INTRODUCTION:
Chronic liver disease is often combined with a morbidity burden that strongly affects the functional domain. In liver cirrhosis (LC), qualitative and quantitative muscle wasting, known as sarcopenia, poses an added clinical burden, together with comorbidities and a poor quality of life.
METHODS:
We conducted a systematic review and meta-analysis of the prevalence of sarcopenia in LC. The literature was screened through 6 electronic databases from the study's inception to January 2023. No exclusion criteria were applied to language, operative tools for diagnosing sarcopenia, population age, general health status, country, and study setting (cohort or cross-sectional). Two independent researchers applied the inclusion criteria in parallel to evaluate the eligibility of the 44 retrieved articles; only 36 met the eligibility requirements.
RESULTS:
The total sample (N = 8,821) was slightly dominated by men (N = 4,941). The cross-sectional design predominated over the longitudinal, and the hospital setting was prevalent. The pooled prevalence of sarcopenia across the selected studies was 33% (95% confidence interval [CI] 0.32–0.34), with high heterogeneity (I2 = 96%). A further meta-analysis using the Child–Pugh (CP) score to stage LC was conducted on 24 entries, and the results showed that for the LC populations classified with the CP-A, CP-B, and CP-C staging, respectively, the overall mean prevalence was 33% (95% CI 0.31–0.35), 36% (95% CI 0.34–0.39) and 46% (95% CI 0.43–0.50). The risk of bias was moderate. In LC, 1 in 3 patients suffers sarcopenia.
DISCUSSION:
Poor management of muscle mass loss plays a role in the prognosis of death and quality of life of patients with LC. Clinicians in the field are recommended, when screening for sarcopenia, to pay close attention by carefully assessing body composition as part of the monitoring scheme.
KEYWORDS: epidemiology, sarcopenia, liver cirrhosis
INTRODUCTION
Chronic diseases currently account for 7 of the top 10 major global causes of death. Likely exacerbated by demographic aging and recent Westernized lifestyle habits, chronic conditions are primarily associated with epigenetic and lifestyle factors, causing a high disease burden, decreased quality of life, and massive healthcare spending. In this context, chronic liver disease (CLD) tops the list of concerns. There is evidence that CLD accounts for 2 million deaths per year worldwide (1), along with a heavy burden of disability, and thus greater disability-adjusted life-years (DALYs) and greater healthcare demands. In 2017, liver cirrhosis (LC) led to more than 1.32 million deaths globally, up from less than 899,000 deaths in 1990 (2,3), accounting for 2.2% of deaths and 1.5% of DALYs worldwide. Epidemiological estimates across several developed countries indicate a prevalence of LC ranging from 4.5% to 9.5% in the general population, approximately 10%–40% of whom undergo a silent, asymptomatic type of LC (4). According to World Health Organization microscopic-level data (5), LC accounts for 1.8% of all deaths in Europe (170,000 per year), with the highest incidence in the southeastern and northeastern regions. However, LC mortality has also increased in the United Kingdom and Ireland in recent years.
Parallel to the increase in life expectancy, the aged population balance is continuously rising (6), along with the burdens of multimorbidity, polypharmacy, and a highly disabling phenotype featuring physical and cognitive decline (6,7). Disease and drug dependency, as well as physiological muscle catabolism and poor taste and smell, play a role in exacerbating a multidimensional aging phenotype featuring loss of physical vigor, muscle mass, and strength, otherwise known as sarcopenia (8). According to the latest 2019 concept, sarcopenia dimensions include low levels of muscle strength, muscle quantity/quality, and of physical performance as an indicator of severity (9). Sarcopenia poses a considerable clinical challenge, especially as the decline approaches multiple irreversible adverse outcomes such as physical disability, dependency, falls, hospitalization, physical frailty, and a reduced quality of life (6,10).
Previous cohort data demonstrated a 2-fold increase in the risk of death and a drop in 5-year survival probability in patients with LC with sarcopenia compared with nonsarcopenic counterparts (11), hence the importance of rapid diagnosis and prevalence data.
In view of the current lack of an epidemiological overview of sarcopenia in CLD, here we conducted a systematic review and meta-analysis on the prevalence of sarcopenia in patients with LC. The aim of this research was to explore the pooled prevalence of sarcopenia in LC settings, with the intent of promoting better clinical management and the implementation of preventive actions, to improve patients' quality of life and reduce healthcare costs.
METHODS
Search strategy and selection criteria
A computerized literature search of MEDLINE and the Cochrane database did not identify any previous systematic reviews on the prevalence of sarcopenia in patients with LC. The present systematic review followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines, adhering to the PRISMA 27-item checklist (12) (Figure 1). An a priori protocol for the search strategy and inclusion criteria was established and recorded, with no particular changes to the information provided at registration on PROSPERO, a prospective international registry of systematic reviews (CRD42023334706). We performed separate searches in the US National Library of Medicine (PubMed), Medical Literature Analysis and Retrieval System Online (MEDLINE), EMBASE, Scopus, Ovid, and Google Scholar, to find original articles investigating the prevalence of sarcopenia in patients with LC, regardless of the liver disease etiology. The primary objective was to assess the pooled prevalence of sarcopenia in cirrhosis phenotypes of CLD. We also considered the gray literature in the study selection phase, using the huge archive of preprints https://arxiv.org/ and the database http://www.opengrey.eu/ to access abstracts of noteworthy conferences and other unreviewed material. No exclusion criteria were applied to article language, operational constructs used to define the condition of sarcopenia, general health status, country, recruitment context (hospital or nursing home), or study setting (cohort or cross-sectional). As inclusion criteria, we retained only original articles on populations diagnosed with LC, providing some prevalence data on sarcopenia. The presence of malignant tumors was used as a covariate to perform a further meta-analysis based on the presence or absence of malignancy that rapidly exacerbates the pathophysiological paths of sarcopenia.
Figure 1.
Flow diagram of the literature screening process.
The research strategy used in PubMed and MEDLINE and adapted to the other 4 electronic sources included the keywords “sarcopenia” and “liver cirrhosis,” combined through the use of Boolean indicators (Table 1). The search strategy used the Boolean indicator NOT to rule out letters, comments, editorials, literature reviews, and meta-analyses. The literature search had no time restrictions, and documents were retrieved until January 31, 2023. No language restrictions were placed. Two researchers (R.Z. and S.M.) searched the articles, reviewed the titles and abstracts of articles retrieved separately and in duplicate, checked the full texts, and selected articles for inclusion in the study. Inter-rater reliability was used to estimate intercoder agreement and then κ statistics to measure accuracy and precision. A κ coefficient of at least 0.9 was obtained in all data extraction steps based on PRISMA concepts and quality assessment steps (13,14).
Table 1.
Search strategy used in the US National Library of Medicine (PubMed) and Medical Literature Analysis and Retrieval System Online (MEDLINE) and adapted to the other sources, according to selected descriptors
| Research question | How prevalent is sarcopenia in liver cirrhosis? |
| Search concepts | Liver cirrhosis, Sarcopenia |
| Sources | PubMed, MEDLINE, EMBASE, Scopus, Ovid, and Google Scholar |
| Limitations | “Human” |
| Grey literature | https://arxiv.org/. Furthermore, https://www.base-search.net/ was used to avoid publication bias in terms of contradictory and negative results' reports, especially in a gray research question such as the one we selected |
| Search date | Inception (2001) to January 2023 |
| N | Searches | Results |
| #1 | Sarcopenia | 15,052 |
| #2 | Liver cirrhosis OR Liver disease | 804,467 |
| #3 | Review OR Systematic Review OR Meta-analysis OR Comment OR Editorial | 5,243,606 |
| #4 | #1 AND #2 NOT #3 | 667 |
Data elaboration and analysis
Two researchers (S.M., A.C., and R.Z.) extracted the following information separately and in duplicate in a piloted form: author(s), year of publication, region (country), study design (longitudinal or cross-sectional), study setting (hospital or care center), type of CLD, and clinical tool (bioimpedance [BIA], computed tomography [CT] scan, dual-energy x-ray absorptiometry [DXA], magnetic resonance imaging [MRI], dynamometry, and others) and operative construct adopted to assess sarcopenia across each selected study. Researchers tabulated data by sarcopenia prevalence in patients with LC to retrieve information on (i) sample size (N); (ii) age (expressed as mean ± SD, or interquartile range, or just as a range); (iii) sarcopenia events (N, %); (iv) male and female representativeness (expressed as N and %) in the whole sample and in the sarcopenia subset; (v) operational construct of sarcopenia used and cutoff values applied for each dimension; and (vi) tools for body composition assessment. All references selected for retrieval from the databases were managed with the MS Excel data collection software platform by an experienced biostatistician (F.C.). Finally, the data extracted from the selected studies and stored in the database were structured as evidence tables.
The tool developed by Hoy et al (14) was adopted in the present research to assess whether the 35 selected research studies were conducted according to the highest possible standards (methodological quality), and the degree of credibility of the results (risk of bias). Each study was assigned a score of 1 (yes) or 0 (no) for each of the 10 criteria. Based on the total score, studies were classified as at low (>8), moderate (6–8), or high (≤5) risk of bias. Disagreements between the 2 researchers on the methodological quality of the included studies were discussed until an agreement with a third researcher (R.S.) was reached.
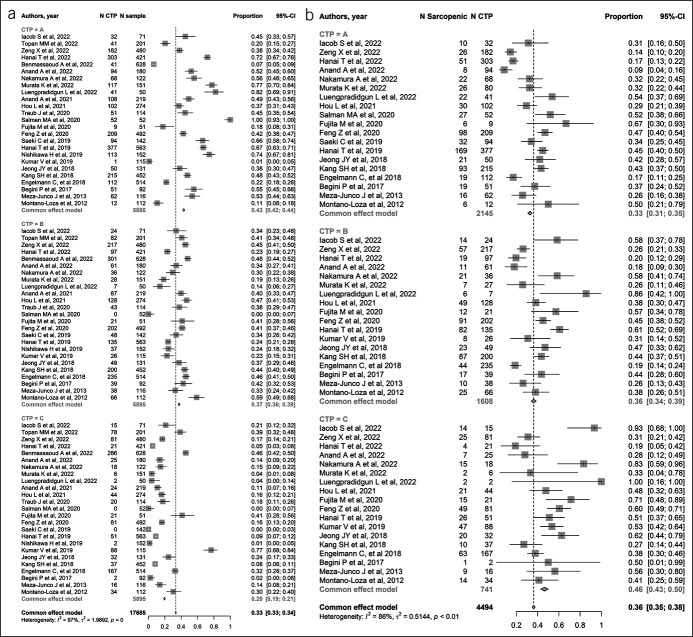
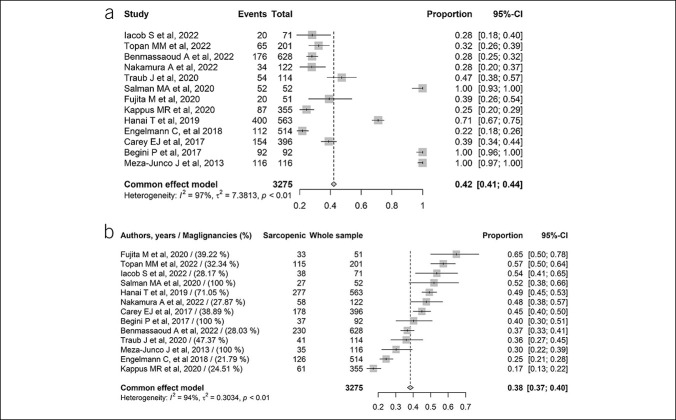
Meta-analyses were performed using the “Metaprop” function in the R package “meta” (version 5.2.0; R Foundation for Statistical Computing, Vienna, Austria). To estimate the overall pooled prevalence of sarcopenia in patients with LC, DerSimonian–Laird random-effect meta-analyses were conducted using the inverse variance method (Figure 2). The logit transformation was applied to stabilize the variance and normalize its distribution. The Clopper–Pearson method was used to calculate point estimates and 95% confidence intervals (CIs) for the prevalence rate of sarcopenia in each study. Statistical heterogeneity was measured by the I2 statistic (15), and values less than 25%, between 25% and 75%, and more than 75% were taken to show low, medium, and high heterogeneity, respectively (15). Subgroup analysis was performed on the basis of potential sources of heterogeneity, such as LC staging, which has been acknowledged as a powerful contributor to the development of sarcopenia. So, we used the Child–Pugh (CP) score to divide the cirrhotic population into 3 groups based on the severity and complexity of the LC status, that is, CP-A, CP-B, and CP-C (Figure 3a). The upward profile of sarcopenia prevalence in relation to the severity of LC by CP was further described for each study using a dodge plot (Figure 3b). Finally, a subgroup of 13 studies that provided prevalence data of sarcopenia in subjects with LC complicated by malignancies allowed us to conduct an additional meta-analysis to appreciate the variation of sarcopenia prevalence in those individuals to compare with the malignancy-free counterparts. Figure 4a shows the proportion of malignancies in LC, whereas Figure 4b shows the proportion of malignancies in LC by subgroups of sarcopenia (presence/absence). All data analyses were performed by a senior biostatistician (F.C.) using R, version 2021.09.1.
Figure 2.
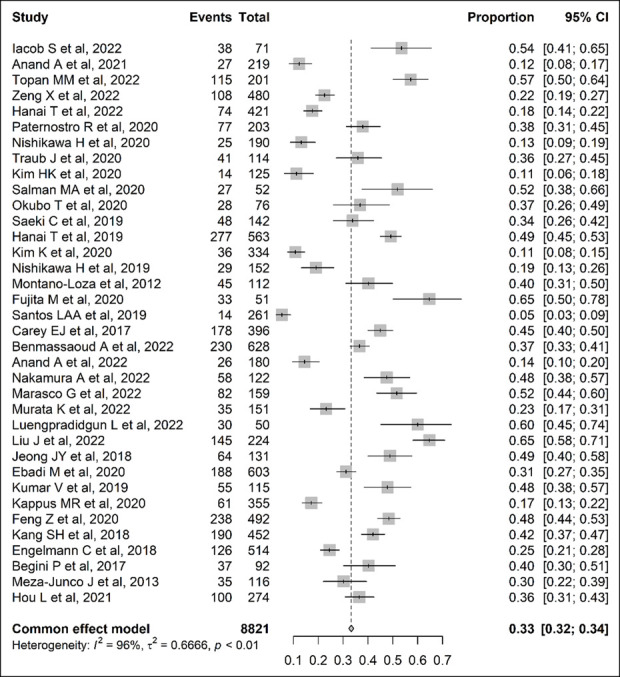
Forest plot of studies estimating the proportion of sarcopenic individuals in patients with cirrhosis. CI, confidence interval.
Figure 3.
(a) Forest plot of studies estimating the proportion of Child–Pugh (A, B, C) in liver cirrhosis individuals. (b) Forest plot of studies estimating the prevalence of sarcopenia in liver cirrhosis according to Child–Pugh (A, B, C) groups. (c) Dodge plot for prevalence of sarcopenia in liver cirrhosis according to Child–Pugh (A, B, C) groups across studies. CI, confidence interval.
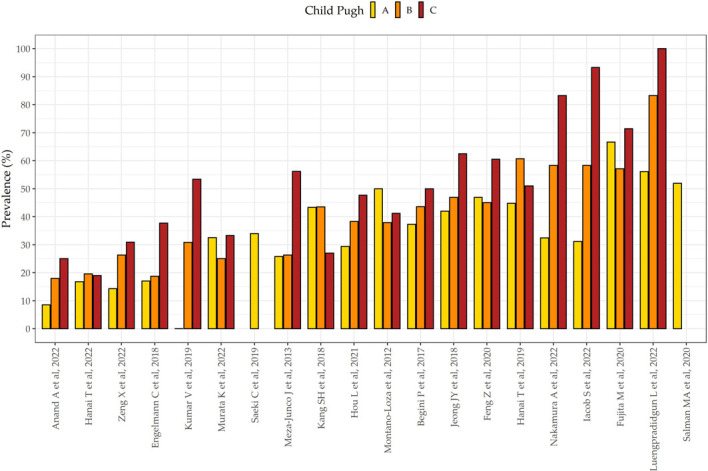
Figure 4.
(a) Forest plot for malignancies prevalence in selected studies. (b) Forest plot for sarcopenia prevalence regarding to malignancy prevalence. CI, confidence interval.
RESULTS
The first systematic literature search yielded 667 entries (Table 1). After excluding duplicates, 77 were classified as potentially relevant and selected for the title and abstract analysis. Then, 37 were excluded because they did not meet the characteristics of the approach or the objective of this research. After reviewing the full text of the remaining 40 articles, only 36 met the inclusion criteria and were included in the meta-analysis (16–51). The PRISMA flow chart illustrating the number of studies at each stage of the review is shown in Figure 1.
The final research focus included 36 articles reporting the prevalence of sarcopenia in LC populations. Table 2 shows details of the design (cohort or cross-sectional), setting (hospital or nursing home), sample size (N), type of sarcopenia construct used to assess the prevalence (including cutoffs for each dimension), and body composition assessment tool (bioimpedance, DXA, CT scan, MRI, and dynamometry). The cross-sectional design (80.5%, N = 29 of 36) predominated over the longitudinal design (19.5%, N = 7 of 36). In all cases, the study setting was the hospital, except for 1 study conducted at a care center. The geographical distribution of the studies favored Asia (61%, N = 22), followed by Europe (22%, N = 8), America (14%, N = 5), and Africa (3%, N = 1). In accordance with the inclusion criteria, all subjects had LC.
Table 2.
Descriptive of selected studies investigating the prevalence of sarcopenia in liver cirrhosis (N = 36)
| Study | Region (country) | Age | Study design | Study setting | Diagnosis of sarcopenia | Method of body composition assessment | Score (risk of bias) |
| Iacob et al, 2022 (16) | Romania (Europe) | 54.5 ± 12.6 | Cross-sectional | Hospital | EWGSOP2 criteria combining low HGS (<27 kg for men and HGS <16 kg for women) with low L3-SMI (<50 cm2/m2 for men and <39 cm2/m2 for women) | CT scan and dynamometry | 7 (moderate) |
| Anand et al, 2022 (17) | India (Asia) | 18–60 (mean 42.6) | Cohort | Care centre | EWGSOP2 criteria combining low HGS (<27 kg for men and HGS < 16 kg for women) with low L3-SMI (<50 cm2/m2 for men and <39 cm2/m2 for women) | CT scan and dynamometry | 7 (moderate) |
| Topan et al, 2022 (18) | Romania (Europe) | 61.65 ± 9.49 | Cohort | Hospital | EWGSOP2 criteria combining low HGS (<27 kg for men and HGS <16 kg for women) with low L3-SMI (<50 cm2/m2 in men and <39 cm2/m2 in women) | CT scan and dynamometry | 7 (moderate) |
| Zeng et al, 2022 (19) | China (Asia) | 18 to 80 | Cross-sectional | Hospital | Low L3-SMI (<44.77 cm2/m2 for men and <32.50 cm2/m2 for women) | CT scan | 7 (moderate) |
| Hanai et al, 2021 (20) | Japan (Asia) | 71 (64–78) | Cross-sectional | Hospital | JSH criteria combining low HGS (<26 kg for men and <18 kg for women) with low SMI (<7.0 kg/m2 for men and <5.7 kg/m2 for women) | CT scan | 7 (moderate) |
| Paternostro et al, 2020 (21) | Austria (Europe) | 18+ | Cohort | Hospital | Low TPMT‐L3 (<12 mm/m for men and <8 mm/m for women) | CT/MRI | 7 (moderate) |
| Nishikawa et al, 2020 (22) | Japan (Asia) | 50–72 | Cross-sectional | Hospital | JSH criteria combining low HGS (<26 kg for men and <18 kg for women) with low SMI (<7.0 kg/m2 for men and <5.7 kg/m2 for women) | BIA and dynamometry | 7 (moderate) |
| Traub et al, 2020 (23) | Austria (Europe) | 65 (61.87–65.97) | Cross-sectional | Hospital | EWGSOP criteria combining low HGS (<30 kg for men and <20 kg for women) with low L3-SMI (<50 cm2/m2 in men and <39 cm2/m2 in women) | CT scan and dynamometry | 7 (moderate) |
| Kim et al, 2020 (24) | Korea (Asia) | 55.9 ± 10.9 | Cross-sectional | Hospital | Low SI (total ASM [kg]/BMI [kg/m2] <0.789 in men and <0.521 in women) | BIA | 7 (moderate) |
| Salman et al, 2020 (25) | Egypt (Africa) | 53.9 ± 5.0 | Cohort | Hospital | Low L3-SMI (≤53 cm2/m2 for men with a BMI ≥25 and ≤43 cm2/m2 for men with BMI <25 and ≤41 cm2/m2 for women, irrespective of BMI) | CT scan | 7 (moderate) |
| Okubo et al, 2020 (26) | Japan (Asia) | 67 (24–8) | Cross-sectional | Hospital | JSH criteria combining low HGS (<26 kg for men and <18 kg for women) with low SMI (<7.0 kg/m2 for men and <5.7 kg/m2 for women) | BIA and dynamometry | 7 (moderate) |
| Saeki et al, 2019 (27) | Japan (Asia) | 70.5 (58.8–76.0) | Cross-sectional | Hospital | JSH criteria combining low HGS (<26 kg for men and <18 kg for women) and/or low gait speed (≤0.8 m/sec both for men and women) and low SMI (<7.0 kg/m2 for men and <5.7 kg/m2 for women) | BIA, dynamometry and gait speed | 7 (moderate) |
| Hanai et al, 2019 (28) | Japan (Asia) | 71 ± 11 | Cohort | Hospital | JSH criteria combining low HGS (<26 kg for men and <18 kg for women) with low L3-SMI (≤42 cm2/m2 for men and ≤38 cm2/m2 for women) | CT scan and dynamometry | 7 (moderate) |
| Kim et al, 2020 (29) | Korea (Asia) | 54.4 ± 12.7 | Cross-sectional | Hospital | Low SI (total appendicular skeletal muscle mass [kg]/BMI [kg/m2] <0.789 for men and <0.521 for women) | BIA | 7 (moderate) |
| Nishikawa et al, 2019 (30) | Japan (Asia) | 61.5 ± 12.7 | Cross-sectional | Hospital | JSH criteria combining low HGS (<26 kg for men and <18 kg for women) and low SMI (<7.0 kg/m2 for men and <5.7 kg/m2 for women) | BIA and dynamometry | 7 (moderate) |
| Montano-Loza et al, 2012 (31) | Canada (America) | 54 ± 1 | Cohort | Hospital | Low L3-SMI (≤52.4 cm2/m2 for men and ≤38.5 cm2/m2 for women) | CT scan | 7 (moderate) |
| Fujita et al, 2020 (32) | Japan (Asia) | Not reported | Cohort | Hospital | Low PMI-L3 (<6.0 for men and <3.4 cm2/m2 for women) | Computed tomography (CT) scan | 7 (moderate) |
| Santos et al, 2019 (33) | China (Asia) | 57 (51.75–63.00) | Cohort | Hospital | EWGSOP criteria combining low HGS (<30 kg for men and <20 kg for women) with low SMI (<7.26 kg/m2 for men and <5.45 kg/m2 for women) | DXA and dynamometry | 7 (moderate) |
| Carey et al, 2017 (34) | USA (America) | 58 (51–62) | Cohort | Hospital | Low L3-SMI (<50 cm2/m2 for men and <39 cm2/m2 for women) | CT scan | 7 (moderate) |
| Benmassaoud et al, 2022 (35) | United Kingdom (Europe) | 54.5 ± 14 | Cohort | Hospital | Low L3-SMI (<50 cm2/m2 for men and <39 cm2/m2 for women) | CT scan | 7 (moderate) |
| Anand et al, 2021 (36) | India (Asia) | 18–60 | Cross-sectional | Hospital | Low L3-SMI (<36.5 cm2/m2 for men and <30.2 cm2/m2 for women) | CT scan | 7 (moderate) |
| Nakamura et al, 2022 (37) | Japan (Asia) | 62 ± 14 | Cross-sectional | Hospital | Low PSMI (<12.62 cm2/m2 for men and <9.77 cm2/m2 for women) | MRI | 7 (moderate) |
| Marasco et al, 2022 (38) | Italy (Europe) | 68 (median) | Cohort | Hospital | Low L3-SMI (<50 cm2/m2 for men and <39 cm2/m2 for women) | CT scan | 7 (moderate) |
| Murata et al, 2022 (39) | Japan (Asia) | 70 ± 10 | Cross-sectional | Hospital | JSH criteria combining low HGS (<26 kg for men and <18 kg for women) with L3-SMI (≤42 cm2/m2 for men and ≤38 cm2/m2 for women) | CT scan and dynamometry | 7 (moderate) |
| Luengpradidgun et al, 2022 (40) | Thailand (Asia) | 63 (54.5–64.5) | Cross-sectional | Hospital | JSH criteria combining low HGS (<26 kg for men and <18 kg for women) with L3-SMI (≤42 cm2/m2 for men and ≤38 cm2/m2 for women) | CT scan and dynamometry | 7 (moderate) |
| Liu et al, 2022 (41) | China (Asia) | 54.3 ± 11.6 | Cohort | Hospital | Low L3-SMI (<50 cm2/m2 for men and <39 cm2/m2 for women) | CT scan | 7 (moderate) |
| Jeong et al, 2018 (42) | Korea (Asia) | 53.7 ± 9.6 | Cohort | Hospital | Low L3-SMI (≤52.4 cm2/m2 for men and ≤38.5 cm2/m2 for women) | CT scan | 6 (moderate) |
| Ebadi et al, 2020 (43) | Canada (America) | 18–40 | Cross-sectional | Hospital | Low L3-SMI (<42 cm2/m2 for men and <30 cm2/m2 for women) | CT scan | 7 (moderate) |
| Kumar et al, 2019 (44) | India (Asia) | 45.75 ± 10.6 | Cross-sectional | Hospital | Low L3-SMI (≤52.4 cm2/m2 for men and ≤38.5 cm2/m2 for women) | CT scan | 7 (moderate) |
| Kappus et al, 2020 (45) | USA (America) | 53.7 ± 12.0 | Cohort | Hospital | Low L3-SMI (<50 cm2/m2 for men and <39 cm2/m2 for women) | CT scan | 7 (moderate) |
| Feng et al, 2020 (46) | China (Asia) | 51 (45–61) | Cohort | Hospital | Low L3-SMI (<50 cm2/m2 for men and <39 cm2/m2 for women, according to EWGSOP2 criteria) or low SMD (myosteatosis) according to SMD <34.1 HU for men and <27.2 HU for women | CT scan | 7 (moderate) |
| Kang et al, 2018 (47) | Korea (Asia) | 51.8 ± 8.8 | Cohort | Hospital | Low L3-SMI (≤52.4 cm2/m2 for men and ≤38.5 cm2/m2 for women) | CT scan | 6 (moderate) |
| Engelmann et al 2018 (48) | Germany (Europe) | 53.7 (mean) | Cohort | Hospital | Low L3-SMI (<41.90 cm2/m2 for men and <35.30 cm2/m2 for women) | CT scan | 7 (moderate) |
| Begini et al, 2017 (49) | Italy (Europe) | 71.6 (30.7–86.4) | Cohort | Care centre | SMI ≤41 cm2/m2 for women and ≤53 cm2/m2 for men with BMI ≥25, and ≤43 cm2/m2 for men and women with BMI <25, respectively | CT scan | 7 (moderate) |
| Meza-Junco et al, 2013 (50) | Canada (America) | 58 ± 6 | Cohort | Hospital | Low L3-SMI: ≤41 cm2/m2 for women and ≤53 cm2/m2 for men with body BMI ≥25 and ≤43 cm2/m2 in patients with BMI <25 | CT scan | 7 (moderate) |
| Hou et al, 2021 (51) | China (Asia) | 62.2 ± 12.9 | Cohort | Hospital | Low L3-SMI (<46.9 cm2/m2 for men and <32.5 cm2/m2 for women) | CT scan | 7 (moderate) |
BIA, bioelectrical impedance analysis; BMI, body mass index; CT, computed tomography; DXA, dual-energy x-ray absorptiometry; EWGSOP, European Working Group on Sarcopenia in Older People; HGS, hand grip strength; JSH, Japan Society of Hepatology; L3, third lumbar vertebra; MRI, magnetic resonance imaging; PMI, psoas muscle index; PSMI, paraspinal muscle index; SI, sarcopenia index; SMD, skeletal muscle radiodensity; SMI, skeletal muscle index; TPMT, transversal psoas muscle thickness.
As regards the concepts of sarcopenia used, most prevalence entries (16–20,22–28,30,31,33–51) were based on deficiency values of the third lumbar vertebra skeletal muscle index (L3-SMI) assessed by CT scan. Eight entries (20,22,26,27,30,39,40) followed the Japan Society of Hepatology guidelines for sarcopenia in liver disease (52), with threshold values for muscle mass depending on the type of clinical device, BIA, DXA, or CT scan. Then, the criteria of the European Working Group on Sarcopenia in Older People, updated to 2019 (9), were applied by 4 studies (16–18,46), whereas 2 (23,33) relied on the first European Working Group on Sarcopenia in Older People consensus (53). Here, cutoffs for muscle mass and strength differed according to the type of population, whether Asian (17) or Western (23). Two entries by Kim et al (24,29) applied the sarcopenia index, that is, the ratio of total appendicular skeletal muscle mass (kg) to body mass index (kg/m2), using consistent cutoffs of <0.789 for men and <0.521 for women. Two sarcopenia entries Resulted from muscle deficits estimated by the psoas muscle index of L3 (32,35). Finally, a single prevalence entry was derived from the construct used by (37), namely, a paraspinal muscle index deficiency, diagnosed on values <12.62 cm2/m2 for men and <9.77 cm2/m2 for women.
As an assessment tool for body composition, most studies used CT scans to quantify muscle mass (Table 2). Only 6 sarcopenia entries were derived from BIA (22,24,26,27,29,30,54) and just 1 from DXA (33) and 2 for MRI (21,37). For handgrip strength estimation, dynamometry was chosen in 12 studies to estimate the prevalence of sarcopenia, combined with appendicular muscle mass. Saeki et al (27) also used walking speed as a substitute estimate for muscle loss/exhaustion.
Table 3 shows data on sample size and sarcopenia prevalence subdivided by gender if provided. A total sample of 8,821 subjects with LC was analyzed, including a slight majority of men (N = 4,941). Of note, 4 studies did not provide quantitative data on the gender ratio and thus were not accounted for in the total female and male subsets' count. Sarcopenia prevalence rates (%) were recorded in Table 3, and the gender ratio and CP (A, B, and C) staging proportion was also provided. The prevalence of sarcopenia across selected studies, accounting for 8,821 patients with LC, showed a pooled average prevalence of 33% (95% CI 0.32–0.34) (Figure 2), but high heterogeneity, I2 = 96%. Sex analysis showed a larger sarcopenia prevalence in males than women, respectively 49.2% and 23.6% (data not shown).
Table 3.
Overview of sample size, gender ratio, the prevalence of sarcopenia, and cirrhosis severity by Child–Pugh scoring across selected studies
| Study | Sarcopenia events (N) | Sample size (N) | Male (N) | Female (N) | Prevalence of sarcopenia (%) | Male sarcopenia events (N) | Female sarcopenia events (N) | Child–Pugh A (N) | Child–Pugh B (N) | Child–Pugh C (N) |
| Iacob et al, 2022 (16) | 38 | 71 | 48 | 23 | 53.5 | Not reported | Not reported | 32 | 24 | 15 |
| Anand et al, 2022 (17) | 27 | 219 | 168 | 51 | 12.3 | 24 | 3 | Not reported | Not reported | Not reported |
| Topan et al, 2022 (18) | 115 | 201 | 127 | 294 | 57.2 | 76 | 39 | 41 | 82 | 78 |
| Zeng et al, 2022 (19) | 108 | 480 | 286 | 184 | 22.5 | 86 | 22 | 182 | 217 | 81 |
| Hanai et al, 2021 (20) | 74 | 421 | Not reported | Not reported | 17.6 | Not reported | Not reported | 303 | 97 | 21 |
| Paternostro et al, 2020 (21) | 77 | 203 | 138 | 65 | 37.9 | 62 | 15 | Not reported | Not reported | Not reported |
| Nishikawa et al, 2020 (22) | 25 | 190 | 103 | 87 | 13.1 | 12 | 13 | Not reported | Not reported | Not reported |
| Traub et al, 2020 (23) | 41 | 114 | 86 | 28 | 36.0 | 36 | 5 | 51 | 43 | 20 |
| Kim et al, 2020 (24) | 14 | 125 | Not reported | Not reported | 11.2 | Not reported | Not reported | Not reported | Not reported | Not reported |
| Salman et al, 2020 (25) | 27 | 52 | 38 | 14 | 51.9 | 18 | 9 | 52 | 0 | 0 |
| Okubo et al, 2020 (26) | 28 | 76 | 41 | 35 | 36.8 | 19 | 9 | Not reported | Not reported | Not reported |
| Saeki et al, 2019 (27) | 48 | 142 | 90 | 52 | 33.8 | 26 | 22 | 94 | 48 | 0 |
| Hanai et al, 2019 (28) | 277 | 563 | 375 | 188 | 49.2 | 221 | 56 | 377 | 135 | 51 |
| Kim et al, 2020 (29) | 36 | 334 | Not reported | Not reported | 10.8 | Not reported | Not reported | Not reported | Not reported | Not reported |
| Nishikawa et al, 2019 (30) | 29 | 152 | Not reported | Not reported | 19.1 | Not reported | Not reported | 113 | 37 | 2 |
| Montano-Loza et al, 2012 (31) | 45 | 112 | 78 | 34 | 40.2 | 39 | 6 | 12 | 66 | 34 |
| Fujita et al, 2020 (32) | 33 | 51 | 26 | 25 | 64.7 | 21 | 12 | 9 | 21 | 21 |
| Santos et al, 2019 (33) | 14 | 261 | 161 | 100 | 5.36 | Not reported | Not reported | Not reported | Not reported | Not reported |
| Carey et al, 2017 (34) | 178 | 396 | 277 | 119 | 45 | 139 | 39 | Not reported | Not reported | Not reported |
| Benmassaoud et al, 2022 (35) | 230 | 628 | 429 | 199 | 36.60 | 177 | 53 | 42 | 300 | 286 |
| Anand et al, 2021 (36) | 26 | 180 | 143 | 37 | 14.4 | 21 | 5 | 94 | 61 | 25 |
| Nakamura et al, 2022 (37) | 58 | 122 | 75 | 47 | 47.5 | Not reported | Not reported | 68 | 36 | 18 |
| Marasco et al, 2022 (38) | 82 | 159 | 128 | 31 | 51.6 | 68 | 14 | |||
| Murata et al, 2022 (39) | 40 | 151 | 95 | 56 | 26.5 | Not reported | Not reported | 117 | 28 | 6 |
| Luengpradidgun et al, 2022 (40) | 30 | 50 | 28 | 22 | 60.0 | 11 | 19 | 41 | 6 | 3 |
| Liu et al, 2022 (41) | 145 | 224 | 159 | 65 | 64.7 | 113 | 32 | Not reported | Not reported | Not reported |
| Jeong et al, 2018 (42) | 64 | 131 | 94 | 37 | 48.9 | 55 | 9 | 50 | 49 | 32 |
| Ebadi et al, 2020 (43) | 188 | 603 | 408 | 195 | 31.2 | 153 | 35 | Not reported | Not reported | Not reported |
| Kumar et al, 2019 (44) | 55 | 115 | 104 | 11 | 47.8 | 51 | 4 | 1 | 26 | 88 |
| Kappus et al, 2020 (45) | 61 | 355 | 232 | 123 | 17.2 | 57 | 4 | Not reported | Not reported | Not reported |
| Feng et al, 2020 (46) | 238 | 492 | 365 | 127 | 48.4 | 183 | 55 | 209 | 202 | 81 |
| Kang et al, 2018 (47) | 190 | 452 | 379 | 73 | 42 | 178 | 12 | 215 | 200 | 37 |
| Engelmann et al 2018 (48) | 126 | 514 | 363 | 151 | 24.50 | — | — | 112 | 235 | 167 |
| Begini et al, 2017 (49) | 37 | 92 | 27 | 65 | 40.20 | 20 | 17 | 51 | 39 | 2 |
| Meza-Junco et al, 2013 (50) | 35 | 116 | 98 | 18 | 30.20 | 30 | 5 | 62 | 38 | 16 |
| Hou et al, 2021 (51) | 100 | 274 | 144 | 130 | 36.50 | 70 | 30 | 102 | 128 | 44 |
Figure 3a showed the prevalence of CP-A, CP-B, and CP-C events in the LC population. The findings reinforced the internal validity of our meta-analysis and showed a downtrend of subjects moving from the less- to the more-complicated LC staging (survival effect) as follows: 43% (95%CI 0.42–0.45), 37% (95%CI 0.36–0.39), and 20% (95%CI 0.19–0.21) of LC subjects fell into the CP-A, CP-B, and CP-C groups, respectively. Then, based on the CP group-scoring meta-analysis, the prevalence of sarcopenia was distributed as follows: 33% (95%CI 0.31–0.35), 36% (95%CI 0.34–0.39), and 46% (95%CI 0.43–0.50) in CP-A, CP-B, and CP-C groups, respectively (Figure 3b), thus showing a slight uptrend in accordance with the severity of the cirrhotic disease.
Figure 4a, b showed the findings of a further meta-analysis on a subgroup of LC subjects whose malignancies and sarcopenia prevalence data were provided contextually by authors. Malignancy prevalence was 42% (95%CI 0.41–44, I2=97%) and the associated prevalence of sarcopenia was increased to 38% (95%CI 0.37–40, I2=94%). However, the amount of data is still sparse (only 13 studies, N=3275) and thus leaves room for further research.
About other results (data not shown), the whole cohort (25 studies, n=6013) MELD score estimation was 12.4. It was increased to 12.8 for the sarcopenic cohort (20 studies, n=1904) and decreased in the non-sarcopenic cohort (20 studies, n=3013) to 11.8. The most common etiology of LC (29 studies, n=7044) was viral (42.1%) followed by alcohol (28.9%), NAFLD (7.8%) then 21.2% for other etiologies. Regarding complications, ascites, upper gastro-instestinal, esophageal varices, hepatic encephalopathy and bacterial peritonitis were 55.4% (20 studies), 43.5% (9 studies), 69.7% (6 studies), 35.3% (21 studies), 15.3% (6 studies) prevalent, respectively.
DISCUSSION
The present research was undertaken to provide a revised estimate of the prevalence of sarcopenia in LC. Given the significantly increased burden of disease-related complications, worse quality of life (and increased DALYs), healthcare costs, and shortened survival in patients with LC, the appraisal of sarcopenia calls for a raised awareness of the importance of screening and clinical management.
Meta-analysis in this study of 36 entries involving 8,821 patients with LC, resulted in an overall estimated prevalence of sarcopenia of 33% (95% CI 0.32–0.34), with a high I2 = 96% heterogeneity, and a moderate risk of bias across selected reports. With a lower population (N=6403) patients, Tantai et al (11) found a higher overall prevalence (37.5%), justifiable by their slight higher proportion of compensated LC (37.5% CP-A; 43.3% CP-B; 19.1% CP-C; N=3048) compared to us (43% CP-A; 37% CP-B; 20% CP-C; N=5895). Thus, our results seem to be closer to a “real world” prevalence whereas theirs seem more “clinical world”. Based on the CP severity of LC, our findings indicated a prevalence of sarcopenia distributed as follows: 33% (95%CI 0.31–0.35), 36% (95%CI 0.34–0.39), and 46% (95%CI 0.43–0.50) in CP-A, CP-B, and CP-C groups, respectively, thus showing a slight uptrend in accordance with the severity of the cirrhotic disease. This result was in accordance with Tantai et al (11): 28.3%, 37.9% and 46.7%. Regarding differences between men and women, our results indicated 23.6% vs 49.2% while Tantai et al (11) found 28.7% vs 41.9% and Kim et al (55) found 36% vs 61.6%. To this observation, a multicentrique study by Michitaka et al (56) showed that alcoholic etiology in LC men is almost fivefold more prevalent than in women, which is more associated with sarcopenia. Furthermore, our overall population was slightly more masculine, which is very common in LC. In our subgroup analysis, malignancy prevalence was 42% (95%CI 0.41–44, I2=97%) representing 1382 subjects, and the associated prevalence of sarcopenia was increased to 38% (95%CI 0.37–40, I2=94%). Despite the amount of data (only 13 studies, N=3275) and a non-exclusively HCC cohort, our results seem to be in accordance with the meta-analysis of Guo et al (57) (41.7%).
From a pathophysiological point of view, sarcopenia is recognized to involve multidomain pathways, ultimately leading to a failure of the balance between protein synthesis and breakdown. Through metabolic and biochemical abnormalities, CLD is known to disrupt whole-body protein homeostasis and directly reduce muscle retention. Multiple metabolic pathways, including those listed as follows, are believed to be incriminated (58). Hyperammonemia, a common feature of LC, has the potential to increase myostatin activity and impair mitochondrial function (59,60). Proinflammatory cytokines, such as tumor necrosis factor-α and nuclear factor-κB signaling, glucocorticoids, and impaired insulin-like growth factor-1 signaling paths are well known to boost proteasome activity and autophagy (61). In addition, the increased hepatic gluconeogenesis shared by LC sufferers, and likely due to limited hepatic glycogen content and insulin resistance, may decrease the availability of branched-chain amino acids and glucose to myocytes. Moreover, recent clinical trials have found a lack of testosterone in subjects with LC, pointing to increased muscle cell apoptosis and myostatin activity (62).
Muscle atrophy may also be induced by chronic catabolic conditions such as cancer cachexia, increased energy expenditure, decreased food intake because of loss of appetite, early satiety, side effects of treatment, or changes in gastrointestinal motility, as well as changes in hormone levels such as insulin and catecholamines (8,63). This latter facet is the most relevant in justifying the prevalence gap in sarcopenia rates found between the malignancy subset of LC and their counterparts. Furthermore, atrophy of type II fast-twitch glycolytic fibers, which underlies the development of sarcopenia, may occur in patients with LC (58). Other lifestyle factors associated with LC may indirectly impair the nitrogen balance, especially a poor diet (low protein and calorie intake), low activity levels, and a sedentary lifestyle (64).
Preservation of muscle mass, avoiding rapid loss of mass and transition to sarcopenia, seems to be crucial for the vital prognosis of patients with LC, and indeed, loss of muscle mass is one of the best predictors of death (31). Among patients with LC, a low total SMI is significantly associated with a worse prognosis, whereas higher SMI scores for the arms and legs are associated with a better prognosis (65). Against this background, it should be a priority in the clinical management of LC to monitor body composition and screen for sarcopenia to improve survival and quality of life.
So far, successful intervention trials performed in these settings have relied primarily on nutritional therapies, exercise programs, and testosterone therapy. In terms of muscle health response, these therapies induced a significant improvement in muscle mass/strength and quality of life in each intervention group (66–68). For practical purposes, Hayashi et al (64) suggested that walking 5,000 or more steps per day and maintaining a total energy intake of 30-kcal/ideal body weight can serve as reference lifestyle guidelines for compensated patients with LC. Tandon et al (69) suggest a personalized, moderately low-calorie diet (∼500–800 kcal/d) in obesity settings. They also suggest a protein intake of 1.2- to 1.5-g/kg body weight per day up to 2.0-g/kg body weight per day, depending on the severity of sarcopenia, emphasizing branched-chain amino acid intake. Regarding exercise, Duarte-Rojo et al (68) suggest that 30- to 60-minute sessions combining both aerobic and resistance training for a total of ≥150 min/wk is a reasonable recommendation. A very recent report from Aamann et al (70) indicated people with LC who train die less and goes less to hospitals.
We acknowledge some limitations of this meta-analysis that may directly impact the prevalence results.
Regarding instruments and diagnosis, it is noteworthy that Sinclair et al (71) found significant differences in the prevalence of sarcopenia when applying CT scan (70.3%) or DXA (38.7%). Furthermore, Buchard et al (72) indicated MRI and CT scan as the only reliable devices for cirrhotic patients with ascites (CP-B and CP-C) and potentially DXA for compensated patients (CP-A). Depending on the definition used, Da Silva et al (73) found more or less incident sarcopenia. However, this overview lacks consistency in the clinical device used for body composition assessment (MRI, DXA, dynamometer, BIA, and CT) and the construct used for diagnosing sarcopenia across studies. Also, we lacked to describe the tools and criteria used for assessing LC.
On the other hand, drugs, operations, or other treatments were rarely reported. In some of our selected studies, patients with LC were a subset of a larger population, which could create a bias. Another significant limitation might be related to the etiology of LC. Finally, Bhanji et al (74) reported that patients with nonalcoholic steatohepatitis, the most advanced stage of nonalcoholic fatty liver disease, have a significantly lower prevalence of sarcopenia (22%) than patients with alcoholic liver disease (47%). In fact, these patients have a higher body mass index and obesity, fat deposits, and utilization that could have a muscle reserve effect and induce an anticatabolic environment (75), and may require more muscle work for the same exercise (76), whereas alcohol consumption leads to anabolic muscle resistance (77). However, patients with nonalcoholic steatohepatitis have a 6-fold higher risk of sarcopenic obesity than alcoholics (78). Therefore, exploring the moderating effect of the nonalcoholic fatty liver disease etiology vs sarcopenia among patients with LC with prevalence data could offer a further study focus.
Avoiding comorbidities in patients with LC may lower the risk of death and disease-related disability, especially muscle wasting, physical decline, and sarcopenia. This research stresses the utility of considering sarcopenia as a critical comorbidity in liver disease because 1 in 3 patients with LC is affected. Furthermore, the severity of LC impacts negatively, indeed, the prevalence increases in CP A-B-C respectively, (33%–36%–46%), likely due to the drug therapy and disease-induced muscle-catabolism environment. There is an urgent necessity to assess sarcopenia systematically in LC patients and to recommend lifestyle interventions to reduce risk.
CONFLICTS OF INTEREST
Guarantors of the article: Fabio Castellana, MS, and Roberta Zupo, MS.
Specific author contributions: S.M., R.S., and R.Z.: designed the study, performed searches, extracted data, assessed data quality, performed statistical analyses, and wrote the manuscript. S.M., F.C., A.C., and R.Z.: performed searches, extracted data, assessed data quality, and reviewed the manuscript. F.C.: assisted with statistical analyses, performed the analysis, and reviewed the manuscript. R.S.: reviewed the manuscript. R.S. provided input into study design and analysis and reviewed the manuscript.
Financial support: None to report.
Potential competing interests: None to report.
Contributor Information
Simon Mazeaud, Email: simon.mazeaud@sfr.fr.
Roberta Zupo, Email: roberta.zupo@irccsdebellis.it.
Alexis Couret, Email: alexis.couret@etu.uca.fr.
Francesco Panza, Email: f_panza@hotmail.com.
Rodolfo Sardone, Email: rodolfo.sardone@irccsdebellis.it.
REFERENCES
- 1.Moon AM, Singal AG, Tapper EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol 2020;18(12):2650–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5(3):245–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mokdad AA, Lopez AD, Shahraz S, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: A systematic analysis. BMC Med 2014;12(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falagas ME, Vardakas KZ, Vergidis PI. Under-diagnosis of common chronic diseases: Prevalence and impact on human health. Int J Clin Pract 2007;61(9):1569–79. [DOI] [PubMed] [Google Scholar]
- 5.Blachier M, Leleu H, Peck-Radosavljevic M, et al. The burden of liver disease in Europe: A review of available epidemiological data. J Hepatol 2013;58(3):593–608. [DOI] [PubMed] [Google Scholar]
- 6.Shafiee G, Keshtkar A, Soltani A, et al. Prevalence of sarcopenia in the world: A systematic review and meta-analysis of general population studies. J Diabetes Metab Disord 2017;16(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castellana F, Lampignano L, Bortone I, et al. Physical frailty, multimorbidity, and all-cause mortality in an older population from Southern Italy: Results from the Salus in Apulia Study. J Am Med Dir Assoc 2021;22(3):598–605. [DOI] [PubMed] [Google Scholar]
- 8.Zupo R, Castellana F, Bortone I, et al. Nutritional domains in frailty tools: Working towards an operational definition of nutritional frailty. Ageing Res Rev 2020;64:101148. [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019;48(4):601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senior HE, Henwood TR, Beller EM, et al. Prevalence and risk factors of sarcopenia among adults living in nursing homes. Maturitas 2015;82(4):418–23. [DOI] [PubMed] [Google Scholar]
- 11.Tantai X, Liu Y, Yeo YH, et al. Effect of sarcopenia on survival in patients with cirrhosis: A meta-analysis. J Hepatol 2022;76(3):588–99. [DOI] [PubMed] [Google Scholar]
- 12.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021;372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belur J, Tompson L, Thornton A, et al. Interrater reliability in systematic review methodology: Exploring variation in coder decision-making. Sociol Methods Res 2021;50(2):837–65. [Google Scholar]
- 14.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012;65(9):934–9. [DOI] [PubMed] [Google Scholar]
- 15.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods 2006;11(2):193–206. [DOI] [PubMed] [Google Scholar]
- 16.Iacob S, Mina V, Mandea M, et al. Assessment of sarcopenia related quality of life using SarQoL questionnaire in patients with liver cirrhosis. Front Nutr 2022;9:774044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anand A, Mohta S, Agarwal S, et al. European Working Group on Sarcopenia in Older People (EWGSOP2) criteria with population-based skeletal muscle index best predicts mortality in Asians with cirrhosis. J Clin Exp Hepatol 2022;12(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topan MM, Sporea I, Dănilă M, et al. Impact of sarcopenia on survival and clinical outcomes in patients with liver cirrhosis. Front Nutr 2021;8:766451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng X, Shi ZW, Yu JJ, et al. Sarcopenia as a prognostic predictor of liver cirrhosis: A multicentre study in China. J Cachexia Sarcopenia Muscle 2021;12(6):1948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanai T, Hiraoka A, Shiraki M, et al. Utility of the SARC-F questionnaire for sarcopenia screening in patients with chronic liver disease: A multicenter cross-sectional study in Japan. J Clin Med Res 2021;10(15):3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paternostro R, Bardach C, Hofer BS, et al. Prognostic impact of sarcopenia in cirrhotic patients stratified by different severity of portal hypertension. Liver Int 2021;41(4):799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishikawa H, Yoh K, Enomoto H, et al. Calf circumference as a useful predictor of sarcopenia in patients with liver diseases. In Vivo 2020;34(5):2561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traub J, Bergheim I, Eibisberger M, et al. Sarcopenia and liver cirrhosis-comparison of the European Working Group on Sarcopenia Criteria 2010 and 2019. Nutrients 2020;12(2):547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim KH, Joo DJ, Lee YH, et al. Association between liver fibrosis and appendicular skeletal muscle mass during antiviral therapy in chronic hepatitis B. Dig Liver Dis 2020;52(11):1338–45. [DOI] [PubMed] [Google Scholar]
- 25.Salman MA, Omar HSE, Mikhail HMS, et al. Sarcopenia increases 1-year mortality after surgical resection of hepatocellular carcinoma. ANZ J Surg 2020;90(5):781–5. [DOI] [PubMed] [Google Scholar]
- 26.Okubo T, Atsukawa M, Tsubota A, et al. Relationship between serum vitamin D level and sarcopenia in chronic liver disease. Hepatol Res 2020;50(5):588–97. [DOI] [PubMed] [Google Scholar]
- 27.Saeki C, Takano K, Oikawa T, et al. Comparative assessment of sarcopenia using the JSH, AWGS, and EWGSOP2 criteria and the relationship between sarcopenia, osteoporosis, and osteosarcopenia in patients with liver cirrhosis. BMC Musculoskelet Disord 2019;20(1):615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanai T, Shiraki M, Imai K, et al. Reduced handgrip strength is predictive of poor survival among patients with liver cirrhosis: A sex-stratified analysis. Hepatol Res 2019;49(12):1414–26. [DOI] [PubMed] [Google Scholar]
- 29.Kim KH, Kim BK, Park JY, et al. Sarcopenia assessed using bioimpedance analysis is associated independently with significant liver fibrosis in patients with chronic liver diseases. Eur J Gastroenterol Hepatol 2020;32(1):58–65. [DOI] [PubMed] [Google Scholar]
- 30.Nishikawa H, Enomoto H, Yoh K, et al. Association between sarcopenia and depression in patients with chronic liver diseases. J Clin Med Res 2019;8(5):634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montano-Loza AJ, Meza-Junco J, Prado CMM, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 2012;10(2):166–73.e1. [DOI] [PubMed] [Google Scholar]
- 32.Fujita M, Abe K, Hayashi M, et al. Skeletal muscle volume loss among liver cirrhosis patients receiving levocarnitine predicts poor prognosis. Medicine 2020;99(28):e21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santos LAA, Lima TB, Ietsugu M, et al. Anthropometric measures associated with sarcopenia in outpatients with liver cirrhosis. Nutr Diet 2019;76(5):613–9. [DOI] [PubMed] [Google Scholar]
- 34.Carey EJ, Lai JC, Wang CW, et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl 2017;23(5):625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benmassaoud A, Roccarina D, Arico FM, et al. Sex is a major effect modifier between body composition and mortality in patients with cirrhosis assessed for liver transplantation. Liver Int 2023;43(1):160–9. [DOI] [PubMed] [Google Scholar]
- 36.Anand A, Nambirajan A, Kumar V, et al. Alterations in autophagy and mammalian target of rapamycin (mTOR) pathways mediate sarcopenia in patients with cirrhosis. J Clin Exp Hepatol 2022;12(2):510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura A, Yoshimura T, Sato T, et al. Diagnosis and pathogenesis of sarcopenia in chronic liver disease using liver magnetic resonance imaging. Cureus 2022;14(5):e24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marasco G, Dajti E, Serenari M, et al. Sarcopenia predicts major complications after resection for primary hepatocellular carcinoma in compensated cirrhosis. Cancers 2022;14(8):1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murata K, Namisaki T, Fujimoto Y, et al. Clinical significance of serum zinc levels on the development of sarcopenia in cirrhotic patients. Cancer Diagn Progn 2022;2(2):184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luengpradidgun L, Chamroonkul N, Sripongpun P, et al. Utility of handgrip strength (HGS) and bioelectrical impedance analysis (BIA) in the diagnosis of sarcopenia in cirrhotic patients. BMC Gastroenterol 2022;22(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Ma J, Yang C, et al. Sarcopenia in patients with cirrhosis after transjugular intrahepatic portosystemic shunt placement. Radiology 2022;303(3):711–9. [DOI] [PubMed] [Google Scholar]
- 42.Jeong JY, Lim S, Sohn JH, et al. Presence of sarcopenia and its rate of change are independently associated with long-term mortality in patients with liver cirrhosis. J Korean Med Sci 2018;33(50):e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebadi M, Bhanji RA, Dunichand-Hoedl AR, et al. Sarcopenia severity based on computed tomography image analysis in patients with cirrhosis. Nutrients 2020;12(11):3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar V, Benjamin J, Shasthry V, et al. Sarcopenia in cirrhosis: Fallout on liver transplantation. J Clin Exp Hepatol 2020;10(5):467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kappus MR, Wegermann K, Bozdogan E, et al. Use of skeletal muscle index as a predictor of wait-list mortality in patients with end-stage liver disease. Liver Transpl 2020;26(9):1090–9. [DOI] [PubMed] [Google Scholar]
- 46.Feng Z, Zhao H, Jiang Y, et al. Sarcopenia associates with increased risk of hepatocellular carcinoma among male patients with cirrhosis. Clin Nutr 2020;39(10):3132–9. [DOI] [PubMed] [Google Scholar]
- 47.Kang SH, Jeong WK, Baik SK, et al. Impact of sarcopenia on prognostic value of cirrhosis: Going beyond the hepatic venous pressure gradient and MELD score. J Cachexia Sarcopenia Muscle 2018;9(5):860–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engelmann C, Schob S, Nonnenmacher I, et al. Loss of paraspinal muscle mass is a gender-specific consequence of cirrhosis that predicts complications and death. Aliment Pharmacol Ther 2018;48(11–12):1271–81. [DOI] [PubMed] [Google Scholar]
- 49.Begini P, Gigante E, Antonelli G, et al. Sarcopenia predicts reduced survival in patients with hepatocellular carcinoma at first diagnosis. Ann Hepatol 2017;16(1):107–14. [DOI] [PubMed] [Google Scholar]
- 50.Meza-Junco J, Montano-Loza AJ, Baracos VE, et al. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol 2013;47(10):861–70. [DOI] [PubMed] [Google Scholar]
- 51.Hou L, Deng Y, Fan X, et al. A sex-stratified prognostic nomogram incorporating body compositions for long-term mortality in cirrhosis. JPEN J Parenter Enteral Nutr 2021;45(2):403–13. [DOI] [PubMed] [Google Scholar]
- 52.Nishikawa H, Shiraki M, Hiramatsu A, et al. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition) Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 2016;46(10):951–63. [DOI] [PubMed] [Google Scholar]
- 53.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010;39(4):412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishikawa H, Yoh K, Enomoto H, et al. Sarcopenia and frailty in chronic liver damage: Common and different points. In Vivo 2020;34(5):2549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim G, Kang SH, Kim MY, et al. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PloS one 2017;12(10):e0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michitaka K, Nishiguchi S, Aoyagi Y, et al. Etiology of liver cirrhosis in Japan: a nationwide survey. J Gastroenterol 2010;45:86–94. [DOI] [PubMed] [Google Scholar]
- 57.Guo Y, Ren Y, Zhu L, et al. Association between sarcopenia and clinical outcomes in patients with hepatocellular carcinoma: an updated meta-analysis. Sci Rep 2023;13(1):934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ebadi M, Bhanji RA, Mazurak VC, et al. Sarcopenia in cirrhosis: From pathogenesis to interventions. J Gastroenterol 2019;54(10):845–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu J, Thapaliya S, Runkana A, et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB–mediated mechanism. Proc Natl Acad Sci USA 2013;110(45):18162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu J, Tsien C, Thapalaya S, et al. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab 2012;303(8):E983–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Egerman MA, Glass DJ. Signaling pathways controlling skeletal muscle mass. Crit Rev Biochem Mol Biol 2014;49(1):59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wing SS, Lecker SH, Jagoe RT. Proteolysis in illness-associated skeletal muscle atrophy: From pathways to networks. Crit Rev Clin Lab Sci 2011;48(2):49–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baracos VE, Martin L, Korc M, et al. Cancer-associated cachexia. Nat Rev Dis Primers 2018;4(1):17105. [DOI] [PubMed] [Google Scholar]
- 64.Hayashi F, Matsumoto Y, Momoki C, et al. Physical inactivity and insufficient dietary intake are associated with the frequency of sarcopenia in patients with compensated viral liver cirrhosis. Hepatol Res 2013;43(12):1264–75. [DOI] [PubMed] [Google Scholar]
- 65.Shimono Y, Enomoto H, Kishino K, et al. Arm skeletal muscle mass is associated with the prognosis of patients with cirrhosis. In Vivo 2020;34(3):1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sinclair M, Grossmann M, Hoermann R, et al. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J Hepatol 2016;65(5):906–13. [DOI] [PubMed] [Google Scholar]
- 67.Tandon P, Ismond KP, Riess K, et al. Exercise in cirrhosis: Translating evidence and experience to practice. J Hepatol 2018;69(5):1164–77. [DOI] [PubMed] [Google Scholar]
- 68.Duarte-Rojo A, Ruiz-Margáin A, Montaño-Loza AJ, et al. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl 2018;24(1):122–39. [DOI] [PubMed] [Google Scholar]
- 69.Tandon P, Montano-Loza AJ, Lai JC, et al. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol 2021;75(Suppl 1):S147–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aamann L, Dam G, Jepsen P, et al. Reduced 3‐year risk of hospital admission and mortality after 12‐week resistance training of cirrhosis patients: A follow‐up of a randomized clinical trial. J Gastroenterol Hepatol 2023. doi: 10.1111/jgh.16141 [DOI] [PubMed] [Google Scholar]
- 71.Sinclair M, Chapman B, Hoermann R, et al. Handgrip strength adds more prognostic value to the model for end-stage liver disease score than imaging-based measures of muscle mass in men with cirrhosis. Liver Transpl 2019;25(10):1480–7. [DOI] [PubMed] [Google Scholar]
- 72.Buchard B, Boirie Y, Cassagnes L, et al. Assessment of malnutrition, sarcopenia and frailty in patients with cirrhosis: Which tools should we use in clinical practice? Nutrients 2020;12(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.da Silva MZC, Vogt BP, Reis NSdC, et al. Update of the European consensus on sarcopenia: What has changed in diagnosis and prevalence in peritoneal dialysis? Eur J Clin Nutr 2019;73(8):1209–11. [DOI] [PubMed] [Google Scholar]
- 74.Bhanji RA, Narayanan P, Moynagh MR, et al. Differing impact of sarcopenia and frailty in nonalcoholic steatohepatitis and alcoholic liver disease. Liver Transpl 2019;25(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peng S, Plank LD, McCall JL, et al. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: A comprehensive study. Am J Clin Nutr 2007;85(5):1257–66. [DOI] [PubMed] [Google Scholar]
- 76.Karakousis ND, Chrysavgis L, Chatzigeorgiou A, et al. Frailty in metabolic syndrome, focusing on nonalcoholic fatty liver disease. Ann Gastroenterol Hepatol 2022;35(3):234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lang CH, Frost RA, Deshpande N, et al. Alcohol impairs leucine-mediated phosphorylation of 4E-BP1, S6K1, eIF4G, and mTOR in skeletal muscle. Am J Physiol Endocrinol Metab 2003;285(6):E1205–15. [DOI] [PubMed] [Google Scholar]
- 78.Carias S, Castellanos AL, Vilchez V, et al. Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J Gastroenterol Hepatol 2016;31(3):628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]