Abstract
Objective
We report a case of additional carotid artery stenting (CAS) for plaque protrusion occurring after initial CAS for radiation-induced common carotid artery (CCA) stenosis.
Case Presentation
A 69-year-old man with a history of radiotherapy for laryngeal cancer presented to our hospital with sudden-onset right hemiparesis. Since vulnerable plaque of the left CCA was considered the embolic source for ischemic stroke, CAS was performed for left CCA stenosis. No perioperative complications were observed and the patient was discharged with a modified Rankin Scale score of 0. However, 1 month after CAS, cerebral embolism recurred. As protruding plaque was found on CTA, additional endovascular treatment was performed with intravascular ultrasonography. He was discharged without complications and showed a good outcome at 3 months.
Conclusion
In CCA stenosis after radiotherapy, accelerated arteriosclerosis may cause drug-resistant cerebral embolism and plaque protrusion after CAS, making determination of the treatment strategy difficult. Appropriate treatment options need to be based on individual underlying diseases and plaque instability.
Keywords: radiation induced, common carotid artery, plaque protrusion, carotid artery stenting
Introduction
Carotid artery stenting (CAS) is often required for radiation-induced carotid artery stenosis, but additional stenting for plaque protrusion occurring after CAS appears rare. Pathologically, the plaque is usually stable and the frequency of ischemic stroke is considered low because inflammatory changes at the lesion site are scarce and usually attributable to fibrosis.1) However, recent reports suggest that carotid artery stenosis after radiotherapy is a form of accelerated arteriosclerosis2) and involves highly unstable plaque,3) with fewer fibrous components than have been considered in the past. Herein, we report an instructive case of plaque protrusion following CAS for left common carotid artery (CCA) stenosis after radiotherapy.
Case Presentation
A 69-year-old man presented to our hospital with sudden onset of right hemiparesis (manual muscle testing: 5-/5). The patient had a medical history of laryngeal microsurgery for laryngeal cancer 11 years earlier, and was subsequently treated with 60 Gy of radiotherapy for 1 month. Since then, no recurrence of cancer has been observed. In addition, the patient had a history of chronic occlusion of the right CCA, hypertension, and dyslipidemia.
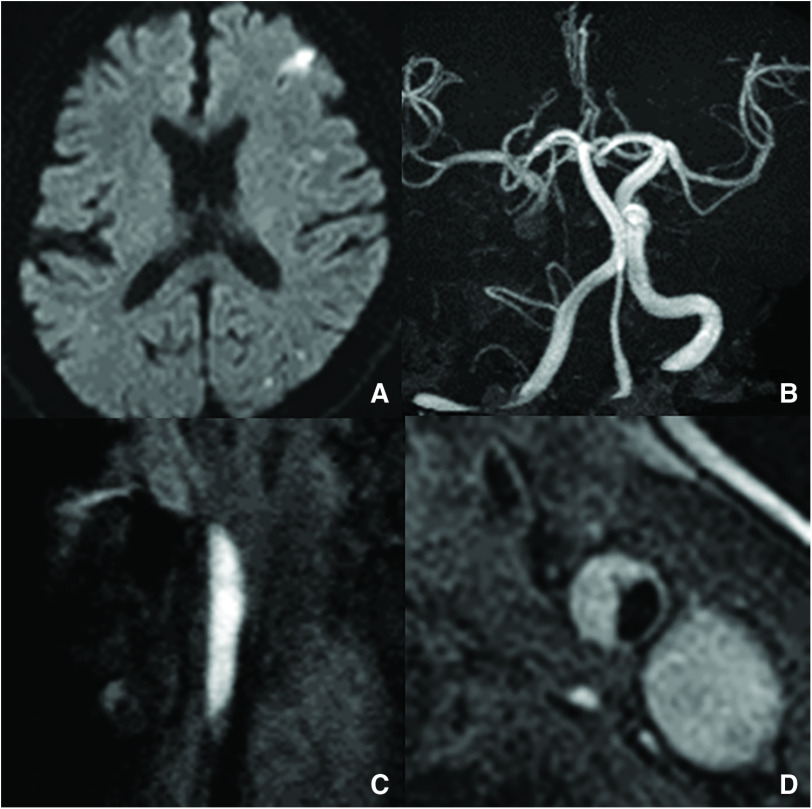
Emergent MRI demonstrated multiple high-intensity signals on diffusion-weighted imaging in the territory of the middle cerebral artery (Fig. 1A). MRA of the head revealed no findings indicative of occlusion or stenosis of the right large vessels, except for the absence of the chronically occluded right internal carotid artery (Fig. 1B). No abnormalities were evident from laboratory findings. According to the European Carotid Surgery Trial criteria, ultrasonography detected 50% stenosis of the left CCA, which showed low-echoic lesions. MRI of neck plaque demonstrated high-intensity signals on magnetization-prepared rapid gradient echo sequences (Fig. 1C) and T1-weighted black-blood imaging (Fig. 1D). Based on these findings, we diagnosed vulnerable plaque of the left CCA as the embolic source of ischemic stroke.
Fig. 1. (A) Diffusion-weighted imaging of the head shows cerebral infarction in the territory of the left middle cerebral artery. (B) MRA of the head reveals no findings suggestive of occlusion or stenosis of the right large vessels, except for the absence of the chronically occluded right internal carotid artery. (C) Magnetization-prepared rapid gradient-echo imaging shows a high-intensity signal of the CCA. (D) T1-weighted black-blood imaging demonstrates a high-intensity signal of the CCA, suggesting vulnerable plaque. CCA: common carotid artery.
On admission, 200 mg of aspirin and 300 mg of clopidogrel were administered. Angiography revealed a long, ulcerated plaque in the left CCA (Fig. 2A), which was located from the upper edge of the 3rd cervical vertebra to the lower edge of the 5th cervical vertebra. In addition, few leptomeningeal collateral pathways from the posterior circulation were present and the territory of the right middle cerebral artery was supplied by cross-circulation through the anterior communicating artery. Symptoms improved markedly and the patient was discharged on hospital day 13 with a modified Rankin Scale score of 0. However, 1.5 months later, he was readmitted with recurrence of cerebral infarction. Since he appeared resistant to the best medical oral treatment, endovascular treatment was performed.
Fig. 2. (A) Left common carotid angiography before CAS (frontal view) shows long stenosis (white arrow). (B) and (C) Pre-dilation performed using a 7.0 mm × 40 mm balloon catheter at the site of stenosis at three locations: distal, middle, and proximal. (D) Fluoroscopic image of first stent placement. A 10 mm × 31 mm Carotid Wallstent is placed at the distal site of stenosis. (E) Subsequently, an additional 10 mm × 31 mm Carotid Wallstent is placed at the proximal site of stenosis. (F) Left common artery injection shows no contrast-enhanced defect. CAS: carotid artery stenting.
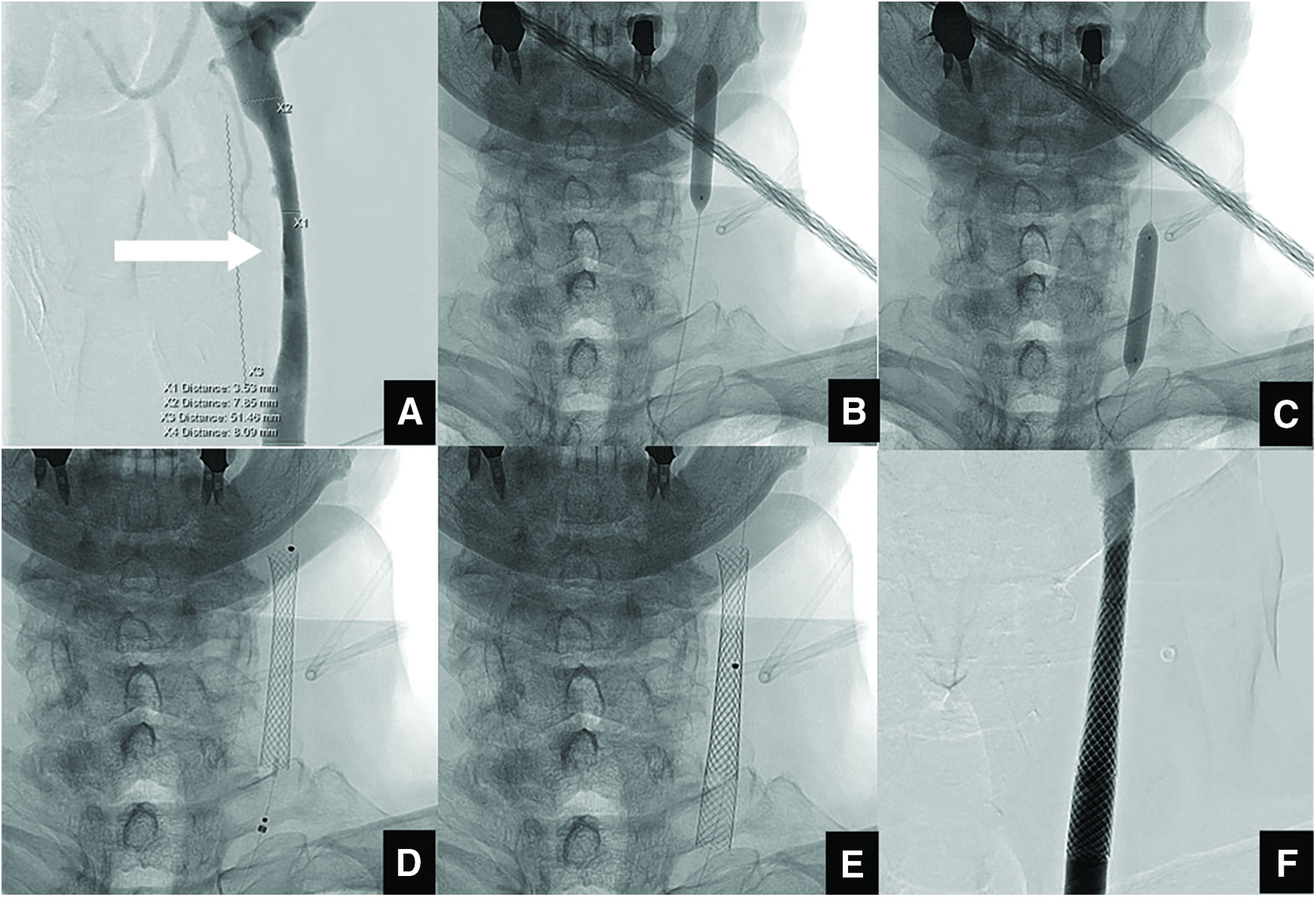
The procedure was performed with the patient awake and under minimal sedation. The right common femoral artery was punctured and an 8-Fr 30-cm sheath was placed. After systemic heparinization, a 4- to 6-Fr JB2 catheter (Medikit, Tokyo, Japan) was guided by a 0.035-inch guidewire to the ascending aorta, and then an 8-Fr SEL-OSP insertion-support guiding catheter (Medikit) was placed at the origin of the left CCA. A PercuSurge GuardWire (Medtronic, Minneapolis, MN, USA) was carefully advanced across the stenotic lesion and blocked the blood stream in the distal internal carotid artery. The most stenotic site of the lesion was 3.4 mm in diameter, the diameter of the distal CCA was 7.8 mm, the diameter of the proximal CCA was 8.2 mm, and the lesion length was 50 mm. Pre-dilation was performed using a 7.0 mm × 40 mm balloon catheter (Sterling; Boston Scientific, Natick, MA, USA) at the site of stenosis at three locations (distal, middle, and proximal) because of the long lesion (Fig. 2B and 2C). Subsequently, two 10 mm × 31 mm carotid stents (Carotid Wallstent; Boston Scientific) were placed and partially overlapped to cover the plaque-rich area (Fig. 2D and 2E). After floating debris was vacuumed out using an aspiration catheter, all procedures were completed. Left common artery injection showed no contrast-enhanced defect (Fig. 2F). No perioperative complications were observed and ultrasonography at 1 and 5 days after CAS showed no plaque protrusion. The patient was discharged with a modified Rankin Scale score of 0 but was readmitted 1 month after CAS because of recurrent cerebral infarction.
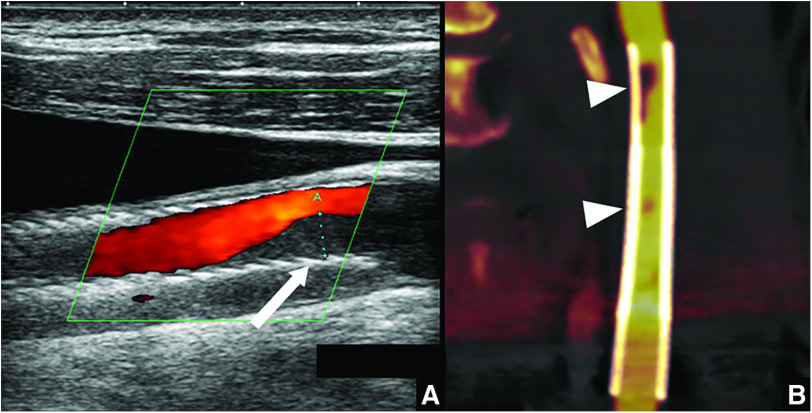
Ultrasonography revealed low-echoic deposits distal to the stent, suggesting plaque protrusion. Moreover, a mobile thrombus was attached to the protruding plaque (Fig. 3A). CTA showed plaque protrusion at the distal end of the Carotid Wallstent and at areas of overlap (Fig. 3B). Warfarin was added and 100 mg of aspirin was discontinued because of insufficient effect on platelet aggregation. Prothrombin time and international normalized ratio (PT-INR) were controlled to within the range of 2.0–3.0 s. Despite aggressive medical treatment, the patient experienced recurrent cerebral infarction and additional endovascular treatment was performed.
Fig. 3. (A) Ultrasonography reveals low-echoic deposits distal to the stent (white arrow), suggesting plaque protrusion. Mobile thrombus is attached to the protruding plaque. (B) CTA shows plaque protrusion at the distal end of the Carotid Wallstent and sites of areas of stent overlap (white arrowheads).
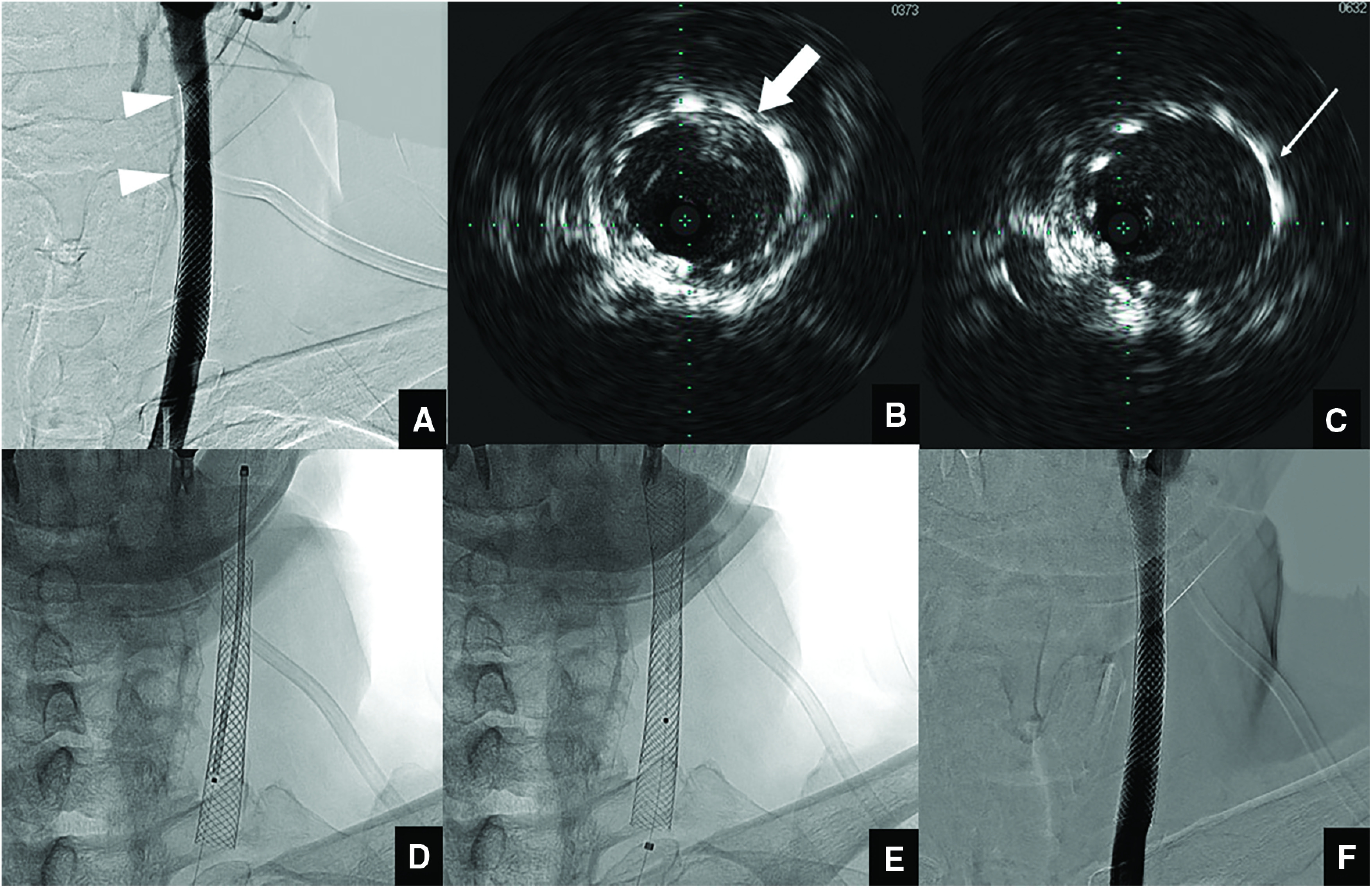
As in the first session, the procedure was performed with an 8-Fr SEL-OSP placed at the origin of the left CCA. Left internal carotid artery injection showed a contrast-enhanced defect on the distal right side of the Carotid Wallstent and on the distomedial side at areas of overlap (Fig. 4A). Before the operative procedure, intravascular ultrasonography (IVUS) (Volcano Visions PV 0.014P catheter with Chroma Flo; Volcano, Rancho Cordova, CA, USA) was navigated to the area of protruding plaque, which clearly indicated the position of the protruding area (Fig. 4B and 4C). Based on these findings, a Filterwire EZ protection device (Boston Scientific) was carefully maneuvered across the stented region, and a 10 mm × 31 mm Carotid Wallstent was placed to cover the protruding plaque (Fig. 4D and 4E). Neither pre- nor post-dilation was performed. After stent placement, IVUS confirmed the disappearance of plaque protrusion. IVUS was useful in determining the position for stent deployment. Left internal carotid artery injection showed disappearance of the protruding plaque (Fig. 4F). During the perioperative period, the patient showed no neurological deficits and plaque protrusion disappeared on ultrasonography. Finally, the patient was discharged with a modified Rankin Scale score of 1. On follow-up at 3 months after discharge, modified Rankin Scale score remained at 1. Ultrasonography 6 and 12 months after additional endovascular treatment did not show any protruding plaque or carotid artery restenosis. We therefore discontinued warfarin and only continued administration of clopidogrel at 12 months after additional CAS. Since then, the patient has been commuting to follow-up at our hospital without recurrence of cerebral infarction. The patient consented to the submission of this report for publication.
Fig. 4. (A) Left common carotid angiography before additional CAS (frontal view) showing plaque protrusion (white arrowheads). (B) IVUS before placement shows plaque protrusion at the distal end of the Carotid Wallstent in the direction of 1 o’clock (thick white arrow). (C) IVUS after stent placement shows plaque protrusion at the site of overlap in the direction of 2 o’clock (thin white arrow). (D) Fluoroscopic image before stent placement. (E) A 10 mm × 31 mm Carotid Wallstent was placed to cover the plaque protruded area. (F) Left common artery injection showed no contrast-enhanced defect. CAS: carotid artery stenting; IVUS: intravascular ultrasonography.
Discussion
To the best of our knowledge, this case represents the rare report of additional CAS for plaque protrusion occurring after initial stent-in-stenting for radiation-induced CCA stenosis due to resistance to pharmacotherapy. In the present case, a large amount of vulnerable plaque was suggested on preoperative examination, so two closed stents were placed in the lesion, but plaque protrusions still occurred. These protrusions were seen to progress during follow-up, and a thrombus was considered to have adhered to the protrusion, so anticoagulant therapy was added. However, since the cerebral infarction subsequently recurred, we decided to perform additional CAS. As no such cases have been reported previously, we report this as a didactic case.
Plaque protrusion is a phenomenon in which plaque protrudes from the mesh of the stent due to the radial force of the stent. Vulnerable plaque with large lipid cores, hemorrhage in the plaque, and the use of open-cell stents have been reported as predictors of plaque protrusion,4) which has a frequency of 2.6%–7.8%.4,5) The clinical course is characterized by the possibility of spontaneous regression, but growth over time leads to intra-stent occlusion and embolic stroke. Retreatment should be considered if narrowing of the stent lumen diameter, an increasing trend in areas of protruding plaque, and mobile plaque are observed. On the other hand, mild narrowing and slight protrusion require pharmacotherapy with frequent follow-up. Whether antiplatelet drugs or anticoagulants are more effective as drug treatments remains unclear.
In general, a history of radiotherapy to the neck represents a risk factor for carotid artery stenosis. In 1962, Lindsay et al. irradiated the abdominal aorta with X-rays in a canine model and discovered arteriosclerotic changes in the aortic wall.6) Morphological characteristics of carotid artery stenosis after radiotherapy have been reported to include presence in the CCA,3) bilateral stenosis,7) a high stenosis rate in the distal part of the lesion,7) and a long distance between lesion sites,7) all of which resemble the pathology in the present case.
We often encounter cases of symptomatic carotid artery stenosis several years after radiotherapy to the neck. A review of cerebrovascular incidents among patients with a history of cervical radiotherapy reported that 18%–38% of patients developed carotid artery stenosis.2) This was explained as accelerated arteriosclerosis due to ischemic necrosis of the blood vessel wall involving vasa vasorum disorder and endothelial damage, and has also been called malignant stenosis. In addition, ischemic stroke was reportedly induced by the accumulation of plaque in the stenotic site after radiotherapy.8) The patient in our case had hypertension and dyslipidemia as underlying diseases, exhibited accelerated arteriosclerosis due to the effects of radiotherapy, and was considered to have developed carotid artery stenosis after 11 years.
No consensus has been established regarding methods for preventing plaque protrusion. As a method to prevent plaque protrusion using standard carotid stents, CASPER micromesh stent (Terumo, Tokyo, Japan) may be effective for plaque protrusion, although plaque protrusion reportedly still developed in 44% of patients treated using CASPER stents.9) On the other hand, the stent-in-stent technique with closed-cell stents has been reported to show no incidence of plaque protrusion or perioperative cerebral infarction.10) However, different methods of evaluating plaque protrusion were used, with optical frequency domain imaging used in the former study and IVUS in the latter. In the future, further cases need to be accumulated regarding preventive measures against plaque protrusion.
The relationship between plaque protrusion and balloon post expansion is controversial. Clinically, excessive post-dilation of vulnerable plaque is recognized to increase the risk of plaque protrusions, but evidence remains lacking. Harada et al. performed pre- and post-dilation evaluations using optical coherence tomography and reported that post-dilation may reduce both the volume of protrusion and late-onset cerebral infarction.11) In this case, post-dilation was not performed in either of the two treatments, but further cases need to be accumulated to clarify this issue.
IVUS before and after stent placement is useful for observing morphological changes in plaque protrusions.12,13) In this case, IVUS used before and after additional stent placement was considered useful for visually confirming morphological changes in plaque protrusions. However, if IVUS had been performed before and after the initial treatment, we might have noticed the plaque protrusion earlier. Plaque protrusion was not detected by ultrasonography at 1 or 5 days after initial CAS in this case, but additional evaluation with CTA was more useful in detecting plaque protrusion.
Whether CAS or carotid endarterectomy (CEA) is more effective for treating carotid artery stenosis after cervical radiotherapy remains contentious. CAS for radiation-induced carotid artery stenosis has been reported to carry a high risk of ischemic stroke within 30 days due to formation of vulnerable plaque.14) In this case, the stenotic lesion was located from the upper edge of the 3rd cervical vertebra to the lower edge of the 5th cervical vertebra, and so could be in a reachable position for CEA. However, this case involved CEA high-risk factors such as contralateral occlusion and a history of radiotherapy, and as the collateral circulation was also underdeveloped, CAS was selected. In general, CEA for carotid artery stenosis after cervical radiotherapy is generally known to be of high risk due to the problem of adhesion around the wound and blood vessels. Since 2004, when the history of cervical radiotherapy was considered a high-risk factor for CEA in the SAPPHIRE trial, CAS has been considered the preferred treatment.15,16) Regarding complications, CEA for carotid artery stenosis after cervical radiotherapy has been reported to be associated with neuropathy, while CAS shows a high frequency of restenosis.17) On the other hand, a recent meta-analysis showed that treatment for carotid artery stenosis after cervical radiotherapy led to similar perioperative complications in both CAS and CEA, with no significant difference in long-term restenosis rates.18) Such reports have been contradictory, so long-term restenosis rates may remain similar for both CAS and CEA. We await the accumulation of more knowledge on this issue in the future.
Relatively few reports have described outcomes for patients with carotid artery stenosis who have undergone CAS and have been treated with radiation. Choy et al.19) followed cases after CAS for carotid artery stenosis with a history of radiotherapy or cervical surgery for 5 years, but no significant difference in adverse events was seen compared to cases with no history of CAS, and the outcomes were also reportedly good. In the present case, no recurrence of cerebral infarction was observed after additional carotid stenting, and the outcome was favorable. However, the timing of surgery needed to be considered while observing responsiveness to antithrombotic therapy. This was one case in which the optimal treatment strategy was also difficult to determine.
Conclusion
CCA stenosis after radiotherapy-accelerated arteriosclerosis may cause drug-resistant cerebral embolism and plaque protrusion after CAS, making determination of the treatment strategy more difficult. Appropriate treatment options need to be considered based on individual underlying diseases and plaque instability.
Disclosure Statement
All authors have no conflicts of interest.
References
- 1). Fokkema M, den Hartog AG, van Lammeren GW, et al. Radiation-induced carotid stenotic lesions have a more stable phenotype than de novo atherosclerotic plaques. Eur J Vasc Endovasc Surg 2012; 43: 643–648. [DOI] [PubMed] [Google Scholar]
- 2). Fernández-Alvarez V, López C, Suárez F, et al. Radiation-induced carotid artery lesions. Strahlenther Onkol 2018; 194: 699–710. [DOI] [PubMed] [Google Scholar]
- 3). Ting ACW, Wu LLH, Cheng SWK. Ultrasonic analysis of plaque characteristics and intimal-medial thickness in radiation-induced atherosclerotic carotid arteries. Eur J Vasc Endovasc Surg 2002; 24: 499–504. [DOI] [PubMed] [Google Scholar]
- 4). Kotsugi M, Takayama K, Myouchin K, et al. Carotid artery stenting: investigation of plaque protrusion incidence and prognosis. JACC Cardiovasc Interv 2017; 10: 824–831. [DOI] [PubMed] [Google Scholar]
- 5). Shinozaki N, Ogata N, Ikari Y. Plaque protrusion detected by intravascular ultrasound during carotid artery stenting. J Stroke Cerebrovasc Dis 2014; 23: 2622–2625. [DOI] [PubMed] [Google Scholar]
- 6). Lindsay S, Kohn HI, Dakin RL, et al. Aortic atherosclerosis in the dog after localized aortic x-irradiation. Circ Res 1962; 10: 51–60. [DOI] [PubMed] [Google Scholar]
- 7). Shichita T, Ogata T, Yasaka M, et al. Angiographic characteristics of radiation-induced carotid arterial stenosis. Angiology 2009; 60: 276–282. [DOI] [PubMed] [Google Scholar]
- 8). Gujral DM, Chahal N, Senior R, et al. Radiation-induced carotid artery atherosclerosis. Radiother Oncol 2014; 110: 31–38. [DOI] [PubMed] [Google Scholar]
- 9). Yamada K, Yoshimura S, Miura M, et al. Potential of new-generation double-layer micromesh stent for carotid artery stenting in patients with unstable plaque: a preliminary result using OFDI analysis. World Neurosurg 2017; 105: 321–326. [DOI] [PubMed] [Google Scholar]
- 10). Myouchin K, Takayama K, Wada T, et al. Carotid artery stenting using a closed-cell stent-in-stent technique for unstable plaque. J Endovasc Ther 2019; 26: 565–571. [DOI] [PubMed] [Google Scholar]
- 11). Harada K, Kajihara M, Sankoda Y, et al. Efficacy of post-dilatation during carotid artery stenting for unstable plaque using closed-cell design stent evaluated by optical coherence tomography. J Neuroradiol 2019; 46: 384–389. [DOI] [PubMed] [Google Scholar]
- 12). Kuroiwa T, Sakai N, Adachi H, et al. Stent-in-stenting for the plaque protrusion after stent deployments. Surg Cereb Stroke 2004; 32: 107–111. (in Japanese) [Google Scholar]
- 13). Taguchi H, Takayama K, Kishida H, et al. A case of intraprocedural plaque protrusion during carotid artery stenting using the stent-in-stent technique for carotid artery stenosis with unstable plaque. JNET J Neuroendovasc Ther 2022; 16: 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Sano N, Satow T, Maruyama D, et al. Relationship between histologic features and outcomes of carotid revascularization for radiation-induced stenosis. J Vasc Surg 2015; 62: 370–377.e1. [DOI] [PubMed] [Google Scholar]
- 15). Yadav JS, Wholey MH, Kuntz RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med 2004; 351: 1493–1501. [DOI] [PubMed] [Google Scholar]
- 16). Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med 2008; 358: 1572–1579. [DOI] [PubMed] [Google Scholar]
- 17). Huang MP, Fang HY, Chen CY, et al. Long-term outcomes of carotid artery stenting for radiation-associated stenosis. Biomed J 2013; 36: 144–149. [DOI] [PubMed] [Google Scholar]
- 18). Giannopoulos S, Texakalidis P, Jonnalagadda AK, et al. Revascularization of radiation-induced carotid artery stenosis with carotid endarterectomy vs. carotid artery stenting: a systematic review and meta-analysis. Cardiovasc Revasc Med 2018; 19: 638–644. [DOI] [PubMed] [Google Scholar]
- 19). Choy HK, Kokkinidis DG, Cotter R, et al. Long-term outcomes after carotid artery stenting of patients with prior neck irradiation or surgery. Cardiovasc Revasc Med 2018; 19: 327–332. [DOI] [PubMed] [Google Scholar]