Abstract
Objectives:
To investigate changes in blood pressure (BP) among pediatric patients with sickle cell disease (SCD) and determine the variables that might influence these changes.
Methods:
A total of 100 pediatric patients with SCD who followed up in the pediatric outpatient clinic were recruited for this retrospective cohort study. Clinical data included anthropometric measures, average systolic and diastolic BP recorded during multiple follow-up visits, hemoglobin (Hb) level, serum creatinine, and hemoglobin S percentage. Blood pressure measurements were categorized according to the guidelines of the American Academy of Pediatrics (AAP, 2017).
Results:
In this cohort, 68% of the patients had normal systolic BP, 13% had elevated systolic BP, 17% had stage 1 hypertension (HTN), while only 2% reported stage 2 HTN. Patients who were overweight had relatively high systolic BP compared to patients who were underweight (p=0.034) or had normal weight (p=0.023). The average systolic BP significantly correlates with body mass index (r= 0.377, p<0.001) and serum creatinine (r=0.369, p<0.001).
Conclusion:
Pediatric overweight SCD patients exhibited higher average systolic BP than those underweight or normal weight. Body mass index and serum creatinine significantly influenced the average systolic BP more than the Hb level or Hb S percentage.
Keywords: blood pressure, body mass index, sickle cell disease, hypertension, overweight
Sickle cell disease (SCD) is a chronic form of hemolytic anemia with an autosomal recessive inheritance. It is caused by a mutation in the beta-globin chain of the hemoglobin molecule with the substitution of glutamic acid with valine, explaining the aberrant development of erythrocytes into a distinctive sickle cell shape. The clinical manifestations of SCD vary according to the mutation type, which includes hemoglobin (Hb) SA disease (sickle cell trait), hemoglobin SS disease, and hemoglobin SC disease. Patients with SCD may present with typical hemolytic anemia and severe symptoms such as vaso-occlusive crisis (painful crisis), splenic sequestration crisis, and end-organ dysfunction.1,2
The interaction between increased blood viscosity due to sickle hemoglobin polymerization, increased stroke volume, and decreased peripheral resistance can significantly determine blood pressure (BP) status in pediatric SCD patients. Blood pressure status is a crucial physiological measure because hypertension and hypotension can negatively affect the health of individuals. In addition to influencing long-term health outcomes, growth, and development, high BP can affect short-term health outcomes.3,4
Despite abundant studies on the adverse effects and treatment of SCD, the association between BP and SCD in pediatric populations needs to be better understood. Several studies have shown that SCD patients have lower BP than the general population.5 Other studies have found no differences in BP between patients with SCD and healthy controls.4 However, some studies reported hypertension in a significant proportion of patients with SCD.6,7 In Saudi Arabia, the prevalence of SCD is significant, with 4.2% of the general population carrying the sickle cell trait while 0.26% carrying SCD.8
Notably, BP fluctuations in pediatric patients with SCD have yet to be studied in Saudi Arabia. This research aimed to investigate changes in BP among pediatric patients with SCD in Saudi Arabia and to determine the variables that might influence these changes.
Methods
The present investigation is a retrospective cohort study carried out at King Abdulaziz University Hospital, Jeddah, Saudi Arabia. The cohort comprised 100 individuals diagnosed with SCD, <18 years and were followed up in the outpatient clinics between January 2015 and December 2022. Patients with cardiovascular or renal structural abnormalities were excluded. The parents provided informed consent, and the ethical board approved the study (Reference No 347-22).
Age, weight, height, body mass index (BMI), pulse rate, systolic, and diastolic BP were collected. The BP measurements were performed with the digital non-invasive automatic monitor for arterial BP during routine follow-up in the pediatric outpatient clinic and not during hospitalization for vaso-occlusive crisis. The systolic and diastolic BP was measured. An average of 2 to 3 readings were taken during outpatient visits within 3 months. Only one reading is recorded for each patient on the medical record system during each visit. The average systolic and diastolic BP were measured, and BP was graded according to the AAP 2017 guidelines.9 Blood pressure measurements were graded as normal, elevated, or stage 1 or 2 hypertension (HTN). The BMI percentiles of patients were then classified as underweight, normal weight, overweight, and obese based on the classification by the Center for Disease Control and Prevention (CDC) of BMI categories for children and teens.10 The most recent results regarding serum creatinine, Hb level, and Hb S percentage (HbS%) were collected. The estimated glomerular filtration rate (eGFR) was computed utilizing the bedside Schwartz formula [eGFR=0.413*height/serum creatinine]. In this formula, height is entered in cm, and creatinine is entered as mg/dl after conversion of the unit of serum creatinine measurement from μmol/L to mg/dl by dividing the creatinine values by 88.4.11
Statistical analysis
Jamovi software (The jamovi project [2022]. jamovi, version 2.3) was used for statistical analysis. The Shapiro-Wilk test tested the normality of numerical variables. Numerical data were presented as mean, standard deviation, median, interquartile range (25th-75th percentile), minimum, and maximum. Numbers and percentages were used to represent nominal variables. To compare 2 unrelated groups, we employed the parametric Student’s t-test for quantitative variables that exhibited normal distribution and the non-parametric Mann-Whitney U test for those that did not. In contrast, we used a one-way ANOVA followed by post hoc tests to compare multiple unrelated groups. Pearson and Spearman correlation coefficients were used to examine the correlation between variables, and a p-value of <0.05 was considered statistically significant.
Results
One hundred patients with sickle cell anemia were enrolled in this retrospective cohort study. The minimum age was 7 years, the maximum was 17 years, and the average was 14.75 years. The average weight was 41.93 kg, the average BMI was 18.5, and the average recent Hb level at the time of BP recording was 8.5 g/dl. The mean Hb S level was 69.4%, the mean serum creatinine level was 36.1 μmol/L, and the mean eGFR was 161. Table 1 shows the patients’ clinical data.
Table 1.
- Numeric clinical data of all patients (N=100).
| Characteristics | Mean±SD | Min | Max | Percentiles | ||
|---|---|---|---|---|---|---|
| 25th | 50th | 75th | ||||
| Age (years) | 14.75±2.60 | 7.00 | 17.0 | 13.00 | 16.00 | 17.00 |
| Weight (kg) | 41.93±12.78 | 14.95 | 81.7 | 31.88 | 41.00 | 50.20 |
| Height (cm) | 149.04±12.38 | 101.00 | 172.0 | 141.38 | 150.00 | 158.00 |
| BMI | 18.52±4.07 | 11.97 | 30.4 | 15.37 | 17.75 | 20.70 |
| BMI percentile | 33.08±33.84 | 1.00 | 97.0 | 2.75 | 20.00 | 63.25 |
| Pulse rate (BPM) | 93.25±12.88 | 64.00 | 131.0 | 85.75 | 94.00 | 101.00 |
| Hb level (gm/dl) | 8.51±1.52 | 5.30 | 13.1 | 7.70 | 8.45 | 9.10 |
| Hb S% | 69.40±20.41 | 11.20 | 94.9 | 54.55 | 78.40 | 85.85 |
| Creatinine (μmol/L) | 36.1±10.6 | 16.0 | 74.0 | 29.0 | 34.0 | 41.5 |
| eGFR (ml/min/1.73m2) | 161.1±45.6 | 82.0 | 357.0 | 128.8 | 155.0 | 185.0 |
| Average systolic BP (mmHg) | 115.06±10.46 | 95.33 | 151.5 | 107.00 | 114.25 | 121.00 |
| Average diastolic BP (mmHg) | 65.94±9.30 | 43.50 | 110.0 | 60.92 | 65.50 | 70.00 |
BMI: body mass index, BP: blood pressure, BPM: beat per minute, Hb: hemoglobin, eGFR: estimated glomerular filtration rate, Min: minimum, Max: maximum
Male patients constituted 55% of all cases. Regarding BMI, 54% of the patients had an average BMI, 30% were underweight, 14% were overweight, and only 2% were obese. Considering average systolic BP and based on the 2017 AAP grading of HTN, most patients (68%) had normal systolic BP, 13% had elevated systolic BP, 17% had stage 1 HTN, 2% had stage 2 HTN. Table 2 presents the categorical clinical information of the study participants.
Table 2.
- Categorical clinical data of all patients.
| Characteristics | n (%) |
|---|---|
| Gender | |
| Male | 55 (55) |
| Female | 45 (45) |
| BMI | |
| Underweight | 30 (30) |
| Normal weight | 54 (54) |
| Overweight | 14 (14) |
| Obese | 2 (2) |
| Systolic BP | |
| Normal | 68 (68) |
| Elevated | 13 (13) |
| Stage 1 HTN | 17 (17) |
| Stage 2 HTN | 2 (2) |
| Diastolic BP | |
| Normal | 89 (89) |
| Elevated | 4 (4) |
| Stage 1 HTN | 6 (6) |
| Stage 2 HTN | 1 (1) |
| BP category | |
| Normal | 68 (68) |
| Abnormally elevated | 32 (32) |
BP: blood pressure, BMI: body mass index, HTN: hypertension
The BMI percentiles categorized the patients into 4 distinct ordinal groups (underweight, average, overweight, and obese). These groups did not differ significantly regarding recent Hb levels or average diastolic BP. The overweight group had substantially higher average systolic BP (123 mmHg) than the underweight and normal weight groups (113.6 mmHg; p=0.019). A comparison between the patient groups based on BMI categories is shown in Table 3.
Table 3.
-Comparison between groups regarding body mass index (BMI) categories.
| BMI Category | n | Mean±SD | SE | P-value |
|---|---|---|---|---|
| Age (years) | ||||
| Underweight | 30 | 14.93±2.333 | 0.426 | 0.920 |
| Normal weight | 54 | 14.57±2.744 | 0.373 | |
| Overweight | 14 | 15.07±2.731 | 0.730 | |
| Obese | 2 | 14.50±3.536 | 2.500 | |
| Average systolic BP (mmHg) | ||||
| Under weight§ | 30 | 113.64±10.151 | 1.853 | 0.019* |
| Normal weight§ | 54 | 113.65±10.024 | 1.364 | |
| Overweight§ | 14 | 123.36±10.282 | 2.748 | |
| Obese | 2 | 116.67±0.943 | 0.667 | |
| Average diastolic BP (mmHg) | ||||
| Underweight | 30 | 64.60±9.216 | 1.683 | 0.739 |
| Normal weight | 54 | 66.01±8.831 | 1.202 | |
| Overweight | 14 | 68.63±11.776 | 3.147 | |
| Obese | 2 | 65.42±1.532 | 1.083 | |
| Pulse rate (BPM) | ||||
| Underweight | 30 | 95.33±12.767 | 2.331 | 0.298 |
| Normal weight | 54 | 90.50±12.589 | 1.713 | |
| Overweight | 14 | 98.86±12.532 | 3.349 | |
| Obese | 2 | 97.00±15.556 | 11.000 | |
| Hemoglobin level (gm/dl) | ||||
| Underweight | 30 | 8.43±1.443 | 0.263 | 0.481 |
| Normal weight | 54 | 8.44±1.447 | 0.197 | |
| Overweight | 14 | 8.64±1.652 | 0.442 | |
| Obese | 2 | 10.70±3.394 | 2.400 | |
SD: standard deviation, SE: standard error, BP: blood pressure, BPM: beat per minute
: Statistically significant,
: post-Hoc test (underweight-overweigh,p=0.034), (normal weight-overweight, p=0.023).
Patients were separated into 2 groups based on their average systolic BP: i) normotensive group, which had a normal average systolic BP, and ii) hypertensive group, which comprised patients with elevated BP, stage 1 HTN, and stage 2 HTN. Age, Hb level, and HbS% did not differ significantly between the normotensive and hypertensive groups. The hypertensive group had considerably greater body weight, BMI, and pulse rate than the normotensive group, as seen in Table 4. The average systolic BP showed a statistically significant positive correlation with weight, height, BMI, heart rate, and creatinine. In contrast, the average systolic BP correlates negatively to the eGFR, as shown in Table 5 and Figure 1.
Table 4.
- Comparison between normotensive and hypertensive groups.
| High BP (yes/no) | Percentiles | |||||
|---|---|---|---|---|---|---|
| n | Mean±SD | 25th | 50th | 75th | P-value | |
| Age (years) | ||||||
| Normotensive | 68 | 14.91±2.45 | 14.00 | 16.00 | 17.00 | 0.552 |
| Hypertensive | 32 | 14.41±2.92 | 12.75 | 16.00 | 17.00 | |
| Weight (kg) | ||||||
| Normotensive | 68 | 39.93±11.96 | 30.00 | 39.25 | 45.75 | 0.017 |
| Hypertensive | 32 | 46.18±13.61 | 37.92 | 48.05 | 56.50 | |
| Height (cm) | ||||||
| Normotensive | 68 | 148.27±11.42 | 140.75 | 150.00 | 156.00 | 0.191 |
| Hypertensive | 32 | 150.67±14.28 | 144.38 | 152.25 | 160.88 | |
| Body mass index | ||||||
| Normotensive | 68 | 17.84±3.74 | 15.32 | 17.26 | 19.28 | 0.022 |
| Hypertensive | 32 | 19.95±4.43 | 16.92 | 19.16 | 23.39 | |
| Pulse rate (BPM) | ||||||
| Normotensive | 68 | 89.93±11.36 | 83.75 | 91.00 | 98.00 | <.001 |
| Hypertensive | 32 | 100.31±13.22 | 93.50 | 98.00 | 108.50 | |
| Serum creatinine (μmol/L) | ||||||
| Normotensive | 66 | 35±10.2 | 29.0 | 33.0 | 40.0 | 0.142 |
| Hypertensive | 29 | 38.7±11.2 | 32.0 | 38.0 | 47.0 | |
| eGFR (ml/min/1.73m2) | ||||||
| Normotensive | 66 | 165.8±48.4 | 134.1 | 155.1 | 193.3 | 0.213 |
| Hypertensive | 29 | 150.6±37.1 | 119.9 | 151.0 | 175.1 | |
| Hb level (gm/dl) | ||||||
| Normotensive | 68 | 8.62±1.60 | 7.77 | 8.50 | 9.15 | 0.311 |
| Hypertensive | 32 | 8.28±1.32 | 7.52 | 8.15 | 9.03 | |
| Normotensive | 50 | 68.75±20.70 | 52.82 | 76.55 | 86.10 | 0.825 |
| Hb S % | ||||||
| Hypertensive | 21 | 70.96±20.11 | 65.60 | 79.50 | 85.70 | |
BP: blood pressure, Hb: hemoglobin, eGFR: estimated glomerular filtration rate, BPM: beat per minute
Table 5.
- Correlation between weight, height, BMI, and average systolic blood pressure.
| Correlated parameters | Correlation coefficient (r) | P-value |
|---|---|---|
| Average Systolic blood pressure | ||
| BMI | 0.377 | <0.001 |
| Weight | 0.525 | <0.001 |
| Height | 0.498 | <0.001 |
| Pulse rate | 0.264 | 0.008 |
| Hb level | 0.122 | 0.226 |
| Serum creatinine | 0.369 | <0.001 |
| eGFR | -270 | 0.008 |
| Hb S% | -0.011 | 0.927 |
BMI: body mass index, Hb: hemoglobin, eGFR: estimated glomerular filtration rate
Figure 1.
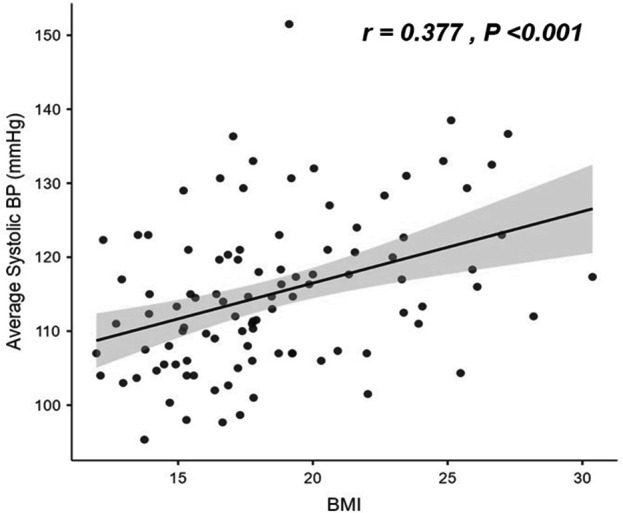
- Correlation between body mas index (MBI) and average systolic blood pressure. BP: blood pressure
Discussion
Changes in BP among patients with SCD have varied in previous studies. In this cohort, 13% of the study population had elevated BP. In comparison, 17% had stage 1 HTN, and 2% had stage 2 HTN. This contrasts with earlier studies that reported lower BP readings in individuals with SCD.5,12
Prehypertension is characterized by BP readings that exceed the 90th percentile but fall below the 95th percentile. Our study revealed a prehypertension prevalence of 13%, consistent with several other studies’ findings. For instance, Shatat et al13 reported a prehypertension prevalence of 10% among adolescent patients with SCA, whereas Becker et al14 found a prevalence of 17% in a similar population.
Previous research has described the prevalence of hypertension among patients with SCD owing to a substantially faster decline in renal function. Because the kidney is a highly vascular organ, it is vulnerable to vaso-occlusive events. Hematuria, proteinuria, concentrating defects, renal insufficiency, and hypertension are symptoms of hemodynamic changes caused by chronic anemia, renal hypoxia from recurrent vaso-occlusion, and hemolysis-related endothelial dysfunction that can progress to chronic kidney disease (CKD).15-17
Although our patients had normal renal function test results, subtle renal dysfunction could not be excluded. In this study, the average systolic BP was positively correlated with serum creatinine while negatively correlated to the eGFR. This finding may be related to the close link between renal function reflected by serum creatinine/eGFR and BP.18,19 Usually, serum creatinine levels do not increase except after a significant loss of renal function. Early dysfunction can be detected by measuring unique markers, namely cystatin C, kidney injury molecule 1 (KIM1), and neutrophil-associated gelatinase lipocalin (NGAL), which were not performed in our cohort. Moreover, unlike our study, previous studies were conducted mainly on adult patients whose kidneys were exposed to the previously mentioned risk factors for a prolonged duration compared to pediatric patients.
Hypertension prevalence was 19% among SCD patients in our study; this aligns with the results of a prior investigation in Ghana.7 Other studies have reported SCD prevalence rates ranging from 3.2% to 10% for hypertension.20,21 Bodas et al22 found that 16.7% of children with SCD had abnormal BP (elevated BP or hypertension). Based on clinical BP measurements, Shatat et al13 reported a lower prevalence of HTN (10.3%) among children with SCD during BP measurement in clinic visits. However, ambulatory BP measurements revealed an elevated prevalence of HTN (43.6% in their study population). This implies that our cohort’s actual hypertension prevalence may have been higher than the 19% observed in our study.
Although most previous reports have demonstrated hypertension in SCD patients, few studies have documented normal BP despite abnormally calculated glomerular filtration rates.23,24
According to our data, age, Hb levels, and HbS% did not differ considerably between the normotensive and hypertension groups. In contrast, the hypertensive group had a substantially higher BMI and pulse rate than the normotensive group, and the average systolic BP was positively correlated with BMI and serum creatinine while negatively correlated to the eGFR. Similar to the results reported by and Pegelow et al5 and Oguanobi et al25 who reported a positive correlation between BMI and systolic and diastolic BP, we found a positive correlation between BMI and systolic BP.
Study limitations
The present study exhibits certain limitations. The study design was retrospective, and the sample size was limited. Furthermore, only one Saudi Arabian center was used for the research, which might determine how broadly the findings could be applied. Additionally, ambulatory BP monitoring, urine analysis, albumin-to-creatinine ratio, renal ultrasound, and markers of renal injuries, such as NGAL, cystatin C, and KIM1, were not performed in all patients to determine the effect of functional renal abnormalities on BP. Future research in Saudi Arabia should use larger sample sizes and prospective study designs to validate these results.
In conclusion, patients with SCD who were overweight or obese had a higher average systolic BP than those underweight or normal. In addition, the patients’ BMI affected the average systolic BP substantially more than the Hb level or HbS percentage.
Disclosure. Authors have no conflict of interests, and the work was not supported or funded by any drug company.
Acknowledgment
We would like to thank Editage (www.editage.com) for the English language editing.
Footnotes
References
- 1.Mousa SA, Al Momen A, Al Sayegh F, Al Jaouni S, Nasrullah Z, Al Saeed H, et al. Management of painful vaso-occlusive crisis of sickle-cell anemia: consensus opinion. Clin Appl Thromb Hemost 2010; 16: 365–376. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico A, Mir N, Wilkerson H, Andrikopoulou E, Definity Kanter J., an affinity for painful crisis: a case series describing vaso-occlusive pain crises in sickle cell patients undergoing echocardiogram with Definity contrast. Eur Heart J Case Rep 2021; 5: ytaa555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley M, Hernandez AK, Kuznia AL.. High BP in children and adolescents. Am Fam Physician 2018; 98: 486–494. [PubMed] [Google Scholar]
- 4.Shrivastava S, Kalvit P.. Blood pressure indices in children with sickle cell disease, of Age 8-18 Yrs. at a Tertiary Care Centre in Chhattisgarh- A Cross Sectional Study. J Evol Med.Dent Sci 2020; 9: 1983–1987. [Google Scholar]
- 5.Pegelow CH, Colangelo L, Steinberg M, Wright EC, Smith J, Phillips G, et al. Natural history of BP in sickle cell disease: risks for stroke and death associated with relative hypertension in sickle cell anemia. Am J Med 1997; 102: 171–177. [DOI] [PubMed] [Google Scholar]
- 6.Kupferman JC, Rosenbaum JE, Lande MB, Stabouli S, Wang Y, Forman D, et al. Blood pressure in children with sickle cell disease is higher than in the general pediatric population. BMC Pediatr 2022; 22: 549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benneh-Akwasi Kuma A, Owusu-Ansah AT, Ampomah MA, Sey F, Olayemi E, Nouraie M, et al. Prevalence of relative systemic hypertension in adults with sickle cell disease in Ghana. PLoS ONE 2018; 13: e0190347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alhamdan NA, Almazrou YY, Alswaidi FM, Choudhry AJ.. Premarital screening for thalassemia and sickle cell disease in Saudi Arabia. Genet Med 2007; 9: 372–377. [DOI] [PubMed] [Google Scholar]
- 9.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Subcommittee on screening and management of high blood pressure in children. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017; 140: e20171904. [DOI] [PubMed] [Google Scholar]
- 10.Hampl SE, Hassink SG, Skinner AC, Armstrong SC, Barlow SE, Bolling CF, et al. Clinical practice guideline for the Evaluation and Treatment of Children and Adolescents With Obesity. Pediatrics 2023; 151: e2022060640. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol 2009; 20: 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Homi J, Homi-Levee L, Gentles S, Thomas P, Serjeant G.. Adolescent blood pressure in a cohort study of sickle cell disease. Arch Intern Med 1993; 153: 1233–1236. [PubMed] [Google Scholar]
- 13.Shatat IF, Jakson SM, Blue AE, Johnson MA, Orak JK, Kalpatthi R.. Masked hypertension is prevalent in children with sickle cell disease: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol 2013; 28: 115–120. [DOI] [PubMed] [Google Scholar]
- 14.Becker AM, Goldberg JH, Henson M, Ahn C, Tong L, Baum M, et al. Blood pressure abnormalities in children with sickle cell anemia. Pediatr Blood Cancer 2014; 61: 518–522. [DOI] [PubMed] [Google Scholar]
- 15.Nath KA, Hebbel RP.. Sickle cell disease: renal manifestations and mechanisms. Nat Rev Nephrol 2015; 11: 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haymann J-P, Stankovic K, Levy P, Avellino V, Tharaux P-L, Letavernier E, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin J Am Soc Nephrol 2010; 5: 756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day TG, Drasar ER, Fulford T, Sharpe CC, Thein SL.. Association between hemolysis and albuminuria in adults with sickle cell anemia. Haematologica 2012; 97: 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ataga KI, Derebail VK, Archer DR.. The glomerulopathy of sickle cell disease. Am J Hematol 2014 89: 907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ataga KI, Orringer EP.. Renal abnormalities in sickle cell disease. Am J Hematol 2000; 63: 205–211. [DOI] [PubMed] [Google Scholar]
- 20.Johnson CS, Giorgio AJ.. Arterial blood pressure in adults with sickle cell disease. Arch Intern Med 1981; 141: 891–893. [PubMed] [Google Scholar]
- 21.Gordeuk VR, Sachdev V, Taylor JG, Gladwin MT, Kato G, Castro OL.. Relative systemic hypertension in patients with sickle cell disease is associated with risk of pulmonary hypertension and renal insufficiency. Am J Hematol 2008; 83: 15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bodas P, Huang A, O’Riordan MA, Sedor JR, Dell KM.. The prevalence of hypertension and abnormal kidney function in children with sickle cell disease- a cross sectional review. BMC Nephrol 2013; 14: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imuetinyan BA-I, Okoeguale MI, Egberue GO.. Microalbuminuria in children with sickle cell anemia. Saudi J Kidney Dis Transpl 2011; 22: 733–788. [PubMed] [Google Scholar]
- 24.Aygun B, Mortier NA, Smeltzer MP, Hankins JS, Ware RE.. Glomerular hyperfiltration and albuminuria in children with sickle cell anemia. Pediatr Nephrol 2011; 26: 1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oguanobi NI, Onwubere BJC, Ibegbulam OG, Ike SO, Anisiuba BC, Ejim EC, et al. Arterial blood pressure in adult Nigerians with sickle cell anemia. J Cardiol 2010; 56: 326–331. [DOI] [PubMed] [Google Scholar]


