Introduction
Hemodialysis (HD) is the cornerstone treatment of kidney dysfunction requiring dialysis (KDRD) in the United States. Notwithstanding this medical success, HD has transformed from a treatment individualized by patients' symptoms to a one-size-HD-fits-all. Here we review the history of HD, focusing on the factors that shaped it into our current undifferentiated care. The future of HD can be brighter, with improved patient outcomes and methods to remove different uremic solutes, if we apply lessons learned from the past, challenge existing preprogrammed care, re-embrace individualized care, and develop new technologies that augment the quality of dialysis.
HD administered thrice-weekly to achieve a minimum target urea removal has been entrenched as the standard treatment of KDRD in the United States for more than four decades. While thrice-weekly HD maintains life and confers operational efficiency, it submits all individuals to uniform therapy. Insufficient appreciation of the approach to HD during its pioneering years and of the contexts that shaped our current adequacy guidelines have unintentionally established uniform HD as the standard of care. In this perspective, we summarize past to present landscapes of HD, examining factors that contributed to current thrice-weekly practice and concluding with the future where individualized care can be the standard (Table 1).
Table 1.
Summary of past, present, and future states of hemodialysis prescription
| Element of HD Prescription | Past | Present | Future |
|---|---|---|---|
| Setting | Home HD more common than facility-based HD | Facility-based HD more common than home HD | On the basis of patient preference with individualized prescription |
| Initiation | Mean eGFRa <5 ml/min per 1.73 m2 | Mean eGFRb >10 ml/min per 1.73 m2 | Mean eGFR <8 ml/min per 1.73 m2 |
| Metric of dialysis adequacy | Symptoms, nerve conduction studies | spKt/Vurea | Symptoms, small and middle-molecular weight solutes |
| Frequency | One to two treatments per week, progressing to three treatments per week on the basis of RKF and symptoms | Three treatments per week regardless of RKF | One to two treatments per week, progressing to three treatments per week on the basis of RKF and symptoms |
| Duration | 6–8 h per treatment at once-weekly or twice-weekly frequency | 3–4 h per treatment at thrice-weekly frequency | Variable, depending on RKF, volume status, and comorbidities |
| Dialyzer | • Plate dialyzers • Coil dialyzers • Cellulosic membrane |
• Hollow-fiber dialyzers • Capillary and biocompatible membrane |
Portable, miniaturized apparatus |
| Dialysate production system | • Recirculated solution • Sorbent systems |
• Single-pass solution • Premanufactured solutions |
• Sorbent systems • Carbon block to regenerate dialysate • Sorbent-loaded mixed-matrix membranes |
| Dialysate base solution | • Acetate | • Bicarbonate (cardiovascular benefit compared with acetate) | • Individualized bicarbonate and electrolyte concentration on the basis of real-time blood measurement during HD treatments |
HD, hemodialysis; spKt/Vurea, single-pool Kt/Vurea; RKF, residual kidney function.
Between early 1960s and early 1980s.
From mid-1980s to present.
The Past: HD Conceptualization
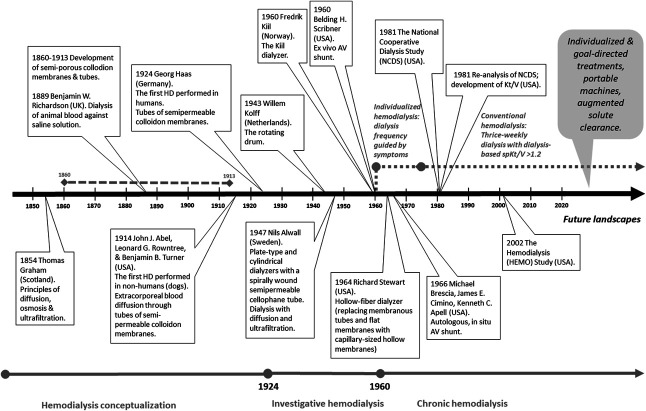
HD was a historical advancement in kidney failure treatment. With the use of assays developed in the early 1900s, kidney function deterioration was assessed by rise in plasma levels of urea and creatinine, hence the term uremia, originating from the Greek ouron (urine) and haima (blood), translating as urine in blood. This recognition of uremic solutes prompted efforts to develop a treatment that removes them. Such was dialysis of blood conceptualized, grounded on the principles of solute diffusion in liquids.1 Decades of pursuits were then invested to develop a reliable apparatus, including HD machines and membranes with biocompatible materials (Figure 1).
Figure 1.
A timeline of the evolution of HD. From conceptualization to contemporary practice, HD transformed from individualized treatments offered to a small patient population to uniform prescription offered to a large patient population. We envision a future that borrows elements from the past (i.e., individualized treatments) and capitalizes on scientific and technological progress. HD, hemodialysis; NCDS, National Cooperative Dialysis Study.
In the mid-1940s, HD was guided by clinical manifestations and was limited to the inpatient setting because of the need for surgical access to the vasculature with each treatment. The introduction of the Scribner shunt in 1960 made chronic HD possible, effecting the first outpatient center in Seattle, WA. Dialysis pioneers initiated HD at very low levels of kidney function, equivalent to present-day GFR estimates of <5 ml/min per 1.73 m2, and adjusted HD intensity on the basis of symptoms. Owing to solute clearance limitations of the available membranes, they prescribed HD as one 10–12-hour session every 5–10 days with increases in frequency if symptoms reemerged. After months of chronic HD, twice-weekly treatment would often become necessary to control symptoms.2 Advancements in membranes allowed treatments to be shortened to 6–8 hours. In parallel, means to identify kidney disease intensified, the number of patients with KDRD increased, and the limited capacity of the outpatient dialysis center was outpaced. This prompted the establishment of the Seattle home HD program, which interestingly started the shift to thrice-weekly regimens. With home HD, patients and their care partners found shorter thrice-weekly treatments to be less onerous than longer twice-weekly treatments.3
A pivotal point in history was the establishment of Medicare funding in 1973, rendering dialysis widely available. Consequently, outpatient centers proliferated and in-center HD steadily became more alluring than home HD. Thrice-weekly schedules used for home HD were adopted in many centers, but it was by no means enforced by dialysis pioneers. Nor did they focus on urea removal as the index of dialysis adequacy. At that time, adequate dialysis was defined as the amount necessary to prevent neuropathy, and solutes with size ranging 500–2000 Dalton (i.e., middle molecules) were implicated, on the basis of observations that patients with residual kidney function (RKF) receiving dialysis did not develop neuropathy compared with anuric patients receiving HD using cellophane membranes with smaller size cutoffs. This led to careful consideration of RKF in removing such solutes4 and therefore the argument that HD be prescribed according to the plasma accumulation of middle molecules.
The Present: Conventional HD
The growth of outpatient dialysis centers necessitated operation-efficient care which, along with reimbursement policies, solidified the movement to preprogrammed thrice-weekly care. Sorbent-regenerated dialysate systems that were once at the core of dialysate production were subsequently abandoned. Many disputed the decision of thrice-weekly HD as an inaugural therapy, pronouncing “there is no reason to assume a priori that every patient requires three instead of two HD per week” and “dialysis times established to prevent all patients in a center from developing chronic neuropathy are excessive for patients with some RKF.” They noted “the prescribed frequency of HD was the result of an incremental process” and cautioned that overdialysis may induce new symptoms.2 However, despite observations linking the contribution of RKF in controlling uremic symptoms, the movement to thrice-weekly HD prevailed.
The growth of chronic HD raised two questions: how to quantify the dose and what dose renders better outcomes. To address these questions, the National Cooperative Dialysis Study (NCDS) and Hemodialysis (HEMO) study tested the effect of HD-based solute targets and outcomes.5 The NCDS showed that targeting a lower plasma urea level reduced morbidity. A reanalysis of the data, however, showed that the marker single-pool Kt/Vurea (spKt/Vurea), which is roughly proportional to the reduction of urea levels by a single HD treatment, was a better predictor of outcomes than absolute urea levels. The HEMO study subsequently showed that intensive spKt/Vurea 1.7 did not reduce mortality versus 1.3, thereby establishing the minimum goal of 1.2 while targeting 1.4.
The contexts of these trials warrant emphasis. First, the main objective in both trials was to determine purely the contribution of HD-based solute targets to outcomes. Patients with RKF, categorized as creatinine clearance >3 ml/min (NCDS) or urea clearance >1.5 ml/min per 35 L (HEMO), were excluded to eliminate confounding by the kidneys' contribution to solute clearance. Second, thrice-weekly HD was the regimen for both trials. These trials therefore established the minimum HD-based solute targets for patients with little to no RKF receiving thrice-weekly HD. Finally, the development of spKt/Vurea solidified thrice-weekly HD. As originally calculated, spKt/Vurea applies only to a single treatment, and adequacy is defined as achieving this spKt/Vurea on each of three-weekly treatments. The weekly adequacy measure standard Kt/Vurea that is routinely used for peritoneal dialysis could be used to incorporate RKF in patients receiving HD of any frequency schedule. This weekly measure, however, was developed after spKt/Vurea-guided thrice-weekly HD had already gained substantial momentum.
A prescription of virtual maximum effectiveness, beyond which few (if any) clinical advantages are derived, thrice-weekly HD targeting spKt/Vurea is recommended as a minimum dialysis prescription—regardless of individual patient circumstances.5 Consequently, patients with appreciable RKF who lacked representation in NCDS and HEMO but now encompass a substantial proportion of incident patients have been absorbed into a system-centered HD practice. The contribution of RKF to solute clearance, while incorporated routinely in the prescription for peritoneal dialysis, has been largely ignored for HD despite data showing that endogenous kidney function affords excretion of complex molecules that are poorly removed by HD.6 Many studies have shown that maintaining full-dose, HD-based urea clearance at thrice-weekly treatments provided no benefit in patients with appreciable volume and solute removal afforded by RKF.7 However, RKF is neither measured nor incorporated routinely in the HD prescription.
The Future: Individualized HD
Awareness of the limitations with one-size-HD-fits-all approach and the biased incorporation of RKF in peritoneal dialysis (but not HD) have been mounting. Updated guidelines and a growing number of studies endorse assessing adequacy as a composite of kidney-based and HD-based urea clearances.7 Beginning in 2000, the Kidney Disease Outcomes and Quality Initiative guidelines allowed HD dose to be reduced (either by duration or frequency) for patients with RKF, defined as GFR >5 ml/min. These recommendations were further refined to reducing HD dose when kidney urea clearance is >2 ml/min in the Kidney Disease Outcomes and Quality Initiative 2006 and 2015 and the European 2019 guidelines. An impressive step forward was made for peritoneal dialysis by abandoning the use of solute-targeted adequacy, placing instead emphasis on patients' well-being.8 Perhaps this is the direction the HD community will follow, starting with incorporation of RKF into the HD prescription and recognition that patients' well-being is not restricted to a specific quantity of HD-based small solute removal. Indeed, in some countries, incremental HD programs are already integrated as a new standard of care; their experiences will enhance the findings from ongoing clinical trials.7,9
Hesitance to abandon entrenched practices and lack of studies to objectively compare different streams of HD care are some of the factors that contribute to the slow progress toward individualized HD. Ongoing and planned clinical trials that compare outcomes between RKF-coupled versus RKF-uncoupled HD prescription will hopefully boost adoption of individualized treatment (NCT05465044 and NCT05828823). Development of electronic platforms that integrate RKF into clinical reports and policy reforms that allow prescription adjustment for RKF could further promote person-centered care. HD prescription should also be adjusted for other individual needs, such as volume control, acute illnesses, or goals of care. In addition, there is renewed interest in the concept of sorbent systems to increase dialysis efficiency while reducing water consumption. Moreover, advances in sorbent technology may allow for removal of solutes which are poorly cleared by conventional HD, such as middle molecules and solutes that bind to plasma proteins.10
Improvement in HD care requires understanding of the underlying contexts from which the treatment was developed and a willingness to adopt new practices. The future of HD will be brighter, with improved patient outcomes and methods to remove nonurea uremic solutes, if we could learn from the past to change the current paradigm.
Disclosures
M. Murea reports the following—advisory or leadership role: ASN Kidney360: Associate Editor. T.L. Sirich reports the following—consultancy: Baxter.
Acknowledgments
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the author(s).
Funding
M. Murea: Patient-Centered Outcomes Research Institute (CER-2022C1-26300). T.L. Sirich: National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK118426) and Neurobiology of Pain and Itch of the NIH (R01 NS054791).
Author Contributions
Conceptualization: Mariana Murea, Tammy L. Sirich.
Writing – original draft: Mariana Murea, Tammy L. Sirich.
Writing – review & editing: Mariana Murea, Tammy L. Sirich.
References
- 1.Graham TX. Liquid diffusion applied to analysis. Philos Trans R Soc Lond. 1861;151:183–224. doi: 10.1098/rstl.1861.0011 [DOI] [Google Scholar]
- 2.Bower JD Berman LB Remmers R, et al. What is adequate dialysis? Proc Clin Dial Transplant Forum. 1971;1:61–72. [PubMed] [Google Scholar]
- 3.Scribner BH, Cole JJ, Ahmad S, Blagg CR. Why thrice weekly dialysis? Hemodialysis Int. 2004;8(2):188–192. doi: 10.1111/j.1492-7535.2004.01094.x [DOI] [PubMed] [Google Scholar]
- 4.Milutinovic J Babb AL Eschbach JW, et al. Uremic neuropathy: evidence of middle molecule toxicity. Artif Organs. 2008;2(1):45–51. doi: 10.1111/j.1525-1594.1978.tb01002.x [DOI] [PubMed] [Google Scholar]
- 5.Murea M Flythe JE Anjay R, et al. Kidney dysfunction requiring dialysis is a heterogeneous syndrome: we should treat it like one. Curr Opin Nephrol Hypertens. 2022;31(1):92–99. doi: 10.1097/mnh.0000000000000754 [DOI] [PubMed] [Google Scholar]
- 6.Sirich TL, Funk BA, Plummer NS, Hostetter TH, Meyer TW. Prominent accumulation in hemodialysis patients of solutes normally cleared by tubular secretion. J Am Soc Nephrol. 2014;25(3):615–622. doi: 10.1681/ASN.2013060597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caton E, Sharma S, Vilar E, Farrington K. Impact of incremental initiation of haemodialysis on mortality: a systematic review and meta-analysis. Nephrol Dial Transplant. 2023;38(2):435–446. doi: 10.1093/ndt/gfac274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown EA Blake PG Boudville N, et al. International Society for Peritoneal Dialysis practice recommendations: prescribing high-quality goal-directed peritoneal dialysis. Perit Dial Int. 2020;40(3):244–253. doi: 10.1177/0896860819895364 [DOI] [PubMed] [Google Scholar]
- 9.Torreggiani M, Fois A, Samoreau C, Santagati G, Piccoli GB. The ABCs of personalized incremental dialysis start, Le Mans style. J Nephrol. 2022;35(9):2417–2423. doi: 10.1007/s40620-022-01507-6 [DOI] [PubMed] [Google Scholar]
- 10.Ash SR. Sorbents in treatment of uremia: a short history and a great future. Semin Dial. 2009;22(6):615–622. doi: 10.1111/j.1525-139X.2009.00657.x [DOI] [PubMed] [Google Scholar]