Abstract
3D printed microfluidics offer several advantages over conventional planar microfabrication techniques including fabrication of 3D microstructures, rapid prototyping, and inertness. While 3D printed materials have been studied for their biocompatibility in cell and tissue culture applications, their compatibility for in vitro biochemistry and molecular biology has not been systematically investigated. Here, we evaluate the compatibility of several common enzymatic reactions in the context of 3D-printed microfluidics: 1) polymerase chain reaction (PCR), 2) T7 in vitro transcription, 3) mammalian in vitro translation, and 4) reverse transcription. Surprisingly, all the materials tested significantly inhibit one or more of these in-vitro enzymatic reactions. Inclusion of BSA mitigates only some of these inhibitory effects. Overall, inhibition appears to be due to a combination of the surface properties of the resins as well as soluble components (leachate) originating in the matrix.
Keywords: Microfluidics, Biomicrofluidics, Enzymatic Reactions, 3D Printing, 3D-Printed Microfluidics, Photocurable Resins
Graphical Abstract
Introduction
Microfluidics is an important tool in areas of biochemistry to miniaturize bulky and costly laboratory processes. By confining reactions and processes within micron-sized channels, microfluidic systems offer high portability, minimal reagent consumption, and sophisticated process integration at low costs.1 - 7 For mass production, microfluidic devices can be manufactured by high-throughput techniques such as injection molding8-10 and hot embossing11-16. For prototyping in research settings, however, microfluidic devices are typically fabricated in small batches through molding elastomers or thermoplastics.17-21 The fabrication process of microfluidic molds are tedious and costly, and the resulting devices are limited to two-dimensional (2D) structures with cumbersome user interfaces.22 Because of these limitations, more and more microfluidic researchers have turned to three-dimensional (3D) printing for microfabrication.6,17,22-28
In 3D-printed microfluidics, the devices are modeled using computer aided design software and then printed additively layer-by-layer. Among all 3D printing techniques, stereolithography (SLA) is the most widely used for microfluidics because of its high printing resolution and capability of fabricating closed microfluidic channels without additional assembly steps.24,29,30 SLA printers selectively polymerize liquid photopolymer resins using a focused laser light source.31 The additive layers of polymerized resin form microchannel walls that are filled with liquid resin, which is flushed out of the microfluidic channels afterwards.24,25 With previously unattainable simplicity, the technique opens the possibility to single-step microfabrication, overcomes the 2D limitations of conventional fabrication methods, and incorporates industry-standard user interfaces that are commercialization-ready.22,24,27,32
Despite its unique advantages, 3D-printed microfluidics have not been readily adopted by the biochemistry community and recent work has been limited to proof-of-concept studies.24 This is in part due to the stringent material compatibility requirements for biochemical reactions. In microfluidic channels, the interaction between biological molecules and the material of the channel wall is especially pronounced because of the high surface-area-to-volume ratio.17,33 Today, most microfluidic devices for biochemical reactions are fabricated by traditional polydimethylsiloxane (PDMS) molding despite its tedious fabrication process and poor user interfaces.7,34-36 Many microfluidic researchers are reluctant to part with PDMS molding because of the optimal material properties: PDMS is optically clear, chemically inert, biocompatible, and easy to mold.37,38 The dissemination of 3D printed microfluidics is therefore challenged by the compatibility of 3D-printable materials with biochemical reactions.17,28-30,33,39-41
Previous investigations on 3D printable photopolymers for biological applications focused on cell culture compatibility41-44, but little has been done to evaluate their inhibitory effects on in-vitro enzymatic reactions. In-vitro enzymatic reactions, such as PCR, are essential for lab-on-a-chip applications, where the transition from traditional microfabrication techniques to 3D printing has been longed for. To help break the barriers of microfluidics application in the biochemistry community, we evaluated some of the most popular SLA-printed materials for their compatibility with polymerase chain reaction (PCR), transcription, translation, and reverse transcription. We designed a 3D printed cone that sits inside conventional conical microcentrifuge tubes to mimic the surface contact of the reactions within microfluidic channels.
Including BSA in PCR solutions as a blocking agent for materials that adsorbs the polymerase is well documented.45-48 For SLA-printed materials, it was speculated that in-vitro enzymatic reactions may be inhibited by the substances, such as uncured resins, leaching out from the printed parts in addition to the effects of polymerase adsorption.49,50 By tuning the concentration of BSA for each reaction, we evaluated current limitations of SLA-printed materials for microfluidics in-vitro enzymatic reactions.
Materials & Methods
3D Printing
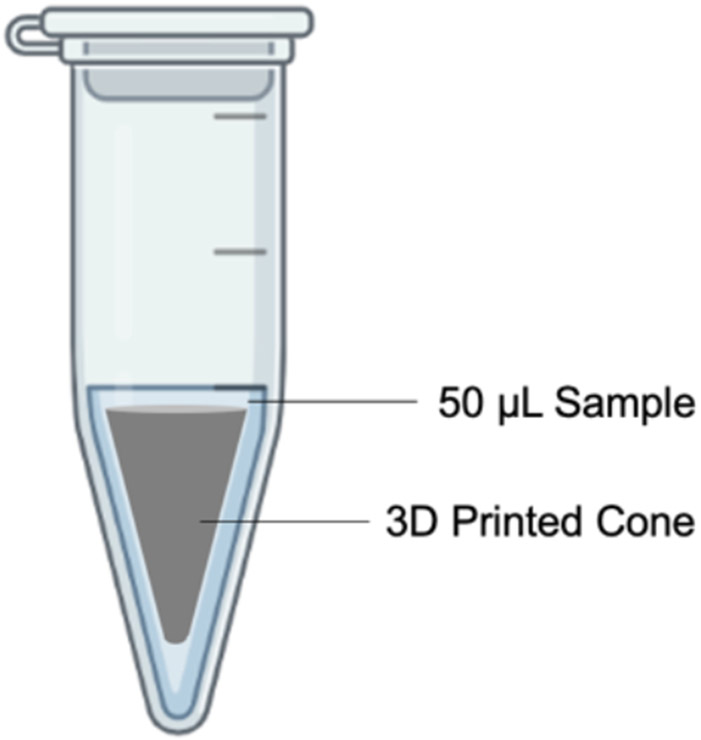
To evaluate the compatibility of SLA printed parts with in-vitro biological reactions, we prepared 3D printed parts of three common SLA materials for microfluidics: WaterShed (Somos WaterShed XC 11122, DSM), Pro3dure GR-1 (Pro3dure Medical GmbH, Dortmund, Germany), and a PEGDA-based resin prepared in-house40. For surface treatment, a commercial Teflon coating (Teflon Non-Stick Dry-Film Lubricant, Dupont, USA) was applied onto parts printed with Pro3dure GR-1. The 3D printed parts were designed with a computer-aided design software (Autodesk Fusion 360). Each part was a designed in cone such that, when placed with 50 μL liquid sample in a 500 μL polypropylene microcentrifuge tube (Eppendorf, Germany), the liquid is in contact with the 3D printed parts at an equivalent surface-area-to-volume ratio to a 1.5 mm-diameter channel (Figure 1).
Figure 1.
Illustration of a 3D-printed cone placed in a microcentrifuge tube.
PCR
PCR was performed using DreamTaq™ Hot Start DNA Polymerase from Thermo Scientific™. We used the template of a previously identified BCL-xL ligand, labeled E1 (amino acid sequence MIETITIYNYKKAADHFSMSM), to test PCR. The resulting product was used to test transcription, translation, and reverse transcription as described in our previous work.6 The PCR reactions were performed in the supplied buffer with 2 mM dNTPs, 100 pM of template DNA, 1 μM primers, and various concentrations of BSA. Standard thermocycling (94 °C - 53 °C - 72 °C cycling at 30 seconds per step) was performed for 10 cycles using polypropylene tubes with or without additional 3D-printed parts in the tube. The samples were run on an agarose gel.
Transcription
Transcription was performed using T7 RNA polymerase that was expressed and purified in house using a his tag. Reactions were performed in 80 mM HEPES pH = 7.4, 2 mM Spermidine, 40 mM DTT, 25 mM MgCl2, 5 mM each NTP, 50 nM of template DNA, and various concentrations of BSA. Incubation was performed on a heating block set to 37 °C for 1 hour in polypropylene tubes with or without additional 3D-printed parts in the tube. The samples were run on a acrylamide urea gel.
Translation
Translation was performed as previously described by Jackson and Hunt using rabbit reticulocyte lysate (RRL)51. The unlabeled methionine was replaced with 35S methionine (Perkin Elmer) for radiolabel the resulting peptides. Reactions were performed in 40% treated RRL, 20 mM HEPES pH = 7.6, 0.5 mM Mg(OAc)2, 8 mM creatine phosphate, 2 mM DTT, 25 μM each amino acid, 400 nM of template ligated mRNA, and various concentrations of BSA. Incubation was performed on a heating block set to 30 °C for 1 hour in polypropylene tubes with or without additional 3D-printed parts in the tube and KCl and MgCl2 salts were added to a final concentration of 470 mM and 60 mM respectively to facilitate fusion formation. Fusions were purified from the translation mixture using dT beads (streptavidin agarose beads, Pierce™, and biotinylated dT25 oligo, IDT), and the beads were counted on a scintillation counter (Beckman LS 6500).
Reverse Transcription
Reverse transcription was performed with Superscript IV in 50 mM Tris-HCl (pH=8.3), 75 mM KCl, 3 mM MgCl2, 10 mM DTT, 500 μM each dNTP, and 1 mM 3’ primer on 200 nM of template mRNA. The reaction was incubated at 42 °C for 1 hour to facilitate reverse transcription, then further incubated at 65 °C for 15 min to inactivate Superscript IV. The sample was PCR amplified and then ran on an agarose gel to evaluate the yield from reverse transcription.
Data Analysis
Each experiment was repeated at least three times. Band intensity analysis was performed by processing the gel electrophoresis images (imaged with Spot RT3, USA) through ImageJ. The intensity data was normalized to the sample with neither BSA nor parts, and the standard error of the mean was reported. The significance of band intensity analysis result was evaluated with 2-way ANOVA test using GraphPad Prism.
Results
PCR
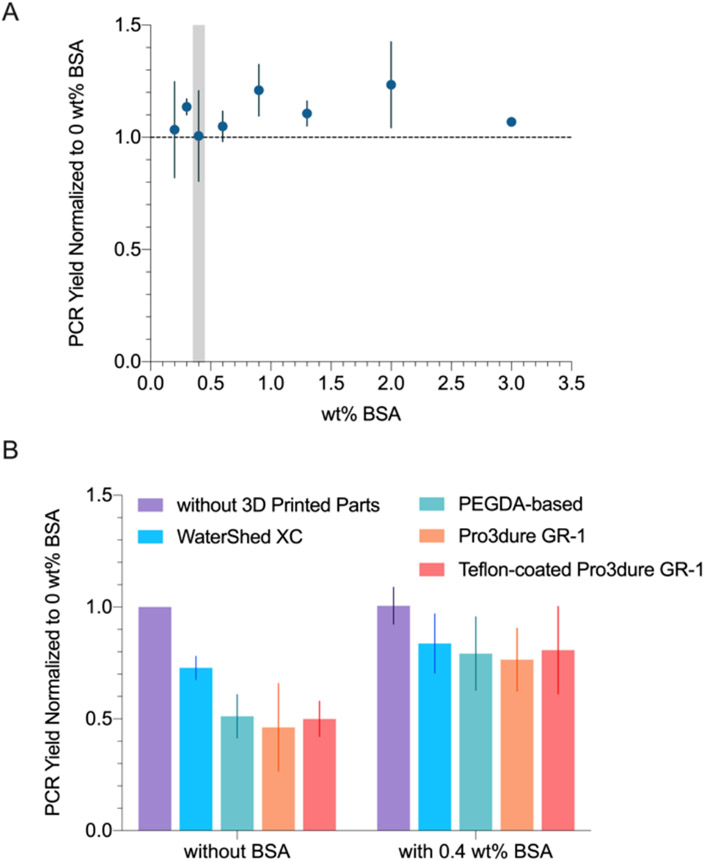
In an attempt to optimize PCR yield with BSA in the presence of 3D printed parts, we first evaluated how PCR reaction yield varies with BSA concentration (Figure 2A). As shown in the figure, PCR yield was consistent among 0 ~ 3 wt% BSA samples. At concentrations above 0.6 wt% BSA, the samples become gel-like after the reaction due to BSA aggregation.52 For microfluidics applications, the increased viscosity can cause the microchannels to clog. Therefore, we proceeded to evaluate the effects of 3D printed parts on PCR with 0.4 wt% BSA, which is the highest BSA concentration deemed suitable for microfluidic applications. We performed two sets of experiments: with and without BSA (0.4 wt%). In each set, PCR reactions were performed in tubes each containing 3D printed cones of different materials. As shown in Figure 2B, samples with 0.4 wt% BSA resulted in significantly higher PCR yields with an average of 20% increase from the sample without BSA or parts.
Figure 2.
(A) PCR yield (normalized to no BSA, no parts sample) at various BSA wt%. 0.4 wt% BSA (highlighted in grey) was chosen for the following experiments. (B) PCR yield (normalized to no BSA, no parts sample) when incubated with different 3D printed materials, performed with and without BSA (0.4 wt%). BSA significantly improved PCR yields when 3D printed parts were present (p < 0.05).
Transcription
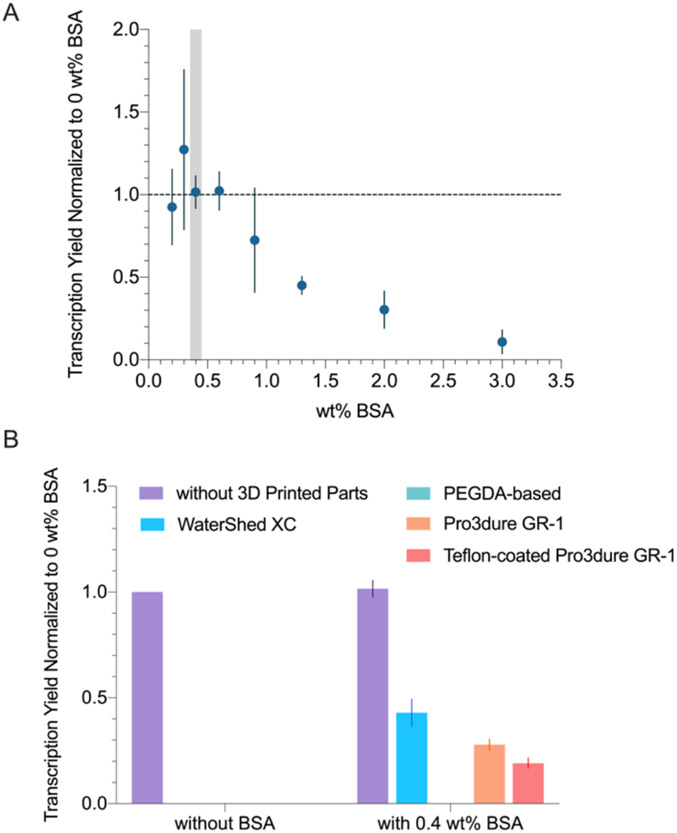
Our early attempts to perform T7 transcription in 3D printed microfluidic channels failed to produce product. While PEG is a more common blocking agent for T7 transcription than BSA, the high viscosity of PEG is unfavorable for microfluidics. To address how BSA might recover activity, we first evaluated how transcription yield varies at different BSA concentrations. As shown in Figure 3A, transcription yield decreased drastically with increased BSA concentration above 0.9 wt%. We suspect that this may be due to BSA competing with the polymerase. Additionally, since we observed sample gelling above 0.6 wt%, we decided to proceed with 0.4 wt% BSA. As shown in Figure 3B, transcription samples that were incubated with 3D printed materials did not result in observable yield. With 0.4 wt% BSA, samples that were incubated with WaterShed XC, Pro3dure GR-1, and Teflon-coated Pro3dure GR-1 showed significant improvements in yield. The samples with parts printed with the PEGDA-based SLA resin failed both with and without BSA. The results suggest that experiments involving T7 polymerase production of RNA may not work well in any of the tested microfluidic materials without taking additional steps.
Figure 3.
(A) Transcription yield (normalized to no BSA, no parts sample) at various BSA wt%. 0.4 wt% BSA (highlighted in grey) was chosen for the following experiments. (B) Transcription yield (normalized to no BSA, no parts sample) when incubated with different 3D printed materials, performed with and without BSA (0.4 wt%). BSA significantly improved the transcription yield when 3D printed parts were present (p < 0.05). The yields of printed parts without BSA and PEGDA-based resin with 0.4 wt% BSA were below the detection limit (Figure S2).
Translation
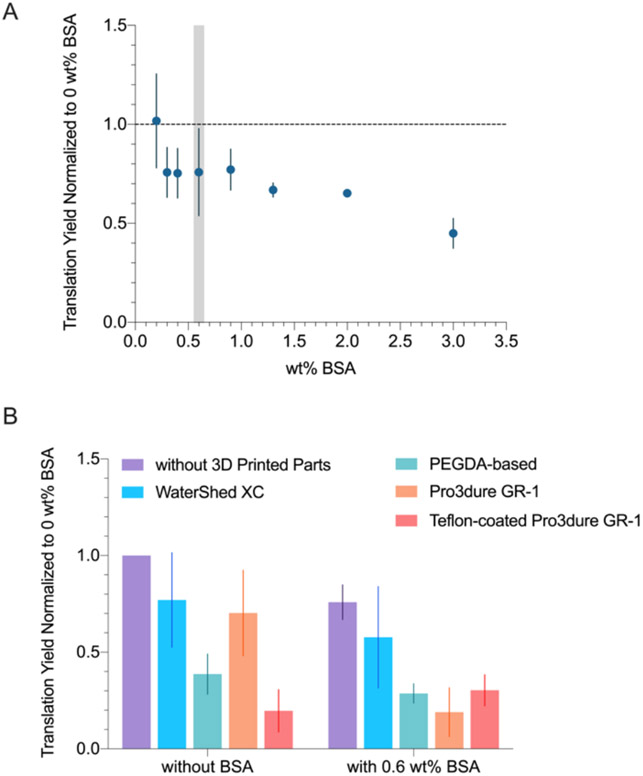
As shown in Figure 4A, translation yield decreases slightly as BSA concentration increased. While the samples remained liquid at all BSA concentrations tested, the viscosity of the sample increased with the BSA content, which would increase the risk of channel fouling and clogging in microfluidics settings. Therefore, to mitigate the inhibiting effects of 3D printed parts without compromising the microfluidic flow performance, we decided to proceed with testing the compatibility of 3D printed materials with 0.6 wt% BSA, a slightly higher concentration than what was used for PCR and transcription (0.4 wt%). The results showed no significant improvement (based on Student’s t-test) of BSA on translation yields across different 3D printed materials.
Figure 4.
(A) Translation yield (normalized to no BSA, no parts sample) at various BSA wt%. 0.6 wt% BSA (highlighted in grey) was chosen for the subsequent experiments. (B) Translation yield (normalized to no BSA, no parts sample) when incubated with different 3D printed materials, performed with and without BSA (0.6 wt%). BSA did not significantly improve translation yield when 3D printed parts were present.
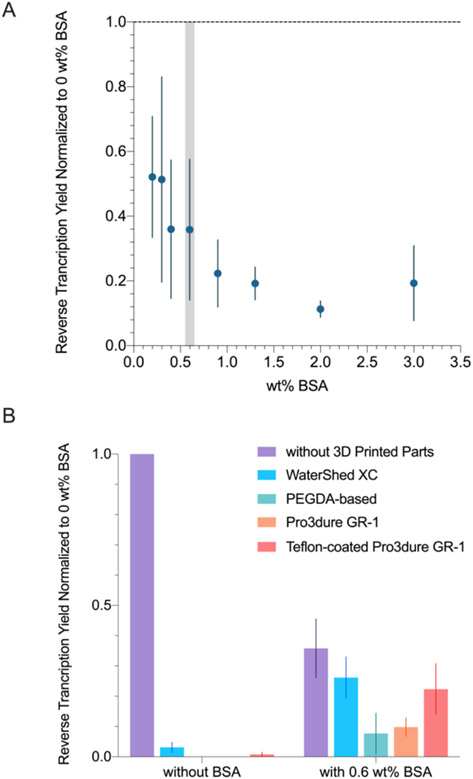
Reverse Transcription
As shown in Figure 5A, reverse transcription yield lowered as BSA concentration increased, and all samples remained liquid after the reaction. Based on the results, we decided to proceed with 0.6 wt% BSA as for the translation experiments so as to not compromise the reaction yield too much. As shown in Figure 5B, 0.6 wt% BSA significantly improved the reverse transcription yield when incubated with all 3D printed materials.
Figure 5.
(A) Reverse transcription yield (normalized to no BSA, no parts sample) at various BSA wt%. 0.4 wt% BSA (highlighted in grey) was chosen for the subsequent experiments. (B) Transcription yield (normalized to no BSA, no parts sample) when incubated with different 3D printed materials, performed with and without BSA (0.6 wt%). BSA significantly improved the reverse transcription yield when 3D printed parts were present (p < 0.05). The yields of PEGDA-based resin without BSA and Pro3dure GR-1 without BSA were below the detection limit (Figure S3).
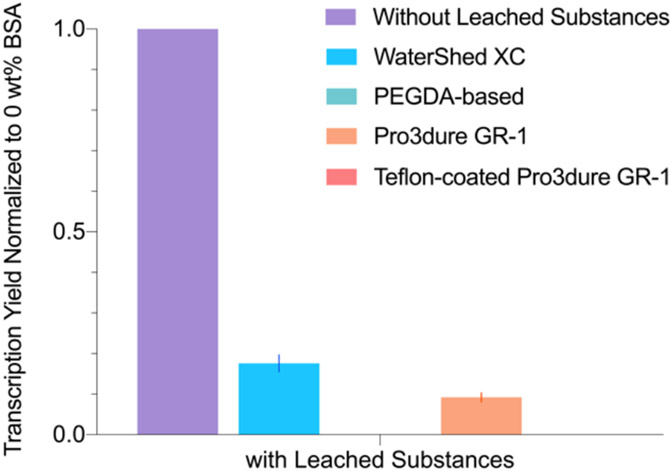
Transcription Using Water with Leachate
To better understand the proportional impact of adsorption versus reaction-inhibiting substances being leached out from the parts, we performed transcription experiments with water that was previously incubated for 12 hours at 37°C with each 3D printed part. Note that all parts were treated in a post-print UV curing chamber, minimizing the amount of unreacted monomer present. During the extended soaking, water-soluble substances leached out. The leachate water collected after extended soaking was diluted 5x in the transcription sample to evaluate its impact on transcription. We chose to test with transcription because it is the easiest to perform among all reactions and was very sensitive to both BSA and the type of substrate material as shown in Figure 3. The amount of water added to each reaction resulted in a 50% dilution of the leachate. In comparison to Figure 3, Figure 6 shows that the reaction yield was slightly improved in the WaterShed XC and Pro3dure GR-1 trials, although still lower than the sample without BSA or parts. This suggests that the inhibiting substances being leached out from the 3D printed parts significantly hindered the reaction.
Figure 6.
Transcription yield (normalized to no BSA, no parts sample) when incubated with parts vs with leachate isolated from parts. The yields from PEGDA-based resin and Teflon-coated Pro3dure GR-1 were below the detection limit (Figure S2).
Discussion
The results shown above indicate that PCR, transcription, and reverse transcription can be improved with the addition of BSA. The addition of BSA did not impact translation yields significantly, perhaps because the reaction uses lysate, which already includes additional proteins that alleviate the inhibiting effects from the leachates. Among all materials tested, we found that WaterShed XC performed the best overall. The PEGDA-based material performed the worst, with non-observable transcription yield even with the addition of BSA. All 3D printed materials assessed in this study significantly inhibits in-vitro enzymatic reactions. This may be due to enzymes being adsorbed onto the 3D printed surfaces and/or inhibiting substances being leached out of the 3D printed materials. When comparing the Pro3dure GR-1 with and without Teflon coating, no significant difference in reaction yields were observed, which suggests that inhibiting substances leaching out of the material during the reaction plays an important role in the low reaction yields.
The transcription experiments performed with only the leachate but not the parts shows low reaction yields relative to the no BSA, no parts samples (Figure 6). This results further confirmed that the leachate from materials have significant inhibiting effects on the reactions, possibly due to their interaction with the polymerase. By comparing the transcription with leachate results (Figure 6) with the transcription with parts results (Figure 3B), we can see that the reaction yield was even lower when incubated with the parts. This indicates that aside from the leachate, transcription was also inhibited by interactions between the polymerase and the parts. Furthermore, the transcription yields with parts and with BSA (Figure 3) were higher than the yields obtained from transcription with leachate (Figure 6), indicating that BSA mitigates the interaction of the polymerase and both the leachate and the materials. We speculate that the mitigating effect of BSA is caused by BSA competing with the polymerase for interactions with the materials.
In order for 3D-printed microfluidic parts to be usable for in-vitro enzymatic reactions, further materials development is needed. Post-print channel modifications with covalent fluorosilane and dimethylsiloxane coatings have been shown to increase PCR yields by minimizing the interactions between the enzymes and 3D printed materials and inhibiting leaching50. New resin formulations for 3D-printed parts that are compatible with in-vitro enzymatic reactions would require elaborate screening of each ingredient. We speculate that the limited photoinitiator selection currently available for transparent prints is incompatible for in-vitro enzymatic reactions and that new photoinitiator should be explored to overcome the challenge. Although rapid prototyping techniques that do not involve photocurable resins (e.g. fused deposition modeling53-56 and 3D CNC milling57) may provide a wider range of materials selection, studies of these materials have also shown decreased enzymatic activities55,57. This suggests the need for the development of dedicated materials specifically designed for biochemical applications.
Conclusions
In this study, we evaluated 3D-printed materials that are popular in microfluidics for their compatibility with in-vitro enzymatic reactions. In the attempt to mitigate the inhibiting effects of the materials to the reactions, we added BSA to the reactions and saw improvements in PCR, transcription, and reverse transcription, but not in translation. We suspect the lysate used in translation already contains proteins that can compete with the polymerase for interactions with the printed materials, and therefore adding more BSA did not further improve the reaction yield.
Based on our findings, WaterShed XC is the most compatible with in-vitro enzymatic reactions, although the reactions were nonetheless significantly inhibited. Since Teflon-coated Pro3dure GR-1 did not show improvement over the non-coated material, we suspect that the inhibiting effects are most likely due to leachate from the materials in addition to adsorption of the enzymes to material surfaces. This speculation was later confirmed in our transcription experiments incubated with only the leachate, without the 3D-printed parts. We conclude that both the leachate and the materials caused significant inhibition to in-vitro enzymatic reactions, and that BSA appears essential to mitigate the inhibiting effects, although the improvements were marginal. The finding is especially concerning as the incompatibly of 3D printed materials with in-vitro enzymatic reaction could be a major barrier for applying 3d printing technology to biomicrofluidics. The development of new SLA materials for biomicrofluidics should focus on reducing leachate and minimizing polymerase interaction.
Supplementary Material
ACKNOWLEDGMENTS
The work was funded by the National Cancer Institute (NCI) of the National Institutes of Health (NIH), award number CA204708.
Footnotes
- Supporting_info.pdf
- Figures showing gel images that resulted from the experiments
- conedesignfile.stl
- Computer-aided design files for the cone test part in .STL format
- printparameters.txt
- 3D printing configuration for the cone test part
REFERENCES
- 1.Sinha A, Gopinathan P, Chung Y. Da, Lin HY, Li KH, Ma HP, Huang PC, Shiesh SC & Lee G. Bin. An integrated microfluidic platform to perform uninterrupted SELEX cycles to screen affinity reagents specific to cardiovascular biomarkers. Biosens. Bioelectron 122, 104–112 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Lou X, Qian J, Xiao Y, Viel L, Gerdon AE, Lagally ET, Atzberger P, Tarasow TM, Heeger AJ & Soh HT Micromagnetic selection of aptamers in microfluidic channels. Proc. Natl. Acad. Sci. U. S. A 106, 2989–2994 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung LY, Wang CH, Fu CY, Gopinathan P & Lee G. Bin. Microfluidics in the selection of affinity reagents for the detection of cancer: Paving a way towards future diagnostics. Lab Chip 16, 2759–2774 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Kinahan DJ, Mangwanya F, Garvey R, Chung DWY, Lipinski A, Julius LAN, King D, Mohammadi M, Mishra R, Al-Ofi M, Miyazaki C & Ducrée J Automation of Silica Bead-based Nucleic Acid Extraction on a Centrifugal Lab-on-a-Disc Platform. J. Phys. Conf. Ser 757, 012013 (2016). [Google Scholar]
- 5.Sanjay ST, Zhou W, Dou M, Tavakoli H, Ma L, Xu F & Li XJ Recent advances of controlled drug delivery using microfluidic platforms. Adv. Drug Deliv. Rev 128, 3–28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evenson WE, Lin WZS, Pang K, Czaja AT, Jalali-Yazdi F, Takahashi TT, Malmstadt N & Roberts RW Enabling Flow-Based Kinetic Off-Rate Selections Using a Microfluidic Enrichment Device. Anal. Chem 92, 10218–10222 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin WZS & Malmstadt N Liposome production and concurrent loading of drug simulants by microfluidic hydrodynamic focusing. Eur. Biophys. J 48, 549–558 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matschuk M & Larsen NB Injection molding of high aspect ratio sub-100 nm nanostructures.J. Micromechanics Microengineering 23, 025003 (2013). [Google Scholar]
- 9.Giboz J, Copponnex T & Mélé P Microinjection molding of thermoplastic polymers:A review. J. Micromechanics Microengineering 17, R96 (2007). [Google Scholar]
- 10.Zhao J, Ong R, Chen G, Juay YK, Ng FL, Lee MW & Kua CH Development of rapid manufacturing technology of polymer microfluidic devices by micro moulding using silicon mould inserts. in Proceedings of the 6th International Conference on Nanochannels,Microchannels,and Minichannels, ICNMM2008 1187–1194 (American Society of Mechanical Engineers Digital Collection, 2008). doi: 10.1115/ICNMM2008-62232. [DOI] [Google Scholar]
- 11.Becker H & Heim U Hot embossing as a method for the fabrication of polymer high aspect ratio structures. Sensors Actuators, A Phys. 83, 130–135 (2000). [Google Scholar]
- 12.Lin TY, Do T, Kwon P & Lillehoj PB 3D printed metal molds for hot embossing plastic microfluidic devices. Lab Chip 17, 241–247 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeon JS, Chung S, Kamm RD & Charest JL Hot embossing for fabrication of a microfluidic 3D cell culture platform. doi: 10.1007/s10544-010-9496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee G. Bin, Chen SH, Huang GR, Sung WC & Lin YH Microfabricated plastic chips by hot embossing methods and their applications for DNA separation and detection. Sensors Actuators, B Chem. 75, 142–148 (2001). [Google Scholar]
- 15.Çoğun F, Yıldırım E & Sahir Arikan MA Investigation on replication of microfluidic channels by hot embossing. Mater. Manuf. Process 32, 1838–1844 (2017). [Google Scholar]
- 16.Shiu PP, Knopf GK, Ostojic M & Nikumb S Rapid fabrication of tooling for microfluidic devices via laser micromachining and hot embossing. J. Micromechanics Microengineering 18, 025012 (2008). [Google Scholar]
- 17.Urrios A, Parra-Cabrera C, Bhattacharjee N, Gonzalez-Suarez AM, Rigat-Brugarolas LG, Nallapatti U, Samitier J, Deforest CA, Posas F, Garcia-Cordero JL & Folch A 3D-printing of transparent bio-microfluidic devices in PEG-DA. Lab Chip 16, 2287–2294 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaegh SAM, Pourmand A, Nabavinia M, Avci H, Tamayol A, Mostafalu P, Ghavifekr HB, Aghdam EN, Dokmeci MR, Khademhosseini A & Zhang YS Rapid prototyping of whole-thermoplastic microfluidics with built-in microvalves using laser ablation and thermal fusion bonding. Sensors Actuators, B Chem. 255, 100–109 (2018). [Google Scholar]
- 19.Sahore V, Doonan SR & Bailey RC Droplet microfluidics in thermoplastics: device fabrication, droplet generation, and content manipulation using integrated electric and magnetic fields. Anal. Methods 10, 4264–4274 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gencturk E, Mutlu S & Ulgen KO Advances in microfluidic devices made from thermoplastics used in cell biology and analyses. Biomicrofluidics 11, 51502 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CF, Liu J, Hromada LP, Tsao CW, Chang CC & DeVoe DL High-pressure needle interface for thermoplastic microfluidics. Lab Chip 9, 50–55 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Au AK, Huynh W, Horowitz LF & Folch A 3D-Printed Microfluidics. Angew. Chemie - Int. Ed 55, 3862–3881 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sochol RD, Sweet E, Glick CC, Wu SY, Yang C, Restaino M & Lin L 3D printed microfluidics and microelectronics. Microelectron. Eng 189, 52–68 (2018). [Google Scholar]
- 24.Waheed S, Cabot JM, Macdonald NP, Lewis T, Guijt RM, Paull B & Breadmore MC 3D printed microfluidic devices: Enablers and barriers. Lab Chip 16, 1993–2013 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Ho CMB, Ng SH, Li KHH & Yoon YJ 3D printed microfluidics for biological applications. Lab Chip 15, 3627–3637 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Au AK, Bhattacharjee N, Horowitz LF, Chang TC & Folch A 3D-printed microfluidic automation. Lab Chip 15, 1934–1941 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Y, Wu Y, Fu JZ, Gao Q & Qiu JJ Developments of 3D Printing Microfluidics and Applications in Chemistry and Biology: a Review. Electroanalysis vol. 28 1658–1678 (2016). [Google Scholar]
- 28.Bhattacharjee N, Urrios A, Kang S & Folch A The upcoming 3D-printing revolution in microfluidics. Lab Chip 16, 1720–1742 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong H, Bickham BP, Woolley AT & Nordin GP Custom 3D printer and resin for 18 μm × 20 μm microfluidic flow channels. Lab Chip 17, 2899–2909 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park HK, Shin M, Kim B, Park JW & Lee H A visible light-curable yet visible wavelength-transparent resin for stereolithography 3D printing. NPG Asia Mater. 10, 82–89 (2018). [Google Scholar]
- 31.Hull C. On Stereolithography. Virtual and Physical Prototyping vol. 7 177 (2012). [Google Scholar]
- 32.Bhargava KC, Ermagan R, Thompson B, Friedman A & Malmstadt N Modular, discrete micromixer elements fabricated by 3D printing. Micromachines 8, 137 (2017). [Google Scholar]
- 33.Gross BC, Erkal JL, Lockwood SY, Chen C & Spence DM Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal. Chem 86, 3240–3253 (2014). [DOI] [PubMed] [Google Scholar]
- 34.McDonald JC & Whitesides GM Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res 35, 491–499 (2002). [DOI] [PubMed] [Google Scholar]
- 35.Folch A, Ayon A, Hurtado O, Schmidt MA & Toner M Molding of deep polydimethylsiloxane microstructures for microfluidics and biological applications. J. Biomech. Eng 121, 28–34 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Ren K, Zhou J & Wu H Materials for microfluidic chip fabrication. Acc. Chem. Res 46, 2396–2406 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Raj M,K & Chakraborty S PDMS microfluidics: A mini review. Journal of Applied Polymer Science vol. 137 48958 (2020). [Google Scholar]
- 38.Fujii T. PDMS-based microfluidic devices for biomedical applications. in Microelectronic Engineering vols 61–62 907–914 (Elsevier, 2002). [Google Scholar]
- 39.Risch P, Kotz F, Helmer D & Rapp BE 3D printing of highly fluorinated methacrylates for the fabrication of transparent and chemically-resistant microfluidic devices. in 22nd International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2018 vol. 4 2163–2165 (2018). [Google Scholar]
- 40.Gong H, Beauchamp M, Perry S, Woolley AT & Nordin GP Optical approach to resin formulation for 3D printed microfluidics. RSC Advances vol. 5 106621–106632 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carve M & Wlodkowic D 3D-printed chips: Compatibility of additive manufacturing photopolymeric substrata with biological applications. Micromachines 9, 91 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu F, Friedrich T, Nugegoda D, Kaslin J & Wlodkowic D Assessment of the biocompatibility of three-dimensional-printed polymers using multispecies toxicity tests. Biomicrofluidics 9, 1–5 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nejedlá Z, Poustka D, Herma R, Liegertová M, Štofik M, Smejkal J, Šícha V, Kaule P & Malý J Class II biocompatible E-Shell 300 3D printing material causes severe developmental toxicity in Danio rerio embryos and reduced cell proliferation in vitro – implications for 3D printed microfluidics. RSC Adv. 11, 16252–16267 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takenaga S, Schneider B, Erbay E, Biselli M, Schnitzler T, Schöning MJ & Wagner T Fabrication of biocompatible lab-on-chip devices for biomedical applications by means of a 3D-printing process. Phys. Status Solidi Appl. Mater. Sci 212, 1347–1352 (2015). [Google Scholar]
- 45.Prakash AR, Amrein M & Kaler KVIS Characteristics and impact of Taq enzyme adsorption on surfaces in microfluidic devices. Microfluid. Nanofluidics 4, 295–305 (2008). [Google Scholar]
- 46.Taylor TB, Winn-Deen ES, Picozza E, Woudenberg TM & Albin M Optimization of the performance of the polymerase chain reaction in silicon-based microstructures. Nucleic Acids Res. 25, 3164–3168 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kodzius R, Xiao K, Wu J, Yi X, Gong X, Foulds IG & Wen W Inhibitory effect of common microfluidic materials on PCR outcome. Sensors Actuators, B Chem. 161, 349–358 (2012). [Google Scholar]
- 48.Wilding P, Shoffner MA & Kricka LJ PCR in a silicon microstructure. in Clinical Chemistry vol. 40 1815–1818 (American Association for Clinical Chemistry Inc., 1994). [PubMed] [Google Scholar]
- 49.Kreader CA Relief of amplification inhibition in PCR with bovine serum albumin or T4 gene 32 protein. Appl. Environ. Microbiol 62, 1102–1106 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tzivelekis C, Sgardelis P, Waldron K, Whalley R, Huo D & Dalgarno K Fabrication routes via projection stereolithography for 3D-printing of microfluidic geometries for nucleic acid amplification. PLoS One 15, 1–21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackson RJ & Hunt T Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 96, 50–74 (1983). [DOI] [PubMed] [Google Scholar]
- 52.Murata M, Tani F, Higasa T, Kitabatake N & Doi E Heat-induced Transparent Gel Formation of Bovine Serum Albumin. Biosci. Biotechnol. Biochem 57, 43–46 (1993). [DOI] [PubMed] [Google Scholar]
- 53.Tothill AM, Partridge M, James SW & Tatam RP Fabrication and optimisation of a fused filament 3D-printed microfluidic platform. J. Micromechanics Microengineering 27, 035018 (2017). [Google Scholar]
- 54.Sirjani E, Migas M, Cragg PJ & Dymond MK 3D printed UV/VIS detection systems constructed from transparent filaments and immobilised enzymes. Addit. Manuf 33, 101094 (2020). [Google Scholar]
- 55.Pantazis AK, Papadakis G, Parasyris K, Stavrinidis A & Gizeli E 3D-printed bioreactors for DNA amplification: application to companion diagnostics. Sensors Actuators, B Chem. 319, 128161 (2020). [Google Scholar]
- 56.Chudobova D, Cihalova K, Skalickova S, Zitka J, Rodrigo MAM, Milosavljevic V, Hynek D, Kopel P, Vesely R, Adam V & Kizek R 3D-printed chip for detection of methicillin-resistant Staphylococcus aureus labeled with gold nanoparticles. Electrophoresis 36, 457–466 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Mulberry G, White KA, Vaidya M, Sugaya K & Kim BN 3D printing and milling a real-time PCR device for infectious disease diagnostics. PLoS One 12, 1–18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.