Abstract
Calcium (Ca2+) uptake by mitochondria is essential in regulating bioenergetics, cell death, and cytosolic Ca2+ transients. Mitochondrial Calcium Uniporter (MCU) mediates the mitochondrial Ca2+ uptake. MCU is a heterooligomeric complex with a pore-forming component and accessory proteins required for channel activity. Though MCU regulation by MICUs is unequivocally established, there needs to be more knowledge of whether divalent cations regulate MCU. Here we set out to understand the mitochondrial matrix Mg2+-dependent regulation of MCU activity. We showed Mrs2 as the authentic mammalian mitochondrial Mg2+ channel using the planar lipid bilayer recordings. Using a liver-specific Mrs2 KO mouse model, we showed that decreased matrix [Mg2+] is associated with increased MCU activity and matrix Ca2+ overload. The disruption of Mg2+dependent MCU regulation significantly prompted mitochondrial permeability transition pore opening-mediated cell death during tissue IR injury. Our findings support a critical role for mMg2+ in regulating MCU activity and attenuating mCa2+ overload.
INTRODUCTION
Calcium (Ca2+) is a unique cellular ion and a critical regulator of multiple cellular processes, including mitochondrial function and dysfunction1. The simultaneous interplay of the on-and-off mechanisms maintains the intracellular Ca2+ (iCa2+) transients2–7. Such a fine regulation is mediated by mitochondria that can decode and transduce iCa2+ signals into an energy output (ATP) to match energetic cellular demand1. The Ca2+ selective channel, the Mitochondrial Ca2+ Uniporter (MCU), precisely controls mitochondrial Ca2+ (mCa2+) uptake and is driven by the considerable electrical gradient (Δψm) across the inner mitochondrial membrane (IMM)8–11. MCU functions as a hetero-oligomeric complex after self-association and interaction with its regulators12–19. Under physiological conditions, MCU-mediated mCa2+ uptake regulates bioenergetics by increasing the supply of reducing equivalents to the electron transport chain and increasing F1-F0 ATP synthase activity1, 3, 20–22. Reciprocally, in ischemia and reperfusion (I/R), dysregulation of mCa2+ cycling is prominent and results in either necrosis or apoptosis through mCa2+ overload23–28. Thus MCU-mediated mCa2+ uptake must be tightly regulated to support both cellular energy demand and also prevent cell death.
Though many Ca2+ channels, including InP3R29, RyRs30, and CRAC31, 32, exhibit Ca2+-dependent feedback mechanisms, whether divalent cations regulate MCU activity is unclear. To understand in depth the regulation of MCU by divalent cations, we resolved the atomic structure of the conserved MCU matrix domain33. Our previous structural analysis of the amino-terminal domain of human MCU revealed a β-grasplike fold containing the MCU regulating acidic patch (MRAP) that binds Mg2+/Ca2+ with ~mM affinity. The binding of Mg2+/Ca2+ to MRAP destabilizes MCU, shifts the self-association equilibrium to monomer, and attenuates mCa2+ uptake33. A seminal work from Foskett’s group showed the Ca2+ that permeates through the channel pore to bind the MRAP region and close the channel despite Ca2+ binding to MICU1/234. Also, mutating the two key aspartic acid residues (D131 and D147) to alanine abolished matrix [Ca2+] dependent channel inhibition, validating divalent cation binding site encompassing D131 and D147 could account for Ca2+ (and Mg2+) dependent MCU inhibition34. Though matrix Ca2+ was shown to inhibit MCU channel activity, the weak binding affinity for divalent cation for MRAP is well suited to the high Mg2+ levels of the mitochondrial matrix (0.5–1.5 mM). Because proteins structurally sensitive to cations have evolved affinities close to the concentration range in the compartment where they function, we anticipate mitochondrial matrix [Mg2+] to regulate MCU channel activity tightly in addition to Ca2+.
Here we set out to understand the matrix [Mg2+] dependent regulation of MCU-channel activity. Using biochemical, lipid bilayer, and patch-clamp recordings, we show Mrs2 as the authentic mammalian mitochondrial Mg2+ channel. Using our newly developed liver-specific mouse model, we illustrate the loss of Mrs2 to drastically alter the matrix [Mg2+]. The decreased matrix [Mg2+] was associated with increased MCU activity and matrix Ca2+ overload. The disruption of Mg2+-dependent MCU activity also significantly prompted mitochondrial permeability transition pore opening (mPTP). We employed a hanging-weight system for liver ischemia/reperfusion (IR) to assess the loss of Mg2+-dependent MCU regulation and mPTP-mediated cell death during tissue IR injury. Our findings support a critical role for mMg2+ in regulating MCU activity and attenuating mCa2+ overload.
RESULTS
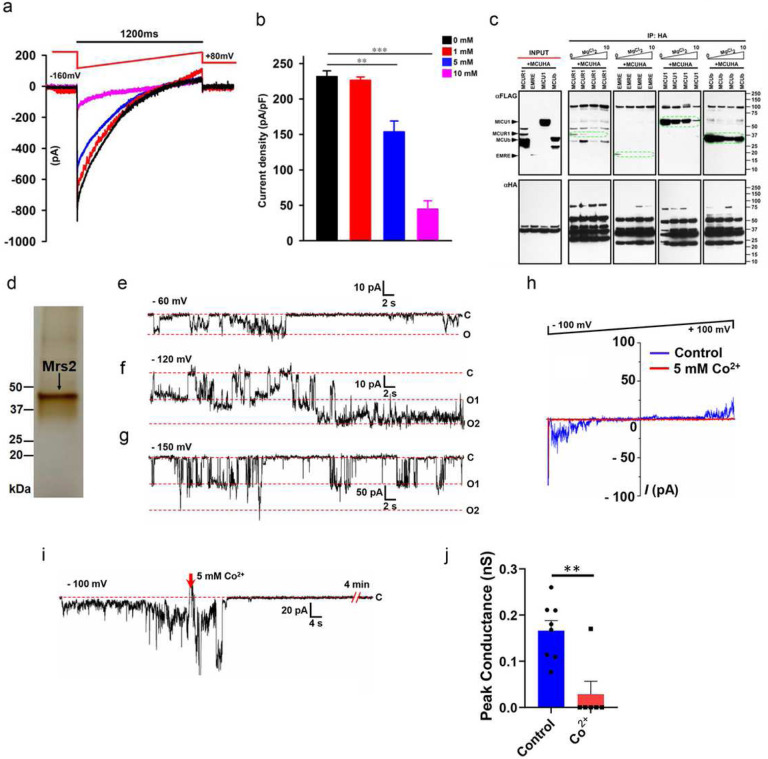
To confirm if matrix [Mg2+] regulates MCU channel activity, we recorded MCU Ca2+ currents (IMCU) in mitoplasts isolated from HeLa cells. We used varying [Mg2+] (0, 1, 5, and 10 mM) in the patch-clamp pipette solution that filled the mitochondrial matrix and measured IMCU. MCU currents were large with pipette solutions containing 0 and 1 mM Mg2+, whereas they were inhibited by ~30 and 65% at 5 and 10 mM Mg2+ (Fig. 1a and 1b). Having observed increasing pipette [Mg2+] to inhibit MCU activity (Fig. 1a and 1b) and Mg2+ to disrupt MCU homo-oligomerization33, we next asked whether increasing [Mg2+] also disrupts the hetero-oligomeric complex of MCU. We ectopically co-expressed MCU-HA with MICU1/MCUR1/EMRE/MCUb – Flag. MICU1/MCUR1/EMRE/MCUb co-immunoprecipitated with MCU-HA in 0 and 1 mM Mg2+. Increasing [Mg2+] disrupted the interaction of MCU with the positive regulators (MCUR1 and EMRE). In contrast, it preserved the interaction with negative regulators (MICU1 and MCUb) (Fig. 1c). Together, these results demonstrate that the mitochondrial matrix [Mg2+] regulates MCU activity by altering both the homo and hetero-oligomeric nature of MCU supercomplex (Fig.1a–1c).
Figure 1. Matrix [Mg2+] regulates MCU activity, and mammalian Mrs2 is the authentic mitochondrial Mg2+ channel.
(a and b) Measurement of IMCU current in mitoplasts prepared from HeLa cells. IMCU was measured with varying [Mg2+] (0, 1, 5, and 10 mM) in the patch-clamp pipette solution that filled the mitochondrial matrix. Traces are a single representative recording of IMCU. (b) IMCU densities (pA/pF). Bar represents Mean ± SEM; **P < 0.01; ***P < 0.001; n = 4–7. (c) HEK293 cells were co-transfected with HAtagged MCU and Flag-tagged MICU1, MCUR1, MCUb, and EMRE. Following immunoprecipitation with HA antibody in the presence of varying concentrations of MgCl2 (0, 1, 5, and 10 mM), total cell lysates and immunoprecipitated materials were subjected to Western blot analysis. Cell lysates were probed with anti-Flag (top left panel) or anti-HA antibodies (bottom left panel) to serve as inputs. Immunoprecipitated samples were probed with anti-Flag (top right panels 1 to 4) and anti-HA antibodies (bottom right panels 1 to 4) (n=3). (d) Silver-stained gel of purified Mrs2 from HEK 293 cells. The single band at ~40 kDa position represents purified Mrs2. SDS-PAGE resolved the sample. (e-g) Representative channel recordings of purified Mrs2 in a lipid bilayer at −60, −120, and −150 mV are displayed in panels (e), (f), and (g), respectively. (h) Representative continuous ramp voltage recording of the Mrs2 channel activity recorded from −100 mV and +100 mV in the presence and absence of 5 mM Cobalt. (i) Displays the continuous recording of Mrs2 at −100mV and channel inhibition with added Cobalt (5 mM). (j) Group data for peak conductances of Mrs2 lipid bilayer recordings in response to Cobalt, unpaired t-test was used, P=0.002. All the channel recordings were performed in 150 mM NMDG and 10 mM HEPES solution buffered at (pH 7.4) and supplemented with 20–104 mM Mg2+ during recordings.
Mitochondrial RNA splicing 2 (Mrs2) was initially considered the primary Mg2+ transporter in Saccharomyces cerevisiae mitochondria35, 36. The electrophysiological studies showed yeast Mrs2 (sMrs2) to form an Mg2+- selective channel (155 pS) and not permeable to Ca2+ 36. Though the core/pore component of the mMg2+ influx machinery was defined in yeast, the physiologic relevance of this observation in higher-order systems remains understudied. A recent study from Madesh and colleagues showed mammalian Mrs2 (mMrs2) to form a selective pore for lactate-mediated mMg2+ uptake37, authenticating Mrs2 as the conserved mMg2+ transport machinery from yeast to mammals. Though mMg2+ influx machinery was defined in mammals, the electrophysiological properties of mMrs2 are not established. To show whether mMrs2 forms an Mg2+-selective channel, we purified mMrs2 from HEK293 cells (Fig. 1d). The highly purified Mrs2 was used in planar lipid bilayer recordings for studying its single-channel activity. Our recordings show mMrs2 to form a voltagegated Mg2+ selective channel with multiple subconductance states (Fig. 1e–g). We recorded channels with the peak conductance activity varying from 80pS-250pS and mean conductance activity of ~150 pS (Fig. 1e–1j). Single-channel currents were recorded in the presence of Mg2+ only in Na+-, K+-, Cl−-, and Ca2+-free buffer and were shown to be inhibited by Co2+ (Fig. 1h–j). The continuous ramp voltage recording of the Mrs2 channel from −100 mV to +100 mV shows that the channel is inhibited by Co2+ at all voltages (Fig. 1h). Fig. 1i shows a continuous lipid bilayer recording of Mrs2 before and after adding Co2+.
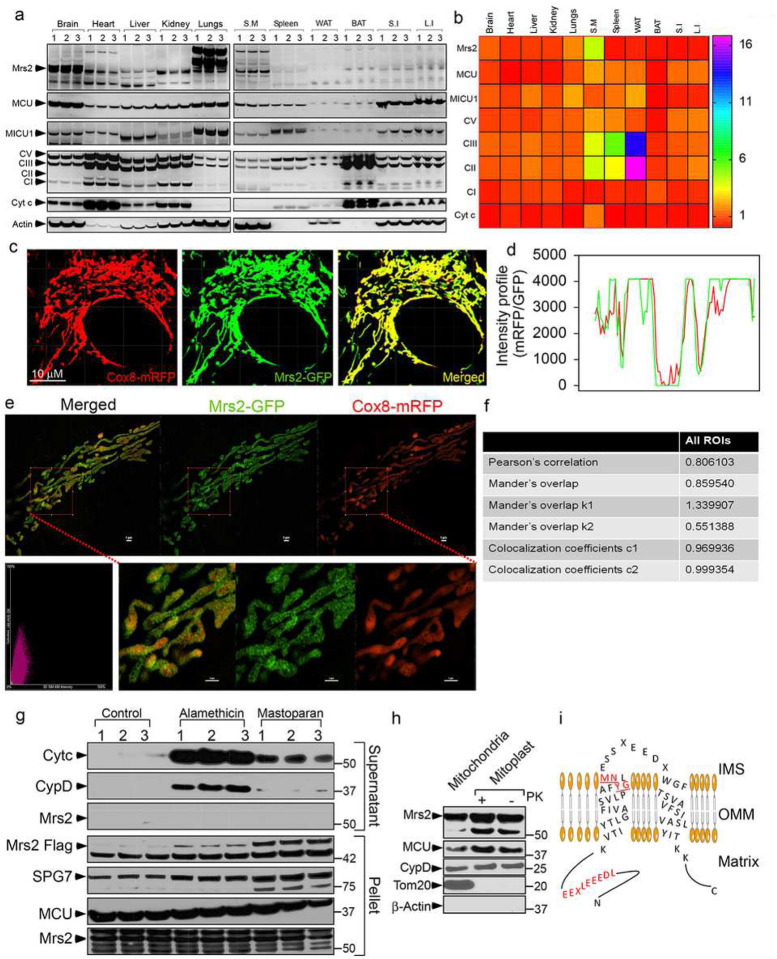
After confirming, Mrs2 channel activity, we extracted RNA and proteins from a panel of adult mouse tissues to verify whether Mrs2 is ubiquitously expressed in all mammalian tissues. Though differentially expressed, qPCR and Western blot analysis show, Mrs2 to be ubiquitously present in all metabolically active tissues (Fig. 2a and 2b; supplementary fig. 1a-1c). After confirming the ubiquitous distribution of Mrs2, we asked whether Mrs2 localizes explicitly to the mammalian mitochondria. To verify the mitochondrial distribution of Mrs2, we performed confocal microscopy and super-resolution structured illumination microscopy (SIM) imaging in HeLa cells expressing Mrs2-GFP and Cox8-mRFP. Confocal images at 488/561 nm and the intensity profile analysis overlap GFP and mRFP signals (Fig. 2c and 2d). Additionally, the SIM images and the correlation analysis substantiate a mitochondrial localization of Mrs2 (Fig. 2e and 2f). After confirming the localization of Mrs2 to the mitochondria, we performed a Western blot analysis to study the sub-organellar localization and orientation of Mrs212. HEK 293 cells stably expressing Mrs2-Flag were permeabilized with digitonin (40 μg/ml). Digitonin-permeabilized cells were incubated with mastoparan or alamethicin to elicit outer or inner mitochondrial membrane permeabilization. OMM permeabilization (with mastoparan) was confirmed by the cytosolic appearance of cytochrome C (Fig. 2g). Permeabilization of both OMM and IMM (with alamethicin) was marked by the cytosolic appearance of cyclophilin D (matrix protein) (Fig. 2g). Mrs2 was observed in the membrane fraction in both mastoparan or alamethicin-treated cells, revealing Mrs2 as an integral membrane protein (Fig. 2g). The orientation of Mrs2 was evaluated by mitochondrial subfractionation18. Mitoplasts prepared from HEK293 cells stably expressing C-terminal Flag-tagged Mrs2 were subjected to proteinase K digestion followed by Western blot analysis with antibodies specific for Flag suggested Mrs2 to be enriched in mitoplasts (Fig. 2h). A mitochondrial matrix protein, CypD, was protected from proteinase K digestion (Fig. 2h). Similarly, the integral membrane protein Mrs2 remained stable with no loss of Flag-tag, suggesting the C-terminal end of Mrs2 to face the mitochondrial matrix. These results suggest that N and C termini face the mitochondrial matrix side, consistent with two transmembrane-spanning regions (Fig. 2i).
Figure 2. Mrs2, the integral membrane protein, localizes to the inner mitochondrial membrane with its N and C-termini in the matrix.
(a and b) Representative Western blot (a) and densitometry quantification (b) show Mrs2 to be expressed ubiquitously in all metabolically active tissues. (c and d) Representative confocal images of HeLa cells transfected with Cox8-mRFP (Red) and Mrs2-GFP (Green) (c) and fluorescence intensity profile (d) show an overlap of RFP and GFP signals authenticating localization of Mrs2 to mitochondria. (e) Representative images of HeLa cells co-transfected with the Cox8-mRFP (Red)/Mrs2-GFP (Green) and imaged using SR-SIM. (f) Pearson, Manders, and overlap coefficients between Mrs2 and COX8-RFP substantiate a mitochondrial localization of Mrs2. (g) To assess the localization of Mrs2, HEK293 cells expressing Mrs2WT-Flag were permeabilized and exposed to mastoparan or alamethicin (20 μg/ml) for 5 min. Cytosolic (supernatant) and mitochondrial (pellet) fractions were subjected to immunoblotting to examine the release of cytochrome c (intermembrane space marker), CypD (matrix marker), Mrs2, SPG7, and MCU from mitochondria. Lanes 1, 2, and 3 refer to triplicate samples. Western blot shows the release of CytC and CypD after OMM/IMM permeabilization but not Mrs2, MCU, or SPG7, confirming Mrs2 as an integral membrane protein. (h) Immunoblot analyses of mitochondria and mitoplast from HEK293 cells expressing Mrs2WT-Flag. The freshly prepared mitoplasts were exposed to Proteinase K for 10 min. The samples were probed using antibodies specific for Flag, MCU, CypD, Tom20, and actin. Enrichment of Mrs2 in the mitoplasts with no loss of Flag tag suggests Mrs2 as an integral membrane protein that localizes to the IMM with its N and C-termini facing the matrix. (i) Schematic representation of Mrs2 with its functional domains in the N-terminus, Transmembrane, and IMS loop.
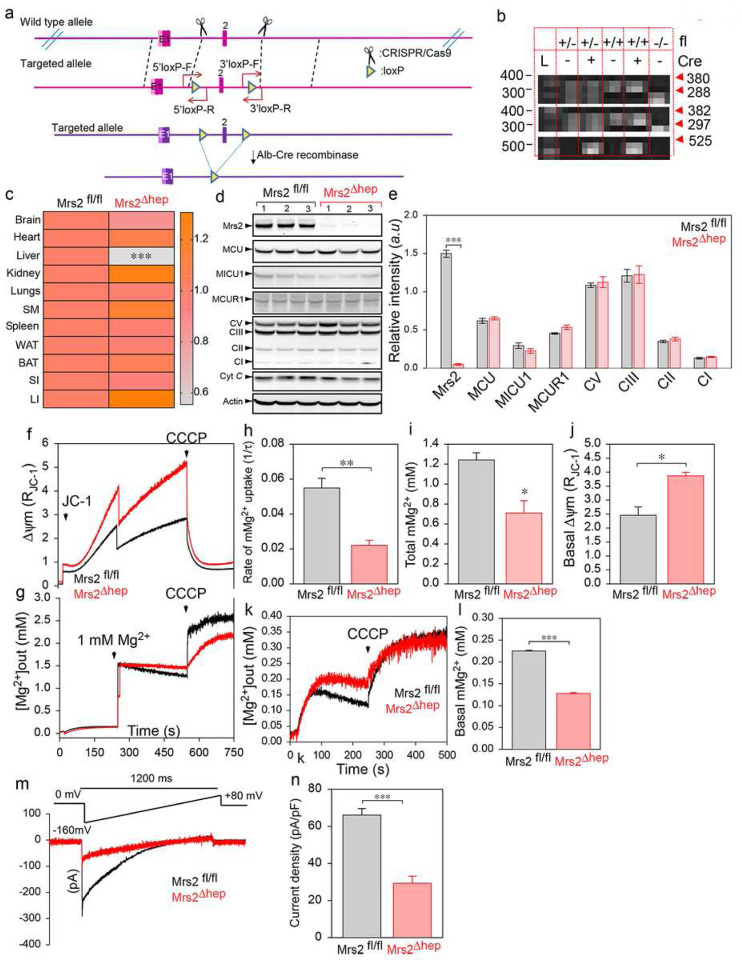
Because we confirmed Mrs2 as the mMg2+ influx machinery, we anticipate losing Mrs2 to decrease matrix [Mg2+] and positively regulate MCU activity. To verify whether matrix [Mg2+] regulates MCU activity, we generated a liver-specific Mrs2 KO mouse model. Loxp/loxp knockin mice (Mrs2fl/fl) were generated using CRISPR/Cas9 strategy. Mrs2fl/fl mice were crossed with Albumin-Cre to allow germline deletion specifically in the liver (Mrs2Δhep) (Fig.3a). The gene targeting was confirmed by genotyping (Fig.3b). The loss of Mrs2 was confirmed using qPCR and Western blot analysis (Fig.3c–3e). The loss of Mrs2 did not alter the expression of other mitochondrial proteins like the OXPHOS complex, MCU, MICU1, and MCUR1 (Fig.3d–3e). We next asked whether loss of Mrs2 alters the mMg2+ uptake. Hepatocytes isolated from Mrs2fl/fl and Mrs2Δhep were permeabilized, and mMg2+ uptake and mitochondrial membrane potential (Δψm) were measured simultaneously (Fig. 3f and 3g) using a multi-wavelength-excitation dual wavelength-emission spectrofluorometer at 340/380 nm. The ratiometric dye, Mag-Fura-2 tetra potassium salt, was calibrated, and the mitochondrial [Mg2+] was determined by where the dissociation constant Kd is 1.98 mM. Mrs2Δhep mitochondria showed no mMg2+ uptake in response to extramitochondrial Mg2+ pulse (Fig. 3g–3i). The loss of mMg2+ uptake was not due to changes in Δψm (Fig. 3f and 3j; supplementary Fig. 2a and 2b). We next asked whether the loss of Mrs2 caused a change in the total matrix [Mg2+] during the resting state. Digitonin permeabilized Mrs2fl/fl and Mrs2Δhep hepatocytes were loaded with Mag-Fura-2 tetra potassium salt. After baseline measurement of fluorescence, CCCP was added to depolarize the mitochondrial membrane (Fig. 3k). Loss of Mrs2 was marked by a decrease in the matrix [Mg2+] (Fig. 3k and 3l).
Figure 3. Loss of Mrs2 alters the mMg2+ uptake and the matrix [Mg2+].
(a) Schematic representation of loxP site insertion by CRISPR/Cas9 strategy and the generation of liver-specific Mrs2 KO (Mrs2Δhep) by CreloxP system. (b) PCR with redundant primers confirmed the genotype of control and KO mice. (c) Quantitative measurement of Mrs2 mRNA levels in different tissues collected from Mrs2fl/fl and Mrs2Δhep show liver-specific loss of Mrs2. (d and e) Representative Western blot (d) and densitometry quantification (e) show complete loss of Mrs2 in hepatocytes with no alterations to other mitochondrial proteins. (f-j) Simultaneous measurement of Δψm and mMg2+ uptake in hepatocytes isolated from Mrs2fl/fl and Mrs2Δhep. Mean traces of Δψm (f) and [Mg2+]out (g) in Mrs2fl/fl (black) and Mrs2Δhep (red). (h) Quantifying the rate of mMg2+ uptake as a function of the decrease in [Mg2+]out after 1 mM Mg2+ pulse. (i) Quantification of the [Mg2+]out after CCCP addition. (j) Quantification of the basal Δψm before adding 1 mM Mg2+ pulse. Data represents Mean ± SEM; *P<0.05, **P <0.01; n = 4. (k) Mean [Mg2+]out traces before and after CCCP (5 μM) addition in Mrs2fl/fl and Mrs2Δhep hepatocytes. (l) Quantification of resting matrix [Mg2+] after the addition of CCCP. (m and n) Measurement of IMrs2 current in mitoplasts prepared from Mrs2fl/fl and Mrs2Δhep hepatocytes. Traces are a single representative recording of IMrs2. (n) IMrs2 densities (pA/pF) in Mrs2fl/fl and Mrs2Δhep. Bar represents Mean ± SEM; ***P < 0.001; n = 4–7.
Because mitochondria can accumulate and release Mg2+ in the presence of stimuli, we then asked whether loss of Mrs2 alters cytosolic Mg2+ (cMg2+) dynamics. Mrs2fl/fl and Mrs2Δhep hepatocytes were loaded with MagGreen-AM and TMRM. cMg2+ and mMg2+ dynamics were measured simultaneously after stimulating the cells with 10 μM glucagon and 2 mM Mg2+ (Supplementary Fig. 2c and 2d). Confocal measurements show Mrs2Δhep hepatocytes to have delayed cMg2+ clearance (Supplementary Fig. 2c-2g) compared to Mrs2fl/fl, confirming mitochondria play a critical role in regulating cellular Mg2+ dynamics. We performed mitoplast patch clamp recordings to verify whether loss of Mrs2 ablates mitochondrial Mg2+ current (IMrs2). Loss of Mrs2 significantly ablated IMrs2 validating Mrs2 as an authentic mammalian mMg2+ channel (Fig. 3m and 3n). Finally, to validate the IMrs2 recordings, we performed mitoplast patch clamp recordings in Mrs2fl/fl mitochondria in the presence of 200 nM ruthenium red (Ru360) and 1 μM hexamine cobalt(III)chloride. Hexamine cobalt(III)chloride, not Ru360, abolished IMrs2, confirming the identity of IMrs2 recordings as Mrs2 currents (Supplementary Fig. 2h-2i).
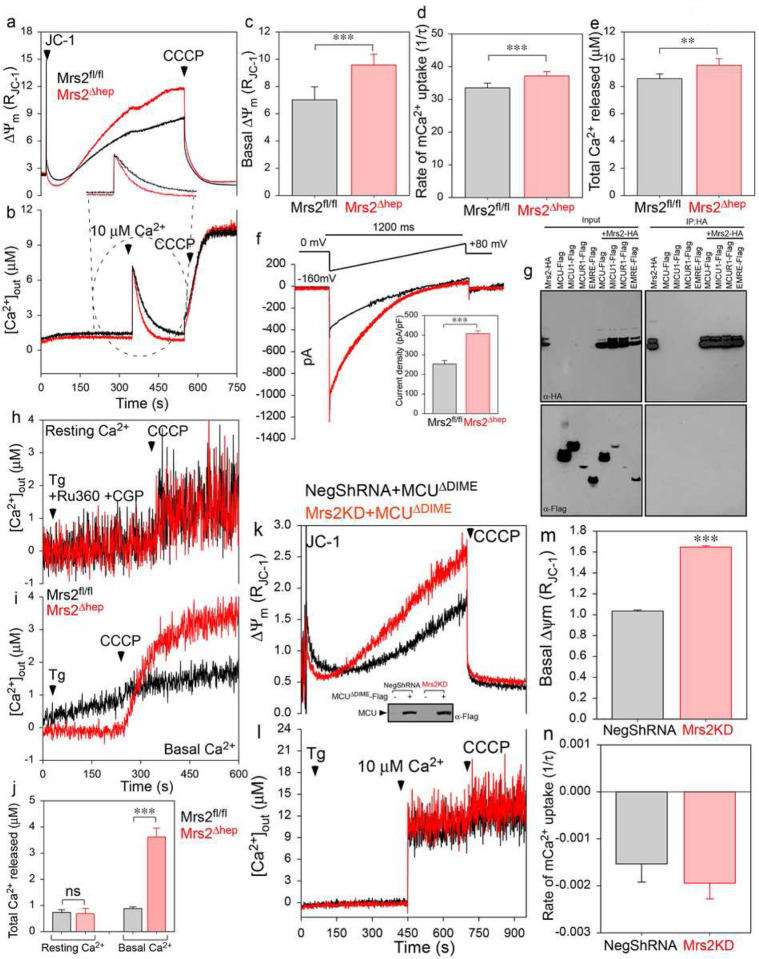
To validate the role of mMg2+ in regulating MCU-mediated mCa2+ uptake, we used our permeabilized cell system to perform simultaneous measurements of mCa2+ uptake and Δψm27, 38 (Fig. 4a and 4b). A single bolus of extramitochondrial Ca2+ pulse (10 μM) was added, and the rate of mCa2+ uptake was measured as a function of the decrease in bath Ca2+ fluorescence. Mrs2Δhep cells could rapidly clear extramitochondrial Ca2+ pulse, indicating increased MCU activity (Fig. 4a–4e). Mitoplast patch-clamp recordings confirmed increased MCU activity (IMCU) in Mrs2Δhep mitoplasts (Fig. 4f and inset). Next, we asked whether Mrs2 binds directly to any of the MCU complex components and increases the MCU activity or is an indirect regulation through altering matrix [Mg2+]. The immunoprecipitation with HA antibody could pull down Mrs2 (Fig. 4g; top right) with no notable interaction of Mrs2 with MCU, MICU1, MCUR1, and EMRE (Fig. 4g; bottom right), confirming no direct interaction between Mrs2 and other MCU complex components. Not just in hepatocytes, we also observed loss of Mrs2 (Supplementary Fig. 3a-3c) and decreased mMg2+ uptake (Supplementary Fig. 3d and 3e) in HEK293 cells to alter MCU activity (Supplementary Fig. 3f-3i). Previously we showed the binding of Mg2+ to the MRAP region to destabilize and shift the self-association equilibrium of MCU to monomer33. Consistent with our previous finding, FPLC analysis showed a heterogenous population of MCU complex (both low and high molecular weight) in HEK 293 NegShRNA cells, whereas MCU assembled as a supercomplex in Mrs2 KD cells (Supplementary Fig. 3j and 3k). Collectively, these results suggest the loss of mMg2+ to stabilize the MCU complex and promote channel activity.
Figure 4. Loss of mMg2+ uptake potentiates MCU-mediated mCa2+ uptake.
(a-e) Simultaneous measurement of Δψm and mCa2+ in hepatocytes isolated from Mrs2fl/fl and Mrs2Δhep. Mean traces of Δψm (a) and [Ca2+]out (b) in Mrs2fl/fl (black) and Mrs2Δhep (red). (c) Quantification of the basal Δψm before the addition of 10 μM Ca2+ pulse. (d) Quantification of the rate of mCa2+ uptake as a function of the decrease in [Ca2+]out after 10 μM Ca2+ pulse. (e) Quantification of the [Ca2+]out after CCCP addition. Data represent Mean ± SEM; **P<0.01, ***P <0.001; n = 4. (f) Measurement of IMcu current in mitoplasts prepared from Mrs2fl/fl and Mrs2Δhep hepatocytes. Traces are a single representative recording of IMcu. (inset) IMrs2 densities (pA/pF) in Mrs2fl/fl and Mrs2Δhep. Bar represents Mean ± SEM; ***P < 0.001; n = 4–7. (g) HEK293 cells were co-transfected with HAtagged Mrs2 and Flag-tagged MCU, MICU1, MCUR1, and EMRE. Following immunoprecipitation with HA antibody, total cell lysates and immunoprecipitated materials were subjected to Western blot analysis. Cell lysates were probed with anti-HA (top left panel) or anti-Flag antibodies (bottom left panel) to serve as inputs. Immunoprecipitated samples were probed with anti-HA (top right panel) and anti-Flag (bottom right panel) antibodies (n=3). (h and i) Mean [Ca2+]out traces before and after CCCP (5 μM) addition in Mrs2fl/fl and Mrs2Δhep hepatocytes in the presence (h) or absence (i) of Ru360 (MCU blocker) and CGP (NCLX blocker). (j) Quantification of resting and basal matrix [Ca2+] after adding CCCP. (k-n) Simultaneous measurement of Δψm and mCa2+ in HEK293 NegShRNA and Mrs2KD cells expressing MCUΔDIME. Mean traces of Δψm (k) and [Ca2+]out (l) in Mrs2fl/fl (black) and Mrs2Δhep (red). (inset) representative Western blot shows the expression of MCUΔDIME in HEK293 NegShRNA and Mrs2KD cells. (m) Quantification of the basal Δψm before adding 10 μM Ca2+ pulse. (n) Quantification of the rate of mCa2+ uptake as a function of the decrease in [Ca2+]out after 10 μM Ca2+ pulse. Bar represents Mean ± SEM; ns non-significant; n = 4–7.
We next asked whether increased MCU activity overloads matrix Ca2+ at the resting state. Permeabilized cells loaded with Fura-FF tetra potassium salt were treated with Ru360 (to block any extramitochondrial Ca2+ uptake) and CGP (to block the efflux of mitochondrial Ca2+). After baseline measurement, an uncoupler CCCP was added to depolarize the mitochondrial membrane (Fig. 4h). Though MCU activity was increased in Mrs2Δhep, we do not observe an increase in resting matrix [Ca2+] (Fig. 4h and 4j). Interestingly, when Ru360 was removed from the experimental condition described above, we observed the matrix [Ca2+] significantly higher in Mrs2Δhep than Mrs2fl/fl (Fig. 4i and 4j). Because the assay buffer contains Thapsigargin (Tg), we anticipated the loss of Mrs2 and the decreased matrix [Mg2+] concentration to activate MCU in the low-[iCa2+] regime39. We used our permeabilized cell system to simultaneously measure mCa2+ uptake and Δψm (Supplementary Fig. 4a-4i). Mrs2Δhep rapidly cleared the 1 μM Ca2+ pulse, whereas the channel gatekeeping function remained intact in Mrs2fl/fl (Supplementary Fig. 4b, 4d-4e). Additionally, the bath Ca2+ before adding Ca2+ pulse was lower in Mrs2Δhep compared to Mrs2fl/fl (Supplementary Fig. 4f and 4g). Also, the bath Ca2+ after CCCP addition was significantly higher in Mrs2Δhep than Mrs2fl/fl (Supplementary Fig. 4h and 4i), confirming the loss of Mrs2 to activate MCU in the low-[iCa2+] regime. We asked whether the loss of Mrs2 alters MCU/MICU1 interaction to activate MCU in the low-[iCa2+] regime. The immunoprecipitation with Flag antibody pulled down MCU and MICU1, indicating MCU/MICU1 complex to be intact in Mrs2 KD cells (Supplementary Fig. 4j).
Since we saw increased MCU activity in the low-[Ca2+]i regime despite stable MCU/MICU1 interaction, we asked whether the increased mCa2+ uptake is strictly MCU-mediated. We stably expressed MCUΔDIME mutant in HEK293 NegShRNA and Mrs2KD cells. MCUΔDIME expression significantly decreased MCU-mediated mCa2+ uptake in both control and KD cells (Fig. 4k–4n), authenticating the regulation of MCU-channel activity by matrix [Mg2+]. Though MICUs sets a [iCa2+] threshold for MCU activation, our results indicate that the regulation of MCU is also to be governed by the matrix [Mg2+] concentration, reinforcing the concept of Mg2+dependent MCU regulation.
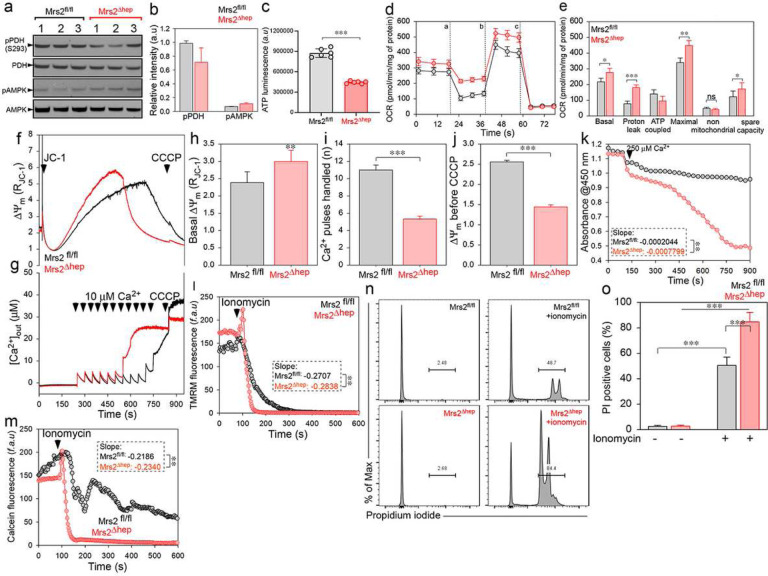
Pyruvate dehydrogenase (PDH) is the enzyme that catalyzes the irreversible conversion of pyruvate to acetyl-CoA. PDH is regulated through the alterations in the phosphorylation state of the enzyme by PDH kinase (PDHK) and PDH phosphatase (PDHP). The major regulators of PDHP are the divalent cations Mg2+ and Ca2+. mMg2+ has been documented to enhance the activity of PDHP40. PDHP is inactive in the absence of Mg2+ and can be activated 10–20 fold by Ca2+ only in the presence of Mg2+. Because we saw decreased and increased matrix [Mg2+] and [Ca2+], we next asked whether the loss of Mrs2 affects the phosphorylation state of PDH. Though insignificant, we saw a trend in the decreased PDH phosphorylation in Mrs2Δhep compared to Mrs2fl/fl (Fig. 5a and 5b). The decrease in PDH phosphorylation can be attributed to increased matrix [Ca2+], but the loss in complete PDH dephosphorylation marks the critical need for matrix Mg2+ to activate PDHP. Because we saw a trend in decreased PDH phosphorylation, we assessed the ATP levels in control and KO/KD cells. ATP was significantly decreased in KO/KD cells compared to control (Fig. 5c, Supplementary Fig. 5a). The decrease in ATP levels was marked by an increase (not significant) in AMPK phosphorylation (Fig. 5a and 5b).
Figure 5. Loss of mMg2+ uptake alters the cellular bioenergetics and induces Ca2+ overload-mediated PTP opening.
(a and b) Representative Western blot was probed with antibodies specific for phospho PDH and phospho AMPK. The phosphorylation of PDH and AMPK was normalized with the total PDH or AMPK. (b) Densitometry quantification show decreased and increased phosphorylation of PDH and AMPK, respectively. (c) Quantifying total cellular ATP levels in Mrs2fl/fl and Mrs2Δhep hepatocytes. (d) Mean traces of oxygen consumption rate (OCR) in Mrs2fl/fl and Mrs2Δhep hepatocytes using pyruvate as a substrate and after sequential exposure to Oligomycin (a), FCCP (b), and rotenone/antimycin (c). (e) Quantification of basal, maximal OCR, proton leak, ATP coupled respiration, spare capacity, and non-mitochondrial respiration. Data indicate Mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001; n = 4–10. (f-j) Simultaneous measurement of Δψm and mCa2+ in Mrs2fl/fl and Mrs2Δhep hepatocytes. Mean traces of Δψm (f) and [Ca2+]out (g) in Mrs2fl/fl (black) and Mrs2Δhep (red) after a series of 10 μM Ca2+ pulse. (h) Quantifying the basal Δψm before adding the first 10 μM Ca2+ pulse. (i) Quantification of the number of Ca2+ pulses handled before the loss of Δψm. (j) Quantification of the Δψm before the addition of CCCP. Bar represents Mean ± SEM; **P < 0.01, ***P < 0.001; n = 4–7. (k) Isolated mitochondria from Mrs2fl/fl and Mrs2Δhep hepatocytes were incubated with Ca2+ (250 μM), and mitochondrial swelling was measured as absorbance at 540 nm. The mean value traces were plotted with a polynomial fit. n=3–6. (l) Mean traces of TMRM fluorescence in Mrs2fl/fl and Mrs2Δhep hepatocytes before and after adding ionomycin (5 μM). (m) Mean traces of mitochondrial calcein fluorescence in Mrs2fl/fl and Mrs2Δhep hepatocytes before and after the addition of ionomycin (5 μM). (n and o) Mrs2fl/fl and Mrs2Δhep hepatocytes were treated with ionomycin (5 μM) for 6 h, stained with propidium iodide, and cell death was measured by flow cytometry. Representative histogram (n) and quantification of PI-positive cells (o) show increased cell death in Mrs2Δhep hepatocytes. Mean ± SEM; ***p<0.001, n=3–4.
Because we saw opposing results with PDH activation and ATP levels, we assessed the oxygen consumption rate (OCR) in control and Mrs2 KO/KD cells (Fig. 5d, 5e, Supplementary Fig. 5b, and 5c). Basal and maximal respiration was increased in KO/KD cells compared to the control (Fig. 5d, 5e, Supplementary Fig. 5b, and 5c). Further analysis of the OCR data showed increased proton leak in KO/KD cells with a concomitant decrease in ATP-coupled respiration (Fig. 5d, 5e, Supplementary Fig. 5b, and 5c). Though the proton leak was increased in Mrs2 KO/KD cells, we do not see a decrease in Δψm. Instead, a significant increase in Δψm was observed (Fig. 3f, 3j, 4a, 4c, 5f, 5h, supplementary fig. 2a and 2b, supplementary fig. 4a and 4b), ruling out the possible contribution of uncoupling proteins (UCP) to proton leak.
According to the chemiosmotic theory, the Δψm can be maintained either by the electron transport chain through respiration or by ATP hydrolysis via the F1-F0 ATPase. Because we observed increased proton leak and decreased ATP, we asked whether the reversal of F1-F0 ATPase contributes to increased Δψm. Using our permeabilized cell system, we measured Δψm in Mrs2fl/fl and Mrs2Δhep in the presence or absence of Oligomycin (ATP synthase and hydrolysis inhibitor) or BTB06584 (selective inhibitor of ATP hydrolysis). Both Oligomycin and BTB treatment depolarized/normalized the Δψm in Mrs2Δhep. Conversely, oligomycin treatment hyperpolarized the Mrs2fl/fl mitochondria, and BTB had no effect (Supplementary Fig. 5d and 5e). Because the reversal of ATP synthase depletes cellular ATP and activates the mitochondrial permeability transition pore (mPTP)41, we next asked whether increased matrix Ca2+ overload and ATP reversal make Mrs2Δhep susceptible to mPTP opening. We simultaneously measured calcium retention capacity (CRC) and Δψm in Mrs2fl/fl and Mrs2Δhep. Mrs2Δhep exhibited decreased CRC associated with an early Δψm collapse (Fig. 5f–5j), indicating matrix Ca2+ overload-induced activation of the mPTP. Increased Ca2+-induced mitochondrial swelling (Fig. 5k), early TMRM/calcein fluorescence loss (Fig. 5l and 5m), and increased ionomycin-mediated cell death confirmed matrix Ca2+ overload-induced mPTP opening in Mrs2Δhep (Fig. 5n and 5o).
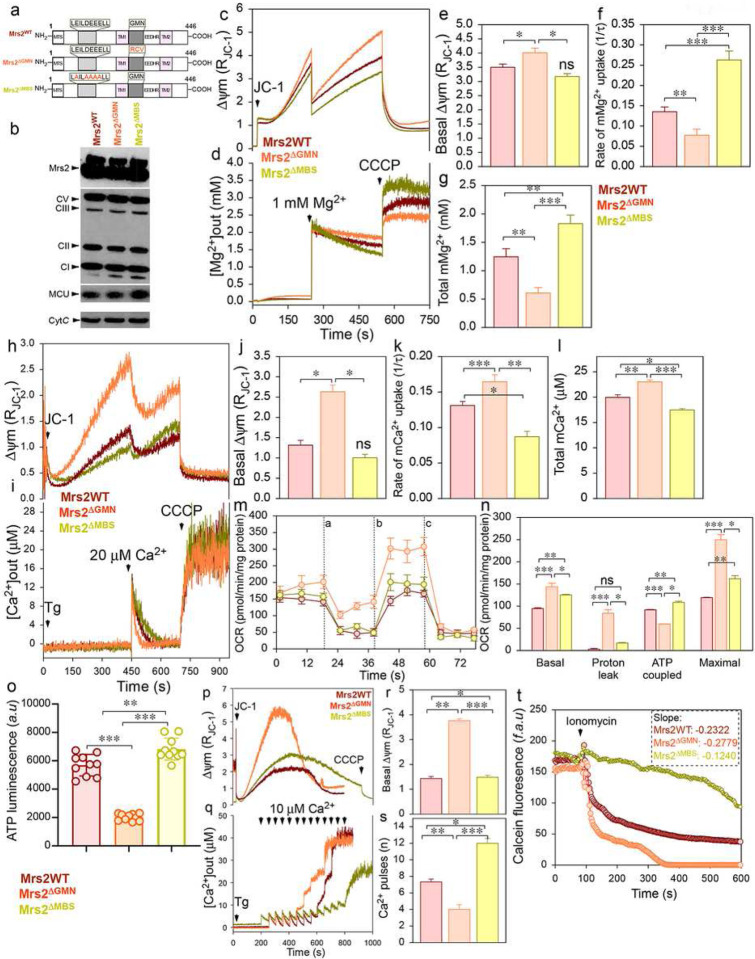
Next, we used gain/loss-of-function Mrs2 mutants to examine whether the increased MCU activity and matrix Ca2+ overload-induced activation of the mPTP are causal effects of decreased mMg2+ uptake. It has been proposed that single amino acid substitutions in the G-M-N motif of Mrs2 are sufficient to abolish Mg2+ transport or profoundly change the ion selectivity of channel42–44. Additionally, reports had shown eventually complete inhibition of CorA-driven Mg2+ currents when the intracellular domain of CorA was exposed to mM concentration of Mg2+ 45, 46. This phenomenon could be due to a self-regulatory mechanism, where an increase in the local [Mg2+] saturates the putative Mg2+ binding site (MBS), triggering channel closure46. We generated loss-of-function (Mrs2ΔGMN) and gain-of-function (Mrs2ΔMBS) mutant constructs (Fig. 6a). Western blot confirms the ectopic expression of Flag-tagged Mrs2WT, Mrs2ΔGMN, and Mrs2ΔMBS in HEK293 WT cells (Fig. 6b). The permeabilized cell system analysis (Fig. 6c–6g) show abolished and increased mMg2+ uptake in Mrs2ΔGMN and Mrs2ΔMBS, respectively (Fig. 6d, 6f, and 6g). The expression of Mrs2WT did not alter the mMg2+ uptake, further authenticating mMg2+ homeostasis to be maintained through a negative feedback loop45, 46. We next sought to define whether altered mMg2+ uptake modifies mCa2+ uptake. Simultaneous measurements of Δψm and mCa2+ (Fig. 6h–6l) show increased and decreased mCa2+ uptake in Mrs2ΔGMN and Mrs2ΔMBS, respectively (Fig. 6i, 6k, and 6l). Similar to Mrs2KO/KD cells, basal and maximal OCR was increased in Mrs2ΔGMN (Fig. 6m and 6n) with a decrease in ATP levels (Fig. 6o). The decrease in ATP levels and increased Δψm was due to increased proton leak (Fig. 6c, 6e, 6h, 6j, and 6n), further confirming the loss of mMg2+ to result in ATP synthase reversal. Our results also show decreased CRC (Fig. 6p–6s) and early calcein quenching (Fig. 6t) in Mrs2ΔGMN. The expression of Mrs2ΔMBS preserved the mitochondrial function (Fig. 6m–6o) and delayed the Ca2+-mediated mPTP opening (Fig. 6p–6t). These data show MCU activity and Ca2+-mediated mPTP opening to be fine-tuned by Mrs2-mediated mMg2+ uptake.
Figure 6. Mrs2 gain-of-function mutant augments mMg2+ uptake and prevents Ca2+-overload mediated PTP opening.
(a) Schematic of full-length Mrs2 with its functional domains (Mrs2WT). Mutations in Mrs2WT are highlighted in red (Mrs2ΔGMN and Mrs2ΔMBS). (b) Representative Western blot probed with antibodies specific for Flag shows the expression of Mrs2WT, Mrs2ΔGMN, and Mrs2ΔMBS in HEK293 WT cells. (c-g) Simultaneous measurement of Δψm and mMg2+ in HEK 293 cells expressing Mrs2WT, Mrs2ΔGMN, and Mrs2ΔMBS. Mean traces of Δψm (c) and [Mg2+]out (d). (e) Quantification of the basal Δψm before the addition of 1 mM Mg2+ pulse. (f) Quantifying the rate of mMg2+ uptake as a function of the decrease in [Mg2+]out after 1 mM Mg2+ pulse. (g) Quantification of the [Mg2+]out after CCCP addition. Data represents Mean ± SEM; *P<0.05, **P <0.01; n = 4. (h-l) Simultaneous measurement of Δψm and mCa2+ in HEK 293 cells expressing Mrs2WT, Mrs2ΔGMN, and Mrs2ΔMBS. Mean traces of Δψm (h) and [Ca2+]out (i). (j) Quantification of the basal Δψm before adding 20 μM Ca2+ pulse. (k) Quantification of the rate of mCa2+ uptake as a function of the decrease in [Ca2+]out after 20 μM Ca2+ pulse. (l) Quantification of the [Ca2+]out after CCCP addition. Data represent Mean ± SEM; **P<0.01, ***P <0.001; n = 4. (m) Mean traces of oxygen consumption rate (OCR) in HEK 293 cells expressing Mrs2WT, Mrs2ΔGMN, and Mrs2ΔMBS using pyruvate as a substrate and after sequential exposure to Oligomycin (a), FCCP (b), and rotenone/antimycin (c). (n) Quantification of basal, maximal OCR, proton leak, and ATP-coupled respiration. Data indicate Mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001; n = 10. (o) Quantification of total cellular ATP levels in HEK 293 cells expressing Mrs2WT, Mrs2ΔGMN, and Mrs2ΔMBS. (p-s) Simultaneous measurement of Δψm and mCa2+ in HEK 293 cells expressing Mrs2WT, Mrs2ΔGMN, and Mrs2ΔMBS. Mean traces of Δψm (p) and [Ca2+]out (q) after a series of 10 μM Ca2+ pulses. (r) Quantification of the basal Δψm before adding the first 10 μM Ca2+ pulse. (s) Quantification of the number of Ca2+ pulses handled before the loss of Δψm. Bar represents Mean ± SEM; **P < 0.01, ***P < 0.001; n = 4–7. (t) Mean traces of mitochondrial calcein fluorescence in HEK 293 cells expressing Mrs2WT, Mrs2ΔGMN, and Mrs2ΔMBS before and after adding ionomycin (5 μM).
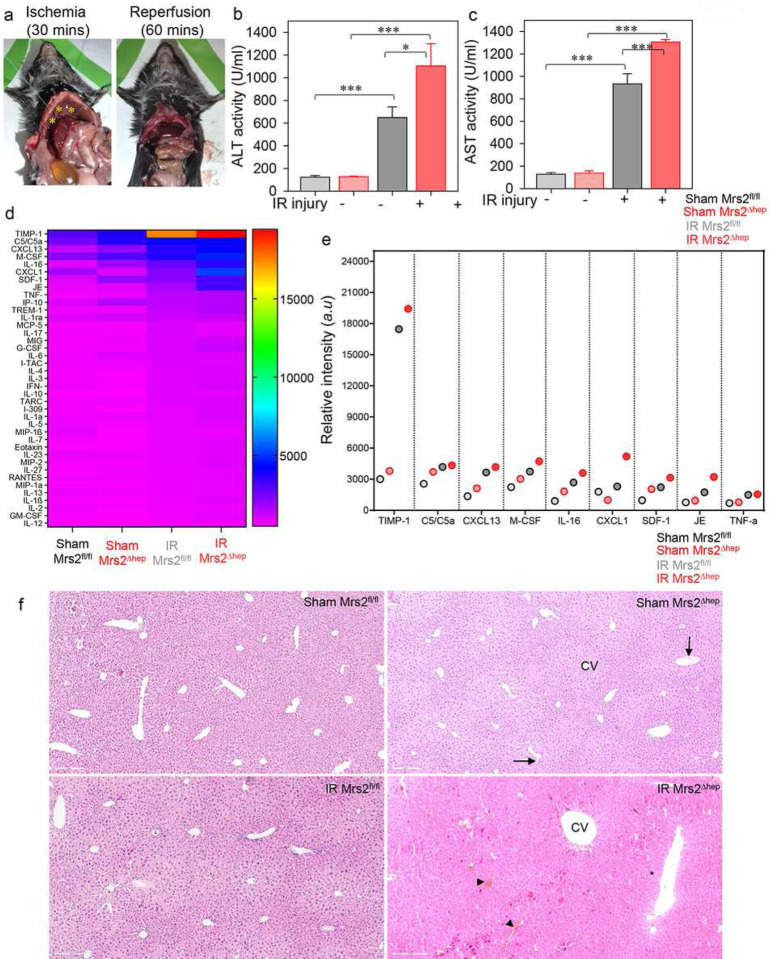
To further confirm whether loss of Mrs2 potentiates MCU-mediated mCa2+ uptake, overloads matrix Ca2+, and induces cell death, we performed an acute liver ischemic reperfusion (IR) injury by hanging weight method. Using this method, we saw the portal triad immediately occluded by hanging the weights over the poles, causing the blood supply to the left, median, and caudate lobes of the liver to be interrupted. Successful occlusion was confirmed by visual inspection of pale blanching in the ischemic lobes (i.e., a change in color from red to a pale color) (Fig. 7a; left panel). In contrast, the change of color immediately disappeared when the hanging weights were removed from the poles, and the liver was reperfused (Fig. 7a, right panel). Because elevated plasma ALT and AST are reliable markers for liver parenchymal cell membrane integrity and liver injury47, 48, we measured ALT and AST activity in plasma after 30 mins of ischemia followed by 1 h of reperfusion. The ALT and AST levels were increased in Mrs2Δhep (Fig. 7b and 7c). Our results also show increased levels of inflammatory cytokines in the plasma of Mrs2Δhep (Fig. 7d and 7e) compared to sham and Mrs2fl/fl IR injured mice. Histological analysis shows hepatocyte liver necrosis, mononucleated cell infiltration, enlarged central vein (cv), and increased congestion in Mrs2Δhep compared to sham and Mrs2fl/fl IR injured mice (Fig. 7f). Taken together; these data show Mrs2-mediated mitochondrial Mg2+ uptake is critical in maintaining MCU activity and protecting mitochondria from Ca2+ overload mediated mPTP opening during IR injury.
Figure 7. Loss of Mrs2 augments ischemic liver injury.
(a and b) Model of ischemic liver reperfusion using the hanging-weight system. (a) Successful occlusion was confirmed by a change of color from red to pale (left panel; marked with a yellow asterisk), and the change of color immediately disappeared when the hanging weights were removed, and the liver was reperfused (right panel). (b and c) The ALT (b) and AST (c) were quantified in the sham and IR injured control and KO mice plasma. Data represent Mean ± SEM; *P<0.05, ***P <0.001; n = 4. (d) Densitometry quantification of the cytokine array and the representative heat map show increased levels of inflammatory cytokines in the plasma of Mrs2Δhep compared to sham and Mrs2fl/fl IR injured mice. (e) Quantification of the inflammatory cytokines significantly altered in the serum of Mrs2Δhep compared to sham and Mrs2fl/fl IR injured mice. The colored circle represents the Mean; *P<0.05, ***P <0.001; n = 4. (f) Histological analysis shows hepatocyte liver necrosis (marked by a black asterisk), mononucleated cell infiltration (arrow), enlarged central vein (cv), and increased congestion (arrowhead) in Mrs2Δhep compared to sham and Mrs2fl/fl IR injured mice.
DISCUSSION
Next to potassium (K+), magnesium (Mg2+) is the abundant intracellular divalent cation. It is an essential co-factor in the machinery replicating, transcribing, and translating genomic information49–52. As a structural cofactor, Mg2+ stabilizes the ribosome, lipid membranes, and nucleic acids49–51, 53. It is pivotal in metabolic networks and signaling cascades where it regulates enzyme activity, especially those requiring ATP54–56. Significantly Mg2+ alters the electrophysiological properties of ion channels55 and can also affect the binding affinity of Ca2+ to specific Ca2+- binding proteins57,58,59,60, 61, thus altering the iCa2+ dynamics and signaling62. Most cell types’ total [iMg2+] is ~20 mM63, 64. The free [Mg2+] is typically in the range of ~0.5–1.0 mM, and a considerable proportion of the iMg2+ pool exists as ATP-bound Mg2+ 63, 64. Remarkably, the free [iMg2+] is 100 fold below the electrochemical potential, theoretically indicating a tight regulation of iMg2+ entry37. Regulation of iMg2+ homeostasis occurs via numerous Mg2+ transport machinery on the plasma membrane, including the ubiquitous MagT1, ACDP, TRPM7, SLC41A1, and tissue-specific TRPM6, as well as cyclin M2 and cyclin M465–69,70–79. Though these findings have enhanced the knowledge of the occurrence of significant Mg2+ fluxes in either direction across the plasma membrane of mammalian cells following metabolic or hormonal stimuli, little is known about the organellar mobilization of Mg2+. Nuclei, mitochondria, and endoplasmic or sarcoplasmic reticulum (ER/SR) compartmentalize iMg2+.
Mitochondrial Mg2+ (mMg2+) is known to significantly impact the metabolic state80–93, mitochondrial Ca2+ (mCa2+) homeostasis94–97, and apoptosis98. Mitochondria can accumulate and release Mg2+ in response to metabolic stimuli, thus representing a critical iMg2+ store37, 99, 100. Mitochondrial RNA splicing 2 (Mrs2) was identified as the primary Mg2+ transporter in yeast mitochondria35, 36 and formed a selective pore for lactatemediated mMg2+ uptake in mammals39, authenticating Mrs2 as the conserved mMg2+ transport machinery from yeast to mammals. Though Mrs2 has been shown as the transport machinery of mammalian mitochondria, Mrs2 has not been characterized. Here using the purified mammalian Mrs2 protein and the state-of-the-art planar lipid bilayer electrophysiological measurements, we characterized Mrs2 as the authentic mammalian mitochondrial Mg2+ channel (Fig. 1). Using biochemical assays, we show Mrs2 to be an integral membrane protein that localizes to the mitochondrial inner membrane with its N and C-termini within the matrix (Fig. 2). Recent structural analysis of the N-terminal domain of Mrs2 identified a novel mechanism by which Mrs2 is autoregulated by matrix [Mg2+] concentration46, implicating the N-terminal domain (NTD) to be critical for Mrs2 regulation and function.
Mitochondria display two different profiles of Ca2+ transients; a fast uptake of Ca2+ followed by a slower and more gradual uptake97, 101–108. Studies show increasing extramitochondrial Mg2+ ([Mg2+]out) to have a differential effect on these two modes of mCa2+ transients. Early initial velocity studies suggest Mg2+ be a competitive inhibitor for mCa2+ uptake where it induces sigmoid kinetics, and the degree of sigmoidicity increases with an increase in [Mg2+]out without altering the maximal activity of the uniporter97, 101–108. However, subsequent studies on kinetics reported a non-competitive inhibition of mCa2+ uptake by [Mg2+]out and are known to decrease the maximal activity of the uniporter97, 101–108. To define the mechanism by which Mg2+ regulates mCa2+ uptake, we previously resolved the atomic structure of the conserved N-terminal domain of MCU33. The crystal structure of the N-terminal domain revealed a β-grasp-like fold containing the MCU regulating acidic patch (MRAP)33. MRAP binds Mg2+/Ca2+ with ~mM affinity. The binding of divalent cations to MRAP destabilizes MCU, shifts the self-association equilibrium to monomer, and attenuates MCU-mediated mCa2+ uptake33. We anticipate divalent cations to regulate MCU activity based on our previous study.
Previously regulation of MCU channel activity was defined by the cooperative activation of MICU1/2 that sets a cytosolic Ca2+ threshold for MCU activity13, 19, 39, 109–111. However, recent seminal work showed that the regulation of MCU activity is set not only by the Ca2+ affinities of the MICU1/2 EF hands but also by matrix [Ca2+] and buffering capacity, allowing for enhanced regulation of mCa2+ homeostasis34. It is known that matrix [Ca2+] regulates MCU channel activity with a biphasic concentration dependence, with potent inhibition at ~100 nM and maximal channel inhibition (~80%) at ~400 nM34. Interestingly, high BAPTA (5 mM) and low matrix [Ca2+] also inhibit MCU activity34. If MCU is regulated by matrix [Ca2+], this result was unpredicted as the matrix Ca2+ regulatory sites should be unoccupied, and MICU1/2 should be fully Ca2+-liganded under the IMCU record conditions, which together would be expected to activate the channel. Nevertheless, channel inhibition was observed.
To increase the electrical stability of the mitoplast, the authors used 2 mM MgCl2 in the pipette solution in all their IMCU current measurements. Remarkably removal of Mg2+ from the pipette solution completely abolished MCU channel inhibition observed in high-[BAPTA]/low matrix [Ca2+] and also in 400 nM Ca2+/1.5 mM EGTA. Therefore, Mg2+ binding to the MRAP region might underlie channel inhibition observed in high-[BAPTA]/low [Ca2+]m. Though the possible role of matrix [Mg2+] in regulating MCU activity was proposed in earlier studies34, the molecular mechanism was not explored in detail. To understand the regulation of MCU by matrix [Mg2+], we generated a liver-specific Mrs2 KO mouse model (Fig. 3). The loss of Mrs2 drastically decreased the matrix [Mg2+] and thus would serve as an ideal model to study MCU regulation. Our results show a loss of Mrs2 to increase the MCU-mediated mCa2+ uptake. We measured the resting matrix [Ca2+] in control and Mrs2 KO cells. We observed no difference in the resting [Ca2+] in the presence of Ru360 and CGP, whereas the absence of both Ru360 and CGP drastically increased the resting matrix [Ca2+] (Fig.4). The increase in the matrix [Ca2+] shows the loss of Mg2+-dependent regulation of MCU. Because we saw an increased matrix [Ca2+] just by adding Tg to the bath, we anticipate the loss of Mrs2 to alter the iCa2+ threshold required to activate MCU. Also, we observed the interaction of MCU/MICU1 to be intact in the Mrs2 KD cells. Further, studies are warranted to understand the MICU1-dependent gating of MCU in the absence of matrix [Mg2+].
Because Ca2+ activates the mitochondrial permeability transition pore, we asked whether loss of MCU regulation in Mrs2 KO cells results in early mPTP opening. We observed early mPTP opening in Mrs2 KO cells (Fig. 5), similar to that observed in HEK cells in which the matrix Ca2+-dependent MCU regulation is abolished34. Our results also show Mrs2 KO mice susceptible to liver ischemia-reperfusion (IR) injury (Fig. 7). We anticipate the loss of Mrs2 to activate mPTP through two modes, direct and indirect regulation. Our results show that loss of Mrs2 positively regulates MCU activity, overloads matrix Ca2+, and triggers mPTP opening. However, Mg2+ is an inhibitor of mPTP opening112. Mg2+ modulates the mPTP pore’s sensitivity to cyclosporine A and ADP113. Additionally, work from the Bernardi laboratory show that the mPTP channel forms from dimers of F-ATP synthase after a conformational change that would follow a replacement of Mg2+ with Ca2+ at the catalytic site. Also, when Ca2+ replaces Mg2+, ATP hydrolysis is not coupled to forming an H+ gradient114.
Interestingly, our data show Mrs2 KO to have decreased ATP levels despite hyperpolarized mitochondria, suggesting an uncoupling of chemical catalysis from H+ translocation and H+ backflow through the mPTP channel (Supplementary Fig. 5). We also anticipate a fall in the electrochemical proton gradient and membrane potential depolarization in Mrs2 KO hepatocytes during early stages of development. The compensatory pathways induced by the loss of Mrs2 during development possibly reversed the direction of ATP synthase rotation, resulting in ATP hydrolysis. We anticipate the reversal of ATP synthase to extrude protons into the intermembrane space and contribute to restoring the proton gradient and the membrane potential while simultaneously resulting in a net loss of ATP115, 116. We used Oligomycin and BTB treatment to show the reverse activity of ATP synthase (Supplementary Fig. 5). Oligomycin blocks ATP hydrolysis and synthesis, whereas BTB is a specific ATP hydrolysis inhibitor. If the respiratory chain can keep up the membrane potential intact, then oligomycin treatment will hyperpolarize the mitochondria; however, if membrane potential is maintained by ATP hydrolysis, oligomycin/BTB will induce depolarization, a phenomenon termed as “oligomycin null-point”117–119. ATP hydrolysis can occur in extreme pathological conditions or normal or mildly compromised mitochondria, such as in humans with mitochondrial genetic disease and myopathies120. Our results demonstrate oligomycin null-point in Mrs2 KO mitochondria (Supplementary Fig. 5). We show in Mrs2 KO mitochondrial membrane potential to be maintained by the reversal of ATP synthase, thus depleting cellular ATP and activating mPTP opening121. We anticipate the increased matrix [Ca2+] to replace the available Mg2+ bound to the F1-F0 ATP synthase, inciting a conformational change in the functional protein dimer to the PTP114. Future studies will be necessary to understand the regulation of mPTP by loss of matrix [Mg2+] and the reversal of ATP synthase.
Our results show expression of gain-of-function Mrs2 mutant to protect cells from mPTP opening (Fig. 6). From our structural insight, we predict that at the physiological range of matrix [Mg2+], ~33 – 60% of MRAP will be occupied. Therefore, even at the highest non-physiological matrix [Mg2+], we do not anticipate a complete loss of MCU activity, thus maintaining the physiological MCU activity and energy homeostasis during pathology. Also, we show that Mrs2-mediated mitochondrial Mg2+ uptake is a critical regulator to prevent MCUmediated Ca2+ overload during I/R injury. However, future studies must study how MCU-mediated Ca2+ overload occurs during I/R injury, despite Mrs2-dependent MCU regulation in reality. We anticipate the loss of Mrs2 (either decreased channel density or decreased activity) to relieve MCU from Mg2+-dependent negative feedback regulation and increase MCU-mediated Ca2+ overload during disease pathology.
CONCLUSION
In conclusion, our results show that regulating MCU by matrix [Mg2+] is pivotal in preserving mitochondrial function and facilitating adaptation to increased workload and disease states. Thus, changes in the free matrix [Mg2+] due to mitochondrial Mg2+ entry or changes in matrix phosphate, ATP, or ADP levels, the MRAP domain of MCU will sense these differences and regulate MCU channel activity.
MATERIALS AND METHODS
Animals:
Lox/loxp knockin mice (Mrs2fl/fl) with loxP sites flanking the exon 2 (ENSMUSE00000251120) were generated using CRISPR/Cas9 strategy. Next, Mrs2fl/fl mice were crossed with liver-specific-Cre transgenic mice, albumin-Cre (B6.Cg-Speer6-ps1Tg(Alb-cre)21Mgn/J) to generate liver-specific Mrs2 knockouts (Mrs2Δhep). The deletion leads to a frameshift and early translation termination, resulting in a 57 amino acid truncated protein. Mrs2fl/fl and Mrs2Δhep mice were maintained in the Penn State College of Medicine animal facility following approval from the Institutional animal care and use committee. All mice were grouped according to sex, age, and genotype and used as required. Both male and female mice were used to isolate primary hepatocytes and mitochondria. For liver IR injury, male mice were used.
Acute liver ischemia and reperfusion injury: hanging weight system:
We used portal triad occlusion and hanging weight system to induce acute liver ischemia-reperfusion injury as described previously122. The peritoneal cavity was exposed after a midline laparotomy and incision of the linea alba. The liver was kept wet and warm during surgery with a wet swab soaked with saline at 37°C. The stomach and duodenum were caudally displaced using a wet cotton tip swab to expose the portal triad and caudate lobe. The caudate lobe was gently separated from the left lobe, and the right lobe was then slightly shifted to clearly view the portal triad above the bifurcation of the right, median, and left lobes. Once visually identified, the needle, followed by a suture (7/0 nylon suture; Ethicon, Norderstedt, Germany), was placed under the portal triad, including the hepatic artery, hepatic vein, and common bile duct. The left end of the suture was then placed over the right pole, whereas the right end was placed over the left pole, and a weight of 1.5 grams was attached to each end. The surgical wound was closed using continuous muscle walls and skin sutures. After surgery, mice were allowed to recover for 1 h of reperfusion under a heating lamp. Sham-operated mice served as the control and underwent anesthesia, laparotomy, and exposure to the portal triad without I/R. All animals survived the surgical procedure, and no complications were observed with portal triad occlusion using the hanging-weight system or in control mice.
Plasmids:
Human Mrs2 full length (Mrs2WT) and its mutants (Mrs2pΔGMN and Mrs2pΔMBS) were custom synthesized as gBlock gene fragments from IDT Inc. and cloned into pCMV6-Entry Cloning Vector (Origene) for expression in mammalian system. The plasmids/clones were confirmed by restriction enzyme analysis (REA) and sequencing before use.
Primary hepatocytes and cell lines:
Primary adult mouse hepatocytes were isolated from 10–12 week-old male and female animals using the two-step collagenase perfusion technique with slight modifications123. In brief, the liver was sequentially perfused with 50 ml of perfusion medium-I (DPBS containing 10mM HEPES, 0.05% w/v KCl, 5mM Glucose, 200 μM EDTA, pH 7.4) and 20 ml of perfusion medium-II (DPBS containing 30mM HEPES, 0.05% w/v KCL, 5mM Glucose, 1mM CaCl2, pH 7.4) containing liberase (250 μg). After perfusion, liver lobes were dissected, dissociated, and crude hepatocyte preparation was passed through a 70 μm cell strainer. The crude hepatocyte preparation was centrifuged at 50xg for 2 mins. The hepatocyte pellet was resuspended in 10 ml of attachment media twice, and viable hepatocytes were separated from dead cells by the Percoll gradient (90%) by centrifugation at 200xg for 20 mins (centrifuged with low acceleration and low brake to minimize trauma to hepatocytes). Following gradient purification, the hepatocytes were plated in culture dishes using the hepatocyte attachment medium (Williams E medium containing 1% (v/v) antibioticantimycotic solution (GIBCO), 1% (v/v) 200mM L-glutamine, 1% (v/v) non-essential amino acids, and 10% fetal bovine serum). After attachment, media was replaced with the hepatocyte culture medium (Williams E medium containing 1% (v/v) antibiotic-antimycotic solution (GIBCO), 1% (v/v) 200mM L-glutamine, and 1% (v/v) nonessential amino acids).
HEK 293 (ATCC# CRL 1573) cells were grown in Dulbecco’s modified Eagle’s medium (DMEM)/10% FBS, supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin at 5% CO2 and 37⁰C. MISSION lentiviral particles (NM_020662.11348s1c1) carrying the shRNA (target sequence: CTTTGTCAGTAT AGGGAATTA) targeting 3’UTR were used to knock down Mrs2 in HEK293 cells. HEK293 cells (5×105 per well) grown in 6-well plates were transduced with a lentivirus. Seventy-two hours post-transduction, cells were selected with puromycin (2 μg/ml) for 6–10 days until clonal. The puromycin-resistant clones were expanded and stored. The level of Mrs2 Knockdown was assessed by qPCR and Western blot analysis.
HEK293 (5×105 per well) cells grown in 6-well plates were transfected with plasmids expressing Mrs2pWT, Mrs2pΔGMN, and Mrs2pΔMBS. Forty-eight hours post-transfection cells were selected with G418 sulfate (2 μg/ml) for 6–10 days until clonal. The G418-resistant clones were expanded and stored. The stable expression of Mrs2 was assessed by Western blot analysis.
Western blotting:
Cell extracts from tissues/control and KO hepatocytes/HEK293 NegShRNA and Mrs2 KD cells were prepared using RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1 mM EDTA, 1% NP-40, protease inhibitor cocktail (Complete, Roche), and Halt phosphatase inhibitor cocktail (Thermo Scientific). Equal amounts of protein (25 μg/lane) were separated on 4–12% Bis-Tris polyacrylamide gel, transferred to a PVDF membrane using iBlot 2 PVDF regular stacks (Thermo Scientific) and probed with antibodies specific for Mrs2 (1:500, Novus biologicals: NBP2–34200), MCU (1:500, Cell signaling technology: 14997), MICU1 (1:500, Cell signaling technology: 12524), cytochrome c (1:5000, Santa Cruz: sc-13156), actin (1:5000, Santa Cruz: sc-47778), Total OXPHOS Rodent Cocktail (1:5000, Abcam: ab110413), MCUR1 (1:500, Cell signaling technology: 13706), HA-Tag-HRP Conjugate (1:2000, Cell signaling technology: 2999), Monoclonal ANTI-FLAG® M2 (1:3000, Millipore Sigma: F1804), Phospho-PDH (1:500, Cell signaling technology: 31866), Pyruvate Dehydrogenase (1:500, Cell signaling technology: 2784) Phospho-AMPKα (Thr172) (1:500, Cell signaling technology: 2535), AMPKα (1:500, Cell signaling technology: 2532).
Localization and orientation of Mrs2:
To test the localization of Mrs2 to the mitochondrial membrane, permeabilized HEK293 cells stably expressing Mrs2WT-Flag were permeabilized with digitonin (40 μg/ml). The permeabilized cells were exposed to mastoparan (20 μg/ml; a wasp venom peptide toxin that induces OMM permeabilization) or alamethicin (20 μg/ml; a fungal peptide that induces large pores in both mitochondrial membranes)12 for 5 mins. After permeabilization and treatment, cytosolic (supernatant) and mitochondrial (pellet) fractions were collected and subjected to Western blotting with antibodies specific for CytC, Mrs2, MCU, SPG7 (1:500, Novus biologicals: NBP2–01860), and CypD (1:1000, Santa Cruz: sc-376061). To test the orientation of Mrs2, mitochondria isolated from HEK293 cells stably expressing Mrs2WT-Flag were suspended in a hypotonic solution (5 mM sucrose, 5 mM HEPES, and 1mM EGTA (pH 7.2)) to swell and rupture the OMM. The suspension was centrifuged at 1000xg, and pelleted mitoplasts were resuspended in mitochondrial resuspension buffer (750 mM KCl, 100 mM HEPES, and 1 mM EGTA (pH 7.2)). The mitoplasts were treated with proteinase K digestion followed by Western blot analysis with antibodies specific for Mrs2, MCU, CypD, Tom20 (1:1000, Santa Cruz: sc-17764), and actin.
Co-immunoprecipitation and Western Blot Analysis:
Cell extracts were prepared from transiently transfected HEK293 cells using RIPA buffer (50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.25% deoxycholic acid, 1 mM EDTA, 1% NP-40, protease inhibitor cocktail (Complete: Roche and 1 mM PMSF). To study the interaction of MCU with MCU complex components in the presence of varying concentrations of Mg2+, HA-tagged MCU was co-transfected with Flag-tagged MCUR1, EMRE, MICU1, and MCUb. Following immunoprecipitation with HA antibody in the presence of varying concentrations of MgCl2, total cell lysates and immunoprecipitated materials were subjected to Western blot analysis. 10% of cell lysates were probed with Flag and HA antibodies to serve as inputs, and similarly, immunoprecipitated samples were probed with their corresponding antibodies to assess protein binding. To study the interaction of Mrs2 with MCU complex components, HAtagged Mrs2 was co-transfected with Flag-tagged MCU, MCUR1, EMRE, and MICU1. Co-immunoprecipitation and western blotting were performed as described above. To study whether the interaction of MCU and MICU1 still exists in the absence of matrix Mg2+, we co-transfected MCU-Flag and MICU1-HA in HEK293 NegShRNA and Mrs2KD cells.
qPCR analysis:
Various mouse tissues were collected from WT/Mrs2fl/fl/Mrs2Δhep mice and preserved in RNA later. mRNA was isolated from tissues using the PureLink RNA Mini Kit (Invitrogen, ThermoFisher Scientific). cDNA was prepared using the iScript™ cDNA Synthesis Kit (BIO-RAD, USA) following the manufacturer’s instructions. qPCR was performed using the PrimeTime™ Gene Expression Master Mix and PrimeTime predesigned probe for mouse Mrs2. Actin was used to normalize the mRNA levels. Mrs2 expression in HEK293 NegShRNA and Mrs2KD was analyzed by qPCR using the PrimeTime predesigned probe for human Mrs2. HPRT was used to normalize the mRNA levels.
Simultaneous measurement of mMg2+/mCa2+ uptake and mitochondrial membrane potential in permeabilized cells:
An equal number of Mrs2fl/fl/Mrs2Δhep /HEK293 NegShRNA/Mrs2KD cells (6 × 106 cells) were washed in Ca2+/Mg2+ free PBS, pH 7.4, resuspended and permeabilized with 40 μg/ml digitonin in 1.5 ml of intracellular medium (ICM) composed of 120 mM KCl, 10 mM NaCl, 1 mM KH2PO4, 20 mM Hepes-Tris, pH 7.2 and 2 μM thapsigargin to block the SERCA pump. All the measurements were performed in the presence of 5 mM succinate. Δψm and extramitochondrial Ca2+ ([Ca2+]out) clearance as an indicator of mCa2+ uptake was achieved by loading the permeabilized cells with JC-1 (800 nM) and Fura2-FF (0.5 μM), respectively. Fluorescence was monitored in a multi-wavelength excitation dual-wavelength emission fluorimeter (Delta RAM, PTI). [Ca2+]out is represented as the excitation ratio (340 nm/380 nm) of Fura2-FF/FA fluorescence, and Δψm as the ratio of the fluorescence of J-aggregate (570 nm excitation/595 nm emission) and monomer (490 nm excitation/535 nm emission) forms of JC-1. A single or series of Ca2+ bolus (20, 10, or 1 μM) and mitochondrial uncoupler, CCCP (2 μM), were added at the indicated time points. All the experiments were performed at 37°C with constant stirring27, 38, 124–128.
To measure mMg2+ uptake, we performed a similar experiment using the ratiometric dye, Mag-Fura-2 tetra potassium salt. Mag-Fura-2 was calibrated, and the [mMg2+] was determined by Where the dissociation constant Kd is 1.98 mM.
Mitoplast patch-clamp recording:
IMCU measurements:
Mitoplast patch-clamp recordings for MCU (IMCU) were performed at 30°C, as reported earlier38, 125, 128. In brief, freshly prepared mitoplasts from HeLa, HEK293 cells, or hepatocytes of Mrs2fl/fl and Mrs2Δhep mice were plated on the Cell-Tak–coated coverslips and mounted on the microscope. The mitoplasts were bathed in a solution containing 150 mM sodium gluconate, 5.4 mM KCl, 5 mM CaCl2, and 10 mM HEPES (pH 7.2). The pipette solution contained 150 mM sodium gluconate, 5 mM NaCl, 135 mM sucrose, 10 mM HEPES, and 1.5 mM EGTA (pH 7.2). After the formation of GΩ seals (20 to 35 MΩ), the mitoplasts were ruptured with a 200 to 400 mV pulse for 2 to 6 ms. After capacitance (2.5 to 3.0 pF) compensation, mitoplasts were held at 0 mV, and IMCU was recorded with a voltage ramp (from −160 to 80 mV, 120 mV/s). The external/bath solution (5 mM Ca2+) was chosen based on our previous measurements38, 125, 128. Samples were discarded if the break-in took longer than 5 s after adding 5 mM Ca2+. IMCU currents were recorded using an Axon200B patch-clamp amplifier with a Digidata 1320A acquisition board (pCLAMP 10.0 software; Axon Instruments). For measuring the effect of matrix Mg2+ on IMCU, varying concentrations of Mg2+ were present in the pipette solution.
IMrs2 measurements:
Mitoplasts were prepared from Mrs2fl/fl and Mrs2Δhep, as mentioned above. The mitoplasts were bathed in a solution containing Na-gluconate (150 mM), HEPES (10 mM) (pH 7.4 with N’-Methyl-Dglucamine (NMDG)), NaCl (5 mM), sucrose (135 mM), and 5 mM MgCl2. In some measurements, 200 nM ruthenium red (Ru360) or 1 μM hexamine cobalt(III)chloride was added to the bath. IMrs2 was recorded as described above.
Protein purification and planar lipid bilayer recordings for single-channel activity measurements:
The construct for Mrs2 was expressed in HEK 293 cells and purified using the EZ view Red ANTI-FLAG M2 Affinity Gel (Sigma), according to the manufacturer’s protocol. A silver-stained gel assessed the purity of isolated Mrs2 protein. Planar lipid bilayer recordings were performed in 150 mM NMDG and 10 mM HEPES solution buffered at (pH 7.4); supplemented with 20–104 mM Mg2+ during recordings on the cis side. α-L-phosphatidylcholine (Sigma) was used for forming the bilayer membrane. ePatch amplifier (Elements) was used for lipid bilayer recordings. Purified Mrs2 (5 μg, final concentration) was used for single-channel recordings, and Co2+ (1–5 mM, final concentration) was added into the bath during the recordings without perfusion. pCLAMP-10 software was used for electrophysiology data acquisition and analysis (Molecular devices). All current measurements were adjusted for the holding voltage assuming a linear current-voltage relationship: The resulting conductances are expressed in pS according to the equation G = I/V where G is conductance in pS, V is the membrane holding voltage in mV, and I is the peak membrane current in pA after subtraction of the baseline electrode leak current. Group data were quantified in terms of conductance. All population data were expressed as mean ± SEM.
ATP Measurement:
Total ATP abundance was assessed in Mrs2fl/fl/Mrs2Δhep /HEK293 NegShRNA/Mrs2KD cells using CellTiter-Glo® luminescent assay as per manufacturer’s instructions27, 38, 125–128.
Mitochondrial Oxygen Consumption Rate:
Intact Mrs2fl/fl/Mrs2Δhep /HEK293 NegShRNA/Mrs2KD cells were subjected to oxygen consumption rate (OCR) measurement at 37°C in an XFe24 extracellular flux analyzer (Seahorse Bioscience). Cells (2.5 × 105) were sequentially exposed to 2 μM oligomycin, 0.5 μM FCCP, and 0.5 μM rotenone plus antimycin A at indicated time points to measure basal and maximal respiration, ATP production, proton leak, spare respiratory capacity, and non-mitochondrial respiration as described previously27, 38, 125–128.
Microscopy, live cell imaging, and confocal measurements:
(i) Mitochondrial localization of Mrs2, confocal imaging: 0.5×106 HeLa cells grown on 0.2% gelatin-coated glass coverslips were co-transfected with Cox8-mRFP and Mrs2-GFP. Forty-eight hours post-transfection, confocal images (Leica SP8) were obtained at 561 and 488 nm excitation using a ×63 oil objective. Overlap of RFP and GFP signals was quantified using Leica LAS-X software. (ii) Mitochondrial localization of Mrs2: SIM super resolution microscopy. 0.5×106 HeLa cells grown on 0.2% gelatin-coated glass coverslips were co-transfected with Cox8-mRFP and Mrs2-GFP. Forty-eight hours post-transfection, cells were fixed with 4% paraformaldehyde and stored at 4°C until imaging. The slides were imaged under a Nikon N-SIM/STORM SR microscope in SIM mode with an SR Apo TIRF100x, NA1.49 objective, and the images were captured with an Andor DU 897x EMCCD camera. The GFP channel was excited by a 488 nm laser to collect an emission peak at 522 nm, while the RFP was excited by a 561 nm laser to obtain an emission peak at 605 nm. Images taken in 3D SIM mode were processed and reconstructed with Nikon Elements. The reconstructed images were analyzed for colocalization in Nikon Elements and ImageJ. (iii) TMRM and Calcein fluorescence (Time-series/intact cell) measurements: 0.5×106 hepatocytes isolated from Mrs2fl/fl and Mrs2Δhep mice grown on 0.1% collagen-coated glass coverslips were stained with the Δψm indicator TMRM or PTP opening indicator calceinAM. Coverslips were mounted in an open perfusion micro incubator (PDMI-2; Harvard Apparatus) and imaged at 561 or 488 excitations using a ×63 oil objective. After baseline measurements, ionomycin (5 μM) was added, and changes in TMRM or calcein fluorescence were recorded every 3 s (Leica SP8) for 10 mins. The loading and imaging buffer contained cobalt chloride (CoCl2; 1mM) for calcein measurement. The presence of CoCl2 quenched the calcein fluorescence in the cytosol and nucleus, leaving the mitochondrial fluorescence intact. As the PTP opens, CoCl2 enters the mitochondrial matrix and quenches calcein fluorescence. The decrease in calcein fluorescence after ionomycin treatment was measured using the Leica LAS-X software. (iv) Confocal imaging of mitochondrial Δψm: 0.5×106 hepatocytes isolated from Mrs2fl/fl and Mrs2Δhep mice grown on 0.1% collagen-coated glass coverslips were stained with the Δψm indicator TMRM and dihydrorhodamine 123 (DHR123). Coverslips were mounted in an open perfusion micro incubator (PDMI-2; Harvard Apparatus) and imaged at 561 or 488 excitations using a ×63 oil objective. Images were analyzed, and the mean TMRM fluorescence was quantified using Image J software (NIH). (v) Measurement of cytosolic Mg2+ dynamics. 0.5×106 hepatocytes isolated from Mrs2fl/fl and Mrs2Δhep mice grown on 0.1% collagen-coated glass coverslips were stained with MagGree-AM and MitoTracker Red. Coverslips were mounted in an open perfusion micro incubator (PDMI-2; Harvard Apparatus) and imaged at 488 and 561 excitations using a ×63 oil objective. After baseline measurements, glucagon (10 μM) and 2 mM Mg2+ were added at the indicated time. MagGreen fluorescence changes (cytosolic and mitochondrial) were recorded every 3 s (Leica SP8) for 10 mins. The rate of cytosolic MagGreen decay and the mitochondrial peak fluorescence was measured using Leica LAS-X software.
Mitochondrial isolation and swelling assay:
Hepatocytes isolated from Mrs2fl/fl and Mrs2Δhep were homogenized in ice-cold mitochondrial isolation buffer (10 mM sucrose, 200 mM mannitol, 5 mM HEPES, and 1 mM EGTA, pH 7.4) containing 1 mg/ml fatty acid-free bovine serum albumin. The homogenate was centrifuged for 10 min at 1000 x g, and the supernatant was centrifuged again at 14,000 x g for 10 min. The mitochondrial pellets were washed twice and centrifuged at 11,200 xg. The isolated mitochondria (1 mg protein) were added to 0.2 ml of mitochondrial swelling buffer (70 mM sucrose, 230 mM mannitol, 3 mM HEPES, 2 mM Trisphosphate, 5 mM succinate). Mitochondrial swelling was measured by the decrease in absorbance at 540 nm after adding Ca2+ (250 μM)27.
Flow Cytometry and PI Staining:
Hepatocytes isolated from Mrs2fl/fl and Mrs2Δhep were treated with ionomycin (25 μM) for 6 hr. For necrotic cell death measurement, cells were stained with propidium iodide. The cells were analyzed with the BD LSR Fortessa (BD Biosciences). Relative PI staining was plotted on a logarithmic scale using Flow-Jo software27, 38.
Size Exclusion Chromatographic Analysis of MCU Complex:
Gel filtration was performed by fast protein liquid chromatography (ÄKTA Pure FPLC; GE Healthcare), and the Superdex 200 10/300 column was equilibrated with PBS. Column calibration was carried out with a gel filtration protein standards kit (Bio-Rad). Cleared lysates from HEK293 NegShRNA and Mrs2KD cells stably expressing MCU-FLAG were directly loaded onto a Superdex 200 FPLC column at a 0.5 ml/min flow rate. Forty fractions were collected, concentrated, and fractionation of MCU complex was assayed by Western blot analysis27, 125.
Plasma enzymatic and cytokine measurements:
Plasma aspartate (AST) and alanine (ALT) aminotransferase activities were measured using a commercially available kit (Cayman) as per the manufacturer’s instruction. We used Mouse Cytokine Array Panel A (Proteome ProfilerTM Array; R&D systems) to determine the relative levels of 40 mouse cytokines.
Histological assessment of damage:
The median and left liver lobes were harvested and fixed in 4% formalin. Fixed tissues were subsequently sectioned, ten μm thick, collected on (+) charge slides, and stained with hematoxylin and eosin. Examination and scoring of each lobe were carried out by a pathologist who was blinded to the experimental group.
Quantification and statistical analysis:
Data from multiple experiments (≥3) were quantified and expressed as Mean ± SE, and differences between groups were analyzed using the two-tailed paired Student’s t-test or, when not normally distributed, a nonparametric Mann–Whitney U-test (Wilcoxon Rank-Sum Test) for two groups. The data were computed with GraphPad Prism version 9.0 or SigmaPlot 11.0 software. Differences in means among multiple datasets were analyzed using one-way ANOVA with Tukey correction performed. A P ≤ 0.05 was considered significant in all analyses.
ACKNOWLEDGEMENTS
This study was supported by the National Institute of Health (R00 HL138268 to SS, R21DA051798 to KN, K01AG054734 and RF1AG072484 to NM, P01HL147841 to SR), the W.W. Smith Charitable Trust (H2103) to SS, Career Development Grant from the American Heart Association (935207) to SS, the Bridge grant from the office of Vice Dean for Research, Penn State College of Medicine to SS, and the start-up funds from the Penn State College of Medicine to SS. This project is funded, in part, under a grant from the Pennsylvania Department of Health using Tobacco CURE Funds. The Department expressly disclaims responsibility for any analyses, interpretations, or conclusions.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Additional Declarations: There is NO Competing Interest.
Supplementary Files
This is a list of supplementary les associated with this preprint. Click to download.
REFERENCES
- 1.Glancy B. & Balaban R.S. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry 51, 2959–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drago I., Pizzo P. & Pozzan T. After half a century mitochondrial calcium in- and efflux machineries reveal themselves. EMBO J 30, 4119–4125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duchen M.R., Verkhratsky A. & Muallem S. Mitochondria and calcium in health and disease. Cell Calcium 44, 1–5 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Nicholls D.G. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem J 176, 463–474 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizzuto R., De Stefani D., Raffaello A. & Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13, 566–578 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Santo-Domingo J. & Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta 1797, 907–912 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Williams G.S., Boyman L., Chikando A.C., Khairallah R.J. & Lederer W.J. Mitochondrial calcium uptake. Proc Natl Acad Sci U S A 110, 10479–10486 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirichok Y., Krapivinsky G. & Clapham D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427, 360–364 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Baughman J.M. et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhuri D., Sancak Y., Mootha V.K. & Clapham D.E. MCU encodes the pore conducting mitochondrial calcium currents. Elife 2, e00704 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Stefani D., Raffaello A., Teardo E., Szabo I. & Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman N.E. et al. MICU1 motifs define mitochondrial calcium uniporter binding and activity. Cell Rep 5, 1576–1588 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antony A.N. et al. MICU1 regulation of mitochondrial Ca(2+) uptake dictates survival and tissue regeneration. Nat Commun 7, 10955 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sancak Y. et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342, 1379–1382 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Csordas G. et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca(2)(+) uniporter. Cell Metab 17, 976–987 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vais H. et al. EMRE Is a Matrix Ca(2+) Sensor that Governs Gatekeeping of the Mitochondrial Ca(2+) Uniporter. Cell Rep 14, 403–410 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vais H. et al. MCUR1, CCDC90A, Is a Regulator of the Mitochondrial Calcium Uniporter. Cell Metab 22, 533–535 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallilankaraman K. et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol 14, 1336–1343 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mallilankaraman K. et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival. Cell 151, 630–644 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denton R.M. & McCormack J.G. The role of calcium in the regulation of mitochondrial metabolism. Biochem Soc Trans 8, 266–268 (1980). [DOI] [PubMed] [Google Scholar]
- 21.McCormack J.G. & Denton R.M. The role of mitochondrial Ca2+ transport and matrix Ca2+ in signal transduction in mammalian tissues. Biochim Biophys Acta 1018, 287–291 (1990). [DOI] [PubMed] [Google Scholar]
- 22.Hajnoczky G., Robb-Gaspers L.D., Seitz M.B. & Thomas A.P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82, 415–424 (1995). [DOI] [PubMed] [Google Scholar]
- 23.Hajnoczky G. et al. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium 40, 553–560 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajnoczky G., Davies E. & Madesh M. Calcium signaling and apoptosis. Biochem Biophys Res Commun 304, 445–454 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Bernardi P. The mitochondrial permeability transition pore: a mystery solved? Front Physiol 4, 95 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernardi P. & Di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J Mol Cell Cardiol 78, 100–106 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shanmughapriya S. et al. SPG7 Is an Essential and Conserved Component of the Mitochondrial Permeability Transition Pore. Mol Cell 60, 47–62 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luongo T.S. et al. The Mitochondrial Calcium Uniporter Matches Energetic Supply with Cardiac Workload during Stress and Modulates Permeability Transition. Cell Rep 12, 23–34 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bezprozvanny I., Watras J. & Ehrlich B.E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351, 751–754 (1991). [DOI] [PubMed] [Google Scholar]
- 30.Meissner G., Darling E. & Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry 25, 236–244 (1986). [DOI] [PubMed] [Google Scholar]
- 31.Hoth M., Fasolato C. & Penner R. Ion channels and calcium signaling in mast cells. Ann N Y Acad Sci 707, 198–209 (1993). [DOI] [PubMed] [Google Scholar]
- 32.Hoth M. & Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol 465, 359–386 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee S.K. et al. Structural Insights into Mitochondrial Calcium Uniporter Regulation by Divalent Cations. Cell Chem Biol (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vais H., Payne R., Paudel U., Li C. & Foskett J.K. Coupled transmembrane mechanisms control MCU-mediated mitochondrial Ca(2+) uptake. Proc Natl Acad Sci U S A 117, 21731–21739 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bui D.M., Gregan J., Jarosch E., Ragnini A. & Schweyen R.J. The bacterial magnesium transporter CorA can functionally substitute for its putative homologue Mrs2p in the yeast inner mitochondrial membrane. J Biol Chem 274, 20438–20443 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Kolisek M. et al. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. EMBO J 22, 1235–1244 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daw C.C. et al. Lactate Elicits ER-Mitochondrial Mg(2+) Dynamics to Integrate Cellular Metabolism. Cell 183, 474–489 e417 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shanmughapriya S. et al. Ca2+ signals regulate mitochondrial metabolism by stimulating CREBmediated expression of the mitochondrial Ca2+ uniporter gene MCU. Sci Signal 8, ra23 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Payne R., Hoff H., Roskowski A. & Foskett J.K. MICU2 Restricts Spatial Crosstalk between InsP(3)R and MCU Channels by Regulating Threshold and Gain of MICU1-Mediated Inhibition and Activation of MCU. Cell Rep 21, 3141–3154 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Midgley P.J., Rutter G.A., Thomas A.P. & Denton R.M. Effects of Ca2+ and Mg2+ on the activity of pyruvate dehydrogenase phosphate phosphatase within toluene-permeabilized mitochondria. Biochem J 241, 371–377 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galkin A., Abramov A.Y., Frakich N., Duchen M.R. & Moncada S. Lack of oxygen deactivates mitochondrial complex I: implications for ischemic injury? J Biol Chem 284, 36055–36061 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palombo I., Daley D.O. & Rapp M. Why is the GMN motif conserved in the CorA/Mrs2/Alr1 superfamily of magnesium transport proteins? Biochemistry 52, 4842–4847 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Merolle L. et al. Overexpression of the mitochondrial Mg channel MRS2 increases total cellular Mg concentration and influences sensitivity to apoptosis. Metallomics 10, 917–928 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Knoop V., Groth-Malonek M., Gebert M., Eifler K. & Weyand K. Transport of magnesium and other divalent cations: evolution of the 2-TM-GxN proteins in the MIT superfamily. Mol Genet Genomics 274, 205–216 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Dalmas O., Sompornpisut P., Bezanilla F. & Perozo E. Molecular mechanism of Mg2+-dependent gating in CorA. Nat Commun 5, 3590 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uthayabalan S., Vishnu N., Madesh M. & Stathopulos P.B. The human MRS2 magnesium-binding domain is a regulatory feedback switch for channel activity. Life Sci Alliance 6 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemasters J.J., Ji S., Stemkowski C.J. & Thurman R.G. Hypoxic hepatocellular injury. Pharmacol Biochem Behav 18 Suppl 1, 455–459 (1983). [DOI] [PubMed] [Google Scholar]
- 48.Iu S. et al. Markers of allograft viability in the rat. Relationship between transplantation viability and liver function in the isolated perfused liver. Transplantation 44, 562–569 (1987). [DOI] [PubMed] [Google Scholar]
- 49.Hartwig A. Role of magnesium in genomic stability. Mutat Res 475, 113–121 (2001). [DOI] [PubMed] [Google Scholar]
- 50.Klein D.J., Moore P.B. & Steitz T.A. The contribution of metal ions to the structural stability of the large ribosomal subunit. RNA 10, 1366–1379 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selmer M. et al. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 313, 1935–1942 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Yang W., Lee J.Y. & Nowotny M. Making and breaking nucleic acids: two-Mg2+-ion catalysis and substrate specificity. Mol Cell 22, 5–13 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Beaven G.H., Parmar J., Nash G.B., Bennett B.M. & Gratzer W.B. Effect of magnesium ions on red cell membrane properties. J Membr Biol 118, 251–257 (1990). [DOI] [PubMed] [Google Scholar]
- 54.de Baaij J.H., Hoenderop J.G. & Bindels R.J. Magnesium in man: implications for health and disease. Physiol Rev 95, 1–46 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Romani A.M. Cellular magnesium homeostasis. Arch Biochem Biophys 512, 1–23 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Romani A.M. Magnesium in health and disease. Met Ions Life Sci 13, 49–79 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Martin S.R., Masino L. & Bayley P.M. Enhancement by Mg2+ of domain specificity in Ca2+ dependent interactions of calmodulin with target sequences. Protein Sci 9, 2477–2488 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ogoma Y. et al. Binding study of metal ions to S100 protein: 43Ca, 25Mg, 67Zn and 39K n.m.r. Int J Biol Macromol 14, 279–286 (1992). [DOI] [PubMed] [Google Scholar]
- 59.Finley N., Dvoretsky A. & Rosevear P.R. Magnesium-calcium exchange in cardiac troponin C bound to cardiac troponin I. J Mol Cell Cardiol 32, 1439–1446 (2000). [DOI] [PubMed] [Google Scholar]
- 60.Cates M.S., Teodoro M.L. & Phillips G.N. Jr. Molecular mechanisms of calcium and magnesium binding to parvalbumin. Biophys J 82, 1133–1146 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwaller B. The continuing disappearance of “pure” Ca2+ buffers. Cell Mol Life Sci 66, 275–300 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grabarek Z. Insights into modulation of calcium signaling by magnesium in calmodulin, troponin C and related EF-hand proteins. Biochim Biophys Acta 1813, 913–921 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maguire M.E. & Cowan J.A. Magnesium chemistry and biochemistry. Biometals 15, 203–210 (2002). [DOI] [PubMed] [Google Scholar]
- 64.Romani A. & Scarpa A. Regulation of cell magnesium. Arch Biochem Biophys 298, 1–12 (1992). [DOI] [PubMed] [Google Scholar]
- 65.Nelson D.L. & Kennedy E.P. Magnesium transport in Escherichia coli. Inhibition by cobaltous ion. J Biol Chem 246, 3042–3049 (1971). [PubMed] [Google Scholar]
- 66.Park M.H., Wong B.B. & Lusk J.E. Mutants in three genes affecting transport of magnesium in Escherichia coli: genetics and physiology. J Bacteriol 126, 1096–1103 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silver L. & Brendler H. Use of magnesium oxide in management of familial hyperoxaluria. J Urol 106, 274–279 (1971). [DOI] [PubMed] [Google Scholar]
- 68.Silver S. Active transport of magnesium in escherichia coli. Proc Natl Acad Sci U S A 62, 764–771 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silver S. & Clark D. Magnesium transport in Escherichia coli. J Biol Chem 246, 569–576 (1971). [PubMed] [Google Scholar]
- 70.Hattori M., Tanaka Y., Fukai S., Ishitani R. & Nureki O. Crystal structure of the MgtE Mg2+ transporter. Nature 448, 1072–1075 (2007). [DOI] [PubMed] [Google Scholar]
- 71.Hattori M. et al. Mg(2+)-dependent gating of bacterial MgtE channel underlies Mg(2+) homeostasis. EMBO J 28, 3602–3612 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lunin V.V. et al. Crystal structure of the CorA Mg2+ transporter. Nature 440, 833–837 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eshaghi S. et al. Crystal structure of a divalent metal ion transporter CorA at 2.9 angstrom resolution. Science 313, 354–357 (2006). [DOI] [PubMed] [Google Scholar]
- 74.Payandeh J. & Pai E.F. A structural basis for Mg2+ homeostasis and the CorA translocation cycle. EMBO J 25, 3762–3773 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Payandeh J. & Pai E.F. Crystallization and preliminary X-ray diffraction analysis of the magnesium transporter CorA. Acta Crystallogr Sect F Struct Biol Cryst Commun 62, 148–152 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guskov A. et al. Structural insights into the mechanisms of Mg2+ uptake, transport, and gating by CorA. Proc Natl Acad Sci U S A 109, 18459–18464 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guskov A. & Eshaghi S. The mechanisms of Mg2+ and Co2+ transport by the CorA family of divalent cation transporters. Curr Top Membr 69, 393–414 (2012). [DOI] [PubMed] [Google Scholar]
- 78.Pfoh R. et al. Structural asymmetry in the magnesium channel CorA points to sequential allosteric regulation. Proc Natl Acad Sci U S A 109, 18809–18814 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nordin N. et al. Exploring the structure and function of Thermotoga maritima CorA reveals the mechanism of gating and ion selectivity in Co2+/Mg2+ transport. Biochem J 451, 365–374 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Denton R.M., McCormack J.G. & Edgell N.J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem J 190, 107–117 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hucho F. Regulation of the mammalian pyruvate dehydrogenase multienzyme complex by Mg2+ and the adenine nucleotide pool. Eur J Biochem 46, 499–505 (1974). [DOI] [PubMed] [Google Scholar]
- 82.Leyssens A., Nowicky A.V., Patterson L., Crompton M. & Duchen M.R. The relationship between mitochondrial state, ATP hydrolysis, [Mg2+]i and [Ca2+]i studied in isolated rat cardiomyocytes. J Physiol 496 (Pt 1), 111–128 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Panov A. & Scarpa A. Mg2+ control of respiration in isolated rat liver mitochondria. Biochemistry 35, 12849–12856 (1996). [DOI] [PubMed] [Google Scholar]
- 84.Panov A. & Scarpa A. Independent modulation of the activity of alpha-ketoglutarate dehydrogenase complex by Ca2+ and Mg2+. Biochemistry 35, 427–432 (1996). [DOI] [PubMed] [Google Scholar]
- 85.Plaut G.W. & Aogaichi T. Purification and properties of diphosphopyridine nuleotide-linked isocitrate dehydrogenase of mammalian liver. J Biol Chem 243, 5572–5583 (1968). [PubMed] [Google Scholar]
- 86.Qi F., Chen X. & Beard D.A. Detailed kinetics and regulation of mammalian NAD-linked isocitrate dehydrogenase. Biochim Biophys Acta 1784, 1641–1651 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rutter G.A. & Denton R.M. Rapid purification of pig heart NAD+-isocitrate dehydrogenase. Studies on the regulation of activity by Ca2+, adenine nucleotides, Mg2+ and other metal ions. Biochem J 263, 445–452 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomas A.P., Diggle T.A. & Denton R.M. Sensitivity of pyruvate dehydrogenase phosphate phosphatase to magnesium ions. Similar effects of spermine and insulin. Biochem J 238, 83–91 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tretter L. & Adam-Vizi V. Alpha-ketoglutarate dehydrogenase: a target and generator of oxidative stress. Philos Trans R Soc Lond B Biol Sci 360, 2335–2345 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Willson V.J. & Tipton K.F. The effect of ligands on the irreversible inhibition of the NAD+-dependent isocitrate dehydrogenase from ox brain. Eur J Biochem 117, 65–68 (1981). [DOI] [PubMed] [Google Scholar]
- 91.Willson V.J. & Tipton K.F. The activation of ox-brain NAD+-dependent isocitrate dehydrogenase by magnesium ions. Eur J Biochem 113, 477–483 (1981). [DOI] [PubMed] [Google Scholar]
- 92.Yamanaka R. et al. Mitochondrial Mg(2+) homeostasis decides cellular energy metabolism and vulnerability to stress. Sci Rep 6, 30027 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McLain A.L., Szweda P.A. & Szweda L.I. alpha-Ketoglutarate dehydrogenase: a mitochondrial redox sensor. Free Radic Res 45, 29–36 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Llorente-Folch I. et al. The regulation of neuronal mitochondrial metabolism by calcium. J Physiol 593, 3447–3462 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rueda C.B. et al. Ca(2+) regulation of mitochondrial function in neurons. Biochim Biophys Acta 1837, 1617–1624 (2014). [DOI] [PubMed] [Google Scholar]
- 96.Williams G.S., Boyman L. & Lederer W.J. Mitochondrial calcium and the regulation of metabolism in the heart. J Mol Cell Cardiol 78, 35–45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boelens A.D. et al. Extra-matrix Mg2+ limits Ca2+ uptake and modulates Ca2+ uptake-independent respiration and redox state in cardiac isolated mitochondria. J Bioenerg Biomembr 45, 203–218 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Seo Y.W. et al. The molecular mechanism of Noxa-induced mitochondrial dysfunction in p53-mediated cell death. J Biol Chem 278, 48292–48299 (2003). [DOI] [PubMed] [Google Scholar]
- 99.Kubota T. et al. Mitochondria are intracellular magnesium stores: investigation by simultaneous fluorescent imagings in PC12 cells. Biochim Biophys Acta 1744, 19–28 (2005). [DOI] [PubMed] [Google Scholar]
- 100.Shindo Y. et al. Newly developed Mg2+-selective fluorescent probe enables visualization of Mg2+ dynamics in mitochondria. PLoS One 6, e23684 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beutner G., Sharma V.K., Giovannucci D.R., Yule D.I. & Sheu S.S. Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem 276, 21482–21488 (2001). [DOI] [PubMed] [Google Scholar]
- 102.Blomeyer C.A., Bazil J.N., Stowe D.F., Dash R.K. & Camara A.K. Mg(2+) differentially regulates two modes of mitochondrial Ca(2+) uptake in isolated cardiac mitochondria: implications for mitochondrial Ca(2+) sequestration. J Bioenerg Biomembr 48, 175–188 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buntinas L., Gunter K.K., Sparagna G.C. & Gunter T.E. The rapid mode of calcium uptake into heart mitochondria (RaM): comparison to RaM in liver mitochondria. Biochim Biophys Acta 1504, 248–261 (2001). [DOI] [PubMed] [Google Scholar]
- 104.Gunter T.E., Buntinas L., Sparagna G.C. & Gunter K.K. The Ca2+ transport mechanisms of mitochondria and Ca2+ uptake from physiological-type Ca2+ transients. Biochim Biophys Acta 1366, 515 (1998). [DOI] [PubMed] [Google Scholar]
- 105.Huser J., Blatter L.A. & Sheu S.S. Mitochondrial calcium in heart cells: beat-to-beat oscillations or slow integration of cytosolic transients? J Bioenerg Biomembr 32, 27–33 (2000). [DOI] [PubMed] [Google Scholar]
- 106.O’Rourke B. & Blatter L.A. Mitochondrial Ca2+ uptake: tortoise or hare? J Mol Cell Cardiol 46, 767–774 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sparagna G.C., Gunter K.K., Sheu S.S. & Gunter T.E. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J Biol Chem 270, 2751027515 (1995). [DOI] [PubMed] [Google Scholar]
- 108.Tewari S.G., Camara A.K., Stowe D.F. & Dash R.K. Computational analysis of Ca2+ dynamics in isolated cardiac mitochondria predicts two distinct modes of Ca2+ uptake. J Physiol 592, 1917–1930 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu J.C. et al. MICU1 Serves as a Molecular Gatekeeper to Prevent In Vivo Mitochondrial Calcium Overload. Cell Rep 16, 1561–1573 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kamer K.J. & Mootha V.K. MICU1 and MICU2 play nonredundant roles in the regulation of the mitochondrial calcium uniporter. EMBO Rep 15, 299–307 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fan M. et al. Structure and mechanism of the mitochondrial Ca(2+) uniporter holocomplex. Nature 582, 129–133 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kwong J.Q. & Molkentin J.D. Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab 21, 206–214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Novgorodov S.A., Gudz T.I., Brierley G.P. & Pfeiffer D.R. Magnesium ion modulates the sensitivity of the mitochondrial permeability transition pore to cyclosporin A and ADP. Arch Biochem Biophys 311, 219–228 (1994). [DOI] [PubMed] [Google Scholar]
- 114.Bernardi P., Di Lisa F., Fogolari F. & Lippe G. From ATP to PTP and Back: A Dual Function for the Mitochondrial ATP Synthase. Circ Res 116, 1850–1862 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rieger B., Arroum T., Borowski M.T., Villalta J. & Busch K.B. Mitochondrial F(1) F(O) ATP synthase determines the local proton motive force at cristae rims. EMBO Rep 22, e52727 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chinopoulos C. & Adam-Vizi V. Mitochondria as ATP consumers in cellular pathology. Biochim Biophys Acta 1802, 221–227 (2010). [DOI] [PubMed] [Google Scholar]
- 117.Vesce S., Jekabsons M.B., Johnson-Cadwell L.I. & Nicholls D.G. Acute glutathione depletion restricts mitochondrial ATP export in cerebellar granule neurons. J Biol Chem 280, 38720–38728 (2005). [DOI] [PubMed] [Google Scholar]
- 118.Rego A.C., Vesce S. & Nicholls D.G. The mechanism of mitochondrial membrane potential retention following release of cytochrome c in apoptotic GT1–7 neural cells. Cell Death Differ 8, 995–1003 (2001). [DOI] [PubMed] [Google Scholar]
- 119.Chen W.W. et al. Inhibition of ATPIF1 ameliorates severe mitochondrial respiratory chain dysfunction in mammalian cells. Cell Rep 7, 27–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Acin-Perez R. et al. Inhibition of ATP synthase reverse activity restores energy homeostasis in mitochondrial pathologies. EMBO J 42, e111699 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mnatsakanyan N. & Jonas E.A. The new role of F(1)F(o) ATP synthase in mitochondria-mediated neurodegeneration and neuroprotection. Exp Neurol 332, 113400 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hart M.L. et al. Use of a hanging-weight system for liver ischemic preconditioning in mice. Am J Physiol Gastrointest Liver Physiol 294, G1431–1440 (2008). [DOI] [PubMed] [Google Scholar]
- 123.Charni-Natan M. & Goldstein I. Protocol for Primary Mouse Hepatocyte Isolation. STAR Protoc 1, 100086 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Woods J.J. et al. A Selective and Cell-Permeable Mitochondrial Calcium Uniporter (MCU) Inhibitor Preserves Mitochondrial Bioenergetics after Hypoxia/Reoxygenation Injury. ACS Cent Sci 5, 153–166 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tomar D. et al. MCUR1 Is a Scaffold Factor for the MCU Complex Function and Promotes Mitochondrial Bioenergetics. Cell Rep 15, 1673–1685 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nemani N. et al. Mitochondrial pyruvate and fatty acid flux modulate MICU1-dependent control of MCU activity. Sci Signal 13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hoffman N.E. et al. SLC25A23 augments mitochondrial Ca(2)(+) uptake, interacts with MCU, and induces oxidative stress-mediated cell death. Mol Biol Cell 25, 936–947 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dong Z. et al. Mitochondrial Ca(2+) Uniporter Is a Mitochondrial Luminal Redox Sensor that Augments MCU Channel Activity. Mol Cell 65, 1014–1028 e1017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]