Abstract
This study establishes the physiological role of Fused in Sarcoma (FUS) in mitochondrial DNA (mtDNA) repair and highlights its implications to the pathogenesis of FUS-associated neurodegenerative diseases such as Amyotrophic lateral sclerosis (ALS). Endogenous FUS interacts with and recruits mtDNA Ligase IIIα (mtLig3) to DNA damage sites within mitochondria, a relationship essential for maintaining mtDNA repair and integrity in healthy cells. Using ALS patient-derived FUS mutant cell lines, a transgenic mouse model, and human autopsy samples, we discovered that compromised FUS functionality hinders mtLig3’s repair role, resulting in increased mtDNA damage and mutations. These alterations cause various manifestations of mitochondrial dysfunction, particularly under stress conditions relevant to disease pathology. Importantly, rectifying FUS mutations in patient-derived induced pluripotent cells (iPSCs) preserves mtDNA integrity. Similarly, targeted introduction of human DNA Ligase 1 restores repair mechanisms and mitochondrial activity in FUS mutant cells, suggesting a potential therapeutic approach. Our findings unveil FUS's critical role in mitochondrial health and mtDNA repair, offering valuable insights into the mechanisms underlying mitochondrial dysfunction in FUS-associated neurodegeneration.
Keywords: Amyotrophic lateral sclerosis, FUS, mitochondria, DNA ligase, DNA damage repair
Introduction
Fused in Sarcoma (FUS) is an important RNA/DNA binding protein involved in cellular metabolism, RNA processing, and DNA repair. It has been linked to neurodegenerative diseases, including Amyotrophic lateral sclerosis (ALS) and Frontotemporal Dementia (FTD). 1, 2, 3, 4, 5. FUS mutations have been identified, contributing to a significant proportion of familial and sporadic ALS cases 6, 7. While FUS mutations can disrupt gene transcription, mRNA splicing, and RNA transport, growing evidence suggests that DNA damage accumulation and repair deficiency also play critical roles in FUS-associated neurodegeneration 8, 9. Studies have documented increased DNA damage in patient tissues and animal models with FUS pathology. Moreover, FUS interacts with various proteins such as XRCC1, PARP1 and HDAC1 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, participating in DNA damage repair processes. Recent research highlights the importance of FUS dependent liquid-liquid phase separation in initiating DNA repair 20.
In our previous investigation, we discovered that FUS forms a complex with PARP1, XRCC1, and DNA ligase IIIα (LigIII/Lig3) to initiate the repair of oxidative DNA damage and single-strand breaks (SSBs) in the nuclear genome 20. FUS plays a role in recruiting and enhancing the break-sealing activity of nuclear Lig3 (nuLig3) in a PARP activity-dependent fashion. While there are two other DNA ligases in the nucleus, namely, Lig1 associated with replicating DNA and Lig4 linked to DNA double-strand break repair, mitochondrial version of Lig3 (mtLig3) is the only DNA ligase found in mammalian mitochondria and is involved in both mitochondrial genome repair and replication 21, 22, 23. Unlike nuLig3, mtLig3 functions independently of XRCC1 and is essential for cell survival 21, 24, 25. The non-essentiality of nuLig3 may be due to the presence of Lig1, which may act as a backup for nuLig3 deficiency in cycling cells 26. Both mtLig3 and nuLig3 are generated by alternative translation initiation, resulting in the presence of N-terminal mitochondrial target sequence (MTS) in mtLig3. Thus, mtLig3, which has extensive sequence homology to nuLig3, is expected to interact with FUS. However, although overexpressed FUS has been shown to localize in mitochondria and abnormalities/damages to mitochondria are observed in FUS-ALS and FUS-FTD 27, 28, 29, its specific functions in mitochondria, particularly with respect to mitochondrial genome maintenance, and its overall native physiological role in mitochondria, remain largely uncharacterized.
This study aims to investigate the role of endogenous wildtype (WT) FUS in mtDNA repair and elucidate the mechanisms by which FUS mutations contribute to mtDNA damage and neurodegeneration. We conducted a series of experiments using multiple cell lines including CRSPR/Cas9 mediated FUS knockout (KO) HEK293, first to establish the localization and DNA repair function of FUS in mitochondria. Subsequently, we investigated various disease-relevant models with FUS mutations/proteinopathy, such as ALS patient-derived cell lines with FUS mutations, their mutation-corrected isogenic control lines, autopsied ALS patient spinal cord tissues, as well as a human FUS R495X (hFUS*R495X) transgenic mouse model to uncover the implications of compromised FUS functions in causing mtDNA damage and overall mitochondrial dysfunction in FUS-associated ALS. Our findings reveal the crucial involvement of FUS in DNA strand break ligation activity by mtLig3. FUS is essential for the recruitment of mtLig3 at the mtDNA damage sites. The mtDNA repair defects observed in FUS KO and mutant cells correlated with the accumulation of damage and mutations in mtDNA, decreased mitochondrial membrane potential, and oxygen consumption rate (OCR), as revealed by DNA integrity assays, membrane potential test, mtDNA sequencing, and Seahorse XFe96 analyses. Furthermore, correction of the FUS mutation by CRISPR/Cas9 mediated knock-in, or the targeted expression of an alternative DNA ligase (Lig1) restored mtDNA fidelity and functions, suggesting a potential for Lig1 in treating FUS associated neurodegeneration. In summary, our study uncovers critical role of FUS in mtDNA repair and provides new insights into the mechanisms of mtDNA damage and repair defects in FUS-associated neurodegenerative diseases. It highlights the potential for therapeutic strategies aimed at correcting mtDNA repair defects and restoring mitochondrial function. This knowledge not only expands our understanding of the normal role of FUS in mitochondrial DNA repair and maintenance but also opens avenues for further understanding the pathogenesis of FUS-related neurodegeneration and developing targeted therapies.
Results
Localization of WT and mutant FUS and their link to mitochondrial abnormalities.
Previous studies have reported the localization of FUS in mitochondria, but these observations were based on the overexpression of exogenous FUS 27, 30. In the current study, we first examined the endogenous levels of FUS in the mitochondria in HEK 293 cells and found that FUS levels were increased after induction of oxidative stress by glucose oxidase (GO) treatment that promotes oxidative DNA damage (Fig. 1a). To demonstrate the purity of the mitochondrial fraction, immunoblotting (IB) was performed using nuclear and cytoplasmic fraction markers. The specificity of the FUS antibody was confirmed as it showed no signal in extracts from knockout (KO) cells (Fig. 1a). We also measured the levels of FUS in ALS patient-derived fibroblasts expressing either the WT or P525L mutant form of FUS. Both the WT and mutant FUS were present in the mitochondrial fraction, and the levels were moderately increased in response to GO treatment (Fig. 1b). Furthermore, we employed a proximity ligation assay (PLA) to visualize the association of FUS and mitochondria using mitochondria-specific makers HSP60 and Tom20 in WT and mutant fibroblasts 31. The results shown in Fig. 1c confirmed the association between FUS and the mitochondrial markers, where the mutant FUS showed a significantly higher count of PLA foci in comparison to the WT FUS. Magnified images related to Fig. 1c are shown in Supplementary Fig. 1a for a more detailed view. Interestingly, FUS P525L cells showed a higher number of PLA foci per cell compared to FUS R521H cells, which could be related to the degree of FUS mislocalization caused by different mutations (Supplementary Fig. 1b). In addition, the overall number of foci were enhanced by GO treatment in both FUS WT and mutant cells (Fig. 1d; magnified images in Supplementary Fig. 1a). Finally, we validated the mitochondrial association of endogenous FUS in iPSC-derived motor neurons by proximity ligation assay (PLA). Supplementary Fig. 1c shows positive PLA foci between FUS and HSP60 in motor neurons.
Figure 1: Localization of endogenous wild type (WT) and mutant FUS to mitochondria: Both ALS associated FUS pathology or glucose oxidase stress increased mitochondrial localization.
a and b Immunoblots (IB) of cellular fractionization showing FUS in mitochondria. Total, cytoplasm and mitochondrial extracts are isolated from HEK 293 WT cells with and without glucose oxidase (GO) 100 ng/ml for 1 hr and from FUS knockout (KO) cells in a. b corresponds to patient-derived fibroblast cells with and without GO treatment. c and d show proximity ligation assay (PLA) performed in patient derived fibroblasts, c corresponds to PLA of FUS with Tom20 and HSP60 without any stress along with quantifications. Scale bar=20μM. d represents the comparison between PLA foci for FUS and HSP60 with and without GO and their quantifications. Nuclei stained with DAPI. Scale bar=20μM. Quantification of PLA foci was derived from 25 fibroblast cells.
To assess mitochondrial function, we conducted measurements of cellular respiration at baseline and after administering mitochondrial inhibitors using Seahorse XF Cell Mito Stress test protocol. Although we did not observe significant differences in non-mitochondrial or basal, maximum and spare oxygen consumption rates (OCR) between untreated and GO treated FUS WT and P525L mutant cells (Fig. 2a, b) 32. However, the ability of cells to recover from GO-induced stress demonstrated significant alterations in non-mitochondrial oxygen consumption, basal, maximal and spare respiration capacity (Fig. 2c). No significant differences were seen in proton leak and ATP production rates. (Supplementary Fig. 2).
Figure 2: Mitochondrial respiration comparison between FUS WT and FUS P525L patient derived fibroblasts by seahorse assay.
Untreated a, GO treated b, recovery after GO treatment c, sodium arsenite treated d and recovery after sodium arsenite treatment e. Oxygen consumption rate (OCR) determined throughout the mitochondrial respiration test in control and patient derived P525L fibroblasts. Arrows indicate the time when mitochondrial inhibitors were added to the media to assess respiratory parameters. Non-mitochondrial oxygen consumption was determined by measuring difference between total oxygen consumption and oligomycin induced reduction in oxygen consumption, maximal respiration was assessed following mitochondria uncoupling by FCCP and spare respiratory capacity was determined by subtracting basal respiration from maximal respiration.
FUS has been shown to colocalize with stress granules (SGs) that have significantly accumulated in FUS mutant cells in response to sodium arsenite treatment, causing oxidative stress and protein misfolding leading to translation stalling 33. This is believed to be a possible pathological mechanism of FUS aggregation formation in ALS patients. Given the localization of FUS in mitochondria and the colocalization of mutant FUS in SGs induced by sodium arsenite, we hypothesized that the accumulation of mutant FUS in SGs results in reduced FUS levels in mitochondria, thereby disturbing its normal function. As shown in (Fig. 2d and 2e), the OCR, basal respiration, maximal respiration and spare respiratory capacity of FUS P525L cells were significantly decreased compared to FUS WT after sodium arsenite treatment.
Taken together, these results suggest that a portion of endogenous FUS is localized in mitochondria and that FUS depletion or mutations lead to alterations in both mitochondrial localization of FUS and mitochondrial function, such as MtMP. These findings are thus highly significant for understanding of the role of FUS in mitochondria and its potential involvement in neurodegenerative diseases, such as ALS.
DNA damage, mutation accumulation as well as repair deficiencies in mitochondria of ALS patient-derived FUS mutant cells, mouse model and patient tissue.
To assess the integrity of the mitochondrial genome, we used a LA-PCR method that detects strand breaks in extracted DNA 34. To normalize the results, the mtDNA content and copy number were first determined by amplifying mtDNA relative to nuclear genes using the comparative Ct method (Supplementary Fig. 3) 35. To do this, real-time PCR was performed to amplify short fragment of three genes: NADH-ubiquinone oxidoreductase chain 1 (ND1) and 16SrRNA, which are mitochondrial genes, B2-microglobulin for human, and Hexokinase II(HK2) for mouse, which are nuclear genes. This experiment was conducted in HEK293 FUS WT and KO cells first to identify the normal role of FUS in mitochondria of healthy cells, and then in mutant FUS fibroblasts derived from ALS patients, and autopsy spinal cord tissues from FUS-ALS patients and hFUS*R495X mice36, to unveil the implications in FUS-ALS..
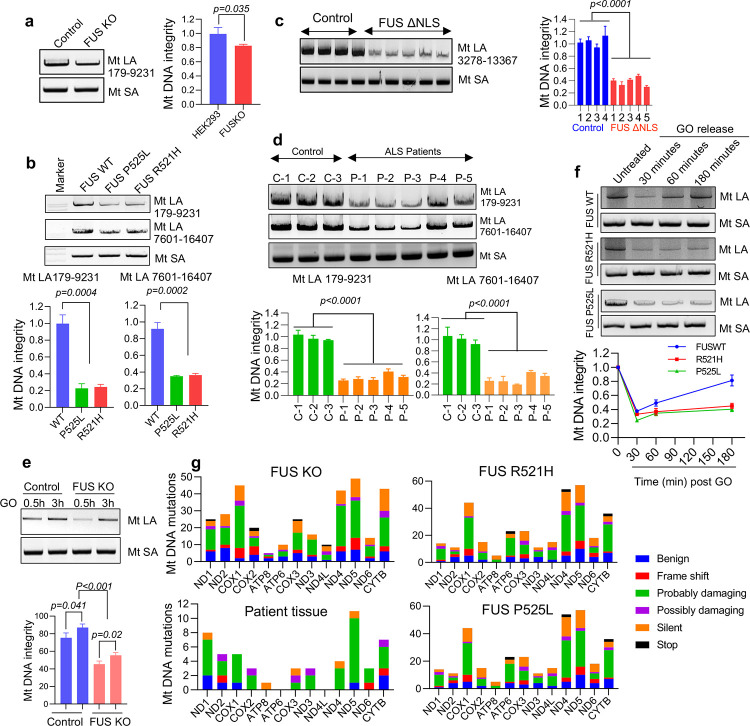
LA-PCR was then performed in FUS WT and KO HEK293 cells by amplifying two long fragments (>8000bp) of mtDNA (179–9231, and 7601–16407), and a short fragment (~200bp) as a control. The results revealed a significant reduction in DNA integrity in FUS KO cells this corelates with decreased membrane potential in FUS KO cells in comparison to WT cells suggesting FUS mediated effect on mitochondrial function (Fig. 3a, Supplementary figure 2f). A similar decrease in mtDNA integrity was observed in fibroblasts derived from ALS patient with FUS R521H and P525L mutations (Fig. 3b).
Figure 3: Accumulation of DNA damage and deficient DNA damage repair in ALS patient-derived FUS mutant cells.
a to f shows long amplification PCR (LA-PCR) to determine genomic integrity of mt DNA, >8000 bp fragment of mtDNA was amplified and separated in 1% agarose gel along with a control PCR of 200 bp (MtSA). Amplified PCR product is quantified by using pico green fluorescence a shows comparison between the control and FUS KO HEK293 cells, b shows comparison of genomic integrity between control and patient derived mutant fibroblasts using two amplicons. c represents the comparison of genomic integrity between WT mice and humanized FUS ΔNLS mice brain tissue at around 12-month age, d corresponds to human spinal cord tissue samples between control and ALS patient spinal cord with FUS pathology. e, and f differentiate the ability of FUS mutated cells and of FUS KO cells to repair oxidative DNA damage e shows patient derived fibroblasts whereas f represents HEK 293 cells. g corresponds to mitochondrial DNA sequencing in HEK 293 FUS KO cells, patient spinal cord tissues and patient derived fibroblasts performed using mitochondrial REPLI-g kit. The unique mutations in protein coding genes are represented based on the kind of mutation and the severity. The frame shift represents either insertions or deletions. Silent mutations showed coding for same amino acid, and the severity of mutation was determined either benign, probably damaging and possibly damaging based on polyphen2 analysis.
To further investigate the association between FUS and mtDNA integrity, we used a FUS transgenic mouse model expressing human FUS protein with the deletion of its NLS 36. The mtDNA extracted from brains of hFUS*R495X mice showed decreased PCR products compared to WT mice, indicating the presence of strand breaks in the mtDNA of mutant mice (Fig. 3c).
Finally, we conducted LA-PCR on mtDNA extracted from five ALS patient spinal cord tissue specimens with FUS pathology and three normal spinal cord samples as controls. The results showed significant accumulation of mtDNA damage in the patient samples (Fig. 3d). The age of the patients and additional information can be found in the Supplementary Table 1.
Several studies have demonstrated that FUS is required for optimal DNA damage repair. Our recent studies have uncovered the role of FUS role in DNA single-strand breaks (SSB) repair by promoting the activity of break-sealing DNA Lig3 in the nuclear genome 11. Based on this evidence, we hypothesized that the accumulation of DNA damage in mitochondria may be caused by a deficiency in DNA damage repair. To test this hypothesis, we evaluated the DNA damage repair kinetics in HEK293 FUS KO cells and FUS-mutant patient fibroblasts. HEK293 cells were treated with a DNA damaging agent (GO), and LA-PCR was performed 30 and 180 minutes after treatment to assess mtDNA damage repair. The results showed that mtDNA integrity increased significantly at 180 minutes in FUS WT cells, indicating successful DNA damage repair, while repair capacity was reduced in FUS KO cells (Fig. 3e). Additionally, FUS P525L mutated cells showed a delayed DNA damage repair response during recovery from the GO treatment, normalized to the relative mtDNA content, at 30, 60, and 180 minutes (Fig. 3f, and Supplementary Fig. 3c).
To further understand the possible connection between DNA damage repair defects and mtDNA instability, we performed mitochondrial DNA sequencing to measure insertions, deletions, and mutations in FUS WT, KO and mutated cells as well as ALS patient spinal cord samples. Due to the challenges of obtaining pure and sufficient mtDNA, we performed PCR to amplify mtDNA before subjecting to sequencing analysis. We identified several unique mutations in FUS KO and mutant cells, as well as in patient tissues, and we evaluated the severity of each mutation using PolyPhen-2 online tool (http://genetics.bwh.harvard.edu/pph2/) Our results showed an increased mtDNA instability in these cells and tissues associated with FUS pathology, as indicated by the high number and severity of mutations (Fig. 3g).
In conclusion, our data show a significant reduction in mtDNA integrity in FUS KO cells, patient fibroblasts, and spinal cord tissue specimens from ALS patients and FUS transgenic mice. This suggests that the accumulation of DNA damage in mitochondria and a deficiency in DNA damage repair are associated with FUS pathology in ALS. Furthermore, our evaluation of DNA damage repair kinetics and mitochondrial DNA sequencing revealed a significant increase in mtDNA instability associated with FUS mutations. These findings revealed a direct link between FUS proteinopathy and mtDNA instability and led us to further explore the relationship between FUS mediated mtDNA instability and parameters of mitochondrial dysfunction in the disease.
Impaired DNA ligation activity and recruitment of Lig3 to mtDNA damage sites in cells with FUS P525L mutation.
Based on our previous study that showed the inhibition of nuclear Lig3 activity in FUS mutant cells, we investigated whether the impaired mtDNA damage repair observed in FUS mutant cells is due to reduced Lig3 activity in mitochondria. As we previously reported the association between FUS and Lig3 in response to oxidative stress in nuclei, we first examined the protein levels of Lig3 in mitochondrial extracts of FUS WT and FUS KO cells 11. We found that the mtLig3 protein levels were not affected by FUS KO (Fig. 4a), and while XRCC1 was not detected, Lig3 was detectable in mitochondria, consistent with previous reports 24. Furthermore, mtLig3 levels were slightly elevated in cells treated to GO (Fig. 4b), and the interaction between FUS and mtLig3 was increased in the presence of GO (Fig. 4c).
Figure 4: FUS mutation impairs the ligation activity as well as the recruitment of Lig3 to DNA damage sites.
a and b immunoblot (IB) showing mitochondrial localization of key DNA repair proteins involved in oxidative damage response in HEK 293 WT and FUS KO cells mitochondria in a and WT cells treated with and without GO treatment (100ng/ml for 1 hr) in b, c represents IB of endogenous FUS co-ip from HEK293 cells for Lig3. The IP was performed with anti-FUS antibody. d PLA of FUS vs Lig3 in control and patient derived fibroblasts with or without GO treatment. Nuclei stained with DAPI. Scale bar=20 μM, quantification of PLA foci from 25 motor neuron cells. e In vitro nick ligation activity assay performed using control and patient derived fibroblast mitochondrial extracts. f ChIP analysis of WT and FUSP525L fibroblast mitochondria with Lig3 Ab reveals reduced enrichment at mitochondrial DNA after GO treatment.
To investigate the interaction between WT or mutant FUS and Lig3, we performed PLA in control and patient fibroblasts. As shown in (Fig. 4d; magnified images in supplementary Fig. 4a), the association between FUS and Lig3 was significantly increased in response to GO treatment, but the interaction was significantly reduced in cells with mutant FUS compared to those with WT FUS. We also compared the DNA ligation activity of Lig3 in mitochondrial extracts by an in vitro ligation activity assay. Although the mitochondrial DNA ligation activity was below the experimental detection level in untreated cells, it increased significantly in GO-treated cells. To compare the ligation activity between FUS P525L mutant and WT cells, we used mitochondrial total lysate from GO-treated fibroblasts. We observed a decrease in ligation activity in FUS mutant cells (Fig. 4e).
To investigate the possibility that the reduced ligation activity in FUS mutant cells was due to the failure of mtLig3 to be recruited to DNA damage sites, we conducted a chromatin immunoprecipitation (ChIP) assay. We amplified short fragments (~200b) of two mitochondria genes, ND1 and 16s rRNA, in mtLig3 immunoprecipitant. We found significantly decreased PCR products in FUS mutant samples (Fig. 4f; Supplementary Fig. 4b), which indicates that mtLig3 was not optimally recruited to DNA damage sites due to the FUS mutation. Taken together, these results suggest that FUS mutation impairs both the ligation activity of mtLig3 and its recruitment to mtDNA damage sites, which could contribute to the observed mtDNA damage repair defects in mutant FUS cells.
Role of FUS P525L mutation in mtDNA damage repair defects and its rescue by targeted expression of Lig1.
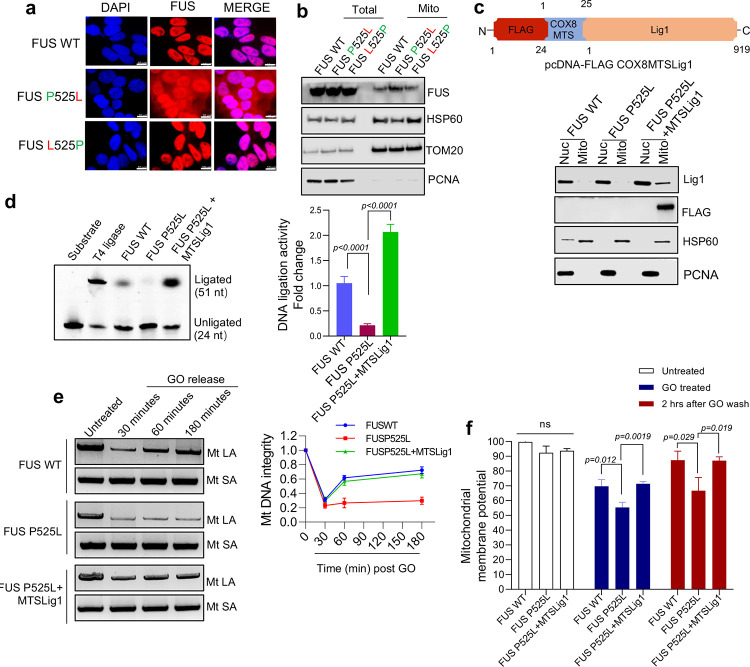
To directly link the deficiency in mtDNA damage repair to the FUS mutation, we measured mtDNA repair in an isogenic iPSC line, in which the P525L mutation was corrected using the CRISPR/Cas9 system 29. Immunofluorescence images showed that the cytoplasmic accumulation of mutant FUS was reduced in the isogenic control cells, closely resembling that of WT cells (Fig. 5a). Moreover, western blot analysis indicated that the total protein level of FUS was unaffected, while the mitochondrial FUS level was slightly decreased in the mutation-corrected isogenic control, likely due to the recovery of the cytoplasmic accumulation (Fig. 5b). We then assessed the DNA repair capacity by LA-PCR at different time points after releasing the cells from GO treatment. Both iPSC P525L and the isogenic control were treated with 100ng/ml of GO for 1hr, followed by recovery for 30, 60 and 180 minutes, respectively. Compared to the mutant, the isogenic control showed a much higher DNA integrity at 180 minutes, which was comparable with FUS WT (Supplementary Fig. 5a).
Figure 5: Mitochondria DNA damage accumulation and DNA repair defects can be rescued by the correction of FUS mutations or targeted Lig1 expression.
a IF of endogenous FUS localization in control and patient derived iPSC cells along with isogenic mutation corrected cells, IF was performed by anti-FUS antibody. DAPI staining indicates nucleus. Scale bar = 10 μm. b IB of endogenous FUS, HSP60, Tom20 and PCNA in FUS WT, FUS P525L and isogenic FUS L525P iPSC cells. c Human DNA ligase 1 (Lig1) localizing in mitochondria (FLAG-MTS) was generated with n-terminal FLAG and mitochondrial targeting sequence (MTS) from Cytochrome c oxidase subunit 8 (COX8) gene, immunoblot (IB) showing MTS-Lig1expression in nucleus and mitochondria in patient derived fibroblasts. d In vitro nick ligation activity assay performed using patient derived fibroblast mitochondrial extracts with MTS-Lig1 expression. e LA-PCR-based DNA damage repair kinetic analysis. Genome DNA extracted from control and patient derived fibroblasts with FUS WT, FUS P525L and FUS P525L MTS-lig1 at indicated time points after release from exposure to GO (100 ng/ml) for 1 hr. Amplification products analyzed by agarose gel electrophoresis and pico green-based quantitation represented. f microplate reader-based analysis of TMRM signal intensity, comparison of control, FUS P525L and FUS P525L cells expressing MTS-Lig1. The cells were treated with GO (100ng/ml) for 1 h and released for 2 hrs post 1 hr treatment.
These data suggest that the DNA damage repair deficiency observed in FUS mutated cells is caused by the P525L mutation. Although correcting the mutation may be a potential therapeutic strategy in treating ALS in the future, currently it is technically difficult to use gene editing tools to modify or correct mutations in patients. Therefore, we hypothesized that the ligation activity of mtLig3 in FUS P525L mutated cells could be rescued by the mitochondria-targeted expression of human DNA Lig1, another major DNA ligase specifically expressed in mammalian nuclei but not in mitochondria and is critical to DNA SSB repair, primarily in dividing cells 37. Notably, Lig1 is minimally expressed in non-dividing cells, including postmitotic neurons, and the absence of Lig1 may worsen Lig3 defects in neurons more than in dividing cells. Additionally, the pathways involving Lig1 recruitment to DNA damage sites are different from those involving Lig3. Lig3 recruitment in nuclear genomes relies on the PARP1 meditated binding with XRCC1 to form a complex, and the ligation activity of Lig3 is promoted by FUS in both nucleus and mitochondria. Conversely, Lig1 requires interaction with proliferating cell nuclear antigen (PCNA) for recruitment to DNA damage sites in nuclear genomes 37. However, studies have shown that mitochondrial localization sequence (MLS) tagged Lig1 (MTS-Lig1) can localize to mitochondria in the absence of PCNA and provide ligase activity in Lig3 KO cells 25. To target Lig1 to mitochondria, we cloned 22-amino acid MLS derived from the precursor of human cytochrome oxidase subunit 8A (COX8) and added it to the N terminal of human Lig1 ORF following a FLAG tag in pcDNA plasmid (Fig. 5c) 38. We verified the specific expression of this plasmid in mitochondria by Western blot (Fig. 5c) after transfecting it into fibroblast cells and immunofluorescence in HEK293 cells transfected with MTS-Lig1 plasmid (Supplementary Fig. 5b).
We first tested the overall ligation activity in mitochondria by in vitro ligation activity assay and found that MTS-Lig1expressing fibroblasts with FUS P525L mutation had a significantly higher ligation activity than the control (Fig. 6d). Next, we compared the oxidative DNA damage repair in MTS-Lig1expressing cells and controls by LA-PCR and observed that Lig1 enabled mitochondria to rescue the DNA damage repair deficiency induced by the FUS P525L mutation (Fig. 5e). We also measured mitochondrial membrane potentials in FUS WT and P525L mutant cells with or without MTS-Lig1 and observed improved membrane potentials in MTS-Lig1 expressing FUS P525L cells (Fig. 5f).
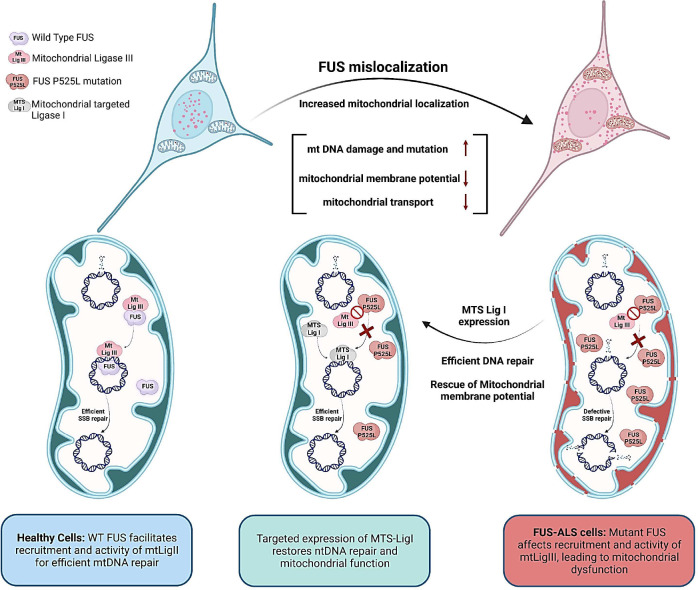
Figure 6: A schematic model summarizing our findings on the role of mutant FUS in inducing mitochondrial dysfunction and mtDNA instability in ALS/FTD, and its amelioration by targeted expression of DNA Lig1.
The optimal FUS recruitment to mitochondria is critical for maintaining mtDNA integrity, as FUS promotes mtDNA Lig3 function through direct interaction, independent of XRCC1 in healthy neurons. However, ALS pathology associated FUS mutations lead to nuclear clearance and increased mitochondrial localization of FUS, and the mutant FUS fails to interact with mtLig3, causing defective recruitment to mtDNA damage sites and increased mutational load, ultimately resulting in mitochondrial dysfunction. The targeted expression of Lig1 in FUS-mutated cell mitochondria restores DNA repair and improves overall mitochondrial function.
Overall, the results suggest that the mtDNA damage repair deficiency observed in FUS P525L mutated cells is linked to the mutation and that the reduced ligation activity of mtLig3 in these cells can be rescued by the expression of MTS-Lig1. This indicates that MTS-Lig1 has therapeutic potential for treating ALS caused by FUS mutations (Schematically shown in Fig. 6).
Discussion
The instability of mtDNA is a hallmark of aging and neurodegeneration 39. As individuals age, mutations accumulate in mtDNA, which leads to decreased mitochondrial function, cellular stress, and inflammation 40, 41. Neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and ALS are also associated with mtDNA instability, which contributes to progressive neuron degeneration 42, 43. The accumulation of mtDNA mutations and impaired mitochondrial function in neurons and other brain cells, can lead to oxidative stress, inflammation, and energy deficits in the brain44, 45, 46. MtDNA damage can cause defective bioenergetics, reduced cell proliferation and apoptosis. The mtDNA is considered more vulnerable to damage than nuclear DNA due to its proximity to the oxidative environment within mitochondria. The absence of protective histones and multiple DNA repair pathways that are present in nucleus but not in mitochondria also contribute to the increased susceptibility to damage. Therefore, preserving mitochondrial function and mtDNA fidelity can potentially delay aging and treat neurodegenerative disorders.
Our studies show that the FUS protein, which is implicated in the etiology of both ALS and FTD, plays a crucial physiological role in repairing mtDNA. FUS was previously reported to localize in mitochondria 29. However, these observations were based on the overexpression of exogenous FUS 27, 30. In this study, we examined the endogenous protein level of FUS in mitochondria in HEK293 cells and showed that mitochondrial localization of endogenous FUS is increased upon induction of oxidative stress by GO treatment. We also observed an interaction between FUS and mtLig3, which was enhanced by treatment with GO, suggesting a functional interaction between FUS and mtLig3. Our data from familial ALS patient-derived fibroblasts indicate that mutant FUS is recruited to mitochondria at a higher level than WT FUS, and that this recruitment is further increased upon DNA damage induction by GO exposure. However, mutant FUS is unable to proficiently interact with mtLig3 compared to WT FUS and this reduced interaction is responsible for reduced mtDNA Lig3 activities. Furthermore, we found that FUS mutations cause defective recruitment of Lig3 to mtDNA, and that this leads to mtDNA damage and accumulation of mutations. Consistently, unlike WT FUS, increased mutant FUS in mitochondria results in DNA repair defects and mitochondrial dysfunction. Mutant FUS iPSC derived motor neurons also show decrease in total mitochondrial content and mitochondrial motility. Additionally, we found that the presence of FUS is required for proper mitochondrial function, as demonstrated by reduction in mitochondrial membrane potential in FUS KO models and iPSC-derived motor neurons. In patient-derived fibroblasts with FUS P525L mutation, we observed reduced mitochondrial function recovery after GO and sodium arsenite treatment in comparison to control cells. To further investigate the effects of the mutation on mitochondrial function, we measured various mitochondrial functional parameters using mitochondrial stress test assay on Seahorse XFe96 platform. We found that mutant cells have defective recovery in various mitochondrial respiratory function parameters in comparison to WT cells after genotoxic and protein aggregation stress. We also observed increased mtDNA damage in spinal cord tissues from ALS patients with FUS pathology, as well as decreased ability to repair damaged DNA in FUS mutant cells compared to WT cells. Our study identified DNA ligation defects associated with FUS pathology as a key driver of DNA damage and defective DNA repair in mitochondria. Furthermore, our results suggest that restoring mitochondrial ligase activity by expressing DNA Lig1 could potentially rescue genomic instability in mitochondria caused by FUS pathology.
The FUS P525L mutation, which is associated with a severe form of juvenile ALS (jALS)47, was found to cause a substantial decrease in mtDNA ligase activity in cells carrying this mutation. The relationship between jALS and mtLig3 activity is an intriguing area warranting further investigation. Understanding the connection between this mutation and mtDNA instability could shed light on the underlying mechanisms of jALS and could lead to new therapeutic strategies.
The mechanisms of mtDNA repair relies on the translocation of nuclear-encoded DNA repair proteins. Recent studies have revealed varying degrees of recruitment of DNA repair proteins to the mitochondria. Notably, mtLig3 is the only DNA ligase that is recruited to mitochondria 48. This is due to mtLig3 being an alternative splice variant of the Lig3 gene that retains a N-terminal mitochondrial targeting sequence (MTS). XRCC1 is another critical protein involved in nuclear SSB repair 22, 24, 49. It coordinates the functions of various reactions involved in DNA end processing, including the DNA nick sealing by Lig3 23. However, XRCC1 is not recruited to mitochondria. In the absence of XRCC1, it is conceivable that FUS acts as a scaffolding factor for mtLig3.
Mutations in mtDNA have been implicated in ALS, that can impair the function of the mitochondria, leading to decreased energy production, increased oxidative stress, and cellular stress 50, 51, 52. This can contribute to the degeneration of motor neurons, which are highly reliant on mitochondrial function to maintain their energy demands. Targeting mtDNA mutations and preserving mitochondrial function could hold promise in delaying the progression of ALS and improving outcomes for affected individuals. However, the underlying mechanisms that generate these mutations are not well understood. Our mtDNA sequencing analyses reveal a substantial increase in mtDNA variations, including base changes, deletions, and insertions in cells affected by the mutant FUS protein. This provides a potential connection between mtDNA repair defects and the accumulation of mtDNA mutations in FUS-related neurodegeneration.
Overall, our study establishes two main outcomes. Firstly, we establish the normal function of FUS in mtDNA repair using WT and FUS KO cells. Through these experiments, we directly demonstrate the crucial role of FUS in maintaining mtDNA integrity through its involvement in repair mechanisms.
Secondly, we uncover the impact of FUS proteinopathy/mutations in causing mtDNA damage, repair defects, and broader mitochondrial dysfunction observed in FUS-associated neurodegeneration. By examining disease-relevant cell lines, mouse models, and autopsy tissues with FUS mutations, we demonstrate the detrimental consequences of compromised FUS functions for mitochondria. These findings add to the growing body of evidence linking mtDNA damage to neurodegenerative diseases.
Finally, we demonstrate that a targeted Lig1 expression offers a promising approach to restore mtDNA integrity and function. The ability to selectively deliver Lig1 to mitochondria to counteract mutant FUS induced Lig3 defects and to reduce the accumulation of mtDNA damage and prevent the onset and progression of mitochondrial dysfunction in FUS-associated neurodegeneration. Moreover, the high specificity and low toxicity of Lig1-based therapy make it a safe and effective treatment option. Although more studies are needed to validate its therapeutic potential, the targeted delivery of Lig1 to mitochondria represents a significant step towards addressing the unmet medical need not only in FUS-associated ALS/FTD, but also in other disorders associated with mitochondrial dysfunction.
Methods
1. Cell lines, cell culture, and tissue origin
Human embryonic kidney HEK293 (ATCC) cell lines were cultivated in Dulbecco’s modified Eagle’s medium (DMEM), containing 10% fetal bovine serum and 100 U/ml penicillin, and 100 U/ml streptomycin. FUS KO HEK293 cell line was described before11. Human fibroblasts were grown in DMEM/F12 medium with 10% fetal bovine serum, 1.6% Sodium Bicarbonate (Corning), and 1% MEM non-essential amino acids (Gibco TM) 29. The origin and the conversion of the patient-derived fibroblasts into Human iPSC cells as well as the mutation corrected lines (VIB-KU Leuven 29) were maintained on Geltrex LDEV-Free, hESC-Qualified, basement membrane matrix cultured with 1X Essential 8 supplement. These cells were maintained at 37 °C in a 5% CO2 atmosphere.
Spinal cord autopsy tissue specimens used for human ALS and control studies were obtained from the Department of Veteran’s Affairs (VA) Biorepository in the USA. These studies were conducted in accordance with the ethics board standards at the VA and the institutional review boards at the Houston Methodist Research Institute (Houston, Texas).
2. Differentiation of motor neurons
Motor neurons were derived from induced pluripotent stem cells (iPSCs) and H9-human embryonic stem cells (H9-hESCs) obtained from WiCell Research Institute and VIB-KU Leuven29. The differentiation process followed established protocols with some modifications. In brief, iPSC clones were transferred from a 60-cm dish to a T-25 flask filled with neuronal basic medium. The medium consisted of a mixture of 50% Neurobasal medium and 50% DMEM/F12 medium, supplemented with N2 and B27 supplements without vitamin A. Collagenase type IV digestion was performed to facilitate suspension of the iPSC clones. Afterward, the suspended cell spheres were subjected to a series of incubations. Initially, they were treated with various inhibitors including 5 μM ROCK Inhibitor (Y-27632), 40 μM TGF-β inhibitor (SB 431524), 0.2 μM bone morphogenetic protein inhibitor (LDN-193189), and 3 μM GSK-3 inhibitor (CHIR99021). This was followed by incubation in a neuronal basic medium containing 0.1 μM retinoic acid (RA) and 500 nM Smoothened Agonist (SAG) for 4 days. Subsequently, the cell spheres were incubated for 2 days in a neuronal basic medium containing RA, SAG, 10 ng/ml Brain-derived neurotrophic factor (BDNF), and 10 ng/ml Glial cell-derived neurotrophic factor (GDNF). To dissociate the cell spheres into single cells, they were exposed to a neuronal basic medium containing trypsin (0.025%)/DNase in a water bath at 37 °C for 20 min. Afterward, the cells were pipetted into a medium containing trypsin inhibitor (1.2 mg/ml) to maintain their viability. Following cell counting, a specific number of cells were seeded onto dishes or chamber slides coated with 20 μg/ml Laminin. These cells were incubated for 5 days in a neuronal basic medium containing RA, SAG, BDNF, GDNF, and 10 μM DAPT. Subsequently, the medium was switched to one containing BDNF, GDNF, and 20 μM Inhibitor of γ-secretase (DAPT) for an additional 2 days. For motor neuron maturation, the cells were cultured in a medium containing BDNF, GDNF, and 10 ng/ml ciliary neurotrophic factor (CNTF) for a period exceeding 7 days.
3. Antibodies and plasmids
Rabbit anti-FUS (Cat# A300-302A) and anti-Lig1 (A301-136A) antibodies were procured from Bethyl Laboratories, Inc. Mouse anti-FLAG antibody (A8592) was obtained from Sigma-Aldrich, and mouse anti-Lig3 antibody (Cat# ab587) was purchased from Abcam. Mouse anti-Tom20 (SC-17764), anti-HSP60 (SC-13115) and anti-PCNA (SC-56) antibodies were procured from Santa Cruz Biotechnology. Fluorescent secondary antibodies, Alexa Fluor 488 anti-mouse (Cat# A28175), and Texas Red anti-rabbit antibody (Cat# T-2767) were obtained from Life Technologies. The antibodies were diluted at 1:1000 for western blotting, 1:500 for immunofluorescence and 1:100 for PLA.
The human Lig1 coding sequence was re-cloned into pCDNA3.1 plasmid as an N-terminal FLAG and COX8 gene mitochondrial targeting sequence containing construct (FLAG-MTS-Lig1) 53.
4. Mouse models and genotyping
Mouse experiments were approved by the institutional ethics review board of Houston Methodist Research Institute. FUS WT (Strain#:017916) and R495X (Strain#:017928) transgenic mice were obtained from the Jackson Laboratory repository. Animals were propagated and genotyped following the guidelines provided by the Jackson Laboratory. The mice were maintained under constant conditions (21 ± 1°C; 60% humidity) with a 12/12-hr of light/dark cycle and given unrestricted access to food and water. Mice were weaned at 21 days and genotyped by ear biopsy.
5. Transfection, immunoblotting, and immunofluorescence
Fibroblast cells were transfected with plasmids using Lipofectamine 3000 (Invitrogen), according to the manufacturer’s instructions. Immunoblotting and immunofluorescence were performed as per standard protocols 54, 55. For immunoblotting, cell lysates extracted with 1X RIPA buffer (Millipore) containing the protease inhibitor cocktail (Roche) were loaded into 4–12% Bis-Tris precast (Bio-Rad) gels for electrophoresis. After transfer to nitrocellulose membranes, the separated proteins were incubated with primary and secondary antibodies, and the protein signals were detected by adding chemiluminescence reagents (Li-cor) and visualized by LI-COR Odyssey imaging system. For immunofluorescence, cells grown on chamber slides were first fixed with 4% paraformaldehyde for 15 minutes, followed by permeabilization in 0.5% Triton X-100 for 15 minutes. The cells were then incubated with primary antibodies overnight and with fluorescently labeled secondary antibodies for 2 hrs. Finally, the immunofluorescent images were captured by ZEISS Axio Observer fluorescence microscope.
6. Co-immunoprecipitation (co-IP)
The co-IP of endogenous FUS was performed using protein A/G PLUS agarose beads (Santa Cruz Biotechnology). Cells were harvested and lysed with a buffer containing 0.2% NP-40, 150 mM NaCl, 25 mM Tris-HCl and 0.1% SDS. The lysate was precleared by adding 1 μg of a control antibody IgG along with the protein A/G beads, which helps to remove non-specific binding. After a 30-minute incubation at 4 °C, the supernatant was collected and incubated with a primary antibody suitable for FUS. Next, the protein A/G beads were added to the mixture and incubated overnight in a rocker platform. The protein-antibody-bead complex was then centrifuged at 600 g to separate the beads from unbound proteins. The beads were washed three times to remove any remaining unbound proteins and the protein complex was eluted from the beads and subjected to IB analysis 11.
7. In situ Proximity Ligation Assay (PLA)
An in-situ PLA assay was performed using a Duolink PLA kit (Sigma) following the manufacturer’s instructions 56, 57. Briefly, cells grown in chamber slides were fixed with 4% formaldehyde for 15 minutes at 37 °C, permeabilized with 0.5% TritonX-100 for 10 minutes, and then incubated with primary antibodies overnight. The cells were then incubated at 37 °C with PLA probes for 1 hr, with ligase for 30 minutes, and with polymerase for 100 minutes. The slides were mounted with mounting medium containing DAPI and the PLA signal was visualized using a fluorescence microscope (ZEISS Axio Observer). The negative control was performed by incubating with IgG.
8. Long amplicon PCR (LA-PCR)
Genomic DNA was extracted using Qiagen Blood and Tissue kit following the manufacturer’s instructions 58. In this study, two sets of primers with a range of 9 kb were used for both human and mouse samples, along with a control amplification of 250 bp short segment. The primer sequences can be found in Supplementary Table S2. The determination of mitochondrial copy number variations among samples was conducted as previously described 59.
9. In vitro ligation activity assay
DNA oligos were synthesized by Sigma using the following the sequences: p24-Cy3-GGCACGGTCTACACGGCACACGAG, p27-TGTACATGATACGATTCCAAGCTAAGC, and p51-CCGTGCCAGATGTGCCGTGTGCTCACATGTACTATGCTAAGGTTCGATTCG. The assay was performed according to a previously published protocol 11. Briefly, 10 pmol of each oligomer was incubated with 50 mM NaCl in a heated water bath until the boiling water cooled to room temperature. Annealed oligomers were mixed with various mitochondrial extracts in 1× T4 ligation buffer and the mixture was incubated in a water bath for 20 minutes at 30 °C. Samples were mixed with 2× TBE buffer, heated for 3 minutes at 100 °C, and cooled down on ice for 3 minutes. Oligomers were then separated by denaturing urea polyacrylamide gel electrophoresis. The band with Cy3 fluorescence was detected by Typhoon FLA 7000 fluorescence imaging system60, 61.
10. Mitochondria protein extraction
Mitochondrial proteins were isolated using differential centrifugation method as described by Ivan Dimauro et al.62. All procedures were performed at 4°C or on ice. The collected cells were homogenized in STM buffer (250 mM Sucrose, 50mM Tris-HCl at pH7.4, 5 mM MgCl2, and a protease inhibitor) using a Glas-Col homogenizer set to 700–1000 rpm. The homogenate was collected in to centrifuge tube and kept on ice for 30 minutes followed by centrifugation at 800 g for 15 minutes. The supernatant was collected and centrifugation was repeated to isolate mitochondrial and cytosolic fractions. The supernatant was then centrifuged at 11,000 g for 15 minutes to pellet mitochondria and supernatant containing cytosolic fraction. The pellet, washed and centrifuged (at 11,000 g for 10 minutes) with STM buffer, was then suspended in 1X RIPA buffer containing 50 mM Tris HCl pH 6.8, 1 mM EDTA, 0.5% Triton-x-100 and a protease inhibitor, for lysis followed by centrifugation for 15 minutes at15,700g.
11. ChIP assay
ChIP assay was performed according to a previously published protocol 60. In brief, patient-derived fibroblasts with FUS WT and FUS P525L mutation were treated with 100 ng/ml glucose oxidase for 1 hr. Mitochondria isolation was performed, as previously described, up to the pellet stage. The isolated mitochondria were crosslinked in 1% formaldehyde for 20 minutes at room temperature. The crosslinking reaction was quenched with 125 mM glycine, and the mitochondria were harvested using cold 1X PBS/1X protease inhibitor buffer. Chromatin was fragmented into average sizes of 250–650 bp by sonication. The Lig3 ChIP assay was performed using cleared lysates, where 5 μg of Lig3 antibody and the Magna ChIP Protein A magnetic beads were incubated overnight at 4 °C. All the ChIP eluates were subjected to reverse-crosslinking, purification by phenol/chloroform extraction, and were finally dissolved in 10 mM Tris-HCl (pH 8). The relative occupancy of the target protein at sites of mtDNA damage was analyzed by qPCR with primers (sequences given in Supplementary Table S2) targeting the mitochondrial ND1 and 16s rRNA genes.
12. Measurement of mitochondrial membrane potential (ΔΨm)
Patient-derived fibroblasts were seeded in 96-well plates and exposed to 100ng/ml glucose oxidase (GO) for 1 hr. After treatment, the cells were allowed to recover for 2 hrs. The mitochondrial membrane potential was then measured using a TMRE mitochondrial membrane potential assay kit, according to the manufacturer’s instructions63.
13. Cellular mitochondrial respiratory metabolic phenotype assessment
Mitochondrial respiration measurement in patient derived fibroblasts was performed in untreated, 1 hr GO treated, recovery followed by GO treatment, 30 minutes NaAs treated, recovery after NaAs treatment. 25,000 cells per well were plated and assessed by measurement of oxygen consumption rate (OCR) in response to inhibitor and uncoupler injections following the Seahorse XF96 Cell Mito Stress Test protocol 64.
14. Mitochondrial DNA sequencing using REPLI-g followed by NGS
The mtDNA genome from the HEK293, Patient derived fibroblasts, and Human tissues were amplified using REPLI-g Mitochondrial DNA kit according to manufacturer’s recommendation (Qiagen, Germantown, MD) 65, 66. The resulting PCR product was sequenced on Illumina HiSeq platform by using GENEWIZ next generation sequencing service.
15. Statistical analysis
At least three independent experiments were performed for each set of presented data. Statistical analysis was performed using GraphPad Prism software. The results were analyzed for significant differences using ANOVA and a student’s t-tests. A p-value of less than 0.05 was considered statistically significant.
Acknowledgments
This research is primarily supported by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute of Aging (NIA) of the National Institutes of Health (NIH) under award number RF1NS112719 to M.L.H. Research in the Hegde laboratory is also supported by the NIH awards R01NS088645, R03AG064266, and R01NS094535, as well as the Sherman Foundation Parkinson’s Disease Research Challenge Fund and Houston Methodist Research Institute’s internal funds. M.L.H. acknowledges the Everett E. and Randee K. Bernal for their support via Centenial Endowed Directorship of DNA Repair. A.E.T. acknowledges the NIH award ES012512. The authors would like to express their gratitude to members of Hegde laboratory for various assistance and Drs. Gillian Hamilton and Anna Dodson at Houston Methodist Research Institute (Houston, TX) for their assistance with document editing.
Footnotes
Competing interests: The authors declare no competing interests.
Additional information
Supplementary Material accompanying this paper includes two Tables and 5 Figures.
Data availability
Upon reasonable request, the corresponding author can provide the data that support the findings of this study.
References
- 1.Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IR. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain 132, 2922–2931 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Langenhove T, van der Zee J, Van Broeckhoven C. The molecular basis of the frontotemporal lobar degeneration-amyotrophic lateral sclerosis spectrum. Ann Med 44, 817–828 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ling SC, Polymenidou M, Cleveland DW. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron 79, 416–438 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vance C, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323, 1208–1211 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwiatkowski TJ Jr., et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 323, 1205–1208 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Dormann D, et al. ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. EMBO J 29, 2841–2857 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Hegde ML. New Mechanisms of DNA Repair Defects in Fused in Sarcoma-Associated Neurodegeneration: Stage Set for DNA Repair-Based Therapeutics? J Exp Neurosci 13, 1179069519856358 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sama RR, Ward CL, Bosco DA. Functions of FUS/TLS from DNA repair to stress response: implications for ALS. ASN Neuro 6, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naumann M, et al. Impaired DNA damage response signaling by FUS-NLS mutations leads to neurodegeneration and FUS aggregate formation. Nat Commun 9, 335 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang WY, et al. Interaction of FUS and HDAC1 regulates DNA damage response and repair in neurons. Nat Neurosci 16, 1383–1391 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, et al. Mutant FUS causes DNA ligation defects to inhibit oxidative damage repair in Amyotrophic Lateral Sclerosis. Nat Commun 9, 3683 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu H, et al. ALS-associated mutation FUS-R521C causes DNA damage and RNA splicing defects. J Clin Invest 124, 981–999 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higelin J, et al. FUS Mislocalization and Vulnerability to DNA Damage in ALS Patients Derived hiPSCs and Aging Motoneurons. Front Cell Neurosci 10, 290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng Q, et al. FUS is phosphorylated by DNA-PK and accumulates in the cytoplasm after DNA damage. J Neurosci 34, 7802–7813 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhoads SN, et al. The prionlike domain of FUS is multiphosphorylated following DNA damage without altering nuclear localization. Mol Biol Cell 29, 1786–1797 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baum N, Suarez G, Appell RA. Urinary incontinence. Not a ‘normal’ part of aging. Postgrad Med 90, 99–102, 107–109 (1991). [DOI] [PubMed] [Google Scholar]
- 17.Rulten SL, et al. PARP-1 dependent recruitment of the amyotrophic lateral sclerosis-associated protein FUS/TLS to sites of oxidative DNA damage. Nucleic Acids Res 42, 307–314 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mastrocola AS, Kim SH, Trinh AT, Rodenkirch LA, Tibbetts RS. The RNA-binding protein fused in sarcoma (FUS) functions downstream of poly(ADP-ribose) polymerase (PARP) in response to DNA damage. J Biol Chem 288, 24731–24741 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singatulina AS, et al. PARP-1 Activation Directs FUS to DNA Damage Sites to Form PARG-Reversible Compartments Enriched in Damaged DNA. Cell Rep 27, 1809–1821 e1805 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Levone BR, et al. FUS-dependent liquid-liquid phase separation is important for DNA repair initiation. J Cell Biol 220, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puebla-Osorio N, Lacey DB, Alt FW, Zhu C. Early embryonic lethality due to targeted inactivation of DNA ligase III. Mol Cell Biol 26, 3935–3941 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakshmipathy U, Campbell C. The human DNA ligase III gene encodes nuclear and mitochondrial proteins. Mol Cell Biol 19, 3869–3876 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katyal S, McKinnon PJ. Disconnecting XRCC1 and DNA ligase III. Cell Cycle 10, 2269–2275 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lakshmipathy U, Campbell C. Mitochondrial DNA ligase III function is independent of Xrcc1. Nucleic Acids Res 28, 3880–3886 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simsek D, et al. Crucial role for DNA ligase III in mitochondria but not in Xrcc1-dependent repair. Nature 471, 245–248 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simsek D, et al. DNA ligase III promotes alternative nonhomologous end-joining during chromosomal translocation formation. PLoS Genet 7, e1002080 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng J, et al. FUS Interacts with HSP60 to Promote Mitochondrial Damage. PLoS Genet 11, e1005357 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng J, et al. FUS interacts with ATP synthase beta subunit and induces mitochondrial unfolded protein response in cellular and animal models. Proc Natl Acad Sci U S A 115, E9678–E9686 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo W, et al. HDAC6 inhibition reverses axonal transport defects in motor neurons derived from FUS-ALS patients. Nat Commun 8, 861 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai YL, et al. ALS/FTD-associated protein FUS induces mitochondrial dysfunction by preferentially sequestering respiratory chain complex mRNAs. Genes Dev 34, 785–805 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalier L, et al. TOM20-mediated transfer of Bcl2 from ER to MAM and mitochondria upon induction of apoptosis. Cell Death Dis 12, 182 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandoorne T, et al. Differentiation but not ALS mutations in FUS rewires motor neuron metabolism. Nat Commun 10, 4147 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang K, et al. Stress Granule Assembly Disrupts Nucleocytoplasmic Transport. Cell 173, 958–971 e917 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santos JH, Meyer JN, Mandavilli BS, Van Houten B. Quantitative PCR-based measurement of nuclear and mitochondrial DNA damage and repair in mammalian cells. Methods Mol Biol 314, 183–199 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Hunt CP, Rooney JP, Ryde IT, Anbalagan C, Joglekar R, Meyer JN. PCR-Based Analysis of Mitochondrial DNA Copy Number, Mitochondrial DNA Damage, and Nuclear DNA Damage. Curr Protoc Toxicol 67, 20 11 21–20 11 25 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tibshirani M, et al. Cytoplasmic sequestration of FUS/TLS associated with ALS alters histone marks through loss of nuclear protein arginine methyltransferase 1. Hum Mol Genet 24, 773–786 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mortusewicz O, Rothbauer U, Cardoso MC, Leonhardt H. Differential recruitment of DNA Ligase I and III to DNA repair sites. Nucleic Acids Res 34, 3523–3532 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu H, et al. Gene delivery to mitochondria by targeting modified adenoassociated virus suppresses Leber’s hereditary optic neuropathy in a mouse model. Proc Natl Acad Sci U S A 109, E1238–1247 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson DM, 3rd, Cookson MR, Van Den Bosch L, Zetterberg H, Holtzman DM, Dewachter I. Hallmarks of neurodegenerative diseases. Cell 186, 693–714 (2023). [DOI] [PubMed] [Google Scholar]
- 40.Linnane AW, Marzuki S, Ozawa T, Tanaka M. Mitochondrial DNA mutations as an important contributor to ageing and degenerative diseases. Lancet 1, 642–645 (1989). [DOI] [PubMed] [Google Scholar]
- 41.Munscher C, Muller-Hocker J, Kadenbach B. Human aging is associated with various point mutations in tRNA genes of mitochondrial DNA. Biol Chem Hoppe Seyler 374, 1099–1104 (1993). [DOI] [PubMed] [Google Scholar]
- 42.Keogh MJ, Chinnery PF. Mitochondrial DNA mutations in neurodegeneration. Biochim Biophys Acta 1847, 1401–1411 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Cha MY, Kim DK, Mook-Jung I. The role of mitochondrial DNA mutation on neurodegenerative diseases. Exp Mol Med 47, e150 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Picca A, et al. Circulating Mitochondrial DNA at the Crossroads of Mitochondrial Dysfunction and Inflammation During Aging and Muscle Wasting Disorders. Rejuvenation Res 21, 350–359 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swerdlow RH. Mitochondria and Mitochondrial Cascades in Alzheimer’s Disease. J Alzheimers Dis 62, 1403–1416 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vielhaber S, et al. Mitochondrial DNA abnormalities in skeletal muscle of patients with sporadic amyotrophic lateral sclerosis. Brain 123 (Pt 7), 1339–1348 (2000). [DOI] [PubMed] [Google Scholar]
- 47.Goldstein O, et al. FUS-P525L Juvenile Amyotrophic Lateral Sclerosis and Intellectual Disability: Evidence for Association and Oligogenic Inheritance. Neurol Genet 8, e200009 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kodavati M, Wang H, Hegde ML. Altered Mitochondrial Dynamics in Motor Neuron Disease: An Emerging Perspective. Cells 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinz KG, Bogenhagen DF. Efficient repair of abasic sites in DNA by mitochondrial enzymes. Mol Cell Biol 18, 1257–1265 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhaliwal GK, Grewal RP. Mitochondrial DNA deletion mutation levels are elevated in ALS brains. Neuroreport 11, 2507–2509 (2000). [DOI] [PubMed] [Google Scholar]
- 51.Warita H, Hayashi T, Murakami T, Manabe Y, Abe K. Oxidative damage to mitochondrial DNA in spinal motoneurons of transgenic ALS mice. Brain Res Mol Brain Res 89, 147–152 (2001). [DOI] [PubMed] [Google Scholar]
- 52.Kikuchi H, Furuta A, Nishioka K, Suzuki SO, Nakabeppu Y, Iwaki T. Impairment of mitochondrial DNA repair enzymes against accumulation of 8-oxo-guanine in the spinal motor neurons of amyotrophic lateral sclerosis. Acta Neuropathol 103, 408–414 (2002). [DOI] [PubMed] [Google Scholar]
- 53.Manohar K, Khandagale P, Patel SK, Sahu JK, Acharya N. The ubiquitin-binding domain of DNA polymerase eta directly binds to DNA clamp PCNA and regulates translesion DNA synthesis. J Biol Chem 298, 101506 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang D, et al. Speckle-type POZ protein, SPOP, is involved in the DNA damage response. Carcinogenesis 35, 1691–1697 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, et al. Human RIF1 encodes an anti-apoptotic factor required for DNA repair. Carcinogenesis 30, 1314–1319 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hegde ML, et al. Prereplicative repair of oxidized bases in the human genome is mediated by NEIL1 DNA glycosylase together with replication proteins. Proc Natl Acad Sci U S A 110, E3090–3099 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gupta A, et al. MOF phosphorylation by ATM regulates 53BP1-mediated double-strand break repair pathway choice. Cell Rep 8, 177–189 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quail MA, Swerdlow H, Turner DJ. Improved protocols for the illumina genome analyzer sequencing system. Curr Protoc Hum Genet Chapter 18, Unit 18 12 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rooney JP, et al. PCR based determination of mitochondrial DNA copy number in multiple species. Methods Mol Biol 1241, 23–38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hegde PM, et al. The C-terminal Domain (CTD) of Human DNA Glycosylase NEIL1 Is Required for Forming BERosome Repair Complex with DNA Replication Proteins at the Replicating Genome: DOMINANT NEGATIVE FUNCTION OF THE CTD. J Biol Chem 290, 20919–20933 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hegde ML, et al. Physical and functional interaction between human oxidized base-specific DNA glycosylase NEIL1 and flap endonuclease 1. J Biol Chem 283, 27028–27037 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dimauro I, Pearson T, Caporossi D, Jackson MJ. A simple protocol for the subcellular fractionation of skeletal muscle cells and tissue. BMC Res Notes 5, 513 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dharmalingam P, et al. Pervasive Genomic Damage in Experimental Intracerebral Hemorrhage: Therapeutic Potential of a Mechanistic-Based Carbon Nanoparticle. ACS Nano 14, 2827–2846 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gu X, Ma Y, Liu Y, Wan Q. Measurement of mitochondrial respiration in adherent cells by Seahorse XF96 Cell Mito Stress Test. STAR Protoc 2, 100245 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dasgupta S, et al. Mitochondrial DNA mutation in normal margins and tumors of recurrent head and neck squamous cell carcinoma patients. Cancer Prev Res (Phila) 3, 1205–1211 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marquis J, et al. MitoRS, a method for high throughput, sensitive, and accurate detection of mitochondrial DNA heteroplasmy. BMC Genomics 18, 326 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon reasonable request, the corresponding author can provide the data that support the findings of this study.