Abstract
Background
Since May 2022, an mpox outbreak affecting primarily men who have sex with men (MSM) has occurred in numerous non-endemic countries worldwide. As MSM frequently reported multiple sexual encounters in this outbreak, reliably determining the time of infection is difficult; consequently, estimation of the incubation period is challenging.
Aim
We aimed to provide valid and precise estimates of the incubation period distribution of mpox by using cases associated with early outbreak settings where infection likely occurred.
Methods
Colleagues in European countries were invited to provide information on exposure intervals and date of symptom onset for mpox cases who attended a fetish festival in Antwerp, Belgium, a gay pride festival in Gran Canaria, Spain or a particular club in Berlin, Germany, where early mpox outbreaks occurred. Cases of these outbreaks were pooled; doubly censored models using the log-normal, Weibull and Gamma distributions were fitted to estimate the incubation period distribution.
Results
We included data on 122 laboratory-confirmed cases from 10 European countries. Depending on the distribution used, the median incubation period ranged between 8 and 9 days, with 5th and 95th percentiles ranging from 2 to 3 and from 20 to 23 days, respectively. The shortest interval that included 50% of incubation periods spanned 8 days (4–11 days).
Conclusion
Current public health management of close contacts should consider that in approximately 5% of cases, the incubation period exceeds the commonly used monitoring period of 21 days.
Keywords: incubation period, monkeypox virus, mpox, MPXV, Germany, Europe
Key public health message.
What did you want to address in this study?
Determining time of infection for mpox among men who have sex with men is challenging as they often report multiple sexual encounters. Data from traditional investigations which determine time of infection ‘backwards’ (from outcome to exposure) can miss exposures further away from disease onset. We applied a forward-looking approach for our incubation period estimates, using data from early mpox outbreaks where infection likely occurred.
What have we learnt from this study?
The majority of cases had disease manifestations within 4–11 days post exposure.
Approximately 5% of cases had incubation periods of less than 3 days and 5% had incubation periods of more than 21 days.
What are the implications of your findings for public health?
For management of close contacts, it should be considered that some cases may not become apparent during the commonly recommended (self-)monitoring period of 21 days. Our findings that short incubation periods are possible support current recommendations that pre-exposure vaccination of high-risk groups should be considered.
Introduction
Mpox is a viral disease endemic to Central and West Africa. It is caused by the monkeypox virus (MPXV), a member of the Orthopoxvirus genus which also includes smallpox [1]. Since May 2022, numerous cases have been reported, primarily in non-endemic countries worldwide [2]; from 23 July 2022 to 11 May 2023 the global outbreak was categorised as a Public Health Emergency of International Concern (PHEIC) [3]. Between 1 January and 8 December 2022, 82,522 mpox cases were reported by 110 countries across six WHO regions [4], and 25,573 cases from 45 countries and areas in the World Health Organization (WHO) European Region have been identified [5]. In the European Region, Germany had, after Spain, France and the United Kingdom (UK), the fourth highest number of reported cases [5,6].
In endemic countries, circulation of MPXV is thought to be maintained through animal reservoirs; contact with infected animals plays an important role in transmission [7]. However, human-to-human transmission as a result of close contact with lesions or body fluids, via exposure to respiratory droplets during face-to-face contact or through contact with contaminated objects (e.g. bedding) also occurs [7]. For the outbreak since May 2022 in non-endemic countries, the majority of reported cases have been due to human-to-human transmission, particularly among men who have sex with men (MSM) [2,4]. For cases with data on sexual orientation, 96% identified themselves as MSM who had been exposed through sexual contact [2].
The incubation period of mpox has historically been reported to range from 5 to 21 days and may vary depending on the type of exposure and transmission route [1,8]. Four early publications focusing on cases in the global outbreak that began in May 2022 have provided incubation period estimates based mostly on small case numbers and the most likely time of infection determined by case investigations (e.g. case interviews or questionnaires) [9-12]. Several larger studies with a main focus on clinical aspects of mpox have also described the incubation period based on exposures reported by cases [13-15]. Due to the sensitive nature of investigating cases with exposure histories involving sexual contact, uncertainty remains regarding the accuracy of self-reported exposures. The reporting of multiple potential exposures also poses a challenge with regards to the identification of transmission events and the use of case investigation data to estimate the incubation period [16]*. Adding to the knowledge base for the global outbreak using estimates from cases with a highly probable time and place of infection is therefore important as it enables more reliable incubation period estimations to inform public health recommendations, in particular for monitoring of close contacts.
We aimed to estimate the incubation period distribution of mpox by using notified cases associated with early outbreaks. To increase sample size, we invited European countries to contribute data on cases who were likely to be part of these outbreaks.
Methods
Case definition
We included laboratory-confirmed mpox cases notified in Europe with a reported symptom onset date and whose probable place of infection (PPOI) was a fetish festival in Antwerp, Belgium (4–9 May 2022), a gay pride festival in Gran Canaria, Spain (5–15 May 2022) or a club in Berlin, Germany (Club C, 10 May–11 June 2022). For both festivals, we included only cases who attended the festival and were exposed for 5 days or less. We assumed that exposure could have occurred shortly before or after the festivals; therefore, cases whose exposure interval overlapped with the festival dates were also included. As most cases attended the festivals for at least several days, an exposure interval cut-off of 5 days was chosen as a compromise between statistical power (i.e. sample size) and uncertainty (i.e. length of exposure interval). For Club C, an additional inclusion criterion was that cases had to be part of a cluster of five or more cases who visited Club C within a time frame of 5 days, corresponding to extended weekends in which the club was open. Cases for any of the three events were excluded if their dates (exposure and/or symptom onset) were incomplete, if they visited more than one of the events above, if their dates of exposure did not overlap with the time frame of the event(s) or if the responsible health authority considered it unlikely that the exposure was associated with infection.
Data sources
The federal public health authority in Germany (Robert Koch Institute) contacted public health authorities from countries within the European region and invited them to send anonymised information on laboratory-confirmed mpox cases meeting the above case definition to the state health authority in Berlin, Germany (State Office for Health and Social Affairs (SOHSA)). Information requested was limited to sex, symptom onset date, first reported symptom(s), exposure date(s) and assessment of whether the reported exposure was the PPOI. The SOHSA contacted the federal states in Germany through established national networks. Countries within the European region were contacted through the European Centre for Disease Prevention and Control European Network for sexually transmitted infections (STI) surveillance, bilaterally based on established networks, or through contact information provided in recent mpox publications. We screened all submitted cases to determine if they met the inclusion criteria described above.
Estimation and analysis of the incubation period
The probable incubation interval for each case was calculated by subtracting the start and end date of the exposure period from the date of symptom onset. To account for uncertainty regarding the exposure date, 0.5 days were subtracted from the lower limit and 0.5 days were added to the upper limit of each incubation interval. The rationale for this was that festival events and club visits often started early in the night (21:00–23:00) and lasted until the morning of the next day. In addition, the exposure for cases with a single exposure date could still have lasted for several hours, e.g. when visiting a club. Consequently, all cases were doubly censored with regards to the probable incubation period. We further assumed that the incubation period was at least 1 day and thus the lower limit of the probable incubation interval was set to 1 day, if smaller.
Since all cases had a range of probable incubation periods, we used a bootstrapping method (resamplings: n = 10,000) to provide a non-parametric estimate of the empirical distribution of the incubation period. For each case in each resampled dataset, a definite incubation period was randomly sampled from the incubation interval of the case using a uniform distribution. The resulting incubation periods were visualised using a density histogram and a smoothed density curve was calculated using a bandwidth of 2 days and a Gaussian kernel.
To estimate the parametric incubation period distribution, we fitted three doubly censored models using the log-normal, Weibull and Gamma distributions. This was done in R (3.1.4) with brms (version 2.17.0) [17] and rstan (version 2.21.2) [18]. Goodness of fit was compared using the widely applicable information criterion (WAIC), the leave-one-out information criterion (LOOIC) and the 10-fold cross-validation information criterion (R package loo version 2.5.1) [19]. The probability density function (PDF) and cumulative distribution function (CDF) were visualised for each of the three distributions based on 500 posterior samples. Credible intervals were calculated as highest density intervals. We carried out a sensitivity analysis to examine the effect of length of exposure, the influence of the case with longest incubation period and the effect of including only cases from the two festivals (fetish festival, Antwerp, Belgium and gay pride festival, Gran Canaria, Spain) and Club C in Berlin, Germany (14/15 May). Details on the sensitivity analysis are provided in Supplementary Tables S3-S7.
To explore the difference in the median incubation time between cases with skin lesions vs prodromal symptoms as the first symptoms, we used the above non-parametric method.
Results
Case description
Within Germany, data were provided by four German federal states. Nine other countries also provided data. In total, we had information on 222 cases: 140 from European countries (excluding Germany), 66 from the state of Berlin, the state with the highest case numbers in this outbreak [20], and 16 from three other German federal states. Overall, we included 122 cases in the analysis. Of the 100 cases excluded, 25 had incomplete exposure data, 17 had exposure dates that did not overlap with the events, seven had visited multiple events (indeterminate PPOI) and 51 had an exposure interval of more than 5 days.
The PPOI was Club C (Berlin, Germany), the gay pride festival (Gran Canaria, Spain) and the fetish festival (Antwerp, Belgium) for 58.2%, 20.5% and 21.3% of cases, respectively. Among the included cases with information on first symptom(s) (n = 69), 30 had prodromal symptoms, 33 had skin lesions and six had both prodromal symptoms and skin lesions (Table 1).
Table 1. Screened mpox cases included and excluded from the analysis, by country, event and first reported symptom, May–June 2022 (n = 222) .
| Included | Excluded | |||||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Number of cases | 122 | 100 | ||||
| Median length of exposure in days (range) | 1.00 (1.00–5.00) | 8.00 (1.00–21.00) | ||||
| Country | 122 | 100.0 | 100 | 100.0 | ||
| Germanya | 71 | 58.2 | 11 | 11.0 | ||
| Belgium | 16 | 13.1 | 21 | 21.0 | ||
| Spain (Madrid only) | 11 | 9.0 | 16 | 16.0 | ||
| France | 9 | 7.4 | 16 | 16.0 | ||
| United Kingdom | 7 | 5.7 | 26 | 26.0 | ||
| The Netherlands | 6 | 4.9 | 4 | 4.0 | ||
| Otherb | 2 | 1.6 | 6 | 6.0 | ||
| Eventc | 122 | 100.0 | 51 | 51.0 | ||
| Club C, Berlin | 71 | 58.2 | 1 | 2.0 | ||
| Fetish festival, Antwerp | 26 | 21.3 | 1 | 2.0 | ||
| Gay pride festival, Gran Canaria | 25 | 20.5 | 49 | 96.1 | ||
| First symptomd | 69 | 56.6 | 50 | 50.0 | ||
| Prodromal | 30 | 43.5 | 31 | 62.0 | ||
| Prodromal/skin lesion | 6 | 8.7 | 6 | 12.0 | ||
| Skin lesion | 33 | 47.8 | 13 | 26.0 | ||
a Includes only data from the following German federal states: Bavaria, Berlin, Brandenburg, North Rhine-Westphalia.
b Ireland, Latvia, Malta and Norway.
c Of the 100 excluded cases, exposure for 49 could not be clearly associated with one of the three events.
d Information on first symptoms was available for 119 of the 222 cases.
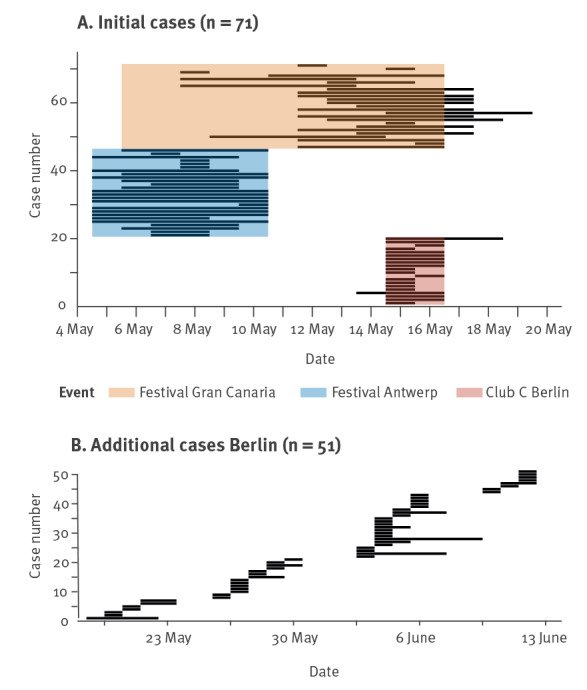
The exposure intervals of all included cases are illustrated in Figure 1. The cases in Figure 1A were from outbreaks in the first half of May 2022 and attended the fetish festival in Antwerp, Belgium (n = 26), the gay pride festival in Gran Canaria, Spain (n = 25) or Club C in Berlin, Germany during the earliest recorded outbreak from 14 to 15 May 2022 (n = 20). Many were international cases that travelled back to their respective home countries (Antwerp, Belgium: n = 12; Gran Canaria, Spain: n = 14; Berlin, Germany: n = 4). Exposure intervals for cases involved in additional outbreaks in Club C between 18 May and 11 June 2022 (n = 51) are illustrated in Figure 1B. The majority of these cases (n = 49) were from Berlin, Germany.
Figure 1.
Exposure periods of included mpox cases, Berlin, May to June 2022 (n = 122)
Each line denotes one case. The start of the line represents the start of the exposure interval and the end of the line the end of the exposure interval. Panel A: Cases that attended the fetish festival in Antwerp, Belgium, gay pride festival in Gran Canaria, Spain or Club C in Berlin, Germany on 14–15 May 2022. Panel B: Cases from additional outbreaks related to Club C in Berlin, Germany.
Empirical incubation periods
Figure 2A shows the empirical distribution of the incubation periods. The histogram shows a plateau for the incubation period of 3–11 days, where each day had more than 5% probability of being the symptom onset. The probability of the incubation period being within this range was 64.2%. Based on the empirical density estimate, the 50% highest density interval, which is the smallest interval containing 50% of probable incubation periods, was 3.7–11.2 days.
Figure 2.
Non-parametric and parametric incubation period distributions, mpox, Berlin, May to June 2022 (n = 122)
Panel A: Empirical distribution of the incubation periods shown as a density histogram and a smoothed density curve. Panels B–C left graph: Posterior samples of the probability density function of the fitted log-normal, Weibull and gamma distributions. The black line denotes the empirical smoothed density curve. Panels B–C right graph: Posterior samples of the cumulative distribution function of the fitted log-normal, Weibull and gamma distributions. The black line denotes the empirical cumulative distribution.
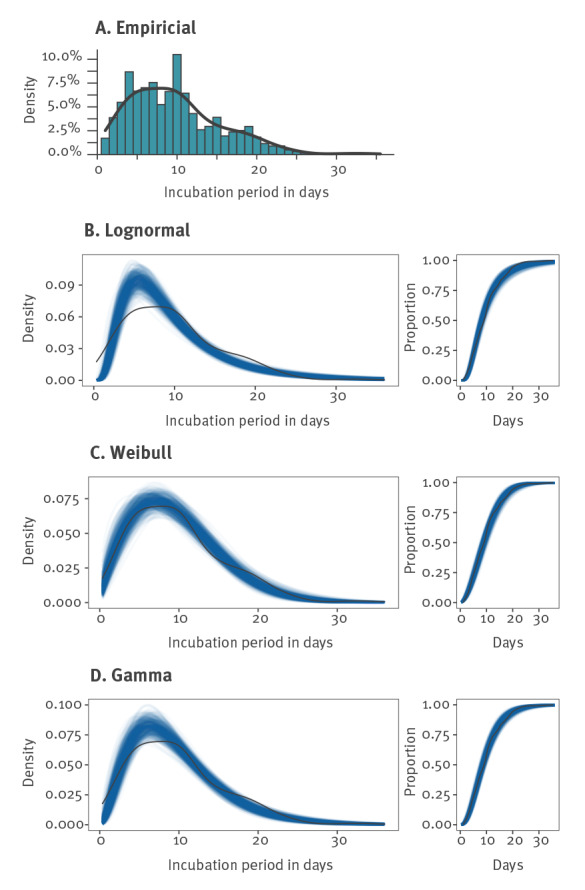
Five per cent of the probable values accounted for an incubation period of 2 days or less. Six cases had a probable incubation period above 21 days. The upper limit of the probable incubation period of five of these cases was shorter than 26 days, whereas one case had a probable incubation period of 30–35 days. We observed a spike in the empirical density for an incubation period of 10 days. Further analysis found only a small possible bias caused by the reported weekday for the nine cases that had an incubation period of exactly 10 days. Only three of the nine cases were exposed on Friday and reported symptoms on Monday 10 days later. This was only marginally more than the one to two randomly expected cases for nine cases spread over seven weekdays.
The percentiles and the medians of the incubation period were similar across the different distributions: 2.1 to 2.9 days for the 5th percentile and 8.1 to 9.0 days for the median (Figure 2B, Table 2). For the 95% percentile, the uncertainty was higher. The Weibull and Gamma distribution estimates were 20.1 and 20.6 days, respectively, whereas the log-normal distribution estimate was 23.1 days.
Table 2. Empirical and estimated percentiles of the incubation period in days for mpox based on different distributions, Berlin, May to June 2022 (n = 122).
| Quantile | Empirical | Lognormal | Weibull | Gamma |
|---|---|---|---|---|
| 2.5% | 1.7 | 2.4 (1.9–2.8) | 1.5 (1.0–2.0) | 1.9 (1.3–2.5) |
| 5% | 2.3 | 2.9 (2.3–3.4) | 2.1 (1.6–2.8) | 2.5 (1.9–3.2) |
| 10% | 3.3 | 3.6 (3.0–4.2) | 3.2 (2.4–3.9) | 3.5 (2.8–4.2) |
| 25% | 5.3 | 5.3 (4.6–6.0) | 5.5 (4.7–6.5) | 5.5 (4.7–6.3) |
| 50% | 9.0 | 8.1 (7.2–9.1) | 9.0 (7.9–10.0) | 8.6 (7.7–9.6) |
| 75% | 12.8 | 12.5 (11.0–14.1) | 13.1 (11.9–14.5) | 12.7 (11.4–14.1) |
| 90% | 18.3 | 18.3 (15.7–21.3) | 17.4 (15.6–19.3) | 17.4 (15.4–19.5) |
| 95% | 20.4 | 23.1 (19.3–27.5) | 20.1 (18.0–22.5) | 20.6 (18.2–23.4) |
| 97.5% | 22.7 | 28.1 (23.0–34.3) | 22.5 (19.9–25.4) | 23.7 (20.7–27.1) |
| 99% | 25.5 | 35.5 (28.3– 44.4) | 25.4 (22.2–29.0) | 27.6 (23.9–31.9) |
Estimates are shown with 95% credible intervals in parentheses.
The three models had similar predictive performances; the difference in goodness of fit was small (i.e. below 4) for all information criteria (IC) [21,22]. The WAIC showed a maximal difference of 2.8, LOOIC of 2.8 and the 10-fold cross-validation information criterion (10-fold IC) of maximal 1.5. In Supplementary Table S2, we provide the values of the WAIC, LOOIC and 10-fold IC for each distribution. The log-normal distribution estimated a lower probability of shorter incubation periods and a higher probability of longer incubation periods. In particular for the CDF, the log-normal distribution lay below the empirical cumulative density function around day 23. In Supplementary Table S1 we provide the estimated distribution parameters.
Explorative analysis of cases with skin lesions vs prodromal symptoms as the first reported symptom showed that the median empirical incubation period of 9.8 days for cases with skin lesions (n = 33) was longer than the median empirical incubation period of 9.0 days for cases with prodromal symptoms (n = 30).
Because we estimated upper 95% limits of the incubation period distribution of more than 20 days, we investigated the influence of the case with the longest reported incubation period interval (30–35 days). Exclusion of this case resulted in a shortening of the 95% quantile by 0.6 to 0.9 days, which was still well within the original credible intervals. Changes in the median and the 5% quantiles were within the rounding error (0.2 days) (see Supplementary Table S3 for the full results). The length of the exposure interval had little effect on the incubation period estimation as the credible intervals had considerable overlap (> 70%) and their width did not vary. Sensitivity analysis results on the length of exposure can be accessed in Supplementary Tables S6-S8.
Discussion
We estimated the incubation period distribution for mpox using cases for whom the time (interval) of infection could be determined with reasonable confidence. All three distributions used fitted the data well. Our study corroborates the wide range of possible mpox incubation periods, which can be as short as 2–3 days (5% percentile of the incubation period distributions) but also longer than 20 days (95% percentile of 20, 21 and 23 days). Half of the cases had incubation periods between 4 and 11 days; the median incubation period for all cases was between 8 and 9 days.
Our estimate of the 95% percentile indicated that incubation periods longer than 21 days occurred in ca 5% of cases. This is comparable to results from previous studies, which estimated 95th percentiles of 17, 18 and 20 days [9-12]. The two studies that estimated a 95th percentile of 17 days were small initial studies from June 2022 that partly shared cases [9,10]. Because these studies aimed to provide early actionable information on the incubation period, they had a short follow-up time. In addition, some cases with longer incubation periods may not have been captured as in a growing outbreak, infected persons with a long incubation period have a lower probability of being included (right-censoring bias) [23]. Furthermore, in routine case investigations by public health personnel, incubation periods are estimated ‘backwards’ from disease onset to possible exposures. This approach, which has also been used in previous studies on the mpox incubation period [9-12], is subject to backward biases, especially when incidence is increasing [23]. In addition, under-ascertainment of exposures that have occurred near or outside presumed maximal incubation periods (e.g. 21 days for mpox) can occur, particularly when plausible exposures were closer to disease onset. To account for this, we conceptually estimated the incubation period ‘forward’, analogous to a retrospective cohort study, starting from the likely time point of infection [23]. We also included only cases from early outbreaks in our study as it was likely that infection occurred in these settings, particularly because MPXV was not widespread at that time and risk of infection outside of these outbreaks was therefore comparatively low. With this approach, it is less likely that long incubation periods were due to unobserved (secondary) exposures that occurred after the presumed time of infection [24]. In addition, as the follow-up time for included cases in our study was at least 45 days, our study was less susceptible to underrepresentation of cases with longer incubation periods.
The 5% percentiles of 2 and 4 days from two studies [9,11] are in line with our estimate of 2–3 days and support our findings that very short incubation periods are possible. This, along with findings of possible presymptomatic transmission of mpox [12,25], has implications for vaccination strategies. In particular, the effectiveness of post-exposure vaccination strategies may be limited and pre-exposure vaccination of high-risk groups should be emphasised, provided that a sufficient number of vaccine doses are available [13,25,26].
The comparatively larger number of cases included in our study enabled more precise estimates of the incubation period than previous studies. This is illustrated by the narrow credible intervals for our distribution estimates, with only ± 1 day uncertainty for the median estimates. In addition, sensitivity analysis showed that removing the case with the highest incubation period had little effect on the incubation period estimates. This underscores that the estimates are robust against single outlier observations.
To evaluate if estimates depend on the distribution(s), different distributions should be used to compare incubation period estimates [27]. Based on our results, the chosen distribution had little effect on the incubation period estimates as all had a similar goodness of fit. The largest difference was observed in the 95% percentile, with 20.1 and 20.6 days for the Weibull and Gamma distributions and 23.1 days for the log-normal distribution due to its long tail. The right tail of the log-normal distribution deviated substantially from the empirical data and estimated the highest possible 95th percentile, representing the most conservative scenario.
Incubation period estimates for cases who had skin lesions as their first symptom vs cases that had prodromal symptoms showed less than a day difference. Our observed median incubation time of 9.8 days for cases with skin lesions as the first symptom was longer than the estimate of 7.8 days in a previous study [10]. Several recent large studies with a broad focus on clinical characteristics of mpox have also reported incubation period results. As they have time frames and methods that are not tailored to only assess the incubation period of mpox, comparing our results with these studies is difficult [13-15].
Our study is subject to limitations. Initial symptoms (e.g. fatigue, fever) can be non-specific; therefore, it is possible that they were not related to mpox illness or that early symptoms were overlooked, potentially biasing our estimates. Notably, published clinical findings for this global mpox outbreak have described previously unreported clinical presentations including single lesions in or around the genitals and anus, oral lesions and symptoms of proctitis [13,15]. This type of clinical presentation might initially have been missed by cases as well as physicians and may have resulted in a slight overestimation of the incubation period in our study and in previous studies, particularly, as we included cases early in the mpox outbreak when knowledge was limited. Furthermore, incomplete recall could have affected our estimates, especially since multiple sexual contacts were commonly reported. Therefore, we cannot completely exclude that the cases, although present at a festival or club with recognised outbreaks, were infected at a later time. Lastly, data regarding smallpox vaccination status was not available for the majority of cases. As a result, we were unable to evaluate the possible influence of vaccination status on incubation period.
Conclusion
Information on the incubation periods for mpox in the global outbreak beginning in May 2022 is important to inform public health recommendations aimed at limiting onward transmission, such as the time period for self-monitoring of contact persons or restrictions for close contacts. Our study illustrates the benefit of collaborative collection and analysis of cases in novel outbreaks in order to build a knowledge base to guide public health recommendation and measures. It also includes the largest number of cases to date used in published studies modelling incubation period estimates for the 2022 global outbreak. Approximately 5% of cases have an incubation period greater than 21 days, and therefore may not become apparent during the commonly recommended monitoring period of close mpox contacts.
Ethical statement
Ethical approval was not needed for this study, which was based on surveillance data only.
Data availability
The data used for the analysis is provided in Supplement Data as a csv file. The file contains information on exposure and symptom onset dates and the first symptom for all 122 included cases. Data on the festival visited and country of origin is omitted for privacy reasons. The results in this paper can be reproduced using the Supplement R Code.
Acknowledgements
We are sincerely indebted to all local public health departments for their diligent work in this outbreak and for providing the data that made this analysis possible.
Supplementary Data
Supplementary Data
Supplementary Data
*Erratum
In the version originally published on 6 July, the references from number [16] onwards were misnumbered. The reference numbering was corrected on 10 July 2023. We apologise for any inconvenience this may have caused.
Conflict of interest: None declared.
Authors’ contributions: SM, UM, LH, FM, JIM, FMM, EGM, EC, AM, YS, NH, AL, NL, WVB, LL, DVB, PC, JW, EPR, TBJ, ABo, TM, CS, LC, RB, AJ, DW, ABa were involved in the collection and evaluation of MPX case data, and critically reviewed and edited the manuscript. ABa conceptualised the study, conducted the analysis and contributed to writing of the manuscript. SM conducted data analysis and wrote the manuscript. DW conceptualised the study, edited and contributed to development of the manuscript. FM reviewed and contributed to the statistical methodology.
References
- 1.World Health Organization (WHO). Mpox (monkeypox). Geneva: WHO. [Accessed: 21 Sep 2022]. Available from: https://www.who.int/news-room/fact-sheets/detail/monkeypox
- 2.European Centre for Disease Prevention and Control (ECDC). Monkeypox multi-country outbreak – second update. Stockholm: ECDC; 2022. Available from: https://www.ecdc.europa.eu/en/publications-data/monkeypox-multi-country-outbreak-second-update
- 3.World Health Organization (WHO). WHO Director-General declares the ongoing monkeypox outbreak a Public Health Emergency of International Concern. Geneva: WHO; 2022. Available from: https://www.who.int/europe/news/item/23-07-2022-who-director-general-declares-the-ongoing-monkeypox-outbreak-a-public-health-event-of-international-concern
- 4.World Health Organization (WHO). 2022-23 Mpox (monkeypox) outbreak: Global trends. Geneva: WHO. [Accessed: 9 Dec 2022]. Available from: https://worldhealthorg.shinyapps.io/mpx_global
- 5.European Centre for Disease Prevention and Control (ECDC) and World Health Organization (WHO). Joint ECDC-WHO Regional Office for Europe monkeypox surveillance bulletin. Stockholm: ECDC: Geneva: WHO. [Accessed: 9 Dec 2022]. Available from: https://monkeypoxreport.ecdc.europa.eu
- 6.Lachmann R, Frank C, Stark K, Falkenhorst G. Kurze Inkubationszeiten bei Affenpockenfällen in Deutschland während des aktuellen Ausbruchsgeschehens. [Short incubation periods for monkeypox cases in Germany during the current outbreak]. EpiBull. 2022;37. German. Available from: https://www.rki.de/DE/Content/Infekt/EpidBull/Archiv/2022/Ausgaben/37_22.pdf?__blob=publicationFile
- 7.European Centre for Disease Prevention and Control (ECDC). Monkeypox multi-country outbreak. Stockholm: ECDC; 2022. Available from: https://www.ecdc.europa.eu/sites/default/files/documents/Monkeypox-multi-country-outbreak.pdf
- 8.Reynolds MG, Yorita KL, Kuehnert MJ, Davidson WB, Huhn GD, Holman RC, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194(6):773-80. 10.1086/505880 [DOI] [PubMed] [Google Scholar]
- 9.Miura F, van Ewijk CE, Backer JA, Xiridou M, Franz E, Op de Coul E, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27(24):2200448. 10.2807/1560-7917.ES.2022.27.24.2200448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charniga K, Masters NB, Slayton RB, Gosdin L, Minhaj FS, Philpott D, et al. Estimating the incubation period of monkeypox virus during the 2022 multi-national outbreak. medRxiv 2022.06.22.22276713 [Preprint]. [posted 28 October2022]. [13 p.]. Available from: https://www.medrxiv.org/content/10.1101/2022.06.22.22276713v2 10.1101/2022.06.22.22276713 10.1101/2022.06.22.22276713 [DOI]
- 11.Guzzetta G, Mammone A, Ferraro F, Caraglia A, Rapiti A, Marziano V, et al. Early estimates of monkeypox incubation period, generation time and reproduction number, Italy, May-June 2022. Emerg Infect Dis. 2022;28(10):2078-81. 10.3201/eid2810.221126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward T, Christie R, Paton RS, Cumming F, Overton CE. Transmission dynamics of monkeypox in the United Kingdom: contact tracing study. BMJ. 2022;379:e073153. 10.1136/bmj-2022-073153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tarín-Vicente EJ, Alemany A, Agud-Dios M, Ubals M, Suñer C, Antón A, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022;400(10353):661-9. 10.1016/S0140-6736(22)01436-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mailhe M, Beaumont AL, Thy M, Le Pluart D, Perrineau S, Houhou-Fidouh N, et al. Clinical characteristics of ambulatory and hospitalized patients with monkeypox virus infection: an observational cohort study. Clin Microbiol Infect. 2023;29(2):233-9. 10.1016/j.cmi.2022.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, et al. Monkeypox virus infection in humans across 16 countries — April–June 2022. N Engl J Med. 2022;387(8):679-91. 10.1056/NEJMoa2207323 [DOI] [PubMed] [Google Scholar]
- 16.United Kingdom Health Security Agency (UKHSA). Investigation into monkeypox outbreak in England: technical briefing 1. Updated 23 September2022. London: UKHSA; 2022. Available from: https://www.gov.uk/government/publications/monkeypox-outbreak-technical-briefings/investigation-into-monkeypox-outbreak-in-england-technical-briefing-1
- 17.Bürkner PS. brms: An R package for Bayesian multilevel models using Stan. J Stat Softw. 2017;80(1):1-28. 10.18637/jss.v080.i01 [DOI] [Google Scholar]
- 18.R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2020. Available from: https://www.R-project.org
- 19.Vehtari A, Gelman A, Gabry J. Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat Comput. 2017;27(5):1413-32. 10.1007/s11222-016-9696-4 [DOI] [Google Scholar]
- 20.Selb R, Werber D, Falkenhorst G, Steffen G, Lachmann R, Ruscher C, et al. A shift from travel-associated cases to autochthonous transmission with Berlin as epicentre of the monkeypox outbreak in Germany, May to June 2022. Euro Surveill. 2022;27(27):2200499. 10.2807/1560-7917.ES.2022.27.27.2200499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sivula T, Magnusson M, Matamoros AA, Vehtari A. Uncertainty in Bayesian leave-one-out cross-validation based model comparison. arXiv2008.10296; 2022;v3.
- 22.Burnham K, Anderson DR. Multimodel inference: Understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33(2):261-304. 10.1177/0049124104268644 [DOI] [Google Scholar]
- 23.Park SW, Sun K, Champredon D, Li M, Bolker BM, Earn DJD, et al. Forward-looking serial intervals correctly link epidemic growth to reproduction numbers. Proc Natl Acad Sci USA. 2021;118(2):e2011548118. 10.1073/pnas.2011548118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werber D, King LA, Müller L, Follin P, Buchholz U, Bernard H, et al. Associations of age and sex with the clinical outcome and incubation period of Shiga toxin-producing Escherichia coli O104:H4 infections, 2011. Am J Epidemiol. 2013;178(6):984-92. 10.1093/aje/kwt069 [DOI] [PubMed] [Google Scholar]
- 25.Freeman EE, Abbott S, Kurpiel B, Okwor T. The dynamics of monkeypox transmission. BMJ. 2022;379:o2504. 10.1136/bmj.o2504 [DOI] [PubMed] [Google Scholar]
- 26.European Centre for Disease Prevention and Control (ECDC). Public health considerations for mpox in EU/EEA countries. Stockholm: ECDC; 2023. Available from: https://data.europa.eu/doi/10.2900/79332
- 27.Nishiura H. Early efforts in modeling the incubation period of infectious diseases with an acute course of illness. Emerg Themes Epidemiol. 2007;4(4):2. 10.1186/1742-7622-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


