Abstract
The management of children with brachial plexus birth injuries is complex and requires a multidisciplinary approach. In the following article, we describe our approach to evaluation and management at Nicklaus Children's Hospital. It is our aim is to elucidate nuances in management.
Keywords: brachial plexus birth injury, microsurgery, nerve grafting, nerve transfers, Erb's palsy
The management of brachial plexus birth injuries is challenging. Controversy surrounds nearly every aspect of patient management, including the relative importance of various clinical exam findings, as well as the perceived benefit of imaging studies and neurophysiology studies. In many practices, decision-making regarding surgical intervention is based solely upon physical exam findings in an infant, who may not be cooperative with examination. Little consensus exists regarding optimal patient age for primary surgical intervention on the nerve injury. In the operating room, there continues to be controversy regarding optimal surgical management of the nerve injury.
History
The multidisciplinary Brachial Plexus Program at Nicklaus Children's Hospital (NCH) was initially established in 1996 by John Grossman (plastic and hand surgeon), Andrew Price (pediatric orthopaedic surgeon), Israel Alfonso (pediatric neurologist), and Lorna Ramos (pediatric occupational therapist). Dr. Berger (plastic and hand surgeon) joined the team in 2014 and Dr. Schreiber (pediatric orthopaedic surgeon) joined the team in 2020. Lorna Ramos passed away in 2010, but left an indelible legacy at the hospital. The current roster of pediatric occupational therapists directly engaged in the care of patients with brachial plexus birth injury at NCH includes Yvette Elias, CHT, OT, Cherise Medina, OT, and Nancy Quinn, OT.
The Program evaluates and provides comprehensive management to over 100 new cases of brachial plexus birth injury each year. This article summarizes our management strategy for brachial plexus birth injury.
Role of Therapy and Shoulder Orthoses
Video 1
Initial and subsequent clinical encounters at the Brachial Plexus Clinic involve evaluation by both the medical/surgical team as well as the pediatric occupational therapist. We strive to have the same clinicians evaluate the patient to assess for signs of improvement over time. For the first 6 months of life, patients are evaluated monthly by the team, and typically semiweekly with the therapist. A single clinical encounter with an infant is rarely sufficient to determine ultimate outcome or surgical plan. We aim to see affected infants as soon after birth as possible.
Early emphasis is placed on shoulder position. We agree with the findings of the group from Helsinki 1 suggesting that early splinting can prevent the need for later operative intervention. Similar findings have been suggested by the group from British Columbia Children's Hospital in Vancouver (the Sup-ER—supination external rotation splint), 2 as well as Texas Scottish Rite in Dallas. 3
In Fig. 1 , we demonstrate how infant patients are typically examined in our Brachial Plexus Clinic. We document each encounter with video. We begin the exam with the patient placed on the parent's lap, straddling one leg, and facing the examiner. In this position, the unaffected arm can easily be restrained by the parent. The patient is then presented with tasks to perform against gravity. Stickers are often employed to assist in determination of functional reach, as well as presence of clarion deformity. The patient is then assessed with gravity eliminated, laying flat on the exam table. Active and passive range of motion is recorded for the shoulder, elbow, forearm, wrist, and hand. Scores are recorded for Active Movement Scale, Toronto Score, and Mallet Score (if age appropriate).
Fig. 1.
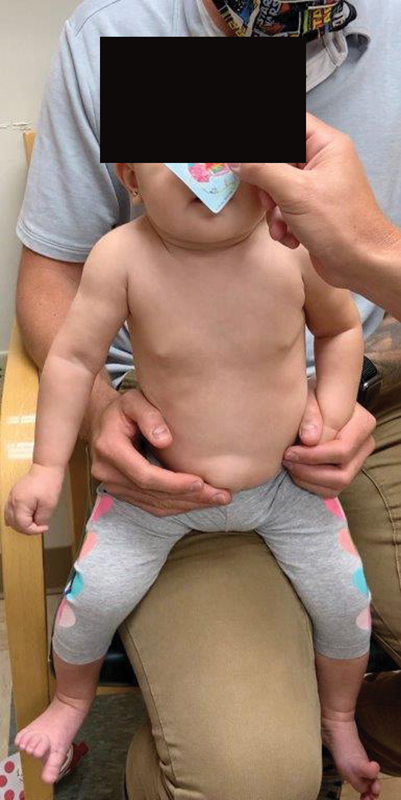
Demonstration of ideal patient position, straddling parent's leg, with unaffected arm restrained by parent. Sticker used to assist in assessment of function.
Early range of motion exercises are taught to the family, especially focused on shoulder external rotation in adduction. Care is first taken to ensure that the clavicle and humerus are stable, and if previously fractured, that they have healed.
If shoulder positioning does not demonstrate spontaneous improvement over the first couple months of life, a splint/harness is fabricated to maintain the shoulder in external rotation ( Fig. 2 ).
Fig. 2.

Examples of orthoses fabricated by our therapists to maintain shoulder in a reduced position. ( A ) Supination-external rotation (Sup-ER) splint and ( B ) spica orthosis that maintains the forearm in a neutral position. ( C ) Two separate examples fabricated for the same patient to be used in an alternating fashion, avoiding elbow flexion contracture and radial head subluxation.
In patients with the more commonly seen upper trunk (shoulder and elbow) and extended upper trunk (shoulder, elbow, and wrist) injuries, therapeutic interventions for the shoulder are started immediately. Strong focus is placed on passive placement of the shoulder into external rotation. If concern for shoulder subluxation/dislocation exists, in-office ultrasound ( Fig. 3 ) is performed to assess for position of the humeral head relative to the glenoid ( Video 1 ).
Fig. 3.
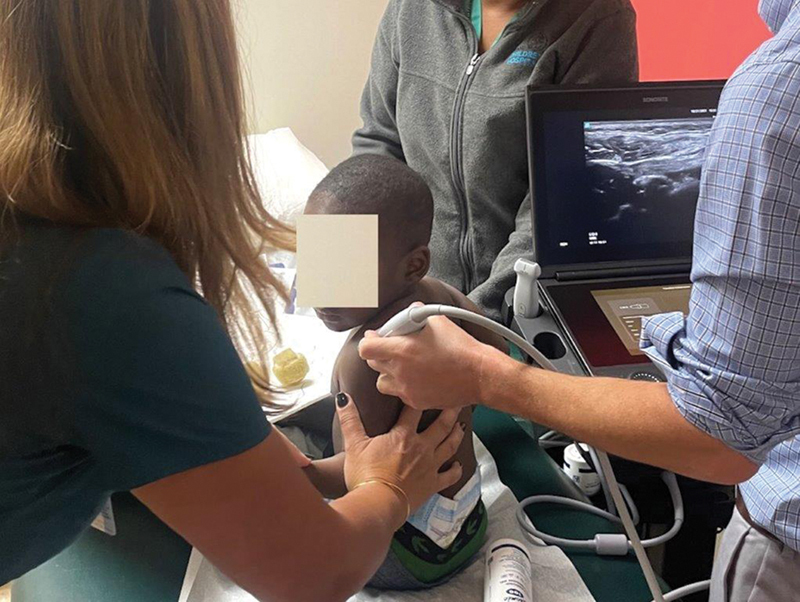
Setup for in-office ultrasound, performed mostly to assess humeral head position relative to glenoid. Video 1 demonstrates a subluxing/dislocating humeral head as it is moved from internal rotation to external rotation.
Surgical decision-making for these patients is based upon clinical examination (e.g., recovery of wrist extension by 4 months of age and biceps-mediated elbow flexion by 6 months of age). We have found that use of shoulder orthoses has allowed us to push the timing of surgery a bit later, and many patients who did not demonstrate biceps-mediated elbow flexion at 6 months may do so at 9 months. As long as the shoulder is protected, that is, able to place the glenohumeral joint into full passive external rotation (while adducted) without a Putti sign (elevation of the medial border of the scapula), we feel that one can wait and see. This is similar to the treatment strategy used in Helsinki. 1
Simultaneous Botulinum Toxin Administration and Magnetic Resonance Imaging
As indicated above, we aim to address the shoulder from initial presentation. The status of the shoulder is a primary concern in patients with upper brachial plexus injury, while the major concern with global injuries is restoration of some basic use of the hand. If the patient with upper brachial plexus injury is not demonstrating improvement in shoulder function, especially passive external rotation, we feel this is an indication for administration of Botulinum toxin (Botox) to adductors and internal rotators (i.e., latissimus dorsi, pectoralis major, teres major, and subscapularis muscles). Our protocol for Botox administration requires a brief course of general anesthesia, as we try to be as accurate as possible in administration, using a Teflon-coated needle attached to a Stimuplex nerve stimulator ( Fig. 4 ), and we also apply a shoulder spica cast in adduction and external rotation, as described by Ezaki et al. 3
Fig. 4.
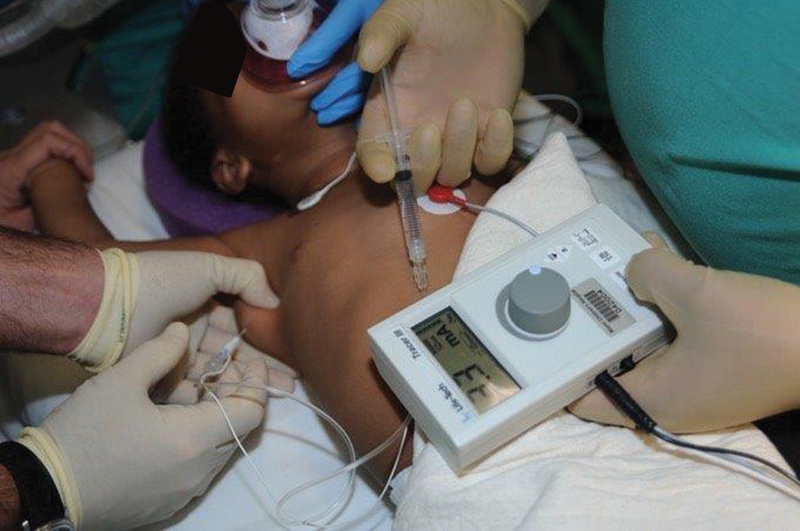
Nerve stimulator guidance for administration of Botox.
We coordinate Botox administration, cast application, and magnetic resonance imaging (MRI) evaluation of the brachial plexus and shoulders with our radiology and anesthesiology departments. This single round of anesthesia allows us to achieve three goals:
Treatment of an impending shoulder deformity with Botox and cast.
Assessment of shoulder anatomy (with a comparison to the opposite side).
Assessment of the nerve roots.
Our MRI protocol includes:
Positioning the shoulders in external rotation, ideally in a cast or orthosis.
Use of a neurovascular coil
Coronal three-dimensional (3D) T2, including both shoulders.
Axial T2 3D of spinal cord only.
Sagittal T2 including C2 to T2 of cervical spine only.
Coronal single-shot fast spin echo with angle parallel and midline with the spinal cord.
Coronal short tau inversion recovery (STIR) of the cervical spine.
Axial T2 including both shoulders.
Axial STIR including both shoulders.
Coronal 3D STIR (to view the nerves).
We aim to assess the overall shoulder joint anatomy with brief sequences devoted to analysis of bilateral shoulders. We find that the most useful sequences for evaluation of the nerves relates to evaluation of the nerve roots, especially CUBE, FIESTA, or DRIVE sequences. Our neuroradiology team specifically documents the presence/absence of pseudomeningoceles, as well as the quality of the anterior and posterior nerve rootlets for each nerve root comprising the brachial plexus ( Fig. 5 ).
Fig. 5.
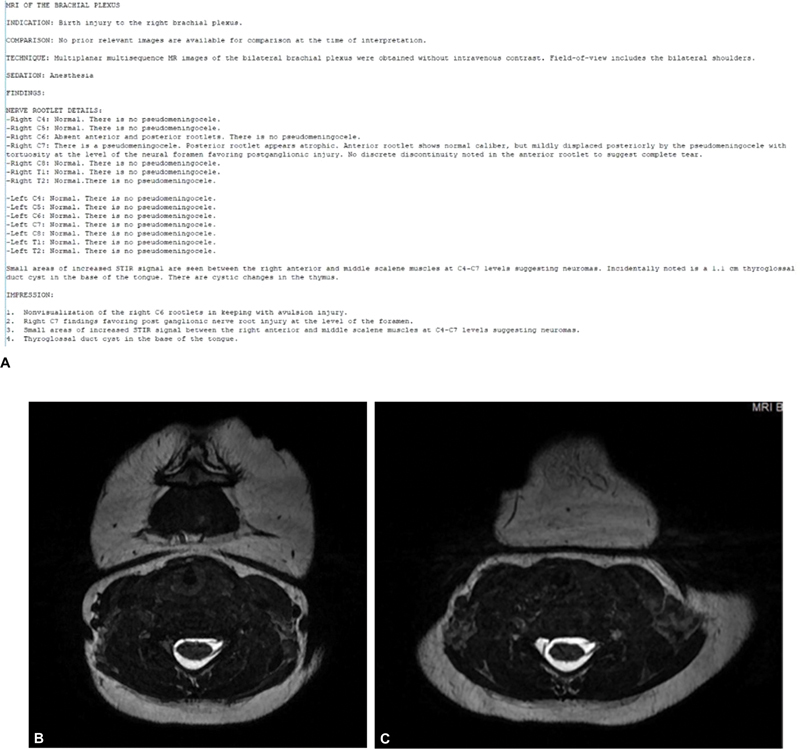
( A ) Typical brachial plexus magnetic resonance imaging (MRI) report detailing pseudomeningocele presence as well as quality of nerve rootlets for each nerve root. Corresponding findings on at C6 ( B ) and C7 ( C ) root levels.
We feel strongly that nerve root quality cannot be reliably assessed with only intraoperative assessment under the operating microscope. As stated in a recent publication by Gilbert and Raimondi, “wherever possible, an MRI can be of use, in order to avoid wrong decisions on the quality of a root,” as grafting to roots that have sustained an unrecognized in situ avulsion may lead to an unfavorable result. 4 In addition to MRI evaluation, intraoperative somatosensory-evoked potentials (SSEPs) can assist in determining the quality of roots at the time of nerve reconstruction.
For Botox, we typically use 10 to 15 units/kg, diluting a 100-unit vial in 4 mL of saline. Each muscle receives 25 to 50 units, depending upon the patient's weight and the apparent degree of involvement of each muscle in the deformity.
Indications for Primary Plexus Surgery
In patients with global injury to the brachial plexus, surgery is indicated earlier, but we see little benefit in operating before 3 months of age. We base our decisions regarding surgery on prior work performed by Gilbert and Narakas, as well as work from our group assessing the natural history of the condition. 5 Generally, in patients with global injuries, if there is no recovery of hand function by 3 months of age or wrist extension by 4 months of age, then surgery is indicated to perform reconstruction of the nerve injury. The goal of surgery is to improve function beyond that which would occur through spontaneous nerve recovery/healing. The impossibility of knowing the end result of conservative management is shared with the patient's parents, as well as the grim reality that patients with global injuries rarely have recovery of normal function in the affected extremity, despite our best efforts.
As noted above, surgical decision-making for our patients is based largely upon clinical examination. In patients with global injuries, the primary goal is restoration of some basic hand function. In patients with good hand function, and predominantly upper trunk or upper and middle trunk involvement, we hope to see recovery of wrist extension by 4 months of age and biceps-mediated elbow flexion by 6 months of age. If these surrogate measures of outcome—wrist extension by 4 months and biceps-mediated elbow flexion by 6 months—are not seen, the discussion regarding nerve surgery is begun with the parents, and the patient is scheduled for high-resolution MRI evaluation of the spinal nerves and shoulders, as well as simultaneous management of shoulder contractures with Botox + external rotation cast placement. The data gleaned from the MRI, along with intraoperative neurophysiology (namely SSEPs), assists the surgical team in intraoperative decision-making regarding root quality. Preoperative workup also includes chest X-ray and ultrasound to assess diaphragm function. The patients are reassessed after 4 weeks of cast immobilization, and the decision for surgical intervention is again discussed with the parents depending on clinical findings over the ensuing weeks.
Surgical Technique
After general endotracheal intubation, and placement of a Foley catheter, the patient is positioned supine with a soft gel roll placed along the midline of the back. The shoulder is assessed first. If we are unable to obtain full passive external rotation in adduction, consideration is given to simultaneous management of the shoulder contracture by performing a subscapularis slide. The details of our technique are described in Immerman et al 6 and detailed later in this article. We have found that this procedure is rarely indicated in younger patients (i.e., < 9 months of age), as they tend to have passively correctable shoulder position. In cases of irreducible shoulder subluxation/dislocation, we will not hesitate to perform this procedure in the same setting as the primary nerve operation. We believe it is imperative to ensure shoulder reduction at the time of nerve surgery.
Open communication is held with the anesthesia team and the intraoperative neurophysiology team throughout the course of the case. The use of paralytics and inhaled anesthesia is held to a minimum to avoid interference with intraoperative nerve stimulation.
The head is turned away from the affected side. The affected arm, chest, and neck are prepared. Bilateral lower extremities are also prepared for sural nerve graft harvest. The incision is marked along the posterior border of the sternocleidomastoid. We have found that this longitudinal incision provides maximal safe exposure, and it also allows for extension to a traditional L- or Z-shaped incision if broader exposure is required.
Electrodes are placed in the scalp and face by the neurophysiology team and remaining needle electrodes are placed in the affected extremity after the sterile field has been established.
Dilute epinephrine (1:500,000) is infiltrated into the subcutaneous tissues of the planned incisions. Needle electrodes are placed in muscles of interest in the affected extremity. After allowing the epinephrine to take effect, the neck incision is carried through the skin and subcutaneous tissues. The platysma is divided. Supraclavicular sensory nerves are identified and preserved. They are followed back to their origin and distally into the skin of the upper chest and neck. They may be used as graft material. The fibrofatty layer is elevated as a flap. The omohyoid is identified and retracted; it may be ligated across its tendinous portion if needed. The transverse cervical vessels are ligated. The phrenic nerve is identified medially, lying on top of the anterior scalene muscle and it is traced proximally. It is protected throughout the course of the case. It may be stimulated to confirm its identity and function. Working laterally, the upper trunk is freed from surrounding scar along its superior surface. A firm neuroma is often encountered within the upper trunk. Working medially and laterally, the epineurium is cleared, identifying structures medially and laterally. With decompression of the neuroma we often see expansion of the nerve with improvements in neurophysiologic and subsequent motor recovery. The anterior scalene is transected with a bipolar electrocautery, taking care to protect the phrenic nerve above. This allows for better identification of the underlying nerve roots, which are identified sequentially, starting at C5. The extent of injury determines the extent of root exposure. If necessary, clavicle osteotomy may be performed, though our current practice is to avoid clavicle osteotomy, exposing the brachial plexus above and below the intact clavicle, with a lateral window created by partial release of the sternal head of the pectoralis major and tenotomy of the pectoralis minor. A cuff of muscle/tendon is left behind to allow for repair of these structures at the conclusion of the procedure ( Fig. 6 ). Once the nerves of interest are exposed, the operating microscope is brought in; we find that it is especially useful at the time of neurotomies and coaptations. We routinely use frozen section pathology to assess the quality of donor/recipient nerves.
Fig. 6.
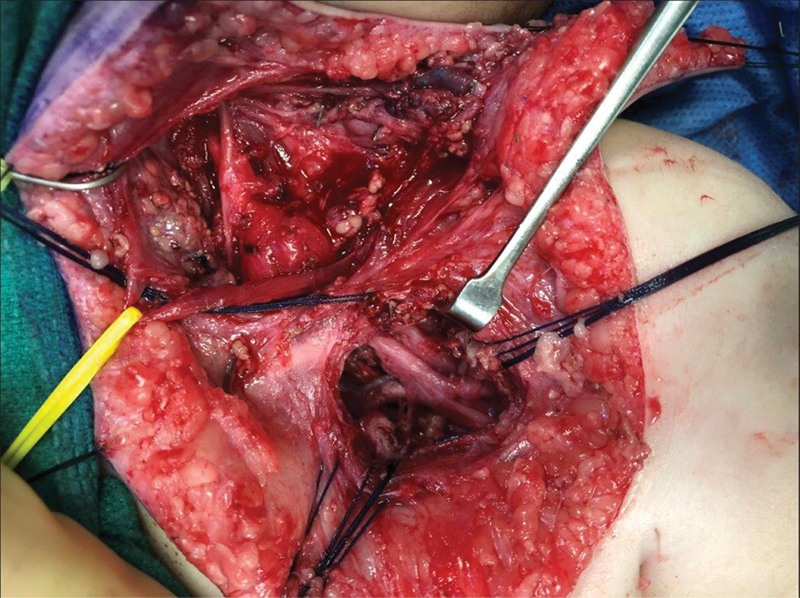
Supra- and infraclavicular exposure of the brachial plexus by partial release of the sternal head of the pectoralis major and tenotomy of the pectoralis minor; both muscles are tagged with braided suture for later repair.
Intraoperative nerve monitoring/stimulation is performed in conjunction with our neurophysiology team, and supplemented with the use of a single-use handheld nerve stimulator (Checkpoint Guardian). We find that assessing the results of stimulation above and below neuroma-in-continuity provides assistance in decision-making regarding management of conducting neuroma-in-continuity. We also find that, combined with high-resolution MRI, we are able to achieve better assessment of questionable nerve roots (i.e., in situ avulsions) with SSEPs. As noted above, we are especially interested in the quality of the nerve rootlets, proximal to the vertebral foramen.
With respect to management of neuroma-in-continuity, in addition to utilization of all available information (i.e., detailed examination of preoperative function, preoperative MRI especially focused on rootlet continuity, intraoperative appearance of the nerves, intraoperative stimulation above and below the neuroma, and assessment of SSEPs), our group has previously described bypass grafting techniques to address management in patients that may present later. 7 8 The technique, which includes partial neurotomies outside the zone of injury, has proven especially useful for augmentation of function to the suprascapular nerve and upper trunk divisions. In long-term follow-up, no patients have experienced a downgrade in function, and clear improvement in motor function was demonstrated in follow-up. While some improvement in function may have been secondary to neurolysis and anticipated recovery, it is possible a component of recovery was related to axonal sprouting/regeneration through the nerve graft.
Nerve graft is typically harvested from the sural nerves, and a sterile tourniquet is applied on the thigh. Harvest is performed through longitudinal incisions directly over the course of the sural nerves, along with the use of a lighted retractor to increase visualization. Although skip incisions have been described for sural nerve harvest, we prefer our approach to avoid traumatic stretch on the grafts, and direct visualization allows for harvest of all branches of this purely sensory nerve. As noted above, the supraclavicular sensory nerves may also provide nerve graft material, though more limited in diameter and length. Rarely, we may also harvest the medial cutaneous nerve of the arm if sural and supraclavicular graft material is insufficient.
Nerve transfers may be intraplexal or extraplexal. Intraplexal transfers include heterotopic reconstruction of the brachial plexus, often required if root avulsion injuries are encountered. With respect to extraplexal transfers, the spinal accessory to suprascapular nerve transfer is the most commonly employed transfer in our practice. We often employ this transfer if one or both of the upper trunk roots have been avulsed. In some cases, especially late-presenting cases, we are able to perform this transfer in an end-to-side fashion. Less commonly employed nerve transfer options, in our practice, include Oberlin/Mackinnon type transfers (uni- or double- fascicular transfers of the ulnar and median nerves to the musculocutaneous nerve), intercostal nerve transfers to the musculocutaneous nerve, and contralateral C7 transfers to the lower trunk. The Oberlin/Mackinnon type transfer requires intact signal to the lower trunk and is typically not an option in global injuries. In our practice, it is rare to see a patient that has not recovered some degree of active elbow flexion.
Nerve coaptation is typically performed with either fibrin glue alone or in conjunction with 9–0 or 10–0 Nylon suture into the epineurium. We try to minimize the use of suture material. After all coaptations have been completed, the shoulder is ranged, typically demonstrating no stress on any of the repair sites. The one movement that has been shown to have a possible negative affect (i.e., tension across a coaptation site), especially on the spinal accessory to suprascapular nerve transfer, is the combined movement of abduction and internal rotation. We avoid this maneuver in the immediate postoperative phase, as well as during the first month after surgery.
If the patient had not received Botox within the preceding 3-month interval, we administer Botox to the muscles of adduction and internal rotation at the conclusion of the surgical procedure. This prevents an uncovering/worsening of the internal rotation forces sometimes seen after neurolysis of the lower trunk. It also protects the shoulder during a time of inevitable loss of external rotation/abduction function if upper trunk neuroma has been excised and grafted.
Postoperatively, the neck is immobilized in a soft cervical collar for 2 weeks and the shoulder is immobilized in a modified shoulder spica cast for 3 to 4 weeks, with the shoulder held in maximal adduction and external rotation ( Fig. 7 ). After removal of the cast, the patient begins a specialized therapy regimen, including the use of an external rotation splint. The patient is followed by the multidisciplinary team monthly for 3 to 6 months, and then every 3 to 6 months. Specialized therapy continues, typically twice a week. We encourage parents to start swimming lessons at/around 1 year of age.
Fig. 7.
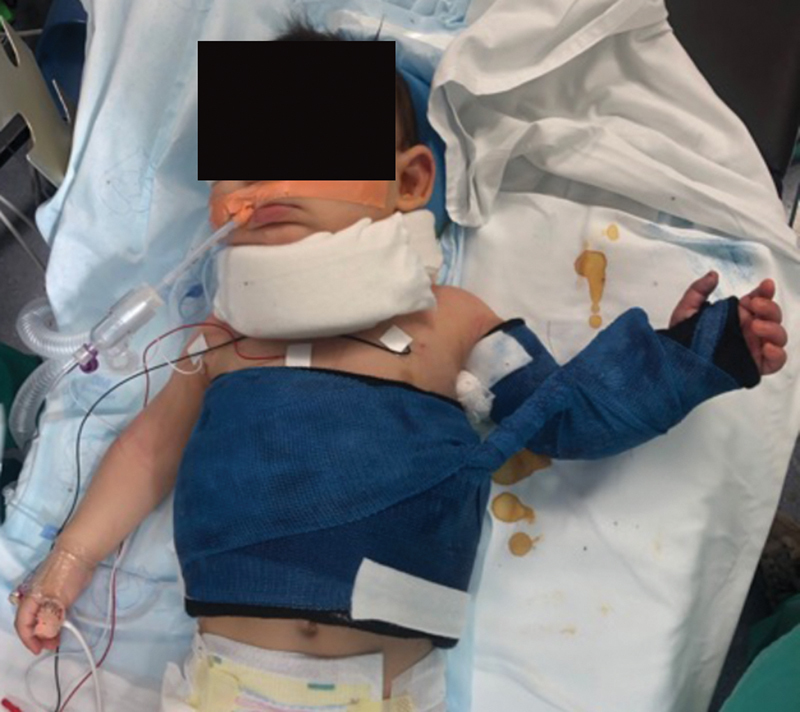
Postoperative immobilization after primary nerve repair, including soft cervical collar and shoulder spica cast in external rotation.
Surgical Management of Shoulder Internal Rotation Contracture
Early management of shoulder internal rotation contractures is initially focused on the use of orthoses and administration of Botox, as noted above. We have started institution of a serial casting protocol that shows promise in addressing the hopefully temporary imbalance of forces across the shoulder. This protocol involves placement of a shoulder spica cast at the time of Botox administration. The shoulder is placed in adduction and maximal external rotation. If less than 70 to 90 degrees of external rotation are achieved, after 1 to 2 weeks, the bar on the cast is cut and we are almost always able to achieve full passive external rotation ( Fig. 8 ).
Fig. 8.
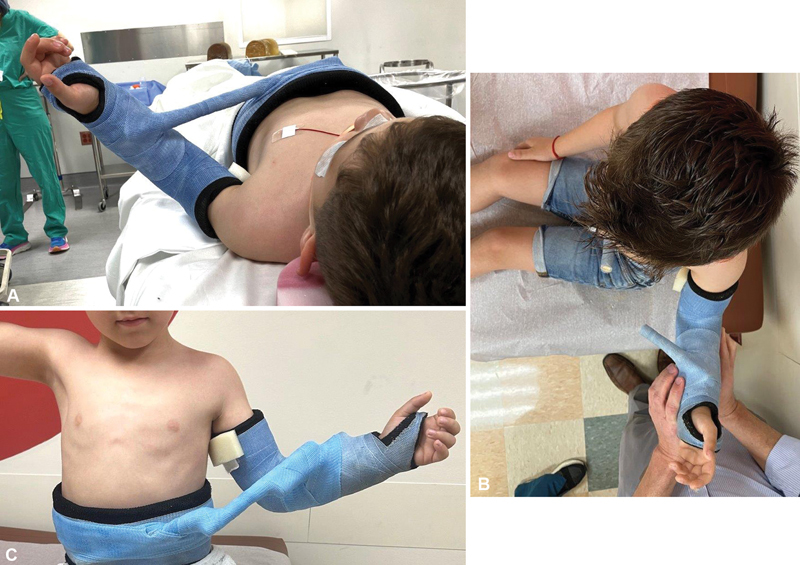
Serial external rotation cast placement after use of Botox. ( A ) At the time of Botox administration, less than 45 degrees of passive isolated external rotation was achieved. ( B ) At 2 weeks after Botox administration, the bar on the cast was cut and full passive external rotation is achieved. ( C ) A new bar is applied to the cast and the cast is maintained for another 4 weeks.
In cases of fixed internal rotation contracture with posterior subluxation/dislocation of the humeral head, in which we are unable to achieve any significant gains in passive range of motion, and in which serial external rotation cast placement fails, an open shoulder procedure is indicated. We typically address these fixed internal rotation contractures with a subscapularis lengthening/slide procedure. The procedure, described in Immerman et al, 6 includes an incision along the posterior axillary line, followed by identification of the lateral border of the latissimus dorsi muscle. The neurovascular pedicle to the latissimus is protected. Deep to this, the lateral edge of the scapula is identified, the arm is hyperabducted, and the tip of the scapula is pulled out of the wound with the aid of a towel clamp. The white raphe of the subscapularis is identified ( Fig. 9A ). It is cut with the cautery to allow a subperiosteal dissection. Using a periosteal elevator, the subscapularis muscle is dissected subperiosteally off the entire anterior surface of the scapula ( Fig. 9B ), as far laterally as the joint capsule. The anterior shoulder structures, including the subscapularis tendon and the joint capsule, are preserved. The passive motion of the shoulder is then tested, and if full passive external rotation is achieved, the procedure is concluded. If residual tightness is present, further release is sequentially performed, including periosteal fasciotomy of the subscapularis. The pectoralis major is assessed, and if tight, it is fractionally lengthened at the musculotendinous junction. If full reduction of the humeral head in the glenoid cannot be achieved due to an overgrown corocoid, the coracohumeral ligament is released with a partial coracoidectomy performed to achieve full reduction into the true glenoid. Anterior release of the joint capsule and, thus, disruption of the subscapularis/capsular relationship is never performed. Botox is always administered to the subscapularis and pectoralis major muscles, and if not transferred, the latissimus dorsi and teres major muscles. The wound is closed in layers followed by administration of local anesthetic. The shoulder is immobilized in a shoulder spica cast in at least 60 degrees of external rotation for approximately 4 weeks.
Fig. 9.
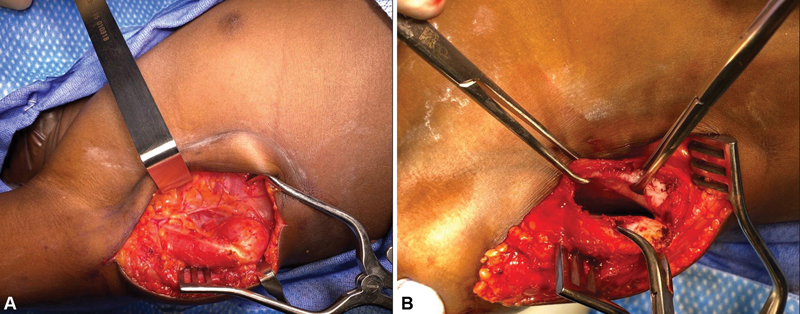
Details of subscapularis slide. ( A ) Exposure of the lateral scapula. ( B ) Elevation of the subscapularis off of the anterior face of the scapula.
In our experience, approximately 25% of children undergoing a primary nerve procedure benefit from an additional shoulder procedure that includes a contracture release, muscle transfer, or both to improve both active elevation and external rotation.
Latissimus dorsi/teres major tendon transfer can be performed through the same incision along the posterior axillary line but it is extended cephalad and caudally. The latissimus dorsi muscle/tendon is separated from the teres major and released off of its insertion on the humerus. Care is taken to identify and protect the neurovascular pedicle to the latissimus dorsi. The latissimus dorsi tendon alone (or together with the teres major) is brought over the long head of the triceps, woven through the infraspinatus tendon at its insertion on the humerus, and then sewn onto itself using nonabsorbable suture. Care must be taken to avoid loss of internal rotation, as loss of midline function is a known complication of this procedure. 9 The incision is closed in layers. Postoperatively, the patient is placed into a shoulder spica cast with the shoulder positioned in 60 degrees of external rotation and 30 degrees of abduction for 6 weeks. Occupational therapy is started after cast removal.
Occasionally, an external rotational osteotomy of the humerus or a glenoid osteotomy is required. We typically perform humeral rotational osteotomy through a lateral approach; the osteotomy is performed above the deltoid insertion. In patients who have previously undergone tendon transfer for external rotation, loss of internal rotation is possible, and for these patients, internal rotation osteotomy is performed below the deltoid insertion.
Management of Elbow Flexion Contractures
The mechanism of elbow flexion contracture development in patients with brachial plexus birth injuries is poorly understood. It is presumed that the disorganized denervation/reinnervation of the biceps/brachialis muscles leads to stunted growth of the muscle. For mild contractures (< 30 degrees), we address this problem with Botox and serial casting followed by serial splinting. More severe contractures can be addressed with Z-lengthening of the biceps and fractional lengthening of the brachialis. Occasionally, dislocation of the radial head occurs and this must be addressed early with lengthening and/or transfer of the biceps to the brachialis.
Management of Forearm Contractures
Limitations and deformities of forearm rotation are common in this population of patients. In more severe cases, supination contracture of the forearm leads to a functional and cosmetic impairment. In cases that are passively correctable, correction can be achieved with a rerouting of the biceps tendon, adding in release of the interosseous membrane (if necessary). In an earlier report our group presented our unique successful experience in the treatment of pronation deformities of the forearm. 10 In recurrent cases, or cases of fixed deformity, rotational osteotomies of the forearm can be performed.
Management of Persistent Hand and Wrist Deficits
Persistent hand and wrist deficits are managed on a case-by-case basis. Tendon transfers can be considered, depending upon available donor muscles. Arthrodesis, especially of the wrist, can be considered in older patients, and functioning free muscle transfers, as described by Doi et al, 11 can be considered.
Footnotes
Conflict of Interest None declared.
References
- 1.Grahn P, Sommarhem A, Nietosvaara Y. A protocol-based treatment plan to improve shoulder function in children with brachial plexus birth injury: a comparative study. J Hand Surg Eur Vol. 2022;47(03):248–256. doi: 10.1177/17531934211056998. [DOI] [PubMed] [Google Scholar]
- 2.Yefet L S, Bellows D, Bucevska M. Shoulder rotation function following the Sup-ER protocol in children with brachial plexus injuries. Hand (N Y) 2022;17(03):549–557. doi: 10.1177/1558944720937365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ezaki M, Malungpaishrope K, Harrison R J. Onabotulinum toxin A injection as an adjunct in the treatment of posterior shoulder subluxation in neonatal brachial plexus palsy. J Bone Joint Surg Am. 2010;92(12):2171–2177. doi: 10.2106/JBJS.I.00499. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert A, Raimondi P. Indications of plexus reconstruction in obstetrical paralysis. J Peripheral Nerve Surg. 2019;2(01):2–9. [Google Scholar]
- 5.DiTaranto P, Campagna L, Price A E, Grossman J AI. Outcome following nonoperative treatment of brachial plexus birth injuries. J Child Neurol. 2004;19(02):87–90. doi: 10.1177/08830738040190020101. [DOI] [PubMed] [Google Scholar]
- 6.Immerman I, Valencia H, DiTaranto P. Subscapularis slide correction of the shoulder internal rotation contracture after brachial plexus birth injury: technique and outcomes. Tech Hand Up Extrem Surg. 2013;17(01):52–56. doi: 10.1097/BTH.0b013e31827b4a23. [DOI] [PubMed] [Google Scholar]
- 7.Grossman J AI, DiTaranto P, Yaylali I, Alfonso I, Ramos L E, Price A E. Shoulder function following late neurolysis and bypass grafting for upper brachial plexus birth injuries. J Hand Surg [Br] 2004;29(04):356–358. doi: 10.1016/j.jhsb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Grossman J AI, Price A E, Tidwell M A, Ramos L E, Alfonso I, Yaylali I. Outcome after later combined brachial plexus and shoulder surgery after birth trauma. J Bone Joint Surg Br. 2003;85(08):1166–1168. doi: 10.1302/0301-620x.85b8.14246. [DOI] [PubMed] [Google Scholar]
- 9.Greenhill D A, Trionfo A, Ramsey F V, Kozin S H, Zlotolow D A. Postoperative loss of midline function in brachial plexus birth palsy. J Hand Surg Am. 2018;43(06):5650–5.65E12. doi: 10.1016/j.jhsa.2017.10.041. [DOI] [PubMed] [Google Scholar]
- 10.Liggio F J, Tham S, Price A, Ramos L E, Mulloy E, Grossman J A. Outcome of surgical treatment for forearm pronation deformities in children with obstetric brachial plexus injuries. J Hand Surg [Br] 1999;24(01):43–45. doi: 10.1016/s0266-7681(99)90023-2. [DOI] [PubMed] [Google Scholar]
- 11.Doi K, Hattori Y, Sakamoto S, Dodakundi C, Satbhai N G, Montales T. Current procedure of double free muscle transfer for traumatic total brachial plexus palsy. JBJS Essential Surg Tech. 2013;3(03):e16. doi: 10.2106/JBJS.ST.M.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]


