Abstract
Niche-conservative species are especially susceptible to changes in their environment, and detecting the negative effects of new stressors in their habitats is vital for safeguarding of these species. In the Arctic, human disturbance including marine traffic and exploration of resources is increasing rapidly due to climate change–induced reduction of sea ice. Here, we show that the narwhal, Monodon monoceros, is extremely sensitive to human-made noise. Narwhals avoided deep diving (> 350 m) with simultaneous reduction of foraging and increased shallow diving activity as a response to either ship sound alone or ship sound with concurrent seismic airgun pulses. Normal behavior decreased by 50 to 75% at distances where received sound levels were below background noise. Narwhals were equally responsive to both disturbance types, hence demonstrating their acute sensitivity to ship sound. This sensitivity coupled with their special behavioral-ecological strategy including a narrow ecological niche and high site fidelity makes them thus especially vulnerable to human impacts in the Arctic.
The narwhal, an endemic Arctic specialist, is extremely sensitive to human-made noise.
INTRODUCTION
A species’ niche is defined as a suite of both abiotic and biotic environmental factors that are required for a species to exist (1). The width of a species’s niche and the ability of a species to disperse are classic concepts in ecology that explain species distribution, and they are also vital for predicting species’ response to changes in their habitat. The degree to which organisms adapt to their niche through space and time, niche conservatism, can be used to assess their capacity to adapt to new environmental conditions (2). Species with a high degree of niche conservatism can be regarded as particularly vulnerable to environmental changes. High site fidelity, which reduces the ability of species to disperse or expand their ranges (1), also adds to the overall sensitivity toward environmental changes.
Narwhals, Monodon monoceros, are characterized by both features; they are niche conservative and exhibit high site fidelity. Narwhals are endemic to the Atlantic sector of the Arctic, and, within this biogeographical region, they prefer deep water areas where they target prey at 300 to 800 m depth (3–6). In addition, narwhals select colder water in their environment, which affects their general distribution and their small-scale habitat preferences within the fjord systems during summer (7, 8). Because of their remarkable philopatry for both summer and winter habitats in this narrow Arctic niche, narwhals maintain a metapopulation structure in which individuals from separate summer stocks can temporally overlap in the wintering areas but return to their separate natal summer areas (9, 10). In addition, narwhals have low genetic diversity that may further limit the physiological and behavioral plasticity and restrain adaptations when predictable Arctic conditions are stressed by rapid changes (11).
Because of polar amplification, the temperatures are changing faster in the Arctic than in other parts of the Earth (12). The temporal and spatial expansion of human activities, as a consequence of warming and subsequent reduction of sea ice, affects narwhals indirectly. Increased activities such as fisheries, tourism, shipping, and oil and gas exploration are altering the acoustic environment in the Arctic by increasing noise in narwhal habitats (13–15). Shipping, which generates low-frequency noise (< 1 kHz), is of special concern because it can ensonify a large spatial area, and, for established shipping routes, the impacts can be nearly uninterrupted (16). Increased shipping is estimated to have contributed to a 32-fold increase in low-frequency noise along major shipping routes globally during the past 50 years (16). This may also occur in the Arctic because shipping, tourism, and seismic exploration are predicted to increase in the region (14, 17, 18). The Northwest Passage and Northern Sea Route are expected to be ice-free by 2050, enabling a near year-round usage of trans-Arctic shipping routes (19, 20) that undoubtedly will increase the ambient noise levels in the region.
Anthropogenic noise has a plethora of negative effects on marine life [e.g., (16)]. Elevated noise levels can cause disturbance and are considered a threat particularly for marine mammals that rely on sound as their main modality for acquiring information about their environment [e.g., (13)]. Responses that affect the energy balance of an individual, such as impaired energy intake due to reduced foraging and/or elevated energy consumption due to higher activity levels, may lead to detrimental population effects. Although the narwhal, as a species with a global population of >80,000 animals, is not generally at risk (21), some local populations are depleted because of hunting (22, 23). Some narwhal populations, such as the Eclipse Sound summer stock, are already experiencing major changes in their environment due to mining development on Baffin Island and the associated vessel traffic in shipping lanes used for exporting mining products (15, 24–26).
While the Arctic is changing from a pristine acoustic environment, where species such as the narwhal evolved in an habitat devoid of anthropogenic noise, and which is now an acoustic habitat increasingly similar to those in lower latitudes, our understanding of the effects of this transformation on endemic Arctic species is largely unknown. To address this, an extensive controlled sound exposure study was conducted in East Greenland in 2017–2018 to explore the reactions of narwhals to sound disturbance (27). In the present study, we combine movement and behavioral data collected by tags deployed on free-ranging narwhals to observe changes in their diving and foraging behavior under two sound conditions that we assumed were affecting the whales: ship sound alone and ship sound coupled with airgun pulses. We hypothesized that the behavioral response variables would show different thresholds of sensitivity and that the changes in the acoustic habitat due to the combined sound condition (ship sound coupled with airgun pulses) would elicit stronger reactions by the whales than ship sound alone. The findings are discussed relative to the special behavioral-ecological adaptations of narwhals.
RESULTS
The behavioral records collected from tags on six males lasted between 4 days 6 hours and 8 days 3 hours in duration. Analyses of the records yielded a total of ~854 hours of depth, acoustic, and accelerometer data (table S1). Analysis of the acoustic data yielded 21,244 signals indicative of foraging behavior [i.e., buzzes; see (28)], excluding 16.2 hours of the available acoustic data (1.9%) that were deemed unsuitable for analysis due to a poor signal-to-noise ratio.
Sound exposure levels
Each animal was exposed to ship noise and airgun pulses an average of five times during “trials” and to ship noise alone an average of three times during “intertrials” [table S2; see (11) for details]. The distance between a whale and the ship ranged from 1 to 63 km during trials and from 1.6 to 56 km during intertrials.
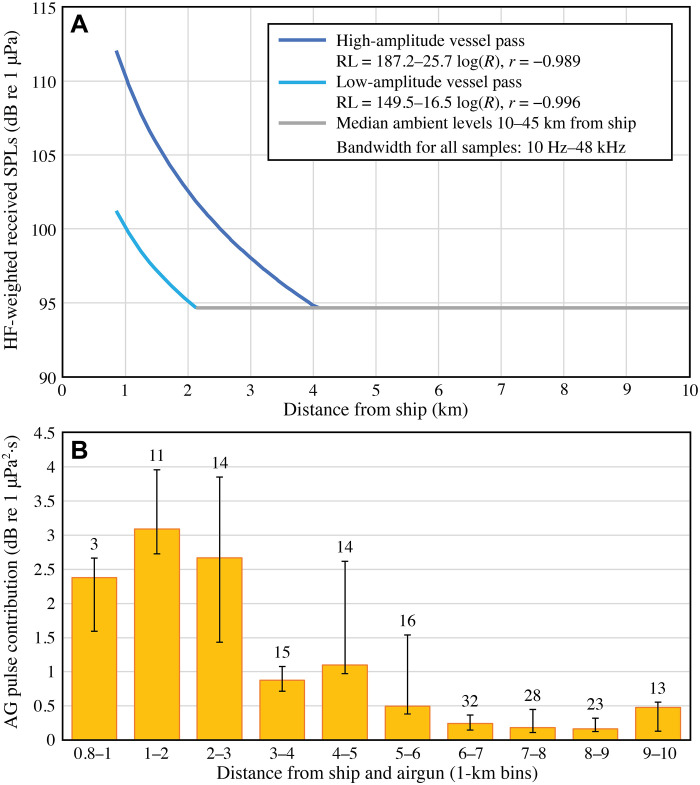
The median high-frequency (HF)–weighted (29) background sound level in the study area, obtained 10 to 45 km from the ship, was 94.6 dB re 1 μPa (1-s sound pressure levels; frequency range, 10 Hz to 48 kHz; Fig. 1A). The asymptotic decline of the ship sound (i.e., during intertrials) intersected with the median background values ~2.1 and 4.1 km from the ship for the low- and high-amplitude vessel recordings, respectively (Fig. 1A). HF-weighted airgun pulse levels barely exceeded ship sound levels, even at the closest distances from the ship [cf. figure 1 of (30)]. As a result, the median decibel contribution of airgun pulses to the sum of airgun and ship sounds during trials (Fig. 1B) was above 2 to 3 dB only within ~3 km of the ship and fell below 0.5 dB at 5 to 6 km distance from the ship.
Fig. 1. Ship and ship and airgun pulse sound levels during intertrials and trials.
(A) Received high-frequency (HF)–weighted (49) sound pressure levels (SPLs) of ship sound during intertrials as a function of range, for high-amplitude (dark blue) and low-amplitude (light blue) ship passes, with corresponding logarithmic fits and correlation coefficient (r) values. Sample duration was 1 s, so SPLs shown are also sound exposure levels (SELs). A total of 1.27 dB [10*Log10(1.34), where 1.34 is the mean duration of the airgun pulses] was added to these values when calculating the airgun contribution shown in (B). (B) Decibel contribution by airgun (AG) pulses to the sum of sounds received by narwhals out to 10 km during trials (see Materials and Methods for details); this illustrates by how many decibel ship or background levels are elevated by the presence of airgun pulses (HF-weighted SELs), for 10 bins of 1 km (sample sizes above each bin, total = 169 airgun pulses). Columns are medians, and the bars delimit the interquartile range (25th to 75th percentiles). Values were calculated using the low-amplitude vessel pass [light blue line in (A)], which results in a higher contribution by airgun pulses and is therefore a more conservative assessment.
Changes in narwhal behavior during sound exposure
Deep diving and buzzing were the activities that were most sensitive to noise exposure: The changes were most pronounced for these two behavioral metrics and were measurable at the greatest distances from the ship (Table 1).
Table 1. Distances from the sound source (kilometers) at which, compared with undisturbed behavior, there was a change (either negative or positive) of 25, 50, and 75% in the four behavioral response variables during the two sound exposure types: trial (ship sound with airgun pulses) and intertrial (ship sound only).
Changes in diving behavior (with mean target depth and dive initiation as behavioral metrics) were estimated for deep dives and medium dives separately. The distances, at which changes in the probability of initiating either a deep or medium dive occurred, are given here for the probability of initiating a dive within 5 min of a previous dive (see Fig. 2). Buzzing bout rate was defined as the rate of initiation of a buzzing bout (1/min). The change from undisturbed behavior as an effect of either exposure was mainly negative, i.e., a decrease in the behavioral metric in question. Only the propensity of initiating a medium dive increased during both exposures compared with undisturbed behavior (marked with ^; Fig. 2B and fig. S1). The most severely affected behavioral metric was the propensity to initiate a deep dive during intertrials; at 40 km distance, the probability of initiating a deep dive within 5 min of a previous dive had already been reduced by 37% compared to undisturbed behavior (marked with #). The least affected metric was the mean target depth of medium dives (indicated with hyphens); the estimated changes never reached the listed values of 25, 50, or 75% (decrease in mean target depth of medium dives was only 3 and 12% during trials and intertrials, respectively).
| Behavior | Behavioral metric | Exposure type | Change from undisturbed behavior, distance in kilometers | ||
|---|---|---|---|---|---|
| 25% | 50% | 75% | |||
| Diving | Mean target depth deep dives | Trial | 24 | 10 | 5 |
| Intertrial | 23 | 10 | 5 | ||
| Mean target depth medium dives | Trial | – | – | – | |
| Intertrial | – | – | – | ||
| Dive initiation deep dives | Trial | 40 | 18 | 9 | |
| Intertrial | >40# | 29 | 13 | ||
| Dive initiation medium dives^ | Trial | 6 | 4 | 3 | |
| Intertrial | 11 | 6 | 4 | ||
| Foraging | Buzzing bout rate | Trial | 20 | 10 | 7 |
| Intertrial | 10 | 6 | 4 | ||
The mean target depth of deep dives decreased with decreasing distance to the sound source. Compared to diving behavior with no sound exposure (undisturbed behavior), the mean target depth of deep dives was halved (from 400 to 200 m) at 10 km range during both trials and intertrials (Fig. 2A and Table 1). Another effect of sound exposure was a decrease in the number of deep dives. The propensity of initiating a deep dive decreased by 68 and 83% for each 0.1 increase in the exposure level during trials and intertrials, respectively (fig. S1). This change was further reflected in the time spent near the surface between deep dives. At 40 km range during both trials and intertrials, the probability that a whale would initiate a deep dive within 5 min of the previous dive was 25% less than with no sound exposure (Table 1 and Fig. 2B). At ~10 km distance during both exposure types, the probability of initiating a deep dive within 5 min of the previous dive was only a few percent (Fig. 2B).
Fig. 2. Estimated effects of sound exposure on the behavior of narwhals.
(A) Estimated distribution of target depth (meters) of dives (N = 1001) during undisturbed behavior (black) and as an effect of trials (red) and intertrials (blue) at specific distances to the ship. The mean target depths of deep dives (component 3; see Materials and Methods) are given for undisturbed behavior (black) and for trials (red) and intertrials (blue) at 10 km distance to the ship. (B) The probability of initiating a dive of deep (solid line) or medium depth (dotted line) as a function of time (minutes) spent at the surface since the last dive during undisturbed behavior (black), trials (red), and intertrials (blue) at specific distances to the ship. The probabilities for initiating a dive within 5 min from the previous dive are given for undisturbed behavior (black) and for trials (red) and intertrials (blue) at 10 km distance to the ship. The top values in the panels are for medium dives (target depth < 350 m), and the bottom values for deep dives (< 350 m). (C) Estimated buzzing bout rate (1/min) per depth during undisturbed behavior (black), trials (red), and intertrials (blue) at specific distances to the ship. The buzzing bout rates at 150 m depth are given for undisturbed behavior and for trials (red) and intertrials (blue) at 10 km distance to the ship. The solid line is the estimate for a random whale and the shaded areas indicate the variation in the estimates among individual whales. The length of the lines in all panels represent the range of available data.
As for deep diving behavior, both sound exposure types negatively affected buzzing bout activity (Fig. 2C). Halving of the buzzing bout rate occurred concurrently with the halving of the target depth of deep dives about 10 km from the ship during trials and at 6 km distance from the ship during intertrials (Table 1).
The aspect of narwhal behavior that was least affected by sound exposure was the target depth of medium dives (20 to 350 m) (Table 1). The mean target depth of medium dives changed only slightly (< 10% compared with behavior without sound exposure) in the presence of either sound exposure type (Table 1 and Fig. 2A). Instead, because of a shift from deep diving to medium diving, the number of medium dives increased with increasing exposure level: For each 0.1 increase in the exposure level, narwhals had a 15 and 41% higher propensity of initiating a medium dive during trials and intertrials, respectively (fig. S1). This increase in medium diving activity could also be seen as a decrease in time spent near the surface between dives with increasing exposure level (Fig. 2B).
DISCUSSION
Both sound exposure types elicited clear responses in the narwhals. Narwhal dives can be coarsely split into three depth categories, in which the deepest dives and, to a lesser extent, medium dives are used for foraging (as indicated by the presence of buzzes), while shallow dives [<100 m; see (6)] are used for traveling and socializing (3, 4, 6, 31). When exposed to either sound type, the whales changed their deep diving behavior by decreasing their propensity of initiating a deep dive while simultaneously reducing the mean target depth. Because buzzing (foraging) is most prominent at depths of 300 to 600 m (4, 6), the change in diving behavior also led to a reduced buzzing activity. Instead of diving deep, narwhals increased the number of shallower dives during both sound exposure types, possibly to accommodate traveling to avoid the exposure (27).
Differences in responses between trial and intertrial exposures were more subtle than hypothesized. The presence of airgun pulses made little difference to the whales’ responses compared to the ship alone, which was unexpected. This may partly be explained by the very small contribution of HF-weighted airgun pulses to the overall sound levels during trials beyond ~3 km distance from the ship. Cetacean responses to human-made noise can be influenced by antipredator adaptations (32). Cessation of foraging (i.e., buzzing bouts), one of the main responses shown here during both trials and intertrials, likely reduces the whales’ risk of acoustically detected (33) and can therefore be considered an antipredator response (33, 34). Similarly, responses to both killer whales (32, 35) and the ship and airgun in another analysis of this dataset (27) led narwhals to head for shore and to be transiting as opposed to feeding. Narwhal responses to both types of sound exposures in this study thus resemble their reactions to a predator. This extreme sensitivity of narwhals to ship noise, with changes in whale behavior at distances of >80 km, was also described in (36).
The observed narwhal responses have two main physiological consequences. First, the reduction of foraging activity, as evidenced by shallower dives and reduced buzzing, will limit energy intake. Second, the shift from deep diving, which includes extended inexpensive periods of gliding (6), to shallower dives with more expensive periods of continuous swimming, will increase energy expenditure (37, 38). Furthermore, at close distance to the ship, the whales showed a higher propensity for initiating a dive after spending less time at the surface. This could leave the whales with the physiological consequences of insufficient dive recovery times.
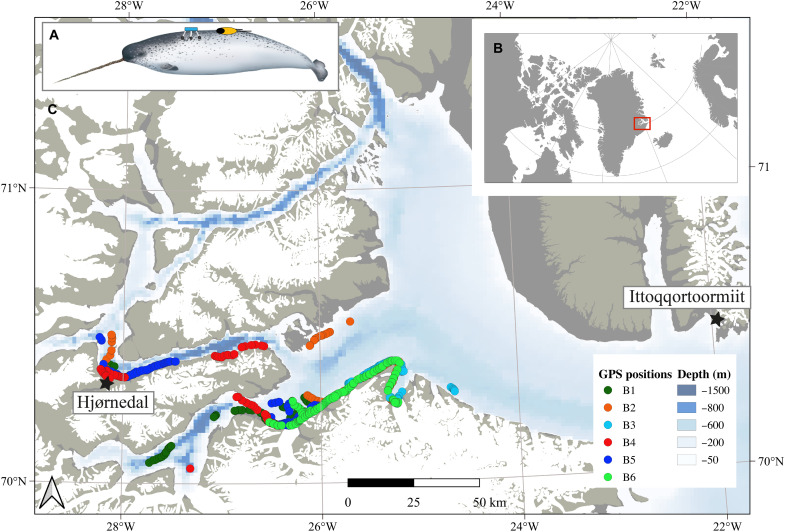
To ascribe behavioral responses to a disturbance, prior knowledge of normal, undisturbed behavior is needed. The Scoresby Sound narwhal population has been the target of an intensive ecological monitoring program for the past decade, providing a solid baseline for diving behavior, acoustic behavior, and movements (4, 6, 8, 28, 31, 39). These data, based on larger sample sizes and spanning several years, verify that our experimental setup captured undisturbed behavior before the start of sound exposure and confirmed that these measurements constitute a reliable baseline. The sound exposures occurred primarily in a deep area of the fjord system previously identified as a foraging area (Fig. 3) (4, 8, 28). This verified that the observed reductions in foraging-related buzzing activity and diving behavior were not due to natural variation between areas but were responses to the sound exposures. These areas are located in the center of the Scoresby Sound fjord system, which allowed the animals to move freely, instead of a “cul-de-sac” situation in which the response distance and duration may be restricted by land [see (27)]. The obvious challenge when observing free-ranging animals is the lack of control over the subjects. Because of this, the number, duration, and intensity of exposures varied among individuals [table S2; see also (27)]. In this study, three individuals provided most of the data for the close encounters (category, 0 to 5 km) (table S2), and the small sample size should be considered when assessing the results. In contrast, all individuals contributed to the data in the remaining distance categories of 5 to 40 km, strengthening the results at these distances. Exposure in this study was defined as the reciprocal of distance between whale and ship resulting in an increasing intensity of exposure with decreasing distance. That the responses of the whales were strongest close to the ship where the sound exposure levels (SELs) were highest supports our definition of exposure; however, other influencing factors cannot be ruled out. These include, for example, the duration of the disturbance, the direction of the disturbance, a whale’s behavioral state, whale’s sex (all whales in this study were males), local environmental conditions, and/or habituation [e.g., (40–42)].
Fig. 3. The study area with tracks of tagged narwhals.
(A) Narwhals were instrumented with backpack-mounted Fastloc GPS receivers (light blue instrument) and suction cup attached Acousonde behavioral tags (black and yellow instrument) (B) in Scoresby Sound fjord in East Greenland. The tagging took place at Hjørnedal field station (marked with a black star). The different colored dots illustrate the tracks of the six male narwhals (table S1) during sound exposure (trials and intertrials). Narwhal illustration by U. Gorter.
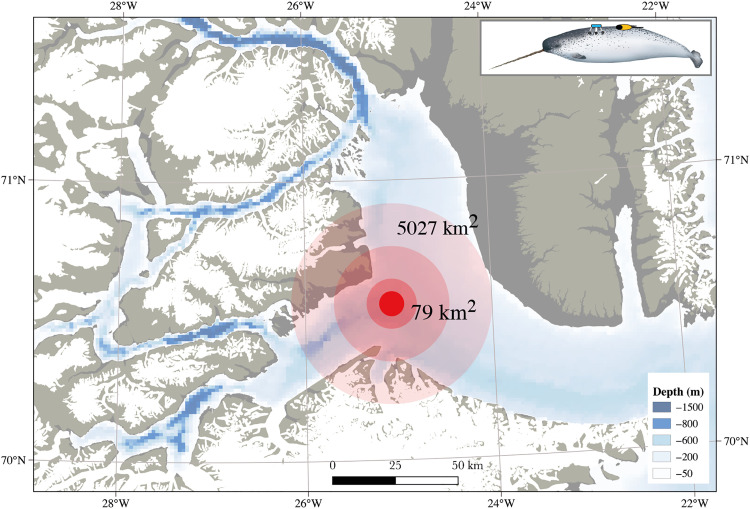
As reported previously (30), narwhals reacted to ship noise with an active airgun at sound levels below background levels. Our results show that the HF-weighted levels of both sound exposure types reached ambient noise levels between ~2 and ~4 km from the ship, meaning that most of the behavioral changes, including changes in diving behavior, in response to either sound disturbance type occurred at received levels below background. In addition, changes in diving behavior could be detected as far as 40 km from the ship, which, in the Scoresby Sound fjord, corresponds to an affected area of >5000 km2 (Fig. 4). Considering that the smallest known narwhal summer stocks in Greenland have habitat sizes of <3000 km2 (43), our results demonstrate how a relatively low-amplitude, short-term disturbance can potentially affect the entire spatial range of a local population. Thus, the sensitivity of narwhals to such a disturbance combined with their limited plasticity in niche selection and high site fidelity makes this species less resilient to human activities. If healthy narwhal populations are to be maintained, then it will be increasingly important to carefully manage human activities in the Arctic. This could include prohibition of noise-generating human activities within important narwhal habitats.
Fig. 4. Spatial extent of sound disturbance.
The effect of sound disturbance from ship noise during intertrials (ship noise only) on diving behavior of narwhals (specifically, the mean target depth of deep dives), converted to area (based on values from Table 1). The light red–to–dark red color progression illustrates the incremental loss of normal behavior; the darkest red area (79 km2) closest to the ship shows where diving behavior has decreased by 75% compared with undisturbed behavior. The effect of ship sound on the diving behavior could be detected 40 km from the ship in an open-water situation, which could lead to a total affected area of 5027 km2. Note that the size of the affected areas depends on the sound speed profile and physical acoustics of the exposed environment. Narwhal illustration by U. Gorter.
MATERIALS AND METHODS
Experimental design and data collection
Live capture of narwhals was carried out from a field station at Hjørnedal in the Scoresby Sound fjord system in East Greenland in collaboration with local hunters (Fig. 3 and tables S1 and S2). The total time from capture in net to release was, on average, 50 min (SD, 22 min) [see (10, 27) for details]. Instrumentation of captured whales lasted, on average, 13 min (SD, 2 min) and was conducted near the shore by four to six persons in survival suits standing next to the whale. Sex of the whales was determined on the basis of the presence (male) or absence (female) of a tusk. Permission for capturing, handling, and tagging of narwhals was provided by the government of Greenland (case ID 2010 ± 035453, document number 429 926). The project was reviewed and approved by the Institutional Animal Care and Use Committee of the University of Copenhagen (17 June 2015). Access and permits to use land facilities in Scoresby Sound were provided by the government of Greenland. No protected species were sampled.
Each study animal was equipped with a Wildlife Computers (Redmond, Seattle, WA, USA) Fastloc GPS receiver and an Acousonde acoustic and orientation recorder (www.acousonde.com). The Fastloc GPS receiver was mounted on the whale’s back, following instrumentation techniques used in similar studies in Canada and West Greenland (44, 45), and was programmed to collect an unrestricted number of Fastloc GPS locations (snapshots) through August. The Fastloc snapshots were transmitted to and relayed through the Argos Location and Data Collection System (Argos-System.cls.fr). Postprocessing of GPS positions was conducted through the Wildlife Computers web portal. Fastloc GPS is a GPS positioning system with the ability to acquire animal positions faster than traditional GPS (29, 46). The Acousonde recorder was mounted on the posterior half of the animal, lateral to the dorsal ridge following the protocol described in (4). The Acousonde float had been modified to accommodate an Argos transmitter (Wildlife Computers SPOT5) in addition to a very high frequency (VHF) transmitter (Advanced Telemetry Systems Inc.) to enable relocation of the tag after release from the whale. Acoustic data were collected with a 25,811-Hz sampling rate providing continuous recordings with 16-bit resolution (HTI-96-MIN hydrophone with a nominal sensitivity of −201 dB re 1 V/μ Pa, a preamp gain of 14 dB, and an anti-aliasing low-pass filter with a 3-dB reduction at 9.2 kHz and a 22-dB reduction at 11.1 kHz). Depth was recorded at a 10-Hz sampling rate.
Sound exposure trials and intertrials were conducted from the offshore patrol vessel HDMS Lauge Koch towing an airgun cluster of two Sercel G-guns (1040 in3 in total) at 6 m depth. During trials, the airguns were fired synchronously every 12 or 80 s, and the ship’s GPS navigation system recorded the location of every shot. Intertrials involved the ship alone. The vessel also included a multibeam echo sounder (MBES), used for mapping the seafloor, which was operating continuously [see (27) for details on MBES]. The acoustic data from the floating autonomous SoundTrap ST202 recorders (ST, Ocean Instruments) deployed at 10 m depth were used to record airgun pulses, ship noise, and background noise levels. The ST202 has a flat frequency response from 20 Hz to 60 kHz (± 3 dB) and internal storage of 256 GB. The sampling rate used during these deployments was 96 kHz.
Data analysis
Sound exposure levels
Airgun pulses were analyzed using the 90% energy approach (47, 48) to define the duration of each pulse, over which the SEL (in dB re 1 μPa2·s) was computed. To provide better estimates of levels perceived by the whales, the data were weighted with a function appropriate for HF cetaceans (49). Received HF-weighted pulse SELs were calculated over the same pulse durations as obtained during the analysis of the unweighted data. Background levels (unweighted and weighted) were subtracted from all pulse measurements, using a 1-s sample selected 3 s before the onset of each pulse.
Ship sound levels during intertrials, as a function of distance to ship, were quantified using HF-weighted sound pressure levels analyzed over 1 s every 12 or 80 s [the firing rates of the airguns; see (27)]. To demonstrate the range of ship sound levels received by the whales, the highest-amplitude and lowest-amplitude passes by the ship were selected [cf. figure 6 of (27)], and the data from each pass were fitted with a logarithmic propagation model (Fig. 1A).
To quantify the average contribution of the airgun pulses to the total sound received by the whale’s ear (as SELs), we summed (in the linear domain) each analyzed airgun pulse value [n = 169; cf. figure 5A of (27)] with the ship sound or ambient sound value at the corresponding distance (see Fig. 1A), after adjusting for the duration of the airgun pulse. This sum was then converted back to decibels. The contribution (in decibels) of the airgun pulses to the total received sound levels was the difference between the total summed value and the ship/background value.
Analyses of behavioral records
Depth measurements (meters) of an animal were down-sampled to 1 Hz and denoted with the distance between the animal and the ship [kilometers; shortest natural distance (avoiding land)] using the whale’s GPS tracks (consisting of GPS hits and additional positions created between subsequent GPS positions through linear interpolation) and the ship’s GPS log. Line-of-sight periods (no land between the ship and whale) were determined visually from maps showing ship and whale positions aligned in time [maps drawn with Scoresby Sound coastline from (50)]. When the ship and whale were in line of sight, the exposure was defined as the reciprocal of the distance between a tagged whale and the ship (kilometers), resulting in a higher exposure with decreasing distance to the ship. Exposure was denoted zero when the ship and the animal were not within line of sight. For each individual, the time-depth data series were further divided into dives. A dive was defined as a continuous period during which the whale reached >20 m in depth. Periods with depths of <20 m were denoted surface. Each dive was denoted with its target depth (meters; the maximum depth of a dive), duration (minutes), and exposure values (1/km at the onset of each dive).
Statistical analysis
Undisturbed behavior of the whales was estimated using behavioral data before sound exposure, excluding the first 24 hours of each record to avoid possible effects of tagging (4, 51). The periods with zero exposure in between intertrials and trials were excluded from all analyses except the Cox survival model that investigated the “dive initiation rate” (see below) to avoid breaking the time dependence used in the model.
Effect of sound exposures on the dive target depth
The target depth of dives performed by narwhals had a trimodal distribution, with local maxima values of 5, 30, and 400 m representing the surface (component 1), medium dives (component 2), and deep dives (component 3), respectively. To analyze how target depth was affected by the two sound exposure types, the log of the target depth was fitted to a mixture of regression models, using the routine flexmix in R (52) [package flexmix; (53)]. Whale ID (allowing for a whale ID–specific intercept) and the two exposure types were entered as explanatory variables. A mixture model assumes that each observation comes from one of K regression models with certain probabilities. Because of the trimodal distribution of the response variable (log of target depth), we used K = 3. We also fitted models with K > 3 that had better Akaike information criterion (AIC) scores than the model with K = 3. The estimates from K > 3 models were, however, not relevant because additional components only fitted to a few outlier observations. The likelihood function is not convex and therefore might include multiple local maxima, which is a known problem in mixture models. The fitting procedure performs a random search, and it was run 20 times, and the run with the maximal log-likelihood value outcome was selected. The number of runs that ended up with the same maximum was also counted, and the parameter estimates of these runs were compared to evaluate the robustness of the model. Of these 20 model runs, 4 runs ended up with the same maximum (probably the global maximum) and had estimates that were equal to three significant digits, which indicates high robustness.
Effect of sound exposures on dive initiation
Dives were categorized as either deep dives (target depth > 350 m) or medium dives (20 m < target depth ≤ 350 m) (6). When a dive is initiated, it can either be a deep or a medium dive, and the dive types can therefore be defined as competing risks used in survival analysis. The time at the surface (≤ 20 m) between dives can be seen as a survival time. The effect of sound disturbance on the propensity of an individual to initiate a dive within a given time since the previous dive was analyzed using two cause-specific Cox models [coxph, package survival; (54)]. Each model included time spent at the surface (≤ 20 m) before initiating a specific dive type (either deep or medium) as the response. The other dive type, deep or medium, that was not used as the response variable was entered as a censoring variable. Covariates were exposure and whale ID. The same model was also run with whale ID as a random effect that resulted in the same outcome, indicating robustness of the chosen method. The results are given as hazard ratios that indicate a proportional change in the propensity of initiating a dive of a given type per 0.1 increase in exposure compared with undisturbed behavior (fig. S1). The cumulative incidence function, which is the probability that a dive of a given type will occur within a given time, was also calculated for each dive type for different distances from the ship (Fig. 2B).
Effect of sound exposures on buzzing bout rate
A previous study on narwhal buzzing behavior showed that the time series of buzzing activity in narwhals is temporally autocorrelated, and, if this is not considered, then effect estimates will be biased (39). An autoregressive memory component was therefore used, and the model with a memory component of length of 60 s was selected by the Bayesian information criterion (BIC) criterion. We thus defined a buzzing bout as a series of buzzes with maximum of 60 s between consecutive buzzes. Using this definition, the median number of buzzes in a bout was six buzzes. Depth was included as an explanatory variable using natural cubic splines with 3 df (ns, package splines). The autoregressive component and depth were fitted to data before the ship’s arrival with a generalized linear model (glm, package stats) with a Poisson response distribution with a log link. The effects of the two exposure types on the buzzing bout rate (the initiation of a buzzing bout at 1-s time bins) were modeled using a generalized linear mixed model [glmer, package lme4; (55)] with a Poisson response distribution with a log link. The fitted values from the generalized linear model were included as an offset. The exposures were entered nonlinearly as explanatory variables using natural cubic splines with 3 df (ns, package splines) with internal knots located at the 33rd and 66th percentiles of the nonzero exposure values. Whale ID was included as a random effect.
Acknowledgments
We thank the hunters from Ittoqqortoormiit and research assistants in Hjørnedal for assistance in catching the whales and deploying and collecting instruments. P. Trinhammer, L. M. Rasmussen, and A. S. Madsen, Aarhus University, are thanked for assistance with seismic operations onboard HDMS Lauge Koch. The crew of HDMS Lauge Koch are acknowledged for skillful navigation in Scoresby Sound. Special thanks to U. Gorter for narwhal illustration. L. Quakenbush and C. W. Clark are thanked for insightful comments that greatly improved the manuscript.
Funding: This study is part of the Northeast Greenland Environmental Study Program, which is a collaboration between DCE - Danish Centre for Environment and Energy at Aarhus University, the Greenland Institute of Natural Resources, and the Environmental Agency for Mineral Resource Activities of the government of Greenland. This work was supported by Carlsberg Foundation, 2013_01_0289 and CF14_0169 (M.P.H.-J.); the Danish Cooperation for the Environment in the Arctic (DANCEA) (M.P.H.-J.); Greenland Institute of Natural Resources (O.M.T., E.G., R.G.H., and M.P.H.-J.); Novo Nordisk Foundation, NNF20OC0062958 (S.D.); and Independent Research Fund Denmark, Natural Sciences, 9040-00215B (S.D.).
Author contributions: Conceptualization: O.M.T., S.B.B., and M.P.H.-J. Methodology: O.M.T., S.B.B., S.D., A.L.S., and A.S.C. Investigation: O.M.T., S.B.B., E.G., R.G.H., and M.P.H.-J. Visualization: O.M.T., S.B.B., and A.L.S. Writing—original draft: O.M.T. Writing—review and editing: O.M.T., S.B.B., S.D., A.S., A.S.C., E.G., R.G.H., and M.P.H.-J.
Competing interests: S.B.B. and A.S.C. were employed by Greeneridge Sciences Inc. that manufactures the Acousonde behavioral tags used in this study. The other authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials and in the Dryad dataset, Tervo et al. (2023), Estimation of changes in narwhal behavior, https://doi.org/10.5061/dryad.8gtht76tq.
Supplementary Materials
This PDF file includes:
Fig. S1
Tables S1 and S2
REFERENCES AND NOTES
- 1.R. D. Holt, Bringing the Hutchinsonian niche into the 21st century: Ecological and evolutionary perspectives. Proc. Natl. Acad. Sci. U.S.A. 106, 19659–19665 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.J. J. Wiens, C. H. Graham, Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 36, 519–539 (2005). [Google Scholar]
- 3.K. L. Laidre, M. P. Heide-Jørgensen, Winter feeding intensity of narwhals (Monodon monoceros). Mar. Mam. Sci. 21, 45–57 (2005). [Google Scholar]
- 4.S. B. Blackwell, O. M. Tervo, A. S. Conrad, M.-H. S. Sinding, R. G. Hansen, S. Ditlevsen, M. P. Heide-Jørgensen, Spatial and temporal patterns of sound production in East Greenland narwhals. PLOS ONE 13, e0198295 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.K. A. Kenyon, D. J. Yurkowski, J. Orr, D. Barber, S. H. Ferguson, Baffin Bay narwhal (Monodon monoceros) select bathymetry over sea ice during winter. Pol. Biol. 41, 2053–2063 (2018). [Google Scholar]
- 6.O. M. Tervo, S. Ditlevsen, M. C. Ngô, N. H. Nielsen, S. B. Blackwell, T. M. Williams, M. P. Heide-Jørgensen, Hunting by the stroke: How foraging drives diving behavior and locomotion of East-Greenland narwhals (Monodon monoceros). Front. Mar. Sci. 7, 596469 (2021). [Google Scholar]
- 7.P. Chambault, O. M. Tervo, E. Garde, R. G. Hansen, S. B. Blackwell, T. M. Williams, R. Dietz, C. M. Albertsen, K. L. Laidre, N. H. Nielsen, P. Richard, M.-H. S. Sinding, H. C. Schmidt, M. P. Heide-Jørgensen, The impact of rising sea temperatures on an Arctic top predator, the narwhal. Nat. Sci. Rep. 10, 18678 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.M. P. Heide-Jørgensen, S. B. Blackwell, T. M. Williams, M.-H. S. Sinding, M. Skovrind, O. M. Tervo, E. Garde, R. G. Hansen, S. Ditlevsen, Some like it cold: Temperature dependent habitat selection by narwhals. Ecol. Evol. 10, 8073–8090 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.M. P. Heide-Jørgensen, P. Richard, R. Dietz, K. L. Laidre, A metapopulation model for Canadian and West Greenland narwhals. Anim. Conserv. 16, 331–343 (2013). [Google Scholar]
- 10.M. P. Heide-Jørgensen, N. H. Nielsen, R. G. Hansen, H. C. Schmidt, S. B. Blackwell, O. Jørgensen, The predictable narwhal: Satellite tracking shows behavioural similarities between isolated subpopulations. J. Zool. 297, 54–65 (2015). [Google Scholar]
- 11.M. Louis, M. Skovrind, J. A. S. Castruita, C. Garilao, K. Kaschner, S. Gopalakrishnan, J. S. Haile, C. Lydersen, K. M. Kovacs, E. Garde, M. P. Heide-Jørgensen, L. Postma, S. H. Ferguson, E. Willerslev, E. D. Lorenzen, Influence of past climate change on phylogeography and demographic history of narwhals, Monodon monoceros. Proc. Biol. Sci. 287, 20192964 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.M. Previdi, K. L. Smith, L. M. Polvani, Arctic amplification of climate change: A review of underlying mechanisms. Environ. Res. Lett. 16, 093003 (2021). [Google Scholar]
- 13.S. E. Moore, R. R. Reeves, B. L. Southall, T. J. Ragen, R. Suydam, C. W. Clark, A new framework for assessing the effects of anthropogenic sound on marine mammals in a rapidly changing. BioScience 62, 289–295 (2012). [Google Scholar]
- 14.Underwater Noise Pollution from Shipping in the Arctic, Protection of the Arctic Marine Environment Working Group (PAME) (2021); https://oaarchive.arctic-council.org/bitstream/handle/11374/2564/PAME%20underwater%20noise%20report_low_res.pdf?sequence=5&isAllowed=y.
- 15.W. D. Halliday, D. Barclayd, A. N. Barkleye, E. Cook, J. Dawson, R. C. Hilliard, N. E. Hussey, J. M. Jones, F. Juanes, M. Marcoux, A. Niemi, S. Nudds, M. K. Pine, C. Richards, K. Scharffenberg, K. Westdal, S. J. Insleyac, Underwater sound levels in the Canadian Arctic, 2014–2019. Mar. Pollut. Bull. 168, 112437 (2021). [DOI] [PubMed] [Google Scholar]
- 16.C. M. Duarte, L. Chapuis, S. P. Collin, D. P. Costa, R. P. Devassy, V. M. Eguiluz, C. Erbe, A. C. Gordon, B. S. Halpern, H. R. Harding, M. N. Havlik, M. Meekan, N. D. Merchant, J. L. Miksis-Olds, M. Parsons, M. Predragovic, A. N. Radford, C. A. Radford, S. D. Simpson, H. Slabbekoorn, E. Staaterman, I. C. Van Opzeeland, J. Winderen, X. Zhang, F. Juanes, The soundscape of the Anthropocene ocean. Science 371, eaba4658 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Arctic Marine Shipping Assessment (AMSA) report. Arctic Council (2009); www.pame.is/index.php/projects/arctic-marine-shipping/amsa.
- 18.R. R. Reeves, P. J. Ewins, S. Agbayanic, M. P. Heide-Jørgensen, K. M. Kovacs, C. Lydersen, R. Suydam, W. Elliott, G. Polet, Y. van Dijk, R. Blijleveni, Distribution of endemic cetaceans in relation to hydrocarbon development and commercial shipping in a warming Arctic. Mar. Policy 44, 375–389 (2014). [Google Scholar]
- 19.L. C. Smith, S. R. Stephenson, New Trans-Arctic shipping routes navigable by midcentury. Proc. Natl. Acad. Sci. U.S.A. 110, E1191–E1195 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.J. E. Overland, M. Wang, When will the summer Arctic be nearly sea ice free? Geophys. Res. Lett. 40, 2097–2101 (2013). [Google Scholar]
- 21.L. Lowry, K. L. Laidre, R. Reeves, Monodon monoceros. The IUCN Red List of Threatened Species 2017: e.T13704A50367651 (2017); 10.2305/IUCN.UK.2017-3.RLTS.T13704A50367651.en [accessed on 15 June 2022]. [DOI]
- 22.M. P. Heide-Jørgensen, E. Garde, R. G. Hansen, O. M. Tervo, M.-H. S. Sinding, L. Witting, M. Marcoux, C. Watt, K. M. Kovacs, R. R. Reeves, Narwhals require targeted conservation. Science 370, 416 (2020). [DOI] [PubMed] [Google Scholar]
- 23.E. Garde, O. M. Tervo, M.-H. S. Sinding, N. H. Nielsen, C. Cornett, M. P. Heide-Jørgensen, Biological parameters in a declining population of narwhals (Monodon monoceros) in Scoresby Sound, southeast Greenland. Arctic Sci. 8, 329–348 (2022). [Google Scholar]
- 24.R. E. A. Stewart, V. Lesage, J. W. Lawson, H. Cleator, K. A. Martin, “Science technical review of the draft Environmental Impact Statement (EIS) for Baffinland’s Mary River Project” (Canadian Science Advisory Secretariat, Fisheries and Oceans Canada, 2011).
- 25.D. D. W. Hauser, K. L. Laidre, H. L. Stern, Vulnerability of Arctic marine mammals to vessel traffic in the increasingly ice-free Northwest Passage and Northern Sea Route. Proc. Natl. Acad. Sci. U.S.A. 115, 7617–7622 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Z. Kochanowicz, J. Dawson, W. D. Halliday, M. Sawada, L. Copland, N. A. Carter, A. Nicolla, S. H. Ferguson, M. P. Heide-Jørgensen, M. Marcoux, C. Watt, D. J. Yurkowski, Using western science and Inuit knowledge to model ship-source noise exposure for cetaceans (marine mammals) in Tallurutiup Imanga (Lancaster Sound), Nunavut, Canada. Mar. Policy 130, 104557 (2021). [Google Scholar]
- 27.M. P. Heide-Jørgensen, S. B. Blackwell, O. M. Tervo, A. L. Samson, E. Garde, R. G. Hansen, M. C. Ngô, A. S. Conrad, P. Trinhammer, H. C. Schmidt, M.-H. S. Sinding, T. M. Williams, S. Ditlevsen, Behavioral response study on seismic airgun and vessel exposures in narwhals. Front. Mar. Sci. 8, 658173 (2021). [Google Scholar]
- 28.M. P. Heide-Jørgensen, N. H. Nielsen, R. G. Hansen, S. B. Blackwell, Stomach temperature of narwhals (Monodon monoceros) during feeding events. Anim. Biotelem. 2, 9 (2014). [Google Scholar]
- 29.E. Bryant, “2D Location accuracy statistics for Fastloc cores running firmware versions 2.2 & 2.3” (Wildtrack Telemetry Systems Ltd., 2007).
- 30.O. M. Tervo, S. B. Blackwell, S. Ditlevsen, A. S. Conrad, A. L. Samson, E. Garde, R. G. Hansen, M. P. Heide-Jørgensen, Narwhals react to ship noise and airgun pulses embedded in background noise. Biol. Lett. 17, 20210220 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.M. C. Ngô, M. P. Heide-Jørgensen, S. Ditlevsen, Understanding narwhal diving behaviour using hidden Markov models with dependent state distributions and long range dependence. PLOS Comput. biol. 15, e1006425 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.G. A. Breed, C. J. D. Matthews, M. Marcoux, J. W. Higdon, B. LeBlanc, S. D. Petersen, J. Orr, N. R. Reinhart, S. H. Ferguson, Sustained disruption of narwhal habitat use and behavior in the presence of Arctic killer whales. Proc. Natl. Acad. Sci. U.S.A. 114, 2628–2633 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.P. M. Miller, S. Isojunno, E. Siegal, F.-P. A. Lam, P. Kvadsheim, C. Cure, Behavioral responses to predatory sounds predict sensitivity of cetaceans to anthropogenic noise within a soundscape of fear. Proc. Natl. Acad. Sci. U.S.A. 119, e2114932119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.N. A. de Soto, F. Visser, P. L. Tyack, J. Alcazar, G. Ruxton, P. Arranz, P. T. Madsen, M. Johnson, Fear of killer whales drives extreme synchrony in deep diving beaked whales. Sci. Rep. 10, 13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.K. L. Laidre, M. P. Heide-Jørgensen, J. R. Orr, Reactions of narwhals, Monodon monoceros, to killer whale, Orcinus orca, attacks in the Eastern Canadian Arctic. Can. Field Nat. 120, 457–465 (2006). [Google Scholar]
- 36.K. J. Finley, G. W. Miller, R. A. Davis, C. R. Greene, Reactions of belugas, Delphinapterus leucas and narwhals, Monodon monoceros, to ice-breaking ships in the Canadian high Arctic. Can. Bull. Fish. Aquat. Sci. 224, 97–117 (1990). [Google Scholar]
- 37.T. M. Williams, T. L. Kendall, B. P. Richter, C. R. Ribeiro-French, J. S. John, K. L. Odell, B. A. Losch, D. A. Feuerbach, M. A. Stamper, Swimming and diving energetics in dolphins: A stroke-by-stroke analysis for predicting the cost of flight responses in wild odontocetes. J. Exp. Biol. 220, 1135–1145 (2017). [DOI] [PubMed] [Google Scholar]
- 38.T. M. Williams, S. B. Blackwell, O. Tervo, E. Garde, M.-H. S. Sinding, B. Richter, M. P. Heide-Jørgensen, Physiological responses of narwhals to anthropogenic noise: A case study with seismic airguns and vessel traffic in the Arctic. Funct. Ecol. 36, 2251–2266 (2022). [Google Scholar]
- 39.A. Søltoft-Jensen, M. P. Heide-Jørgensen, S. Ditlevsen, Modelling the sound production of narwhals using a point process framework with memory effect. Ann. Appl. Stat. 14, 2037–2052 (2020). [Google Scholar]
- 40.W. J. Richardson, C. R. Greene Jr, C. I. Malme, D. H. Thomson, Marine Mammals and Noise (Academic Press, 2013). [Google Scholar]
- 41.D. Wartzok, A. N. Popper, J. Gordon, J. Merrill, Factors affecting the responses of marine mammals to acoustic disturbance. Mar. Tech. Soc. J. 37, 6–15 (2003). [Google Scholar]
- 42.W. T. Ellison, B. L. Southall, C. W. Clark, A. S. Frankel, A new context-based approach to assess marine mammal behavioral responses to anthropogenic sounds. Conserv. Biol. 26, 21–28 (2012). [DOI] [PubMed] [Google Scholar]
- 43.M. P. Heide-Jørgensen, K. L. Laidre, M. L. Burt, D. L. Borchers, T. A. Marques, R. G. Hansen, M. Rasmussen, S. Fossette, Abundance of narwhals (Monodon monoceros) on the hunting grounds in Greenland. J. Mammal. 91, 1135–1151 (2010). [Google Scholar]
- 44.M. P. Heide-Jørgensen, R. Dietz, K. L. Laidre, P. Richard, J. Orr, H. C. Schmidt, The migratory behaviour of narwhals (Monodon monoceros). Can. J. Zool. 81, 1298–1305 (2003). [Google Scholar]
- 45.R. Dietz, M. P. Heide-Jørgensen, P. Richard, J. Orr, K. Laidre, H. C. Schmidt, Movements of narwhals (Monodon monoceros) from Admiralty Inlet monitored by satellite telemetry. Polar Biol. 31, 1295–1306 (2008). [Google Scholar]
- 46.S. M. Tomkiewicz, M. R. Fuller, J. G. Kie, K. K. Bates, Global positioning system and associated technologies in animal behaviour and ecological research. Philos. Trans. R. Soc. B Biol. Sci. 365, 2163–2176 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.R. D. McCauley, J. Fewtrell, A. J. Duncan, C. Jenner, M.-N. Jenner, J. D. Penrose, R. I. T. Prince, A. Adhiyta, J. Murdoch, K. McCabe, Marine seismic surveys—A study of environmental implications. APPEA J. 40, 692–706 (2000). [Google Scholar]
- 48.S. B. Blackwell, J. W. Lawson, M. T. Williams, Tolerance by ringed seals (Phoca hispida) to impact pipe-driving and construction sounds at an oil production island. J. Acoust. Soc. Am. 115, 2346–2357 (2004). [DOI] [PubMed] [Google Scholar]
- 49.B. L. Southall, J. J. Finneran, C. Reichmuth, P. E. Nachtigall, D. R. Ketten, A. E. Bowles, W. T. Ellison, D. P. Nowacek, P. L. Tyack, Marine mammal noise exposure criteria: Updated scientific recommendations for residual hearing effects. Aquat. Mamm. 45, 125–232 (2019). [Google Scholar]
- 50.H. F. Jepsen, J. R. Ineson, N. Mikkelsen, S. Piasecki, F. Platen-Hallermund, F. Schjøth, Kortlægning. Danmarks og Grønlands Geologiske Undersøgelse Rapport 2005/28 (2005), p. 50.
- 51.C. R. Shuert, M. Marcoux, N. E. Hussey, C. A. Watt, M. Auger-Méthé, Assessing the post-release effects of capture, handling and placement of satellite telemetry devices on narwhal (Monodon monoceros) movement behaviour. Conserv. Physiol. 9, coaa128 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.R Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2019); www.R-project.org/.
- 53.B. Gruen, F. Leisch, FlexMix Version 2: Finite mixtures with concomitant variables and varying and constant parameters. J. Stat. Soft. 28, 1–35 (2008). [Google Scholar]
- 54.T. Therneau, A Package for Survival Analysis in S, version 2.38 (2015); https://CRAN.R-project.org/package=survival.
- 55.D. Bates, M. Maechler, B. Bolker, S. Walker, Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1–48 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1
Tables S1 and S2