Abstract
Secular variations in the major ion chemistry and isotopic composition of seawater on multimillion-year time scales are well documented, but the causes of these changes are debated. Fluid inclusions in marine halite indicate that the Li concentration in seawater [Li+]SW declined sevenfold over the past 150 million years (Ma) from ~184 μmol/kg H2O at 150 Ma ago to 27 μmol/kg H2O today. Modeling of the lithium geochemical cycle shows that the decrease in [Li+]SW was controlled chiefly by long-term decreases in ocean crust production rates and mid-ocean ridge and ridge flank hydrothermal fluxes without requiring changes in continental weathering fluxes. The decrease in [Li+]SW parallels the 150 Ma increase in seawater Mg2+/Ca2+ and 87Sr/86Sr, and the change from calcite to aragonite seas, KCl to MgSO4 evaporites, and greenhouse to icehouse climates, all of which point to the importance of plate tectonic activity in regulating the composition of Earth’s hydrosphere and atmosphere.
Declining hydrothermal fluxes drove a 7-fold decrease in seawater [Li+] over the last 150 Ma.
INTRODUCTION
The major ion (Mg2+, Ca2+, Na+, K+, SO42−, Cl−, HCO3−) (1) and isotopic composition (i.e., δ7Li, 87Sr/86Sr) (2, 3) of seawater have varied in the Phanerozoic Eon. Systematic changes in the Mg2+, Ca2+, and SO42− concentrations in Phanerozoic seawater regulate the alternating MgSO4 and KCl mineralogies of marine potash evaporites (4). Variations in the Mg2+ and Ca2+ concentrations may have influenced the evolution of marine shell-building organisms, including dominant reef-builders and skeletal algae, because the Mg2+/Ca2+ ratio of seawater controls which carbonate, calcite, or aragonite precipitates during so-called calcite seas and aragonite seas (5). The Mg2+/Ca2+ ratio of seawater from the Jurassic to the present closely matches the marine 87Sr/86Sr curve, and both show a sharp rise in the Cenozoic similar to that exhibited by the marine lithium isotope (δ7Li) record from 60 to 0 million years (Ma) ago (Fig. 1) (3, 6, 7). Long-term changes in the major ion chemistry of seawater broadly correlate with variations in atmospheric CO2 and icehouse-greenhouse climate, suggesting links to the carbon cycle (8).
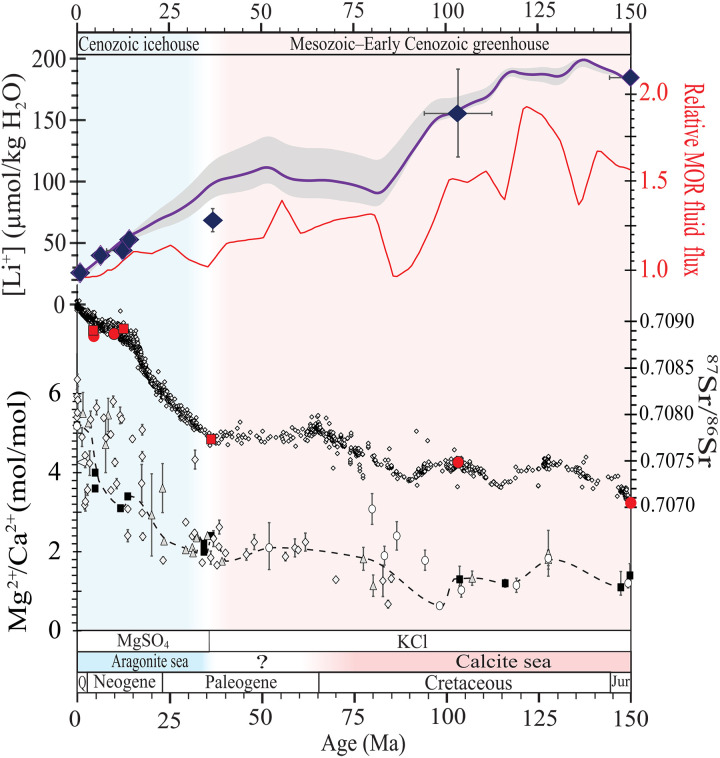
Fig. 1. Seawater [Li+] over the past 150 Ma compared to MOR fluid flux and other seawater records.
From top to bottom: seawater [Li+] from fluid inclusions in halite, global mean MOR fluid flux (38), 87Sr/86Sr from marine skeletal carbonates (2), Mg2+/Ca2+ from fluid inclusions in halite (black squares) (7), skeletal carbonates (ovals) (66), calcite veins (triangles) (67), and corals (diamonds) (68), MgSO4 and KCl evaporites (4), calcite and aragonite seas (5), and greenhouse (peach) and icehouse (blue) climates (37). Indigo curve shows the modeling results for [Li+]SW. Gray shading around the modeled [Li+]SW curve shows the uncertainty from high-temperature axial (MOR) and low-temperature off-axis (AOC) hydrothermal fluid flux estimates. Error bars are the uncertainty of the data (minimum/maximum for [Li+]SW and Mg2+/Ca2+). Red circles show 87Sr/86Sr values from fluid inclusions in marine halite (table S2) (30); red squares show 87Sr/86Sr values in sulfates interbedded with halite (31–33).
Long-term variations in seawater composition are now well documented, but there is disagreement over which input or output fluxes were responsible for those changes. Proposed controls of temporal changes in seawater chemistry include variations in the rates of (i) ocean crust production and mid-ocean ridge (MOR) hydrothermal discharge (4), (ii) river flux (RIV) and terrestrial weathering intensity (9), (iii) deposition of marine carbonates and dolomitization (10), (iv) low-temperature, off-axis alteration of oceanic crust (AOC) (11), and (v) formation of marine authigenic aluminosilicate clays (MAAC) (12). AOC and MAAC together can be considered reverse weathering (3).
Recent advances in laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) now allow quantitative measurement of minor and trace elements in fluid inclusions in halite, including lithium (Li+) (13). Li+, a trace element in seawater, is particularly useful for understanding the drivers of long-term changes in seawater chemistry. It is a conservative element not incorporated to any significant degree in minerals precipitated from evaporating seawater, such as calcite, aragonite, gypsum, or halite (fig. S1). Li+ is entirely derived from terrestrial silicate mineral weathering and seafloor-basalt interactions with hydrothermal fluids at MORs, and its concentration in seawater is not significantly influenced by biological processes or precipitation of carbonates (3). Li+ today is supplied to the oceans by MOR hydrothermal fluids (FMOR = 13.7 ± 2.8 × 109 mol/year, 58 ± 12% of global input flux) and rivers (FRIV = 9.9 × 109 mol/year, ~42%) (14, 15). [Li+] in MOR hydrothermal fluids are three orders of magnitude higher than rivers (457 ± 66 versus 0.265 μmol/kg of H2O, here termed μmolal) and ~17 ± 3 times that of seawater (27 μmolal) (15–17). Small fluctuations in ocean crust production rates and hydrothermal fluxes could therefore change seawater Li+ ([Li+]SW). Li+ is removed from seawater during low-temperature (off-axis) AOC (FAOC = 2 to 21 × 109 mol/year) (18, 19) and MAAC formation (FMAAC = 11 to 20 × 109 mol/year) (3, 20).
Recent records of δ7LiSW in Cenozoic seawater from foraminifera (3) and brachiopods (21) show an increase of ∼8 to 9‰ over the past 60 Ma, which is thought to be due to (i) increased tectonic uplift that increased continental clay mineral formation (3), (ii) increased diagenetic reactions of marine clays (20), (iii) increased land surface reactivity and erosion (22), (iv) increased Li isotopic fractionation between seawater and oceanic crust (18), and (v) decreased continental soil formation (23). Modeling and interpretation of the Cenozoic δ7LiSW record, however, are based on the assumption that the [Li+]SW has been roughly constant (3, 18, 22, 23). Li and West (20) tested 12 model scenarios to explain the Cenozoic δ7LiSW record and suggested that knowledge of [Li+] in ancient seawater would help distinguish the major controls responsible for changes in the global Li cycle. Here, we show from measurements of fluid inclusions in marine halite that [Li+]SW in Jurassic and Cretaceous seawater was up to seven times greater than in modern seawater. Such variations are not included in any past modeling of the global geochemical cycle of Li reconstructed from Li isotopes. We present a 150 Ma record of [Li+]SW and test the hypothesis that the decrease of [Li+]SW since the Jurassic was produced by a decline of MOR and ridge flank hydrothermal fluxes.
RESULTS AND DISCUSSION
Li+/Mg2+ ratios in fluid inclusions
A total of 639 fluid inclusions along growth bands (fig. S2) in 65 halite samples (seven ages from 13 Mesozoic and Cenozoic marine evaporite basins; fig. S3) were analyzed using LA-ICP-MS to obtain Li+/Mg2+ ratios (Fig. 2, Table 1, table S1, and data S1; see Materials and Methods). Fluid inclusions from the same halites were used to document the major ion chemistry (7, 24–29) and Sr concentration (30) of Mesozoic and Cenozoic seawater. The 87Sr/86Sr ratios from these fluid inclusions (30) and associated sulfate minerals (31–33) lie on the marine curve, supporting the seawater origin of the brines from which halite precipitated (Fig. 1 and table S2; see Materials and Methods).
Fig. 2. Histograms and frequency distribution curves of Li+/Mg2+ ratios measured in (n) fluid inclusions from Cenozoic and Mesozoic halite.
(A) Normal distribution of the Li+/Mg2+ ratios for modern (0 Ma), Messinian (5.61 to 5.55 Ma), Serravallian-Tortonian (11.8 to 7.2 Ma), Langhian-Serravallian (13.81 to 13.1 Ma), Albian-Cenomanian (112.2 to 93.5 Ma), and Kimmeridgian-Tithonian (155.7 to 145.5 Ma) inclusion brines. (B) Bimodal distribution of Priabonian (~36 Ma) inclusions. Bin sizes were calculated using the Sturges’ Rule [log2(n) + 1, where n is the number of data points]. Small vertical dashed lines indicate the mode of Kernel density estimate. Outliers (red) outside of ±3σ (99.7% confidence interval) at the head and tail of the histograms (Serravallian-Tortonian, Langhian-Serravallian, Kimmeridgian-Tithonian, and Priabonian) were excluded from statistical analysis. Such outliers are due to poorly controlled laser ablation of fluid inclusions (13).
Table 1. [Li+] calculated for Mesozoic and Cenozoic seawater from measured [Li+]/[Mg2+] ratios and published [Mg2+].
[Li+] (μmol/kg H2O) calculated for modern salinas (solar evaporation ponds) and evaporite basins/subbasins using [Li+]/[Mg2+] ratios (μmol/mmol) measured in (n) number fluid inclusions in (N) number of halite intervals and [Mg2+] (mmol/kg H2O) in seawater from published fluid inclusion data (7). One SE was calculated from the average of (n) fluid inclusion analyses in halite of the same age. [Li+]/[Mg2+] ratios and [Li+] values for individual fluid inclusions are given in data S1.
| Age | Age (Ma ago) | Basins/Subbasins/Salinas | Locality/Country | N | n | [Mg2+]calc | [Li+]/[Mg2+]measured | [Li+]calc | ||
|---|---|---|---|---|---|---|---|---|---|---|
| (mmol/kg H2O) | (μmol/mmol) | 1 SD | (μmol/kg H2O) | 1 SD | ||||||
| Modern | 0 | Seawater | Atlantic and Pacific | 2 | 47 | 55 | 0.49 | 0.0005 | 27 | 0 |
| Conti Vecchi | Mediterranean Salinas, Italy | 1 | 18 | 0.52 | 0.05 | 29 | 3 | |||
| Sant’Antioco | Mediterranean Salinas, Italy | 1 | 10 | 0.50 | 0.05 | 28 | 3 | |||
| Cabo de Gata | Mediterranean Salinas, Spain | 1 | 6 | 0.58 | 0.05 | 32 | 3 | |||
| Great Inagua Island* | Caribbean Salinas, Bahamas | 1 | 16 | 0.57 | 0.06 | 31 | 3 | |||
| Average | 0.54 | 0.05 | 29 | 3 | ||||||
| Messinian | ~5.61–5.55 (5.6) | Caltanissetta | Sicily, Italy | 1 | 10 | 51 | 0.85 | 0.05 | 43 | 3 |
| Serravallian-Tortonian | ~11.8–7.2 (11.6) | Gulf of Suez | Hurghada, Egypt | 17 | 150 | 45 | 1.01 | 0.09 | 45 | 4 |
| Langhian-Serravallian | ~13.81–13.1 (13.6) | Carpathian Foredeep | Tarnow, Poland | 6 | 64 | 43 | 1.24 | 0.13 | 53 | 6 |
| Carpathian Foredeep | Wieliczka, Poland | 2 | 13 | 1.12 | 0.11 | 48 | 5 | |||
| Carpathian Foredeep | Bochnia, Poland | 4 | 31 | 1.28 | 0.06 | 55 | 3 | |||
| Carpathian Foredeep | Hrynivka, Ukraine | 2 | 15 | 1.38 | 0.12 | 59 | 5 | |||
| Carpathian Foredeep | Slanic-Prahova, Romania | 4 | 30 | 1.23 | 0.06 | 53 | 3 | |||
| East Slovakian | Zbuzda, Slovakia | 2 | 19 | 1.27 | 0.08 | 55 | 3 | |||
| Transcarpathian | Solotvyno, Ukraine | 1 | 8 | 1.22 | 0.02 | 52 | 1 | |||
| Average | 1.24 | 0.08 | 53 | 3 | ||||||
| Priabonian | ~36 | Catalan | Catalonia, Spain | 6 | 64 | 35 | 2.85 | 0.87 | 100 | 31 |
| Navarra | Navarra, Spain | 2 | 25 | 1.93 | 0.14 | 67 | 5 | |||
| Average | 1.93 | 0.24 | 68 | 8 | ||||||
| Albian-Cenomanian | 112.2–93.5 (103) | Sakon Nakhon | Vientiane, Laos | 3 | 50 | 33 | 4.74 | 1.01 | 156 | 34 |
| Kimmeridgian-Tithonian | ~155.7–145.5 (150) | East Kuban | Northern Caucasus, Russia | 2 | 25 | 38 | 4.98 | 0.62 | 189 | 24 |
| Foredobrogean | Izmail, Ukraine | 8 | 77 | 4.81 | 0.67 | 183 | 26 | |||
| Average | 4.85 | 0.66 | 184 | 25 | ||||||
*Data from von Borstel et al. (69).
Histograms and frequency distribution curves of Li+/Mg2+ ratios in halite inclusions for six of the seven ages reported show a normal distribution, with most data symmetrically distributed around the mean (Fig. 2A). The Li+/Mg2+ ratios of these inclusion brines fall within 7 and 21% RSD (relative standard deviation) equivalent to the LA-ICP-MS analytical uncertainty (<10% RSD) (13) and variability in the Li+/Mg2+ ratios of natural brines (<20% RSD). Modern brines from solar evaporation ponds at Atlit salt plant, Israel (34, 35) and Great Inagua Morton salt plant, Bahamas (36) show up to 20% variability in Li+/Mg2+ ratios of 0.5 ± 0.1, ranging between 0.4 and 0.6 μmol/mmol (fig. S1). Late Priabonian (~36 Ma old) fluid inclusions, however, show a bimodal distribution of Li+/Mg2+ ratios from the two main evaporite depocenters (300 km apart) in the Eocene South Pyrenean basin (26). Fluid inclusions from the Navarra subbasin have a mean Li+/Mg2+ ratio of 1.93 ± 0.24, whereas the mean Li+/Mg2+ ratio in fluid inclusions from the Catalan subbasin is 3.51 ± 0.24 (Fig. 2B). Fluid inclusions in halite from the Catalan subbasin contain variable Ca2+ and SO42+ concentrations, and associated anhydrite layers exhibit δ34S values slightly higher than expected from Eocene seawater. Such data were interpreted by Cendón et al. (26) to reflect continental recharge and less seawater inflow to the Catalan subbasin, which was further from the open ocean than the Navarra subbasin. For these reasons, we only use Li+/Mg2+ ratios from the Navarra subbasin here.
Li+ concentrations in paleoseawater
Li+/Mg2+ ratios measured from fluid inclusions require known Mg2+ concentrations [Mg2+] to convert ratios into absolute [Li+]. The conversion is straightforward because Mg2+ and Li+ are conservative ions in seawater and inclusion brines measured in this study (fig. S1; see Materials and Methods). [Li+] of paleoseawater, therefore, is calculated using Li+/Mg2+ ratios and published paleoseawater [Mg2+] (Fig. 1, fig. S4, Table 1, table S1, and data S1) (7). Over the past 150 Ma, [Li+]SW declined from 184 ± 25 to 27 μmolal. [Li+]SW was high (156 to 184 μmolal) during the Kimmeridgian-Tithonian (155.7 to 145.5 Ma ago) and Albian-Cenomanian (112.2 to 93.5 Ma ago) stages and then decreased [~1.2 μmolal/Ma, r2 = 0.99 (n = 558)] over the past ~150 Ma. [Li+]SW decreased significantly (up to a factor of ~3 to 4) during the Cenozoic. [Li+]SW during the Eocene (~36 Ma ago) was 68 ± 8 μmolal (n = 25), which decreased to 45 to 53 ± 3 μmolal (n = 330) during the Serravallian-Tortonian (~14 to 11 Ma ago), 43 ± 3 μmolal (n = 10) during the Messinian (~6 to 5 Ma ago), to 27 μmolal today.
Overall, the 150 Ma decrease in [Li+]SW parallels (Fig. 1) (i) the increase in Mg2+/Ca2+ ratio from 1.3 ± 0.25 in the Jurassic to the modern value of 5.2, as seawater [Mg2+] increased and [Ca2+] decreased (7); (ii) the change from calcite seas in the Jurassic/Cretaceous to the aragonite seas of the late Cenozoic with the rise of Mg2+/Ca2+ (5); (iii) the change from KCl potash evaporites of the Mesozoic to the MgSO4 type of the late Cenozoic as seawater [Mg2+] and [SO42−] rose and [Ca2+] decreased (4); (iv) the increase in 87Sr/86Sr from the Late Jurassic minimum of 0.7069 to the maximum today of 0.7092 (2); and (v) the change from the greenhouse climates of the Mesozoic–Early Cenozoic to the icehouse of the late Cenozoic accompanied by lower atmospheric CO2 (37).
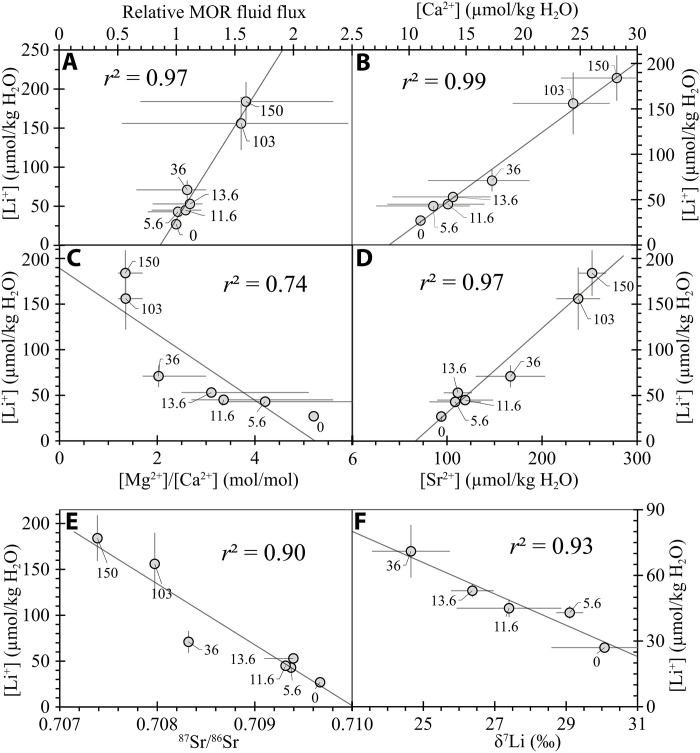
The 150 Ma record of [Li+]SW is plotted against the mean MOR fluid flux (Fig. 3A) (38), recently estimated from oceanic crust reconstructions and data from modern hydrothermal systems. The strong positive correlation between [Li+]SW and variations in MOR fluid flux (r2 = 0.97) over the past 150 Ma suggests that long-term changes in [Li+] SW were produced by variations in plate tectonic activity, primarily from fluctuations in ocean crust production and hydrothermal fluid flux. To test this hypothesis, we constructed a 150 Ma forward model of the global Li cycle to investigate the controls on [Li+]SW.
Fig. 3. Covariation of [Li+] with MOR fluid flow and seawater constituents.
[Li+] in seawater from fluid inclusions versus relative MOR fluid flux (A) (38), seawater [Ca2+] (B) (7, 24, 25), Mg2+/Ca2+ (C) (7, 24, 25), [Sr2+] (D) (30), 87Sr/86Sr (E) (2), and δ7Li (F) (3). Ages in million years adjacent to symbols show average values of seawater [Li+], [Ca2+], Mg2+/Ca2+, [Sr2+], 87Sr/86Sr, and δ7Li with 1σ (2SE for [Sr2+]) and 2σ (δ7Li) error bars. Linear regressions show the relationship between [Li+]SW and MOR fluid flow and other marine records.
[Li+]SW model
The forward model equation (39) used for estimating [Li+] in paleoseawater can be represented by
| (1) |
| (2) |
where fRIV, fMOR, fAOC, and fMAAC are water fluxes (in kilograms per year) associated with RIV, the high-temperature axial portions of the MOR, off-axis low-temperature hydrothermal exchange with upper oceanic crust (AOC), and MAAC, respectively. [LiSW]t, [LiRIV]t, [LiMOR]t, [LiAOC]t, and [LiMAAC]t are the concentrations (in micromoles per kilogram) of Li+ in seawater, river water, MOR hydrothermal vent fluids, off-axis hydrothermal fluids, and marine aluminosilicate clay pore fluids, respectively, at time t. M is the total mass of seawater (~1.38 × 1021 kg) assumed constant in the past. Δt is the time step, 10 thousand years (ka), used in this model. FRIV, FMOR, FAOC, and FMAAC in Eq. 2 represent Li+ input and output fluxes (in moles per year) to the oceans associated with rivers, high-temperature MORs, off-axis ocean crust alteration, and marine clay diagenesis, respectively, used in calculating the seawater Li budget from Eq. 1. LiSW is total Li+ in seawater; modern LiSW(0) ≈ 3.5 × 1016 mol. Li+ inputs are MOR fluids (FMOR) and river water (FRIV), and the outputs are uptake during off-axis low-temperature weathering of oceanic crust (FAOC) and diagenetic reactions between seawater and marine aluminosilicate clays (FMAAC). Long-term variations of [LiSW]t reflect changes in the mass balance between the input and output fluxes given the residence time of Li+ (~1.5 Ma) (40) in the modern ocean.
[Li+]SW model parameters and assumptions
The constraints and assumptions of the input and output fluxes used to model [Li+]SW are shown in Fig. 4 and Table 2:
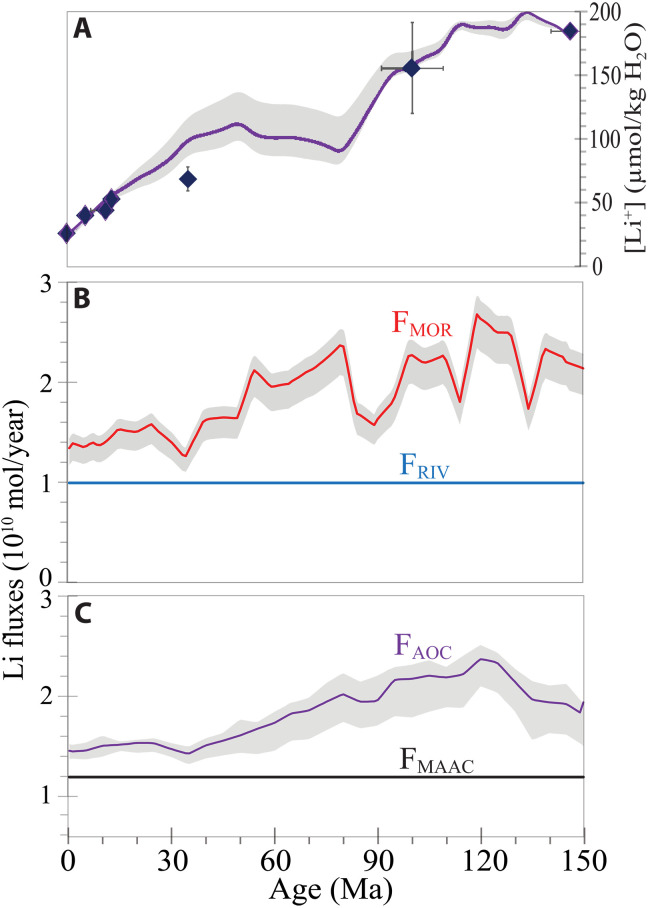
Fig. 4. [Li+] mass balance model parameters and [Li+] model output.
(A) [Li+] from fluid inclusions in halite and the [Li+]SW model. Model input (B) and output (C) fluxes for [Li+] in seawater, using (i) variable high-temperature hydrothermal flux along MORs (FMOR), with maximum at 120 Ma ago, (ii) variable low-temperature hydrothermal flux involving off-axis AOC (FAOC), (iii) constant RIV equal to modern global riverine inflow (FRIV), and (iv) constant uptake flux of Li by marine clays (FMAAC). Gray shading shows the full range of uncertainty for AOC and ±15% uncertainty from the mean MOR Li fluxes (fig. S6).
Table 2. Parameters used to model [LiSW].
| Symbol | Description | Present-day values | 150 Ma ago values | Units | References for present-day values |
|---|---|---|---|---|---|
| [LiSW] | Lithium concentration in seawater | 27 | 184 | μmol/kg H2O | (36) |
| [LiRW] | Lithium concentration in river water | 0.265 | 0.265 | μmol/liter | (15) |
| [LiMOR] | Lithium concentration in MOR hydrothermal vents fluids | 457 ± 66 | 457 ± 66 | μmol/kg H2O | fig. S5 |
| [LiAOC] | Lithium concentration in off-axis hydrothermal fluids | 9–16 (13.4 ± 3.7) | 166 ± 46 | μmol/kg seawater | (46) |
| [LiMAAC] | Lithium concentration in marine aluminosilicate clay diagenetic fluids | 18–26 | μmol/kg H2O | (3) | |
| f RW | River water flux | 3.75 × 1016 | 3.75 × 1016 | liter/year | (39) |
| f MOR | Water flux through axial portions of the MORs | (3 ± 1.5) × 1013 | (4.7 ± 0.5) × 1013 | kg/year | (16) |
| f AOC | Water flux through off-axis oceanic crust | 1.1–24 (1.1) × 1015 | (1.5 ± 0.2) × 1015 | kg/year | (44, 45) |
| f MAAC | Water flux through marine aluminosilicate clays | ||||
| M | Mass of seawater | 1.38 × 1021 | 1.38 × 1021 | kg | Calculated using (39) and average density of seawater (1.025 kg/liter) |
| LiSW | Total Li in seawater | 3.73 × 1016 | 2.54 × 1017 | mol | Calculated using (36) and (39) |
| FRW | Riverine Li flux | 9.94 × 109 | 9.94 × 109 | mol/year | (15) |
| FMOR | High-temperature MOR hydrothermal Li flux | (13.7 ± 2.8) × 109 | (21.6 ± 2.1) × 109 | mol/year | Calculated using (16) and [LiMOR] |
| FAOC | Li flux related to low-temperature altered oceanic crust | 11–41 (14.7 ± 0.8) × 109 | (19.6 ± 2.5) × 109 | mol/year | Calculated using (44) and (46) |
| FMAAC | Li flux related to MAACs | 11.9 × 109 | 11.9 × 109 | mol/year | Based on mass balance |
1. MOR hydrothermal Li+ flux (FMOR): Modern FMOR was calculated from compilation of data on lithium concentrations in MOR hydrothermal vent fluids ([LiMOR] = 457 ± 66 μmol/kg) along active spreading centers (fig. S5) and estimated global mean hydrothermal water flux [fMOR = (3 ± 1.5) × 1013 kg/year] derived from geophysical and geochemical methods (16). We used a modern mean FMOR of (13.7 ± 2.8) × 109 mol/year, the same FMOR used in previous δ7LiSW models (3, 20, 22).
Reconstruction of past FMOR requires knowledge of axial hydrothermal fluid flux, fMOR, through time. Müller et al. (38) calculated fMOR for the past 200 Ma from oceanic heat flow derived from reconstructions of the area of 0 to 1 Ma ocean crust (41) and data from modern hydrothermal systems (42), normalized to mean modern fMOR of 3 × 1013 kg/year (fig. S6). In modern hydrothermal systems, about 30% of the total global hydrothermal water flux occurs in ocean crust younger than 1 Ma along active vents of MOR crests, and the remaining flux occurs in cooler, off-axis regions (38, 42). The uncertainties in estimating past fMOR over the past 150 Ma increase by up to a factor of 4 (fig. S6) (38). These uncertainties are from (i) reconstructions of the age-area distribution of global ocean floor, including subducted oceanic crust (41), (ii) mapping of hydrothermal fluid flux as a function of crustal age (43), and (iii) partitioning of axial heat flux into high-temperature flow and diffuse flow (16). The impact of uncertainties in the estimation of fMOR on the modeling of [LiSW]t was tested in the sensitivity analysis discussed below. FMOR (11 × 109 to 29 × 109 mol/year) over the past 150 Ma was calculated here using variable fMOR (38) and mean [LiMOR] of 457 μmol/kg (Fig. 4B, fig. S6, and data S3).
Lower global mean hydrothermal fluid flux [fMOR = (8 ± 2.1) × 1012 kg/year] and FMOR = (5.16 ± 1.43) × 109 mol/year was estimated from a compilation of vent fluid compositions and altered sheeted dikes (14). This FMOR is similar to the FMOR (6.6 × 109 mol/year) estimated from hydrothermal leaching of all Li in 1 km of ocean crust (3.4 km2) produced annually at the MOR (18). An alternative 150 Ma [LiSW]t model with lower modern fMOR and FMOR of 5.16 × 109 mol/year is shown on fig. S7.
2. Off-axis Li+ uptake flux (FAOC): The uptake of Li+ during off-axis low-temperature hydrothermal exchange {FAOC = fAOC × ([LiAOC] – [LiSW])} between seawater and upper ocean crust is estimated to be 11 × 109 to 41 × 109 mol/year based on the modern low-temperature off-axis hydrothermal fluid flux (fAOC = 1.1 × 1015 to 24 × 1015 kg/year) (44, 45), and pore fluid/spring compositions ([LiAOC] = 9 to 16 μmol/kg) from warm (25° to 63°C) seawater circulating within 3.5-Ma-old ocean crust of the Juan de Fuca Ridge at Baby Bare and 5.9-Ma-old crust from the Costa Rica Rift (58°C) (46). The contribution of cool versus warm off-axis hydrothermal fluids to ridge-flank Li sinks, however, is not well known. Experimental studies show that Li loss from seawater into secondary phases occurs below 150°C (47). Warm springs (25° to 63°C) from the Juan de Fuca Ridge show a positive linear correlation between Li and Mg (46), indicating removal of both species from seawater. More than 80% of seawater Mg is removed by secondary alteration phases at temperatures above 45°C, and <10% is lost at temperatures below 25°C (48). Here, we similarly assume that warm springs (>25°C) remove 80 to 90% of the Li in seawater, and 10% is lost at lower temperatures (<25°C) on ridge flanks.
Independent estimates of FAOC, based on steady-state mass balance models (3, 20) and Li− enrichment in altered off-axis ophiolites (18), dredged samples from the Atlantic seafloor (49), Ocean Drilling Program (ODP) sites 504, 896, and 1256 (19, 50), and Deep Sea Drilling Project (DSDP) data (51), together give a range of 2 × 109 to 21 × 109 mol/year. This wide range of FAOC is due to the variable [Li] [5.2 to 75 parts per million (ppm)] measured from submarine basalts variably buried by marine sediments and subjected to different water/rock ratios, duration of reactions, and hydrothermal temperatures (18, 19, 51). For example, dredged basalts from the Atlantic seafloor exposed to seawater for ~46 Ma have higher [Li] (up to 75 ppm) than basalts in ocean drilling cores (ODP sites 504 and 896 and international ODP site 1256) (~9 ppm), which underwent low-temperature hydrothermal alteration for only ~5.9 Ma before they were buried by marine sediments (smaller water/rock ratio) (18, 51). Here, modern FAOC (14.7 ± 0.8 × 109 mol/year) is calculated using fAOC of 1.1 × 1015 kg/year and mean warm spring composition ([LiAOC] = 13.4 ± 3.7 μmol/kg), which is ~50% lower than seawater Li ([LiSW] = 27 μmol/kg H2O). This modern FAOC is slightly higher than that used for a recent steady-state mass balance model (11 × 109 to 12 × 109 mol/year) (20, 22) but lower than mean FAOC estimates from Li enrichment (23.1 to 27 ppm) in altered off-axis ocean crust from the Cretaceous Troodos ophiolite (14 × 109 to 21 × 109 mol/year) (18). The FAOC estimates from the Troodos ophiolite are probably an upper limit for modern FAOC because [Li+]SW in late Cretaceous seawater, measured here from fluid inclusions in halite (156 ± 34 μmolal), contained far more Li+ than modern seawater (27 μmol/kg H2O).
FAOC (13.4 × 109 to 25.4 × 109 mol/year) over the past 150 Ma was calculated using the variable fAOC (1.1 × 1015 to 1.9 × 1015 kg/year) (38), [LiSW]t, and [LiAOC]t (Fig. 4C and data S3). [LiAOC]t depends on [LiSW]t, fAOC, and FMOR (Eq. 1). fAOC was determined from reconstructions of the area of 1 to 65 Ma old ocean crust and data from modern hydrothermal systems for ocean crust–seawater interactions between 30° and 60°C, normalized to mean modern fAOC of 1.1 × 1015 kg/year (Fig. 4C and fig. S6) (38, 42). Hydrothermal flux in ocean crust older than 65 Ma old approaches zero because impermeable marine sediments disconnect the ocean from the underlying crust (43). The uncertainties involved in estimation of fAOC are 20%, which are then propagated through the calculations for modeling of [LiSW]t (Eq. 1) (Figs. 1 and 4C and fig. S6).
Low-temperature spring fluids venting at 12.3° ± 0.01°C from a basaltic edifice on 23 Ma old seafloor at the Dorado outcrop on the Cocos Plate, eastern Pacific Ocean (52), are suggested to be more representative of Li removal from seawater into secondary phases than warmer pore and spring fluids from the Juan de Fuca Ridge at Baby Bare and the Costa Rica Rift (46). Dorado spring fluid composition ([LiAOC] = 25.1 ± 0.1 μmol/kg) differs from seawater ([LiSW] = 27 μmol/kg H2O) by only ~4 to 7% (52). An alternative 150 Ma [LiSW]t model using larger fluid fluxes (fAOC = 5.8 × 1015 to 9.9 × 1015 kg/year) and Dorado-like spring fluid composition [LiAOC]t is shown in fig. S7.
3. Riverine Li+ flux (FRIV): Rivers, the conduit for global continental weathering, supply average annual dissolved Li+ (FRIV = 9.94 × 109 mol/year) to the ocean, calculated from modern river water flux (fRIV = 3.75 × 1016 kg/year) and average [LiRIV] = 0.265 μmol/kg (15). The modern flux of Li from rivers may not be completely representative because above average Li input from rapid chemical weathering during the last deglaciation has not yet declined to the Pleistocene average [LiRIV] (53). Changes in RIV over the past 150 Ma are uncertain. Previous estimates, obtained from paleoclimate reconstructions, predicted decreased runoff from 150 to 0 Ma ago (54), whereas increased runoff and increased continental silicate weathering flux from 100 to 0 Ma ago were calculated from inverse modeling of the marine Sr, Os, C, and Li isotope records (20, 23, 55). Still, other models assumed constant riverine Li+ flux from the continents over the Cenozoic (3). There are no empirical data available to independently estimate changes in FRIV over time, so here, FRIV was assumed to be constant to test the sensitivity of the model to other parameters (FMOR and FAOC) (Fig. 4B and data S3).
4. Uptake of Li by MAAC (FMAAC): Li enrichment in marine clay occurs when Li+ in seawater is incorporated in detrital and authigenic clays (56). The magnitude of removal of Li from seawater into aluminosilicate clays is poorly known in the modern ocean and even less well known in the past. Global rates of burial diagenesis of detrital clays and formation of authigenic minerals are unknown (18, 23, 51). A previous estimate of FMAAC (20 × 109 mol/year) (3) was based on the input of continental sediments to the ocean (13.5 × 1012 kg/year), assuming that the average clay content of suspended sediment was 53% and that marine clays were enriched in Li+ by ~10 to 60 ppm (1 to 9 μmol/kg). Those assumptions gave an FMAAC four times higher than that estimated from the mass-weighted average marine sedimentary flux of Li to subduction zones (FMAAC = 5 × 109 mol/year) (56). Uncertainties in the global flux of suspended sediment to the oceans, its clay/sand ratio, and the rate of formation of authigenic clays have complicated the calculation of global removal of Li+ into marine sediments. No empirical data are available to independently estimate present-day FMAAC. Modern FMAAC was estimated here to be 11.9 × 109 mol/year, similar to that used recently for steady-state mass balance calculations (FMAAC = 11 × 109 mol/year) (20). FMAAC in the past is further complicated because the change in terrestrial clay flux to the ocean and authigenic clay formation through time are unknown. Here, FMAAC is assumed to be constant (11.9 × 109 mol/year) over the past 150 Ma (Fig. 4C and data S3).
[Li+]SW model implementation
The 150 Ma forward model for [Li+]SW (Fig. 1) assumes the following: (i) variable Li fluxes from high-temperature hydrothermal fluids through the axial portions of the MOR systems (FMOR) for crust 0 to 1 Ma old (38); (ii) variable removal rates of Li into off-axis (FAOC) portions of the ridge flank crust, 1 to 65 Ma old (38); (iii) constant riverine Li flux (fRIV) equal to modern global river discharge; and (iv) constant uptake flux of Li into marine clays (FMAAC), which then allowed testing of model sensitivity to changes in FMOR and FAOC (Fig. 4). The forward model starts at steady state (total mass of Li input fluxes equals total output) at 150 Ma and then calculates [Li+]SW at each 10 ka time step using the input and output fluxes of [Li+] from the sources and sinks until the present (0 Ma). The model agrees with measured [Li+]SW from fluid inclusions (Fig. 1), which shows that the drop in [Li+]SW over the past 150 Ma can be produced by decreasing high- and low-temperature seafloor hydrothermal fluxes without requiring changes in river input and MAAC uptake fluxes.
Given the uncertainties in estimating fMOR (200 to 400%) and fAOC (<20%) (fig. S6), the sensitivity of the model result is considered by propagating the fMOR and fAOC uncertainties through the calculations for [LiSW]t in Eq. 1. The uncertainty associated with fAOC narrows the possible fMOR range needed for the Li+ mass balance to within ±15% of the mean fMOR (Eq. 1). These uncertainties (gray band around the mean fMOR and fAOC curves in Fig. 1 and fig. S6) replicate the [LiSW]t model result without requiring changes in the FRIV and FMAAC (Fig. 4). Modeling of [LiSW]t using fMOR above or below the ±15% mean fMOR range, however, requires changes in FRIV and FMAAC to reproduce the [Li]SW record. A range of fMOR, greater than ±15% of the mean fMOR range (fig. S6), for example, produces an exceedingly large Li+ flux from MOR input, which requires an additional sink (FMAAC) to remove the excess Li+. On the other hand, a lower range of fMOR, less than ±15% of the mean fMOR range, is compatible with interpretations that MOR activity has not varied significantly in the past (57). Such a lower range of fMOR then requires higher FRIV and FMAAC to explain the [Li+]SW record.
Covariation of [Li+]SW with [Ca2+]SW, [Mg2+]SW, [Mg2+/Ca2+]SW, and 87Sr/86Sr
The 150 Ma decrease of [Li+]SW and the modeling results presented here have implications for interpreting secular changes in the major ion chemical composition of seawater. The positive correlation between [Li+]SW and [Ca2+]SW (r2 = 0.99) (Fig. 3B) suggests that declining seafloor hydrothermal fluxes and concomitant lower rates of Ca2+ released to seawater via albitization and chloritization reactions (17, 58) produced the threefold decrease in [Ca2+]SW over the past 150 Ma. The threefold decrease in [Ca2+] and the 1.6× increase in [Mg2+]SW over the past 150 Ma are responsible for the negative correlation between [Li+]SW and [Mg2+/Ca2+]SW (Fig. 3C). The Mesozoic-Cenozoic increase in [Mg2+]SW may be explained by decreased removal of Mg2+ from seawater at the MOR (4, 17, 58), a decrease in the rate of Mg2+ removal into marine clays attributable to Cenozoic cooling of ocean bottom waters by 10° to 15°C (8, 11) or even 20°C (59), and a decrease in the rate of dolomitization on the seafloor (60). The positive correlation between [Li+]SW and [Sr2+]SW (r2 = 0.97) (Fig. 3D) suggests that declining seafloor hydrothermal fluxes over the past 150 Ma may be responsible for lower fluxes of Sr2+ and Li+ released to seawater because MOR hydrothermal brines are enriched in [Li+], [Sr2+], and [Ca2+] via reactions between MOR basalts and seawater. The increase in 87Sr/86Sr from the Late Jurassic (0.7069) to today (0.7092) and the negative correlation (r2 = 0.90) between [Li+]SW and 87Sr/86Sr (Fig. 3E) suggest that the progressive decline of MOR hydrothermal fluxes decreased input of low 87Sr/86Sr hydrothermal fluids (61). Last, the increase of δ7LiSW by ∼5 to 6‰ over the past 36 Ma and the strong negative correlation (r2 = 0.93) between [Li+]SW and δ7LiSW (Fig. 3F) suggest that decline of MOR hydrothermal fluxes decreased input of low δ7Li fluids (3). In addition, the Cenozoic increase in δ7LiSW may also be explained by an increase in isotopic fractionation between seawater and oceanic Li sinks (ocean crust and marine clays) as ocean bottom water temperatures decreased (18). In summary, the previously unknown and unexpectedly large decrease in [Li]sw over the past 150 Ma reported here has important implications for better understanding the global geochemical cycle of Li, and more broadly, how plate tectonic activity influences the long-term chemical composition of seawater, greenhouse-icehouse climate states, and the global carbon cycle.
MATERIALS AND METHODS
Sample selection
Fluid inclusions in halite were used to reconstruct the major ion chemistry of seawater in the Phanerozoic (1, 7, 29). Primary fluid inclusions along growth bands in bottom-grown crystals of marine halite (chevron and/or cornet structures) and cumulate crystals (hoppers, rafts, and sunken cubes formed at the air-brine interface and in the water column) record the chemistry of evaporating seawater (fig. S2) (62). A total of 639 fluid inclusions in 65 halite crystals from 3 Mesozoic [Kimmeridgian-Tithonian (155.7 to 145.5 Ma) and Albian-Cenomanian (112.2 to 93.5 Ma)] and 10 Cenozoic [Priabonian (~36 Ma), Langhian-Serravallian (13.81 to 13.1 Ma), Serravallian-Tortonian (11.8 to 7.2 Ma), Messinian (5.61 to 5.55 Ma), and modern (0 Ma)] marine evaporite formations (fig. S3), well documented for major ion chemistry (Mg2+, Ca2+, Na+, K+, SO42−, Clˉ) (7, 24–28), were reanalyzed to obtain Li+/Mg2+ ratios and Li+ concentrations (table S1 and data S1). Petrographic, geochemical, and paleontological criteria used to characterize the marine origin of the fluid inclusions in the halites studied include the following: (i) Brˉ concentrations in halite (24–29), (ii) δ34S in sulfate minerals interbedded with halite (24–27, 31, 63), (iii) 87Sr/86Sr in sulfate minerals (31–33) and in fluid inclusions in halite (30), and (iv) marine fossils preserved in underlying carbonate/marl units (24, 25, 64). These records show that the parent waters of the inclusion brines reported here were marine in origin, especially because the 87Sr/86Sr ratio in halite fluid inclusions is the same as contemporaneous seawater (Fig. 1 and table S2).
Analytical methods
Fluid inclusions in halite were analyzed using an NWR193 (ESL, Bozeman, MT) ArF excimer laser ablation system coupled to an Agilent 7900 quadrupole mass spectrometer. Halite crystals were first cleaved with a razor blade into tabular fragments, approximately 8 mm by 5 mm by 1 mm in size, to expose fresh surfaces and to fit on glass slides within the laser ablation sample chamber. Each cleaved halite crystal was examined to document the primary nature of fluid inclusions along halite growth bands using a transmitted light Zeiss Axio Imager.A1 (Carl Zeiss AG, Oberkochen, Germany) at magnifications between 25× and 100× (fig. S2). Well-defined bands of single-phase fluid inclusions along primary growth zones were selected for LA-ICP-MS analysis. Recently developed analytical methods were used to obtain Li+/Mg2+ ratios of individual fluid inclusions in halite (13).
Typical analytical accuracy for Li+/Mg2+ ratios was within 7% of the expected value based on measurements of fluid inclusions with known chemical compositions, and precision was <10% RSD (13). Controlled and optimized laser ablation of fluid inclusions improved the analytical accuracy, precision, and lowered detection limits for Li+ and Mg2+to values below 50 and 100 μmolal, respectively. The accuracy of the method was verified using modern halite samples from the Dead Sea. Mg2+ and Li+ concentrations in modern Dead Sea brine measured using ICP–optical emission spectroscopy and the LA-ICP-MS analyses of fluid inclusions in Dead Sea halite formed from those brines show that fluid inclusion and brine chemistry agree within 7% error (13).
Calculation of [Li+]
Li+/Mg2+ ratios measured from fluid inclusions require known Mg2+ to convert ratios into absolute [Li+]. The conversion is straightforward because the Li+/Mg2+ ratio remains unchanged during evaporation of seawater through aragonite, gypsum, and halite precipitation until the first Mg-bearing salts form. For example, the Li+/Mg2+ ratio in modern seawater is 0.49 μmol/mmol (36), and this ratio remains constant until the Mg-bearing salt, polyhalite [K₂Ca₂Mg(SO₄)₄·2H₂O], precipitates at a degree of evaporation (DE) of 38.7. The formation of polyhalite applies to conditions where early formed minerals (gypsum or anhydrite) are allowed to back-react with the brine. In contrast, during “fractional crystallization,” which occurs in man-made solar evaporation systems, brines are removed from contact with precipitated minerals as evaporated seawater flows from pond to pond. In this case, the Li+/Mg2+ ratio remains conservative until epsomite (MgSO₄·7H₂O) and/or kainite (KMgSO4Cl·3H2O) precipitate at a DE of 60.5 and ~70, respectively. That is because gypsum, precipitated in ponds during the early stages of evaporation, never comes in direct contact with highly evolved seawater and so cannot react with brine to form polyhalite (fig. S1). Li+/Mg2+ ratios change slightly from 0.49 to 0.59 μmol/mmol between a DE of 0 and 100 due to a small amount of MgSO₄ salt precipitation between a DE of 70 and 100. This change is indistinguishable from the observed variability in Li+/Mg2+ ratios of 0.4 to 0.6 μmol/mmol during the early stages (DE <10) of solar evaporation ponds (fig. S1B).
Evaporative concentration of seawater to extreme levels beyond a DE of 100 causes the seawater Li+/Mg2+ ratio of 0.9 to increase to a maximum of 3.9 at DE of 870 as Mg2+ is removed from the brine to form kieserite, carnallite, and finally bischofite (35). In contrast, Li+ is conservative during the complete evaporation of seawater because there are no naturally occurring Li salts that incorporate significant amounts of Li (36). Experiments with solar evaporation of Black Sea water and Mediterranean seawater showed that Li+ increased conservatively up to a DE of 117 (65) and 870 (35), respectively. Halite analyzed in this study was not associated with Mg-bearing salts (30); thus, the Li+/Mg2+ ratio measured in fluid inclusions in halite is assumed to be the same as the ratio in unevaporated seawater.
The major ion chemistry of Phanerozoic seawater, including Mg2+, was previously estimated from chemical analyses of fluid inclusions in marine halite (7, 24, 25, 29). Here, we reanalyzed the same halites earlier used to evaluate the major ion chemistry of ancient seawater. Li+/Mg2+ ratios and published paleoseawater [Mg2+] allowed calculation of [Li+] in Mesozoic and Cenozoic seawater (Table 1, table S1, Figs. 1 and 2, and fig. S4) (7, 24, 25, 29). For example, the [Li+] of modern seawater (29 ± 3 μmolal) was calculated using the present-day [Mg2+] of 55 mmolal and the average Li+/Mg2+ ratio from 50 fluid inclusions in halite from three solar evaporation ponds on the coast of the Mediterranean Sea and the Caribbean Sea. For comparison, modern seawater has measured [Li+] of 27 ± 0.2 μmolal (Table 1 table S1, and data S1). The close agreement between modern seawater [Li+] and the [Li+] calculated from the Li+/Mg2+ ratio of fluid inclusions in halite from modern solar evaporation ponds shows the validity of calculated [Li+] in paleoseawater using Li+/Mg2+ ratios and published paleoseawater [Mg2+] (Figs. 1 and 2 and fig. S4).
Acknowledgments
We thank J. García-Veigas, D. Cendón, V. M. Kovalevych, T. M. Peryt, J. Horita, J. Rae, and H. Jurikova for providing samples. We thank D. Collins for assistance in maintaining the LA-ICP MS and R.V. Demicco for help with the lithium model. We thank P. Froelich and three anonymous reviewers for the careful review of the manuscript and comments that helped improve the paper.
Funding: This research was supported by graduate student research grant funds from the Society of Economic Geologists Foundation (2018); the American Association of Petroleum Geologists Foundation (2018); and the Mineralogy, Geochemistry, Petrology, Volcanology (MGPV) Division of the Geological Society of America (2019). The Binghamton University LA-ICP-MS system was purchased using National Science Foundation (NSF) grant EAR-1463936. Additional funds came from the Binghamton University Mileur Faculty Development Grant.
Author contributions: M.F.W. and T.K.L. designed the research. M.F.W. conducted the fluid inclusion analyses and prepared figures and tables. M.F.W. and T.K.L. discussed and interpreted results. M.F.W. modeled the data. M.F.W. and T.K.L. wrote the original draft, and T.K.L. reviewed and edited the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S7
Tables S1 and S2
Legends for data S1 to S3
References
Other Supplementary Material for this manuscript includes the following:
Data S1 to S3
REFERENCES AND NOTES
- 1.T. K. Lowenstein, M. N. Timofeeff, S. T. Brennan, L. A. Hardie, R. V. Demicco, Oscillations in Phanerozoic seawater chemistry: Evidence from fluid inclusions. Science 294, 1086–1088 (2001). [DOI] [PubMed] [Google Scholar]
- 2.J. Veizer, D. Ala, A. Karem, P. Bruckschen, D. Buhl, F. Bruhn, G. A. F. Carden, A. Diener, S. Ebnethag, Y. Godderis, T. Jasper, C. Korte, F. Pawellek, O. G. Podlaha, H. Strauss, 87Sr/86Sr, δ13C and δ18O evolution of Phanerozoic seawater. Chem. Geol. 161, 55–88 (1999). [Google Scholar]
- 3.S. Misra, P. N. Froelich, Lithium isotope history of Cenozoic seawater: Changes in silicate weathering and reverse weathering. Science 335, 818–823 (2012). [DOI] [PubMed] [Google Scholar]
- 4.L. A. Hardie, Secular variation in seawater chemistry: An explanation for the coupled secular variation in the mineralogies of marine limestones and potash evaporites over the past 600 m.y. Geology 24, 279–283 (1996). [Google Scholar]
- 5.S. M. Stanley, L. A. Hardie, Secular oscillations in the carbonate mineralogy of reef-building and sediment-producing organisms driven by tectonically forced shifts in seawater chemistry. Palaeogeogr. Palaeoclimatol. Palaeoecol. 144, 3–19 (1998). [Google Scholar]
- 6.J. M. Edmond, Himalayan tectonics, weathering processes, and the strontium isotope record in marine limestones. Science 258, 1594–1597 (1992). [DOI] [PubMed] [Google Scholar]
- 7.M. F. Weldeghebriel, T. K. Lowenstein, J. García-Veigas, D. I. Cendón, [Ca2+] and [SO42−] in Phanerozoic and terminal Proterozoic seawater from fluid inclusions in halite: The significance of Ca-SO4 crossover points. Earth Planet. Sci. Lett. 594, 117712 (2022). [Google Scholar]
- 8.J. A. Higgins, D. P. Schrag, The Mg isotopic composition of Cenozoic seawater—Evidence for a link between Mg-clays, seawater Mg/Ca, and climate. Earth Planet. Sci. Lett. 416, 73–81 (2015). [Google Scholar]
- 9.M. E. Raymo, W. F. Ruddiman, Tectonic forcing of late Cenozoic climate. Nature 359, 117–122 (1992). [Google Scholar]
- 10.H. D. Holland, Sea level, sediments and the composition of seawater. Am. J. Sci. 305, 220–239 (2005). [Google Scholar]
- 11.L. A. Coogan, K. M. Gillis, Evidence that low-temperature oceanic hydrothermal systems play an important role in the silicate-carbonate weathering cycle and long-term climate regulation. Geochem. Geophys. Geosyst. 14, 1771–1786 (2013). [Google Scholar]
- 12.R. H. James, M. R. Palmer, Marine geochemical cycles of the alkali elements and boron: The role of sediments. Geochim. Cosmochim. Acta 64, 3111–3122 (2000). [Google Scholar]
- 13.M. F. Weldeghebriel, T. K. Lowenstein, J. García-Veigas, D. Collins, E. Sendula, R. J. Bodnar, J. R. Graney, D. I. Cendón, N. G. Lensky, Z. Mor, I. Sirota, Combined LA-ICP-MS and cryo-SEM-EDS: An improved technique for quantitative analysis of major, minor, and trace elements in fluid inclusions in halite. Chem. Geol. 551, 119762 (2020). [Google Scholar]
- 14.L. A. Coogan, S. Dasso, An internally consistent, probabilistic, determination of ridge-axis hydrothermal fluxes from basalt-hosted systems. Earth Planet. Sci. Lett. 323–324, 92–101 (2012). [Google Scholar]
- 15.J. Gaillardet, J. Viers, B. Dupré, Trace elements in river waters, in Treatise on Geochemistry, H. D. Holland, K. K. Turekian, Eds. (Pergamon, 2003), pp. 225–272. [Google Scholar]
- 16.H. Elderfield, A. Schultz, Mid-ocean ridge hydrothermal fluxes and the chemical composition of the ocean. Annu. Rev. Earth Planet. Sci. 24, 191–224 (1996). [Google Scholar]
- 17.K. L. Von Damm, Seafloor hydrothermal activity: Black smoker chemistry and chimneys. Annu. Rev. Earth Planet. Sci. 18, 173–204 (1990). [Google Scholar]
- 18.L. A. Coogan, K. M. Gillis, M. Pope, J. Spence, The role of low-temperature (off-axis) alteration of the oceanic crust in the global Li-cycle: Insights from the Troodos ophiolite. Geochim. Cosmochim. Acta 203, 201–215 (2017). [Google Scholar]
- 19.L. H. Chan, J. C. Alt, D. A. H. Teagle, Lithium and lithium isotope profiles through the upper oceanic crust: A study of seawater-basalt exchange at ODP Sites 504B and 896A. Earth Planet. Sci. Lett. 201, 187–201 (2002). [Google Scholar]
- 20.G. Li, A. J. West, Evolution of Cenozoic seawater lithium isotopes: Coupling of global denudation regime and shifting seawater sinks. Earth Planet. Sci. Lett. 401, 284–293 (2014). [Google Scholar]
- 21.K. E. Washington, A. J. West, B. Kalderon-Asael, J. A. R. Katchinoff, E. I. Stevenson, N. J. Planavsky, Lithium isotope composition of modern and fossilized Cenozoic brachiopods. Geology 48, 1058–1061 (2020). [Google Scholar]
- 22.J. K. Caves Rugenstein, D. E. Ibarra, F. von Blanckenburg, Neogene cooling driven by land surface reactivity rather than increased weathering fluxes. Nature 571, 99–102 (2019). [DOI] [PubMed] [Google Scholar]
- 23.N. Vigier, Y. Goddéris, A new approach for modeling Cenozoic oceanic lithium isotope paleo-variations: The key role of climate. Clim. Past 11, 635–645 (2015). [Google Scholar]
- 24.S. T. Brennan, T. K. Lowenstein, D. I. Cendón, The major-ion composition of Cenozoic seawater: The past 36 million years from fluid inclusions in marine halite. Am. J. Sci. 313, 713–775 (2013). [Google Scholar]
- 25.M. N. Timofeeff, T. K. Lowenstein, M. A. M. Da Silva, N. B. Harris, Secular variation in the major-ion chemistry of seawater: Evidence from fluid inclusions in Cretaceous halites. Geochim. Cosmochim. Acta 70, 1977–1994 (2006). [Google Scholar]
- 26.D. I. Cendón, C. Ayora, J. J. Pueyo, C. Taberner, The geochemical evolution of the Catalan potash subbasin, South Pyrenean foreland basin (Spain). Chem. Geol. 200, 339–357 (2003). [Google Scholar]
- 27.D. I. Cendón, T. M. Peryt, C. Ayora, J. J. Pueyo, C. Taberner, The importance of recycling processes in the Middle Miocene Badenian evaporite basin (Carpathian foredeep): Palaeoenvironmental implications. Palaeogeogr. Palaeoclimatol. Palaeoecol. 212, 141–158 (2004). [Google Scholar]
- 28.J. Garcia-Veigas, F. Orti, L. Rosell, C. Ayora, J. M. Rouchy, S. Lugli, The Messinian salt of the Mediterranean: Geochemical study of the salt from the Central Sicily Basin and comparison with the Lorca Basin (Spain). Bull. Soc. Geol. Fr. 166, 699–710 (1995). [Google Scholar]
- 29.J. Horita, H. Zimmermann, H. D. Holland, Chemical evolution of seawater during the Phanerozoic: Implications from the record of marine evaporites. Geochim. Cosmochim. Acta 66, 3733–3756 (2002). [Google Scholar]
- 30.M. F. Weldeghebriel, T. K. Lowenstein, Z. Xia, W. Li, Plate tectonic control of strontium concentration in Phanerozoic and Neoproterozoic seawater: Evidence from fluid inclusions in marine halite. Geochim. Cosmochim. Acta 346, 165–179 (2023). [Google Scholar]
- 31.J. García-Veigas, D. I. Cendón, L. Gibert, T. K. Lowenstein, D. Artiaga, Geochemical indicators in Western Mediterranean Messinian evaporites: Implications for the salinity crisis. Mar. Geol. 403, 197–214 (2018). [Google Scholar]
- 32.T. M. Peryt, R. Anczkiewicz, Strontium isotope composition of Middle Miocene primary gypsum (Badenian of the Polish Carpathian Foredeep Basin): Evidence for continual non-marine inflow of radiogenic strontium into evaporite basin. Terra Nov. 27, 54–61 (2014). [Google Scholar]
- 33.C. Taberner, J. Dinarès-Turell, J. Giménez, C. Docherty, Basin infill architecture and evolution from magnetostratigraphic cross-basin correlations in the southeastern Pyrenean foreland basin. Geol. Soc. Am. Bull. 111, 1155–1174 (1999). [Google Scholar]
- 34.Y. O. Rosenberg, Z. Sade, J. Ganor, The precipitation of gypsum, celestine, and barite and coprecipitation of radium during seawater evaporation. Geochim. Cosmochim. Acta 233, 50–65 (2018). [Google Scholar]
- 35.N. Shalev, B. Lazar, M. Köbberich, L. Halicz, I. Gavrieli, The chemical evolution of brine and Mg-K-salts along the course of extreme evaporation of seawater—An experimental study. Geochim. Cosmochim. Acta 241, 164–179 (2018). [Google Scholar]
- 36.M. A. McCaffrey, B. Lazar, H. D. Holland, The evaporation path of seawater and the coprecipitation of Br− and K+ with halite. J. Sediment. Petrol. 57, 928–938 (1987). [DOI] [PubMed] [Google Scholar]
- 37.G. L. Foster, D. L. Royer, D. J. Lunt, Future climate forcing potentially without precedent in the last 420 million years. Nat. Commun. 8, 14845 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R. D. Müller, A. Dutkiewicz, M. Seton, C. Gaina, Seawater chemistry driven by supercontinent assembly, breakup, and dispersal. Geology 41, 907–910 (2013). [Google Scholar]
- 39.R. V. Demicco, T. K. Lowenstein, L. A. Hardie, R. J. Spencer, Model of seawater composition for the Phanerozoic. Geology 33, 877–880 (2005). [Google Scholar]
- 40.Y. Huh, L. H. Chan, L. Zhang, J. M. Edmond, Lithium and its isotopes in major world rivers: Implications for weathering and the oceanic budget. Geochim. Cosmochim. Acta 62, 2039–2051 (1998). [Google Scholar]
- 41.M. Seton, C. Gaina, R. D. Müller, C. Heine, Mid-Cretaceous seafloor spreading pulse: Fact or fiction? Geology 37, 687–690 (2009). [Google Scholar]
- 42.C. A. Stein, S. Stein, Constraints on hydrothermal heat flux through the oceanic lithosphere from global heat flow. J. Geophys. Res. 99, 3081–3095 (1994). [Google Scholar]
- 43.H. P. Johnson, M. J. Pruis, Fluxes of fluid and heat from the oceanic crustal reservoir. Earth Planet. Sci. Lett. 216, 565–574 (2003). [Google Scholar]
- 44.R. N. Harris, A. T. Fisher, D. S. Chapman, Fluid flow through seamounts and implications for global mass fluxes. Geology 32, 725–728 (2004). [Google Scholar]
- 45.A. Schultz, H. Elderfield, Controls on the physics and chemistry of seafloor hydrothermal circulation. Philos. Trans. R. Soc. A 355, 387–425 (1997). [Google Scholar]
- 46.C. G. Wheat, M. J. Mottl, Composition of pore and spring waters from Baby Bare: Global implications of geochemical fluxes from a ridge flank hydrothermal system. Geochim. Cosmochim. Acta 64, 629–642 (2000). [Google Scholar]
- 47.W. E. Seyfried Jr., D. R. Janecky, M. J. Mottl, Alteration of the oceanic crust: Implications for geochemical cycles of lithium and boron. Geochim. Cosmochim. Acta 48, 557–569 (1984). [Google Scholar]
- 48.M. J. Mottl, C. G. Wheat, Hydrothermal circulation through mid-ocean ridge flanks: Fluxes of heat and magnesium. Geochim. Cosmochim. Acta 58, 2225–2237 (1994). [Google Scholar]
- 49.L. H. Chan, J. M. Edmond, G. Thompson, K. Gillis, Lithium isotopic composition of submarine basalts: Implications for the lithium cycle in the oceans. Earth Planet. Sci. Lett. 108, 151–160 (1992). [Google Scholar]
- 50.Y. Gao, F. Vils, K. M. Cooper, N. Banerjee, M. Harris, J. Hoefs, D. A. H. Teagle, J. F. Casey, T. Elliott, C. Laverne, J. C. Alt, K. Muehlenbachs, Downhole variation of lithium and oxygen isotopic compositions of oceanic crust at East Pacific Rise, ODP site 1256. Geochem. Geophys. Geosyst. 13, 10.1029/2012GC004207, (2012). [Google Scholar]
- 51.P. Stoffynegli, F. T. Mackenzie, Mass balance of dissolved lithium in the oceans. Geochim. Cosmochim. Acta 48, 859–872 (1984). [Google Scholar]
- 52.C. G. Wheat, A. T. Fisher, J. McManus, S. M. Hulme, B. N. Orcutt, Cool seafloor hydrothermal springs reveal global geochemical fluxes. Earth Planet. Sci. Lett. 476, 179–188 (2017). [Google Scholar]
- 53.D. Vance, D. A. H. Teagle, G. L. Foster, Variable Quaternary chemical weathering fluxes and imbalances in marine geochemical budgets. Nature 458, 493–496 (2009). [DOI] [PubMed] [Google Scholar]
- 54.R. A. Berner, A model for atmospheric CO2 over Phanerozoic time. Am. J. Sci. 291, 339–376 (1991). [DOI] [PubMed] [Google Scholar]
- 55.G. Li, H. Elderfield, Evolution of carbon cycle over the past 100 million years. Geochim. Cosmochim. Acta 103, 11–25 (2013). [Google Scholar]
- 56.L.-H. Chan, W. P. Leeman, T. Plank, Lithium isotopic composition of marine sediments. Geochem. Geophys. Geosyst. 7, 10.1029/2005GC001202, (2006). [Google Scholar]
- 57.D. B. Rowley, Rate of plate creation and destruction: 180 Ma to present. Bull. Geol. Soc. Am. 114, 927–933 (2002). [Google Scholar]
- 58.M. A. Antonelli, N. J. Pester, S. T. Brown, D. J. DePaolo, Effect of paleoseawater composition on hydrothermal exchange in midocean ridges. Proc. Natl. Acad. Sci. U.S.A. 114, 12413–12418 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.A. N. Meckler, P. F. Sexton, A. M. Piasecki, T. J. Leutert, J. Marquardt, M. Ziegler, T. Agterhuis, L. J. Lourens, J. W. B. Rae, J. Barnet, A. Tripati, S. M. Bernasconi, Cenozoic evolution of deep ocean temperature from clumped isotope thermometry. Science 377, 86–90 (2022). [DOI] [PubMed] [Google Scholar]
- 60.Z. Hu, Z. Shi, G. Li, Z. Xia, L. Yi, C. Liu, W. Li, The Cenozoic seawater conundrum: New constraints from Mg isotopes in island dolostones. Earth Planet. Sci. Lett. 595, 117755 (2022). [Google Scholar]
- 61.L. A. Coogan, S. E. Dosso, Alteration of ocean crust provides a strong temperature dependent feedback on the geological carbon cycle and is a primary driver of the Sr-isotopic composition of seawater. Earth Planet. Sci. Lett. 415, 38–46 (2015). [Google Scholar]
- 62.T. K. Lowenstein, L. A. Hardie, Criteria for the recognition of salt-pan evaporites. Sedimentology 32, 627–644 (1985). [Google Scholar]
- 63.C. Ayora, C. Taberner, C. Pierre, J.-J. Pueyo, Modeling the sulfur and oxygen isotopic composition of sulfates through a halite-potash sequence: Implications for the hydrological evolution of the upper Eocene Southpyrenean basin. Geochim. Cosmochim. Acta 59, 1799–1808 (1995). [Google Scholar]
- 64.L. Rosell, J.-J. Pueyo, Second marine evaporitic phase in the south Pyrenean Foredeep: The Priabonian potash basin (Late Eocene: autochthonous-allochthonous Zone), in Sedimentary Deposition in Rift and Foreland Basins in France and Spain (Paleogene and Neogene), G. Busson, B. C. Schreiber, Eds. (Columbia Univ. Press, 1997), pp. 358–387. [Google Scholar]
- 65.I. K. Zherebtsova, N. N. Volkova, Experimental study of behavior of trace elements in the process of natural solar evaporation of Black Sea water and Sasyk-Sivash brine. Geochem. Int. 3, 656–670 (1966). [Google Scholar]
- 66.A. M. Gothmann, J. Stolarski, J. F. Adkins, B. Schoene, K. J. Dennis, D. P. Schrag, M. Mazur, M. L. Bender, Fossil corals as an archive of secular variations in seawater chemistry since the Mesozoic. Geochim. Cosmochim. Acta 160, 188–208 (2015). [Google Scholar]
- 67.J. A. D. Dickson, Fossil echinoderms as monitor of the Mg/Ca ratio of Phanerozoic oceans. Science 298, 1222–1224 (2002). [DOI] [PubMed] [Google Scholar]
- 68.R. M. Coggon, D. A. H. Teagle, C. E. Smith-Duque, J. C. Alt, M. J. Cooper, Reconstructing past seawater Mg/Ca and Sr/Ca from mid-ocean ridge flank calcium carbonate veins. Science 327, 1114–1117 (2010). [DOI] [PubMed] [Google Scholar]
- 69.L. E. von Borstel, H. Zimmermann, H. Ruppert. Fluid inclusion studies in modern halite from the Inagua solar saltwork, in Eighth World Salt Symposium, R. M. Geertman, Ed. (Elsevier Sci. BV, 2000), pp. 673–678. [Google Scholar]
- 70.F. Risacher, A. Clement, A computer program for the simulation of evaporation of natural waters to high concentration. Comput. Geosci. 27, 191–201 (2001). [Google Scholar]
- 71.M. J. Mottl, VentDB: An online database of hydrothermal vent chemistry. Online database (2012). https://ecl.earthchem.org/search.php
- 72.C. R. German, K. L. Von Damm, Hydrothermal processes in Treatise on Geochemistry, H. D. Holland, K. K. Turekian, Eds. (Pergamon, 2003), pp. 181–222. [Google Scholar]
- 73.F. Cilkin, R. A. Cox, Sodium, potassium, magnesium, calcium and strontium in seawater. Deep Sea Res. Oceanogr. Abstr. 13, 789–804 (1966). [Google Scholar]
- 74.S. deVilliers, B. K. Nelson, Detection of low-temperature hydrothermal fluxes by seawater Mg and Ca anomalies. Science 285, 721–723 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S7
Tables S1 and S2
Legends for data S1 to S3
References
Data S1 to S3