Abstract
Objective
Coil compaction after aneurysm embolization is one of the major issues associated with aneurysm recurrence. On the presumption that pulsatile stress to the aneurysm is responsible for coil compaction, we developed an experimental model in vitro to visualize the mechanical stresses exerted by blood pressure and pulse and their relation to coil compaction.
Methods
A closed-type non-circulation system was developed by installing a syringe that generated pressure at one end of a tube, along with a spherical aneurysm made of silicone and a pressure sensor in the bifurcated end. We installed a fixed-pressure model under a steady pressure of 300 mmHg while the pressure-fluctuation model simulated the pressure variations using a plunger (in a syringe) by using a motor that applied pulsatile stress in the range of 50 mmHg for a 10-ms cycle. We devised four types of aneurysms with different depths and the same coil length. After coil packing, the aneurysms were observed for 3 days (the observation period in the pressure-fluctuation model corresponded to approximately 300 days in real time). The distance from the datum point to the observable coil loops was determined as the initial position, and the temporal change in the distance from that position was measured.
Results
In the fixed-pressure model, the average distance of coil movement was very small (less than ±0.1 mm). In the pressure-fluctuation model, the movement of coils was observed to be significant for the two longest depths (0.11 and 0.14 mm). The maximal dynamic change in coil movement was observed on the second day. The range of movement was observed to decrease thereafter.
Conclusion
Our experimental study enabled the observation of coil movement within a short duration. It examined coil compaction by applying pulsed pressure to the coils at high speeds. Consequently, a shift of the coil loops inside the incompletely occluded aneurysms was detected on applying a pulsed pressure.
Keywords: aneurysm, embolization, coil, compaction, pulse
Introduction
Endovascular coil embolization is used for the treatment of cerebral aneurysms. Coil compaction can occur in larger aneurysms, especially when the volume embolization ratio (VER) is low.1) However, the mechanism underlying coil compaction has not been determined yet.2,3) On the presumption that pulsatile stress exerted from the neck of the aneurysm inwards is responsible for coil compaction, we developed an in vitro experimental model to visualize the mechanical stresses exerted by blood pressure and pulse and their relation to coil compaction. The model helped achieve results earlier by shortening the research duration. The aim of this study was to determine whether pulsatile stresses affected the movement of the coils by using an incomplete occlusion model.
Materials and Methods
Description of the experimental device
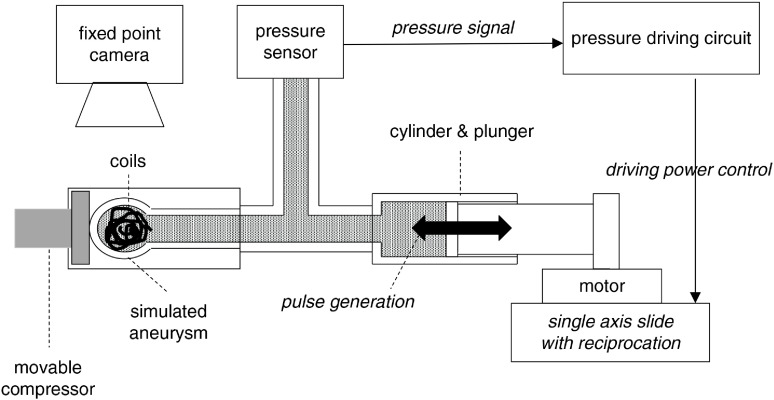
A closed-type non-circulation system was developed by installing a syringe that generated pressure fluctuations at one end of a silicone tube, 2 mm in diameter. The other end of the tube was bifurcated, and a simulated aneurysm and pressure sensor were placed in them (Fig. 1). Distilled water was used as the internal liquid in the system. The motor attached to the syringe produced pressure fluctuations, and the internal pressure of the tube was detected by the pressure sensor that was fixed to monitor the conditions accurately.
Fig. 1. The scheme of the experimental system. A closed-type non-circulation system was developed by installing a syringe that generated pressure at one end of a tube, along with a spherical aneurysm made of silicone and a pressure sensor at the other, bifurcated end. The motor attached to the syringe produced pressure fluctuations, and the internal pressure of the tube was detected by the pressure sensor to monitor the conditions accurately.
Simulated aneurysms
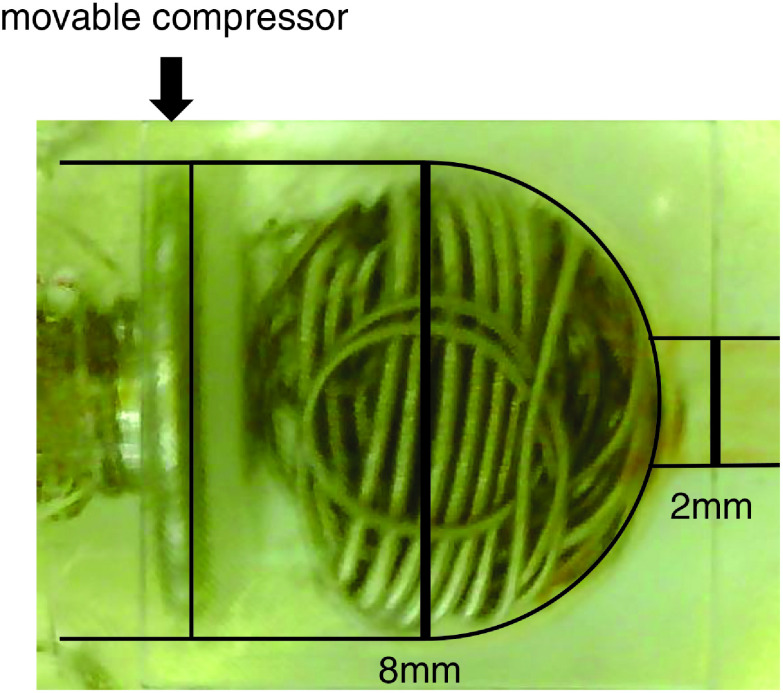
To simulate a terminal-type aneurysm, a spherical aneurysm made of silicone with a diameter of 8 mm was attached to the tip of the tube. A mechanism to change the depth of the aneurysm by compressing and releasing the aneurysm to the bottom of the aneurysmal dome with a movable compressor was devised (Fig. 2).
Fig. 2. Image of the simulated aneurysm model.
Experimental methods
Stresses applied on the aneurysm
To perform comparisons with the pressure fluctuation model, a fixed-pressure model was installed. This model produced a steady pressure of 300 mmHg. In contrast, the pressure fluctuation model simulated the pressure variations with the aid of a plunger (in a syringe) by using a motor at high speeds. Stresses corresponding to a systolic pressure of 300 mmHg and a diastolic pressure of 250 mmHg were applied for a 10-ms cycle to generate a pulsatile wave. This stress was applied 100 times faster than the actual pulse cycle (and its pressure fluctuations) at an interval of approximately 1 s.
Coil embolization model
After inserting nine coils (30 cm of GDC18, 31 cm of GDC10 Soft, and 27 cm of GDC10 Ultrasoft; Stryker, Kalamazoo, MI, USA), the movable bottom of the aneurysmal dome was repositioned to compress the coil mass and reduce the volume of the aneurysm to the limiting point. After compressing for 1 min, an incomplete occlusion model was created by slightly shifting the movable wall back and restoring the volume of the aneurysm. The same coil volume and coil varieties were used to create identical experimental conditions in the model. There were four measures of volume expansion depending on the depth achieved from the neck of the aneurysm (accomplished by compressing the movable wall). The depth was set at 5.6 mm and then extended to 6.5 mm and 7.4 mm at 0.9-mm intervals. The final measure of expansion achieved was 8.0 mm, which was the maximum limit. According to the calculation, the VER of the 5.6 mm depth model was approximately 1.4 times that of the 8.0 mm depth model.
Measurement methods
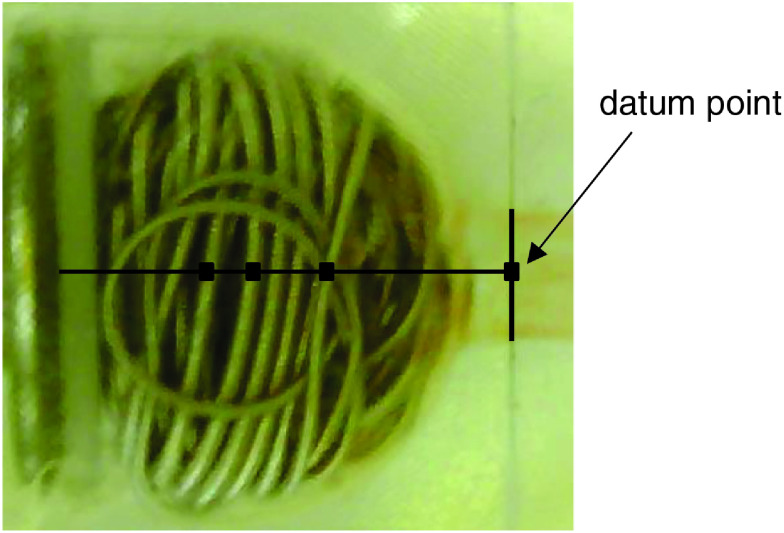
The prepared aneurysms were observed for 3 days (assuming a heart rate of 60 beats per minute in an individual, the observation period corresponded to approximately 300 days, since the frequency used in the model was 100 times faster than that of the actual heart rate). The changes were captured after every 50000 beats using a fixed-point camera. On the screen, a datum point was placed at the neck of the aneurysm, and a line passing through the center of the aneurysm was drawn. The distance from the datum point to the observable coil loops was determined as the initial position, and the temporal change in the distance from that position was measured (Fig. 3).
Fig. 3. The method for measurement of coil movement. A datum point was placed at the neck of the aneurysm. A line was drawn passing through the center of the aneurysm from the point. The change in the distance from the datum point to the nine observable shifts of the coil loop was measured.
Results
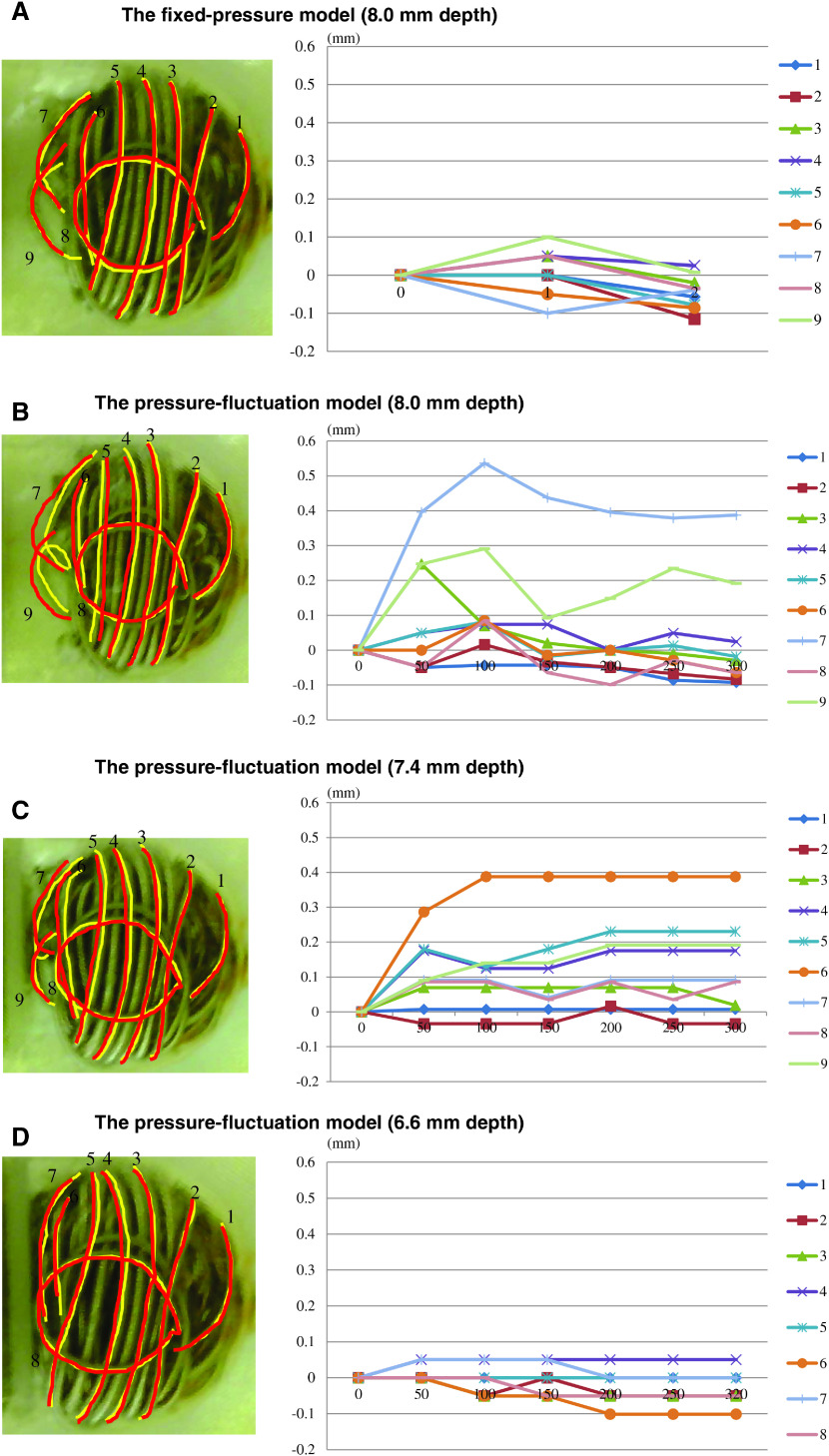
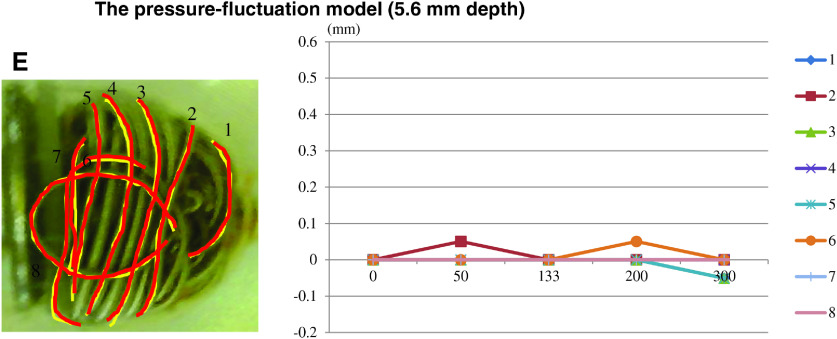
Figure 4 depicts the change in the volume in each case. In the fixed-pressure model, the average distance of coil movement was 0.05 mm (range: 0.007–0.115 mm). Thus, the change was perceived to be very small (less than ±0.1 mm). In the pressure-fluctuation model, a greater movement of the coils was confirmed for the two longest depths (the distance repositioned for a depth of 8.0 mm: average = 0.11 mm [range: 0.018–0.39 mm] and for a depth of 7.4 mm: average = 0.14 mm [range: 0.007–0.39 mm]). In contrast, the pressure-fluctuation model with the two shorter depths (6.5 mm and 5.6 mm) exhibited lesser coil movement (for a depth of 6.5 mm: average = 0.03 mm [range: 0–0.10 mm] and for a depth of 5.6 mm: average = 0.02 mm [range: 0–0.05 mm]). The longest distance of coil movement was achieved when the coil loops were placed at the bottom of the aneurysm. The maximal dynamic change in coil movement was observed on the second day. The range of movement was observed to decrease thereafter.
Fig. 4. The results of coil movement. The values in the vertical axis show the distance of temporary change (mm), and the values in the horizontal axis indicate the actual days along with the conversion of the observed period. The fixed-pressure model (8 mm depth) shows little movement of the coils (A). In the pressure-fluctuation model, significant coil movement is observed in the 8.0 mm and 7.4 mm depth models (B and C). A lesser coil movement was seen in the models with shorter depths (6.6 mm and 5.6 mm) (D and E).
Discussion
In the procedure of endovascular coiling for a cerebral aneurysm, the packed coils in the aneurysm occupy approximately 30% of the space, and the remaining 70% of the space is empty. Owing to the shape memory of the coils and the friction among them, the empty space is permeated by thrombosis, and organization occurs inside the aneurysm that retains the initial shape of the coils. Through this process, a stable coil mass made of metal and living tissue is achieved inside the aneurysm. However, maldistributed coils, partial thrombosis, and extension of an aneurysmal wall can lead to movement of the coil mass that could result in the filling of a newly created space or for them to seek another gap. This process eventually results in compaction and reopening.1) Even if a contrast medium cannot enter the aneurysm after treatment, the coil loops can sink into the slime-like soft thrombus. This phenomenon is especially remarkable in cases of incomplete occlusions that contain large gaps.
Recurrence of a cerebral aneurysm after coil embolization is a clinically crucial adverse event that can induce rupture, and its reported incidence is 12%–40%.4) Previous reports have shown that large, wide-necked, or ruptured aneurysms with low VER, and large remnants exhibit a high risk of recurrence.5–7) Among these reports, there are several studies on VER. Some of them reported that the VER should be higher than 25%, as recurrence of an aneurysm is frequently observed when the VER is below 20%.8) Similarly, other studies have reported that many clinical cases with coil compaction exhibit lower VER values.1,9) Complex coils4,10,11) and hydro coils12) were effective in achieving higher VERs. In addition, a stent-assisted technique also enabled tight coil packing with high density.13,14)
However, the precise mechanism of coil compaction is unknown.2,3) Hence, we aimed to explore the mechanism by simulating conditions that enabled the prediction of changes in the coils in a shorter time. We developed an in vitro experimental model that visualized how the different intensities of hemodynamic stress generated by vibration pressures were associated with coil compaction. The model helped achieve results earlier by shortening the research duration. The present study was conducted as an experiment to observe the movement of coil loops inserted inside a simulated aneurysm. Due to the application of faster pulsations, the observation period was shorter than the actual time taken to obtain the results in other similar studies. This experimental system was useful for investigating the changes in the coils under various conditions.
In this study, the space was designed to simulate aneurysms where the packed coils were able to move around by altering their volume. Even with abundant space inside the aneurysm, the application of steady pressure alone did not affect the shape of the coils. In contrast, the movement of the coils occurred on application of pulsations that simulated the pulse in a living body. However, the application of a similar pressure did not cause any significant changes in the filling coils if the space was narrow. Thus, coil compaction requires sufficient space for expansion and pulsed vibrations, instead of a constant pressure alone. Clinically, coil movement is expected to largely rely on the presence of additional space at the bottom of the aneurysm rather than the space in the embolized part. A similar movement may occur, especially in a partially thrombosed aneurysm due to the presence of a soft thrombus or dissolution of the thrombus. The two models with shorter depths exhibited coil movements of less than ±0.1 mm. Based on the findings from the non-pulsatile fixed-pressure models, a movement of less than ±0.1 mm could be considered as that within the margin of error, and therefore, the incidence of coil compaction is unlikely.
This experiment confirms the occurrence of movement in the outermost coil loops as well as the deformation of the coil loops inside the aneurysm. These findings might be attributed to coordinated changes among the coil loops, including a chain reaction involving all loops due to interferences among them. Such phenomena may be more likely to occur if the filling coils are loosely packed inside the aneurysm. In contrast, there were no changes in the coils around the neck of the aneurysm, where there was a strong pressure due to blood flow. In this experiment, the bottom wall was repositioned to the designated position after coil packing. The space for the coils to move was wider at the bottom than in the neck portion, resulting in an artificial and disproportionate coil distribution. This experimental design could cause an unbalanced shifting, but it seems possible that a persistent enlargement of the neck due to coil movements could occur upon longer observation. Interestingly, the most drastic movement of the coils was observed within 1 day (simulating 100 days) and stabilized thereafter. This result seems to have some discrepancy with the clinical evidence because we often encounter coil movement after a longer duration. However, delayed movement of the coils may be affected by an increase in the aneurysm volume.15)
Since the experimental system employed in this study was different from a living body, there were some limitations to this study. The pressure was set significantly higher than that experienced in normal physiological conditions to determine coil movement in a short observation period. Thus, it is possible that the effects of liquid flow from and into the aneurysm were not considered in this study. In addition, the model in this study produced high-speed vibrations to shorten the duration of the experiment. Therefore, it is necessary to verify whether a pulse in an actual cycle can result in similar phenomena. Moreover, the simulated aneurysm was a terminal type aneurysm, and the extension of the aneurysm was in the same direction as that of the axis of the parent vessel. Deviation of the axis can induce disequilibrium in pressure toward the packed coils inside the aneurysm. However, the present model could have failed to reproduce this imbalance, and thus, the accompanied shift in movements, including rotation of the coils, particularly in aneurysms with a lateral axis, was not realized in this experiment.
This study attempted to restore the coil mass that was compressed earlier. The distance was measured from the neck of the aneurysm to determine the movement of the coils. However, this measurement may have included the movement at the center of the loops, but failed to include the 3D or cumulative movement of the loops. The silicone aneurysmal wall might offer strong resistance, and the stiff structure could lead to disturbances in the movement of the filling coils. Thus, the movement of the coils may have been underestimated in this study. Moreover, the viscosity of the water that was used in this experiment is different from that of the blood. Hence, it is a non-physiological environment wherein the mechanisms of thrombosis and organization observed in vivo may not work. The influence of pulsatile stress on the coil mass detected in this experiment is only one of the factors of coil movement. In future studies, it will be necessary to develop an aneurysmal model that is more similar to a living body. In addition, a longer observation period might be required to unmask and verify the mechanism of coil compaction.
Conclusion
The present study developed an experimental system that could observe the movement of the coils within a short duration of time and examined coil compaction by applying pulsed pressure to the coils at high speeds. The shift in the coil loops was detected by creating an empty space in the simulated aneurysm with intentional incomplete occlusion by applying pulsed pressure. This experimental system can aid in determining the tendency for coil compaction in various types of coils, based on their shape, elasticity, surface modification, or coiling strategy, as well as by evaluating the effect of the assisted stent and flow diverter. Analyses of different data may be useful for determining the most effective and perfect coil embolization strategy.
Funding
This work was supported by JSPS KAKENHI Grant Number JP18K08954.
Disclosure Statement
There are no conflicts of interest to declare concerning with this article.
References
- 1).Kai Y, Hamada J, Morioka M, et al. : Evaluation of the stability of small ruptured aneurysms with a small neck after embolization with Guglielmi detachable coils: correlation between coil packing ratio and coil compaction. Neurosurgery 2005; 56: 785–792. [DOI] [PubMed] [Google Scholar]
- 2).Abdihalim M, Watanabe M, Chaudhry S, et al. : Are coil compaction and aneurysmal growth two distinct etiologies leading to recurrence following endovascular treatment of intracranial aneurysm? J Neuroimaging 2014; 24: 171–175. [DOI] [PubMed] [Google Scholar]
- 3).Hoppe Al, Raghavan ML, Hasan DM: Comparison of the association of sac growth and coil compaction with recurrence in coil embolized cerebral aneurysms. PLoS One 2015; 10: e0123017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Ortiz R, Song J, Niimi Y, et al. : Rate of recanalization and safety of endovascular embolization of intracranial saccular aneurysms framed with GDC 360 coils. Interv Neuroradiol 2008; 14: 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Ries T, Siemonsen S, Thomalla G, et al. : Long-term follow-up of cerebral aneurysms after endovascular therapy prediction and outcome of retreatment. AJNR Am J Neuroradiol 2007; 28: 1755–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Lee JY, Kwon BJ, Cho YD, et al. : Reappraisal of anatomic outcome scales of coiled intracranial aneurysms in the prediction of recanalization. J Korean Neurosurg Soc 2013; 53: 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Sadato A, Hayakawa M, Adachi K, et al. : Large residual volume, not low packing density, is the most influential risk factor for recanalization after coil embolization of cerebral aneurysms. PLoS One 2016; 11: e0155062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Satoh K, Satomi J, Matsubara S, et al. : Measurement of volume ratio to predict coil compaction, on aneurysmal embolization. Interv Neuroradiol 1998; 4: 179–182. [DOI] [PubMed] [Google Scholar]
- 9).Kawanabe Y, Sadato A, Taki W, et al. : Endovascular occlusion of intracranial aneurysms with Guglielmi detachable coils: correlation between coil packing density and coil compaction. Acta Neurochir (Wien) 2001; 143: 451–455. [DOI] [PubMed] [Google Scholar]
- 10).Wakhloo AK, Gounis MJ, Sandhu JS, et al. : Complex-shaped platinum coils for brain aneurysms: higher packing density, improved biomechanical stability, and midterm angiographic outcome. AJNR Am J Neuroradiol 2007; 28: 1395–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Neki H, Kohyama S, Otsuka T, et al. : Optimal first coil selection to avoid aneurysmal recanalization in endovascular intracranial aneurysmal coiling. J Neurointerv Surg 2018; 10: 50–54. [DOI] [PubMed] [Google Scholar]
- 12).Ding YH, Dai D, Lewis DA, et al. : Angiographic and histologic analysis of experimental aneurysms embolized with platinum coils, Matrix, and HydroCoil. AJNR Am J Neuroradiol 2005; 26: 1757–1763. [PMC free article] [PubMed] [Google Scholar]
- 13).Raymond J, Darsaut TE, Bing F, et al. : Stent-assisted coiling of bifurcation aneurysms may improve endovascular treatment: a critical evaluation in an experimental model. AJNR Am J Neuroradiol 2013; 34: 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Griessenauer CJ, Adeeb N, Foreman PM, et al. : Impact of coil packing density and coiling technique on occlusion rates for aneurysms treated with stent-assisted coil embolization. World Neurosurg 2016; 94: 157–166. [DOI] [PubMed] [Google Scholar]
- 15).Hasan DM, Nadareyshvili AI, Hoppe AL, et al. : Cerebral aneurysm sac growth as the etiology of recurrence after successful coil embolization. Stroke 2012; 43: 866–888. [DOI] [PMC free article] [PubMed] [Google Scholar]