenarios in patients with advanced disease33. However, the experiment had to be unexpectantly terminated for ethical reasons, as per our ACUC approved animal protocol, after two cycles of ABT-263 because >50% of the ABT-263 treated mice died (Fig.2B). ABT-263 treated mice had no changes in BCA lesion size (Fig.2C–2D), lumen area and outward remodeling (EEL-external elastic lamina area) (Fig.2E–2F), and no change in aortic root lesion area (Fig.2G–H). However, ABT-263 treated mice showed multiple detrimental changes in lesion composition including reduced BCA lesion collagen content (Fig.2I–2J), a reduced α-SMA+ fibrous cap area (Fig.3A–3C), and a reduced fraction of α-SMA+ or α-SMA− SMC-derived cells (Myh11-eYFP+/DAPI) in lesions and the 30um fibrous cap area (Fig.3B and 3D–3E, and Figure IIA-IIC in the data supplement). Surprisingly, ABT-263 treatment did not change the frequency of apoptotic cells (TUNEL+/DAPI) in the BCA lesions as would be expected based on its mechanism of action (Figure IIIA-C in the data supplement). To determine if the non-SMC-derived α-SMA+ fibrous cap cells in this study were derived from EC, we treated EC-lineage tracing mice with the same dose of ABT-263 and assessed (Fig.3F) BCA lesions for eYFP, α-SMA and DAPI (Fig.3G). The ABT-263 treatment also reduced the α-SMA+ cap area and the fraction of α-SMA+ (α-SMA+/DAPI) cells in the 30um fibrous cap area of these mice (Fig.3H and Figure IID-IIE in the data supplement). Interestingly, ABT-263 treatment increased endothelial cells (Cdh5-eYFP+/DAPI) in the fibrous cap and lesions but did not increase endothelial-derived α-SMA+ (Cdh5-eYFP+ α-SMA+/ α-SMA+) cells (Fig.3I–3J and Figure IIF-IIH in the data supplement). Taken together, results show that ABT-263 treatment at 100mg/kg/bw had no effect on lesion size but markedly reduced the probability of survival. This treatment regimen was also associated with major detrimental changes in lesion composition including reduced α-SMA+ fibrous cap thickness and SMC investment into the fibrous cap. In addition, ABT-263 treatment prevented adaptive increases in investment of EC-derived cells into the fibrous cap via EndoMT to myofibroblast transitions that we have shown normally occur when SMC investment into the fibrous cap of lesions is impaired9.
Figure 2. Treatment of SMC (Myh11-CreERT2-eYFP) and EC (Cdh5-CreERT2-eYFP)-lineage tracing Apoe−/− mice with advanced atherosclerotic lesions with ABT-263 had no effect on lesion size but increased mortality.
(A) Experimental design, SMC- and EC-lineage tracing Apoe−/− mice were fed a WD for 18 weeks followed by ABT-263 treatment on western diet (WD) for 6 weeks. (B) Probability of survival (Kaplan-Meier curve). (C) Representative 10x images with 100μm scale bar of MOVAT staining on Brachiocephalic Artery (BCA). (D) Lesion area from D. (E) Lumen area from D. (F) External elastic lamina (EEL) area from D, for outward remodeling. (G) Aortic roots stained with MOVAT. (H) Lesion area quantification from G. (I) Representative 10x images with 100μm scale bar of Picrosirius red staining on Brachiocephalic Artery (BCA). (J) Quantification of matured (red) collagen content normalized to lesion area from figure I. A repeated measures Two-way ANOVA method was used for statistical analysis in D-F whereas Mann-Whitney U-tests were used for H and J. Biologically independent animals are indicated as individual dots, error bars show the SEM, Mantel-Cox test used for B. The p-values are indicated on the respective graphs.
Figure 3. Treatment of SMC (Myh11-CreERT2-eYFP) and EC (Cdh5-CreERT2-eYFP)-lineage tracing Apoe−/− mice with advanced atherosclerotic lesions with the senolytic drug ABT-263 (100mg/kg/bw) was associated with a marked reduction in the number of SMC within BCA lesions but an increase in EC-derived cells undergoing EndoMT although the latter did not result in increased investment of EC-derived α-SMA+ cells into the fibrous cap.
(A) Experimental design for figures B-E, SMC-lineage tracing Apoe−/−mice were fed a WD for 18 weeks followed by 100mg/kg/bw ABT-263 treatment on WD for 6 weeks. (B) Representative confocal images of co-staining for eYFP (for detecting SMC), α-SMA+, and DAPI in advanced BCA lesions from experiment A. The confocal images show a maximum intensity projection ×20 zoom and a scale bar of 100μm. (C) α-SMA+ cap area normalized to lesion area (α-SMA+ cap area/Lesion area). (D) Quantification of the percentage of SMC-derived (Myh11-eYFP+/DAPI+) cells in the fibrous cap, and (E) quantification of the % SMC-derived α-SMA+ (Myh11-eYFP+ α-SMA+/α-SMA+) cells in the fibrous cap. (F) The experimental design for figures H-J, EC-lineage tracing Apoe−/− mice were fed a WD for 18 weeks followed by 100mg/kg/bw ABT-263 treatment on WD for 6 weeks. (G) Probability of survival (Kaplan-Meier curve). (H) Representative confocal images of co-staining for eYFP (for detecting EC), α-SMA+, and DAPI in advanced BCA lesions from experiment F. (I) Quantification of the percentage of EC-derived Cdh5-eYFP+ DAPI+ cells in the fibrous cap, and (J) quantification of the percentage of EC-derived α-SMA+ (Cdh5-eYFP+ α-SMA+/ α-SMA+) cells in the fibrous cap. The two-way ANOVA method was used for statistical analyses in C-E and H-J. Biologically independent animals are indicated as individual dots. Error bars are shown with the SEM. A Mantel-Cox test was used for G. The p-values are indicated on the figures.
Apoe−/− mice with advanced lesions treated with a reduced dose of ABT-263 also had increased mortality and detrimental changes in lesion composition.
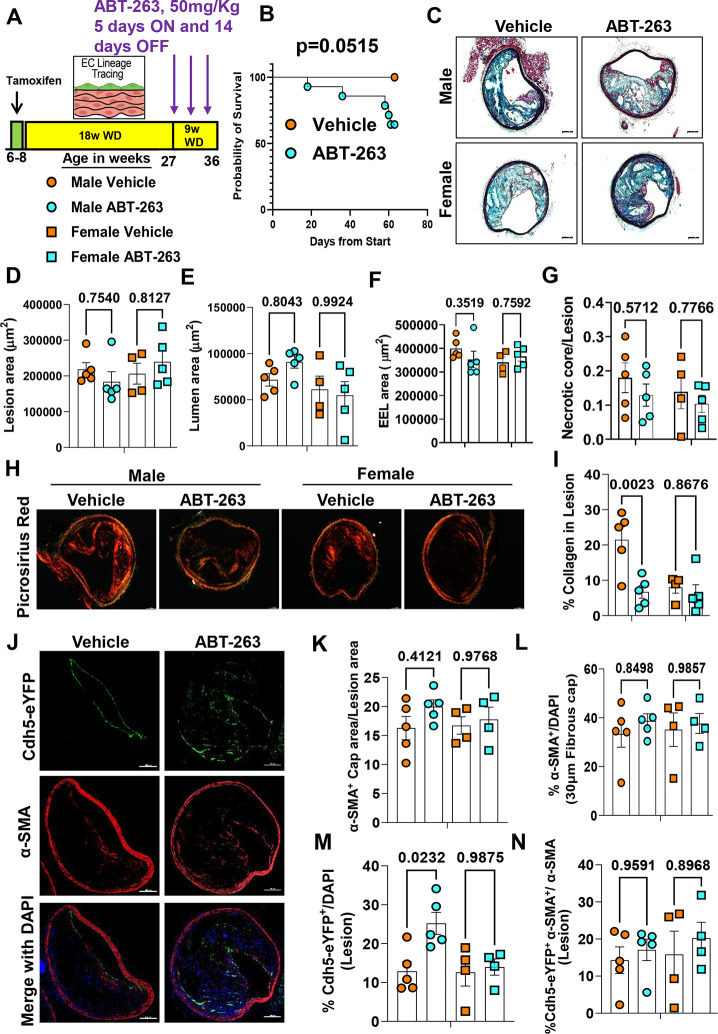
Given the multiple detrimental effects of treating WD-fed Apoe−/− mice with ABT-263 at 100mg/kg/bw we repeated the preceding studies with 50mg/kg/bw of ABT-263. EC-lineage tracing Apoe−/− mice were fed a WD for 18 weeks followed by treatment with ABT-263 at half our original dose (i.e., 3 cycles of 50mg/kg/bw) (Fig.4A). Contrary to our expectations, even this lower dose of ABT-263 was associated with increased mortality (Fig.4B). Similar to the higher dose of ABT-263, there were no changes in lesion size, lumen area, outward remodeling, and necrotic core area of BCA lesions (Fig.4B–G). There were also no reductions in aortic root lesion area (Figure IIID-IIIF in the data supplement) in male or female mice. Surprisingly, the lower dose of ABT-263 reduced collagen content in male Apoe−/− mice (Fig.4H–4I). Unlike the 100mg/kg dose, treatment at the lower dose did not reduce α-SMA+ cap thickness, area, or α-SMA+ cells in the fibrous cap or lesions (Fig. 4J–4L and Figure IVA-IVB in the data supplement). The lower dose of ABT-263 was associated with increased EndoMT (Cdh5-eYFP+/DAPI) in male Apoe−/− mice but no change in endothelial-derived α-SMA+ (Cdh5-eYFP+ α-SMA+/ α-SMA) cells in the lesions or 30um fibrous cap area (Fig.4M–4N and Figure IVC-IVD in the data supplement). Taken together, even the reduced dose of ABT-263 increased mortality and did not show beneficial changes in plaque size and composition.
Figure 4. 263 Treatment of Apoe−/− mice with advanced lesions with a reduced dose (50mg/kg/bw) of ABT- decreased collagen content within BCA lesions and was also associated with increased mortality.
(A) Experimental design, EC-lineage tracing Apoe−/− mice were fed a WD for 18 weeks followed by 50mg/kg/bw ABT-263 treatment on WD for 9 weeks. (B) Probability of survival (Kaplan-Meier curve). (C) Representative 10x images with 100μm scale bar of MOVAT staining of the BCA. (D) Lesion area from C. (E) Lumen area from C. (F) External elastic lamina (EEL) area from C, for outward remodeling. (G) Necrotic core area normalized to lesion size. (H) Representative 10x images with 100μm scale bar of Picrosirius red staining on Brachiocephalic Artery (BCA). (I) Quantification of mature (red) collagen content normalized to lesion area from figure H. (J) Representative confocal images of co-staining for eYFP (for detecting EC), α-SMA+, and DAPI in advanced BCA lesions. (K) α-SMA+ cap area normalized to lesion area (α-SMA+ cap area/Lesion area). (L) Quantification of the percentage α-SMA+ (α-SMA+/DAPI) cells in the fibrous cap. (M) Quantification of the percentage EC-derived (Cdh5-eYFP+/DAPI+ cells in the lesion, and (N) quantification of the percentage EC-derived α-SMA+ (Cdh5-eYFP+ α-SMA+/α-SMA+) cells in lesions. The two-way ANOVA method was used for statistical analysis in D, G, and K-L, and biologically independent animals are indicated as individual dots. Error bars show the SEM. A Mantel-Cox test used for statistical analysis in B. The p-values are indicated on the respective graphs.
Low dose ABT-263 treatment of WD-fed Apoe−/− mice reduced plasma Cxcl5 and was associated with an abnormal fibrous liver phenotype.
To determine if ABT-263 treatment reduces senescence-associated secretory phenotypes (SASPs) and pro-inflammatory cytokine levels, we performed luminex assays on plasma samples. Here we discovered that ABT-263 did not reduce SASPs and cytokines including, IL-1β, IL-1α, IL-6, Mcp-1, Tnf-α, and Ifn-γ (Figure V in the data supplement). However, ABT-263 treatment reduced LIX (Cxcl5) levels in female Apoe−/− mice (Figure V in the data supplement). Cxcl5 has an atheroprotective role in that inhibition of Cxcl5 has previously been shown to induce significant macrophage foam cell accumulation in murine atherosclerotic plaques34.
Mice treated with ABT-263 also showed an abnormal liver phenotype with increased Masson trichrome positive fibrous tissue. However, ABT-263 treatment did not result in changes in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in the plasma (Figure 5F–5G) suggesting that the ABT-263 induced increase in liver damage and fibrosis may be associated with other factors or mechanisms. Although ABT-263 did not reduce lesion size, it reduced cholesterol and LDL levels in male but not female mice Apoe−/− mice (Figure VIB-VIC in the data supplement). Triglycerides levels were not changed with ABT-263 treatment (Figure VID in the data supplement). ABT-263 treatment did not increase thrombocytopenia (Figure VIE in the data supplement). Moreover, ABT-263 did not result in changes in basophils, lymphocytes, WBC, RBC, nucleated RBC, hemoglobin, monocytes, and neutrophils in the blood (Figure VII in the data supplement). Also, ABT-263 treatment did not change the body weights (Figure VIII in the data supplement).
Figure 5. Low dose (50mg/kg/bw) ABT-263 treatment of Apoe−/− mice with advanced lesions was associated with increased hepatic fibrosis.
(A) Experimental design, EC-lineage tracing Apoe−/− mice were fed a WD for 18 weeks followed by 50mg/kg/bw ABT-263 treatment on WD for 9 weeks. (B) Percentage of mice showing an abnormal liver phenotype. (C) Representative photographs of mice showing an abnormal live phenotype with ABT-263 treatment. (D) Masson trichrome stain to detect the fibrous tissue (blue) in the liver sections from the vehicle and ABT-263 treated mice. (E) Percentage mice showing fibrous tissue (blue) in the liver sections from the vehicle and ABT-263 treated mice. (F) ALT and (G) AST levels measured in plasma. Mann-Whitney U-tests were used for statistical analysis in F and G, and biologically independent animals are indicated as individual dots. Error bars show the SEM. The p-values are indicated on the respective graphs.
Discussion
Senescent cells contribute to age-associated diseases35 and recent murine studies have tested the ability of senolytic agents to remove these senescent cells and to potentially treat a number of major diseases including atherosclerosis25,26,28,29 neurodegeneration36, pulmonary fibrosis37, diabetic chronic kidney disease38, and cancer39. Indeed, multiple ongoing clinical trials with senolytic drugs are in progress targeting various diseases, including Alzheimer’s (NCT0463124), and diabetic chronic kidney disease (NCT02848131)40. These clinical trials are based on the belief that selective removal of these senescent cells will be beneficial. However, the results of the present study show that one of the most popular senolytic drugs, ABT-263, has multiple detrimental effects on Apoe−/− mice with advanced atherosclerosis including a reduced probability of survival by 50–60%, that may be due to a reduced α-SMA+ fibrous cap thickness and a 90% reduction in SMC within the lesions. Indeed, our results suggest that the majority of SMC-derived cells within lesions, including those that are critical for the formation and maintenance of the protective fibrous cap, are particularly sensitive to ABT-263-induced clearance. Consistent with this possibility, Leeper and co-workers demonstrated that clonal expansion of lesion SMC is dependent on them escaping efferocytosis at least in part by activating the anti-phagocytic molecule CD4741. As such, results indicate that ABT-263 may be inducing apoptosis of non-senescent SMC and that with long-term use may increase the risk for the development of unstable advanced atherosclerotic lesions and the incidence of MI or stroke. This may be particularly severe since our data also showed that ABT-263 prevented adaptive increases in investment of EC-derived cells into the fibrous cap via beneficial EndoMT to myofibroblast transitions that we have shown normally occur when SMC investment into fibrous cap of lesions is impaired9.
At first glance, our results showing multiple detrimental changes in lesion pathology and a marked increase in mortality appear to be at odds with previous studies in the field reporting beneficial effects of ABT-263 treatment of atherosclerotic mice25,26. The reasons for these differences are unclear, but likely include the following key variables between studies. First, to better match clinical paradigms of treating elderly patients with advanced disease, we did a late-stage intervention study33 involving initiation of ABT-263 treatment in Apoe−/− mice after 18-weeks of WD (TD.88137; 42% calories from fat) feeding so that they already have highly advanced BCA lesions closely resembling advanced human lesions both morphologically2 and similar cellular composition of lesions as determined by scRNAseq analysis15. In contrast, previous studies showing beneficial effects of ABT-263 were either prevention studies where treatment was initiated in very young mice at the time of beginning WD feeding25, or an intervention study following just 12–14 weeks of WD feeding of Ldlr−/− mice at which time lesions are still early stage26. Second, to model patients with undiagnosed advanced disease or with poor lipid management we elected to continue mice on a WD during the 9-week treatment period (i.e. 27 weeks of WD +/− ABT-263 treatment during the last 9 weeks). In contrast, to mimic the clinically relevant context of patients with highly effective medical management of pro-atherogenic lipids, Childs et al26 fed a WD to Ldlr−/− mice for 12 weeks followed by 9 weeks of low fat diet (LFD) +/− ABT-263. They observed multiple beneficial effects of ABT-263 treatment including thickening of the fibrous cap of aortic lesions. Given that we modeled our intermittent ABT-263 dosing regimen to that of Childs et al., it is likely that the high mortality observed in our studies with ABT-263 is due, at least in part, to prolonged hyperlipidemia. However, it remains to be determined if our ABT-263-induced increase in mortality is due to thromboembolic events secondary to plaque de-stabilization, increased liver toxicity, and/or other mechanisms. Nevertheless, no matter what the cause of death with ABT-263 (Navitoclax), it is concerning given the extensive clinical42,43 testing in progress. Indeed, results suggest that it may be prudent to exclude subjects with poorly controlled lipids, systemic inflammation, and/or advanced atherosclerosis from these studies.
Previous studies reported that EC-like24 and SMC-like20 cells undergo senescence that is associated with telomere shortening in human atherosclerotic lesions. The results of the present study showed that KO of Klf4 in SMC was associated with reduced expression of pro-senescence markers, but preserved expression of the anti-senescence marker, telomerase reverse transcriptase (Tert+). It is not clear how the loss of Klf4 in SMC preserved Tert+ expression. However, it is probably the result of loss of Klf4-induced repression of Tert given previous studies showing that Klf4 downregulates hTERT expression and telomerase activity to inhibit lung carcinoma growth44. Also the knockdown of Klf4 in HUVECs in hyperglycemic conditions increased hTERT expression45. Telomerase maintains the telomere length and the telomerase deficiency (Trf1- and Tert-deficient mice) in mice lead to aplastic anemia and Tert gene therapy improved telomere length and blood count in these Tert-deficient mice46. Furthermore, recent Tert gene therapy in the mice, reduced vascular senescence and increased lifespan47, improved myocardial revascularization and tissue repair48,49, and reduced neurodegeneration associated with short telomeres50. Given the multiple beneficial effects of Tert therapy in the pre-clinical mouse models, future studies need to evaluate if the Tert therapy would show beneficial effects on advanced atherosclerosis.
In conclusion, ABT-263 treatment of WD-fed Apoe−/− mice with advanced atherosclerosis and persistent hyperlipidemia had multiple unexpected detrimental effects including it inducing reduced Myh11-eYFP+ SMC-derived cells within lesions and the fibrous cap and decreased α-SMA+ fibrous cap thickness. ABT-263 treatment also prevented adaptive increases in investment of EC-derived cells into the fibrous cap via beneficial EndoMT to myofibroblast transitions and increased mortality. Removing the senescent cells from the plaque is conceptually a good idea but given the multiple detrimental effects of ABT-263 in our WD fed Apoe−/− mice, further pre-clinical studies with senolytic drugs are needed to identify factors and mechanisms that modulate their biological effects before planning a large clinical trial targeting atherosclerosis.
Supplementary Material
Highlights:
Treatment of Apoe−/− mice with advanced atherosclerosis with the senolytic agent ABT-263 increased mortality by >50%.
ABT-263 showed a 90% reduction in SMC but a 60% increase in endothelial cell (EC) contributions to lesions via EC to mesenchymal transition (EndoMT) but prevented adaptive increases in investment of EC-derived cells into the fibrous cap via beneficial EndoMT to myofibroblast transitions that we have shown normally occur when SMC investment into fibrous cap of lesions is impaired.
Knock out (KO) of Klf4 in SMC, which results in smaller but more stable atherosclerotic lesions, was associated with reduced expression of pro-senescence markers, but preserved expression of the anti-senescence marker, telomerase reverse transcriptase although it is unclear if the latter is causal or an effect.
Sources of funding:
This work was supported by National Institutes of Health grants R01 HL156849, R01 HL136314, and R01 HL141425 to GKO. Leducq Foundation Transatlantic Network of Excellence (‘PlaqOmics’) to GKO, and PlaqOmics Junior Investigator award to SK, and LSS.
Footnotes
Disclosures: None.
References:
- 1.Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics—2021 Update. Circulation. 2021;143(8). doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 2.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons From Sudden Coronary Death. Arterioscler Thromb Vasc Biol. 2000;20(5). doi: 10.1161/01.ATV.20.5.1262 [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–325. doi: 10.1038/nature10146 [DOI] [PubMed] [Google Scholar]
- 4.Shankman LS, Gomez D, Cherepanova OA, et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. Published online 2015. doi: 10.1038/nm.3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feil S, Fehrenbacher B, Lukowski R, et al. Transdifferentiation of Vascular Smooth Muscle Cells to Macrophage-Like Cells During Atherogenesis. Circ Res. 2014;115(7):662–667. doi: 10.1161/CIRCRESAHA.115.304634 [DOI] [PubMed] [Google Scholar]
- 6.Wirka RC, Wagh D, Paik DT, et al. Atheroprotective roles of smooth muscle cell phenotypic modulation and the TCF21 disease gene as revealed by single-cell analysis. Nat Med. 2019;25(8):1280–1289. doi: 10.1038/s41591-019-0512-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan H, Xue C, Auerbach BJ, et al. Single-Cell Genomics Reveals a Novel Cell State During Smooth Muscle Cell Phenotypic Switching and Potential Therapeutic Targets for Atherosclerosis in Mouse and Human. Circulation. 2020;142(21):2060–2075. doi: 10.1161/CIRCULATIONAHA.120.048378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Zhu H, Zhang Q, et al. Smooth muscle-derived macrophage-like cells contribute to multiple cell lineages in the atherosclerotic plaque. Cell Discov. 2021;7(1):111. doi: 10.1038/s41421-021-00328-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman AAC, Serbulea V, Baylis RA, et al. Multiple cell types contribute to the atherosclerotic lesion fibrous cap by PDGFRβ and bioenergetic mechanisms. Nat Metab. 2021;3(2). doi: 10.1038/s42255-020-00338-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanters E, Pasparakis M, Gijbels MJJ, et al. Inhibition of NF-κB activation in macrophages increases atherosclerosis in LDL receptor–deficient mice. J Clin Invest. 2003;112(8). doi: 10.1172/JCI18580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winkels H, Ehinger E, Vassallo M, et al. Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry. Circ Res. 2018;122(12). doi: 10.1161/CIRCRESAHA.117.312513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srikakulapu P, McNamara CA. B cells and atherosclerosis. Am J Physiol Circ Physiol. 2017;312(5):H1060–H1067. doi: 10.1152/ajpheart.00859.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Döring Y, Drechsler M, Soehnlein O, Weber C. Neutrophils in Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(2):288–295. doi: 10.1161/ATVBAHA.114.303564 [DOI] [PubMed] [Google Scholar]
- 14.Cherepanova OA, Gomez D, Shankman LS, et al. Activation of the pluripotency factor OCT4 in smooth muscle cells is atheroprotective. Nat Med. 2016;22(6). doi: 10.1038/nm.4109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alencar GF, Owsiany KM, K S, et al. The Stem Cell Pluripotency Genes Klf4 and Oct4 Regulate Complex SMC Phenotypic Changes Critical in Late-Stage Atherosclerotic Lesion Pathogenesis. Circulation. Published online July 17, 2020. doi: 10.1161/CIRCULATIONAHA.120.046672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan H, Xue C, Auerbach BJ, et al. Single-Cell Genomics Reveals a Novel Cell State During Smooth Muscle Cell Phenotypic Switching and Potential Therapeutic Targets for Atherosclerosis in Mouse and Human. Circulation. 2020;142(21). doi: 10.1161/CIRCULATIONAHA.120.048378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tabas I, García-Cardeña G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209(1):13–22. doi: 10.1083/jcb.201412052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Harst P, Verweij N. Identification of 64 Novel Genetic Loci Provides an Expanded View on the Genetic Architecture of Coronary Artery Disease. Circ Res. 2018;122(3):433–443. doi: 10.1161/CIRCRESAHA.117.312086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coppé J-P, Desprez P-Y, Krtolica A, Campisi J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu Rev Pathol Mech Dis. 2010;5(1):99–118. doi: 10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews C, Gorenne I, Scott S, et al. Vascular Smooth Muscle Cells Undergo Telomere-Based Senescence in Human Atherosclerosis. Circ Res. 2006;99(2):156–164. doi: 10.1161/01.RES.0000233315.38086.bc [DOI] [PubMed] [Google Scholar]
- 21.Ogami M, Ikura Y, Ohsawa M, et al. Telomere Shortening in Human Coronary Artery Diseases. Arterioscler Thromb Vasc Biol. 2004;24(3):546–550. doi: 10.1161/01.ATV.0000117200.46938.e7 [DOI] [PubMed] [Google Scholar]
- 22.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White Cell Telomere Length and Risk of Premature Myocardial Infarction. Arterioscler Thromb Vasc Biol. 2003;23(5):842–846. doi: 10.1161/01.ATV.0000067426.96344.32 [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Uryga AK, Reinhold J, et al. Vascular Smooth Muscle Cell Senescence Promotes Atherosclerosis and Features of Plaque Vulnerability. Circulation. 2015;132(20):1909–1919. doi: 10.1161/CIRCULATIONAHA.115.016457 [DOI] [PubMed] [Google Scholar]
- 24.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial Cell Senescence in Human Atherosclerosis. Circulation. 2002;105(13):1541–1544. doi: 10.1161/01.CIR.0000013836.85741.17 [DOI] [PubMed] [Google Scholar]
- 25.Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science (80- ). 2016;354(6311). doi: 10.1126/science.aaf6659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Childs BG, Zhang C, Shuja F, et al. Senescent cells suppress innate smooth muscle cell repair functions in atherosclerosis. Nat Aging. 2021;1(8). doi: 10.1038/s43587-021-00089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang J, Wang Y, Shao L, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22(1):78–83. doi: 10.1038/nm.4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garrido AM, Kaistha A, Uryga AK, et al. Efficacy and limitations of senolysis in atherosclerosis. Cardiovasc Res. Published online June 17, 2021. doi: 10.1093/cvr/cvab208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roos CM, Zhang B, Palmer AK, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15(5):973–977. doi: 10.1111/acel.12458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karnewar S, Neeli PK, Panuganti D, et al. Metformin regulates mitochondrial biogenesis and senescence through AMPK mediated H3K79 methylation: Relevance in age-associated vascular dysfunction. Biochim Biophys Acta - Mol Basis Dis. 2018;1864(4). doi: 10.1016/j.bbadis.2018.01.018 [DOI] [PubMed] [Google Scholar]
- 31.Yoshida T, Kaestner KH, Owens GK. Conditional Deletion of Krüppel-Like Factor 4 Delays Downregulation of Smooth Muscle Cell Differentiation Markers but Accelerates Neointimal Formation Following Vascular Injury. Circ Res. 2008;102(12):1548–1557. doi: 10.1161/CIRCRESAHA.108.176974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uryga AK, Bennett MR. Ageing induced vascular smooth muscle cell senescence in atherosclerosis. J Physiol. 2016;594(8). doi: 10.1113/JP270923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baylis RA, Gomez D, Owens GK. Shifting the Focus of Preclinical, Murine Atherosclerosis Studies From Prevention to Late-Stage Intervention. Circ Res. 2017;120(5). doi: 10.1161/CIRCRESAHA.116.310101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rousselle A, Qadri F, Leukel L, et al. CXCL5 limits macrophage foam cell formation in atherosclerosis. J Clin Invest. 2013;123(3):1343–1347. doi: 10.1172/JCI66580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care. 2014;17(4):324–328. doi: 10.1097/MCO.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 36.Musi N, Valentine JM, Sickora KR, et al. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. 2018;17(6):e12840. doi: 10.1111/acel.12840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hickson LJ, Langhi Prata LGP, Bobart SA, et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine. 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tse C, Shoemaker AR, Adickes J, et al. ABT-263: A Potent and Orally Bioavailable Bcl-2 Family Inhibitor. Cancer Res. 2008;68(9):3421–3428. doi: 10.1158/0008-5472.CAN-07-5836 [DOI] [PubMed] [Google Scholar]
- 40.Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. 2020;288(5):518–536. doi: 10.1111/joim.13141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Nanda V, Direnzo D, et al. Clonally expanding smooth muscle cells promote atherosclerosis by escaping efferocytosis and activating the complement cascade. Proc Natl Acad Sci. 2020;117(27):15818–15826. doi: 10.1073/pnas.2006348117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potluri J, Harb J, Masud AA, Hutti JE. A Phase 3, Double-Blind, Placebo-Controlled, Randomized Study Evaluating Navitoclax in Combination with Ruxolitinib in Patients with Myelofibrosis (TRANSFORM-1). Blood. 2020;136(Supplement 1):4–4. doi: 10.1182/blood-2020-13975832614961 [DOI] [Google Scholar]
- 43.Dilley K, Harb J, Jalaluddin M, Hutti JE, Potluri J. A Phase 3, Open-Label, Randomized Study Evaluating the Efficacy and Safety of Navitoclax Plus Ruxolitinib Versus Best Available Therapy in Patients with Relapsed/Refractory Myelofibrosis (TRANSFORM-2). Blood. 2020;136(Supplement 1):8–8. doi: 10.1182/blood-2020-13924732614959 [DOI] [Google Scholar]
- 44.Hu W, Jia Y, Xiao X, et al. KLF4 downregulates hTERT expression and telomerase activity to inhibit lung carcinoma growth. Oncotarget. 2016;7(33):52870–52887. doi: 10.18632/oncotarget.9141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang G, Han B, Zhang R, et al. C1q/TNF-Related Protein 9 Attenuates Atherosclerosis by Inhibiting Hyperglycemia-Induced Endothelial Cell Senescence Through the AMPKα/KLF4 Signaling Pathway. Front Pharmacol. 2021;12. doi: 10.3389/fphar.2021.758792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bär C, Povedano JM, Serrano R, et al. Telomerase gene therapy rescues telomere length, bone marrow aplasia, and survival in mice with aplastic anemia. Blood. 2016;127(14):1770–1779. doi: 10.1182/blood-2015-08-667485 [DOI] [PubMed] [Google Scholar]
- 47.Mojiri A, Walther BK, Jiang C, et al. Telomerase therapy reverses vascular senescence and extends lifespan in progeria mice. Eur Heart J. 2021;42(42):4352–4369. doi: 10.1093/eurheartj/ehab547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madonna R, Pieragostino D, Rossi C, et al. Transplantation of telomerase/myocardin-co-expressing mesenchymal cells in the mouse promotes myocardial revascularization and tissue repair. Vascul Pharmacol. 2020;135:106807. doi: 10.1016/j.vph.2020.106807 [DOI] [PubMed] [Google Scholar]
- 49.Madonna R. Vascular rejuvenation: a new therapeutic target? Eur Heart J. 2021;42(42):4370–4372. doi: 10.1093/eurheartj/ehab587 [DOI] [PubMed] [Google Scholar]
- 50.Whittemore K, Derevyanko A, Martinez P, et al. Telomerase gene therapy ameliorates the effects of neurodegeneration associated to short telomeres in mice. Aging (Albany NY). 2019;11(10):2916–2948. doi: 10.18632/aging.101982 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.