Abstract
Background
Disentangling the effects of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants and vaccination on the occurrence of post-acute sequelae of SARS-CoV-2 (PASC) is crucial to estimate and reduce the burden of PASC.
Methods
We performed a cross-sectional analysis (May/June 2022) within a prospective multicenter healthcare worker (HCW) cohort in north-eastern Switzerland. HCWs were stratified by viral variant and vaccination status at time of their first positive SARS-CoV-2 nasopharyngeal swab. HCWs without positive swab and with negative serology served as controls. The sum of 18 self-reported PASC symptoms was modeled with univariable and multivariable negative-binomial regression to analyze the association of mean symptom number with viral variant and vaccination status.
Results
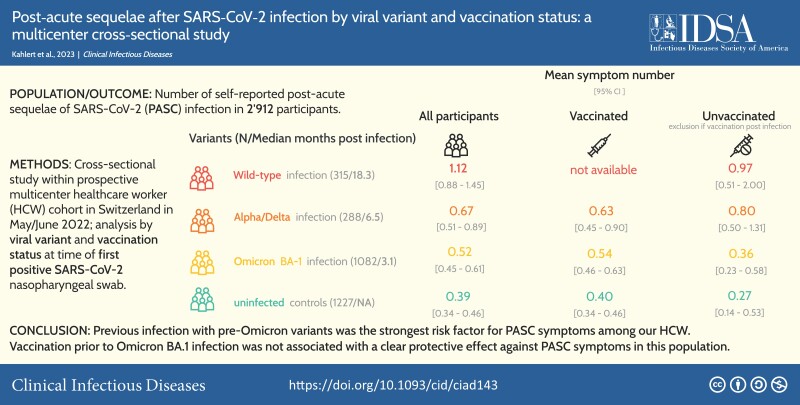
Among 2912 participants (median age: 44 years; 81.3% female), PASC symptoms were significantly more frequent after wild-type infection (estimated mean symptom number: 1.12; P < .001; median time since infection: 18.3 months), after Alpha/Delta infection (0.67 symptoms; P < .001; 6.5 months), and after Omicron BA.1 infections (0.52 symptoms; P = .005; 3.1 months) versus uninfected controls (0.39 symptoms). After Omicron BA.1 infection, the estimated mean symptom number was 0.36 for unvaccinated individuals versus 0.71 with 1–2 vaccinations (P = .028) and 0.49 with ≥3 prior vaccinations (P = .30). Adjusting for confounders, only wild-type (adjusted rate ratio [aRR]: 2.81; 95% confidence interval [CI]: 2.08–3.83) and Alpha/Delta infections (aRR: 1.93; 95% CI: 1.10–3.46) were significantly associated with the outcome.
Conclusions
Previous infection with pre-Omicron variants was the strongest risk factor for PASC symptoms among our HCWs. Vaccination before Omicron BA.1 infection was not associated with a clear protective effect against PASC symptoms in this population.
Keywords: long COVID, post-acute sequelae of SARS-CoV-2, healthcare workers, viral variant, vaccination
Within this healthcare worker cohort, the frequency of post-acute sequelae of SARS-CoV-2 symptoms was highest after wild-type infection. In contrast, symptoms were only slightly more common after Omicron BA.1 infection compared with uninfected controls, independent of SARS-CoV-2 vaccination status.
Graphical Abstract
Graphical Abstract.
This graphical abstract is also available at Tidbit: https://tidbitapp.io/tidbits/post-acute-sequelae-after-sars-cov-2-infection-by-viral-variant-and-vaccination-status-a-multicenter-cross-sectional-study
Post-acute sequelae after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; PASC), or long coronavirus disease (COVID), represent a significant challenge to healthcare systems [1]. The estimates on the frequency of long COVID vary greatly, depending on definitions used, geographical region, and time elapsed since infection [2–5]. Omicron, the predominating SARS-CoV-2 variant in 2022, spreads even more efficiently but seems less pathogenic in acute disease [6]. As the severity of acute infection has been directly associated with the risk of developing PASC [7, 8], this condition might be less common after Omicron infection compared with previous variants. Indeed, previous data show that, compared with previous variants, the risk of PASC is reduced after Omicron [9, 10]. Similarly, SARS-CoV-2 vaccination before infection has been associated with reduced risk for PASC after infection with pre-Omicron variants or mixed infections [10–13], whereas there is a scarcity of data looking at PASC after Omicron infections specifically [14]. Furthermore, an important limitation of many previous studies evaluating the burden of PASC is the lack of a control group, which is crucial to correctly attribute the often nonspecific PASC symptoms to coronavirus disease 2019 (COVID-19) [15].
In this study, we aimed to compare symptoms compatible with PASC between healthcare workers (HCWs) after infection with different SARS-CoV-2 variants and uninfected, seronegative controls. Also, we assessed the impact of mRNA COVID-19 vaccine on these symptoms.
METHODS
Setting and Participants
The Ethics Committee of Eastern Switzerland approved the study. Written informed consent was obtained. The observational multicenter study included volunteer HCWs from 9 healthcare networks in northern/eastern Switzerland [16]. The study population consisted mostly of participants from a previous HCW cohort, which had been launched in July/August 2020, but also of newly recruited participants (Supplementary Figure 1).
Study Procedures
Participants from the original cohort were prospectively followed between July/August 2020 and May/June 2022 with questionnaires capturing SARS-CoV-2–positive swabs and vaccinations; repetitive SARS-CoV-2 serologies were also performed (Supplementary Figure 2) [17].
In May/June 2022, the cross-sectional study was performed including SARS-CoV-2 serology and an electronic questionnaire, asking about personal characteristics, risk factors (eg, comorbidities, medications, work type, COVID-19 patient exposure), dates of and acute symptoms associated with previous SARS-CoV-2–positive nasopharyngeal swabs, vaccinations, and PASC symptoms. Participants with multiple positive swabs and those with a first positive swab within 4 weeks or vaccination within 1 week prior to the questionnaire were excluded.
SARS-CoV-2 Diagnostics
Participants were asked to get tested for SARS-CoV-2 in case of compatible symptoms either by rapid antigen test or polymerase chain reaction, according to national recommendations. Self-reported nasopharyngeal swab results were validated as previously described [17]. In May/June 2022, participants were screened for anti-spike (anti-S) and anti-nucleocapsid (anti-N) antibodies, to identify pauci-/asymptomatic infection [16]. The Roche Elecsys (Roche Diagnostics, Rotkreuz, Switzerland) electro-chemiluminescence immunoassay was used. For anti-S, the sensitivity and specificity are 99.9% and 97.9%, respectively [18]; for anti-N, the sensitivity at 18 months after infection is 92% [19].
Definition of Viral Variant and Vaccination Status
We used sequencing data from northeastern Switzerland to infer the most likely viral variant infecting a participant at the time of the first positive swab [20]: wild-type infections (February 2020 to January 2021); Alpha variant (February–June 2021); Delta variant (July–December 2021); Omicron variant (B.1.1.529.1; BA.1; January–June 2022) (Supplementary Figure 2). Infections during the Alpha period were merged with the Delta period due to the small number. Participants without a previous positive SARS-CoV-2 swab and with negative anti-N in May/June 2022 were considered as uninfected controls. Those without a positive SARS-CoV-2 swab but positive anti-N were excluded. Vaccination status was assessed based on self-reported data; more than 99% of vaccinated participants indicated receipt of either the mRNA-1273 (Moderna) or the BNT162b2 (Pfizer BioNTech) vaccine.
Outcomes
At the time of the questionnaire, participants were asked about the presence of any of the following symptoms for more than 7 days but not before the pandemic: loss of smell/taste, shortness of breath, chest pain, hair loss, brain fog, tiredness/weakness, skin rash, muscle/limb pain, joint point, headache, nausea/anorexia, dizziness, stomachache, diarrhea, burnout/exhaustion, fever more than 38°C/feverish feeling, chills, and cough. The main outcome was the total symptom number; individual symptoms were also analyzed.
Additionally, participants answered the 9-item Fatigue Severity Scale (FSS) [21], the 7-item Generalized Anxiety Disorder (GAD-7) Scale [22], and the 9-item Patient Health Questionnaire (PHQ-9) for depression [23]; reported their self-rated health (SRH) on a 5-point scale (“poor” to “excellent”) [24]; and indicated whether they think they would suffer from long COVID (yes vs no). If yes, the severity of long COVID was assessed using the Post-COVID-19 Functional Status Scale (PCFS), a 5-point scale from “no restrictions in daily life” to “severe restrictions” (Supplementary Table 1) [25, 26].
Statistical Analysis (see also Supplementary Material)
We used descriptive statistics to compare baseline characteristics between those infected with the wild-type virus, Alpha/Delta variant, or Omicron BA.1 variant and uninfected controls. For the main analysis, mean PASC symptom scores were compared between each of the infected groups and controls, respectively, using univariable negative-binomial models. The same analyses were performed separately by vaccination status: unvaccinated, vaccinated after infection (controls with any vaccination), and vaccinated before infection with 1 or more vaccine dose (controls with any vaccination). Also, we compared the PASC symptom scores with the number of symptoms at the time of acute infection.
Second, the frequency of individual symptoms was compared between each of the infected groups and controls, respectively, using logistic regression. This analysis was performed separately for those who were unvaccinated at the time of infection (ie, never vaccinated individuals or vaccination after infection) and those with 1 or more vaccine dose before infection. Controls were uninfected individuals without and with any vaccination, respectively.
Third, we compared the mean symptom score between boosted (≥3 vaccinations at least 7 days before infection) and nonboosted (1–2 vaccinations) participants compared with unvaccinated (but also infected) participants using negative-binomial models. This analysis was restricted to participants infected in the Delta (without Alpha) and Omicron period, because booster vaccination was not available before then.
Multivariable Analysis
To assess the independent association of viral variant and vaccination status on symptom number, we used multivariable negative-binomial regression. Potential confounders included baseline health, social determinants, job details, and receipt of any COVID-19 vaccine, which were all included in the model (Supplementary Table 1) [7, 27]. A complete case analysis was performed.
Sensitivity Analyses
To minimize the risk of incorrect group assignment due to undetected earlier SARS-CoV-2 infection, we performed a sensitivity analysis including only individuals with all previous serology data available and excluding those with any positive anti-N before the first positive SARS-CoV-2 swab or before the survey (for controls). Because the variable burnout/exhaustion is similar to tiredness, we excluded burnout in another sensitivity analysis. To account for missing data, we conducted a model using multiple imputation.
Additional Analyses
Additional outcomes were analyzed between each of the infected group and controls. For this purpose, we used a negative-binomial model (FSS, GAD-7, PHQ-9), logistic regression (self-classification of having long COVID), and proportional-odds logistic regression (distribution of SRH ratings and PCFS). These analyses were not stratified by vaccination status. This report follows the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guidelines.
RESULTS
Study Population and SARS-CoV-2 Infections
Of approximately 19 600 eligible HCWs, 3870 answered the questionnaire in May/June 2022, and 2912 (median age: 44 years; 81% women) were included (Supplementary Figure 1). SARS-CoV-2 infection was reported by 1685 (57.9%) participants: 315 (18.7%) during wild-type, 288 (17.1%) during Alpha/Delta, and 1082 (64.2%) during Omicron periods. The median time since infection was 18.3 (interquartile range [IQR]: 17.5–19.2) months for wild-type, 6.5 (IQR: 6.0–9.0) months for Alpha/Delta, and 3.1 (IQR: 2.6–4.0) months for Omicron BA.1 infection. The group of uninfected controls consisted of 1227 individuals. Of the 2912 individuals, 2570 reported baseline characteristics (Table 1). Comparing included and excluded HCWs with available baseline data, excluded HCWs were slightly younger, had fewer comorbidities, were taking fewer medications, and had more patient contact (Supplementary Table 2).
Table 1.
Characteristics of 2570 Participants Answering the Study Questionnaire by Time of First Positive SARS-CoV-2 Swab
| Baseline Characteristics | Wild-type Infection (n = 283) | Alpha/Delta Infection (n = 268) | Omicron Infection (n = 963) | No Infection (n = 1056) | P a |
|---|---|---|---|---|---|
| Age, median (IQR), y | 44 (33–54) | 42 (32–49) | 42 (34–52) | 48 (37–56) | <.001 |
| Male gender | 54 (19.1%) | 47 (17.5%) | 173 (18.0%) | 206 (19.5%) | .787 |
| BMI >30 kg/m2 | 40 (14.1%) | 23 (8.6%) | 97 (10.1%) | 134 (12.7%) | .055 |
| White ethnicity | 275 (97.2%) | 259 (96.6%) | 946 (98.2%) | 1024 (97.0%) | .442 |
| Child ≤6 y in household | 36 (12.7%) | 36 (13.4%) | 150 (15.6%) | 91 (8.6%) | <.001 |
| Any comorbidity | 143 (50.5%) | 116 (43.3%) | 467 (48.5%) | 514 (48.7%) | .343 |
| Pollen allergy | 86 (30.4%) | 77 (28.7%) | 322 (33.4%) | 331 (31.3%) | .438 |
| Other comorbidities | 86 (30.4%) | 58 (21.6%) | 254 (26.4%) | 299 (28.3%) | .085 |
| Any medication | 97 (34.3%) | 66 (24.6%) | 271 (28.1%) | 335 (31.7%) | .026 |
| Active smoking | 41 (14.5%) | 44 (16.4%) | 154 (16.0%) | 200 (18.9%) | .186 |
| Alcohol (>1 drink/wk) | 113 (39.9%) | 103 (38.4%) | 424 (44.0%) | 419 (39.7%) | .089 |
| Profession | <.001 | ||||
| Nurse | 175 (61.8%) | 149 (55.6%) | 459 (47.7%) | 476 (45.1%) | |
| Physician | 27 (9.5%) | 23 (8.6%) | 127 (13.2%) | 163 (15.4%) | |
| Other | 81 (28.6%) | 96 (35.8%) | 377 (39.1%) | 417 (39.5%) | |
| Cumulative contact duration to patients with COVID-19, median (IQR). min | 400 (30–2250) | 285 (1–1200) | 150 (1–1000) | 130 (1–750) | <.001 |
| Intensive care | 52 (18.4%) | 32 (11.9%) | 133 (13.8%) | 140 (13.3%) | .113 |
| Full-time work (>0.8 FTE) | 147 (51.9%) | 128 (47.8%) | 475 (49.3%) | 561 (53.1%) | .239 |
| Vaccinationb | <.001 | ||||
| Before infection | 0 (0.0%) | 165 (61.6%) | 876 (91.0%) | 0 (0.0%) | |
| After or without infection | 255 (90.1%) | 35 (13.1%) | 3 (0.3%) | 1022 (96.8%) | |
| No vaccination | 28 (9.9%) | 68 (25.4%) | 84 (8.7%) | 34 (3.2%) |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; FTE, full-time equivalent; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Global test for the significance of group differences. Kruskal-Wallis test for numeric variables (age, cumulative patient contact duration) and chi-square test for categorical variables (all others).
Note that numbers are slightly lower than in Figure 1 because 342 individuals only reported data on infections and vaccinations but no further data.
PASC Symptoms and Viral Variant
The 2912 participants reported a mean of 0.55 symptoms (including those without symptoms). The mean symptom number in the 3 groups of previously infected participants significantly exceeded that of uninfected controls (.39; 95% CI: .34–.45), but decreased with recency of the viral variant: 1.12 (95% CI: .88–1.45; P < .001) for wild-type, .67 (95% CI: .51–.89; P < .001) for Alpha/Delta, and .52 (95% CI: .45–.61; P = .005) for participants with Omicron BA.1 (Figure 1A, Supplementary Table 3, Supplementary Figure 3). Similar decreasing trends across viral variants were observed for unvaccinated participants (Figure 1B), for those with vaccination before or after infection (Figure 1C), and in the sensitivity analysis considering only participants with previous serologies (Supplementary Figure 4). The frequency of PASC symptoms among excluded participants was similar to that in noninfected controls. This was also true for seropositive HCWs without positive swab results. However, HCWs who were excluded due to reinfection had a symptom score similar to those with wild-type infection (Supplementary Figure 5). The number of symptoms at the time of acute infection decreased from wild-type to Omicron BA.1 infection and was associated with the number of PASC symptoms. Even when adjusting for this association, more PASC symptoms were reported after wild-type than after Alpha/Delta and Omicron BA.1 infection (Supplementary Figure 6).
Figure 1.
A–C, Cross-sectional analysis of May/June 2022. Means and 95% confidence intervals of post-acute sequelae of SARS-CoV-2 symptom score by vaccination status and viral variant dominating at the time of infection. Asterisks above the bars indicate statistical significance in reference to uninfected participants with the same vaccination status, respectively, as obtained through Wald tests on coefficients of negative binomial models with uninfected participants as the reference group: ***P < .001; **P < .01; *P < .05; nsP ≥ .05. The numbers at the bottom of the bars designate the number of participants. Note: there were no vaccinations before wild-type infection and only 3 individuals were vaccinated after Omicron BA.1 infection (not shown in panel C). Abbreviations: ns, not significant; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
At least 1 symptom was reported by 695 (23.9%) participants: 38.7% of participants with wild-type infection (P < .001), 31.6% of those with Alpha/Delta (P = .001), and 23.8% of those with Omicron BA.1 (P = .001), compared with 18.3% for uninfected individuals. The most commonly reported symptom was tiredness/weakness (14.7% in infected; 9.4% in uninfected participants). Symptoms consistently associated with SARS-CoV-2 infection across all variants were loss of smell/taste and hair loss for unvaccinated participants (Supplementary Figure 7) and loss of smell/taste and brain fog for vaccinated participants (Supplementary Figure 8).
PASC Symptoms and Vaccination Status
Among participants who were infected during the Delta period, vaccinated individuals (±booster) reported, on average, fewer symptoms than unvaccinated participants; however, the differences were not statistically significant. After Omicron BA.1 infection, the mean reported symptom number was .49 (95% CI: .41–.58; P = .30) for those with 3 or more prior vaccinations and .71 (95% CI: .53–.95; P = .028) for those with 1–2 previous vaccinations compared with .36 (95% CI: .22–.60) for unvaccinated individuals (Figure 2, Supplementary Table 3).
Figure 2.
Means and 95% confidence intervals of post-acute sequelae of SARS-CoV-2 symptom score in relation to number of vaccine doses received before positive swab. Left: Participants after infection in the Delta period (1 July to 31 December 2021). Right: Participants after infection in the Omicron BA.1 period (1 January to 30 June 2022). The asterisk above the bar indicates statistical significance in reference to unvaccinated participants infected in the same period, respectively, as obtained through Wald tests on coefficients of negative binomial models with group “none” as the reference: *P < .05; nsP ≥ .05. Abbreviations: ns, not significant; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Multivariable Analysis
Excluding those with missing data, we performed multivariable analysis including 2452 individuals. Infections with wild-type (adjusted rate ratio [aRR]: 2.81; 95% CI: 2.08–3.83) and with Alpha/Delta (aRR: 1.93; 95% CI: 1.10–3.46) were positively associated with the number of symptoms reported, whereas infection during the Omicron period (aRR: 1.29; 95% CI: .69–2.43) and vaccination before infection (aRR: 1.27; 95% CI: .82–1.94) were not. Other variables associated with the outcome were body mass index (BMI) greater than 30 m/kg2 (aRR: 1.43; 95% CI: 1.08–1.92), having a pre-existing comorbidity (RR: 1.35; 95% CI: 1.11–1.65), taking any medication (aRR: 1.49; 95% CI: 1.20–1.86), and cumulative COVID-19 patient contact (aRR: 1.11; 95% CI: 1.01–1.21) (Figure 3, Supplementary Table 4). Sensitivity analyses and data imputation showed mostly similar results. However, when including only participants with all previous serology results being negative for anti-N, the effect sizes for viral variants increased slightly, whereas other covariables, except taking any medication, were no longer associated with the outcome (Supplementary Table 5).
Figure 3.
Results of a multivariable negative binomial model regarding number of symptoms compatible with post-acute sequelae of SARS-CoV-2. For numeric values of point estimates and 95% confidence intervals, see Supplementary Table 4. Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; FTE, full-time equivalent; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Additional Analyses
The FSS and PHQ-9 were highest and the SRH lowest for those after wild-type infection, while no difference was observed between those with Alpha/Delta or Omicron BA.1 infection and controls. For the GAD-7, no difference was observed between groups (Table 2). The percentage of participants who considered themselves having long COVID was substantially higher after wild-type (17.1%; P < .001), Alpha/Delta (10.4%; P < .001), and Omicron BA.1 (4.8%; P < .001) infection compared with controls (0.9%). Those with self-reported long COVID had a mean of 3.2 (95% CI: 2.4–4.4) PASC symptoms compared with .41 (95% CI: .37–.44) in those without long COVID (P < .001). Functional impairment in daily life (ie, PCFS score) was similar between groups experiencing long COVID.
Table 2.
Results of Additional Outcomes by Time of First Infection (or No Infection) Among 2912 Participants
| Wild-type Infection (n = 315) |
Alpha/Delta Infection (n = 288) |
Omicron Infection (n = 1082) |
No Infection (n = 1227) |
|
|---|---|---|---|---|
| FSS score, mean | 22.5** | 20.4ns | 20.4ns | 20.5ref |
| GAD-7 anxiety score, mean | 2.8ns | 2.0ns | 2.3ns | 2.4ref |
| PHQ-9 depression score, mean | 3.6** | 2.5ns | 2.7ns | 3.0ref |
| SRH, mean | 4.18* | 4.36* | 4.30ns | 4.30ref |
| 1 (Very bad) | 0.6% | 1.0% | 0.5% | 0.3% |
| 2 (Bad) | 0.3% | 0.3% | 0.7% | 0.4% |
| 3 (Average) | 14.3% | 7.6% | 8.1% | 8.0% |
| 4 (Good) | 49.5% | 43.4% | 49.5% | 51.4% |
| 5 (Very good) | 35.2% | 47.6% | 41.1% | 39.9% |
| Having long COVID, no. (%) | 54 (17.1%)*** | 30 (10.4%)*** | 52 (4.8%)*** | 11 (0.9%)ref |
| PCFS, mean | 2.25ns | 2.14* | 2.32ns | 2.73ref |
| 1 (No restrictions)a | 14.8% | 36.7% | 17.3% | 0% |
| 2 (Negligible restrictions)a | 53.7% | 33.3% | 44.2% | 45.5% |
| 3 (Restrictions, able to fulfill daily activities)a | 24.1% | 13.3% | 26.9% | 36.4% |
| 4 (Restrictions, not able to fulfill daily activities)a | 7.4% | 16.7% | 11.5% | 18.2% |
| 5 (Severe restrictions)a | 0% | 0% | 0% | 0% |
Asterisks indicate statistically significant differences in outcome distribution between each infected group and uninfected controls, as given by P values from Wald tests on the coefficients of a negative binomial model for FSS, proportional-odds logistic regression for SRH and PCFS, and ordinary logistic regression for having long COVID: ***P < .001; **P < .01; *P < .05; nsP ≥ .05.
Abbreviations: COVID, coronavirus disease; FSS, Fatigue Severity Scale; GAD-7, 7-item Generalized Anxiety Disorder Scale; ns, not significant; PCFS, Post-COVID-19 Functional Status Scale (assessed only by those with self-reported long COVID); PHQ-9, 9-item Patient Health Questionnaire; ref, reference group; SRH, self-reported health.
Percentages refer to the number of participants with self-reported long COVID.
DISCUSSION
Within a well-defined HCW population, we show that the most decisive risk factor for reporting symptoms compatible with PASC is the viral variant causing the primary infection. Participants after SARS-CoV-2 wild-type and Alpha/Delta infections reported the highest numbers, even 18 and 6 months after infection, respectively. In contrast, those at 3 months after Omicron BA.1 infection were only minimally affected compared with uninfected controls. SARS-CoV-2 vaccination including receipt of booster vaccine before Omicron BA.1 infection was not associated with fewer PASC symptoms.
The viral variant was the most important risk factor associated with the presence of PASC symptoms, even after adjusting for important covariables, such as age, sex, and BMI. Individuals after wild-type infection reported significantly more PASC symptoms and had higher fatigue and depression scores and a lower SRH than uninfected controls. This effect is partly related to the higher number of symptoms experienced during acute infection, as shown in our data and by others [7, 8]. These findings are particularly worrying considering that wild-type infections occurred a median of 18 months before this survey. Few studies have reported such a long duration of PASC. The persistence of symptoms for 12 months or longer has been reported by several others, both in hospitalized and nonhospitalized populations [28–32]. In addition, some authors have suggested that loss of smell/taste, which was one of the symptoms showing a strong association with PASC in our study, might be a permanent sequela after COVID-19 [33]. Further research aimed at mitigating the PASC burden in those infected early on in the pandemic is urgently needed.
The frequency of long COVID after infection with the Omicron variant has been reported to be 5% at 4 weeks or more after infection, which is approximately half that of the Delta variant [9]. Comparing people infected with Omicron with previous variants, 5 versus 55% reported 1 or more PASC symptom in another study [34]. However, both studies did not include an uninfected control group. In our study, participants after Omicron BA.1 infection still reported more symptoms than uninfected controls. However, we found that many symptoms typically associated with PASC, such as fatigue, exhaustion, or muscle/limb pain, were slightly or not at all more common after Omicron BA.1 infection compared with uninfected controls. Also, the high prevalence of some of these symptoms in uninfected individuals highlights the importance to consider other causes, besides COVID-19, underlying these symptoms. This includes conditions such as chronic fatigue syndrome/myalgic encephalomyelitis, which have been shown to be often related to other viral infections [35–37]. Importantly, in contrast to participants after wild-type infection, FSS, PHQ-9, and SRH were not any different between individuals with Omicron BA.1 and controls.
Although the numbers were too small to reach statistical significance, vaccination prior to infection with the Delta variant was associated with decreased PASC symptom score. This finding is in line with several other studies, which included at least some infections from the pre-Omicron period [10–12]. However, with regard to the effect of previous vaccination on PASC after Omicron BA.1 infection, our results were ambiguous. Individuals with booster vaccination had a symptom score similar to that of unvaccinated individuals, but both groups had a lower score compared with those with only 1 or 2 vaccinations (Figure 2). These findings contrast results from a study specifically looking at Omicron infections [14], where a positive effect of previous vaccination on risk of PASC was found. In contrast to our study, Nehme et al [14] looked at adult outpatient populations of any age, including people with more severe disease receiving antiviral treatment. Also, partly vaccinated individuals were regarded as unvaccinated. We believe that further study is needed to better understand these contradictory results.
Important strengths of our study are the clearly defined infection and vaccine status of every individual study participant and the relatively large share of previously unvaccinated participants. This enabled us to adequately disentangle the effects of viral variant and of SARS-CoV-2 vaccination on the occurrence of PASC symptoms, a task which is increasingly becoming unrealizable due to high vaccination and infection rates [38]. Probably the most important strength is the inclusion of uninfected controls using (repetitive) SARS-CoV-2 serologies. Many previous studies on long COVID completely lacked a control group [15], relied on electronic health record data for their case definition [39], and/or were unable to differentiate between truly noninfected and asymptomatically infected individuals [39, 40].
Our study has limitations. First, the definition of viral variants was based on population data and not on individual sequencing data. This might have led to misclassification of viral variants in any direction and therefore to an underestimation of the observed differences. Second, the choice of our outcome definition can be debated. The large number of symptoms and the less strict time criterion (symptoms had to be present at least for 7 days) compared with other studies might overestimate, whereas the lack of some symptoms (eg, palpitations) could underestimate, the PASC prevalence. Third, our data lack generalizability—that is, Omicron BA.1 infections occurring in the elderly or more comorbid populations, where acute infection might manifest itself more severely, might confer an increased risk of PASC. Also, our results are not necessarily applicable to newer Omicron variants. Fourth, we relied on anti-N negativity to exclude previous infection. We acknowledge that this definition may have missed a certain proportion of infected individuals due to lack of seroconversion or waning titers over time [19]. By considering both criteria, anti-N negativity and lack of a positive swab, we believe that our approach of defining controls is reasonable. Furthermore, results of the sensitivity analysis, which also considered previous serology results and excluded those with waning immunity over time, largely confirmed our main findings. Finally, PASC symptoms among only seropositive participants (who were excluded from the main analysis) were similar to that in controls, confirming our previous finding that asymptomatic SARS-CoV-2 infection is only weakly associated with long COVID [7].
To conclude, these data suggest that, in an HCW population of predominantly young, healthy White females, individuals infected with the wild-type virus still report symptoms compatible with PASC after a median of 18 months, whereas the burden at 3 months after Omicron BA.1 infection is considerably lower and comparable to that in uninfected individuals. Vaccination prior to Omicron BA.1 infection was not associated with fewer PASC symptoms. Future research should primarily address the needs of individuals infected early on in the pandemic, whereas in individuals who experience symptoms compatible with PASC after Omicron BA.1 infection, other etiologies besides COVID-19 should be actively sought.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Christian R Kahlert, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St. Gallen, Switzerland; Department of Infectious Diseases and Hospital Epidemiology, Children’s Hospital of Eastern Switzerland, St. Gallen, Switzerland.
Carol Strahm, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St. Gallen, Switzerland.
Sabine Güsewell, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St. Gallen, Switzerland.
Alexia Cusini, Division of Infectious Diseases, Cantonal Hospital of Grisons, Chur, Switzerland.
Angela Brucher, Ambulatory Services, Psychiatry Services of the Canton of St. Gallen (South), St. Gallen, Switzerland.
Stephan Goppel, Ambulatory Services, Psychiatry Services of the Canton of St. Gallen (North), St. Gallen, Switzerland.
Elisabeth Möller, Department of Psychiatry, Clienia Littenheid, Littenheid, Switzerland.
J Carsten Möller, Center for Neurological Rehabilitation, Zihlschlacht, Switzerland.
Manuela Ortner, Rheintal Werdenberg Sarganserland Hospital Group, Grabs, Switzerland.
Markus Ruetti, Fuerstenland Toggenburg Hospital Group, Wil, Switzerland.
Reto Stocker, Hirslanden Clinic, Zurich, Switzerland.
Danielle Vuichard-Gysin, Division of Infectious Diseases and Hospital Epidemiology, Thurgau Hospital Group, Muensterlingen, Switzerland; Department of Research and Development, Swiss National Centre for Infection Prevention (Swissnoso), Berne, Switzerland.
Ulrike Besold, Geriatric Clinic St. Gallen, St. Gallen, Switzerland.
Allison McGeer, Sinai Health System, Toronto, Canada.
Lorenz Risch, Labormedizinisches Zentrum Dr Risch Ostschweiz AG, Buchs, Switzerland; Private Universität im Fürstentum Liechtenstein, Triesen, Liechtenstein; Center of Laboratory Medicine, University Institute of Clinical Chemistry, University of Bern, Inselspital, Bern, Switzerland.
Andrée Friedl, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital Baden, Baden, Switzerland.
Matthias Schlegel, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St. Gallen, Switzerland.
Pietro Vernazza, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St. Gallen, Switzerland.
Stefan P Kuster, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St. Gallen, Switzerland.
Philipp Kohler, Division of Infectious Diseases and Hospital Epidemiology, Cantonal Hospital St Gallen, St. Gallen, Switzerland.
for the SURPRISE (SURveillance of infectious diseases among health PRofessionals In SwitzErland) Study Group:
Ulrike Besold, Angela Brucher, Alexia Cusini, Thomas Egger, Andrée Friedl, Stephan Goppel, Fabian Grässli, Christian R Kahlert, Joelle Keller, Simone Kessler, Philipp Kohler, Stefan P Kuster, Onicio Leal, Eva Lemmenmeier, Allison McGeer, Dorette Meier Kleeb, Elisabeth Möller, J Carsten Möller, Maja F Müller, Vaxhid Musa, Manuela Ortner, Philip Rieder, Lorenz Risch, Markus Ruetti, Matthias Schlegel, Hans-Ruedi Schmid, Reto Stocker, Pietro Vernazza, Matthias von Kietzell, Danielle Vuichard-Gysin, and Benedikt Wiggli
Notes
Acknowledgments. The authors thank the participants of the SURPRISE study and the members of the study group (in alphabetical order): Ulrike Besold, MD (Geriatric Clinic St. Gallen); Angela Brucher, MD (Psychiatry Services South, St. Gallen); Alexia Cusini, MD (Cantonal Hospital Graubünden); Thomas Egger, MSc (Cantonal Hospital St. Gallen); Andrée Friedl, MD (Cantonal Hospital Baden); Stephan Goppel, MD (Psychiatry Services North, St. Gallen); Fabian Grässli, MSc (Cantonal Hospital St. Gallen); Christian R. Kahlert, MD (Children's Hospital of Eastern Switzerland, St. Gallen); Joelle Keller (Hirslanden Clinic Zurich); Simone Kessler (Cantonal Hospital St. Gallen); Philipp Kohler, MD, MSc (Cantonal Hospital St. Gallen); Stefan P. Kuster, MD, MSc (Cantonal Hospital St. Gallen); Onicio Leal, PhD (University of Zurich); Eva Lemmenmeier, MD (Clienia Littenheid); Allison McGeer, MD, MSc (Mount Sinai Hospital, Toronto); Dorette Meier Kleeb, MD (Cantonal Hospital Baden); Elisabeth Möller (Clienia Littenheid); J. Carsten Möller, MD (Clinic Zihlschlacht); Maja F. Müller (Hirslanden Clinic Zurich); Vaxhid Musa (Cantonal Hospital St. Gallen); Manuela Ortner (Rheintal Werdenberg Sarganserland Hospital Group, Grabs); Philip Rieder, PhD (Hirslanden Clinic Zurich); Lorenz Risch, MD, PhD (Laboratory Risch Buchs); Markus Ruetti, MD (Fuerstenland Toggenburg Hospital Group Wil); Matthias Schlegel, MD (Cantonal Hospital St. Gallen); Hans-Ruedi Schmid, PhD (Cantonal Hospital Baden); Reto Stocker, MD (Hirslanden Clinic Zurich); Pietro Vernazza, MD (Cantonal Hospital St. Gallen); Matthias von Kietzell, MD (Clinic Stephanshorn St. Gallen); Danielle Vuichard-Gysin, MD, MSc (Thurgau Hospital Group Muensterlingen); and Benedikt Wiggli, MD (Cantonal Hospital Baden).
Disclaimer. The funders played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Swiss National Sciences Foundation (grant number 31CA30_196544; grant number PZ00P3_179919 to P. K.), the Federal Office of Public Health (grant number 20.008218/421-28/1), and the research fund of the Cantonal Hospital of St. Gallen. D. V.-G. reports nonfinancial support from Roche Diagnostics (Switzerland) AG. The company provided the Elecsys anti–SARS-CoV-2 assays and additional ancillary products to the Thurgau Hospital Group (one of the study sites).
References
- 1. Phillips S, Williams MA. Confronting our next national health disaster—long-haul Covid. N Engl J Med 2021; 385:577–9. [DOI] [PubMed] [Google Scholar]
- 2. Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open 2021; 4:e2111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis 2022; 226:1593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Global Burden of Disease Long COVID Collaborators . Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 2022; 328: 1604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peter RS, Nieters A, Kräusslich H-G, et al. Post-acute sequelae of COVID-19 six to 12 months after infection: population based study. BMJ 2022; 379:e071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and Delta (B.1.617.2) variants in England: a cohort study. Lancet 2022; 399:1303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strahm C, Seneghini M, Güsewell S, et al. Symptoms compatible with long-COVID in healthcare workers with and without SARS-CoV-2 infection—results of a prospective multicenter cohort. Clin Infect Dis 2022; 75:e1011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bahmer T, Borzikowsky C, Lieb W, et al. Severity, predictors and clinical correlates of post-COVID syndrome (PCS) in Germany: a prospective, multi-centre, population-based cohort study. EClinicalMedicine 2022; 51:101549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antonelli M, Pujol JC, Spector TD, Ourselin S, Steves CJ. Risk of long COVID associated with Delta versus Omicron variants of SARS-CoV-2. Lancet 2022; 399:2263–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Azzolini E, Levi R, Sarti R, et al. Association between BNT162b2 vaccination and long COVID after infections not requiring hospitalization in health care workers. JAMA 2022; 328:676–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med 2022; 28:1461–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Antonelli M, Penfold RS, Merino J, et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID symptom study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis 2022; 22:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Perlis RH, Santillana M, Ognyanova K, et al. Prevalence and correlates of long COVID symptoms among US adults. JAMA Netw Open 2022; 5:e2238804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nehme M, Vetter P, Chappuis F, Kaiser L, Guessous I; CoviCare Study Team . Prevalence of post-COVID condition 12 weeks after Omicron infection compared to negative controls and association with vaccination status. Clin Infect Dis 2023; 76:1567–75. [DOI] [PubMed] [Google Scholar]
- 15. Amin-Chowdhury Z, Ladhani SN. Causation or confounding: why controls are critical for characterizing long COVID. Nat Med 2021; 27:1129–30. [DOI] [PubMed] [Google Scholar]
- 16. Kahlert CR, Persi R, Güsewell S, et al. Non-occupational and occupational factors associated with specific SARS-CoV-2 antibodies among hospital workers—a multicentre cross-sectional study. Clin Microbiol Infect 2021; 27:1336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kohler P, Güsewell S, Seneghini M, et al. Impact of baseline SARS-CoV-2 antibody status on syndromic surveillance and the risk of subsequent COVID-19—a prospective multicenter cohort study. BMC Med 2021; 19:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Riester E, Findeisen P, Hegel JK, et al. Performance evaluation of the Roche Elecsys anti-SARS-CoV-2 S immunoassay. J Virol Methods 2021; 297:114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakagama Y, Komase Y, Kaku N, et al. Detecting waning serological response with commercial immunoassays: 18-month longitudinal follow-up of anti-SARS-CoV-2 nucleocapsid antibodies. Microbiol Spectr 2022; 10:e00986-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Federal Office of Public Health . Virus variants overview. Available at: https://www.covid19.admin.ch/en/epidemiologic/virus-variants. Accessed 1 April 2021.
- 21. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 1989; 46:1121–3. [DOI] [PubMed] [Google Scholar]
- 22. Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006; 166:1092–7. [DOI] [PubMed] [Google Scholar]
- 23. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med 2001; 16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav 1997; 38:21–37. [PubMed] [Google Scholar]
- 25. Klok FA, Boon GJAM, Barco S, et al. The post-COVID-19 Functional Status Scale: a tool to measure functional status over time after COVID-19. Eur Respir J 2020; 56:2001494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Machado FVC, Meys R, Delbressine JM, et al. Construct validity of the post-COVID-19 Functional Status Scale in adult subjects with COVID-19. Health Qual Life Outcomes 2021; 19:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021; 27:626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pazukhina E, Andreeva M, Spiridonova E, et al. Prevalence and risk factors of post-COVID-19 condition in adults and children at 6 and 12 months after hospital discharge: a prospective, cohort study in Moscow (StopCOVID). BMC Med 2022; 20:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seeßle J, Waterboer T, Hippchen T, et al. Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): a prospective cohort study. Clin Infect Dis 2022; 74:1191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haddad A, Janda A, Renk H, et al. Long COVID symptoms in exposed and infected children, adolescents and their parents one year after SARS-CoV-2 infection: a prospective observational cohort study. EBioMedicine 2022; 84:104245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fjelltveit EB, Blomberg B, Kuwelker K, et al. Symptom burden and immune dynamics 6 to 18 months following mild severe acute respiratory syndrome coronavirus 2 infection (SARS-CoV-2): a case-control study. Clin Infect Dis 2023; 76:e60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Helmsdal G, Hanusson KD, Kristiansen MF, et al. Long COVID in the long run—23-month follow-up study of persistent symptoms. Open Forum Infect Dis 2022; 9:ofac270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mendes Paranhos AC, Nazareth Dias ÁR, Machado da Silva LC, et al. Sociodemographic characteristics and comorbidities of patients with long COVID and persistent olfactory dysfunction. JAMA Netw Open 2022; 5:e2230637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morioka S, Tsuzuki S, Suzuki M, et al. Post COVID-19 condition of the omicron variant of SARS-CoV-2. J Infect Chemother 2022; 28:1546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Magnus P, Gunnes N, Tveito K, et al. Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is associated with pandemic influenza infection, but not with an adjuvanted pandemic influenza vaccine. Vaccine 2015; 33:6173–7. [DOI] [PubMed] [Google Scholar]
- 36. Petersen I, Thomas JM, Hamilton WT, White PD. Risk and predictors of fatigue after infectious mononucleosis in a large primary-care cohort. QJM 2006; 99:49–55. [DOI] [PubMed] [Google Scholar]
- 37. Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med 2022; 28:911–23. [DOI] [PubMed] [Google Scholar]
- 38. Adriaenssens N, Scholtes B, Bruyndonckx R, et al. Prevalence, incidence and longevity of antibodies against SARS-CoV-2 among primary healthcare providers in Belgium: a prospective cohort study with 12 months of follow-up. BMJ Open 2022; 12:e065897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 2021; 18:e1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Robertson MM, Qasmieh SA, Kulkarni SG, et al. The epidemiology of long COVID in US adults. Clin Infect Dis 2023; 76:1636–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.