Abstract
The conventional wisdom that chimeric RNAs being peculiarity of carcinoma, and the products of chromosomal rearrangement is being challenged, However, experimental evidence supporting chimeric RNAs in normal physiology being functional is scarce. We decided to focus on one particular chimeric RNA, CTNNBIP1-CLSTN1. We examined its expression among various tissues and cell types, and compared quantitatively among cancer and non-cancer cells. We further investigated its role in a panel of non-cancer cells and probed the functional mechanism. We found that this fusion transcript is expressed in almost all tissues, and a wide range of cell types including fibroblasts, epithelial, stem, vascular endothelial cells, and hepatocytes. The expression level in non-cancerous cell lines is also not evidently different from that in the cancer cell lines. Furthermore, silencing CTNNBIP1-CLSTN1 significantly reduces cell proliferation rate, by inducing G2/M arrest in cell cycle progress and apoptosis in at least three cell types. Importantly, rescue experiments confirmed that the cell cycle arrest can be regained by exogenous expression of the chimera, but not the wild type parental gene. Further evidence is provided that CTNNBIP1-CLSTN1 regulates cell proliferation through SERPINE2. Thus, CTNNBIP1-CLSTN1 represents an example of a new class of fusion RNA, dubbed “housekeeping chimeric RNAs”.
1. BACKGROUND
The discovery of the BCR-ABL fusion gene by Nowell et al. (1) during 1960s, as a result of chromosomal translocation in chronic myelogenous leukemia, has provided a strategy to those who are in search of gene fusions involved in various neoplasm(2, 3). Even since then, the prevailing view is that gene fusions are cancer specific, and they then make fusion products (RNAs and proteins), which can be also used as specific biomarkers and drug targets (4, 5). This assumption has resulted in the explosion of Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer in the Cancer Genome Anatomy Project. However, this very dogma has been challenged, when more and more studies and researches led to the continuous finding of chimeric RNAs in the normal physiology, especially with the availability of a large number of transcriptome sequencing (RNA-seq) databases (6). The prevalence of chimeric RNA has so far exceeds our expectations (7–9), with several characteristic modalities(7, 10), and, at least some of them also play key roles in various settings (7, 8, 11, 12). In particular, a group of chimeric RNAs are suggested to serve a housekeeping role, in that their expression are generally ubiquitous, and are indispensible for cell survival and maintenance.
We previously implemented and analyzed approximately 300 RNA sequencing libraries established from 30 different non-neoplastic human tissues and cell lines, which led to the discovery of 9,778 chimeric RNAs that are potential products of cis-splicing of adjacent genes (cis-SAGe) or RNA trans-splicing (7). In that study, the great majority of the chimeric RNAs are tissue-specific, and around 10% occur in more than one sample, of which 51 in more than five different samples. Among these recurrent chimeras, CTNNBIP1-CLSTN1 was found in a large number of tissues, and seemed to be indispensible for the culture of an immortalized astrocyte cell line (7). In addition, we demonstrated that it is a cis-SAGe chimeric RNA(13), composed of two neighboring parental genes located on chr.1p36 (7, 13), and induced when transcription factor CTCF is silenced (9). However, the exact role the chimeric RNA plays, and the mechanism of its functionality are not clear.
We hypothesize the existence of a group of chimeric RNAs functioning as “housekeeping” chimeric RNAs, i.e., playing an universal role in maintaining physiological functions in various non-cancer human cell types. In this study, we aim to investigate whether CTNNBIP1-CLSTN1 chimeric RNA represents a member of this new class of chimeric RNAs, and to further investigate its downstream pathway. We first confirmed the presence of this transcript in different types of tissues and cells, and from these, three different cell lines were further selected to study the role of the chimera related to cell proliferation, migration and apoptosis. Transcriptome sequencing and downstream analyses were further conducted to uncover its functional mechanism.
2. METHODS
2.1. Cell culture, siRNA and transfections
HEK-293T, HUVEC, and LO2 cells were maintained in DMEM/HIGH GLUCOSE medium with 4500 mg/L Glucose and 4.0 mM L-Glutamine (HyClone™, USA), supplemented with 10% fetal bovine serum (LONSERA, Uruguay), and 1% penicillin and streptomycin (HyClone™, USA). The cells were incubated in 37°C, 5% CO2, with medium changes every other day. Cells were digested by 0.25% trypsin with 1g/L EDTA (HyClone™, USA). siRNAs were synthesized by Sangon Biotech (Shanghai, China) and transfected with Lipofectamine RNAiMAX (Life Technologies, USA) following the manufacture’s protocols. Transfection efficiencies were evaluated 48 hours after siRNA transfection.
The targeting sequences are:
si-Negative Control, CGTACGCGGAATACTTCGA;
siCTNNBIP1, GGAAGAGTCCGGAGGAGAT;
siCTNNBIP1-CLSTN1, TGCTTGTTAACCTGGTCGA.
2.2. RNA extraction, quantitative reverse transcription PCR (qRT-PCR) and sanger sequencing
RNA was extracted from cells with BEI-BEI BIOTECH Total RNA Isolation Kit (Zhengzhou, China) and reverse-transcribed by TIANGEN FastKing cDNA Kit (Beijing, China) according to the manufacture’s instructions. Primers used for the fusion have been described earlier (13). qRT-PCR was carried out using the ABI StepOne Plus Real-Time PCR system (Life Technologies) with TB Green® Premix Ex Taq™ (TaKaRa, Japan), and followed by gel electrophoresis with Gel-Red™ Nucleic Acid Gel Stain (Biotium, California, USA). Data were analyzed using ΔΔCt statistical method, with the expressions was normalized against internal control GAPDH. Axygen® AxyPrep DNA Gel Extraction Kit (USA) was used for DNA purification, and followed by Sanger sequencing validation at Sangon Biotech.
The primer sequences for qRT-PCR are:
WT-CTNNBIP1:F:5’-CTCATGCTGCGGAAGATGGGAT-3’; R:5’-CTGGAAAACGCCATCACCACGT-3’;
FN1: F:5’-CCACCCAATGTTCAGCTCAC-3’; R:5’-GTAGCATCTGTCACACGAGC-3’;
SERPINE2: F:5’-CTTCCTCTTGGCCTCTGTGA-3’; R:5’-ACGCCGTATCTCATCACCAT-3’
2.3. Vector construction of CTNNBIP1-CLSTN1 and plasmid transfection
The full length cDNA of CTNNBIP1-CLSTN1, and the wild type CTNNBIP1 open reading frame were cloned into pCDNA3.1 plasmid vector system and verified by Sanger sequencing. For transient expression, the plasmids were transfected into cells with Lipofectamine 2000 (Life Technologies, USA) following the manufacture’s instruction.
2.4. Cell proliferation assay
HEK-293T, HUVEC and LO2 were seeded on 96-well plates with appropriate confluence. 72 hours after siRNAs or plasmids transfections, cells proliferation was measured with DOJINDO Cell Counting Kit-8 (Japan) and incubated at 37°C, 5% CO2 for two hours. Cell viability in each well was determined by O.D. value at 450 nm.
2.5. Rescue experiment
For CCK8 assay, HEK-293T cells were seeded in 96-well plates with 2,000 per well. Cells were first transfected with pCDNA3.1 plasmids expressing CTNNBIP1-CLSTN1, CTNNBIP1, SERPINE2, FN1 or empty control respectively, all plasmids were synthesized by Genscript and confirmed by Sanger sequencing; 24 hours later, the cells were transfected with siCTNNBIP1-CLSTN1, siCTNNBIP1 or NC; 48 hours after siRNA transfection, 10 μl CCK8 was added in each well and incubated for two hours at 37°C, 5% CO2. Cell viability in each well was determined by O.D. value at 450 nm. In live cell imaging, HEK-293T were seeded and transfected the same way as cell proliferation assay, then the plates were placed under a live-cell-imaging microscope (JULI Stage, NanoEnTek, Seoul, South Korea) after siRNA transfection for 60 hours. Images were captured every six hours for measurement of cell proliferation. A total of 10 cycles were captured. Image analysis was performed for cell proliferation analysis using JULI Stat (NanoEnTek, Seoul, South Korea), and a cell growth curve was plotted according to the cell growth density.
2.6. Wound healing assay
HEK-293T, HUVEC and LO2 cells were seeded in 6-well plates and transfected with siRNAs or plasmids with proper reagents. Around 48 hours after transfections, when cell confluence reached more than 90%, a scratch was made by manual scratching longitudinally on the bottom of each well with a 10 μl plastic pipette tip. The plates were rinsed twice with PBS, and replaced with fresh DMEM complete media. After that, the plates were placed under a live-cell-imaging microscope (JULI Stage, NanoEnTek, Seoul, South Korea) and incubated at 37°C, 5% CO2. Images were captured every two hours and the gap distance after the scratch was documented, with a total of 12 cycles captured. Picture analysis was performed by Wound healing analysis using JULI Stat (NanoEnTek, Seoul, South Korea). A wound density curve according to the percentage of cell migration measured as the recovered wound area relative to the original wound area was plotted.
2.7. Flow cytometry
72 hours after siRNA transfections, HEK-293T, HUVEC and LO2 cells were digested with 0.25% trypsin and washed twice with PBS, and cell density was adjusted to 1×106 cells/ml. Next, the cells were fixed with pre-cooled 70% alcohol overnight at 4°C, washed twice with PBS, and treated with 500 μl propidium iodide (PI) (KeyGEN BioTECH, Nanjing, China) reaction mixture containing RNase under dark conditions for 60 minutes at room temperature. Cell cycle stages were detected by flow cytometer (FACS Calibur, Becton, Dickinson and Company, New Jersey, USA) and analyzed by Flow Jo software. For cell apoptosis detection, the cells were collected and washed as above, then mixed with 500 μl binding buffer containing 5 μl Annexin V-FITC and 5 μl PI stain (KeyGEN BioTECH, Nanjing, China), reacted in dark place for 15 minutes at room temperature. The flow cytometer was applied for detecting cell apoptosis and analyzed by Flow Jo.
2.8. Cell fractionation
HEK-293T, HUVEC and LO2 cells were digested with 0.25% trypsin, washed once with PBS, then separated into two fractions using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Fisher, USA) following the manufacturer’s instructions. RNA from each part was extracted respectively with BEI-BEI BIOTECH Total RNA Isolation Kit, followed by qRT-PCR with MALAT1 and GAPDH as controls.
3. RESULTS
3.1. CTNNBIP1-CLSTN1 is widely expressed among human tissues and cell types.
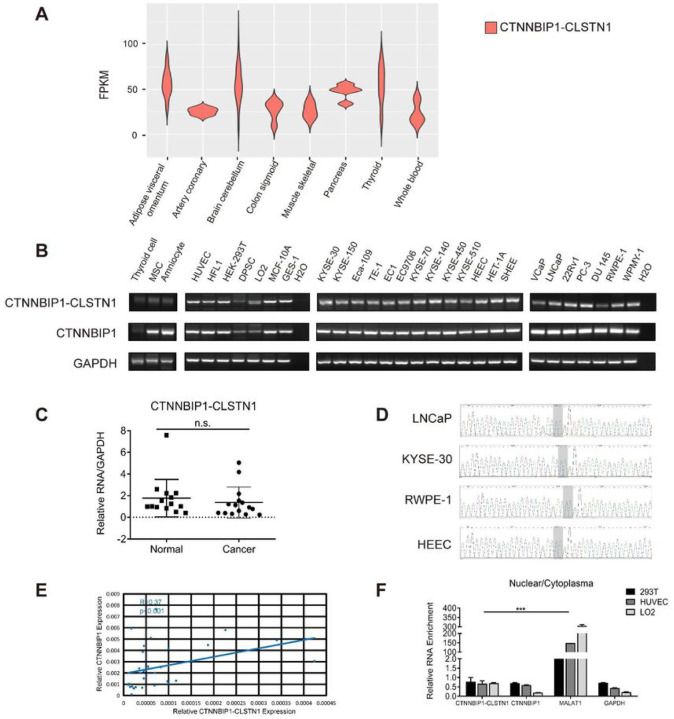
Previously, we identified 291 recurrent chimeric RNAs by analyzing RN-Seq data from around 300 non-cancer tissues and cells (7). CTNNBIP1-CLSTN1 is one of them, detected by RT-PCR in liver, lung, kidney etc. We recently expanded our chimeric RNA search to the whole Genotype-Tissue Expression (GTEx), which contains 9,495 non-diseased human tissue samples from 53 different tissues (14, 15). From this study, we noticed that CTNNBIP1-CLSTN1 was detected in almost all samples (Fig. 1A and Fig. S1).
Figure 1. The expression of CTNNBIP1-CLSTN1 in various human tissues and cell lines.
(A) The expression of CTNNBIP1-CLSTN1 in whole Genotype-Tissue Expression (GTEx). FPKM of the chimeric RNA in various tissues were plotted. (B) RT-PCR detecting the expression of CTNNBIP1-CLSTN1 in several human cell lines, followed by gel-electrophoresis. Internal control, GAPDH, and wild type parental gene CTNNBIP1 were also included. (C) The expression level of the chimera was measured in 15 non-cancer cell lines and 15 cancer cell lines using qRT-PCR. The expression of the fusion was normalized against internal control, GAPDH. (D) Sanger sequencing of RT-PCR products in cancer cell lines, LNCaP, KYSE-30, and non-cancer cell lines, RWPE-1, HEEC. Light gray area marks the fusion junction site. (E) Correlation between the relative expression level of fusion and CTNNBIP1 was plotted (R=0.37, P<0.001). (F) HEK-293T, HUVEC and LO2 cells were fractioned into nuclear and cytoplasmic parts. Expression of the chimera and CTNNBIP1 in each part were measured by qRT-PCR. GAPDH and known long non-coding RNA MALAT1 were used as controls. Ratios of expression in nuclear and cytoplasm parts were plotted. Asterisks indicate statistical significance: *P < 0.05. **P < 0.01.***P < 0.001.
We then performed RT-PCR to detect the fusion and its parental genes in 15 non-cancer cell lines belonging to 12 different tissue of origins, including cell types of fibrocytes, amniocytes, epithelial cells, stem cells, and vascular endothelial cells, followed by gel-electrophoresis (Fig. 1B). CTNNBIP1-CLSTN1 was discovered in all the samples. Using qRT-PCR, we quantified its expression in 15 non-cancer cells, and 15 cancer cell lines of esophageal and prostate cancer. There was no statistically significant difference in the expression level between cancer and normal lines (Fig. 1C, and Fig. S2-S3). The junction sequence was shown exact the same among different cancer cell lines, LNCaP and KYSE-30, and non-cancer lines, RWPE-1 and HEEC by Sanger sequencing (Fig. 1D). We also monitored the wild type parental transcripts using primers covering at least one exon not shared with the fusion transcript. Even though the wild type CTNNBIP1 transcript was readily detectable, the wild type CLSTN1 was not. We also found that the relative expressions of the chimeric fusion and CTNNBIP1 are positively correlated (Pearson’s correlation R = 0.37, P < 0.001) (Fig. 1E, and Fig. S2-S3), consistent with the chimeric RNA being a product of 5’ gene read-through, and suggesting that the great majority of CLSTN1 transcripts is used to form chimeric RNA.
This chimeric RNA is predicted to encode an in-frame chimeric protein. Here, we decided to take advantage of the fact that regular protein-coding mRNAs are mainly present in the cytoplasm, while long-non-coding RNAs often reside in the nucleus to regulate transcription (16–18). We chose human embryonic kidney cell HEK-293T, human umbilical vein endothelial cell HUVEC, and hepatocyte line LO2 cells for fractionation assay and extracted RNA to detect the chimeric RNA by qRT-PCR. Classic protein-coding gene GAPDH and long non-coding RNA MALAT1 were used as controls. Not surprisingly, MALAT1 was found mostly in the nuclear fraction. On the contrary, CTNNBIP1-CLSTN1, wild type CTNNBIP1 and GAPDH were all enriched in the cytoplasmic part, (Fig. 1F). This observation is consistent with the previous experiment of Western blot (7), further supporting that the fusion has a protein-coding function.
3.2. Silencing CTNNBIP1-CLSTN1 reduced cell proliferation and cell motility
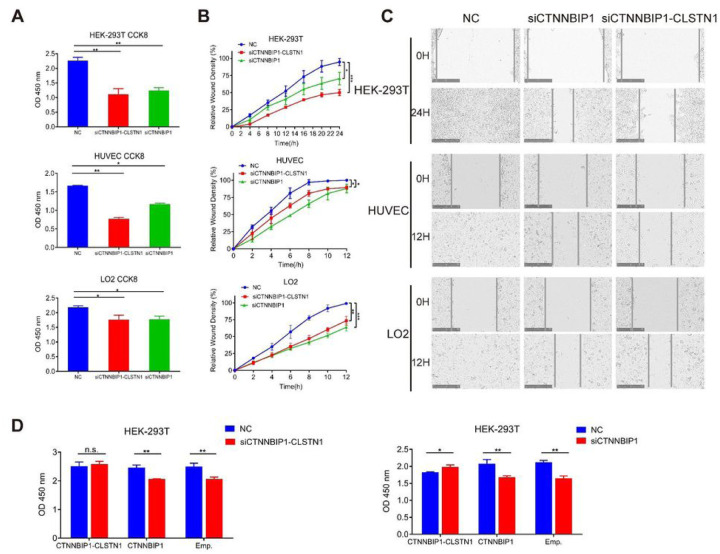
Based on its ubiquitous expression pattern, we hypothesized that the chimeric RNA may belong to the group of chimeric RNAs, which we call “housekeeping chimeric RNAs”. Indeed, previous report has documented its indispensible role in immortalized astrocytes (7). To further investigate the function of CTNNBIP1-CLSTN1, and support its basic role of principle cell maintenance, we transfected siRNAs targeting the chimera in multiple human non-cancer cell lines, HEK-293T, HUVEC and LO2 cell lines. The siRNA, siCTNNBIP1-CLSTN1 targeting the fusion junction sequence resulted in significant reduction of the fusion transcript, but not the wild-type CTNNBIP1 in all three cell lines (Fig. S4 and S5). Since, we could not design a siRNA that specifically silences the wild type CTNNBIP1, as a control, we designed a siRNA, siCTNNBIP1 that targets the common region sequence in the fusion and wild type CTNNBIP1. As predicted, this siRNA lead to the silencing of both the fusion and wild type CTNNBIP1 (Fig. S5). Both siCTNNBIP1-CLSTN1 and siCTNNBIP1 significantly decreased cell proliferation based on CCK8 measurement (Fig. 2A). Cell migration was monitored by wound healing assay with live-cell-imaging microscopy after scratching. The percent relative wound density was calculated by measuring the density of cells that migrated into the original wound. Both siCTNNBIP1-CLSTN1 and siCTNNBIP1 significantly inhibited cell migration compared to the negative control (Fig. 2B). Figure 2C shows representative microscopic images of cells across a wound at zero and 12 hours in HUVEC and LO2 cells, and zero and 24 hours in HEK-293T cells. These results suggest that CTNNBIP1-CLSTN1 plays a significant role in general cell growth and movement, regardless of cell types.
Figure 2. Knocking down CTNNBIP1-CLSTN1 results in significance reduction in cell proliferation and cell motility in HEK-293T, HUVEC and LO2 cell lines.
(A) CCK8 assay was used to measure the cell proliferation rate 72 hours after siRNA transfection. Both siRNAs resulted in significant cell growth suppression in three cell lines. (B) Both siRNAs resulted in significant reduction of cell motility in three cell lines. (C) Representative microscopy images of cells transfected NC, siCTNNBIP1-CLSTN1 and siCTNNBIP1 showed movement across a wound at zero (upper panel) and 12 hours (lower panel) in HUVEC and LO2, zero (upper panel) and 24 hours (lower panel) in HEK-293T. Scale bars represent 250 μm. (D) In a rescue experiment, HEK-293T were transfected with plasmid expressing CTNNBIP1-CLSTN1, CTNNBIP1 or empty control, and 24 hours later, the cells were transfected with siCTNNBIP1-CLSTN1 or siCTNNBIP1 and negative control (NC). Cell viability was measured by CCK8 after 48 additional hours. Asterisks indicate statistical significance: *P < 0.05. **P < 0.01.***P < 0.001.
To rule out the off-target effect of siRNA, we performed rescue experiments by transfecting in the construct of CTNNBIP1-CLSTN1 expressing plasmid and construct encoding the wild type CTNNBIP1. The reduced cell proliferation was indeed rescued by the CTNNBIP1-CLSTN1 construct in both siCTNNBIP1-CLSTN1 and siCTNNBIP1 groups. In contrast, the wild type CTNNBIP1 failed to rescue in either groups (Fig. 2D), suggesting the effect of reduced cell proliferation was caused by the fusion silencing, but the wild type CTNNBIP1.
3.3. Silencing CTNNBIP1-CLSTN1 resulted in cell cycle arrest and apoptosis
To investigate the mechanism of the reduced cell proliferation caused by silencing the fusion, we performed flow cytometry to evaluate cell cycle with propidium iodide staining after siRNA transfection. Cell numbers in G2/M phase were notably increased in both siRNA groups that silenced the fusion, compared to that in the NC group (Fig. 3A). This G2/M arrest was observed in all three cell lines (Fig. 3B).
Figure 3. Knocking down CTNNBIP1-CLSTN1 resulted in G2/M cell cycle arrest and apoptosis.
(A) Cell cycle stages 72 hours after siRNAs transfection were examined by flow cytometry and analyzed by FlowJo. Representative pictures were shown. (B) Silencing CTNNBIP1-CLSTN1 induced G2/M arrest. (C) Cell apoptosis was examined at 72 hours after siRNAs transfection by flow cytometry and analyzed by FlowJo. Representative pictures were shown. (D) Silencing CTNNBIP1-CLSTN1 induced apoptosis. Data were presented as mean±SD. Asterisks indicate statistical significance: *P < 0.05.
We then evaluated cell apoptosis using Annexin V-FITC and propidium iodide staining. The results from the flow cytometry assay showed that siCTNNBIP1 and siCTNNBIP1-CLSTN1 transfection noticeably increased apoptosis in all three cell lines, compared to the control (Fig. 3C and Fig. 3D).
3.4. Overexpression of CTNNBIP1-CLSTN1, but not the wild type CTNNBIP1, promotes cell growth and cell migration
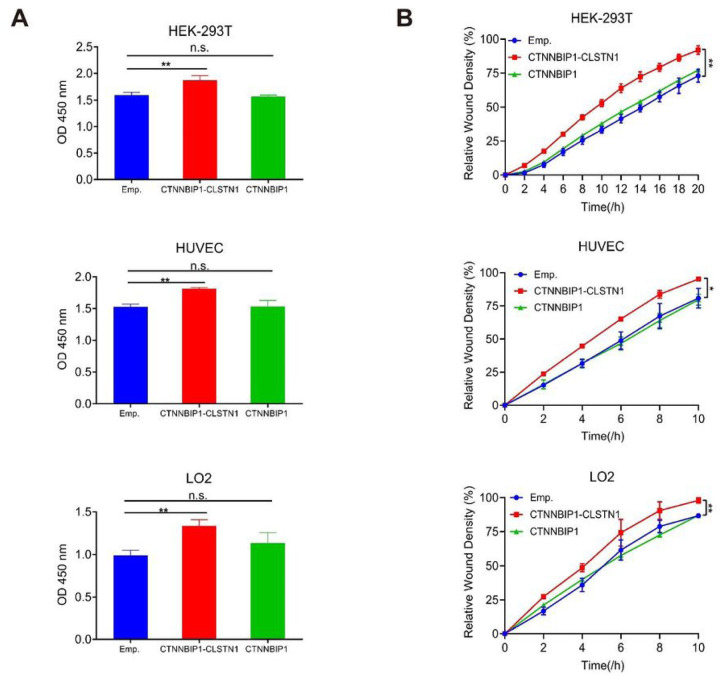
In this experiment, HEK-293T, HUVEC, and LO2 cells were transfected with either pCDNA3.1 plasmid that encodes the full-length CDS of CTNNBIP1-CLSTN1, or CTNNBIP1, or the empty vector respectively. In contrast to the loss-of-function experiments, CCK8 assay showed that the expression of the chimera, but not the wild type CTNNBIP1, promoted cell proliferation in all three cell lines (Fig. 4A). Consistently, wound healing assay also demonstrated that the overexpression of CTNNBIP1-CLSTN1, but not CTNNBIP1 enhanced the cell migration ability in all three cell lines (Fig. 4B).
Figure 4. Overexpression of CTNNBIP1-CLSTN1, but not wild type CTNNBIP1, promotes cell proliferation and cell motility.
(A) HEK-293T, HUVEC and LO2 were transfected with pCDNA3.1 plasmids transcribing full-length coding regions of CTNNBIP1-CLSTN1 (CTNNBIP1-CLSTN1), CTNNBIP1 (CTNNBIP1) or empty vector (Emp.). CCK8 was used to measure the cell proliferation rate 72 hours after plasmid transfection. Overexpression of the fusion promoted cell proliferation rate, whereas overexpression of the wild type CTNNBIP1 had no such effect. (B) Overexpression of the fusion also promoted cell motility, whereas overexpression of the wild type CTNNBIP1 had no such effect.
3.5. CTNNBIP1-CLSTN1 influences cell proliferation by regulating autocrine signaling factors SERPINE2
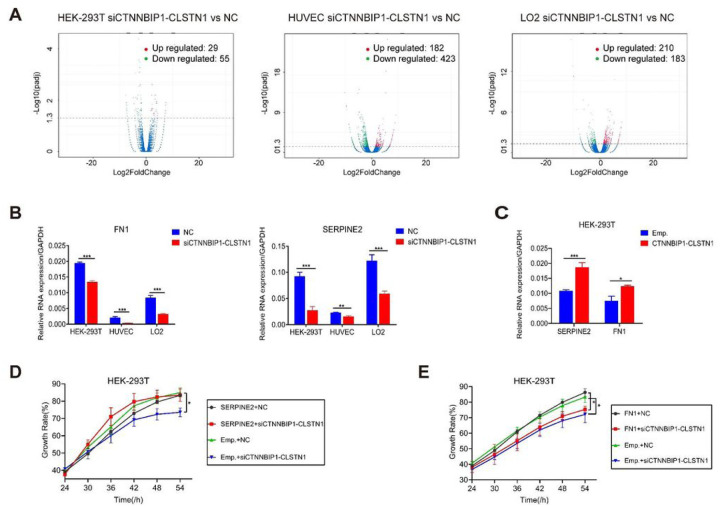
To investigate the downstream pathway mediating the effect of CTNNBIP1-CLSTN1 on cell proliferation, we performed transcriptome sequencing analysis of HEK-293T, HUVEC and LO2 cells transfected with siCTNNBIP1-CLSTN1 and negative control siRNA. Differential expression analysis between the two groups in three cell lines was conducted, obtaining 84, 605, and 393 differentially expressed genes (DEGs) (Supplementary Table 1), respectively. Volcano plot of DEGs in three cell lines are shown in Fig. 5A (padj < 0.05). Gene Ontology (GO) terms for DEGs were also examined. 135 enriched GO terms in HEK-293T, 286 enriched GO terms in HUVEC, and 137 enriched GO terms in LO2 were found (Supplementary Table 1). Several terms showed unconformity in different cells, and were consistent with the general role the fusion plays. However, there are also specific GO terms unique to individual cell lines, suggesting that the fusion may have additional roles that are cell type specific. The top 13 for HEK-293T, the top 23 for HUVEC, and the top 14 for LO2 in the GO term list are displayed (Fig. S6).
Figure 5. CTNNBIP1-CLSTN1 regulates cell proliferation through SERPINE2.
(A) Volcano plots of DEGs in HEK-293T, HUVEC, and LO2. Red dots indicate significantly up-regulated genes. Green dots indicate significantly down-regulated genes. Blue dots indicate no significant difference (P value<0.05). (B) qRT-PCR detecting expression of FN1 and SERPINE2 at 48 hours after siRNAs transfection. Silencing CTNNBIP1-CLSTN1 significantly suppressed FN1 and SERPINE2. (C) Overexpression of CTNNBIP1-CLSTN1 increased the expression of FN1 and SERPINE2. (D) In a rescue experiment, the reduced cell growth rate caused by siCTNNBIP1-CLSTN1 was recovered by transfecting the cells with SERPINE2 expression plasmid. (E) Overexpression of FN1 had no such rescue effect. The horizontal axis represents the time after plasmid transfection. Asterisks indicate statistical significance: *P < 0.05. **P < 0.01.***P < 0.001.
Out of the DEGs, Fibronectin 1 (FN1) and Serine protease inhibitor E2 (SERPINE2) stand out, owing to the fact that they both were down-regulated in siCTNNBIP1-CLSTN1 group of all three cell lines. Furthermore, they were included in several GO terms related to cell growth (GO:0001558, GO:0040008, and GO:0016049). Fibronectin 1(FN1)(19, 20), is a member of the glycoprotein family that is widely expressed by multiple cell types (GTEx data, Fig. S7A). Serine protease inhibitor E member 2 (SERPINE2) (21, 22), also called Protease Nexin-1(PN-1) belongs to the Serpin gene super family, is also ubiquitously expressed in human tissues and cells (GTEx, Fig. S7B). We first confirmed their expression by qRT-PCR (Fig. 5B). Both were reduced upon the silencing of the fusion. In contrast, overexpression of CTNNBIP1-CLSTN1 up-regulated both genes (Fig. 5C). We then investigated whether the effect of the fusion is mediated by these two genes. To do so, we transfected in expression plasmids encoding FN1 or SERPINE2 in cells with siCTNNBIP1-CLSTN1 or siCT. As indicated in Fig. 5D, SERPINE2 can restore the cell proliferation rate to a normal level. In contrast, FN1 failed to rescue the reduced cellular growth induced by siCTNNBIP1-CLSTN1 (Fig. 5E), suggesting that the effect of the chimeric RNA on cell proliferation is mostly mediated by SERPINE2.
4. DISCUSSION
In recent years, exploration of gene fusions, and chimeric RNAs in various carcinomas has progressed by leaps and bounds (23). Numerous examples were found by high throughput approaches, including microarray and next generation sequencing, for instance, TMPRSS2-ETS (24, 25) and D2HGDH-GAL3ST2 (26) in prostate cancer, LHX6-NDUFA8 and SLC2A11-MIF in cervical cancer (27), GOLM1-MAK10 in esophageal squamous cell carcinoma (ESCC) (28), EML4-ALK in non-small cell lung (NSCLC) (29), CHFR-GOLGA3 in Bladder cancer (30), RRM2-C2orf48 in colorectal cancer (CRC) (31), and ASTN2-PAPPAas in esophageal cancer (32). Most of these gene fusions are significantly over-expressed in cancer, compared to non-cancer tissues and cells. In addition, some of them were verified to have clinical correlations between their expression level and cancer stage and/or patient survival (26). Without doubt, they represent effective markers for clinical diagnosis/prognosis, and/or drug targets.
However, in the recent years, continuous discoveries have demonstrated that chimeric RNA is not a unique phenomenon to cancer, nor is it all due to the composition of gene fusion. It is widespread in normal human tissues and cells (7, 8, 33–36). JAZF1-JJAZ1 is observed in endometrial stromal cells, and instead of chromosome rearrangement, it is derived from RNA trans-splicing there (37). Similarly DUS4L-BCAP29, which is a product of cis-splicing of adjacent genes exists not only in prostate cancer and gastric cancer as previously reported (38, 39), but is also present in various normal tissues (40). In a previous study, 291 fusion transcripts were found by analyzing nearly 300 RNA-Seq libraries, with CTNNBIP1-CLSTN1 being observed in five non-neoplastic tissues (7). In another study, Singh et al. explored the landscape of chimeric RNAs in 9,495 Genotype-Tissue Expression (GTEx) samples and established a dataset containing a total of 7,193 chimeric RNAs (8), where CTNNBIP1-CLSTN1 being detected in the majority of samples. The results we presented here provide further evidences that CTNNBIP1-CLSTN1 is commonly expressed in miscellaneous cell types, and to a similar level in normal and cancer cells. Furthermore, this fusion also plays basic physiological roles that sustain cell growth, and cell migration. These evidences support the classification of this fusion as a member of housekeeping chimeric RNAs.
Transcriptome sequencing exploration connects the fusion with downstream targets including FN1, and SERPINE2. FN1 is an adhesive glycoprotein of the extracellular matrix with a variety of binding domains for cell surface and extracellular ligands (19, 20), and involved in some biological processes including cell vitality and apoptosis through these multiple interaction sites (41, 42). SERPINE2 is an extracellular plasminogen activator inhibitor with the effect of inhibiting various protease activities (21, 22), and function in many physiological processes including inflammation, cell growth and metastasis (43, 44). They are both ubiquitously expressed in human tissues and cells (Fig. S7). Rescue experiments confirmed that the expression of SERPINE2 can restore the cell proliferation to a normal level, suggesting its role in mediating the effect of CTNNBIP1-CLSTN1 on cell proliferation. However, the expression of FN1 failed to have any rescue effect on cell proliferation, suggesting that it may play other roles (Fig. 6).
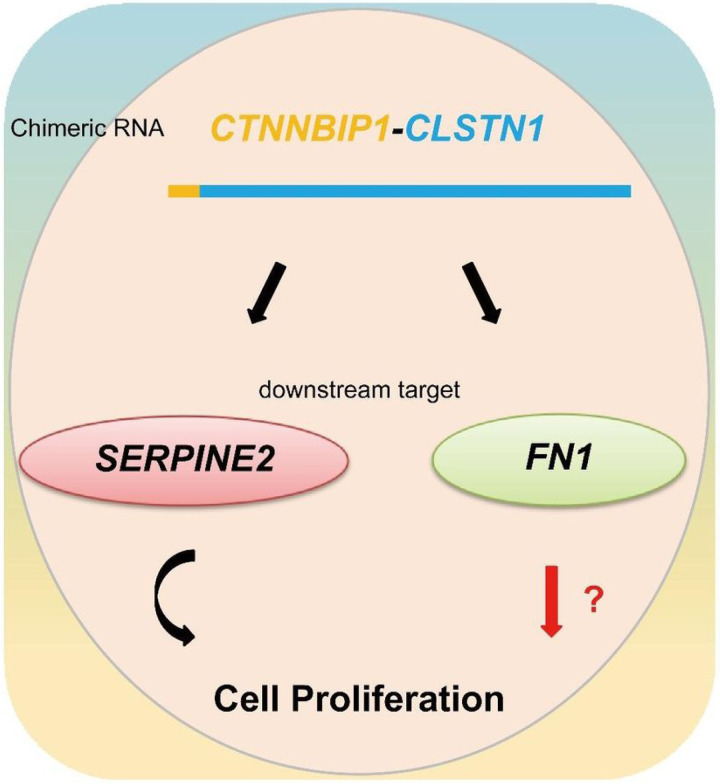
Figure 6. An illustration of chimeric RNA CTNNBIP1-CLSTN1, and its functional mechanism.
CTNNBIP1-CLSTN1 regulates the expression of two downstream target genes, SERPINE2 and FN1. Silencing the fusion reduces cell proliferation rate, which can be rescued by SERPINE2, but not FN1. It is possible that FN1 mediates other cell physiological roles of the fusion.
Besides the common DEGs among the three different cell models, there are groups of differentially expressed genes that are found in only two or one cell models, suggesting that the fusion may also have cell-specific roles. The exact mechanism of how the fusion RNA regulates the expression of its downstream genes remains unclear, and will be the directions of our future studies.
CONCLUSION
In summary, CTNNBIP1-CLSTN1 is ubiquitously expressed in normal and cancer cells. It regulates cell proliferation in various cell types, and thus represents a new class of RNA, housekeeping chimeric RNA. We believe more of such chimeric RNAs exist. Their presence challenges the traditional dogma that chimeric fusion RNAs are cancer specific, and sounds the alarm for the practice of rushing fusion RNAs discovered from cancer tissue/cells into biomarkers and/or therapeutic targets.
ACKNOWLEDGEMENTS
We thank Sangeen Khan for his help on editing of the manuscript.
Funding
Y.T. is supported by Key R&D and Promotion Project (No.202102310097) in Henan Province, China. H.L. is supported by NIGMS grant GM132138.
LIST OF ABBREVIATIONS
- cis-SAGe
GTEx, ESCC, NSCLC, CRC
Funding Statement
Y.T. is supported by Key R&D and Promotion Project (No.202102310097) in Henan Province, China. H.L. is supported by NIGMS grant GM132138.
Footnotes
Competing Interests
The authors declare no competing interests.
Supplementary Files
Contributor Information
Chen Chen, School of Basic Medical Sciences.
Yue Tang, Zhengzhou University.
Fujun Qin, Zhengzhou University.
Sandeep Singh, University of Virginia.
Availability of Data and Materials
The Raw and processed RNA-Sequencing data from this study have been submitted to the NCBI Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE165479. The security token for reviewers to access the data before it is publicly available is gjqlgkamtxgltgn.
Flow cytometry data has been deposited into Flow Repository. The ID and URL with secret code for reviewers are listed below:
Cell cycle: FR-FCM-Z3E3
http://flowrepository.org/id/RvFrm5qG1S7akXGqSKb85uWhbRTZ0ACn5uCIPoGiulAygdG2Lq0SnQRYez7L5oHB
Cell apoptosis: FR-FCM-Z3E4
http://flowrepository.org/id/RvFrzD1pnAaXP44GVUKW0wVl1QimdHNOKR1hXyc07ao0xIZODy0tzrEgOykrnMUg
All the other data supporting the findings of this study are available within the article and its supplementary information files and from the corresponding author upon reasonable request. A reporting summary for this article is available as a Supplementary Information file.
REFERENCES
- 1.Nowell P.C. (1962) The minute chromosome (Phl) in chronic granulocytic leukemia. Blut, 8, 65–66. [DOI] [PubMed] [Google Scholar]
- 2.Rabbitts T.H. (1994) Chromosomal translocations in human cancer. Nature, 372, 143–149. [DOI] [PubMed] [Google Scholar]
- 3.Rowley J.D. (1999) The role of chromosome translocations in leukemogenesis. Seminars in hematology, 36, 59–72. [PubMed] [Google Scholar]
- 4.Druker B.J., Talpaz M., Resta D.J., Peng B., Buchdunger E., Ford J.M., Lydon N.B., Kantarjian H., Capdeville R., Ohno-Jones S. et al. (2001) Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. The New England journal of medicine, 344, 1031–1037. [DOI] [PubMed] [Google Scholar]
- 5.Shaw A.T., Yeap B.Y., Solomon B.J., Riely G.J., Gainor J., Engelman J.A., Shapiro G.I., Costa D.B., Ou S.H., Butaney M. et al. (2011) Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. The Lancet. Oncology, 12, 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin J.Z., Berger M.F., Adiconis X., Rogov P., Melnikov A., Fennell T., Nusbaum C., Garraway L.A. and Gnirke A. (2009) Targeted next-generation sequencing of a cancer transcriptome enhances detection of sequence variants and novel fusion transcripts. Genome biology, 10, R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babiceanu M., Qin F., Xie Z., Jia Y., Lopez K., Janus N., Facemire L., Kumar S., Pang Y., Qi Y. et al. (2016) Recurrent chimeric fusion RNAs in non-cancer tissues and cells. Nucleic acids research, 44, 2859–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh S., Qin F., Kumar S., Elfman J., Lin E., Pham L.P., Yang A. and Li H. (2020) The landscape of chimeric RNAs in non-diseased tissues and cells. Nucleic acids research, 48, 1764–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin F., Song Z., Babiceanu M., Song Y., Facemire L., Singh R., Adli M. and Li H. (2015) Discovery of CTCF-sensitive Cis-spliced fusion RNAs between adjacent genes in human prostate cells. PLoS genetics, 11, e1005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim R.N., Kim A., Choi S.H., Kim D.S., Nam S.H., Kim D.W., Kim D.W., Kang A., Kim M.Y., Park K.H. et al. (2012) Novel mechanism of conjoined gene formation in the human genome. Functional & integrative genomics, 12, 45–61. [DOI] [PubMed] [Google Scholar]
- 11.Yuan H., Qin F., Movassagh M., Park H., Golden W., Xie Z., Zhang P., Sklar J. and Li H. (2013) A chimeric RNA characteristic of rhabdomyosarcoma in normal myogenesis process. Cancer discovery, 3, 1394–1403. [DOI] [PubMed] [Google Scholar]
- 12.Jividen K. and Li H. (2014) Chimeric RNAs generated by intergenic splicing in normal and cancer cells. Genes, chromosomes & cancer, 53, 963–971. [DOI] [PubMed] [Google Scholar]
- 13.Chwalenia K., Qin F., Singh S. and Li H. (2019) A cell-based splicing reporter system to identify regulators of cis-splicing between adjacent genes. Nucleic acids research, 47, e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ardlie K.G., DeLuca D.S., Segre A.V., Sullivan T.J., Young T.R., Gelfand E.T., Trowbridge C.A., Maller J.B., Tukiainen T., Lek M. et al. (2015) The Genotype-Tissue Expression (GTEx) pilot analysis: Multitissue gene regulation in humans. Science, 348, 648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lonsdale J., Thomas J., Salvatore M., Phillips R., Lo E., Shad S., Hasz R., Walters G., Garcia F., Young N. et al. (2013) The Genotype-Tissue Expression (GTEx) project. Nature genetics, 45, 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown C.J., Hendrich B.D., Rupert J.L., Lafreniere R.G., Xing Y., Lawrence J. and Willard H.F. (1992) The human XIST gene: analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell, 71, 527–542. [DOI] [PubMed] [Google Scholar]
- 17.Hutchinson J.N., Ensminger A.W., Clemson C.M., Lynch C.R., Lawrence J.B. and Chess A. (2007) A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC genomics, 8, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sone M., Hayashi T., Tarui H., Agata K., Takeichi M. and Nakagawa S. (2007) The mRNA-like noncoding RNA Gomafu constitutes a novel nuclear domain in a subset of neurons. Journal of cell science, 120, 2498–2506. [DOI] [PubMed] [Google Scholar]
- 19.Pankov R. and Yamada K.M. (2002) Fibronectin at a glance. Journal of cell science, 115, 3861–3863. [DOI] [PubMed] [Google Scholar]
- 20.Gao W.W., Liu Y., Qin R.L., Liu D.J. and Feng Q.Q. (2016) Silence of fibronectin 1 increases cisplatin sensitivity of non-small cell lung cancer cell line. Biochemical and biophysical research communications, 476, 35–41. [DOI] [PubMed] [Google Scholar]
- 21.Valiente M., Obenauf A.C., Jin X., Chen Q., Zhang X.H., Lee D.J., Chaft J.E., Kris M.G., Huse J.T., Brogi E. et al. (2014) Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell, 156, 1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heit C., Jackson B.C., McAndrews M., Wright M.W., Thompson D.C., Silverman G.A., Nebert D.W. and Vasiliou V. (2013) Update of the human and mouse SERPIN gene superfamily. Human genomics, 7, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar-Sinha C., Kalyana-Sundaram S. and Chinnaiyan A.M. (2015) Landscape of gene fusions in epithelial cancers: seq and ye shall find. Genome Med, 7, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomlins S.A., Laxman B., Varambally S., Cao X., Yu J., Helgeson B.E., Cao Q., Prensner J.R., Rubin M.A., Shah R.B. et al. (2008) Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia, 10, 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomlins S.A., Rhodes D.R., Perner S., Dhanasekaran S.M., Mehra R., Sun X.W., Varambally S., Cao X., Tchinda J., Kuefer R. et al. (2005) Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science, 310, 644–648. [DOI] [PubMed] [Google Scholar]
- 26.Qin F.J., Song Z.G., Chang M., Song Y.S., Frierson H. and Li H. (2016) Recurrent cis-SAGe chimeric RNA, D2HGDH-GAL3ST2, in prostate cancer. Cancer Lett, 380, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu P., Yang S., Singh S., Qin F., Kumar S., Wang L., Ma D. and Li H. (2018) The Landscape and Implications of Chimeric RNAs in Cervical Cancer. EBioMedicine, 37, 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H., Lin W., Kannan K., Luo L., Li J., Chao P.W., Wang Y., Chen Y.P., Gu J. and Yen L. (2013) Aberrant chimeric RNA GOLM1-MAK10 encoding a secreted fusion protein as a molecular signature for human esophageal squamous cell carcinoma. Oncotarget, 4, 2135–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soda M., Choi Y.L., Enomoto M., Takada S., Yamashita Y., Ishikawa S., Fujiwara S., Watanabe H., Kurashina K., Hatanaka H. et al. (2007) Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature, 448, 561–566. [DOI] [PubMed] [Google Scholar]
- 30.Zhu D. and Li H. (2020) Case Study: Landscape of Chimeric RNAs in Bladder Cancer. Methods Mol Biol, 2079, 233–241. [DOI] [PubMed] [Google Scholar]
- 31.Wu H., Singh S., Xie Z., Li X. and Li H. (2020) Landscape characterization of chimeric RNAs in colorectal cancer. Cancer Lett, 489, 56–65. [DOI] [PubMed] [Google Scholar]
- 32.Wang L., Xiong X., Yao Z., Zhu J., Lin Y., Lin W., Li K., Xu X., Guo Y., Chen Y. et al. (2020) Chimeric RNA ASTN2-PAPPAas aggravates tumor progression and metastasis in human esophageal cancer. Cancer Lett, 501, 1–11. [DOI] [PubMed] [Google Scholar]
- 33.Chase A., Ernst T., Fiebig A., Collins A., Grand F., Erben P., Reiter A., Schreiber S. and Cross N.C. (2010) TFG, a target of chromosome translocations in lymphoma and soft tissue tumors, fuses to GPR128 in healthy individuals. Haematologica, 95, 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finta C. and Zaphiropoulos P.G. (2002) Intergenic mRNA molecules resulting from trans-splicing. The Journal of biological chemistry, 277, 5882–5890. [DOI] [PubMed] [Google Scholar]
- 35.Wu C.S., Yu C.Y., Chuang C.Y., Hsiao M., Kao C.F., Kuo H.C. and Chuang T.J. (2014) Integrative transcriptome sequencing identifies trans-splicing events with important roles in human embryonic stem cell pluripotency. Genome Res, 24, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balamurali D., Gorohovski A., Detroja R., Palande V., Raviv-Shay D. and Frenkel-Morgenstern M. (2020) ChiTaRS 5.0: the comprehensive database of chimeric transcripts matched with druggable fusions and 3D chromatin maps. Nucleic acids research, 48, D825–D834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H., Wang J., Mor G. and Sklar J. (2008) A neoplastic gene fusion mimics trans-splicing of RNAs in normal human cells. Science, 321, 1357–1361. [DOI] [PubMed] [Google Scholar]
- 38.Nacu S., Yuan W.L., Kan Z.Y., Bhatt D., Rivers C.S., Stinson J., Peters B.A., Modrusan Z., Jung K., Seshagiri S. et al. (2011) Deep RNA sequencing analysis of readthrough gene fusions in human prostate adenocarcinoma and reference samples. Bmc Med Genomics, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim H.P., Cho G.A., Han S.W., Shin J.Y., Jeong E.G., Song S.H., Lee W.C., Lee K.H., Bang D., Seo J.S. et al. (2014) Novel fusion transcripts in human gastric cancer revealed by transcriptome analysis. Oncogene, 33, 5434–5441. [DOI] [PubMed] [Google Scholar]
- 40.Tang Y., Qin F., Liu A. and Li H. (2017) Recurrent fusion RNA DUS4L-BCAP29 in non-cancer human tissues and cells. Oncotarget, 8, 31415–31423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goossens K., Van Soom A., Van Zeveren A., Favoreel H. and Peelman L.J. (2009) Quantification of Fibronectin 1 (FN1) splice variants, including two novel ones, and analysis of integrins as candidate FN1 receptors in bovine preimplantation embryos. Bmc Dev Biol, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai X., Liu C., Zhang T.N., Zhu Y.W., Dong X. and Xue P. (2018) Down-regulation of FN1 inhibits colorectal carcinogenesis by suppressing proliferation, migration, and invasion. J Cell Biochem, 119, 4717–4728. [DOI] [PubMed] [Google Scholar]
- 43.Vaillant C., Valdivieso P., Nuciforo S., Kool M., Schwarzentruber-Schauerte A., Mereau H., Cabuy E., Lobrinus J.A., Pfister S., Zuniga A. et al. (2015) Serpine2/PN-1 Is Required for Proliferative Expansion of Pre-Neoplastic Lesions and Malignant Progression to Medulloblastoma. Plos One, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mao M.Z. and Wang W.C. (2016) SerpinE2 promotes multiple cell proliferation and drug resistance in osteosarcoma. Mol Med Rep, 14, 881–887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Raw and processed RNA-Sequencing data from this study have been submitted to the NCBI Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE165479. The security token for reviewers to access the data before it is publicly available is gjqlgkamtxgltgn.
Flow cytometry data has been deposited into Flow Repository. The ID and URL with secret code for reviewers are listed below:
Cell cycle: FR-FCM-Z3E3
http://flowrepository.org/id/RvFrm5qG1S7akXGqSKb85uWhbRTZ0ACn5uCIPoGiulAygdG2Lq0SnQRYez7L5oHB
Cell apoptosis: FR-FCM-Z3E4
http://flowrepository.org/id/RvFrzD1pnAaXP44GVUKW0wVl1QimdHNOKR1hXyc07ao0xIZODy0tzrEgOykrnMUg
All the other data supporting the findings of this study are available within the article and its supplementary information files and from the corresponding author upon reasonable request. A reporting summary for this article is available as a Supplementary Information file.