INTRODUCTION:
Mirikizumab, a monoclonal antibody targeting the p19 subunit of interleukin (IL)-23, demonstrated efficacy and was well-tolerated in a phase 2 randomized clinical trial in patients with moderate-to-severe ulcerative colitis (UC) (NCT02589665). We explored gene expression changes in colonic tissue from study patients and their association with clinical outcomes.
METHODS:
Patients were randomized to receive intravenous placebo or 3 mirikizumab induction doses. Patient biopsies were collected at baseline and week 12, and differential gene expression was measured using a microarray platform and compared in all treatment groups to determine differential expression values between baseline and week 12.
RESULTS:
The greatest improvement in clinical outcomes and placebo-adjusted change from baseline in transcripts at week 12 was observed in the 200 mg mirikizumab group. Transcripts significantly modified by mirikizumab correlate with key UC disease activity indices (modified Mayo score, Geboes score, and Robarts Histopathology Index) and include MMP1, MMP3, S100A8, and IL1β. Changes in transcripts associated with increased disease activity were decreased after 12 weeks of mirikizumab treatment. Mirikizumab treatment affected transcripts associated with resistance to current therapies, including IL-1β, OSMR, FCGR3A and FCGR3B, and CXCL6, suggesting that anti-IL23p19 therapy modulates biological pathways involved in resistance to antitumor necrosis factor and Janus kinase inhibitors.
DISCUSSION:
This is the first large-scale gene expression study of inflamed mucosa from patients with UC treated with anti-IL23p19 therapy. These results provide molecular evidence for mucosal healing from an extensive survey of changes in transcripts that improve our understanding of the molecular effects of IL-23p19 inhibition in UC.
KEYWORDS: p-19 targeted IL23, anti-TNF resistance, molecular healing
INTRODUCTION
Ulcerative colitis (UC) is a chronic relapsing immune-mediated inflammatory bowel disease (IBD) characterized by mucosal inflammation of the colon. Substantial morbidity and impaired quality of life (1) result from typical symptoms such as stool frequency (SF), rectal bleeding, and bowel urgency (BU). Treatment aims include achieving symptom control, suppressing intestinal inflammation leading to mucosal healing, and preserving gut functionality (2). Current treatment options include 5-aminosalicylates, glucocorticoids, thiopurines, and biologics that antagonize tumor necrosis factor alpha (TNFα), the p40 subunit of interleukin (IL)-12/IL-23, and a4b7 integrin (3–9). However, up to one-third of patients do not initially respond to treatment, and approximately 40% of patients who respond subsequently lose response. Therefore, despite multiple treatment options for IBD, there remains an unmet need for new therapies.
IL-23, a heterodimeric cytokine composed of a unique p19 subunit and a p40 subunit that it shares with IL-12, is a novel therapeutic target in IBD. IL-23 receptor engagement leads to activation of Janus kinases (JAKs) (mainly TYK2 and JAK2) and signal transducer and activator of transcription 3 and 4 (STAT3 and STAT4), triggering transcription of downstream target genes. IL-23 promotes the differentiation, maintenance, and stabilization of pathogenic T-cell lineages, including populations that simultaneously produce multiple proinflammatory cytokines, such as interferon-γ, IL-17A, IL-17F, and IL-22 (10,11), as well as activation and induction of the effector function of colitogenic innate lymphoid cells (12,13). Therapeutic blockade of p40 is effective in both UC and CD (14,15), and a biologic that targets p40 has been approved for both CD and UC. It has been hypothesized that IL-12 mediates more systemic effects while IL-23 is more specifically implicated in mucosal immunology, but these notions remain unproven in humans.
We studied colonic transcripts modulated by mirikizumab, an anti-IL-23p19 antibody, in patients with moderately to severely active UC to identify the molecular basis for the observed improvements in signs and symptoms of UC associated with mirikizumab treatment (16).
METHODS
Study design and participants
I6T-MC-AMAC was a multicenter, randomized, double-blinded, parallel-arm, placebo-controlled phase 2 trial in patients with moderately to severely active UC (see Supplemental Figure 1 for study design, Supplementary Digital Content 1, http://links.lww.com/CTG/A925) (16,17). This study was compliant with the International Conference on Harmonisation guidelines on good clinical practice. All informed consent forms and protocols were approved by appropriate ethical review boards before initiation of the study. All patients gave written informed consent before the first screening procedure for the study.
Procedures and outcomes
Endoscopic findings were scored by 1 of 2 blinded central readers. Histologic disease activity was assessed by a central reader using 2 biopsy samples obtained during endoscopy at baseline and study week 12. All biopsy specimens were collected at least 30 cm from the anal verge.
Where discrete lesions were present, biopsies were obtained preferentially at the edge of ulcers or if ulcers were not present, from the edge of erosions. Where visible macroscopic disease was present but without discrete lesions, biopsies were obtained spaced throughout the affected mucosa. In the absence of macroscopic disease, biopsies were obtained from throughout the segment.
See the Supplementary Methods (Supplementary Digital Content 1, http://links.lww.com/CTG/A925) for histopathology details.
RNA extraction and gene array methodology
Gene expression was measured in 553 colonic tissue biopsies from I6T-MC-AMAC using the Affymetrix WT protocol on the GeneChip HTA 2.0 arrays. Biopsies from the same subject for each time point were pooled together to get a total of 277 biopsy RNA samples. These were subjected to quality control (QC) for RNA sample quality/quantity and amplification before HTA 2.0 processing.
See the Supplementary Methods (Supplementary Digital Content 1, http://links.lww.com/CTG/A925) for RNA sample preparation and QC.
Gene chip array analysis
Two hundred twenty-four week 0 time point and 220 week 12 time point sets of probe-level data that passed QC were preprocessed with background correction and quantile normalization per standard robust multi-array average methods and summarized to the level of probe sets as defined by the Affymetrix NetAffxTM NA35/GRCh37 human reference genome release. The data were then summarized to the level of exon groups, data-defined clusters of highly correlated exon-based probe sets, as described in Supplementary Methods (see Supplementary Digital Content 1, http://links.lww.com/CTG/A925).
Differential expression statistical analyses
A mixed-effect repeated measurements model was fit to each filtered exon group separately to calculate fold changes between the week 0 and week 12 time points using age, sex, batch, body mass index at baseline, previous biologics therapy, and modified Mayo score (MMS) at baseline as covariates. Crossed time point contrast models compared the differential expression of each exon group in a dosed treatment group with its differential expression in the placebo group. In all models, multiplicity corrections were applied to the resulting P values to account for the number of comparisons made across all tested exon groups using the Benjamini-Hochberg method. Exon groups with fold changes greater than 0.5 log2 units (∼1.41× change) and false discovery rate (FDR)-adjusted q-values less than 0.05 were classified as differentially expressed.
Correlation of gene expression to SF and BU
SF was reported by patients and transformed to a 4-level ordinal scale (0–3) representing increased SF above their normal baseline. Absence of BU within 3 days before each visit was reported by patients and then converted to the 4-level ordinal scale (0: absence of urgency in all 3 days; 3: presence of urgency in all 3 days). Correlation between gene expression and SF or BU was calculated with nonparametric Kendall tau, with gene expression and SF/BU data pooled from week 0 and week 12 time points. Multitesting correction was applied to resulting P values of correlation by using the Benjamini-Hochberg procedure. Associated genes were identified by |tau| > 0.3 and adjusted P value < 0.001 for SF and |tau| > 0.225 and adjusted P value < 0.001 for BU. For each of these associated genes, the mirikizumab treatment effect was determined by comparing the expression values at week 0 and week 12 using gene expression data from patients in the 200 mg treatment group. P values of such comparison were calculated by a paired t test.
Correlation statistical analyses
Pearson correlation coefficients between the crossed time point differential expression of exon groups and the change in clinical metrics between week 0 and week 12 were calculated using age, sex, and array chip batch as covariates. The clinical metrics included MMS, total Mayo score, Mayo endoscopic subscore, Ulcerative Colitis Endoscopic Index of Severity (UCEIS) total score, Geboes score, and Robarts Histopathology Index (RHI). Correlations and associated P values were determined using the Pearson option in the R cor() function. A significantly correlated gene is defined as having a multiplicity-corrected q-value of the correlation coefficient of ≤0.05. This q-value is obtained from the residuals of the linear model that produced the correlation coefficient and is false discovery rate-adjusted for the number of genes evaluated. The laboratory results included were fecal calprotectin (fCLP) and peripheral blood concentrations of IL-17A, IL-22, and C-reactive protein (CRP).
This study is registered with ClinicalTrials.gov, number NCT02589665.
RESULTS
Patients, study outline, and biological samples
Between December 2015 and September 2017, 358 patients were screened for eligibility and 249 randomized. Of these, 224 patients had a baseline biopsy and 220 had a week 12 biopsy (see Supplemental Figure 1, Supplementary Digital Content 1, http://links.lww.com/CTG/A925). More than 62% of patients had previously received treatment with a biologic, and 49% were using corticosteroids at baseline. Baseline disease characteristics were well-balanced across treatment arms, with similar disease activity (modified Mayo score) and mucosal inflammation (Mayo endoscopy subscore). Histology measures (Geboes Index and RHI) were lower in the placebo group but similar among mirikizumab treatment groups (Table 1).
Table 1.
Baseline demographics and disease characteristics in biopsy patients
| Placebo IV Q4W (N = 62) | Miri 50 mg EB IV Q4W (N = 63) | Miri 200 mg EB IV Q4W (N = 62) | Miri 600 mg IV Q4W (N = 60) | |
| Age, yr | 42.8 (13.5) | 41.8 (14.1) | 43.4 (14.8) | 42.3 (13.4) |
| Disease duration, yr | 9.6 (9.7) | 8.2 (7.2) | 9.0 (9.0) | 6.1 (5.7) |
| Modified Mayo scorea | 6.6 (1.2) | 6.6 (1.4) | 6.4 (1.4) | 6.5 (1.3) |
| Mayo endoscopic subscore | 2.7 (0.4) | 2.8 (0.4) | 2.7 (0.5) | 2.7 (0.5) |
| Geboes Indexb | 4.4 (1.2) | 4.2 (1.3) | 3.9 (1.7) | 4.0 (1.4) |
| Geboes gradesc | ||||
| Grade 0 | 1 (1.6) | 2 (3.2) | 4 (6.5) | 3 (5.1) |
| Grade 1 | 3 (4.8) | 3 (4.8) | 8 (12.9) | 7 (11.67) |
| Grade 2A | 26 (41.94) | 23 (36.51) | 24 (38.71) | 32 (53.33) |
| Grade 2B | 4 (6.5) | 4 (6.4) | 9 (14.5) | 5 (8.3) |
| Grade 3 | 4 (6.5) | 5 (7.9) | 10 (16.1) | 5 (8.5) |
| Grade 4 | 36 (58.1) | 36 (57.1) | 35 (56.5) | 35 (59.3) |
| Grade 5 | 19 (30.65) | 22 (34.9) | 26 (41.9) | 25 (41.7) |
| RHId | 18.0 (7.8) | 18.1 (9.1) | 15.7 (9.3) | 16.8 (9.1) |
| Concomitant therapies | ||||
| 5-ASA | 46 (74.2) | 42 (66.7) | 56 (90.3) | 39 (65.0) |
| Corticosteroids | 33 (53.2) | 29 (46.0) | 25 (40.3) | 35 (58.3) |
| Thiopurines | 25 (40.3) | 15 (23.8) | 18 (29.0) | 11 (18.3) |
| Prior biologic use | 39 (62.9) | 39 (61.9) | 40 (64.5) | 38 (63.3) |
| Prior anti-TNF use | 36 (58.1) | 35 (55.6) | 37 (59.7) | 35 (58.3) |
| Fecal calprotectin, mg/kg | 1,392 (3,450) | 1,945 (3,868) | 1,560 (2,964) | 1,523 (6,197) |
| CRP, mg/L | 8.8 (13.5) | 9.3 (12.9) | 9.1 (12.9) | 14.6 (25.9) |
Data are presented as mean (SD) or n (%) unless otherwise noted. Analysis population included those patients who received both week 0 and week 12 biopsies.
5-ASA, 5-aminosalicylic acid; CRP, C-reactive protein; EB, exposure-based; IV, intravenous; RHI, Robarts Histopathology Index; TNF, tumor necrosis factor.
Modified Mayo score = rectal bleeding, stool frequency, and endoscopy subscores.
Geboes Index = average of all Geboes grades.
Geboes grades (grades for each patient where a level of abnormality is seen in that grade): 0 = structural (architectural change), 1 = chronic inflammatory infiltrate, 2A = lamina propria eosinophils, 2B = lamina propria neutrophils, 3 = neutrophils in epithelium, 4 = crypt destruction, and 5 = erosion or ulceration; a subscore of 0 indicates normal appearance, with higher subscores indicating increasingly abnormal appearance.
RHI scores from 0 (no disease activity) to 33 (high disease activity).
Mirikizumab-mediated changes in transcripts involved in mucosal healing
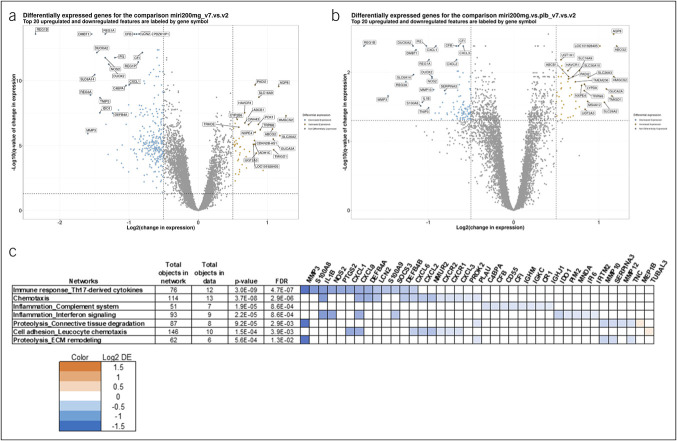
The 200 mg mirikizumab treatment group demonstrated the greatest efficacy at week 12 compared with placebo of all 3 treatment arms (15.9% [P = 0.066], 22.6% [P = 0.004], and 11.5% [P = 0.142] of patients in the 50, 200, and 600 mg groups achieved clinical remission, respectively) (16). To better discern the effect of mirikizumab treatment from the effect of the standard of care, we evaluated the change in gene expression between baseline and week 12 in the 200 mg mirikizumab group both without (Figure 1a) and with (Figure 1b) normalization to placebo. As expected, normalization to placebo provides a higher degree of confidence in identifying the transcripts that are likely to be regulated by mirikizumab.
Figure 1.
Differentially expressed genes after mirikizumab treatment. (a, b) Differentially expressed (log2 fold change ≥0.5 and false discovery rate [FDR] ≤0.05) genes in the 200 mg mirikizumab group before (a) and after (b) normalizing for placebo are shown as blue circles if decreased and orange circles if increased. The 20 most increased and decreased are labeled. (c) The most differentially expressed genes in the 200 mg mirikizumab group, after normalizing for placebo, grouped by gene networks as defined by the MetaCore database (Clarivate Analytics, Philadelphia, PA).
The transcripts with the greatest change at week 12, after normalization for changes in the placebo group, are provided in Table 2. The most significant increases in expression are seen for genes encoding proteins that are common in epithelial cells found in healthy mucosa, including AQP8, ABCG2, HMGCS2, SLC26A3, and GUCA2A, and could reflect a recovery of the epithelial lining and barrier integrity. TMIGD1, an adhesion molecule that protects epithelial cells from oxidative injury, is similarly significantly upregulated after mirikizumab treatment (18). These robustly upregulated transcripts represent both structural components seen in a healthy mucosa and critical functional proteins, which may provide some evidence of return to function in these patients. The most significantly upregulated transcript after placebo normalization (Figure 1b) was AQP8 (aquaporin-8), which codes for a critical water transporter in the apical colon surface that mediates retrieval of water from fecal content; this may contribute a beneficial effect to patients experiencing watery and frequent diarrhea (19,20). Efflux transporters, such as ABCG2, are reduced in UC and, when upregulated as given in Table 2, offer improved mucosal function in patients with UC (21).
Table 2.
Genes with the greatest fold change in expression from baseline at week 12 in the 200 mg mirikizumab group, adjusted for placebo
| Most decreaseda | Most increaseda |
| REG1B** | ABCG2 ++ |
| MMP3** | HMGCS2 ++ |
| REG3A** | AQP8 ++ |
| DUOXA2** | TMIGD1 ++ |
| SLC6A14** | GUCA2A ++ |
| DMBT1** | SLC26A2 ++ |
| MMP1** | LOC101928405 ++ |
| REG1P** | SLC26A3 + |
| S100A8** | MS4A12 + |
| IL1β** | UGT2A3 + |
| IGKV2D-40** | TRPM6 + |
| PI3** | NXPE4 + |
| TNIP3* | SLC16A9 + |
| REG1A* | ADH1C + |
| IDO1* | PCK1 + |
| DUOX2* | CDKN2B-AS1 + |
| NOS2* | TMEM236 + |
| MMP10* | CD177P1 + |
| CXCL1* | SLC17A4 + |
| PTGS2* | ZG16 + |
Genes ordered by greatest to least magnitude of fold change.
PBO, placebo; less than PBO by at least 0.5 log2 units-fold; **less than PBO by at least 1 log2 unit-fold; + greater than PBO by at least 0.5 log2 units-fold; ++ greater than PBO by at least 1 log2 unit-fold.
Conversely, the list of genes showing the greatest decreases in expression (Figure 1c) includes genes that have been associated with IBD activity by their functions in tissue remodeling (MMP3 and MMP10), oxidative stress (DUOX2, DUOXA2, and NOS2), inflammation (S100A8 and IL1β), and chemotaxis (CXCL1) or by their negative effects on epithelial cell growth (REG1B and REG3A) (22). SLC6A14, a sodium and chloride ion-dependent amino acid transporter that is involved with solute flux across the epithelial barrier and has been shown to be upregulated in patients with UC (23), is similarly strongly downregulated by mirikizumab.
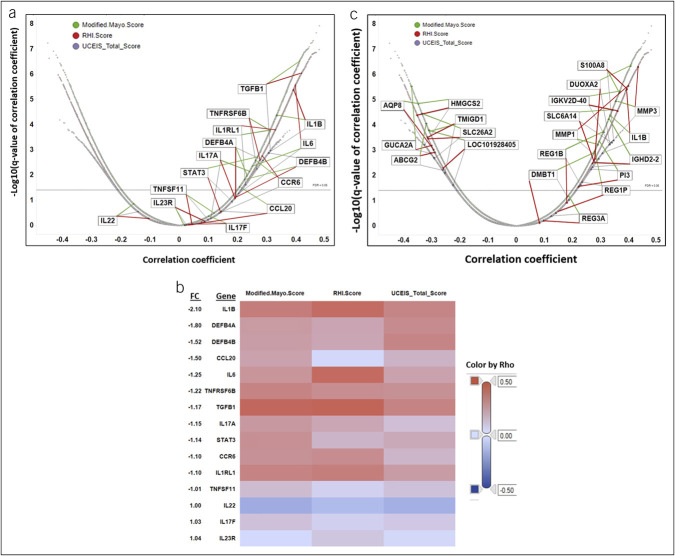
Correlation between IL-23 pathway transcripts, disease activity scores, and effects of mirikizumab at week 12
Changes in expression levels of genes that are upregulated by IL-23 receptor activation, as defined in the MetaCore (Clarivate, Philadelphia, PA) database, were correlated with changes in disease activity scales MMS, UCEIS, and RHI at week 12 (Figure 2a). All the genes that are upregulated by IL-23/IL-23R engagement and significantly correlated with changes in disease activity metrics have positive correlations, with the degree of gene expression change correlating with disease activity metrics, with the exception of IL22, which did not pass the false discovery rate ≤0.05 significance threshold. This may be because of the hierarchy of the IL-23 pathway in the colon. The most highly correlated genes include transforming growth factor β1, IL1β and its receptor, and IL6, all of which are associated with inflammation. Of these, IL1β is also one of the genes most reduced in expression between baseline and week 12 in the placebo-adjusted 200 mg mirikizumab group (Figure 1b).
Figure 2.
Correlation between IL-23 pathway transcripts and changes in disease activity scores. (a) Pearson correlation coefficients for the differential expression of mirikizumab-regulated genes at week 12 with change over the same period in modified Mayo score (green), RHI (red), and UCEIS (gray). Genes that are identified by the MetaCore database (Clarivate Analytics, Philadelphia, PA) as being upregulated by IL-23 are labeled. (b) Heat map of Pearson correlation coefficients (Rho) for the differential expression of each gene identified above. Fold change values for each gene in the 200 mg mirikizumab dose group after normalization with the change in the placebo group are labeled on the vertical axis. (c) Correlation between changes in mirikizumab-regulated genes and changes in disease activity scores. Pearson correlation coefficients for the differential expression of mirikizumab-regulated genes at week 12 with change over the same period in modified Mayo score (green), RHI (red), and UCEIS (gray). Genes with a differential expression of ≥2-fold in the 200 mg mirikizumab dose group after normalization with change in the placebo group are labeled. All labeled genes that were positively correlated with changes in disease activity scores decreased between baseline and week 12, and those that were negatively correlated with changes in disease activity scores increased in expression over that period. RHI, Robarts Histopathology Index; UCEIS, Ulcerative Colitis Endoscopic Index of Severity.
Figure 2b presents a heat map of Pearson correlation coefficients (Rho) for the placebo-adjusted differential expression of each gene between baseline and week 12 with the change over the same period of modified Mayo score, RHI, and UCEIS sorted vertically by the fold changes of these genes. This allows identification of genes with both high correlation to disease activity and high differential expression after mirikizumab treatment. Mirikizumab-regulated genes correlate more strongly with the disease indices for clinical activity (MMS) and with histopathology (RHI) than with endoscopy alone (UCEIS), which may reflect the functional contributions made by these molecular changes or the lower background noise and greater breadth of score of the MMS compared with UCEIS. The highest differential expression is found in those genes that are the most strongly correlated with disease activity and histology. For instance, we see IL1β having both high correlation to disease activity and high differential expression relative to the other genes while IL22 had a nonsignificant negative correlation with disease activity.
To evaluate the effect of mirikizumab on gene expression and disease activity holistically, we assessed correlation between changes in disease activity and changes in gene expression from baseline to week 12. Figure 2c shows changes in individual transcripts due to mirikizumab treatment, correlated with changes in disease activity as defined by MMS, RHI, and UCEIS. Several transcripts that are most strongly modulated by mirikizumab (Table 2) are also strongly correlated with disease activity. All the transcripts that positively correlate with disease are uniformly and consistently decreased by mirikizumab (Figure 2c). By contrast, all transcripts that negatively correlate with disease are increased with mirikizumab treatment. This is consistent with the observation that changes in mirikizumab-regulated transcripts demonstrate a profile of attenuation of disease, suggesting preliminary evidence of molecular healing as early as 12 weeks after initiation of treatment.
Regulation of gene expression by mirikizumab associated with BU and SF
To evaluate the correlation of individual transcripts regulated by mirikizumab with symptomatic outcomes such as SF and BU, we used the patient-reported SF and BU response over the 12-week period from all 4 treatment arms. The top 20 transcripts that correlate with either SF or BU, as defined by absolute correlation coefficients and q-values, are provided in Table 3. After the transcripts that correlated with each symptom were identified, the effect of mirikizumab (200 mg dose) on these genes was determined. All transcripts that were positively correlated with SF or BU were decreased after mirikizumab treatment, whereas all transcripts that were negatively correlated with SF and BU were increased with 200 mg mirikizumab treatment (Table 4). It is noteworthy that the individual genes (S100A8 and S100A9) that most robustly positively correlated with the SF response were strongly decreased by mirikizumab (200 mg). These genes code for calprotectin, which is a clinical biomarker of UC.
Table 3.
Top 20 genes that correlate with stool frequency or bowel urgency
| Top 20 genes correlated with stool frequency | Top 20 genes correlated with bowel urgency | ||||
| Symbol | Name | tau | Symbol | Name | tau |
| S100A8 | S100 calcium binding protein A8 | 0.379 | CCDC175 | Coiled-coil domain containing 175 | −0.297 |
| S100A12 | S100 calcium binding protein A12 | 0.371 | TNFRSF17 | TNF receptor superfamily member 17 | 0.277 |
| CDHR1 | Cadherin-related family member 1 | −0.370 | CFB | Complement factor B | 0.273 |
| S100A9 | S100 calcium binding protein A9 | 0.363 | FBXW7 | F-box and WD repeat domain containing 7 | 0.271 |
| TRIB2 | Tribbles pseudokinase 2 | 0.360 | LIPA | Lipase A, lysosomal acid type | 0.269 |
| PTAFR | Platelet-activating factor receptor | 0.359 | CEP128 | Centrosomal protein 128 | 0.268 |
| AIFM3 | Apoptosis-inducing factor mitochondria associated 3 | −0.359 | BIRC3 | Baculoviral IAP repeat containing 3 | 0.267 |
| FCGR3B | Fc fragment of IgG receptor IIIb | 0.357 | IFNAR2 | Interferon alpha and beta receptor subunit 2 | 0.267 |
| CSF3R | Colony-stimulating factor 3 receptor | 0.356 | PSAT1 | Phosphoserine aminotransferase 1 | 0.267 |
| LYN | LYN proto-oncogene, Src family tyrosine kinase | 0.356 | SNX25 | Sorting nexin 25 | 0.266 |
| IFITM2 | Interferon-induced transmembrane protein 2 | 0.356 | HSPA13 | Heat shock protein family A (Hsp70) member 13 | 0.263 |
| CAPN13 | Calpain 13 | −0.356 | CLDN2 | Claudin 2 | 0.263 |
| ELL2 | Elongation factor for RNA polymerase II 2 | 0.356 | LY96 | Lymphocyte antigen 96 | 0.262 |
| PROK2 | Prokineticin 2 | 0.355 | SEC11C | SEC11 homolog C, signal peptidase complex subunit | 0.261 |
| AQP9 | Aquaporin 9 | 0.355 | DRAM1 | DNA damage-regulated autophagy modulator 1 | 0.260 |
| IL1A | Interleukin 1 alpha | 0.355 | CPEB4 | Cytoplasmic polyadenylation element-binding protein 4 | 0.260 |
| FCGR2A | Fc fragment of IgG receptor IIa | 0.354 | PCK1 | Phosphoenolpyruvate carboxykinase 1 | −0.259 |
| TIMP1 | TIMP metallopeptidase inhibitor 1 | 0.354 | ELL2 | Elongation factor for RNA polymerase II 2 | 0.259 |
| TCN1 | Transcobalamin 1 | 0.353 | CTSH | Cathepsin H | 0.259 |
| CKB | Creatine kinase B | −0.353 | CAPN13 | Calpain 13 | −0.259 |
Top 20 genes correlated with stool frequency or bowel urgency. Tau value (Kendall rank correlation) indicates the strength of correlation.
Table 4.
Mirikizumab effect on genes correlated with stool frequency or bowel urgency
| Genes correlated with stool frequencya | Baseline vs week 12 200 mg mirikizumabb |
| 212 (+) | 212 ↓ |
| 55 (−) | 55 ↑ |
| Genes correlated with bowel urgencya | Baseline vs week 12 200 mg mirikizumabb |
| 296 (+) | 296 ↓ |
| 24 (−) | 24 ↑ |
| Genes correlated with both stool frequency and bowel urgency | Baseline vs week 12 200 mg mirikizumab |
| 107 (+) | 107 ↓ |
| 15 (−) | 15 ↑ |
For stool frequency, 212 genes were positively correlated (increased gene expression and greater stool frequency) and 55 negatively correlated (increased gene expression and lesser stool frequency). For bowel urgency, 296 genes were positively correlated and 24 negatively correlated. One hundred seven genes were positively correlated with both stool frequency and bowel urgency while 15 genes were negatively correlated with both.
Number of genes correlated with stool frequency or bowel urgency: |tau| >0.3 and q-value <0.001. For stool frequency (baseline N = 224, week 12 N = 219), |tau| >0.225 and q-value <0.001; for bowel urgency (baseline N = 223, week 12 N = 219). All patients included in the analysis had both gene expression and stool frequency or bowel urgency data at the given time point.
The effect of mirikizumab treatment on these genes are indicated with arrows. N = 53 patients in the mirikizumab 200 mg IV dose group who had biopsies at both baseline and week 12.
Pathway analysis of top mirikizumab-regulated genes most highly correlated with change in disease scores
We then focused on the most highly modulated of the transcripts identified in Figure 2c, with the stringent cutoff of r values greater than ±0.4. The 2 disease indices chosen were RHI and MMS, which offered the highest -log10 q values for these correlations. As shown in Supplemental Figure 2 (see Supplementary Digital Content 1, http://links.lww.com/CTG/A925), these transcripts provide a glimpse into the common pathways that are altered with change in disease activity and include cell adhesion and extracellular matrix remodeling, followed by features seen in profibrotic pathways and tissue repair.
Correlation of mirikizumab-induced changes in gene expression with systemic biomarkers
We then determined correlation between transcripts for IL1β, IL6, S100A8, and S100A9 to systemic biomarkers IL-17, IL-22, CRP, and fCLP (Table 5, see Supplemental Figure 3, Supplementary Digital Content 1, http://links.lww.com/CTG/A925) and found that changes in colonic expression of S100A8/9, IL1β, and IL6 strongly correlated with levels of fCLP as well as serum IL-17A and less strongly, yet still significantly, with serum CRP levels. Circulating levels of IL-22 were significantly correlated with changes in S100A9 and IL1β, but not S100A8 or IL6. These correlations suggest that accessible biomarkers such as fCLP, IL-1β, and IL-6 are correlated with the changes in the transcripts encoding these proteins and reflect p19 engagement in the colonic mucosa.
Table 5.
Correlation matrix of protein biomarkers with change from baseline in expression of key transcripts
| Gene | Protein | |||||||
| CRP | fCLP | IL-17A | IL-22 | |||||
| P | Rho | P | Rho | P | Rho | P | Rho | |
| S100A8 | 0.012 | 0.345 | <0.001 | 0.515 | <0.001 | 0.505 | 0.120 | 0.235 |
| S100A9 | 0.008 | 0.362 | <0.001 | 0.494 | <0.001 | 0.534 | 0.044 | 0.301 |
| IL1β | 0.004 | 0.389 | 0.009 | 0.377 | <0.001 | 0.545 | 0.033 | 0.319 |
| IL6 | 0.016 | 0.332 | 0.005 | 0.399 | <0.001 | 0.499 | 0.059 | 0.283 |
Pearson correlation of change from baseline in gene expression with log2 (protein) levels from the residuals of the linear mixed-effects models that each adjusts for batch, age, and sex.
CRP, C-reactive protein; fCLP, fecal calprotectin; IL, interleukin.
Mirikizumab modulates core TNF-resistance transcripts
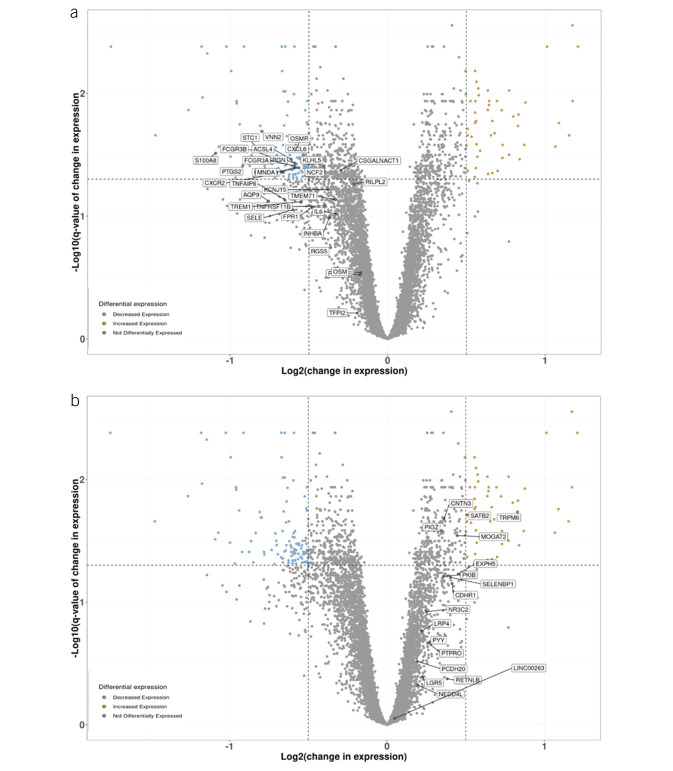
Smillie et al found that inflammation-associated fibroblasts, monocytes, and dendritic cells showed a high relative expression of genes that were part of a drug-resistance signature identified using a meta-analysis of bulk expression data from 60 responders and 57 nonresponders to anti-TNF inhibitor therapy (24). In contrast to the drug-resistant phenotype, the anti-TNF inhibitor-sensitive genes in the colon tissue were most enriched in epithelial cells. In Figure 3a, we show the transcripts included in the meta-analysis that reflect key drivers of TNFR. It is notable that the number of transcripts regulated by mirikizumab in the TNF-sensitive cluster (Figure 3b) are fewer than those seen in the TNFR cluster (Figure 3a). This is consistent with the distinct mechanism of action for mirikizumab that targets the p19-IL23 protein compared with antibodies that target TNFα. However, interestingly, mirikizumab-regulated genes are predominant in the TNFR transcript cluster. Collectively, these results suggest that mirikizumab treatment may serve to reduce the transcripts associated with TNFR and may offer a different phenotype in the colonic tissue than those seen in TNFR patients.
Figure 3.
Mirikizumab-regulated genes associated with anti-TNF inhibitor resistance and response. Change in expression of each gene in the 200 mg mirikizumab dose group between baseline and week 12 normalized for change in expression in the placebo group over the same period. Differentially expressed genes (log2 fold change ≥0.5 and false discovery rate ≤0.05) are shown with blue circles if decreased and orange circles if increased. Genes associated with resistance (a) and response (b) to anti-TNF inhibitor treatment are labeled. TNF, tumor necrosis factor.
DISCUSSION
Robust changes in transcript expression levels were observed in the 200 mg mirikizumab group, consistent with previously published improvements in disease end points at this dose in patients with UC (16). By examining the Pearson correlation coefficients for the differential expression of these genes in the context of UC disease indices (Figure 2b), we are able to identify those genes that are the most strongly correlated with disease activity and histology. The genes increased by mirikizumab are consistent with those expressed in healthy mucosa, with the top regulated transcripts representing improvements in barrier integrity and those that provide functional transporters in the colon. For example, the increase in aquaporin-8, a water transporter, may limit the water content in the fecal matter; increases in anion transporters, SLC26A2 and SLC16A9, reaffirm that mirikizumab treatment may lead to a mucosa capable of retaining functional transporters as early 12 weeks after initiation of treatment during the induction phase. Some of the genes most robustly decreased by mirikizumab, such as REG1A and REG1B, have been shown to be increased in UC in previous studies conducted in UC mucosal biopsies (25). Of note, IL1β was the only IL-23 pathway gene identified to be both significantly downregulated by mirikizumab and significantly correlated with changes in disease activity. IL1β, which has been shown to be upregulated in active UC (26), also had strong associations with all 4 systemic biomarkers analyzed, suggesting that it may be a useful indicator of UC disease activity. Furthermore, recent reports have identified an IL-1R-mediated mucosal fibroblast pathway that serves as a chemoattractant to neutrophils and is enriched in nonresponders to several current therapies such as anti-TNF and JAK inhibitors (27).
Of relevance is the change in the S100A8 and S100A9 transcripts that contribute to the calprotectin protein derived from the colonic mucosa. It has been previously reported that mirikizumab treatment leads to dose-dependent changes in fCLP (16); these changes are likely a result of reductions in calprotectin transcripts derived from the colon mucosa. Consistent with this finding, we observe a statistically significant correlation with the decreased levels of fCLP in those patients in whom the S100A8 and S100A9 transcripts are downregulated in the colon with mirikizumab treatment. Overall, the most highly mirikizumab-regulated genes reflect a reduction in inflammatory signals (IL1β, CXCL1, CXCL2, and CXCL3), attenuation of the transcripts that mediate matrix disruption of the colonic mucosa (matric metalloproteases 10 and 3), and an increase in anion and water transporters. These molecular changes are suggestive of mucosal healing (28). It is noteworthy that the transcripts coding for calprotectin (S100A8 and S100A9) are in the top 20 genes that correlate with the patient-centric clinical response of SF. Mirikizumab-mediated decreases in these transcripts may provide the mechanistic basis for the observed improvements in SF and BU (16,29).
The IL23 pathway has been implicated in genetic predisposition for UC, confirmed by a meta-analysis of the association of 10 polymorphisms in the IL23R gene (except rs10489629) with UC risk (30). Our analysis of IL23-regulated transcripts overlaid onto the change in transcripts correlated with disease activity indicates a consistent downregulation of IL23 pathway genes (Figure 2). Analysis of patients with UC shows that inflammation-associated fibroblasts, monocytes, and tissue-resident antigen-presenting cells exhibit high relative expression of genes that are enriched in biopsy samples from patients who represent primary and secondary failures from anti-TNF inhibitor therapies (16,19). By contrast, the anti-TNF inhibitor-sensitive genes in the colon tissue originated from epithelial cells. In a meta-analysis of the transcripts that are enriched in such TNFR colon mucosa (30), several transcripts given in Table 2 as being regulated by mirikizumab appear. These transcripts are potentially represented in those cell types identified by the previous studies. However, one would require future single-cell RNASeq to elucidate the reduction in such cell types in the mirikizumab-treated colon mucosa.
Of importance is the change in oncostatin M receptor (OSMR) expression, which is downregulated by mirikizumab. It has been previously hypothesized that OSM represents a potential divergent disease pathway as the colonic mucosa acquires TNFR32. OSM, a gene showing significantly increased expression in the mucosa of TNFR patients with UC compared witih the mucosa in anti-TNF inhibitor-responsive patients (19), was enriched in inflammation-associated monocytes and antigen-presenting cells, whereas its receptor, OSMR, is enriched in inflammation-associated fibroblasts (24). The finding that mirikizumab downregulates OSMR in the colon mucosa within 12 weeks of mirikizumab treatment suggests a suppression of this pathway's activation. Moreover, a number of genes associated with OSM activity (31) are also regulated by mirikizumab (see Supplemental Table 3, Supplementary Digital Content 1, http://links.lww.com/CTG/A925).
The changes observed within 12 weeks of mirikizumab treatment are distinct from those seen after vedolizumab (VDZ) treatment (32). However, the VDZ analysis of the transcriptome changes were not normalized for changes to their placebo group, which we believe is a more stringent method to the evaluation of treatment-related transcriptome effects because the lack of placebo adjustment may result in some transcript changes that associate with the edge of the q = 0.05 threshold. As such, we can assert with greater confidence that the gene expression changes seen in the placebo-adjusted 200 mg mirikizumab group are associated with mirikizumab treatment rather than being associated with spontaneous mucosal healing. There is very little in common between the top 10 transcripts altered by VDZ vs the control group and the top 10 in our analyses, possibly because of both the lack of placebo normalization in the VDZ study and the use of healthy controls instead of diseased baseline patients for the comparison. These results suggest a more robust and rapid change in transcripts with mirikizumab treatment at 12 weeks, but subsequent head-to-head studies with VDZ would be required to confirm this finding.
Collectively, our study provides evidence of a distinct pattern of transcriptional changes as early as 12 weeks after mirikizumab treatment in a randomized controlled Ph2 clinical trial using well-annotated disease activity indices. These transcriptional changes correlate with disease activity and demonstrate a profile of attenuation of disease, consistent with molecular healing early in the initiation of treatment with mirikizumab. The changes mediated by mirikizumab include transcripts that are enriched in TNFR mucosa, suggesting an opportunity to intervene in this pathway with mirikizumab in patients in whom anti-TNF inhibitors have failed. Finally, the transcript changes observed at week 12 are mediated by mirikizumab with a high degree of confidence and may be distinct from those seen with VDZ.
CONFLICTS OF INTEREST
Guarantor of the article: Venkatesh Krishnan, PhD.
Specific author contributions: J.L.T., V.K., J.S., and W.J.S.: contributed to the study conception or design. J.S. and B.J.: contributed to data acquisition. V.K., J.S., Y.L., W.J.S., B.J., N.P., G.D., and B.S.: contributed to the data analysis. J.L.T., B.E.S., V.K., J.S., Y.L., R.H., W.J.S., B.S., W.R., K.G., G.D., and N.P.: contributed to data interpretation. J.L.T., B.E.S., V.K., J.S., Y.L., R.H., W.J.S., B.J., B.S., W.R., K.G., G.D., and N.P.: manuscript writing and/or critical review/analysis of the manuscript. V.K. wrote the first draft. All authors have approved the final version of the manuscript.
Financial support: This study was funded by Eli Lilly and Company.
Potential competing interests: B.S., R.H., K.G., Y.L., B.J., J.L.T., and V.K. are current employees and shareholders of Eli Lilly and Company. J.S. is a former employee of Eli Lilly and Company. W.R. reports grant support and personal fees from Abbott Laboratories, AbbVie, Aesca, Centocor, Falk Pharma GmbH, Immundiagnostik, and Merck Sharp & Dohme and personal fees from Aptalis, Celltrion, Danone Austria, Elan, Ferring, Mitsubishi Tanabe Pharma Corporation, Otsuka, PDL BioPharma, Pharmacosmos, Schering-Plough, Shire, Takeda, Therakos, Vifor, Yakult, Amgen, AM Pharma, Astellas, AstraZeneca, Avaxia, Bioclinica, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Cellerix, Chemocentryx, Celgene, Covance, Galapagos, Genentech, Gilead Sciences, Grünenthal, ICON, Index Pharma, Inova, Janssen, Johnson and Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millennium, Nestle, Novartis, Ocera, Pfizer, Procter & Gamble, Prometheus, Robarts Clinical Trial, Second Genome, SetPoint Medical, TiGenix, UCB, Zyngenia, and 4SC. W.J.S. reports research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, AbbVie, Janssen, Takeda, Lilly, Celgene/Receptos,Pfizer, Prometheus Laboratories (now Prometheus Biosciences); consulting fees from AbbVie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Progenity, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust, HART), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, and Vivelix Pharmaceuticals; and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, Vimalan Biosciences. Spouse: Opthotech—consultant and stock options; Progenity—consultant and stock; Oppilan Pharma—employee and stock options; Escalier Biosciences—employee and stock options; Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories)—employee and stock options; Ventyx Biosciences—employee and stock options; and Vimalan Biosciences—employee and stock options. B.E.S. has received fees for consulting and speaking from Abivax, Bristol Myers Squibb, Janssen, Lilly Pfizer, and Takeda; for consulting from AbbVie, Adiso Therapeutics, Alimentiv, Amgen, Arena Pharmaceuticals, Artizan Biosciences, Artugen Therapeutics, AstraZeneca; Bacainn Therapeutics, Boehringer-Ingelheim, Boston Pharmaceuticals, Calibr, Celltrion Healthcare, ClostraBio, Cytoki Pharma, Connect Biopharma, Entera, Evommune, Fresenius Kabi, Galapagos, Genentech, Gilead Sciences, GlaxoSmithKline, Gossamer Bio, Imhotex, Immunic, Index Pharmaceuticals, Inotrem, Innovation Therapeutics, Ironwood Pharmaceuticals, Kaleido, Kallyope, MiroBio, Morphic Therapeutics, MRM Health, OSE Immunotherapeutics, Progenity, Prometheus Biosciences, Protagonist Therapeutics, Q32 Bio, Redhill Biopharma, Sun Pharma, Surrozen, Synlogic, Target RWE, Teva, Theravance Biopharma, TLL Pharmaceutical, USWM Enterprises, VielaBio: VTA Labs; and personal fees and stock options for consulting from Ventyx Biosciences. N.P. has received consultancy fees, speaker fees and/or research grants from AbbVie, Allergan, AstraZeneca, Bristol-Myers Squibb, Celgene, Dr Falk Pharma UK, Ferring, Galapagos, Janssen, Roche, Pfizer, Sobi, Takeda, Tillotts, and Vifor.
IRB statement: All informed consent forms and protocols were approved by appropriate ethical review boards before initiation of the study. All patients gave written informed consent before the first screening procedure for the study.
Data and materials availability: Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Study Highlights.
WHAT IS KNOWN
✓ Interleukin 23 is a novel therapeutic target in inflammatory bowel disease.
✓ Mirikizumab demonstrated efficacy and safety in patients with moderate-to-severe ulcerative colitis in a clinical trial.
WHAT IS NEW HERE
✓ Changes in colonic transcripts occur as early as 12 weeks after mirikizumab induction treatment.
✓ These transcriptional changes correlate with molecular healing early in the initiation of treatment with mirikizumab.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Taku Kobayashi of the Center for Advanced IBD Research and Treatment, Kitasato University, Kitasato Institute Hospital, Tokyo, Japan, for his insightful comments and discussion of the data, and the following employees of Eli Lilly: Linden Green for providing writing and editorial support, Meenu Kaur for providing analyst support, Catherine Milch for providing medical peer review and strategic analysis, and Ernst Dow for providing statistical and data review.
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A925, http://links.lww.com/CTG/A926
Denotes affiliation at time of study.
Contributor Information
Boyd Steere, Email: steere_boyd_a@lilly.com.
Jochen Schmitz, Email: jochen.schmitz@gmail.com.
Nick Powell, Email: nicholas.powell@imperial.ac.uk.
Richard Higgs, Email: higgs_richard_e_jr@lilly.com.
Klaus Gottlieb, Email: klaus.gottlieb@lilly.com.
Yushi Liu, Email: liu_yushi@lilly.com.
Bochao Jia, Email: jia_bochao@lilly.com.
Jay L. Tuttle, Email: tuttle_jay@lilly.com.
William J. Sandborn, Email: wjsandborn.md@gmail.com.
Bruce E. Sands, Email: bruce.sands@mssm.edu.
Geert D'Haens, Email: g.dhaens@amsterdamumc.nl.
Walter Reinisch, Email: walter.reinisch@meduniwien.ac.at.
REFERENCES
- 1.Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017;389(10080):1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): Determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015;110(9):1324–38. [DOI] [PubMed] [Google Scholar]
- 3.Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 2012;380(9853):1606–19. [DOI] [PubMed] [Google Scholar]
- 4.Rubin DT, Ananthakrishnan AN, Siegel CA, et al. ACG clinical guideline: Ulcerative colitis in adults. Am J Gastroenterol 2019;114(3):384–413. [DOI] [PubMed] [Google Scholar]
- 5.Sandborn WJ, Su C, Panes J. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377(5):496–7. [DOI] [PubMed] [Google Scholar]
- 6.Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369(8):699–710. [DOI] [PubMed] [Google Scholar]
- 7.Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353(23):2462–76. [DOI] [PubMed] [Google Scholar]
- 8.Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146(1):85–95; quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 9.Sandborn WJ, van Assche G, Reinisch W, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142(2):257–65.e3. [DOI] [PubMed] [Google Scholar]
- 10.Harbour SN, Maynard CL, Zindl CL, et al. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci USA 2015;112(22):7061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee YK, Turner H, Maynard CL, et al. Late developmental plasticity in the T helper 17 lineage. Immunity 2009;30(1):92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buonocore S, Ahern PP, Uhlig HH, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 2010;464(7293):1371–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powell N, Walker AW, Stolarczyk E, et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity 2012;37(4):674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandborn WJ, Rutgeerts P, Gasink C, et al. Long-term efficacy and safety of ustekinumab for Crohn's disease through the second year of therapy. Aliment Pharmacol Ther 2018;48(1):65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381(13):1201–14. [DOI] [PubMed] [Google Scholar]
- 16.Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with ulcerative colitis. Gastroenterology 2020;158(3):537–49.e10. [DOI] [PubMed] [Google Scholar]
- 17.Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of continued treatment with mirikizumab in a phase 2 trial of patients with ulcerative colitis. Clin Gastroenterol Hepatol 2022;20(1):105–15.e14. [DOI] [PubMed] [Google Scholar]
- 18.Arafa E, Bondzie PA, Rezazadeh K, et al. TMIGD1 is a novel adhesion molecule that protects epithelial cells from oxidative cell injury. Am J Pathol 2015;185(10):2757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Planell N, Lozano JJ, Mora-Buch R, et al. Transcriptional analysis of the intestinal mucosa of patients with ulcerative colitis in remission reveals lasting epithelial cell alterations. Gut 2013;62(7):967–76. [DOI] [PubMed] [Google Scholar]
- 20.Zahn A, Moehle C, Langmann T, et al. Aquaporin-8 expression is reduced in ileum and induced in colon of patients with ulcerative colitis. World J Gastroenterol 2007;13(11):1687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Englund G, Jacobson A, Rorsman F, et al. Efflux transporters in ulcerative colitis: Decreased expression of BCRP (ABCG2) and Pgp (ABCB1). Inflamm Bowel Dis 2007;13(3):291–7. [DOI] [PubMed] [Google Scholar]
- 22.Hill T, Krougly O, Nikoopour E, et al. The involvement of interleukin-22 in the expression of pancreatic beta cell regenerative Reg genes. Cell Regen 2013;2(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eriksson A, Flach CF, Lindgren A, et al. Five mucosal transcripts of interest in ulcerative colitis identified by quantitative real-time PCR: A prospective study. BMC Gastroenterol 2008;8(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smillie CS, Biton M, Ordovas-Montanes J, et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell 2019;178(3):714–30 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinozaki S, Nakamura T, Iimura M, et al. Upregulation of Reg 1α and GW112 in the epithelium of inflamed colonic mucosa. Gut 2001;48(5):623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranson N, Veldhuis M, Mitchell B, et al. NLRP3-dependent and -independent processing of interleukin (IL)-1β in active ulcerative colitis. Int J Mol Sci 2018;20(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedrich M, Pohin M, Jackson MA, et al. IL-1-driven stromal-neutrophil interactions define a subset of patients with inflammatory bowel disease that does not respond to therapies. Nat Med 2021;27(11):1970–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danese S, Roda G, Peyrin-Biroulet L. Evolving therapeutic goals in ulcerative colitis: Towards disease clearance. Nat Rev Gastroenterol Hepatol 2020;17:1–2. [DOI] [PubMed] [Google Scholar]
- 29.Dubinsky M, Lee SD, Panaccione R, et al. P068: Mirikizumab treatment improves bowel movement urgency in patients with moderately to severely active ulcerative colitis. Gastroenterology 2020;158:S17–8. [Google Scholar]
- 30.Peng LL, Wang Y, Zhu FL, et al. IL-23R mutation is associated with ulcerative colitis: A systemic review and meta-analysis. Oncotarget 2017;8(3):4849–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.West NR, Hegazy AN, Owens BMJ, et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med 2017;23(5):579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arijs I, De Hertogh G, Lemmens B, et al. Effect of vedolizumab (anti-α4β7-integrin) therapy on histological healing and mucosal gene expression in patients with UC. Gut 2018;67(1):43–52. [DOI] [PubMed] [Google Scholar]