Abstract
Objectives:
Intracerebral hemorrhage (ICH) has the highest morbidity and mortality rate of any stroke subtype and clinicians often administer prophylactic antiseizure medications (ASMs) as a means of preventing post-stroke seizures, particularly following lobar ICH. However, evidence for ASM efficacy in preventing seizures and reducing disability is lacking given limited randomized trials. Herein, we report analysis from a large prospective observational study that evaluates the effect of primary prophylactic ASM administration on seizure occurrence and disability following ICH.
Materials and methods:
Primary analysis was performed on 1630 patients with ICH enrolled in the ERICH study. A propensity score for administration of prophylactic ASM was developed and patients were matched by the closest propensity score (difference < 0.1). McNemar’s test was used to compare occurrence of in-hospital seizure and disability, defined by modified Rankin Score (mRS) ≥ 3 at 3 months post ICH.
Results:
Of the 815 matched pairs of patients treated with primary prophylactic ASM, there was no significant difference in seizure occurrence (p = 0.4631) or disability (p = 0.4653). Subset analysis of 280 matched pairs of patients with primary lobar ICH similarly revealed no significant difference in seizure occurrence (p = 0.1011) or disability (p = 1.00) between prophylactically treated and untreated patients.
Conclusions:
Although current guidelines do not recommend primary prophylactic ASM following ICH, clinical use remains widespread. Data from the ERICH study did not find an association between administering primary prophylactic ASM and preventing seizures or reducing disability following ICH, thus providing evidence to influence clinical practice and patient care.
Keywords: Stroke, Intracerebral hemorrhage, Seizure, Prospective cohort study, Antiseizure medicine, outcome
Introduction
The current utilization of primary prophylactic antiseizure medication (ASM) following intracerebral hemorrhage (ICH) is controversial given the lack of randomized, controlled trials. Although ICH has been shown to have the highest morbidity and mortality rate of any stroke subtype, the prognostic contribution of seizures is largely unknown. Previous reports have identified an independent association, though not causality, between seizures and early ICH expansion,2 midline shift3 and functional outcomes.4 Therefore, clinical practice has often implemented primary prophylactic ASM and widespread use (estimated between 20% and 40%)5–8 continues despite current guidelines. Our aim in this study was to analyze data from the Ethnic/Racial Variations of Intracerebral Hemorrhage Study (ERICH), one of the largest prospective observational studies of ICH, to evaluate the effect of primary prophylactic ASM administration on seizure occurrence and disability following ICH. The study’s large sample size from 19 independent centers afforded us the ability to perform propensity score analysis in a multi-ethnic population with sufficient power for subset analysis in patients with primary lobar hemorrhage. The data presented, herein, provides an evidence-based evaluation of primary prophylactic ASM following ICH and specifically, lobar ICH.
Seizure onset has been shown to occur at various time points after ICH (i.e. weeks, months, or years post-injury). Prevalence of clinically observed seizures has been documented to range from 1.7% to 31% despite primary prophylactic ASM administration2,3,9 and between 28% and 42% when electrographic seizures were considered.3,10 Location of ICH, particularly primary lobar hemorrhage which has closer proximity to the cortex, is thought to be correlated with increased risk of seizure occurrence.1,3,11–18 Meanwhile, size of hemorrhage has been controversially related to seizure occurrence with some reports identifying potential association19–20 and others not.14,16 Patients who require neurosurgical interventions after ICH, such as placement of external ventricular drains or surgical evacuation, may have increased risk for seizures.21,22 Other confounding factors include sepsis, which may itself, in addition to commonly prescribed antibiotics, lower seizure threshold,23,24 though previous data have not been conclusive. Similarly, heavy alcohol use or illicit drug use may concurrently lower seizure threshold in patients at time of presentation. The variable prevalence of seizure after ICH and diverse clinical presentations complicate establishing set criteria for initiating a randomized control trial. However, the large multiethnic patient population in the ERICH study allows for propensity score analysis of matched pairs as a means of emulating a randomized trial. In this prospective observational study, we were also able to identify and account for certain confounding factors thought to influence administration of primary prophylactic ASM as well as exclude patients with prior history of epilepsy or ASM use.
Though the use of primary prophylactic ASM has not been recommended by the AHA (Class III; Level B) since 2010, widespread use continues and remains controversial. There appears to be an increased preference for levetiracetam given that prior studies have reported an association between phenytoin administration and worse cognitive outcomes when compared with levetiracetam in patients with ICH.8,23,25,26 However, clinical practice regarding implementation of ASM prophylaxis should be determined by evidence-based evaluation of treatment efficacy in preventing seizures and ultimately reducing disability. The analyses presented in this paper address the question of whether primary prophylactic ASM can decrease seizure occurrence after ICH and improve long-term outcome, as measured by the modified Rankin Scale (mRS), using the largest dataset available to date.
Methods
The Ethnic/Racial Variations of Intracerebral Hemorrhage (ERICH) study was a multicenter, prospective, case-control study of self-reported non-Hispanic white, non-Hispanic black and Hispanic patients with ICH that has been previously well described.27 The study utilized 19 clinical recruitment centers with prior approval by the Institutional Review Board to enroll 3000 patients with ICH from September 2010 to October 2015. A total of 42 sites were involved and recruitment was facilitated by “hot-pursuit”: a strategy which involves early identification of cases through active screening of admission logs from the emergency department, general admission logs, neurological intensive care unit, neurology and neurosurgery services to minimize the effect of early mortality following ICH and survival bias. Study participants were ≥ 18 years of age residing within 75 miles of one of the recruitment centers. Participants or legal representatives were provided with informed consent in English or Spanish prior to enrollment and subjects were matched 1:1 with control cases by age (+/− 5 years), sex, ethnicity/race, and metropolitan area.
ICH was defined in the study as a spontaneous focal blood collection within the brain parenchyma causing abrupt onset of a severe headache, focal neurologic deficit, or altered level of consciousness.27,28 Exclusion criteria for enrollment included ICH attributable to trauma, hemorrhagic conversion of ischemic stroke, aneurysm-related, dural venous sinus thrombosis, vascular malformation, malignancy-related coagulopathy, or tumor with hemorrhage. Among items on baseline interview, participants (both cases and controls) were asked to provide information regarding prior diagnosis of epilepsy as well as a list of all medications including use of prior ASMs. Patients who self-reported (or by proxy) prior history of seizure or patients who were first administered ASM for a documented seizure were excluded from the analysis. ASMs were initiated at the discretion of clinicians at each study site. Data for the analyses presented in this manuscript were obtained from chart abstraction into form sections that distinguished ASM administration for seizure prophylaxis or for a documented seizure. Seizure prevalence during the hospitalization was recorded based on the standard practice of the treatment teams and included either clinically witnessed seizures and/or those captured by electroencephalogram (EEG). Seizure prevalence at 90 days was assessed by questionnaire.
Participant characteristics
Researchers across the centers or their proxies were required to interview enrolled participants using a standardized form as well as chart abstraction. Participants were contacted at 90 days for evaluation of mRS which was used to define disability with modified Rankin Score (mRS) ≥ 3. They were also asked about seizure occurrence at 90 days and other complications. From the data collected, characteristics of interest for the present analysis included age, sex, race/ethnicity, location and volume of hemorrhage, presence of intraventricular hemorrhage (IVH), ventriculostomy or surgery during hospitalization, history of ischemic stroke, diabetes, or dementia, sepsis and Glasgow Coma Scale (GCS) on admission, heavy alcohol use or illicit drug use. The following surgical interventions were grouped together for analysis: craniotomy for clot evacuation, stereotactic clot aspiration, and certain other procedures. Heavy alcohol use was defined on the standardized form as self-reported (or by proxy) more than two alcoholic beverages on average per day, drinking ≥ 5 drinks in a single setting within the last 90 days or prior history of alcohol abuse. Self-reported (or by proxy) use of illicit drugs or a positive urine toxicology screen on admission was used to define illicit drug use.
Imaging
Neuroimaging obtained from study participants were de-identified at study sites prior to storage on Digital Imaging and Communications in Medicine (DICOM) software and electronic upload to a dedicated workstation at the neuroimaging repository (Massachusetts General Hospital and University of Arizona - Tucson). Centralized analysis was performed by reviewers who were blinded to all clinical information and using Alice software (Parexel Corporation, Waltham, MA). Hemorrhage volume, hemorrhage location (deep (i.e. basal ganglia or thalamus), lobar, brainstem, cerebellum or primary IVH), as well as presence of IVH was measured by planimetric analysis of initial diagnostic CT images as previously published.29–31
Outcomes
The primary outcome in this study was defined as documented seizure occurrence, while hospitalized after ICH, in study groups treated with primary prophylactic ASM (Prophylactic ASM) or without primary prophylactic ASM (No Prophylactic ASM). Secondary outcome was defined as disability (loss of functional independence), defined as mRS ≥ 3 at 90 days after ICH onset.
Statistical analysis
A propensity score for administration of prophylactic ASM was based on covariates selected a priori based on variables which were thought to influence the clinical decision of providing primary prophylactic ASM (e.g. age, sex, race/ethnicity, ICH volume, location of ICH, presence of IVH, ventriculostomy, surgery, GCS, history of ischemic stroke, diabetes, dementia, sepsis, alcohol use and illicit drug use). A stepwise modeling approach was used to match those individuals, without prior documented or witnessed seizure(s), who received prophylactic ASM with those who did not receive prophylactic ASM. Patients were matched by the closest propensity score with no more than a difference of 0.1. To check that the matches were appropriate, descriptive statistics are presented as mean ± SD, median (IQR) for continuous variables and percentages for categorical variables. Patient characteristics are compared between Prophylactic ASM and No Prophylactic ASM groups with t-test (age) and Wilcoxon rank-sum test (e.g. volume of ICH, GCS). Categorical variables (e.g. sex, race/ethnicity, presence of IVH, ventriculostomy, surgery, history of ischemic stroke, heavy alcohol use, diabetes, dementia, sepsis, and illicit drug use) are compared with Chi-squared test between the groups. Primary analysis was performed on 1630 patients with ICH enrolled in the ERICH study with subset analysis completed in patients with primary lobar hemorrhage (n = 560). McNemar’s test was used to compare the occurrence of in-hospital seizure and disability, defined by mRS ≥ 3 at 90 days post ICH, in the Prophylactic ASM group and No Prophylactic ASM group (P value < 0.05). A similar process was used to calculate the propensity for receiving primary prophylactic ASM and matching among patients with primary lobar hemorrhages, resulting in 280 pairs. McNemar’s test was again used to compare the occurrence of in-hospital seizure and disability, defined by mRS ≥ 3 at 90 days post ICH, in the Prophylactic ASM group and No Prophylactic ASM group (P value < 0.05). Given that mRS data was not available for all study participants at 3-month follow-up, dichotomized analysis of disability (mRS ≥ 3) was only completed for matched pairs with available data (n = 1208 patients with ICH; n = 436 patients with primary lobar hemorrhage). Additional analyses of disability outcome were performed on all patients with available mRS scores using the Cochran Armitage trend test and an ordinal logistical regression analysis with increasing mRS as an outcome measure.
Results
Three thousand participants enrolled in the ERICH study from September 2010 to October 2015. A total of 2540 patients were available for present analysis after excluding patients with prior history of seizure, documented seizure on admission and those patients who were previously taking ASM as secondary prophylaxis or for any underlying condition(s). Of the final ERICH cohort, 815 case-control pairs were matched based on primary prophylactic treatment with ASM (Prophylactic ASM) or no primary prophylactic ASM (No Prophylactic ASM). Among the study participants, 537 were non-Hispanic black, 555 were Hispanic, and 538 were non-Hispanic white.
We first evaluated the covariates thought to influence the propensity to receive primary prophylactic ASM (Table 1). There were no significant differences, after matching, in the following study characteristics among subjects who received ASM prophylaxis (n = 815) versus those who did not (n = 815): age, volume of ICH, race, sex, lobar location of ICH, presence of IVH, ventriculostomy or surgical intervention, history of ischemic stroke, heavy alcohol use, diabetes, dementia, sepsis at time of intake, illicit drug use or GCS on admission. Using a similar approach to calculate the propensity score, we were able to match those who received primary prophylactic ASM to those who did not in order to emulate a randomized trial among patients who had a primary lobar hemorrhage (Table 2). Table 2 demonstrates excellent matching on those variables for the propensity for using primary prophylactic ASM in patients with primary lobar hemorrhage (n = 560), as there was no significant difference between the matched groups.
Table 1.
Demographic data and clinical characteristics of total cohort (n = 1630) after propensity score modeling in Prophylactic ASM and No Prophylactic ASM groups. Data represents n (%) unless otherwise noted. Abbreviations: SD – standard deviation, cc – cubic centimeter volume, IQR – interquartile range, IVH – intraventricular hemorrhage, GCS – Glasgow coma scale.
| Variable | Prophylactic ASM (n = 815) |
No Prophylactic ASM (n = 815) |
P-value |
|---|---|---|---|
| Age: Mean ± SD | 62.0 ± 14.9 | 61.4 ± 14.8 | 0.43 |
| Sex | 0.29 | ||
| Female | 348 (42.7) | 327 (40.1) | |
| Male | 467 (57.3) | 488 (59.9) | |
| Race/Ethnicity | 0.97 | ||
| White non-Hispanic | 270 (33.1) | 268 (32.9) | |
| Black non-Hispanic | 270 (33.1) | 267 (32.8) | |
| Hispanic | 275 (33.7) | 280 (34.4) | |
| Volume (cc): Median; IQR | 15.1; 6.0–32.5 | 13.9; 6.5–30.3 | 0.64 |
| Lobar Location | 307 (37.7) | 296 (36.3) | 0.57 |
| Presence of IVH | 380 (46.6) | 393 (48.2) | 0.52 |
| Ventriculostomy | 191 (23.4) | 196 (24.0) | 0.77 |
| Surgery | 74 (9.1) | 73 (9.0) | 0.93 |
| History of Ischemic Stroke | 82 (10.1) | 98 (12.0) | 0.21 |
| Heavy Alcohol Use | 88 (11.0) | 73 (9.2) | 0.23 |
| Diabetes | 231 (28.4) | 208 (25.6) | 0.20 |
| Dementia | 33 (4.0) | 35 (4.3) | 0.80 |
| Sepsis | 41 (5.0) | 35 (4.3) | 0.48 |
| Illicit Drug Use | 95 (11.7) | 89 (10.9) | 0.64 |
| GCS: Median; IQR | 14; 10—15 | 14; 10—15 | 0.70 |
Table 2.
Demographic data and clinical characteristics of matched pairs after propensity score modeling of patients with primary lobar hemorrhage (n = 560) in Prophylactic ASM and No Prophylactic ASM groups. Data represents n (%) unless otherwise noted. Abbreviations: SD – standard deviation, cc – cubic centimeter volume, IQR – interquartile range, IVH – intraventricular hemorrhage, GCS – Glasgow coma scale.
| Variable | Prophylactic ASM (n = 280) |
No Prophylactic ASM (n = 280) |
P-value |
|---|---|---|---|
| Age: Mean ± SD | 66.3 ± 15.0 | 68.0 ± 14.9 | 0.17 |
| Sex | 1.00 | ||
| Female | 141 (50.4) | 141 (50.4) | |
| Male | 139 (49.6) | 139 (49.6) | |
| Race/Ethnicity | 0.06 | ||
| White non-Hispanic | 123 (43.9) | 146 (52.1) | |
| Black non-Hispanic | 77 (27.5) | 55 (19.6) | |
| Hispanic | 80 (28.6) | 79 (28.2) | |
| Volume (cc): Median; IQR | 24.2; 11.2–49.6 | 22.9; 10.9–50.8 | 0.71 |
| Presence of IVH | 92 (32.9) | 80 (28.6) | 0.27 |
| Ventriculostomy | 16 (5.7) | 15 (5.4) | 0.85 |
| Surgery | 31 (11.1) | 23 (8.2) | 0.25 |
| History of Ischemic Stroke | 32 (11.4) | 37 (13.2) | 0.52 |
| Heavy Alcohol Use | 18 (6.5) | 13 (4.7) | 0.35 |
| Diabetes | 71 (25.4) | 80 (28.6) | 0.39 |
| Dementia | 22 (7.9) | 27 (9.6) | 0.45 |
| Sepsis | 4 (1.4) | 9 (3.2) | 0.16 |
| Illicit Drug Use | 18 (6.4) | 18 (6.4) | 1.00 |
| GCS: Median; IQR | 14; 12—15 | 15; 12—15 | 0.61 |
Among the 815 patients in the Prophylactic ASM group, 85.3% received levetiracetam (n = 695) and 8.5% received phenytoin (n = 69). Patients who received both levetiracetam and phenytoin accounted for 3.7% patients (n = 30). Meanwhile, 2.6% patients in the Prophylactic ASM group (n = 21) received alternative ASM.
In the matched pair analysis (Fig. 1A), there was no significant difference in seizure prevalence when comparing hospitalized patients who were receiving primary prophylactic ASM versus those who did not receive primary prophylactic ASM (p = 0.4631). Of the 1630 patients in total cohort, 97 individuals (6.0%) had documented seizure(s). Among those patients treated with primary prophylactic ASM, 52 patients (6.4%) had seizures. Meanwhile, 45 patients (5.5%) had seizures in the group of patients not treated with primary prophylactic ASM. Subsequent subset analysis of patients with primary lobar hemorrhage (n = 560) was performed in matched pairs (Fig. 1B). Seizure(s) were reported in 51 individuals with primary lobar hemorrhage (9.1%). Among the matched pairs, seizures were documented in 20 patients (7.1%) in the Prophylactic ASM group and 31 patients in the No Prophylactic ASM group (11.1%). There was no significant difference (p = 0.1011) between the matched pairs when evaluating seizure activity in patients with primary lobar hemorrhage treated with primary prophylactic ASM and those without primary prophylactic ASM.
Fig. 1.
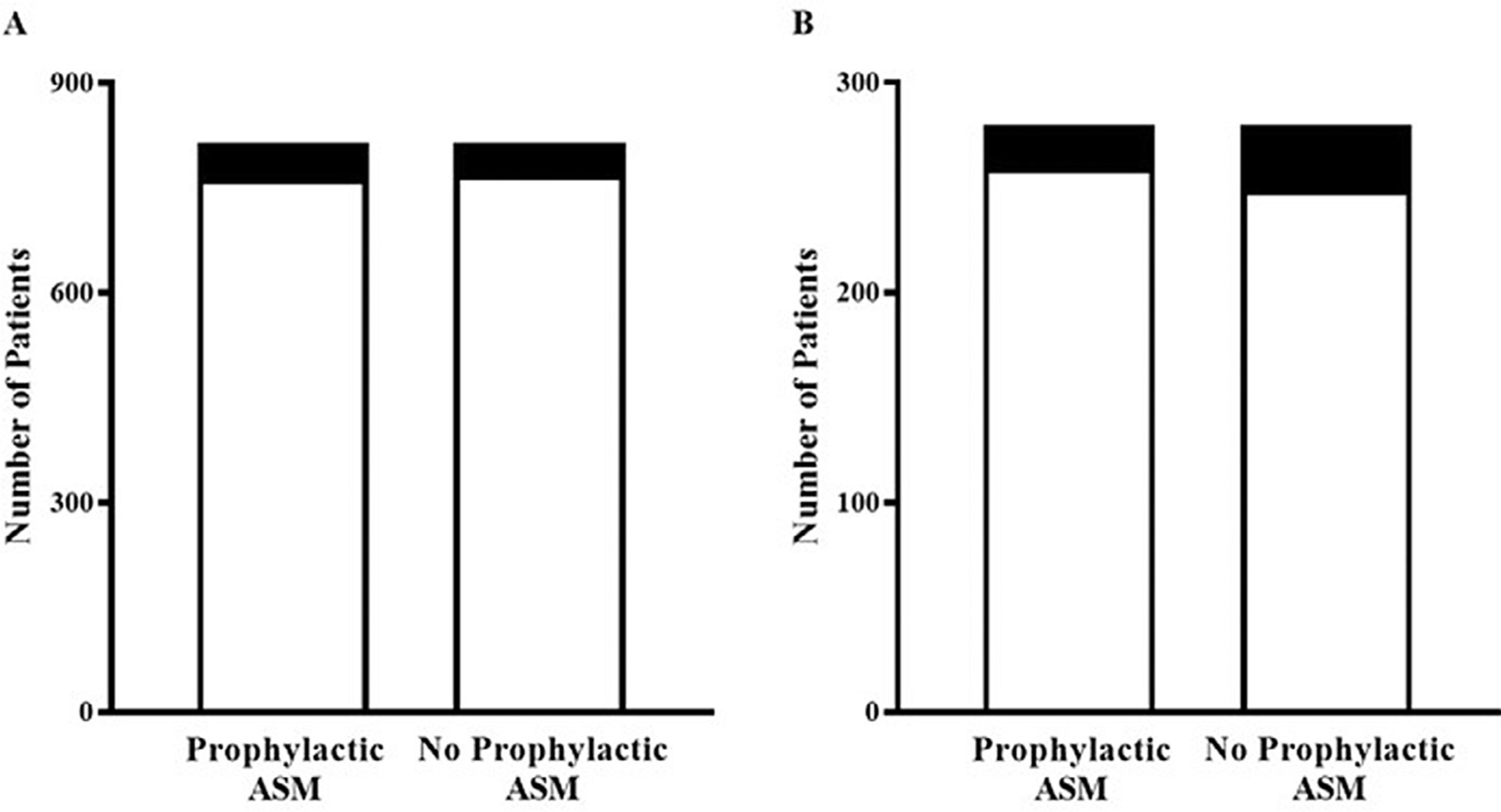
Primary prophylactic ASM did not reduce seizure occurrence among (A) total ICH patients (n = 1630) or in a subset analysis of (B) primary lobar hemorrhage patients (n = 560) during their initial hospitalization.
Legend: □ No Seizure ■ Seizure
Unfavorable outcome was defined by the loss of functional independence mRS ≥ 3 at 90 days post ICH. Of the 1208 patients with ICH (Fig. 2A), 401 patients (33.2%) were functionally independent (mRS < 3), and 807 patients (66.8%) were no longer functionally independent (mRS ≥ 3) at 90 days post ICH. Analysis was performed on matched pairs of patients in the Prophylactic ASM and No Prophylactic ASM groups; there was no significant difference between the matched pairs with regards to disability at 90 days post ICH (p = 0.4653). Similar analysis was performed on subset population of patients with primary lobar hemorrhage (Fig. 2B). Among 436 patients with lobar ICH, 172 patients (39.4%) were functionally independent (mRS < 3), and 264 patients (60.6%) were no longer functionally independent (mRS ≥ 3) at 90 days post ICH. No significant difference was found with occurrence of disability at 90 days between the matched pairs in patients with primary lobar hemorrhage (p = 1.000). Analysis of mRS scores using the Cochran Armitage trend test revealed no significant difference between Prophylactic ASM and No Prophylactic ASM groups for the total cohort (p = 0.8551) and primary lobar hemorrhage subset (p = 0.6698). Furthermore, ordinal logistic regression models revealed no significant difference favoring Prophylactic ASM over No Prophylactic ASM in the total cohort (odds ratio 0.99; 95% confidence interval: 0.82–1.18) or primary lobar hemorrhage subset (odds ratio 0.93; 95% confidence interval: 0.68–1.28).
Fig. 2.
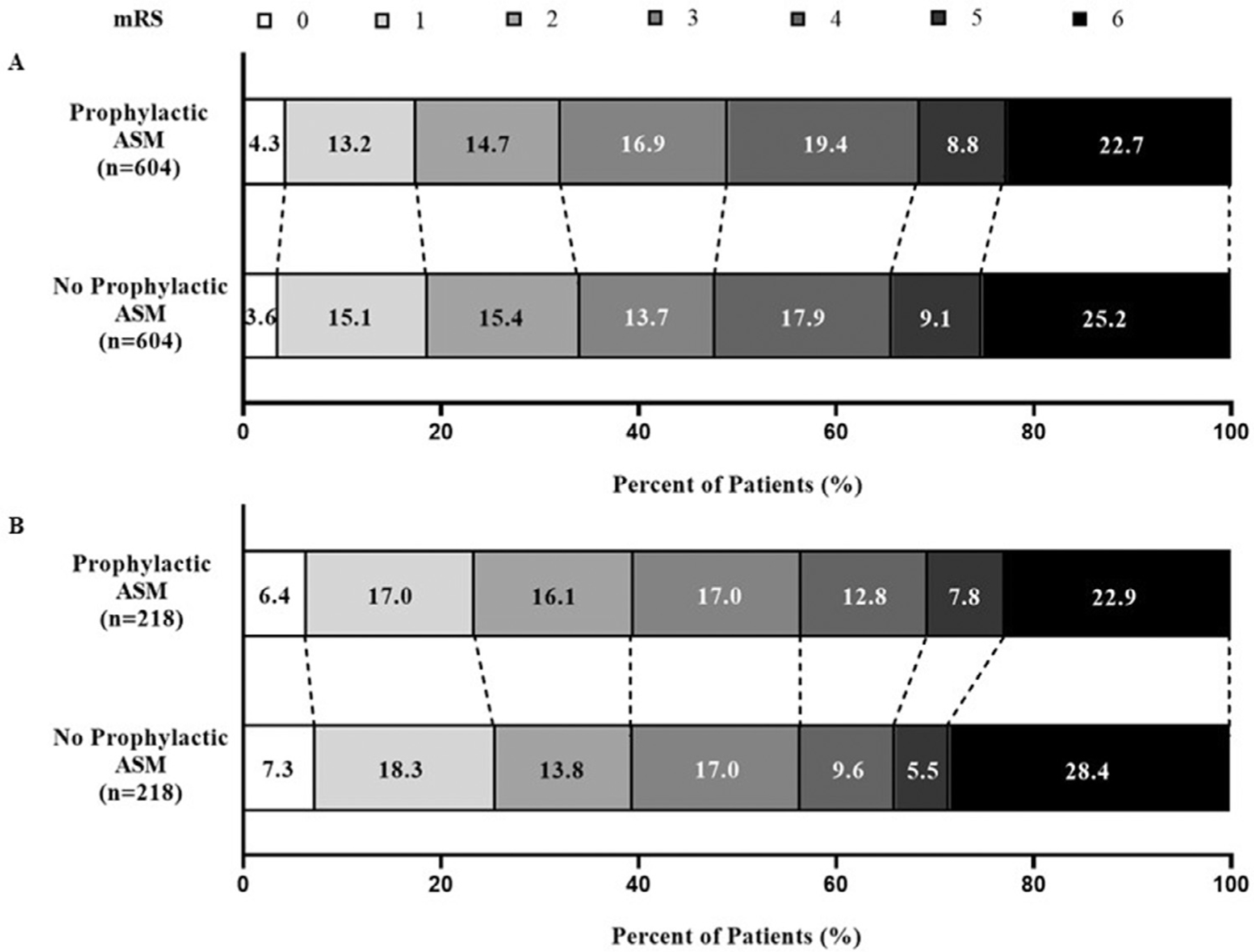
Primary prophylactic ASM administration did not significantly influence disability (mRS ≥ 3) at 90 days among total ICH patients (A; n = 1208) or patients with primary lobar hemorrhage (B; n = 436).
Discussion
As the largest and most ethnically diverse prospective observational study of patients with ICH to date, the present study evaluated the effect of primary prophylactic ASM administration on in-hospital seizure occurrence and 90-day disability by comparing matched pairs (Prophylactic ASM versus No Prophylactic ASM) using propensity scores calculated from multivariable logistic regression. Although primary prophylactic ASM use is often implemented in clinical practice to prevent seizures in patients with ICH, our data reveal no significant difference in seizure occurrence between Prophylactic ASM and No Prophylactic ASM groups (Fig. 1A). Additionally, our data indicate no significant influence of primary prophylactic ASM on 90-day disability after ICH (Fig. 2A). Though the analyses presented herein are consistent with prior studies, the large multi-ethnic patient population in the ERICH study further allowed for exclusion of patients with prior history of epilepsy or ASM use and propensity score analysis was utilized to account for certain confounding factors thought to influence administration of primary prophylactic ASM (Table 1). Furthermore, the analysis of matched pairs allowed us to emulate a randomized trial among patients who had primary lobar hemorrhage (Table 2) which revealed no significant difference for in-hospital seizure occurrence (Fig. 1B) or 90-day disability (Fig. 2B) in patients treated with primary prophylactic ASM.
There are several potential limitations to the analysis presented in this study. First, despite using prospectively collected data, our current study is limited by the retrospective nature of data analysis. Data were obtained from chart abstraction and only available if reported into the ERICH study data collection form for individually enrolled patients by each respective site. Second, there is a possibility of survival bias given the high mortality in ICH patients and the uncertainty of developing post-injury seizures in this population. Of the 1630 patients incorporated into our study analyses, there was no appreciable difference between treatment groups in the “do not resuscitate” code status (Prophylactic ASM: 15.8% yes, 83.2% no, 1.0% unknown; No Prophylactic ASM: 16.0% yes, 82.1% no, 2.0% unknown) nor comfort care designation (Prophylactic ASM: 11.7% yes, 87.1% no, 1.2% unknown; No Prophylactic ASM: 11.5% yes, 86.4% no, 2.1% unknown). The methodology implemented in the ERICH study attempts to reduce survival bias using “hot pursuit” which required patients to be screened and enrolled within 48 hours of admission. While the study’s exclusion criteria (e.g., ICH attributable to trauma, hemorrhagic conversion of ischemic stroke, aneurysm-related, dural venous sinus thrombosis, vascular malformation, malignancy-related coagulopathy, or tumor with hemorrhage) was established to avoid confounding variables, it is plausible that patients at risk for seizure development or increased disability were excluded from our data analyses. Furthermore, patients who were lost to follow-up limited the sample size of patients evaluated for 90-day disability between Prophylactic ASM and No Prophylactic ASM groups.
Our present study was not designed to stratify seizure occurrence into categories such as early, late, convulsive or non-convulsive status epilepticus. Previous studies have reported 4.0% to 23.5% early seizures10–12,14,23,32–39 compared with 2.6% to 15.6% late seizures10,14,17,18,20,23,32,38–42 in ICH patients. Prevalence of convulsive status epilepticus in ICH patients has been estimated between 4% and 60%.1,15,23,37 However, approximately 22% to 76% of ICH patients are thought to have non-convulsive seizures which would be difficult to identify without EEG monitoring.2,3 Seizure occurrence was identified and documented at the discretion of providers at each study site. Therefore, a documented seizure may have been a witnessed clinical seizure, clinical seizure captured on EEG, or a subclinical seizure captured on EEG. The inconsistent use of electroen-cephalogram (EEG) across the ERICH study may not have captured a proportion of patients having subclinical seizures of uncertain significance. As a result, it is possible that the prevalence of seizures may be underestimated in our sample for this study. Furthermore, the variable prevalence and categorization of seizure subtypes may have implications for determining the utility of primary prophylactic ASM after ICH for a specific subset of patients and their prognosis.
Substantial clinical curiosity has warranted development of scoring systems to help with prognostication. For example, the CAVE Score has been validated to estimate the risk of late seizures > 7 days after ICH based on cortical involvement (1 point), age < 65yrs (1 point), volume > 10cc (1 point) and early seizure < 7 days of ICH (1 point).43,44 The data analysis presented herein did allow for subset analysis in a large group of patients with primary lobar hemorrhage and revealed no significant difference in seizure occurrence while hospitalized, despite administration of primary prophylactic ASM. While the etiology of ICH was not always reported or determined at the time of chart abstraction, future studies accounting for the etiology of ICH as a determinant of seizure prophylaxis initiation or efficacy may yield useful information. Our data set unfortunately does not allow us to distinguish whether patients had seizure onset early or late in their clinical course, nor the duration of ASM administration in this context of seizure timing as it relates to injury. Subset analysis of ERICH study participants by Kwon et al. found that the risk of developing late seizures following ICH is increased by younger age, large volume hematoma, surgical evacuation and lobar ICH.44 Future studies may wish to clarify the timing of seizure occurrence to further investigate the utility of the CAVE score in helping predict a subset of patients who may benefit from primary prophylactic ASM after ICH.
Although it has previously been considered that certain factors may increase seizure risk following ICH (i.e. ICH volume and location, surgical intervention, sepsis, heavy alcohol or illicit drug use, etc.), our analysis of data from the ERICH study did not identify a subpopulation of ICH patients which would benefit from primary prophylactic ASM administration. The current study did account for craniotomy for clot evacuation, stereotactic clot aspiration, and certain other procedures. Though surgical interventions have previously been thought to be a risk factor for seizures, likely given the presumption of cortical white matter disruption, our data does not indicate a significant influence of surgical intervention on in-hospital seizure occurrence between the groups. However, our analysis did group various surgical interventions into either surgery or ventriculostomy subsets. Perhaps future studies with larger sample sizes may be able to stratify data based on the specific surgical intervention implemented.
Post hoc analysis of the ERICH study revealed no independent association of primary prophylactic ASM with unfavorable mRS at 90 days,23 and our data presented herein similarly suggests the same. As a prospective observational study, we were unable to control for ASM selection, dosing range, time of medication initiation (e.g., within 24 hours of admission) or scheduled duration of treatment throughout hospitalization or at the 90-day timepoint. Data entry in this study additionally did not require study sites to report continuous sedative infusions (e.g. propofol, midazolam or pentobarbital) which may have been implemented as antiseizure treatment or for sedative purposes. The use of prophylactic ASM based on admission protocols certainly has the potential to add bias to the study; however, there was only one site of the 19 enrollment centers that administered prophylactic ASM to all lobar hemorrhage patients (n = 20) enrolled in the ERICH study. Otherwise, the administration of prophylactic ASM was variable among the centers (Supplemental Table 1).
Most patients enrolled in this study were administered levetiracetam (n = 695; 85.3%), though other ASM in descending order of use includes phenytoin, lorazepam, phosphenytoin, and midazolam. There have been reports of phenytoin being associated with worse cognitive and functional outcomes when compared with levetiracetam in patients with ICH.8,23,25,26 Additional favorable features that have likely contributed to the increased popularity of levetiracetam include more predictable pharmacokinetics and therapeutic range, less reported drug interactions or allergies, and predominantly renal clearance. However, Naidech et al. more recently reported that the administration of prophylactic levetiracetam during hospitalization for ICH was independently associated with lower cognitive function at follow-up when assessed by Neuro-QOL™ (Quality of Life in Neurologic Disorders) as a determinant of health-related quality of life (HRQoL).45 Although domain-specific HRQoL assessment tools, such as the validated46 Neuro-QOL™ or PROMIS® (NIH Patient-Reported Outcomes Measurement Information System), provide meaningful information that may not be captured by ordinal versus dichotomous forms of the mRS, widespread utilization into clinical trials may be limited due to the self-reported subjectivity and cumbersome administration. Future prospective trials may benefit from incorporating diverse patient-centered outcome measurement tools and may also consider either dose-response or dose-escalation study designs to further evaluate the effect of ASM on seizure occurrence and disability following ICH.
Summary (Conclusions)
The variable onset and prevalence of seizure after ICH undeniably complicates establishing set criteria for treatment guidelines. In 1999, the AHA guidelines proposed 1-month administration of primary prophylactic ASM followed by a taper or discontinuation if there was no clinical evidence of seizure activity during that time (Level of Evidence V, Grade C Recommendation).47 Almost 10 years later, in 2007, the AHA recommendation for primary prophylactic ASM after ICH was primarily thought to reduce the risk of early seizures and specifically in those patients with lobar ICH.48 Updated guidelines in 2010 suggested that data to support primary prophylactic ASM administration after ICH was limited in terms of long-term benefit of neurologic outcome, prevention of seizures and mortality.9,25,49 Thus, the AHA’s only Class I recommendations for ASM implementation is in patients with seizures (Class I; Level A) and patients with a change in mental status with electrographic evidence of seizures on EEG (Class I; Level C).49
Gilmore et al proposed an algorithmic approach to implementing seizure prophylaxis with risk stratification based on clinical, radiographic and encephalographic parameters and repeated assessments to guide therapy initiation or duration.23 This algorithm aligns with the AHA recommendation and is further supported by the analyses presented in this paper. Our clinical practice regarding implementation of ASM should be based on evidence-based data regarding treatment efficacy in preventing seizures and ultimately reducing disability. Data from the large prospective ERICH study did not find an association between administering primary prophylactic ASM and preventing seizure prevalence or reducing disability following ICH and even more specifically, primary lobar hemorrhage. Thus, the analysis presented from the largest and most ethnically diverse prospective observational study of patients with ICH to date provides additional evidence to influence clinical practice and patient care.
Supplementary Material
Grant Support:
The study was supported by the National Institute of Neurological Diseases and Stroke (NINDS U01NS069763). The funding entity had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Sources of funding
The study was supported by the National Institute of Neurological Diseases and Stroke (NINDS U01NS069763). The funding entity had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Declaration of Competing Interest
Dr. Moomaw is supported by U01NS069763. Dr. Sheth is supported by U24NS107215, U24NS107136, U01NS106513, R01NR018335, and AHA17CSA33550004; received research funding from Hyperfine, Bard, Novartis, and Biogen; received consulting fees from Zoll Medical Corporation; and holds equity in Alva Health and Astrocyte Pharmaceuticals Inc. Dr. Woo is supported by U01NS036695, T32NS047996, U01NS069763, and R01NS100417. The other authors report no conflicts or disclosures.
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jstrokecerebrovasdis.2021.106143.
References
- 1.DeHerdt V, Dumont F, Henon H, et al. Early seizures in intracerebral hemorrhage: incidence, associated factors, and outcome. Neurology 2011;77:1794–1800. [DOI] [PubMed] [Google Scholar]
- 2.Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology 2007;69:1356–1365. [DOI] [PubMed] [Google Scholar]
- 3.Vespa PM, O’Phelan K, Shah M, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology 2003;60:1441–1446. [DOI] [PubMed] [Google Scholar]
- 4.Arntz RM, Maaijwee NA, Rutten-Jacobs LC, et al. Epilepsy after TIA or stroke in young patients impairs long-term functional outcome: the FUTURE Study. Neurology 2013;81:1907–1913. [DOI] [PubMed] [Google Scholar]
- 5.Battey TW, Falcone GJ, Sheth KN, et al. Authors’ reply: confounding by indication in retrospective studies of intracerebral hemorrhage: antiepileptic treatment and mortality. Neurocrit Care 2013;18:287–288. [DOI] [PubMed] [Google Scholar]
- 6.Naidech AM, Beaumont J, Jahromi B, et al. Evolving use of seizure medications after intracerebral hemorrhage: a multicenter study. Neurology 2017;88:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddig RT, Nixdorf KE, Jensen MB. The prophylactic use of an antiepileptic drug in intracerebral hemorrhage. Clin Neurol Neurosurg 2011;113:895–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheth KN, Martini SR, Moomaw CJ, et al. Prophylactic antiepileptic drug use and outcome in the ethnic/racial variations of intracerebral hemorrhage study. Stroke 2015;46:3532–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messe SR, Sansing LH, Cucchiara BL, et al. Prophylactic antiepileptic drug use is associated with poor outcome following ICH. Neurocrit Care 2009;11:38–44. [DOI] [PubMed] [Google Scholar]
- 10.Garrett MC, Komotar RJ, Starke RM, et al. Predictors of seizure onset after intracerebral hemorrhage and the role of long-term antiepileptic therapy. J Crit Care 2009;24:335–339. [DOI] [PubMed] [Google Scholar]
- 11.Alberti A, Paciaroni M, Caso V, et al. Early seizures in patients with acute stroke: frequency, predictive factors, and effect on clinical outcome. Vasc Health Risk Manag 2008;4:715–720. [PMC free article] [PubMed] [Google Scholar]
- 12.Beghi E, D’Alessandro R, Beretta S, et al. Incidence and predictors of acute symptomatic seizures after stroke. Neurology 2011;77:1785–1793. [DOI] [PubMed] [Google Scholar]
- 13.Berger AR, Lipton RB, Lesser ML, et al. Early seizures following intracerebral hemorrhage: implications for therapy. Neurology 1988;38:1363–1365. [DOI] [PubMed] [Google Scholar]
- 14.Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol 2000;57:1617–1622. [DOI] [PubMed] [Google Scholar]
- 15.DeReuck J, Hemelsoet D, VanMaele G. Seizures and epilepsy in patients with a spontaneous intracerebral haematoma. Clin Neurol Neurosurg 2007;109:501–504. [DOI] [PubMed] [Google Scholar]
- 16.Passero S, Rocchi R, Rossi S, et al. Seizures after spontaneous supratentorial intracerebral hemorrhage. Epilepsia 2002;43:1175–1180. [DOI] [PubMed] [Google Scholar]
- 17.Woo KM, Yang SY, Cho KT. Seizures after spontaneous intracerebral hemorrhage. J Korean Neurosurg Soc 2012;52:312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang TM, Lin WC, Chang WN, et al. Predictors and outcome of seizures after spontaneous intracerebral hemorrhage. Clinical article. J Neurosurg 2009;111:87–93. [DOI] [PubMed] [Google Scholar]
- 19.Kammersgaard LP, Olsen TS. Poststroke epilepsy in the Copenhagen stroke study: incidence and predictors. J Stroke Cerebrovasc Dis 2005;14:210–214. [DOI] [PubMed] [Google Scholar]
- 20.Okuda S, Takano S, Ueno M, et al. Clinical features of late-onset poststroke seizures. J Stroke Cerebrovasc Dis 2012;21:583–586. [DOI] [PubMed] [Google Scholar]
- 21.Foy PM, Copeland GP, Shaw MD. The natural history of postoperative seizures. Acta Neurochir 1981;57:15–22. (Wien). [DOI] [PubMed] [Google Scholar]
- 22.Foy PM, Copeland GP, Shaw MD. The incidence of postoperative seizures. Acta Neurochir 1981;55:253–264. (Wien). [DOI] [PubMed] [Google Scholar]
- 23.Gilmore EJ, Maciel CB, Hirsch LJ, et al. Review of the utility of prophylactic anticonvulsant use in critically Ill patients with intracerebral hemorrhage. Stroke 2016;47:2666–2672. [DOI] [PubMed] [Google Scholar]
- 24.Krakow K, Sitzer M, Rosenow F, et al. Predictors of acute post stroke seizures. Cerebrovasc Dis 2010;30:584–589. [DOI] [PubMed] [Google Scholar]
- 25.Naidech AM, Garg RK, Liebling S, et al. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke 2009;40:3810–3815. [DOI] [PubMed] [Google Scholar]
- 26.Taylor S, Heinrichs RJ, Janzen JM, et al. Levetiracetam is associated with improved cognitive outcome for patients with intracranial hemorrhage. Neurocrit Care 2011;15: 80–84. [DOI] [PubMed] [Google Scholar]
- 27.Woo D, Rosand J, Kidwell C, et al. The ethnic/racial variations of intracerebral hemorrhage (ERICH) study protocol. Stroke 2013;44:e120–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh KB, Sekar P, Langefeld CD, et al. Monocyte count and 30-day case fatality in intracerebral hemorrhage. Stroke 2015;46:2302–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flibotte JJ, Hagan N, O’Donnell J, et al. Warfarin, hematoma expansion, and outcome of intracerebral hemorrhage. Neurology 2004;63:1059–1064. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein JN, Fazen LE, Snider R, et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology 2007;68:889–894. [DOI] [PubMed] [Google Scholar]
- 31.Kumar MA, Rost NS, Snider RW, et al. Anemia and hematoma volume in acute intracerebral hemorrhage. Crit Care Med 2009;37:1442–1447. [DOI] [PubMed] [Google Scholar]
- 32.Berges S, Moulin T, Berger E, et al. Seizures and epilepsy following strokes: recurrence factors. Eur Neurol 2000;43:3–8. [DOI] [PubMed] [Google Scholar]
- 33.Conrad J, Pawlowski M, Dogan M, et al. Seizures after cerebrovascular events: risk factors and clinical features. Seizure 2013;22:275–282. [DOI] [PubMed] [Google Scholar]
- 34.Goswami RP, Karmakar PS, Ghosh A. Early seizures in first-ever acute stroke patients in India: incidence, predictive factors and impact on early outcome. Eur J Neurol 2012;19:1361–1366. [DOI] [PubMed] [Google Scholar]
- 35.Hundozi Z, Shala A, Boshnjaku D, et al. Hypertension on admission is associated with a lower risk of early seizures after stroke. Seizure 2016;36:40–43. [DOI] [PubMed] [Google Scholar]
- 36.Labovitz DL, Hauser WA, Sacco RL. Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology 2001;57:200–206. [DOI] [PubMed] [Google Scholar]
- 37.Mecarelli O, Pro S, Randi F, et al. EEG patterns and epileptic seizures in acute phase stroke. Cerebrovasc Dis 2011;31:191–198. [DOI] [PubMed] [Google Scholar]
- 38.Serafini A, Gigli GL, Gregoraci G, et al. Are early seizures predictive of epilepsy after a stroke? Results of a population-based study. Neuroepidemiology 2015;45:50–58. [DOI] [PubMed] [Google Scholar]
- 39.Wang G, Jia H, Chen C, et al. Analysis of risk factors for first seizure after stroke in Chinese patients. Biomed Res Int 2013;2013:702871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilad R, Boaz M, Dabby R, et al. Are post intracerebral hemorrhage seizures prevented by anti-epileptic treatment? Epilepsy Res 2011;95:227–231. [DOI] [PubMed] [Google Scholar]
- 41.Neshige S, Kuriyama M, Yoshimoto T, et al. Seizures after intracerebral hemorrhage; risk factor, recurrence, efficacy of antiepileptic drug. J Neurol Sci 2015;359:318–322. [DOI] [PubMed] [Google Scholar]
- 42.Rossi C, DeHerdt V, Dequatre-Ponchelle N, et al. Incidence and predictors of late seizures in intracerebral hemorrhages. Stroke 2013;44:1723–1725. [DOI] [PubMed] [Google Scholar]
- 43.Haapaniemi E, Strbian D, Rossi C, et al. The CAVE score for predicting late seizures after intracerebral hemorrhage. Stroke 2014;45:1971–1976. [DOI] [PubMed] [Google Scholar]
- 44.Kwon SY, Obeidat AZ, Sekar P, et al. Risk factors for seizures after intracerebral hemorrhage: ethnic/racial variations of intracerebral hemorrhage (ERICH) study. Clin Neurol Neurosurg 2020;192:105731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Naidech AM, Beaumont J, Muldoon K, et al. Prophylactic seizure medication and health-related quality of life after intracerebral hemorrhage. Crit Care Med 2018;46:1480–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cella D, Lai JS, Nowinski CJ, et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology 2012;78:1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Broderick JP, Adams HP, Barsan W, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the stroke council, American heart association. Stroke 1999;30:905–915. [DOI] [PubMed] [Google Scholar]
- 48.Adams HP, delZoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American heart association/American stroke association stroke council, clinical cardiology council, cardiovascular radiology and intervention council, and the atherosclerotic peripheral vascular disease and quality of care outcomes in research interdisciplinary working groups: the American academy of neurology affirms the value of this guideline as an educational tool for neurologists. Stroke 2007;38:1655–1711. [DOI] [PubMed] [Google Scholar]
- 49.Hemphill JC, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the american heart association/American stroke association. Stroke 2015;46:2032–2060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


