Abstract
Naltrexone (NTX), a homologue of the opiate antidote naloxone, is an orally active long-acting mu-opioid receptor (MOP) antagonist used in the treatment of opiate dependence. NTX is also found to relieve craving for alcohol and is one of the few FDA-approved drugs for alcohol use disorder (AUD). Reports that NTX blocks the actions of endogenous opioids released by alcohol are not convincing, suggesting that NTX interferes with alcohol actions by affecting opioid receptors. MOP and kappa-opioid receptor (KOP) are structurally related but functionally different. MOP is mainly located in interneurons activated by enkephalins while KOP is located in longer projections activated by dynorphins. While the actions of NTX on MOP are well established, the interaction with KOP and addiction is not well understood. We used sensitive fluorescence-based methods to study the influence of alcohol on KOP and the interaction between KOP and NTX. Here we report that alcohol interacts with KOP and its environment in the plasma membrane. These interactions are affected by NTX and are exerted both on KOP directly and on the plasma membrane (lipid) structures (“off-target”). The actions of NTX are stereospecific. Selective KOP antagonists, recently in early clinical trials for major depressive disorder, block the receptor but do not show the full action profile of NTX. The therapeutic effect of NTX treatment in AUD may be due to direct actions on KOP and the receptor environment.
INTRODUCTION
Moderate consumption of alcohol (i.e., social drinking) gives a sensation of elatedness and relaxation. This is very different from the drive to binge drinking, to get intoxicated. Whereas the euphoric effects of alcohol have been well studied, much less is known about factors driving to alcohol abuse. We have decided to assess the actions of alcohol at the molecular level, using sensitive methods and fluorescent molecular markers. A focus is naltrexone (NTX) which is approved by the Food and Drug Administration (FDA) for the treatment of alcohol use disorder (AUD). Recently, NTX has been advocated for the treatment of alcohol misuse as “one of the most underutilized interventions in medicine” [1].
Early experimental studies reporting the potential therapeutic effects of NTX in alcohol dependence [2, 3] were followed by clinical studies in human subjects. For example, NTX was found to reduce the feeling of “high” induced by alcohol in alcohol-dependent individuals [4]. However, the response was not universal and it has been suggested that differences in response may be genetically determined. The potential therapeutic effects of NTX for the treatment of AUD eventually led to an FDA approval for this indication, which is significant since very few medications for this condition are available. NTX was initially assumed to counteract alcohol-induced release of endogenous opioids acting on the MOP [5, 6].
It is commonly assumed that AUD is a consequence of the euphoriant activity of alcohol (positive reinforcement) and of craving, the urge to resume consumption in abstinence (negative reinforcement). It has also been proposed that positive reinforcement is mainly exerted via MOP and negative reinforcement is mainly exerted via kappa-opioid receptors (KOP) [7]. Although this model may seem rational for therapeutic developments, there are principal difficulties. In classic binding analysis, NTX is assumed to primarily act on MOP and has lower affinity and activity for KOP. This has been an opening for the clinical use of compounds with overlapping affinities for MOP and KOP such as pentazocine or buprenorphine. Our studies show that NTX has a significant influence on alcohol interactions with KOP – an activity that may be particularly relevant in binge drinking. In a series of publications using sensitive imaging technologies, we have observed that alcohol (ethanol, EtOH) in pharmacologically relevant concentrations directly affects glycosylphosphatidylinositol-enriched membrane domains, MOP, and KOP; these effects were largely blocked by NTX [8, 9]. A behavioral/neurochemistry analysis in mice observed a KOP supersensitivity and a hypodynamic side of the nucleus accumbens [10].
A valuable asset for drug development is the reported structural characterization of the dynorphin/KOP system. The X-ray structure of KOP with the antagonist JDTic was one of the first in the opioid receptor family [11]. The dynamics of the interaction between dynorphin and KOP was followed using nuclear magnetic resonance (NMR) [12]. Membrane lipids have been seen as a catalyst for dynorphin – KOP interaction: membrane attraction results in lower energy needed for the ligand to bind to the receptor [13]. These observations are probably also relevant for the NTX-KOP interaction and may be sensitive to the presence of alcohol. To probe the receptor binding sites, fluorescent NTX has been used previously [14]. Here we used a newly designed fluorescent NTX derivative (fNTX) and a fluorescent protein conjugate of KOP to assess the ethanol effects on KOP and KOP-NTX.
MATERIALS AND METHODS
Chemical reagents
Ethanol (EtOH, purity ≥ 99.5%) and NTX were purchased from VWR and Tocris. Methyl-β-cyclodextrin (mβCD) and Nalfulrafine (NFF) were purchased from Sigma-Aldrich. The less active opioid enantiomer of NTX, (+)-NTX was kindly provided by Dr. Kenner C. Rice [15]. The fluorescent NTX derivative (fNTX) with Alexa Fluor 633 was synthesized as detailed in the Supplement Information (fig. S15). The KOP selective antagonist, LY2444296 was supplied by Eli Lilly. All chemical compounds except for EtOH and (+)-NTX was suspended in dimethyl-sulfoxide (DMSO). (+)-NTX was suspended with MQ water. 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) was conjugated with Abberior Star Red with a polyethylene glycol (PEG) linker (ASR-DOPE) [16]. MemGlow Nail Red 12S (NR12S) for lipid fluidity studies was purchased from Cytoskeleton, Inc.
Cell culture
PC12 cells (American Type Culture Collection) were maintained in a humidified atmosphere containing 5% CO2 at 37°C in RPMI1640 medium (Gibco) supplemented with 10% horse serum (Gibco), 5% fetal bovine serum (Gibco) and 1% penicillin-streptomycin (10,000 U/mL, Gibco). We also transformed PC12 cells with a plasmid DNA encoding human KOP tagged with enhanced green fluorescent protein (PC12/hKOP-eGFP) as described previously [17]. For fluorescence measurements, PC12 cells were seeded in Lab-Tek 8-well chambered cover glass (Thermo Fisher Scientific) with 4.0 ×104 cells/well.
For FLIM/FRAP measurements, PC12 cells were pre-treated at 37°C with antagonists (NTX, (+)-NTX for 30 min; LY2444296 for 15 min) or an agonist (NFF for 30 min), then treated with EtOH + antagonists/agonist for 1 h. The EtOH alone cells were pretreated with vehicle for 30 min, then treated with EtOH for 1 h. For cholesterol depletion, cells were treated for 3 h with 2.5 mM mβCD in serum free medium at 37°C [18]. Antagonists, agonist, mβCD and EtOH were diluted with FluoroBrite RPMI1640 (Gibco).
For dual-color Fluorescence Correlation Spectroscopy (dcFCS) of lipid fluidity and KOP dynamics, PC12 cells were treated with antagonists/EtOH as described above. Cells were further stained with ASR-DOPE for 5 min. To obtain eGFP brightness, untransfected PC12 cells were transfected with 100 ng of plasmid encoding eGFP, peGFP-N1 with 0.2 μL of lipofectamine 2000 (Thermo Fisher Scientific). After the transfection, PC12/eGFP cells were cultured for 24 h.
For Ca2+ imaging, PC12 cells were stained with 10 μM Fura Red in non-serum FluoroBrite RPMI1640 with 0.1% Pluronic F-127 (Invitrogen) for 3 h. Antagonists and EtOH were diluted with Dulbecco’s Phosphate Buffered Saline supplemented with 2.2 mM CaCl2, and 3.5 mM KCl. PC12 cells were treated with antagonists/EtOH as described above.
Microscopic techniques and their data analysis
Detailed descriptions of microscopy techniques and data analysis form a separate chapter in the Supplementary Information.
RESULTS
Impact of ethanol on the KOP-surrounding environment
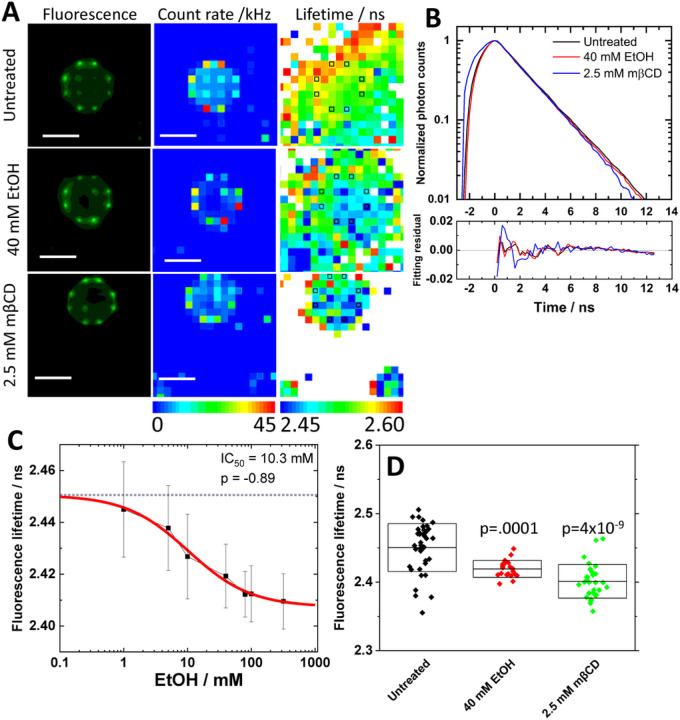
Ethanol is known to affect membrane lipid structures [8, 19, 20]. The size of nano-scale clusters and receptor density of KOP in the plasma membrane are also affected under EtOH treatment [9]. These studies indicate that EtOH may change the environment surrounding KOP and receptor self-organization in the plasma membrane. To further characterize the receptor environment, Fluorescence Lifetime Imaging Microscopy (FLIM) was carried out in PC12 cells expressing KOP-eGFP using our in-house instrument [21]. KOP-eGFP fluorescence was localized in the plasma membrane with and without the treatment of 40 mM EtOH (Fig. 1A, top and middle rows), suggesting EtOH treatment does not induce receptor internalization as observed with agonist (fig. S2A). FLIM curves in the plasma membrane, showed a small change (Fig. 1B). Fluorescence Lifetime (FL) was determined in several positions in the plasma membrane. A spatial FL map indicated shorter FL in the plasma membrane (Fig. 1A, right). This change in FL depended on the EtOH concentration with an IC50 value, 10.3 mM (Fig. 1C). Since dynamic properties and nano-scale clusters of KOP-eGFP are influenced by cholesterol-enriched membrane domains [18], FLIM measurement was performed in the plasma membrane under the cholesterol depletion by 2.5 mM mβCD. FL was significantly decreased (Fig. 1D). This suggests that EtOH affects dose-dependently the receptor-surrounding membrane environment including a change in the cholesterol-enriched membrane domain.
Figure 1. Impact of ethanol on the surrounding environment assessed with fluorescence lifetime (FL) analysis.
(A) Fluorescence images/photon counts map taken with CMOS camera (left column) and spc3 SPAD camera (middle column). FL map (right column) generated by fitting analysis on FLIM curves shown in (B). Black squares indicate plasma membrane positions assigned by photon count map. Scale bar: 10 μm. (B) FLIM curves and fit residuals. Black: untreated, Red: 40 mM EtOH, Blue: 2.5 mM mβCD. (C) Dose response of FL against EtOH concentration. Best fit of dose-response curve determined 10.3 mM and −0.89 as IC50 value and allosteric factor (p), respectively. (D) Average ± Standard deviation of FL under the treatment with 40 mM EtOH and 2.5 mM mβCD. Statistical analysis was performed by two-tailed student’s t-test against untreated.
Specificity of NTX binding to the KOP receptor and influence of EtOH
We have previously observed that NTX enhances formation of a larger nano-scale cluster of KOP [9]. To confirm the NTX effect on KOP in the plasma membrane, FLIM was also performed under the treatment with NTX or NTX + EtOH. NTX decreased FL in a dose-dependent manner, with a IC50 value 7.6 nM (Fig. 2A). To confirm that NTX binding triggered a change in the FL, we also tested two related compounds; 1) the inactive optical isomer, (+)-NTX [22] and 2) the KOP-selective agonist, nalfurafine (NFF) [23]. Each compound caused changes in FL, with the IC50 value 19 μM with (+)-NTX and 0.15 nM with NFF (fig. S1A and S2B). These values coincide with the binding affinity of these compounds to the KOP receptor estimated in other studies [22, 24, 25] (Fig. 2C, black). Interestingly, the dose-response curve with NTX in presence of 40 mM EtOH was shifted to a higher concentration (Fig. 2B), whereas dose dependency disappeared in the treatment with (+)-NTX and 40 mM EtOH (fig. S1B and S1C). On the other hand, 40 mM EtOH did not change the IC50 value of NFF (fig. S2C).
Figure 2. Effect of EtOH on NTX interaction with the KOP receptor.
(A, B) Dose-response of FL under NTX (A) and NTX with 40 mM EtOH (B). Best fit of dose-response curve determined 7.6 nM and 1.0 (A) and 730 nM and −0.66 (B) as IC50 and allosteric factor (p), respectively. (C) Relationship of IC50 value in FL with binding affinity of agonist/antagonist to KOP; Nalfurafine (NFF), naltrexone (NTX) and (+)-NTX, respectively. Reported binding affinity was referred from [22, 24, 25]. Binding affinity of (+)-NTX to KOP was estimated from binding affinity of (+)-NTX to MOP and ratio of binding affinity of NTX. Black: Vehicle, Red: 40 mM EtOH. (D, E) Dose-dependency of fluorescence intensity ratio in the plasm membrane (fNTX/KOP-eGFP) in the absence (D) and presence (E) of 40 mM EtOH. Best fit of dose-response curves determined EC50 and allosteric factor (p). (F) Immobile fraction of KOP-eGFP in the plasma membrane assessed by FRAP. Statistical analysis was performed by two-tailed student’s t-test.
To further address whether the higher IC50 value of FL is caused by the lower binding affinity of NTX in the presence of 40 mM EtOH, a fluorescently labeled NTX (fNTX) was synthesized and tested in PC12 cells (fig. S3A). fNTX binding was reduced under the treatment with 40 mM EtOH, and was blocked by a large excess of non-labeled NTX and the KOP-selective antagonist, LY2444296 (fig. S3A, B), suggesting KOP-specific binding of fNTX in the plasma membrane. Dose-response curves of fluorescence intensity ratio (fNTX/KOP-eGFP) were determined to be 15.4 nM (fNTX) and 93 nM (fNTX + 40 mM EtOH) as EC50 values (Fig. 2D and 2E). NTX binding affinity to KOP was lower under the 40 mM EtOH, in agreement with our FLIM data (Fig. 2A–C). Fluorescence Recovery After Photobleaching (FRAP) analysis demonstrated that the immobile fraction of KOP-eGFP was the same with fNTX and NTX (Fig. 2F), suggesting that fNTX retained similar functionality.
NTX reduces EtOH effect on lipid dynamics
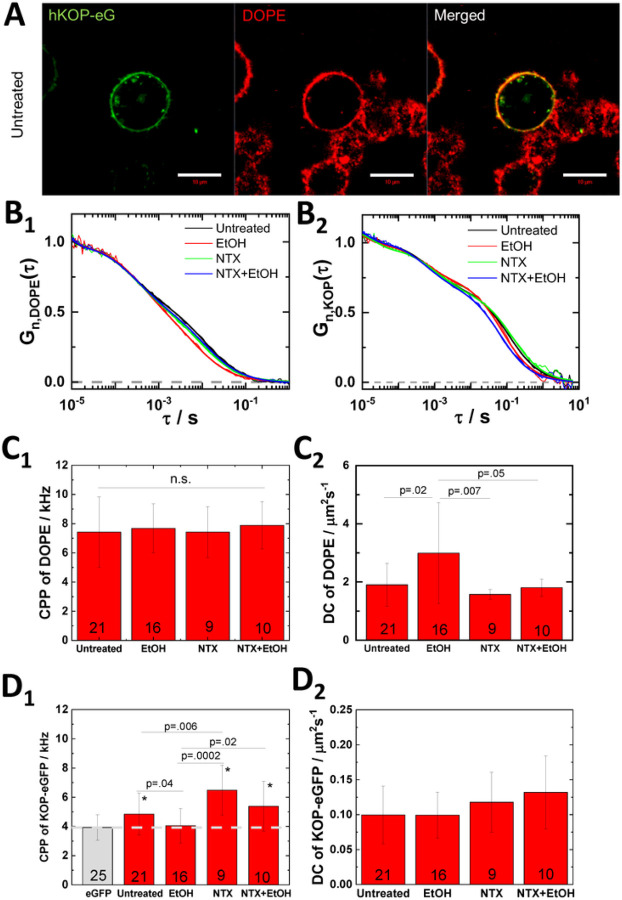
To investigate the EtOH effect on lipid and receptor dynamics, we stained PC12 cells expressing KOP-eGFP with a lipid marker Abberior Star Red-labeled DOPE (ASR-DOPE) which clearly stained the plasma membrane and colocalized with KOP-eGFP in control cells (Fig. 3A) and EtOH-treated cells (fig. S4). Dual-color Fluorescence Correlation Spectroscopy (dcFCS) was carried out in the plasma membrane. Autocorrelation curves recorded with ASR-DOPE (Fig. 3B1) showed two distinct decay curves corresponding to diffusions of ASR-DOPE in the medium and in the plasma membrane, respectively. The counts per particle of ASR-DOPE (CPP; particle brightness in FCS) was not significantly changed under any condition (Fig. 3C1), whereas diffusion in the plasma membrane was significantly faster in the presence of 40 mM EtOH (Fig. 3C2). Pretreatment with 200 nM NTX significantly reduced the EtOH effect (Fig. 3C2). To address the NTX effect on EtOH-modulated lipid dynamics, we also tested (+)-NTX and the selective KOP antagonist, LY2444296 (LY) [26, 27]. As expected from FL measurement (fig. S1A), 200 nM (+)-NTX did not suppress the EtOH effect on lipid dynamics (fig. S5C). To study whether this is an NTX-specific or general effect of KOP antagonists, PC12 cells were treated with 100 nM LY2444296. There was no suppression of the EtOH effect (fig. S6B). This suggests that NTX modulates specifically lipid dynamics in a KOP-unrelated pathway. ASR-DOPE diffusion (fig. S7) and membrane fluidity using General Polarization (GP) analysis (fig. S8) in untransfected PC12 cells supported the EtOH and NTX effect on lipid dynamics. These results indicate that EtOH enhances lipid fluidity and that NTX reduces the EtOH effect in a KOP-independent manner.
Figure 3. NTX reduces EtOH effect on lipid dynamics and KOP oligomerization.
(A) Fluorescence images of untreated PC12 cells expressing KOP-eGFP (KOP-eG, green) and staining with ASR-DOPE (DOPE, red). Scale bar: 10 μm. (B) Autocorrelation curves of ASR-DOPE (B1) and KOP-eGFP (B2) in the plasma membrane under the treatments. (C) Fitting results from ASR-DOPE. Counts per particle (CPP) (C1) and diffusion coefficient (DC) (C2) of membrane-bound component of ASR-DOPE. (D) Fitting results from KOP-eGFP and eGFP (grey). CPP (D1) and DC of slow component (D2) of KOP-eGFP. eGFP was used as brightness standard for the monomeric form of KOP-eGFP. Number of measured single cells is shown at the bottom of bars in (C) and (D). Statistical analysis was performed by two-tailed student’s t-test in (C) and (D). *p<0.01 against eGFP.
Effects of EtOH and NTX on KOP dynamics and dimerization
Receptor homodimerization has key roles for receptor functionality [28–30]. We computed counts per particle (CPP) of KOP-eGFP for analysis of its homodimerization. Recorded in untreated cells, CPP was significantly higher than eGFP (Fig. 3D1), suggesting that KOP is partially homodimerized. EtOH significantly reduced the CPP of KOP-eGFP to the level of eGFP, suggesting that KOP homodimers dissociate to the monomeric state. On the other hand, NTX enhanced KOP homodimerization and suppressed the EtOH effect during combined treatment. This effect was also observed under the treatment with 100 μM (+)-NTX, but not with 200 nM (+)-NTX (fig. S5D). The selective KOP-antagonist, LY2444296 did not interfere with EtOH-induced dissociation of KOP dimer (fig. S6C). We suggest that EtOH and NTX modulate nano-scale cluster formation and number of KOP in the nano-scale cluster, affecting KOP homodimerization.
Our previous data indicate that KOP is non-randomly organized into nano-domains [18]. We also performed total internal reflection microscopy-based FCS (TIR-FCS) to determine the KOP-eGFP confinement in membrane domains by diffusion law analysis (fig. S9D). The intercept of linear regression under 40 mM EtOH was significantly reduced, suggesting that EtOH reduces KOP-eGFP confined to the membrane domains. Diffusion coefficient (DC) was insensitive under each treatment in PC12 cells, whereas significant difference in U2OS cells (Fig. 3D2 and fig. S9C). FRAP analysis addressed a reduction of the immobile fraction of KOP-eGFP with EtOH and an increase of that fraction under the treatment with NTX and NTX + EtOH (fig. S10). This may suggest that membrane domain localization of KOP-eGFP contributes to generate immobile receptor in the plasma membrane and EtOH redistributes KOP-eGFP to mobile fraction.
EtOH and NTX modulate calcium signaling via KOP-dependent and independent pathways
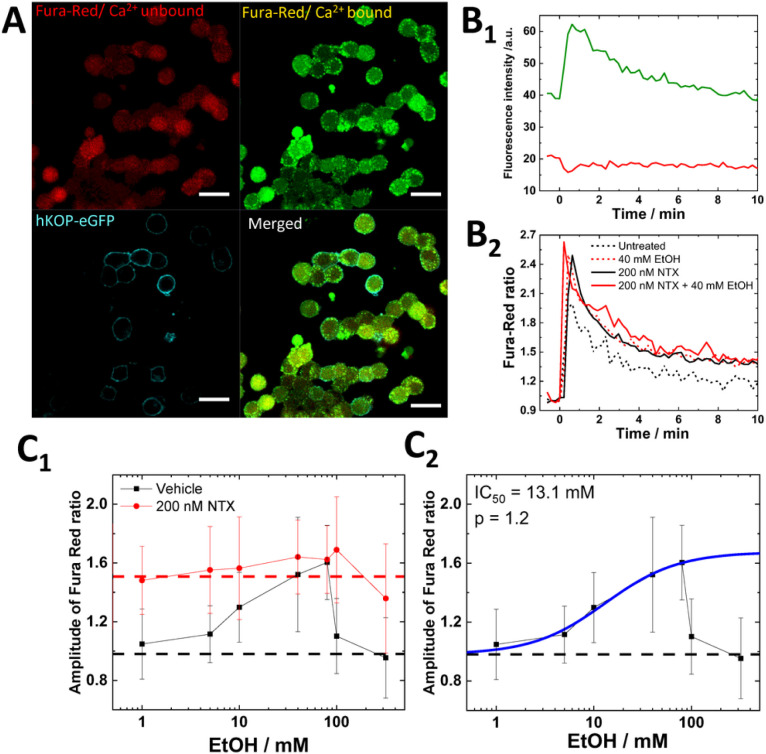
To address the impact of lipid dynamics modulated by EtOH and NTX for the function of KOP, we performed Ca2+ imaging in KOP-transfected cells with Fura Red (Fig. 4A). After K+ depolarization, fluorescence intensity of Ca2+-bound Fura Red dramatically increased while fluorescence intensity of Ca2+-unbound Fura Red was decreased (Fig. 4B1). The Fura Red ratio was calculated as the ratio of Ca2+-bound intensity and Ca2+-unbound intensity (Fig. 4B2). EtOH-treated KOP cells showed dose-dependent increment of the Fura Red ratio up to 80 mM EtOH, followed by a sudden drop to untreated levels with over 100 mM EtOH (Fig. 4C1). The IC50 value was determined to be 13.1 mM EtOH (Fig. 4C2), in good agreement with the IC50 value in FL (10.3 mM) (Fig. 1C). Cholesterol depletion caused similar changes of the Fura Red ratio as EtOH treatment (fig. S11), suggesting that the EtOH modulated Ca2+ influx via the modulation of the environment of KOP-eGFP including deformation of cholesterol-enriched membrane domains. To clarify whether this was a KOP-mediated pathway or not, Ca2+ imaging was performed in untransfected PC12 cells, showing a higher Fura Red ratio in untreated (black dashed line) compared with EtOH-untreated KOP-expressing cells (Fig. 4C1) and a gradual decrease of Fura Red ratio (fig. S12). This is in good agreement with previous studies that a L-type channel is inhibited and a non-L-type channel is partially inhibited by EtOH [31]. This suggests that KOP inhibits Ca2+ signaling at a basal level and that the enhancement of Ca2+ influx with EtOH treatment is KOP-mediated. To clarify the impact of NTX on Ca2+ influx, we pre-treated KOP-expressing cells and untransfected cells with 200 nM NTX. EtOH-untreated KOP-expressing cells showed higher Fura Red ratio and a constant Fura Red ratio even under the over 100 mM EtOH conditions (Fig. 4C1). The constant Fura Red ratio was also confirmed in untransfected cells (fig. S12). These data suggest that NTX affects live cells in two aspects; 1) direct binding to KOP in the plasma membrane and 2) KOP-unmediated modulation of cholesterol-enriched membrane domains which is in good agreement with our FCS data (Fig. 3). To further characterize the two effects of NTX on Ca2+ influx, we tested (+)-NTX (fig. S13) and LY2444296 (fig. S14). 200 nM (+)-NTX clearly showed no effect on Ca2+ influx (fig. S13, red), while 100 μM (+)-NTX slightly increased Ca2+ uptake without EtOH treatment, following similar changes under the co-treatment with EtOH (fig. S13, blue). LY2444296 (100 nM) was only found to enhance Ca2+ influx as a direct KOP antagonistic effect (Fig S14), via direct binding to KOP, but no KOP-unmediated effect (Fig. S6). Altogether FL data (Figs. 1 and 2) and FCS data (Fig. 3), suggest that EtOH modulates Ca2+ influx via changes of membrane environment, in particular deformation of cholesterol-enriched membrane domains. NTX does not only show the KOP-mediated antagonistic effects on Ca2+ influx, but also KOP-unmediated effects linking lipid dynamics and the receptor surrounding environment.
Figure 4. EtOH and NTX modulate calcium signaling via KOP-dependent and -independent pathways.
Fluorescence images of untreated PC12 cells expressing KOP-eGFP and staining with Fura Red. Red: Ca2+-unbound Fura Red, Green: Ca2+-bound Fura Red, Cyan: KOP-eGFP. Scale bar: 20 μm. (B1) Time-series of fluorescent intensity of Fura Red during K+ depolarization. Green: Ca2+-bound Fura Red, Red: Ca2+-unbound Fura Red. (B2) Fura Red ratio normalized before the K+ stimulation. Black dashed line: Untreated, Red dashed line: 40 mM EtOH, Black solid line: 200 nM NTX, Red solid line: 200 nM NTX and 40 mM EtOH. (C1) Dose-dependency of amplitude of Fura Red ratio. Black: vehicle with EtOH, Red: 200 nM NTX with EtOH. Dashed line: mean value under the vehicle without EtOH (black) and with the 200 nM NTX alone (red). (C2) Best fit of dose-response curve (blue) to the data with EtOH gave 13.1 mM and 1.2 as IC50 and allosteric factor (p), respectively.
DISCUSSION
Alcohol abuse and dependence remain the most significant substance abuse problems worldwide and in the US alone, more than 140,000 people are dying from alcohol-related causes annually [32]. There is a choice whether psychotherapy or medication have superiority for treatment, the majority of cases with AUD receive no treatment at all.
Earlier studies of medication in AUD have been subjected to a meta-analysis, showing the NTX and acamprosate are superior to placebo; NTX is particularly effective in heavy drinking and prevention of recurrence [33]. A more recent survey supports the efficacy of NTX. Moreover, effectiveness of extended-release NTX medication [34] was recently demonstrated. However, NTX-based prescriptions were primarily given to higher income males with private insurance, leaving women and minorities without such intervention [35]. A certain rise in AUD have been recorded during the covid-19 pandemic [36], prompting an editorial advocating higher rate of NTX prescriptions [1].
Behavioral effects mediated by KOP differ markedly from those of the other opioid receptors, MOP and the delta-opioid receptor (DOP). Kappa-agonists are not self-injected and clinical use is compromised by psychotomimetic side effects. It has even been proposed that the overt euphorigenic effects of MOP and DOP pathways are related to positive reinforcement whereas effects on KOP are balancing and related to the negative reinforcement (craving) [37]; both effects have been related to AUD.
NTX has found a therapeutic niche for the treatment of AUD and is one of the very few medications that can be prescribed for this indication. One characteristic of AUD in humans is that dependent subjects will consume alcohol to relieve or avoid withdrawal symptoms. Similarly, in preclinical studies, alcohol postdependent rats exhibit an alcohol dependence syndrome that is characterized by both somatic and motivational withdrawal symptoms that usually begin after 6 to 8 hours of abstinence and engage in excessive drinking when alcohol is made available again. Using a rat model of alcohol dependence (i.e, chronic intermittent alcohol vapor exposure) we showed that NTX decreased alcohol intake in nondependent rats, regardless of sex and abstinence time point [38]. In postdependent rats, NTX significantly decreased the exaggerated alcohol intake only at a delayed abstinence time point (i.e., 6 weeks) in males, whereas it similarly reduced alcohol drinking in females at 8 h, 2 weeks, and 6 weeks abstinence time points. These findings further support targeting the endogenous opioid system to prevent excessive drinking that is characteristic of AUD, even after long periods of abstinence and further suggest that alcohol dependence causes neuroadaptations [38].
The access of a fluorescent derivative of NTX was a priority in the study. While fluorescent NTX derivatives have been described before [14], the strategy here was to extend the separation of the fluorescent marker to NTX by a longer linker, considering the X-ray analysis data showing that the JDTic binds in a deep pocket [11] and NMR analysis of the KOP/dynorphin interaction [12].
NTX has been reported to have lower affinity for KOP as compared to MOP and may therefore not be considered a KOP antagonist. However, under our conditions, affinity is strong and more in line with previous studies using competition assays in transfected cell cultures that identified approximately equal affinity of NTX for KOP and MOP [39]. Constitutive activity and inverse agonism was also observed, which increased after agonist pretreatment [39]. To approach the effects of alcohol on both MOP and KOP at a molecular level, we introduced high-resolution molecular imaging with FCS to follow the dynamics in cell culture of MOP and KOP labeled with fluorescent tags. The addition of pharmacologically relevant concentrations of EtOH influenced their lateral movements in the plasma membrane [40]. Significantly, EtOH-induced effects showed differences between MOP and KOP, with higher presence of MOP in the membrane, whereas KOP presence declined. Differences related to EtOH-induced effects were also observed with super-resolution microscopy [8, 9].
In our studies, we have also used high-resolution technologies to investigate the effects of EtOH on both MOP and KOP. As expected, NTX blocks the activation of MOP. In cell culture at the ultrastructure level, EtOH affects the distribution of both MOP and KOP (induces the formation of smaller and less occupied MOP and KOP nanodomains) [9]. These studies also revealed that NTX induces formation of larger and more occupied KOP nanodomains and that NTX pretreatment has protective effects against EtOH-induced changes in nano-organization of both receptors [9].
Another approach to the specificity of the studies effects is the use of stereoisomers. The (+) isomer of NTX ((+)-NTX) was available to us. This isomer has its own pharmacologic profile and shows equipotent binding for MOP and the toll-like receptor TR4, and interacts with opioid (morphine) analgesia [41, 42] as well as drug reward [43] The results are clear, the (+) isomer is much less active demonstrating that the NTX effects we observe are mediated by interaction with KOP and not due to off-target effects (Fig. 2).
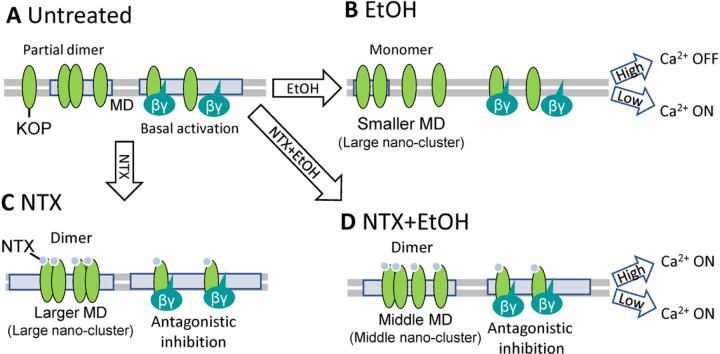
Based on this work, we have developed a model of direct/indirect actions of NTX to KOP (Fig. 5). In untreated condition, KOP has constitutive activity. It is partly in a homodimer form and causes Ca2+ channel inhibition. EtOH treatment dissociates KOP to monomers and induces deformation of cholesterol-enriched membrane domains. Since the Ca2+ channel may be effectively inhibited by KOP in the cholesterol-enriched membrane domains, Ca2+ influx is enhanced under the lower EtOH condition (1 ~ 80 mM). On the other hand, higher EtOH concentration (> 100 mM) distorts the membrane structure and induces Ca2+ channel inhibition.
Figure 5. Modeling interaction of NTX, ethanol (EtOH) and the kappa opioid receptor (KOP).
(A) KOP diffuses freely and partially localizes in the cholesterol-enriched membrane domains (MDs) and forms partially homodimers. MD-localized KOP is constitutively active and inhibits a Ca2+ channel. (B) Under treatment with EtOH, KOP dissociates to monomers in the fluidic plasma membrane. The Ca2+ channel is ON-state under lower EtOH concentration (0 mM – 80 mM). The Ca2+ channel may be effectively inhibited in MDs. Under high EtOH concentration (> 100 mM), Ca2+ channel may be inhibited generally. (C) Under treatment with NTX, larger nano-scale KOP clusters are formed*. NTX-liganded KOP forms preferentially homodimers in the larger MDs. Ca2+ channel is released from constitutive inhibition of KOP by NTX. (D) EtOH-modulated lipid dynamics is suppressed by NTX. Middle-sized of nano-scale clusters are formed. NTX also plays a role of the antagonist at KOP, thus the Ca2+ channel is released by KOP inhibition. * Model based on present data and superresolution analysis [9].
Under the NTX condition, KOP forms larger nano-clusters and KOP is preferably present as homodimers. NTX also shows antagonistic effect on KOP, following the enhancement of Ca2+ influx. These NTX effects are sustained even with EtOH. Both NTX and EtOH affect the cholesterol-enriched membrane domains (KOP nano-clusters) as observed in previous study [9]. With EtOH cholesterol-enriched membrane domains of intermediate size are formed, and KOP is preferentially in homodimers even though to a lesser extent than with NTX alone. The Ca2+ influx remains the same in the whole EtOH concentration range, and lipid dynamics does not change. The EtOH-induced enhancement of Ca2+ influx was suppressed by NTX. Also, EtOH-induced distortion of Ca2+ influx under higher EtOH concentration was inhibited by NTX.
For comparison, we also included a known KOP antagonist a homologue of JNJ-67953964, LY2444296, and a NTX-related agent, nalfurafine, a KOP agonist recently introduced in the treatment of itch (as developed in patients receiving opiates chronically) [44]. We confirm that nalfurafine induces KOP internalization as a KOP agonist and like the natural ligand dynorphin. The observed activity of the LY2444296 confirmed KOP antagonism and as is shown here, NTX and LY2444296 share binding sites.
It can be noted that KOP has been identified as one of the strongest genetic linkages in major depressive disorder along with the D2R (dopamine receptor 2) [45]. It is noticeable that there is a striatonigral dynorphin pathway reciprocal to the classic nigrostriatal dopamine pathway [46, 47]. The close connection between two potentially relevant neurotransmitter systems may be an indication of a functional relationship. A recent “fast-fail” study of JNJ-67953964 (a.k.a. CERC-501 and LY2456302) in major depressive disorder showed activity in anhedonia [48]. It is now in Phase III trial as Aticaprant. Another chemically distinct KOP antagonist BTRX-335140, Navacaprant is in Phase II clinical trial in depression [49]. To our knowledge, there are so far no clinical studies of KOP antagonists in AUD.
Concluding remarks
NTX is an analog of naloxone – a well-known opiate antidote in emergencies. NTX was developed as a long-acting MOP antagonist to protect against further intoxication. Its activity in AUD has been hard to conceptualize. As shown here, NTX has potent activity on KOP as an inverse agonist and takes actions on MOP. These receptors are structurally related. However, the neuronal pathways with both receptors are functionally highly different and there is no reason to see them as connected. A formal model with alcohol activating MOP (and euphoria) has been linked to release of endogenous opioids (enkephalins). The KOP system is seen as the origin of craving (and release of dynorphin peptides). Our model suggests that alcohol acts directly on KOP and that NTX acts as an inverse agonist blocking constitutive and alcohol-induced activity. Binge drinking (to intoxication) which is of medical concern is very different from social alcohol use [50].
The interactions of alcohol and NTX and the opioid receptors, particularly MOP, have been studied with a variety of technologies [6, 51]. The current data illustrates that the KOP receptor is also a relevant target.
ACKNOWLEDGEMENTS
We gratefully acknowledge the supply of (+) NTX from Dr. Kenner Rice, Drug Design and Synthesis Section, MTMDB, was supported by the NIH Intramural Research Programs of the NIDA and the NIAAA, Bethesda, MD. Compound LY2444296 was generously donated by Eli Lilly and Company with help from Dr. Linda Rorick-Kehn. We thank Dr. Barbara Mason for valuable comments regarding an earlier version of this paper.
FUNDING
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (grant no. AA006420, AA026999 to RM-F, and AA028549 to LT, TJ-T and RM-F).
S.O. acknowledges the financial support by Nakatani Foundation for Advancement of Measuring Technologies in Biomedical Engineering and the Strategic Research Program in Neuroscience (StratNeuro) at the Karolinska Institutet. Research reported in this publication included work performed in the City of Hope Drug Discovery and Structural Biology Core supported by the National Cancer Institute of the National Institutes of Health under grant number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Statement
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (grant no. AA006420, AA026999 to RM-F, and AA028549 to LT, TJ-T and RM-F).
S.O. acknowledges the financial support by Nakatani Foundation for Advancement of Measuring Technologies in Biomedical Engineering and the Strategic Research Program in Neuroscience (StratNeuro) at the Karolinska Institutet. Research reported in this publication included work performed in the City of Hope Drug Discovery and Structural Biology Core supported by the National Cancer Institute of the National Institutes of Health under grant number P30CA033572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The authors have no competing interests.
Supplementary Files
Contributor Information
Lars Terenius, Karolinska Institutet.
Sho Oasa, Karolinska Institutet.
Erdinc Sezgin, Karolinska Institute.
Remi Martin-Fardon, The Scripps Research Institute.
Vladana Vukojevic, Karolinska Institute.
DATA AND MATERIALS AVAILABILITY
Raw data can be provided for investigations from S.O.
References
- 1.Avery J. Naltrexone and Alcohol Use. Am J Psychiatry 2022; 179: 886–87. [DOI] [PubMed] [Google Scholar]
- 2.Altshuler HL, Phillips PE and Feinhandler DA. Alteration of ethanol self-administration by naltrexone. Life Sci 1980; 26: 679–88. [DOI] [PubMed] [Google Scholar]
- 3.Volpicelli JR, Davis MA and Olgin JE. Naltrexone blocks the post-shock increase of ethanol consumption. Life Sci 1986; 38: 841–7. [DOI] [PubMed] [Google Scholar]
- 4.Volpicelli JR, Watson NT, King AC, Sherman CE and O’Brien CP. Effect of naltrexone on alcohol “high” in alcoholics. Am J Psychiatry 1995; 152: 613–5. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell JM et al. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med 2012; 4: 116ra6. [DOI] [PubMed] [Google Scholar]
- 6.Nutt DJ. The role of the opioid system in alcohol dependence. J Psychopharmacol 2014; 28: 8–22. [DOI] [PubMed] [Google Scholar]
- 7.Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 2003; 27: 232–43. [DOI] [PubMed] [Google Scholar]
- 8.Tobin SJ et al. Nanoscale effects of ethanol and naltrexone on protein organization in the plasma membrane studied by photoactivated localization microscopy (PALM). PLoS One 2014; 9: e87225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tobin SJ, Wakefield DL, Terenius L, Vukojevic V and Jovanovic-Talisman T. Ethanol and Naltrexone Have Distinct Effects on the Lateral Nano-organization of Mu and Kappa Opioid Receptors in the Plasma Membrane. ACS Chem Neurosci 2019; 10: 667–76. [DOI] [PubMed] [Google Scholar]
- 10.Rose JH et al. Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. Int J Neuropsychopharmacol 2016; 19: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu H et al. Structure of the human kappa-opioid receptor in complex with JDTic. Nature 2012; 485: 327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor C et al. NMR structure and dynamics of the agonist dynorphin peptide bound to the human kappa opioid receptor. Proc Natl Acad Sci U S A 2015; 112: 11852–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sargent DF and Schwyzer R. Membrane lipid phase as catalyst for peptide-receptor interactions. Proc Natl Acad Sci U S A 1986; 83: 5774–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolb VM, Koman A and Terenius L. Fluorescent probes for opioid receptors. Life Sci 1983; 33 Suppl 1: 423–6. [DOI] [PubMed] [Google Scholar]
- 15.Selfridge BR et al. Structure-Activity Relationships of (+)-Naltrexone-Inspired Toll-like Receptor 4 (TLR4) Antagonists. J Med Chem 2015; 58: 5038–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider F et al. Diffusion of lipids and GPI-anchored proteins in actin-free plasma membrane vesicles measured by STED-FCS. Mol Biol Cell 2017; 28: 1507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vukojevic V et al. Mu-opioid receptor activation in live cells. FASEB J 2008; 22: 3537–48. [DOI] [PubMed] [Google Scholar]
- 18.Rogacki MK et al. Dynamic lateral organization of opioid receptors (kappa, muwt and muN40D) in the plasma membrane at the nanoscale level. Traffic 2018; 10.1111/tra.12582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein DB. Effect of alcohol on cellular membranes. Ann Emerg Med 1986; 15: 1013–8. [DOI] [PubMed] [Google Scholar]
- 20.Sergent O et al. Role for Membrane Fluidity in Ethanol-Induced Oxidative Stress of Primary Rat Hepatocytes. J. Pharmacol. Exp. Ther. 2005; 313: 104–11. [DOI] [PubMed] [Google Scholar]
- 21.Oasa S et al. Dynamic Cellular Cartography: Mapping the Local Determinants of Oligodendrocyte Transcription Factor 2 (OLIG2) Function in Live Cells Using Massively Parallel Fluorescence Correlation Spectroscopy Integrated with Fluorescence Lifetime Imaging Microscopy (mpFCS/FLIM). Anal Chem 2021; 93: 12011–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theberge FR et al. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry 2013; 73: 729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagase H, Imaide S, Hirayama S, Nemoto T and Fujii H. Essential structure of opioid kappa receptor agonist nalfurafine for binding to the kappa receptor 2: synthesis of decahydro(iminoethano)phenanthrene derivatives and their pharmacologies. Bioorg Med Chem Lett 2012; 22: 5071–4. [DOI] [PubMed] [Google Scholar]
- 24.Nagase H and Fujii H. Essential structure of the kappa opioid receptor agonist nalfurafine for binding to the kappa receptor. Curr Pharm Des 2013; 19: 7400–14. [DOI] [PubMed] [Google Scholar]
- 25.Yamagami C et al. Quantitative structure-activity relationship analyses of antioxidant and free radical scavenging activities for hydroxybenzalacetones. Bioorg Med Chem Lett 2004; 14: 5629–33. [DOI] [PubMed] [Google Scholar]
- 26.Rorick-Kehn LM et al. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology 2014; 77: 131–44. [DOI] [PubMed] [Google Scholar]
- 27.Valenza M, Butelman ER and Kreek MJ. “Effects of the novel relatively short-acting kappa opioid receptor antagonist LY2444296 in behaviors observed after chronic extended-access cocaine self-administration in rats”. Psychopharmacology (Berl) 2017; 234: 2219–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan BA and Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature 1999; 399: 697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milligan G, Ward RJ and Marsango S. GPCR homo-oligomerization. Curr Opin Cell Biol 2019; 57: 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cechova K et al. Kappa but not delta or mu opioid receptors form homodimers at low membrane densities. Cell Mol Life Sci 2021; 78: 7557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walter HJ and Messing RO. Regulation of neuronal voltage-gated calcium channels by ethanol. Neurochem Int 1999; 35: 95–101. [DOI] [PubMed] [Google Scholar]
- 32.Centers for Dsease Control and Prevention (CDC). Alcohol and Public Health: Alcohol-Related Disease Impact. https://nccd.cdc.gov/DPH_ARDI/Default/Report.aspx?T=AAM&P=612EF325-9B55-442B-AE0C-789B06E3A8D5&R=C877B524-834A-47D5-964D-158FE519C894&M=DB4DAAC0-C9B3-4F92-91A5-A5781DA85B68&F=&D=].
- 33.Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K and Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction 2013; 108: 275–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy CEt, Wang RC, Montoy JC, Whittaker E and Raven M. Effect of extended-release naltrexone on alcohol consumption: a systematic review and meta-analysis. Addiction 2022; 117: 271–81. [DOI] [PubMed] [Google Scholar]
- 35.Qeadan F et al. Trends in the Use of Naltrexone for Addiction Treatment among Alcohol Use Disorder Admissions in U.S. Substance Use Treatment Facilities. Int J Environ Res Public Health 2021; 18: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos GM et al. Targeted Oral Naltrexone for Mild to Moderate Alcohol Use Disorder Among Sexual and Gender Minority Men: A Randomized Trial. Am J Psychiatry 2022; 179: 915–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koob GF and Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci 2005; 8: 1442–4. [DOI] [PubMed] [Google Scholar]
- 38.Matzeu A, Terenius L and Martin-Fardon R. Exploring Sex Differences in the Attenuation of Ethanol Drinking by Naltrexone in Dependent Rats During Early and Protracted Abstinence. Alcohol Clin Exp Res 2018; 42: 2466–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D, Sun X and Sadee W. Different effects of opioid antagonists on mu-, delta-, and kappa-opioid receptors with and without agonist pretreatment. J Pharmacol Exp Ther 2007; 321: 544–52. [DOI] [PubMed] [Google Scholar]
- 40.Vukojevic V et al. Ethanol/naltrexone interactions at the mu-opioid receptor. CLSM/FCS study in live cells. PLoS One 2008; 3: e4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hutchinson MR et al. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal 2007; 7: 98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X et al. Pharmacological characterization of the opioid inactive isomers (+)-naltrexone and (+)-naloxone as antagonists of toll-like receptor 4. Br J Pharmacol 2016; 173: 856–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Northcutt AL et al. DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol Psychiatry 2015; 20: 1525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schattauer SS, Kuhar JR, Song A and Chavkin C. Nalfurafine is a G-protein biased agonist having significantly greater bias at the human than rodent form of the kappa opioid receptor. Cell Signal 2017; 32: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levey DF et al. Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in >1.2 million individuals highlight new therapeutic directions. Nat Neurosci 2021; 24: 954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.You ZB et al. The striatonigral dynorphin pathway of the rat studied with in vivo microdialysis--I. Effects of K(+)-depolarization, lesions and peptidase inhibition. Neuroscience 1994; 63: 415–25. [DOI] [PubMed] [Google Scholar]
- 47.You ZB et al. The striatonigral dynorphin pathway of the rat studied with in vivo microdialysis--II. Effects of dopamine D1 and D2 receptor agonists. Neuroscience 1994; 63: 427–34. [DOI] [PubMed] [Google Scholar]
- 48.Krystal AD et al. A randomized proof-of-mechanism trial applying the ‘fast-fail’ approach to evaluating kappa-opioid antagonism as a treatment for anhedonia. Nat Med 2020; 26: 760–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Study in major depressive disorder with BTRX-335140 vs placebo. ClinicalTrials.gov Identifier: NCT04221230. Ongoing study; [Google Scholar]
- 50.Koob GF and Colrain IM. Alcohol use disorder and sleep disturbances: a feed-forward allostatic framework. Neuropsychopharmacology 2020; 45: 141–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris LS et al. Naltrexone ameliorates functional network abnormalities in alcohol-dependent individuals. Addict Biol 2018; 23: 425–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data can be provided for investigations from S.O.