Abstract
Human cytomegalovirus (CMV) is a highly prevalent herpesvirus that is often transmitted to the neonate via breast milk. Postnatal CMV transmission can have negative health consequences for preterm and immunocompromised infants, but any effects on healthy term infants are thought to be benign. Furthermore, the impact of CMV on the composition of the hundreds of bioactive factors in human milk has not been tested. Here, we utilize a cohort of exclusively breastfeeding full term mother-infant pairs to test for differences in the milk transcriptome and metabolome associated with CMV, and the impact of CMV in breast milk on the infant gut microbiome and infant growth. We find upregulation of the indoleamine 2,3- dioxygenase (IDO) tryptophan-to-kynurenine metabolic pathway in CMV+ milk samples, and that CMV+ milk is associated with decreased Bifidobacterium in the infant gut. Our data indicate a complex relationship between milk CMV, milk kynurenine, and infant growth; with kynurenine positively correlated, and CMV viral load negatively correlated, with infant weight-for-length at 1 month of age. These results suggest CMV transmission, CMV-related changes in milk composition, or both may be modulators of full term infant development.
Introduction
Human Cytomegalovirus (CMV) is a member of the herpesvirus family with a global seroprevalence of ~85% in women of childbearing age1. CMV is a double-stranded DNA virus that can infect multiple cell types including epithelial, endothelial, and immune cells2. Initial infection in healthy individuals is often asymptomatic, followed by lifelong viral latency. The most common mode of CMV transmission in infants is through breast milk, as during lactation CMV locally reactivates in the mammary gland in virtually all seropositive women3-6. Following mammary CMV reactivation, the presence of viral DNA in milk can be detected in both milk cells and whey7-9.
Postnatal CMV transmission via breast milk is thought to be benign in full-term, non-immunocompromised infants10. However, for preterm infants, postnatal CMV can have serious clinical consequences including sepsis, thrombocytopenia, and long-term neurodevelopmental impairment10. Among preterm and very low birthweight infants fed CMV+ breast milk, about 20% are estimated to acquire CMV10,11. The rate of transmission in full-term infants breastfed by seropositive mothers is estimated at up to 70%12-14.
Despite the prevalence and clinical importance of mammary CMV reactivation, little is known about its relationship to human milk composition. CMV reactivation could lead to a local immune response and viral regulation of host metabolism that could impact milk composition. Conversely, differences in milk composition could modify the risk of CMV reactivation, also leading to associations between CMV reactivation and milk composition. Associations between mammary CMV reactivation and the hundreds of nutritive and bioactive components of human milk have mostly not been assessed, but one study found an increase in pro-inflammatory cytokines in the setting of maternal CMV reactivation during lactation5. If CMV reactivation does alter human milk composition, it would be important to understand the impact of these changes on the infant. Variation in milk composition is associated with infant development, including the gut microbiome and immune system15-17. For preterm infants, who strongly benefit from human milk feeding18, an understanding of CMV-related changes in milk composition and their impact on infant health outcomes is critical.
One approach to understanding the mechanism by which CMV affects host physiology is to quantify the host transcriptional response and the metabolome in the context of CMV infection. The impact of CMV on host gene expression has been examined in cultured cells19-24 and in the blood of kidney transplant recipients21, but not in the context of mammary reactivation. Similarly, the metabolome during CMV infection has been described in cultured cells25,26 and infant urine27, but not in milk. Milk transcriptome and metabolome provide complementary profiles of the physiology of the lactating mammary gland and milk composition15,28-30. Although the clinical impact of postnatal CMV transmission is far greater for preterm than for term infants, the mechanisms by which CMV alters or is altered by human milk composition can be studied using milk from term mother-infant dyads.
In this study, we aimed to identify differences in human milk composition and infant outcomes associated with CMV reactivation in a deeply phenotyped cohort of lactating mothers and their full term infants. Leveraging multi-omics data from mother-infant dyads, we tested for differences in the milk transcriptome, milk metabolome, and infant fecal metagenome associated with milk CMV reactivation (Figure 1). Further, we utilized anthropometric data to characterize differences in infant growth associated with milk CMV reactivation. Our results indicate that there are previously unappreciated differences in milk composition, infant gut microbiome composition, and growth in healthy full-term infants exposed to CMV through breast milk.
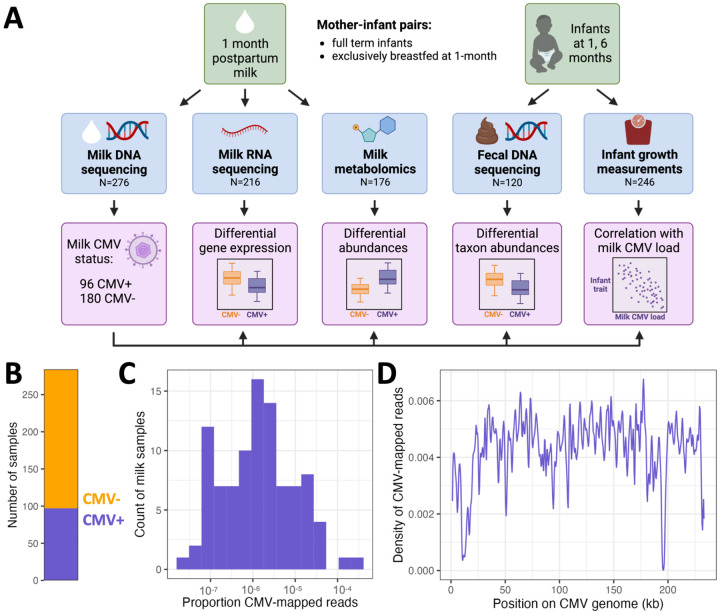
Figure 1.
(A) Study overview. (B) Count of milk samples identified as CMV+ (N=97, purple) or CMV− (N=187, orange). (C) The distribution of CMV-mapped DNA reads, as a proportion of all DNA reads, across milk samples that had at least one read mapped to the CMV genome. (D) Density of CMV-aligned reads across the CMV genome from all CMV+ milk samples. The density refers to the fraction of all CMV-mapped reads aligned to a particular region of the CMV genome. The density dips close to zero at repetitive regions in the CMV genome31.
Results
Identifying CMV-positive samples from shotgun DNA sequencing of human milk
As CMV is a DNA virus, its presence can be detected in the lactating mammary gland by measuring CMV DNA in milk8. Viral shedding into breast milk typically begins within one week postpartum, and peaks 1-2 months postpartum5. We leveraged existing shotgun DNA sequencing data from 1-month postpartum milk samples15 (N=276) to identify milk samples with CMV viral shedding (Figure 1A). We mapped milk-derived DNA sequencing reads to the CMV genome and designated any sample with at least one read mapped to the CMV genome as CMV+ (97/284, 34% CMV+; Figure 1B, Table S1). Hereafter, samples with no CMV-mapped reads were designated as CMV−. To ensure our results were not dependent on this choice of threshold, we repeated the main analyses in this manuscript using a series of higher thresholds for the required proportion of CMV-mapped reads to designate a sample as CMV+. We saw no qualitative difference in our results across the range of tested thresholds (Table S2; but see infant growth section below). The mean proportion of CMV-mapped reads in samples designated as CMV+ was 1.0x10−5, or about 1 per 100,000 sequenced reads (Figure 1C), reflecting the fact that the vast majority of DNA in these milk samples comes from human cells.
Milk DNA was extracted and sequenced using two approaches for two distinct original goals: low-pass human whole genome sequencing (WGS) or shotgun metagenomic sequencing (SMS). The main difference between these approaches was the extraction protocol (see details in Methods). Within samples that had CMV-mapped reads from both datasets (N=24), there was a positive correlation in the proportion of CMV-mapped reads (Spearman’s rho=0.81, P=3.47x10−5; Figure S1). Mapped reads were widely distributed across the CMV genome (Figure 1D). There was no significant difference in the mean total read count for CMV+ vs. CMV− samples (two-sided t-test, P=0.26; Figure S2), suggesting that read depth did not bias our approach to detect CMV+ samples. Within CMV+ samples, there was no significant difference in the mean proportion of reads that mapped to the CMV genome between the two sources of DNA sequencing data (two-sided t-test, P=0.23; Figure S3). Taken together, these results suggest that our detection of CMV+ samples is not biased by technical factors or sequencing approach.
Comparing the maternal characteristics of CMV+ vs. CMV− milk samples, we observed that CMV+ milk samples were less likely to come from mothers who self-identified as White/European-American (74% in CMV+ vs. 91% in CMV−, P=3.1x10−4, q-value=3.7x10−3, Fisher’s exact test; Table S3). This is consistent with previous epidemiological estimates that CMV seropositivity is higher in non-white than white populations worldwide32-34. All other tested maternal traits were not significantly different between CMV+ and CMV− groups (q-value>0.25 for all other tests; Table S3).
Immune response genes are upregulated in CMV+ milk samples
Human milk contains RNA from the milk-producing mammary epithelial cells and immune cells35-38. Thus, gene expression analyses of human milk provide a profile of the lactating mammary gland28,29. Using RNA-sequencing data we previously generated from the same milk samples studied here (N=221)15, we tested for differential expression of 17,675 genes in CMV+ vs. CMV− milk samples (Figure 2A). 36 genes were significantly differentially expressed (q-value<0.05), 34 of which were upregulated in CMV+ milk (Figure 2B, Table S4). These 34 upregulated genes were enriched for pathways related to the immune response to viral infections (Table S5), with “cellular response to interferon-gamma” as the most significant pathway (GO:0071346, odds ratio = 74.5, P = 5.22x10−15, q-value = 2.70x10−12). Upregulation of interferon-stimulated genes is a typical feature of the immune response to CMV infection22,39,40 (Figure S4). Within CMV+ milk samples, the proportion of CMV-mapped DNA reads and expression of the differentially expressed genes was significantly positively correlated for two genes: BATF2 and IDO1 (q-value<0.05, Table S6).
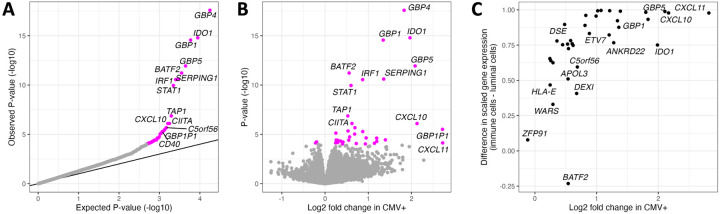
Figure 2.
Differential gene expression analysis comparing CMV− to CMV+ milk samples. (A) QQ-plot from the results of differential gene expression analysis. The x-axis plots the expected P-value for the number of genes tested following a uniform distribution of P-values from 0 to 1, and the y-axis plots the observed P-values. Genes whose P-value was below the false discovery rate threshold of 5% are colored in magenta. (B) Volcano plot comparing estimated effect sizes of CMV+ on milk gene expression (x-axis) with each gene’s P-value (y-axis). Genes whose P-value was below the false discovery rate threshold of 5% are colored in magenta. (C) Comparison of log fold change in CMV+ samples from our bulk RNA-seq data (x-axis) vs. gene expression in a publicly available human milk single cell RNA-seq dataset36 (y-axis). Gene expression from milk single cells is plotted as the difference between scaled gene expression in immune cells and mammary luminal cells, to display that genes more highly expressed in our CMV+ milk samples tended to be more highly expressed in the immune cells in milk.
As our bulk milk RNA sequencing data derives from all the cells in our milk samples, we leveraged publicly available single cell RNA-sequencing data from human milk36 to explore the expression patterns of the 36 differentially expressed genes across milk cell types. We observed that genes more highly expressed in our CMV+ milk samples tended to also be more highly expressed in immune cells in milk in the single cell data (Spearman’s rho= 0.72, P= 1.7x10−6; Figure 2C). CMV+ milk samples also had a higher estimated proportion of immune cells (mean 16.5% in CMV+ vs. 12.6% in CMV−, P=0.041, Wilcoxon rank sum test; Figure S5; see Methods). We note that the CMV status of the milk samples in the reference single cell dataset (N=15) is unknown, but given the high prevalence of CMV it likely includes both CMV+ and CMV− samples. These results suggest that the elevated expression of these genes in CMV+ milk samples stems from an increased proportion of immune cells in CMV+ milk. This is potentially consistent with previous studies showing an increase in T cells in CMV+ human milk40,41, though we only tested the estimated proportion of all immune cells here due to the imprecision of cell-type deconvolution of bulk RNA-seq data.
Differentially abundant metabolites in CMV+ samples indicate higher activity of the IDO tryptophan-to-kynurenine metabolic pathway
The human milk metabolome reflects cellular processes in the mammary gland and the composition of nutritive and bioactive components delivered to the infant42. We tested for differential abundance of 458 metabolites between 58 CMV+ and 84 CMV− milk samples in a regression model including study site, parity, maternal age, maternal pre-pregnancy BMI, maternal self-identified race, maternal gestational diabetes status, and maternal Healthy Eating Index score as covariates (Figure S6, see Methods). Two metabolites were significantly differentially abundant after correcting for multiple tests (q-value<0.05, Table S7): kynurenine (CMV+ estimated effect = 0.81, P= 1.3x10−6, q-value= 6.1x10−4; Figure 3A) and its metabolite kynurenic acid (CMV+ estimated effect = 0.75, P= 1.6x10−5, q-value= 6.6x10−3; Figure S7A).
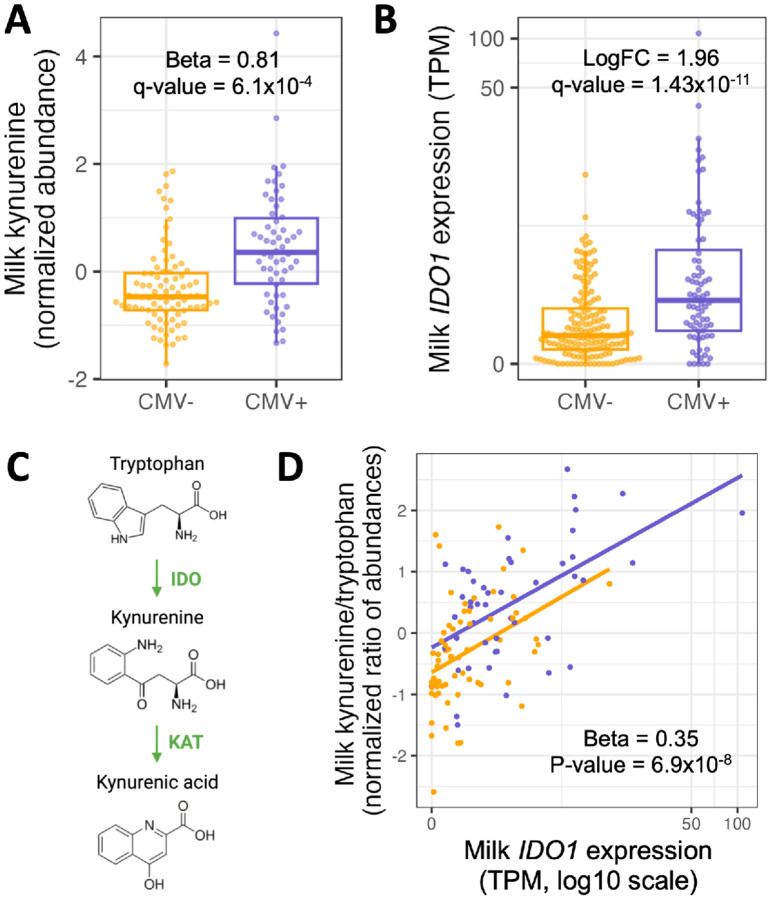
Figure 3.
(A) Kynurenine abundances in CMV− (orange) vs. CMV+ (purple) milk samples. Each dot represents a milk sample. Plotted kynurenine levels (y-axis) are residuals after correcting for covariates included in the differential abundance analysis (see Methods). (B) IDO1 expression in CMV− (orange) vs. CMV+ (purple) milk samples. Each dot represents a milk sample. LogFC: log fold-change between CMV+ and CMV− samples. (C) IDO1 encodes the enzyme indoleamine 2,3-dioxygenase (IDO), which performs the rate-limiting step converting tryptophan to kynurenine. Kynurenic acid is metabolized from kynurenine by the KAT enzyme. (D) Correlation between IDO1 expression (x-axis) and the ratio of kynurenine and tryptophan abundances (y-axis) in milk samples, stratified by CMV status. Each dot represents a milk sample.
The increased abundance of kynurenine and kynurenic acid in CMV+ samples is concordant with the upregulation of the IDO1 gene we observed in our gene expression data (Figure 3B). IDO1 encodes indoleamine 2,3-dioxygenase (IDO), the rate-limiting enzyme in the tryptophan-to-kynurenine metabolic pathway (Figure 3C). The kynurenine/tryptophan ratio was more significantly associated with CMV status than kynurenine alone (CMV+ estimated effect = 0.82, P= 9.4x10−7; Figure S7B). Within CMV+ milk samples, the kynurenine/tryptophan ratio was positively correlated with the proportion of CMV-mapped reads (Beta = 0.19, P = 6.3x10−3; Figure S7C). We did not observe a difference in the abundance of tryptophan by CMV status (CMV+ estimated effect = −0.22, P= 0.20, q-value= 0.85). Milk IDO1 expression was also positively correlated with the kynurenine/tryptophan ratio of abundances in milk, independent of milk CMV status (Beta= 0.35, P=6.9x10−8; Figure 3D), illustrating the strong link between expression of IDO1 and the abundance of these metabolites.
Milk CMV status is correlated with composition of the infant gut microbiome
Variation in human milk composition has been previously associated with the infant gut microbiome15,43,44. Motivated by the differences in milk composition we observed between CMV+ and CMV− milk samples, we next tested for associations between milk CMV status and composition of the infant gut microbiome. We previously generated shotgun metagenomic data from infant feces collected at 1 and 6 months postpartum (N=127 mother/infant pairs at 1 month, N=120 at 6 months)15,45. To explore a potential relationship between milk CMV status and the overall structure of the infant fecal microbiome, we first reduced the dimensionality of the microbial taxon abundance table using principal component analysis (each time point analyzed separately, see Methods). We then tested for associations between milk CMV status and the microbial principal components (PCs). Milk CMV status was significantly correlated with PC3 of the 1-month infant fecal metagenomes (Beta=1.79, P=1.1x10−3, q-value = 5.6x10−3; Figure 4A, Table S8). The top-loading taxa in 1-month PC3 were species of Bifidobacterium (negatively correlated with PC3; Figure S8). PC3 was not correlated with milk kynurenine abundance (P=0.12); and within infants fed CMV+ milk, PC3 was not correlated with the proportion of CMV-mapped reads in milk (P=0.79). Milk CMV status was not associated with the 6-month taxon abundance PCs (Table S8). Separately, we performed principal component analysis on the microbial genetic pathway abundances estimated from shotgun metagenomic data, and milk CMV status was not associated with any of the pathway PCs (Table S9). Milk CMV status was not associated with infant fecal alpha diversity at 1 month (Beta=0.29, P=0.15) or 6 months (Beta=0.06, P=0.70).
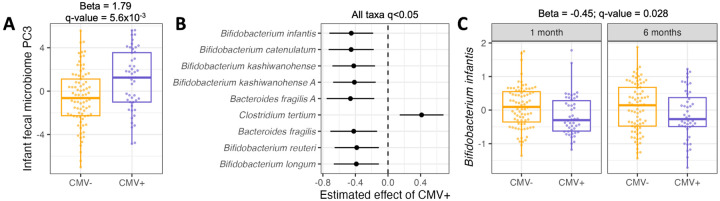
Figure 4.
(A) Comparison of PC3 values for infant fecal samples fed CMV− (orange) vs. CMV+ (purple) breastmilk. Principal component analysis was performed on the taxon abundance table for infant fecal samples at 1 month of age. Each dot represents an infant fecal sample. Plotted PC3 levels are residuals after correcting for covariates included in the association analysis with milk CMV status (see Methods). (B) Estimated effect of CMV+ milk on normalized microbial taxa abundances in the infant gut, including samples from 1 and 6 months of age. All taxa listed had a P-value below a false discovery rate of 5%. Taxa are arranged from smallest (top) to largest (bottom) P-value. (C) The distribution of Bifidobacterium infantis abundances in the infant fecal microbiome, for infants fed CMV− (orange) or CMV+ (purple) milk, at 1 and 6 months of age. Plotted B. infantis levels are residuals after correcting for covariates included in the association analysis with milk CMV status (see Methods). In (B) and (C), taxon relative abundances were centered log ratio transformed and scaled to mean 0, standard deviation 1 before association analysis.
We next tested for associations between milk CMV status and abundances of individual microbial taxa. We modeled 56 microbial species’ abundances in both 1 and 6 month old infants in a linear mixed effects model (see Methods). Abundances of nine taxa were significantly correlated with milk CMV status (q-value<0.05), including six species of Bifidobacterium that were less abundant in the gut metagenomes of infants fed CMV+ milk; Clostridium tertium, which was more abundant in infants fed CMV+ milk; and Bacteroides fragilis, which was less abundant in infants fed CMV+ milk (Figure 4B, Table S10). The taxon with the strongest association with milk CMV status was Bifidobacterium infantis (Beta = −0.45, P = 1.4x10−3, q-value = 0.028; Figure 4C).
Milk CMV status is correlated with infant growth
Finally, we tested if exposure to CMV+ milk was associated with infant growth, measured as weight-for-length Z-score (WLZ), a commonly used nutritional status metric to assess adequacy of weight relative to length and age in infants46. Infants fed CMV+ milk had on average approximately one-third of a Z-score greater weight-for-length at 1 month of age compared to infants fed CMV− milk (Beta = 0.38, P=0.011, N=246; linear regression including WLZ at birth and additional covariates, see Methods; Figure 5A, Table S11). This relationship between WLZ and 1 month milk CMV status was not present at birth or at 6 months of age (Figure 5A, Figure S9A). Infants fed CMV+ milk had somewhat lower mean length-for-age Z-score at 1 month (Beta = −0.27, P=0.025, Figure 5A, Figure S9B), and no difference in weight-for-age Z-score at 1 month (Beta = −0.012, P=0.89, Figure 5A, Figure S9C). These results indicate that infants fed CMV+ milk in the first month of life tended to have weight growth that exceeded their length growth in the first month. However, this difference did not persist to 6 months of age.
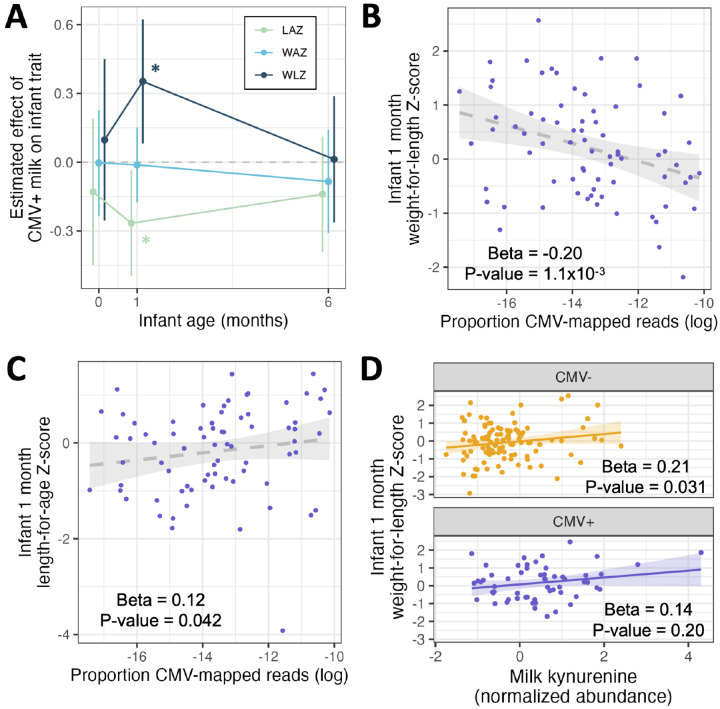
Figure 5.
Results of multivariate regressions of infant anthropometric measurements vs. milk CMV status, proportion CMV-mapped reads in milk, or milk kynurenine. All regression models included the equivalent Z-score at birth as a covariate (except when the Z-score at birth was the response variable). (A) Estimated effect of CMV+ milk on infant growth metrics at birth, 1 month, and 6 months of age. Error bars represent 95% confidence intervals. *P<0.05; LAZ: length-for-age Z-score; WAZ: weight-for-age Z-score; WLZ: weight-for-length Z-score. (B) Within infants fed CMV+ milk, there was a negative correlation between the proportion of CMV-mapped reads and infant WLZ at 1 month of age. (C) Within infants fed CMV+ milk, there was a positive correlation between the proportion of CMV-mapped reads and infant WLZ at 1 month of age. (D) There was a positive correlation between the abundance of kynurenine in milk and infant WLZ at 1 month, when tested for infants fed CMV+ (orange, top) or CMV− (purple, bottom) milk separately. All plotted infant growth metrics in panels B-D are residuals after correcting for covariates included in the association analyses with milk CMV status (see Methods).
Within infants fed CMV+ milk, we observed a negative correlation between the proportion of CMV-mapped reads in milk and infant WLZ at 1 month (Beta = −0.20, P = 1.1x10−3, N=74; Figure 5B), the opposite direction of the relationship when comparing CMV− and CMV+ groups. We also observed a positive correlation between the CMV-mapped read proportion in milk and infant length-for-age at one month (Beta = 0.12, P = 0.042; Figure 5C), and no correlation with infant weight-for-age at one month (Beta = −0.035, P = 0.46; Figure S10). The relationship between milk CMV load and infant growth can be seen in our sensitivity analysis of escalating thresholds to designate milk samples as CMV+: as the threshold increases, only the milk samples with the highest proportion of CMV-mapped reads are designated CMV+, and the effect estimate of CMV+ milk on infant WLZ and milk CMV status reverses direction from positive to negative (Table S2). These results suggest that a factor other than CMV viral load itself is driving the CMV group differences in WLZ at 1 month.
Hypothesizing that the relationship between CMV status and infant growth could be due to CMV-related differences in milk composition, we tested for a relationship between milk kynurenine abundance and infant 1-month WLZ. Kynurenine was positively correlated with WLZ (Beta = 0.21, P = 1.9x10−3; Figure S11), a relationship that persisted when milk CMV status was added as a covariate (Beta = 0.20, P = 0.011). Further, when testing the relationship between milk kynurenine and infant WLZ in CMV+ and CMV− groups separately, there was a positive correlation for both groups; though, it was only significant in the CMV− group (CMV+: Beta = 0.14, P = 0.20; CMV−: Beta = 0.24, P = 0.031; Figure 5D). Within infants fed CMV+ milk, when including both milk kynurenine and the proportion of CMV-mapped reads in milk, both terms were correlated with infant WLZ in opposing directions (kynurenine: beta = 0.22, P = 0.047; CMV read proportion: beta = −0.15, P = 0.011). Given that (1) accounting for milk kynurenine levels removes the association between milk CMV status and infant WLZ at one month; and (2) CMV viral load is correlated with WLZ in the opposite direction as milk CMV status, even when including milk kynurenine levels; we conclude that increased kynurenine in CMV+ milk samples, or a correlated factor, is responsible for the positive association between milk CMV status and infant WLZ at 1 month.
Discussion
In this study, we found that the presence of CMV DNA in human milk is associated with milk gene expression and metabolite abundances, altered composition of the infant gut microbiome, and potential disruptions to infant growth in the first month of life. Notably, our study utilized a cohort of healthy, full-term infants in the U.S.; a population where the impact of CMV presence in breast milk or postnatal CMV transmission was largely thought to be negligible.
We utilized shotgun DNA sequencing from the cell pellet of human milk to identify samples with the presence of CMV at 1 month postpartum. Our study demonstrates that non targeted DNA sequencing of human milk can be used to identify CMV+ samples. We identified CMV DNA in 32% of 1 month milk samples, which is lower than the estimated prevalence for US adults of childbearing age (~50%)32,47. Given that virtually all seropositive women will have CMV reactivation in the mammary gland during lactation48, and CMV viral loads are estimated to peak around 4-6 weeks postpartum5,8, we likely were unable to detect CMV in some samples with a low viral load. We also acknowledge that while viral reactivation during lactation is likely the primary cause of CMV DNA in breast milk, CMV could also be shed through breast milk in the context of primary infections or re-infections occurring late in gestation.
Using complementary milk RNA sequencing and metabolomics approaches, we identified an upregulation of the IDO1 tryptophan-to-kynurenine metabolic pathway in CMV+ milk samples. This pathway has previously been implicated in the immune response to CMV in studies of human cells and primary tissues49,50, suggesting this association may be a response to mammary CMV reactivation. Additionally, one study found that providing kynurenine to human fibroblasts promoted CMV replication, and blocking IDO1 decreased CMV replication51. Given our observational study design, we cannot determine if the association with increased IDO1/kynurenine is a cause or consequence of mammary CMV reactivation. Overall, the impact of CMV on milk composition was notably narrow, with a handful of genes and two metabolites differentially abundant between CMV+ and CMV− milk samples.
Under conditions of chronic viral infection, activation of the IDO pathway can lead to a more tolerogenic immune state49, but the impact of elevated milk kynurenine and its metabolites on the infant is unknown. Kynurenine induction of the aryl hydrocarbon receptor (AHR) can cause immunosuppression via generation of regulatory T-cells52, and AHR activation may protect against necrotizing enterocolitis and inflammation in the infant gut53,54. Whether kynurenine metabolites in milk are at high enough concentrations to have physiological effects in the infant, and the potential impacts of CMV on this pathway, are possible areas of future investigation. We observed a positive association between milk kynurenine and infant growth at 1 month, with higher milk kynurenine correlated with lower length-for-age and greater weight-for-length Z-scores, suggesting milk kynurenine levels could impact growth in early life independent of CMV status. It is important to note that while the impact of kynurenine on weight-for-length was of moderate effect statistically, differences of this magnitude are not generally of clinical significance for healthy term infants.
We also observed that within infants fed CMV+ milk, higher CMV-mapped read proportion (as a proxy for viral load) was negatively correlated with infant weight-for-length and positively correlated with length-for-age at 1 month of age. Previous research on the impact of postnatal CMV transmission on infant growth has primarily focused on two contexts: (1) very low birth weight infants in the NICU setting, and (2) in perinatally HIV-exposed but uninfected infants. Studies focused on very low birth weight infants have found mixed evidence for impacts of postnatal CMV on anthropometric measures55. The largest study to date in very low birth weight infants found that postnatal CMV acquisition was associated with lower weight-for-age Z-score at discharge, but no difference in length-for-age in a U.S. population56. In HIV-exposed but uninfected Malawian infants, breast milk CMV DNA load was negatively correlated with infant weight-for-length, length-for-age, and weight-for-age at 6 months (infant CMV status was unknown in this study)57. In addition, a study of Zambian infants found that postnatal CMV acquisition was associated with lower length-for-age Z-score at 18 months in both HIV-exposed and HIV-unexposed infants, but no difference in weight-for-age by CMV status58. The context of our cohort is quite different from these previous analyses, yet cumulatively, these studies suggest that postnatal exposure and/or acquisition of CMV can impact infant growth.
We observed that exposure to CMV+ milk was associated with the composition of the infant gut microbiome in our cohort of breastfed babies. Specifically, CMV+ milk-exposed infants had lower abundances of Bifidobacterium species and higher abundances of Clostridium tertium. Lower Bifidobacterium, particularly B. infantis, in the infant gut microbiome is associated with adverse health outcomes59-61. C. tertium has been reported as potentially pathogenic in the infant gut62,63. Notably, milk kynurenine was not associated with the infant gut microbiome in our study, indicating that the potential effects of CMV viral load on infant growth and the infant gut microbiome may act through distinct pathways. A previous study by Sbihi et al. examined the impact of CMV acquisition on the infant gut microbiome. In a population-based birth cohort, early CMV acquisition (in the first 3 months of life) but not later CMV acquisition (between 3-12 months) was associated with lower alpha diversity (i.e. within-sample diversity)64. While our study is not directly comparable as we do not know infant CMV status, we did not observe a significant difference in alpha diversity in CMV-exposed vs. unexposed infants. Sbihi et al. also observed increased incidence of childhood allergy with early CMV acquisition64, a phenotype not currently assessed in our cohort.
A limitation of our study is the unknown serostatus of the infants at birth and subsequent timepoints, as infant blood samples were not available. As previous studies estimate up to 70% of breastfed term babies of seropositive mothers acquire CMV postnatally12-14, it is possible that a substantial fraction of the babies fed CMV+ milk in our study had postnatally acquired CMV by 1 month of age. The infants in our study were also not tested for congenital CMV, which has a prevalence of about 4.5 per 1000 births in the US65,66 and is often asymptomatic and undetected67. Further studies are required to characterize the impacts of CMV+ milk on growth and the gut microbiome in infants with and without CMV transmission.
While there is growing awareness and understanding of the negative impacts of breastmilk-acquired CMV in preterm infants10, it is generally thought to be benign in healthy term infants. Some have even speculated that there may be an evolutionary advantage to postnatal CMV acquisition, in the form of a ‘natural immunization’ or other immune-boosting effect for the infant12. We find that exposure to CMV+ milk is associated with reduction in beneficial microbes in the infant gut. Given the high prevalence of CMV globally, impacts on infant microbiome development could have a substantial impact at the population level. This study highlights not only these CMV-related changes but also more generally, how ‘normal’ variation in human milk impacts healthy infant development.
Materials and Methods
Description of study population
This study made use of existing data from the Mothers and Infants LinKed for Healthy Growth (MILK) study. Recruitment protocols and study characteristics have previously been extensively described15,45,68-71. This study recruited mothers intending to exclusively breastfeed their infants prenatally. Study visits occurred at two sites: the University of Minnesota (MN) or the University of Oklahoma Health Sciences Center (OK). All included infants were born at full term. The milk samples utilized in this manuscript were collected during a study visit about 1 month postpartum via a full breast milk expression two hours after a complete infant feed. Expressed milk volume and weight was recorded, milk was gently mixed, aliquots were made, and then stored at −80°C within 20 minutes of collection and kept at −80°C until thawed for RNA/DNA extraction or metabolomics analysis.
Human milk RNA extraction, sequencing, and gene expression quantification
The human milk RNA extraction protocol, sequencing, and gene expression quantifications used in this study have been previously described15. RNA extraction, library preparation, and sequencing was performed at the University of Minnesota Genomics Center (UMGC). Briefly, bulk RNA was extracted from the whole milk cell pellet to profile gene expression of all cell types present in the milk sample. RNA was extracted from the cell pellet using the RNeasy Plus Universal HTP following the manufacturer’s instructions. RNA libraries were prepared with the TakaraBio Stranded Total RNA Pico Mammalian kit and sequenced on an Illumina NovaSeq 6000 S2 flow cell with 2x150 paired-end reads in two pools. Gene-level quantifications were generated using RNA-SeQC v2.3.472.
Analyses with publicly available single cell RNA-seq data from human milk
Raw gene counts (MIT_Milk_Study_Raw_counts.txt.gz) and metadata (MIT_milk_study_metadata.csv.gz) were downloaded for the Nyquist et al. study73 from the Broad Insitute Single Cell Portal (https://singlecell.broadinstitute.org/single_cell/study/SCP1671/cellular-and-transcriptional-diversity-over-the-course-of-human-lactation) on 6/3/2022. Gene counts for each cell were scaled to log(x/s + 1), where x was the gene count in a cell and s was a scaling factor. s was calculated as the total counts per cell divided by the mean of total counts across all cells. For each of the 36 differentially expressed genes in our CMV+ milk samples, the scaled expression for each cell type was calculated as the mean scaled expression across all cells of that cell type, divided by the gene’s mean scaled expression in the cell type with the highest mean expression. In Figure 2C, immune cell expression included six cell types (T cells, eosinophils, dendritic cells, B cells, neutrophils, macrophages) and mammary luminal cell expression included two cell types (luminal cell 1 and luminal cell 2).
Cell type proportions were estimated for each milk sample with bulk RNA-sequencing data as previously described15, using a publicly available single cell RNA sequencing dataset from human milk36 cells and Bisque74. Proportions of 8 cell types were estimated: two types of mammary epithelial cells (luminal cell 1, luminal cell 2) and six immune cell types (T cells, eosinophils, dendritic cells, B cells, neutrophils, macrophages). The estimated immune cell proportion was calculated as the sum of the six immune cell types.
Human milk DNA extraction and sequencing
DNA was extracted and sequenced from human milk using separate protocols for different initial applications:
Human low-pass whole genome sequencing (WGS): The DNA extraction protocol and sequencing for this application has been previously described15. In brief, DNA was extracted from the cell pellet at UMGC with the QIAamp 96 DNA Blood Kit, and sequenced by Gencove, Inc. for target sequencing depth of ~1x for the human genome.
Shotgun metagenomic sequencing (SMS): DNA extraction and sequencing from milk samples for this application has also been previously described15,45. DNA was extracted using the PowerSoil kit, libraries constructed for metagenomics sequencing using the Illumina Nextera XT kit, and sequenced on an Illumina NovaSeq system using the S4 flow cell with the 2x150 bp paired end V4 chemistry kit at UMGC.
Identification of CMV-positive milk samples
We mapped DNA sequencing reads generated from human milk with the above two approaches to the human cytomegalovirus genome to identify milk samples with CMV DNA. Starting with the WGS DNA reads, we mapped the reads from each milk sample to seven CMV genome isolates from human milk 75 accessed from NCBI Genbank (https://www.ncbi.nlm.nih.gov/genbank/, MW528458 – MW528464) using Bowtie276. Finding that the number of aligned reads across reference CMV isolates was in strong agreement, we continued with the aligned read count for each sample from isolate BM1 (accession MW528458) for reads from both WGS and SMS. We called milk samples as CMV+ if they had at least one concordantly mapped read pair with MAPQ>5 from either WGS or SMS. Of the 276 milk samples utilized in this study, 86 had both WGS and SMS (n=34 CMV+), 132 only had WGS (n=40 CMV+), and 58 had only SMS (n=22 CMV+). The proportion of CMV-mapped reads was calculated for each CMV+ sample as the number of reads mapped to the CMV genome divided by the total number of sequencing reads, with counts from SMS and WGS data summed if both were available.
Identification of differentially expressed genes by milk CMV status
Differential gene expression analysis between CMV− and CMV+ milk samples was performed in DESeq277 using the gene-level read count matrix generated with RNA-SeQC72. 17,675 genes were included in differential gene expression analysis. Maternal age, maternal pre-pregnancy BMI, maternal self-reported race, maternal parity, infant age in days, sample RIN, RNA sequencing pool, and the mass RNA extracted from the sample were included as covariates. None of the individuals with transcriptomic data had gestational diabetes, so this was not included as a covariate. P-values were adjusted for multiple tests using the default Benjamini and Hochberg method in DESeq277,78. Enrichment analysis of upregulated genes was performed with EnrichR79, using “GO_Biological_Process_2021” as the reference gene ontology. To test for a correlation between CMV-mapped read proportion and gene expression, the same DESeq2 model was used, replacing CMV status with the CMV-mapped read proportion (logged and scaled to mean 0, s.d. 1) and including only CMV+ samples.
Human milk metabolomics and identification of differentially abundant metabolites
Samples for milk metabolomics were prepared and analyzed as previously described80 from frozen milk samples at BERG health (Framingham, MA). For each of 458 metabolites, the association between metabolite abundance and milk CMV status was estimated using a multivariate regression with ‘lm’ in R. Metabolite abundances were log(x+1) transformed and scaled to mean 0, standard deviation 1. Additional included covariates were the study center (MN vs. OK), parity, maternal age, maternal pre-pregnancy BMI, maternal gestational diabetes (yes/no), maternal self-reported race (white vs. non-white) and maternal Healthy Eating Index total score81 (averaged from three timepoints: prenatal, 1 month postpartum, and 3 months postpartum). P-values were corrected for multiple tests using the Benjamini-Hochberg false discovery rate78 with ‘p.adjust’ in R. To test for a correlation between CMV-mapped read proportion and metabolite abundance, the same multivariate model was used, replacing CMV status with the CMV-mapped read proportion (logged and scaled to mean 0, s.d. 1) and including only CMV+ samples.
Infant fecal metagenomics and comparison with milk CMV status
Infant fecal sample collection, DNA extraction, metagenomic sequencing, and estimation of microbial taxon and pathway abundances from 1 and 6 month samples has been previously described15,45. Principal components analysis of 1 and 6 month infant metagenomes, summarized as taxon or pathway abundances, was performed separately. Data were filtered to include only taxa/pathways with relative abundance >0.001 in at least 10% of 1-month or 6-month samples. A centered log-ratio transformation was performed on the relative abundances of each sample, and principal components were calculated with the ‘prcomp’ command in R. Associations between the metagenomic PCs that explained at least 5% of the variance in the data (5 PCs each for 1 and 6 month taxa abundances, 3 PCs each for pathway abundances at 1 and 6 months) and milk CMV status were calculated using linear regression with the ‘glm’ command in R. Infant delivery mode (cesarean vs. vaginal), maternal parity, maternal age, maternal self-identified race, maternal pre-pregnancy BMI, maternal gestational diabetes (yes/no), maternal Group B streptococcus status, fecal sample collection site (home vs. study visit), and maternal Healthy Eating Index total score81 (averaged from three timepoints: prenatal, 1 month postpartum, and 3 months postpartum) were included as covariates. Two additional covariates were included in the regression models for 6 month infant fecal samples: exclusive breastfeeding status at 6 months (yes/no), and if complementary foods had been introduced at 6 months (yes/no). At 1 month, all infants were exclusively breastfed with no complementary foods. Additional variables about antibiotics use were not included (beyond Group B Streptococcus status, which is treated with antibiotics during labor) because there was too much missing data that would vastly reduce the sample size for these analyses.
Alpha diversity was calculated for each infant fecal sample from 1 or 6 months with the inverse Simpson index with the unfiltered taxon count matrix using the vegan82 package in R. Alpha diversity was scaled to mean 0, s.d. 1 and tested for association with milk CMV status in a multivariate regression model including the same covariates described above for the microbiome PCs.
Associations between individual taxon abundances and milk CMV status were estimated using a linear mixed effects model with the ‘lmerTest’ package83 in R. Using taxon abundances (centered log-transformed and scaled to mean 0, standard deviation 1 within each timepoint) from both 1 and 6 month timepoints as the response variable; fixed effects variables were milk CMV status, sample time point (1 or 6 months, coded as 0 or 1), infant delivery mode (cesarean or vaginal), maternal parity, maternal self-reported race, maternal pre-pregnancy BMI, maternal Group B streptococcus status, fecal sample collection site (home vs. study visit), maternal gestational diabetes (yes/no), and exclusive breastfeeding status at 6 months; and the mother-infant pair ID was included as a random variable. Only species-level taxa with relative abundance >0.001 in at least 10% of samples in both 1 and 6 month samples were included (56 species). P-values were corrected using the Benjamini-Hochberg false discovery rate with ‘p.adjust’ in R.
Infant growth measurement and comparison with milk CMV status
Infant growth measurements and Z-score calculation from this cohort have been previously described70,84. Age and sex-specific length-for-age, weight-for-age, and weight-for-length Z-scores (WLZ) were calculated using the World Health Organization standards for term infants46. Association between infant 1-month WLZ and milk CMV status was calculated in a regression model including WLZ at birth, infant race (parental report), maternal pre-pregnancy BMI, maternal gestational diabetes (yes/no), household income, and delivery mode (cesarean vs. vaginal) as covariates with the ‘lm’ command in R. Associations between milk CMV status and 3- and 6-month WLZ were calculated in the same model, replacing the outcome (1-month WLZ) with the 3- or 6-month WLZ. Associations with length-for-age or weight-for age Z-scores used the same covariates, replacing WLZ at birth with the respective Z-score at birth. To test for a correlation between CMV-mapped read proportion and WLZ, CMV status was replaced in the model with the CMV-mapped read proportion (logged and scaled to mean 0, s.d. 1) and including only CMV+ samples.
Supplementary Material
Acknowledgements
We would like to thank Katy Duncan, Laurie Foster, Tipper Gallagher, and all MILk study staff and participants for their contributions, and members of the Albert and Blekhman labs for helpful discussions related to this project. This work was supported by the resources and staff at the University of Minnesota Genomics Center (https://genomics.umn.edu). This work was carried out in part by resources provided by the Minnesota Supercomputing Institute (https://www.msi.umn.edu/).
Funding
This study was supported by a University of Minnesota Department of Pediatrics Masonic Cross-Departmental Research Grant (FWA, RB, EWD, CAG), University of Minnesota Masonic Children’s Hospital Research Fund Award (CAG, EWD, and DK), NIH/NICHD grant R01HD109830 (RB, EWD, CAG), NIH/NICHD grant R21HD099473 (CAG), and a University of Minnesota Office of Academic and Clinical Affairs Faculty Research Development Grant (CAG, EWD, KMJ, and DK). The MILK Study which provided the cohort and milk samples for this study was supported by NIH/NICHD grant R01HD080444 (EWD and DAF). KEJ was supported by NIH/NICHD F32HD105364 and NIH/NIDCR T90DE0227232.
Footnotes
Declaration of interests
The authors declare no competing interests.
Data availability
Milk metabolite abundances, gene expression matrices, and microbial abundance tables are available as extended data tables (see descriptions in supplementary material). Raw sequencing data will be available at dbGaP prior to publication.
References
- 1.Zuhair M. et al. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 29, e2034 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Sinzger C. et al. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J. Gen. Virol. 76 (Pt 4), 741–750 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Hamprecht K. & Goelz R. Postnatal Cytomegalovirus Infection Through Human Milk in Preterm Infants: Transmission, Clinical Presentation, and Prevention. Clin. Perinatol. 44, 121–130 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Hamprecht K., Witzel S., Maschmann J., Speer C. P. & Jahn G. Transmission of Cytomegalovirus Infection Through Breast Milk in Term and Pretern Infants. in Short and Long Term Effects of Breast Feeding on Child Health (eds. Koletzko B., Michaelsen K. F. & Hernell O.) 231–239 (Springer US, 2002). [Google Scholar]
- 5.Lazar K., Rabe T., Goelz R. & Hamprecht K. Human Cytomegalovirus Reactivation During Lactation: Impact of Antibody Kinetics and Neutralization in Blood and Breast Milk. Nutrients 12, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meier J. et al. Human cytomegalovirus reactivation during lactation and mother-to-child transmission in preterm infants. J. Clin. Microbiol. 43, 1318–1324 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maschmann J. et al. Characterization of human breast milk leukocytes and their potential role in cytomegalovirus transmission to newborns. Neonatology 107, 213–219 (2015). [DOI] [PubMed] [Google Scholar]
- 8.Hamprecht K. et al. Rapid detection and quantification of cell free cytomegalovirus by a high-speed centrifugation-based microculture assay: comparison to longitudinally analyzed viral DNA load and pp67 late transcript during lactation. J. Clin. Virol. 28, 303–316 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Hamprecht K. et al. Detection of cytomegaloviral DNA in human milk cells and cell free milk whey by nested PCR. J. Virol. Methods 70, 167–176 (1998). [DOI] [PubMed] [Google Scholar]
- 10.Osterholm E. A. & Schleiss M. R. Impact of breast milk-acquired cytomegalovirus infection in premature infants: Pathogenesis, prevention, and clinical consequences? Rev. Med. Virol. 30, 1–11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanzieri T. M., Dollard S. C., Josephson C. D., Schmid D. S. & Bialek S. R. Breast milk-acquired cytomegalovirus infection and disease in VLBW and premature infants. Pediatrics 131, e1937–45 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stagno S., Reynolds D. W., Pass R. F. & Alford C. A. Breast milk and the risk of cytomegalovirus infection. N. Engl. J. Med. 302, 1073–1076 (1980). [DOI] [PubMed] [Google Scholar]
- 13.Dworsky M., Yow M., Stagno S., Pass R. F. & Alford C. Cytomegalovirus infection of breast milk and transmission in infancy. Pediatrics 72, 295–299 (1983). [PubMed] [Google Scholar]
- 14.Minamishima I. et al. Role of breast milk in acquisition of cytomegalovirus infection. Microbiol. Immunol. 38, 549–552 (1994). [DOI] [PubMed] [Google Scholar]
- 15.Johnson K. E. et al. Human milk variation is shaped by maternal genetics and impacts the infant gut microbiome. bioRxiv 2023.01.24.525211 (2023) doi: 10.1101/2023.01.24.525211. [DOI] [PubMed] [Google Scholar]
- 16.Garwolińska D., Namieśnik J., Kot-Wasik A. & Hewelt-Belka W. Chemistry of Human Breast Milk-A Comprehensive Review of the Composition and Role of Milk Metabolites in Child Development. J. Agric. Food Chem. 66, 11881–11896 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Järvinen K. M. Variations in Human Milk Composition: Impact on Immune Development and Allergic Disease Susceptibility. Breastfeed. Med. 13, S11–S13 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Neu J. & Walker W. A. Necrotizing enterocolitis. N. Engl. J. Med. 364, 255–264 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shnayder M. et al. Defining the Transcriptional Landscape during Cytomegalovirus Latency with Single-Cell RNA Sequencing. MBio 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tirosh O. et al. The Transcription and Translation Landscapes during Human Cytomegalovirus Infection Reveal Novel Host-Pathogen Interactions. PLoS Pathog. 11, e1005288 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahn R. et al. Acute and Chronic Changes in Gene Expression After CMV DNAemia in Kidney Transplant Recipients. Front. Immunol. 12, 750659 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hein M. Y. & Weissman J. S. Functional single-cell genomics of human cytomegalovirus infection. Nat. Biotechnol. 40, 391–401 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Hertel L. & Mocarski E. S. Global analysis of host cell gene expression late during cytomegalovirus infection reveals extensive dysregulation of cell cycle gene expression and induction of Pseudomitosis independent of US28 function. J. Virol. 78, 11988–12011 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcinowski L. et al. Real-time transcriptional profiling of cellular and viral gene expression during lytic cytomegalovirus infection. PLoS Pathog. 8, e1002908 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munger J., Bajad S. U., Coller H. A., Shenk T. & Rabinowitz J. D. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog. 2, e132 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vastag L., Koyuncu E., Grady S. L., Shenk T. E. & Rabinowitz J. D. Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog. 7, e1002124 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fanos V. et al. Urinary metabolomics in newborns infected by human cytomegalovirus: a preliminary investigation. Early Hum. Dev. 89 Suppl 1, S58–61 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Lemay D. G. et al. RNA sequencing of the human milk fat layer transcriptome reveals distinct gene expression profiles at three stages of lactation. PLoS One 8, e67531 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lemay D. G. et al. Sequencing the transcriptome of milk production: milk trumps mammary tissue. BMC Genomics 14, 872 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poulsen K. O. & Sundekilde U. K. The Metabolomic Analysis of Human Milk Offers Unique Insights into Potential Child Health Benefits. Curr. Nutr. Rep. 10, 12–29 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Dolan A. et al. Genetic content of wild-type human cytomegalovirus. J. Gen. Virol. 85, 1301–1312 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Cannon M. J., Schmid D. S. & Hyde T. B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 20, 202–213 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Mentzer A. J. et al. Identification of host-pathogen-disease relationships using a scalable multiplex serology platform in UK Biobank. Nat. Commun. 13, 1818 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lantos P. M., Permar S. R., Hoffman K. & Swamy G. K. The Excess Burden of Cytomegalovirus in African American Communities: A Geospatial Analysis. Open Forum Infect Dis 2, ofv180 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Twigger A.-J. et al. Transcriptional changes in the mammary gland during lactation revealed by single cell sequencing of cells from human milk. Nat. Commun. 13, 562 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nyquist S. K. et al. Cellular and transcriptional diversity over the course of human lactation. Proc. Natl. Acad. Sci. U. S. A. 119, e2121720119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gleeson J. P. et al. Profiling of mature-stage human breast milk cells identifies six unique lactocyte subpopulations. Sci Adv 8, eabm6865 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin Carli J. F. et al. Single Cell RNA Sequencing of Human Milk-Derived Cells Reveals Sub-Populations of Mammary Epithelial Cells with Molecular Signatures of Progenitor and Mature States: a Novel, Non-invasive Framework for Investigating Human Lactation Physiology. J. Mammary Gland Biol. Neoplasia (2020) doi: 10.1007/s10911-020-09466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lueder Y. et al. Control of primary mouse cytomegalovirus infection in lung nodular inflammatory foci by cooperation of interferon-gamma expressing CD4 and CD8 T cells. PLoS Pathog. 14, e1007252 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moylan D. C. et al. Breast Milk Human Cytomegalovirus (CMV) Viral Load and the Establishment of Breast Milk CMV-pp65-Specific CD8 T Cells in Human CMV Infected Mothers. J. Infect. Dis. 216, 1176–1179 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazar K. et al. Immunomonitoring of Human Breast Milk Cells During HCMV-Reactivation. Front. Immunol. 12, 723010 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ojo-Okunola A., Cacciatore S., Nicol M. P. & du Toit E. The Determinants of the Human Milk Metabolome and Its Role in Infant Health. Metabolites 10, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pace R. M. et al. Variation in Human Milk Composition Is Related to Differences in Milk and Infant Fecal Microbial Communities. Microorganisms 9, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kijner S., Kolodny O. & Yassour M. Human milk oligosaccharides and the infant gut microbiome from an eco-evolutionary perspective. Curr. Opin. Microbiol. 68, 102156 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Heisel T. et al. Bacterial, fungal, and interkingdom microbiome features of exclusively breastfeeding dyads are associated with infant age, antibiotic exposure, and birth mode. Front. Microbiol. 13, 1050574 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WHO MULTICENTRE GROWTH REFERENCE STUDY GROUP & de Onis M. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. 95, 76–85 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Bate S. L., Dollard S. C. & Cannon M. J. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin. Infect. Dis. 50, 1439–1447 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamprecht K. et al. Epidemiology of transmission of cytomegalovirus from mother to preterm infant by breastfeeding. Lancet 357, 513–518 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Mehraj V. & Routy J.-P. Tryptophan Catabolism in Chronic Viral Infections: Handling Uninvited Guests. Int. J. Tryptophan Res. 8, 41–48 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sadeghi M. et al. Strong association of phenylalanine and tryptophan metabolites with activated cytomegalovirus infection in kidney transplant recipients. Hum. Immunol. 73, 186–192 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Wise L. M., Xi Y. & Purdy J. G. Hypoxia-Inducible Factor 1α (HIF1α) Suppresses Virus Replication in Human Cytomegalovirus Infection by Limiting Kynurenine Synthesis. MBio 12, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mezrich J. D. et al. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 185, 3190–3198 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lu P. et al. Maternal aryl hydrocarbon receptor activation protects newborns against necrotizing enterocolitis. Nat. Commun. 12, 1042 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meng D. et al. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr. Res. 88, 209–217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stark A., Cantrell S., Greenberg R. G., Permar S. R. & Weimer K. E. D. Long-term Outcomes after Postnatal Cytomegalovirus Infection in Low Birthweight Preterm Infants: A Systematic Review. Pediatr. Infect. Dis. J. 40, 571–581 (2021). [DOI] [PubMed] [Google Scholar]
- 56.Weimer K. E. D., Kelly M. S., Permar S. R., Clark R. H. & Greenberg R. G. Association of Adverse Hearing, Growth, and Discharge Age Outcomes With Postnatal Cytomegalovirus Infection in Infants With Very Low Birth Weight. JAMA Pediatr. 174, 133–140 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meyer S. A. et al. Postnatal cytomegalovirus exposure in infants of antiretroviral-treated and untreated HIV-infected mothers. Infect. Dis. Obstet. Gynecol. 2014, 989721 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gompels U. A. et al. Human cytomegalovirus infant infection adversely affects growth and development in maternally HIV-exposed and unexposed infants in Zambia. Clin. Infect. Dis. 54, 434–442 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramani S. et al. Human milk oligosaccharides, milk microbiome and infant gut microbiome modulate neonatal rotavirus infection. Nat. Commun. 9, 5010 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ismail I. H. et al. Early gut colonization by Bifidobacterium breve and B. catenulatum differentially modulates eczema risk in children at high risk of developing allergic disease. Pediatr. Allergy Immunol. 27, 838–846 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Henrick B. M. et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 184, 3884–3898.e11 (2021). [DOI] [PubMed] [Google Scholar]
- 62.Kiu R. et al. Preterm Infant-Associated Clostridium tertium, Clostridium cadaveris, and Clostridium paraputrificum Strains: Genomic and Evolutionary Insights. Genome Biol. Evol. 9, 2707–2714 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheah F. C., Lim K. E. & Boo N. Y. Clostridium tertium in cerebrospinal fluid of a premature neonate with necrotizing enterocolitis: contamination or real? Acta Paediatr. 90, 704–705 (2001). [PubMed] [Google Scholar]
- 64.Sbihi H. et al. Early-life cytomegalovirus infection is associated with gut microbiota perturbations and increased risk of atopy. Pediatr. Allergy Immunol. 33, e13658 (2022). [DOI] [PubMed] [Google Scholar]
- 65.Dollard S. C. et al. Sensitivity of Dried Blood Spot Testing for Detection of Congenital Cytomegalovirus Infection. JAMA Pediatr. 175, e205441 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fowler K. B. et al. Racial and Ethnic Differences in the Prevalence of Congenital Cytomegalovirus Infection. J. Pediatr. 200, 196–201.e1 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Kenneson A. & Cannon M. J. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 17, 253–276 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Isganaitis E. et al. Maternal obesity and the human milk metabolome: associations with infant body composition and postnatal weight gain. Am. J. Clin. Nutr. 110, 111–120 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Whitaker K. M. et al. Associations of Maternal Weight Status Before, During, and After Pregnancy with Inflammatory Markers in Breast Milk. Obesity 25, 2092–2099 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fields D. A. et al. Associations between human breast milk hormones and adipocytokines and infant growth and body composition in the first 6 months of life. Pediatr. Obes. 12 Suppl 1, 78–85 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sadr Dadres G. et al. Relationship of Maternal Weight Status Before, During, and After Pregnancy with Breast Milk Hormone Concentrations. Obesity 27, 621–628 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DeLuca D. S. et al. RNA-SeQC: RNA-seq metrics for quality control and process optimization. Bioinformatics 28, 1530–1532 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nyquist S. K. et al. Cellular and transcriptional diversity over the course of human lactation. bioRxiv 2021.11.13.468496 (2021) doi: 10.1101/2021.11.13.468496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jew B. et al. Accurate estimation of cell composition in bulk expression through robust integration of single-cell information. Nat. Commun. 11, 1971 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Götting J. et al. Human Cytomegalovirus Genome Diversity in Longitudinally Collected Breast Milk Samples. Front. Cell. Infect. Microbiol. 11, 664247 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Langmead B. & Salzberg S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Love M. I., Huber W. & Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benjamini Y. & Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. 57, 289–300 (1995). [Google Scholar]
- 79.Kuleshov M. V. et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 44, W90–7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolfs D. et al. Brown Fat-Activating Lipokine 12,13-diHOME in Human Milk Is Associated With Infant Adiposity. J. Clin. Endocrinol. Metab. 106, e943–e956 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Krebs-Smith S. M. et al. Update of the Healthy Eating Index: HEI-2015. J. Acad. Nutr. Diet. 118, 1591–1602 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oksanen J. et al. vegan: Community Ecology Package. Preprint at https://CRAN.R-project.org/package=vegan (2022). [Google Scholar]
- 83.Kuznetsova A., Brockhoff P. B. & Christensen R. H. B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 82, 1–26 (2017). [Google Scholar]
- 84.Tahir M. J. et al. Higher Maternal Diet Quality during Pregnancy and Lactation Is Associated with Lower Infant Weight-For-Length, Body Fat Percent, and Fat Mass in Early Postnatal Life. Nutrients 11, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Milk metabolite abundances, gene expression matrices, and microbial abundance tables are available as extended data tables (see descriptions in supplementary material). Raw sequencing data will be available at dbGaP prior to publication.