Abstract
Objective
CT perfusion (CTP) provides various hemodynamic parameters. However, it is unclear which CTP parameters are useful in predicting clinical outcome in patients with acute ischemic stroke (AIS).
Methods
Between February 2019 and June 2021, patients with anterior circulation large-vessel occlusion who achieved successful recanalization within 8 hours after stroke onset were included. The relative CTP parameter values analyzed by the reformulated singular value decomposition (SVD) method in the affected middle cerebral artery territories compared to those in the unaffected side were calculated. In addition, the ischemic core volume (ICV) was evaluated using a Bayesian Vitrea. The final infarct volume (FIV) was assessed by 24-hour MRI. The correlation between these CTP-derived values and clinical outcome was assessed.
Results
Forty-two patients were analyzed. Among the CTP-related parameters, the ICV, relative cerebral blood volume (rCBV), and relative mean transit time (rMTT) showed a strong correlation with the FIV (ρ = 0.74, p <0.0001; ρ = −0.67, p <0.0001; and ρ = −0.66, p <0.0001, respectively). In multivariate analysis, rCBV, rMTT, and ICV were significantly associated with good functional outcome, which was defined as a modified Rankin Scale score ≤2 (OR, 6.87 [95% CI, 1.20–39.30], p = 0.0303; OR, 11.27 [95% CI, 0.97–130.94], p = 0.0269; and OR, 36.22 [95% CI, 2.78–471.18], p = 0.0061, respectively).
Conclusion
Among the CTP parameters analyzed by the SVD deconvolution algorithms, rCBV and rMTT could be useful imaging predictors of response to recanalization in patients with AIS, and the performances of these variables were similar to that of the ICV calculated by the Bayesian Vitrea.
Keywords: CT perfusion, acute ischemic stroke, mechanical thrombectomy, cerebral perfusion parameter, Bayesian Vitrea
Introduction
CT perfusion (CTP) has been a commonly used imaging modality in the management of patients with acute ischemic stroke (AIS) because of its high availability and fast acquisition.1,2) It provides quantitative hemodynamic parameters to assess the volumes and spatial locations of ischemic core and penumbra lesions using automated CTP analysis software.3,4) The ischemic core is irreversibly damaged tissue, and its volume is considered to be one of the predictors associated with good clinical outcome in patients with AIS.5) Penumbra represents the ischemic tissue that is salvageable by immediate recanalization.6) An accurate estimation of the ischemic core and penumbra volume is essential in the selection of optimal candidates who would benefit from reperfusion therapy, especially in the era of extending the indication for endovascular treatment (EVT) beyond the conventional therapeutic time window.3,4) However, it requires the CTP mismatch analyzer, and the results of the analysis may differ among the software programs because of their specific postprocessing algorithms and due to the differences in their thresholds.7) Moreover, these analyzers are only used in a limited number of institutions because of their additional cost.
Physicians often visually evaluate the result of a CTP analysis based on the various color-coded CTP maps in the acute clinical setting. In patients with acute anterior circulation large-vessel occlusion (LVO), including internal carotid artery (ICA) or middle cerebral artery (MCA) occlusion, the cerebral hemodynamics of the ipsilateral MCA territory might be affected, and the changes in the cerebral hemodynamics represent the difference in the color tone between the affected and unaffected MCA territories on the color-coded CTP maps. Physicians may focus on the abnormal color tone asymmetries of the MCA territory. Several authors have reported the utility of the Alberta stroke program early CT score (ASPECTS) based on the CTP images for predicting the clinical outcome, and it is a qualitative evaluation method focusing on the affected MCA territory.8,9) However, there are few reports on the quantitative and easy-to-use CTP assessments without using CTP mismatch analyzers. Moreover, it is not clear which CTP parameters are useful in predicting the clinical outcome in patients with AIS after successful recanalization.
In this study, we provide a quantitative evaluation method of measuring the ratio of the CTP hemodynamic parameters in the affected MCA territories to the parameters in the unaffected side, and we assess the performance of these parameters in predicting the final infarct volume (FIV) in anterior circulation LVO patients who achieved successful recanalization. Additionally, we compared these parameters with the ischemic core volume (ICV) that was estimated by a Bayesian Vitrea in predicting the clinical outcome.
Materials and Methods
Study subjects and data collection
A single-center retrospective cohort study was performed based on a prospectively built stroke database. Between February 2019 and June 2021, we included consecutive patients with AIS who met the following criteria: 1) had anterior circulation LVO (MCA M1 segment or ICA) that presented within 8 hours of stroke onset; 2) had a baseline noncontrast CT (NCCT) and CTP; 3) had successful recanalization by EVT; and 4) had follow-up MRI or NCCT data that were obtained within 24 hours after recanalization to calculate the FIV. We excluded patients who met the following criteria: 1) poor radiological imaging due to motion artifacts; 2) preexisting large parenchymal damage or other coexisting intracranial LVO; 3) hemorrhagic transformation that precluded an accurate estimation of the infarct volume; and 4) reocclusion of the recanalized vessels on follow-up MRA.
The neurological status was assessed upon each patient’s arrival using the National Institutes of Health Stroke Scale (NIHSS) score at baseline. Intravenous tissue plasminogen activator (IV-tPA) treatment was administered to all eligible patients.10) EVT was performed on the patients within 8 hours of either stroke onset or the last known time the patient was well, with a baseline NIHSS score >4 and a baseline ASPECTS system ≥7 but was not performed in the patients with rapid neurological improvement after IV-tPA.
The angiographic outcomes were assessed using the modified thrombolysis in cerebral infarction (mTICI) grade, and successful recanalization was defined as an mTICI grade 2b to 3. The recanalization status was confirmed by 24-hour MRA. The durations of two key workflows, from symptom onset to CT imaging and from symptom onset to recanalization, were reviewed. The functional outcome 3 months after stroke onset was assessed based on the modified Rankin Scale (mRS) score, which we collected by face-to face or telephone clinical interviews of the patients, and a good outcome was defined as an mRS score of ≤2.
Acute CT imaging protocol
All patients were subjected to acute CT-based protocols, including NCCT and CTP, using a 320-detector row CT scanner (Aquilion ONE; Canon Medical Systems, Tokyo, Japan). NCCT of the head was performed with the following imaging parameters: 120 kVp, 352.5 mAs, 0.5-mm section collimation, and 0.75-second rotation. The early ischemic changes on NCCT were evaluated by using the ASPECTS system. The CTP imaging acquisition was performed as a dynamic contrast agent-enhanced scan covering the entire brain with a 0.5-mm section thickness, a 512 × 512 matrix, and a 200-mm axial field of view. A 40-mL aliquot of nonionic iodinated contrast agent (iopamidol, 370 mg/mL; Hikari Pharmaceutical, Tokyo, Japan) was injected intravenously at a rate of 5 mL/sec, followed by a 20-mL saline chaser using a power injector and via a 20-gauge intravenous catheter that had been inserted into the antecubital vein. The dynamic volume scanning started 5 seconds after the injection of the contrast material. The scanning protocol was as follows: 35-second continuous dynamic scan for the arterial phase, 7 intermittent scans at 2-second intervals for the venous phase, and 5 intermittent scans at 5-second intervals for the late venous phase. The scanning speed was 1 second per rotation, and the total scan time was 76.0 seconds, with 80 kVp and 100 mA. The volumetric CT dose index for this protocol was 159 mGy. The occluded vessels were assessed on the dynamic 3D-CTA images of the head created from the CTP volume data using a 3D image analysis workstation (Ziostation 2, version 2.3.4.0; Ziosoft, Tokyo, Japan).
CTP data analysis with 2 postprocessing algorithms and image analysis
The CTP data were analyzed using built-in CTP analysis software with the reformulated singular value decomposition (SVD) method, which is theoretically delay-insensitive, equal to the block-circulant SVD method, and the most widely used method.11) The arterial input function was manually set in the MCA M1 segment on the unaffected side, and the venous output function was manually set in the superior sagittal sinus. Subsequently, maps of the CTP parameters, including the cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT), time-to-peak (TTP), and time to maximum of the residue function (Tmax), were obtained.5,12,13)
The imaging processing of the CTP data and a region of interest (ROI)-based evaluation were performed as previously described.14,15) After the CTP data were transferred to a computer that had installed software that could automatically determine the ROI (three-dimensional stereotactic region of interest template [3D-SRT]; FUJIFILM RI Pharma, Tokyo, Japan), the regional CTP parameters were calculated.15,16) Briefly, the quantitative CTP maps were registered anatomically to the standard brain atlas, and then the ROIs were automatically placed over the whole brain. The 3D-SRT software has predefined ROIs for the standard brain atlas and provides quantitative CTP values for each of the following 12 regions for both the right and left sides: callosomarginal, precentral, central, parietal, angular, temporal, posterior cerebral, pericallosal, basal ganglia, thalamus, hippocampus, and cerebellum. We determined each of the CTP parameters in the territory of the MCA using the precentral, central, parietal, angular, and temporal ROIs, as described previously.17,18) We defined the ratio of the average values of the CTP parameters, including CBF, CBV, MTT, TTP, and Tmax, in the affected MCA territories, divided by the average of the respective CTP parameters in the unaffected side as the relative cerebral blood flow (rCBF), relative cerebral blood volume (rCBV), relative mean transit time (rMTT), relative time-to-peak (rTTP), and relative time to maximum of the residue function (rTmax), respectively. After transferring the CTP data to a computer installed with the 3D-SRT software, we calculated these relative CTP parameter values over an approximately 5-minute time course.
We also used automated perfusion analyzer software (Vitrea Workstation 7.11; Canon Medical Systems) for our research. The reconstructed CTP volume data were transferred to a Vitrea workstation using a Bayesian algorithm for postprocessing. The arterial input function and the venous output function were manually set as described above. The default Vitrea setting (a 38% reduction in the CBV with a 5.3-second increase in the TTP compared to the contralateral hemisphere) was applied for the calculation of the baseline ICV, while a 5.3-second increase in the TTP indicated the baseline hypoperfusion volume.
The FIV was calculated through manual outlines based on the hyperintense lesions on diffusion-weighted imaging (DWI) with a maximal visual extent window using Ziostation 2 software. When a 24-hour MRI examination was not available, the follow-up CT within 24 hours of stroke onset was used to determine the FIV. For these patients, the infarcted volumes were calculated based on the hypodense lesions that were manually delineated on CT.
All imaging analyses were used by Ziostation 2 software. The clinical data collections were performed in consensus by two or three stroke neurologists with at least 5 years of experience, and these neurologists were blinded to all of the patients’ clinical information except for the stroke side. This study protocol was approved by the Institutional Review Board, and all patients or their surrogates provided written informed consent.
MRI acquisition
MRI scans, including the DWI and 3D time-of-flight flow-compensated MRA sequences, were performed with a 1.5 T Vantage Titan whole body scanner (Canon Medical Systems) with a 12-channel head coil. DWI was performed with a single-shot spin-echo echo-planar imaging sequence. Twenty images were acquired at b = 0 seconds/mm3 followed by 20 at b = 1000 seconds/mm3. The imaging parameters were as follows: repetition time (TR)/echo time (TE) 6000/100 milliseconds, a field of view of 22 cm, a matrix of 128 × 128 zero filled to a 256 × 256, 5-mm section thickness, and a 1-mm gap. The MRAs of the patients’ heads were performed using a 3D time-of-flight technique with an 18° flip angle, TR/TE 28/3.4 milliseconds, a 1-mm section thickness covering 7.2 cm, and a 224 × 160 matrix encoding a 21 cm field of view.
Statistical analysis
Continuous variables were summarized using the median and interquartile range (IQR), and categorical variables were summarized using frequencies and proportions. The correlations between the CTP-derived variables and the FIV were assessed with Spearman’s ρ. To assess the impact of the CTP-derived variables, which had moderate or strong correlation with the FIV (|ρ| >0.5), on having a good outcome, univariate and multivariate logistic regression analyses were performed with adjustments for age, sex, NIHSS score, and time from onset to recanalization. The odds ratio (OR) and 95% confidence interval (CI) for each variable were estimated. The cutoff values for each score including age, NIHSS score, time from onset to recanalization, and ICV for predicting good functional outcome in univariate and multivariate logistic regression analysis were used as the values or the values that were close to the respective medians in this study population. The cutoff values of the relative CTP parameter values were defined as 1.00 for focusing on the degree of the increase in the CTP parameter values of the affected MCA side compared to unaffected side. A P value <0.05 was considered statistically significant. All statistical analyses were performed with JMP Pro version 15.0.0 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Fifty-seven patients with acute anterior circulation LVO received EVT, and immediate successful recanalization was achieved for 51 patients (89.5%). After excluding the patients without optimal imaging (n = 3), those without follow-up imaging (n = 1), those with preexisting large parenchymal damage (n = 1), those with coexisting contralateral M1 occlusion (n = 1), and those with reocclusion of the recanalized vessels on 24-hour MRA (n = 3), 42 patients were ultimately included in this analysis.
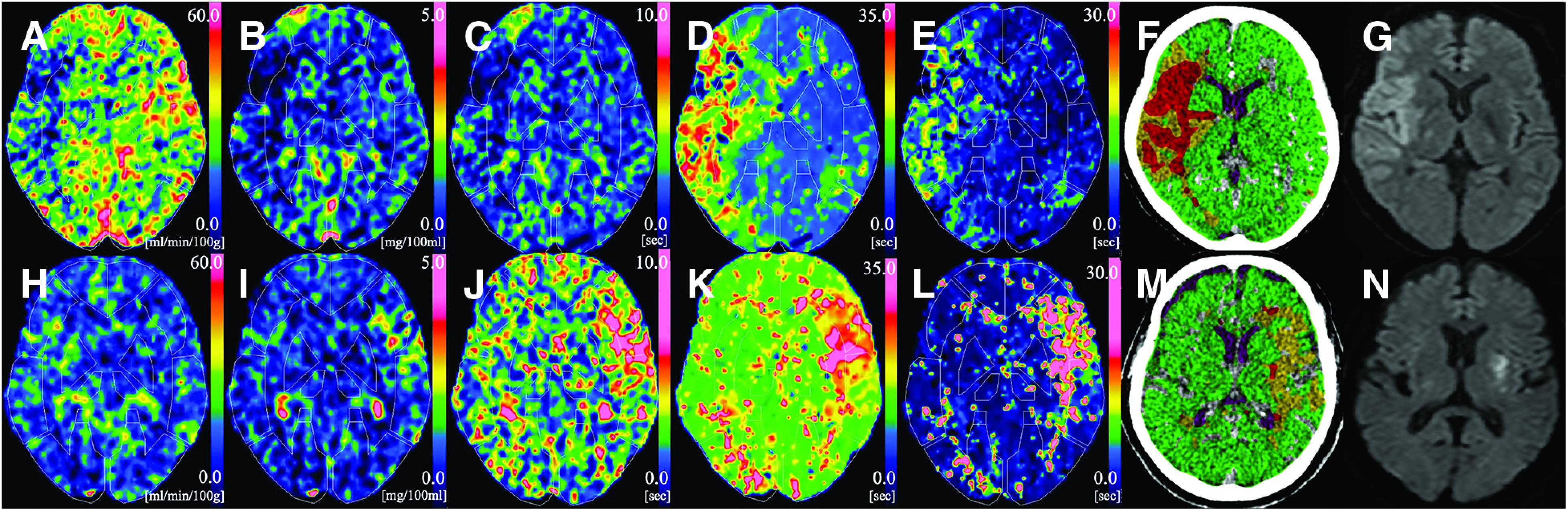
The baseline clinical characteristics are shown in Table 1. Nineteen (45.2%) patients were females, and their median (IQR) age was 72 years (65–81 years), their median (IQR) baseline NIHSS score was 18 (12–23), and the median (IQR) time from onset to recanalization was 215 minutes (162–303 minutes). The median (IQR) FIV was 46.01 mL (12.75–91.45 mL). Two patients who were confirmed to have a massive infarcted area on CT within 12 hours of stroke onset did not undergo MRI examinations, and these patients’ infarcted volumes were calculated based on the NCCT. Of all the patients, 22 patients (52.4%) had a good outcome. Figure 1 shows two representative cases with CTP parameter maps, including the CBF, CBV, MTT, TTP, and Tmax, with an automatic ROI placement by the 3D-SRT software, a mismatch map analyzed by Bayesian Vitrea, and the 24-hour MRI-DWI at the same representative slice. One is a large FIV after successful recanalization (Fig. 1a–1g), and the other is the small one (Fig. 1h–1n).
Table 1. Baseline characteristics of the study population.
| Variables | Overall (n = 42) |
|---|---|
| Age, years, median (IQR) | 72 (65–81) |
| Female sex, n (%) | 19 (45.2) |
| Baseline NIHSS score, median (IQR) | 18 (12–23) |
| Time from onset to CT imaging, minutes, median (IQR) | 103 (66–199) |
| Baseline radiological findings | |
| ASPECTS, median (IQR) | 10 (9–10) |
| ICA occlusion, n (%) | 17 (40.5) |
| CTP analysis | |
| SVD method with 3D-SRT | |
| rCBF, median (IQR) | 0.85 (0.79–0.97) |
| rCBV, median (IQR) | 1.07 (0.97–1.16) |
| rMTT, median (IQR) | 1.21 (1.15–1.27) |
| rTTP, median (IQR) | 1.42 (1.30–1.51) |
| rTmax, median (IQR) | 2.57 (2.15–2.92) |
| Bayesian Vitrea | |
| ICV, mL, median (IQR) | 15.90 (7.75–49.31) |
| IV-tPA, n (%) | 41 (97.6) |
| Time from onset to recanalization, min, median (IQR) | 215 (162–303) |
| Radiological outcome | |
| FIV, mL, mean (SD) | 46.01 (12.75–91.45) |
| Functional outcome | |
| mRS (=0, 1, 2) at 3 months, n (%) | 22 (52.4) |
Data are presented as the median (IQR) or numbers (%).
ASPECTS: Alberta stroke program early CT score; CTP: CT perfusion; FIV: final infarct volume; ICA: internal carotid artery; ICV: ischemic core volume; IQR: interquartile range; IV-tPA: intravenous tissue plasminogen activator; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; rCBF: relative cerebral blood flow; rCBV: relative cerebral blood volume; rMTT: relative mean transit time; rTmax: relative time to maximum of the residue function; rTTP: relative time-to-peak; SD: standard deviation; SVD: singular value decomposition
Fig. 1. Graphic representation of 2 cases. (A)–(G): A 58-year-old female presented with right ICA occlusion, and acute CTP imaging was obtained 108 minutes after symptom onset. Successful recanalization was achieved by EVT 173 minutes after symptom onset. (A) CBF map (rCBF = 0.71). (B) CBV map (rCBV = 0.78). (C) MTT map (rMTT = 0.97). (D) TTP map (rTTP = 1.66). (E) Tmax map (rTmax = 2.03). (F) The ICV that was calculated by the Bayesian Vitrea was 121.46 mL. (G) MRI-DWI indicates a hyperintense lesion in the right MCA territory (FIV = 89.84 mL). (H)–(N): A 71-year-old male presented with left MCA occlusion, and acute CTP imaging was obtained at 178 minutes after symptom onset. Successful recanalization was achieved by EVT 240 minutes after symptom onset. (H) CBF map (rCBF = 0.96). (I) CBV map (rCBV = 1.25). (J) MTT map (rMTT = 1.27). (K) TTP map (rTTP = 1.24). (L) Tmax map (rTmax = 2.57). (M) The ICV that was calculated by the Bayesian Vitrea was 13.3 mL. (G) MRI-DWI indicates a hyperintense lesion in the left putamen (FIV = 10.21 mL). CBF: cerebral blood flow; CBV: cerebral blood volume; CTP: CT perfusion; DWI: diffusion-weighted imaging; EVT: endovascular treatment; FIV: final infarct volume; ICA: internal carotid artery; ICV: ischemic core volume; MCA: middle cerebral artery; MTT: mean transit time; rCBF: relative cerebral blood flow; rCBV: relative cerebral blood volume; rMTT: relative mean transit time; rTmax: relative time to maximum of the residue function; rTTP: relative time-to-peak; Tmax: time to maximum of the residue function; TTP: time-to-peak.
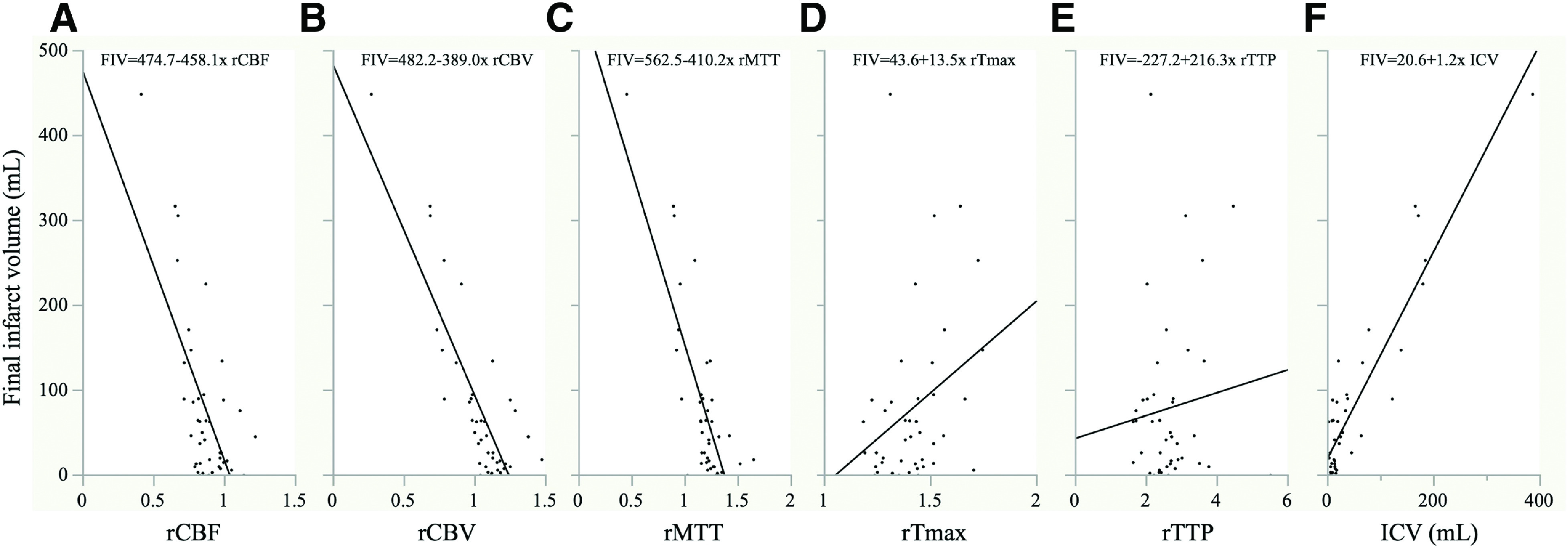
Correlation between the CTP-derived parameters and the FIV
Figure 2 demonstrates the scatterplot and regression lines of the FIV and CTP parameters, including the ICV. Among the CTP-related parameters, the ICV, rCBV, and rMTT showed a strong correlation with the FIV (ρ = 0.74, p <0.0001 for ICV; ρ = −0.67, p <0.0001 for rCBV; and ρ = −0.66, p <0.0001 for rMTT). The rCBF showed a moderate (ρ = −0.48, p = 0.0013) and the rTTP and rTmax showed a weak (ρ = 0.33, p = 0.0321 for rTTP and ρ = −0.02, p = 0.9225 for rTmax) correlation with the FIV.
Fig. 2. Scatterplot and regression lines of the FIV and CTP-derived parameters. (A) vs. rCBF (ρ = −0.48, p = 0.0013), (B) vs. rCBV (ρ = −0.67, p <0.0001), (C) vs. rMTT (ρ = −0.66, p <0.0001), (D) vs. rTmax (ρ = −0.02, p = 0.9225), (E) vs. rTTP (ρ = 0.33, p = 0.0321), and (F) vs. ICV (ρ = 0.74, p <0.0001). CTP: CT perfusion; FIV: final infarct volume; ICV: ischemic core volume; rCBF: relative cerebral blood flow; rCBV: relative cerebral blood volume; rMTT: relative mean transit time; rTmax: relative time to maximum of the residue function; rTTP: relative time-to-peak.
Comparing the Vitrea ICV with the CTP parameters for predicting good clinical outcome
Table 2 shows the results of the univariate and multivariate logistic regression analyses with good clinical outcomes. In the univariate analyses, rCBV ≥1.00, rMTT ≥1.00, and ICV ≤27.6 mL were significantly associated with good clinical outcomes (OR, 6.33 [95% CI, 1.37–29.20], p = 0.0179 for rCBV; OR, 9.00 [95% CI, 0.98–83.06], p = 0.0216 for rMTT; and OR, 9.5 [95% CI, 2.10–43.40], p = 0.0035 for ICV). Similar results were also observed in the multivariate analyses (OR, 6.87 [95% CI, 1.20–39.30], p = 0.0303 for rCBV; OR, 11.27 [95% CI, 0.97–130.94], p = 0.0269 for rMTT; and OR, 36.22 [95% CI, 2.78–471.18] for ICV). Age <70 years, female sex, an NIHSS score of ≤18, and a time from onset to recanalization of ≤210 minutes were not associated with good clinical outcomes in the univariate and multivariate analyses.
Table 2. Univariate and multivariate logistic regression analyses for each score to predict good functional outcome.
| Variable | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| rCBV ≥1.00 | 6.33 (1.37, 29.20) | 0.0179 | 6.87 (1.20, 39.30) | 0.0303 | - | - | - | - |
| rMTT ≥1.00 | 9.00 (0.98, 83.06) | 0.0216 | - | - | 11.27 (0.97, 130.94) | 0.0269 | - | - |
| ICV ≤27.6 | 9.5 (2.10, 43.04) | 0.0035 | - | - | - | - | 36.22 (2.78, 471.18) | 0.0061 |
| Age <70 | 0.86 (0.25, 2.98) | 0.8086 | 2.28 (0.42, 12.27) | 0.3384 | 1.22 (0.27, 5.43) | 0.7947 | 5.92 (0.59, 59.89) | 0.1315 |
| Female | 1.5 (0.44, 5.10) | 0.5162 | 1.26 (0.28, 5.58) | 0.7596 | 1.51 (0.36, 6.40) | 0.5767 | 0.91 (0.18, 4.51) | 0.9054 |
| NIHSS score ≤18 | 2.62 (0.74, 9.21) | 0.1334 | 2.29 (0.49, 10.66) | 0.2923 | 1.87 (0.44, 8.04) | 0.3979 | 0.95 (0.18, 5.13) | 0.9565 |
| Time from onset to recanalization ≤210 | 2.17 (0.63, 7.44) | 0.2194 | 3.43 (0.74, 15.88) | 0.1141 | 3.74 (0.87, 16.15) | 0.0767 | 4.10 (0.83, 20.17) | 0.083 |
CI: confidence interval; ICV: ischemic core volume; NIHSS: National Institutes of Health Stroke Scale; OR: odds ratio; rCBV: relative cerebral blood volume; rMTT: relative mean transit time
Discussion
In the present study, the rCBV and rMTT analyzed by the SVD method had a strong correlation with the FIV in patients with acute anterior circulation LVO after they had successful recanalization. In the multivariate analysis, these CTP-derived variables were stronger predictors of the patient outcome than the standard clinical variables, including the baseline stroke severity and early reperfusion. These results might suggest that the rCBV and rMTT analyzed by the SVD method can predict the FIV after successful recanalization and had similar performance to the ICV that was calculated by the Bayesian Vitrea.
Recently, CT-based stroke protocols, including CTP, have often been the preferred diagnostic imaging method because of their widespread availability and rapid acquisition.19) CTP provides quantitative perfusion parameter maps, including CBF, CBV, MTT, TTP, and Tmax. Based on these parameter maps, the spatial and volumetric ischemic core and penumbra lesion can be visually estimated. Although the utility of semiquantitative CBV-ASPECTS, which applies the ASPECTS to the color-coded CBV parameter map, has been reported in previous studies, the quantitative volumetric evaluation of ischemic core and penumbra lesions cannot be obtained without an automated perfusion mismatch analyzer.8,20) The baseline infarct volume, which is calculated by automated perfusion analysis software, as represented by RAPID (iSchema View, Menlo Park, CA, USA) software, is considered a strong predictor of the clinical outcome in patients with anterior circulation LVO.3,21) The utility of a Bayesian Vitrea, based on a probabilistic approach, has been reported in the evaluation of the ICV, similar to RAPID software.22,23) In the present study, the baseline infarct volume that was calculated by the Bayesian Vitrea had a strong correlation with the FIV; it was an independent predictor of patient outcome after the patient had a successful recanalization; and this result is consistent with the results of previous studies.22) Although the hemodynamic status in patients with acute anterior circulation LVO may be heterogeneous, our method provides the quantitative evaluation of each parameter value in the affected MCA territories compared to the unaffected side, by considering the affected MCA territories as the total hypoperfused area, instead of a volumetric assessment of the ICV. Moreover, its performance in predicting the response to recanalization is similar to the ICV that was calculated by the Bayesian Vitrea. Our method might be useful in clinical practice, especially in the institutions where CTP is used as the first-line stroke diagnostic modality but the automated mismatch analyzers are not equipped.
CTP has a variety of algorithms and postprocessing analysis software, and the quantitative evaluation of the CTP parameters can differ, depending on these algorithms and software.12) The CTP imaging in this study was performed by delay-insensitive SVD deconvolution algorithms, which are postprocessing software that are currently widely used in clinical practice.11) The CBF and MTT values obtained from the SVD deconvolution algorithms were considered inaccurate, while the CBV was generally accurate.24) Moreover, a paradoxically shortened MTT in patients with AIS with the use of SVD deconvolution algorithms has been reported.25,26) Although the present study showed that the rCBV and rMTT were useful predictors of patient outcome, the rCBV might be considered a suitable parameter in the prediction of patient outcome when taking these matters of measuring the MTT through the SVD deconvolution algorithms into consideration. The CBF is reported as the optimal CTP-derived parameter for predicting the FIV, and the infarct core volume was calculated by the automated perfusion analysis software using the threshold of the relative CBF value.27,28) However, the rCBF value in this study was calculated as the ratio of the average CBF value in the affected MCA territories to that of the unaffected side, and a volumetric assessment using the relative CTP parameter values could not be obtained. Therefore, the different CBF assessments might be the main reason why the rCBF was not strongly correlated with the FIV in this study. TTP and Tmax are commonly used to identify tissue with hypoperfusion, and the relative values that were calculated in this study were not appropriate for the prediction of the FIV.6)
The CTP-derived CBV is considered as the quantitative evaluation of the collateral status using a mismatch analyzer and is used as a predictor of the response to recanalization in patients with anterior circulation LVO.2,29) We previously reported that the relative CBV values in the affected MCA territories, as compared to those in the unaffected side, which were calculated by the same methodology as the present study, were the quantitative parameter of the collateral status.14) The CBV value reflects the extent of the vascular bed. The increase in the rCBV was considered to be secondary to the development of the patient’s collateral status to preserve the CBF, while the decrease in the rCBV was considered to be due to a poor collateral status, which caused the patient to not maintain their CBF despite the maximal dilation of the vascular bed. The cutoff value of the rCBV was 1, which might be considered to represent the degree of the increase or decrease in the CTP parameter values of the affected MCA side compared to the unaffected side, and the rCBV was an independent predictor of patient outcome in this study. In emergent clinical practice, the visual assessment of the CBV map focusing on the abnormal color tone asymmetries of the right and left MCA territories is considered a reasonable method of predicting the response to recanalization in patients with anterior circulation LVO.
There are several limitations of this study. First, this study was a retrospective design with a small sample size and was conducted at a single center. A potential selection bias should be considered when interpreting the results of this study. Second, the calculation of the FIV was performed by manually drawing the ROIs on the hyperintense lesions on DWI. Moreover, the lesion volumes for the two patients who lacked MRI data were calculated based on their CT imaging. All of these factors might have resulted in inconsistencies in the analysis. Third, the infarct growth during the interval between the imaging acquisition and recanalization should be considered when assessing the correlation between the baseline CTP parameters and the FIV obtained by 24-hour MRI-DWI. Almost all of the patients in the study population achieved successful recanalization within 5 hours; therefore, it could be considered that the influence of the infarct growth could potentially be minimized.
Conclusion
Among the CTP parameters analyzed by the SVD method, the relative CBV and MTT values in the affected MCA territories compared to the unaffected side had a strong correlation with the FIV and were considered useful predictors of clinical outcome in patients with acute anterior circulation LVO after successful recanalization. Their correlation with the FIV and predicting patient outcome was similar to the ICV calculated by the Bayesian Vitrea. Our method is useful in predicting the response to recanalization in patients with acute anterior circulation LVO.
Disclosure Statement
The authors have no relevant financial or non-financial interests to disclose.
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1). Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 2). Arenillas JF, Cortijo E, García-Bermejo P, et al. Relative cerebral blood volume is associated with collateral status and infarct growth in stroke patients in SWIFT PRIME. J Cereb Blood Flow Metab 2018; 38: 1839–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; 378: 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; 378: 11–21. [DOI] [PubMed] [Google Scholar]
- 5). Sakai Y, Delman BN, Fifi JT, et al. Estimation of ischemic core volume using computed tomographic perfusion. Stroke 2018; 49: 2345–2352. [DOI] [PubMed] [Google Scholar]
- 6). Olivot JM, Mlynash M, Thijs VN, et al. Optimal Tmax threshold for predicting penumbral tissue in acute stroke. Stroke 2009; 40: 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Koopman MS, Berkhemer OA, Geuskens R, et al. Comparison of three commonly used CT perfusion software packages in patients with acute ischemic stroke. J Neurointerv Surg 2019; 11: 1249–1256. [DOI] [PubMed] [Google Scholar]
- 8). Padroni M, Bernardoni A, Tamborino C, et al. Cerebral blood volume ASPECTS is the best predictor of clinical outcome in acute ischemic stroke: A retrospective, combined semi-quantitative and quantitative assessment. PLoS One 2016; 11: e0147910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Sillanpaa N, Saarinen JT, Rusanen H, et al. CT perfusion ASPECTS in the evaluation of acute ischemic stroke: Thrombolytic therapy perspective. Cerebrovasc Dis Extra 2011; 1: 6–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Toyoda K, Koga M, Iguchi Y, et al. Guidelines for intravenous thrombolysis (recombinant tissue-type plasminogen activator), the third edition, March 2019: A guideline from the Japan Stroke Society. Neurol Med Chir (Tokyo). 2019; 59: 449–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Konstas AA, Goldmakher GV, Lee TY, et al. Theoretic basis and technical implementations of CT perfusion in acute ischemic stroke, part 2: Technical implementations. AJNR Am J Neuroradiol 2009; 30: 885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12). Kudo K, Sasaki M, Ogasawara K, et al. Difference in tracer delay-induced effect among deconvolution algorithms in CT perfusion analysis: Quantitative evaluation with digital phantoms. Radiology 2009; 251: 241–249. [DOI] [PubMed] [Google Scholar]
- 13). Shinohara Y, Ibaraki M, Ohmura T, et al. Whole-brain perfusion measurement using 320-detector row computed tomography in patients with cerebrovascular steno-occlusive disease: Comparison with 15O-positron emission tomography. J Comput Assist Tomogr 2010; 34: 830–835. [DOI] [PubMed] [Google Scholar]
- 14). Hirai S, Tanaka Y, Sato H, et al. Quantitative collateral assessment evaluated by cerebral blood volume measured by CT perfusion in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 2021; 30: 105797. [DOI] [PubMed] [Google Scholar]
- 15). Inoue Y, Tanaka Y, Hata H, et al. Arterial spin-labeling evaluation of cerebrovascular reactivity to acetazolamide in healthy subjects. AJNR Am J Neuroradiol 2014; 35: 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Takeuchi R, Yonekura Y, Matsuda H, et al. Usefulness of a three-dimensional stereotaxic ROI template on anatomically standardised 99mTc-ECD SPET. Eur J Nucl Med Mol Imaging 2002; 29: 331–341. [DOI] [PubMed] [Google Scholar]
- 17). Marushima A, Tsurushima H, Suzuki K, et al. Time-course analysis of brain perfusion single photon emission computed tomography using a three-dimensional stereotactic region-of-interest template in patients with moyamoya disease. World Neurosurg 2011; 76: 304–310. [DOI] [PubMed] [Google Scholar]
- 18). Takahashi S, Tanizaki Y, Kimura H, et al. Prediction of cerebrovascular reserve capacity by computed tomography perfusion using 320-row computed tomography. J Stroke Cerebrovasc Dis 2015; 24: 939–945. [DOI] [PubMed] [Google Scholar]
- 19). Martinez G, Katz JM, Pandya A, et al. Cost-effectiveness study of initial imaging selection in acute ischemic stroke care. J Am Coll Radiol 2021; 18: 820–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Psychogios MN, Sporns PB, Ospel J, et al. Automated perfusion calculations vs. visual scoring of collaterals and CBV-ASPECTS: Has the machine surpassed the eye? Clin Neuroradiol 2021; 31: 499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Goyal M, Ospel JM, Menon B, et al. Challenging the ischemic core concept in acute ischemic stroke imaging. Stroke 2020; 51: 3147–3155. [DOI] [PubMed] [Google Scholar]
- 22). Rava RA, Snyder KV, Mokin M, et al. Assessment of a Bayesian Vitrea CT perfusion analysis to predict final infarct and penumbra volumes in patients with acute ischemic stroke: A comparison with RAPID. AJNR Am J Neuroradiol 2020; 41: 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Nael K, Tadayon E, Wheelwright D, et al. Defining ischemic core in acute ischemic stroke using CT perfusion: A multiparametric Bayesian-based model. AJNR Am J Neuroradiol 2019; 40: 1491–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Sasaki M, Kudo K, Boutelier T, et al. Assessment of the accuracy of a Bayesian estimation algorithm for perfusion CT by using a digital phantom. Neuroradiology 2013; 55: 1197–1203. [DOI] [PubMed] [Google Scholar]
- 25). Murayama K, Katada K, Hayakawa M, et al. Shortened mean transit time in CT perfusion with singular value decomposition analysis in acute cerebral infarction: Quantitative evaluation and comparison with various CT perfusion parameters. J Comput Assist Tomogr 2017; 41: 173–180. [DOI] [PubMed] [Google Scholar]
- 26). Doucet C, Roncarolo F, Tampieri D, et al. Paradoxically decreased mean transit time in patients presenting with acute stroke. J Comput Assist Tomogr 2016; 40: 409–412. [DOI] [PubMed] [Google Scholar]
- 27). Amukotuwa S, Straka M, Aksoy D, et al. Cerebral blood flow predicts the infarct core: New insights from contemporaneous diffusion and perfusion imaging. Stroke 2019; 50: 2783–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Campbell BC, Christensen S, Levi CR, et al. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke 2011; 42: 3435–3440. [DOI] [PubMed] [Google Scholar]
- 29). Rao VL, Mlynash M, Christensen S, et al. Collateral status contributes to differences between observed and predicted 24-h infarct volumes in DEFUSE 3. J Cereb Blood Flow Metab 2020; 40: 1966–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


