Keywords: glomerular and tubulointerstitial diseases, AKI, casts, dysmorphic RBC, microscopy, nephrologist, urinalysis, urine microscopy
Abstract
Key Points
A nephrologist is more likely to recognize the presence of pathologic casts and dysmorphic red blood cells.
Nephrologist-performed urine sediment analysis is also highly accurate in diagnosing acute tubular injury or glomerulonephritis when compared with kidney biopsy.
Introduction
Automated urine technology is becoming the standard for urinalysis microscopy. We sought to compare urine sediment analysis performed by a nephrologist with the analysis performed by the laboratory. When available, we also compared the suggested diagnosis per nephrologists' sediment analysis with the biopsy diagnosis.
Methods
We identified patients with AKI who had urine microscopy with sediment analysis performed by the laboratory (Laboratory-UrSA) and by a nephrologist (Nephrologist-UrSA) within 72 hours of each other. We collected data to determine the following: number of red blood cells (RBCs) and white blood cells (WBCs) per high-power field, presence and types of casts per low-power field, and presence of dysmorphic RBCs. We evaluated agreement between the Laboratory-UrSA and the Nephrologist-UrSA using cross-tabulation and the Kappa statistic. When available, we categorized the nephrologist sediment findings into four categories: (1) bland, (2) suggestive of acute tubular injury (ATI), (3) suggestive of glomerulonephritis (GN), and (4) suggestive of acute interstitial nephritis (AIN). In a group of patients with kidney biopsy within 30 days of the Nephrologist-UrSA, we assessed agreement between the nephrologist diagnosis and the biopsy diagnosis.
Results
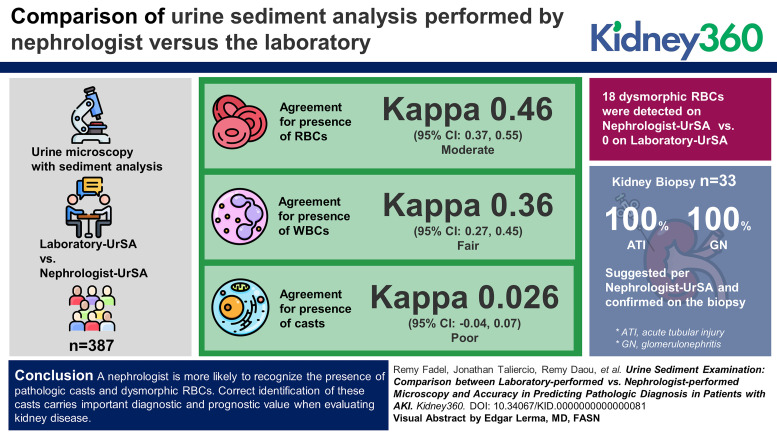
We included 387 patients with both Laboratory-UrSA and Nephrologist-UrSA. The agreement was moderate for the presence of RBCs (Kappa, 0.46; 95% CI, 0.37 to 0.55) and fair for WBCs (Kappa, 0.36; 95% CI, 0.27 to 0.45). There was no agreement for casts (Kappa, 0.026; 95% CI, −0.04 to 0.07). Eighteen dysmorphic RBCs were detected on Nephrologist-UrSA compared with zero on Laboratory-UrSA. Among the 33 patients with kidney biopsy, 100% ATI and 100% GN suggested per Nephrologist-UrSA were confirmed on the biopsy. Of the five patients with bland sediment on the Nephrologist-UrSA, 40% showed ATI pathologically while the other 60% demonstrated GN.
Conclusion
A nephrologist is more likely to recognize the presence of pathologic casts and dysmorphic RBCs. Correct identification of these casts carries important diagnostic and prognostic value when evaluating kidney disease.
Introduction
AKI is a major public health problem that affects millions of patients and leads to increased mortality and development and progression of CKD.1 The etiology of AKI is often identified through history taking, focusing on exposure to nephrotoxic medications, contrast exposure, prerenal causes, lower urinary tract symptoms, changes in hemodynamics, and systemic manifestations suggestive of an autoimmune process.2 In addition to history taking, urinalysis with microscopic examination is an inexpensive and critical test in developing a differential diagnosis for AKI, which when performed by a nephrologist (Nephrologist-UrSA) offers a great deal of information beyond what is yielded solely by automated urinalysis.3 Sediment examination has been shown to be more accurate in predicting the etiology of AKI than the more expensive and less available biomarkers, such as neutrophil gelatinase-associated lipocalin.3
Owing to high volumes and workflow standardization, most laboratories have converted to automated urine flow cytometry or digital imaging systems (Laboratory-UrSA),4 making the examination of urine sediment by nephrologists and training programs a relatively rare event. Therefore, we sought to evaluate the agreement between the Nephrologist-UrSA and Laboratory-UrSA in recognizing the presence or absence of urinary cells and casts. We further sought to evaluate the suggested diagnosis per Nephrologist-UrSA results compared with diagnosis from kidney biopsy, the gold standard diagnostic tool of kidney injury.5
Methods
Study Population
We compiled records from patients seen by our inpatient nephrology consult team at the Cleveland Clinic for evaluation of AKI having a urine sediment analysis performed by a nephrologist from December 2019 to December 2020. AKI was defined as per KDIGO definition2: an increase in serum creatinine by ≥0.3 mg/dl within 48 hours or decrease in urine output of <0.5 ml/kg per hour for 6 hours. We searched for laboratory evaluation of the urine sediment within 3 days of the nephrologist's sediment analysis and included patients who had both analyses completed.
For our secondary analysis, we identified a group of patients with a kidney biopsy and suggested diagnosis obtained from Nephrologist-UrSA performed within 30 days. Some of these were not part of our primary analysis because they did not have nephrologist and automated laboratory analyses within 3 days of each other.
The institutional review board approved this study with a waiver for informed consent.
Technical Performance of Urine Sediment Performed by Nephrologists
Nephrologists performed urine microscopy for evaluation of red blood cell (RBC) and white blood cell (WBC) counts, as well as assessment of morphology of RBC and detection of casts. Urine was centrifuged at 2500 rpm for 5 minutes within 1 hour of voiding. Supernatant was decanted, leaving 1 ml in the tube. A single drop of the suspended pellet was transferred to a glass slide and placed under a glass cover slip for bright-field microscopic examination under low-power field (LPF) (magnification ×100) and high-power field (HPF) (magnification ×400). Microscopy was performed within 30 minutes of centrifugation of urine samples. The nephrologist recorded their findings using the laboratory's standard report form. Elements of urine sediment listed in the standard laboratory form include the presence and quantification of WBCs per HPF, RBCs per HPF, epithelial cells per LPF, crystals (LPF), casts (LPF), and absence and/or presence of bacteriuria and yeast (LPF). The sediments from every urine sample were examined by microscopy using the following optical illumination techniques: bright field, phase contrast, and polarized microscopy. The nephrologists performing the procedure at our institution undergo an online course and testing with CLIA (Clinical Laboratory Improvement Amendments)-certified Medialab before starting and then are tested annually to check their competency. The AxioScope A1microscope was used for all the sediment examination in our study. Both experienced nephrologists and trainees performed the evaluations.
Technical Performance of Urine Sediment Performed by Laboratory Technicians
The laboratory operating procedure requires all UA should be performed within 2 hours of collection if not refrigerated, within 8 hours of collection if refrigerated, and within 3 days if immediately put in a preservative. Our laboratory procedure includes an automated portion that uses the iQ 200 automatic urine analyzer (IRIS Diagnostics, Chatsworth, CA), and a manual portion, which consists in a technician-performed review. The iQ 200 automatic urine analyzer is a commonly used automated analysis system that aspirates 1 ml of unspun urine and passes 2 μl through a flow cell in the object plane of a microscope; in this plane, using stroboscopic illumination to prevent blurring, 500 frames are captured on a charge-coupled device digital camera. Individual particles are isolated in each frame. Proprietary software analyzes the captured images and splits them into 11 categories: RBCs, WBCs, WBC clumps, squamous epithelial cells, nonsquamous epithelial cells, hyaline casts, unclassified casts, crystals, yeast, bacteria, and sperm. However, the identification of unclassified cast, nonsquamous epithelial cells, and other unknown particles still requires the visual review by a skilled technologist to inspect before the analyzer reports the results.6 At our institution, all medical laboratory technologists are trained at the bench before starting. They are competencied at 6 months, 12 months, and annually thereafter. Training is CLIA-approved.
RBC, WBC, and Cast Evaluation
For both RBCs and WBCs, more than five cells/HPF was considered positive. We grouped casts into the following categories: RBC, WBC, muddy brown and coarse granular (MBC/CG), and fine granular (FG)/hyaline. Patients with no sediment casts of any kind had their samples categorized as none. We combined epithelial casts with MBC/CG. Bilirubin casts, waxy casts, and fatty casts were combined into the FG/hyaline category. Cellular casts and mixed cellular casts were combined with RBC casts. The presence of crystals without casts was categorized as none.
In general, when two or more types of casts were observed, we categorized them on the basis of the most clinically relevant type of cast observed: RBC and mixed cellular casts, followed by WBCs, followed by the MBC/CG category and finally the FG/hyaline category. Casts of type RBC, WBC, and MBC/CG were considered indicative, respectively, of glomerulonephritis (GN), acute interstitial nephritis (AIN), and acute tubular necrosis (ATN) while hyaline/FG was not considered indicative of any specific kidney injury.
Statistical Methods
We summarized the age, sex, and race of patients with samples included in the study. We cross-tabulated the presence of WBCs and RBCs and the types of casts observed by laboratory analysis and nephrologist assessment. We used the Cohen Kappa statistic to evaluate agreement. The Kappa statistic evaluates agreement beyond that expected by chance, and in certain situations where expected agreement is high, Kappa can be low, so Kappa should be interpreted along with the observed agreement. We used the following general guidelines on interpreting the kappa statistic: values ≤0 as indicating no agreement, 0.01–0.20 as none to slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1.00 as almost perfect agreement.
We performed a sensitivity analysis of agreement including only samples that were drawn within 24h of each other. For samples with renal biopsy diagnosis, we evaluated agreement between the diagnosis per nephrologist's urine sediment examination and the biopsy diagnosis. We also estimated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for each diagnosis as yes/no separately. All analyses were conducted using Linux SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline characteristics for the included patients are summarized in Table 1. From the 387 patients included, 52% patients were female with a mean age of 59.6 years. Racial distribution was as follows: 1% Asian, 28% Black, 67% White, and 2% multiracial (patient's ancestry comes from multiple races). Of note, race was self-reported by the patients per our institute guidelines. The most frequent etiology of AKI in those patients was acute tubular necrosis (56%), followed by GN (17%) and prerenal acute kidney injury (15%).
Table 1.
Baseline characteristics of included patients
| Variable | Included Patients (N=388) |
|---|---|
| Age in years, mean (SD) | 59.6 (16) |
| Female sex, n (%) | 201 (52) |
| Diagnosis per nephrologist, n (%) | |
| Acute interstitial nephritis | 12 (3.1) |
| Acute tubular necrosis | 216 (55.7) |
| Cardiorenal syndrome | 8 (2.1) |
| Diabetic kidney disease | 2 (0.5) |
| Glomerulonephritis | 64 (16.5) |
| Hepatorenal syndrome | 6 (1.5) |
| Prerenal AKI | 59 (15.2) |
| Postrenal AKI | 4 (1.0) |
| Othersa | 17 (4.4) |
Others include light chain nephropathy, hypertensive nephropathy, lymphoproliferative disorder, tumor lysis syndrome, and unclear diagnosis.
The kappa agreement between findings seen by Nephrologist-UrSA and Laboratory-UrSA was 0.36 (95% confidence interval (CI), 0.27 to 0.45) for WBCs with 72% of samples in agreement and 0.46 (95% CI, 0.37 to 0.55) for RBCs with 75% of samples in agreement. Nephrologist-UrSA found 18 dysmorphic RBCs while Laboratory-UrSA found 0 with 95% of samples in agreement (i.e., all dysmorphic negative samples). Kappa agreement was 0.02 (95% CI, −0.04 to 0.07) for casts, including hyaline or FG, coarse granular or mixed cellular, WBC, or RBC casts (Tables 2 and 3). Course granular casts were reported in 36% of Nephrologist-UrSA compared with none on Laboratory-UrSA. Similarly, WBC casts were reported in 2% of Nephrologist-UrSA but none on Laboratory-UrSA. Finally, RBC casts were seen on 5% of nephrology-UrSA compared with 0.8% on Laboratory-UrSA.
Table 2.
Finding per laboratory analysis versus nephrologist analysis
| Laboratory-UrSA | Nephrologist-UrSA | ||
|---|---|---|---|
| WBC Absent | WBC Present | Total | |
| WBC absent | 221 | 16 | 237 |
| WBC present | 91 | 59 | 150 |
| Total | 312 | 75 | 387 |
| Kappa 0.36 (95% CI, 0.27 to 0.45) | |||
| Laboratory-UrSA | Nephrologist-UrSA | ||
|---|---|---|---|
| RBC Absent | RBC Present | Total | |
| RBC absent | 197 | 31 | 228 |
| RBC present | 67 | 92 | 159 |
| Total | 264 | 123 | 387 |
| Kappa 0.46 (95% CI, 0.37 to 0.55) | |||
| Laboratory-UrSA | Nephrologist-UrSA | ||
|---|---|---|---|
| Dysmorphic RBC Absent | Dysmorphic RBC Present | Total | |
| Dysmorphic RBC absent | 369 | 18 | 387 |
| Dysmorphic RBC present | 0 | 0 | 0 |
| Total | 369 | 18 | 387 |
| Kappa not reported because Laboratory-UrSA always showed absent | |||
WBC, white blood cell; RBC, red blood cell; CI, confidence interval.
Table 3.
Types of casts found per laboratory analysis versus nephrologist analysis
| Laboratory-UrSA | Nephrologist-UrSA | |||||
|---|---|---|---|---|---|---|
| None | Hyaline or FG | CG or MBC | WBC | RBC | Total | |
| None | 68 | 60 | 74 | 5 | 6 | 213 |
| Hyaline or FG | 48 | 49 | 63 | 2 | 9 | 171 |
| CG or MBC | 0 | 0 | 0 | 0 | 0 | 0 |
| WBC | 0 | 0 | 0 | 0 | 0 | 0 |
| RBC | 1 | 0 | 1 | 1 | 0 | 3 |
| Total | 117 | 109 | 138 | 8 | 15 | 387 |
Kappa 0.02 (95% CI, −0.04 to 0.07). FG, fine granular; MBC/CG, muddy brown and coarse granular; WBC, white blood cell; RBC, red blood cell.
We had 298 samples collected within 24 hours of each other for our sensitivity analysis. Results in this subset was comparable with what we found in our main analysis. Seventy-four percent of the samples were in agreement for WBCs (Kappa, 0.40; 95% CI, 0.29 to 0.50), and 75% were in agreement for RBCs (Kappa, 0.47; 95% CI, 0.37 to 0.57) (Supplemental Appendix Table 1). The classification of casts was in agreement for 34% of samples (Kappa, 0.05; 95% CI, −0.02 to 0.11, Supplemental Appendix Table 2).
Thirty-three patients had biopsy and nephrologist urine sediment analysis. Eleven of these were not in the main analysis of nephrologist versus automated laboratory analysis because they did not have both of those measures within 3 days of each other. Baseline characteristics for the 33 patients with kidney biopsies included for agreement analysis with nephrologist urine sediment showed a mean age of 56.6 years (SD 18.4) and 55% female. Racial distribution was 27% Black, 70% White, and 3% multiracial. The negative predictive value of Nephrologist-UrSA to detect GN and acute tubular necrosis was 73% and 93%, respectively, and the sensitivity was 88% and 75%, respectively. Specificity and positive predictive value were 100% for both diagnoses. Furthermore, kappa agreement between diagnoses performed by nephrologists on the basis of urine sediment and renal biopsies was 0.66 (95% CI, 0.44 to 0.89, Table 4). In fact, Nephrologist-UrSA suggested a diagnosis of ATN in 18% of patients compared with 24% ATN determined by kidney biopsy. In addition, a diagnosis of GN was suggested in 67% of patients on the basis of nephrology-UrSA compared with a determined GN diagnosis by kidney biopsy in 76% of patients.
Table 4.
Diagnosis by nephrologist interpretation of urine sediment versus diagnosis by biopsy
| Sediment Interpretation by Nephrologist | Diagnosis by Biopsy | |||
|---|---|---|---|---|
| Frequency | ATN | GN | Normal | Total |
| ATN | 6 | 0 | 0 | 6 |
| GN | 0 | 22 | 0 | 22 |
| Bland | 2 | 3 | 0 | 5 |
| Total | 8 | 25 | 0 | 33 |
Kappa 0.66 (95% CI, 0.44 to 0.89). ATN, acute tubular necrosis; GN, glomerulonephritis.
Discussion
Performance of urine sediment analysis is critical in the assessment of patients with AKI.7 Our study shows significant disagreement between interpretation of URsAs performed by nephrologists and those performed by certified laboratory technicians. Nephrologists were more likely to report the presence of coarse granular, muddy brown, WBC and RBC casts and dysmorphic RBCs in urine and less likely to report squamous epithelial cells.
Several studies assessed the accuracy of the iQ200 automated system. Lamchiagdhase et al.6 in a 2004 study aimed to compare the routine manual microscopic urine sediment examination to those from the iQ200 analyzer. They reported that iQ200 is less definitive in the presence of pathological casts than the detection of cellular elements, thus manual review was recommended in those cases. Similarly, in a study in 2006, Linko et al.8 found on 167 urine specimens that Iris IQ200 was capable of counting reliably RBCs, WBCs, and squamous epithelial cells; however, identification of casts required a trained technician’s assistance for better accuracy. Our study showed that the automated system was able to reliably count RBC and WBC numbers compared with manual microscopy. However, nephrology-UrSA was far superior at detecting casts and dysmorphic RBCs among other pathologies that carry a significant diagnostic effect.
The diagnostic utility of urine sediment analysis in identifying ATN is well established because it can detect hallmarks of this injury, namely renal tubular epithelial (RTE) cells, RTE casts, granular casts, and/or muddy brown casts.9 Our study showed that nephrologists were more adept at identifying these elements of the urine sediment. Given that ATN was the primary cause of AKI in our study and affected >50% of the patients, Nephrologist-UrSA will inevitably lead to greater accuracy in making the correct diagnosis.
The efficacy of a urine microscopy in recognizing RBC casts and/or dysmorphic RBCs for the diagnosis of GN is also established in the renal community.10,11 Our study showed that Laboratory-UrSA did not comment on the morphological characteristics of the RBCs, whereas Nephrologist-UrSA noted the presence of dysmorphic RBCs. Similarly, Nephrologist-UrSA was almost 5 times more likely to find RBC casts compared with Laboratory-UrSA. Identifying these elements is essential to determine the next step in diagnosis and prevent delay in treatment. Furthermore, WBC casts—suggestive of AIN11—were only detected by the Nephrologist-UrSA.
Interestingly, the iQ200 automated system had significantly greater reporting of squamous epithelial cells and hyaline casts. We suspect that those were overlooked by nephrologists as clinically nonrelevant, and so they may not have been mentioned in their report.
The secondary analysis that compared biopsy findings with the diagnosis made by nephrologists on the basis of their findings on the urine microscopy showed that the urine sediment has a very high specificity and PPV in diagnosing GN and ATN. Our nephrologists were able to get the accurate diagnosis on the basis of the findings on the urine sediment analysis in 90% of the cases. Despite the relatively small biopsy sample, our study demonstrates that urine sediment analysis remains crucial in establishing an accurate etiology of an AKI—when the accurate elements are identified—and guides clinicians in decision making.
Our study has several limitations. First, urine sediment was not evaluated by the same nephrologist for every patient, therefore affecting the internal validity of our study. We recognize that interobserver variability of different urine sediment findings can be considerable.12 However, the portion of the laboratory evaluation that is performed by the human technician is subject to the same interobserver variability, making our comparison valid. In addition, the nephrologists and technicians undergo the same CLIA-approved testing to ascertain and maintain competence. Second, nephrologists were not blinded when looking at urine sediment given that they knew the clinical history of the patients, which might lead to an observer bias. However, we think that knowing the clinical scenario is in line with clinical practice and is precisely what makes the nephrologists' analyses so crucial and irreplaceable. Third, we recognize that specimens sent to the laboratory may have been reviewed after a longer wait time, which is problematic given the fact that casts can denature overtime.13 In comparison, nephrologist-performed sediment analyses occur within the hour. Despite our strict laboratory operating procedure to preserve and minimize cast denaturation (detailed in methods), this could have given the nephrologist-performed analyses a considerable advantage. Although we recognize this limitation, we argue that this is in line with clinical practice, and perhaps yet another reason why nephrologists should continue to perform and rely on their own urine sediment analyses.
Nephrologist-performed UrSA is superior to laboratory-performed UrSA as correct identification of urinary casts carries important diagnostic and prognostic value when evaluating kidney disease. Nephrologist-performed UrSA is also highly accurate in diagnosing acute tubular injury or GN when compared with kidney biopsy, which is particularly important in patients in whom a kidney biopsy might be contraindicated. Our findings highlight the importance of urine sediment analysis in the diagnosis of AKI and should encourage physicians to incorporate the art of urine microscopy in the evaluation of their patients.
Supplementary Material
Acknowledgments
Portions of this article appeared as an abstract at ASN Kidney Week 2022.
Disclosures
L. Herlitz reports the following: Consultancy: ChemoCentryx; Honoraria: Novartis; and Other Interests or Relationships: Kidney360 editorial board member. A. Mehdi reports the following: Consultancy: AstraZeneca and Fresenius Kidney Care; Honoraria: AstraZeneca and Fresenius Kidney Care; and Speakers Bureau: AstraZeneca. G. Nakhoul reports the following: Consultancy: Chemocentryx, GSK, Otsuka, and Taiho Oncology; and Speakers Bureau: ChemoCentryx. J.D. Schold reports the following: Consultancy: eGenesis, NephroSant, and Sanofi Corporation; Research Funding: One Legacy Foundation; Honoraria: eGenesis, NephroSant, and Sanofi Inc; Advisory or Leadership Role: Data Safety Monitoring Board Member—Bristol Myers Squibb; Board of Directors of Lifebanc organ procurement organization; Vice Chair of UNOS Data Advisory Committee; and Speakers Bureau: Sanofi. J.F. Simon reports the following: Consultancy: Reata—one-time consulting agreement to discuss CARDINAL study results, January 2021; Patents or Royalties: UpToDate; and Advisory or Leadership Role: Alport Syndrome Foundation—Medical Advisory Committee. J.J. Taliercio reports the following: Employer: Cleveland Clinic and Glickman Urological and Kidney Institute; Consultancy: AstraZeneca, Merck & Co Inc., and Otsuka; Research Funding: Pfizer; and Speakers Bureau: Merck & Co Inc. All remaining authors have nothing to disclose.
Funding
None.
Author Contributions
Conceptualization: Remy Fadel, Leal Herlitz, Ali Mehdi, Georges Nakhoul, James F. Simon, Jonathan J. Taliercio.
Data curation: Elias Bassil, Remy Fadel, Adam Fawaz, Leal Herlitz, Habib Layoun, Georges Nakhoul.
Formal analysis: Susana Arrigain, Habib Layoun, Jesse D. Schold.
Investigation: Susana Arrigain, Elias Bassil, Remy Daou, Remy Fadel, Adam Fawaz, Habib Layoun, Ali Mehdi, Georges Nakhoul, Jonathan J. Taliercio.
Methodology: Susana Arrigain, Elias Bassil, Remy Fadel, Habib Layoun, Jesse D. Schold, Jonathan J. Taliercio.
Project administration: Remy Fadel, Ali Mehdi, Georges Nakhoul.
Resources: Elias Bassil, Remy Daou, Remy Fadel, Adam Fawaz, Jesse D. Schold, James F. Simon.
Supervision: Ali Mehdi, Georges Nakhoul, Jonathan J. Taliercio.
Validation: Susana Arrigain, Remy Daou, Ali Mehdi, Georges Nakhoul, Jesse D. Schold, James F. Simon, Jonathan J. Taliercio.
Visualization: Susana Arrigain.
Writing – original draft: Remy Fadel, Habib Layoun.
Writing – review & editing: Susana Arrigain, Elias Bassil, Remy Daou, Remy Fadel, Leal Herlitz, Ali Mehdi, Georges Nakhoul, Jesse D. Schold, James F. Simon, Jonathan J. Taliercio.
Supplemental Material
This article contains the following supplemental material online at http://links.lww.com/KN9/A319.
Supplemental Appendix Table 1. Sensitivity Analysis: Finding per laboratory analysis vs. nephrologist analysis for samples drawn within 24h.
Supplemental Appendix Table 2. Sensitivity Analysis: Types of casts found per laboratory analysis vs. nephrologist analysis for samples drawn within 24h.
References
- 1.Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012;81(9):819-825. doi: 10.1038/ki.2011.339 [DOI] [PubMed] [Google Scholar]
- 2.Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (part 1). Crit Care. 2013;17(1):204. doi: 10.1186/cc11454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavanaugh C, Perazella MA. Urine sediment examination in the diagnosis and management of kidney disease: core curriculum 2019. Am J Kidney Dis. 2019;73(2):258-272. doi: 10.1053/j.ajkd.2018.07.012 [DOI] [PubMed] [Google Scholar]
- 4.Shaikh S, Seltzer JR. The resurgence of urine microscopy. Kidney News. 2021;13(7):20. [Google Scholar]
- 5.Waikar SS, McMahon GM. Expanding the role for kidney biopsies in acute kidney injury. Semin Nephrol. 2018;38(1):12-20. doi: 10.1016/j.semnephrol.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamchiagdhase P Preechaborisutkul K Lomsomboon P, et al. Urine sediment examination: a comparison between the manual method and the iQ200 automated urine microscopy analyzer. Clin Chim Acta. 2005;358(1-2):167-174. doi: 10.1016/j.cccn.2005.02.021 [DOI] [PubMed] [Google Scholar]
- 7.Makris K, Spanou L. Acute kidney injury: diagnostic approaches and controversies. Clin Biochem Rev. 2016;37(4):153-175 [PMC free article] [PubMed] [Google Scholar]
- 8.Linko S, Kouri TT, Toivonen E, Ranta PH, Chapoulaud E, Lalla M. Analytical performance of the Iris iQ200 automated urine microscopy analyzer. Clin Chim Acta. 2006;372(1-2):54-64. doi: 10.1016/j.cca.2006.03.015 [DOI] [PubMed] [Google Scholar]
- 9.Kanbay M, Kasapoglu B, Perazella MA. Acute tubular necrosis and pre-renal acute kidney injury: utility of urine microscopy in their evaluation—a systematic review. Int Urol Nephrol. 2010;42(2):425-433. doi: 10.1007/s11255-009-9673-3 [DOI] [PubMed] [Google Scholar]
- 10.Nagahama D, Yoshiko K, Watanabe M, Morita Y, Iwatani Y, Matsuo S. A useful new classification of dysmorphic urinary erythrocytes. Clin Exp Nephrol. 2005;9(4):304-309. doi: 10.1007/s10157-005-0380-9 [DOI] [PubMed] [Google Scholar]
- 11.Raghavan R, Eknoyan G. Acute interstitial nephritis—a reappraisal and update. Clin Nephrol. 2014;82(09):149-162. doi: 10.5414/cn108386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palsson R Colona MR Hoenig MP, et al. Assessment of interobserver reliability of nephrologist examination of urine sediment. JAMA Netw Open. 2020;3(8):e2013959. doi: 10.1001/jamanetworkopen.2020.13959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delanghe JR, Speeckaert MM. Preanalytics in urinalysis. Clin Biochem. 2016;49(18):1346-1350. doi: 10.1016/j.clinbiochem.2016.10.016 [DOI] [PubMed] [Google Scholar]