Abstract
Background:
Competing priorities make using a transparent and evidence-based approach important when deciding to recommend new vaccines. We conducted a literature review to document the processes and frameworks for national decision-making on new vaccine introductions and explored which key features have evolved since 2010.
Methods:
We searched literature published on policymaking related to vaccine introduction from March 2010 to August 2020 in six databases. We screened articles for eligibility with the following exclusion criteria: non-human or hypothetical vaccines, the sole focus on economic evaluation or decision to adopt rather than policy decision-making. We employed nine broad categories of criteria from the 2012 review for categorization and abstracted data on the country, income level, vaccine, and other relevant criteria.
Results:
Of the 3808 unique references screened, 116 met eligibility criteria and were classified as: a) framework of vaccine adoption decision-making (27), b) studies that analyse empirical data on or examples of vaccine adoption decision-making (45), c) theoretical and empirical articles that provide insights into the vaccine policymaking process (44 + 17 already included in the previous categories). Commonly reported criteria for decision-making were the burden of disease; vaccine efficacy/effectiveness, safety; impact on health and non-health outcomes; economic evaluation and cost-effectiveness of alternative interventions. Programmatic and acceptability aspects were not as often considered. Most (50; 82%) of the 61 articles describing the process of vaccine introduction policymaking highlighted the role of country, regional, or global evidence-informed recommendations and a robust national governance as enabling factors for vaccine adoption.
Conclusions:
The literature on vaccine adoption decision-making has expanded since 2010. We found that policymakers and expert advisory committee members (e.g., National Immunization Technical Advisory Group [NITAG]) increasingly value the interventions based on economic evaluations. The results of this review could guide discussions on evidence-informed immunization decision-making among country, sub-regional, and regional stakeholders.
Keywords: Infectious diseases, Immunization programs, Vaccine policy, Evidence-based decision-making, Health system strengthening, Systematic review
1. Introduction
Competing priorities and limited resources highlight the importance of using a transparent and evidence-based approach by decision-makers to introduce new vaccines into national programs. Under the Global Immunization Vision and Strategy (GIVS), the World Health Organization (WHO) developed guidelines for vaccine introduction, aiming to guide country-level decision-makers [1].
In May 2012, the 194 countries that are members of the United Nations endorsed the Global Vaccine Action Plan, “commit [ting] to immunization as a priority” [2]. The principle of country ownership of immunization programs was defined as having primary ownership of and responsibility for establishing good governance and providing effective and quality immunization services for all. For more than 10 years, WHO has been recommending that countries establish national immunization technical advisory groups (NITAG) or equivalent independent expert advisory groups as a way to improve quality and ownership of national immunization programs [3].
Burchett et al. (2012) conducted a systematic review of the literature until 2010, identified a total of 85 studies, and outlined the processes and frameworks used by the countries for national decision-making when adopting new vaccines [4]. They found a paucity of published literature describing the actual process of decision-making for vaccine adoption. Ten years after Burchett et al. (2012) review, we anticipated that the landscape had changed knowing the breadth of vaccines that have entered the market over the same period. Building on Burchett et al. (2012) findings, we conducted a systematic review, as defined in the PRISMA statement [5], to document the evolution of criteria that affect vaccine policy decisions since 2010 and pinpoint the enabling factors for vaccine policymaking.
2. Methods
2.1. Search methods
A search strategy was developed and used in MEDLINE. It included both keyword and free text (title and abstract) terms relating to ‘vaccines’ or ‘immunization’ AND ‘policy’ or ‘policy making’ AND ‘introduction’ or ‘adoption’ from March 2010 to June 2017 with an additional update in August 2020 (Appendix 1). This strategy was then adapted for use in the additional five databases: EMBASE, Global Health, Cochrane Library, CINAHL, and Scopus. The searches did not include any language limitations although they were conducted in English only. Abstracts and full text articles were screened, and data were abstracted in English.
2.2. Data collection
Two reviewers for the March 2010 to June 2017 literature search and one reviewer for the June 2017 to August 2020 literature search independently screened titles and abstracts of the references and assessed the full-text articles for eligibility, using the following exclusion criteria: 1) Did not focus on human vaccination; 2) Did not focus on policy decision-making around vaccine adoption (i.e., considered decision-making at the clinical, individual level, or focused on implementation issues only); 3) Focused on hypothetical vaccines (e.g., HIV vaccine) and did not present a framework for decision-making (i.e., a hypothetical study would only be included if it presented a decision-making framework); 4) Did not consider factors that directly affected decisions (e.g. assessed which macro-level factors, such as the Gross Domestic Product (GDP) were associated with decisions to adopt, rather than focusing on the actual criteria considered within decision-making processes); 5) Focused on the economic evaluation of introducing a new vaccine and did not present any other factors that affected decisions; 6) Was published in a language other than English, Spanish, or French.
We classified the articles identified into the following groups based on the type of article: 1) Articles that present a framework of decision-making for vaccine adoption (article type 1); 2) Studies that collect or analyze empirical data on decision-making for vaccine adoption and relevant examples of such decision-making (article type 2); and 3) Theoretical and empirical articles that provide insights into the process of vaccine policymaking (article type 3) (Appendix 2). Burchett et al (2012) found the published literature describing the process of decision-making for vaccine adoption to be limited. Thus, we specifically added article type 3 to our systematic review. Any disagreement between reviewers in the processes of screening and classification was discussed and resolved through consensus.
2.3. Data abstraction
We abstracted data based on the type of article, the country income level and Gavi, the Vaccine Alliance (Gavi) eligibility status, the type of vaccine, and the categories and criteria included in the decision-making frameworks or mentioned in the studies. Country income levels were defined, using the 2018 World Bank definitions, based on country Gross National Income (GNI) per capita and the World Bank Atlas method3 [6]. Gavi-eligible countries were defined as those that were eligible for Gavi funding in Phase 3, commencing in 2010, which includes 74 countries globally [7]. The eligibility threshold was defined as country GNI per capita ≤ US$ 1000, based on 1998 World Bank data. Data abstraction for article type 1 and 2 was conducted by the same reviewer. Another reviewer abstracted article type 3.
We performed a quality appraisal of the studies that collected or analyzed empirical data on decision-making for vaccine adoption (Article type 2), using the Mixed Methods Appraisal Tool (MMAT) (Appendix 3). This tool was chosen because many different study designs were used across the studies [8]. Answers to the two initial screening questions determined whether the article could be appraised using the MMAT. The MMAT score ranges from 0 to 100% and is based on meeting certain methodological quality criteria, depending on the type of study (i.e., quantitative, qualitative, or mixed methods). Articles that passed the two screening questions with a quality score of ≥ 25% were included in the analysis.
2.4. Data analysis
We used the nine broad categories of criteria that Burchett et al. 2012 identified in their analysis [4]. The categories were: the importance of the health problem, vaccine characteristics, programmatic considerations, acceptability, accessibility, equity and ethics, financial/economic issues, the impact of vaccination, consideration of alternative interventions, and the decision-making process. When analyzing the studies conducted on decision-making and examples of vaccine adoption decision-making (Article type 2), we focused on obtaining a more robust database and compared the criteria identified in these articles to those described in the frameworks (Article type 1). To analyze Type 3 articles, one reviewer abstracted the main themes or factors that the authors mentioned as being important in facilitating successful vaccine policymaking in a specific setting (i.e., country).
3. Results
The searches identified a total of 5,621 references, of which 1,813 were duplicates, leaving 3,808 unique references (Fig. 1). Table 1 lists the number of articles excluded based on each of the exclusion criterion. Of the 3,808 unique references, most articles were excluded because they did not focus on decision-making for national adoption. Of the 520 full-text articles assessed for eligibility, 116 were selected for our review (27 frameworks that were used for immunization-related decision-making, 45 studies that collected or analyzed empirical data on vaccine adoption decision-making and examples of vaccine adoption decision-making, and 44 theoretical and empirical articles provided insights into the vaccine policymaking process) (Appendix 4). Seventeen references included in the article Types 1 and 2 provided insights into the policymaking process; therefore, their findings were also used to document the enabling factors to vaccine policymaking (Type 3 articles), providing for a total of 61 articles in that group.
Fig. 1.
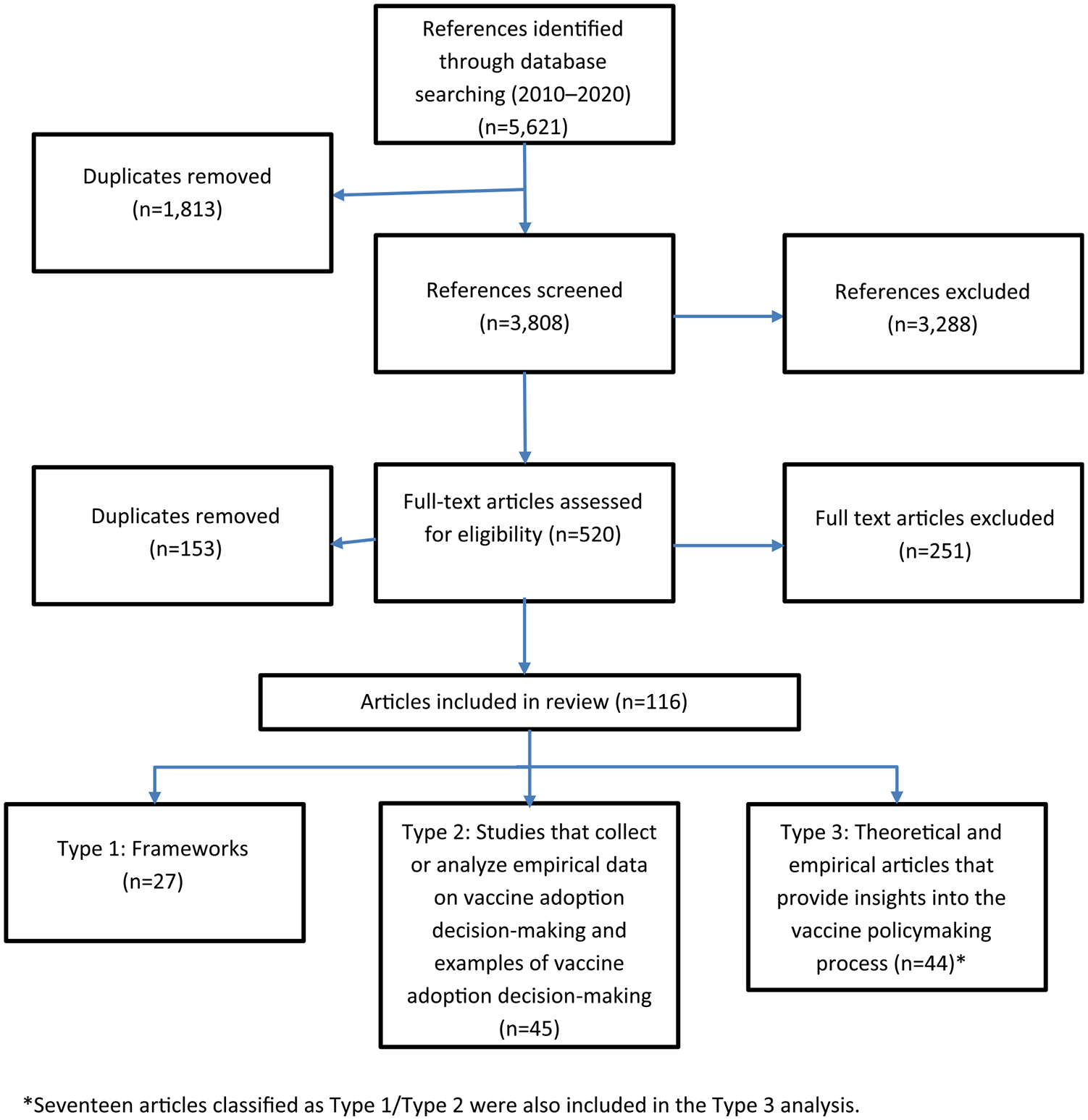
Flow chart of the systematic review of national decision-making 2010–2020.
Table 1.
Reasons for excluding articles for the systematic review, 2010–2020.
| Exclusion criteria | Number of references |
|---|---|
| Did not focus on human vaccination | 83 |
| Did not focus on decision-making for national vaccine introduction | 3,005 |
| Focused on hypothetical decision-making | 206 |
| Did not focus on direct influencing factors | 54 |
| Focused on economic evaluation of the vaccination | 149 |
| Language of the article other than English, Spanish, or French | 42 |
| Total | 3,539 |
Of the 116 articles included, 20% (23/116) reported data from countries of the WHO Region of the Americas (AMR), 16% (18/116) from the WHO European Region (EUR), 15% (17/116) from the WHO African Region (AFR), 12% (14/116) from the WHO South-East Asia Region (SEAR) and 6% (7/116) from the WHO Western Pacific Region (WPR). There were no articles reflecting the situation in the WHO Eastern Mediterranean Region (EMR). The rest of the articles compiled data from countries from more than one WHO region (37/116; 32%). Of the 116 articles included, 27 (23%) provided data from high-income countries (HIC), 14 (12%) from upper-middle-income countries (UMIC), 20 (17%) from lower-middle-income countries (LMIC), and four (3%) from low-income countries (LIC). Fifty-one (44%) articles focused on a mix of economies while providing a regional or global perspective (Appendix 4).
Thirty-eight percent of the articles (44/116) did not consider a specific vaccine. Of those that did, most focused on one vaccine. The human papillomavirus, the rotavirus, the pneumococcal conjugate, and Haemophilus influenzae type b vaccines were the most commonly reported vaccines (Appendix 4).
3.1. Frameworks of decision-making for vaccine introduction (Article type 1)
Of the 27 articles that were categorized as frameworks of decision-making for vaccine introduction, 20 provided a country perspective, two provided a regional perspective, and five gave a global perspective [9–35]. Most articles focused on a HIC (n = 9) or a UMIC (n = 8); many (n = 18) focused exclusively on countries that are not eligible for Gavi support (Table 2). Eleven articles did not consider a specific vaccine. Of those that did, most focused on one vaccine, and others focused on multiple vaccines. The categories of criteria for decision-making within the frameworks are described below.
Table 2.
Characteristics of articles included under the framework article type* (n = 27) by country focus and vaccine, systematic review of national decision-making 2010–2020.
| Frameworks | Country focus and income level | Vaccine | Category | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine characteristics | The importance of the health problem | Financial/economic issues | Considerations of the alternative interventions | Decision making process | Impact of vaccination | Programmatic considerations | Acceptability | Accessibility, equity, and ethics | |||
| Ceyhan, 2010 [8] | Turkey, UMIC | None specifically | X | X | X | X | X | X | |||
| Houweling et al., 2010 [9] | The Netherlands, HIC | HBV | X | X | X | X | X | X | X | ||
| John, 2010 [10] | India, LMIC Gavi | None specifically | X | X | X | X | X | ||||
| La Torre et al., 2010 [11] | Italy, HIC | HPV | X | X | X | X | X | X | X | X | |
| Levine et al., 2010 [12] | LIC and LMIC, all Gavi supported countries | Hib | X | X | X | X | X | X | |||
| Levine et al., 2010 [13] | Global, Gavi and non-Gavi supported countries | Hib | X | X | X | X | X | X | X | ||
| Ahmed et al., 2011 [14] | USA, HIC | None specifically | X | X | X | X | X | X | X | X | |
| Blecher et al., 2012 [15] | South Africa, UMIC | PCV, Rotavirus | X | X | X | X | X | X | X | ||
| Brooks et al., 2012 [16] | Global, Gavi and non-Gavi supported countries | Malaria | X | X | X | X | X | X | X | ||
| Cho, 2012 [17] | South Korea, HIC | None specifically | X | X | X | X | X | ||||
| Georgousakis et al., 2012 [18] | Australia, HIC | HPV | X | X | X | X | X | X | X | X | |
| Ngcobo et al., 2012 [19] | South Africa, UMIC | None specifically | X | X | X | X | X | X | |||
| Schoub, 2012 [20] | South Africa, UMIC | IPV | X | X | X | X | X | X | X | ||
| Ahout et al., 2014 [21] | The Netherlands, HIC | Influenza | X | X | X | X | X | X | |||
| Betancourt-Cravioto et al., 2014 [22] | Mexico, UMIC | Dengue | X | X | X | X | X | X | |||
| Domingues et al., 2014 [23] | Brazil, UMIC | IPV | X | X | X | X | X | X | X | X | |
| Falleiros-Arlant et al., 2014 [24] | Global, Gavi and non-Gavi supported countries | IPV | X | X | X | ||||||
| Rao et al., 2014 [25] | India, LMIC Gavi | Rotavirus | X | X | X | X | X | X | |||
| Stecher et al., 2014 [26] | Argentina, UMIC | None specifically | X | X | X | X | X | X | X | X | |
| Phelps et al., 2016 [27] | Global, Gavi and non-Gavi supported countries | None specifically | X | X | X | X | X | X | X | X | |
| Seib et al., 2017 [28] | USA, UK, Canada, Australia | None specifically | X | X | X | X | X | X | X | X | X |
| Dawa et al., 2019 [29] | Kenya | Seasonal influenza | X | X | X | X | X | X | X | X | X |
| De Wals et al., 2019 [30] | Canada | None specifically | X | X | X | X | X | X | X | X | |
| Floret 2019 [31] | France | None specifically | X | X | X | X | X | X | X | X | |
| Rattanavipapong et al., 2020 [32] | Thailand | Rotavirus | X | X | X | X | X | X | X | ||
| Sekhar et al., 2020 [33] | India | Cholera | X | X | X | X | X | X | X | X | |
| Taychakhoonavudh et al., 2020 [34] | Association of Southeast Asian Nations (ASEAN) countries | None specifically | X | X | X | X | X | X | |||
| Total (n) | 27 | 26 | 26 | 25 | 24 | 20 | 15 | 11 | 13 | ||
Article type 1, Frameworks.
Definitions: LIC, Low Income Country; LMIC, Lower-Middle Income Country; UMIC, Upper-Middle Income Country; HIC, High Income Country; Gavi, Gavi, the Vaccine Alliance; HBV, Hepatitis B vaccine; HPV, Human Papillomavirus vaccine; Hib, Haemophilus influenzae type b vaccine; PCV, pneumococcal conjugate vaccine; IPV, Inactivated Polio vaccine.
The vaccine characteristics were reported in all 27 frameworks and were most frequently captured as vaccine efficacy or effectiveness (26/27; 96%) followed by vaccine safety (Table 3). Delivery issues and other vaccine characteristics, including vaccine wastage, vaccine presentation, and the infrastructure required to implement the new intervention were inconsistently considered throughout the frameworks (Table 3).
Table 3.
Criteria for decision-making included in the framework articles and related studies* (n = 72), systematic review of national decision-making 2010–2020.
| Category | Criteria | Frameworks including criterion (n = 27) | Studiesa mentioning criterion (n = 45) | ||
|---|---|---|---|---|---|
| % (n) | Total n of articles including at least one criteria of the category | % (n) | Total n of articles including at least one criteria of the category | ||
| Vaccine characteristics | Efficacy/effectiveness | 96 (26) | (n = 27) | 93 (41) | (n = 44) |
| Vaccine safety | 93 (25) | 80 (35) | |||
| Delivery issues | 41 (11) | 66 (29) | |||
| Other characteristics | 37 (10) | 30 (13) | |||
| The importance of the health problem | Burden of disease | 100 (26) | (n = 26) | 95 (40) | (n = 42) |
| Political priority | 31 (8) | 62 (26) | |||
| Costs of disease | 50 (13) | 40 (17) | |||
| Perceptions of importance | 38 (10) | 38 (16) | |||
| Other | 23 (6) | 14 (6) | |||
| Financial/economic issues | Economic evaluation | 96 (25) | (n = 26) | 83 (34) | (n = 41) |
| Incremental costs | 27 (7) | 20 (8) | |||
| Funding sources | 12 (3) | 46 (19) | |||
| Vaccine price | 62 (16) | 51 (21) | |||
| Financial sustainability | 31 (8) | 32 (13) | |||
| Other (including affordability) | 35 (9) | 29 (12) | |||
| Consideration of alternative interventions | Cost-effectiveness of alternatives | 96 (24) | (n = 25) | 100 (35) | (n = 35) |
| Effectiveness of alternatives | 40 (10) | 34 (12) | |||
| Other considerations | 16 (4) | 3 (1) | |||
| Decision-making process | Evidence sources/quality of evidence | 75 (18) | (n = 24) | 61 (23) | (n = 38) |
| Stakeholders involved | 67 (16) | 89 (34) | |||
| Procedures | 42 (10) | 66 (25) | |||
| Cues to action | 14 (4) | 29 (11) | |||
| Impact of vaccination | Impact on health outcomes | 90 (19) | (n = 21) | 93 (28) | (n = 30) |
| Impact on non-health outcomes | 67 (14) | 67 (20) | |||
| Effects of coadministration | 5 (1) | 23 (7) | |||
| Risks of serotype replacement | 19 (4) | 20 (6) | |||
| Other impact | 0 (0) | 3 (1) | |||
| Programmatic considerations | Feasibility | 73 (11) | (n =15) | 50 (13) | (n = 26) |
| Vaccine supply | 47 (7) | 69 (18) | |||
| Acceptability | Acceptability of vaccine | 100 (11) | (n = 11) | 100 (20) | (n = 20) |
| Accessibility, equity and ethics | Accessibility, equity and ethics | 100 (13) | (n = 13) | 100 (15) | (n = 15) |
Article type 1, Frameworks and article type 2, Studies that collect or analyze empirical data on vaccine adoption decision-making and examples of vaccine adoption decision-making.
Studies include the 31 articles that collected empirical data on decision-making and the 14 examples of vaccine introduction decision-making.
The importance of the health problem was reported in 26 of the 27 frameworks. The criteria described under the importance of the health problem in this type of article varied: the burden of the disease was most commonly reported, followed by the cost or economic burden of the disease and the public perception of the disease (Table 3). Political priority was considered to a lesser extent (8/26; 31%).
Financial and economic issues were reported in 26 of the 27 frameworks (Table 2), mostly via the inclusion of an economic evaluation (25/26; 96%) or consideration of the vaccine price (16/26; 62%). The incremental costs induced by the introduction of the new strategy, its financial sustainability, its affordability, and the funding sources were considered to a lesser extent (Table 3). The program implementation costs (i.e., the cost of vaccination, the demand forecast) were the additional economic issues considered.
Alternative interventions were reported in 25 of the 27 frameworks. In all but one of those, the influence of alternative interventions was captured via cost-effectiveness analysis, rather than via the effectiveness of the interventions alone (Table 3).
The decision-making process was reported in 24 of the 27 frameworks. The procedures and enabling factors for decision-making were not considered often.
The impact of vaccination was reported in 21 of the 27 frameworks. In most articles, the impact of vaccination was captured by the impact of the intervention on health outcomes, followed by the impact on non-health outcomes (14/21; 67%). The risks of serotype replacement and the effects of co-administration with other vaccines were reported to a lesser extent (Table 3).
Immunization program considerations and more specifically, the programmatic aspects of introducing a new vaccine were reported to a lesser extent (15/27 frameworks) than the previous categories.
Finally, the acceptability of the vaccine was considered in 11 frameworks while the accessibility, equity, and ethics related to vaccination were considered in 13 of the 27 frameworks.
In summary, the criteria most commonly reported in the frameworks were the burden of disease, vaccine efficacy/ effectiveness, vaccine safety, economic evaluation, impact on health and non-health outcomes, cost-effectiveness of alternatives, and the evidence sources. The public perception of the disease, acceptability of the vaccine, its accessibility, as well as equity and ethical issues, delivery issues, feasibility aspects, and the procedures involved were considered to a lesser extent.
3.2. Empirical studies and examples of decision-making for vaccine introduction (Article type 2)
Of the 45 studies categorized as Article type 2, 14 were examples of decision-making for vaccine introduction [36–49], and 31 collected empirical data on decision-making [50–80]. Of the 31 empirical studies, 21 used qualitative methods, three used mixed methods (quantitative and qualitative), three were quantitative, and four were literature reviews.
When we used the MMAT tool, the quality of the 27 empirical studies (excluding the literature reviews) varied, with scores ranging between 25 and 100% and a median of 75% (Appendix 5). Of the 27 empirical studies, four studies scored 100%, 13 studies scored 75%, six studies scored 50%, and four studies scored 25 percent. Considering that all studies passed the two screening questions and had a quality rating of ≥ 25%, we decided that none of the studies would be excluded. Therefore, the analysis is based on all of the empirical studies and examples of decision-making. We did not find any difference in the categories of criteria reported after stratification of empirical studies by MMAT score.
Almost a third of these studies focused on HICs (14/45; 31%), four focused on UMICs, nine focused on LMICs, and only two focused on a LIC; sixteen studies reported on multiple countries’ income-levels. Eleven studies focused on a Gavi-eligible country, while 13 compiled data from a mix of countries (i.e., Gavi-eligible and not eligible). Twenty-one studies focused exclusively on countries not eligible for Gavi support (Appendix 4). Of the 32 studies that focused on a specific vaccines(s), most focused on one vaccine, and five focused on multiple vaccines.
The topics of the studies varied. Some used qualitative or quantitative data collection methods to assess the decision-making process; others presented the criteria valued by the national immunization stakeholders for introducing new vaccines or reviewing immunization policy.
The vaccine characteristics were reported in 34 of the 45 studies and were most frequently stated via the inclusion of vaccine efficacy and/or effectiveness (41/44; 93%), followed by vaccine safety (35/44; 80%) (Table 3). We observed the same pattern in the frameworks. However, the delivery issues were more frequently explored and reported in the studies (29/44; 66%) than in the frameworks (11/27; 41%). Other vaccine characteristics, such as the vaccine wastage; the vaccine presentation and formulation; the vaccine viability under adverse conditions, and the cold chain capacity were considered inconsistently as was the case with the frameworks (Table 3).
The importance of the health problem was reported in 42 of the 45 studies included (Table 3). Similar to the frameworks, the burden of the disease was most commonly mentioned (40/42; 95%). However, the political priority for implementing such vaccine intervention was more frequently explored in the studies (26/42; 62%) than in the frameworks (8/26; 31%) (Table 3).
Financial and economic issues were reported in 41 of the 45 studies (Table 3), which were mostly stated via the inclusion of an economic evaluation (34/41; 83%), and this was similar to what we found in the framework articles. However, the vaccine price (21/41; 51%) and incremental costs (8/41; 20%) were reported to a lesser extent in the studies than in the framework articles: (16/26; 62%) and (7/26; 27%), respectively. However, the funding source was more likely to be considered in the studies (19/41; 46%) compared to the framework articles (3/26; 12%) (Table 3). Other economic issues considered were program implementation costs, programmatic strength, and budget impact analysis.
Alternative interventions were reported in 35 of the 45 studies (Table 3). As found in the framework articles, those that provided information on alternative interventions, the focus was mostly on the cost-effectiveness of the alternatives and less frequently on the effectiveness (Table 3).
The decision-making process was reported in 38 of the 45 studies (Table 3). The sources of evidence and/or the quality of the evidence were reflected similarly in the studies (20/27; 74%) and the frameworks (13/17; 76%). However, the stakeholders involved (34/38; 89%) and the procedures required for decision-making (25/38; 66%) were more likely to be considered in the studies than in the frameworks: (16/24; 67%) and (10/24; 42%) respectively.
The impact of vaccination was reported in 30 of the 45 studies (Table 3). As for the frameworks, it was most frequently captured through the impact of the intervention on health outcomes (28/30; 93%), followed by the impact on non-health outcomes (20/30; 67%). However, the effects of co-administration with other vaccines were questioned to a larger extent in the studies (7/30; 23%) than in the frameworks (1/21; 5%) (Table 3).
Immunization program considerations, and more specifically, the programmatic aspects of introducing a new vaccine were represented to a lesser extent than the previous categories in 26 of the 45 studies (Table 3). The programmatic aspects were captured to a larger extent by vaccine supply in the studies (18/26; 69%) than in the frameworks (7/15; 47%), whereas it was the other way around with the feasibility issue: (13/26; 50%) in the studies and (11/15; 73%) in the frameworks (Table 3).
The acceptability of the vaccine was considered in 20 studies while the accessibility, equity, and ethics related to vaccination were considered in 15 of the 45 studies (Table 3).
In summary, the most common criteria of interest in the studies were the burden of disease, vaccine efficacy/effectiveness, vaccine safety, economic evaluation, the cost-effectiveness of alternatives, and the stakeholders involved. Public perception of the disease, accessibility, equity, and ethical issues regarding the new vaccine, the effects of vaccine co-administration, and risks of serotype replacement and feasibility aspects were considered to a lesser extent.
3.3. Theoretical and empirical articles that provide insights into the policymaking process for vaccine introduction
A total of 61 references were identified as relevant to document decision-making processes for vaccine introduction: seventeen articles already included in either type 1 or type 2 articles [9,11,25,34,35,45–49,59,70,75–78,80] and 44 additional articles [81–124]. Findings are summarized in Table 4. Enabling factors for vaccine introduction include recommendations made from global committees such as the Strategic Advisory Group of Experts on Immunization (SAGE)/ WHO or other guidance developed by UNICEF, Gavi, the World Bank, or the Bill and Melinda Gates Foundation (BMGF). Recommendations made by the Regional Technical Advisory Group on Immunization and Vaccine-Preventable Diseases (TAG) and national bodies, such as the NITAG and the National Regulatory Agency (NRA) have facilitated national policymaking as reported in most references. In many LMICs, Gavi, has partnered with governments to strengthen decision-making and has played a leading role in vaccine introductions. Most references (70%) mentioned country ownership of the immunization programs as a facilitator to vaccine introduction policymaking. It was described as a strong level of governance, political will, and allocation of financial resources. Other key features of decision-making for vaccine adoption include robust policy dialogue among the different national stakeholders and institutions (57%), strong partnerships between the public and the private sectors (57%), primarily to address issues related to purchasing and reimbursement mechanisms for the vaccine. Additionally, most references (52%) mentioned strong health systems as facilitators to vaccine adoption decision-making, especially in the presence of adequate operational capacity to add another vaccine into the national schedule. Finally, lessons learned and experiences from other countries, especially neighboring countries benefited vaccine policy decisions (43%). Having a robust institutionalized process for vaccine introduction was cited as an enabling factor in most references (56%). Relying on the enactment of immunization laws for vaccine introduction was mentioned in less than a quarter of the references. Several references (18%) indicated that the collaboration between the NITAG and the NRA helped with fast-tracking the vaccine introduction and address issues, such as the off-label use of vaccines in a timely manner.
Table 4.
Enabling factors for vaccine-related policymaking mentioned in theoretical and empirical articles* (n = 61), systematic review of national decision-making 2010–2020.
| Enabling factor | % (n) references where factor is mentioned |
|---|---|
| Recommendations (NITAG/NRA/ICC/TAG/WHO/SAGE/UNICEF/WB/Gavi/BMGF/CDC) | 82 (50) |
| Country ownership (i.e., governance, political will, financial resources) | 70 (43) |
| Policy dialogue, networks, champions | 57 (35) |
| Public private partnerships (i.e., purchasing mechanisms, vaccine price) | 57 (35) |
| Vaccine introduction model/structure (i.e., process in place) | 56 (34) |
| Integration of EPI within strong health system (i.e., operational capacity) | 52 (32) |
| Lessons learned from other countries or regions | 43 (26) |
| Immunization law or health legislation | 21 (13) |
| NITAG and NRA integration/collaboration | 18 (11) |
Article type 3, Theoretical and empirical articles that provide insights into the vaccine policymaking process.
Definitions: NITAG, National Immunization Technical Advisory Group; NRA, National Regulatory Authority; Gavi, Gavi, the Vaccine Alliance; TAG: Regional Technical Advisory Group on immunization and VPD; SAGE: Strategic Advisory Group of Experts on Immunization; WHO: World Health Organization; UNICEF: the United Nations Children’s Fund; ICC: National Inter-Agency Co-ordinating Committee; CDC: the U.S. Centers for Disease Control and Prevention; WB: the World Bank; BMGF: The Bill and Melinda Gates Foundation ; EPI: Expanded Programme on Immunization.
4. Discussion
Our findings suggest an increased global interest in strengthening evidence-based policymaking for vaccines. Our literature review identified more than 100 new articles that have been published since 2010. Most of the literature had a global perspective rather than a country perspective, with particular interest in addressing the high price of new vaccines (i.e., the rotavirus, the pneumococcal conjugate, and the human papillomavirus vaccines) and other competing health priorities, especially in LMICs.
A framework of evidence for decision-making is critical to reaching informed decisions for allocating and prioritizing scarce resources. The most common criteria included in the national frameworks were the burden of disease, vaccine efficacy/effectiveness, vaccine safety, economic evaluation, the vaccine impact on health and non-health outcomes, and cost-effectiveness of the alternatives. Political priority, vaccine delivery issues, vaccine supply, funding sources, and the procedures and stakeholders involved were not considered frequently within the country frameworks. However, these criteria were more likely to be included in the studies on decision-making for vaccine introduction, which suggests the need to expand considerations of the programmatic implications when introducing a new vaccine. Although we did not identify any additional criteria compared to Burchett et al.’s review, we noted increased considerations in valuation and comparing interventions via economic evaluations [4]. Cost-effectiveness analyses were the most common economic evaluations undertaken and published, whereas cost and budget impact analyses were not as widely published. Another significant difference observed over the past decade is the role of Health Technology Assessment agencies in evaluating new vaccines.
Research on vaccine decision-making processes has also expanded, providing insights into the enabling factors for vaccine introduction. Most of the references that provided insights into the vaccine policymaking process reported the critical role of the country ownership of immunization programs (70%) and the recommendations made by national advisory committees, including NITAGs when deciding on vaccine introduction. Although not all NITAGs are completely independent from the government, NITAGs are comprised of national experts, and their mandate is to be independent of the government, with the technical expertise to systematically evaluate vaccine introduction, priorities, schedules, target groups, immunization strategies, and safety issues to guide national policies and strategies based on local epidemiology and cost-effectiveness [3]. Such expertise, however, may be limited in some settings and individual countries could consider providing ability to interpret cost-effectiveness studies via the secretariat and/or expertise beyond that of the core group of NITAG members [3]. The collective expertise should be adjusted to the specific terms of reference (TORs) for the NITAG. A systematic and transparent process for decision-making enables NITAGs to undertake a rigorous review of the evidence related to the criteria that NITAG members have agreed upon to consider in making an informed decision, and issue independent recommendations to the national government [125]. Therefore, the integration of NITAGs into the national policy process is important to ensure that sound decisions follow recommendations on vaccine introduction and deployment [105]. As supported by WHO, the establishment and functionality of NITAGs should be central to making evidence-based recommendations on immunization policy [3,114]. NITAG’s role at the national level mirrors the role of SAGE at the global level [89,126]. Indeed, recent efforts by WHO and partners to build immunization decision-making capacity of NITAGs in the European Region have highlighted the importance of understanding the SAGE Evidence to Recommendation Framework, within the country context [127].
Factors and processes influencing country decisions in LMICs include the burden of vaccine-preventable disease, vaccine characteristics (e.g., effectiveness, safety, and programmatic suitability), economic evaluations, financing for immunization, and delivery systems. However, policy decisions are not only shaped by data and evidence but also by an array of social, institutional, economic, and political factors [107]. The WHO Principles and considerations for adding a vaccine to a national immunization program offer national decision-makers a structured and comprehensive framework when deciding to introduce a new vaccine, and for planning, managing, and monitoring the introduction, and evaluating its impact [128].
In recent years, because of the higher price of the new vaccines compared to earlier vaccines, health economics evaluations played an essential role in deciding which vaccine should be developed or routinely used [129]. Authors posit that economic evaluations that capture the full benefits of the vaccination should be included in the multicriteria decision-making together with additional core concepts, such as the severity of the disease (mortality and morbidity rates), and the vaccine safety considerations [130]. However, the use of models and cost-effectiveness analysis of vaccines to better inform vaccine introduction decisions at the local level requires capacity strengthening via technical assistance, especially in low-capacity settings [84,86,131]. Standardization of economic evaluation methods and adherence to guidelines should be promoted [82,85,132] as well as raising awareness and a better understanding of what economic evaluation findings mean and how they should be interpreted, including their limitations (e.g., model assumptions).
Understanding the value of the vaccines, the health system characteristics, and the policy issues are significant barriers to new vaccine introduction [94]. With support from the BMGF from 2008 to 2017, the Supporting Independent Immunization and Vaccine Advisory Committees (SIVAC) Initiative provided technical assistance to LMICs to establish and strengthen NITAG functionality and integration into the national policy process [111,133]. An important goal of SIVAC was to ensure that NITAG recommendations contributed to optimization of the immunization policies and programs based on scientific evidence and local characteristics [112]. The lessons learned from establishing NITAGs in LMICs and strengthening their capacity in the use of evidence-based processes for decision-making were shared in publications [134,135]. A critical issue that NITAGs need to address in order to fulfill their role is the proper management of conflicts of interest to ensure the independence of their recommendations. This is to promote independence from all external influences including vaccine manufacturers, the Ministry of Health, the WHO and its secretariat. In addition and to prevent any undue influence of the secretariat on the NITAG, a working group should be established to address a specific policy question. Comprised of a minimal number of NITAG core members with additional subject-matter experts, its role is to gather, analyze and prepare information for presentation and for decision making by the full NITAG [3]. In some countries, the working group chair is a NITAG member and is appointed by the NITAG chair, which further promotes independence from the secretariat. Vaccine manufacturer’s representatives should not serve on the working groups although they could be asked to provide specific information to the working groups [3]. To limit the influence of outside entities on the NITAG, NITAG non-core members that represent government entities, professional societies and technical partners such as WHO, should not be directly involved in deciding on the final set of recommendations. Their role should be limited to contributing to the discussion, to help provide background information, and to help ensure smooth implementation of the new recommendations [3]. Many of the national experts in the field of immunization and vaccines will have some relationship with various interest groups, including industry, professional associations, and governments. The goal is not to include only persons in the NITAG with absolutely no relevant interests but to manage potential conflicts of interest in a transparent and ethical fashion [3]. NITAG members who have a conflict of interest should be requested to leave the room during discussions in which they have a declared interest and/or should be asked to withhold from voting. It is critical that the NITAG develops detailed TORs and standard operating procedures that define 1) the role of its secretariat and the scope of the secretariat’s input, 2) the processes to preserve NITAG transparency and independence including the processes to manage conflicts of interest, 3) how the NITAG operates: open vs. closed meetings, participation of industry and of professional societies and associations, role of each member type (i.e., participate in the discussions vs. present data vs. decide on recommendations), process to share and review the evidence, process for decision-making of core members, establishment of working groups and their mode of operation, framework for decision-making, process for deciding on agenda items [3,134]. As a NITAG develops and matures into using its own evidence to recommendation framework, the committee is able to take into account the diversity of available information through an objective, transparent, and well documented process.
Since 2004, the ProVac Global Initiative aims to strengthen national decision-making for new vaccine introduction in LMICs of the WHO Region of the Americas by strengthening infrastructure for decision-making and developing tools for economic analyses, and providing training to national multidisciplinary teams [97–99]. There are additional tools to support vaccine decision-making, such as the Strategic Multi-Attribute Ranking Tool for Vaccines (SMART Vaccines) developed by the U.S. Institute of -Medicine. This tool helps decision-makers prioritize vaccine adoption by specifying their value structure, and selecting attributes relevant to the ranking of vaccine candidates [136].
Despite these efforts, many NITAGs continue to face challenges in fulfilling their roles, especially related to data generation, interpretation, and use. Since 2016, the Global NITAG Network (GNN) has provided “a global platform to enable NITAGs to efficiently share and access knowledge, technical reviews, data, lessons learned, trends, and innovations” [137,138]. NITAG functionality, quality, and integration into the policy process have been evaluated to facilitate NITAG effectiveness and sustainability [138,139]. The WHO and the U.S. CDC have jointly developed competency-based trainings, relevant materials, and tools to fill identified capacity gaps.
4.1. Limitations
There were several limitations to this review. Our search strategy did not include grey literature as a source of information, which limits the comprehensiveness of our findings. The incomplete retrieval of identified research and publication bias may have affected the robustness of our findings. The screening of the references that came from the June 2017 to August 2020 literature search, the data abstraction, and the analysis of the references were done by one researcher. Although extra checks were performed to improve the analysis and all results were discussed by the research team, some bias may have resulted. Twenty-three percent of the articles included in our review reported on a HIC, while only 3% reported on a LIC; therefore, our findings are biased towards the frameworks used and studies performed in these settings. Additionally, we structured our review on the nine broad categories of criteria that Burchett et al. 2012 developed; we used those for evaluating the frameworks and the studies on decision-making [4]. This approach enabled the comparison of results and assessment of trends in this area of research although it may have driven our analysis away from a different taxonomy of frameworks.
5. Conclusions
Although we did not identify any additional important criteria, we found that policymakers and NITAG committee members increasingly value the interventions based on economic evaluations. The results of this review could guide vaccine introduction discussions among country, sub-regional, and regional stakeholders. The sharing of resources could be particularly useful in microstates or countries that currently lack capacity or diversity of expertise.
Supplementary Material
Acknowledgements
The authors express their gratitude to Mrs. Joanna Taliano for developing the literature search strategy and conducting the search and to the CDC librarians for retrieving the electronic copies of the articles that were unavailable through the CDC library network.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2021.02.059.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
In 2018, low-income countries were defined as those with a GNI per capita of $1005 or less, lower middle-income countries were those with a GNI per capita between $1006 and $3955, upper middle-income countries were those with a GNI per capita between $3956 and $12,235, and high-income countries were those with a GNI per capita of $12,236 or more.
Availability of data and materials
The datasets used and/or analyzed during this review are available from the corresponding author on reasonable request.
References
- [1].World Health Organisation. Vaccine introduction guidelines. Adding a vaccine to a national immunisation program: decision and implementation; 2005. [Google Scholar]
- [2].World Health Organisation. Global Vaccine Action Plan 2011–2020; 2013, June 27 2018. Available: http://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/
- [3].Duclos P. National Immunization Technical Advisory Groups (NITAGs): guidance for their establishment and strengthening. Vaccine 2010;28(Suppl 1):A18–25 (in eng). [DOI] [PubMed] [Google Scholar]
- [4].Burchett HE, Mounier-Jack S, Griffiths UK, Mills AJ. National decision-making on adopting new vaccines: a systematic review. Health Policy Plann 2012;27 (Suppl 2):ii62–76. [DOI] [PubMed] [Google Scholar]
- [5].Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration (in eng). PLoS Med 2009; 6(7): p. e1000100, Jul 21 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].World Bank. Data: Country and Lending Groups; June 2017, 24 July 2017. Available: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- [7].Gavi - the Vaccine Alliance. Countries eligible for support; 2018, 24 July 2017. Available: http://www.gavi.org/support/sustainability/countries-eligible-for-support/
- [8].Pluye P, Robert E, Cargo M, Bartlett G, O’Cathain A, Griffiths F, et al. Proposal: A mixed methods appraisal tool for systematic mixed studies reviews; 2011. (June, 29th 2018). Available: http://mixedmethodsappraisaltoolpublic.pbworks.com
- [9].Ceyhan M. Recent improvements in the Turkish Childhood National Immunization Program. Turkish J Pediat 2010;52(6):563–9. [PubMed] [Google Scholar]
- [10].Houweling H, Wittevrongel CF, Verweij M, Ruitenberg EJ, N. National Immunisation Programme Review Committee of the Health Council of the, “Public vaccination programmes against hepatitis B in The Netherlands: assessing whether a targeted or a universal approach is appropriate. Vaccine 2010; 28(49): pp. 7723–30. [DOI] [PubMed] [Google Scholar]
- [11].John TJ. India’s National Technical Advisory Group on Immunisation. Vaccine 2010;28(Suppl 1):A88–90. [DOI] [PubMed] [Google Scholar]
- [12].La Torre G, de Waure C, Chiaradia G, Mannocci A, Capri S, Ricciardi W. The Health Technology Assessment of bivalent HPV vaccine Cervarix in Italy. Vaccine 2010;28(19):3379–84. [DOI] [PubMed] [Google Scholar]
- [13].Levine OS et al. A policy framework for accelerating adoption of new vaccines. Human Vaccines 2010;6(12):1021–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Levine OS, Knoll MD, Jones A, Walker DG, Risko N, Gilani Z. Global status of Haemophilus influenzae type b and pneumococcal conjugate vaccines: evidence, policies, and introductions. Curr Opin Infect Dis 2010;23 (3):236–41. [DOI] [PubMed] [Google Scholar]
- [15].Ahmed F, Temte JL, Campos-Outcalt D, Schunemann HJ, A. E. B. R. W. Group. Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine 2011; 29(49): pp. 9171–6. [Review] [DOI] [PubMed] [Google Scholar]
- [16].Blecher MS, et al. Financing vaccinations - the South African experience. (Special Issue: Introducing new vaccines into the South African national immunisation programme - a case study.). Vaccine 2012; 30(Suppl. 3): pp. C79–C86. [DOI] [PubMed] [Google Scholar]
- [17].Brooks A, Ba-Nguz A. Country planning for health interventions under development: lessons from the malaria vaccine decision-making framework and implications for other new interventions. Health Policy Plann 2012;27 (Suppl 2):ii50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cho HY. An overview of the national immunization policy making process: the role of the Korea expert committee on immunization practices. Korean J Pediatrics 2012;55(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Georgousakis M, Jayasinghe S, Brotherton J, Gilroy N, Chiu C, Macartney K. Population-wide vaccination against human papillomavirus in adolescent boys: Australia as a case study. Lancet Infect Dis 2012;12(8):627–34. [DOI] [PubMed] [Google Scholar]
- [20].Ngcobo NJ, Cameron NA. The decision making process on new vaccines introduction in South Africa. Vaccine 2012;30(Suppl 3):C9–C13. Review. [DOI] [PubMed] [Google Scholar]
- [21].Schoub BD. Introduction of inactivated polio vaccine (IPV) into the routine immunization schedule of South Africa. Vaccine 2012;30(Suppl 3):C35–7. Review. [DOI] [PubMed] [Google Scholar]
- [22].Ahout I, Ferwerda G, de Groot R. Influenza vaccination in kids, are you kidding me?. J Infect 2014;68(Suppl 1):S100–7. Review. [DOI] [PubMed] [Google Scholar]
- [23].Betancourt-Cravioto M, Kuri-Morales P, Gonzalez-Roldan JF, Tapia-Conyer R, G. Mexican Dengue Expert. Introducing a dengue vaccine to Mexico: development of a system for evidence-based public policy recommendations. PLoS Neglected Tropical Diseases [electronic resource] 2014; 8(7): p. e3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Domingues CM, de Fatima Pereira S, Cunha Marreiros AC, Menezes N, Flannery B. Introduction of sequential inactivated polio vaccine-oral polio vaccine schedule for routine infant immunization in Brazil’s National Immunization Program. J Infect Dis 2014;210(Suppl 1):S143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Falleiros-Arlant LH, Avila-Aguero ML, Brea del Castillo J, Marino C. The challenge of changing the inactivated poliomyelitis vaccine in Latin America: declaration of the Latin American Society of Pediatric Infectious Diseases (SLIPE). Revista Chilena de Infectologia 2014;31(5):590–603. [DOI] [PubMed] [Google Scholar]
- [26].Rao TS, Rashmi A, Ajay K, Tate JE, Parashar U, Gagandeep K. Insights from global data for use of rotavirus vaccines in India. (Special Issue: Rotavirus in India: an update on epidemiology and vaccines.). Vaccine 2014; 32(Suppl.1): pp. A171–A178. [DOI] [PubMed] [Google Scholar]
- [27].Stecher D et al. National Immunization Commission: strengthening evidence-based decision making in Argentina. Vaccine 2014;32(16):1778–80. [DOI] [PubMed] [Google Scholar]
- [28].Phelps C, Madhavan G, Rappuoli R, Levin S, Shortliffe E, Colwell R. Strategic planning in population health and public health practice: A call to action for higher education. Milbank Q 2016;94(1):109–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Seib K et al. Policy making for vaccine use as a driver of vaccine innovation and development in the developed world. Vaccine 2017;35(10):1380–9. Review. [DOI] [PubMed] [Google Scholar]
- [30].Dawa J et al. Developing a seasonal influenza vaccine recommendation in Kenya: Process and challenges faced by the National Immunization Technical Advisory Group (NITAG). Vaccine 2019;37(3):464–72. Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.. [DOI] [PubMed] [Google Scholar]
- [31].De Wals P, Espinoza-Moya ME, Beland D. Kingdon’s multiple streams framework and the analysis of decision-making processes regarding publicly-funded immunization programs. Expert Rev Vaccines 2019;18 (6):575–85. Research Support, Non-U.S. Gov’t Review. [DOI] [PubMed] [Google Scholar]
- [32].Floret D The development of vaccination policy. Rev Mal Respir 2019;36 (9):1038–46. Elaboration de la politique vaccinale Review. [DOI] [PubMed] [Google Scholar]
- [33].Rattanavipapong W et al. Comparing 3 approaches for making vaccine adoption decisions in Thailand. Int J Health Policy Manage 2020;20:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sekhar A, Kang G. Pathways to a policy for cholera control in India. Vaccine 2020;38(Suppl 1):A157–9. Review. [DOI] [PubMed] [Google Scholar]
- [35].Taychakhoonavudh S, Chumchujan W, Hutubessy R, Chaiyakunapruk N. Landscape of vaccine access and health technology assessment role in decision-making process in ASEAN countries. Human Vaccines Immunotherap 2020;16(7):1728–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hajjeh RA et al. Supporting new vaccine introduction decisions: lessons learned from the Hib Initiative experience. Vaccine 2010;28(43):7123–9. [DOI] [PubMed] [Google Scholar]
- [37].Jacobs P, Ohinmaa A. A comparison of the use of economics in vaccine expert reviews. Vaccine 2010;28(16):2841–5. Comparative Study. [DOI] [PubMed] [Google Scholar]
- [38].Madhi SA, Cohen C, Gottberg Av. Introduction of pneumococcal conjugate vaccine into the public immunization program in South Africa: translating research into policy. (Special Issue: Introducing new vaccines into the South African national immunisation programme - a case study.). Vaccine 2012; 30 (Suppl. 3): pp. C21–C27. [DOI] [PubMed] [Google Scholar]
- [39].Perez Schael I et al. Clinical development, registration, and introduction of human rotavirus vaccine: The Latin American experience. Trials in Vaccinol 2012;1:10–20. [Google Scholar]
- [40].Koch J et al. Background paper to the recommendation for routine rotavirus vaccination of infants in Germany. Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz 2013;56(7):957–84. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- [41].Whitney CG, Parashar UD. What do policy makers need to know? Lessons from the decision to add pneumococcal conjugate and rotavirus vaccines to the US immunization program. (Special Issue: Evidence Base for Vaccine Introduction in Latin America and the Caribbean.). Vaccine 2013; 31(Suppl. 3): pp. C6–C7. [DOI] [PubMed] [Google Scholar]
- [42].Nelson CB, Mogasale V, Bari TI, Clemens JD. Considerations around the introduction of a cholera vaccine in Bangladesh. Vaccine 2014;32 (52):7033–6. [DOI] [PubMed] [Google Scholar]
- [43].Rivero-Santana A, Cuellar-Pompa L, Sanchez-Gomez LM, Perestelo-Perez L, Serrano-Aguilar P. Effectiveness and cost-effectiveness of different immunization strategies against whooping cough to reduce child morbidity and mortality. Health Policy 2014;115(1):82–91. [DOI] [PubMed] [Google Scholar]
- [44].Limia-Sanchez A, Andreu MM, Torres de Mier Mde V, Navarro-Alonso JA, S. Working Group on Review of the Immunization. [Toward a New Immunization Schedule in Spain, 2016 (Part 1)]. Revista Espanola de Salud Publica 2016; 90: p. E2. [PubMed] [Google Scholar]
- [45].Ba-Nguz A, Adjagba A, Hendrarto TW, Sewankambo NK, Nalwadda C, Kisakye A. The role of National Immunization Technical Advisory Groups (NITAGs) in the introduction of inactivated polio vaccine: experience of the Indonesia and Uganda NITAGs. (Special Issue: Polio endgame & legacy-implementation, best practices, and lessons learned.). J Infect Dis 2017; 216 (Suppl. 1): pp. S109–S113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Scotney S et al. Succeeding in New Vaccine Introduction: Lessons Learned from the Introduction of Inactivated Poliovirus Vaccine in Cameroon, Kenya, and Nigeria. J Infect Dis 2017;216:S130–6. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hasan AZ, et al. Using pneumococcal and rotavirus surveillance in vaccine decision-making: A series of case studies in Bangladesh, Armenia and the Gambia. Vaccine 2018; 36(32 Pt B): pp. 4939–4943. [DOI] [PubMed] [Google Scholar]
- [48].Malik A et al. Introducing rotavirus vaccine in the Universal Immunization Programme in India: From evidence to policy to implementation. Vaccine 2019;37(39):5817–24. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mandal S Introduction of universal infant hepatitis B immunisation in the UK- paving the way to elimination. Human Vaccines Immunotherap 2019; 15(2):440–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Arbyn M, Simoens C, Van Damme P, Scharpantgen A, Meijer CJ, Beutels P. Introduction of human papillomavirus vaccination in Belgium, Luxembourg and the Netherlands. Gynecol Obstet Invest 2010;70(4):224–32. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- [51].Blume S, Tump J. Evidence and policymaking: The introduction of MMR vaccine in the Netherlands. Soc Sci Med 2010;71(6):1049–55. Research Support, Non-U.S. Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nguyen Quy N, et al. Human papillomavirus vaccine introduction in Vietnam: formative research findings. (Special Issue: Human papillomavirus.). Sexual Health 2010; 7(3): pp. 262–270. [DOI] [PubMed] [Google Scholar]
- [53].Pineros M, Wiesner C, Cortes C, Trujillo LM. HPV vaccine introduction at the local level in a developing country: attitudes and criteria among key actors. Cadernos de Saude Publica 2010;26(5):900–8. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- [54].Piso B, Zechmeister I, Geiger-Gritsch S. Criteria for vaccine introduction: Results of a DELPHI discussion among international immunisation experts on a stepwise decision-making procedure. J Public Health 2011;19(1):73–80. [Google Scholar]
- [55].Burchett HED et al. New vaccine adoption: Qualitative study of national decision-making processes in seven low- and middle-income countries. Health Policy Plann 2012;27(SUPPL 2):ii5–ii16. Article. [DOI] [PubMed] [Google Scholar]
- [56].Makinen M, Kaddar M, Molldrem V, Wilson L. New vaccine adoption in lower-middle-income countries. Health Policy Plann 2012;27(SUPPL 2):ii39–49. Article. [DOI] [PubMed] [Google Scholar]
- [57].Vikas B, Anoop S. Agenda setting in vaccine policy and social relevance of the emerging vaccine technologies from public health perspective - Part 1. Int J Med Public Health 2012;2(1):7–15. [Google Scholar]
- [58].de Oliveira LH et al. Systematic documentation of new vaccine introduction in selected countries of the Latin American Region. Vaccine 2013;31(Suppl 3): C114–22. [DOI] [PubMed] [Google Scholar]
- [59].Douglas DL, DeRoeck DA, Mahoney RT, Wichmann O. Will Dengue Vaccines Be Used in the Public Sector and if so, How? Findings from an 8-country Survey of Policymakers and Opinion Leaders. PLoS Neglected Tropical Dis 2013; 7(3): Art. no. e2127 [Article]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nohynek H, Wichmann O, F DA, Gatekeepers VN. National Advisory Groups and their role in immunization policy-making processes in European countries. Clin Microbiol Infect 2013; 19(12): pp. 1096–105 [Review]. [DOI] [PubMed] [Google Scholar]
- [61].Rosella LC et al. Pandemic H1N1 in Canada and the use of evidence in developing public health policies - a policy analysis. Soc Sci Med 2013;83:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Samaan G, McPherson M, Partridge J. A review of the evidence to support influenza vaccine introduction in countries and areas of WHO’s Western Pacific Region. PLoS ONE [Electronic Resource] 8(7), p. e70003 [Review]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Uddin J, Sarma H, Bari TI, Koehlmoos TP. Introduction of new vaccines: decision-making process in Bangladesh. J Health Popul Nutr 2013;31 (2):211–7. Research Support, Non-U.S. Gov’t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Choe YJ et al. Prioritization of the introduction of new vaccines to the national immunization program in the Republic of Korea. Vaccine 2014;32 (46):6049–53. Article. [DOI] [PubMed] [Google Scholar]
- [65].Nyirenda LJ, Sandberg KI, Justice J. When are health systems ready for new vaccines? the introduction of pneumococcal vaccine in Malawi. Forum Develop Stud 2014;41(2):317–36. Article. [Google Scholar]
- [66].Ozawa S et al. Evidence-to-policy gap on hepatitis A vaccine adoption in 6 countries: Literature vs. policymakers’ beliefs. Vaccine 2014;32(32):4089–96. Review. [DOI] [PubMed] [Google Scholar]
- [67].Panda S, Das A, Samanta S. Synthesizing evidences for policy translation: a public health discourse on rotavirus vaccine in India. Vaccine 2014;32 (Suppl 1):A162–70. Review. [DOI] [PubMed] [Google Scholar]
- [68].González-Lorenzo M et al. Conceptual frameworks and key dimensions to support coverage decisions for vaccines. Vaccine 2015;33(9):1206–17. Article. [DOI] [PubMed] [Google Scholar]
- [69].Van Der Putten IM, Evers SMAA, Deogaonkar R, Jit M, Hutubessy RCW. Stakeholders’ perception on including broader economic impact of vaccines in economic evaluations in low and middle income countries: A mixed methods study. BMC Public Health 2015; 15(1): Art. no. 356 [Article]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Hadisoemarto PF, Reich MR, Castro MC. Introduction of pentavalent vaccine in Indonesia: a policy analysis. Health Policy Plann 2016;31 (8):1079–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Parrella A et al. Prioritizing government funding of adolescent vaccinations: Recommendations from young people on a citizens’ jury. Vaccine 2016;34 (31):3592–7. Article. [DOI] [PubMed] [Google Scholar]
- [72].Pooripussarakul S, Riewpaiboon A, Bishai D, Muangchana C, Tantivess S. What criteria do decision makers in Thailand use to set priorities for vaccine introduction?. BMC Public Health 2016; 16(1): Art. no. 684 [Article]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Romore I et al. Policy analysis for deciding on a malaria vaccine RTS, S in Tanzania. Malar J 2016;15:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Williams EA, Lewis DJ, Bertholet S, Zazzi M. Anticipating policy considerations for a future HIV vaccine: a preliminary study. Vaccine 2016;34(32):3697–701. [DOI] [PubMed] [Google Scholar]
- [75].Caro Martinez A, et al. Adoption of the HPV vaccine: A case study of three emerging countries. J Comparat Effectiveness Res 2017; 6(3): pp. 195–204. [DOI] [PubMed] [Google Scholar]
- [76].Wallace L, Kapirir L. How are new vaccines prioritized in low-income countries? A case study of human papilloma virus vaccine and pneumococcal conjugate vaccine in Uganda. Int J Health Policy Manage 2017;6(12):707–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Howard N, Walls H, Bell S, Mounier-Jack S. The role of National Immunisation Technical Advisory Groups (NITAGs) in strengthening national vaccine decision-making: A comparative case study of Armenia, Ghana, Indonesia, Nigeria, Senegal and Uganda. Vaccine 2018; 36(37): pp. 5536–5543 [Comparative Study Research Support, Non-U.S. Gov’t]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].St-Martin G, Lindstrand A, Sandbu S, Fischer TK. Selection and Interpretation of Scientific Evidence in Preparation for Policy Decisions: A Case Study Regarding Introduction of Rotavirus Vaccine Into National Immunization Programs in Sweden, Norway, Finland, and Denmark. Front Public Health 2018;6:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chen R, Wong E. The feasibility of universal HPV vaccination program in Shenzhen of China: a health policy analysis. BMC Public Health 2019;19 (1):781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].van der Putten IM, Paulus ATG, Hiligsmann M, Hutubessy RCW, Evers S. Evidence-informed vaccine decision making: The introduction of Human Papilloma Virus (HPV) vaccination in the Netherlands. Health Policy 2019;123(3):260–6. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- [81].Baleta AF, Heever Jvd, Burnett RJ. Meeting the need for advocacy, social mobilisation and communication in the introduction of three new vaccines in South Africa - successes and challenges. (Special Issue: Introducing new vaccines into the South African national immunisation programme - a case study.). Vaccine 2012; 30(Suppl. 3): pp. C66–C71. [DOI] [PubMed] [Google Scholar]
- [82].Barnighausen T et al. Rethinking the benefits and costs of childhood vaccination: the example of the Haemophilus influenzae type b vaccine. Vaccine 2011;29(13):2371–80. [DOI] [PubMed] [Google Scholar]
- [83].Bartolini RM et al. Formative research to shape HPV vaccine introduction strategies in Peru. Salud Publica Mex 2010;52(3):226–33. [DOI] [PubMed] [Google Scholar]
- [84].Bloom DE. Valuing vaccines: deficiencies and remedies. Vaccine 2015;33 (Suppl 2):B29–33. Review. [DOI] [PubMed] [Google Scholar]
- [85].Bloom DE, Madhavan G. Vaccines: from valuation to resource allocation. Vaccine 2015;33(Suppl 2):B52–4. Review. [DOI] [PubMed] [Google Scholar]
- [86].Chauke-Moagi BE, Mumba M. New vaccine introduction in the East and Southern African sub-region of the WHO African region in the context of GIVS and MDGs. Vaccine 2012;30(Suppl 3):C3–8. Review. [DOI] [PubMed] [Google Scholar]
- [87].Chocarro L, Duclos P, Senouci K, Southern J. Consultation on interactions between National Regulatory Authorities and National Immunization Technical Advisory Groups. Expert Rev Vaccines 2011;10(9):1265–70. Congresses. [DOI] [PubMed] [Google Scholar]
- [88].de Quadros C Historical perspectives on new vaccine introduction in Latin America and the Caribbean. Vaccine 2013;31(Suppl 3):C4–5. [DOI] [PubMed] [Google Scholar]
- [89].Duclos P, Okwo-Bele JM, Salisbury D. Establishing global policy recommendations: the role of the Strategic Advisory Group of Experts on immunization. Expert Rev Vaccines 2011;10(2):163–73. Review. [DOI] [PubMed] [Google Scholar]
- [90].Gatera M et al. Successive introduction of four new vaccines in Rwanda: High coverage and rapid scale up of Rwanda’s expanded immunization program from 2009 to 2013. Vaccine 2016;34(29):3420–6. [DOI] [PubMed] [Google Scholar]
- [91].Glassman A Beyond methods and studies: building institutions for better public spending on vaccination. (Special Issue: Evidence Base for Vaccine Introduction in Latin America and the Caribbean.). Vaccine 2013; 31: (Suppl. 3): pp. C10–C11. [DOI] [PubMed] [Google Scholar]
- [92].Glatman-Freedman A et al. Factors affecting the introduction of new vaccines to poor nations: a comparative study of the Haemophilus influenzae type B and hepatitis B vaccines. PLoS ONE [Electronic Resource] Comparative Study 2010;5(11):e13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Gordon WS, Jones A, Wecker J. Introducing multiple vaccines in low- and lower-middle-income countries: issues, opportunities and challenges. Health Policy Plann 2012;27(Suppl 2):ii17–26. Research Support, U.S. Gov’t, Non-P. H.S.. [DOI] [PubMed] [Google Scholar]
- [94].Hajjeh R Accelerating introduction of new vaccines: barriers to introduction and lessons learned from the recent Haemophilus influenzae type B vaccine experience. Philos Trans Roy Soc London - Series B: Biol Sci 2011;366 (1579):2827–32. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Hawkes S, Kismodi E, Larson H, Buse K. Vaccines to promote and protect sexual health: policy challenges and opportunities. Vaccine 2014;32 (14):1610–5. Review. [DOI] [PubMed] [Google Scholar]
- [96].Hinman AR. Perspectives on sustainable vaccine introduction. Vaccine 2013;31(Suppl 3):C8–9. [DOI] [PubMed] [Google Scholar]
- [97].Jauregui B et al. Strengthening the technical capacity at country-level to make informed policy decisions on new vaccine introduction: lessons learned by PAHO’s ProVac Initiative. Vaccine 2011;29(5):1099–106. [DOI] [PubMed] [Google Scholar]
- [98].Jauregui B et al. Evidence-based decision-making for vaccine introductions: Overview of the ProVac International Working Group’s experience. Vaccine 2015;33(Suppl 1):A28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Jauregui B et al. ProVac Global Initiative: a vision shaped by ten years of supporting evidence-based policy decisions. Vaccine 2015;33(Suppl 1): A21–7. [DOI] [PubMed] [Google Scholar]
- [100].Kaddar M, Schmitt S, Makinen M, Milstien J. Global support for new vaccine implementation in middle-income countries. Vaccine 2013;31(Suppl 2): B81–96. Review. [DOI] [PubMed] [Google Scholar]
- [101].Kane MA, Serrano B, de Sanjose S, Wittet S. Implementation of human papillomavirus immunization in the developing world. Vaccine 2012;30 (Suppl 5):F192–200. Review. [DOI] [PubMed] [Google Scholar]
- [102].Koon AD, Rao KD, Tran NT, Ghaffar A. Embedding health policy and systems research into decision-making processes in low- and middle-income countries. Health Res Policy Syst 2013; 11(1): Art. no. 30 [Review]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].La Torre G et al. Guidance for future HTA applications to vaccines: the HPV lesson. Human Vaccines 2011;7(9):900–4. Review. [DOI] [PubMed] [Google Scholar]
- [104].Laurent-Ledru V, Thomson A, Monsonego J. Civil society: a critical new advocate for vaccination in Europe. Vaccine 2011;29(4):624–8. Review. [DOI] [PubMed] [Google Scholar]
- [105].Levine OS et al. The future of immunisation policy, implementation, and financing. Lancet 2011;378(9789):439–48. [DOI] [PubMed] [Google Scholar]
- [106].Madhavi Y et al. Evidence-based National Vaccine Policy. Indian J Med Res 2010;131:617–28. [PubMed] [Google Scholar]
- [107].Mantel C, Wang SA. The privilege and responsibility of having choices: Decision-making for new vaccines in developing countries. Health Policy Plann 2012;27(SUPPL 2):ii1–4. Review. [DOI] [PubMed] [Google Scholar]
- [108].Nghi NQ et al. Human papillomavirus vaccine introduction in Vietnam: formative research findings. Sexual Health 2010;7(3):262–70. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- [109].Perronne C, et al. Implementing efficient and sustainable collaboration between National Immunization Technical Advisory Groups: Report on the 3rd International Technical Meeting, Paris, France, 8–9 December 2014. Vaccine 2016; 34(11): pp. 1325–306 [Research Support, Non-U.S. Gov’t]. [DOI] [PubMed] [Google Scholar]
- [110].Sandberg KI, Andresen S, Bjune G. A new approach to global health institutions? A case study of new vaccine introduction and the formation of the GAVI Alliance. Soc Sci Med 2010;71(7):1349–56. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- [111].Senouci K, et al. The Supporting Independent Immunization and Vaccine Advisory Committees (SIVAC) Initiative: a country-driven, multi-partner program to support evidence-based decision making. (Special Issue: The role of national advisory committees in supporting evidence-based decision making for national immunisation programs.). Vaccine 2010; 28(Supplement 1): pp. A26–A30. [DOI] [PubMed] [Google Scholar]
- [112].Senouci K, Faye PC, Blau J, Silva Ad, Gessner B. Implementation of national immmunization technical advisory groups: countries’ ownership of priority setting and decision-making for immunization programs and policies. Revue Med Trop 2011;71(4):363–6. [Google Scholar]
- [113].Shearer JC, Stack ML, Richmond MR, Bear AP, Hajjeh RA, Bishai DM. Accelerating policy decisions to adopt haemophilus influenzae type B vaccine: a global, multivariable analysis. PLoS Med/Public Library Sci 2010;7(3):. Research Support Non-U.S. Gov’te1000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Tapia-Conyer R, Betancourt-Cravioto M, Saucedo-Martinez R, Motta-Murguia L, Gallardo-Rincon H. Strengthening vaccination policies in Latin America: an evidence-based approach. Vaccine 2013;31(37):3826–33. Review. [DOI] [PubMed] [Google Scholar]
- [115].Toscano CM et al. Cost analysis of an integrated vaccine-preventable disease surveillance system in Costa Rica. Vaccine 2013;31(Suppl 3): C88–93. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Walton LR, Orenstein WA, Pickering LK. The history of the United States Advisory Committee on Immunization Practices (ACIP). Vaccine 2015;33 (3):405–14. Historical Article. [DOI] [PubMed] [Google Scholar]
- [117].Wang SA et al. New vaccine introductions: assessing the impact and the opportunities for immunization and health systems strengthening. Vaccine 2013;31(Suppl 2):B122–8. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Wonodi CB et al. Using social network analysis to examine the decision-making process on new vaccine introduction in Nigeria. Health Policy Plann 2012;27(Suppl 2):ii27–38. Research Support, Non-U.S. Gov’t. [DOI] [PubMed] [Google Scholar]
- [119].Yamashiro H, Cutcliffe N, Dobson S, Fisman D, Gold R. The role of pediatricians as key stakeholders in influencing immunization policy decisions for the introduction of meningitis B vaccine in Canada: The Ontario perspective. Can J Infect Dis Med Microbiol 2015;26(4):183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Zuber PL et al. Sustaining GAVI-supported vaccine introductions in resource-poor countries. Vaccine 2011;29(17):3149–54. [DOI] [PubMed] [Google Scholar]
- [121].de Oliveira LH, Trumbo SP, Ruiz Matus C, Sanwogou NJ, Toscano CM. Pneumococcal conjugate vaccine introduction in Latin America and the Caribbean: progress and lessons learned. Expert Rev Vaccines 2016;15 (10):1295–304. [DOI] [PubMed] [Google Scholar]
- [122].Abdoulaye Alfa D, Houngnihin RA, Ilboudo GP, Dick N, Kaucley L, Essoh TA. Introduction of multi-dose PCV 13 vaccine in Benin: from the decision to vaccinators experience. BMC Public Health 2020;20(1):1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Date K, et al. Decision making and implementation of the first public sector introduction of typhoid conjugate vaccine-Navi Mumbai, India, 2018. Clin Infectious Dis 2020; 71(Supplement_2): pp. S172–S178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lang S, Loving S, McCarthy ND, Ramsay ME, Salisbury D, Pollard AJ. Two centuries of immunisation in the UK (part II). Arch Dis Child 2020;105 (3):216–22. Historical Article. [DOI] [PubMed] [Google Scholar]
- [125].World Health Organization. A guide to introducing a second dose of measles vaccine into routine immunization schedules, ed. Geneva; 2013. [Google Scholar]
- [126].Duclos P, Durrheim DN, Reingold AL, Bhutta ZA, Vannice K, Rees H. Developing evidence-based immunization recommendations and GRADE. Vaccine 2012;31(1):12–9. [DOI] [PubMed] [Google Scholar]
- [127].Mosina L et al. Building immunization decision-making capacity within the World Health Organization European Region. Vaccine 2020;38(33):5109–13. 2020/07/14/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].World Health Organization. Principles and considerations for adding a vaccine to a national immunization programme: from decision to implementation and monitoring. vol. 126; 2014. [Google Scholar]
- [129].Black S The role of health economic analyses in vaccine decision making. Vaccine 2013;31(51):6046–9. Review. [DOI] [PubMed] [Google Scholar]
- [130].Barocchi MA, Black S, Rappuoli R. Multicriteria decision analysis and core values for enhancing vaccine-related decision-making. Sci Transl Med, Review 2016; 8(345), p. 345ps14. [DOI] [PubMed] [Google Scholar]
- [131].Hutubessy R, Henao AM, Namgyal P, Moorthy V, Hombach J. Results from evaluations of models and cost-effectiveness tools to support introduction decisions for new vaccines need critical appraisal. BMC Med 2011;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Hutubessy R Q&A–Economic analyses for vaccine introduction decisions in low- and middle- income countries. BMC Med 2013;11:71. Interview. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Senouci K, et al. Enhancing the use of evidence-based decision-making processes to establish immunization policies and programmes at national level: What should be the roe of Pasteur Institutes?, BMC Proceedings. Conference: Institut Pasteur International Network Annual Scientific Meeting, vol. 5, no. no pagination; 2010. [Google Scholar]
- [134].Adjagba A et al. Supporting countries in establishing and strengthening NITAGs: lessons learned from 5 years of the SIVAC initiative, (in eng). Vaccine 2015;33(5):588–95. [DOI] [PubMed] [Google Scholar]
- [135].Blau J et al. Establishment of a National Immunization Technical Advisory Group in Côte d’Ivoire: process and lessons learned. Vaccine 2012;30 (15):2588–93 (in eng). [DOI] [PubMed] [Google Scholar]
- [136].Madhavan G, Phelps C, Sangha K, Levin S, Rappuoli R. Bridging the gap: need for a data repository to support vaccine prioritization efforts. Vaccine 2015;33(Suppl 2):B34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Global NITAG Network. Strategic document of the Global NITAG Network; 2017, Available: https://www.nitag-resource.org/media-center/document/3864-strategic-document-of-the-global-nitag-network.
- [138].Adjagba A, MacDonald NE, Ortega-Pérez I, Duclos P. “trengthening and sustainability of national immunization technical advisory groups (NITAGs) globally: Lessons and recommendations from the founding meeting of the global NITAG network. Vaccine 2017;35(23):3007–11 (in eng). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Dabanch J et al. Chile’s National Advisory Committee on Immunization (CAVEI): Evidence-based recommendations for public policy decision-making on vaccines and immunization. Vaccine 2019;37(32):4646–50 (in eng). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during this review are available from the corresponding author on reasonable request.


