Abstract
Background:
sodium-dependent glucose cotransporter 1 and 2 (SGLT1/2) belong to the family of glucose transporters, encoded by SLC5A1 and SLC5A2, respectively. SGLT-2 is almost exclusively expressed in the renal proximal convoluted tubule cells. SGLT-1 is expressed in the kidneys but also in other organs throughout the body. Many SGLT inhibitor drugs have been developed based on the mechanism of blocking glucose (re)absorption mediated by SGLT1/2, and several have gained major regulatory agencies’ approval for treating diabetes. Intriguingly these drugs are also effective in treating diseases beyond diabetes, for example heart failure and chronic kidney disease. We recently discovered that SGLT-1 is upregulated in the airway epithelial cells derived from patients of cystic fibrosis (CF), a devastating genetic disease affecting greater than 70,000 worldwide.
Results:
in the present work, we show that the SGLT-1 upregulation is coupled with elevated endoplasmic reticulum (ER) stress response, indicated by activation of the primary ER stress senor inositol-requiring protein 1a (IRE1a) and the ER stress-induced transcription factor X-box binding protein 1 (XBP1), in CF epithelial cells, and in epithelial cells of other stress conditions. Through biochemistry experiments, we demonstrated that XBP1 acts as a transcription factor for SLC5A1 by directly binding to its promoter region. Targeting this ER stress → SLC5A1 axis by either the ER stress inhibitor Rapamycin or the SGLT-1 inhibitor Sotagliflozin was effective in attenuating the ER stress response and reducing the SGLT-1 levels in these cellular model systems.
Conclusions:
the present work establishes a causal relationship between ER stress and SGLT-1 upregulation and provides a mechanistic explanation why SGLT inhibitor drugs benefit diseases beyond diabetes.
Keywords: ER stress, XBP1, SLC5A1, SGLT1, Epithelial cells
Introduction
Sodium-dependent glucose cotransporter 1 and 2 (SGLT1/2) belong to the family of glucose transporters, encoded by SLC5A1 and SLC5A2, respectively. SGLT-2 is almost exclusively expressed in the renal proximal convoluted tubule cells, whereas SGLT-1 is expressed throughout the body. SGLT inhibitor (SGLTi) drugs were originally developed based on the mechanism of blocking glucose (re)absorption mediated by SGLT1/2. Several SGLTi drugs, including Canagliflozin, Dapagliflozin, Empagliflozin, and Sotagliflozin (1–3), have gained major regulatory agencies’ approval for treating diabetes. Intriguingly, accumulating clinical data show that these SGLTi drugs provide benefits to diseases beyond diabetes, such as heart failure and renal diseases (4, 5). In preclinical model systems, beneficial effects of SGLTi drugs were reported on a wide spectrum of diseases that include cancer, Alzheimer’s, atherosclerosis, and others (6–9). Considering that SGLT-1 is expressed globally but SGLT-2’s expression is limited to the kidney, it has been speculated that the beneficial effects by SGLTi drugs in non-renal organs may be attributed to the SGLT-1 inhibition.
In a previous work, we examined the SGLT-1 expression levels in cystic fibrosis (CF) patient-derived airway lineage cells (10). Loss of function mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene lead to CF, a devastating genetic disease affecting greater than 70,000 patients worldwide (11). CFTR-F508del (dF) is the most common mutation type in CF which causes the deletion of phenylalanine at position 508 (F508) of the CFTR protein (12). We revealed that SGLT-1 is upregulated in CF bronchial epithelial (CFBE-dF) cells carrying the homozygous dF mutation (10), as well as in dF patient cell derived lung organoids (10). These findings indicate that SGLT-1 may be a therapeutic target for CF. However, the molecular basis for SGLT-1 upregulation in CF conditions remains to be determined. These findings also promoted us to ask the question whether SGLT-1 upregulation takes place in other human disease conditions.
Endoplasmic reticulum (ER) stress is triggered when the ER protein folding capacity is overwhelmed, often due to the excess accumulation of unfolded or misfolded proteins (13). In the context of CF, the CFTR-F508del mutant protein misfolds (14), and is associated with elevated ER stress (15). There is mounting evidence that ER stress response contributes to the development and progression of many diseases, including diabetes, atherosclerosis, neurodegeneration, liver diseases, and cancer (16–21). ER stress engages the unfolded protein response (UPR), an adaptive response that reduces unfolded protein load to maintain cell viability and function (13). The UPR is a complex signal transduction pathway that is initiated by the activation of one or more UPR transducers which include at least three: inositol-requiring protein 1a (IRE1a), protein kinase RNA-like ER kinase (PERK), and activating transcription factor 6 (ATF6). IRE1a is the most conserved UPR transducer (22). Activated IRE1a processes the pre-matured mRNA (XBP1u) of X-box binding protein 1 (XBP1), after which the spliced transcripts (XBP1s) are translated into XBP1 protein, a transcription factor that regulates many genes involved in ER protein folding, secretion, and ER-associated degradation (ERAD) as part of a concerted effort to increase the capacity of the ER to cope with stress (23), as well as some non-canonical targets such as IL-6 in plasma cells, C/EBPα in adipocytes, and proinflammatory cytokines in macrophages (24). XBP1 was reported to bind to cAMP-responsive elements (CRE) sites and CRE-like elements in which the core “ACGT” is highly conserved (25). In most of its target genes, XBP1 binding occurred within 200 bp of transcriptional start sites (24). But XBP1 targets were also enriched in additional distinct motifs including the UPR element (TGACGTG(G/A)) and the CCACG box (26, 27).
In the present work, we show that the upregulation of SGLT-1 correlates with elevated ER stress markers IRE1a and XBP1 in CFBE-dF cells, and that XBP1 regulates the transcription of SLC5A1. We also examined other epithelial cell models under ER stress and revealed similar trend of upregulation of SGLT-1. Pharmacological interference of this ER stress → SGLT-1 axis by either ER stress inhibitor Rapamycin or SGLT inhibitor Sotagliflozin were effective in attenuating the ER stress response, and reduced the SGLT-1 levels in these cellular models. Our work demonstrates a crosstalk between ER stress response and SGLT-1 and suggests targeting this interaction for treating CF and other human diseases.
Results
SGLT-1 and ER stress markers are upregulated in the CFBE-dF cells
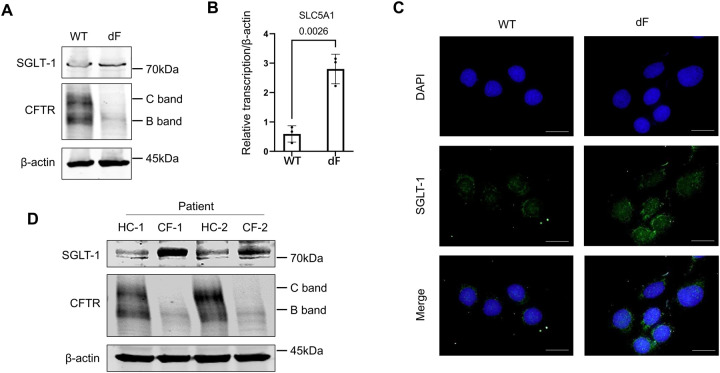
We previously reported that SGLT-1 is upregulated in different types of dF patient-derived airway lineage cells including the iPS cell-derived lung organoids, the CFBE-dF cells, and the primary airway epithelial cells (10). In the present work, we confirmed that the levels of SLC5A1 mRNA and SGLT-1 protein levels were upregulated in the CFBE-dF cells compared with those in the cells of wild-type CFTR genotype (CFBE-WT) by RT-qPCR, Western blot and immunofluorescence staining (Figure 1A to C). Consistently, the SGLT-1 protein expressions were higher in the primary airway epithelial cells derived from two dF patients, comparing with those in two healthy control subjects (Figure 1D).
Figure 1. SGLT-1 is upregulated in CF patient-derived airway lineage cells.
(A) Western blot of SGLT-1 and CFTR in the CFBE-WT cells and the CFBE-dF cells. (B) RT-qPCR of SLC5A1 in the CFBE-WT cells and the CFBE-dF cells. (C) Immunofluorescence staining of SGLT-1 in the CFBE-WT cells and the CFBE-dF cells. Scale bar: 20μm. (D) Western blot of SGLT-1 in CF patient-derived airway epithelial cells. HC-1 and −2: healthy control; CF-1 and −2: CF patients of homozygous dF508 mutation.
We next determined the expression levels of major ER stress markers in the IRE1a-XBP1 pathway in the CFBE cells, as previous studies have suggested that this pathway is activated in CF cells (15). Western blot data showed that GRP78, phosphorylated IRE1a (p-IRE1a), IRE1a, and XBP1s were all of higher levels in the CFBE-dF cells than those in the CFBE-WT cells (Figure 2A). Similarly, the transcript levels of these ER stress markers were all upregulated in the CFBE-dF cells compared to the CFBE-WT cells (Figure 2B).
Figure 2. Elevation of ER stress markers in CFBE cells.
(A) Western blot of GRP78, p-IRE1a, IRE1a, and XBP1s in the CFBE-WT cells and the CFBE-dF cells. (B) RT-qPCR of GRP78, IRE1a, and XBP1s in the CFBE-WT cells and the CFBE-dF cells.
These data suggest a correlation between the IRE1a-XBP1 ER stress pathway markers and the SGLT-1 in the CFBE-dF cells.
XBP1 transcriptionally upregulates SLC5A1 expression in dF epithelial cells
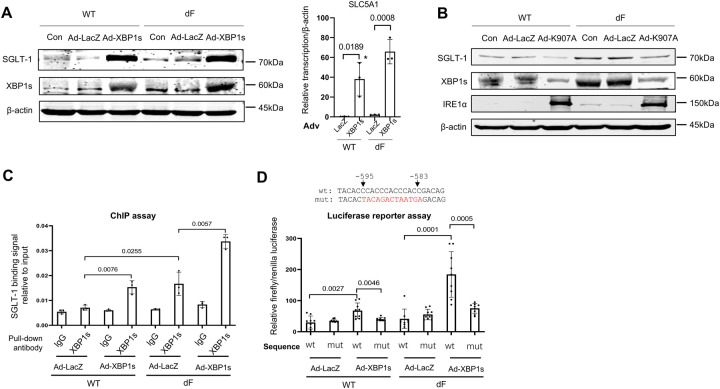
We then asked the question whether XBP1 directly regulates the transcription of SLC5A1. In both the CFBE-WT and the CFBE-dF cells, overexpression of XBP1s by adenovirus (Ad-XBP1s) resulted in dramatically increased levels of SLC5A1 transcripts and SGLT-1 proteins, whereas minimal effects were seen when a control adenovirus overexpressing β-galactosidase (Ad-LacZ) was used (Figure 3A).
Figure 3. XBP1 upregulates the expression of SLC5A1.
(A) Left: western blot of SGLT-1 and XBP1s in CFBE-WT (WT) and CFBE-dF (dF) cells transduced with Ad-LacZ or Ad-XBP1s or without any viral infection (Con). Right: SLC5A1 mRNA levels in WT and dF cells transduced with Ad-LacZ or Ad-XBP1. (B) Western blot of SGLT-1 XBP1s in CFBE-WT (WT) and CFBE-dF (dF) cells transduced with Ad-LacZ or AD-K907A (the dominant negative IRE1a) or without any viral infection (Con). (C) ChIP assay of CFBE-WT (WT) and CFBE-dF (dF) cells transduced with Ad-LacZ or Ad-XBP1s. The binding of XBP1 to the SLC5A1 promoter was determined by qPCR. (D) Top: illustration of the putative wild-type (wt) binding sequence (from −595 to −583) or the mutated binding sequence (mut) of the reporter plasmid. Red colored letters indicated mutated sequences. Bottom: relative firefly/renilla luciferase signal levels in CFBE-WT (WT) and CFBE-dF (dF) cells transfected with different combinations of the reporter plasmids (wt or mut) and the Ad viruses (Ad-LacZ or Ad-XBP1s).
On the other hand, overexpression of the dominant negative IRE1a carrying a K907A mutation by adenovirus (Ad-K907A) led to decreased protein levels of XBP1s and SGLT-1 in both CFBE-WT and CFBE-dF cells (Figure 3B). This is as expected based on the knowledge that IRE1a is an upstream activator of XBP1 and that the Ad-K907A could effectively silence the expression of XBP1 (28).
We then verified these findings in another cell line, the dF patient-derived pancreatic ductal epithelial (CFPAC-1-dF) cells (Supplementary Figure 1). Similar to the observations in CFBE-dF cells, transduction by Ad-XBP1s dramatically elevated the protein levels of SGLT-1, whereas transduction by Ad-K907A reduced the protein levels of XBP1 and SGLT-1 in CFPAC-1-dF cells. Similarly when human kidney 2 (HK-2) cells, a proximal tubular cell (PTC) line derived from normal kidney, were transfected with the SGLT-1 overexpression vector, both the SLC5A1 transcript and the SGLT-1 protein levels were upregulated (Supplementary Figure 2).
Together, these results suggest a IRE1a → XBP1 →SGLT-1 regulatory axis in human epithelial cells, and that XBP1 directly regulate the transcription of SLC5A1.
XBP1 binds to the 5’-upstream region of SLC5A1 gene promoter
We hence hypothesized that XBP1 is a transcription factor of SLC5A1. To test this, we worked to determine if XBP1 binds to the promoter of SLC5A1 (Figure 3C). Chromatin immunoprecipitation (ChIP) assay on the SLC5A1 promoter region revealed that there are minimal XBP1 binding signals in the CFBE-WT cells (Figure 3C). Moderate XBP1 binding signals were detected in the CFBE-dF cells, as well as in the CFBE-WT cells transduced with Ad-XBP1s (Figure 3C). The highest XBP1 binding signals were detected, as expected, in the CFBE-dF cells transduced with Ad-XBP1s. These results show that XBP1 binds to the promoter of SLC5A1 in CFBE cells, and such binding is activated in CF and ER stress conditions.
A previous study has suggested that XBP1 binds to the “CCACC” motif in human cells (29). We identified a sequence “5’-CCACCCACCCACC-3’” at the −595 to −583 bp position of the SLC5A1 gene that contains the “CCACC” motif. To test if XBP1 binds to this sequence to exert its transcriptional regulation of SLC5A1, we constructed a reporter plasmid consisting of the SLC5A1 promoter sequence (from position −1087 to position −21) with either the wild-type putative sequence (wt-luc) or a mutant sequence “5’-TACAGACTAATGA-3’” (mut-Luc) followed by a luciferase coding sequence. We transfected the CFBE-WT and the CFBE-dF cells with the wt-Luc or the mut-Luc plasmids, followed by transduction of these cells with Ad-XBP1s or the vehicle control Ad-LacZ. The relative luciferase signals from each of the combinations between the cell types (dF and WT) and the adenoviruses (Ad-XBP1s and Ad-LacZ) were quantified, and used as a proxy for the level of transcription induced by the XBP1’s binding to the SLC5A1 promoter.
As expected, Ad-LacZ had no effects on the luciferase signal levels in all groups, regardless of the CFTR genotype of the cells (WT or dF) or the binding motif sequence type (wt-luc or mutluc) of the plasmids. On the other hand, transduction of Ad-XBP1s increased the luciferase signal levels in both the CFBE-WT and the CFBE-dF cells that were transfected with wt-Luc. Such effects were abolished, however, when the cells were transfected with mut-Luc (Figure 3D).
These data suggest that the “5’-CCACCCACCCACC-3’” sequence at the −595 to −583 bp position of the SLC5A1 promoter is essential for XBP1’s binding and the subsequent activation of transcription.
Effects of targeting the ER stress → SGLT-1 axis in CFBE-dF cells
We next tested whether Rapamycin or Sotagliflozin, two pharmacological inhibitors of the ER stress → SGLT-1 axis, has any effects on the CFBE-dF cells. Rapamycin is an inhibitor of the mammalian target of Rapamycin (mTOR) complex 1 (mTORC1). It is known that Rapamycin, through inhibiting mTOR, effectively attenuates ER stress, and furthermore, selectively suppresses the IRE1 signaling without affecting the other two, i.e., PERK and ATF6, pathways in human cells (30). Sotagliflozin (Sota) is an SGLT inhibitor. Several SGLT inhibitor drugs, including Empagliflozin, Dapagliflozin and Sota, have gained major agencies’ approval for clinical applications (31–33). Among them, Sota is the most potent SGLT-1 inhibitor (Supplementary Table 1).
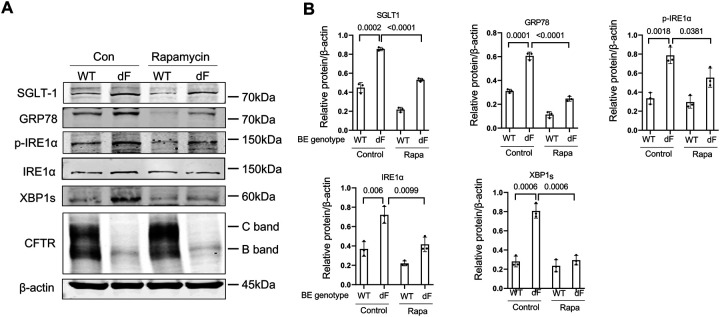
Applying Rapamycin (20 nM) to the culture medium of CFBE-dF cells indeed reduced the protein levels of GRP78, phosphorylated IRE1a (p-IRE1a), total IRE1a and XBP1s, and consequently SGLT-1 (Figure 4A and B). Similarly, when Sota (20 uM) was used to treat CFBE-dF cells, Western blot and RT-qPCR data showed that there were attenuated ER stress markers GRP78, IRE1a, and XBP1s at both the protein and the transcription levels in CFBE cells (Figure 5A to B). The levels of SGLT-1 protein and SLC5A-1 mRNAs were also reduced. Immunofluorescence (IF) staining confirmed this finding (Figure 5C).
Figure 4. Rapamycin attenuates SGLT-1 and ER stress in CFBE cells.
(A) Representative Western blots of SGLT-1, GRP78, p-IRE1a, IRE1a, XBP1s and CFTR in the CFBE-WT cells and the CFBE-dF cells treated with or without Rapamycin. (B) Quantification of Western blots of SGLT-1, GRP78, p-IRE1a, IRE1a and XBP1s in the CFBE-WT cells and the CFBE-dF cells treated with or without Rapamycin.
Figure 5. Sotagliflozin attenuates ER stress in CFBE cells.
(A) Western blot of SGLT-1, GRP78, p-IRE1a, IRE1a, and XBP1s in the CFBE-WT cells and the CFBE-dF cells treated with or without Sota. (B) qRT-PCR of SLC5A1, GRP78, IRE1a, and XBP1s in the CFBE-WT cells and the CFBE-dF cells treated with or without Sota. (C) Immunofluorescence staining of SGLT-1 in the CFBE-WT cells and the CFBE-dF cells treated with or without Sota. Scale bar: 20μm.
These data demonstrate potential beneficial effects of Rapamycin and Sotagliflozin on CFBE-dF cells through inhibiting the CF →ER stress → XBP1 → SLC5A1/SGLT-1 axis.
Effects of targeting the ER stress → SGLT-1 axis in squamous epithelial cells generated in the air-liquid interface culture
ER stress is a common etiology in many human diseases. On the other hand, SGLTi drugs appear to be effective on different human diseases. Hence, we speculated that the Disease → ER stress → XBP1 → SLC5A1/SGLT-1 axis is not limited to CF; rather, it might exist in different human diseases as long as they are associated with ER stress in epithelial cells.
Squamous metaplasia (SQM) refers to a pre-neoplastic change of the bronchial epithelium in the lungs in response to toxic injury such as that induced by cigarette smoke (34). During SQM, quiescent basal stem cells within the pseudostratified epithelium re-enter the cell cycle, become hyperproliferative, and begin to express markers of a squamous phenotype rather than those of the normal pseudostratified epithelium. SQM is associated with chronic obstructive pulmonary disease (COPD), and it has been reported that SQM amplifies the pathologic epithelial-mesenchymal interactions in COPD patients (35).
In a recent work, we established an air-liquid interface (ALI) culture system using patient derived airway basal cells, and demonstrated that the WNT signaling agonists CHIR-99021, when supplemented in the ALI culture (CHIR-ALI), suppressed the generation of AcTub+ ciliated cells and MUC5AC+ goblet cells, but drastically induced KRT13+ squamous epithelial cells, thus recapitulating key molecular and cellular changes of the SQM process in COPD (36).
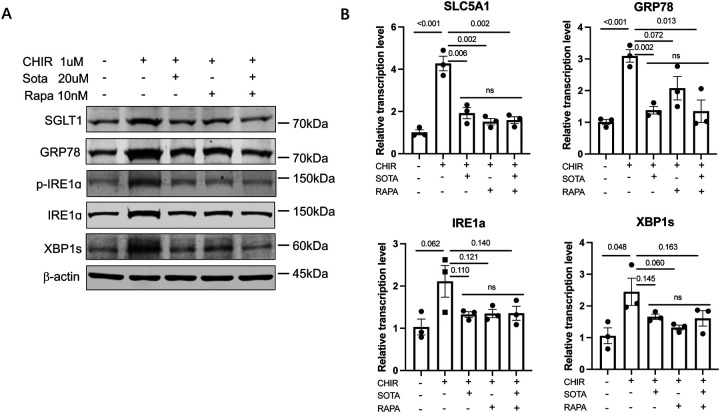
Comparison between the CHIR-ALI culture with the ALI culture without the supplementation of CHIR-99021 (ALI-no-CHIR), both derived from the airway basal cells of the same patient, revealed higher protein levels of SGLT-1, and ER stress markers GRP78, p-IRE1a, total IRE1a, and XBP1s, as well as their corresponding mRNA levels (Figure 6), similar to the patterns that we have observed in CFBE-dF cells.
Figure 6. Effects of Sotagliflozin and Rapamycin on the CHIR-ALI culture.
(A) Western blot of SGLT-1, GRP78, p-IRE1a, IRE1a, and XBP1s in the CHIR-ALI cells treated with different combinations of Sota and Rapamycin (Rapa). (B) qRT-PCR of SLC5A1, GRP78, IRE1a, and XBP1s in in the CHIR-ALI cells treated with different combinations of Sota and Rapamycin (Rapa). Control (the leftmost bar in each panel) are ALI-no-CHIR cells without Sota or Rapa supplementation to the culture medium. ns: not statistically different among these three groups.
We next examined the effects of Rapamycin and Sotagliflozin on the CHIR-ALI culture. As expected, applying Rapamycin (10 nM) or Sota (20 μM) to the system both led to reduced protein and transcription levels of GRP78, p-IRE1a, total IRE1a and XBP1s, as well as SGLT-1/SLC5A1 (Figure 6), again a similar trend to what we have observed in CFBE-dF cells. No additive effects were observed when Rapamycin (10 nM) or Sota (20 μM) were added together.
These results confirmed that the ER stress → SGLT-1 axis exists in the CHIR-99021 induced squamous epithelial cell model and demonstrate potential beneficial effects of Rapamycin and Sotagliflozin to suppress ER stress in squamous remodeling.
Effects of targeting the ER stress → SGLT-1 axis palmitic acid treated hepatocytes
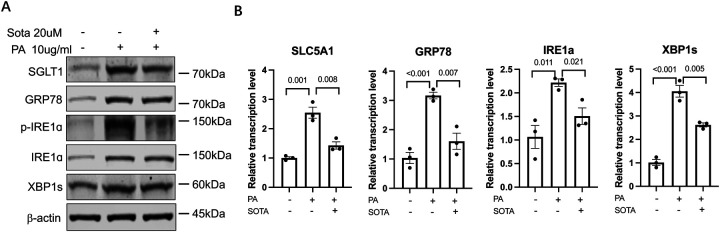
Non-alcoholic fatty liver disease (NAFLD) refers to a spectrum of diseases ranging from steatosis to non-alcoholic steatohepatitis (NASH). ER stress response in the hepatocytes, the epithelial cells of the liver, plays a crucial role in both the onset of steatosis and progression to NASH (37, 38). In particular, certain saturated fatty acids, such as palmitic acid (PA), can induce endoplasmic reticulum (ER) stress in human hepatocyte cell lines such as Huh-7 cells (39, 40). We first evaluated if PA treatment (10 μg/mL) to the Huh-7 cells would upregulate SLC5A-1/SGLT-1 levels, along with the anticipated activation of ER stress markers. Indeed, higher protein levels of SGLT-1, GRP78, total IRE1a, phosphorylated IRE1a (p-IRE1a) and spliced XBP1 (XBP1s), the indicators of ER stress and activation of IRE1α-XBP1 UPR pathway, and the corresponding mRNA levels were observed in the PA treated Huh-7 (PA-Huh7) cells in comparison to vehicle control treated (veh-Huh7) cells (Figure 7). Importantly, when Sota (20 uM) were added to the PA-Huh7 cells, reduced protein and mRNA levels of GRP78, p-IRE1a, total IRE1a and XBP1s, as well as SGLT-1/SLC5A1 were observed (Figure 7), similar to what we have observed in the CFBE-dF and CHIR-ALI cells.
Figure 7.
(A) Western blot of SGLT-1, GRP78, p-IRE1a, IRE1a, and XBP1s in the PA-Huh7 cells treated or without Sota. (B) qRT-PCR of SLC5A1, GRP78, IRE1a, and XBP1s in the PA-Huh7 cells treated or without Sota. Huh-7 cells were treated with BSA-complexed PA (10 μg/mL) in the presence or absence of Sota (50μg/mL) for 36 h. Control are Huh7 cells treated with BSA vehicle without any PA or Sota treatment.
These results demonstrated the existence of hepatic ER stress → SGLT-1 regulatory axis in the PA-Huh7 model and the potential beneficial effect of Sotagliflozin on attenuating PA-induced hepatic ER stress response.
Discussion
ER stress contributes to the development and progression of many diseases, including diabetes, atherosclerosis, neurodegeneration, liver diseases, and cancer (16–21). In the context of CF, mitigating ER stress and potentially its related inflammatory responses in CF represents a major research direction in the drug development for this devastating disease (41, 42). In the present work, we confirmed the existence of ER stress in CF patient-derived cells, both the airway lineage CFBE-dF cells and the pancreatic ductal epithelial CFPAC-1-dF cells. There is a prominent activation of the IRE1a-XBP1 pathway in these cells. These data corroborate with our findings in CF rabbit models, in which the IRE1a-XBP1 pathway markers are activated in all major CF affected organs including the lungs, pancreas, intestine and liver (43). It should be noted although we focus on the IRE1a-XBP1 pathway in the present work, future work should investigate if/how other ER stress pathways (e.g., PERK and ATF6) play roles in the CF pathogenesis.
A major contribution of the current work is the establishment of the causal relationship between ER stress and SGLT-1 upregulation in and beyond CF. We previously reported that SGLT-1 is activated in the CFBE-dF, the CF patient iPSC-derived lung organoids, and the CF patient primary airway epithelial cells (10). However, it is not known how SGLT-1 is activated in CF conditions. Here we provided evidence that XBP1 is a transcription factor of SLC5A1. Our data further suggest that upregulated SGLT-1 may in turn aggravate ER stress, as shown in Figure 5 where inhibiting SGLT-1 by Sota resulted in reduced ER stress markers. We hence expand our hypothesized regulatory axis to a regulatory loop: ER stress → XBP1 → SLC5A1/SGLT-1 → ER stress. Disrupting a node in this loop breaks this vicious cycle, which may yield benefits to CF cells and ultimately CF individuals. This idea is supported by our findings that Rapamycin and Sotagliflozin are both effective in attenuating ER stress as well as SGLT-1 in the CFBE-dF cells.
Our data in CHIR-ALI and PA-Huh7 cells suggest that such regulatory loop formed by ER stress members and SGLT-1 may be present in other ER stress associated diseases. Accumulated clinical data demonstrate that SGLT-inhibiting drugs such as Empagliflozin and Dapagliflozin provided benefits to a wide range of diseases, including diabetes, heart failure, and acute kidney failure (44–46). Emerging data suggest the benefits may be broader, including inflammatory liver diseases, dementia, and stroke (47–50). How SGLT-inhibiting drugs provide therapeutic benefits to such a wide spectrum of diseases is exciting and enigmatic. The present work provides one possible mechanistic explanation, i.e., SGLT inhibition attenuates ER stress, a common etiology for many diseases (51–53).
We need to point out that while we attribute the observed effects of Sota to its SGLT-1 inhibiting capacity, we cannot exclude the possibility that its SGLT-2 inhibiting capacity (Supplementary Table 1) may also have contributed. Follow-up work is warranted to delineate the roles of SGLT-1 and SGLT-2 in attenuating ER stress in these cellular models, for example through generating SLC5A1 and SLC5A2 knockout CFBE-dF cells and subject these cells to different gliflozin drugs.
In summary, the present work reports that XBP1 is a transcription factor for SLC5A1. Our data provide a mechanistic explanation for the SGLT-1 upregulation in CF and in other human disease cellular models, and provide support to target the ER stress→XBP1→SLC5A1/SGLT-1 axis in these diseases.
Materials and Methods
Cell culture and treatment
The CFBE-WT and the CFBE-dF cells were gifts from Dr. Fei Sun’s laboratory at Wayne State University (54). The CFBE-WT cells were cultured in MEM media (#11095–80, Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (#10438026, Gibco, Waltham, MA, USA) and 0.5μg/ml puromycin (#P9620, Sigma-Aldrich, St. Louis, MO, USA) at 37°C with 5% CO2 in a humidified incubator. The CFBE-dF cells were cultured in MEM media with 10% FBS and 1μg/ml puromycin. The CFPAC-1 (#CRL-1918) cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA), and cultured in IMDM media (#12440053, Gibco, Waltham, MA, USA) with 10% FBS and 100U/ml penicillin/streptomycin (#15140122, Gibco, Waltham, MA, USA). Human embryonic kidney (HEK-293) cells were obtained from ATCC (#CRL-1573), and were cultured in DMEM media (#11995073, Gibco, Waltham, MA, USA) with 10% FBS.
Human kidney 2 (HK-2) cells were obtained from ATCC (#CRL-2190), and were cultured in DMEM-F12 media (#11320033, Gibco, Waltham, MA, USA) with 10% FBS.
The hepatocarcinoma cell line Huh-7 was originally provided by Dr. Charles Rice at Rockefeller University (55) and cultured in DMEM/High Glucose media containing 10% FBS.
To evaluate the effects of Sotagliflozin and Rapamycin, the CFBE-WT cells and the CFBE-dF cells were treated with 20μM Sotagliflozin (#17011711, Sun-Shine Chemical Technology Co., Ltd, Shanghai, China.) in serum-free MEM media for 24 hours, or were treated with 20nM Rapamycin (#S1039, SelleckChem, Houston, TX, USA) in serum-free MEM media for 48 hours. To assess the effects of Sotagliflozin on attenuating hepatic ER stress response and elevation of SGLT, Huh-7 cells were treated with 10 μg/mL fatty acid-free BSA-complexed palmitate acids (PA, #P0500, Sigma-Aldrich, St. Louis, USA) in the presence or absence of Sotagliflozin (50μg/mL) for 36 hours.
Airway epithelial cells from CF patients and healthy control individuals
Human airway basal cells from healthy control (HC) and CF patients carrying the CFTR-F508del homozygous mutation were isolated from freshly discarded lung tissues at Massachusetts General Hospital under Institutional Review Board (IRB) protocol approval (#2017P001479 and #2013P002332). The airway basal cells were used to generate matured airway epithelial cells in the air-liquid interface (ALI) culture, as previously described (56). The matured airway epithelial cells from HC and CF individuals were used for the analysis of gene and protein expressions. to promote squamous remodeling, 1 micro-M CHIR99021 (#4423, Tocris Bioscience, Minneapolis, MN) was added to the ALI medium as previously described (36). After 14 days of differentiation, epithelial cells were treated with vehicle, 20 μM Sotagliflozin and 10 nM Rapamycin, individually or in combination, for 3 days before cell harvest for protein and RNA extraction.
Antibodies
Antibodies against Bip/GRP78 (#3177), IRE1a (#3294), and β-actin (#3700) were obtained from Cell Signaling Technologies (Danvers, MA, USA). The CFTR antibody (#217 and #596) was from the Cystic Fibrosis Foundation Therapeutics (Bethesda, MD). The SGLT-1 antibodies were from Invitrogen (PA5–88282, Waltham, MA, USA; for Western blot) and Abcam (ab14686, Waltham, MA, US; for immunofluorescence staining). The p-IRE1a antibody (#AP0878) was from ABclonal Technology (Woburn, MA, USA). The XBP1s antibody (#619502) was from BioLegend (San Diego, CA, USA). The secondary antibodies were from LI-COR Biosciences (#D01216–10 and #D00226–05, Lincoln, NE, USA; for Western blot) and Jackson ImmunoResearch Laboratories (#147158, West Grove, PA, USA; for immunofluorescence staining).
Protein extraction and Western blot
Cells were lysed in RIPA lysis buffer (#89900, ThermoFisher Scientific, Waltham, MA, USA) supplemented with protease inhibitor and phosphatase inhibitor cocktails (#11873580001, Roche, Penzberg, Germany). Proteins were resolved in 10% SDS–PAGE gels and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). Membranes were blocked in TBST containing 5% non-fat milk at room temperature for 2 hours, and were incubated with primary antibodies (1:1000 dilution) at 4°C overnight. After washing with TBST, membranes were incubated with secondary antibodies (1:8000 dilution) at room temperature for 1 hour. After TBST wash, bands were scanned and quantified using an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA).
RNA isolation and quantitative real-time PCR (qRT-PCR)
Total RNA from cell samples was extracted using the Trizol reagent (#15596018, ThermoFisher Scientific) and purified with the RNeasy kit (#74106, QIAGEN, Hilden, Germany). RNAs were reverse transcribed into cDNA with the SuperScript III kit (#18080051, ThermoFisher Scientific). The target gene expression at the transcription level was assessed by the quantitative real-time PCR system (Bio-Rad, Hercules, CA, USA) using iQ SYBR Green Supermix (#1708884, Bio-Rad, Hercules, CA, USA). β-actin was used as the internal control. Primers for qRT-PCR are listed in Supplemental Table 2.
Immunofluorescence (IF) staining
The CFBE-WT cells and the CFBE-dF cells were fixed on Nunc Lab-Tek Chamber Slides (Thermo Fisher Scientific) with 4% paraformaldehyde in PBS for 15 minutes. After washing with PBS, the slides were blocked with 5% donkey serum for 1 hour at room temperature and then incubated with primary antibody against SGLT-1 (1:50 dilution). After PBS washing, the slides were incubated with Alexa Fluor–labeled secondary antibody (1:1000 dilution) at room temperature for 1 hour. IF slides were washed with PBS and mounted before image collection. IF images were acquired using an Olympus IX73 microscope.
Adenoviral infection
Adenovirus expressing spliced XBP1 (Ad-XBP1s) and adenovirus expressing mutant IRE1 K907A (Ad-K907A) were produced as previously described (28). For XBP1s overexpression, the CFBE-WT cells and the CFBE-dF cells at approximately 70–80% confluency were infected with Ad-XBP1s at a MOI of 100 for 48 hours. To suppress XBP1 expression, the cells were transduced with Ad-K907A at an MOI of 100 for 48 hours. The adenovirus encoding LacZ (Ad-LacZ) infection or non-infection served as controls.
Chromatin immunoprecipitation (ChIP) assay
The CFBE-WT cells and the CFBE-dF cells were transduced with Ad-XBP1s at an MOI of 100 for 48 hours to overexpress XBP1s. Ad-LacZ was used as the vehicle control. ChIP assay was performed with the SimpleChIP® Enzymatic Chromatin IP Kit (#9003, Cell Signaling Technologies, Danvers, MA, USA), following the manufacturer’s instructions. The DNA/protein complex was immunoprecipitated with control IgG or with anti-XBP1s antibody. Purified precipitated DNA was used as the template for qPCR. Primers used to detect the putative XBP1s binding motif on human SLC5A1 promoter are listed in Supplemental Table 2.
Plasmid construction and transfection
The putative promoter region of the human SLC5A1 gene (from position 22121087 to position −21) was PCR-amplified from human genomic DNA by using forward primer 5’TTTCTGTGGTCCTCTGCCTC-3’ and reverse primer 5’-TCCTTATACGGCCTCCTGGT-3’. The amplified promoter region was inserted into the pGL4.10 luciferase reporter vector (#E1910, Promega, Madison, WI, USA) by using In-Fusion® HD Cloning Kit (#102518, Takara, CA, USA). The 5’-CCACCCACCCACC-3’ box on the human SLC5A1 promoter (−595 to −583), which is the putative XBP1s binding site, was mutated to 5’-TACAGACTAATGA-3’ by using Q5 SiteDirected Mutagenesis Kit (#E0554S, New England Biolabs, Ipswich, MA). All plasmids were validated by Sanger sequencing. The plasmids were named wt-Luc and mut-Luc, respectively.
The CFBE-WT cells and the CFBE-dF cells at 70–80% confluence were transfected with either the wt-Luc or the mut-Luc plasmids by lipofectamine 2000 (#11668019, ThermoFisher Scientific) according to the manufacturer’s protocol. After 24 hours, these cells were infected with Ad-LacZ or Ad-XBP1s (MOI, 100). Luciferase activity was detected using Dual Luciferase Reporter Assay System (#E1910, Promega, Madison, WI, USA).
Statistics analysis
Statistical analyses were performed using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA). Data were reported as mean ± SEM (standard error of means) with three replicates for each data point. Comparison between two groups was analyzed by unpaired, 2-tailed Student’s t test (GraphPad).
Supplementary Material
Acknowledgements
We thank members of the Center for Advanced Models for Translational Sciences and Therapeutics at the University of Michigan for providing support to this project.
Funding
This work was supported by National Institutes of Health grants HL133162 (to JPJ and JX), DK134361 (to JX and KZ) and DK126908 and DK090313 (to KZ); Cystic Fibrosis Foundation grants XU19XX0 (to JX); and University of Michigan Medical Center internal fund to CAMTraST (to YEC).
Funding Statement
This work was supported by National Institutes of Health grants HL133162 (to JPJ and JX), DK134361 (to JX and KZ) and DK126908 and DK090313 (to KZ); Cystic Fibrosis Foundation grants XU19XX0 (to JX); and University of Michigan Medical Center internal fund to CAMTraST (to YEC).
Footnotes
Competing interests
The authors declare no conflicts of interest.
Availability of data and materials
All data analyzed in this study are provided in this article and its additional files. All data used in this study are available from the corresponding author on reasonable request.
References
- 1.Deeks E. D., Sotagliflozin: A Review in Type 1 Diabetes. Drugs 79, 1977–1987 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Hassanabad M. F., Abad Z. F. H., Are SGLT2 inhibitors joining the mainstream therapy for diabetes type 2? Diabetes Metab Syndr 13, 1893–1896 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Chilton R. J., Effects of sodium-glucose cotransporter-2 inhibitors on the cardiovascular and renal complications of type 2 diabetes. Diabetes Obes Metab 22, 16–29 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Szarek M. et al. , Effect of Sotagliflozin on Total Hospitalizations in Patients With Type 2 Diabetes and Worsening Heart Failure : A Randomized Trial. Ann Intern Med 174, 10651072 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Bhatt D. L. et al. , Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N Engl J Med 384, 129–139 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Jojima T. et al. , The SGLT2 Inhibitor Canagliflozin Prevents Carcinogenesis in a Mouse Model of Diabetes and Non-Alcoholic Steatohepatitis-Related Hepatocarcinogenesis: Association with SGLT2 Expression in Hepatocellular Carcinoma. Int J Mol Sci 20 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng C. H. et al. , Antidiabetic Medications for Type 2 Diabetics with Nonalcoholic Fatty Liver Disease: Evidence From a Network Meta-Analysis of Randomized Controlled Trials. Endocr Pract 28, 223–230 (2022). [DOI] [PubMed] [Google Scholar]
- 8.Liu Z. et al. , Impact of sodium glucose cotransporter 2 (SGLT2) inhibitors on atherosclerosis: from pharmacology to pre-clinical and clinical therapeutics. Theranostics 11, 4502–4515 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mui J. V. et al. , Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors vs. Dipeptidyl Peptidase-4 (DPP4) Inhibitors for New-Onset Dementia: A Propensity Score-Matched Population-Based Study With Competing Risk Analysis. Front Cardiovasc Med 8, 747620 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirai H. et al. , The sodium/glucose cotransporters as potential therapeutic targets for CF lung diseases revealed by human lung organoid swelling assay. Mol Ther Methods Clin Dev 24, 11–19 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riordan J. R. et al. , Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245, 1066–1073 (1989). [DOI] [PubMed] [Google Scholar]
- 12.Rowe S. M., Miller S., Sorscher E. J., Cystic fibrosis. N Engl J Med 352, 1992–2001 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Hetz C., The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13, 89–102 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Fiedorczuk K., Chen J., Mechanism of CFTR correction by type I folding correctors. Cell 185, 158–168 e111 (2022). [DOI] [PubMed] [Google Scholar]
- 15.Bartoszewski R. et al. , Activation of the unfolded protein response by deltaF508 CFTR. Am J Respir Cell Mol Biol 39, 448–457 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oakes S. A., Papa F. R., The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol 10, 173–194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yong J., Johnson J. D., Arvan P., Han J., Kaufman R. J., Therapeutic opportunities for pancreatic beta-cell ER stress in diabetes mellitus. Nat Rev Endocrinol 17, 455–467 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren J., Bi Y., Sowers J. R., Hetz C., Zhang Y., Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat Rev Cardiol 18, 499–521 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Uddin M. S. et al. , Molecular Mechanisms of ER Stress and UPR in the Pathogenesis of Alzheimer’s Disease. Mol Neurobiol 57, 2902–2919 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Ajoolabady A. et al. , Endoplasmic reticulum stress in liver diseases. Hepatology 77, 619639 (2023). [Google Scholar]
- 21.Chen X., Cubillos-Ruiz J. R., Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat Rev Cancer 21, 71–88 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y., Brandizzi F., IRE1: ER stress sensor and cell fate executor. Trends Cell Biol 23, 547–555 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Read A., Schroder M., The Unfolded Protein Response: An Overview. Biology (Basel) 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S. M., Kang T. I., So J. S., Roles of XBP1s in Transcriptional Regulation of Target Genes. Biomedicines 9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanemoto S. et al. , XBP1 activates the transcription of its target genes via an ACGT core sequence under ER stress. Biochem Biophys Res Commun 331, 1146–1153 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Acosta-Alvear D. et al. , XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27, 53–66 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Fink E. E. et al. , XBP1-KLF9 Axis Acts as a Molecular Rheostat to Control the Transition from Adaptive to Cytotoxic Unfolded Protein Response. Cell Rep 25, 212–223 e214 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J. M., Qiu Y., Yang Z. Q., Li L., Zhang K., Inositol-Requiring Enzyme 1 Facilitates Diabetic Wound Healing Through Modulating MicroRNAs. Diabetes 66, 177–192 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ning J. et al. , Constitutive role for IRE1alpha-XBP1 signaling pathway in the insulinmediated hepatic lipogenic program. Endocrinology 152, 2247–2255 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato H. et al. , mTORC1 serves ER stress-triggered apoptosis via selective activation of the IRE1-JNK pathway. Cell Death Differ 19, 310–320 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg S. K. et al. , Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes. N Engl J Med 377, 2337–2348 (2017). [DOI] [PubMed] [Google Scholar]
- 32.McCrimmon R. J., Henry R. R., SGLT inhibitor adjunct therapy in type 1 diabetes. Diabetologia 61, 2126–2133 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caruso I., Giorgino F., SGLT-2 inhibitors as cardio-renal protective agents. Metabolism 127, 154937 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Rigden H. M. et al. , Squamous Metaplasia Is Increased in the Bronchial Epithelium of Smokers with Chronic Obstructive Pulmonary Disease. PLoS One 11, e0156009 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Araya J. et al. , Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest 117, 3551–3562 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y. et al. , Human Airway Basal Cells Undergo Reversible Squamous Differentiation and Reshape Innate Immunity. Am J Respir Cell Mol Biol 68, 664–678 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J. S. et al. , Pharmacologic ER stress induces non-alcoholic steatohepatitis in an animal model. Toxicol Lett 211, 29–38 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng Z., Zhang C., Zhang K., Measurement of ER stress response and inflammation in the mouse model of nonalcoholic fatty liver disease. Methods Enzymol 489, 329–348 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Chen X. et al. , Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci 203, 291–304 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Zhang C. et al. , Endoplasmic reticulum-tethered transcription factor cAMP responsive element-binding protein, hepatocyte specific, regulates hepatic lipogenesis, fatty acid oxidation, and lipolysis upon metabolic stress in mice. Hepatology 55, 1070–1082 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribeiro C. M. P., Hull-Ryde E. A., Functional role of the ER stress transducer IRE1alpha in CF airway epithelial inflammation. Curr Opin Pharmacol 65, 102258 (2022). [DOI] [PubMed] [Google Scholar]
- 42.Tang A. C. et al. , Endoplasmic Reticulum Stress and Chemokine Production in Cystic Fibrosis Airway Cells: Regulation by STAT3 Modulation. J Infect Dis 215, 293–302 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Wu Q. et al. , Cystic fibrosis rabbits develop spontaneous hepatobiliary lesions and CFassociated liver disease (CFLD)-like phenotypes. PNAS Nexus 2, pgac306 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nevola R. et al. , Cardiorenal Impact of SGLT-2 Inhibitors: A Conceptual Revolution in The Management of Type 2 Diabetes, Heart Failure and Chronic Kidney Disease. Rev Cardiovasc Med 23, 106 (2022). [DOI] [PubMed] [Google Scholar]
- 45.Tentolouris A., Vlachakis P., Tzeravini E., Eleftheriadou I., Tentolouris N., SGLT2 Inhibitors: A Review of Their Antidiabetic and Cardioprotective Effects. Int J Environ Res Public Health 16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pistelli L. et al. , Gliflozins: From Antidiabetic Drugs to Cornerstone in Heart Failure Therapy-A Boost to Their Utilization and Multidisciplinary Approach in the Management of Heart Failure. J Clin Med 12 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nasiri-Ansari N. et al. , Empagliflozin Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) in High Fat Diet Fed ApoE((−/−)) Mice by Activating Autophagy and Reducing ER Stress and Apoptosis. Int J Mol Sci 22 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ala M., SGLT2 Inhibition for Cardiovascular Diseases, Chronic Kidney Disease, and NAFLD. Endocrinology 162 (2021). [DOI] [PubMed] [Google Scholar]
- 49.Secnik J. et al. , Glucose-Lowering Medications and Post-Dementia Survival in Patients with Diabetes and Dementia. J Alzheimers Dis 86, 245–257 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsapas A. et al. , Comparative Effectiveness of Glucose-Lowering Drugs for Type 2 Diabetes: A Systematic Review and Network Meta-analysis. Ann Intern Med 173, 278–286 (2020). [DOI] [PubMed] [Google Scholar]
- 51.Dandekar A., Mendez R., Zhang K., Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol Biol 1292, 205–214 (2015). [DOI] [PubMed] [Google Scholar]
- 52.Garg A. D. et al. , ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol Med 18, 589–598 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Cao S. S., Luo K. L., Shi L., Endoplasmic Reticulum Stress Interacts With Inflammation in Human Diseases. J Cell Physiol 231, 288–294 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hou X. et al. , CK19 stabilizes CFTR at the cell surface by limiting its endocytic pathway degradation. FASEB J 33, 12602–12615 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blight K. J., McKeating J. A., Rice C. M., Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol 76, 13001–13014 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Levardon H., Yonker L. M., Hurley B. P., Mou H., Expansion of Airway Basal Cells and Generation of Polarized Epithelium. Bio Protoc 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed in this study are provided in this article and its additional files. All data used in this study are available from the corresponding author on reasonable request.