Abstract
Hippo pathway is a master regulator of development, cell proliferation, stem cell function, tissue regeneration, homeostasis, and organ size control. Hippo pathway relays signals from different extracellular and intracellular events to regulate cell behavior and functions. Hippo pathway is conserved from Protista to eukaryotes. Deregulation of the Hippo pathway is associated with numerous cancers. Alteration of the Hippo pathway results in cell invasion, migration, disease progression, and therapy resistance in cancers. However, the function of the various components of the mammalian Hippo pathway is yet to be elucidated in detail especially concerning tumor biology. In the present review, we focused on the Hippo pathway in different model organisms, its regulation and deregulation, and possible therapeutic targets for cancer treatment.
Keywords: Hippo pathway, Regulation, Cancer metabolism, Immunity, Epigenetics
Introduction
It’s really interesting to imagine the regulation of cell growth and division in a developing organism. During development, how a cell knows that it’s the time to stop growth or to divide to attain a correct body size and shape, and how organ size is specified and maintained in adult life are still a mystery to biologists. Hippo pathway is one of the most important pathways that regulate organ size and maintain tissue homeostasis. Hippo pathway was first identified in Drosophila melanogaster through genetic mosaic screens for the tumor suppressor genes that are involved in the regulation of organ size [1]. The name of the Hippo pathway was coined based on the key component (Hippo kinase) of this pathway. Initially, the Hippo pathway was thought to be well-conserved in mammals [2]. Now, this pathway is also found to exist in other eukaryotes. The Hippo pathway plays a vital role in relaying various signals from upstream cues to regulate cell behavior, growth, proliferation, and tissue homeostasis [3]. Hippo pathway is also activated by contact inhibition when cells reach the highest confluency to inhibit cell growth and to regulate organ size [4].
Dysregulation of the Hippo pathway is apparent in many cancers. Mutations and alterations in the key components of this pathway result in tumorigenesis, aggressiveness, invasion, migration, metastasis, and resistance to treatments [5]. In this review, we envision giving a comparative outline of the Hippo pathway in Drosophila, mammals, C. elegans, and yeast, and its regulation, deregulation, role in different cancers, and possible therapeutic targets for cancer treatment.
Comparative study of Hippo pathway in different model organisms
The Hippo pathway is evolutionarily conserved in different organisms (Figure 1) (Table 1). The core components of the Hippo pathway are kinases, adaptor proteins, and transcription coactivators. The core kinase of this pathway is Hippo in Drosophila [6], mammalian STE20–like kinases (MST1/2)- (Hippo homolog) in mammals [7] and CST1/2 (Hippo homolog) in C. elegans [2]. The two adaptor proteins of the Drosophila Hippo pathway are Salvador (Sav) [8] and Mats (mob as tumor suppressor) [9]. In mammals, these two adaptors are Salvador-1 (Sav homolog of Drosophila) [10] and MOB1 (Mats homolog of Drosophila) [11]. In C. elegans, these are SAV-1 (Sav homolog of Drosophila) and F09A5.4 (Mats homolog of Drosophila) [2]. In Drosophila, the second kinase of the Hippo pathway is Warts (Wts) [12, 13]. In mammals, the Warts homolog is large tumor suppressor kinases 1/2 (LATS1/2) [14], and in C. elegans it is WTS-1 [2]. In Drosophila and C. elegans, the Hippo pathway has only one transcriptional coactivator, named Yorkie (in Drosophila) [15] and YAP-1 (in C. elegans) [2], whereas, mammalian Hippo pathway has two transcriptional coactivators, named YAP (yes-associated protein) and its paralog TAZ (Transcriptional activator with PDZ-binding domain) [16]. Both YAP and TAZ have TEAD binding domain, WW domain, and a transactivation domain (include coil-coil regions, phosphodegron motif, and PDZ binding motif). YAP also has a proline-rich region close to TEAD binding domain, PXXØP site in the TEAD binding domain, and SH3 binding motif [17] (Figure 2).
Figure 1: Components of the Hippo pathway in Drosophila/Mammals, C. elegans and Yeast (S. cerevisiae):
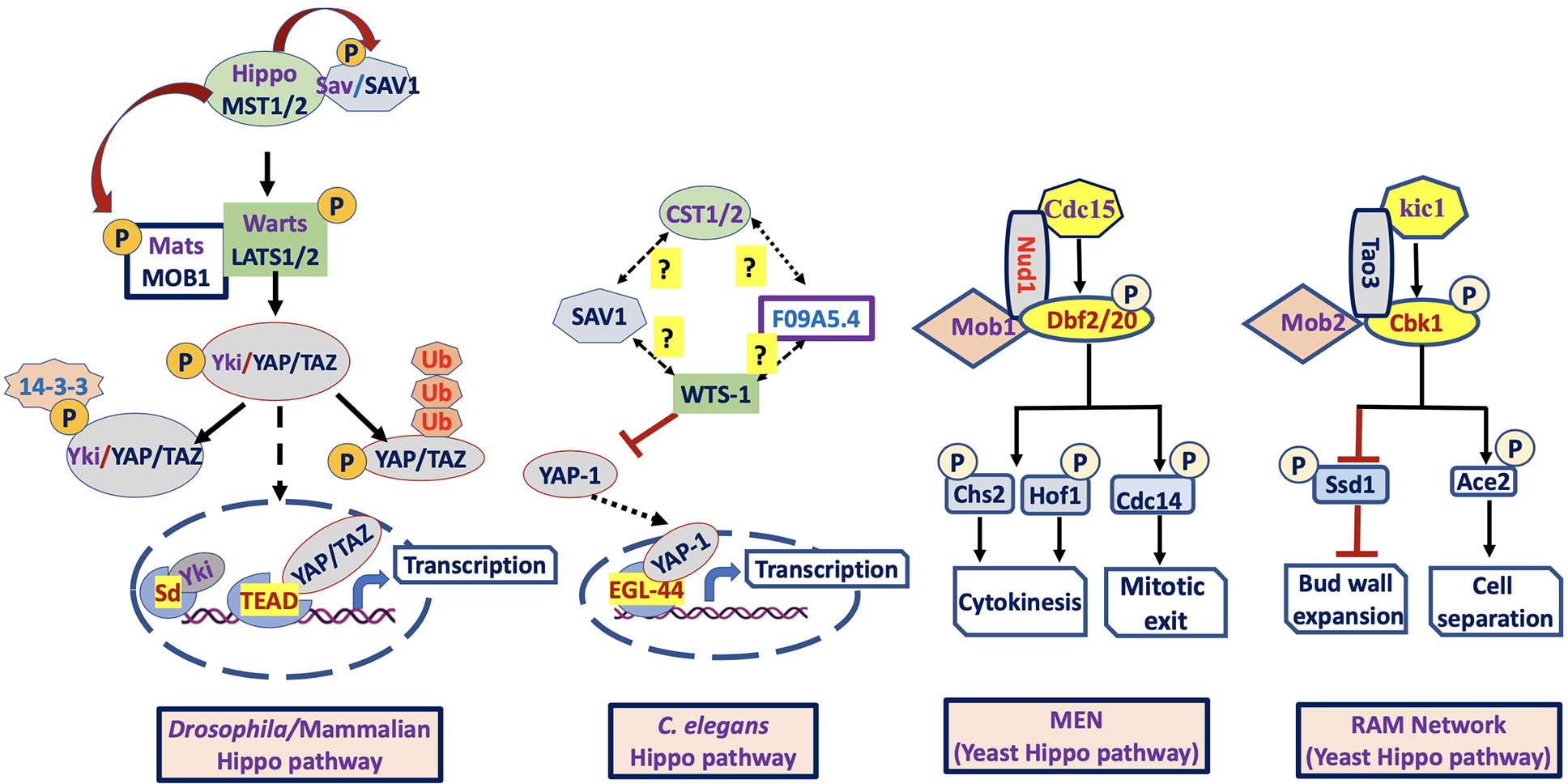
In Drosophila/Mammals (left figure), together with Sav/SAV1 and Mats/MOB1, the Hippo/MST1/2 (kinase) phosphorylates and activates Warts/LATS1/2 (kinase) that in turn phosphorylates and inactivates Yki/YAP/TAZ (downstream effector). Inactivation of hippo pathway results in Yki/YAP/TAZ nuclear translocation and interaction with Sd/TEAD or other transcription factors to induce target gene expression. In C. elegans (middle figure), the role of upstream regulator CST1/2 (Hippo homolog) on the activation of F09A5.4 (Putative Mats homolog), SAV1 (Salvador homolog) and WTS-1 (Warts homolog) is unknown. However, the WTS-1_YAP-1_EGL-44 axis is conserved, supported by the same role as Warts_Yorkie_Scalloped or LATS_YAP_TEAD axis of Hippo pathway. In Yeast (S. cerevisiae), mitotic exit network (MEN) is similar to canonical LATS pathway (Salvador/warts/Hippo system), whereas RAM network is similar to non-canonical NDR pathway (furry/tricomered/Hippo system). The core components of MEN include, Cdc15 (MST/Hippo -related kinase domain), and the protein kinases Dbf2 and Dbf20 (Paralogous protein LATS-family kinases) and Mob1 (co-activator). The core components of RAM network include Kic1 (MST/Hippo related protein kinase), Cbk1 (Ndr/LATS family-related protein kinase), Tao3 (scaffold protein), Mob2, and Hym1 (activator), Ace2 and Ssd1 (downstream effectors).
Table 1:
Core components of the Hippo pathway are conserved in various organisms
| Hippo pathway components in different species | ||||
|---|---|---|---|---|
| Drosophila | Mammals | S. cerevisiae | C. elegans | |
| MEN network | RAN network | |||
| Hippo | MST1/2 | Cdc15 | Kic1 | CST1/2 |
| Sav | SAV1 | Nud1 | Tao3 (not well defined) | SAV-1 |
| Mob | MOB1A/B | Mob1 | Mob2 | F09A5.4 |
| Warts | LATS1/2 | Dbf2/20 | Cbk1 | WTS-1 |
| Yorkie | YAP/TAZ | ? | ? | YAP-1 |
| Scalloped | TEAD | ? | ? | EGL-44 |
Same row-wise components represent functional similarity
Figure 2: Regulatory domains of YAP/TAZ (Hippo pathway effector proteins):
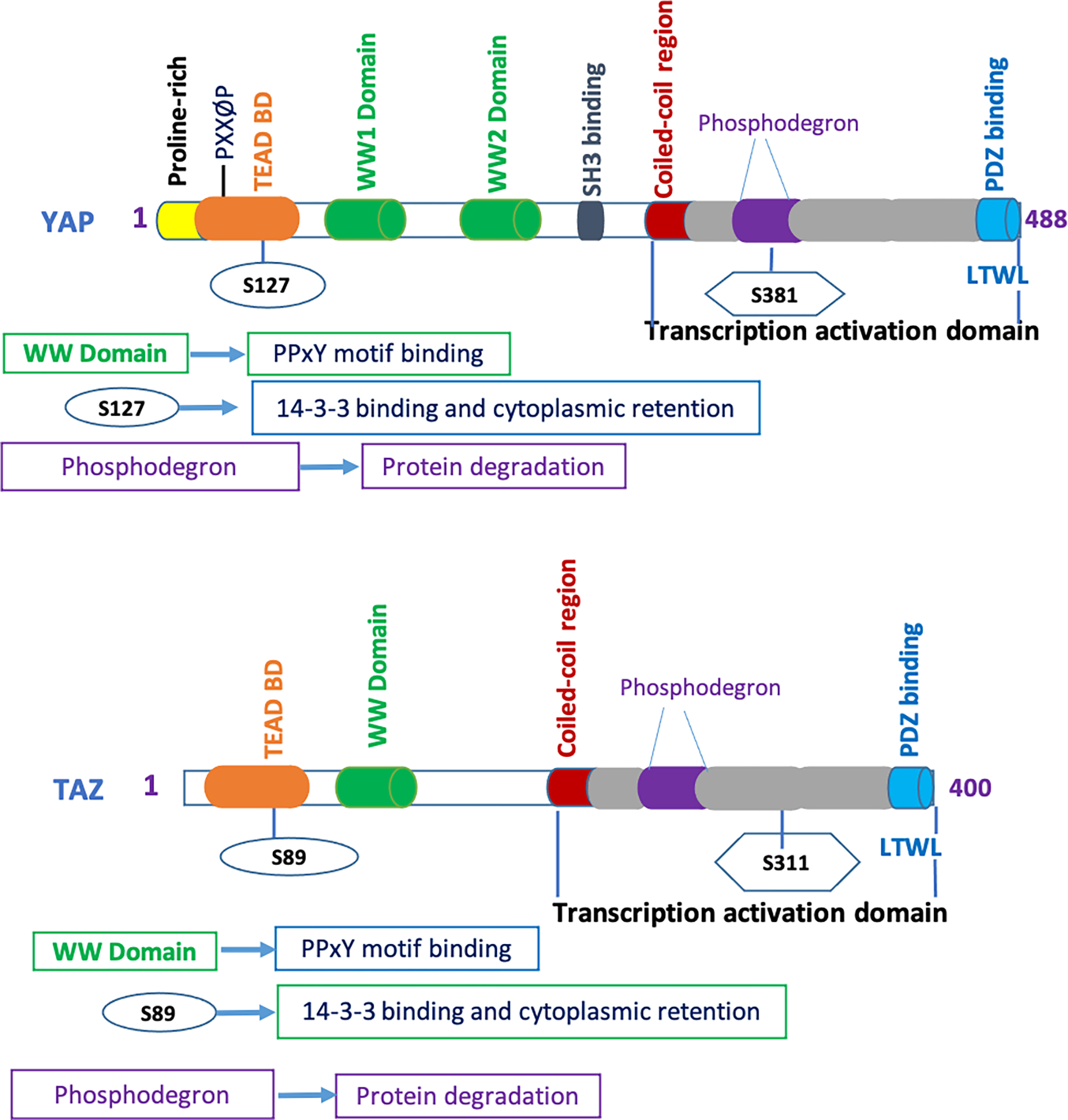
Domains of YAP effector include the Proline-rich domain (P-rich), TEAD binding domain (TEAD BD), two WW domain, SH3-binding domain, coiled-coil domain (CC), transcriptional activation domain (TAD) and PDZ-binding motif. TAZ has TEAD binding domain (TEAD BD), one WW domain, coiled-coil domain (CC), transcriptional activation domain (TAD), and PDZ-binding motif.
In Drosophila/mammals, along with the Sav/SAV1 and Mats/MOB1, Hippo/MST directly phosphorylates and activates Warts/LATS [6, 9, 11, 14, 18, 19] which in turn phosphorylates and inactivates Yki/YAP/TAZ by either cytoplasmic retention or lysosomal/proteosomal degradation [15, 20–22]. Inactivation of the Hippo pathway leads to the activation and nuclear localization of Yki/YAP/TAZ where it interacts with Scalloped (Sd)/TEAD or other transcription factors that ultimately induce expression of target genes that are involved in proliferation, differentiation, and development [23–26]. In C. elegans, the role of core kinase CST1/2 on WTS-1 is still unknown. WTS-1 inhibits the nuclear localization of YAP-1. Inactivation of WTS-1 results in nuclear localization and interaction of YAP-1 with the EGL-44 and induces target gene expression [2].
Hippo pathway exists in budding yeast as well. In budding yeast, the mitotic exit network (MEN) is similar to the canonical LATS pathway (Salvador/warts/Hippo system), whereas the RAM network is similar to the non-canonical NDR pathway (furry/tricomered/Hippo system). In MEN, Cdc15 (Mst/Hippo-related kinase domain) phosphorylates the Nud1 (scaffold protein) and creates a docking site for Dbf2 and Dbf20 (Paralogous protein LATS-family kinases) to be activated [27]. Activated Dbf2 (Kinase)/Mob1(co-activator) activates Cdc14, Chs2 and Hof1 that ultimately regulate mitotic exit and cytokinesis [28–30]. The core components of the RAM network include Kic1 (Mst/Hippo-related protein kinase), Cbk1-Mob2 (Ndr/LATS kinase-Mob complex), Tao3 (scaffold protein), and Hym1 (activator) [31, 32]. Cbk1-Mob2 phosphorylates Ace2 and drives its nuclear localization [33]. Cbk1-Mob2 also allows bud wall expansion by inhibiting RNA binding protein Ssd1 [34].
Regulation of Hippo Signaling Pathway
Hippo signaling pathway is regulated by different intracellular, extracellular, and non-cellular events that modulate the subcellular localization and protein stability of its effector YAP/TAZ to regulate cell behavior, growth, and proliferation (Figure 3).
Figure 3: Regulators and downstream targets of the Hippo pathway:
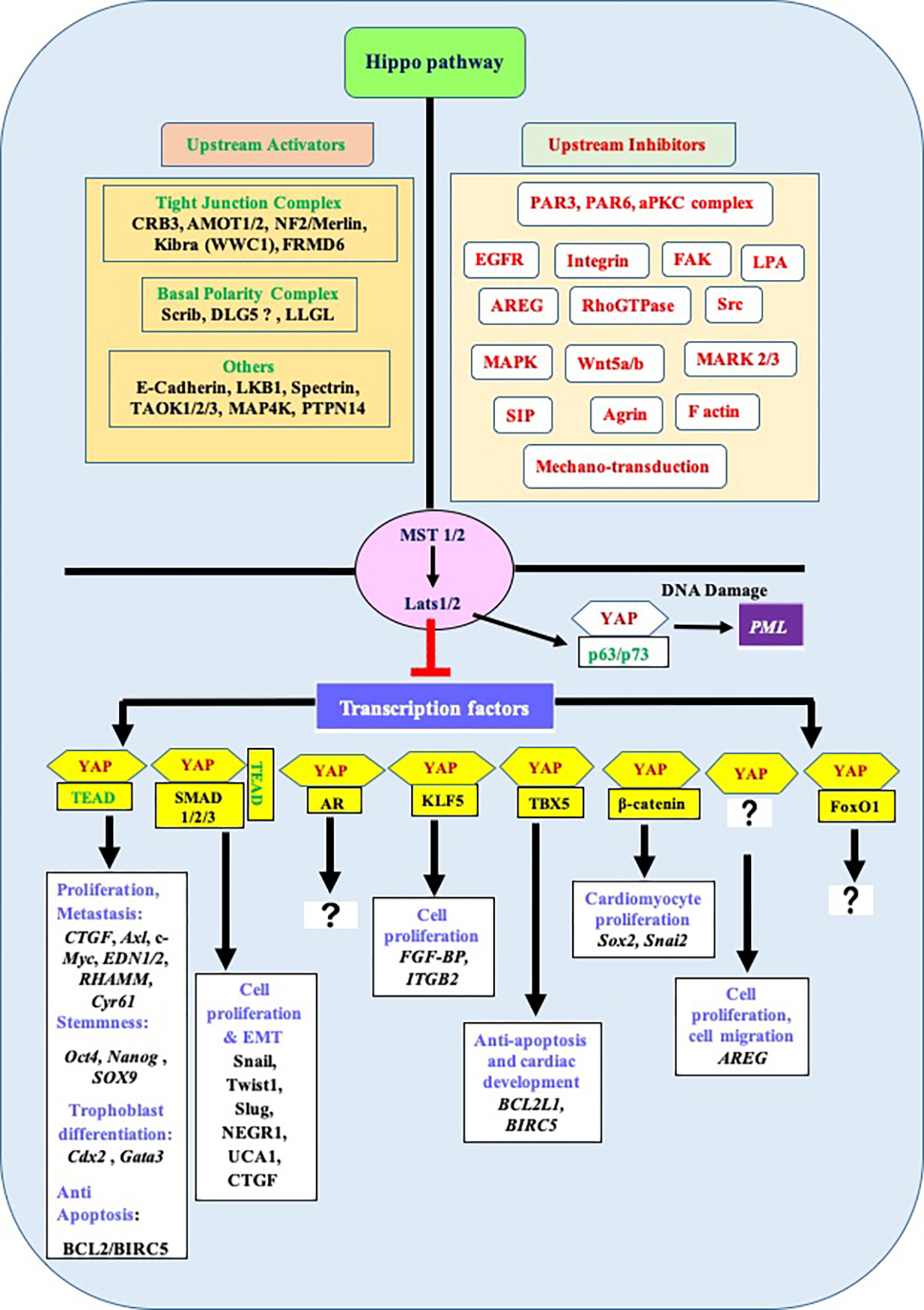
The figure shows upstream activators and inhibitors of the Mammalian Hippo pathway, their binding partners and downstream target genes. Apical polarity complex (Crumbs complex) and basal polarity complex (Scribble complex) as well as cell adhesion molecules such as E-cadherin and signaling molecules positively regulate Hippo pathway (Left side of the figure), whereas, PAR complex and various signaling molecules negatively regulate Hippo pathway (Right side of the figure). These upstream regulators regulate nuclear translocation and binding of YAP/TAZ with different transcription factors that induce expression of genes involved in cell proliferation and migration, stemness, differentiation, apoptosis, DNA damages response etc.
Regulation by cell polarity and cell adhesion molecules
Hippo pathway is regulated by the apical-basal cell polarity and cell adhesion molecules. For example, Crumbs complex (CRB/PATJ/PALS1) that forms apical polarity of cells activates the Hippo pathway by interacting with Angiomotin (AMOTs) family of proteins, FERM domain-containing protein 6 (FRMD6), Kibra (WW domain-containing protein), and Merlin [35]. Scribble complex (SCRIB/DLG/LGL) that form basal polarity regulates Hippo pathway by interacting with the hippo pathway components [36]. E-cadherin, a transmembrane protein of adherens junction, interacts with the cytoskeleton through alpha-catenin and negatively regulates YAP/TAZ function [37]. Protein-tyrosine phosphatase type 14 (PTPN14), another adherens junction component, activates the Hippo pathway by sequestering YAP in the cytoplasm [38].
Regulation by mechanical cues
Hippo pathway is also regulated by different mechanical cues such as cell geometry, extracellular matrix stiffness, cell adhesiveness, mechanical strain, or distortion [39]. For example, ECM stiffness or mechanical stress induces nuclear localization of YAP/TAZ and thus negatively regulates Hippo pathway [40, 41], whereas cell density positively regulates the Hippo pathway by preventing nuclear localization of YAP/TAZ [42].
Regulation by signaling pathways
Apart from cell polarity, mechanical cues, numerous extracellular ligands, and signaling pathways also regulate the Hippo pathway. For example, lysophosphatidic acid (LPA) and sphingosine1-phosphate (S1P) inactivate the Hippo pathway via activation of GPCR [43]. EGFR-Ras-Raf-Mitogen-activated protein kinases (MAPK) signaling axis also regulates Hippo signaling through activation of YAP/TAZ [44]. Amphiregulin (AREG) inhibits the Hippo pathway via EGFR mediated signaling pathway [45]. Integrin-linked kinase (ILK) inactivates the Hippo pathway via inactivation of the merlin through the phosphorylation of MYPT1-PP1 [46]. Integrin- Src signaling axis inactivates the Hippo pathway via triggering translocation of YAP/TAZ to the nucleus [47]. Mitogen-activated protein kinase kinase kinase kinase (MAP4K) activates the Hippo pathway by activating LATS1/LATS2 [48]. Thousand-and-one amino acid kinases (TAOK1/2/3) activate the Hippo pathway via phosphorylating core kinase MST1/MST2 [49]. Furthermore, autophosphorylation at the multiple sites on the MST linker also regulates the Hippo pathway [50] via MST1/2 activation.
Recently a study reported that STK25 suppresses Hippo pathway in human by phosphorylating SAV1 and in turn diminishes the inhibitory action of SAV1 to STRIPAK that ultimately blocks MST1/2 activation [51]. Recently another study reported that the TLK1-NEK1 signaling pathway can also regulate hippo pathway by phosphorylating and stabilizing YAP1, thereby promoting CRPC progression [52]. Our recent study shows that expression of Prostate-Derived ETS Factor (PDEF) in prostate cancer (PC3and DU145) cell lines resulted in increased phospho-YAP1 (Ser127) levels and inhibition of YAP1 activity [53]. To the best of our knowledge, this result provides the first demonstration of inhibiting YAP1 activity by PDEF in any system and suggests crosstalk between PDEF and the Hippo signaling pathway. However, the mechanism by which PDEF regulates the Hippo pathway is yet to be elucidated. Therefore, multiple cellular crosstalks and signaling pathways regulate the Hippo pathway to maintain homeostasis of cellular growth and proliferation.
Regulation by Endosomes
Several studies reported the role of endosomes in regulating the Hippo pathway. In Drosophila, the endosome regulates cytoplasmic Yki levels by concentrating Yki at the late endosome and subsequent lysosome-mediated degradation of Yki. Two endosomal adaptor proteins, Myopic (Mop) (co-localized with Rab7-positive endosomes) [54] and Leash (co-localized with Rab9 late endosome) [55] directly interact with the Yki and drive the endo-lysosomal pathway for Yki degradation [56].
In human, YAP activity is regulated by the phosphatidylserine in the recycling endosomes (REs) as evidenced by the suppression of YAP1 nuclear localization upon knockdown of ATP8A1 (an RE PS-flippase) or evectin-2 (a RE-resident protein) and masking of PS in the cytoplasmic leaflet of membranes [57]. Recycling endosome also regulates Hippo pathway in the mature epithelia as evidence by the disruption of Rab11a directed recycling endosome that results in the activation of Yki/YAP [58]. Recently a study by Block et al. reported that late endosomal/lysosomal adaptor MAPK and mTOR activator (LAMTOR) complex promotes SRC- positive late endosome recycling and subsequent activation of YAP protein. Moreover, LAMTOR complex can also be activated by β1 integrin engagement and mechano-sensitive cues suggesting late endosomal recycling is an important regulator of YAP activity and YAP mechano-sensitivity [59]
Hippo pathway is also regulated by endosomal integral membrane protein, endotubin (EDTB) that binds to the AMOT in sub-confluent cells, and ultimately drives translocation of YAP into the nucleus. In the confluence cells, contact inhibition is mediated by the AMPT: YAP interaction because EDTB preferentially binds to occluding, suggesting a role of the endosomal membrane protein in regulating tight junction protein trafficking and hippo pathway [60].
Several studies reported that α-arrestins domain-containing protein 1 and 3 (ARRDC1 and ARRDC3), members of the α-arrestins family, also regulate Hippo pathway by directly interacting with YAP1, and E3 ubiquitin ligase Itch-mediated ubiquitination, followed by degradation of YAP [61, 62].
Hippo pathway and organ size regulation
Hippo signaling pathway plays an important role in regulating organ size and tissue homeostasis, as evidenced by the overgrowth of Drosophila’s wings, eyes, or other appendages after mutation of Hippo pathway components and upstream regulators. A similar overgrowth phenotype was also observed by transgenic overexpression of Hippo downstream regulator Yki [63, 64]. In transgenic mice, liver-specific expression of Yap causes an increase in liver size and has long-term effects on liver cancer [23, 65]. Similar changes have been observed in liver-specific knockouts of Mst1 and Mst2, Sav1 or Mer [66–70]. Several studies showed enlargement of mouse embryos after the knockout of Hippo upstream regulators such as SAV1, MST1/2, or LATS1/2 [71–73]. Recently a study reported that O-GlcNAcylation on LATS2 disrupts the interaction with MOB1 and thereby activation of YAP/TAZ that promotes cell proliferation and tumor development [74]. Collectively, these compelling genetic studies and post-translational modification of Hippo components depict that the Hippo pathway is indubitably a key regulator of organ size.
Hippo pathway and regulation of stem cell behavior
Hippo pathway regulates stem cell renewal, proliferation, and differentiation. For example, activation of YAP results in reprogramming of the somatic cells into induced pluripotent stem cells, whereas knockdown of YAP causes loss of stem cell pluripotency. Inactivation of YAP triggers Embryonic Stem Cells (ESCs) differentiation [75]. A recent study reported that YAP plays an important role in maintaining trophoblast stemness of the developing placenta in human [76]. An in-vitro study showed that knockdown of TAZ (paralog of YAP) results in the differentiation of human CA1 stem cells [77].
Overexpression of YAP in mouse intestine expands undifferentiated progenitor cells [65]. A similar expansion of neural progenitor cells is also observed when YAP and TEAD are activated in the chicken neural tube model [78]. Higher expression of YAP is also observed in the perivascular cancer stem cell compartment in the subtypes of medulloblastomas indicating that YAP plays an important role in the proliferation and maintenance of cancer stem cells [79]. The role of the Hippo pathway in regulating cancer stem cell proliferation is also shown by the knockout of upstream hippo components such as Merlin, or double knockout of Mst1 and Mst2, or knockout of Sav1 in mice liver which results in accumulation of oval cells and adult liver stem cells that subsequently induce tumor formation [66, 67, 70]. These pieces of evidence support the role of the Hippo pathway in regulating stem cell behavior.
Hippo pathway and cancer metabolism
Energy metabolism is significantly different in cancer cells than the normal cells. Cancer cells have a high demand for glucose, amino acid, and fatty acid for their unrestricted growth and proliferation. This phenomenon is more apparent in the Warburg effect where cancer cells rely on aerobic glycolysis to obtain more energy by converting the glucose to lactate synthesis; whereas, normal tissues depend on mitochondrial oxidative phosphorylation [80].
Hippo pathway is regulated by glucose metabolism and energy levels. Cellular energy stress activates the Hippo pathway by AMP-activated protein kinase (AMPK) (an energy sensor) mediated LATS activation that leads to YAP phosphorylation and inactivation. AMPK can also inactivate YAP, independent of Hippo activation, by disrupting the interaction between YAP and TEAD through direct phosphorylation of YAP at ser 89 that are necessary for their interaction [81]. Glucose metabolism also regulates YAP/TAZ transcriptional activity, as evidenced by the study conducted by Enzo et al. where the main rate-limiting enzyme of glycolysis, phosphofructokinase-1 (PFK-1) was shown to bind to TEAD and enhance the interaction between TEAD and YAP/TAZ which in turn increases the gene transcription [82]. In liver cancer, the high glucose level activates YAP through O-GlcNAcylation at T241 by O-GlcNAC transferase (OGT) [83].
Fat metabolism also regulates YAP/TAZ activity. Mevalonate pathway regulates YAP/TAZ by activating Rho GTPase mediated inhibition of YAP/TAZ phosphorylation and promoting nuclear localization of YAP/TAZ. In Breast cancer, the mevalonate/YAP/TAZ axis stimulates the proliferation and self-renewal of breast cancer cells [84]. During development and tumorigenesis, YAP reprograms glutamine metabolism by inducing glutamine synthase expression and subsequent increase in glutamine levels for nucleotide biosynthesis that acts as the building block for liver growth and tumorigenesis [85]. A recent study suggests that YAP/TAZ coordinates the interplay between metastasis and metabolic alternation [86]. Therefore, understanding the interplay between the Hippo pathway and metabolism in cancer could open a new avenue for cancer treatment.
Hippo pathway and cancer immunity
Several studies have demonstrated the role of the Hippo pathway in regulating host immune response and tumor immunity. Tumor initiating cells utilize Hippo effector YAP to escape from immune clearance by recruiting M2 macrophages [87]. A previous study showed that Hippo signaling impairs tumor progression by infiltrating polymorphonuclear myeloid-derived suppressor cells (MDSC) through up-regulation of TEAD dependent CXCL5 expression [88]. However, an in-vivo study by Moroishi et al. showed that depletion of LATS1/2 induces antitumor immunity by stimulating the host TLRs-IFN pathway through the secretion of nucleic-acid-rich extracellular vesicles [89].
Recently, immunotherapy against programmed cell death protein 1 ligand (PD-L1) opened a new avenue for treating metastatic cancer. A recent study showed that YAP upregulates the PD-L1 expression in many solid tumors. In EGFR-TKI-resistant lung adenocarcinoma, YAP induces expression of PD-L1 at the transcription level and thus stimulates cell proliferation and migration [90]. TAZ activation is known to up-regulate PD-L1 expression through the GPR81 receptor in human lung cancer cells. Moreover, activation of GPR81 induces TAZ activation by inhibiting protein kinase A activity by decreasing intracellular cAMP levels [91]. These studies suggest an interplay between YAP/TAZ and programmed cell death protein 1 ligand in the regulation of cancer immunity.
Hippo pathway also plays an important role in regulating inflammation and T cell differentiation. Contact-dependent activation of the Hippo pathway by Cytotoxic T lymphocyte antigen 4 (CTLA-4) and CD80 ligand drives terminal differentiation of the CD8 T cells via regulating Blimp-1 expression through degradation of YAP in the CD8+ T cells [92]. Hippo pathway effector, TAZ, reciprocally regulates the differentiation of inflammatory Th17, subset of T helper cells and Treg cells, immunosuppressive regulatory T cell [93]. A recent study reported that YAP attenuates activated cytotoxic T cell (CD8 T cell) mediated anti-tumor immunity in the tumor microenvironment [94]. Therefore, future research will help to understand the role of YAP/TAZ and the Hippo pathway in cancer immunity and to identify new targets for cancer treatment.
Hippo pathway and cancer biology
The deregulation of the Hippo pathway results in an overgrowth of cells and organs, thus highlighting the significance of the Hippo pathway in cancer biology. Numerous studies have revealed the connection of YAP/TAZ/TEAD overexpression with cell proliferation, invasion and migration, metastasis, cancer stem cell characteristics, and drug resistance [95, 96].
An In-vivo study showed that knockdown of upstream Hippo regulators or overexpression of YAP in mouse liver leads to the abnormal growth of liver tissue and enlargement of the liver that subsequently developed into liver cancer [23, 65]. Another study reported that knockdown of MST1/2 or SAV1 increased cryptic hyperplasia and tumorigenesis by up-regulation of YAP [18]. Hippo-independent inactivation of YAP/TAZ is also observed by the APC tumor suppressor, as evidenced by the deletion of APC results in activation of YAP/TAZ in colon cancer [97].
Hippo pathway effector YAP also promotes resistance of cancer cells with BRAF, KRAS, and NRAS mutations to anticancer drugs such as RAF and MEK inhibitors [98]. Overexpression of YAP/TAZ is detected in many solid tumors [3, 99–101] and positively associated with the poor survival rate of cancer patients [95]. Here, we describe the role of YAP/TAZ in the top five cancers according to Global cancer incidence in both sexes (Table 2).
Table 2:
Deregulation of YAP/TAZ in top 5 cancers
Non-small cell lung cancer (NSCLC)
A deregulated Hippo pathway is observed in NSCLC. Overexpression of YAP/TAZ is associated with the development, progression, and poor prognosis of the disease. In NSCLC, YAP is frequently mutated which leads to the formation and progression of lung cancer [102]. Knockdown of YAP/TAZ is shown to decrease the tumor cell mass, migration, and metastasis of lung cancer cells [103]. Recently, a study reported that in NSCLC, YAP/TAZ and EZH2 act together to suppress tumor suppressor activity of TGFBR2 [104]. In their previous study, they also reported that many signaling molecules that induce YAP/TAZ nuclear translocation are increased and those are involved in cytoplasmic retention and degradation of YAP/TAZ are mostly suppressed in NSCLC. [105]. For example, several studies reported upregulation of oncogenic ABL1/2 kinases and downregulation of LATS1/2 in NSCLC [103, 105]. In vitro and in vivo experiments showed that expression of the MST1, upstream regulator of YAP/TAZ, inhibits the NSCLC growth [106]. During DNA damage response or other cellular stress, RASSF1A (Ras-association domain family 1 isoform A), an upstream regulator of the Hippo pathway, activates MST1/2 and LATS1 in NSCLC [107]. Other signaling pathway components, such as TGF-β and ERK1/2 can interplay with the Hippo pathway in NSCLC affecting the development and progression of lung cancer [108, 109].
Breast cancer
Deregulated Hippo pathway is also observed in breast cancer [110, 111]. The two effectors of the Hippo pathway, YAP/TAZ commonly overexpressed in breast cancer and could be used as therapeutic targets [112, 113]. YAP overexpression in mammary epithelia causes a defect in the terminal differentiation; whereas, loss of YAP suppresses the growth of oncogenic mammary tumors [114]. Recently it is reported that RING finger protein RNF181 regulates Hippo pathway and induces triple-negative breast cancer progression, as evidenced by the depletion of RNF181 that results in decreased level of YAP, suggesting the role of YAP in TNBC [110].
In highly invasive breast cancer, TAZ is overexpressed and induces migration, metastasis, and chemo-resistance of breast cancer cells. In a hypoxic microenvironment, TAZ interacts with hypoxic-inducing factor (HIF)-1α and stimulates bone metastasis in breast cancer [115, 116]. Overexpressed TAZ also reduces disease-free survival, overall survival, and increases the recurrence of cancer in breast cancer patients; while the loss of TAZ has opposite effects [112, 117]. Moreover, in triple-negative breast cancer (TNBC), TAZ is highly overexpressed in both the cytoplasmic and nuclear compartments [118, 119]. Generally, the nuclear expression of TAZ was reported in triple-negative breast cancer (60.5%), basal-like subtype (70.8%), and metaplastic breast carcinomas (90%) [112].
Measurement of the YAP/TAZ expression has a predictive and prognostic value to assess the effectiveness of chemotherapy on triple-negative breast cancer [120]. For example, In paclitaxel-treated breast cancer cells, overexpression of TAZ, Cyr61, and CTGF- the Hippo pathway downstream targets, is a good indication of paclitaxel-resistant breast cancer cells [121]. Furthermore, downregulation of YAP/TAZ expression by siRNA or small molecule inhibitors increases the sensitivity of breast cancer cells to drug lapatinib in mice [122].
Colorectal cancer
Deregulation of the Hippo pathway is associated with the progression and metastasis of colorectal cancer (CRC) [123, 124]. Progression of CRC from colorectal adenomas is associated with suppression of the Hippo pathway and the increased expression of YAP, TAZ, TEAD, and OCT4 [125]. In CRC patients, expression of YAP and TAZ is highly associated with lymph node metastasis [126]. Higher expression of YAP and TAZ is also positively correlated with their downstream target genes AXL and CTGF in the CRC patients [127].
Recently it is reported that in human CRC, YAP drives epithelial-mesenchymal transition (EMT) by inducing Slug expression that subsequently inhibits E-cadherin expression [128]. In human CRC liver metastases, a higher expression of YAP is correlated with CRC relapse [123]. Also, in the familial adenomatous polyposis (FAP), activation of YAP is confirmed to be related to the development of Adenomatous polyposis coli-deficient adenomas [97]. In CRC patients, nuclear localization of YAP and positive expression of β-catenin are indicators of shorter overall survival and progression-free survival [126]. YAP/TAZ is also highly expressed in chemo-resistance liver metastasis in colon cancer. YAP inactivation increases the sensitivity to cetuximab monotherapy in colorectal patients and longer progression-free survival [129].
Prostate cancer
Both YAP and TAZ are believed to be involved in tumorigenesis and metastasis of prostate cancer [130, 131]. However, the exact contribution of each of these orthologues to prostate cancer progression is not clearly understood. Our analysis of the TCGA prostate cancer data set revealed that YAP1 expression is lower in high-grade prostate cancer [53]. Overexpressed TAZ is involved in EMT transition, cell migration, anchorage-independent growth, and metastasis in prostate cancer with a high Gleason score [132]. TAZ can regulate epigenetic changes by interacting with other transcription factors such as NURD that can recruit HDAC and reduce chromatin accessibility. This leads to the suppression of gene expression [133–135], which plays an important role in prostate cancer progression [136]. Our studies for over the last decade have shown an inverse relationship of PDEF with prostate cancer progression and a complete loss of PDEF in NEPC [137–140]. Moreover, we demonstrated that PDEF expression suppresses genes associated with tumor progression and metastasis [139, 140] and that PDEF serves as a negative regulator of YAP1/TAZ transcriptional networks in prostate cancer is quite exciting [53].
Gastric cancer
Hippo pathway associates with the development, progression, and metastasis of gastric cancer as evidenced by the upregulation of YAP/TAZ/TEAD and the downregulation of MST1 and LATS1 in gastric cancer. All of these Hippo components are found to closely correlate with lymphatic metastasis and tumor TNM stage in gastric cancer [141]. YAP1 is also highly activated in dysplasia, gastric adenocarcinoma, and metastatic stomach disease [142], and is associated with the poor outcome of the early stages in gastric carcinoma.
Down-regulation of YAP1 expression correlates with the reduced cell proliferation, invasion, migration, and single layer colony formation in gastric cancer cell lines. Ectopic expression of YAP1 in MKN45 cells, which have low endogenous YAP1 expression, showed increased cell proliferation and colony formation [143]. In gastric adenocarcinoma cells, YAP1 activates the receptor tyrosine kinase AXL, and thus promotes the growth and metastasis of adenocarcinoma [144]. A recent study reported that YAP1 drives gastric adenocarcinoma peritoneal metastases, as evidenced by the attenuation of peritoneal carcinomatosis upon inhibition of YAP1, suggesting YAP1 could be a good target for treating gastric adenocarcinoma patients [145]
In gastric carcinoma, YAP1 also induces the expression of survivin, an inhibitor of apoptosis. Both YAP1 and Survivin markers together could help in the early diagnosis of gastric carcinoma [146]. YAP1 is used as a biomarker for the diagnosis and prediction of the disease progression in patients with gastric cancers. Increased expression of YAP1 positively correlated with progression, lymph node metastasis, and lower survival in gastric carcinoma [147]. TAZ is positively associated with the abnormal expression of β-catenin, a critical component of the Wnt pathway. Both are positive regulators of tumor development and progression in the adenocarcinoma of the esophagogastric junction (AEG) and could be potential therapeutic targets for the treatment of AEG [148].
Epigenetic regulation of Hippo pathway in cancer
Cancer development follows a multi-stage process in which epigenetic modifications play decisive roles. The connection between epigenetic hallmarks (DNA methylation and histone modifications, as well as non-coding RNAs) and the gene expression regulation is shown in most of the proteins involved in the cancer hallmarks (proliferation, apoptosis resistance, immune checkpoint bypassing, etc.). The Hippo signaling, as a well-defined functional pathway controlling cancer initiation and progression, is also a subject of the epigenetic machinery.
Our recent bioinformatics analysis revealed that the promoter of YAP/TAZ, as the final targets of the Hippo pathway in the nucleus, is highly enriched in binding sites for various transcription factors (TFs). Interestingly, most of these TFs were shown to be either the direct members of the epigenetic machine (e.g. P300 acts as a histone acetyl-transferase (HAT) at YAP1 promoter) or act indirectly by recruiting chromatin remodelers of other DNA or histone-modifying components (e.g. CTCF that recruits HDAC and HAT enzymes at the TAZ promoter) in human cancer cells. In this in-silico study, a long list of more than 2000 different binding sites analyzed and confirmed to be mostly associated with active epigenetic components regulating the YAP/TAZ transcriptional activity as the functional outcomes of the Hippo pathway (unpublished data). On the other hand, YAP/TAZ affects the activity of their target genes through epigenetic modifications exerted by chromatin-remodeling complex proteins [149].
Other contributors of the Hippo pathway (from the ligand-receptor interaction to the TFs in the nucleus), show a tight regulation of these proteins by various epigenetic signatures that ultimately change the cancer phenotypes [150, 151]. Specifically, the DNA methylation-based regulation of RASSF1A, a Hippo pathway scaffold protein that enhances the tumor-suppressive function of YAP/p73, in multiple human cancer types is well known [152, 153]. RASSF1A has been frequently silenced epigenetically in cancer, leading to numerous phenotypic consequences including cancer cell migration and chemo-resistance [154]. The reactivation of the silenced RASSF1A using a combination of chemotherapeutic agents with decitabine, a demethylase compound, was shown to be beneficial in bladder cancer. This treatment restores the RASSF1A and Hippo pathway activity and suppresses the oncogenic activity of its downstream targets [155].
The WW-domain containing protein KIBRA, an upstream of the Hippo pathway, is deregulated in response to aberrant DNA methylation in various cancer cell lines. As reported by Hill et al., while the cell lines representing common epithelial cancers showed an un-methylated pattern for KIBRA, those representing acute lymphoblastic leukemia (ALL) undergo frequent silencing of KIBRA by DNA hypermethylation, suggesting a dynamic DNA methylation pattern governing Hippo signaling in various cancers [156]. A genome-wide CRISPR screen revealed that the transcriptional repressor protein Trichorhinophalangeal Syndrome 1 (TRPS1) serves as an epigenetic modulator of YAP activity in breast cancer. TRPS1 binds to a large set of enhancer regions and represses the transcriptional activity of its targets including YAP. This was shown to be associated with suppressed tumor-infiltrating immune cells in breast cancer [157].
Interestingly, the C4–2 cells, a human prostate carcinoma cell line, harbor a silenced MST1 kinase signaling. MST is epigenetically down-regulated in castration-resistant prostate carcinoma (CRPC) patients [158]. Cellular myelocytomatosis (c-MYC), a well-described tumor initiator, is highly expressed in prostate cancer. Mechanistically, the c-MYC regulates the enhancer of EZH2, a histone methyltransferase that catalyzes H3K27 trimethylation (H3K27me3) [159], and result in the MST1 promoter silencing via suppressing microRNA - miR-26a/b, which leads to increased survival in human prostate cancer cells [158].
A large effort has also been undertaken to dissect the impact of small non-coding RNAs in the Hippo signaling and their tumorigenic potentials. Using the microarray technology, Pinto et al. evaluated miRNA profiling in familial breast cancer and revealed 287 differentially expressed miRNA molecules that are associated mostly with MAPK and Hippo signaling pathways. Among these, miR-152 and miR-497 up-regulation showed an indirect interaction with RASSF1A and NORE1A gene expressions [160]. In the esophageal squamous cell carcinoma (ESCC) cells, low promoter methylation of miR-373-3p correlated with ESCC development. A bioinformatics analysis detected LATS2 and OXRI genes as the main targets of this miRNA [161]. Besides, MicroRNA-135b targets Hippo signaling components, thereby accelerating lung cancer metastasis [162].
The role of long- noncoding RNAs (lncRNAs) via Hippo signaling was also verified in cancer development. Using an integrative Bioinformatics assessment, James et al. reported 1235 deregulated lncRNAs and proposed a strong correlation between their promoter methylation pattern with the TGF-β and Hippo signaling-related genes in certain subtypes of acute lymphoblastic leukemia (ALL) cells [163]. Furthermore, long intergenic non-coding RNA 673 (LINC00673) was shown to accelerate the proliferation of breast cancer cells via miR-515-5p/MARK4/Hippo signaling pathway [164].
Collectively, not only the role of epigenetic mechanisms in controlling various layers of the Hippo signaling pathway has been clearly defined, but also, it is considered as a potential therapeutic target for the cancers in which Hippo signaling plays a decisive role.
Hippo pathway regulation mediated by exosomes
Tumor-derived exosomes (TDEs) engage in the initiation and progression of tumors, and associate with different cancer hallmarks including the modulation of tumor immunity, promoting angiogenesis, invasion, metastasis, as well as drug-resistance [165] [166]. Numerous regulatory mechanisms are identified in the oncogenic generation of exosomes involved in tumor progression. By carrying different biological molecules including lipids, DNA, mRNA, miRNA, and lncRNA, exosomes can regulate different signaling pathways including the Hippo pathway in stromal and cancer cells [167] [168]. For instance, Moroishi et al. showed that the nucleic acid-rich extracellular vesicles from tumor cells depleted for LATS1/2 improves the immunogenicity by inducing the interferon response and Toll-like receptors-MYD88/TRIF pathway. Consistent with this, the anti-tumor response leads to tumor suppression by targeting LATS1/2 [169]. In addition, the miR-145 in exosomes generated by the ovarian cancer cells targets connective tissue growth factor (CTGF) as a downstream target of the Hippo pathway. Therefore, the down-regulation of miR-145 in ovarian cancer cells might accelerate further phenotypic development [170]. Interestingly, exosomes from stromal cells can regulate the Hippo pathway in tumor cells. For example, adipocyte exosomes could activate the Hippo pathway in MCF7 breast cancer cells to promote the malignant phenotypes including proliferation, migration, and resistance to apoptosis [171]. In contrast, tumor-derived exosomes can regulate stromal cells by modulating the Hippo pathway. In a recent study, Yoshida et al. showed that exosomal miR-7977 released by AML can regulate the Hippo-YAP signaling pathway in bone marrow mesenchymal stromal cells (BM-MSCs) and this might exert leukemia-favoring stromal phenotypes [172], [173]. Together these studies suggest that the components of exosomes mediate a path to regulate the Hippo pathway in supporting cancer cells in the tumor microenvironment. However, this topic warrants further investigation in addition to elucidation of the underlying mechanisms.
Hippo pathway as a therapeutic target
Considering the roles of the Hippo signaling pathway in diverse pathophysiological processes, targeting different components of this pathway could open a new window for cancer treatment. Core components of the Hippo pathway are kinases, thus different therapeutics might target these kinases, such as XMU-MP-1 an inhibitor of MST1/2 kinases [174]. Inhibitors of serine-threonine kinases, MST1/2 or LATS1/2 may up-regulate the transcriptional co-activator YAP/TAZ that facilitates tissue repair in regenerative medicine.
As most cancers have elevated YAP/TAZ expression, inhibitors of these two transcriptional activators may be used as cancer therapeutics. Recently, Wu et al. showed that peptide inhibitors that disrupt the interaction between TEAD and YAP/TAZ could be used as a potential therapeutic option for gastric cancer [175]. Hippo effector Yki/YAP promotes transcription by modifying chromatin through recruitment of the Trr/MLL H3K4 methyltransferase complex [176]. It is possible to develop Inhibitors against these downstream components of Yki/YAP as a therapeutic intervention.
In addition to targeting the downstream regulators of the Hippo pathway, activating upstream regulators may also be beneficial in cancer therapy. For example, Tankyrase inhibitors stabilize Hippo upstream regulator angiomotin family proteins (AMOT) and thus suppress Yap activity [177]. Statins inhibit the mevalonate cascade that is necessary for the activation of Rho GTPase and thereby suppress YAP/TAZ activity [84]. Simvastatin negatively modulates transcription of the receptor for hyaluronan-mediated motility (RHAMM), a downstream effector of the mevalonate pathway, by regulating YAP phosphorylation and cytoplasmic localization and subseconsequently inhibit breast cancer cell migration and invasion [84, 178]. Moreover, rolipram and ibudilast inhibit the phosphodiesterase, thus activate cAMP-dependent protein kinase A signaling that ultimately suppresses Yap activity [179]. It is also reported that metformin activates Hippo upstream regulator AMPK [180]. Activated AMPK then phosphorylates and inactivates YAP. Recently Slemmons et al reported that YAP is highly expressed in Rhabdosarcoma. DNA methyltransferase inhibitor (DNMTi) can be used to activates Hippo pathway upstream regulators RASSF1 and RASSF5 by demethylating their promoter sequence that lead to the activation of Hippo pathway and reduction of tumor growth [181]. Very recently, another study reported that inhibition of the YAP/TAZ/YEAD complex by Verteporfin may prevent Ewing sarcoma cell migration and metastasis [182]. These reports highlight the importance of having better insights into the regulation of the Hippo pathway to open the new therapeutic window that will maximize the effects of anticancer agents and minimize unwanted side effects.
Summary and future directions:
Hippo pathway is one of the most conserved pathways among the different species from Protista to eukaryotes and plays an important role in the regulation of cell growth/proliferation, cellular homeostasis, and by extension in cancer. Different intracellular and extracellular signaling events regulate the Hippo pathway. Much information regarding the mechanism of regulation of the Hippo signaling pathway and the deregulation of this pathway in cancers remains to be fully elucidated. In mammalian systems, Hippo signaling regulates YAP and TAZ transcriptional networks. We discovered that PDEF, a tumor metastasis suppressor, positively regulates Hippo signaling as evidenced by downregulation of YAP1/TAZ transcriptional output. These findings could open a new window for prostate cancer therapy, but the molecular mechanisms are far from understood. Insights learned from the Hippo pathway will likely pave the way toward better therapeutic targets for many human cancers. Targeting the YAP/TAZ transcriptional network with small molecule inhibitors might bring much needed promising advances in overcoming cancer therapeutic resistance.
Highlights:
Hippo pathway is a master regulator of development, cell proliferation, stem cell function, tissue regeneration, homeostasis, and organ size.
Deregulation of the Hippo pathway is associated with numerous cancers.
Alteration of the Hippo pathway results in cell invasion, migration, disease progression, and therapy resistance in cancers.
Ttargeting Hippo pathway offers a new paradigm in therapeutic intervention in cancer.
Financial support:
The studies were supported in part by NCI 7R01CA242839 (Koul H-PI). Carroll W. Feist endowed Chair Funds (Koul H) and startup funds (Koul H) from LSUHSC School of Medicine and Stanley Scott Cancer Center, New Orleans.
Footnotes
Statement of interest
Conflict of interests: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Snigdha K, Gangwani KS, Lapalikar GV, Singh A, Kango-Singh M, Hippo Signaling in Cancer: Lessons From Drosophila Models, Front Cell Dev Biol, 7 (2019) 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yang Z, Hata Y, What is the Hippo pathway? Is the Hippo pathway conserved in Caenorhabditis elegans?, J Biochem, 154 (2013) 207–209. [DOI] [PubMed] [Google Scholar]
- [3].Yu FX, Zhao B, Guan KL, Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer, Cell, 163 (2015) 811–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gumbiner BM, Kim NG, The Hippo-YAP signaling pathway and contact inhibition of growth, J Cell Sci, 127 (2014) 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Han Y, Analysis of the role of the Hippo pathway in cancer, J Transl Med, 17 (2019) 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wu S, Huang J, Dong J, Pan D, hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts, Cell, 114 (2003) 445–456. [DOI] [PubMed] [Google Scholar]
- [7].Callus BA, Verhagen AM, Vaux DL, Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation, FEBS J, 273 (2006) 4264–4276. [DOI] [PubMed] [Google Scholar]
- [8].Harvey KF, Pfleger CM, Hariharan IK, The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis, Cell, 114 (2003) 457–467. [DOI] [PubMed] [Google Scholar]
- [9].Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y, Control of cell proliferation and apoptosis by mob as tumor suppressor, mats, Cell, 120 (2005) 675–685. [DOI] [PubMed] [Google Scholar]
- [10].Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber D, Hariharan IK, salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines, Cell, 110 (2002) 467–478. [DOI] [PubMed] [Google Scholar]
- [11].Praskova M, Xia F, Avruch J, MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation, Curr Biol, 18 (2008) 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ, The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation, Genes Dev, 9 (1995) 534–546. [DOI] [PubMed] [Google Scholar]
- [13].Xu T, Wang W, Zhang S, Stewart RA, Yu W, Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase, Development, 121 (1995) 1053–1063. [DOI] [PubMed] [Google Scholar]
- [14].Chan EH, Nousiainen M, Chalamalasetty RB, Schäfer A, Nigg EA, Silljé HH, The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1, Oncogene, 24 (2005) 2076–2086. [DOI] [PubMed] [Google Scholar]
- [15].Huang J, Wu S, Barrera J, Matthews K, Pan D, The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP, Cell, 122 (2005) 421–434. [DOI] [PubMed] [Google Scholar]
- [16].Kodaka M, Hata Y, The mammalian Hippo pathway: regulation and function of YAP1 and TAZ, Cell Mol Life Sci, 72 (2015) 285–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen YA, Lu CY, Cheng TY, Pan SH, Chen HF, Chang NS, WW Domain-Containing Proteins YAP and TAZ in the Hippo Pathway as Key Regulators in Stemness Maintenance, Tissue Homeostasis, and Tumorigenesis, Front Oncol, 9 (2019) 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee JH, Kim TS, Yang TH, Koo BK, Oh SP, Lee KP, Oh HJ, Lee SH, Kong YY, Kim JM, Lim DS, A crucial role of WW45 in developing epithelial tissues in the mouse, EMBO J, 27 (2008) 1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wei X, Shimizu T, Lai ZC, Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila, EMBO J, 26 (2007) 1772–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hao Y, Chun A, Cheung K, Rashidi B, Yang X, Tumor suppressor LATS1 is a negative regulator of oncogene YAP, J Biol Chem, 283 (2008) 5496–5509. [DOI] [PubMed] [Google Scholar]
- [21].Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, Zhao S, Xiong Y, Lei QY, Guan KL, The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase, J Biol Chem, 285 (2010) 37159–37169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhao B, Li L, Tumaneng K, Wang CY, Guan KL, A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP), Genes Dev, 24 (2010) 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D, Elucidation of a universal size-control mechanism in Drosophila and mammals, Cell, 130 (2007) 1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML, TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm, Genes Dev, 15 (2001) 1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhao B, Ye X, Yu J, Li L, Li W, Li S, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL, TEAD mediates YAP-dependent gene induction and growth control, Genes Dev, 22 (2008) 1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kim MK, Jang JW, Bae SC, DNA binding partners of YAP/TAZ, BMB Rep, 51 (2018) 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rock JM, Lim D, Stach L, Ogrodowicz RW, Keck JM, Jones MH, Wong CCL, Yates JR, Winey M, Smerdon SJ, Yaffe MB, Amon A, Activation of the Yeast Hippo Pathway by Phosphorylation-Dependent Assembly of Signaling Complexes, Science, 340 (2013) 871–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mohl DA, Huddleston MJ, Collingwood TS, Annan RS, Deshaies RJ, Dbf2-Mob1 drives relocalization of protein phosphatase Cdc14 to the cytoplasm during exit from mitosis, J Cell Biol, 184 (2009) 527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Meitinger F, Boehm ME, Hofmann A, Hub B, Zentgraf H, Lehmann WD, Pereira G, Phosphorylation-dependent regulation of the F-BAR protein Hof1 during cytokinesis, Genes Dev, 25 (2011) 875–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Oh Y, Chang KJ, Orlean P, Wloka C, Deshaies R, Bi E, Mitotic exit kinase Dbf2 directly phosphorylates chitin synthase Chs2 to regulate cytokinesis in budding yeast, Mol Biol Cell, 23 (2012) 2445–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hsu J, Weiss EL, Cell cycle regulated interaction of a yeast Hippo kinase and its activator MO25/Hym1, PLoS One, 8 (2013) e78334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Weiss EL, Mitotic exit and separation of mother and daughter cells, Genetics, 192 (2012) 1165–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mazanka E, Alexander J, Yeh BJ, Charoenpong P, Lowery DM, Yaffe M, Weiss EL, The NDR/LATS family kinase Cbk1 directly controls transcriptional asymmetry, PLoS Biol, 6 (2008) e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jansen JM, Wanless AG, Seidel CW, Weiss EL, Cbk1 regulation of the RNA-binding protein Ssd1 integrates cell fate with translational control, Curr Biol, 19 (2009) 2114–2120. ‘ [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, Wu S, Pan D, The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded, Proc Natl Acad Sci U S A, 107 (2010) 10532–10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mohseni M, Sun J, Lau A, Curtis S, Goldsmith J, Fox VL, Wei C, Frazier M, Samson O, Wong KK, Kim C, Camargo FD, A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway, Nat Cell Biol, 16 (2014) 108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Robinson BS, Moberg KH, Cell-cell junctions: α-catenin and E-cadherin help fence in Yap1, Curr Biol, 21 (2011) R890–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang W, Huang J, Wang X, Yuan J, Li X, Feng L, Park JI, Chen J, PTPN14 is required for the density-dependent control of YAP1, Genes Dev, 26 (2012) 1959–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dupont S, Regulation of YAP/TAZ Activity by Mechanical Cues: An Experimental Overview, Methods Mol Biol, 1893 (2019) 183–202. [DOI] [PubMed] [Google Scholar]
- [40].Kuroda M, Wada H, Kimura Y, Ueda K, Kioka N, Vinculin promotes nuclear localization of TAZ to inhibit ECM stiffness-dependent differentiation into adipocytes, Journal of Cell Science, 130 (2017) 989–1002. [DOI] [PubMed] [Google Scholar]
- [41].Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, Oria R, Kechagia JZ, Rico-Lastres P, Le Roux AL, Shanahan CM, Trepat X, Navajas D, Garcia-Manyes S, Roca-Cusachs P, Force Triggers YAP Nuclear Entry by Regulating Transport across Nuclear Pores, Cell, 171 (2017) 1397–1410.e1314. [DOI] [PubMed] [Google Scholar]
- [42].Fletcher GC, Elbediwy A, Khanal I, Ribeiro PS, Tapon N, Thompson BJ, The Spectrin cytoskeleton regulates the Hippo signalling pathway, EMBO J, 34 (2015) 940–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL, Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling, Cell, 150 (2012) 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Reddy BV, Irvine KD, Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins, Dev Cell, 24 (2013) 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R, Brugge JS, Dyson NJ, Haber DA, YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway, Nat Cell Biol, 11 (2009) 1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Serrano I, McDonald PC, Lock F, Muller WJ, Dedhar S, Inactivation of the Hippo tumour suppressor pathway by integrin-linked kinase, Nat Commun, 4 (2013) 2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Elbediwy A, Vincent-Mistiaen ZI, Spencer-Dene B, Stone RK, Boeing S, Wculek SK, Cordero J, Tan EH, Ridgway R, Brunton VG, Sahai E, Gerhardt H, Behrens A, Malanchi I, Sansom OJ, Thompson BJ, Integrin signalling regulates YAP and TAZ to control skin homeostasis, Development, 143 (2016) 1674–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S, Flores F, Yu FX, Halder G, Guan KL, MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway, Nat Commun, 6 (2015) 8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Boggiano JC, Vanderzalm PJ, Fehon RG, Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway, Dev Cell, 21 (2011) 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Zheng Y, Liu B, Wang L, Lei H, Pulgar Prieto KD, Pan D, Homeostatic Control of Hpo/MST Kinase Activity through Autophosphorylation-Dependent Recruitment of the STRIPAK PP2A Phosphatase Complex, Cell Rep, 21 (2017) 3612–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bae SJ, Ni L, Luo X, STK25 suppresses Hippo signaling by regulating SAV1-STRIPAK antagonism, Elife, 9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Khalil MI, Ghosh I, Singh V, Chen J, Zhu H, De Benedetti A, NEK1 Phosphorylation of YAP Promotes Its Stabilization and Transcriptional Output, Cancers (Basel), 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jaiswal PK, Mohajan S, Koul S, Wang F, Shi R, Koul HK, Prostate-Derived ETS Factor (PDEF) Modulates Yes Associated Protein 1 (YAP1) in Prostate Cancer Cells: A Potential Cross-Talk between PDEF and Hippo Signaling, Pharmaceuticals (Basel), 12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Huang HR, Chen ZJ, Kunes S, Chang GD, Maniatis T, Endocytic pathway is required for Drosophila Toll innate immune signaling, Proc Natl Acad Sci U S A, 107 (2010) 8322–8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kwon Y, Vinayagam A, Sun X, Dephoure N, Gygi SP, Hong P, Perrimon N, The Hippo signaling pathway interactome, Science, 342 (2013) 737–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Verghese S, Moberg K, Roles of Membrane and Vesicular Traffic in Regulation of the Hippo Pathway, Front Cell Dev Biol, 7 (2019) 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Matsudaira T, Mukai K, Noguchi T, Hasegawa J, Hatta T, Iemura S.-i., Natsume T, Miyamura N, Nishina H, Nakayama J, Semba K, Tomita T, Murata S, Arai H, Taguchi T, Endosomal phosphatidylserine is critical for the YAP signalling pathway in proliferating cells, Nature Communications, 8 (2017) 1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].D’Agostino L, Nie Y, Goswami S, Tong K, Yu S, Bandyopadhyay S, Flores J, Zhang X, Balasubramanian I, Joseph I, Sakamori R, Farrell V, Li Q, Yang CS, Gao B, Ferraris RP, Yehia G, Bonder EM, Goldenring JR, Verzi MP, Zhang L, Ip YT, Gao N, Recycling Endosomes in Mature Epithelia Restrain Tumorigenic Signaling, Cancer Res, 79 (2019) 4099–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Block MR, Brunner M, Ziegelmeyer T, Lallemand D, Pezet M, Chevalier G, Rondé P, Gauthier-Rouviere C, Wehrle-Haller B, Bouvard D, The mechano-sensitive response of β1 integrin promotes SRC-positive late endosome recycling and activation of Yes-associated protein, J Biol Chem, 295 (2020) 13474–13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cox CM, Mandell EK, Stewart L, Lu R, Johnson DL, McCarter SD, Tavares A, Runyan R, Ghosh S, Wilson JM, Endosomal regulation of contact inhibition through the AMOT:YAP pathway, Mol Biol Cell, 26 (2015) 2673–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Xiao J, Shi Q, Li W, Mu X, Peng J, Li M, Chen M, Huang H, Wang C, Gao K, Fan J, ARRDC1 and ARRDC3 act as tumor suppressors in renal cell carcinoma by facilitating YAP1 degradation, Am J Cancer Res, 8 (2018) 132–143. [PMC free article] [PubMed] [Google Scholar]
- [62].Shen X, Sun X, Sun B, Li T, Wu G, Li Y, Chen L, Liu Q, Cui M, Zhou Z, ARRDC3 suppresses colorectal cancer progression through destabilizing the oncoprotein YAP, FEBS Lett, 592 (2018) 599–609. [DOI] [PubMed] [Google Scholar]
- [63].Pan D, The hippo signaling pathway in development and cancer, Dev Cell, 19 (2010) 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Halder G, Johnson RL, Hippo signaling: growth control and beyond, Development, 138 (2011) 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR, YAP1 increases organ size and expands undifferentiated progenitor cells, Curr Biol, 17 (2007) 2054–2060. [DOI] [PubMed] [Google Scholar]
- [66].Benhamouche S, Curto M, Saotome I, Gladden AB, Liu CH, Giovannini M, McClatchey AI, Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver, Genes Dev, 24 (2010) 1718–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM, Lim DS, The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis, Proc Natl Acad Sci U S A, 107 (2010) 8248–8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, Johnson RL, Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver, Proc Natl Acad Sci U S A, 107 (2010) 1437–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, Chen Y, Park O, Chang J, Simpson RM, Wang CY, Gao B, Jiang J, Yang Y, Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression, Proc Natl Acad Sci U S A, 107 (2010) 1431–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D, The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals, Dev Cell, 19 (2010) 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF, Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size, Science, 332 (2011) 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN, Hippo pathway effector Yap promotes cardiac regeneration, Proc Natl Acad Sci U S A, 110 (2013) 13839–13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, Yau AL, Buck JN, Gouin KA, van Gorp PR, Zhou B, Chen J, Seidman JG, Wang DZ, Pu WT, Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model, Circ Res, 115 (2014) 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Kim E, Kang JG, Kang MJ, Park JH, Kim YJ, Kweon TH, Lee HW, Jho EH, Lee YH, Kim SI, Yi EC, Park HW, Yang WH, Cho JW, O-GlcNAcylation on LATS2 disrupts the Hippo pathway by inhibiting its activity, Proc Natl Acad Sci U S A, 117 (2020) 14259–14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R, Ding S, Guan KL, The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation, Genes Dev, 24 (2010) 1106–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Meinhardt G, Haider S, Kunihs V, Saleh L, Pollheimer J, Fiala C, Hetey S, Feher Z, Szilagyi A, Than NG, Knöfler M, Pivotal role of the transcriptional co-activator YAP in trophoblast stemness of the developing human placenta, Proceedings of the National Academy of Sciences, 117 (2020) 13562–13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW, Wrana JL, TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal, Nat Cell Biol, 10 (2008) 837–848. [DOI] [PubMed] [Google Scholar]
- [78].Cao X, Pfaff SL, Gage FH, YAP regulates neural progenitor cell number via the TEA domain transcription factor, Genes Dev, 22 (2008) 3320–3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Fernandez-L A, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, Taylor MD, Kenney AM, YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation, Genes Dev, 23 (2009) 2729–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Vander Heiden MG, Cantley LC, Thompson CB, Understanding the Warburg effect: the metabolic requirements of cell proliferation, Science, 324 (2009) 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, Lim DS, Guan KL, Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway, Nat Cell Biol, 17 (2015) 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, Forcato M, Grifoni D, Pession A, Zanconato F, Guzzo G, Bicciato S, Dupont S, Aerobic glycolysis tunes YAP/TAZ transcriptional activity, EMBO J, 34 (2015) 1349–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Peng C, Zhu Y, Zhang W, Liao Q, Chen Y, Zhao X, Guo Q, Shen P, Zhen B, Qian X, Yang D, Zhang JS, Xiao D, Qin W, Pei H, Regulation of the Hippo-YAP Pathway by Glucose Sensor O-GlcNAcylation, Mol Cell, 68 (2017) 591–604.e595. [DOI] [PubMed] [Google Scholar]
- [84].Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S, Rosato A, Piccolo S, Del Sal G, Metabolic control of YAP and TAZ by the mevalonate pathway, Nat Cell Biol, 16 (2014) 357–366. [DOI] [PubMed] [Google Scholar]
- [85].Cox AG, Hwang KL, Brown KK, Evason K, Beltz S, Tsomides A, O’Connor K, Galli GG, Yimlamai D, Chhangawala S, Yuan M, Lien EC, Wucherpfennig J, Nissim S, Minami A, Cohen DE, Camargo FD, Asara JM, Houvras Y, Stainier DYR, Goessling W, Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth, Nat Cell Biol, 18 (2016) 886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Yamaguchi H, Taouk GM, A Potential Role of YAP/TAZ in the Interplay Between Metastasis and Metabolic Alterations, Frontiers in Oncology, 10 (2020) 928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Guo X, Zhao Y, Yan H, Yang Y, Shen S, Dai X, Ji X, Ji F, Gong XG, Li L, Bai X, Feng XH, Liang T, Ji J, Chen L, Wang H, Zhao B, Single tumor-initiating cells evade immune clearance by recruiting type II macrophages, Genes Dev, 31 (2017) 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wang G, Lu X, Dey P, Deng P, Wu CC, Jiang S, Fang Z, Zhao K, Konaparthi R, Hua S, Zhang J, Li-Ning-Tapia EM, Kapoor A, Wu CJ, Patel NB, Guo Z, Ramamoorthy V, Tieu TN, Heffernan T, Zhao D, Shang X, Khadka S, Hou P, Hu B, Jin EJ, Yao W, Pan X, Ding Z, Shi Y, Li L, Chang Q, Troncoso P, Logothetis CJ, McArthur MJ, Chin L, Wang YA, DePinho RA, Targeting YAP-Dependent MDSC Infiltration Impairs Tumor Progression, Cancer Discov, 6 (2016) 80–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Moroishi T, Hayashi T, Pan WW, Fujita Y, Holt MV, Qin J, Carson DA, Guan KL, The Hippo Pathway Kinases LATS1/2 Suppress Cancer Immunity, Cell, 167 (2016) 1525–1539.e1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lee BS, Park DI, Lee DH, Lee JE, Yeo MK, Park YH, Lim DS, Choi W, Yoo G, Kim HB, Kang D, Moon JY, Jung SS, Kim JO, Cho SY, Park HS, Chung C, Hippo effector YAP directly regulates the expression of PD-L1 transcripts in EGFR-TKI-resistant lung adenocarcinoma, Biochem Biophys Res Commun, 491 (2017) 493–499. [DOI] [PubMed] [Google Scholar]
- [91].Feng J, Yang H, Zhang Y, Wei H, Zhu Z, Zhu B, Yang M, Cao W, Wang L, Wu Z, Tumor cell-derived lactate induces TAZ-dependent upregulation of PD-L1 through GPR81 in human lung cancer cells, Oncogene, 36 (2017) 5829–5839. [DOI] [PubMed] [Google Scholar]
- [92].Thaventhiran JE, Hoffmann A, Magiera L, de la Roche M, Lingel H, Brunner-Weinzierl M, Fearon DT, Activation of the Hippo pathway by CTLA-4 regulates the expression of Blimp-1 in the CD8+ T cell, Proc Natl Acad Sci U S A, 109 (2012) E2223–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Geng J, Yu S, Zhao H, Sun X, Li X, Wang P, Xiong X, Hong L, Xie C, Gao J, Shi Y, Peng J, Johnson RL, Xiao N, Lu L, Han J, Zhou D, Chen L, Publisher Correction: The transcriptional coactivator TAZ regulates reciprocal differentiation of T, Nat Immunol, 19 (2018) 1036. [DOI] [PubMed] [Google Scholar]
- [94].Lebid A, Chung L, Pardoll DM, Pan F, YAP Attenuates CD8 T Cell-Mediated Anti-tumor Response, Front Immunol, 11 (2020) 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zanconato F, Cordenonsi M, Piccolo S, YAP/TAZ at the Roots of Cancer, Cancer Cell, 29 (2016) 783–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kim MH, Kim J, Role of YAP/TAZ transcriptional regulators in resistance to anti-cancer therapies, Cell Mol Life Sci, 74 (2017) 1457–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Cai J, Maitra A, Anders RA, Taketo MM, Pan D, β-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis, Genes Dev, 29 (2015) 1493–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, Pham L, Wang MM, Karachaliou N, Cao MG, Manzano JL, Ramirez JL, Torres JM, Buttitta F, Rudin CM, Collisson EA, Algazi A, Robinson E, Osman I, Muñoz-Couselo E, Cortes J, Frederick DT, Cooper ZA, McMahon M, Marchetti A, Rosell R, Flaherty KT, Wargo JA, Bivona TG, The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies, Nat Genet, 47 (2015) 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Moroishi T, Hansen CG, Guan KL, The emerging roles of YAP and TAZ in cancer, Nat Rev Cancer, 15 (2015) 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Park JH, Shin JE, Park HW, The Role of Hippo Pathway in Cancer Stem Cell Biology, Mol Cells, 41 (2018) 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Thompson BJ, YAP/TAZ: Drivers of Tumor Growth, Metastasis, and Resistance to Therapy, BioEssays, 42 (2020) 1900162. [DOI] [PubMed] [Google Scholar]
- [102].Chen HY, Yu SL, Ho BC, Su KY, Hsu YC, Chang CS, Li YC, Yang SY, Hsu PY, Ho H, Chang YH, Chen CY, Yang HI, Hsu CP, Yang TY, Chen KC, Hsu KH, Tseng JS, Hsia JY, Chuang CY, Yuan S, Lee MH, Liu CH, Wu GI, Hsiung CA, Chen YM, Wang CL, Huang MS, Yu CJ, Chen KY, Tsai YH, Su WC, Chen HW, Chen JJ, Chen CJ, Chang GC, Yang PC, Li KC, R331W Missense Mutation of Oncogene YAP1 Is a Germline Risk Allele for Lung Adenocarcinoma With Medical Actionability, J Clin Oncol, 33 (2015) 2303–2310. [DOI] [PubMed] [Google Scholar]
- [103].Yeung B, Yu J, Yang X, Roles of the Hippo pathway in lung development and tumorigenesis, Int J Cancer, 138 (2016) 533–539. [DOI] [PubMed] [Google Scholar]
- [104].Lo Sardo F, Pulito C, Sacconi A, Korita E, Sudol M, Strano S, Blandino G, YAP/TAZ and EZH2 synergize to impair tumor suppressor activity of TGFBR2 in non-small cell lung cancer, Cancer Lett, 500 (2021) 51–63. [DOI] [PubMed] [Google Scholar]
- [105].Lo Sardo F, Strano S, Blandino G, YAP and TAZ in Lung Cancer: Oncogenic Role and Clinical Targeting, Cancers (Basel), 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Xu CM, Liu WW, Liu CJ, Wen C, Lu HF, Wan FS, Mst1 overexpression inhibited the growth of human non-small cell lung cancer in vitro and in vivo, Cancer Gene Ther, 20 (2013) 453–460. [DOI] [PubMed] [Google Scholar]
- [107].Agathanggelou A, Honorio S, Macartney DP, Martinez A, Dallol A, Rader J, Fullwood P, Chauhan A, Walker R, Shaw JA, Hosoe S, Lerman MI, Minna JD, Maher ER, Latif F, Methylation associated inactivation of RASSF1A from region 3p21.3 in lung, breast and ovarian tumours, Oncogene, 20 (2001) 1509–1518. [DOI] [PubMed] [Google Scholar]
- [108].Saito A, Nagase T, Hippo and TGF-β interplay in the lung field, Am J Physiol Lung Cell Mol Physiol, 309 (2015) L756–767. [DOI] [PubMed] [Google Scholar]
- [109].You B, Yang YL, Xu Z, Dai Y, Liu S, Mao JH, Tetsu O, Li H, Jablons DM, You L, Inhibition of ERK1/2 down-regulates the Hippo/YAP signaling pathway in human NSCLC cells, Oncotarget, 6 (2015) 4357–4368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zhou R, Ding Y, Xue M, Xiong B, Zhuang T, RNF181 modulates Hippo signaling and triple negative breast cancer progression, Cancer Cell International, 20 (2020) 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Wei C, Wang Y, Li X, The role of Hippo signal pathway in breast cancer metastasis, Onco Targets Ther, 11 (2018) 2185–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Díaz-Martín J, López-García M, Romero-Pérez L, Atienza-Amores MR, Pecero ML, Castilla M, Biscuola M, Santón A, Palacios J, Nuclear TAZ expression associates with the triple-negative phenotype in breast cancer, Endocr Relat Cancer, 22 (2015) 443–454. [DOI] [PubMed] [Google Scholar]
- [113].Maugeri-Saccà M, Barba M, Pizzuti L, Vici P, Di Lauro L, Dattilo R, Vitale I, Bartucci M, Mottolese M, De Maria R, The Hippo transducers TAZ and YAP in breast cancer: oncogenic activities and clinical implications, Expert Rev Mol Med, 17 (2015) e14. [DOI] [PubMed] [Google Scholar]
- [114].Chen Q, Zhang N, Gray RS, Li H, Ewald AJ, Zahnow CA, Pan D, A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis, Genes Dev, 28 (2014) 432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Bendinelli P, Maroni P, Matteucci E, Luzzati A, Perrucchini G, Desiderio MA, Hypoxia inducible factor-1 is activated by transcriptional co-activator with PDZ-binding motif (TAZ) versus WWdomain-containing oxidoreductase (WWOX) in hypoxic microenvironment of bone metastasis from breast cancer, Eur J Cancer, 49 (2013) 2608–2618. [DOI] [PubMed] [Google Scholar]
- [116].Xiang L, Gilkes DM, Hu H, Luo W, Bullen JW, Liang H, Semenza GL, HIF-1α and TAZ serve as reciprocal co-activators in human breast cancer cells, Oncotarget, 6 (2015) 11768–11778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Bartucci M, Dattilo R, Moriconi C, Pagliuca A, Mottolese M, Federici G, Benedetto AD, Todaro M, Stassi G, Sperati F, Amabile MI, Pilozzi E, Patrizii M, Biffoni M, Maugeri-Saccà M, Piccolo S, De Maria R, TAZ is required for metastatic activity and chemoresistance of breast cancer stem cells, Oncogene, 34 (2015) 681–690. [DOI] [PubMed] [Google Scholar]
- [118].Frangou C, Li YW, Shen H, Yang N, Wilson KE, Blijlevens M, Guo J, Nowak NJ, Zhang J, Molecular profiling and computational network analysis of TAZ-mediated mammary tumorigenesis identifies actionable therapeutic targets, Oncotarget, 5 (2014) 12166–12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Li YW, Shen H, Frangou C, Yang N, Guo J, Xu B, Bshara W, Shepherd L, Zhu Q, Wang J, Hu Q, Liu S, Morrison CD, Sun P, Zhang J, Characterization of TAZ domains important for the induction of breast cancer stem cell properties and tumorigenesis, Cell Cycle, 14 (2015) 146–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Vici P, Ercolani C, Di Benedetto A, Pizzuti L, Di Lauro L, Sperati F, Terrenato I, Gamucci T, Natoli C, Di Filippo F, Botti C, Barba M, Mottolese M, De Maria R, Maugeri-Saccà M, Topographic expression of the Hippo transducers TAZ and YAP in triple-negative breast cancer treated with neoadjuvant chemotherapy, J Exp Clin Cancer Res, 35 (2016) 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lai D, Ho KC, Hao Y, Yang X, Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF, Cancer Res, 71 (2011) 2728–2738. [DOI] [PubMed] [Google Scholar]
- [122].Lin CH, Pelissier FA, Zhang H, Lakins J, Weaver VM, Park C, LaBarge MA, Microenvironment rigidity modulates responses to the HER2 receptor tyrosine kinase inhibitor lapatinib via YAP and TAZ transcription factors, Mol Biol Cell, 26 (2015) 3946–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wierzbicki PM, Rybarczyk A, The Hippo pathway in colorectal cancer, Folia Histochem Cytobiol, 53 (2015) 105–119. [DOI] [PubMed] [Google Scholar]
- [124].Mouillet-Richard S, Laurent-Puig P, YAP/TAZ Signalling in Colorectal Cancer: Lessons from Consensus Molecular Subtypes, Cancers (Basel), 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Liang K, Zhou G, Zhang Q, Li J, Zhang C, Expression of hippo pathway in colorectal cancer, Saudi J Gastroenterol, 20 (2014) 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wang L, Shi S, Guo Z, Zhang X, Han S, Yang A, Wen W, Zhu Q, Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells, PLoS One, 8 (2013) e65539. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [127].Yuen HF, McCrudden CM, Huang YH, Tham JM, Zhang X, Zeng Q, Zhang SD, Hong W, TAZ expression as a prognostic indicator in colorectal cancer, PLoS One, 8 (2013) e54211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Cheng D, Jin L, Chen Y, Xi X, Guo Y, YAP promotes epithelial mesenchymal transition by upregulating Slug expression in human colorectal cancer cells, Int J Clin Exp Pathol, 13 (2020) 701–710. [PMC free article] [PubMed] [Google Scholar]
- [129].Lee KW, Lee SS, Kim SB, Sohn BH, Lee HS, Jang HJ, Park YY, Kopetz S, Kim SS, Oh SC, Lee JS, Significant association of oncogene YAP1 with poor prognosis and cetuximab resistance in colorectal cancer patients, Clin Cancer Res, 21 (2015) 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Salem O, Hansen CG, The Hippo Pathway in Prostate Cancer, Cells, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Coffey K, Targeting the Hippo Pathway in Prostate Cancer: What’s New?, Cancers, 13 (2021) 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Liu CY, Yu T, Huang Y, Cui L, Hong W, ETS (E26 transformation-specific) up-regulation of the transcriptional co-activator TAZ promotes cell migration and metastasis in prostate cancer, J Biol Chem, 292 (2017) 9420–9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Xue Y, Wong J, Moreno GT, Young MK, Côté J, Wang W, NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities, Mol Cell, 2 (1998) 851–861. [DOI] [PubMed] [Google Scholar]
- [134].Beyer TA, Weiss A, Khomchuk Y, Huang K, Ogunjimi AA, Varelas X, Wrana JL, Switch enhancers interpret TGF-β and Hippo signaling to control cell fate in human embryonic stem cells, Cell Rep, 5 (2013) 1611–1624. [DOI] [PubMed] [Google Scholar]
- [135].Kim M, Kim T, Johnson RL, Lim DS, Transcriptional co-repressor function of the hippo pathway transducers YAP and TAZ, Cell Rep, 11 (2015) 270–282. [DOI] [PubMed] [Google Scholar]
- [136].Abbas A, Gupta S, The role of histone deacetylases in prostate cancer, Epigenetics, 3 (2008) 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Johnson TR, Koul S, Kumar B, Khandrika L, Venezia S, Maroni PD, Meacham RB, Koul HK, Loss of PDEF, a prostate-derived Ets factor is associated with aggressive phenotype of prostate cancer: regulation of MMP 9 by PDEF, Mol Cancer, 9 (2010) 148. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [138].Steffan JJ, Koul HK, Prostate derived ETS factor (PDEF): a putative tumor metastasis suppressor, Cancer Lett, 310 (2011) 109–117. [DOI] [PubMed] [Google Scholar]
- [139].Steffan JJ, Koul S, Meacham RB, Koul HK, The transcription factor SPDEF suppresses prostate tumor metastasis, J Biol Chem, 291 (2016) 20826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Wang F, Koul S, Shanmugam PST, Dong Q, Koul HK, Prostate-Derived Ets Factor (PDEF) Inhibits Metastasis by Inducing Epithelial/Luminal Phenotype in Prostate Cancer Cells, Mol Cancer Res, 16 (2018) 1430–1440. [DOI] [PubMed] [Google Scholar]
- [141].Zhou GX, Li XY, Zhang Q, Zhao K, Zhang CP, Xue CH, Yang K, Tian ZB, Effects of the hippo signaling pathway in human gastric cancer, Asian Pac J Cancer Prev, 14 (2013) 5199–5205. [DOI] [PubMed] [Google Scholar]
- [142].Lam-Himlin DM, Daniels JA, Gayyed MF, Dong J, Maitra A, Pan D, Montgomery EA, Anders RA, The hippo pathway in human upper gastrointestinal dysplasia and carcinoma: a novel oncogenic pathway, Int J Gastrointest Cancer, 37 (2006) 103–109. [DOI] [PubMed] [Google Scholar]
- [143].Kang W, Cheng AS, Yu J, To KF, Emerging role of Hippo pathway in gastric and other gastrointestinal cancers, World J Gastroenterol, 22 (2016) 1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Cui ZL, Han FF, Peng XH, Chen X, Luan CY, Han RC, Xu WG, Guo XJ, YES-associated protein 1 promotes adenocarcinoma growth and metastasis through activation of the receptor tyrosine kinase Axl, Int J Immunopathol Pharmacol, 25 (2012) 989–1001. [DOI] [PubMed] [Google Scholar]
- [145].Ajani JA, Xu Y, Huo L, Wang R, Li Y, Wang Y, Pizzi MP, Scott A, Harada K, Ma L, Yao X, Jin J, Zhao W, Dong X, Badgwell BD, Shanbhag N, Tatlonghari G, Estrella JS, Roy-Chowdhuri S, Kobayashi M, Vykoukal JV, Hanash SM, Calin GA, Peng G, Lee JS, Johnson RL, Wang Z, Wang L, Song S, YAP1 mediates gastric adenocarcinoma peritoneal metastases that are attenuated by YAP1 inhibition, Gut, 70 (2021) 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Da CL, Xin Y, Zhao J, Luo XD, Significance and relationship between Yes-associated protein and survivin expression in gastric carcinoma and precancerous lesions, World J Gastroenterol, 15 (2009) 4055–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Hu X, Xin Y, Xiao Y, Zhao J, Overexpression of YAP1 is correlated with progression, metastasis and poor prognosis in patients with gastric carcinoma, Pathol Oncol Res, 20 (2014) 805–811. [DOI] [PubMed] [Google Scholar]
- [148].Sun L, Chen F, Shi W, Qi L, Zhao Z, Zhang J, Prognostic impact of TAZ and β-catenin expression in adenocarcinoma of the esophagogastric junction, Diagn Pathol, 9 (2014) 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Hillmer RE, Link BA, The Roles of Hippo Signaling Transducers Yap and Taz in Chromatin Remodeling, Cells, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Song JY, Lee JH, Joe CO, Lim DS, Chung JH, Retrotransposon-specific DNA hypomethylation and two-step loss-of-imprinting during WW45 haploinsufficiency-induced hepatocarcinogenesis, Biochem Biophys Res Commun, 404 (2011) 728–734. [DOI] [PubMed] [Google Scholar]
- [151].Vatanmakanian M, Tavallaie M, Ghadami S, Imatinib independent aberrant methylation of NOV/CCN3 in chronic myelogenous leukemia patients: a mechanism upstream of BCR-ABL1 function?, Cell Commun Signal, 17 (2019) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Jiménez AP, Traum A, Boettger T, Hackstein H, Richter AM, Dammann RH, The tumor suppressor RASSF1A induces the YAP1 target gene, Oncotarget, 8 (2017) 88437–88452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Malpeli G, Innamorati G, Decimo I, Bencivenga M, Nwabo Kamdje AH, Perris R, Bassi C, Methylation Dynamics of, Cancers (Basel), 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].García-Gutiérrez L, McKenna S, Kolch W, Matallanas D, RASSF1A Tumour Suppressor: Target the Network for Effective Cancer Therapy, Cancers (Basel), 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Khandelwal M, Anand V, Appunni S, Seth A, Singh P, Mathur S, Sharma A, Decitabine augments cytotoxicity of cisplatin and doxorubicin to bladder cancer cells by activating hippo pathway through RASSF1A, Mol Cell Biochem, 446 (2018) 105–114. [DOI] [PubMed] [Google Scholar]
- [156].Hill VK, Dunwell TL, Catchpoole D, Krex D, Brini AT, Griffiths M, Craddock C, Maher ER, Latif F, Frequent epigenetic inactivation of KIBRA, an upstream member of the Salvador/Warts/Hippo (SWH) tumor suppressor network, is associated with specific genetic event in B-cell acute lymphocytic leukemia, Epigenetics, 6 (2011) 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Elster D, Tollot M, Schlegelmilch K, Ori A, Rosenwald A, Sahai E, von Eyss B, TRPS1 shapes YAP/TEAD-dependent transcription in breast cancer cells, Nat Commun, 9 (2018) 3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Kuser-Abali G, Alptekin A, Cinar B, Overexpression of MYC and EZH2 cooperates to epigenetically silence MST1 expression, Epigenetics, 9 (2014) 634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, Wu X, Stack EC, Loda M, Liu T, Xu H, Cato L, Thornton JE, Gregory RI, Morrissey C, Vessella RL, Montironi R, Magi-Galluzzi C, Kantoff PW, Balk SP, Liu XS, Brown M, EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent, Science, 338 (2012) 1465–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Pinto R, De Summa S, Danza K, Popescu O, Paradiso A, Micale L, Merla G, Palumbo O, Carella M, Tommasi S, MicroRNA expression profiling in male and female familial breast cancer, Br J Cancer, 111 (2014) 2361–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Wang L, Chang W, Li Y, MicroRNA-373 promotes the development of esophageal squamous cell carcinoma by targeting, Int J Biol Markers, 34 (2019) 148–155. [DOI] [PubMed] [Google Scholar]
- [162].Lin CW, Chang YL, Chang YC, Lin JC, Chen CC, Pan SH, Wu CT, Chen HY, Yang SC, Hong TM, Yang PC, MicroRNA-135b promotes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1, Nat Commun, 4 (2013) 1877. [DOI] [PubMed] [Google Scholar]
- [163].James AR, Schroeder MP, Neumann M, Bastian L, Eckert C, Gökbuget N, Tanchez JO, Schlee C, Isaakidis K, Schwartz S, Burmeister T, von Stackelberg A, Rieger MA, Göllner S, Horstman M, Schrappe M, Kirschner-Schwabe R, Brüggemann M, Müller-Tidow C, Serve H, Akalin A, Baldus CD, Long non-coding RNAs defining major subtypes of B cell precursor acute lymphoblastic leukemia, J Hematol Oncol, 12 (2019) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Qiao K, Ning S, Wan L, Wu H, Wang Q, Zhang X, Xu S, Pang D, Correction to: LINC00673 is activated by YY1 and promotes the proliferation of breast cancer cells via the miR-515–5p/MARK4/Hippo signaling pathway, J Exp Clin Cancer Res, 39 (2020) 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Mashouri L, Yousefi H, Aref AR, mohammad Ahadi A, Molaei F, Alahari SK, Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance, Molecular cancer, 18 (2019) 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK, Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance, Mol Cancer, 18 (2019) 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Yousefi H, Maheronnaghsh M, Molaei F, Mashouri L, Aref AR, Momeny M, Alahari SK, Long noncoding RNAs and exosomal lncRNAs: classification, and mechanisms in breast cancer metastasis and drug resistance, Oncogene, (2019) 1–22. [DOI] [PubMed] [Google Scholar]
- [168].Yousefi H, Maheronnaghsh M, Molaei F, Mashouri L, Reza Aref A, Momeny M, Alahari SK, Long noncoding RNAs and exosomal lncRNAs: classification, and mechanisms in breast cancer metastasis and drug resistance, Oncogene, 39 (2020) 953–974. [DOI] [PubMed] [Google Scholar]
- [169].Moroishi T, Hayashi T, Pan W-W, Fujita Y, Holt MV, Qin J, Carson DA, Guan K-L, The Hippo pathway kinases LATS1/2 suppress cancer immunity, Cell, 167 (2016) 1525–1539. e1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Hang W, Feng Y, Sang Z, Yang Y, Zhu Y, Huang Q, Xi X, Downregulation of miR-145–5p in cancer cells and their derived exosomes may contribute to the development of ovarian cancer by targeting CT, International journal of molecular medicine, 43 (2019) 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Wang S, Su X, Xu M, Xiao X, Li X, Li H, Keating A, Zhao RC, Exosomes secreted by mesenchymal stromal/stem cell-derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway, Stem Cell Res Ther, 10 (2019) 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Yoshida M, Horiguchi H, Kikuchi S, Iyama S, Ikeda H, Goto A, Kawano Y, Murase K, Takada K, Miyanishi K, miR-7977 inhibits the Hippo-YAP signaling pathway in bone marrow mesenchymal stromal cells, PloS one, 14 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Yoshida M, Horiguchi H, Kikuchi S, Iyama S, Ikeda H, Goto A, Kawano Y, Murase K, Takada K, Miyanishi K, Kato J, Kobune M, miR-7977 inhibits the Hippo-YAP signaling pathway in bone marrow mesenchymal stromal cells, PLoS One, 14 (2019) e0213220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Fan F, He Z, Kong LL, Chen Q, Yuan Q, Zhang S, Ye J, Liu H, Sun X, Geng J, Yuan L, Hong L, Xiao C, Zhang W, Li Y, Wang P, Huang L, Wu X, Ji Z, Wu Q, Xia NS, Gray NS, Chen L, Yun CH, Deng X, Zhou D, Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration, Sci Transl Med, 8 (2016) 352ra108. [DOI] [PubMed] [Google Scholar]
- [175].Wu D, Luo L, Yang Z, Chen Y, Quan Y, Min Z, Targeting Human Hippo TEAD Binding Interface with YAP/TAZ-Derived, Flexibility-Reduced Peptides in Gastric Cancer, International Journal of Peptide Research and Therapeutics, 27 (2021) 119–128. [Google Scholar]
- [176].Oh H, Slattery M, Ma L, White KP, Mann RS, Irvine KD, Yorkie promotes transcription by recruiting a histone methyltransferase complex, Cell Rep, 8 (2014) 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Wang W, Li N, Li X, Tran MK, Han X, Chen J, Tankyrase Inhibitors Target YAP by Stabilizing Angiomotin Family Proteins, Cell Rep, 13 (2015) 524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Wang Z, Wu Y, Wang H, Zhang Y, Mei L, Fang X, Zhang X, Zhang F, Chen H, Liu Y, Jiang Y, Sun S, Zheng Y, Li N, Huang L, Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility, Proc Natl Acad Sci U S A, 111 (2014) E89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Yu FX, Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, Taylor SS, Lai ZC, Guan KL, Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation, Genes Dev, 27 (2013) 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Hawley SA, Gadalla AE, Olsen GS, Hardie DG, The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism, Diabetes, 51 (2002) 2420–2425. [DOI] [PubMed] [Google Scholar]
- [181].Slemmons KK, Yeung C, Baumgart JT, Juarez JOM, McCalla A, Helman LJ, Targeting Hippo-Dependent and Hippo-Independent YAP1 Signaling for the Treatment of Childhood Rhabdomyosarcoma, Cancer Research, 80 (2020) 3046–3056. [DOI] [PubMed] [Google Scholar]
- [182].Bierbaumer L, Katschnig AM, Radic-Sarikas B, Kauer MO, Petro JA, Högler S, Gurnhofer E, Pedot G, Schäfer BW, Schwentner R, Mühlbacher K, Kromp F, Aryee DNT, Kenner L, Uren A, Kovar H, YAP/TAZ inhibition reduces metastatic potential of Ewing sarcoma cells, Oncogenesis, 10 (2021) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, Marotz C, Giannopoulou E, Chakravarthi BV, Varambally S, Tomlins SA, Nanus DM, Tagawa ST, Van Allen EM, Elemento O, Sboner A, Garraway LA, Rubin MA, Demichelis F, Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer, Nat Med, 22 (2016) 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]



 Yap/Taz
Yap/Taz  Lats1/2
Lats1/2