Abstract
Background
People with human immunodeficiency virus (PWH) are at increased risk for comorbidities, and plasma interleukin 6 (IL-6) levels are among the most robust predictors of these outcomes. Tocilizumab (TCZ) blocks the receptor for IL-6, inhibiting functions of this cytokine.
Methods
This was a 40-week, placebo-controlled, crossover trial (NCT02049437) where PWH on stable antiretroviral therapy (ART) were randomized to receive 3 monthly doses of TCZ or matching placebo intravenously. Following a 10-week treatment period and a 12-week washout, participants were switched to the opposite treatment. The primary endpoints were safety and posttreatment levels of C-reactive protein (CRP) and CD4+ T-cell cycling. Secondary endpoints included changes in inflammatory indices and lipid levels.
Results
There were 9 treatment-related toxicities of grade 2 or greater during TCZ administration (mostly neutropenia) and 2 during placebo administration. Thirty-one of 34 participants completed the study and were included in a modified intent-to-treat analysis. TCZ reduced levels of CRP (median decrease, 1819.9 ng/mL, P < .0001; effect size, 0.87) and reduced inflammatory markers in PWH, including D-dimer, soluble CD14, and tumor necrosis factor receptors. T-cell cycling tended to decrease in all maturation subsets after TCZ administration, but was only significant among naive CD4 T cells. Lipid levels, including lipid classes that have been related to cardiovascular disease risk, increased during TCZ treatment.
Conclusions
TCZ is safe and decreases inflammation in PWH; IL-6 is a key driver of the inflammatory environment that predicts morbidity and mortality in ART-treated PWH. The clinical significance of lipid elevations during TCZ treatment requires further study.
Clinical Trials Registration. NCT02049437.
Keywords: interleukin-6, HIV-1, inflammation, tocilizumab, lipid profiling
Inhibition of IL-6 activity by tocilizumab had profound effects on immune activation/inflammation and lipid profiles in people with treated HIV infection.
Antiretroviral therapy (ART) blocks viral replication and usually improves CD4+ T-cell counts in people with human immunodeficiency virus (PWH), preventing opportunistic infection and death. Nonetheless, heightened immune activation, inflammation, and coagulation persist in ART-treated PWH [1] and many comorbidities, including cardiovascular disease (CVD), type 2 diabetes mellitus, and frailty, are linked to these indices and are seen increasingly in aging ART-treated PWH. Interventional studies to reduce inflammation, through modulation of putative drivers [1] or through targeting specific inflammatory pathways, have generated mixed results, with none yet conferring clinical benefit. The biological mechanisms that underpin increased age-related comorbidities in PWH are not clear. As interleukin 6 (IL-6) is a robust predictor of morbidity and mortality in both aging [2] and PWH [3–5], we asked if blockade of the biological functions of IL-6 by tocilizumab (TCZ) could provide novel insights into the mechanisms of disease pathogenesis in ART-treated human immunodeficiency virus (HIV) infection.
Numerous studies, including our own [3, 5], have reported that higher plasma levels of IL-6, C-reactive protein (CRP), D-dimer, and soluble CD14 (sCD14) are powerful predictors of morbidity and mortality in PWH [4, 6], and IL-6 levels are among the most robust [3]. It is not clear whether IL-6 drives disease or whether it is simply a marker of risk reflecting its proximity to the actual drivers. Previously, we demonstrated that ART-treated PWH with persistently low CD4 counts showed higher levels of T-cell activation (HLA-DR+CD38+ expression) and dysregulated CD4 T-cell cycling (Ki67+) [7]. We also found that IL-6 can drive T-cell activation, cycling, and senescence in vitro [8–10]. T-cell activation, measured by coexpression of CD38 and HLA-DR, is predictive of CD4+ T-cell decline pre-ART [11] and CD4+ T-cell recovery following ART initiation [12]. We report the results of a randomized, placebo-controlled, crossover trial of TCZ administration in ART-treated PWH. The primary objectives were to determine the safety of IL-6 blockade in PWH and to determine if IL-6 blockade would decrease plasma levels of CRP and decrease the dysregulated cycling/turnover of central memory CD4+ T cells. Secondary objectives included examining the effects of TCZ on lipid and metabolic profiles and on a variety of inflammation and immune activation indices, including effects on the interleukin 7 (IL-7) receptor and downstream readouts of IL-7 response. IL-7 is a quintessential cytokine for T-cell homeostasis and survival. We previously demonstrated that in vitro exposure of T cells to IL-6 inhibits IL-7 responses at multiple points in the pathway to CD4 T-cell recovery [6, 8].
METHODS
Study Design
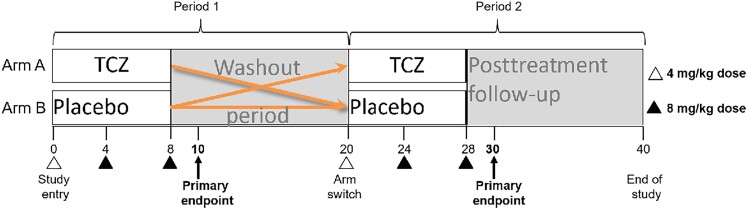
Eligibility in this randomized, double-blind, placebo-controlled crossover trial of TCZ administration (Figure 1) included HIV infection controlled by ART (plasma HIV RNA <200 copies/mL) and CD4+ T-cell counts between 350 and 1000 cells/μL. Participants were randomized to receive 3 monthly doses of TCZ or matching placebo intravenously, at doses of 4 mg/kg at entry and then 8 mg/kg for the next 2 doses. Participants receiving TCZ first were in arm A, and those receiving placebo first were in arm B. After a washout of 12 weeks, participants received the other treatment on the same schedule. The primary endpoints were measured 2 weeks after the last dose of treatment in each study period. Participants were enrolled after providing written informed consent between 23 October 2014 and 8 December 2016 at University Hospitals, Cleveland Medical Center. This trial is registered at ClinicalTrials.gov (identifier NCT02049437).
Figure 1.
Cross-over trial schema for interleukin 6 blockade with tocilizumab (TCZ) in people with human immunodeficiency virus (HIV). All participants had HIV, were aged 18–60 years, and had suppressed viremia on stable antiretroviral therapy. Study participants were randomized to receive TCZ (arm A) or placebo (arm B) intravenously during the first treatment period (10 weeks). The initial TCZ dose was 4 mg/kg; the second and third doses were 8 mg/kg and were given every 4 weeks. After a washout period, participants were switched to the opposite treatment and were followed through the end of study at 40 weeks. Three of 34 participants discontinued treatment, 1 due to low plasma antiviral drug levels (while receiving placebo, before receiving TCZ) and 2 due to dose limiting toxicities (1 grade 3 rash that occurred after the second dose of TCZ and 1 absolute neutrophil count <500 cells/µL after the first dose of TCZ).
To determine the safety of TCZ administration, toxicities grade 2 or greater were documented.
Blood was collected in ethylenediaminetetraacetic acid tubes at baseline; at weeks 4, 8, and 10; at switch (week 20); and at weeks 24, 28, 30, and 40. Peripheral blood mononuclear cells (PBMCs) were collected by centrifugation with Ficoll and cryopreserved at −180°C and then analyzed in batches. Plasma was obtained after centrifugations at 400g and 800g, then cryopreserved at −80°C.
Plasma Marker and Lipid Measurements
Plasma markers were measured by enzyme-linked immunosorbent assay (ELISA) for high-sensitivity IL-6, IL-7, sCD14, soluble CD163 (sCD163), tumor necrosis factor receptor (TNFR)-1, TNFR-2, interferon-γ–induced protein 10 (IP-10), soluble CD40L (sCD40L), high-sensitivity CRP, vascular cell adhesion molecule (VCAM)-1, P-selectin, E-selectin, and intestinal fatty acid–binding protein using kits from R&D Systems. Zonulin kits were from Alpco, and D-dimer kits were from Diagnostica Stago. Interleukin-21 and IL-22 kits were from Invitrogen. Therapeutic drug monitoring was performed in real time in the study by Edmund Caparelli at the University of California, San Diego Antiviral Research Center.
Total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and calculated low-density lipoprotein (LDL) were measured using a Beckman AU 5800 analyzer. Oxidized LDL (oxLDL) levels were measured by ELISA (Mercodia). Apolipoprotein A (Apo-A) and apolipoprotein B (Apo-B) were measured by ELISA (R&D Systems). Additional plasma markers of cardiometabolic risk included 3 adipokines (resistin, leptin, adiponectin) that were measured by ELISA (R&D Systems) and lipoprotein-associated phospholipase A2 (Lp-PLA2) measured by ELISA (Plac Test, DiaDexus).
Plasma lipids were also analyzed using the direct infusion–tandem mass spectrometry Lipidyzer platform (Sciex) that quantifies approximately 1200 lipids covering 13 lipid classes: cholesterol esters (CE), ceramides (CER), diacylglycerols (DAG), dihydroceramide (DCER), free fatty acids, hexosylceramides (HCER), lactosylceramides (LCER), lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), phosphatidylcholine (PC), phosphatidylethanolamine (PE), sphingomyelin (SM), and triacylglycerols (TAG) [13]. Data were generated using the Lipidomics Workflow Manager software (Sciex).
Flow Cytometric Phenotyping
PBMCs were thawed and stained as described in the Supplementary Methods. Samples were examined using a BD LSR Fortessa and analyzed with BD FACSDiva software.
Endothelial Function Testing
Brachial artery reactivity testing (BART) was performed at baseline, week 10, week 20, and week 30 as described previously [14]. Participants were fasting and had refrained from exercise and smoking for 8 hours. BART outcome measures were flow-mediated dilation (FMD) and hyperemic velocity-time integral (VTI).
Statistical Methods
The target sample size was 30, which provided adequate power under a range of plausible effect sizes. Thirty-one participants were included in a modified intent-to-treat analysis. Mann-Whitney U tests were used to compare age and CD4 and CD8 counts between arms, and Fisher exact tests to compare other variables. To investigate the effect of TCZ, we followed the nonparametric method in Jones and Kenward [15]. We conducted Wilcoxon rank-sum tests to assess carry-over or period effects. Only sCD40L showed a carry-over effect (P = .006). We applied Wilcoxon signed-rank tests to test for differences between changes from period baselines during the treatment periods and the placebo periods. Between-treatment-group comparison was performed according to the standard 2 × 2 crossover method based on a 2-sample Wilcoxon rank-sum test. For more details, see the Supplementary Methods.
To assess changes in the vascular parameters FMD and VTI, after adjustment for concurrent changes in atherogenic lipids, LDL, or non-HDL cholesterol, we applied linear models with panel data where vascular parameters were the outcome variables; treatment, time, interaction between treatment and time, lipid variables, and interaction between lipid variables and time were the covariates.
To assess the correlation between PD-1 and Ki67 changes, we applied linear models. Ki67 was the outcome variable; PD-1, treatment, time, and interaction between treatment and time were the covariates.
For lipidomics data, we conducted exploratory Wilcoxon signed-rank tests using differences in treatment and placebo changes as described in the Supplementary Materials.
P values of the 2 primary outcomes were adjusted by Bonferroni correction, for mean CRP and the proportion of central memory (CD45RA−, CD27+) CD4+ T lymphocytes that had entered cell cycle in vivo as measured by expression of the nuclear protein Ki67. Two-sided type I error was 0.05, and 95% confidence intervals (CIs) were estimated. Statistical analysis was completed using R 4.0.1 software.
RESULTS
Overall there were 9 treatment-related toxicities of grade 2 or greater during TCZ administration (mostly neutropenia) and 2 during placebo administration. Three of 34 participants discontinued treatment before completing the study, 1 due to low plasma antiviral drug levels (while receiving placebo, before receiving TCZ) and 2 due to dose-limiting toxicities (1 grade 3 rash that occurred after the second dose of TCZ and 1 absolute neutrophil count <500 cells/µL after the first dose of TCZ). Demographic information is included in Table 1. Participants randomized to arms A (n = 15) and B (n = 16) were similar for biological sex, race/ethnicity, age, CD4+ and CD8+ T-cell counts, and ART regimen.
Table 1.
Demographics of Study Participants
| Characteristic | Arm | |||
|---|---|---|---|---|
| A (n = 15) | B (n = 16) | Total | P Valuea | |
| Age, y, median (IQR) | 47 (38.5–54.5) | 50.5 (45.5–55) | 49 (43.5–54.5) | .46 |
| Sex | .65 | |||
| Male | 12 (80.0) | 14 (87.5) | 26 (83.9) | |
| Female | 3 (20.0) | 2 (12.5) | 5 (16.1) | |
| Other | 0 (0) | 0 (0) | 0 (0) | |
| Race | .16 | |||
| Black | 10 (66.7) | 6 (37.5) | 16 (51.6) | |
| Not Black | 5 (33.3) | 10 (62.5) | 15 (48.4) | |
| CD4+ T-cell count, cells/µL, median (IQR) | 608 (513–756) | 556 (470–829) | 583 (479–803) | .92 |
| CD8+ T-cell count, cells/µL, median (IQR) | 704 (506–878) | 852.5 (638–989) | 711 (547–916) | .55 |
| Statin user | 4 (26.7) | 5 (31.2) | 9 (29.0) | 1.00 |
| ART class (in addition to NRTI backbone) | ||||
| PI | 6 (40.0) | 5 (31.2) | 11 (35.5) | .72 |
| INSTI | 3 (20.0) | 8 (50.0) | 11 (35.5) | .14 |
| NNRTI | 6 (40.0) | 6 (37.5) | 12 (38.7) | 1.00 |
| Viral load, copies/mL | ||||
| <20 | 14 (93.3) | 14 (87.5) | 28 (90.3) | 1.00 |
| 20 | 1 (6.7) | 2 (12.5) | 3 (9.7) | |
Data are presented as No. (%) unless otherwise indicated. Patients were randomized so any differences in variables would be by chance.
Abbreviations: ART, antiretroviral therapy; INSTI, integrase strand transfer inhibitor; IQR, interquartile range; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Comparisons were performed between arms using Mann-Whitney U test for continuous variables and Fisher exact tests for categorical variables.
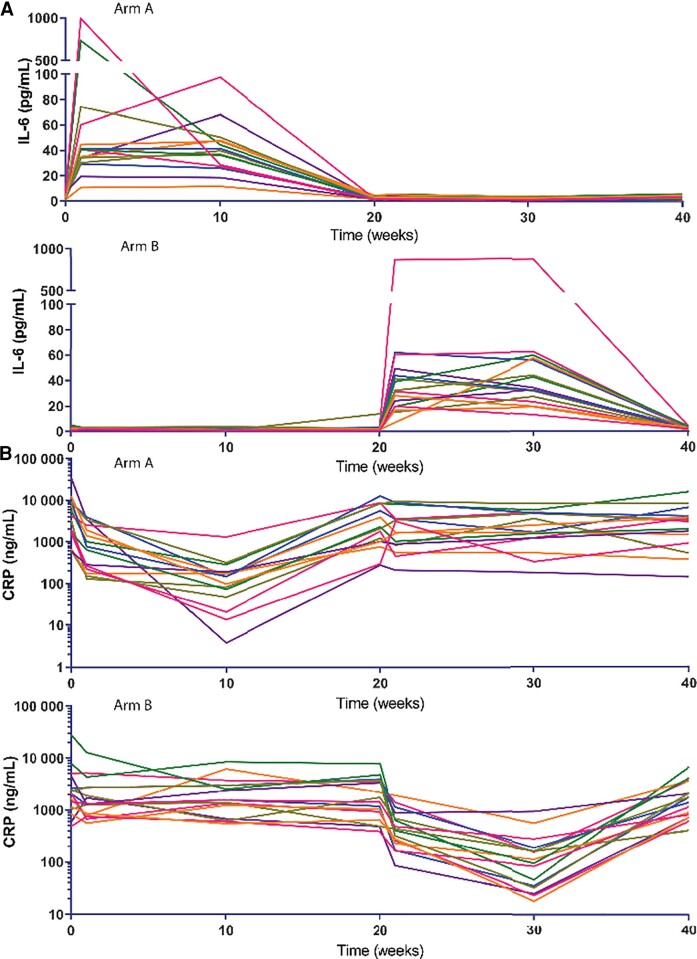
Levels of CRP fell dramatically during TCZ treatment (P < .001) and returned to baseline levels 12 weeks after the last infusion. The median decrease in CRP after treatment was 1820 ng/mL (95% CI, −2506 to −1232; effect size = 0.87, adjusted P < .0001; Figure 2A). As TCZ blocks access to the IL-6 receptor, treatment increased IL-6 levels (median increase, 41.4 pg/mL [95% CI, 33.8–51.1]; effect size = 0.87, P < .0001; Figure 2B); IL-6 levels remained stable during placebo administration.
Figure 2.
Tocilizumab (TCZ) treatment decreases plasma C-reactive protein (CRP) levels and increases levels of interleukin 6 (IL-6). Study participants in arm A received TCZ treatment in the first period (week 0); participants in arm B received TCZ in the second treatment period (week 20). A, Plasma levels of CRP decreased significantly during TCZ treatment in both arms. B, Levels of IL-6 increased significantly during TCZ treatment in both arms. After confirming the absence of carry-over effects and period effect, Wilcoxon signed-rank methods were used to test for differences between changes from baselines during the treatment periods and the placebo periods.
As mentioned above, IL-6 may drive T-cell activation. Treatment with TCZ did not reduce the proportion of CD4+ or CD8+ T cells that expressed CD38 and HLA-DR, in total populations or within maturation subsets (Supplementary Figure 1A). T-cell cycling was quantified by Ki67 expression among T-lymphocyte subsets. There was a trend to greater decreases in T-cell cycling during TCZ administration among all CD4 and CD8 maturation subsets; effect sizes range from 0.06 to 0.57); the changes were significant only among naive (CD45RA+, CD27+) CD4+ T cells (median change, −0.13% [95% CI, −.19% to −.06%]; effect size = 0.57, P = .0013; Supplementary Figure 1B).
We also measured T-cell homeostatic potential and regulation by measuring expression of the IL-7 receptor and the immune checkpoint element PD-1. Tocilizumab treatment tended to increase expression of the IL-7 receptor alpha chain (CD127) on naive (CD45RA+CD27+; effect size = 0.22, P = .24) and terminally differentiated (CD45RA+CD27−; median change, 2.35% [95% CI, .12%–6.08%]; effect size = 0.37; P = .045) CD8+ T cells (Supplementary Figure 1C) and decreased the expression of PD-1 on naive (median change, −1.27% [95% CI, −1.82% to −.67%]; effect size = 0.68, P < .001) and central memory (CD45RA−CD27+, (median change, −2.38% [95% CI, −3.95% to −1.07%]; effect size = 0.58, P < .001) CD4+ T cells (Supplementary Figure 1D). Expression of PD-1 and Ki67 are often related and here, there was a direct relationship between Ki67 expression and PD1 expression on CD4+ T cells (estimate, 0.15 [95% CI, .05–.25]; P = .0047). There was no significant change in circulating CD4+ T-cell counts during TCZ treatment (estimate, 0.027 [95% CI, −.043 to .096]; P = .45).
Tocilizumab reduced levels of biomarkers associated with mortality in PWH, including sCD14 (median change, −312.8 ng/mL; P < .0001), D-dimer (median change, −47.1 ng/mL; P < .0001), sTNFR1 (median change, −105.9 pg/mL; P = .0019), and sTNFR2 (median change, −168.5 pg/mL; P = .036) (Table 2). Levels of sCD40L also decreased (median change, −466.5 pg/mL; P < .001), as did plasma levels of IL-7 (median change, −1.17 pg/mL; P < .001) during TCZ treatment. Soluble CD163 tended to increase (median change, 60.6 ng/mL; effect size = 0.35, P = .06). Levels of Lp-PLA2 increased (median change, 22.3 ng/mL; P < .001; Table 3) and P-selectin decreased (median change, −16.0 ng/mL; P < .001; Table 2).
Table 2.
Tocilizumab Treatment Decreases Plasma Levels of Several Markers of Immune Activation and Coagulation That Are Associated With Morbidity and Mortality in People With Human Immunodeficiency Virus
| Analyte | Estimated Effect (Median, 95% CI) | Effect Size | P Value |
|---|---|---|---|
| Soluble CD14, ng/mL | −312.8 (−473.9 to −210.5) | 0.81 | <.001 |
| Soluble CD40L, pg/mL | −466.5 (−758.7 to −272.0) | 0.69 | <.001 |
| D-dimer, ng/mL | −47.1 (−70.0 to −34.4) | 0.85 | <.001 |
| IL-7, pg/mL | −1.17 (−2.09 to −.74) | 0.79 | <.001 |
| Soluble TNFR-1, pg/mL | −106.0 (−165.5 to −42.3) | 0.55 | .0019 |
| Soluble TNFR-2, pg/mL | −168.5 (−309.2 to −6.021) | 0.38 | .036 |
| Soluble CD163, ng/mL | 60.6 (−1.6 to 121.7) | 0.35 | .058 |
| IP-10, pg/mL | 14.5 (−6.5 to 40.5) | 0.28 | .13 |
| IL-22, pg/mL | −0.44 (−5.11 to 4.22) | 0.04 | .83 |
| I-FABP, pg/mL | 171.1 (−299.3 to 670.9) | 0.13 | .48 |
| Zonulin, ng/mL | −1.33 (−3.90 to 1.60) | 0.18 | .33 |
| P-selectin, ng/mL | −16.0 (−26.0 to −9.0) | 0.69 | <.001 |
| E-selectin, ng/mL | 2.74 (−1.46 to 8.79) | 0.26 | .15 |
| VCAM, ng/mL | 35.1 (−19.1 to 80.6) | 0.25 | .17 |
Plasma samples were thawed in batch and levels of biomarkers were measured by enzyme-linked immunosorbent assay. Except for soluble CD40L (sCD40L), after confirming the absence of carry-over effects and period effect, Wilcoxon signed-rank methods were used to test on differences between changes from baselines during the treatment periods and the placebo periods. For sCD40L, there was a period effect. Intergroup comparisons were performed according to the standard 2 × 2 crossover method based on a 2-sample Wilcoxon rank-sum test [15]. The response variable compared by arm is the differences between first-period change and second-period change from their corresponding baselines.
Abbreviations: I-FABP, intestinal fatty acid–binding protein; IL-7, interleukin 7; IL-22, interleukin 22; IP-10, interferon-γ–induced protein 10; TNFR, tumor necrosis factor receptor; VCAM, vascular cell adhesion molecule.
Table 3.
Lipid Profiles and Metabolism Are Altered by Tocilizumab Treatment in People With Human Immunodeficiency Virus
| Analyte | Estimated Effect (Median, 95% CI) | Effect Size | P Value |
|---|---|---|---|
| Total cholesterol, mg/dL | 18.0 (7.5–27.0) | 0.59 | .0028 |
| LDL cholesterol, mg/dL | 9.0 (1.5–18.0) | 0.44 | .025 |
| HDL cholesterol, mg/dL | 3.50 (.65–6.50) | 0.43 | .03 |
| VLDL cholesterol, mg/dL | 3.0 (−1.0 to 7.5) | 0.26 | .17 |
| Triglycerides, mg/dL | 12.0 (−4.5 to 32.0) | 0.26 | .19 |
| Lp-PLA2, ng/mL | 22.3 (11.3–35.2) | 0.64 | <.001 |
| Oxidized LDL, U/L | 5.11 (.95–9.51) | 0.42 | .022 |
| Apo-A, ng/mL | −0.18 (−.92 to .46) | 0.11 | .54 |
| Apo-B, µg/mL | 37.9 (−47.5 to 107.9) | 0.19 | .31 |
| Resistin, ng/mL | 0.70 (.26–1.16) | 0.51 | .004 |
| Leptin, ng/mL | 5.7 (−1608.6 to 2157.6) | 0.00 | 1.00 |
| Adiponectin, ng/mL | 526.5 (−88.6 to 950.6) | 0.29 | .11 |
| CE, µM | 541.4 (361.9–712.1) | 0.73 | <.0001 |
| CER, µM | 0.56 (.086–1.14) | 0.43 | .017 |
| DAG, µM | 6.22 (2.32–12.08) | 0.55 | .0015 |
| DCER, µM | 0.13 (.036–.23) | 0.47 | .0076 |
| FFA, µM | 55.4 (−38.3 to 158.80) | 0.21 | .25 |
| HCER, µM | 0.52 (.31–.73) | 0.74 | <.0001 |
| LCER, µM | 0.16 (−.073 to .39) | 0.23 | .20 |
| LPC, µM | 56.5 (29.4–83.1) | 0.6 | .0005 |
| LPE, µM | 1.24 (.43–2.30) | 0.49 | .0051 |
| PC, µM | 386.8 (249.2–517.1) | 0.76 | <.0001 |
| PE, µM | 20.4 (7.9–34.5) | 0.54 | .0022 |
| SM, µM | 61.8 (24.0–107.2) | 0.56 | .0012 |
| TAG, µM | 365.0 (164.8–617.8) | 0.59 | .0005 |
Cholesterol, HDL cholesterol, triglycerides, and calculated LDL were measured using a Beckman AU 5800 analyzer. Plasma levels of Lp-PLA2, oxidized LDL, Apo-A, Apo-B, resistin, leptin, and adiponectin were measured by enzyme-linked immunosorbent assay. Plasma samples were thawed in batches and the concentrations of 13 lipid classes were measured by the direct infusion-tandem mass spectrometry and the Lipidyzer platform. After confirming the absence of carry-over effects and period effect, Wilcoxon signed-rank methods were used to test for differences between changes from baselines during the treatment periods and the placebo periods.
Abbreviations: Apo-A, apolipoprotein A; Apo-B, apolipoprotein B; CE, cholesterol esters; CER, ceramides; CI, confidence interval; DAG, diacylglycerols; DCER, dihydroceramide; FFAs, free fatty acids; HCER, hexosylceramides; HDL, high-density lipoprotein; LCER, lactosylceramides; LDL, low-density lipoprotein; LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; Lp-PLA2, lipoprotein-associated phospholipase A2; PC, phosphatidylcholine; PE, phosphatidylethanolamine; SM, sphingomyelin; TAG, triacylglycerols; VLDL, very low-density lipoprotein.
Sparse sampling of the pharmacokinetic profiles of ART medications demonstrated no significant changes in pharmacokinetic profiles between the placebo and TCZ administration periods (data not shown).
Treatment with TCZ had no effect on brachial artery FMD (effect size = 0, P = 1.00) or hyperemic flow (effect size = 0.23, P = .24), which remained negative even after adjusting for LDL and non-HDL cholesterol levels.
Treatment with TCZ has increased lipid levels in patients with rheumatoid arthritis [16–18] and COVID-19 [19]. Here, total cholesterol (TC), and both LDL and HDL increased during TCZ treatment (Table 3), as did levels of oxLDL (median change, 5.11 U/L; P = .02). Apo-A decreased slightly and Apo-B increased slightly during TCZ treatment (P = .54 and P = .31, respectively; Table 3). The adipokines resistin (median change, 0.70 ng/mL; P = .004) and adiponectin (median change, 526.5 ng/mL; P = .11) tended to increase. Traditional lipid panels (ie, LDL, TC) may not adequately represent lipid profiles in PWH that are associated with cardiometabolic risk and inflammation [20–24]. Following TCZ treatment, plasma concentrations of total lipids (P = .0001) as well as 11 of the 13 measured lipid classes (CE, CER, DAG, DCER, HCER, LPC, LPE, PC, PE, SM, TAG) were increased (P < .05 for all; Table 3). Multiple individual lipid species were also increased following TCZ treatment (129 lipid species; P < .05). Several of these are associated with type 2 diabetes mellitus and CVD in the general population, including LPC(16:0) (151 µM vs 117 µM; P = 0.007) and LPC(18:0) (64 µM vs 47 µM; P = .005). Tocilizumab treatment increased lipid levels similarly among all participants, irrespective of ART class or statin treatment.
DISCUSSION
Plasma IL-6 levels are among the most robust predictors of morbidity/mortality in ART-treated PWH, but the role of IL-6 in driving these outcomes is uncertain [3, 5]. We explored, in a focused clinical trial, the role of IL-6 in HIV pathogenesis by blocking its biological activity in vivo with TCZ. This cytokine exerts activity by binding to receptors CD126 and CD130. Soluble CD130 can bind IL-6 and inhibit its biologic activity, while IL-6 bound to soluble CD126 can interact with cellular CD130 and induce both JAK/STAT3 and ERK 1/2 MAP kinase activation. IL-6 induces acute phase reactant (eg, CRP) expression in the liver and can act as an endogenous pyrogen. IL-6 has also been implicated in demargination of circulating neutrophils and induction of adhesion molecules VCAM-1 and ICAM-1 on endothelial cells [25, 26].
In this study of IL-6 receptor blockade, CRP levels fell dramatically as did additional markers of inflammation and coagulation, including sCD14, sTNFR1, sTNFR2, and D-dimer, each of which is predictive of morbidity and mortality in PWH [3–6, 27]. We also report a significant decrease in levels of sCD40L during TCZ treatment. Soluble CD40L levels are increased in HIV-uninfected individuals with unstable coronary artery disease and predict future CVD events [28], perhaps through induction of tissue factor expression on monocytes [29]. Reduction in sCD40L may limit tissue factor expression and clot formation in vivo as reflected here by decreased levels of the fibrin degradation product D-dimer. We also observed an increase in soluble CD163, possibly an indication of alternatively activated macrophages [30]. The impressive reduction in plasma markers of immune activation, inflammation, and coagulation with TCZ treatment implicates IL-6 as a key driver of pathogenesis in ART-treated PWH.
We demonstrated previously that circulating CD4+ and CD8+ T cells in ART-treated PWH were more often in cell cycle [7], yet this cycling appeared dysregulated, as cycling cells failed to complete cell division [31]. CD4+ and CD8+ T-cell cycling can be driven in vitro by IL-6 [10]. After in vivo blockade of IL-6 signaling by tocilizumab, in vivo cycling of naive CD4+ T cells fell significantly and cycling of most CD4+ and CD8+ T-cell maturation subsets tended to diminish (Supplementary Figure 1). Furthermore, in vitro exposure to IL-6 decreased T-cell expression of the IL-7 receptor alpha chain (CD127), impairing Stat5 phosphorylation and induction of pro-survival Bcl2 in response to IL-7 [10]. We hypothesized that IL-6 blockade would help correct the dysregulated entry of T cells into the cell cycle and would permit more T cells to successfully complete cell division in vivo in response to endogenous IL-7. TCZ administration increased expression of CD127 on naive and terminally differentiated CD8+ T cells and tended to do the same on all CD8+ T-cell maturation subsets and on terminally differentiated CD4+ T cells. After treatment with TCZ there was also a decrease in circulating levels of IL-7, likely because systemic IL-7 levels are largely regulated by CD127 binding [32]. During this 10-week treatment period, circulating CD4+ T-cell numbers were not affected. Indeed, one study that looked at CD4 T-cell turnover using heavy water labeling found that CD4 T-cell turnover rates increased as cells matured from naive to effector memory phenotype and the median half-life of naive CD4 T cells was 2.9 years [33]. Whether more sustained blockade of IL-6 activity would result in restoration of CD4+ T-cell numbers in PWH with persistent CD4+ T-cell cytopenia is not known.
A broad decrease in T-cell expression of the checkpoint inhibitor PD-1 may reflect the anti-inflammatory effects of IL-6 blockade, enhancing T-cell responses to receptor engagement, but this was not tested. As Ki67 and PD-1 expression were correlated, these data may also simply reflect decreased cellular cycling.
Treatment with tocilizumab reduces inflammation and coagulopathy in PWH; however, targeting host elements may have unintended consequences. Interleukin-6 reduces plasma lipid concentrations by increasing LDL receptor expression in tissues [18, 34]. Receptor blockade with TCZ raises lipid levels in rheumatoid arthritis [16–18], and LDL and total cholesterol increased during TCZ treatment here. Inflammatory oxLDL levels rose, as did Lp-PLA2, levels of which are predictive of CVD events in the general population [35]. We have reported that statin therapy reduces levels of Lp-PLA2 and oxLDL in PWH [36]. We show also that TCZ increased levels of multiple lipid classes and species, including DAG, TAG, CER, and LPC, that have been associated with inflammation and cardiometabolic risk in other populations [37, 38] and plausibly, may directly contribute to inflammation and cardiometabolic risk in PWH. Ceramides can activate the inflammasome, increasing IL-1β production, can reduce mitochondrial function, and can decrease insulin sensitivity through blockade of the AKT pathway [39]. Elevated levels of CER are associated with progression of carotid artery atherosclerosis [24] and pro-inflammatory, pro-coagulant macrophage profiles in PWH [22]. Both HIV and ART induce lipid changes that are linked to cardiometabolic risk in PWH [24], and we have linked perturbations in the lipidomes of PWH to indices of immune activation [21–23]. The mechanisms by which blockade of the IL-6 receptor by TCZ would induce such profound increases in multiple lipid classes remain unclear, yet these increases may confound the benefits of TCZ on CVD risk. Further investigation into the relationships among viral infections, immune function, and lipid profiles is needed. Although we saw no effect on brachial artery FMD or hyperemic flow, the small sample size and short treatment duration may have limited power to detect clinically significant changes.
While our results implicate IL-6 as a key driver of immune activation/inflammation in PWH, long-term treatment with parenteral TCZ among persons with modestly increased long-term risks for morbid outcomes may be limited by cost, logistics, and adverse effects. Future studies of IL-6 blockade could target participants with higher risk for clinical comorbidities or immune nonresponders to ART [7]. Beyond the primary objectives, our findings should be considered exploratory.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Nicholas T Funderburg, Division of Medical Laboratory Science, School of Health and Rehabilitation Sciences, Ohio State University, Columbus, Ohio, USA.
Carey L Shive, Department of Medicine, Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA; Cleveland Veterans Affairs Medical Center, Cleveland, Ohio, USA.
Zhengyi Chen, Department of Population and Quantitative Health Sciences, Case Western Reserve University, Cleveland, Ohio, USA.
Curtis Tatsuoka, Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Emily R Bowman, Division of Medical Laboratory Science, School of Health and Rehabilitation Sciences, Ohio State University, Columbus, Ohio, USA.
Chris T Longenecker, Department of Medicine and Department of Global Health, University of Washington, Seattle, Washington, USA.
Grace A McComsey, Department of Medicine, Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA; Department of Pediatrics, Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Brian M Clagett, Department of Medicine, Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Dominic Dorazio, Department of Medicine, Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Michael L Freeman, Department of Medicine, Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Scott F Sieg, Department of Medicine, Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Daniela Moisi, Department of Medicine, Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Donald D Anthony, Department of Medicine, Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA; Cleveland Veterans Affairs Medical Center, Cleveland, Ohio, USA; Rheumatology Section, MetroHealth Medical Center, Cleveland, Ohio, USA.
Jeffrey M Jacobson, Department of Medicine, Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Sharon L Stein, Department of Surgery, Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Leonard H Calabrese, Immunology and Rheumatology, Cleveland Clinic, Cleveland, Ohio, USA.
Alan Landay, Department of Internal Medicine, Rush University Medical Center, Chicago, Illinois, USA.
Charles Flexner, Divisions of Clinical Pharmacology and Infectious Diseases, School of Medicine and Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA; Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Keith W Crawford, Therapeutic Research Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Edmund V Capparelli, Clinical Pediatrics and Pharmacy, University of California, San Diego, La Jolla, California, USA.
Benigno Rodriguez, Department of Medicine, Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Michael M Lederman, Department of Medicine, Case Western Reserve University, University Hospitals Cleveland Medical Center, Cleveland, Ohio, USA.
Notes
Author contributions. All authors contributed to the review and editing of the manuscript. Conceptualization of the study: B. R., M. M. L., L. H. C., A. L., J. M. J., C. F., K. W. C. Selection of methodology: D. M., B. M. C., D. D., C. L. S., N. T. F., E. R. B., M. L. F., S. F. S. Statistical support: Z. C., C. T. Funding acquisition and supervision of the study: M. M. L., B. R. Writing–original draft: M. M. L., N. T. F., C. L. S.
Acknowledgments. The authors thank the Nutrient and Phytochemical Shared Resource at The Ohio State University for lipidomics analyses.
Financial support. This work was supported by the National Institutes of Health (NIH) (award number U01AI105937 to M. M. L., C. T., S. F. S., J. M. J., B. R., and C. F.); the National Institute of Allergy and Infectious Diseases (P30AI036219 to C. T.); the Fasenmeyer Foundation; Veterans Affairs (VA) Clinical Science Research and Development (CSR&D) (CDA2 CX001471 to C. L. S. and M. M. L.), and VA Merit (CX001791, BX001894, and BX005480 to D. D. A.).
References
- 1. Gabuzda D, Jamieson BD, Collman RG, et al. Pathogenesis of aging and age-related comorbidities in people with HIV: highlights from the HIV ACTION workshop. Pathog Immun 2020; 5:143–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology (Bethesda) 2008; 23:64–74. [DOI] [PubMed] [Google Scholar]
- 3. Hunt PW, Sinclair E, Rodriguez B, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tenorio AR, Zheng Y, Bosch RJ, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lederman MM, Calabrese L, Funderburg NT, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis 2011; 204:1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shive CL, Clagett B, McCausland MR, et al. Inflammation perturbs the IL-7 axis, promoting senescence and exhaustion that broadly characterize immune failure in treated HIV infection. J Acquir Immune Defic Syndr 2016; 71:483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shive CL, Funderburg NT, Mudd JC, et al. Acute phase cytokine down-regulates IL-7Rα and induces CD4 T cell cycling without proliferation or induction of the survival factor Bcl2. In: Keystone Conference on Immune Activation in HIV Infection: basic mechanisms and clinical implications. Breckenridge, CO, 2013.
- 10. Shive CL, Mudd JC, Funderburg NT, et al. Inflammatory cytokines drive CD4+ T-cell cycling and impaired responsiveness to interleukin 7: implications for immune failure in HIV disease. J Infect Dis 2014; 210:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis 1999; 179:859–70. [DOI] [PubMed] [Google Scholar]
- 12. Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 2004; 104:942–7. [DOI] [PubMed] [Google Scholar]
- 13. Ubhi BK. Direct infusion-tandem mass spectrometry (DI-MS/MS) analysis of complex lipids in human plasma and serum using the Lipidyzer platform. Methods Mol Biol 2018; 1730:227–36. [DOI] [PubMed] [Google Scholar]
- 14. Longenecker CT, Jiang Y, Orringer CE, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS 2014; 28:969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones B, Kenward MG. Design and analysis of cross-over trials. 3rd ed. Boca Raton: CRC Press/Taylor & Francis, 2014. [Google Scholar]
- 16. Kawashiri SY, Kawakami A, Yamasaki S, et al. Effects of the anti-interleukin-6 receptor antibody, tocilizumab, on serum lipid levels in patients with rheumatoid arthritis. Rheumatol Int 2011; 31:451–6. [DOI] [PubMed] [Google Scholar]
- 17. McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis 2015; 74:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Strang AC, Bisoendial RJ, Kootte RS, et al. Pro-atherogenic lipid changes and decreased hepatic LDL receptor expression by tocilizumab in rheumatoid arthritis. Atherosclerosis 2013; 229:174–81. [DOI] [PubMed] [Google Scholar]
- 19. Meoni G, Ghini V, Maggi L, et al. Metabolomic/lipidomic profiling of COVID-19 and individual response to tocilizumab. PLoS Pathog 2021; 17:e1009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cassol E, Misra V, Holman A, Kamat A, Morgello S, Gabuzda D. Plasma metabolomics identifies lipid abnormalities linked to markers of inflammation, microbial translocation, and hepatic function in HIV patients receiving protease inhibitors. BMC Infect Dis 2013; 13:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Belury MA, Bowman E, Gabriel J, et al. Prospective analysis of lipid composition changes with antiretroviral therapy and immune activation in persons living with HIV. Pathog Immun 2017; 2:376–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bowman ER, Cameron CM, Richardson B, et al. Macrophage maturation from blood monocytes is altered in people with HIV, and is linked to serum lipid profiles and activation indices: a model for studying atherogenic mechanisms. PLoS Pathog 2020; 16:e1008869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bowman ER, Kulkarni M, Gabriel J, et al. Altered lipidome composition is related to markers of monocyte and immune activation in antiretroviral therapy treated human immunodeficiency virus (HIV) infection and in uninfected persons. Front Immunol 2019; 10:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao W, Wang X, Deik AA, et al. Elevated plasma ceramides are associated with antiretroviral therapy use and progression of carotid artery atherosclerosis in HIV infection. Circulation 2019; 139:2003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Modur V, Li Y, Zimmerman GA, Prescott SM, McIntyre TM. Retrograde inflammatory signaling from neutrophils to endothelial cells by soluble interleukin-6 receptor alpha. J Clin Invest 1997; 100:2752–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Romano M, Sironi M, Toniatti C, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity 1997; 6:315–25. [DOI] [PubMed] [Google Scholar]
- 27. Kalayjian RC, Machekano RN, Rizk N, et al. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis 2010; 201:1796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heeschen C, Dimmeler S, Hamm CW, et al. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med 2003; 348:1104–11. [DOI] [PubMed] [Google Scholar]
- 29. Mach F, Schonbeck U, Bonnefoy JY, Pober JS, Libby P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40: induction of collagenase, stromelysin, and tissue factor. Circulation 1997; 96:396–9. [DOI] [PubMed] [Google Scholar]
- 30. Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015; 2015:816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Younes SA, Freeman ML, Mudd JC, et al. IL-15 promotes activation and expansion of CD8+ T cells in HIV-1 infection. J Clin Invest 2016; 126:2745–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sasson SC, Zaunders JJ, Zanetti G, et al. Increased plasma interleukin-7 level correlates with decreased CD127 and increased CD132 extracellular expression on T cell subsets in patients with HIV-1 infection. J Infect Dis 2006; 193:505–14. [DOI] [PubMed] [Google Scholar]
- 33. Bacchus-Souffan C, Fitch M, Symons J, et al. Relationship between CD4 T cell turnover, cellular differentiation and HIV persistence during ART. PLoS Pathog 2021; 17:e1009214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hashizume M, Yoshida H, Koike N, Suzuki M, Mihara M. Overproduced interleukin 6 decreases blood lipid levels via upregulation of very-low-density lipoprotein receptor. Ann Rheum Dis 2010; 69:741–6. [DOI] [PubMed] [Google Scholar]
- 35. White HD, Simes J, Stewart RA, et al. Changes in lipoprotein-associated phospholipase A2 activity predict coronary events and partly account for the treatment effect of pravastatin: results from the long-term intervention with pravastatin in ischemic disease study. J Am Heart Assoc 2013; 2:e000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hileman CO, Turner R, Funderburg NT, Semba RD, McComsey GA. Changes in oxidized lipids drive the improvement in monocyte activation and vascular disease after statin therapy in HIV. AIDS 2016; 30:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fernandez C, Sandin M, Sampaio JL, et al. Plasma lipid composition and risk of developing cardiovascular disease. PLoS One 2013; 8:e71846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Razquin C, Toledo E, Clish CB, et al. Plasma lipidomic profiling and risk of type 2 diabetes in the PREDIMED trial. Diabetes Care 2018; 41:2617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chaurasia B, Talbot CL, Summers SA. Adipocyte ceramides—the nexus of inflammation and metabolic disease. Front Immunol 2020; 11:576347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.