Abstract
Aims
There is still no non-invasive septal reduction therapy for patients with hypertrophic obstructive cardiomyopathy (HOCM). This study aimed to investigate the feasibility, safety, and efficacy of stereotactic body radiotherapy (SBRT) in patients with drug-refractory symptomatic HOCM.
Methods and results
The radiation target of ventricular septum was determined by multiple anatomical imaging. Stereotactic body radiotherapy was performed with standard techniques. Patients were treated with a single fraction of 25 Gy, followed up at 1, 3, 6, and 12 months by clinical visit. Five patients were enrolled and completed the 12 months follow-up. The mean radioablation time was 21.6 min, and the mean target volume was 10.5 cm3. All five patients survived and showed improvements in symptoms after SBRT. At 12 months post-SBRT, the echocardiography-derived left ventricular outflow tract gradient decreased from 88 mmHg (range, 63–105) to 52 mmHg (range, 36–66) at rest and from 101 mmHg (range, 72–121) to 74 mmHg (range, 65–100) after Valsalva. The end-diastolic thickness of the targeted septum reduced from 23.7 mm (range, 20.3–29) to 22.4 mm (range, 19.7–26.5); 6 min walking distance increased from 190.4 m (range, 50–370) to 412.0 m (range, 320–480). All patients presented with new fibrosis in the irradiated septum area. No radiation-related complications were observed during SBRT and up to 12 months post procedure.
Conclusion
The current study suggests that SBRT might be a feasible radioablation therapeutic option for patients with drug-refractory symptomatic HOCM.
Trial registration
ClinicalTrials.gov Identifier: NCT04686487
Keywords: Stereotactic body radiotherapy, Hypertrophic obstructive cardiomyopathy, Left ventricular outflow tract, Cardiac fibrosis
Structured Graphical Abstract
Structured Graphical Abstract.
Introduction
Hypertrophic obstructive cardiomyopathy (HOCM) is an autosomal dominant disease, characterized by asymmetric hypertrophy of the ventricular septum and left ventricular outflow tract (LVOT) obstruction. It can cause dizziness, angina pectoris, dyspnoea, and even sudden cardiac death (SCD).1 It is now clear that the vast majority of mutations in HOCM involve cardiac β-myosin heavy chain and myosin-binding protein C.2 Despite therapeutic advancements, SCD is still the most dangerous complication of HOCM, especially in younger patients. Macroscopically, HOCM is characterized by thickened left ventricular walls and reduced left ventricular volume, whereas at the tissue level, the HOCM myocardium typically shows interstitial fibrosis and fibre disarray.3 At the cellular level, cardiac myocytes are hypertrophied, disorganized, and separated by areas of interstitial fibrosis.
According to current evidence and guidelines,4,5 pharmacologic therapy with β-blockers, non-dihydropyridine calcium-channel blockers and disopyramide is the mainstay of treatment in HOCM. Mavacamten, a selective inhibitor of cardiac myosin, has recently emerged as a novel medical therapy for HOCM.6 Invasive or surgical operation is often applied to patients with HOCM refractory to medical management to mitigate LVOT obstruction and ameliorate symptoms.
Currently, there are three main invasive methods aimed at reducing septum volume for HOCM.7 Myectomy and alcohol septal ablation (ASA) are the most popular procedures for patients with drug-refractory HOCM, and their success is highly dependent on the experience of operators. The long-term mortality rates of myectomy and ASA are 8.2 and 26.1%, respectively, based on a recent study.8 Compared with myectomy, ASA is also associated with a higher rate of complete heart block, repeated procedures, a greater residual outflow pressure gradient, and a possible increased risk of scar-related ventricular arrhythmias, which limit the broad application of this invasive procedure in the treatment of patients with HOCM. Notably, intramyocardial septal radiofrequency ablation is a new and less invasive myocardial reduction option for patients with HOCM.9,10 However, its efficacy needs to be confirmed in large-scale clinical studies.
Despite encouraging advances in the treatment of HOCM, less invasive septal reduction therapy options remain absent.2 Stereotactic body radiotherapy (SBRT) is a well-established therapeutic option for patients with tumours.11 Recently, SBRT has been applied to patients with refractory ventricular tachycardia12 and achieved significant therapeutic effects in selected patients, highlighting the possibility of using SBRT to treat patients with other cardiac diseases.
Recently, we confirmed the safety of SBRT in swine.13 In this first-in-human study, we tested the hypothesis that SBRT could be a feasible radioablation option to target hypertrophied septum and reduce LVOT obstruction in drug-refectory HOCM patients.
Methods
Study patients
Patients aged ≥18 years with a confirmed diagnosis of HOCM4 were screened for SBRT if they had obstructive symptoms including chest pain, chest distress, palpitations, shortness of breath, or syncope despite optimal medication for >3 months, with a maximal septum thickness of ≥15 mm and peak LVOT gradient ≥50 mmHg at rest measured by echocardiography. Patients did not want to receive surgery or ASA and agreed to participate in this study and provided written informed consent. Patients who were enrolled in other clinical studies, unable to tolerate lying flat for 1 h, pregnant or lactating, and with other contraindications to stereotactic ablative radiotherapy were excluded. This trial (SELECT study) was registered at ClinicalTrials.gov (NCT04686487). The study protocol was approved by the Institutional Ethics Committee of the Second Xiangya Hospital of Central South University (#2018044) and was performed in accordance with the ethical standards of the Declaration of Helsinki.
Procedural workflow
The procedural workflow of SBRT is shown in Figure 1. The patients underwent echocardiography, computed tomography (CT), and cardiovascular magnetic resonance (CMR) prior to SBRT. The clinical symptoms, New York Heart Association (NYHA) classification, Kansas City Cardiomyopathy Questionnaire (KCCQ), and 6 min walking distance (6MWD) test were also assessed before SBRT, and the radiation target volume was comprehensively determined by multiple-image matching.
Figure 1.
Procedure workflow. Patients undergo non-invasive visualization with computed tomography, echocardiography, and cardiovascular magnetic resonance according to clinical routine. Anatomical information obtained from the above imaging procedures is used to develop the radiation volume, which is called ‘multi-image matching’. The target volume is transferred by the radiation oncologist onto a respiratory-correlated, four-dimensional computed tomography scan, which allows an assessment of the total cardiac and pulmonary motion. In this example, a dose of 25 Gy in a single fraction is prescribed for delivery to the target volume, with the goal of achieving maximal coverage inside the volume while avoiding exposure to the surrounding organs at risk. The target volume is indicated in a red cube in the figure panel showing the treatment plan; a green dot indicates the His bundle. The patient is immobilized with the use of a vacuum-assisted device, and stereotactic radiation is performed by means of an image-guided, radiotherapy-equipped linear accelerator. After treatment, the clinical and haemodynamic conditions are monitored by electrocardiogram, echocardiography, or cardiovascular magnetic resonance during 12 months follow-up.
Multi-image matching
The anatomic information (magnetic resonance imaging, CT, and/or echocardiography) were used to build a target for radioablation. In general, the targeted area was 2–4 cm below the non-coronary cusp along the long axis of the interventricular septum, with a 2 cm width and 1 cm depth, avoiding the aortic valve, His bundle, and papillary muscles of left ventricle. The total contoured volume was 4 cm3. To confirm the absence of His bundle in the defined target radiation area, His-bundle mapping was performed in two out of five patients. Briefly, a 7 F arterial sheath was placed in the femoral artery using Seldinger’s method, and a 10-polar diagnostic catheter (Inquiry™ steerable diagnostic catheter; Abbott, USA) was delivered retrogradely across aortic valves and into left ventricle. The biggest His-bundle potential was usually found in the area of left ventricular superior septum, near the root of aorta by three-dimensional reconstruction (EnSite™ Velocity™ Cardiac Mapping System; Abbott). As expected, absence of His bundle was confirmed by His-bundle mapping in the defined target radiation area.
Target volume margining
The treatment target volume was defined as described above, and the location and shape of the target were outlined on the free-breathing CT scan using the treatment planning system (TPS). The target is referred to as the gross target volume (GTV). After outlining the GTV, an additional safety margin of 3 mm around the GTV was added to account for internal motion of the GTV caused by breathing and cardiac motion, as assessed by review of the 4D-CT. This is called the planning target volume (PTV). Based on our pre-clinical research,13 the SBRT radiation treatment plan was generated in the TPS to deliver a total dose of 25 Gy in a single treatment fraction to cover the entire region of the PTV.
Radioablation delivery
The radiation treatment was delivered using an image-guided radiotherapy-equipped linear accelerator (Trilogy, Varian Medical Systems, USA).12 Before radiation, a custom immobilization device for the patient was created to prohibit the patient from moving and to restrict the breath extent during treatment. A series of CT scans are acquired including a free-breathing CT and a respiration-correlated CT (4D-CT), which provides information about the sum of cardiac and pulmonary motion. A cone-beam CT was performed before treatment and was compared with the reference CT from the TPS. Automatic fusion was performed by Siemens Syngo (Malverne, PA, USA) software, and correctional couch shifts were implemented to achieve millimetre accuracy in positioning. Computed tomography contrast was used during the free-breathing CT to facilitate definition of cardiac structures when not otherwise contraindicated. A total dose of 25 Gy in a single fraction was administered to the pre-defined target volume with the goal of achieving sufficient dose coverage while avoiding or limiting radiation injury to the surrounding organs, including the oesophagus, stomach, lungs, and spinal cord. The entire procedure was performed on conscious patients without sedation or anaesthesia.
Outcome assessments
The primary safety endpoint was defined as serious adverse events (SAEs) that are possibly or probably or definitely related to SBRT, including radiation-induced pneumonitis, atrioventricular block, and dysphagia. The secondary outcome was the assessment of major adverse cardiac events (MACE) endpoints including death, heart failure, myocardial infarction and stroke, change in LVOT gradient, exercise capacity, and ventricular septum thickness post-SBRT.
After treatment, patients were followed up by clinical visits according to the standard of care protocol established for patients undergoing SBRT. Serum concentrations of N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity cardiac troponin T (cTnT), 6MWD, NYHA classification, KCCQ, electrocardiogram (ECG), 24 h Holter, echocardiography, CMR, and pulmonary CT were assessed before SBRT and at 1, 3, 6, and 12 months follow-ups. All clinical data assembled from the study population are maintained in an institutional database.
Transthoracic echocardiographic examinations were performed in all patients with the EPIQ7C ultrasound systems (Philips Medical Systems, USA) according to standard protocol.14 All echocardiographic recordings consisted of three cardiac cycles with a frame rate >60 frames/s. Chamber and wall dimensions of the left ventricle were measured in the two-dimensional parasternal images. Two-dimensional and Doppler echocardiographies were acquired based on the guidelines of the American Society of Echocardiography. Images were stored and analysed by two independent observers (N.L. and F.X.) blinded to clinical data. Subsequently, chamber and wall dimensions of the left ventricle were measured in the two-dimensional parasternal long-axis images. Left ventricular ejection fraction and left atrial volume were calculated from the apical views using the Simpson biplane method with left atrial volume indexed to body surface area. Left ventricular outflow tract obstruction was assessed from a modified five-chamber view optimized to align the continuous wave Doppler with flow direction in LVOT. Maximum flow velocity was measured at rest and during the Valsalva manoeuvre and was automatically converted into the LVOT pressure gradient by using the modified Bernoulli formula. For the Valsalva manoeuvre, patients were carefully instructed to increase their intrathoracic pressure to a maximum at a midrange volume to maintain an apical window for echocardiographic imaging. The Valsalva manoeuvre was repeated at least three times, and the highest outflow gradient was registered. The presence of systolic anterior motion of the mitral valve together with mitral valve regurgitation was assessed in both parasternal and apical views.
Cardiovascular magnetic resonance was performed using a 3.0 T scanner (Skyra, Siemens Healthcare, Germany) with the following protocol: balanced steady-state free precession for long- and short-axis cine; standard late gadolinium enhancement (LGE) imaging matching long- and short-axis cine slices for the evaluation of fibrosis burde; T1-modified Look-Locker inversion recovery acquisition with a 5(3)3 acquisition scheme for T1 mapping and T2-prepared steady-state free-precession sequence for T2 mapping. T1 and T2 mapping were performed before gadolinium chelate injection. Late gadolinium enhancement images were obtained 10–15 min after the administration of a gadolinium-based contrast agent (Magnevist, Bayer, Germany) at a dose of 0.2 mmol/kg. Image post processing was performed on a commercially available software (Cvi42, Version 5.13.5; Circle Cardiovascular Imaging Inc, Canada) by an experienced CMR operator. Morphological and functional parameters were calculated from cine images. Late gadolinium enhancement area was identified semi-automatically using full-width half-maximum approach. All the CMR analyses were performed by a third organization (Department of Magnetic Resonance Imaging, Fuwai Hospital, National Center for Cardiovascular Diseases) with blind method.
Results
Patients
From December 2020 to August 2022, five drug-refractory symptomatic HOCM patients (two females, three males) were enrolled in this first-in-human study. In this study, all five patients were applied with β-blocker and only one of them could tolerate another calcium antagonist. Complete clinical data before the procedure and follow-up results up to 1 year after the procedure for individual patients are presented in the Supplementary material online, Appendix. Table 1 outlines the demographic and clinical data for each patient. The mean age of enrolled patients was 52 years (range, 33–72). The echocardiography-derived baseline LVOT gradient was 88 mmHg (range, 63–105) at rest, 101 mmHg (range, 72–121) after the Valsalva manoeuvre. All patients suffered from chest distress or shortness of breath, two out of five patients suffered from chest pain and one out of five patients suffered from syncope or palpitation, even with regular medication therapy.
Table 1.
Demographic and clinical characteristics of the patients and treatment details
| Variable | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 |
|---|---|---|---|---|---|
| Age (year) | 72 | 33 | 67 | 52 | 38 |
| Sex | Male | Male | Female | Female | Male |
| Height (cm) | 166 | 178 | 153 | 158 | 172 |
| Weight (kg) | 67 | 82 | 58 | 65 | 74 |
| BSA (kg/m2) | 1.75 | 2.0 | 1.55 | 1.66 | 1.87 |
| Resting LVOT-PG (mmHg) | 63 | 75 | 100 | 105 | 97 |
| Provocation LVOT-PG (mmHg) | 72 | 82 | 121 | 112 | 118 |
| SBP (mmHg) | 120 | 92 | 112 | 99 | 120 |
| DBP (mmHg) | 70 | 64 | 74 | 70 | 73 |
| NYHA class | IV | III | III | III | III |
| LVEF (%) | 64 | 54 | 58 | 55 | 65 |
| Ablation volume (mL) | 8.35 | 12.75 | 10.48 | 10.16 | 10.75 |
| Treatment time (min) | 20.7 | 22.1 | 22.6 | 23.3 | 21 |
| Symptoms | |||||
| Chest pain | Yes | Yes | No | No | No |
| Chest distress | Yes | Yes | Yes | Yes | Yes |
| Shortness of breath | Yes | Yes | Yes | Yes | Yes |
| Palpitations | No | Yes | No | No | No |
| Syncope | Yes | No | No | No | No |
| Medical history | β-Blocker, calcium antagonist | β-Blocker | β-Blocker | β-Blocker | β-Blocker |
BSA, body surface area; DBP, diastolic blood pressure; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; NYHA, New York Heart Association; PG, peak gradient; SBP, systolic blood pressure.
Stereotactic body radiotherapy
All the patients completed the SBRT procedure without complications. The procedural details for each patient are provided in the Supplementary material online, Appendix. The treatment characteristics are presented in Table 1. The mean radioablation time was 21.6 min (range, 20–23). The mean clinical target volume was 10.5 cm3 (range, 8.35–12.75 cm3).
Clinical and haemodynamic changes after the procedure
It seems the myocardium did not suffer an intense and acute injury from the radiation. The serum levels of cTnT increased from 31.8 pg/mL (range, 10–60.9) at baseline to 39.28 pg/mL (range, 11.8–60.1) at 1 day post-SBRT and returned to 31.56 pg/mL (range, 11.7–62.4) at 12 months after SBRT. The NT-proBNP level increased from 3191 pg/mL (range, 1481–6616) at baseline to 6722 pg/mL (range, 1811–11 238) at 1 day post-SBRT, and returned to 3655 pg/mL (range, 1863–6972) at 12 months after SBRT (Figure 2).
Figure 2.
Assessment of clinical improvement after cardiac radiation therapy. (A) The serum level of cardiac troponin before and after stereotactic body radiotherapy. (B) The serum level of N-terminal pro-B-type natriuretic peptide before and after stereotactic body radiotherapy. (C) New York Heart Association classification of cardiac function before and after stereotactic body radiotherapy. (D) Six minute walking distance test before and after stereotactic body radiotherapy. (E) Kansas City Cardiomyopathy Questionnaire before and after stereotactic body radiotherapy.
All five patients involved in this study survived and showed improvements in symptoms. Furthermore, 12 months after SBRT, NYHA classification improved from III to II in two patients and from IV to III in one patient. The other two patients remained with Class III heart function, while the KCCQ improved. The mean KCCQ of these 5 patients increased from 61 (range, 47.3–70) to 77 (range, 55–88.3). Six-minute walking distance increased in all patients: the mean value was 190.4 m (range, 50–370) at baseline, 396.2 m (range, 300–540) at 1 month follow-up, 382.2 m (range, 312–420) at 3 months follow-up, 401.6 m (range, 280–540) at 6 months follow-up and 412 m (range, 320–480) at 12 months follow-up (Figure 2). Importantly, the Patient 1 had syncope episodes two to three times per year before SBRT, whereas no syncope was observed during the 1-year follow-up period after SBRT.
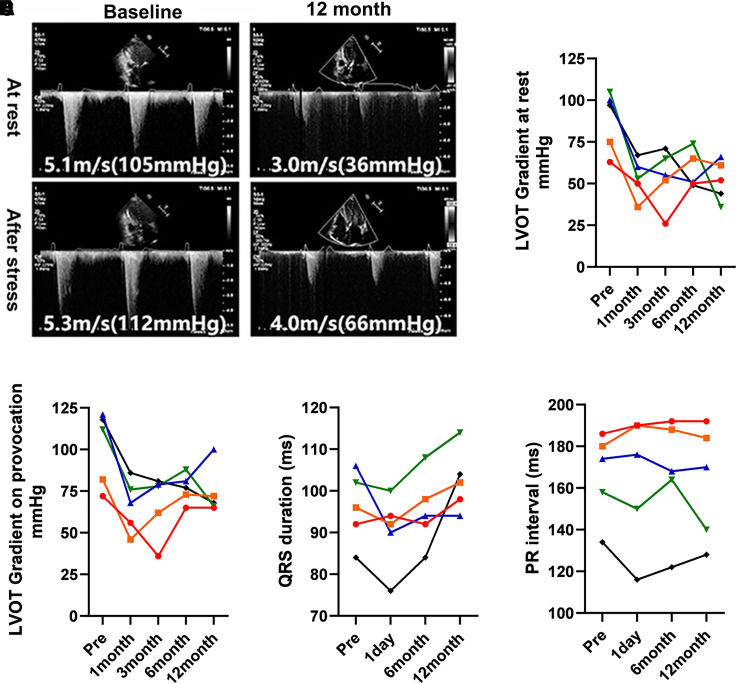
To clarify the LVOT gradient before and after SBRT, echocardiography assessment was applied during follow-up. As shown in Figure 3, the LVOT gradient decreased in all patients after SBRT. The echocardiography-derived LVOT gradient decreased from 88 mmHg (range, 63–105) to 52 mmHg (range, 36–66) at rest and 101 mmHg (range, 72–121) to 74 mmHg (range, 65–100) after Valsalva manoeuvre at 12 months follow-up. However, there is a trend of bouncing back of both LVOT gradient at rest and after Valsalva during follow-up. The inversion point usually occurred between 1 and 3 months post-SBRT. In this study, we monitored the mitral regurgitation volume before and after SBRT, the specific data were described in the Supplementary material online, Appendix. Generally, the mitral regurgitation volume was slightly reduced post-SBRT.
Figure 3.
Assessment of left ventricular outflow tract gradient and cardiac conduction after cardiac radiation therapy. (A) Presentive images of the left ventricular outflow tract gradient from one patient 12 months post-stereotactic body radiotherapy. (B) Left ventricular outflow tract gradient at rest by echocardiography before and 12 months post-stereotactic body radiotherapy. (C) Left ventricular outflow tract gradient after Valsalva manoeuvre by echocardiography before and 12 months post-stereotactic body radiotherapy. (D) QRS duration before and after stereotactic body radiotherapy. (E) PR interval before and after stereotactic body radiotherapy.
To identify the myocardium change after radiation, CMR was applied before and after SBRT. As shown in Figure 4, new cardiac fibrosis did not appear until 3 months after SBRT, which can be observed with LGE and T1 mapping. In addition, the newly emerged fibrosis was most obvious at 6 and 12 months follow-up post-SBRT. And with the help of segment analysis of LGE and T1 mapping, fibrosis was observed to be primarily located in segments 3 and 9, corresponding to the targeted septum area. Moreover, myocardial oedema was quantified by T2 mapping, which showed a persistent high signal in the targeted septum of SBRT, indicating a chronic and durable injury of the radiated myocardium.
Figure 4.
Assessment of cardiac fibrosis after cardiac radiation therapy. Analysis of (A) late gadolinium enhancement, (B) T1 mapping in different segments of the septum before and after stereotactic body radiotherapy (the American Heart Association standard 16-segment model was used). (C) Representative short axis (upper panel), long axis (middle panel) of late gadolinium enhancement picture, and T1 mapping (lower panel) before and post-stereotactic body radiotherapy by cardiovascular magnetic resonance. (Red arrows showed the fibrosis area.) (D) Representative T2 mapping images of five patients 12 months post-stereotactic body radiotherapy.
According to the newly developed fibrosis area (targeted septum), end-diastolic thickness of the targeted septum was measured by CMR. Septum thickness was measured by CMR imaging (Figure 5), mean value decreased from 23.7 mm (range, 20.3–29) at baseline to 22.4 mm (range, 19.7–26.5) at 12 months follow-up. Notably, the thickness of the septal wall was 23.7 mm at baseline and 22.4 mm at 1 year post-SBRT; however, this minor change could not be clearly visualized in CMR imaging.
Figure 5.

Assessment of targeted septum thickness by cardiovascular magnetic resonance. Analysis of targeted septum thickness at diastolic end before and after stereotactic body radiotherapy.
Serious adverse events related with stereotactic body radiotherapy
No SAEs related to SBRT, including pneumonitis, atrioventricular block, or dysphagia, were observed during the 12 months follow-up (see Supplementary material online, Appendix). Moreover, pulmonary CT images were performed to observe if there was newly developed pulmonary fibrosis after SBRT. Our results showed that SBRT did not induce pulmonary fibrosis. As shown in Figure 3D, the mean QRS duration was 96 ms (range, 84–106) at baseline, 90.4 ms (76–100) at 1 day post-SBRT, 95.2 ms (84–108) at 6 months post-SBRT and 100.4 (88–114) at 12 months follow-up. As shown in Figure 3E, the PR interval was 166.4 ms (134–186) at baseline, 164.4 ms (116–190) at 1 day post-SBRT, 166.8 ms (122–192) at 6 months post-SBRT and 162.8 ms (128–192) at 12 months follow-up. No MACE occurred among the five patients during the 12 months follow-up.
Discussion
In this first-in-human study, a septal radioablation procedure was applied for patients with drug-refractory HOCM, who did not wish to be treated with surgery or ASA. The LVOT gradient was slightly reduced and the symptom was relieved during the 12 months follow-up after SBRT. Importantly, no unwanted complications occurred during the procedure or the follow-up period.
Stereotactic body radiotherapy is usually used in cancer treatment to kill cancer cells and reduce tumour volume. Some pioneering pre-clinical and clinical studies were conducted several years ago to investigate the radiobiological effect and feasibility or efficacy of SBRT in cardiovascular disease.12,15–18 Intrigued by the previous studies, we investigated the effects of septum radiation in swine,13 which showed that after 25 or 40 Gy radiation on the ventricular septum, serum cTnT level increase at 1 day post-SBRT and the degree of necrosis in the irradiated interventricular septum at 6 months after SBRT were observed in both groups and which were more obvious in the 40 Gy dose group as compared with the 25 Gy group. Based on the above work, for the first time, we applied SBRT at a dose of 25 Gy to patients with drug-refractory HOCM, who did not wish to be treated with surgery or ASA. Although the reduction of LVOT gradient and septum thickness after SBRT is not as significant as that after ASA or myectomy, septal radioablation results in significant symptom relief and exercise capacity increase in our patient cohort, the primary results hinted that SBRT might be a feasible radioablation therapeutic option for patients with drug-refractory symptomatic HOCM.
In this study, it is interesting that the LVOT gradient mainly decreased at 1 and 3 months follow-up; however, it increased 3–6 months post-SBRT. The 6MWD showed a similar trend. We speculate that SBRT may have two kinds of effects on myocardium: pathological effect and functional effect. The pathological effect might lead to cell apoptosis or death and form fibrosis. Currently, the functional effect, to date, has not been fully understood. Radiation may inhibit the myocardial function without causing pathological injury to the myocardium. The dysfunction of the septum myocardium may also contribute to the LVOT gradient reduction. Unlike pathological injury, the functional effect might gradually fade with time, which might cause the ‘bounce back’ curve of the LVOT gradient. This phenomenon looks like the ‘myocardial stunning and hibernation’ after myocardial ischaemia,19 which is also a kind of functional inhibition of the myocardium.
In this study, CMR provided in-depth observation of radiation-induced injury in the myocardium. Gradually increased fibrosis was evidenced by CMR imaging in all patients (especially in the last four patients) in the targeted septum at 6 and 12 months post-SBRT. The T2 mapping of CMR showed increased oedema of the targeted area even at 12 months post-SBRT, which indicated a sustained injury after radiation. This finding is consistent with previous studies in cerebral radiation, in that the mean and/or median time to the onset of oedema were within the range of ∼3–9 months and can endure up to 3–4 years after radiation.20,21 Although the T2 mapping data were missed in the early follow-up, we can speculate myocardium oedema occurred late after SBRT. The delayed oedema may also contribute to the gradient rebound in this study. Besides, the delayed oedema combined with the nearly unchanged serum level of cTnT confirmed the chronic injury post-SBRT.
The major mechanism accounting of radiation-induced cell death is DNA damage, with double-strand breaks (DSBs) being the most lethal form. Oncogene-induced hyper-proliferation and DNA replication stress are characteristic of cancers, making cancer cells vulnerable to radiation. Similarly, it has been reported that pressure overload or mutations might lead to replication stress response and DNA DSBs in cardiomyocytes,22 which may be attributable to the vulnerability of hypertrophic cardiomyocytes to ionizing radiation injury.
Safety is of the utmost importance for a novel therapeutic option. The greatest challenge of cardiac SBRT is to prevent the potential risk of injuring the atrioventricular conduction system. In our study, several efforts were made to avoid inflicting unwanted radiation-induced injury on the conduction system, especially on the His bundle. Defining the target volume was achieved with the help of three-dimensional CT reconstruction together with electrophysiological mapping. The location and shape of the pre-defined radiation target were then outlined on a free-breathing CT scan using the TPS. In fact, no significant conduction abnormalities were observed during SBRT or subsequent follow-up.
Study limitations
This study had certain limitations. First, this first-in-human study enrolled only five patients. Randomized clinical trials with a larger patient cohort are needed to validate the promising results of this study. Second, the molecular mechanisms of SBRT in HOCM should be further investigated in basic experimental studies. Third, prolonged Holter monitoring, post-procedural EPS, and/or event/loop recorder were not performed in our study, which should be part of the future study protocol. Fourth, in this FIM study, we did not apply the ECG gating during SBRT. Cardiac motion, including the changes derived from differences in heart rate, pre-load and afterload, may considerably change the target locations in these millimetric targets. We will improve the SBRT procedure in future studies. Last but not least, potential peripheral organ damage post-SBRT, such as objective assessment of oesophageal function, should be evaluated in future studies.
Conclusion
Ventricular septal SBRT might be a feasible radioablation therapeutic option for patients with drug-refractory HOCM.
Supplementary Material
Acknowledgements
The authors thank Prof. Shihua Zhao and Dr Jiaxin Wang from the Department of Magnetic Resonance Imaging, Fuwai Hospital, National Center for Cardiovascular Diseases for performing the CMR analysis. The authors thank Dr Emily L. Gilbert from Remission Medical, Jacksonville Beach, FL, for her assistance in animation dubbing.
Contributor Information
Xuping Li, Department of Cardiovascular Medicine, The Second Xiangya Hospital, Central South University, 139 Mid-Renmin Road, Changsha, Hunan 410011, China.
Zhaowei Zhu, Department of Cardiovascular Medicine, The Second Xiangya Hospital, Central South University, 139 Mid-Renmin Road, Changsha, Hunan 410011, China.
Jun Liu, Radiology Department, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China.
Yawen Gao, Oncology Department, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China.
Yichao Xiao, Department of Cardiovascular Medicine, The Second Xiangya Hospital, Central South University, 139 Mid-Renmin Road, Changsha, Hunan 410011, China.
Zhenfei Fang, Department of Cardiovascular Medicine, The Second Xiangya Hospital, Central South University, 139 Mid-Renmin Road, Changsha, Hunan 410011, China.
Qiming Liu, Department of Cardiovascular Medicine, The Second Xiangya Hospital, Central South University, 139 Mid-Renmin Road, Changsha, Hunan 410011, China.
Xianling Liu, Oncology Department, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China.
Chunhong Hu, Oncology Department, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China.
Fang Ma, Oncology Department, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China.
Mu Zeng, Radiology Department, The Second Xiangya Hospital, Central South University, Changsha, Hunan, China.
Zhi Liu, Anesthesiology Department, Hunan Provincial People’s Hospital, Changsha, Hunan, China.
Lin Hu, Department of Cardiovascular Medicine, The Second Xiangya Hospital, Central South University, 139 Mid-Renmin Road, Changsha, Hunan 410011, China.
Na Liu, Department of Cardiovascular Medicine, The Second Xiangya Hospital, Central South University, 139 Mid-Renmin Road, Changsha, Hunan 410011, China.
Fan Xiang, Department of Cardiovascular Medicine, The Second Xiangya Hospital, Central South University, 139 Mid-Renmin Road, Changsha, Hunan 410011, China.
Xinqun Hu, Department of Cardiovascular Medicine, The Second Xiangya Hospital, Central South University, 139 Mid-Renmin Road, Changsha, Hunan 410011, China.
Lihong Huang, Zhongshan Hospital Affiliated to Fudan University, Shanghai, China.
Shenghua Zhou, Department of Cardiovascular Medicine, The Second Xiangya Hospital, Central South University, 139 Mid-Renmin Road, Changsha, Hunan 410011, China.
Lead author biography
 Dr Shenghua Zhou, MD, FACC, FHRS Director, Department of Cardiology of the Second Xiangya Hospital, Central South University, China. He is a member of the Standing Committee of Chinese Society of Pacing and Electrophysiology and Chinese Society of Cardiology, and chairman of the Hunan provincial Society of Cardiology. His specialty and research interests are interventional cardiology and anticoagulation therapy in acute coronary syndrome.
Dr Shenghua Zhou, MD, FACC, FHRS Director, Department of Cardiology of the Second Xiangya Hospital, Central South University, China. He is a member of the Standing Committee of Chinese Society of Pacing and Electrophysiology and Chinese Society of Cardiology, and chairman of the Hunan provincial Society of Cardiology. His specialty and research interests are interventional cardiology and anticoagulation therapy in acute coronary syndrome.
Supplementary material
Supplementary material is available at European Heart Journal Open online.
Funding
This work was supported by the National Nature Science Foundation of China (NSFC) Project 82150006 (to S.Z.).
Data availability
The data underlying this article cannot be shared publicly to protect the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
References
- 1.Ommen SR, Semsarian C. Hypertrophic cardiomyopathy: a practical approach to guideline directed management. Lancet 2021;398:2102–2108. [DOI] [PubMed] [Google Scholar]
- 2.Tuohy CV, Kaul S, Song HK, Nazer B, Heitner SB. Hypertrophic cardiomyopathy: the future of treatment. Eur J Heart Fail 2020;22:228–240. [DOI] [PubMed] [Google Scholar]
- 3.Cui H, Schaff HV, Lentz CJ, Nishimura RA, Geske JB, Dearani JA, Lahr BD, Lee AT, Bos JM, Ackerman MJ, Ommen SR, Maleszewski JJ. Myocardial histopathology in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2021;77:2159–2170. [DOI] [PubMed] [Google Scholar]
- 4.Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, Evanovich LL, Hung J, Joglar JA, Kantor P, Kimmelstiel C, Kittleson M, Link MS, Maron MS, Martinez MW, Miyake CY, Schaff HV, Semsarian C, Sorajja P. 2020 AHA/ACC guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2020;76:e159–e240. [DOI] [PubMed] [Google Scholar]
- 5.Dybro AM, Rasmussen TB, Nielsen RR, Andersen MJ, Jensen MK, Poulsen SH. Randomized trial of metoprolol in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2021;78:2505–2517. [DOI] [PubMed] [Google Scholar]
- 6.Spertus JA, Fine JT, Elliott P, Ho CY, Olivotto I, Saberi S, Li W, Dolan C, Reaney M, Sehnert AJ, Jacoby D. Mavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): health status analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2021;397:2467–2475. [DOI] [PubMed] [Google Scholar]
- 7.Fifer MA. Septal reduction therapy for hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 2018;72:3095–3097. [DOI] [PubMed] [Google Scholar]
- 8.Cui H, Schaff HV, Wang S, Lahr BD, Rowin EJ, Rastegar H, Hu S, Eleid MF, Dearani JA, Kimmelstiel C, Maron BJ, Nishimura RA, Ommen SR, Maron MS. Survival following alcohol septal ablation or septal myectomy for patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2022;79:1647–1655. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Li J, Zuo L, Zhang J, Zhou M, Xu B, Hahn RT, Leon MB, Hsi DH, Ge J, Zhou X, Zhang J, Ge S, Xiong L. Percutaneous intramyocardial septal radiofrequency ablation for hypertrophic obstructive cardiomyopathy. J Am Coll Cardiol 2018;72:1898–1909. [DOI] [PubMed] [Google Scholar]
- 10.Zhou M, Ta S, Hahn RT, Hsi DH, Leon MB, Hu R, Zhang J, Zuo L, Li J, Wang J, Wang B, Zhu X, Liu J, Han Y, Li X, Xu B, Zhang L, Hou L, Han C, Liu J, Liu L. Percutaneous intramyocardial septal radiofrequency ablation in patients with drug-refractory hypertrophic obstructive cardiomyopathy. JAMA Cardiol 2022;7:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo SS, Fakiris AJ, Chang EL, Mayr NA, Wang JZ, Papiez L, Teh BS, McGarry RC, Cardenes HR, Timmerman RD. Stereotactic body radiation therapy: a novel treatment modality. Nat Rev Clin Oncol 2010;7:44–54. [DOI] [PubMed] [Google Scholar]
- 12.Cuculich PS, Schill MR, Kashani R, Mutic S, Lang A, Cooper D, Faddis M, Gleva M, Noheria A, Smith TW, Hallahan D, Rudy Y, Robinson CG. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Med 2017;377:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu ZW, Li XP, Gao YW, Xiao YC, Ma F, Hu CH, Liu XL, Liu J, Zeng M, Tang L, Huang YY, Zou P, Liu ZJ, Zhou SH. [Safety and feasibility of stereotactic radiation therapy on porcine ventricular septum: a preliminary study]. Zhonghua Xin Xue Guan Bing Za Zhi 2022;50:907–912. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 15.Cai X, Yang Y, Shen Y, Wang W, Qian L, Cai J, Chi R, Fei Y, Yu S, Wei L, Hou J, Wang Q, Zhang J, Wang D, Jiang M, Li YG. Noninvasive stereotactic radiotherapy for renal denervation in a swine model. J Am Coll Cardiol 2019;74:1697–1709. [DOI] [PubMed] [Google Scholar]
- 16.Blanck O, Bode F, Gebhard M, Hunold P, Brandt S, Bruder R, Grossherr M, Vonthein R, Rades D, Dunst J. Dose-escalation study for cardiac radiosurgery in a porcine model. Int J Radiat Oncol Biol Phys 2014;89:590–598. [DOI] [PubMed] [Google Scholar]
- 17.Refaat MM, Ballout JA, Zakka P, Hotait M, Al Feghali KA, Gheida IA, Saade C, Hourani M, Geara F, Tabbal M, Sfeir P, Jalbout W, Al-Jaroudi W, Jurjus A, Youssef B. Swine atrioventricular node ablation using stereotactic radiosurgery: methods and in vivo feasibility investigation for catheter-free ablation of cardiac arrhythmias. J Am Heart Assoc 2017;6:e007193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang DM, Navara R, Yin T, Szymanski J, Goldsztejn U, Kenkel C, Lang A, Mpoy C, Lipovsky CE, Qiao Y, Hicks S, Li G, Moore KMS, Bergom C, Rogers BE, Robinson CG, Cuculich PS, Schwarz JK, Rentschler SL. Cardiac radiotherapy induces electrical conduction reprogramming in the absence of transmural fibrosis. Nat Commun 2021;12:5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heusch G. Myocardial stunning and hibernation revisited. Nat Rev Cardiol 2021;18:522–536. [DOI] [PubMed] [Google Scholar]
- 20.Milano MT, Sharma M, Soltys SG, Sahgal A, Usuki KY, Saenz JM, Grimm J, El Naqa I. Radiation-induced edema after single-fraction or multifraction stereotactic radiosurgery for meningioma: a critical review. Int J Radiat Oncol Biol Phys 2018;101:344–357. [DOI] [PubMed] [Google Scholar]
- 21.Daou BJ, Palmateer G, Wilkinson DA, Thompson BG, Maher CO, Chaudhary N, Gemmete JJ, Hayman JA, Lam K, Wahl DR, Kim M, Pandey AS. Radiation-induced imaging changes and cerebral edema following stereotactic radiosurgery for brain AVMs. AJNR Am J Neuroradiol 2021;42:82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakada Y, Nhi NN, Xiao F, Savla JJ, Lam NT, Abdisalaam S, Bhattacharya S, Mukherjee S, Asaithamby A, Gillette TG, Hill JA, Sadek HA. DNA damage response mediates pressure overload-induced cardiomyocyte hypertrophy. Circulation 2019;139:1237–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly to protect the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.