Abstract
Objective
Posterior condylar canal dural arteriovenous fistula (PCC DAVF) is extremely rare, with only four previously reported cases in the English literature. Cases may present tinnitus and radiculopathy. In cases where the drainer is around the brainstem, subarachnoid and intraventricular hemorrhages (IVHs) may occur. We describe the clinical presentation, angiographic imaging, and endovascular treatment strategy of a PCC DAVF.
Case Presentation
A 30-year-old woman presented to our hospital with tinnitus and stiffness of the shoulder. Neuroimaging studies showed DAVF with fistulous points around right PCC consisted of a high-flow shunt, fed mainly by the occipital artery, and drained to the suboccipital cavernous sinus (SCS) and internal jugular vein. The lesion was treated with a combination of transvenous coil embolization and transarterial Onyx injection. The patient recovered immediately after intervention and had no neurological deficits in the follow-up visit.
Conclusion
In this case, endovascular treatment was performed safely without recurrence so far. A strategy combining transvenous coil embolization and transarterial Onyx injection may be an effective treatment for PCC DAVF with high-flow shunt. Further case accumulation is desired.
Keywords: transarterial embolization, transvenous embolization, dural arteriovenous fistula, endovascular therapy, condylar canal
Introduction
Dural arteriovenous fistula (DAVF) of the posterior condylar canal (PCC) is rare. To the best of our knowledge, only four cases have been previously reported in English,1–4) and all four cases were treated with either transarterial or transvenous embolization alone. We report a case of PCC DAVF with a high-flow shunt that was treated with transvenous coil embolization followed by transarterial Onyx injection (Medtronic, Minneapolis, MN, USA). This case report emphasizes the scarcity, angiographic findings, and anatomical characteristics of PCC DAFV, and discusses endovascular treatment strategies.
Case Presentation
A 30-year-old woman was admitted to our hospital with right-sided pulsatile tinnitus that had been occurring for 1 month. Around the time of tinnitus onset, she had also developed a very stiff right shoulder, but had no neurological deficits. She had no history of head trauma, cerebrovascular disease, or previous therapeutic interventions.
MRA revealed an abnormal flow signal inside the dilated right PCC, which implied DAVF. Cone-beam CT demonstrated that the location of the shunt point was inside the PCC (Fig. 1). A DSA of the right external carotid artery revealed that the DAVF was fed by multiple fistulous feeders; the right occipital artery, vertebral artery (meningeal branch from the V3–4 portion), ascending pharyngeal artery (jugular and hypoglossal branches), posterior auricular artery, and middle meningeal artery (convexity posterior and petrosquamous branches). Highly dilated feeders and drainers were depicted in the early arterial phase of DSA, implying high-flow shunt. The DAVF was drained by the right suboccipital cavernous sinus (SCS), followed by the deep cervical vein and the vertebral venous plexus without cortical venous reflux (Fig. 2). Symptoms of stiff shoulder might have been caused by C5 radiculopathy due to the blood stagnation and dilation of the epidural veins.
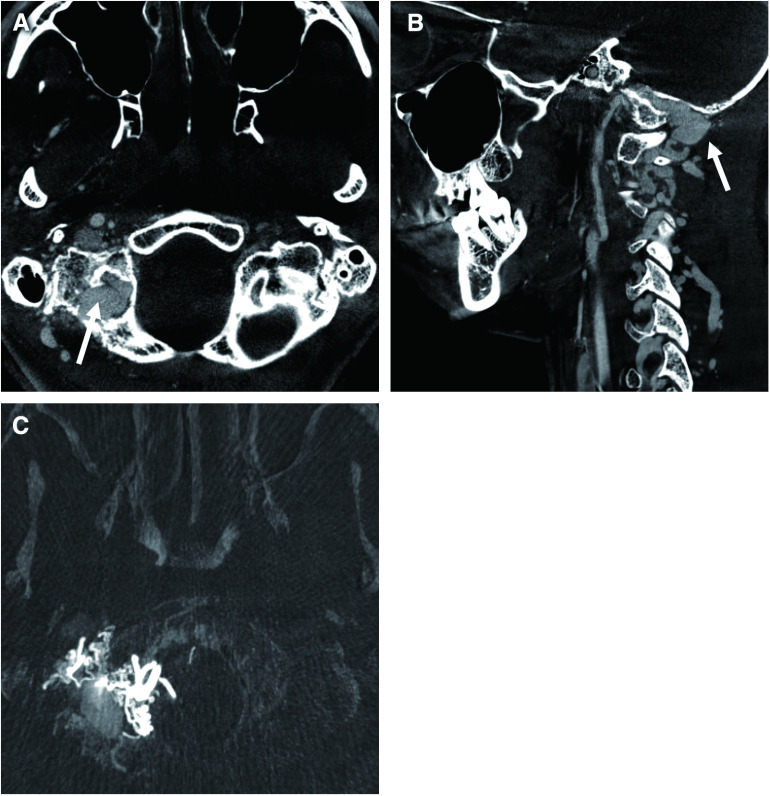
Fig. 1. Frontal (A) and lateral (B) views of a cone-beam CT angiogram of the right side VA shows the dilated posterior condylar canal and vein (arrow). (C) Cone-beam CT implies shunt point to be at the posterior condylar vein. VA: vertebral artery.
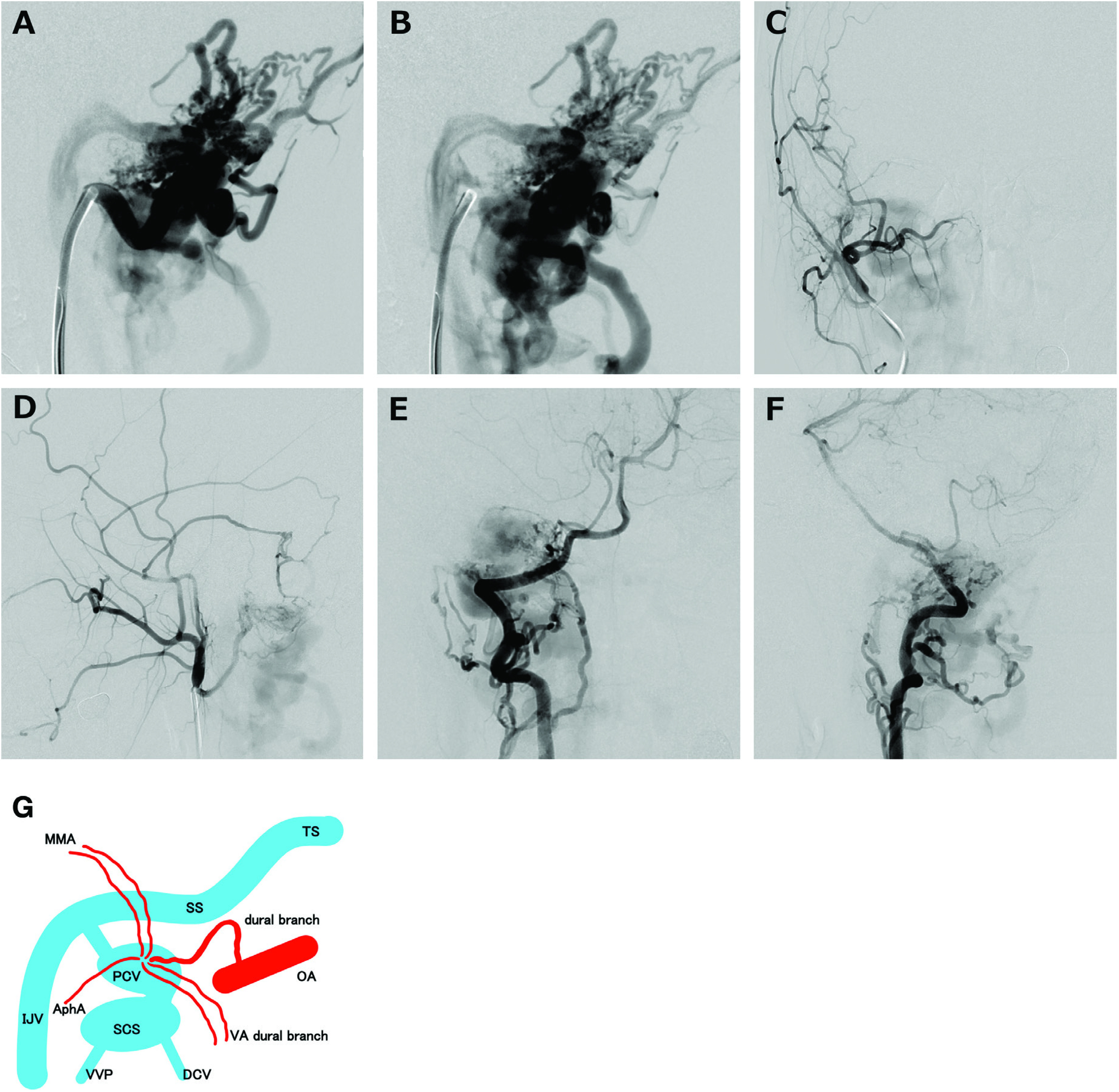
Fig. 2. Arterial (A) and venous (B) phase of lateral views of the right external carotid arteriogram show the feeders and drainers appearing simultaneously, implying high-flow shunt. The DAVF is mainly fed by the occipital artery and drained by the SCS, followed by the deep cervical vein and vertebral venous plexus without cortical venous reflux. Frontal (C and E) and lateral (D and F) views of the angiogram of the external carotid artery just distal to occipital artery/VA show the DAVF being fed by several feeders from the VA (meningeal branch from the V3–4 portion), ascending pharyngeal artery (jugular and hypoglossal branches), posterior auricular artery, and middle meningeal artery (convexity posterior and petrosquamous branches). (G) Schema of the present case. AphA: ascending pharyngeal artery; DAVF: dural arteriovenous fistula; DCV: deep cervical vein; IJV: internal jugular vein; MMA: middle meningeal artery; OA: occipital artery; PCV: posterior condylar vein; SCS: suboccipital cavernous sinus; SS: sigmoid sinus; TS: transverse sinus; VA: vertebral artery; VVP: vertebral venous plexus.
Transvenous coil embolization and transarterial Onyx embolization were performed (Fig. 3). Using a 6-Fr guiding catheter (ENVOY DA; Codman & Shurtleff, Raynham, MA, USA) placed in the right internal jugular vein, a microcatheter (Excelsior SL-10; Stryker, Kalamazoo, MI, USA) was advanced into the venous pouch of the right posterior condylar vein (PCV), passing through the SCS. A balloon catheter (Scepter XC; MicroVention, Aliso Viejo, CA, USA) was navigated distally into the dural branch of the right occipital artery for flow control. Bare-platinum coils were placed into the pouch without compromising the jugular bulb. After coil embolization of the PCC and a part of the SCS, there was slight residual shunt flow (Fig. 4). A 4-Fr diagnostic catheter was then placed in the right vertebral artery to confirm that there was no untoward reflux of Onyx. Onyx injection (Onyx-18, 0.91 mL) was added with the Scepter XC balloon inflated. The arteriovenous shunt had completely disappeared by the final angiogram. Cone-beam CT (Artis zee; Siemens Healthcare, Erlangen, Germany) after embolization showed coils placed inside the right PCC, and the Onyx cast, which occluded from the occipital artery to the posterior meningeal artery, distributed in the dura mater.
Fig. 3. (A) Microcatheter is navigated from the internal jugular vein to the PCV, making a loop at the SCS to embolize the SCS in addition to the PCV packing. A balloon catheter is advanced into the dural branch of the right occipital artery for flow control. (B) Platinum coils are placed at the venous pouch and part of the SCS to prevent onyx migration into distal vessels. (C) High-flow shunt is decreased in the right VA angiogram. (D) Coil protrusion to the internal jugular vein is denied. (E) Schema of the strategy in our case. Transvenous coil embolization of the PCV and part of the SCS was performed, following the Onyx injection from the meningeal branch of the right occipital artery. DCV: deep cervical vein; IJV: internal jugular vein; OA: occipital artery; PCV: posterior condylar vein; SCS: suboccipital cavernous sinus; SS: sigmoid sinus; VA: vertebral artery; VVP: vertebral venous plexus.
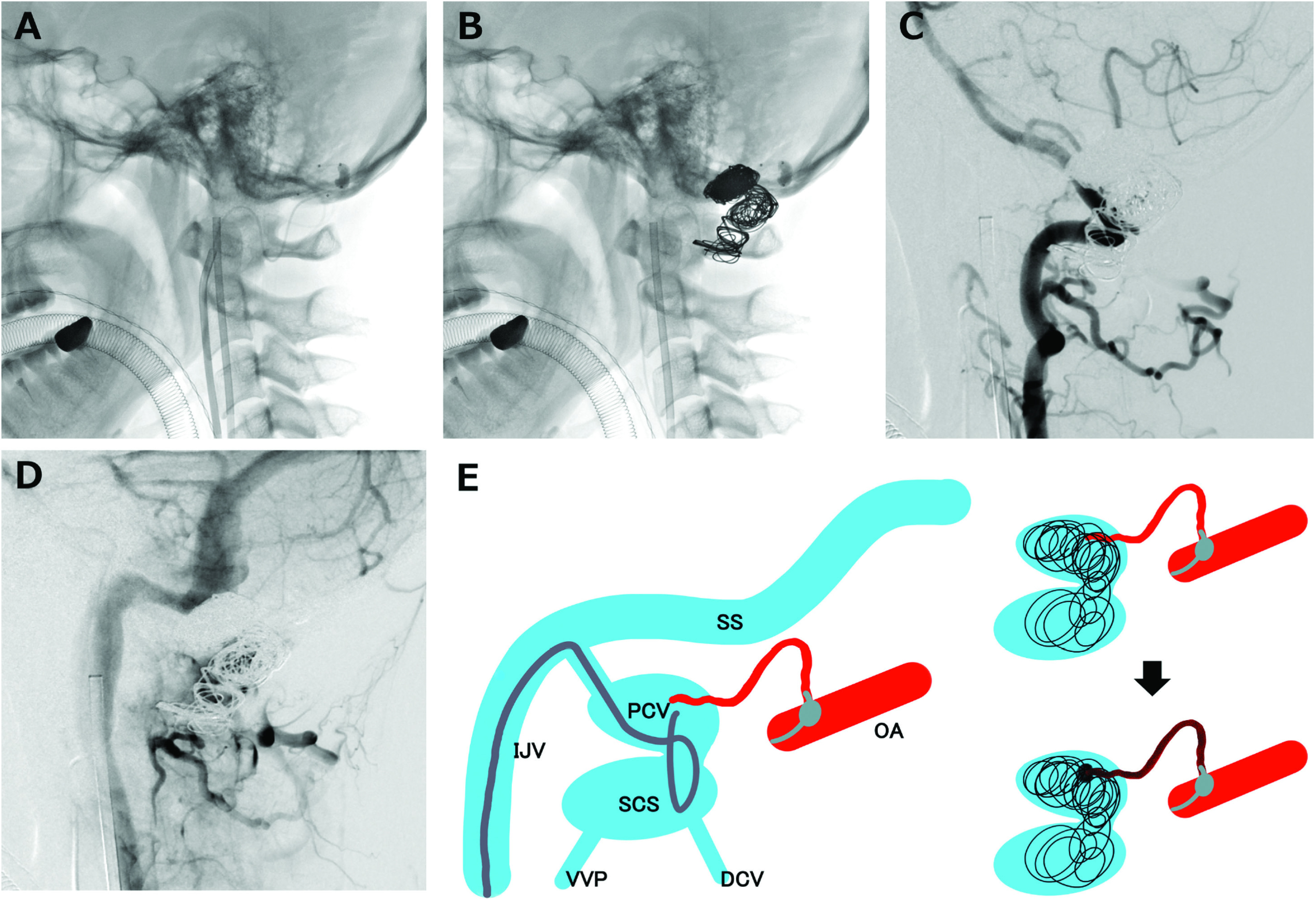
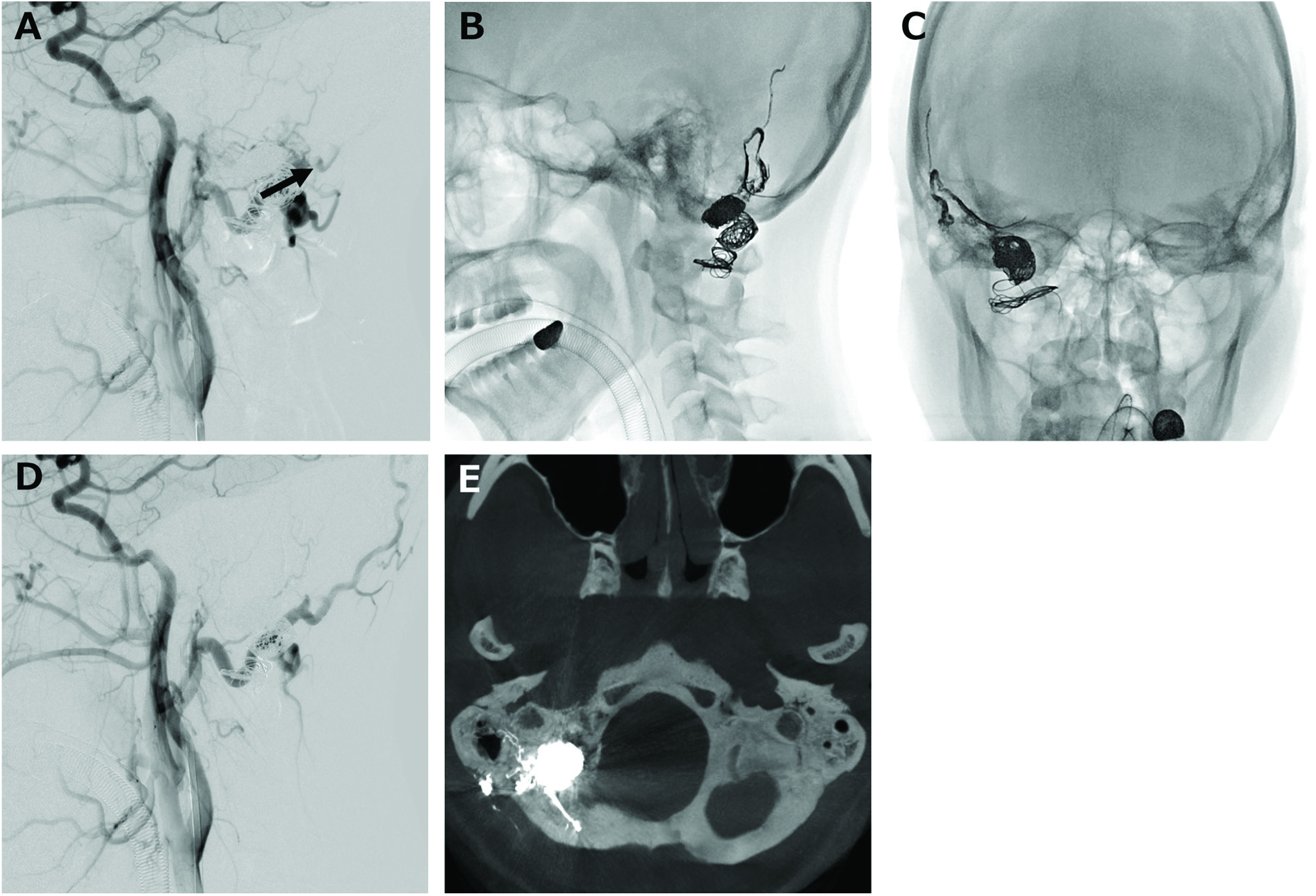
Fig. 4. (A) Lateral view of the common carotid arteriogram shows the feeders from the meningeal branch of the right occipital artery remaining after the coil embolization. Frontal (B) and lateral (C) views of the craniogram. Onyx was injected from the inflated balloon catheter placed at the dural branch of the occipital artery. (D) Lateral view of the common carotid arteriogram shows the DAVF being completely disappeared. (E) CT demonstration of the onyx cast filling the occipital artery and posterior meningeal artery. DAVF: dural arteriovenous fistula.
The tinnitus disappeared immediately after treatment and the shoulder stiffness gradually resolved over 1 month. The patient was discharged without any neurological deficits, and no complications were observed during or after the procedure. A follow-up MRI at 3 months revealed stable occlusion of the fistula, without any other neurological symptoms.
Discussion
The PCC is the largest emissary foramen in the human skull, located posterior–inferior to the jugular foramen and posterior to the hypoglossal canal.5) It transmits an emissary vein called as PCV, which originates from the anterior condylar confluence, jugular bulb, and sigmoid sinus, transmitting into the SCS and paravertebral vein with a few variations.6,7) Matsushima et al. classified into four types in their cadaveric study according to intracranial orifice: the sigmoid sinus type (25%), the jugular bulb type (67%), the occipital cavernous sinus type (6%), and the anterior condylar emissary vein type (3%).8) In the sigmoid sinus type, the intracranial orifice of PCC opened on the posterojugular ridge, which was the boundary edge between the distal end of the sigmoid sulcus and jugular foramen. PCV originated from the distal end of sigmoid sinus. In the jugular bulb type, PCC opened in the medial wall of the posterior part of jugular foramen, and PCV originated from jugular bulb. In the occipital cavernous sinus type, the large occipital cavernous sinus descended along the posterolateral edge of the foramen magnum. PCV originated from occipital cavernous sinus before draining to jugular bulb. In the anterior condylar emissary vein type, PCV in PCC connected to anterior condylar vein in hypoglossal canal intracranially. The difficulty of catheter inducibility is important to perform transvenous coil embolization safely, thus venous anatomy is one of the great factors in developing a strategy. The cases introduced by Kiyosue et al.1) and Maus et al.3) were the jugular bulb type, which was easy to advance and stabilize catheter inside the PCV. They succeeded in diminishing the DAVF by transvenous coil embolization safely. Our case was also the jugular bulb type, which had a good catheter inducibility; however, the high-flow shunt required a flow control to place the coils safely.
PCC DAVF is extremely rare (Table 1).1–4) Among the four previous cases and the present case, ages ranged from 30–72 years (mean 49.8 ± 15.4). There were three men and two women. Two cases had no history of head trauma, cerebrovascular disease, or therapeutic intervention, whereas the others did not mention this in their clinical history. Clinical presentations included tinnitus, radiculopathy, subarachnoid hemorrhage (SAH), and intraventricular hemorrhage (IVH). In the case described by Kiyosue et al., the patient had presented with pulsatile tinnitus alone and no intracranial hemorrhage. The PCC DAVF was fed by the occipital artery, ascending pharyngeal artery, clival artery, and anterior meningeal artery. It drained through the PCV into the sigmoid sinus, paravertebral vein, and occipital cavernous sinus without draining normal cerebral parenchyma. A transvenous approach was used and successfully treated without complication. Maus et al. also report a case onset of tinnitus treated by transvenous approach, which was fed by the vertebral artery, ascending pharyngeal artery, and external carotid artery, and drained into the SCS, sigmoid sinus, and internal jugular vein (IJV). Both cases had a similar presentation and venous drainage pattern as ours; however, our case had a high-flow shunt, which was difficult to treat by transvenous coil embolization alone. Mondel et al.2) presented a PCC DAVF patient onset of fourth ventricular SAH and IVH. It was fed by the neuromeningeal branch of the ascending pharyngeal artery, cervical branch of the vertebral artery, and posterior meningeal artery, drained by the medulla bridging vein with reflux into the anterior medullary anterior pontomosencephalic venous system and anterior spinal vein. Shambanduram et al.4) also reported a case with fourth ventricular SAH and IVH which had a similar venous drainage pattern as the case Mondel et al. presented. These two cases had basal vein reflux in common, that is anatomically dangerous feature. All cases were completely treated using endovascular surgery. A combined transarterial and transvenous strategy was only used in our case. No complications were reported, aside from brainstem, dorso-lateral medulla infarction in the case of Shambanduram et al. reported.
Table 1. Summary of the cases of dural arteriovenous fistulas in the posterior condylar canal.
| Symptom | Side | Drainer | Therapeutic access | Embolic material | Angiographic outcome | Complication | |
|---|---|---|---|---|---|---|---|
| Kiyosue (2007)1) | Tinnitus | L | JV, VVP | TVE | Coils | Diminished | – |
| Mondel (2014)2) | SAH, IVH | R | VVP, SPS, APMV | TAE | Onyx | Diminished | – |
| Maus (2014)3) | Tinnitus | L | JV | TVE | Coils | Diminished | – |
| Shambanduram (2017)4) | SAH, IVH | L | SPS, APMV | TAE | NBCA | Diminished | Brainstem infarction |
| Our case | Tinnitus, radiculopathy | R | JV, DCV, VVP | TAE, TVE | Onyx, Coils | Diminished | – |
APMV: anterior pontomesencephalic vein; DCV: deep cervical vein; IVH: intraventricular hemorrhage; JV: jugular vein; NBCA: n-butyl-2-cyanoacrylate; SAH: subarachnoid hemorrhage; SPS: superior petrosal sinus; TAE: transarterial embolization; TVE: transvenous embolization; VVP: vertebral venous plexus
Various embolic agents are available to treat DAVF, including coils, particle agents (e.g., polyvinyl alcohol microspheres), and liquid agents (e.g., n-butyl-2-cyanoacrylate [NBCA] or Onyx). Among the many options, it is important to select appropriate strategy in treating high-flow shunt DAVF. Endovascular embolization with Onyx is increasingly useful; however, in high-flow lesions, embolization with Onyx alone may risk its distal migration into the venous system, resulting in unexpected venous outflow obstruction or pulmonary embolism.9) Transvenous coil embolization technique is also effective; however, it may require a large number of coils with a risk of nerve compression. Endovascular flow control techniques using coils or balloons can reduce the rapid flow within the fistulous connections, thus decreasing the risk of coil deviation and distal Onyx migration into the venous system; they can also prevent unintended Onyx reflux.10) In the present case, several arteries fed the fistulous pouch with the high-flow shunt. Furthermore, a preoperative angiogram suggested that there were several shunt lesions inside the PCC. We therefore used a two-step strategy to treat the lesion. First, a retrograde transvenous approach with coil embolization was used to pack the fistulous pouch and reduce the high flow. Next, transarterial Onyx injection was used to embolize the remaining shunt lesions. The selective injection from the mastoid branch under flow control allowed for efficient onyx embolization of remaining shunt lesions and shunted pouches, resulting in complete occlusion. However, it seems necessary to pay attention to the cerebral nerve palsy and embolic complications caused by onyx migration into the vasa nervorum via a potential anastomosis from the mastoid branch.
Conclusion
PCC DAVF is extremely rare. They may present with tinnitus or radiculopathy, such as a stiff shoulder. The existence of cortical venous reflex around the brainstem may result in acute SAH. PCC DAVF with high-flow shunts may be treated by a combination of transvenous coil embolization and transarterial Onyx injection. A successful strategy relies on careful analysis of the vascular anatomy of the region, and further accumulation of cases is needed.
Ethics Approval
Ethics approval was obtained from the Ethics Committee, Kyoto University.
Acknowledgment
We thank Bronwen Gardner, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/) for editing a draft of this manuscript.
Disclosure Statement
We declare no conflicts of interest.
References
- 1).Kiyosue H, Okahara M, Sagara Y, et al. : Dural arteriovenous fistula involving the posterior condylar canal. AJNR Am J Neuroradiol 2007; 28: 1599–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Mondel PK, Saraf R, Limaye US: Acute subarachnoid hemorrhage in posterior condylar canal dural arteriovenous fistula: imaging features with endovascular management. J Neurointerv Surg 2015; 7: e26. [DOI] [PubMed] [Google Scholar]
- 3).Maus V, Söderman M, Rodesch G, et al. : Endovascular treatment of posterior condylar canal dural arteriovenous fistula. J Neurointerv Surg 2017; 9: e7. [DOI] [PubMed] [Google Scholar]
- 4).Shambanduram SS, Devarajan Sebastian LJ, Jain N, et al. : Management of a rare case of posterior condylar canal dural arteriovenous fistula presenting with subarachnoid haemorrhage: a case report and review of literature. Interv Neuroradiol 2018; 24: 206–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Boyd GI: The emissary foramina of the cranium in man and the anthropoids. J Anat 1930; 65: 108–121. [PMC free article] [PubMed] [Google Scholar]
- 6).San Millán Ruíz D, Gailloud P, Rüfenacht DA, et al. : The craniocervical venous system in relation to cerebral venous drainage. AJNR Am J Neuroradiol 2002; 23: 1500–1508. [PMC free article] [PubMed] [Google Scholar]
- 7).Takahashi S, Sakuma I, Omachi K, et al. : Craniocervical junction venous anatomy around the suboccipital cavernous sinus: evaluation by MR imaging. Eur Radiol 2005; 15: 1694–1700. [DOI] [PubMed] [Google Scholar]
- 8).Matsushima K, Kawashima M, Matsushima T, et al. : Posterior condylar canals and posterior condylar emissary veins-a microsurgical and CT anatomical study. Neurosurg Rev 2014; 37: 115–126. [DOI] [PubMed] [Google Scholar]
- 9).Cognard C, Januel AC, Silva NA, et al. : Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: new management using Onyx. AJNR Am J Neuroradiol 2008; 29: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Shi ZS, Loh Y, Gonzalez N, et al. : Flow control techniques for Onyx embolization of intracranial dural arteriovenous fistulae. J Neurointerv Surg 2013; 5: 311–316. [DOI] [PubMed] [Google Scholar]