Abstract
Collapsing Focal Segmental Glomerulosclerosis (FSGS) has been reported relatively frequently in African American (AA) patients with coronavirus disease 2019 (COVID-19), and it is associated almost always with Apolipoprotein L gen 1 (APOL1) high-risk variants. We reviewed the published literature from April 2020 to November 2022 searching for non–African American (non-AA) patients with FSGS associated with COVID-19 (eight White patients, six Hispanic patients, three Asian patients, one Indian patient, and one Asian Indian patient). The following histologic patterns were found: collapsing (n=11), not otherwise specified (n=5), tip (n=2), and perihilar (n=1). Fifteen of the 19 patients had AKI. The APOL1 genotype was reported in only six of the 19 non-AA patients. Three of them (two Hispanic patients and one White patient) with collapsing FSGS had high-risk APOL1 variants. The other three patients (two White patients and one Hispanic patient with the collapsing variant, tip variant, and not otherwise specified) had low-risk APOL1 variants. Among 53 African American patients with collapsing FSGS associated with COVID-19, 48 had high-risk APOL1 variants and five had low-risk APOL1 variants. We conclude that in non-AA patients, FSGS is a rare complication of COVID-19. FSGS associated with COVID-19 can occur rarely with low-risk APOL1 variants in non-AA and AA patients. Non-AA patients reported to be associated with high-risk APOL1 variants possibly reflect inaccuracy of self-reported race with AA admixture because of unknown ancestry. Given the importance of APOL1 in the pathogenesis of FSGS associated with viral infection and to avoid racial bias, it seems appropriate that APOL1 testing be considered in patients with FSGS associated with COVID-19, regardless of self-reported race.
Keywords: FSGS, collapsing, APOL1, COVID-19, SARS-CoV-2, kidney biopsy
Introduction
In patients with coronavirus disease 2019 (COVID-19) and clinical signs of kidney involvement, kidney biopsy usually shows features of acute proximal tubular injury consistent with the AKI clinical presentation.1–4 However, FSGS, particularly of the collapsing variant, has been reported relatively frequently in African American (AA) patients with COVID-19.5–14 Noncollapsing variants of FSGS have also been reported, albeit rarely.1 Collapsing glomerulopathy is often associated with certain viral infections, such as HIV.15 This glomerulopathy when linked to COVID-19 has been termed COVID-19 associated nephropathy (COVAN) by Velez et al.16 Histologically, there are no apparent differences between collapsing glomerulopathy caused by HIV and COVID-19 infections.17
Approximately 91%–100% of AA patients with COVID-19 with collapsing glomerulopathy carry high-risk Apolipoprotein L gen 1 (APOL1) variants.1,9 The APOL1 risk genotypes, defined by the G1 and G2 risk alleles, are believed to be absent in populations without African ancestry and likely to have evolved to protect carriers in West Africa from African sleeping sickness (trypanosomiasis).18,19 The dispersion of people from West and Central Africa to the Americas and the Caribbean islands because of the trans-Atlantic slave trade and more recent migration has led to the global distribution of APOL1 variants.19 Some studies identified two types of Hispanics: the continental one from mainland Latin America and the other from the Caribbean, with the latter group having an increased frequency up to 10 times higher of carrying APOL1 risk alleles (0.1% vs 1%).20 Very little is known about the occurrence of FSGS in non-AA patients, and the impression is that this is a rare lesion because APOL1 high-risk variants are usually absent.7,21 The purpose of this review was to survey the literature for non-AA patients with FSGS since the COVID-19 pandemic started and examine the characteristics of such patients and, when possible, their association with APOL1 variants.
APOL1 is a gene that represents an important risk factor for the development of podocytopathies, mainly in those with collapsing glomerulopathy and of African ancestry.7,22 Cell culture studies suggest that APOL1 variants cause cell dysfunction through several processes, including alterations in cation channel activity—the G1 and G2 variants form cytotoxic cation channels at the surface of cells, which triggers an influx of Na+ and Ca+ across the plasma membrane and leads to cell death.23,24 Other mechanisms are inflammasome activation, increased endoplasmic reticulum stress, activation of protein kinase R, mitochondrial dysfunction, and disruption of APOL1 ubiquitination.18,24
APOL1 is encoded in the gene APOL1 (locus 22q12.3). G0 is called the reference sequence APOL1 allele or wild-type.25 In the kidney, G0 expression contributes to maintaining podocyte phenotype and integrity.24 There are two genetic variants for two different isoforms of the APOL1, G1 and G2, that are associated with kidney disease,26 FSGS,27 HIV nephropathy,28 and other forms of nondiabetic kidney disease in patients of African ancestry.29 We analyzed cases of FSGS reported in association with COVID-19 infection from the beginning of the pandemic until November 2022. We were able to review individual data from 19 non–African American patients. In addition, data were included from 94 African American patients (for the purpose of this review article by an African American, we included Black patients from the United States, Africa, and worldwide) with FSGS who had a kidney biopsy after COVID-19 infection. From the individual data available, we examined clinical characteristics and APOL1 status when available in non-AA and AA patients.
Methods
We performed a PubMed search from December 2019 to November 13, 2022 with the words “COVID-19 and biopsy and kidney,” “glomerulosclerosis and COVID-19,” “COVID-19 and FSGS,” “Collapsing glomerulopathy and COVID-19,” and “MCD and COVID-19” and found 1425 articles. Conference abstracts from the American Society of Nephrology (ASN) (2020–2022) and the European Renal Association (2020–2022) were also reviewed. From those, we identified a total of only 19 non–African American patients with FSGS of any variant. In this search, an additional seven patients with minimal change disease were identified who were not included in our analysis, except for one who converted to FSGS during COVID-19.
In all articles describing patients with information on APOL1 status, we noted whether they had high-risk or low-risk genotype variants for FSGS. In total, this information was available in 55 of 94 African American patients (here referring to Black people from the United States, Africa, and worldwide). Patients reported as non–African American were specified as White, Hispanic, Asian, or Indian; when it was not specified, the patients were assumed to be non-AA because the reports came from China (n=2) and Croatia (n=3). Of the 19 non–African American patients, only six had APOL1 status reported.
In transplant patients, we considered APOL1 genotype from the donor as the risk factor, although it is still controversial which confers the most risk between the donor and recipient genotypes. We did not include patients from large series where ancestry was mentioned as a percentage of patients, but two studies1,2 are mentioned in the discussion as they had a significant number of non-AA patients.1,2
Results
Non–African American Patients
General Characteristics
In total, we found 19 non–African American patients with FSGS associated with COVID-19 reported in the literature (11 male and eight female): eight White patients, six Hispanic patients, three Asian patients, one Indian patient, and one Asian Indian patient. Each patient demographic and clinical characteristics are summarized in Table 1.
Table 1.
FSGS and coronavirus disease 2019 infection in non–African American patientsa
| Patient | Sex | Age | Race | APOL1 | FSGS (Type) | Glomerulopathy | History | AKI/Dialysis | COVID-19 | Steroid | Transplant/Autopsy | Proteinuria | Serum Creatinine (mg/dl) | Time to Biopsy | Author |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 64 yo | Hispanic | N/A | Collapsing | De novo | DM, transplant source deceased | No/No | Severe | No | Yes/No | 9.8 g/g | 2.5 | ≤1 mo | Daniel54 |
| 2 | M | 42 yo | Indian | N/A | Collapsing | De novo | Previously healthy | No/No | Moderate | No | No/No | 8 g/g | 1.0 | ≤1 mo | Deshmukh59 |
| 3 | M | 48 yo | White | N/A | Collapsing | Progression | FSGS noncollapsing second renal transplant | Yes/No | N/A | N/A | Yes/No | 11.51 g/g | 2.65 | ≈1 mo | Thorburn60 |
| 4 | F | 56 yo | White | High-risk N/A | Collapsing | De novo | HTN, obesity, gastric bypass, hypothyroidism, and CKD | Yes/Yes | Mild | No | No/No | 7.2 g/g | 8.6 | ≤2 wk | Kudose9 |
| 5 | M | 69 yo | White | Collapsing | De novo | HTN, chronic lower extremity cellulitis, BPH, GERD, and CKD | Yes/Yes | Moderate | No | No/No | >1000 mg/dl | 13.2 | ≤2 wk | ||
| 6 | M | 54 yo | Croatian | N/A | Collapsing | De novo | Nephroangiosclerosis, HTN, 2nd COVID-19 infection, and hepatitis B | Yes/No | Mild | No | Yes/No | 7.44 g/d | N/A | N/A | Basic61 |
| 7 | M | 44 yo | Hispanic | G2/G2 | Collapsingb | Relapsing | FSGS collapsing | Yes/Yes | Mild | No | No/No | 11.4 g/g | 12.00 | 6 wk | Akilesh8 |
| 8 | M | 41 yo | Hispanic | G0/G0 | Collapsing | De novo | N/A | Yes/N/A | N/A | N/A | No/No | 10.4 g/g | 8.6 | N/A | Nystrom12 |
| 9 | F | 51 yo | Hispanic | G1/G1 | Collapsing | De novo | N/A | Yes/Yes | N/A | N/A | No/No | 9.3 g/g | 4.5 | 1.5 mo | |
| 10 | M | 34 yo | White | Low-risk (NS) | NOS | De novo | HTN, anabolic steroids, and high-protein diet | Yes/No | Critical | Yes | No/No | 26.5 g/d | 6.45 | 17 d | Nowak62 |
| 11 | F | 56 yo | Hispanic | N/A | NOS | De novo | HTN, DLP, and depression | No/No | Mild | Yes | No/No | 10.39 mg/g | 0.63 | N/A | Román63 |
| 12 | F | 87 yo | Chinese | N/A | NOS | De novo | DM, HTN, and CKD | Yes/No | Critical | No | No/Yes | 3+ protein | N/A | N/A | Su64 |
| 13 | M | 87 yo | Chinese | N/A | NOS | De novo | DM, HTN, and CKD | Yes/No | Critical | No | No/Yes | N/A | 2.6 | N/A | |
| 14 | F | 77 yo | Hispanic | N/A | NOSb | De novo | DM and HTN | Yes/Yes | Asymptomatic | No | No/No | 13.41 g/g | 3.99 | 3 d | Akilesh8 |
| 15 | F | 40 yo | Croatian | N/A | Tip | De novo | Lupus nephropathy and HTNc | Yes/Yes | Moderate | Yes | Yes/No | 2.05 g/d | N/A | N/A | Basic61 |
| 16 | F | 20 yo | White | G0/G0 | Tip | De novo | None | Yes/No | Mild | Yes | No/No | 9 g/g | 2.80 | ≤2 wk | Kudose9 |
| 17 | M | 60 yo | Croatian | N/A | Perihilar | De novo | Nephroangiosclerosis, HTN, and BKVANc | No/No | Moderate | No | Yes/No | 2.91 g/d | N/A | N/A | Basic61 |
| 18 | M | 71 yo | Asian Indian | N/A | 1st Bx: MCD 2nd Bx: Collapsing |
De novo | DM2, HTN, and BPH | Yes/Yes | Mild | Yes | No/No | 18.46 g/g; 16g/d |
4.49 | 1st Bx: 21 d 2nd Bx: 77 d |
Gupta65 |
| 19 | F | 42 yo | Asian | N/A | Collapsing | De novo | Noned | Yes/No | Asymptomatic | Yes | No/No | 11 g | 2.5 | N/A | Ganglam66 |
APOL1, Apolipoprotein L gen 1; COVID-19, coronavirus disease 2019; M, male; N/A, not available; F, female; DM, diabetes mellitus; HTN, hypertension; BPH, benign prostatic hyperplasia; GERD, gastroesophageal reflux disease; NS, not specified; NOS, not otherwise specified; Protein; DLP, dyslipidemia; Urine Protein-Creatinine Ratio (g/g) or 24-hours urine protein (g/d) or dipstick proteinuria; Bx, biopsy.; MCD, minimal change disease; BKVAN, BK virus–associated nephropathy (BK virus is an abbreviation of the name of the first patient, from whom the virus was isolated).
The term “non–African American” included patients reported as non-Black from Asia, India, and Europe on the basis of the description used in the publications.
TMA, thrombotic microangiopathy.
Viral reactivation of Epstein-Barr virus detected in hospitalization.
Crescentic glomerulonephritis and class IV lupus nephritis at the same time of diagnosis of the collapsing variant.
The mean age of patients was 55 (20–87) years. Ten of 19 patients had mild-to-moderate COVID-19, one severe, three critical, and two asymptomatic per the National Institutes of Health COVID-19 severity classification30; in the remaining three patients, the information was not available. Hypertension was the most prevalent comorbidity; one transplant patient had chronic hepatitis B infection and a history of an episode of COVID-19 3 months ago.
Renal Findings
Most of the kidney biopsies were performed within 4–6 weeks of a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PCR test. FSGS was reported in native kidneys (n=12), kidney allografts (n=5), and postmortem (n=2). De novo FSGS was observed in 17 patients, relapsing in one patient, and progression from minimal change disease to FSGS in the remaining patient.
Plasma creatinine was elevated (mean, 5.1±3.86 mg/dl; median, 3.99 mg/dl; range, 0.63–13.2). Fifteen of the 19 patients had AKI, and seven required hemodialysis. Nephrotic range proteinuria (defined as more than 3.5 g) was reported in 15 patients. Of the remaining four patients, three had 2.05, 2.91 g/d, and 3+ protein on a dipstick, in one patient (autopsy patient), the information on proteinuria was not available.
Type of FSGS and APOL1 Status
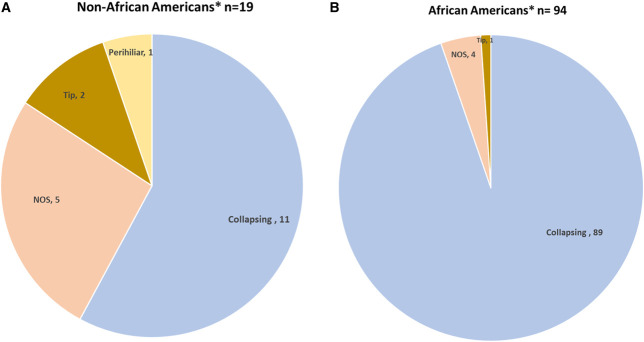
Eleven of the 19 non-AA patients had FSGS of the collapsing variant (Figure 1A). Eight had noncollapsing FSGS. APOL1 was evaluated in six of the 19 non-AA patients (Figure 2A).
Figure 1.
FSGS variants and coronavirus disease 2019 in non–African American and African American patients. (A) There were 19 non–African American patients: 11 with collapsing FSGS, five with NOS, one with the tip variant, and one with perihilar. (B) There were 94 African American patients: 89 were collapsing FSGS, four NOS, and one tip variant. *The term “non–African American” included patients reported from Asia, India, and Europe on the basis of the description in the articles, and “African American” included patients described as Black, including patients outside of the United States (see Methods). NOS, not otherwise specified.
Figure 2.
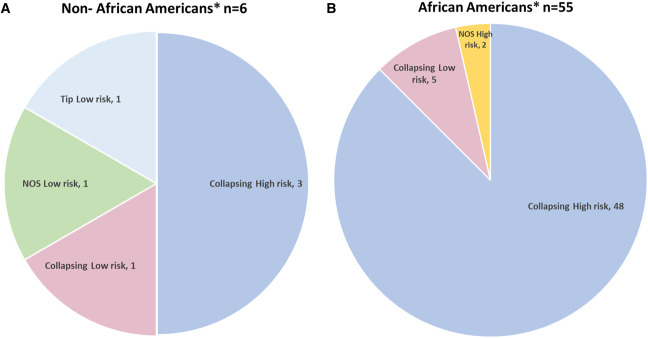
APOL1 Genotype. (A) In non-African Americans patients 6 were genotyped for APOL1; 3 had collapsing and APOL1-high risk, 1 had collapsing and APOL1-low risk; 1 had the tip variant and APOL1-low risk and the last patient had NOS and APOL1-low risk. (B) In African Americans patients 55 were genotyped for APOL1: 48 had collapsing and APOL1-high risk, 5 had collapsing and APOL1-low risk, and 2 had NOS and APOL1- high risk genotype. *The term “non–African American” included patients reported from Asia, India, and Europe, and “African American” included patients described as Black, including patients outside of the United States (see Methods). APOL1, Apolipoprotein L gen 1; NOS, not otherwise specified.
Of the patients with noncollapsing FSGS, five of eight had the not otherwise specified (NOS) variant as per the Columbia pathological classification of FSGS lesions.31 The tip variant was found in two patients and perihilar in one patient (Figure 1A, Table 1). In addition, one patient with the NOS variant had biopsy findings of thrombotic microangiopathy. APOL1 was evaluated in only two of the eight patients with noncollapsing FSGS: one patient had a tip lesion and the other had NOS, both with APOL1 low-risk variants.
One of the 11 AA patients with the collapsing variant had biopsy findings of thrombotic microangiopathy. APOL1 was evaluated in four of the 11 non-AA patients with the collapsing FSGS variant: three patients had high-risk variants (one with G1/G1, one G2/G2, and it was not specified in one) and one patient with the collapsing variant had the low-risk variant G0/G0.
African American Patients
General Characteristics
We found a total of 94 AA patients (55 men, 34 women, five not specified), as summarized in Tables 2. The mean age of patients was 52±11 years (range 16–79 years). Of the 94 AA patients with COVID-19, 33 had mild disease, 25 moderate, 15 severe, nine critical, three asymptomatic, one was reported as hospitalized, and eight did not specify a score or any symptom per the National Institutes of Health COVID-19 severity classification.30
Table 2.
FSGS associated with COVID-19 in African American patientsa
| Patient | Sex | Age | Race | APOL1 | FSGS (Type) | Glomerulopathy | History | AKI/Dialysis | COVID-19 | Steroid | Proteinuria | Serum Creatinine (mg/dl) | Time | Author |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 63 | African | G1/G2 | Collapsing | De novo | CKD 3, SLE, and APS | Yes/No | Mild | Yes | 6.8 g/g | 4.6 | N/A | Hoilat67 |
| 2 | M | 64 | African | G1/G0 | Collapsing | De novo | HTN, DM, CKD 3, HIV controlled | Yes/Yes | Critical | Yes | 2.74 | 2.3 | 11 d | Malhotra37 |
| 3 | F | 77 | African | N/A | Collapsing | De novo | HTN, cardiac disease, and AFib | Yes/Yes | Severe | Yes | 1.5 g/d | 8.3 | Third week | Sharma68 |
| 4 | F | 67 | African | G1/G2 | Collapsing | De novo | HTN, DM 2, DLP, OSA, and GERD | Yes/Yes | Mild | Yes | 3276 mg/g | 8.27 | N/A | Sharma14 |
| 5 | M | 49 | African | G1/G2 | Collapsing | De novo | HTN, cardiomyopathy, peripheral vascular disease, and arthritis | Yes/Yes | Severe | Yes | 2598 mg/g | 10.1 | N/A | |
| 6 | F | 44 | African | G1/G1 | Collapsing | De novo | DM, HTN, DLP, and CKD | Yes/Yes | Severe | No | 25 g/g | 11.4 | N/A | Larsen69 |
| 7 | F | 49 | African | G1/G1 | Collapsing | De novo | CKD, heart allograft, DM2, HTN, and obesity | Yes/No | Moderate | Yes | 6.6 g/d | 8.63 | N/A | Izzedine13 |
| 8 | F | 38 | African | G1/G1 | Collapsing | De novo | CKD, HTN, obesity, and SLE (class IV glomerulonephritis) | No/No | Moderate | Yes | 2.16 g/mmol | 11.68 | N/A | |
| 9 | F | 63 | African | G1/G1 | Collapsing | De novo | HTN | Yes/No | Severe | No | 5 g/L | 8.4 | 8 d | Kissling48 |
| 10 | M | 46 | African | Two with G1/G1 and one with G1/G2 the two patient's N/A | Collapsing | De novo | OSA and obesity | Yes/Yes | Mild | Yes | 5.8 g/g o g/d | 12.5 | N/A | Kudose5 |
| 11 | M | 62 | African | Collapsing | De novo | HTN, prostate carcinoma, and CKD | Yes/No | Mild | No | 12.1 g/g o g/d | 10.7 | N/A | ||
| 12 | M | 62 | African | Collapsing | De novo | HTN, DM, and prostate carcinoma | Yes/No | Moderate | Yes | 19 g/g o g/d | 11.6 | N/A | ||
| 13 | M | 57 | African | Collapsing | De novo | HTN and untreated hepatitis C virus | Yes/No | Moderate | No | 6.2 g/g o g/d | 4.9 | N/A | ||
| 14 | M | 61 | African | Collapsing | De novo | HTN and obesity | Yes/Yes | Mild | No | 9 g/g o g/d | 15 | N/A | ||
| 15 | M | 64 | African | G1/G2 | Collapsing | De novo | Not known | Yes/No | Severe | No | 7.6 g/d | 7.2 | ≤1 mo | Shetty10 |
| 16 | F | 54 | African | N/A | Collapsing | De novo | HTN | Yes/No | Mild | Yes | 11.7 g/d | 2.3 | ≤2 wk | |
| 17 | N/A | 57 | African | Rare APOL1 genotype G1GM/G1G+ | Collapsing | De novo | HTN | Yes/Yes | Mild | No | 4.9 g/g | 30.3 | N/A | Laboux70 |
| 18 | M | 56 | African | N/A | Collapsing | De novo | Ischemic cardiomyopathy and CKD and heart transplantation | Yes/No | Mild | No | 7354 mg/dl | 7.78 | N/A | Kadosh22 |
| 19 | M | 48 | African | N/A | Collapsing | De novo | DM 2, HTN, and CKD | Yes/Yes | Moderate | Yes | 18 g/d | 15.9 | ≥1 mo | Nlandu71 |
| 20 | M | 63 | African | G1/G1 | Collapsing | De novo | Acquired solitary kidney because of donation to his sister | Yes/Yes | Moderate | N/A | 12.7 g/g | 4.9 | N/A | Wu7 |
| 21 | F | 64 | African | G2/G2 | Collapsing | De novo | Three patients had CKD stage 3A, four patients HTN, and three patients DM2 | Yes/No | Moderate | N/A | 4.6 g/g | 4.2 | N/A | |
| 22 | F | 65 | African | G1/G1 | Collapsing | De novo | Yes/Yes | Critical | N/A | 13.6 g/g | 2.9 | N/A | ||
| 23 | M | 44 | African | G1/G1 | Collapsing | De novo | Yes/Yes | Moderate | N/A | 25 g/g | 11.4 | N/A | ||
| 24 | M | 37 | African | G1/G2 | Collapsing | De novo | Yes/Yes | Critical | N/A | 3+ protein | 9 | N/A | ||
| 25 | M | 56 | African | G1/G1 | Collapsing | De novo | Yes/Yes | Moderate | N/A | 3.6 g/g | 6.7 | N/A | ||
| 26 | M | 46 | African | G1/G1 | Collapsing | De novo | Obesity and OSA | Yes/Yes | Mild | Yes | 10.376 g/d | 19.9 | ≤1 mo | Peleg72 |
| 27 | M | 54 | African | N/A | Collapsing | De novo | DM2, HTN, and smoking | Yes/No | Moderate | No | 16 g/g | 4.67 | ≤1 mo | Gupta65 |
| 28 | M | 54 | African | N/A | Collapsing | De novo | HTN, obesity, and CKD G2 | Yes/Yes | Mild | No | 359 mg/mmol | 13.59 | ≤1 mo | Noble73 |
| 29 | F | 28 | African | G1/G1 | Collapsing | De novo | Asthma | Yes/Yes | Severe | No | 2 g/g | 6.5 | N/A | Magoon11 |
| 30 | M | 56 | African | G1/G2 | Collapsing | De novo | Uncontrolled HTN and CKD 3 | Yes/Yes | Severe | No | >21 g/g | 7.72 | N/A | |
| 31 | M | 57 | African | N/A | Collapsing | De novo | Not known | Yes/Yes | Severe | No | 14,865 mg/g | 10.2 | 2 mo | Malik74 |
| 32 | M | 53 | African | G1/G1 | Collapsing | De novo | HTN and acute cardiac failure | Yes/No | Critical | No | 1870 mg/mmol | 2.19 | ≤1 mo | Couturier75 |
| 33 | M | 53 | African | G1/G2 | Collapsing | De novo | HTN and untreated chronic hepatitis B | Yes/No | Severe | No | 2.65 g/d | 5.98 | ≤1 mo | |
| 34 | M | 65 | African | G1/G2 | Collapsing | De novo | HTN, DM2, DLP, and CKD 3A | Yes/Yes | Severe | Yes | 15.8 g/g | 6.5 | N/A | Roy76 |
| 35 | M | 79 | African | N/A | Collapsing | De novo | Hemorrhage stroke, MGUS, CKD 3 due to HTN | Yes/Yes | Mild | Yes | 11.4 g/g | 2.53 | 5 d | Gaillard77 |
| 36 | F | 50 | African | G1/G1 | Collapsing | De novo | HTN, hypothyroidism, depression, OSA, and obesity | Yes/Yes | Mild | No | 12.5 g/g | 6.08 | ≥1 mo | Meliambro78 |
| 37 | F | 38 | African | N/A | Collapsing | De novo | Morbid obesity, status post gastric bypass, DM2, asthma, and a sister with membranous nephritis | Yes/No | Moderate | No | 6.7 g/d | 7.08 | N/A | Tancredi79 |
| 38 | F | 61 | African | N/A | Collapsing | De novo | HTN, obesity, and DM2 | N/A | Severe | N/A | 14 g/d | N/A | N/A | Gambella80 |
| 39 | M | 45 | African | N/A | Collapsing | De novo | HTN and DM2 | N/A | Moderate | N/A | 30 g/d | N/A | N/A | |
| 40 | F | 49 | African | N/A | Collapsing | De novo | CKD 4, HTN, DM 2, heart failure, and obesity | Yes/Yes | Mild | No | 19 g/g | 7.17 | ≤1 mo | Kesiena81 |
| 41 | F | 21 | African | G2/G0 | Collapsing | De novo | SLE and active class IV lupus nephritis | Yes/No | Mild | Yes | 6.0 g/g | 1.3 | ≤1 mo | Masset38 |
| 42 | M | 52 | African | G1/G1 | Collapsing | De novo | N/A | Yes/Yes | N/A | N/A | 1.7 g/g | 2.68 | 10 mo | Nystrom12 |
| 43 | M | 44 | African | G1/G1 | Collapsing | De novo | N/A | Yes/N/A | N/A | N/A | 14 g/g | 1.9 | N/A | |
| 44 | M | 37 | African | G1/G1 | Collapsing | De novo | N/A | No/No | N/A | N/A | 13 g/g | 2.8 | 2 mo | |
| 45 | M | 42 | African | G2/G2 | Collapsing | De novo | N/A | Yes/Yes | N/A | N/A | 1.4 g/g | 11.3 | ≤1 mo | |
| 46 | M | 57 | African | G1/G1 | Collapsing | De novo | N/A | Yes/Yes | N/A | N/A | N/A | 16.1 | 9 d | |
| 47 | M | 60 | African | G1/G2 | Collapsing | De novo | N/A | Yes/No | N/A | N/A | 7.9 g/g | 6.1 | ≤1 mo | |
| 48 | M | 58 | African | G0/G0 | Collapsing | De novo | N/A | Yes/Yes | N/A | N/A | 6 g/g | 3.53 | ≤1 mo | |
| 49 | M | 49 | African | N/A | Collapsing | De novo | Multifactorial liver cirrhosis (alcoholic and 1b genotype HCV infection) and active cocaine | Yes/No | Mild | Yes | 22 g/12 h | 2.27 | ≤1 mo | Papalia82 |
| 50 | N/A | N/A | African | N/A | Collapsing | N/A | N/A | N/A | Critical | N/A | 8.5 g/g | N/A | Postmortem | Santoriello4 |
| 51 | M | 46 | African | G1/G1 | Collapsing | De novo | HTN | Yes/Yes | Critical | No | 13.7 g/g | 8.7 | 2 wk | Akilesh8 |
| 52 | F | 60 | African | N/A | Collapsing | De novo | HTN | Yes/N/A | Asymptomatic | No | 21 g/g | 5.7 | 4 wk | |
| 53 | F | 58 | African | N/A | Collapsing | De novo | HTN | Yes/Yes | Asymptomatic | No | 20 g/g | 10.2 | 8 d | |
| 54 | M | 58 | African | N/A | Collapsingb | De novo | Non-HTN and DM | Yes/Yes | Severe | No | 4 g/d | 11.3 | 4 d | |
| 55 | M | 47 | African | N/A | Collapsingb | De novo | HTN | Yes/Yes | Asymptomatic | No | 7.6 g/g | 6.6 | 25 d | |
| 56 | F | 63 | African | N/A | Collapsingb | De novo | HTN and adenocarcinoma | Yes/Yes | Mild | No | 20 g/g | 6 | 10–14 d | |
| 57 | M | 56 | African | N/A | Collapsing | De novo | Hyperlipidemia | Yes/Yes | Mild | No | 15 g/d | 4.97 | N/A | Hale83 |
| 58 | M | 58 | African | G1/G1 | Collapsing | De novo | HTN | Yes/Yes | Moderate | N/A | 9.3 g/d | 21.5 | N/A | Saleem84 |
| 59 | F | 29 | African | G1/G1 | Collapsing | Relapsing | Sickle cell disease, FSGS collapsing | Yes/No | Moderate | Yes | 28 g/d | 3.6 | N/A | |
| 60 | M | 53 | African | G1/G1 | Collapsing | De novo | HTN, new diagnosis of HIV, and syphilis | Yes/No | Mild | N/A | 5.6 g/d | 3.2 | N/A | |
| 61 | N/A | 42 | African | N/A | Collapsing | De novo | Not known | Yes/Yes | Mild | N/A | 5.6 g | 12.5 | N/A | Pendyala85 |
| 62 | N/A | 50 | African | N/A | Collapsing | De novo | HTN | Yes/Yes | Mild | N/A | 4.8 g | 14 | N/A | |
| 63 | N/A | 55 | African | N/A | Collapsing | De novo | Not known | Yes/Yes | Mild | N/A | 6.2 g | 9.6 | N/A | |
| 64 | F | 48 | African | N/A | Collapsing | De novo | HTN and CKD | Yes/No | Mild | N/A | 6.15 g/g | 9.9 | N/A | Akrawi86 |
| 65 | F | 32 | African | High risk | Collapsing | De novo | LN class 3 + class V partial remission, SLE, and current pregnancy (12 wk) | Yes/No | Mild | Yes | 13.6 g/d | 4.7 | N/A | Santosh87 |
| 66 | F | 57 | African | N/A | Collapsing | De novo | DM2, HTN, and small cell lung cancer | Yes/No | Mild | Yes | 12 g/g | 1.96 | N/A | Spinella88 |
| 67 | F | 51 | African | N/A | Collapsing | De novo | Not known | Yes/No | Moderate | Yes | 3.0 g/g | 5.3 | N/A | Zemke89 |
| 68 | F | 42 | African | N/A | Collapsing | De novo | HTN and DM | Yes/Yes | Moderate | N/A | 15.4 g/g | 12.7 | N/A | Scherchan90 |
| 69 | M | 48 | African | N/A | Collapsing | De novo | Not known | Yes/No | N/A | No | 1.4 g/g | 4.8 | N/A | Gallagher91 |
| 70 | M | 54 | African | 12 Patients at high risk | Collapsing | De novo | HTN and prostate carcinoma | Yes/Yes | Moderate | Yes | 20 g/g or g/d | 5.1 | ≤2 wk | Kudose9 |
| 71 | M | 66 | African | Collapsing | De novo | HTN, obesity, and CKD | Yes/Yes | Mild | No | 10 g/g | 7.0 | ≤2 wk | ||
| 72 | M | 57 | African | Collapsing | De novo | Not known | Yes/Yes | Moderate | Yes | 10 g/g | 3.0 | 1–2 mo | ||
| 73 | M | 68 | African | Collapsing | De novo | HTN, DM, obesity, gout, BPH, and CKD | Yes/No | Moderate | Yes | 6.6 g/g | 6.8 | ≤2 wk | ||
| 74 | M | 64 | African | Collapsing | De novo | HTN and CKD | No/Yes | N/A hospitalized | No | 0.8 g/g | 1.8 | 4 mo | ||
| 75 | F | 58 | African | Collapsing | De novo | HTN | Yes/Yes | Moderate | Yes | 4 g/g | 7.4 | ≤2 wk | ||
| 76 | M | 52 | African | Collapsing | De novo | HTN, DM, and carcinoid tumor | Yes/No | Moderate | Yes | 30 g/g | 10.6 | ≤2 wk | ||
| 77 | M | 55 | African | Collapsing | De novo | HTN and obesity | Yes/No | Mild | Yes | 18 g/g | 2.6 | ≤2 wk | ||
| 78 | F | 56 | African | Collapsing | De novo | HTN, DM, and obesity | Yes/No | Severe | No | 3 g/g | 3.4 | ≤2 wk | ||
| 79 | M | 57 | African | Collapsing | De novo | HTN, obesity, and CKD | Yes/No | Mild | Yes | 11 g/g | 2.5 | At 1 mo | ||
| 80 | F | 37 | African | Collapsing | De novo | HTN, DM, and obesity | Yes/No | Mild | No | 23 g/g | 4.5 | ≤2 wk after resolution | ||
| 81 | F | 72 | African | Collapsing | De novo | HTN, obesity, and CAD | Yes/Yes | Mild | No | 13 g/g | 11.5 | ≤2 wk | ||
| 82 | F | 59 | African | Collapsing | De novo | HTN, obesity, untreated HCV, RA, and CKD | Yes/Yes | Severe | Yes | 5 g/g | 3.8 | 3 mo | ||
| 83 | F | 54 | African | Collapsing | De novo | HTN, SLE, CVA, and CKD | Yes/Yes | Mild | Yes | 2.2 g/g | 12.9 | ≤2 wk | ||
| 84 | M | 52 | African | Collapsing | De novo | HTN, DM, and ethanol abuse | Yes/Yes | Critical | No | 3+ protein | 31 | ≤2 wk | ||
| 85 | M | 35 | African | Collapsing | De novo | Obesity and CNS vasculitis | Yes/Yes | Critical | Yes | 16 g/g | 10.9 | 3 wk | ||
| 86 | F | 50 | Hispanic donor African recipient | Transplanted kidney G1/G0, the recipient was G1/G1 | Collapsing | De novo | Two kidney transplants, the last was a deceased Hispanic donor. Banff 1B acute cellular rejection and CKD stage 4. | Yes/No | Moderate | No | 6.11 g/d | 5.99 | ≤2 wk | Shetty10 |
| 87 | M | 29 | African | Low-risk G0/G2 genotype donor, the recipient was G0/G0 | Collapsing | De novo | Urinary schistosomiasis, kidney transplant from a deceased donor—ABMR diagnosis | Yes/N/A | Mild | No | 0.8 g/mmol | 6.03 | 7 d | Lazareth39 |
| 88 | M | 16 | African | N/A | Collapsing | De novo | CVA and CKD due to microscopic polyangiitis, living-related donor kidney transplant | Yes/No | Mild | Yes | Prot 17 mg/mg Album 8.8 mg/mg |
4.7 | 13 d | Levenson92 |
| 89 | M | 49 | African | G2/G2 donor recipient N/A | NOS | De novo | HTN associated with hypertrophic cardiomyopathy, kidney transplant | Yes/No | Moderate | Yes | 3270 mg/g | 2.17 | ≤1 mo | Oniszczuk93 |
| 90 | M | 45 | African | N/A | Collapsing | De novo | DM2, obesity, CKD secondary to malignant hypertension, living donor transplant | Yes/Yes | Severe | Yes | 8.0 g/g | 10.27 | N/A Autopsy |
Noble73 |
| 91 | M | 36 | African | G1/G2 | NOS | De novo | HTN, DM, and obesity | Yes/No | Moderate | No | 4.9 g/g | 5.4 | 2–3 wk | Kudose9 |
| 92 | F | 52 | African | N/A | NOS | De novo | Previously healthy | Yes/Yes | Critical | Yes | 5.8 g/d | 2.0 | ≥1 mo | Shabaka94 |
| 93 | M | 59 | African | N/A | NOS | De novo | HTN and DM | Yes/N/A | Mild | No | >12 g/d | 11.9 | 11 d | Akilesh8 |
| 94 | F | 43 | African | N/A | Tip | De novo | Not known | Yes/No | Moderate | No | 13.445 g/d | 1.6 | N/A | Afonso95 |
APOL1, Apolipoprotein L gen 1; COVID-19, coronavirus disease 2019; F, female; SLE, systemic lupus erythematosus; APS, antiphospholipid syndrome; M, male; Urine Protein-Creatinine Ratio (g/g) or 24-hours urine protein (g/d) or dipstick proteinuria; N/A, not available; HTN, hypertension; DM, diabetes mellitus; AFib, atrial fibrillation; DLP, dyslipidemia; OSA, obstructive sleep apnea; GERD, gastroesophageal reflux disease; MGUS, monoclonal gammopathy of unknown significance; HCV, hepatitis C virus; LN, lupus nephritis; BPH, benign prostatic hyperplasia; CAD, coronary artery disease; RA, rheumatoid arthritis; CVA, cerebrovascular accident; CNS, central nervous system; ABMR, antibody-mediated rejection; NOS, not otherwise specified.
The term “African American” included Black people, including patients outside of the United States, on the basis of the descriptions in the references cited.
TMA, thrombotic microangiopathy.
In 59 patients, hypertension was found to be the most frequent comorbidity. CKD was present in 25 patients, type 2 diabetes in 25, obesity in 21, and systemic lupus erythematosus in five (three with nonactive disease, one on partial remission, and one with active nephritis); four patients had untreated hepatitis (three with hepatitis C and one with hepatitis B); and two patients had HIV, one controlled and the other with active disease. The remaining nine patients has no comorbidities.
Renal Findings
In AA patients, FSGS was reported in native kidneys (n=89), one of those was from autopsy; the reminding cases of FSGS were from kidney allografts (n=5). Most of the biopsies were performed within 4 weeks of a positive SARS-CoV-2 PCR test (ranging from 5 days to 10 months). De novo FSGS was observed in 92 patients; relapsing collapsing FSGS was observed in one patient; and it was not specified in the remaining patient. Among the 94 AA patients with COVID-19 and FSGS, 89 had the collapsing variant, four had NOS, and the remaining one had tip (Figure 1B). In addition, three patients had thrombotic microangiopathy and collapsing glomerulopathy.
Of all 94 AA patients with FSGS and COVID-19, 88 patients had AKI, and no information was provided for three patients. The mean creatinine was 7.81±5.47 mg/dl, with a median of 6.6 mg/dl (range 1.30–31 mg/dl). Of the 94 patients, 50 required hemodialysis, and in seven patients, this was not specified.
Nephrotic range proteinuria was reported in 77 of the 94 patients. Of the remaining 17 patients, 15 had subnephrotic range proteinuria, one had +3 protein on a dipstick, and the remaining patient had no information available (autopsy patient).
Type of FSGS and APOL1 Status
Of 94 AA patients, five had noncollapsing FSGS, and all the remaining 89 AA patients had collapsing FSGS (Figure 1B). APOL1 status was reported in 55 of the 94 AA patients (Figure 2B).
In noncollapsing FSGS, four of five patients had the NOS variant and one had the tip variant. APOL1 alleles were reported in two of five patients. In the NOS variant, two of four patients had APOL1 genotyping: one with high-risk native kidney G1/G2 and the other with donor high-risk G2/G2; the recipient information, however, was not available; and the remaining two patients had no information available.
Of the 89 AA patients with collapsing FSGS, 53 were genotyped (Figure 2C). Forty-eight of them had a high-risk variant, all from native kidneys: 22 G1/G1, 10 G1/G2, 2 G2/G2, and one G1GM/G1G+. The remaining 13 patients had high-risk alleles that, however, were not specified. Five of 53 patients with collapsing FSGS had low-risk variants: G1/G0, G2/G0, and one without risk G0/G0. The remaining two patients had kidney transplants: one with donor low-risk G0/G2 and the other recipient G0/G0. The remaining patient had been transplanted with a combination of alleles: The African ancestry recipient had high-risk G1/G1, and the Hispanic donor had low-risk G1/G0 (Figure 2D, Table 2).
Discussion
In this review of the literature on FSGS associated with COVID-19, we found only 19 patients reported as non–African American. Of note, 15 of the 19 patients had AKI, and seven required dialysis. In this regard, non-AA patients present with AKI similar to African American patients, although the reported creatinine values were not as high. Nephrotic range proteinuria was found in non-AA similar to AA patients. The relatively rare occurrence of FSGS in non–African American patients is in contrast with that in African American patients where this entity is found much more frequently in COVID-19–afflicted patients. We included data from 94 AA patients in this review, but this figure is clearly underestimated because we did not include, by our study design, studies that reported a series of patients with FSGS associated with COVID-19 without individual data that could be used for our analysis. For instance, Ferlicot et al.2 reported 17 patients with FSGS but did not specify the race. May et al.1 reported the largest multicenter retrospective cohort, including 107 genotyped patients. Giannini et al.6 reported 56 patients, but AA and Hispanic patients' characteristics were combined.
In addition to these reports, in an oral presentation during the last ASN conference (November 2022), Pourmehdi et al.32 reported the largest cohort in North America of kidney biopsies from fatal cases of COVID-19 (from April 2020 to July 2021). Unfortunately, of their 40 patients with COVID-19 with collapsing FSGS, none of them were genotyped for APOL1, and we were not able to include them because of the limited data provided in the abstract. We would like to highlight, however, that 40 of the 82 patients with COVID-19 had collapsing FSGS and that as many as 26 of these patients were non-AA. This large number of non-AA patients in this preliminary report is indeed surprising. This likely reflects, in part, the demographics of the overall Texas population with a predominance of Hispanic and White patients. Notwithstanding the large proportion of non-AA patients in this study is in contrast to our findings of only 19 patients worldwide, and of those patients, only 11 had collapsing FSGS. Other pathologic patterns of FSGS found were NOS (n=5), tip (n=2), and perihilar (n=1) (Table 1). This spectrum of patterns is in contrast with AA patients where the collapsing FSGS variant is, by far, the dominant pattern (89 of the 94 patients) (cf. Figure 1, A and B).
The proportion of African genetic ancestry among Hispanic/Latino patients has been associated with CKD risk.33,34 It is believed that because of the trans-Atlantic slave trade, there has been dispersion of high-risk APOL1 genotype into Latin American and Caribbean populations.20 Self-identified race or ethnicity is not a reliable criterion to exclude the possibility that individuals carry APOL1 risk alleles.35 Unfortunately, in research, unintentional racism might have a role in self-reported cases of race because of a taxonomy that categorized phenotypically defined humans.36 This is likely relevant to the APOL1 status reported in non-AA patients with FSGS associated with COVID-19 in our review of the literature. We found that in non-AA patients, information on APOL1 was found only for six patients (Figure 2A). Three of them were part of the 11 patients with collapsing FSGS, and all three had APOL1 high-risk variants. Of the remaining eight patients, one had low-risk variants. It is well-known that the predominance of the collapsing variant in AA patients is attributable to the almost universal presence of high-risk variants for APOL1 in AA patients. Consistent with this notion, 48 of the 53 AA patients with collapsing FSGS in whom APOL1 was performed had high-risk variants (Figure 2B). Of note, however, there were five AA patients with collapsing FSGS associated with low-risk variants. In non-AA patients, we found one of four patients with the collapsing variant who also had low-risk variants (Figure 2A). The patients with collapsing FSGS with low-risk variants might provide clues for pathophysiological mechanisms involved in the development of FSGS in patients with COVID-19. Collapsing FSGS may occur even in lower-risk heterozygous APOL1 variants.37,38
In patients with a low-risk genotype, but with at least one G2 risk allele, combined with viral infection and a hyperinflammatory state, podocyte damage may increase.39 Among individuals with collapsing glomerulopathy and APOL1 risk alleles, other gene–gene interactions may contribute to glomerulopathy.40 Systemic inflammation due to cytokine storm and interferon-mediated inflammatory signaling has been incriminated in COVID-19–associated FSGS.12 This is similar to other high interferon states that have been associated with collapsing FSGS, such as HIV, SLE, and treatment with interferon of hepatitis B and other conditions.12,41–44 Renal tropism and direct viral infection45–47 were speculated as the triggers for collapsing FSGS.22,48 This speculation of viral renal tropism is difficult to support given the fact that SARS-CoV-2 kidney invasion in patients with COVID-19 is usually not demonstrable.49,50 Other possible pathological mechanisms like “the three hit hypothesis for collapsing glomerulopathy” in the context of viral infection where the first hit is the high-risk APOL1 genotype, second hit being the inflammatory reaction and cytokines and third hit, modifier genes.46 COVID-19–associated nephropathy without the high-risk APOL1 genotype suggests other mechanisms triggered by viral infections that might be independent of APOL1 genotype.12,37–39,51 A role for non–renal cell APOL1 variant expression in COVID-19–associated FSGS has also been suggested.10,52 Regarding the similarities between COVAN and HIV-associated nephropathy (HIVAN), some studies found a high percentage (72%) of APOL1 high-risk alleles in HIVAN-associated FSGS.53 The effect of carrying two APOL1 risk alleles explains 18% of FSGS in general and 35% of HIVAN.28
Regardless of the mechanisms, the findings that there are cases of collapsing FSGS in AA and non-AA patients with COVID-19 and low risk APOL1 variants suggest that it would be misleading to assume that everyone with this disease has APOL1 high-risk variants. We corroborate that in the AA patients with collapsing FSGS, the percentage of patients with COVID-19 and high-risk alleles is more than 90%.1,2,9 On comparison, among the 11 non-AA patients with collapsing FSGS with known APOL1 status, we found three of four (75%) with high-risk variants (Figure 2). Considering the abstract by Pourmehdi et al.32 reporting 26 of 40 non-AA patients with collapsing FSGS and COVID-19, it seems likely that this lesion occurs much more frequently in patients without high-risk APOL1 variants than in AA patients. There are four reported cases of collapsing FSGS in Hispanic patients: one from Mexico with APOL1 high-risk G2/G2, during infection with COVID-19, had a relapse of the disease8 and three patients had de novo glomerulopathy—one with APOL1 high-risk (G1/G1), one low-risk G0/G0,12 and the other transplant patient not genotyped.54 APOL1 testing is available in some clinical laboratories and should be increasingly used in the evaluation of kidney disease and most definitely, in our opinion, in patients with FSGS associated with viral infections, regardless of the reported race. A significant bias has been noted in the field of genome-wide association studies, with the majority of discovery efforts conducted in populations of European ancestry. By contrast, individuals of African or Latin American ancestry accounted for only 4.2% of samples analyzed.55
Follow-up of renal function in COVID-19 patients with FSGS is limited, in general, and similarly, very little information was available for the 19 non-AA patients reviewed. Some reports indicated that half of the patients with FSGS who initially required dialysis achieved dialysis independence6,9 while others required dialysis at follow-up, but not at presentation.6 Some patients with collapsing FSGS and COVID-19 developed ESKD and/or died, which is similar to collapsing FSGS without COVID-19.6,56 In a recent review by Giannini et al.,6 nearly all patients with COVAN had advanced CKD at follow-up, with most showing no remission or disease progression. Similarly, in HIVAN, ESKD developed in more than half of the patients.57,58
In summary, non-African American patients, collapsing FSGS has been reported rarely as a complication of COVID-19, and it can be associated with high-risk and a minority of low-risk APOL1 variants. In African American patients, more than 90% of cases reported are associated with high-risk APOL1 variants but it can also occur with low-risk variants. APOL1 genotyping in non-AA patients associated with COVID-19 was rarely performed, and ancestry was not always specified in the reviewed literature. Considering potential issues with race admixture and inaccuracy of self-reported race and also to avoid racial bias, it seems appropriate that APOL1 testing, which is increasingly available, be considered in all patients with FSGS associated with COVID-19.
Disclosures
D. Batlle reports the following: Consultancy: AstraZeneca; Ownership Interest: Angiotensin Therapeutics Inc.; Research Funding: AstraZeneca, Feinberg Foundation, and NIDDK; Patents or Royalties: Founder and main owner of Angiotensin Therapeutics Inc. No royalties or income at this time; and Other Interests or Relationships: Dr. Batlle is the coinventor of an issued patent: “Active Low Molecular Weight Variants of Angiotensin Converting Enzyme 2,” and provisional patents “Active low molecular weight variants of Angiotensin Converting Enzyme 2 (ACE2) for the treatment of diseases and conditions of the eye” and “Soluble ACE2 variants and uses therefore.” E. Medina-Hernandez reports the following: Research Funding: Participation in the study “Safety and Efficacy of Maraviroc and/or Favipiravir With Standard Therapy in Severe COVID-19 Adults,” ClinicalTrials.gov Identifier: NCT04475991 (COMVIVIR), and GlaxoSmithKline, GSK donated the study drug. The remaining author has nothing to disclose.
Funding
None.
Author Contributions
Data curation: Elba Medina
Formal analysis: Elba Medina
Methodology: Elba Medina, Daniel Batlle
Writing – original draft: Carlos Rueda, Daniel Batlle
Writing – review & editing: Daniel Batlle, Elba Medina-Hernández, Carlos Rueda-Mantilla
References
- 1.May RM, Cassol C, Hannoudi A, Larsen CP, Lerma EV, Haun RS. A multi-center retrospective cohort study defines the spectrum of kidney pathology in coronavirus 2019 disease (COVID-19). Kidney Int. 2021;100(6):1303–1315. doi: 10.1016/j.kint.2021.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlicot S, Jamme M, Gaillard F, Oniszczuk J, Couturier A, May O. The spectrum of kidney biopsies in hospitalized patients with COVID-19, acute kidney injury, and/or proteinuria. Nephrol Dial Transplant. 2021;36(7):gfab042. doi: 10.1093/ndt/gfab042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nava-Santana C, Rodriguez-Armida M, Jimenez JV, Vargas-Parra N, Leon DEA, Campos-Murguia A. Clinicopathologic characteristics of severe COVID-19 patients in Mexico City: a post-mortem analysis using a minimally invasive autopsy approach. PLoS One. 2022;17(3):e0262783. doi: 10.1371/journal.pone.0262783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santoriello D, Khairallah P, Bomback AS, Xu K, Kudose S, Batal I. Postmortem kidney pathology findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):2158–2167. doi: 10.1681/ASN.2020050744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):1959–1968. doi: 10.1681/ASN.2020060802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giannini G Carlos Q Velez J, et al. Renal prognosis of COVID-19 associated nephropathy. Kidney Int Rep. 2022;7(12):2722–2725. doi: 10.1016/j.ekir.2022.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu H, Larsen CP, Hernandez-Arroyo CF, Mohamed MMB, Caza T, Sharshir M. AKI and collapsing glomerulopathy associated with COVID-19 and APOL 1 high-risk genotype. J Am Soc Nephrol. 2020;31(8):1688–1695. doi: 10.1681/ASN.2020050558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akilesh S, Nast CC, Yamashita M, Henriksen K, Charu V, Troxell ML. Multicenter clinicopathologic correlation of kidney biopsies performed in COVID-19 patients presenting with acute kidney injury or proteinuria. Am J Kidney Dis. 2021;77(1):82–93.e1. doi: 10.1053/j.ajkd.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kudose S, Santoriello D, Bomback AS, Sekulic M, Batal I, Stokes MB. Longitudinal outcomes of COVID-19-associated collapsing glomerulopathy and other podocytopathies. J Am Soc Nephrol. 2021;32(11):2958–2969. doi: 10.1681/ASN.2021070931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shetty AA, Tawhari I, Safar-Boueri L, Seif N, Alahmadi A, Gargiulo R. COVID-19-Associated glomerular disease. J Am Soc Nephrol. 2021;32(1):33–40. doi: 10.1681/ASN.2020060804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magoon S, Bichu P, Malhotra V, Alhashimi F, Hu Y, Khanna S. COVID-19-Related glomerulopathy: a report of 2 cases of collapsing focal segmental glomerulosclerosis. Kidney Med. 2020;2(4):488–492. doi: 10.1016/j.xkme.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nystrom SE, Li G, Datta S, Soldano KL, Silas D, Weins A. JAK inhibitor blocks COVID-19 cytokine-induced JAK/STAT/APOL1 signaling in glomerular cells and podocytopathy in human kidney organoids. JCI Insight. 2022;7(11):e157432. doi: 10.1172/jci.insight.157432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Izzedine H, Brocheriou I, Arzouk N, Seilhean D, Couvert P, Cluzel P. COVID-19-associated collapsing glomerulopathy: a report of two cases and literature review. Intern Med J. 2020;50(12):1551–1558. doi: 10.1111/imj.15041 [DOI] [PubMed] [Google Scholar]
- 14.Sharma Y, Nasr SH, Larsen CP, Kemper A, Ormsby AH, Williamson SR. COVID-19-Associated collapsing focal segmental glomerulosclerosis: a report of 2 cases. Kidney Med. 2020;2(4):493–497. doi: 10.1016/j.xkme.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg AZ, Naicker S, Winkler CA, Kopp JB. HIV-associated nephropathies: epidemiology, pathology, mechanisms and treatment. Nat Rev Nephrol. 2015;11(3):150–160. doi: 10.1038/nrneph.2015.9 [DOI] [PubMed] [Google Scholar]
- 16.Velez JCQ, Caza T, Larsen CP. COVAN Is the New HIVAN: the re-emergence of collapsing glomerulopathy with COVID-19. Nat Rev Nephrol. 2020;16(10):565–567. doi: 10.1038/s41581-020-0332-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith KD, Prince DK, Henriksen KJ, Nicosia RF, Alpers CE, Akilesh S. Digital spatial profiling of collapsing glomerulopathy. Kidney Int. 2022;101(5):1017–1026. doi: 10.1016/j.kint.2022.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanwalleghem G, Fontaine F, Lecordier L, Tebabi P, Klewe K, Nolan DP. Coupling of lysosomal and mitochondrial membrane permeabilization in trypanolysis by APOL1. Nat Commun. 2015;6(1):8078. doi: 10.1038/ncomms9078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daneshpajouhnejad P, Kopp JB, Winkler CA, Rosenberg AZ. The evolving story of apolipoprotein L1 nephropathy: the end of the beginning. Nat Rev Nephrol. 2022;18(5):307–320. doi: 10.1038/s41581-022-00538-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer HJ, Stilp AM, Laurie CC, Reiner AP, Lash J, Daviglus ML. African ancestry-specific alleles and kidney disease risk in hispanics/latinos. J Am Soc Nephrol. 2017;28(3):915–922. doi: 10.1681/ASN.2016030357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholas Cossey L, Larsen CP, Liapis H. Collapsing glomerulopathy: a 30-year perspective and single, large center experience. Clin Kidney J. 2017;10(4):443–449. doi: 10.1093/ckj/sfx029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadosh BS, Pavone J, Wu M, Reyentovich A, Gidea C. Collapsing glomerulopathy associated with COVID-19 infection in a heart transplant recipient. J Heart Lung Transplant. 2020;39(8):855–857. doi: 10.1016/j.healun.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedman BI, Limou S, Ma L, Kopp JB. APOL1-Associated nephropathy: a key contributor to racial disparities in CKD. Am J Kidney Dis. 2018;72(5):S8–S16. doi: 10.1053/j.ajkd.2018.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar V, Singhal PC. APOL1 and kidney cell function. Am J Physiol Ren Physiol. 2019;317(2):F463–F477. doi: 10.1152/ajprenal.00233.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Toole JF, Bruggeman LA, Madhavan S, Sedor JR. The cell biology of APOL1. Semin Nephrol. 2017;37(6):538–545. doi: 10.1016/j.semnephrol.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128(3):345–350. doi: 10.1007/s00439-010-0861-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. doi: 10.1126/science.1193032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22(11):2129–2137. doi: 10.1681/ASN.2011040388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23):2183–2196. doi: 10.1056/nejmoa1310345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.NIH. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/ [Google Scholar]
- 31.Tsuchimoto A, Matsukuma Y, Ueki K, Tanaka S, Masutani K, Nakagawa K. Utility of Columbia classification in focal segmental glomerulosclerosis: renal prognosis and treatment response among the pathological variants. Nephrol Dial Transplant. 2020;35(7):1219–1227. doi: 10.1093/ndt/gfy374 [DOI] [PubMed] [Google Scholar]
- 32.Pourmehdi Lahiji A Ariele-Gietzen R Walker Aronson, et al. Renal Pathology of Fatal Cases of COVID-19: A Study of 94 Autopsies. ASN Meeting. 2022; Abstract SA-0R02. [Google Scholar]
- 33.Pabon-Nau LP, Cohen A, Meigs JB, Grant RW. Hypertension and diabetes prevalence among U.S. Hispanics by country of origin: the National health interview survey 2000-2005. J Gen Intern Med. 2010;25(8):847–852. doi: 10.1007/s11606-010-1335-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.González Burchard E, Borrell LN, Choudhry S, Naqvi M, Tsai H-J, Rodriguez-Santana JR. Latino populations: a unique opportunity for the study of race, genetics, and social environment in epidemiological research. Am J Public Health. 2005;95(12):2161–2168. doi: 10.2105/ajph.2005.068668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kopp JB, Winkler CA, Zhao X, Radeva MK, Gassman JJ, D'Agati VD. Clinical features and histology of apolipoprotein L1-associated nephropathy in the FSGS clinical trial. J Am Soc Nephrol. 2015;26(6):1443–1448. doi: 10.1681/ASN.2013111242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohottige D, Boulware LE, Ford CL, Jones C, Norris KC. Use of race in kidney research and medicine: concepts, principles, and practice. Clin J Am Soc Nephrol. 2022;17(2):314–322. doi: 10.2215/CJN.04890421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malhotra V, Magoon S, Troyer DA, McCune TR. Collapsing focal segmental glomerulosclerosis and acute oxalate nephropathy in a patient with COVID-19: a double Whammy. J Investig Med High Impact Case Rep. 2020;8:232470962096363. doi: 10.1177/2324709620963635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masset C, Renaudin K, Kervella D, Chapelet A, Deltombe C, Ville S. Collapsing glomerulopathy in a patient with APOL1 intermediate-risk genotype triggered by lupus nephritis and SARS-CoV-2 infection: lessons for the clinical nephrologist. J Nephrol. 2022;35(1):347–350. doi: 10.1007/s40620-021-01144-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazareth H, Pere H, Binois Y, Chabannes M, Schurder J, Bruneau T. COVID-19-related collapsing glomerulopathy in a kidney transplant recipient. Am J Kidney Dis. 2020;76(4):590–594. doi: 10.1053/j.ajkd.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Divers J, Palmer ND, Lu L, Langefeld CD, Rocco MV, Hicks PJ. Gene-gene interactions in APOL1-associated nephropathy. Nephrol Dial Transplant. 2014;29(3):587–594. doi: 10.1093/ndt/gft423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rönnblom L, Leonard D. Interferon pathway in SLE: one key to unlocking the mystery of the disease. Lupus Sci Med. 2019;6(1):e000270. doi: 10.1136/lupus-2018-000270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goyal R, Singhal PC. APOL1 risk variants and the development of HIV-associated nephropathy. FEBS J. 2021;288(19):5586–5597. doi: 10.1111/febs.15677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kayar Y, Bayram Kayar N, Alpay N, Hamdard J, Ekinci I, Emegil S. Interferon induced focal segmental glomerulosclerosis. Case Rep Nephrol. 2016;2016:1–3. doi: 10.1155/2016/6967378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abid Q, Best Rocha A, Larsen CP, Schulert G, Marsh R, Yasin S. APOL1-associated collapsing focal segmental glomerulosclerosis in a patient with stimulator of interferon genes (STING)-Associated vasculopathy with onset in infancy (SAVI). Am J Kidney Dis. 2020;75(2):287–290. doi: 10.1053/j.ajkd.2019.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puelles VG, Lutgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi: 10.1056/nejmc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savedchuk S, Raslan R, Nystrom S, Sparks MA. Emerging viral infections and the potential impact on hypertension, cardiovascular disease, and kidney disease. Circ Res. 2022;130(10):1618–1641. doi: 10.1161/circresaha.122.320873 [DOI] [PubMed] [Google Scholar]
- 47.Braun F, Lutgehetmann M, Pfefferle S, Wong MN, Carsten A, Lindenmeyer MT. SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet. 2020;396(10251):597–598. doi: 10.1016/s0140-6736(20)31759-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kissling S, Rotman S, Gerber C, Halfon M, Lamoth F, Comte D. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98(1):228–231. doi: 10.1016/j.kint.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hassler L, Reyes F, Sparks MA, Welling P, Batlle D. Evidence for and against direct kidney infection by SARS-CoV-2 in patients with COVID-19. Clin J Am Soc Nephrol. 2021;16(11):1755–1765. doi: 10.2215/CJN.04560421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hassler L, Batlle D. Potential SARS-CoV-2 kidney infection and paths to injury. Nat Rev Nephrol. 2022;18(5):275–276. doi: 10.1038/s41581-022-00551-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sabaghian T, Kharazmi AB, Ansari A, Omidi F, Kazemi SN, Hajikhani B. COVID-19 and acute kidney injury: a systematic review. Front Med (Lausanne). 2022;9:705908. doi: 10.3389/fmed.2022.705908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z, Hao K, Ross MJ, Murphy B, Menon MC. APOL1 G2 risk allele-clarifying nomenclature. Kidney Int. 2017;92(2):518–519. doi: 10.1016/j.kint.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 53.Kopp JB, Heymann J, Winkler CA. APOL1 renal risk variants: fertile soil for HIV-associated nephropathy. Semin Nephrol. 2017;37(6):514–519. doi: 10.1016/j.semnephrol.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daniel E, Sekulic M, Kudose S, Kubin C, Ye X, Shayan K. Kidney allograft biopsy findings after COVID-19. Am J Transplant. 2021;21(12):4032–4042. doi: 10.1111/ajt.16804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wojcik GL Graff M Nishimura KK, et al. The PAGE study: how genetic diversity improves our understanding of the architecture of complex traits. bioRxiv.2018:188094. doi: 10.1101/188094 [DOI] [Google Scholar]
- 56.D'Agati VD, Alster JM, Jennette JC, Thomas DB, Pullman J, Savino DA. Association of histologic variants in FSGS clinical trial with presenting features and outcomes. Clin J Am Soc Nephrol. 2013;8(3):399–406. doi: 10.2215/CJN.06100612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen A, Yin L, Lee K, He JC. Similarities and differences between COVID-19-associated nephropathy and HIV-associated nephropathy. Kidney Dis. 2022;8(1):1–12. doi: 10.1159/000520235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bige N, Lanternier F, Viard JP, Kamgang P, Daugas E, Elie C. Presentation of HIV-associated nephropathy and outcome in HAART-treated patients. Nephrol Dial Transplant. 2012;27(3):1114–1121. doi: 10.1093/ndt/gfr376 [DOI] [PubMed] [Google Scholar]
- 59.Deshmukh S, Zhou XJ, Hiser W. Collapsing glomerulopathy in a patient of Indian descent in the setting of COVID-19. Ren Fail. 2020;42(1):877–880. doi: 10.1080/0886022x.2020.1811122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thorburn CA, Samarapungavan D, Kanaan HD, Cohn S, Jabbar KJ, Li W. Focal segmental glomerulosclerosis (FSGS) progressing to collapsing glomerulopathy in renal transplant recipients with and without COVID-19 infection. Transpl Proc. 2022;54(6):1465–1470. doi: 10.1016/j.transproceed.2022.02.010 [DOI] [PubMed] [Google Scholar]
- 61.Basic-Jukic N, Coric M, Bulimbasic S, Dika Z, Juric I, Furic-Cunko V. Histopathologic findings on indication renal allograft biopsies after recovery from acute COVID-19. Clin Transplant. 2021;35(12):e14486. doi: 10.1111/ctr.14486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nowak PJ Forycka J Cegielska N, et al. Glucocorticoids induce partial remission of focal segmental glomerulosclerosis but not interstitial nephritis in COVID-19 acute kidney injury in an APOL1 low-risk genotype white patient. Am J Case Rep. 2021;22:e933462. doi: 10.12659/ajcr.933462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roman JL, Vergara A, Agraz I, Garcia-Carro C, Bermejo S, Gabaldon A. Focal and segmental glomerulosclerosis associated with COVID-19 infection. Nefrologia (Engl Ed). 2021;41(6):706–708. doi: 10.1016/j.nefroe.2021.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gupta RK, Bhargava R, Shaukat AA, Albert E, Leggat J. Spectrum of podocytopathies in new-onset nephrotic syndrome following COVID-19 disease: a report of 2 cases. BMC Nephrol. 2020;21(1):326. doi: 10.1186/s12882-020-01970-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ganglam FBAB, Miick R, Chewaproug D, Pedroza MA. A Rare Case of Crescentic Glomerulonehritis, Diffuse Proliferative Class IV Lupus Nephritis, and Collapsing Glomerulopathy in a COVID, P-ANCA, and Myeloperoxidase-Positive Patient. ASN. 2021; Abstract PO1617. [Google Scholar]
- 67.Hoilat GJ, Das G, Shahnawaz M, Shanley P, Bukhari SH. COVID-19 induced collapsing glomerulopathy and role of APOL1. QJM. 2021;114(4):263–264. doi: 10.1093/qjmed/hcaa335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma P, Uppal NN, Wanchoo R, Shah HH, Yang Y, Parikh R. COVID-19-Associated kidney injury: a case series of kidney biopsy findings. J Am Soc Nephrol. 2020;31(9):1948–1958. doi: 10.1681/ASN.2020050699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int Rep. 2020;5(6):935–939. doi: 10.1016/j.ekir.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laboux T, Gibier JB, Pottier N, Glowacki F, Hamroun A. Correction to: COVID-19-related collapsing glomerulopathy revealing a rare risk variant of APOL1: lessons for the clinical nephrologist. J Nephrol. 2021;34(2):379. doi: 10.1007/s40620-021-01037-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nlandu YM, Makulo JR, Pakasa NM, Sumaili EK, Nkondi CN, Bukabau JB. First case of COVID-19-associated collapsing glomerulopathy in sub-saharan Africa. Case Rep Nephrol. 2020;2020:1–5. doi: 10.1155/2020/8820713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peleg Y, Kudose S, D'Agati V, Siddall E, Ahmad S, Nickolas T. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep. 2020;5(6):940–945. doi: 10.1016/j.ekir.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Noble R, Tan MY, McCulloch T, Shantier M, Byrne C, Hall M. Collapsing glomerulopathy affecting native and transplant kidneys in individuals with COVID-19. Nephron. 2020;144(11):589–594. doi: 10.1159/000509938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Malik IO, Ladiwala N, Chinta S, Khan M, Patel K. Severe acute respiratory syndrome coronavirus 2 induced focal segmental glomerulosclerosis. Cureus. 2020;12(10):e10898. doi: 10.7759/cureus.10898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Couturier A, Ferlicot S, Chevalier K, Guillet M, Essig M, Jaureguiberry S. Indirect effects of severe acute respiratory syndrome coronavirus 2 on the kidney in coronavirus disease patients. Clin Kidney J. 2020;13(3):347–353. doi: 10.1093/ckj/sfaa088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roy S, Kunaparaju S, Koduri NM, Sangani V, Pokal M, Konala VM. COVID-19 and APOL-1 high-risk genotype-associated collapsing glomerulonephritis. Case Rep Nephrol. 2021;2021:3737751. doi: 10.1155/2021/3737751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gaillard F, Ismael S, Sannier A, Tarhini H, Volpe T, Greze C. Tubuloreticular inclusions in COVID-19-related collapsing glomerulopathy. Kidney Int. 2020;98(1):241. doi: 10.1016/j.kint.2020.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meliambro K, Li X, Salem F, Yi Z, Sun Z, Chan L. Molecular analysis of the kidney from a patient with COVID-19-associated collapsing glomerulopathy. Kidney Med. 2021;3(4):653–658. doi: 10.1016/j.xkme.2021.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tancredi T, DeWaters A, McGillen KL. Renal ultrasound findings secondary to COVID-19 related collapsing focal segmental glomerulosclerosis—a case report. Clin Imaging. 2021;71:34–38. doi: 10.1016/j.clinimag.2020.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gambella A, Barreca A, Biancone L, Roccatello D, Peruzzi L, Besso L. Spectrum of kidney injury following COVID-19 disease: renal biopsy findings in a single Italian pathology service. Biomolecules. 2022;12(2):298. doi: 10.3390/biom12020298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kesiena O, Papadopoulos P, Amakye D, Hama E, Mackay R. COVID-19 associated collapsing glomerulopathy presenting as acute kidney injury on chronic kidney disease: a case report and review of the literature. CEN Case Rep. 2022;11(2):273–277. doi: 10.1007/s13730-021-00667-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Papalia G, Barbuto S, Campus A, Vischini G. Double glomerulopathies or two-faced janus? A challenging case in the COVID-19 era. J Nephrol. 2022;36(1):225-228. doi: 10.1007/s40620-022-01351-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muna Hale EJB, Jean Calude YD, Vanbeek CA. Collapsing FSGS in COVID-19. ASN Kidney Week. 2020; Abstract PO0806. [Google Scholar]
- 84.Maryam Saleem BNR, Stockholm SC. COVID-19-Associated Collapsing Glomerulopathy: A Report of Three Patients with African Ancestry. ASN Kidney Week. 2022; Abstract PUB013. [Google Scholar]
- 85.Reshub Pendyala RPP. Features and Long Term Follow up of Collapsing Glomerulopathy in Three Patients of COVID-19 Infection Presenting with Severe AKI. ASN Meeting. 2022; Abstract PUB038. [Google Scholar]
- 86.Samer Akrawi TS, Janom K, Rao PS, Lapedis CJ. Catastrophic COVID-19-Associated Nephropathy (COVAN) in an Asymptomatic Patient. ASN Meeting. 2021; Abstract PO1414. [Google Scholar]
- 87.Ramchandani Santosh SA, Maynard SE. COVID-19-Associated Collapsing Focal Segmental Glomerulosclerosis during Pregnancy in a Woman with Lupus Nephritis. ASN Kidney Week. 2021; Abstract PO1487. [Google Scholar]
- 88.Kaitlyn E, Spinella RGM. Treatment Outcome of New-Onset Collapsing Focal Segmental Glomerulosclerosis in a Patient with COVID-19. ASN Kidney Week. 2021; Abstract PUB004. [Google Scholar]
- 89.Anna M, Zemke PVB, Rodby RA. Corticosteroid Treatment in a Case of COVID-19-Associated Collapsing FSGS. ASN Kidney Week. 2020; Abstract PO0795. [Google Scholar]
- 90.Sunil Sherchan MK Durrani JK Puri I, et al. Collapsing/Sclerosing Glomerulopathy (CSG) and Acute Tubular Injury (ATI) in Patients with COVID-19. ASN Kidney Week. 2020; Abstract PO0770. [Google Scholar]
- 91.Megan K, Gallagher DSD, Wang D, Saha S. Kidney Disease in the Aftermath of COVID-19 Infection. ASN Kidney Week. 2021; Abstract PUB003. [Google Scholar]
- 92.Levenson E, Shepherd TN, Aviles D, Craver R, Ehlayel A, Love GL. De novo collapsing glomerulopathy in a pediatric kidney transplant recipient with COVID-19 infection. Pediatr Transplant. 2021;25(4):e14013. doi: 10.1111/petr.14013 [DOI] [PubMed] [Google Scholar]
- 93.Oniszczuk J, Moktefi A, Mausoleo A, Pallet N, Malard-Castagnet S, Fourati S. De novo focal and segmental glomerulosclerosis after COVID-19 in a patient with a transplanted kidney from a donor with a high-risk APOL1 variant. Transplantation. 2021;105(1):206–211. doi: 10.1097/tp.0000000000003432 [DOI] [PubMed] [Google Scholar]
- 94.Shabaka A, Rovirosa-Bigot S, Guerrero Marquez C, Alonso Riano M, Fernandez-Juarez G. Acute Kidney Injury and Nephrotic Syndrome Secondary to COVID-19-Associated Focal Segmental Glomerulosclerosis. Nefrologia (Engl Ed); 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rita S. Afonso AC Silva AP. Tip Lesion Variant of Focal and Segmental Glomerulosclerosis (FSGS): A Case Report in Patient with COVID-19. ASN Kidney Week. 2021; Abstract PO0116. [Google Scholar]