Abstract
CDK4/6 inhibitors (CDK4/6i) have improved survival of patients with estrogen receptor-positive (ER+) breast cancer. However, patients treated with CDK4/6i eventually develop drug resistance and progress. RB1 loss-of-function alterations confer acquired resistance to CDK4/6i, but the optimal therapy for these patients is unclear. Using a genome-wide CRISPR screen, we identified protein arginine methyltransferase 5 (PRMT5) as a molecular vulnerability in ER+/RB1-knockout (RBKO) breast cancer cells. PRMT5 inhibition blocked cell cycle G1-to-S transition independent of RB, thus arresting growth of RBKO cells. Proteomics analysis uncovered fused in sarcoma (FUS) as a downstream effector of PRMT5. Pharmacological inhibition of PRMT5 resulted in dissociation of FUS from RNA polymerase II (Pol II), Ser2 Pol II hyperphosphorylation, and intron retention in genes that promote DNA synthesis. Treatment with the PRMT5i inhibitor pemrametostat and fulvestrant synergistically inhibited growth of ER+/RB-deficient patient-derived xenografts, suggesting dual ER and PRMT5 blockade as a novel therapeutic strategy to treat ER+/RB-deficient breast cancer.
Main
Estrogen receptor-positive (ER+) breast cancer is the most common breast cancer subtype and, as such, is the leading cause of death from this disease [1, 2]. Recently, the approval and clinical use of CDK4/6 inhibitors (CDK4/6i) in combination with antiestrogen therapy has significantly improved progression-free and overall survival of patients with ER+ metastatic breast cancer (MBC) [3–6]. Despite these advances, virtually all tumors eventually acquire resistance to this therapy, leaving patients with limited therapeutic options. The efficacy of CDK4/6i relies on an intact retinoblastoma protein (RB)/E2F transcription axis. Inhibition of CDK4/6 activity suppresses RB phosphorylation, enabling RB to couple to E2F transcription factors and block entry into the S-phase of the cell cycle [7]. Several clinical studies have reported an association with and enrichment of RB1 loss-of-function genomic alterations with resistance to CDK4/6i in patients with ER+ MBC [8–12]. Recently, the CDK4/6i abemaciclib was approved as adjuvant therapy for high-risk ER+ breast cancer [13]. With the increased use of CDK4/6i as the standard-of-care for ER+ breast cancer, it is anticipated that RB-deficient breast cancer will become a rising patient population in need of novel therapeutic strategies. To discover actionable vulnerabilities in this refractory breast cancer subtype, we performed a genome-wide CRISPR screen using ER+/RB1-knockout (RBKO) cells and identified protein arginine methyltransferase 5 (PRMT5) as a novel dependency in these cells.
Arginine methylation is an ubiquitous and key post-translational modification (PTM) catalyzed by the PRMT family of enzymes. This family comprises three types of PRMTs, all of which catalyze the formation of ω-NG-monomethyl arginine (MMA). While type I and type II PRMTs are responsible for the formation of ω-NG,NG-asymmetric dimethylarginine (ADMA) and ω-NG,N’G-symmetric dimethylarginine (SDMA), respectively, a type III PRMT catalyzes the formation of only MMA [14]. In mammalian cells, PRMT5 is the primary type II PRMT that methylates arginine residues in the RGG/RG motif [15, 16]. Functionally, SDMA catalyzed by PRMT5 is recognized by Tudor domains in proteins, thus facilitating protein-protein interactions [17]. Emerging evidence has demonstrated that PRMT5 plays an important role in epigenetic regulation, RNA processing, DNA repair, and cell cycle progression [14, 18]. Currently, multiple PRMT5 small molecule inhibitors have entered early-phase clinical trials in solid tumors and hematological malignancies (https://clinicaltrials.gov).
In this study, genetic and pharmacological inhibition of the methyltransferase activity of PRMT5 in ER+/RB-deficient human breast cancer cells blocked the G1-to-S cell cycle transition and arrested their proliferation despite loss of RB – the canonical mechanism regulating arrest of cells in G1 phase of the cell cycle. Similar results were observed in lung, prostate, and triple-negative breast cancer cells harboring natural RB1 loss-of-function mutations or deletion. Mass spectrometry analysis identified the protein fused in sarcoma (FUS) as a downstream effector of PRMT5. Inhibition of PRMT5 resulted in dissociation of FUS from RNA polymerase II (Pol II), leading to Pol II Ser2 hyperphosphorylation and intron retention in multiple genes that promote DNA synthesis in S phase. Finally, treatment with the PRMT5 small molecule inhibitor pemrametostat and the ER degrader fulvestrant synergistically suppressed growth of ER+/RB-deficient xenografts derived from cell lines and patients, suggesting dual ER and PRMT5 blockade as a novel therapeutic strategy to treat ER+/RB-deficient breast cancer.
Results
Genome-wide CRISPR screen identifies PRMT5 as a molecular vulnerability in ER+/RB1-deficient breast cancer cells
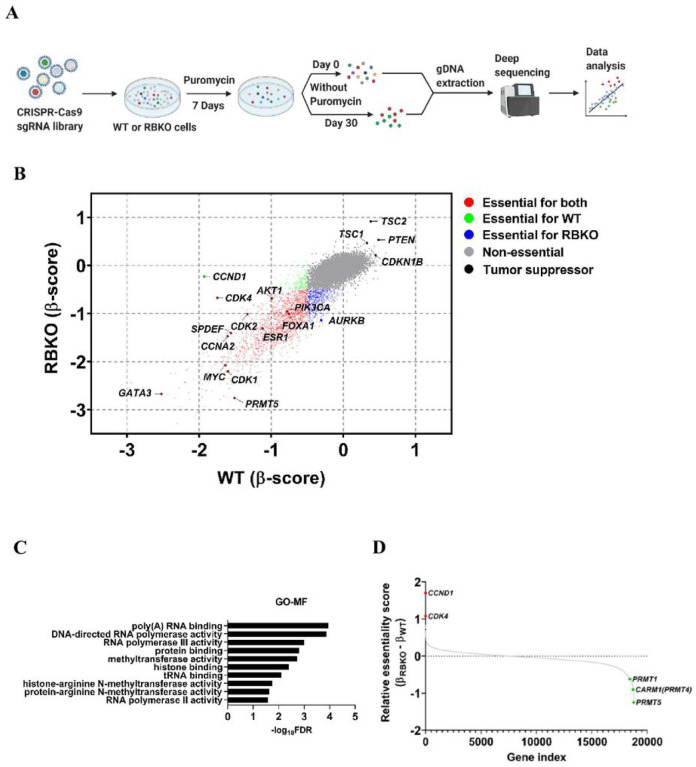
To mimic RB1 loss-of-function alterations, we used CRISPR-Cas9 to delete RB1 in CDK4/6i sensitive MCF-7 and T47D breast cancer cells (Supplementary Fig. 1A). MCF-7_RBKO and T47D_RBKO cells were resistant to treatment with the CDK4/6i abemaciclib, palbociclib and ribociclib, exhibiting a 10- to 300-fold higher IC50 of these drugs compared to that of their isogenic parental cells (Supplementary Fig. 1B–E). Next, we performed a genome-wide CRISPR dropout screen using T47D_WT and _RBKO cells (Fig. 1A). We analyzed the deep sequencing data using MAGeCK [19] and stratified gene essentiality with a false discovery rate (FDR) <0.05 and β-score <−0.5 (Supplementary Table 1) as previously described [20]. The β-score represents the degree of sgRNA depletion or enrichment, with essential genes having a more negative β-score. For example, CCND1 (βWT = −1.93; βRBKO = −0.23) and CDK4 (βWT = −1.75; βRBKO = −0.67) were not essential in RBKO compared to WT cells, in line with the notion that loss of RB1 uncouples the CDK4/Cyclin D1 complex from E2F-regulated transcription and the G1-to-S transition [21]. Conversely, CDK2 and CCNA2, both involved in S phase progression, were essential in both cell types (Fig. 1B, bottom half for RBKO and left half for WT cells). We also observed enrichment of sgRNAs against CDKN1B, PTEN, TSC1 and TSC2 in both cell types, suggesting deletion of these tumor suppressors provides a survival advantage. In contrast, sgRNAs targeting known oncogenic drivers of ER+ breast cancer were depleted in both WT and RBKO cells. These included essential genes like MYC, PIK3CA, and AKT1 (T47D cells harbor an activating PIK3CA mutation [22]). Further, sgRNAs targeting essential transcription factors that drive ER signaling, such as FOXA1, GATA3, MYC, SPDEF and ESR1 itself, were evenly depleted in both WT and RBKO cells (Fig. 1B), suggesting that the ERα pathway may still be essential in these cells irrespective of RB1 status. We next ranked the essential genes in RBKO cells by selecting the top 50 genes whose corresponding sgRNAs were statistically more depleted in RBKO over WT cells and subjected them to gene ontology (GO) analysis [23] to investigate whether these hits converge on a defined molecular function. This gene list was enriched for molecules involved in arginine methyltransferase activity, primarily due to significant depletion of sgRNAs targeting PRMT5 (βWT = −1.51; βRBKO = −2.76) and CARM1 (βWT = −0.06; βRBKO = −0.97) (Fig. 1B–D). We first focused on PRMT5 because 1) PRMT5 was the top-scoring and druggable hit, 2) literature supports a role for PRMT5 in the progression of various cancer types, including breast cancer [24, 25], and 3) PRMT5 small molecule inhibitors (PRMT5i) are in clinical development, thus allowing us to test the antitumor effects of PRMT5 pharmacological inhibition.
Figure 1. Genome-wide CRISPR dropout screen identifies PRMT5 as an essential gene for survival of ER+/RB1-deficient breast cancer cells.
A) Schematic of the CRSIPR screen. B) Comparison of the β-scores of the CRISPR screen in T47D_WT and _RBKO cells. The β-score represents the degree of sgRNA depletion or enrichment, with essential genes having a more negative β-score. C) Gene Ontology-molecular function (GO-MF) analysis using the top 50 genes of which the corresponding sgRNAs were more significantly depleted in T47D_RBKO cells over WT cells. D) Gene relative essentiality score ranked by the differences of the β-scores in RBKO and WT cells (βRBKO – βWT).
Genetic and pharmacological inhibition of PRMT5 suppress growth of ER+/RB-deficient breast cancer cells
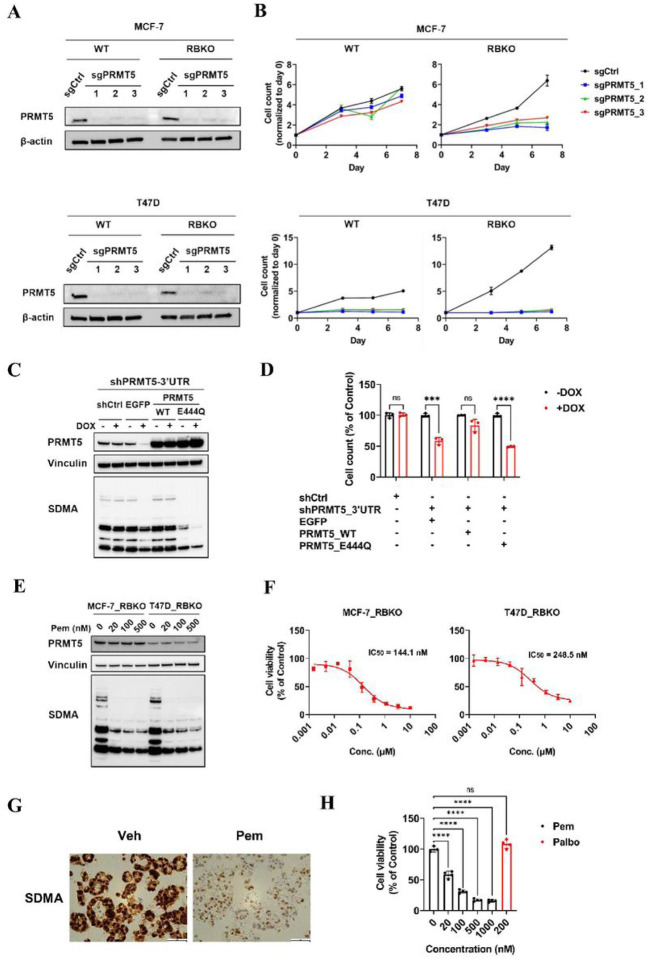
To validate whether PRMT5 was essential for survival of ER+/RBKO cells, we depleted PRMT5 using CRISPR-Cas9 in WT and RBKO cells in each MCF-7 and T47D lines (Fig. 2A). Concordant with the CRISPR screening results, PRMT5 depletion resulted in growth inhibition in isogenic T47D_WT and _RBKO cells (Fig. 2B). MCF-7_RBKO cells were sensitive to PRMT5 depletion whereas MCF-7_WT cells were not (Fig. 2B). Next, we asked if inhibition of the methyltransferase activity of PRMT5 was required to block growth of ER+/RB-deficient breast cancer cells. To test this, we knocked down PRMT5 using a doxycycline (DOX)-inducible shRNA targeting the 3’UTR of PRMT5 in T47D_RBKO cells and then rescued the effect of the shRNA by exogenous expression of either WT PRMT5 or enzymatically dead PRMT5_E444Q. Immunoblot analysis of DOX-treated T47D_RBKO cells showed that expression of WT PRMT5 rescued symmetric dimethylarginine (SDMA) levels that had been suppressed upon induction of the PRMT5 shRNA. By contrast, expression of PRMT5_E444Q failed to rescue SDMA levels (Fig. 2C). Furthermore, expression of WT PRMT5 but not PRMT5_E444Q rescued the growth inhibition induced by PRMT5 knockdown (Fig. 2D). These results suggested the potential of inhibiting ER+/RB-deficient breast cancer cell growth by pharmacological inhibition of the PRMT5 methyltrasferase activity. Thus, we next examined the effect of pemrametostat, a competitive inhibitor that binds to PRMT5 substrate binding pocket [26], on growth of RB-deficient cells and a patient-derived xenograft-derived organoid (PDxO; HCI-018) [27]. In a concentration-dependent manner, treatment with pemrametostat markedly decreased SDMA levels in RBKO cells (Fig. 2E), suggesting the inhibitor was engaging its molecular target. Consistent with these results, pemrametostat used over a dose range inhibited growth of MCF-7_RBKO and T47D_RBKO cells with an IC50 of 144.1 and 248.5 nM, respectively (Fig. 2F). Pemrametostat treatment of PDxO HCI-018 also decreased SDMA levels measured by immunohistochemistry (IHC; Fig. 2G) and inhibited up to 85% growth of the organoids in a concentration-dependent manner (Fig. 2H). Treatment of MCF-7_RBKO and T47D_RBKO cells with sub-μM concentrations of the PRMT5i JNJ64619178 and of CAMA1_RBKO and KPL1_RBKO cells with the PRMT5i GSK591 [28] also resulted in growth inhibition (Supplementary Fig. 2), suggesting the results with pemrametostat also apply to other PRMT5 substrate binding inhibitors. Collectively, these results suggest that PRMT5 is an actionable molecular vulnerability in ER+/RB-deficient breast cancer cells.
Figure 2. Targeting PRMT5 inhibits growth of ER+/RBKO breast cancer cells.
A) Immunoblot analysis of MCF-7 and T47D cell lysates. Non-targeting control sgRNA (sgCtrl) or three sgRNAs targeting PRMT5 (sgPRMT5) were individually transduced to the cells. Lysates were probed with the indicated antibodies. B) Monolayer growth of sgCtrl- or sgPRMT5-transduced MCF-7 and T47D WT and RBKO cells. Cells were seeded in 12-well plates with estrogen-deprived IMEM. Cells were counted on days 3, 5 and 7 using a Coulter counter. Data represent mean ± SD (n = 3). C) Immunoblot analysis of T47D_RBKO cells transduced with a doxycycline (DOX)-inducible control shRNA (shCtrl) or a shRNA targeting PRMT5 3’UTR (shPRMT5–3’UTR). The cells transduced with the shPRMT5–3’UTR were further transduced with pLX304-zeo carrying EGFP, WT PRMT5, or PRMT5_E444Q. Cells were treated with or without 200 ng/mL DOX for 4 days; cell lysates were collected and subjected to immunoblot with the indicated antibodies. D) Monolayer growth of T47D_RBKO cells as described in (C). Cells were treated with or without 200 ng/mL DOX for 8 days. Cell number was counted using a Coulter counter. Data represent mean ± SD (n = 3); *** (P <.001), **** (P <.0001), ns: P not significant, Student’s t test. E) Immunoblot analysis of MCF-7_ and T47D_RBKO lysates. Cells were treated with different concentrations of pemrametostat (Pem) for 3 days. Lysates were collected and subjected to immunoblot with the indicated antibodies. F) Dose response curve of Pem. MCF-7_RBKO and T47D_RBKO cells were treated with Pem (0–10 μM) for 6 days. Cell viability was measured by the CyQuant assay. Data represent mean ± SD (n = 3). G) IHC of SDMA. The ER+/RB1-deleted organoid HCI-018 was treated with vehicle control (Veh) or 500 nM Pem for 6 days. The organoids were then harvested, fixed, and then embedded for IHC. H) Viability of HCI-018. The organoids were treated with Veh, Pem, or palbociclib (Palbo) for 20 days and then subjected to the 3D CellTiter-Glow assay. Data represent mean ± SD (n = 4); **** (P <.0001), ns: P not significant, one-way ANOVA with a Dunnett’s post-hoc test.
PRMT5 inhibition blocks G1-to-S cell cycle transition in an RB-independent manner
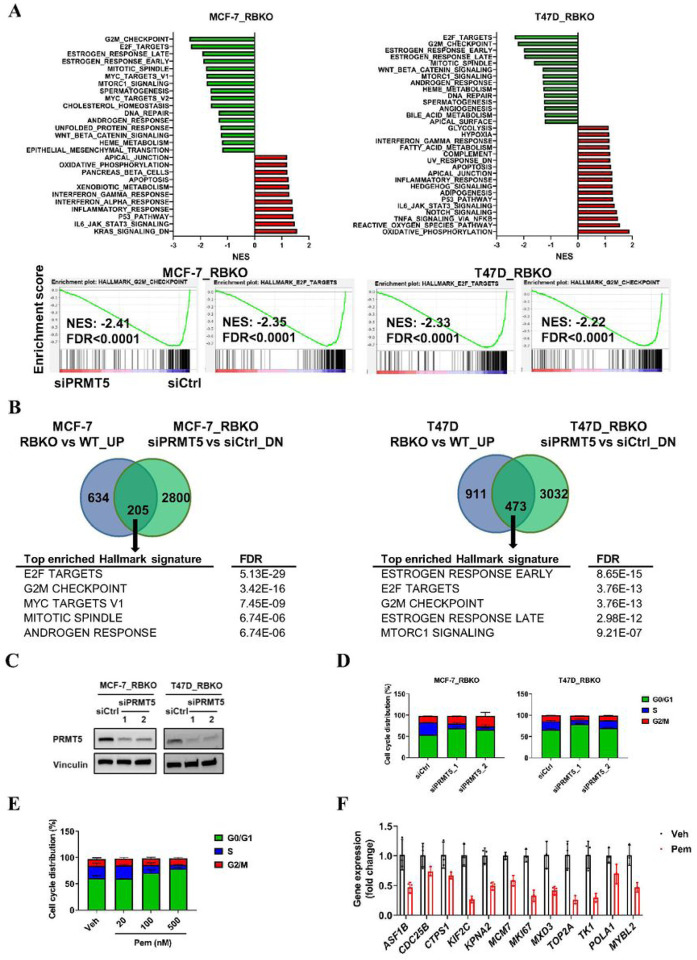
To investigate the mechanisms underlying the inhibition of cell growth upon silencing of PRMT5, we performed RNA-seq of both MCF-7 and T47D cells and compared gene expression of 1) RBKO vs. WT cells, and 2) in RBKO cells transfected with a PRMT5 siRNA vs. control siRNA. As expected, Gene Set Enrichment Analysis (GSEA) of RNA-seq from RBKO vs. WT cells showed significant upregulation of cell cycle-related Hallmark gene signatures, including E2F targets and G2/M gene signatures in RBKO cells (Supplementary Fig. 3). By contrast, PRMT5 knockdown in RBKO cells resulted in downregulation of E2F targets, G2/M checkpoint, and mitotic spindle Hallmark gene signatures (Fig. 3A). Further, silencing of PRMT5 in RBKO cells downregulated 205 genes (in MCF-7) and 473 genes (in T47D) whose expression had been increased by RB1 knockout. GSEA of those (205 and 473) genes in both cell lines showed highly statistical enrichment of E2F targets and G2/M checkpoint gene signatures (Fig. 3B), suggesting that silencing of PRMT5 reversed the changes on cell cycle gene expression induced by RB1 loss.
Figure 3. Silencing of PRMT5 downregulates E2F gene signature and blocks G1-to-S-phase progression.
A) Gene set enrichment analysis (GSEA) of the Hallmark gene signatures. MCF-7_ RBKO and T47D_RBKO cells were transfected with control siRNA (siCtrl) or a PRMT5 siRNA (siPRMT5) for 3 days. Total RNA was extracted from the cells and then subjected to RNA-seq. NES: normalized enrichment score; FDR: false discovery rate. B) Venn diagram showing differentially expressed genes in RBKO vs WT cells and siPRMT5 vs siCtrl in RBKO cells. Cutoff FDR <0.01 and fold change >0.2. C) Immunoblot analysis of MCF-7_RBKO cells. Cell lysates were collected 3 days after transfection of siCtrl or two individual siPRMT5. The lysates were probed with antibodies as indicated. D-E) Cell cycle analysis of MCF-7_RBKO cells. Cells were fixed 3 days after transfection of siCtrl or two individual siPRMT5 (D) or treatment of vehicle control (Veh) or Pemrametostat (Pem) (E). The fixed cells were stained with propidium iodide (PI) and then analyzed using flow cytometry. Data represent mean ± SD (n = 3). F) Expression of E2F target genes in MCF-7_RBKO cells. Cells were treated with Veh or 500 nM Pem for 3 days and then subjected to total RNA extraction, reverse transcription, and qRT-PCR. Data represent mean ± SD (n = 3).
Next, we sought to examine whether PRMT5 inhibition resulted in dysregulation of the cell cycle. We employed siRNAs to knockdown PRMT5 in MCF-7_RBKO and T47D_RBKO cells and then performed cell cycle analysis of propidium iodide-stained cells. PRMT5 knockdown led to accumulation of cells in G1 phase and reduction of cells in S phase (Fig. 3C,D). Similar to PRMT5 siRNA, treatment with pemrametostat of MCF-7_RBKO cells also hampered the G1-to-S phase transition in a dose-dependent manner (Fig. 3E) and suppressed the expression of E2F target genes as measured by qRT-PCR (Fig. 3F).
Since RB1 loss-of-function mutations occur across various tumor types, we also examined the effect of PRMT5 silencing in other RB-deficient cancer cell lines. PRMT5 knockdown in lung cancer (H596, H1048 and H1155), prostate cancer (Du-145) and triple-negative breast cancer (MDA-MB-436) cell lines, all harboring natural RB1 loss-of-function mutations or deletion, resulted in significant growth inhibition, accumulation of cells in G1, and a decrease in cells in S phase (Supplementary Fig. 4), further suggesting the potential applicability of therapeutic targeting PRMT5 across various types of cancer with RB deficiency.
FUS is a functional substrate of PRMT5
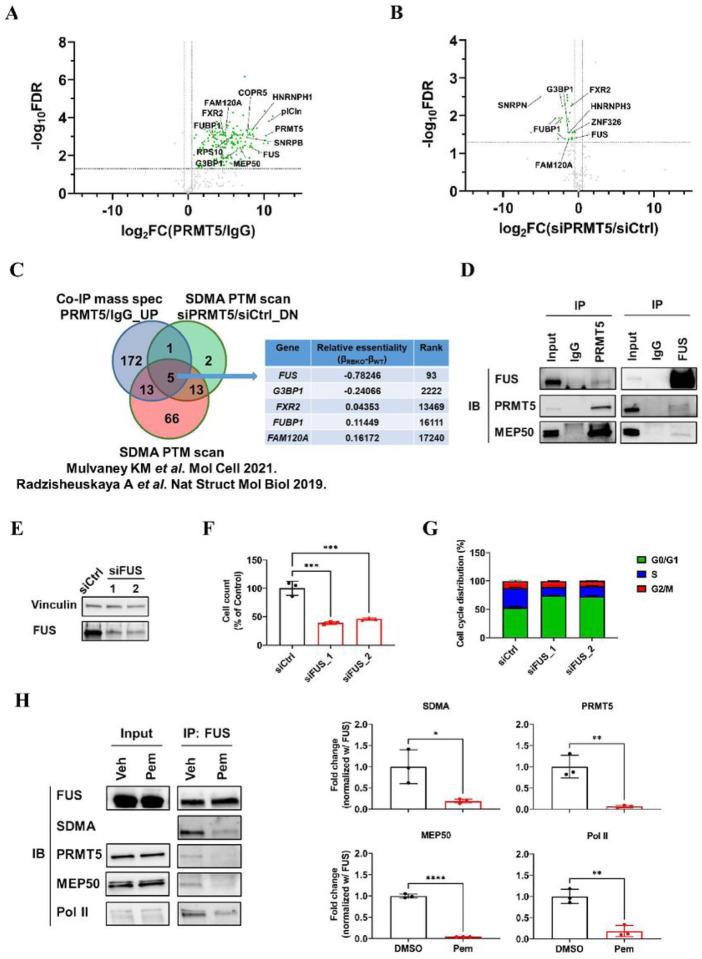
RB plays a pivotal role in the G1-to-S checkpoint during cell cycle progression [29, 30]. The effect of PRMT5 inhibition on blockade of the G1-to-S transition in RB1-deleted cells suggested a novel approach to suppress cancer cell proliferation. Since the methyltransferase activity of PRMT5 was essential to support growth of ER+/RBKO cells (Fig. 2C,D), we aimed to identify downstream effectors of PRMT5. Thus, we employed co-immunoprecipitation (Co-IP) mass spectrometry (MS) and found 192 proteins that were significantly enriched in PRMT5 antibody pulldowns (over control IgG) (Supplementary Table 2). As expected, PRMT5 was one of the most significantly enriched proteins identified by MS. In line with previous studies [16, 31], we also found significant enrichment of the PRMT5 hetero-octamer partner MEP50 (also known as WDR77) and substrate adaptors COPR5 and plCln (Fig. 4A). To determine putative substrates of PRMT5’s enzymatic activity, we performed a SDMA post-translational modification (PTM) analysis using LC-MS/MS to examine SDMA level changes in PRMT5-associated proteins as a result of PRMT5 knockdown. The PTM analysis identified 165 SDMA peptides, of which 21 peptides exhibited significant downregulation of SDMA levels when PRMT5 was knocked down (Fig. 4B; Supplementary Table 3). Integration of the results from the Co-IP MS and the SDMA PTM analysis with those PRMT5 substrates reported in published studies [16, 31] identified five common hits (FAM120A, FUBP1, FUS, FXR2 and G3BP1) (Fig. 4C). Among these five candidates, FUS (fused in sarcoma) was the most essential gene (βWT = 0.17; βRBKO = −0.61) in the initial CRISPR screen of ER+/RBKO cells (Fig. 4C; Supplementary Table 1).
Figure 4. Proteomics analysis identifies FUS as a downstream effector of PRMT5.
A) Co-IP MS analysis. MCF-7_RBKO cell lysates were subjected to immunoprecipitation (IP) with a PRMT5 antibody or rabbit IgG control and then subjected to MS analysis. Green: proteins that shows significant enrichment in PRMT5 antibody pulldowns (log2FC ≥0.5 and FDR <0.05). Grey: proteins that shows non-significant enrichment in the PRMT5 antibody pulldowns (log2FC <0.5 or FDR >0.05). B)SDMA PTM analysis. MCF-7_RBKO cells were transfected with siPRMT5 or siCtrl for three days. Cell lysates were collected in urea buffer and precipitated with a SDMA antibody; antibody pulldowns were then subjected to LC-MS/MS analysis. Green: peptides that showed significant SDMA downregulation (log2FC ≤0.5 and FDR <0.05). Grey: peptides that showed non-significant SDMA downregulation (log2FC >0.5 or FDR >0.05). C) Venn diagram integrating PRMT5 interacting proteins identified by Co-IP MS analysis, proteins where the PRMT5 siRNA reduced SDMA levels, and PRMT5 substrates identified by SDMA PTM analysis in published literature. The table shows the common hits and their essentiality scores and ranking in the initial CRISPR screen. D) Co-IP of MCF-7_RBKO lysates using a PRMT5 or a FUS antibody followed by immunoblot analysis. E) Immunoblot analysis of MCF-7_RBKO cells. Cell lysates were collected 3 days after transfection of control siRNA (siCtrl) or two individual FUS siRNAs (siFUS) and then probed with antibodies as indicated. F) Monolayer growth of MCF-7_RBKO cells. Cell number was counted using a Coulter counter five days after the siRNA transfection. Data represent mean ± SD (n = 3); *** (P <.001), one-way ANOVA with a Dunnett’s post-hoc test. G) Cell cycle analysis of MCF-7_RBKO cells. Cells were fixed 3 days after the transfection of siCtrl or two individual siFUS. The fixed cells were stained with propidium iodide (PI) and then analyzed by flow cytometry. Data represent mean ± SD (n = 3). H) Co-IP of MCF-7_RBKO lysates followed by immunoblot analysis. Cells were treated with DMSO or 500 nM Pemrametostat (Pem) for three days and then subjected to Co-IP using a FUS antibody. Immunoblot analysis of the FUS antibody pulldowns was conducted (left) and quantified (right) using the indicated antibodies. Data represent mean ± SD (n = 3); * (P <.05), ** (P <.01), **** (P <.0001), Student’s t test.
FUS is a DNA/RNA-binding protein involved in the regulation of gene transcription, DNA repair, and RNA processing [14]. We confirmed the interaction between PRMT5 and FUS by reciprocal Co-IP of endogenous PRMT5 and of FUS followed by immunoblot analysis with FUS and PRMT5 antibodies, respectively (Fig. 4D). In addition to PRMT5, FUS also interacted with MEP50, suggesting that FUS may be involved in the PRMT5 methylosome (Fig. 4D). We then monitored cell proliferation and performed cell cycle analysis upon knockdown of FUS with siRNAs in MCF-7_RBKO cells. Knockdown of FUS significantly decreased RBKO cell viability and their G1-to-S cell cycle transition (Fig. 4E–G), thus phenocopying the effects of PRMT5 silencing (Figs. 2,3). Taken together, these results were consistent with the proteomics analysis and supported FUS as a functional substrate of PRMT5 in ER+/RBKO cells.
PRMT5 inhibition results in Pol II Ser2 hyperphosphorylation and dysregulation of RNA splicing
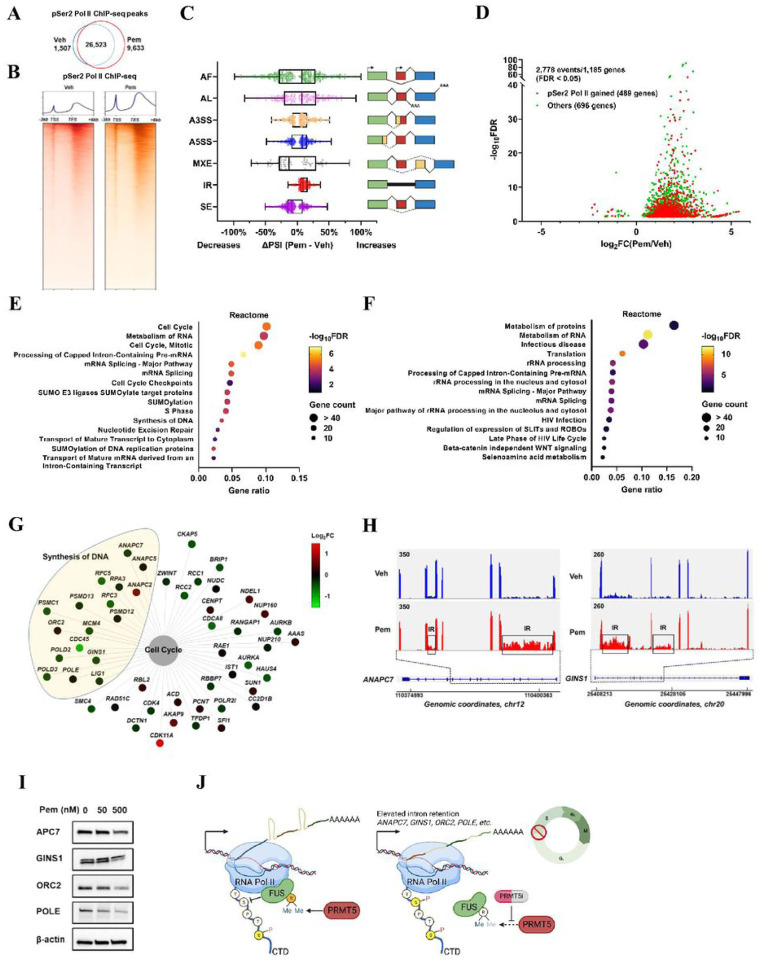
FUS is known to form a liquid droplet phase-separated structure that mediates bindings with the carboxyl-terminal domain (CTD) in RNA polymerase II (Pol II) [32–34]. Depletion of FUS has been reported to derepress Ser2 phosphorylation (pSer2) in the CTD of Pol II, resulting in abnormal accumulation of pSer2 Pol II and dysregulation of gene transcription and RNA splicing [35, 36]. Therefore, we speculated that 1) PRMT5-catalyzed SDMA levels in FUS are necessary for the interaction between FUS and Pol II, and 2) PRMT5 inhibition results in dissociation of FUS from Pol II. Supporting this hypothesis, treatment of pemrametostat in MCF-7_RBKO cells significantly suppressed SDMA in FUS and reduced the association of FUS with PRMT5, MEP50, and Pol II as shown by FUS antibody pulldowns followed by immunoblot analysis (Fig. 4H). Next, we asked whether PRMT5 inhibition increased pSer2 Pol II as a result of uncoupling FUS from Pol II. To this end, we investigated the effects of pemrametostat on the distribution of pSer2 Pol II by chromatin immunoprecipitation-sequencing (ChIP-seq) with a pSer2 Pol II-specific antibody. ChIP-seq analysis identified 26,523 common peaks of pSer2 Pol II chromatin binding (FDR <0.05) in MCF-7_RBKO cells treated with pemrametostat or DMSO. Pemrametostat-mediated inhibition of PRMT5 in MCF-7_RBKO cells resulted in gains of 9,633 unique pSer2 Pol II chromatin binding peaks as opposed to a 6-fold lower loss in 1,507 binding peaks (Fig. 5A,B). This global increase of pSer2 Pol II chromatin binding was in line with a previous study that showed accumulation of pSer2 Pol II as a result of loss of FUS [35].
Figure 5. PRMT5 inhibition results in Pol II Ser2 hyperphosphorylation and intron retention of cell cycle regulating genes.
A) Venn diagram of Pol II pSer2 peaks identified by ChIP-seq in MCF-7_RBKO cells treated with 500 nM pemrametostat (Pem) or vehicle (Veh) for 72 hours (n = 2). B) Heatmap displaying Pol II pSer2 binding intensity based on ChIP-seq in MCF-7_RBKO cells. TSS: transcription start sites; TES: transcription end sites. C) Differences in percentage spliced in index (ΔPSI) between Pem and Veh treated MCF-7_RBKO cells. Analysis was conducted using SUPPA2 with RNA-seq data from MCF-7_RBKO cells treated with 500 nM Pem or Veh for 72 hours (n = 3). AF: alternative first exon; AL: alternative last exon; A3SS: alternative 3’ splice-site; A5SS: alternative 5’ splice-site; MXE, mutually exclusive exon; IR, intron retention; SE: skipped exon. Vertical lines within boxes represent median, edges of boxes represent the first or fourth quartiles, and whiskers represent the minimum or maximum values, outliers (greater or less than 1.5× interquartile range) were excluded. D) IR events identified using IRFinder. The analysis was conducted with the same dataset as described in (C). Transcripts with significant changes in IR were stratified based on whether their corresponding genes gained (red) or not (green) Pol II pSer2 chromatin bindings upon treatment of pemrametostat. E-F) Reactome pathway analysis using the gene stratification as described in (D). Pathway enrichment of genes that gained or not Pol II pSer2 chromatin bindings was shown in (E) and (F), respectively. G) Visualization of genes enriched for cell cycle pathway as described in (E). Color code represents differential gene expression of Pem vs Veh. H) Schematic of representative genes with IR induced by Pem. Upper left numbers denote exon/intron coverage on the same scale for each gene. X axis denotes genomic coordinates. I) Immunoblot analysis of MCF-7_RBKO lysates. Cells were treated with different concentrations of Pem for 3 days. Lysates were collected and subjected to immunoblot with the indicated antibodies. J) Schematic of the proposed model.
Since the function of PRMT5, FUS, and Pol II pSer2 converges on regulation of RNA splicing [16, 35–41], we next performed RNA-seq of MCF-7_RBKO cells treated with pemrametostat to examine changes in RNA splicing. Pemrametostat-mediated inhibition of PRMT5 resulted in significant changes in RNA splicing, particularly intron retention (IR) events (Fig. 5C). Transcripts with IR are either degraded by nonsense-mediated decay (NMD) or detained in the nucleus (also known as detained intron) and then degraded before protein translation [37, 42]. Either NMD or a detained intron will result in lower protein translation. To assess this, we next used IRFinder, an algorithm designed to identify IR events with higher precision and accuracy than MISO and DEXseq [43]. In RNA from cells treated with pemrametostat vs. DMSO, we identified 2,778 significant IR events (FDR <0.05) in 1,185 genes; 41% (489/1,185) of these genes also gained pSer2 Pol II chromatin binding (Fig. 5D) in line with the notion that pSer2 Pol II is associated with regulation of RNA splicing [35, 44]. Finally, we performed pathway analysis with these 1,185 genes stratified as whether they gained (489 genes) or did not gain (696 genes) pSer2 Pol II binding. Among the genes that gained pSer2 Pol II chromatin binding, cell cycle-related pathways were among the top enriched pathways (Fig. 5E), whereas the group of genes without gain in pSer2 Pol II binding was mainly enriched for RNA processing-related pathways (Fig. 5F). This gene-specific pattern suggested that the increase in pSer2 Pol II as a result of inhibition of PRMT5 is associated with intron retention of genes that regulate cell cycle progression.
The pathway analysis also identified multiple genes with pemrametostat-induced IR that are involved in DNA replication (e.g., ANAPC7, CDC45, GINS1, LIG1, MCM4, ORC2, POLD2, POLD3, POLE, RFC3, etc.) (Fig. 5G,H). The majority of these genes had minor changes in gene expression (log2FC within ±0.5). Thus, we next inquired if IR in these genes (upon inhibition of PRMT5) is associated with a reduction of their corresponding protein levels. Treatment of pemrametostat in MCF-7_RBKO cells resulted in a clear downregulation of APC7, GINS1, ORC2, and POLE as measured by immunoblot (Fig. 5I), suggesting a causal association between the PRMT5-FUS-Pol II axis and proper RNA splicing of genes involved in DNA replication (Fig. 5J). We propose this as a mechanism by which PRMT5 inhibition impedes entry into S phase irrespective of RB1 status. Supporting this hypothesis, cell cycle analysis also showed accumulation of RBKO cells in G1 phase and a decrease in S phase upon PRMT5 genetic and pharmacological inhibition (Fig. 3D,E; Supplementary Fig. 4C).
Therapeutic inhibition of PRMT5 synergizes with antiestrogens against ER+/RB-deficient breast cancer
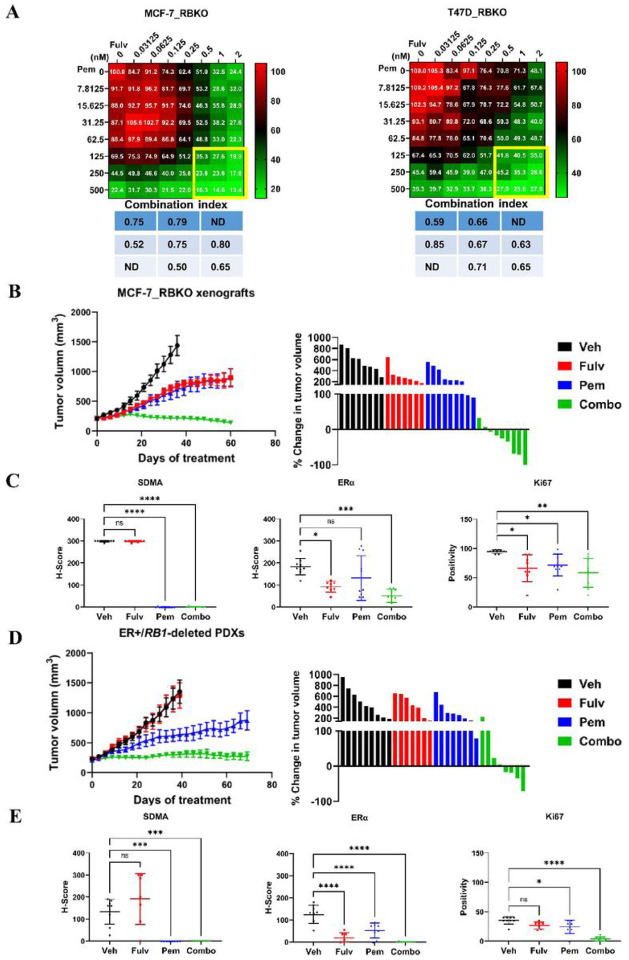
Finally, we investigated effective therapeutic combinations to treat ER+/RB-deficient breast cancer. Our CRISPR screen identified that drivers of ERα signaling remained essential for the viability of ER+ breast cancer cells irrespective of RB1 status (Fig. 2B), suggesting that ER+/RB-deficient breast cancer cells may still be dependent on ERα signaling. Thus, to test whether antiestrogens can be leveraged for treatment of ER+/RB-deficient breast cancer, we treated MCF-7 and T47D, both WT and RBKO cells with fulvestrant (a selective ERα degrader) or by switching them to estrogen-free media to mimic estrogen suppression with aromatase inhibitors as is done in patients. Both treatments with fulvestrant and estrogen deprivation inhibited 60–80% of cell growth, blocked the G1-to-S transition, and reduced ER transcriptional activity, as assayed with an estrogen response element (ERE) luciferase reporter, in both WT and RBKO cells (Supplementary Fig. 5). Addition of 17β-estradiol rescued from the inhibitory effects of estrogen deprivation on cell growth, ER transcriptional reporter activity, and cell cycle progression (Supplementary Fig. 5). Since estrogen suppression, fulvestrant, and PRMT5i exerted blockade of the G1-to-S transition independent of RB, we reasoned that anti-ER therapy plus a PRMT5i may serve as an effective combination strategy to treat ER+/RB-deficient breast cancer. To evaluate the antitumor action of the combination, we treated MCF-7_RBKO and T47D_RBKO cells with fulvestrant and pemrametostat, each over a dose range, and then calculated the combination index using the Chou-Talalay method [45]. After 6 days of treatment, the drug combination resulted in greater growth inhibition of RBKO cells than either fulvestrant or pemrametostat alone, with combination indices ranging between 0.50 to 0.85, suggesting a synergistic effect of the combination (Fig. 6A).
Figure 6. Combination of fulvestrant and pemrametostat synergistically inhibits growth of ER+/RB1-deficient breast cancer in vitro and in vivo.
A) Monolayer growth of MCF-7_RBKO and T47D_RBKO cells treated with a dose response of fulvestrant (Fulv) and pemrametostat (Pem) for 6 days. Cell viability was determined by the CyQuant assay. The numbers in the heatmap represent averaged cell viability (% of control, n = 3). The table lists the combination indices of the highlighted region, with the index <1 representing synergistic effects. ND: not determined. B) Tumor volume of MCF-7_RBKO xenografts in female nude mice. The mice were treated with vehicle control (Veh), Fulv (5 mg/kg/week, s.c.), Pem (200 mg/kg/day, p.o.), or the combination of both drugs (Combo). Data represent mean ± SD (n = 8–10). C) Quantification of IHC of FFPE sections from MCF-7_RBKO xenografts. Data represent mean ± SD; * (P <.05), ** (P <.01), *** (P <.001), **** (P <.0001), ns, P not significant, one-way ANOVA with a Dunnett’s post-hoc test. D) Tumor volume of ER+/RB1-deleted PDXs in female NSG mice. The mice were treated as described in (C). E) Quantification of IHC using FFPE sections from the PDXs. Data represent mean ± SD; * (P <.05), *** (P <.001), **** (P <.0001), ns, P not significant, one-way ANOVA with a Dunnett’s post-hoc test.
We then tested the effect of dual blockade of ER and PRMT5 in vivo. We treated female nude mice bearing established MCF-7_RBKO xenografts with fulvestrant, pemrametostat or the combination for 60 days. Treatment of fulvestrant and pemrametostat each alone delayed tumor growth compared to the control arm whereas the combination of both drugs arrested tumor growth, with 6/9 mice exhibiting a partial tumor remission and 1/9 a complete remission (Fig. 6B). ERα and SDMA expression as measured by IHC were significantly downregulated in tumors treated with fulvestrant and pemrametostat, respectively, supporting drug target inhibition; treatment with each inhibitor alone or in combination, significantly decreased Ki67 positive cells compared to the control arm (Fig. 6C). Similar results with the combination of fulvestrant and pemrametostat were observed in mice bearing RB1-deleted patient-derived xenografts (PDX) derived from a patient with ER+ MBC that progressed clinically on palbociclib plus the aromatase inhibitor letrozole. RB1 deletion in the PDX was confirmed by exome sequencing (data not shown) and loss of RB protein expression, by immunoblot analysis and IHC with an RB antibody (Supplementary Fig. 6). Single agent pemrametostat but not fulvestrant delayed growth of the RB1-deleted PDXs compared to vehicle control. However, the combination of fulvestrant and pemrametostat induced durable tumor suppression over 70 days of treatment, with 4/8 mice exhibiting a partial tumor remission (Fig. 6D). Treatment with the combination also suppressed expression of ERα, SDMA, and Ki67 in the RB1-deleted PDXs (Fig. 6E; Supplementary Fig. 7). Collectively, our data suggest that dual blockade of ER and PRMT5 can effectively suppress tumor growth of ER+/RB-deficient breast cancer, thus providing the basis to testing this novel therapeutic combination in patients with this refractory breast cancer subtype.
Discussion
In this study using a genome-wide CRISPR screen with ER+/RB-deficient cells, we identified PRMT5 as a novel dependency in breast cancer. Loss of the tumor suppressor RB is an established mechanism of de novo and acquired resistance to CDK4/6i and with the wider use of these agents as standard of care, the population of patients with RB-deficient breast tumors is likely to rapidly increase. To the best of our knowledge, a targeted therapeutic approach against these cancers once they progress on a CDK4/6i has not yet been established. Thus, PRMT5 is an actionable therapeutic vulnerability in breast cancers of this genotype and potentially fill an unmet need for patients with acquired resistance to CDK4/6i.
Inhibition of PRMT5 blocked the G1-to-S cell cycle transition in ER+/RBKO breast cancer cells and other cancer cells with natural loss-of-function alterations of RB1. Of relevance to our results, AbuHammad et al. recently reported that PRMT5 is an indirect target of CDK4 and is required for the sensitivity of RB-competent melanoma cells to palbociclib [46]. In this study, treatment of melanoma cells with palbociclib resulted in suppression of PRMT5 activity, which altered pre-mRNA splicing of MDM4 and downregulated MDM4 protein levels. This in turn led to activation of p53, induction of p21 expression, and subsequent inhibition of CDK2 as a mechanism of action of CDK4/6i in RB-proficient cells. This study, though not focusing on RB-deficient cancers, provided important insights as to the mechanism of how PRMT5 regulates cell cycle progression. Our data, however, show that silencing of PRMT5 resulted in suppression of cell growth and entry into S phase in both p53 mutant (T47D) and p53 wild-type (MCF-7) CDK4/6-resistant cells lacking RB, thus suggesting that in addition to MDM4-p53, other regulatory axes may play a role in PRMT5-mediated regulation of the G1-to-S transition independent of RB and CDK4/6.
An unbiased proteomics approach (e.g., Co-IP MS and SDMA PTM analysis) allowed us to identify the DNA/RNA binding protein FUS as a putative substrate of PRMT5. Importantly, FUS knockdown phenocopied the effects of PRMT5 silencing in RBKO cells. Previous studies have reported that silencing of FUS results in abnormal accumulation of pSer2 Pol II and RNA splicing defects [35, 36]. In line with these studies, our ChIP-seq and RNA-seq analysis revealed that treatment with pemrametostat induced a gain of pSer2 Pol II associated with intron retention (IR) within genes significantly enriched for cell cycle-related pathways, thus supporting a regulatory role of the PRMT5-FUS-Pol II axis in RNA splicing and cell cycle progression. Additionally, FUS itself is essential to bridge the interaction between the splicing factor U1 snRNP with Pol II [47]. Since inhibition of PRMT5 uncoupled FUS from Pol II, further studies are warranted to decipher if U1 snRNP or other splicing factors contribute to RNA splicing of cell cycle regulators. Of note, previous studies have reported that PRMT5 regulates RNA splicing via arginine methylation of splicing factors and subunits of the spliceosome [16, 31, 38]. We thus recognize that inhibition of PRMT5 may directly affect splicing of cell cycle regulators independent of the PRMT5-FUS-Pol II axis.
The activating cofactor S-adenosyl-l-methionine (SAM) is the methyl group donor required for PRMT5’s methyltransferase activity. Because of this, first generation PRMT5i in clinical development are either SAM-cooperative or SAM-competitive [48]. Therapeutic inhibition of PRMT5 has been proposed as synthetically lethal in cancers with loss of MTAP. In MTAP-deleted tumors, increased intracellular concentrations of methylthioadenosine (MTA), the metabolite cleaved by the MTAP enzyme, couple with PRMT5, compete with SAM and, as a result, inhibit PRMT5’s enzymatic activity and contribute to the proposed synthetic lethality in these cancers [49, 50]. Since first generation PRMT5i do not target the PRMT5-MTA complex, it is not clear yet whether they will be clinically active in patients with MTAP-deficient cancers. It has been proposed that treatment of these tumors may require a selective binder to the PRMT5/MTA complex [51]. In our study herein, we show that both SAM-cooperative (e.g., pemrametostat) and SAM-competitive (e.g., JNJ-64619178) PRMT5i block growth of ER+/RB-deficient breast cancer in vitro and/or in vivo, thus providing a rationale for the testing of first generation PRMT5i in patients with CDK4/6i-refractory breast cancers.
Our data also suggest that the ERα pathway may still be essential in ER+ breast cancer cells lacking RB and as such remain sensitive to estrogen suppression and ER antagonists. This finding is in line with a recent study by Wander SA et al., which showed growth of RB1-deleted breast cancer cells is still inhibited by fulvestrant [11]. In this study, the combination of PRMT5i plus fulvestrant exhibited superior antitumor activity compared to either monotherapy alone against xenografts of this breast cancer subtype. Hence, we propose that the combination of ER and PRMT5 inhibitors can synergistically block the G1-to-S transition in ER+/RB-deficient breast cancer, and this effect is independent of the CDK4/6/Cyclin D1 complex.
In summary, our results provide evidence that targeting the arginine methyltransferase activity of PRMT5 blocks the G1-to-S cell cycle transition independent of RB. We also show a novel link between the PRMT5-FUS-Pol II axis and intron retention within genes enriched for cell cycle progression in ER+/RB-deficient breast cancer cells. Taken together, these data support PRMT5 as a therapeutically actionable vulnerability to overcome resistance to CDK4/6 inhibitors in ER+/RB-deficient breast cancer.
Materials and Methods
Cell lines and organoids
MCF-7, T47D and MDA-MB-436 cell lines were purchased from ATCC. 293FT cells were purchased from Invitrogen. H596, H1048 and H1155 cell lines were kindly provided by Dr. John Minna. Du-145 cells were kindly provided by Dr. Ganesh Raj. MCF-7, MDA-MB-436 and 293FT cells were maintained in DMEM containing 10% FBS. T47D and Du-145 cells were maintained in RPMI containing 10% FBS. H596, H1048 and H1155 cells were maintained in RPMI containing 5% FBS. All culture media was supplemented with 1x antibiotic-antimycotic (Invitrogen). The PDxOs were maintained in Matrigel dome (Corning) supplemented with DMEM/F12 containing 250 ng/ml R-Spondin 3, 5 nM Heregulin β1, 5 ng/ml FGF7, 20 ng/ml FGF10, 5 ng/ml EGF, 100 ng/ml Noggin, 500 nM A83–01, 5 μM Y-27632, 500 nM SB202190, 1X B27 supplement, 1.25 mM N-Acetylcysteine, 5 mM Nicotinamide, 1X GlutaMax, 10 mM HEPES, 50 μg/ml primocin and 100 U/ml penicillin/100 μg/ml streptomycin.
Plasmids
pX458 and plentiCRISPR_v2 were gifts from Feng Zhang (Addgene plasmid #48138 and #52961). sgRNAs targeting RB1 were subcloned into pX458 for the establishment of RB1 knockout cells. Briefly, pX458 was digested with BbsI and ligated with oligonucleotides containing sgRNA sequences targeting RB1. For PRMT5 depletion, sgRNAs targeting PRMT5 were subcloned into plentiCRISPR_v2, which was digested with Esp3I and then ligated with oligonucleotides containing sgRNA sequences targeting PRMT5. Tet-pLKO-puro and Tet-pLKO-puro-Scrambled were gifts from Dmitri Wiederschain (Addgene plasmid #21915 and #110470). pLX304-zeo and pLX304-zeo-eGFP were gifts from Rizwan Haq (Addgene plasmid #160092 and #160095). shRNA targeting PRMT5 3’UTR was subcloned into Tet-pLKO-puro. Tet-pLKO-puro was digested with AgeI and EcoRI and then ligated with oligonucleotides containing shRNA sequence targeting PRMT5 3’UTR. pDONR221_PRMT5_WT was purchased from DNASU. Enzymatically dead pDONR221_PRMT5_E444Q was generated using the Q5 site-directed mutagenesis kit (NEB BioLabs). PRMT5_WT and PRMT5_E444Q open reading frames were subcloned into pLX304-zeo using LR Gateway clonase (Invitrogen). Virus packaging vectors psPAX2 and pMD2.G were gifts from Didier Trono (Addgene plasmid #12260 and #12259). Primers used for cloning are listed in Supplementary Table 4.
RB1 knockout
To mimic RB1 loss-of-function alterations, we used CRISPR-Cas9 to knockout RB1 in MCF-7 and T47D cells. This was achieved by transient transfection of pX458 plasmid carrying individual sgRNAs targeting RB1 and followed by sorting for green fluorescence protein (GFP)-positive single cells using flow cytometry. RBKO single clones were validated by PCR-based genotyping, Sanger sequencing, and immunoblot analysis. GFP negativity was confirmed in the RBKO clones to ensure that the plasmid did not randomly integrate into the genome.
siRNA transfection
Silencer Select siRNAs targeting PRMT5 (ID: s20375 and s20377) and FUS (ID: s5401 and s533595) were purchased from Invitrogen. All Stars negative control siRNA was purchased from Qiagen. siRNA transfection was conducted using Lipofectamine RNAiMAX (Invitrogen).
Lentiviral transduction
Virus packaging was conducted by co-transfection of psPAX2 and pMD2.G plasmids along with viral vectors into 293FT cells. Media was replenished 24 hours after transfection, and virus supernatant was collected 24 hours later. Target cells were transduced with virus supernatant in the presence of 8 μg/mL polybrene and then selected with puromycin (1 μg/mL) or zeocin (100 μg/mL) according to the selection marker carried in the viral vectors.
Genome-wide CRISPR screen
Human Brunello CRISPR knockout pooled library was a gift from David Root and John Doench (Addgene #73178). The CRISPR screen was performed using T47D_WT and T47D _RBKO cells, each in two replicates. The lentiviral sgRNA library was transduced into T47D_WT and two independent RBKO clones at low multiplicity of infection (MOI = 0.3) and at a coverage of ≥500×. Non-transduced cells were eliminated by puromycin (1 μg/mL) selection for 7 days. After puromycin removal, cells were collected on days 0 and 30. Genomic DNA from both time points was extracted. The sequences encoding the sgRNAs were PCR-amplified and then subjected to deep sequencing at the UTSW Next Generation Sequencing Core to determine sgRNA abundance.
Cell viability assays
Cell viability of 2D-cultured cells was determined using a Z2 Coulter Counter Analyzer (Beckman) or by the CyQuant cell proliferation assay (Invitrogen). Cell viability of 3D-cultured organoids was determined by the 3D Cell TiterGlo assay (Promega) on a GloMax plate reader (Promega).
Cell cycle analysis
Cells were fixed with ice-cold 70% ethanol and stored at −20 °C. On the day of cell cycle analysis, fixed cells were washed twice with ice-cold PBS and then stained with 50 μg/mL propidium iodide (Invitrogen) in PBS supplemented with 10 μg/mL RNase A (Invitrogen). Cell cycle analysis was performed using a LSRFortessa flow cytometer (BD Biosciences).
RNA-seq
Total RNA was extracted using Maxwell RSC simply RNA kit (Promega). mRNA libraries were prepared using TruSeq Stranded mRNA Library prep kit (Illumina) and sequenced on NextSeq 550 sequencer (Illumina) in a PE75 run. RNA-Seq analysis was conducted by aligning sequencing reads to the human reference genome GRCh38 (hg38) using STAR [52], and gene expression levels were estimated as row read counts. DESeq2 was used to assess the statistical significance of differentially expressed genes [53]. Gene set enrichment analysis (GSEA) and gene ontology (GO) analysis were conducted using the GSEA software [54] and DAVID [55], respectively. RNA splicing analysis was first conducted using SUPPA2 [56], and further analysis to identify IR was conducted using IRFinder [43].
qRT-PCR
Total RNA was reversely transcribed to cDNA using iScript kit (Bio-Rad). qRT-PCR was performed using SYBR Green master mix (ThermoFisher) on a QuantStudio 3 Real-Time PCR System (ThermoFisher). Expression of YWHAZ was used as internal control for normalization. Sequences of primers are listed in Supplementary Table 4.
Immunoblot analysis and co-immunoprecipitation
For immunoblot analysis, cells were lysed with RIPA buffer supplemented with proteinase and phosphatase inhibitors (Roche). Protein concentration was determined using Gold Rapid BCA (Thermo Fisher). Proteins were separated by 4–20% gradient SDS-PAGE (Bio-Rad) or 4–12% NuPage gradient gels (Invitrogen), transferred onto nitrocellulose membrane, blocked with 5% non-fat milk, and then probed with primary antibodies. HRP-conjugated anti-rabbit or anti-mouse were used as secondary antibodies. Antibodies are listed in Supplementary Table 4. For Co-IP experiments, cells were lysed with NP-40 lysis buffer (20 mM Tris-HCl pH 7.6, 150 mM NaCl, 0.1% NP-40, 1 mM EDTA) supplemented with proteinase and phosphatase inhibitors. Protein concentration was determined using the Gold Rapid BCA. Lysates were pre-cleared with protein-G-conjugated Dyna beads (Invitrogen) and then incubated with either a PRMT5 or a FUS antibody or a control IgG overnight at 4°C. Next day, lysates were incubated with protein-G-conjugated Dyna beads for 2 hours at 4°C, washed three times with NP-40 lysis buffer, and then eluted with 0.2 M Glycine (pH 2.0). Eluates were subjected to SDS-PAGE and immunoblot analysis. For Co-IP MS analysis, immunoprecipitated proteins were further processed by running in a SDS-PAGE gel followed by Coomassie blue staining, tryptic digestion, reduction with DTT, alkylation with iodoacetamide, and finally cleanup with Oasis MCX solid-phase extraction cartridges (Waters).
Immunoaffinity enrichment of peptides containing SDMA
Cells were lysed with PTMScan® Urea Lysis Buffer (9 M urea, 20 mM HEPES pH 8.0; Cell Signaling) and then sonicated at 25% amplitude using a microtip sonifier (Branson 150). Protein concentration was determined using Gold Rapid BCA, and lysates were reduced and alkylated with DTT and iodoacetamide, respectively. Next, lysates were subjected to tryptic digestion, solid-phase cleanup and SDMA enrichment following the manual instruction of PTMScan® Symmetric Di-Methyl Arginine Motif [sdme-RG] Kit (Cell Signaling). SDMA enriched samples were subjected to secondary digestion, solid-phase cleanup, and finally analyzed by LC-MS/MS.
MS and data analysis
MS data were acquired using a Q-Exactive HF Quadrupole-Orbitrap mass spectrometer (Thermo Fisher) for the PRMT5 antibody pulldowns and an Orbitrap Fusion Lumos Tribrid mass spectrometer (Thermo Fisher) for the SDMA enriched samples. Data were analyzed using Proteome Discoverer 2.4 and were searched using the human protein database from UniProt. Proteins were filtered for downstream analysis using a cutoff FDR <0.01 with at least two peptides being mapped.
ChIP and ChIP-seq
Cells were fixed with 1% formaldehyde for 10 min and then quenched with 125 mM glycine for 5 min at room temperature. Cells were next washed twice with ice-cold PBS and harvested by scrapping. Cells were lysed with PIPES buffer (5 mM PIPES pH 8.0, 85 mM KCl, 0.5% NP-40; Santa Cruz Biotechnology), and nuclei were isolated by centrifugation and then lysed with ChIP high salt lysis buffer (PBS, 1% NP-40, 0.5% Sodium Deoxycholate, 0.1% SDS; Santa Cruz Biotechnology). Both PIPES and ChIP high salt lysis buffer were supplemented with proteinase and phosphatase inhibitors. Chromatin was sheared using a microtip sonicator (Branson 150) to an average fragment size of 100–300 bp. Sheared chromatin containing 25 μg DNA was diluted with IP buffer (0.01% SDS, 1.1% Triton-X, 1.2 mM EDTA, 16.7 mM Tris-HCL pH 8.0, 167 mM NaCl) and incubated overnight at 4 °C with 10 μg of precipitating antibodies. The next day, antibody pulldowns were incubated with protein-G-conjugated Dyna beads for 2 hours at 4°C. The beads were washed once with each of the following buffers in sequence: low salt (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl pH 8.0, 150 mM NaCl), high salt (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl pH 8.0, 500 mM NaCl), LiCl wash (0.25 M LiCl, 1% NP40, 1% deoxycholate, 1 mM EDTA, 10 mM Tris-HCl pH 8.0), and Tris-EDTA (pH 8.0). DNA was eluted with elution buffer (0.1 M NaHCO3, 1% SDS) for 1 hour at 65°C and then incubated with 200 mM NaCl overnight at 65°C to reverse crosslinking. Next day, the elution was treated with RNase A (Thermo Fisher) and Proteinase K (Thermo Fisher); DNA was then purified using ProNex Size-Selective Purification System (Promega). For ChIP-seq, libraries were prepared using the Kapa HyperPlus Kit (Roche) and sequenced using Illumina NextSeq 550 sequencer with PE-75.
ChIP-Seq analysis was conducted by aligning sequencing reads to the human reference genome GRCh38 (hg38) using Bowtie2 [57] with default parameters. Peaks were called using Model-based Analysis of ChIP-Seq (MACS) software [58] with default parameters and FDR <0.05 as cutoff.
Xenograft studies
Animal experiments were approved by the UTSW Institutional Animal Care and Use Committee (IACUC protocol 2018–102359). An estrogen pellet (0.25 mg/pellet, 21-day release; Innovative Research of America) was implanted s.c. in the mouse dorsum one day before tumor inoculation. One million MCF-7_RBKO cells were mixed in PBS:matrigel (1:1) and then injected s.c. into 6-week-old female nude mice. For PDXs, ER+/RB1-deleted PDX fragments were implanted s.c. into 6-week-old female NOD scid gamma (NSG) mice. Once tumors reached ≈200 mm3, mice were randomized to receive treatment with vehicle (5% DMSO, 40% PEG-300 and 5% Tween-80 in sterilized water), fulvestrant (5 mg/mouse/week, s.c.), pemrametostat (200 mg/kg/day, via orogastric gavage). or combination both drugs. Tumor size was serially measured with calipers and calculated every three days with the formula: volume = width2 × length/2. At the end of the treatment, tumors were harvested and then snap frozen in liquid N2 or fixed in 10% neutral-buffered formalin.
IHC
Formalin-fixed tumors were embedded in paraffin; 5-μm tumor sections were used for IHC (antibodies listed in Supplementary Table 4). Nuclear positivity of Ki67 and H-scores of SDMA and ERα were quantified by an expert breast pathologist blinded to treatment arms and using standard CAP breast tumor biomarkers scoring guidelines. For cells from the PDxOs, cells in a Matrigel dome were fixed in 10% neutral-buffered formalin for 1 hour at room temperature, pelleted in 2.5% low-melt agarose, and then subjected to paraffin embedding, sectioning, and IHC.
Supplementary Material
Acknowledgements
We acknowledge the assistance of the Tissue Management Shared Resource of the Simmons Comprehensive Cancer Center. This work is supported by, National Cancer Institute P30 CA142543, DOD BC 210406 (C.C. Lin), NCI R01CA224899 (C.L. Arteaga and A.B. Hanker), NCI Breast SPORE P50 CA098131 (C.L. Arteaga and A.B. Hanker), CPRIT RR170061 (C.L. Arteaga), Susan G. Komen Breast Cancer Foundation SAB1800010 (C.L. Arteaga), Breast Cancer Research Foundation DRC-20–001 (C.L. Arteaga and A.B. Hanker), CPRIT RP220309 (J.T. Mendell), and The Welch Foundation I-1961–20210327 (J.T Mendell). J.T Mendell is an Investigator of the Howard Hughes Medical Institute.
Additional Declarations:
Yes there is potential Competing Interest. J.T.M. is a scientific advisor for Ribometrix, Inc., and owns equity in Orbital Therapeutics, Inc.. H.A. receives honoraria from Boehringer Ingelheim Japan Inc., Eli Lilly Japan K.K., and Taiho Pharmaceuticals. A.B.H. received or has received research grants from Takeda and Lilly and nonfinancial support from Puma Biotechnology and Daiichi Sankyo. C.L.A. receives or has received research grants from Pfizer, Lilly, and Takeda; holds minor stock options in Provista; serves or has served in an advisory role to Novartis, Merck, Lilly, Daiichi Sankyo, Taiho Oncology, OrigiMed, Puma Biotechnology, Immunomedics, AstraZeneca, Arvinas, and Sanofi; and reports scientific advisory board remuneration from the Susan G. Komen Foundation. D.W.C. reports advisory services to AstraZeneca, Exact Sciences, Eisai, Gilead, GlaxoSmithKline, Inivata, Merck, Novartis, Pfizer, and Roche; reports research funding (to institution) from AstraZeneca, Gilead, GlaxoSmithKline, Inivata, Merck, Pfizer, and Roche; and is a member of a trial steering committee for AstraZeneca, Merck, and GlaxoSmithKline.
Footnotes
Supplementary Files
References
- 1.Siegel R.L., Miller K.D., and Jemal A., Cancer statistics, 2019. CA Cancer J Clin, 2019. 69(1): p. 7–34; https://www.ncbi.nlm.nih.gov/pubmed/30620402. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance Epidemiology, and Results End (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, 8 Registries, Nov 2021 Sub (1975–2019) - Linked To County Attributes - Time Dependent (1990–2019) Income/Rurality, 1969–2020 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2022, based on the November 2021 submission.
- 3.Slamon D.J., Neven P., Chia S., Fasching P.A., De Laurentiis M., Im S.A., Petrakova K., Bianchi G.V., Esteva F.J., Martin M., Nusch A., Sonke G.S., De la Cruz-Merino L., Beck J.T., Pivot X., Sondhi M., Wang Y., Chakravartty A., Rodriguez-Lorenc K., Taran T., and Jerusalem G., Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N Engl J Med, 2020. 382(6): p. 514–524; https://www.ncbi.nlm.nih.gov/pubmed/31826360. [DOI] [PubMed] [Google Scholar]
- 4.Slamon D.J., Neven P., Chia S., Fasching P.A., De Laurentiis M., Im S.A., Petrakova K., Bianchi G.V., Esteva F.J., Martin M., Nusch A., Sonke G.S., De la Cruz-Merino L., Beck J.T., Pivot X., Vidam G., Wang Y., Rodriguez Lorenc K., Miller M., Taran T., and Jerusalem G., Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J Clin Oncol, 2018. 36(24): p. 2465–2472; https://www.ncbi.nlm.nih.gov/pubmed/29860922. [DOI] [PubMed] [Google Scholar]
- 5.Turner N.C., Slamon D.J., Ro J., Bondarenko I., Im S.A., Masuda N., Colleoni M., DeMichele A., Loi S., Verma S., Iwata H., Harbeck N., Loibl S., Andre F., Puyana Theall K., Huang X., Giorgetti C., Huang Bartlett C., and Cristofanilli M., Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med, 2018. 379(20): p. 1926–1936; https://www.ncbi.nlm.nih.gov/pubmed/30345905. [DOI] [PubMed] [Google Scholar]
- 6.Sledge G.W. Jr., Toi M., Neven P., Sohn J., Inoue K., Pivot X., Burdaeva O., Okera M., Masuda N., Kaufman P.A., Koh H., Grischke E.M., Conte P., Lu Y., Barriga S., Hurt K., Frenzel M., Johnston S., and Llombart-Cussac A., The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol, 2019; https://www.ncbi.nlm.nih.gov/pubmed/31563959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otto T. and Sicinski P., Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer, 2017. 17(2): p. 93–115; https://www.ncbi.nlm.nih.gov/pubmed/28127048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condorelli R., Spring L., O’Shaughnessy J., Lacroix L., Bailleux C., Scott V., Dubois J., Nagy R.J., Lanman R.B., Iafrate A.J., Andre F., and Bardia A., Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann Oncol, 2018. 29(3): p. 640–645; https://www.ncbi.nlm.nih.gov/pubmed/29236940. [DOI] [PubMed] [Google Scholar]
- 9.Li Z., Razavi P., Li Q., Toy W., Liu B., Ping C., Hsieh W., Sanchez-Vega F., Brown D.N., Da Cruz Paula A.F., Morris L., Selenica P., Eichenberger E., Shen R., Schultz N., Rosen N., Scaltriti M., Brogi E., Baselga J., Reis-Filho J.S., and Chandarlapaty S., Loss of the FAT1 Tumor Suppressor Promotes Resistance to CDK4/6 Inhibitors via the Hippo Pathway. Cancer Cell, 2018. 34(6): p. 893–905 e8; https://www.ncbi.nlm.nih.gov/pubmed/30537512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Leary B., Cutts R.J., Liu Y., Hrebien S., Huang X., Fenwick K., Andre F., Loibl S., Loi S., Garcia-Murillas I., Cristofanilli M., Huang Bartlett C., and Turner N.C., The Genetic Landscape and Clonal Evolution of Breast Cancer Resistance to Palbociclib plus Fulvestrant in the PALOMA-3 Trial. Cancer Discov, 2018. 8(11): p. 1390–1403; https://www.ncbi.nlm.nih.gov/pubmed/30206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wander S.A., Cohen O., Gong X., Johnson G.N., Buendia-Buendia J.E., Lloyd M.R., Kim D., Luo F., Mao P., Helvie K., Kowalski K.J., Nayar U., Waks A.G., Parsons S.H., Martinez R., Litchfield L.M., Ye X.S., Yu C., Jansen V.M., Stille J.R., Smith P.S., Oakley G.J., Chu Q.S., Batist G., Hughes M.E., Kremer J.D., Garraway L.A., Winer E.P., Tolaney S.M., Lin N.U., Buchanan S.G., and Wagle N., The Genomic Landscape of Intrinsic and Acquired Resistance to Cyclin-Dependent Kinase 4/6 Inhibitors in Patients with Hormone Receptor-Positive Metastatic Breast Cancer. Cancer Discov, 2020; https://www.ncbi.nlm.nih.gov/pubmed/32404308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kan Z, I. S., Park K, Wen J, Lee KH, Choi YL, Lee WC, Min A, Bonato V, Park S, Ram S, Lee DW, Kim JY, Lee SK, Lee WW, Lee J, Kim M, Weinrich SL, Ryu HS, Kim TY, Dann S, Fernandez D, Koh J, Park SY, Deng S, Powell E, Ravi RK, Bienkowska J, Rejto PA, Park WY, Park YH., Serial genomic profiling reveals molecular mechanisms of breast cancer resistance to palbociclib. Cancer Res, 2022. 82(4 Suppl): p. Abstract nr PD2–08. [Google Scholar]
- 13.Johnston S.R.D., Toi M., O’Shaughnessy J., Rastogi P., Campone M., Neven P., Huang C.S., Huober J., Jaliffe G.G., Cicin I., Tolaney S.M., Goetz M.P., Rugo H.S., Senkus E., Testa L., Del Mastro L., Shimizu C., Wei R., Shahir A., Munoz M., San Antonio B., Andre V., Harbeck N., Martin M., and monarch E.C.M., Abemaciclib plus endocrine therapy for hormone receptor-positive, HER2-negative, node-positive, high-risk early breast cancer (monarchE): results from a preplanned interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol, 2023. 24(1): p. 77–90; https://www.ncbi.nlm.nih.gov/pubmed/36493792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarrold J. and Davies C.C., PRMTs and Arginine Methylation: Cancer’s Best-Kept Secret? Trends Mol Med, 2019. 25(11): p. 993–1009; https://www.ncbi.nlm.nih.gov/pubmed/31230909. [DOI] [PubMed] [Google Scholar]
- 15.Thandapani P., O’Connor T.R., Bailey T.L., and Richard S., Defining the RGG/RG motif. Mol Cell, 2013. 50(5): p. 613–23; https://www.ncbi.nlm.nih.gov/pubmed/23746349. [DOI] [PubMed] [Google Scholar]
- 16.Radzisheuskaya A., Shliaha P.V., Grinev V., Lorenzini E., Kovalchuk S., Shlyueva D., Gorshkov V., Hendrickson R.C., Jensen O.N., and Helin K., PRMT5 methylome profiling uncovers a direct link to splicing regulation in acute myeloid leukemia. Nat Struct Mol Biol, 2019. 26(11): p. 999–1012; https://www.ncbi.nlm.nih.gov/pubmed/31611688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C., Nott T.J., Jin J., and Pawson T., Deciphering arginine methylation: Tudor tells the tale. Nat Rev Mol Cell Biol, 2011. 12(10): p. 629–42; https://www.ncbi.nlm.nih.gov/pubmed/21915143. [DOI] [PubMed] [Google Scholar]
- 18.Raposo A.E. and Piller S.C., Protein arginine methylation: an emerging regulator of the cell cycle. Cell Div, 2018. 13: p. 3; https://www.ncbi.nlm.nih.gov/pubmed/29568320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang B., Wang M., Zhang W., Xiao T., Chen C.H., Wu A., Wu F., Traugh N., Wang X., Li Z., Mei S., Cui Y., Shi S., Lipp J.J., Hinterndorfer M., Zuber J., Brown M., Li W., and Liu X.S., Integrative analysis of pooled CRISPR genetic screens using MAGeCKFlute. Nat Protoc, 2019. 14(3): p. 756–780; https://www.ncbi.nlm.nih.gov/pubmed/30710114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeselsohn R., Bergholz J.S., Pun M., Cornwell M., Liu W., Nardone A., Xiao T., Li W., Qiu X., Buchwalter G., Feiglin A., Abell-Hart K., Fei T., Rao P., Long H., Kwiatkowski N., Zhang T., Gray N., Melchers D., Houtman R., Liu X.S., Cohen O., Wagle N., Winer E.P., Zhao J., and Brown M., Allele-Specific Chromatin Recruitment and Therapeutic Vulnerabilities of ESR1 Activating Mutations. Cancer Cell, 2018. 33(2): p. 173–186 e5; https://www.ncbi.nlm.nih.gov/pubmed/29438694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukas J., Bartkova J., Rohde M., Strauss M., and Bartek J., Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol, 1995. 15(5): p. 2600–11; https://www.ncbi.nlm.nih.gov/pubmed/7739541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zardavas D., Phillips W.A., and Loi S., PIK3CA mutations in breast cancer: reconciling findings from preclinical and clinical data. Breast Cancer Res, 2014. 16(1): p. 201; https://www.ncbi.nlm.nih.gov/pubmed/25192370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang da W., Sherman B.T., and Lempicki R.A., Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res, 2009. 37(1): p. 1–13; https://www.ncbi.nlm.nih.gov/pubmed/19033363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pastore F., Bhagwat N., Pastore A., Radzisheuskaya A., Karzai A., Krishnan A., Li B., Bowman R.L., Xiao W., Viny A.D., Zouak A., Park Y.C., Cordner K.B., Braunstein S., Maag J.L., Grego A., Mehta J., Wang M., Lin H., Durham B.H., Koche R.P., Rampal R.K., Helin K., Scherle P., Vaddi K., and Levine R.L., PRMT5 Inhibition Modulates E2F1 Methylation and Gene-Regulatory Networks Leading to Therapeutic Efficacy in JAK2(V617F)-Mutant MPN. Cancer Discov, 2020. 10(11): p. 1742–1757; https://www.ncbi.nlm.nih.gov/pubmed/32669286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chiang K., Zielinska A.E., Shaaban A.M., Sanchez-Bailon M.P., Jarrold J., Clarke T.L., Zhang J., Francis A., Jones L.J., Smith S., Barbash O., Guccione E., Farnie G., Smalley M.J., and Davies C.C., PRMT5 Is a Critical Regulator of Breast Cancer Stem Cell Function via Histone Methylation and FOXP1 Expression. Cell Rep, 2017. 21(12): p. 3498–3513; https://www.ncbi.nlm.nih.gov/pubmed/29262329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerhart S.V., Kellner W.A., Thompson C., Pappalardi M.B., Zhang X.P., Montes de Oca R., Penebre E., Duncan K., Boriack-Sjodin A., Le B., Majer C., McCabe M.T., Carpenter C., Johnson N., Kruger R.G., and Barbash O., Activation of the p53-MDM4 regulatory axis defines the anti-tumour response to PRMT5 inhibition through its role in regulating cellular splicing. Sci Rep, 2018. 8(1): p. 9711; https://www.ncbi.nlm.nih.gov/pubmed/29946150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guillen K.P., Fujita M., Butterfield A.J., Scherer S.D., Bailey M.H., Chu Z., DeRose Y.S., Zhao L., Cortes-Sanchez E., Yang C.H., Toner J., Wang G., Qiao Y., Huang X., Greenland J.A., Vahrenkamp J.M., Lum D.H., Factor R.E., Nelson E.W., Matsen C.B., Poretta J.M., Rosenthal R., Beck A.C., Buys S.S., Vaklavas C., Ward J.H., Jensen R.L., Jones K.B., Li Z., Oesterreich S., Dobrolecki L.E., Pathi S.S., Woo X.Y., Berrett K.C., Wadsworth M.E., Chuang J.H., Lewis M.T., Marth G.T., Gertz J., Varley K.E., Welm B.E., and Welm A.L., A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nat Cancer, 2022. 3(2): p. 232–250; https://www.ncbi.nlm.nih.gov/pubmed/35221336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soria-Bretones I., Thu K.L., Silvester J., Cruickshank J., El Ghamrasni S., Ba-Alawi W., Fletcher G.C., Kiarash R., Elliott M.J., Chalmers J.J., Elia A.C., Cheng A., Rose A.A.N., Bray M.R., Haibe-Kains B., Mak T.W., and Cescon D.W., The spindle assembly checkpoint is a therapeutic vulnerability of CDK4/6 inhibitor-resistant ER(+) breast cancer with mitotic aberrations. Sci Adv, 2022. 8(36): p. eabq4293; https://www.ncbi.nlm.nih.gov/pubmed/36070391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dick F.A., Goodrich D.W., Sage J., and Dyson N.J., Non-canonical functions of the RB protein in cancer. Nat Rev Cancer, 2018. 18(7): p. 442–451; https://www.ncbi.nlm.nih.gov/pubmed/29692417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kent L.N. and Leone G., The broken cycle: E2F dysfunction in cancer. Nat Rev Cancer, 2019. 19(6): p. 326–338; https://www.ncbi.nlm.nih.gov/pubmed/31053804. [DOI] [PubMed] [Google Scholar]
- 31.Mulvaney K.M., Blomquist C., Acharya N., Li R., Ranaghan M.J., O’Keefe M., Rodriguez D.J., Young M.J., Kesar D., Pal D., Stokes M., Nelson A.J., Jain S.S., Yang A., Mullin-Bernstein Z., Columbus J., Bozal F.K., Skepner A., Raymond D., LaRussa S., McKinney D.C., Freyzon Y., Baidi Y., Porter D., Aguirre A.J., Ianari A., McMillan B., and Sellers W.R., Molecular basis for substrate recruitment to the PRMT5 methylosome. Mol Cell, 2021. 81(17): p. 3481–3495 e7; https://www.ncbi.nlm.nih.gov/pubmed/34358446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwon I., Kato M., Xiang S., Wu L., Theodoropoulos P., Mirzaei H., Han T., Xie S., Corden J.L., and McKnight S.L., Phosphorylation-regulated binding of RNA polymerase II to fibrous polymers of low-complexity domains. Cell, 2013. 155(5): p. 1049–1060; https://www.ncbi.nlm.nih.gov/pubmed/24267890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burke K.A., Janke A.M., Rhine C.L., and Fawzi N.L., Residue-by-Residue View of In Vitro FUS Granules that Bind the C-Terminal Domain of RNA Polymerase II. Mol Cell, 2015. 60(2): p. 231–41; https://www.ncbi.nlm.nih.gov/pubmed/26455390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murthy A.C., Tang W.S., Jovic N., Janke A.M., Seo D.H., Perdikari T.M., Mittal J., and Fawzi N.L., Molecular interactions contributing to FUS SYGQ LC-RGG phase separation and co-partitioning with RNA polymerase II heptads. Nat Struct Mol Biol, 2021. 28(11): p. 923–935; https://www.ncbi.nlm.nih.gov/pubmed/34759379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz J.C., Ebmeier C.C., Podell E.R., Heimiller J., Taatjes D.J., and Cech T.R., FUS binds the CTD of RNA polymerase II and regulates its phosphorylation at Ser2. Genes Dev, 2012. 26(24): p. 2690–5; https://www.ncbi.nlm.nih.gov/pubmed/23249733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masuda A., Takeda J., Okuno T., Okamoto T., Ohkawara B., Ito M., Ishigaki S., Sobue G., and Ohno K., Position-specific binding of FUS to nascent RNA regulates mRNA length. Genes Dev, 2015. 29(10): p. 1045–57; https://www.ncbi.nlm.nih.gov/pubmed/25995189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braun C.J., Stanciu M., Boutz P.L., Patterson J.C., Calligaris D., Higuchi F., Neupane R., Fenoglio S., Cahill D.P., Wakimoto H., Agar N.Y.R., Yaffe M.B., Sharp P.A., Hemann M.T., and Lees J.A., Coordinated Splicing of Regulatory Detained Introns within Oncogenic Transcripts Creates an Exploitable Vulnerability in Malignant Glioma. Cancer Cell, 2017. 32(4): p. 411–426 e11; https://www.ncbi.nlm.nih.gov/pubmed/28966034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fong J.Y., Pignata L., Goy P.A., Kawabata K.C., Lee S.C., Koh C.M., Musiani D., Massignani E., Kotini A.G., Penson A., Wun C.M., Shen Y., Schwarz M., Low D.H., Rialdi A., Ki M., Wollmann H., Mzoughi S., Gay F., Thompson C., Hart T., Barbash O., Luciani G.M., Szewczyk M.M., Wouters B.J., Delwel R., Papapetrou E.P., Barsyte-Lovejoy D., Arrowsmith C.H., Minden M.D., Jin J., Melnick A., Bonaldi T., Abdel-Wahab O., and Guccione E., Therapeutic Targeting of RNA Splicing Catalysis through Inhibition of Protein Arginine Methylation. Cancer Cell, 2019. 36(2): p. 194–209 e9; https://www.ncbi.nlm.nih.gov/pubmed/31408619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalev P., Hyer M.L., Gross S., Konteatis Z., Chen C.C., Fletcher M., Lein M., Aguado-Fraile E., Frank V., Barnett A., Mandley E., Goldford J., Chen Y., Sellers K., Hayes S., Lizotte K., Quang P., Tuncay Y., Clasquin M., Peters R., Weier J., Simone E., Murtie J., Liu W., Nagaraja R., Dang L., Sui Z., Biller S.A., Travins J., Marks K.M., and Marjon K., MAT2A Inhibition Blocks the Growth of MTAP-Deleted Cancer Cells by Reducing PRMT5-Dependent mRNA Splicing and Inducing DNA Damage. Cancer Cell, 2021. 39(2): p. 209–224 e11; https://www.ncbi.nlm.nih.gov/pubmed/33450196. [DOI] [PubMed] [Google Scholar]
- 40.Fedoriw A., Rajapurkar S.R., O’Brien S., Gerhart S.V., Mitchell L.H., Adams N.D., Rioux N., Lingaraj T., Ribich S.A., Pappalardi M.B., Shah N., Laraio J., Liu Y., Butticello M., Carpenter C.L., Creasy C., Korenchuk S., McCabe M.T., McHugh C.F., Nagarajan R., Wagner C., Zappacosta F., Annan R., Concha N.O., Thomas R.A., Hart T.K., Smith J.J., Copeland R.A., Moyer M.P., Campbell J., Stickland K., Mills J., Jacques-O’Hagan S., Allain C., Johnston D., Raimondi A., Porter Scott M., Waters N., Swinger K., Boriack-Sjodin A., Riera T., Shapiro G., Chesworth R., Prinjha R.K., Kruger R.G., Barbash O., and Mohammad H.P., Anti-tumor Activity of the Type I PRMT Inhibitor, GSK3368715, Synergizes with PRMT5 Inhibition through MTAP Loss. Cancer Cell, 2019. 36(1): p. 100–114 e25; https://www.ncbi.nlm.nih.gov/pubmed/31257072. [DOI] [PubMed] [Google Scholar]
- 41.Tan D.Q., Li Y., Yang C., Li J., Tan S.H., Chin D.W.L., Nakamura-Ishizu A., Yang H., and Suda T., PRMT5 Modulates Splicing for Genome Integrity and Preserves Proteostasis of Hematopoietic Stem Cells. Cell Rep, 2019. 26(9): p. 2316–2328 e6; https://www.ncbi.nlm.nih.gov/pubmed/30811983. [DOI] [PubMed] [Google Scholar]
- 42.Boutz P.L., Bhutkar A., and Sharp P.A., Detained introns are a novel, widespread class of post-transcriptionally spliced introns. Genes Dev, 2015. 29(1): p. 63–80; https://www.ncbi.nlm.nih.gov/pubmed/25561496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Middleton R., Gao D., Thomas A., Singh B., Au A., Wong J.J., Bomane A., Cosson B., Eyras E., Rasko J.E., and Ritchie W., IRFinder: assessing the impact of intron retention on mammalian gene expression. Genome Biol, 2017. 18(1): p. 51; https://www.ncbi.nlm.nih.gov/pubmed/28298237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu B., Eick D., and Bensaude O., CTD serine-2 plays a critical role in splicing and termination factor recruitment to RNA polymerase II in vivo. Nucleic Acids Res, 2013. 41(3): p. 1591–603; https://www.ncbi.nlm.nih.gov/pubmed/23275552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou T.C., Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res, 2010. 70(2): p. 440–6; https://www.ncbi.nlm.nih.gov/pubmed/20068163. [DOI] [PubMed] [Google Scholar]
- 46.AbuHammad S., Cullinane C., Martin C., Bacolas Z., Ward T., Chen H., Slater A., Ardley K., Kirby L., Chan K.T., Brajanovski N., Smith L.K., Rao A.D., Lelliott E.J., Kleinschmidt M., Vergara I.A., Papenfuss A.T., Lau P., Ghosh P., Haupt S., Haupt Y., Sanij E., Poortinga G., Pearson R.B., Falk H., Curtis D.J., Stupple P., Devlin M., Street I., Davies M.A., McArthur G.A., and Sheppard K.E., Regulation of PRMT5-MDM4 axis is critical in the response to CDK4/6 inhibitors in melanoma. Proc Natl Acad Sci U S A, 2019. 116(36): p. 17990–18000; https://www.ncbi.nlm.nih.gov/pubmed/31439820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y. and Reed R., FUS functions in coupling transcription to splicing by mediating an interaction between RNAP II and U1 snRNP. Proc Natl Acad Sci U S A, 2015. 112(28): p. 8608–13; https://www.ncbi.nlm.nih.gov/pubmed/26124092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X., Wang C., Jiang H., and Luo C., A patent review of arginine methyltransferase inhibitors (2010–2018). Expert Opin Ther Pat, 2019. 29(2): p. 97–114; https://www.ncbi.nlm.nih.gov/pubmed/30640571. [DOI] [PubMed] [Google Scholar]
- 49.Kryukov G.V., Wilson F.H., Ruth J.R., Paulk J., Tsherniak A., Marlow S.E., Vazquez F., Weir B.A., Fitzgerald M.E., Tanaka M., Bielski C.M., Scott J.M., Dennis C., Cowley G.S., Boehm J.S., Root D.E., Golub T.R., Clish C.B., Bradner J.E., Hahn W.C., and Garraway L.A., MTAP deletion confers enhanced dependency on the PRMT5 arginine methyltransferase in cancer cells. Science, 2016. 351(6278): p. 1214–8; https://www.ncbi.nlm.nih.gov/pubmed/26912360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mavrakis K.J., McDonald E.R. 3rd, Schlabach M.R., Billy E., Hoffman G.R., deWeck A., Ruddy D.A., Venkatesan K., Yu J., McAllister G., Stump M., deBeaumont R., Ho S., Yue Y., Liu Y., Yan-Neale Y., Yang G., Lin F., Yin H., Gao H., Kipp D.R., Zhao S., McNamara J.T., Sprague E.R., Zheng B., Lin Y., Cho Y.S., Gu J., Crawford K., Ciccone D., Vitari A.C., Lai A., Capka V., Hurov K., Porter J.A., Tallarico J., Mickanin C., Lees E., Pagliarini R., Keen N., Schmelzle T., Hofmann F., Stegmeier F., and Sellers W.R., Disordered methionine metabolism in MTAP/CDKN2A-deleted cancers leads to dependence on PRMT5. Science, 2016. 351(6278): p. 1208–13; https://www.ncbi.nlm.nih.gov/pubmed/26912361. [DOI] [PubMed] [Google Scholar]
- 51.Smith C.R., Aranda R., Bobinski T.P., Briere D.M., Burns A.C., Christensen J.G., Clarine J., Engstrom L.D., Gunn R.J., Ivetac A., Jean-Baptiste R., Ketcham J.M., Kobayashi M., Kuehler J., Kulyk S., Lawson J.D., Moya K., Olson P., Rahbaek L., Thomas N.C., Wang X., Waters L.M., and Marx M.A., Fragment-Based Discovery of MRTX1719, a Synthetic Lethal Inhibitor of the PRMT5*MTA Complex for the Treatment of MTAP-Deleted Cancers. J Med Chem, 2022. 65(3): p. 1749–1766; https://www.ncbi.nlm.nih.gov/pubmed/35041419. [DOI] [PubMed] [Google Scholar]
- 52.Dobin A., Davis C.A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., and Gingeras T.R., STAR: ultrafast universal RNA-seq aligner. Bioinformatics, 2013. 29(1): p. 15–21; https://www.ncbi.nlm.nih.gov/pubmed/23104886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Love M.I., Huber W., and Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol, 2014. 15(12): p. 550; https://www.ncbi.nlm.nih.gov/pubmed/25516281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., and Mesirov J.P., Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A, 2005. 102(43): p. 15545–50; https://www.ncbi.nlm.nih.gov/pubmed/16199517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang da W., Sherman B.T., and Lempicki R.A., Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc, 2009. 4(1): p. 44–57; https://www.ncbi.nlm.nih.gov/pubmed/19131956. [DOI] [PubMed] [Google Scholar]
- 56.Trincado J.L., Entizne J.C., Hysenaj G., Singh B., Skalic M., Elliott D.J., and Eyras E., SUPPA2: fast, accurate, and uncertainty-aware differential splicing analysis across multiple conditions. Genome Biol, 2018. 19(1): p. 40; https://www.ncbi.nlm.nih.gov/pubmed/29571299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langmead B. and Salzberg S.L., Fast gapped-read alignment with Bowtie 2. Nat Methods, 2012. 9(4): p. 357–9; https://www.ncbi.nlm.nih.gov/pubmed/22388286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y., Liu T., Meyer C.A., Eeckhoute J., Johnson D.S., Bernstein B.E., Nusbaum C., Myers R.M., Brown M., Li W., and Liu X.S., Model-based analysis of ChIP-Seq (MACS). Genome Biol, 2008. 9(9): p. R137; https://www.ncbi.nlm.nih.gov/pubmed/18798982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.