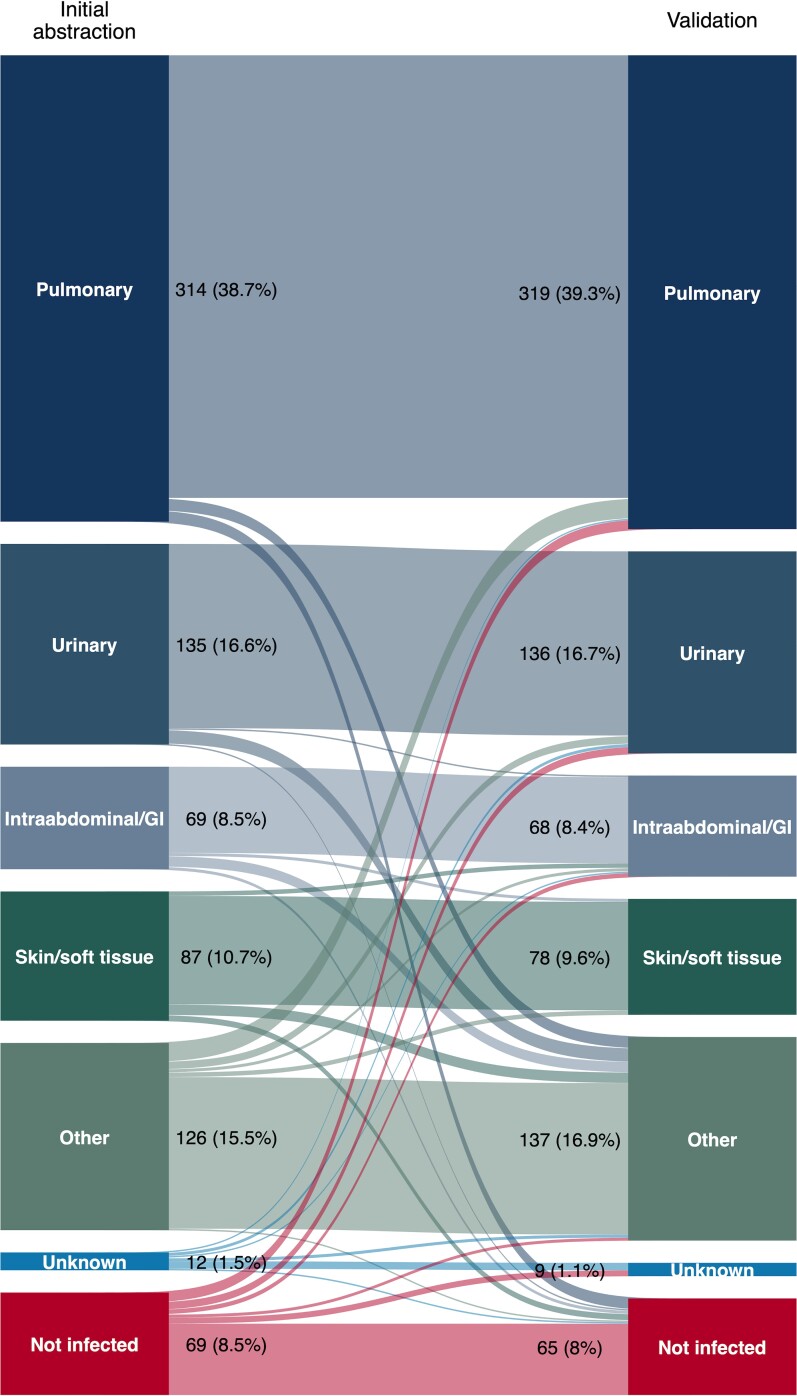
To the Editor—We appreciate Adelman and colleagues’ [1] thoughtful response to our study [2]. We agree that sepsis diagnosis is complex and that it is important to consider whether our adjudication protocol yielded reproducible and accurate results. One potential limitation noted is that the final presence of infection was adjudicated by more than 1 person for only a subset of our cohort. However, interrater agreement was high and comparable to past studies [3, 4]—κ = 0.69 (95% confidence interval [CI]: .60–.78) for the binary determination of infection presence and κ = 0.83 (95% CI: .80–.86) for infection presence and source [5] — and there was not a systematic pattern of between-rater disagreement (Figure 1). As such, it seems unlikely that additional two-reviewer adjudications would have substantially altered our findings.
Figure 1.
Alluvial diagram illustrating the final presence and source of infection for 812 emergency department patients who met Sepsis-3 criteria adjudicated by independent reviewers using structured medical record review. Block sizes are proportional to the number of patients with each infection status/source during each round of adjudication. “Streams” between initial abstraction and validation results depict patterns and proportions of interrater agreement and disagreement for each infection status/source. Abbreviation: GI, gastrointestinal.
We employed a panel of trained research assistants and medical students for most chart adjudication and validation. While this pragmatic strategy allowed us to evaluate a cohort substantially larger than the cited studies that used multiphysician adjudication (n = 211 [6], n = 447 [7], and n = 2579 [8]), we concur that the use of nonclinician adjudicators is a possible limitation of our study. However, as noted by Adelman and colleagues, even physician adjudication is not perfect, with the determination of the presence of sepsis varying substantially between physicians. In one study, clinicians who were given a series of case vignettes but no structured adjudication criteria exhibited poor agreement regarding the presence or absence of sepsis (κ only 0.18) [9]. By contrast, a study that used structured adjudication criteria had interrater agreement similar to the agreement in our study (κ = 0.79) between a two-physician panel with all available discharge information and a “gold standard” external 3-physician panel [3]. Taken together, these data suggest that expert judgment is insufficient for reliable and reproducible sepsis adjudication, while reproducible adjudication criteria like those used in our study are critical. It is also worth considering whether physicians can bring systematic biases to the adjudication task. A recent study found that infectious diseases specialists had higher thresholds for recognizing and treating infection than critical care and emergency medicine physicians [10], suggesting that the training background of physician adjudicators could influence study findings.
Overall, we believe that structured sepsis adjudication by comprehensively trained nonclinician personnel using objective criteria formulated by a panel of expert infectious diseases, emergency department, and critical care physicians provided an unbiased and reproducible estimate of false-positive presumptive infection diagnosis rates among emergency department patients meeting sepsis criteria.
Contributor Information
Gabriel A Hooper, University of Utah School of Medicine, Salt Lake City, Utah, USA.
Edward A Stenehjem, Division of Infectious Diseases and Epidemiology, Department of Medicine, Intermountain Medical Center, Salt Lake City, Utah, USA.
Joseph R Bledsoe, Department of Emergency Medicine, Intermountain Medical Center, Murray, Utah, USA; Department of Emergency Medicine, Stanford University, Palo Alto, California, USA.
Samuel M Brown, Department of Pulmonary and Critical Care Medicine, Intermountain Medical Center, Murray, Utah, USA; Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, Utah, USA.
Ithan D Peltan, Department of Pulmonary and Critical Care Medicine, Intermountain Medical Center, Murray, Utah, USA; Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, Utah, USA.
Notes
Financial support. This work was funded by grants from the Intermountain Research and Medical Foundation and the National Institute of General Medical Sciences (K23GM129661 to I. D. P.) and the National Heart, Lung, and Blood Institute (T35HL007744 to G. A. H.).
References
- 1. Adelman MW, Septimus EJ, Arias CA. The accuracy of infection diagnoses among patients meeting Sepsis-3 criteria in the emergency department. Clin Infect Dis 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hooper GA, Klippel CJ, McLean SR, et al. Concordance between initial presumptive versus final adjudicated diagnoses of infection among patients meeting Sepsis-3 criteria in the emergency department. Clin Infect Dis 2023; 76:2047–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lopansri BK, Miller Iii RR, Burke JP, et al. Physician agreement on the diagnosis of sepsis in the intensive care unit: estimation of concordance and analysis of underlying factors in a multicenter cohort. J Intensive Care 2019; 7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dregmans E, Kaal AG, Meziyerh S, et al. Analysis of variation between diagnosis at admission vs discharge and clinical outcomes among adults with possible bacteremia. JAMA Netw Open 2022; 5:e2218172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–74. [PubMed] [Google Scholar]
- 6. Heffner AC, Horton JM, Marchick MR, Jones AE. Etiology of illness in patients with severe sepsis admitted to the hospital from the emergency department. Clin Infect Dis 2010; 50:814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller RR 3rd, Lopansri BK, Burke JP, et al. Validation of a host response assay, SeptiCyte LAB, for discriminating sepsis from systemic inflammatory response syndrome in the ICU. Am J Respir Crit Care Med 2018; 198:903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klein Klouwenberg PM, Cremer OL, van Vught LA, et al. Likelihood of infection in patients with presumed sepsis at the time of intensive care unit admission: a cohort study. Crit Care 2015; 19:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rhee C, Kadri SS, Danner RL, et al. Diagnosing sepsis is subjective and highly variable: a survey of intensivists using case vignettes. Crit Care 2016; 20:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Taylor SP, Weissman GE, Kowalkowski M, et al. A quantitative study of decision thresholds for initiation of antibiotics in suspected sepsis. Med Decis Making 2023; 43:175–82. [DOI] [PubMed] [Google Scholar]