Abstract
Objective
To examine the effectiveness of a newly developed emergency room (ER) protocol to treat patients with stroke and control the spread of SARS-CoV-2 by evaluating the door-to-picture time.
Methods
We retrospectively enrolled 126 patients who were transported to our ER by ambulance with suspected stroke between April 15 and October 31, 2020 (study group). A risk judgment system named the COVID level was introduced to classify the risk of infection as follows: level 0, no infection; I, infection unlikely; II, possible; III, probable; and IV, definite. Patients with COVID levels 0, I, or II and a Glasgow Coma Scale (GCS) score >10 were placed in a normal ER (nER) without atmospheric pressure control; the medical staff wore standard personal protective equipment (PPE) in such cases. Patients with COVID level II, III, or IV, and a GCS score of ≤10 were assigned to the negative pressure ER (NPER); the medical staff wore enhanced PPE for these cases. The validity of the protocol was assessed. The door-to-picture time of the study group was compared with that of 114 control patients who were transported with suspected stroke during the same period in 2019 (control group). The difference in the time for CT and MRI between the two groups was also compared. In the study group, the time spent in the nER and NPER was evaluated.
Results
In all, 118 patients (93.7%) were classified as level I, 6 (4.8%) as level II, and 2 (1.6%) as level III. Only five patients (4.0%) were treated with NPER. Polymerase chain reaction tests were performed on 118 out of 126 patients (93.7%) and were negative. No significant differences were observed in age, sex, neurological severity, modalities of diagnostic imaging, and diagnosis compared with the control group. The median door-to-picture time was 18 (11–27.8) min in the study group and 15 (10–25) min in the control group (p = 0.08). No delay was found on CT (15 [10–21] vs. 14 [9–21] min, p = 0.24). In contrast, there was an 8-min delay for MRI (30 [21.8–50] vs. 22 [14–30] min, p = 0.01). The median door-to-picture time was 29 min longer in patients treated with NPER than in those treated with nER, although the difference was not significant due to the small number of patients (47 [27–57] vs. 18 [11–26] min, p = 0.07).
Conclusion
Our protocol could optimize the use of medical resources with only a 3-min delay in the door-to-picture time in an area without explosive outbreak. Unfortunately, the effectiveness of the protocol in preventing infection could not be verified because of the low incidence of COVID-19. When developing and modifying an institutional protocol, recognizing the outbreak status surrounding each institution is important.
Keywords: coronavirus disease 2019, initial treatment, mechanical thrombectomy, protocol, stroke
Introduction
Stroke is the most common cause of a bedridden state in Japan.1) Acute large vessel occlusion (LVO) stroke has benefited from recent advances in treatment strategies, namely mechanical thrombectomy (MT).2–6) As better outcomes are achieved when recanalization is performed as soon as possible, medical institutes focus exclusively on achieving rapid recanalization. However, the emergence of the lethal virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has had a major impact on treatment. Coronavirus disease 2019 (COVID-19), the disease caused by SARS-CoV-2 infection, was initially confirmed in Wuhan, China at the end of 2019.7) SARS-CoV-2 has since spread worldwide at an incredible speed. In Japan, cities such as Tokyo, Yokohama, and Sapporo initially reported cases of the virus in January 2020.7)
COVID-19 is spread by asymptomatic and presymptomatic carriers and its transmission route is via small, aerosolized particles in addition to respiratory droplets and person-to-person contact.8) Therefore, stroke patients now have to be treated under the strict infection control of SARS-CoV-2.9–12) In emergent cases, such as stroke, there is an insufficient amount of time to assess the COVID-19 status of patients. The early stage of the SARS-CoV-2 spread caused panic worldwide, and, as a consequence, personal protection equipment (PPE) was in short supply. Therefore, we had to achieve a balance between infection control and stroke treatment.
Our hospital is located in Tsuchiura, one of the commuter towns of Tokyo in Ibaraki prefecture, in which the number of COVID-19 patients was small, but steadily increasing at that time. Therefore, we developed an emergency room (ER) protocol to treat stroke patients under the strict control of SARS-CoV-2 spread prevention, which saves both time and medical resources. Once a patient starts to undergo cerebral angiography or is on the operating table, the time for treatment does not differ significantly between infected and uninfected patients, except for the requirement for PPE. However, the preparation time for interventions under infection control increases. Therefore, we introduced our protocol and evaluated its usefulness by comparing the door-to-picture time with that before SARS-CoV-2.
Materials and Methods
Patient population
The present study was approved by the Institutional Ethical Review Board and was conducted in accordance with the Declaration of Helsinki. We retrospectively enrolled patients who were transported to our ER by ambulance with suspected stroke and treated according to the institutional protocol for stroke patients under the strict control of SARS-CoV-2 spread between April 15, 2020 and October 31, 2020. Patients who were transferred from other hospitals after diagnosis using diagnostic imaging were excluded. Patients who visited by themselves and those who were not hospitalized were also excluded. Therefore, 126 patients were finally examined in the present study. As a control group, 114 patients who were transported to our ER with suspected stroke during the same period in 2019 were also extracted with identical exclusion criteria.
Methods
To clarify whether our protocol was effective, the following data on study subjects were retrospectively recorded: age, sex, the COVID level (described below), the Glasgow Coma Scale (GCS) score and National Institute of Health stroke score (NIHSS) on arrival, the use of the normal ER with no atmospheric pressure control (nER) or the negative pressure ER (NPER), with or without intubation, modality of diagnostic imaging, diagnosis, last known well (LKW) time, symptom onset time, onset (or LKW)-to-door time, and door-to-picture time. The results of SARS-CoV-2 polymerase chain reaction (PCR) tests were also recorded.
To confirm that our protocol effectively balances safety for infection control with treatment times, the door-to-picture time was compared with that of the control group. The door-to-picture times were compared using diagnostic imaging modalities, and a comparison was also performed between groups treated with nER or NPER within the study group.
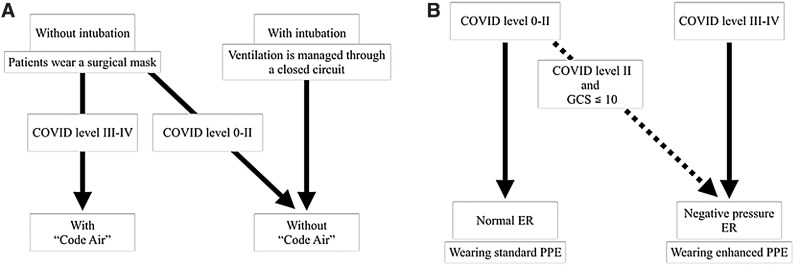
ER protocol to treat stroke patients under the strict control of SARS-CoV-2 spread prevention that saves both time and medical resources (Figs. 1 and 2)
Fig. 1. Flowcharts for accepting emergency patients with suspected stroke (A) and for the in-hospital transportation of patients (B) in the COVID-19 setting. (A) As a general rule of the institute, according to the COVID level, which is an institutional risk judgment system shown in Table 1, patients classified as level II or less are taken to the normal ER and treated with standard PPE precautions. Patients classified as level III or IV are treated with the NPER and treated with enhanced PPE precautions. Patients with suspected stroke are more likely to have compromised upper airways due to vomiting or impaired consciousness and may require suction or tracheal intubation, which results in the aerosolization of respiratory secretions. Patients with suspected stroke with a level II risk and a disturbance of consciousness of 10 or lower in GCS are also taken to the NPER. (B) Patients without intubation wear a surgical mask and are transported to the diagnostic imaging room via a cleared route to manage airborne infection when they have a COVID level III or IV risk. This countermeasure against airborne infection in transportation is called code air. Patients without intubation who have a COVID level 0–II risk also wear a surgical mask, but are transported without code air. Patients with intubation are transported without code air because their ventilation is managed through a closed circuit, and the risk of aerosol infection is reduced. COVID-19: coronavirus disease 2019; ER: emergency room; GCS: Glasgow Coma Scale; NPER: negative pressure emergency room; PPE: personal protective equipment.
Fig. 2. Illustrations of the intra-hospital transportation routes with (A) or without (B) code air. The numbers following CT, MRI, or EV indicate the individual room numbers. Code air is the institutional countermeasure against airborne infection in transportation. The dashed lines indicate the routes in which patients are transported. At our hospital, both CT and MRI are available, regardless of the suspected infection. CT 3 or MRI 2 is used when code air is announced, while CT 1 or MRI 1 is used when it is not. After diagnostic imaging, if mechanical thrombectomy is indicated, patients are transported to the angiography suite on the third floor using separate elevators. ER: emergency room; EV: elevator; NPER; negative pressure emergency room.
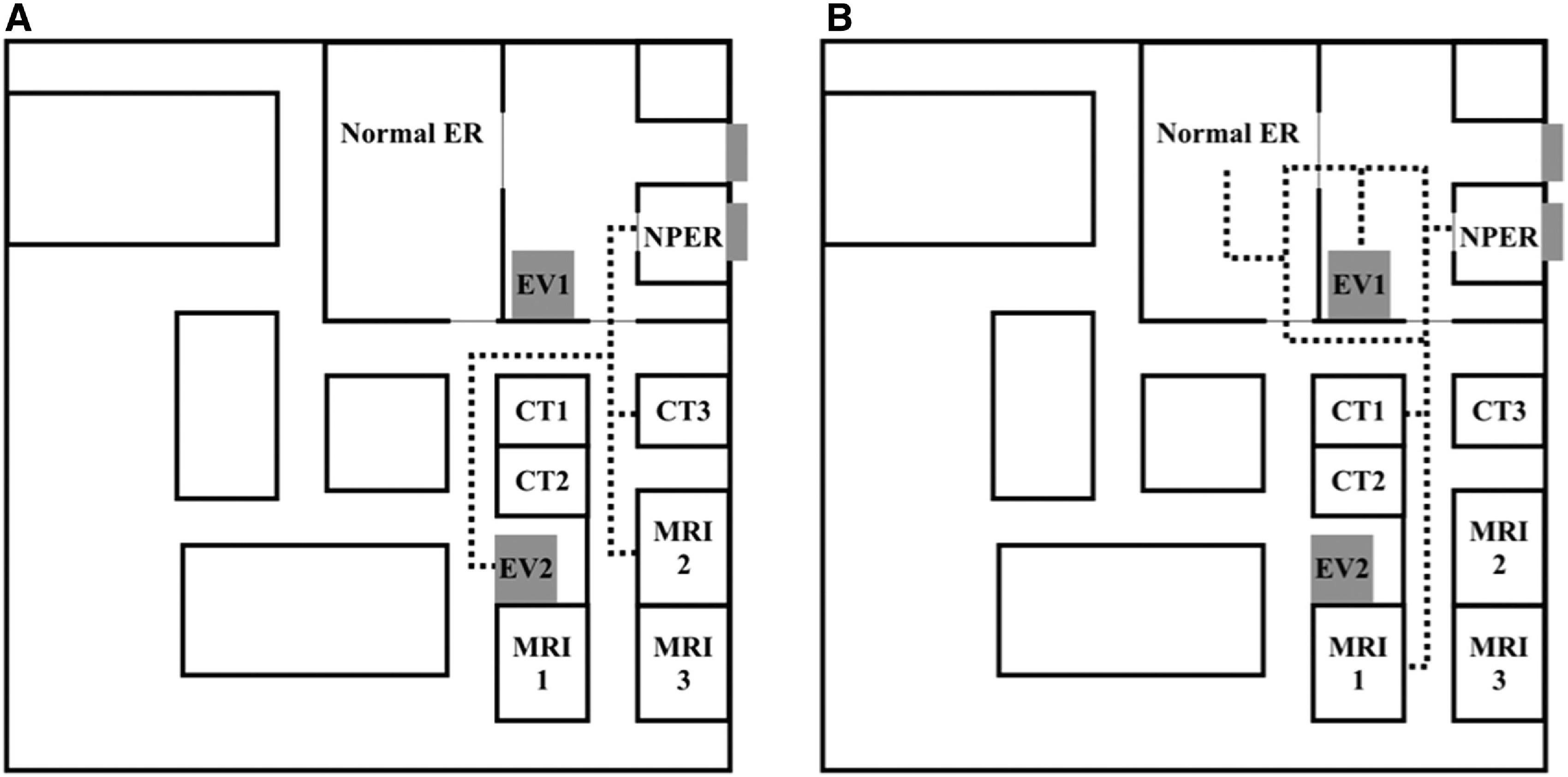
In our hospital, an institutional risk judgment system named the COVID level was introduced on April 10, 2020, to classify the risk of infection (Table 1). The COVID level evaluates a patient’s infectious risk on a scale of 0 to IV based on epidemiological and symptomatic information and the result of a PCR test for SARS-CoV-2. If a patient has a negative PCR test and has neither an epidemiological risk nor symptoms of COVID-19, the COVID level is defined as level 0. Conversely, if a patient has a positive PCR test, the COVID level is defined as level IV, regardless of the epidemiological risk or symptoms. Regarding patients with an undocumented COVID status, if a patient has neither an epidemiological risk nor symptoms of COVID-19, the COVID level is defined as level I; if the patient has either of them, the COVID level is defined as level II; and if the patient has both, the COVID level is defined as level III. The judgment on the COVID level is made by an on-duty doctor using information from paramedics. Both the doctor and the nurse wait in the ER, wearing proper personal protective equipment (PPE), before the patient arrives. As a general rule, patients who are classified as level II or lower are taken to the nER, and the attending medical staff wear standard PPE, comprising a surgical mask, gloves, and a vinyl apron. If a patient has a higher risk of aerosolization, an N95 mask is used under the surgical mask. Patients classified as level III or IV are taken to the NPER, and staff wear enhanced PPE, comprising a surgical cap, goggles, a face shield, an N95 mask under the surgical mask, a full gown, and double gloves. In addition, level II patients with suspected stroke with GCS ≤10 are taken to the NPER in order to reduce the risk of aerosol infection during transportation from the ER to the diagnostic imaging room or angiography suite. Patients with suspected stroke are more likely to have a compromised upper airway due to vomiting or impaired consciousness; these patients sometimes require suction or tracheal intubation, which results in the aerosolization of respiratory secretions. The Society of Neurointerventional Surgery (SNIS) recommends the use of standard institutional protocols with a low threshold for intubation of stroke patients prior to transport to the angiography suite, ideally in a negative pressure environment.9) The physical and neurological findings of patients taken to the NPER are assessed by a neurologist or neurosurgeon in enhanced PPE with the assistance of a nurse in enhanced PPE, and the patients are intubated by an emergency physician in enhanced PPE where necessary. Patients taken to the nER wear a surgical mask and are assessed by a physician and nurse wearing standard PPE. If patients require intubation, it is performed in the nER by an emergency doctor with enhanced PPE before or after imaging with the assistance of a nurse in enhanced PPE. Level III or IV patients without intubation wear a surgical mask and are transported to the diagnostic imaging room by a physician and nurse in enhanced PPE; the route is first secured and cleared in order to manage airborne infection. This countermeasure against airborne infection in transportation is called code air in our hospital. Patients without intubation who have a COVID level 0–II risk also wear a surgical mask, but are transported by a small team of staff in standard PPE, without code air. Patients with intubation are transported without code air because their ventilation is managed through a closed circuit and the risk of aerosol infection is reduced.9) At our hospital, both CT and MRI are available, regardless of the suspected infection. Chest and head CT are taken simultaneously to assess pneumonia.10,11) Chest radiography is performed when MRI is selected. If MT is indicated after diagnostic imaging, patients are transported to the angiography suite on the third floor by using elevator 1 for no code air and elevator 2 for code air. The routes are illustrated in Fig. 2. MT is performed by a minimum number of providers wearing enhanced PPE in all cases regardless of the COVID level in accordance with the SNIS recommendations.9) A non-operating physician outside the angiography suite ensures proper technique and inspection of the equipment as a safety leader.10,12)
Table 1. COVID level: an institutional risk judgment system.
| Level | Meaning | Definition |
|---|---|---|
| 0 | No infection | With documented COVID-negative status in PCR test and with neither an epidemiological risk nor symptoms of COVID-19 |
| I | Unlikely | With undocumented COVID status and with neither an epidemiological risk nor symptoms of COVID-19 |
| II | Possible | With undocumented COVID status and with either an epidemiological risk or symptoms of COVID-19 |
| III | Probably | With undocumented COVID status and with both an epidemiological risk and symptoms of COVID-19 |
| IV | Definite | With documented COVID-positive status, regardless of the epidemiological risk or symptoms of COVID-19 |
Epidemiological risk: contact with COVID-19-positive patients or close contacts of COVID-19-positive patients and history of going out to outbreak areas. Symptoms of COVID-19: fever of ≥37.5° or airway symptoms or loss of smell or taste. COVID-19: coronavirus disease 2019; PCR: polymerase chain reaction
This protocol was initiated at our institution from April 15, 2020.
Statistical analysis
Continuous variables were analyzed using the Mann–Whitney U-test, and categorical variables were analyzed using Fisher’s exact test for comparisons between two groups. Statistical analyses were performed using R 3.3.3 GUI 1.69 Mavericks build (7328) (the R Foundation, Vienna, Austria. https://www.R-project.org/). The significance level was set at p <0.05.
Results
The patient characteristics are summarized in Table 2. The median age of the 126 study patients was 75 (interquartile range: 62.3–82) years, which was not significantly different from that in the control group (77 [67–83] years, p = 0.31). There were 68 men (54.0%) and 58 women (46.0%). None of the patients were classified as having COVID level 0 or IV before admission; 118 patients (93.7%) were classified as level I, 6 (4.8%) as level II, and 2 (1.6%) as level III. The median GCS score was 14 (10–15), which was not significantly different from that in the control group (13 [10–15], p = 0.71]. In addition, 34 patients (27.0%) presented with a GCS score of ≤10 and no significant difference was observed compared to those in the control group (32 patients [28.1%], p = 0.89). The NIHSS was available in 67 out of 126 patients, with a median of 4 (2–13.5), which was not significantly different from that in the control group (available in 51 of 114 patients; 6 [3–14], p = 0.32). Among 126 patients, only 5 (4.0%) were taken to the NPER; 2 (40.0%) were level III, and 3 (60.0%) were level II and a GCS score lower than 10. Sixteen patients (12.7%) were intubated in the ER, 7 (43.8%) before diagnostic imaging, and 9 (56.2%) after diagnostic imaging. Three of these patients (18.8%) were intubated in a negative pressure environment. In the control group, 13 patients (11.4%) were intubated in the ER, 1 (7.7%) before diagnostic imaging, and 12 (92.3%) after diagnostic imaging. Although no significant difference was observed in intubation itself, significantly more patients were intubated before diagnostic imaging in the study group (p = 0.04). The modalities of diagnostic imaging were CT in 94 patients (74.6%) and MRI in 32 patients (25.4%). No significant difference was observed in the fraction of diagnostic imaging modalities compared to that in the control group. Diagnoses included cerebral infarction in 58 patients (46.0%), transient ischemic attack in 6 (4.8%), intracerebral hemorrhage in 34 (27.0%), subarachnoid hemorrhage in 10 (7.9%), and others in 18 (14.3%). Ten patients (7.9%) were treated with intravenous tissue plasminogen activator (IV-tPA) and 14 (11.1%) were treated with MT. No significant differences were observed neither in the diagnosis nor in the application of IV-tPA or MT compared to the control group.
Table 2. Patient characteristics.
| Variables | Study group (n = 126) | Control group (n = 114) | p value |
|---|---|---|---|
| Age (years) | 75 (62.3–82) | 77 (67–83) | 0.31 |
| Female gender | 58 (46.0%) | 46 (40.4%) | 0.43 |
| GCS score | 14 (10–15) | 13 (10–15) | 0.71 |
| GCS ≤10 | 34 (27.0%) | 32 (28.1%) | 0.89 |
| NIHSS | 4 (2–13.5) | 6 (3–14) | 0.32 |
| COVID level 0 | 0 (0%) | NA | |
| COVID level I | 118 (93.7%) | ||
| COVID level II | 6 (4.8%) | ||
| COVID level III | 2 (1.6%) | ||
| COVID level IV | 0 (0%) | ||
| Taken to the negative pressure ER | 5 (4.0%) | NA | |
| With a higher COVID level (level III) | 2 (40.0%)* | ||
| With a level II risk and a lower GCS score | 3 (60.0%)* | ||
| Tracheal intubation | 16 (12.7%) | 13 (11.4%) | 0.84 |
| Before diagnostic imaging | 7 (43.8%)* | 1 (7.7%)* | 0.04† |
| After diagnostic imaging | 9 (56.2%)* | 12 (92.3%)* | |
| Done in the negative pressure | 3 (18.8%)* | NA | |
| Done in the normal pressure | 13 (81.3%)* | ||
| Diagnostic imaging modality | 0.47 | ||
| CT | 94 (74.6%) | 80 (70.2%) | |
| MRI | 32 (25.4%) | 34 (29.8%) | |
| Stroke classification | 0.71 | ||
| Cerebral infarction | 58 (46.0%) | 62 (54.4%) | |
| Transient ischemic attack | 6 (4.8%) | 6 (5.3%) | |
| Intracerebral hemorrhage | 34 (27.0%) | 25 (21.9%) | |
| Subarachnoid hemorrhage | 10 (7.9%) | 6 (5.3%) | |
| Other disease | 18 (14.3%) | 15 (13.2%) | |
| IV-tPA | 10 (7.9%) | 17 (14.9%) | 0.1 |
| MT | 14 (11.1%) | 17 (14.9%) | 0.44 |
Continuous variables are presented as median (interquartile range).
* Percentages are shown as ratios within the above items.
† Statistically significant in Fisher’s exact test.
ER: emergency room; GCS: Glasgow Coma Scale; IV-tPA: intravenous tissue plasminogen activator; MT: mechanical thrombectomy; NIHSS: National Institute of Health stroke score
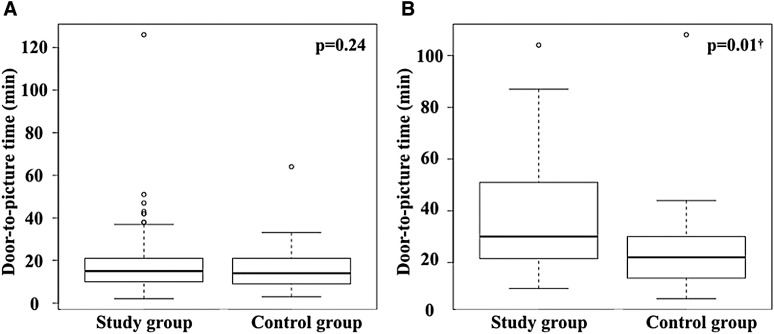
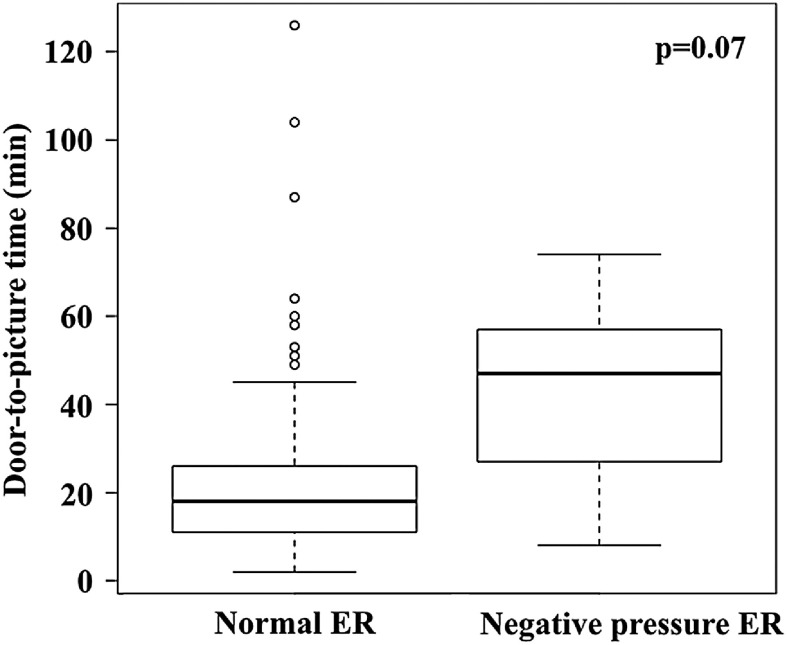
The items related to treatment time are summarized in Table 3. The symptom onset time was unclear in 43 patients (34.1%), and the LKW time was unknown in 14 patients (11.1%); no significant differences were observed between the study and the control groups. In the study group, the median onset-to-door time was 131.5 (69–346.3) min and the median door-to-picture time was 18 (11–27.8) min. In the control group, the median onset-to-door time was 125 (62–362) min, and the median door-to-picture time was 15 (10–25) min. No significant difference was observed in the onset-to-door time between the two groups (p = 0.97), whereas an almost significant difference was observed in the door-to-picture time (p = 0.08). The median door-to-picture time did not differ significantly between patients who were diagnosed using CT (15 [10–21] vs. 14 [9–21] min, p = 0.24) (Fig. 3A). In contrast, among patients who were diagnosed by MRI, the median door-to-picture time in the study group was significantly longer than that in the control group by 8 min (30 [21.8–50] vs. 22 [14–30] min, p = 0.01) (Fig. 3B). The median door-to-picture time was longer in patients treated with NPER than in those treated with nER within the study group (47 [27–57] vs. 18 [11–26] min, p = 0.07) (Fig. 4). PCR tests were performed on 118 out of 126 patients (93.7%) and were negative in all the tested cases.
Table 3. Comparisons of the items related to treatment time.
| Variables | Study group (n = 126) | Control group (n = 114) | p value |
|---|---|---|---|
| Unknown symptom onset time | 43 (34.1%) | 49 (43.0%) | 0.18 |
| Unknown last known well time | 14 (11.1%) | 13 (11.4%) | 1 |
| Onset-to-door time (min) | 131.5 (69–346.3) | 125 (62–362) | 0.97 |
| Door-to-picture time (min) | 18 (11–27.8) | 15 (10–25) | 0.08 |
Continuous variables are presented as median (interquartile range).
Fig. 3. Comparisons of the door-to-picture time by the modality of diagnostic imaging. (A) Comparison of patients with CT. Box-and-whisker plots are presented. The median door-to-picture time did not differ significantly between the groups (15 [10–21] vs. 14 [9–21], p = 0.24). (B) Comparison of patients who underwent MRI. Box-and-whisker plots are presented. The median door-to-picture time in the study group was significantly longer than that in the control group by 8 min (30 [21.8–50] vs. 22 [14–30], p = 0.01). † Significantly different according to the Mann–Whitney U-test.
Fig. 4. Comparison of the door-to-picture time between patients who were treated in the nER and those who were treated in the NPER within the study group. Box-and-whisker plots are presented. The median door-to-picture time was longer in patients treated with the NPER than in those treated with the nER (47 [27–57] vs. 18 [11–28.3], p = 0.07). nER: normal emergency room; NPER: negative pressure emergency room.
Discussion
We developed an ER protocol to treat patients with stroke under the strict control of SARS-CoV-2 spread prevention, which saves both time and medical resources. In this protocol, patients with a moderate risk of COVID-19 and a GCS ≤10, or those with a high risk regardless of GCS were managed in the NPER. The patients were intubated before leaving the NPER to control aerosol infection where necessary. None of the patients were diagnosed with COVID-19 in this study, and almost all patients were treated with a similar initial treatment pathway as that used during the pre-COVID period. Although the difference was not statistically significant, the door-to-picture time tended to be 3 min longer in the study group than in the control group.
Impact of the low incidence of COVID-19
During the study period, no COVID-19-positive patients were observed. Only 5 of 126 patients (4.0%) were taken to the NPER, and 3 (60%) had COVID level II with a GCS ≤10. Thirty-four patients (27.0%) had a GCS score ≤10; however, only six of them had COVID level II due to the low incidence of COVID-19 in the area. Thus, the effectiveness of this protocol from the viewpoint of infection prevention could not be verified. Still, the protocol was useful in that it avoided excessive precautions and saved medical resources.
Interpretation of the 3-min delay in the door-to-picture time
The initial treatment pathways were similar between the two groups. However, the proper donning and doffing of PPE, which was initially unfamiliar for most medical staff, may have affected to this 3-min delay, particularly MRI, which resulted in an 8-min delay. Both patients and medical staff are prohibited from wearing metallic or magnetic materials in the MRI room, and checking and removing magnetic materials require partial donning and doffing of PPE with proper hand hygiene.
According to highly effective reperfusion evaluated in multiple endovascular stroke trials (HERMES) collaborators, with every 4-min delay in the door-to-reperfusion time, 1 out of 100 treated patients had a worse disability outcome.13) When applying this statistic to our study, a 3-min delay in the door-to-picture time may result in worse outcomes. Previous studies on the time frames of stroke treatment in the setting of COVID-19 reported that the door-to-picture time was 12–34 min in the pre-COVID-19 period.14–20) Our door-to-picture time of 18 min in the study group was within this range, which is considered acceptable. However, it may differ in an area with a particularly severe outbreak of COVID-19; thus, further preparation is required. Studies on workflow time intervals and the functional outcomes of patients with LVO are accumulating.14–20) Contrary to expectations, there is no certain tendency on the door-to-picture times. Chowdhury et al. performed an international global survey to gather and summarize information from tertiary care stroke centers on periprocedural pathways and the endovascular management of patients with acute ischemic stroke during the COVID-19 pandemic.21) They received 114 responses from 25 different countries across all five continents. Only 16% of the participating centers used a negative pressure room for thrombectomy. Regarding anesthetic management, 50% of the participating centers remained unchanged from that used pre-COVID. In addition, COVID-19 testing was performed after the procedure in 31% of centers, and 20% of centers did not perform COVID testing at all, suggesting no time delay before door-to-picture time. These variabilities in infection management will likely lead to inconsistencies in door-to-picture times.
Effects of lowering the threshold for considering intubation
The SNIS recommends lowering the threshold for intubation of MT patients to reduce the risk of aerosol infection.9) As there is no clear indication for intubation in stroke patients, we referred to trauma guidelines, which recommend intubation of traumatic patients with a GCS score of ≤8.22) We lowered the threshold for considering intubation to a GCS of 10 for level II risk patients, and they were taken to the NPER to manage the risk of aerosolization. In the present study, 16 patients (12.7%) in the study group were intubated in the ER, which was not significantly different from that in the control group (11.4% in the pre-COVID period). In contrast, intubation was performed significantly more often before diagnostic imaging in the study group despite similar neurological severities to the control group. Considering that intubation was mostly performed in the nER in the study group, these results suggest that prevention of aerosol infection by intubation before diagnostic imaging was emphasized in cases requiring intubation, even if the COVID level was low.
Considerations for the future
Strict infection control is crucial because the nosocomial spread of SARS-CoV-2 infection results in hospital function outages. Ensuring the safety of healthcare workers and other patients is of utmost importance, and our goal is to achieve similar outcomes for patients with stroke to those in the pre-COVID era. In the present study, the median door-to-picture time was extended by 29 min, with a minimum of 8 min, when patients were taken to the NPER. Unfortunately, the error was large due to the small number of patients, and no significant difference was found between the NPER and nER groups. Therefore, if the number of patients with COVID-19 increases in the near future, the delay in the door-to-picture time will be greater, resulting in worse clinical outcomes. Furthermore, general anesthesia is necessary for intubated patients, which further worsens the clinical outcomes due to the time needed for preparation.23) Therefore, further training to shorten the treatment time in the NPER while also ensuring safety is vital.11)
Our protocol worked well in that it resulted in an acceptable time delay and saved medical resources in terms of stroke treatments performed in the absence of an explosive COVID-19 outbreak. However, the effectiveness of infection prevention could not be verified because of the low incidence of COVID-19. The application of this protocol during an explosive outbreak may cause a significant time delay and/or nosocomial infections. Therefore, the accurate recognition of an outbreak status and modifications to the protocol in advance are critical. Infection control is the highest priority to ensure the safety of healthcare workers and other patients and to prevent hospital function outages. Improvements in the time delay of stroke treatments are needed, but without the detriment of nosocomial infections.
Limitations
This study has several limitations that should be discussed. This was a retrospective analysis with a short study period, and the number of MT patients in the study group was too small to verify our findings. Moreover, the study group included miscellaneous stroke types, such as subarachnoid hemorrhage and suspected mild cerebral infarction, which were delayed until examinations were performed. Ideally, the study should be performed with MT patients to verify the effect of the protocol. For the same reason, we could not survey functional outcomes that showed a strong correlation with the time frame of stroke treatments. Moreover, the lack of COVID-19-positive patients prevented us from verifying the effectiveness of infection prevention. Further studies are needed to examine the effects of this new protocol on functional outcomes.
Conclusion
Our protocol for the initial treatment of patients with suspected stroke in the setting of COVID-19 was developed in consideration of epidemiological and symptomatic risks of COVID-19 infection, using the NPER as needed. This protocol worked well in saving medical resources with only a 3-min delay in the door-to-picture time in an area without an explosive outbreak. However, the effectiveness of infection prevention could not be verified because of the low incidence of COVID-19. When developing and modifying an institutional protocol, it is important to recognize the outbreak status of each institution and shorten the treatment time while also prioritizing infection control.
Acknowledgment
We would like to thank Editage (www.editage.com) for English language editing.
Disclosure Statement
The authors declare that they have no conflicts of interest.
References
- 1). Ministry of Health, Labor and Welfare . Comprehensive survey of living conditions 2016. https://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa16/dl/06.pdf (Accessed: January 25, 2021)
- 2). Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 3). Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 4). Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 5). Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 6). Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 7). Watanabe M. The COVID-19 pandemic in Japan. Surg Today 2020; 50: 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Azuma K, Yanagi U, Kagi N, et al. Environmental factors involved in SARS-CoV-2 transmission: effect and role of indoor environmental quality in the strategy for COVID-19 infection control. Environ Health Prev Med 2020; 25: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Fraser JF, Arthur AS, Chen M, et al. Society of neurointerventional surgery recommendations for the care of emergent neurointerventional patients in the setting of COVID-19. J Neurointerv Surg 2020; 12: 539–541. [DOI] [PubMed] [Google Scholar]
- 10). Japanese Stroke Society PCS Working Group . Protocol for stroke management during COVID-19 pandemic: protected code stroke, Japan Stroke Society edition (JSS-PCS). Jpn J Stroke 2020; 42: 315–343. (in Japanese) [Google Scholar]
- 11). Srivatanakul K, Asai S, Hirayama A, et al. A protocol of infection control for mechanical thrombectomy in possible COVID-19 patients: Tokai University COVID-19 manual. JNET J Neuroendovasc Ther 2020; 14: 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. Stroke 2020; 51: 1891–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Saver JL, Goyal M, van der Lugt A, et al. Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016; 316: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 14). Candeloro E, Carimati F, Tabaee Damavandi P, et al. An example of a stroke unit reshaping in the context of a regional hub and spoke system in the COVID-19 era. Front Neurol 2020; 11: 1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Wu Y, Chen F, Wang Z, et al. Reductions in hospital admissions and delays in acute stroke care during the pandemic of COVID-19. Front Neurol 2020; 11: 584734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Agarwal S, Scher E, Rossan-Raghunath N, et al. Acute stroke care in a New York City comprehensive stroke center during the COVID-19 pandemic. J Stroke Cerebrovasc Dis 2020; 29: 105068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Uchino K, Kolikonda MK, Brown D, et al. Decline in stroke presentations during COVID-19 surge. Stroke 2020; 51: 2544–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18). Plumereau C, Cho TH, Buisson M, et al. Effect of the COVID-19 pandemic on acute stroke reperfusion therapy: data from the Lyon Stroke Center Network. J Neurol 2020; 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Siegler JE, Zha AM, Czap AL, et al. Influence of the COVID-19 pandemic on treatment times for acute ischemic stroke: the society of vascular and interventional neurology multicenter collaboration. Stroke 2021; 52: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Kim TJ, Kim BJ, Gwak DS, et al. Modification of acute stroke pathway in Korea after the coronavirus disease 2019 outbreak. Front Neurol 2020; 11: 597785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Chowdhury T, Rizk AA, Daniels AH, et al. Management of acute ischemic stroke in the interventional neuroradiology suite during the COVID-19 pandemic: a global survey. J Neurosurg Anesthesiol 2021; 33: 44–50. [DOI] [PubMed] [Google Scholar]
- 22). ATLS Subcommittee; American College of Surgeons’ Committee on Trauma; International ATLS Working Group . Advanced trauma life support (ATLS®): the ninth edition. J Trauma Acute Care Surg 2013; 74: 1363–1366. [DOI] [PubMed] [Google Scholar]
- 23). Campbell BCV, van Zwam WH, Goyal M, et al. Effect of general anaesthesia on functional outcome in patients with anterior circulation ischaemic stroke having endovascular thrombectomy versus standard care: a meta-analysis of individual patient data. Lancet Neurol 2018; 17: 47–53. [DOI] [PubMed] [Google Scholar]