INTRODUCTION:
Water-assisted colonoscopy increases left colon mucus production; however, the effect of saline on mucus production is unclear. We tested the hypothesis that saline infusion may reduce mucus production in a dose-related manner.
METHODS:
In a randomized trial, patients were assigned to colonoscopy with CO2 insufflation, water exchange (WE) with warm water, 25% saline, or 50% saline. The primary outcome was the Left Colon Mucus Scale (LCMS) score (5-point scale). Blood electrolytes were measured before and after saline infusion.
RESULTS:
A total of 296 patients with similar baseline demographics were included. The mean LCMS score for WE with water was significantly higher than that for WE with saline and CO2 (1.4 ± 0.8 [WE water] vs 0.7 ± 0.6 [WE 25% saline] vs 0.5 ± 0.5 [WE 50% saline] vs 0.2 ± 0.4 [CO2]; overall P < 0.0001), with no significant difference between the 25% and 50% saline groups. The left colon adenoma detection rate (ADR) was highest in the 50% saline group, followed by the 25% saline and the water groups (25.0% vs 18.7% vs 13.3%), but the difference was not significant. Logistic regression showed water infusion as the only predictor of moderate mucus production (odds ratio 33.3, 95% confidence interval 7.2–153.2). No acute electrolyte abnormalities were documented indicating a safe modification.
DISCUSSION:
The use of 25% and 50% saline significantly inhibited mucus production and numerically increased ADR in the left colon. Evaluation of the impact of mucus inhibition by saline on ADR may refine the outcomes of WE.
KEYWORDS: randomized trial, colon mucus, water, saline, water exchange colonoscopy
INTRODUCTION
Water-assisted colonoscopy (WAC) reduces insertion discomfort, facilitates cecal intubation in non-sedated or minimally sedated patients, enhances completion in challenging cases, and allows more complete resection of nonpedunculated lesions (1–5). Therefore, it is recommended that endoscopists become familiar with WAC during their clinical training (6,7). Water exchange (WE) colonoscopy, one of the WAC techniques, maximizes colon cleanliness during insertion and improves mucosal inspection during withdrawal, increasing the adenoma detection rate (ADR) and reducing the adenoma miss rate (AMR) (8–11). WE has been endorsed to increase the ADR in clinical practice (12–14).
In WE, colonoscope withdrawal is performed with gas insufflation. Total underwater colonoscopy, another WAC technique, applies water infusion with partial removal during insertion, and withdrawal is also performed with a water-filled lumen (15). The authors observed that the infusion of large amounts (>1,600 mL) of room temperature water induced colon mucus production, with mucus causing distraction during withdrawal inspection and potentially contributing to a higher AMR (15). An observational study reported that WAC with room temperature normal saline (NS) reduced mucus production (16). Whether mucus production can be minimized by altering the salt content or temperature of the water should be investigated.
We hypothesized that saline infusion reduces mucus production in a dose-dependent manner. We aimed to evaluate the use of warm saline solutions for WE colonoscopy and compare differences in left colon mucus production in patients undergoing warm water–infused WE and standard CO2 insufflated colonoscopy.
METHODS
Between August 2021 and March 2022, we conducted a prospective randomized controlled trial (RCT) to compare the effects of WE performed under various conditions on left colon mucus production. The study was approved by the institutional review board and was registered at ClinicalTrials.gov (NCT04769739). All patients provided written informed consent. This study is presented according to the Consolidated Standards of Reporting Trials guidelines for reporting RCTs.
Patients
Adults aged 20–75 years undergoing colonoscopy for screening, surveillance, or positive fecal immunochemical tests were recruited. Exclusion criteria included hereditary colorectal cancer syndrome, inflammatory bowel disease, previous colorectal resection, therapeutic colonoscopy, American Society of Anesthesiology classification of patient physical status grade III or higher, and refusal to provide written informed consent.
WE-infused solutions and randomization
We used sterile water, 25% saline (250 mL NS + 750 mL sterile water), and 50% saline (500 mL NS + 500 mL sterile water) for WE infusion. All infusates were warmed to 32°C and stored in a 1,000 mL container (for the purpose of blinding). The participants were randomized to undergo colonoscopy insertion with CO2 insufflation, WE with water, 25% saline, or 50% saline. Randomization was computer generated using a random-block size (4 or 8) to maintain concealed allocation and stratified by colonoscopists. Sealed, opaque envelopes for each participant were opened at the time of the procedure.
Study procedures
Split-dose bowel preparation with polyethylene glycol, moderate sedation (fentanyl and midazolam), and standard colonoscopes (CF-Q260AL/I; Olympus Medical Systems, Tokyo, Japan) were used. Colonoscopies were performed by 2 experienced colonoscopists (C.-L.C. and Y.-L.K.). CO2 insufflation was used for the CO2 group and during withdrawal in the WE groups. In the WE groups, the air pump was turned off, and the colon was irrigated with warm fluid using a flushing pump (Olympus AFU-100) during insertion as previously described (8–11). The colon was cleansed to reproduce the large amounts of infused water (>1,600 mL) in the total underwater colonoscopy study (15). Three stopwatches were used to record the time taken for cleansing, inspection, and polyp treatment during withdrawal. A difficult colonoscopy was defined as a colonoscopy in which the colonoscopist had difficulty getting through the entire colon, and considerable efforts were required to reach the cecum, including multiple attempts to change the abdominal pressure and patient position (17,18).
The following information was collected: bowel preparation quality (Boston Bowel Preparation Scale score) (19), volume of fluid infused and suctioned during insertion and withdrawal, insertion and withdrawal times, and polyp number, size, histology, and location. Moreover, serum sodium, chloride, and potassium levels were measured 15 minutes before and 60 minutes after the procedure in the WE saline group to detect any. The timing of blood tests allowed detecting the acute effect of saline infusion, if present.
Mucus scores
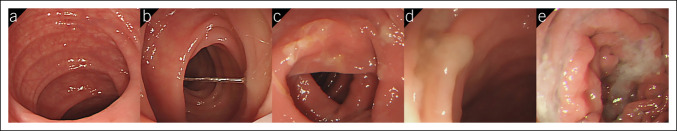
Mucus production was evaluated during real-time colonoscopy withdrawal by the colonoscopist who performed the procedure and a research assistant. Both blinded observers independently rated the amount of mucus in the left colon (descending colon, sigmoid colon, and rectum) with a 5-point Left Colon Mucus Scale (LCMS) score (Figure 1): score 0: no visible mucus, score 1: clear mucus, score 2: opaque mucus in thin strands, score 3: opaque mucus in thicker clumps covering one side of the surface, and score 4: opaque mucus in thick clumps covering more views of the lumen. The raters validated the method by reviewing and discussing 10 videos to reach a consensus. The raters also guessed the fluid used during WE insertion. Blinding was considered successful if ≤67% of guesses were correct (20).
Figure 1.
Left Colon Mucus Scale score: (a) no visible mucus (score 0), (b) clear mucus (score 1), (c) mild opaque mucus presenting as thin strands (score 2), (d) moderate opaque mucus presenting as thicker clumps covering one side of the surface (score 3), and (e) more opaque mucus presenting as thick clumps covering more views of the lumen (score 4).
Study outcomes
The primary outcome was the average LCMS score rated by the colonoscopist and assistant. Mucus was considered a plausible source of interference during inspection (15), although there are no published data supporting this hypothesis. Our preliminary data (see Supplementary Table, Supplementary Digital Content 1, http://links.lww.com/CTG/A939) showed that the LCMS score in the WE water group was 1.88 ± 0.78. We postulated that the LCMS score ≥2, that is, presence of any opaque mucus, was clinically significant with the ability to obscure inspection.
Secondary outcomes included changes in the serum electrolytes in the saline groups, ADR (percentage of patients with ≥1 adenoma), and clinically significant serrated polyp (CSSP) detection rate (percentage of patients with ≥1 CSSP). Adenomas included tubular, villous, and tubulovillous adenomas. CSSPs included all sessile serrated adenomas/polyps, traditional serrated adenomas, hyperplastic polyps ≥10 mm in the colon, or hyperplastic polyps ≥6 mm in the proximal colon (colon segment proximal to the left colon) (21).
Sample size calculation
Our preliminary data suggested that the proportion of participants with LCMS score <2 would be 50% in the WE water group. The sample size needed to show an increase to 80% in the WE 50% saline group, and a 5% alpha error level with 90% power would be 68 patients per group. To account for dropouts, 300 patients (75 per group) were enrolled.
Statistical analysis
Categorical variables are presented as numbers (percentages) and continuous variables as means and standard deviations. Overall P values for categorical and continuous parameters were obtained using the Fisher exact test and the Kruskal-Wallis test, respectively. Pairwise comparisons were performed when the overall group effect was significant. The permutation resampling method for the Fisher exact test and the Dwass-Steel-Critchlow-Fligner method for the Kruskal-Wallis test were applied for the adjusted P values on pairwise comparisons. Agreement between the blinded raters was tested with Fleiss κ. Univariate logistic regression analysis was performed to determine which of the demographic and procedural data were potential predictors of moderate mucus production (LCMS score ≥2). Factors with P value <0.1 on univariate analysis were analyzed with multivariate logistic regression analysis. Odds ratio with 95% confidence interval (CI) was used to describe the influence of various factors on mucus production. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC). A P value <0.05 was considered significant.
RESULTS
Patient demographics
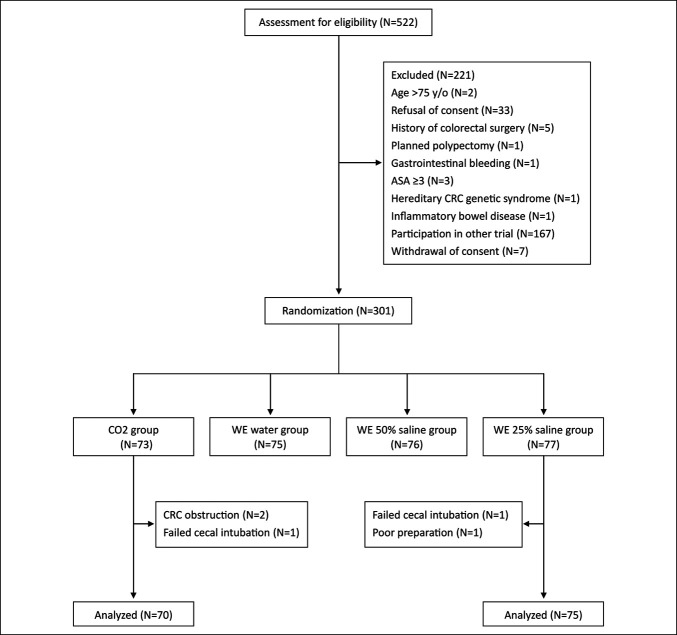
Patient enrollment, allocation, and exclusion are shown in Figure 2. Overall, 301 patients were randomized. Five were excluded due to failed cecal intubation (n = 2), colorectal cancer obstruction (n = 2), and poor bowel preparation (n = 1). In total, 296 patients completed the study (WE water group = 75, WE 25% saline group = 75, WE 50% saline group = 76, and CO2 group = 70). Table 1 summarizes the demographic features and procedure indications of the 4 groups. No significant differences were noted in baseline data.
Figure 2.
Flow diagram of enrollment, intervention allocation, and exclusion. ASA, American Society of Anesthesiology; CRC, colorectal cancer; WE, water exchange.
Table 1.
Demographic details and procedure indications
| Full analysis seta | Study groups | Overall P value | |||
| Variable | CO2 (n = 70) | WE water (n = 75) | WE 25% saline (n = 75) | WE 50% saline (n = 76) | |
| Female, n (%) | 37 (52.9) | 32 (42.7) | 38 (50.7) | 43 (56.6) | 0.3771 |
| Age, yr, mean (SD) | 51.2 (10.0) | 51.4 (10.7) | 52.0 (10.9) | 53.0 (11.5) | 0.8346 |
| Body mass index, kg/m2, mean (SD) | 25.0 (4.0) | 24.9 (3.5) | 24.6 (3.1) | 24.8 (4.2) | 0.8677 |
| Previous abdominal surgery, n (%) | 29 (41.4) | 22 (29.3) | 19 (25.3) | 28 (36.8) | 0.1607 |
| Family history of colorectal cancer, n (%) | 4 (5.7) | 4 (5.3) | 3 (4.0) | 2 (2.6) | 0.7822 |
| Indications for colonoscopy, n (%) | 0.9955 | ||||
| Screening | 41 (58.6) | 46 (61.3) | 42 (56.0) | 43 (56.6) | |
| Surveillance | 24 (34.3) | 24 (32.0) | 27 (36.0) | 28 (36.8) | |
| Positive fecal immunochemical test | 5 (7.1) | 5 (6.7) | 6 (8.0) | 5 (6.6) | |
WE, water exchange.
Analyses performed in the full analysis set considered the intention-to-treat principle and considered patients' allocations to the study groups as randomized.
Primary outcome
The LCMS scores are presented in Table 2. The mean combined LCMS score was highest in the WE water group, followed by the 25% saline, 50% saline, and CO2 groups (1.4 ± 0.84 [WE water], 0.7 ± 0.63 [WE 25% saline], 0.5 ± 0.50 [WE 50% saline], and 0.2 ± 0.38 [CO2]; overall P < 0.0001) (Figure 3). However, the mean LCMS score was not significantly different between the WE 25% and 50% saline groups (P = 0.4325). The details of LCMS score distribution are shown in Supplementary Table (see Supplementary Digital Content 2, http://links.lww.com/CTG/A939). The proportion of patients with moderate mucus production was significantly higher in the WE water group than in the saline groups (42.7% [WE water] vs 8.0% [WE 25% saline] vs 2.6% [WE 50% saline], overall P < 0.0001). In the WE water group, significantly more patients required additional mucus cleansing during withdrawal than those in the WE 50% saline group (6.7% vs 0%, P = 0.032). Statistical details of pairwise comparisons are shown in Supplementary Table (see Supplementary Digital Content 3, http://links.lww.com/CTG/A939).
Table 2.
Left colon mucus production and related data
| Variable | CO2 (n = 70) | WE water (n = 75) | WE 25% saline (n = 75) | WE 50% saline (n = 76) | P value | |||
| Overall | Water vs 50% saline | Water vs 25% saline | 25% saline vs 50% saline | |||||
| Combined LCMS scorea by blinded observers, mean (SD) [95% CI] | 0.2 (0.38) [0.10–0.28] | 1.4 (0.84) [1.23–1.61] | 0.7 (0.63) [0.52–0.81] | 0.5 (0.50) [0.38–0.62] | <0.0001 | <0.0001 | <0.0001 | 0.4325 |
| LCMS score by endoscopists, mean (SD) [95% CI] | 0.2 (0.46) [0.12–0.34] | 1.3 (0.92) [1.13–1.56] | 0.7 (0.66) [0.53–0.83] | 0.5 (0.55) [0.39–0.64] | <0.0001 | <0.0001 | <0.0001 | 0.4582 |
| LCMS score by the study assistant, mean (SD) [95% CI] | 0.2 (0.40) [0.06–0.25] | 1.5 (0.84) [1.30–1.69] | 0.7 (0.69) [0.50–0.81] | 0.5 (0.58) [0.35–0.62] | <0.0001 | <0.0001 | <0.0001 | 0.4940 |
| Patients with LCMS score <2, n (%) | 70 (100) | 43 (57.3) | 69 (92.0) | 74 (97.4) | <0.0001 | <0.0001 | <0.0001 | 0.4640 |
| Patients with LCMS score ≥1, n (%) | 8 (11.4) | 59 (78.7) | 36 (48.0) | 26 (34.2) | <0.0001 | <0.0001 | 0.0010 | 0.3140 |
| Patients with moderate mucus production (LCMS score ≥2), n (%) | 0 (0) | 32 (42.7) | 6 (8.0) | 2 (2.6) | <0.0001 | <0.0001 | <0.0001 | 0.4640 |
| Patients who required additional mucus cleansing during withdrawal, n (%) | 0 (0) | 5 (6.7) | 2 (2.7) | 0 (0) | 0.0136 | 0.0320 | 0.7040 | 0.4440 |
| Correct guess of infused fluid by colonoscopists, n (%) | NA | 47 (62.7) | 39 (52.0) | 28 (36.8) | 0.0062 | 0.0030 | 0.3810 | 0.1210 |
| Correct guess of infused fluid by study assistant, n (%) | NA | 60 (80.0) | 55 (73.3) | 40 (52.6) | 0.0009 | 0.0010 | 0.6810 | 0.0270 |
CI, confidence interval; LCMS, left colon mucus scale; NA, not available; WE, water exchange.
The combined LCMS score was the average of scores by the blinded colonoscopist and study assistant.
Figure 3.
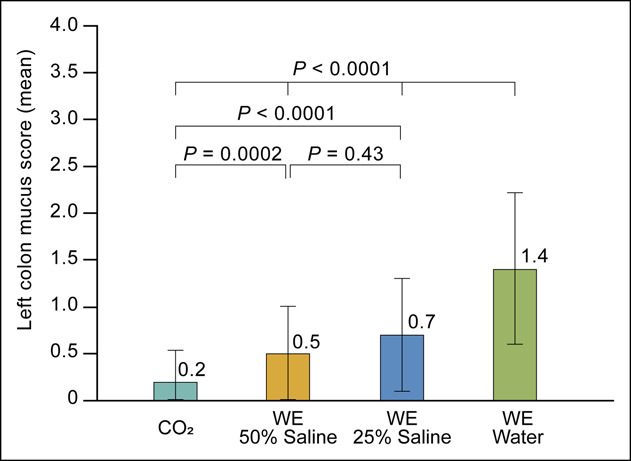
Mean combined Left Colon Mucus Scale score of each study group. WE, water exchange.
Agreement among blinded observers was substantial (Fleiss κ 0.636, 95% CI 0.560–0.713, P < 0.0001) (see Supplementary Table, Supplementary Digital Content 4, http://links.lww.com/CTG/A939). Correct guesses by colonoscopists regarding fluid used during WE insertion were <67% in all groups, indicating adequate blinding.
Secondary outcomes
Procedure and polyp detection data are summarized in Table 3. The total procedure time was comparable, and the volumes of fluid infused and aspirated during WE insertions were similar. WE showed a significantly longer insertion time and significantly shorter withdrawal cleansing time than CO2. Withdrawal inspection time was significantly longer in the CO2 than in the WE groups. The overall adenoma and CSSP data were not significantly different among the 4 groups. The left colon ADR was highest in the WE 50% saline, followed by WE 25% saline and WE water group (25.0% vs 18.7% vs 13.3%), but the difference did not reach statistical significance. There was a significant difference in overall ADR between the 2 participating endoscopists (see Supplementary Table, Supplementary Digital Content 5, http://links.lww.com/CTG/A939). Multivariate logistic regression analysis showed that WE with water infusion was the only predictor of moderate left colon mucus production (odds ratio 33.3; 95% CI 7.2–153.2) (Table 4). Acute abnormal serum sodium, chloride, and potassium levels were not observed in the WE saline groups (Table 5).
Table 3.
Colonoscopy procedure and detection data
| Variable | Study group | Overall P value | |||
| CO2 (n = 70) | WE water (n = 75) | WE 25% saline (n = 75) | WE 50% saline (n = 76) | ||
| Cecal intubation rate, n (%) | 70 (100) | 75 (100) | 75 (100) | 76 (100) | |
| Patients with difficult colonoscopy, n (%) | 12 (17.1) | 16 (21.3) | 10 (13.3) | 13 (17.1) | 0.6506 |
| Patients requiring the change of position, n (%) | 27 (37.1) | 16 (21.3) | 16 (21.3) | 21 (27.6) | 0.1142 |
| Patients requiring abdominal compression, n (%) | 58 (82.9) | 49 (65.3) | 45 (60.0) | 40 (52.6) | 0.0009 |
| Insertion time, min, mean (SD) | 10.0 (6.0) | 18.0 (6.2) | 18.6 (9.1) | 18.4 (7.1) | <0.0001 |
| Withdrawal cleansing time, min, mean (SD) | 4.2 (3.7) | 2.5 (2.0) | 2.7 (2.5) | 2.1 (1.8) | 0.0024 |
| Withdrawal inspection time, min, mean (SD) | 20.2 (6.4) | 17.1 (5.4) | 16.6 (5.4) | 17.2 (5.3) | 0.0005 |
| Withdrawal treatment time, min, mean (SD) | 3.3 (5.1) | 3.1 (3.5) | 2.5 (3.5) | 3.2 (3.7) | 0.2013 |
| Total withdrawal time, min, mean (SD) | 27.7 (9.7) | 22.6 (7.8) | 21.8 (7.9) | 22.4 (8.5) | <0.0001 |
| Total procedure time, min, mean (SD) | 37.6 (11.4) | 40.6 (10.6) | 40.0 (12.8) | 41.1 (11.6) | 0.0740 |
| Infused fluid during insertion, mL, mean (SD) | 82.7 (199.7) | 1,702 (587.1) | 1,708 (840.2) | 1,644 (612.4) | <0.0001 |
| Aspirated fluid during insertion, mL, mean (SD) | 183.4 (153.3) | 1,643 (582.8) | 1,641 (710.4) | 1,552 (564.1) | <0.0001 |
| Infused fluid during withdrawal, mL, mean (SD) | 390.4 (369.8) | 208.5 (240.7) | 216.5 (329.9) | 163.0 (230.3) | <0.0001 |
| Aspirated fluid during withdrawal, mL, mean (SD) | 378.0 (317.9) | 296.7 (216.8) | 327.1 (317.3) | 256.2 (199.9) | 0.3229 |
| Overall BBPS score, mean (SD) | 7.4 (0.8) | 7.4 (0.8) | 7.5 (0.8) | 7.8 (0.9) | 0.0633 |
| Patients with excellent bowel preparation,a n (%) | 21 (30.0) | 23 (30.7) | 31 (41.3) | 35 (46.1) | 0.1139 |
| Overall ADR, n (%) [95% CI] | 35 (50.0) [37.8–62.2] | 31 (41.3) [30.1–53.3] | 35 (46.7) [35.1–58.6] | 41 (53.9) [42.1–65.5] | 0.4674 |
| Proximal colon ADR, n (%) [95% CI] | 27 (38.6) [27.2–51.0] | 26 (34.7) [24.0–46.5] | 26 (34.7) [24.0–46.5] | 33 (43.4) [32.1–55.3] | 0.6533 |
| Left colon ADR, n (%) [95% CI] | 17 (24.3) [14.8–36.0] | 10 (13.3)b [6.6–23.2] | 14 (18.7) [10.6–29.3] | 19 (25.0)b [15.8–36.3] | 0.2392 |
| Overall APC (SD) | 1.1 (1.7) | 1.0 (2.4) | 0.8 (1.2) | 1.0 (1.7) | 0.4608 |
| Proximal colon APC (SD) | 0.8 (1.4) | 0.8 (1.8) | 0.5 (0.8) | 0.8 (1.4) | 0.6787 |
| Left colon APC (SD) | 0.3 (0.6) | 0.2 (0.8) | 0.3 (0.7) | 0.3 (0.5) | 0.2764 |
| CSSP detection rate, n (%) [95% CI] | 12 (17.1) [9.2–28.0] | 10 (13.3) [6.6–23.2] | 10 (13.3) [6.6–23.2] | 9 (11.8) [5.6–21.3] | 0.8467 |
| Mean number of CSSP per colonoscopy (SD) | 0.3 (0.9) | 0.1 (0.4) | 0.2 (0.5) | 0.4 (1.8) | 0.8268 |
ADR, adenoma detection rate; APC, adenoma per colonoscopy; BBPS, Boston Bowel Preparation Scale; CI, confidence interval; CSSP, clinically significant serrated polyp; WE, water exchange.
Excellent bowel preparation was defined as an overall BBPS score ≥8.
P = 0.270 between WE water and WE 50% saline group.
Table 4.
Multivariate logistic regression analysis of moderate mucus production in the left colon
| Patients with a combined LCMS score ≥2 | Univariate | Multivariate | ||
| Variable | OR (95% CI) | P value | OR (95% CI) | P value |
| Female (male reference) | 1.008 (0.558–2.120) | 0.8040 | ||
| Body mass index (for a 1 kg/m2 increment) | 0.966 (0.880–1.060) | 0.4655 | ||
| Previous abdominal surgery | 1.251 (0.626–2.497) | 0.5262 | ||
| Endoscopist (endoscopist 1 as reference) | 1.272 (0.653–2.480) | 0.4792 | ||
| Difficult colonoscopy (nondifficult as reference) | 2.374 (1.113–5.062) | 0.0253 | 1.506 (0.495–4.586) | 0.4709 |
| Colonoscopy insertion time (for a 1 min increment) | 1.068 (1.029–1.110) | 0.0007 | 1.031 (0.954–1.115) | 0.4390 |
| Infused fluid volume during colonoscopy insertion (for a 100 mL increment) | 1.095 (1.050–1.141) | <0.0001 | 1.048 (0.973–1.129) | 0.2190 |
| WE water group (WE 50% saline as reference) | 27.535 (6.286–120.617) | <0.0001 | 33.273 (7.227–153.186) | <0.0001 |
CI, confidence interval; LCMS, Left Colon Mucus Scale; OR, odds ratio; WE, water exchange.
Table 5.
Changes of serum electrolyte levels within the WE saline groups
| Variable | Study group | |
| WE with 25% saline (n = 75) | WE with 50% saline (n = 76) | |
| Sodium | ||
| Precolonoscopy level, mEq/L, mean (SD) | 143.9 (2.9) | 144.8 (2.0) |
| Postcolonoscopy level, mEq/L, mean (SD) | 142.8 (2.5) | 143.6 (1.7) |
| Change during procedure, mEq/L, mean (SD) | −1.1 (1.9) | −1.2 (1.2) |
| Chloride | ||
| Precolonoscopy level, mEq/L, mean (SD) | 102.4 (2.9) | 103.0 (2.2) |
| Postcolonoscopy level, mEq/L, mean (SD) | 104.5 (2.6) | 104.7 (1.9) |
| Change during procedure, mEq/L, mean (SD) | 2.1 (1.8) | 1.7 (1.6) |
| Potassium | ||
| Precolonoscopy level, mEq/L, mean (SD) | 4.0 (0.3) | 4.0 (0.3) |
| Postcolonoscopy level, mEq/L, mean (SD) | 4.1 (0.4) | 4.1 (0.4) |
| Change during procedure, mEq/L, mean (SD) | 0.1 (0.4) | 0.1 (0.4) |
WE, water exchange.
DISCUSSION
The American Society of Gastrointestinal Endoscopy recommends using sterile water during endoscopy, particularly in immunocompromised patients (22). Sterile water is infused during WE, and its use in safely maintaining serum electrolytes has been demonstrated (23,24). However, increased mucus production in the left colon has been reported with total underwater colonoscopy using room temperature water (15). This could impair mucosal visualization, potentially contributing to an overall higher AMR. Furthermore, NS may reduce mucus production (16), an observation that warrants further evaluation.
This RCT used the LCMS score to evaluate whether warm water could increase mucus production and whether the use of warm 25% and 50% saline could significantly reduce mucus production and decrease the proportion of patients requiring additional cleansing for mucus removal. To the best of our knowledge, this study is the first to compare the effects of different warm infusates for WE colonoscopy on mucus in the left colon. Our findings suggest that the use of 25% and 50% saline can significantly reduce the LCMS score.
The novelty of our study lies in the fact that it compared all strategies used during WE colonoscopy. In multivariate logistic regression, only water was an independent predictor of mucus production. Our observations are similar to those in the total underwater colonoscopy study (15) suggesting that water temperature was not the determining factor. Consistent with the previous finding with NS (16), we showed that mucus production could be inhibited by 25% and 50% saline solutions.
The use of sterile water in lengthy and large-volume underwater duodenal mucosal resection (2.5-hour therapy with intraprocedural infusion of 5 L of water) has been associated with water intoxication initiated by the absorption of infused water that leads to dilutional hyponatremia (25). This complication has been rarely reported in gastroenterology but is a well-known complication of transurethral resection of the prostate (26). The risk of water intoxication with transurethral resection of the prostate has been largely eliminated by replacing water with NS (26). Similarly, NS and 50% saline are being used for many underwater endoscopic therapies of the gastrointestinal tract (27–32).
The mucolytic mechanism of NS involves reducing the entanglement of the mucus gel, thereby reducing the active degree of crosslinking and lowering mucus viscosity (33).
Although not statistically significant, our data implied that 50% saline is superior to 25% saline in eliminating mucus production. Both can be used and the choice will depend on convenience and availability.
The use of NS may result in channel damage within endoscopes (16). We heeded this advice and tested 25% and 50% saline instead. However, there are no objective data to support the possibility of channel damage by salt-containing solutions (34). Furthermore, under current high-level infection control processes (22), obstruction of any endoscope channel seems unlikely.
In this study, difficult colonoscopy was associated with a LCMS score ≥2 in the univariate analysis. Difficult colonoscopy cases usually occur in the left colon and involve multiple attempts of transabdominal compression and changes in the patient position (17,18). A prolonged insertion time (>10 minutes) has been used to define a difficult colonoscopy during air insufflation insertion (35), but the corresponding threshold for WE colonoscopy is unclear. We speculate that increased left colon mucus production may result from increased friction between the instrument and mucosa and greater amounts of fluid instilled during difficult insertion; these fluids tend to accumulate in the left colon, the dependent part of the colon when the patient is the left decubitus position. On the contrary, the right colon is in a higher position and less friction occurs there, which may explain why mucus production is less common in the right colon.
Our data suggest that mucus production does not influence overall ADR during WE colonoscopy. We did not record polyp detection after mucus cleansing. The small sample size was adequate to show a difference in the mucus score but insufficient to show significant differences in left colon ADR. Although not statistically significant, the highest left colon ADR was observed in the WE 50% saline group, followed by the WE 25% saline and WE water groups. These results suggest that colon mucus may obscure adenoma detection and warrants further investigation.
Contrary to previous studies (9–11), the ADR for the WE groups was not higher than that of the CO2 group in this study. This was probably due to a significantly longer withdrawal inspection time in the CO2 group. Withdrawal inspection time is known to directly affect the quality of colonoscopy and ADR (12–14). For every 1 minute increase in withdrawal time, there are 6% higher odds of detecting an additional subject with an adenoma (36).
The inhibition of mucus production by saline in WE colonoscopy has implications in WE and artificial intelligence (AI). Mucus as false positive has moderate-to-severe clinical relevance in approximately 20% of cases (37,38). The strengths of AI and water-infused WE complement the weaknesses of each other in the optimization of polyp detection (39). Such interactions with saline-infused WE should be studied further.
Our study has several strengths. First, this is the first RCT to confirm the effect of saline on reduced left colon mucus production in a dose-related manner. WAC may be challenging in some cases, particularly when the procedure is deterred by excess production of mucus in the colon. We compared 2 different strengths of saline solution and found that 50% saline produced the least amount of mucus. Second, we tested warm water and found that this also contributed to mucus production. Third, we provided the first safety data regarding serum electrolyte levels while performing WE with 25% and 50% saline. No acute electrolyte change with saline infusion indicated a safe modification of WE. Fourth, we recorded the number of patients who required additional mucus cleansing and the amount of fluid instilled during withdrawal. We found that, in the saline groups, fewer patients needed mucus cleansing, and less fluid was infused during withdrawal, suggesting that WE with saline is a more efficient procedure. Fifth, the correct guesses by the colonoscopists regarding which fluid was used were less than 67% in all groups, suggesting adequate blinding. Finally, the substantial agreement between raters validated the reliability of the LCMS scoring system.
Some limitations should be noted. The LCMS score used in this study is a subjective evaluation of mucus production and has not been previously validated, but the agreement between raters was substantial indicating its reliability. We tested 25% and 50% saline, although the preliminary data did not support a difference between the groups. The 25% saline group was intended to be a “control” group to show that some amount of saline is better than none. Moreover, the nonsignificant difference between 25% and 50% saline was likely a type II error. Although we found that the withdrawal cleansing time was not significantly different among the 3 WE groups, we did not record the time necessary for mucus cleansing, partly because the cleansing of mucus and debris occurred simultaneously. Moreover, we did not record whether additional polyps were identified after the mucus was removed and were unable to examine the impact of mucus on polyp detection. The sample size calculation was based on the LCMS score rather than the ADR, and thus, this study was underpowered for assessing the left colon ADR. The question of mucus-obscured polyp/adenoma requires evaluation in a properly designed trial with adequate sample size. In addition to acute electrolyte changes, other clinical outcomes, including cardiovascular events and hemodynamic changes, should be examined to further evaluate the use of saline infusion for WE colonoscopy. The effect of using saline in WE on polypectomy success and recurrence should be further investigated. Finally, this is a single-center study; its generalizability to other settings is unknown. These results were obtained from experienced colonoscopists and would not be generalizable to procedures performed by less experienced operators although trainees can still benefit from learning WE.
In conclusion, this RCT with blinded raters confirmed that 25% and 50% saline infusion significantly reduced left colon mucus production without interfering with serum electrolytes, indicating a safe modification of WE colonoscopy. The reduction of mucus by saline for WE raises important questions regarding the complementary relationship between AI and WE in enhancing polyp detection. Although not statistically significant, left colon ADR was numerically increased by 25% and 50% saline. Evaluation of the impact of mucus by saline on the ADR may refine the outcomes with WE.
CONFLICTS OF INTEREST
Guarantor of the article: Chi-Liang Cheng, MD.
Specific author contribution: C.-L.C.: conception and design of the study, analysis, and interpretation of the data, drafting of the article, and critical revision of the article for important intellectual content. Y.-L.K.: conception of the study, acquisition of data, and drafting of the manuscript. N.-J.L.: conception of the study, interpretation of data, and drafting of the manuscript. J.-M.L.: conception and design of the study, interpretation of data, and critical revision of the article for important intellectual content. I.-C.S.: conception of the study and drafting of the manuscript. C.-P.T.: interpretation of data and critical revision of the manuscript for important intellectual content. Y.-H.H.: interpretation of data and critical revision of the manuscript for important intellectual content. F.W.L.: conception and design of the study, interpretation of data, and critical revision and editing of the manuscript for important intellectual content. All authors read and approved the final manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial support: F.W.L. is supported in part by Veterans Affairs (VA) and American Society of Gastrointestinal Endoscopy (ASGE) research funds. The other authors declare no funding or financial support.
Potential competing interests: None to report.
Clinical trial registration: This trial has been registered at ClinicalTrials.gov (NCT04769739) on February 24, 2021.
IRB approval statement: The study has been approved by the Joint Institutional Review Board of Taiwan (JIRB No.: 21-002-T-1) on April 23, 2021.
Study Highlights.
WHAT IS KNOWN
✓ Water exchange (WE) colonoscopy increases adenoma detection.
✓ WE is associated with increased mucus production in the left colon.
✓ Potential predictors and solutions for mucus production are poorly documented.
WHAT IS NEW HERE
✓ Water infusion was the only predictor of moderate mucus production with WE.
✓ Saline infusion (25% and 50%) during WE insertion inhibited left colon mucus production.
✓ Saline infusion is a safe modification of WE colonoscopy.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Shih-Tsung Huang for sharing his experience from a urological perspective. A part of the study data had been reported in the annual scientific meeting of American College of Gastroenterology 2022 (Abstract ID 1295619).
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/A939
Contributor Information
Yen-Lin Kuo, Email: zooropa1121@yahoo.com.tw.
Nai-Jen Liu, Email: naijen.liu@gmail.com.
Jau-Min Lien, Email: lienosky@yahoo.com.
I-Chia Su, Email: sophiasu911@gmail.com.
Chia-Pei Tang, Email: franktg@hotmail.com.
Yu-Hsi Hsieh, Email: hsieh.yuhsi@msa.hinet.net.
Felix W. Leung, Email: felixleung@socal.rr.com.
REFERENCES
- 1.Hsieh YH, Koo M, Leung FW. A patient-blinded randomized, controlled trial comparing air insufflation, water immersion, and water exchange during minimally sedated colonoscopy. Am J Gastroenterol 2014;109(9):1390–400. [DOI] [PubMed] [Google Scholar]
- 2.Cadoni S, Falt P, Gallittu P, et al. Water exchange is the least painful colonoscope insertion technique and increases completion of unsedated colonoscopy. Clin Gastroenterol Hepatol 2015;13(11):1972–80.e3. [DOI] [PubMed] [Google Scholar]
- 3.Luo H, Zhang L, Liu X, et al. Water exchange enhanced cecal intubation in potentially difficult colonoscopy. Unsedated patients with prior abdominal or pelvic surgery: A prospective, randomized, controlled trial. Gastrointest Endosc 2013;77(5):767–73. [DOI] [PubMed] [Google Scholar]
- 4.Vemulapalli KC, Rex DK. Water immersion simplifies cecal intubation in patients with redundant colons and previous incomplete colonoscopies. Gastrointest Endosc 2012;76(4):812–7. [DOI] [PubMed] [Google Scholar]
- 5.Spadaccini M, Fuccio L, Lamonaca L, et al. Underwater EMR for colorectal lesions: A systematic review with meta-analysis (with video). Gastrointest Endosc 2019;89(6):1109–16.e4. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JC. Use of total underwater colonoscopy to navigate endoscopic challenges. Clin Gastroenterol Hepatol 2020;18(7):1427–30. [DOI] [PubMed] [Google Scholar]
- 7.Rex DK. Achieving cecal intubation in the difficult colon (with videos). Gastrointest Endosc 2022;95(3):568–72. [DOI] [PubMed] [Google Scholar]
- 8.Cadoni S, Ishaq S, Hassan C, et al. Water-assisted colonoscopy: An international modified Delphi review on definitions and practice recommendations. Gastrointest Endosc 2021;93(6):1411–20.e18. [DOI] [PubMed] [Google Scholar]
- 9.Cadoni S, Falt P, Rondonotti E, et al. Water exchange for screening colonoscopy increases adenoma detection rate: A multicenter, double-blinded, randomized controlled trial. Endoscopy 2017;49(5):456–67. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh YH, Tseng CW, Hu CT, et al. Prospective multicenter randomized controlled trial comparing adenoma detection rate in colonoscopy using water exchange, water immersion, and air insufflation. Gastrointest Endosc 2017;86(1):192–201. [DOI] [PubMed] [Google Scholar]
- 11.Cheng CL, Kuo YL, Hsieh YH, et al. Comparison of right colon adenoma miss rates between water exchange and carbon dioxide insufflation: A prospective randomized controlled trial. J Clin Gastroenterol 2021;55(10):869–75. [DOI] [PubMed] [Google Scholar]
- 12.May FP, Shaukat A. State of the science on quality indicators for colonoscopy and how to achieve them. Am J Gastroenerol 2020;115(8);1183–90. [DOI] [PubMed] [Google Scholar]
- 13.Keswani RN, Crockett SD, Calderwood AH. AGA clinical practice update on strategies to improve quality of screening and surveillance colonoscopy: Expert review. Gastroenterology 2021;161(2):701–11. [DOI] [PubMed] [Google Scholar]
- 14.Shaukat A, Tuskey A, Rao VL, et al. ; (ASGE Quality Assurance in Endoscopy Committee Chair). Interventions to improve adenoma detection rates in colonoscopy. Gastrointest Endosc 2022;96(2):171–83. [DOI] [PubMed] [Google Scholar]
- 15.Anderson JC, Kahi CJ, Sullivan A, et al. Comparing adenoma and polyp miss rates for total underwater colonoscopy versus standard CO2: A randomized controlled trial using a tandem colonoscopy approach. Gastrointest Endosc 2019;89(3):591–8. [DOI] [PubMed] [Google Scholar]
- 16.El Rahyel A, McWhinney CD, Parsa N, et al. Room temperature water infusion during colonoscopy insertion induces rectosigmoid colon mucus production. Endoscopy 2020;52(12):1118–21. [DOI] [PubMed] [Google Scholar]
- 17.Witte TN, Enns R. The difficult colonoscopy. Can J Gastroenterol 2007;21(8):487–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson RR. Accessing a difficult colon. Gastroenterol Hepatol 2021;17(2):79–81. [PMC free article] [PubMed] [Google Scholar]
- 19.Lai EJ, Calderwood AH, Doros G, et al. The Boston bowel preparation scale: A valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 2009;69(3 Pt 2):620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sang CN, Booher S, Gilron I, et al. Dextromethorphan and memantine in painful diabetic neuropathy and postherpetic neuralgia: Efficacy and dose-response trials. Anesthesiology 2002;96(5):1053–61. [DOI] [PubMed] [Google Scholar]
- 21.Anderson JC, Butterly LF, Weiss JE, et al. Providing data for serrated polyp detection rate benchmarks: An analysis of the New Hampshire Colonoscopy Registry. Gastrointest Endosc 2017;85(6):1188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ASGE Quality Assurance in Endoscopy Committee, Calderwood AH, Day LW, Muthusamy VR, et al. ASGE guideline for infection control during GI endoscopy. Gastrointest Endosc 2018;87(5):1167–79. [DOI] [PubMed] [Google Scholar]
- 23.Leung JW, Siao-Salera R, Abramyan O, et al. Impact of water exchange colonoscopy on serum sodium and potassium levels: An observational study. Dig Dis Sci 2014;59(3):653–7. [DOI] [PubMed] [Google Scholar]
- 24.Leung JW, Yen AW, Jia H, et al. A prospective RCT comparing combined chromoendoscopy with water exchange (CWE) vs water exchange (WE) vs air insufflation (AI) in adenoma detection in screening colonoscopy. United European Gastroenterol J 2019;7(4): 477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binmoeller KF, Shah JN, Bhat YM, et al. “Underwater” EMR of sporadic laterally spreading nonampullary duodenal adenomas (with video). Gastrointest Endosc 2013;78(3):496–502.e1. [DOI] [PubMed] [Google Scholar]
- 26.Issa MM. Technological advances in transurethral resection of the prostate: Bipolar versus monopolar TURP. J Endourol 2008;22(8):1587–96. [DOI] [PubMed] [Google Scholar]
- 27.Yamashina T, Uedo N, Akasaka T, et al. Comparison of underwater vs conventional endoscopic mucosal resection of intermediate-size colorectal polyps. Gastroenterology 2019;157(2):451–61.e2. [DOI] [PubMed] [Google Scholar]
- 28.Nagl S, Ebigbo A, Goelder SK, et al. Underwater vs conventional endoscopic mucosal resection of large sessile or flat colorectal polyps: A prospective randomized controlled trial. Gastroenterology 2021;161(5):1460–74.e1. [DOI] [PubMed] [Google Scholar]
- 29.Yamasaki Y, Uedo N, Akamatsu T, et al. Nonrecurrence rate of underwater EMR for ≤20-mm nonampullary duodenal adenoma: A multicenter prospective study (D-UEMR Study). Clin Gastroenterol Hepatol 2022;20(5):1010–8.e3. [DOI] [PubMed] [Google Scholar]
- 30.Nagata M. Usefulness of underwater endoscopic submucosal dissection in saline solution with a monopolar knife for colorectal tumors (with videos). Gastrointest Endosc 2018;87(5):1345–53. [DOI] [PubMed] [Google Scholar]
- 31.Kato M, Takatori Y, Sasaki M, et al. Water pressure method for duodenal endoscopic submucosal dissection (with video). Gastrointest Endosc 2021;93(4):942–9. [DOI] [PubMed] [Google Scholar]
- 32.Okimoto K, Maruoka D, Matsumura T, et al. Utility of underwater EMR for nonpolypoid superficial nonampullary duodenal epithelial tumors ≤20 mm. Gastrointest Endosc 2022;95(1):140–8. [DOI] [PubMed] [Google Scholar]
- 33.King M, Rubin BK. Pharmacological approaches to discovery and development of new mucolytic agents. Adv Drug Deliv Rev 2002;54(11):1475–90. [DOI] [PubMed] [Google Scholar]
- 34.Rockey DC. Endoscopy: Dollars and sense. Gastroenterology 1995;108(6):1957. [DOI] [PubMed] [Google Scholar]
- 35.Moon SY, Kim BC, Sohn DK, et al. Predictors for difficult cecal insertion in colonoscopy: The impact of obesity indices. World J Gastroenterol 2017;23(13):2346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Desai M, Rex DK, Bohm ME, et al. Impact of withdrawal time on adenoma detection rate: Results from a prospective multicenter trial. Gastrointest Endosc 2023;97(3):537–43.e2. [DOI] [PubMed] [Google Scholar]
- 37.Hassan C, Badalamenti M, Maselli R, et al. Computer-aided detection-assisted colonoscopy: Classification and relevance of false positives. Gastrointest Endosc 2020;92(4):900–4.e4. [DOI] [PubMed] [Google Scholar]
- 38.Spadaccini M, Hassan C, Alfarone L, et al. Comparing the number and relevance of false activations between 2 artificial intelligence computer-aided detection systems: The NOISE study. Gastrointest Endosc 2022;95(5):975–81.e1. [DOI] [PubMed] [Google Scholar]
- 39.Tang CP, Lin TL, Hsieh YH, et al. Polyp detection and false-positive rates by computer-aided analysis of withdrawal-phase videos of colonoscopy of the right-sided colon segment in a randomized controlled trial comparing water exchange and air insufflation. Gastrointest Endosc 2022;95(6):1198–206.e6. [DOI] [PubMed] [Google Scholar]