Abstract
The care of children with brachial plexus birth injuries (BPBI) is a complex multidisciplinary endeavor. At the Shriners Hospital for Children in Philadelphia, we have sought to elevate the quality of care delivered to patients through outcomes research and collaboration with colleagues around the world. Our approach to the management of this challenging pathology has evolved time and again. Here, we describe our current approach to patient assessment and operative management in patients with BPBI and its many sequelae.
Keywords: brachial plexus birth palsy, nerve grafting, nerve transfers, Erb's palsy, contralateral C7 transfer
The Shriners Hospital for Children in Philadelphia was first established in 1926. Since 2000, our group has been devoted to advancing care for pediatric upper extremity pathology, including brachial plexus birth injury (BPBI). In total we have cared for more than 4,000 children with BPBI who come to us from all over the world. Our management of BPBI has evolved over time with experience and continues to change as we learn from our colleagues and our outcomes.
Assessment
History
During the initial evaluation, a thorough history of the pregnancy, delivery, and perinatal period is collected. Maternal health issues, such as gestational diabetes, can increase fetal size (increased abdominal circumference) and weight. 1 The use of oxytocin to induce and augment labor can cause uterine tachysystole and intensify contractions. 2 Together, these forces can disturb the cardinal movements of labor, increasing the likelihood of shoulder dystocia. Because parents are often unfamiliar with the term “shoulder dystocia,” we simply ask if their child was “stuck” during the delivery. We also ask about the application of a vacuum or forceps, as an instrumented delivery also suggests a shoulder dystocia.
The initial status of the infant is important information. We inquire about APGAR scores, which are often unknown. We ask about the lack of respiratory effort, ventilatory resuscitation, oxygen supplementation, and/or neonatal intensive care unit (NICU) admission. Ventilatory support, poor feeding, and difficulty latching during breast feeding should raise concern for phrenic nerve injury and diaphragm paralysis. Infants with hemidiaphragmatic palsy cannot breathe and suck at the same time.
The immediate status of the limb and any improvement since birth are also important. Parents are more reliable in recalling obvious movements such as shoulder elevation and grasp, but are less consistent with finger extension, wrist extension, and elbow extension. Parents can sometimes be deceived regarding the presence of elbow flexion, as the supine infant will extend their elbow against gravity and then relax their triceps, allowing their elbow to bend. We ask about the presence of facial asymmetry, such as an eyelid droop, that may signify a Horner's syndrome.
Parents often seek multiple opinions concerning their child's injury and care may have been provided earlier in life, often delaying definitive management. A detailed summary of their previous treatment including therapy, surgery, and diagnostics testing should be pursued.
Physical Examination
Examination of the infant is performed in a calm environment, with the parents close by and visible to the child. All clothing above the waist should be removed. The infant is positioned laying supine to obtain gravity-eliminated motion, and then sitting on a parent's lap to obtain antigravity motion. Ideally, the examiner should be down at the level of the child, and not standing over them. Infants cannot follow commands; motor activity is assessed by encouraging spontaneous movements via tactile stimulation of the limb. The Active Movement Scale (AMS) is an invaluable tool in infants and can be repeated allowing longitudinal assessment of any changes in motor function. Fifteen movement patterns are assessed. The scores range from 0 to 7 with lower scores grading gravity-eliminated movement and the higher scores grading motion against gravity ( Fig. 1 ).
Fig. 1.
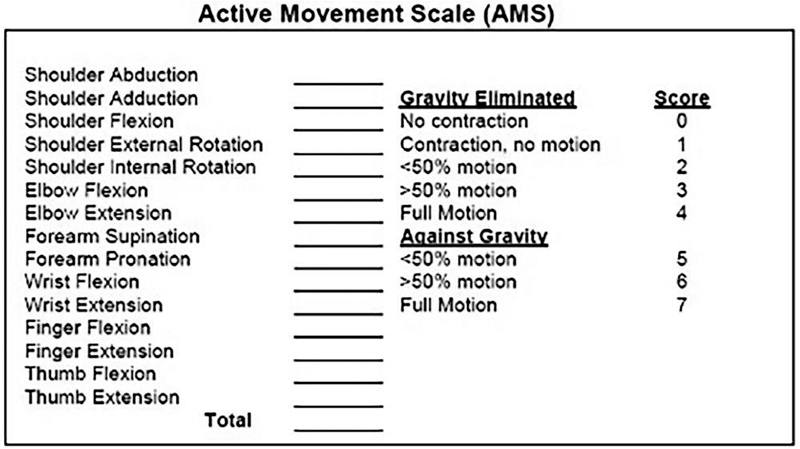
The Active Movement Scale (AMS) is our preferred tool for the assessment of motor function in patients with brachial plexus birth palsy.
An assortment of age-appropriate toys is instrumental when examining infants and young children. In preschool-aged children, stickers can also be useful in provoking movements. For example, forearm supination can be elicited by placing a sticker on the child's palm with the forearm in pronation. The inquisitive child will supinate their forearm to visualize the sticker. This process can be reversed to assess for active forearm pronation.
Children older than 3 years can more reliably follow commands, allowing the use of the Mallet scoring system. The initial Mallet classification assessed global shoulder function which scored global shoulder abduction, global shoulder external rotation, hand-to-neck activity, hand-to-spine movement, and hand-to-mouth capability. We previously published a modification to the Mallet, which additionally measured hand-to-navel ability ( Fig. 2 ). 3 We noted that hand-to-spine motion required shoulder extension in addition to internal rotation, and is therefore a poor gauge of internal rotation. Many children with BPBI lack shoulder extension and are therefore unable to reach their spine despite full internal rotation. Passive shoulder external rotation should be checked with the scapula stabilized to isolate glenohumeral joint motion. Passive elbow extension should also be measured, as children with BPBI are prone to develop elbow flexion contractures.
Fig. 2.

With the addition of a sixth category assessing internal rotation, the modified Mallet scale provides a reliable and repeatable evaluation of shoulder function in patients with brachial plexus birth palsy.
Patient-reported outcomes are measured using the pediatric upper extremity PROMIS in all patients older than 5 years. Recently, we have validated the use of motion analysis in the BPBI population. This technology provides an objective assessment of a patient's ability to reach in space (a.k.a. reachable workspace) and can differentiate glenohumeral and scapulothoracic joint motion. 4 5 Motion analysis yields a more accurate and nuanced assessment of shoulder function than can be obtained from a Mallet score. 5
Infants are followed up on a monthly basis to track their progress and to determine the necessity of microsurgical intervention. We work closely with a team of dedicated pediatric occupational therapists who are experienced in examining and treating infants and adolescents. The therapy team is an integral part of the brachial plexus program, providing measurements (strength, sensibility, and range of motion) as well as their opinion regarding intervention.
Imaging
We do not routinely perform imaging of the brachial plexus prior to surgery except in cases of global injury. While the presence of pseudomeningoceles on magnetic resonance imaging (MRI) can suggest nerve root avulsion, we consider intraoperative evaluation to be the gold standard. Many of our patient referrals come from a distance and even abroad, which makes routine imaging difficult. In global cases, the MRI can assist in planning for a potential contralateral C7 (CC7) nerve root transfer if there are three or more root avulsions seen on MRI. We rely primarily on our clinical judgment and avoid delaying surgery to obtain an MRI if it cannot be obtained in a timely manner. We do believe that as MRI speed and resolution improves, this modality will become a more important diagnostic tool that will aid in reconstructive planning.
We routinely evaluate the diaphragm using ultrasound or fluoroscopy to check for phrenic nerve palsy in all patients. In the presence of a phrenic nerve palsy, we avoid using intercostal nerve transfers as part of our reconstructive strategy.
X-rays have a very limited role in BPBI. In patients who develop an internal rotation contracture of the shoulder, X-rays do not visualize cartilage and are unreliable in the assessment of shoulder subluxation in infants. We perform a screening ultrasound of the shoulder in the clinic to evaluate for posterior subluxation of the humeral head. Ultrasound visualizes the cartilaginous humeral head relative to the glenoid. Ultrasound is also a dynamic study that allows an assessment of the humeral head position in internal and external rotation. Ultrasound is also a dynamic study that allows an assessment of the humeral head position in internal and external rotation. A humeral head that realigns within the glenoid during external rotation is deemed “reducible.” A humeral head that remains posterior to the glenoid in internal and external rotation is considered “irreducible.” In our experience, ultrasound fails to reliably assess the glenoid configuration. 6
Electrodiagnostic Studies
Routine electrodiagnostic studies in infants with brachial plexus injuries are unnecessary. In fact, studies have shown electrodiagnostic studies to be overly optimistic concerning recovery and may delay treatment. 7 Infants cannot comply with the examination and require sedation, further hindering its applicability. Early in our experience, we attempted somatosensory evoked potentials (SSEPs) during surgery as an adjunct tool for intraoperative decision making. The recordings were inconsistent and added uncertainty to our reconstructive strategy. While we are not utilizing SSEPs at the current time, better techniques and routine use at other centers to assess the nerve roots via these recordings have prompted us to reexplore this modality. A thorough preoperative evaluation and careful assessment of the plexus at the time of surgery remains our most reliable method for reconstructive planning. We do use a nerve stimulator (Checkpoint Surgical, Independence, OH) to facilitate nerve identification and to assist in the formulation of a nerve reconstruction plan.
Early Management of Shoulder Contractures
Patients with isolated upper trunk injuries (C5 and C6 nerve roots) or those with upper/middle trunk (C5, C6, and C7 nerve roots) injuries have a tendency to develop internal rotation contractures of the shoulder. The etiology is multifactorial but primarily related to a more robust recovery of internal rotation versus external rotation. Early shoulder stretching in infants with a BPBI is crucial to prevent an internal rotation contracture. The detection of a developing internal rotation contracture can avoid surgical intervention. Focused stretching and nighttime bracing with a teapot brace ( Fig. 3 ) can prevent the internal rotation contracture if used diligently. If the contracture is not passively correctible, an ultrasound is performed to assess the glenohumeral joint alignment. A reducible humeral head can be managed by botulinum toxin injections to the internal rotators of the shoulder (subscapularis, latissimus dorsi, teres major, pectoralis major), and application of a shoulder spica cast for 4 weeks. Following cast removal, a regimen of stretching and splinting with a teapot brace is implemented. If the shoulder joint is irreducible, we perform an open reduction of the shoulder with coracoidectomy, subscapularis lengthening, and anterior capsulotomy. We no longer perform a joint release from the posterior axillary approach, as this does not address the tightness of the subscapularis tendon or the bony impingement from the coracoid. Arthroscopic releases are limited to the few children with internal rotation contractures and a near-normal glenohumeral joint with no coracoid deformity seen on MRI. Shoulder reduction can be performed at the time of nerve surgery.
Fig. 3.

A teapot splint is used to hold the shoulder in external rotation and adduction. This posture provides the most stable reduction of the glenohumeral joint. (Courtesy of Shriners Hospital for Children – Philadelphia.)
Surgical Indications
C5 and C6 Nerve Roots (Upper Trunk Injury or Erb's Palsy)
Infants with a C5 and C6 nerve root injury present with absent shoulder abduction, shoulder external rotation, elbow flexion, and forearm supination. If wrist extension is weak or absent, the injury includes the C7 nerve root. The presence of triceps function and finger extension does not exclude a C7 injury, as C8 can power these movements. A simple stretch injury that results primarily in demyelination and minimal axotomy will improve by 6 to 8 weeks of life, and full recovery of function can be expected. Failure to recover by 3 months of age signifies that less than 20% of the axons of the injured nerve remains intact. 8 If the baby regains considerable antigravity elbow flexion by 3 months of age, there is good evidence that he or she will recover normal or near-normal shoulder and elbow function. 9 If an infant with an upper trunk injury fails to recover antigravity elbow flexion to at least 90 degrees by 3 months of age, we proactively schedule the patient for nerve surgery around 6 months of age. Age at surgery tends to range from 4 to 9 months of age based on operating room/surgeon's availability and patient's travel. A preoperative evaluation is mandatory close to the date of surgery and on the morning of surgery to assess any changes that may alter the surgical plan. If the preoperative evaluation finds robust gains in elbow and shoulder function, surgery is cancelled.
Due to the fact that we receive referrals from far away, infants can present after the optimum time window. In those older infants (9–15 months of age), we will plan distal nerve transfers to decrease the time to reinnervation. In adults, nerve transfers for upper trunk lesions outperform nerve grafting. 10 In infants, the results of nerve grafting are superior compared with adults. Sensory recovery in nerve transfers may also be inferior to grafting. Hence, there is controversy regarding the use of nerve transfers in isolated upper trunk lesions. Clear indications for nerve transfers include late presentation, and as a salvage technique after failed nerve grafting. We have had favorable outcomes following nerve transfers in upper trunk injuries, with consistent recovery of useful elbow flexion and shoulder abduction/external rotation. 11 In recent years, however, it has become more evident that sensation plays a large role in patients' long-term functional recovery. For this reason, we currently try to schedule surgery in a timely manner for nerve grafting. More investigation is needed to determine whether nerve grafting does indeed provide better sensory recovery compared with transfers in isolated upper trunk injuries.
C5, C6, and C7 Nerve Roots (Upper and Middle Trunk Injury or Extended Erb's Palsy)
Infants with isolated upper trunk injuries must be distinguished from infants with upper and middle trunk involvement. Commonly, infants with upper and middle trunk injuries will lack wrist extension but retain elbow extension. Elbow extension is thought to be primarily innervated by the C7 nerve root, but there is routinely a contribution from C8 and even T1. For patients who fail to recover wrist extension by 3 months of age, we plan for brachial plexus exploration and nerve grafting by 5 months of age. Nerve grafting is the only chance for reinnervation of wrist extension, as reliable nerve transfer donors are unavailable.
Global Injuries
The definition of global injuries is confusing, as the lower trunk is often partially injured with preservation of T1 function. Unlike upper trunk injures, lower trunk injuries may completely rupture or avulse the C8 nerve root while sparing the T1 root. Patients with global brachial plexus birth injuries have a flail extremity but may retain or recover finger and thumb flexion from T1. In severe cases, a Horner's sign may be present, as well as difficulties with nursing or feeding from a bottle due to compromised phrenic nerve function. Infants with global injuries are followed up closely within the couple of months of life for return of motor function. Early return of full synchronized finger flexion (metacarpophalangeal and interphalangeal joint flexion), within the first 6 to 8 weeks of life, indicates that T1 will likely recover. Lack of elbow and finger extension by 2 months suggests that not only is C7 injured but C8 has also sustained a greater than 80% axotomy injury. If there is no return or limited recovery of hand function or elbow extension by 2 months of age, surgery is scheduled for 3 months of age. Early surgery is critical, to provide the best possibility of recovery of hand function.
Unfortunately, some patients with global injuries present late due to geographic limitations or other barriers to care. If an infant presents after 6 months of age with compromised hand function, nerve grafting is unlikely to restore useful hand function. These infants still undergo surgical intervention, but priority is shifted from lower trunk reconstruction to upper and middle trunk and C8 reconstruction.
Primary Plexus Surgery—Surgical Technique
Most cases start with the infant in the prone position to allow for fast and efficient harvest of bilateral sural nerves using two teams. The main benefit is the surgical ease and to reduce the length of anesthesia. We do not utilize nerve allograft for any mixed motor-sensory nerve reconstructions, as we have seen reliably better results with autograft. The sural nerve is first identified at the posterolateral ankle, between the lateral malleolus and Achilles tendon. Once identified, the nerve is dissected in a proximal direction via a series of transverse incisions. Transverse incisions are made in the calf at two or three intervals, terminating in the posterior crease of the knee, to maximize graft length. Several small transverse incisions placed along Langer's lines in the skin will lead to superior scars compared with a single longitudinal incision ( Fig. 4 ).
Fig. 4.

Multiple transverse incisions are designed on the posterior aspect of the leg. This design optimizes long-term scar appearance and allows access to the proximal takeoff of the sural nerve from the peroneal and tibial nerves. (Courtesy of Shriners Hospital for Children – Philadelphia.)
The infant is then positioned supine, with an “A-frame” created using two gel-rolls placed under the scapulae. Both the head and the endotracheal tube are turned and taped away from the affected side. If planning a CC7 transfer, a nasal intubation may be used with the tube turned toward the top of the head.
Dilute epinephrine is injected along the incision site prior to draping. Supraclavicular dissection of the brachial plexus is performed through a transverse incision made within a natural crease/fold in the infant's neck. The sternocleidomastoid and trapezius muscles form the medial and lateral borders of the incision, respectively ( Fig. 5 ). Early in our group's practice, a more extensile Z-type incision was utilized. However, the resultant scar was unsightly, and provided no benefit in terms of exposure.
Fig. 5.

The neck incision is designed within an existing skin crease. The sternocleidomastoid and trapezius muscles form the medial and lateral borders, respectively. (Courtesy of Shriners Hospital for Children – Philadelphia.)
The surgical dissection is performed in a stepwise fashion. The mantra “the same way every time” allows an efficient dissection to facilitate reconstruction in a timely manner. We aim to have both sural nerves harvested, the brachial plexus exposed, and a reconstructive plan in place by 12:00 noon at the latest.
After the skin is incised, monopolar electrocautery is used to dissect through the subcutaneous tissue. Infants do not have a well-developed platysma, and care must be taken to control the depth of dissection. Once the supraclavicular nerves are encountered, a combination of blunt dissection using Littler scissors and bipolar electrocautery is used. The omohyoid is identified and protected. The omohyoid is rarely divided and provides an important measure of the depth of dissection and location of the suprascapular nerve.
The anterior scalene, C5 root, and subsequently the phrenic nerve are first identified and preliminarily isolated ( Fig. 6 ). The anesthesia team is notified to hold ventilation before the phrenic nerve is stimulated to confirm its identity via chest movement. With the phrenic nerve protected, the fat pad overlying the plexus can then be elevated in a lateral direction. The phrenic nerve can be in a nonanatomic position in an injured plexus, translated laterally along with the neuroma. Because of this potential, we do not ligate the major traversing vessels—the transverse cervical (coursing over the C5 and C6 roots) and the dorsal scapular (coursing between the upper and middle trunks) vessels—until we have identified and protected the phrenic nerve.
Fig. 6.

The phrenic nerve is seen on the anterior surface of the anterior scalene. The phrenic nerve must be identified prior to proceeding with deeper dissection to prevent injury. (Courtesy of Shriners Hospital for Children – Philadelphia.)
If exposure is needed beneath the clavicle to address more distal ruptures, a greenstick fracture is created in the clavicle to improve exposure. The periosteum is split longitudinally, and a bone biter is used to create a partial osteotomy involving roughly 50% of the clavicle. An Army-Navy or vein retractor is then used to pull on the clavicle, creating a greenstick fracture. Because the periosteum and near cortex remains intact, the fracture is length stable and does not require any internal fixation.
We isolate the nerve roots cephalad to caudad in sequential fashion from C5 to C6 to C7 to C8 and to T1 (if necessary) ( Fig. 7A ). The subclavian artery is identified and protected anterior to the lower trunk prior to lower trunk dissection. Generous use of vascular clips is recommended near the subclavian artery and vein. With the roots identified, we will proceed with nerve stimulation at 0.5 mA, mid-pulse width (100 ms), and assess for any distal motor function. In rare instances, we will perform a neurolysis on conducting neuromas that have a normal or near-normal motor response when stimulated at 0.5 mA. When in doubt, however, we will not hesitate to resect the neuroma and “start over” ( Fig. 7B ). A combination of intraoperative findings, such as visualization of an avulsed roots, the presence of a neuroma, and response to hand-held nerve stimulation is used to determine the injury pattern and avulsed/ruptured status of nerve roots. In our practice, intraoperative neurophysiology testing with SSEPs/motor evoked potentials has been unreliable in differentiating root avulsion versus rupture. Once the nerve roots are exposed, the neuroma is resected back to healthy-appearing fascicles using a circumferential nerve cutting device ( Fig. 7 ). We do not use intraoperative frozen sections or an operative microscope. Cabled nerve autograft is then used to reconstruct the defect ( Fig. 8 ). We strongly prefer to use only fibrin glue to secure the reconstruction if there is no tension on the grafts, although we will use 9–0 nylon suture in select cases in which a primary coaptation is possible, such as a direct repair of a C6 root to the middle trunk.
Fig. 7.

( A ) A neuroma in continuity is seen between the C5 and C6 nerve roots and the trifurcation of the upper trunk into the anterior and posterior divisions, as well as the suprascapular nerve. ( B ) The neuroma is excised sharply using a circumferential nerve cutting device until healthy-appearing fascicles are seen mushrooming out from the nerve end. (Courtesy of Shriners Hospital for Children – Philadelphia.)
Fig. 8.

Cabled sural nerve grafts are used to reconstruct the nerve defects. (Courtesy of Shriners Hospital for Children – Philadelphia.)
When possible, we aim to achieve reconstruction across the entire plexus as opposed to nerve transfers, as we believe the number of motor axons provided is far higher with root-to-trunk or root-to-division level grafting compared with distal nerve transfers. However, root avulsions are common, and concomitant nerve transfers are performed when a complete reconstruction cannot be performed in the neck. We commonly graft C5 into suprascapular nerve and the posterior division of the upper trunk, but if the C5 nerve root is diminutive, a spinal accessory to suprascapular nerve transfer is utilized and C5 is grafted solely to the posterior division. The spinal accessory to suprascapular nerve transfer is performed through the supraclavicular incision. We also release the suprascapular ligament from this anterior approach to alleviate any potential sites of compression. 12
In extended upper injuries, where there are ruptures of the C5 and C6 nerve roots and an avulsion of the C7 nerve root, we will attempt a primary coaptation between the C6 root and the C7 root/middle trunk. If this coaptation cannot be accomplished without tension, a short interposition graft is placed. In this situation, elbow flexion must be restored with a nerve transfer to the musculocutaneous nerve or its branches. Our preference is to use the ulnar and median nerves as donors when the lower trunk is intact. Intraoperative nerve stimulation is used to identify one or two ulnar nerve fascicles, depending on the size of the nerve, which predominantly produces wrist flexion. In our experience, it is rare to find a fascicle that does not produce some hand/intrinsic function as well. The same is true for the median nerve; a true flexor carpi radialis fascicle is rare. We will not use the median nerve as a donor if C7 is avulsed or severely injured. Likewise, the lower trunk must be intact for us to use a fascicle from the ulnar nerve. If there is concern about the state of the ulnar nerve, then three ipsilateral intercostal nerves can be transferred to the musculocutaneous nerve. We harvest intercostal nerves using a curved incision placed along the inframammary crease. The pectoralis major and minor muscles are elevated superomedially, and the serratus is elevated laterally, exposing the ribs. The fascia on the inferior margins of ribs 3 to 5 are opened using bipolar cautery. A combination of blunt dissection and bipolar cautery is used to cautiously dissect the nerve from costochondral junction to the posterior axillary fold.
In global injuries, the number of available roots without avulsion injury determines the reconstructive plan. Hand and wrist function is prioritized first in the reconstructive ladder. The largest available root (usually C6) is used to provide axonal inflow into the lower trunk; the second largest available root is then grafted to the middle trunk. Shoulder function is restored most commonly by grafting C5 to the posterior division of upper trunk and the suprascapular nerve, with augmentation from the spinal accessory nerve if the C5 root is small or in poor condition. Elbow flexion is restored with nerve transfers as described earlier if there are one or more avulsions or one or more of the roots are deemed to be unusable. We will utilize a CC7 transfer in injuries with three or more avulsions ( Fig. 9 ). The CC7 is used as axonal inflow to either the middle or lower trunk or both depending on the circumstances. Our priority, particularly in late presenting injuries, is to deliver axons to C8 and the posterior division of the middle trunk if there are limited available roots. Unless absolutely necessary for the primary reconstruction, we try to preserve the spinal accessory nerve for a future free functional muscle transfer if elbow flexion does not recover.
Fig. 9.

( A ) The C7 nerve root of the donor side has been transected. A cabled sural nerve graft is coapted to the end of the nerve root in preparation for delivery to the other side of the neck. ( B ) The end of the nerve graft is protected with a nonadherent gauze, and the graft is delivered to the injury side. The graft is delivered across the neck in the prevertebral plane. (Courtesy of Shriners Hospital for Children – Philadelphia.)
Postoperatively, if a shoulder release was performed, or if the shoulder is tight but reducible, the patient is placed into a shoulder spica cast, with the shoulder positioned in adduction and external rotation for 4 weeks. Otherwise a soft dressing is used as a sling and swathe to immobilize the affected arm. This immobilization prevents excessive traction on the reconstruction during the immediate postoperative period. We do not immobilize the infant's neck.
Secondary Procedures
Internal Rotation Contracture of Shoulder and Glenohumeral Dysplasia
As reinnervation occurs following upper trunk BPBI, a combination of muscle imbalance favoring internal rotation and stunted growth of the rotator cuff leads to an internal rotation contracture. This contracture occurs most commonly in patients with upper trunk injuries but may be seen in all injury types. When the contracture is detected early, botulinum toxin injection and casting is an effective treatment as described earlier. In our office, patients with internal rotation contractures of the shoulder are evaluated by ultrasound as a preliminary screening for posterior subluxation of the humeral head. If these patients are indicated for nerve surgery, a more detailed ultrasound is performed with the patient under anesthesia, and reduction is attempted via external rotation and adduction of the shoulder. If the joint can be reduced without undue force, the patient is placed into a shoulder spica cast for 4 weeks. If we are unable to manually reduce the joint, an open shoulder release is performed at the same time as the brachial plexus exploration.
For patients older than a year who develop internal rotation contractures of the shoulder, we obtain an MRI to evaluate the configuration of the glenohumeral joint, as well as the muscles of the rotator cuff. In the absence of dysplasia, patients are candidates for an open or arthroscopic shoulder release regardless of age. If the glenohumeral joint is moderately to severely dysplastic, we perform only an open release via an anterior approach with a coracoidectomy. We do not routinely perform tendon transfers at the time of shoulder release. Preoperative examination and imaging are determining factors. If the joint is minimally dysplastic and the patient can maintain external rotation of the shoulder within their available range, an isolated shoulder release is typically sufficient. If the patient's arm drops into internal rotation due to weakness of external rotation, he or she is considered for a latissimus dorsi and/or teres major transfer can be used to supplement external rotation strength. We have seen that patients with C5, C6, and C7 nerve root injuries who undergo latissimus dorsi/teres major transfers are prone to losing internal rotation and midline function following surgery, making zippering, buttoning, and pulling up pants more difficult. 13 Thus, if shoulder tendon transfers are indicated in a patient with an upper and middle trunk injury, we transfer only one tendon, either the latissimus dorsi or teres major, to preserve an internal rotator. The treatment of patients who have moderate to severe is more complex. If there is good muscle bulk of the infra- and supraspinatus, and the child is younger than 4 years, an anterior release with a coracoidectomy is usually sufficient as long as there is an adequate anterior facet to support the humeral head and allow the remainder of the glenoid to remodel.
Patients with severe glenoid dysplasia and older than 4 years are not candidates for open shoulder release or tendon transfers, as the surgery could result in pain and decreased range of motion. In older patients with a biconcave glenoid and a reducible joint, a glenoid osteotomy can be performed to reform the glenoid and prevent posterior subluxation of the humeral head.
Open Shoulder Release Technique
The patient is placed in the supine or lateral position. An incision is designed within the deltopectoral interval ( Fig. 10A ). The incision begins just above the coracoid, which is palpated. The incision is infiltrated with 0.25% bupivacaine and epinephrine prior to draping to lessen the bleeding and decrease postoperative pain. The skin is incised, and monopolar cautery is used to dissect through the subcutaneous tissue. The cephalic vein is identified and retracted in a medial direction. Deep within this interval, the coracoid is visualized, along with the conjoint tendon and origin of the pectoralis minor ( Fig. 10B ). Both the conjoint tendon and pectoralis minor are released from the coracoid with a bipolar electrocautery. The coracoid is excised from its base using a bone biter ( Fig. 10C ). With the shoulder in external rotation, the rolled border of the subscapularis is identified and the joint entered within the rotator interval. A right-angle forceps placed within the joint ensures that the biceps tendon is out of harm's way. The rolled border of the subscapularis and anterior capsule are transected using bipolar cautery ( Fig. 10D ). The shoulder is continually held in external rotation as the shoulder is released to ascertain whether a sufficient release has been performed. The pectoralis major can also be lengthened via fractional lengthening if there is ongoing tightness following capsular release.
Fig. 10.

( A ) The incision for an open shoulder release is designed in the deltopectoral interval, with the superior aspect ending at the coracoid. ( B ) The coracoid is visualized along with the conjoint tendon (10 o'clock position) and pectoralis minor tendon (8 o'clock position). ( C ) The tendons are released using bipolar cautery, and the coracoid is isolated so that it can be excised using a bone biter. ( D ) A right angle forceps is placed into the glenohumeral joint to aid in the visualization of the joint space, and to facilitate division of the superior rolled border of the subscapularis. (Courtesy of Shriners Hospital for Children – Philadelphia.)
The skin is closed with absorbable suture. The patient is then placed into a shoulder spica cast with the shoulder in external rotation and adduction. The cast is maintained for 4 weeks, before transitioning to a teapot splint and initiating a stretching regimen with an occupational therapist.
Latissimus Dorsi and/or Teres Major Transfer for External Rotation—Technique
The latissimus dorsi/teres major transfer is performed via a separate posterior axillary incision. The anterior limit is within the armpit, anterior to the palpable latissimus tendon. The posterior limit is about halfway up the posterior shoulder, in the interval between the deltoid and triceps. This procedure is performed in the lateral decubitus position. The incision is injected with dilute epinephrine prior to draping. The skin is incised with a scalpel, and monopolar cautery dissection is performed down to the level of the fascia overlying the latissimus dorsi. There are multiple layers of “confusing” fascia in this region, and dissection through these layers must be performed to identify the actual latissimus dorsi tendon, which lies deep to these fascial layers. The latissimus dorsi and teres major tendons become conjoined as they course toward the humerus. The radial nerve is identified travelling down the arm anterior to the latissimus dorsi and protected. The axillary nerve courses in a transverse direction, deep to the latissimus dorsi/teres major, and should be identified before circumferential dissection around the muscles. Once the muscles have been dissected in a circumferential fashion, a Penrose drain is placed for retraction and the latissimus dorsi tendon is traced distal toward its insertion into the humerus. The insertion of the latissimus dorsi and teres major tendons is better visualized by placing the shoulder in maximal internal rotation and 90 degrees of abduction. The tendon origins are released from the humerus.
Proximal dissection of the muscle bellies enhances excursion and improves line of pull ( Fig. 11A ). At this point, the arm is rotated into external rotation, and dissection is performed in the interval between the deltoid and triceps. Deeper dissection reveals greater tuberosity of the humerus, which will be the insertion site. Two permanent sutures are placed in a transosseous manner through the greater tuberosity to firmly secure the tendon transfer ( Fig. 11B ). The released latissimus dorsi and/or teres major tendons are transferred to the recipient site. The tendons are secured using the previously placed suture in a locking Krackow fashion. The shoulder must be maintained in external rotation during this process and the remainder of the procedure to prevent tearing of the tendon transfer. Following skin closure, the patient is placed into a shoulder spica cast, with the shoulder in external rotation and approximately 90 degrees of abduction. After 4 weeks, the cast is removed, and therapy is begun. A removable brace is used for an additional 4 to 6 weeks' time.
Fig. 11.

( A ) The conjoint tendon of the latissimus dorsi and teres major muscles has been released, and proximal dissection of the muscle has been performed to increase excursion. ( B ) The tendon is inset into the greater tuberosity of the humerus using two permanent sutures placed in transosseous fashion. (Courtesy of Shriners Hospital for Children – Philadelphia.)
Elbow Flexion Contractures
Infant muscles that have previously been denervated and subsequently underwent reinnervation display impaired growth compared with normal muscle. As a result, patients with BPBI can develop joint contractures with skeletal growth. 14 This commonly occurs in the elbow, where stunted growth of the biceps and brachialis can cause a flexion contracture. In our practice, we use 30 degrees as a threshold for intervention. If a patient develops 30 degrees of flexion contracture, botulinum toxin injection of the elbow flexors and serial casting is recommended. For patients with severe contractures, we still recommend an attempt at botulinum toxin injection and serial casting to lessen the contracture. Subsequently, an elbow release with Z-lengthening of the biceps, fractional lengthening of the brachialis, and myotomy of the brachioradialis can be performed.
Forearm Contractures
The most common forearm deformity in patients with a global BPBI is a supination contracture. Recovery of the C5 and C6 innervated supinator muscles (biceps and supinator muscles) and deficient recovery of the pronator muscles (pronator teres and quadratus muscles) create imbalance resulting in a sustained supination posture of the forearm. Patients with forearm supination deformities are divided into two categories, those with passively correctible deformities and those with fixed deformities. Passively correctible deformities can be treated with a biceps rerouting that changes the biceps tendon from a supinator into a pronator. For patients with a fixed deformity, a one-bone forearm procedure that affixes the distal radius to the proximal ulna in a functional forearm position is preferred ( Fig. 12 ).
Fig. 12.

( A ) A patient with a supination contracture of the forearm is seen. ( B ) A volar incision is created to expose the radius and ulna. ( C ) The radial osteotomy is placed 1 cm distal to the ulnar osteotomy. ( D ) The distal end of the radius is fixed to the proximal end of the ulna in a functional amount of pronation using limited contact dynamic compression plate. ( E ) Postoperative position of the patient's forearm. (Courtesy of Shriners Hospital for Children – Philadelphia.)
Lack of Wrist Extension
Infants with deficient recovery in the C5, C6, and C7 nerve roots often lack wrist extension, and a tendon transfer to restore wrist extension can be beneficial. The pronator teres (a C7-predominant muscle) is also weakened in these patients and not available as a donor for transfer. Our preferred donor is the flexor digitorum superficialis (FDS) to the ring and middle fingers. The FDS tendons have adequate power and excursion, and finger flexion is a synergistic motion with wrist extension. In global injuries, there are often no donors available.
Clinical examination is imperative to ensure adequate FDS and flexor digitorum profundus (FDP) function. Strong FDS and FDP tendons are a prerequisite for transfer. To test FDS function to the ring finger, the examiner holds the index, middle, and small finger in full extension and asks the child to bend his or her finger through the proximal interphalangeal joint. The examiner must ensure that there is no obligate distal interphalangeal joint flexion that would suggest that the FDS is weak, and/or that the FDP tendon is the only tendon providing finger flexion. This test is repeated on the middle finger, with the index, ring, and small finger held in extension.
The transfer is performed using a transverse incision in the palm, an anterior forearm incision to harvest the two FDS tendons, a dorsal forearm incision, and a dorsal wrist incision centered over the third metacarpal base. One FDS is routed from volar to dorsal through the interosseous membrane (IOM), and the other tendon routed around the radial border of the wrist to provide a slight pronation force in addition to wrist extension ( Fig. 13 ). Early in our group's experience, both tendons were passed through the IOM. However, this technique resulted in an unwanted supination force leading to a supination contracture in patients who already lacked pronation.
Fig. 13.

( A ) The flexor digitorum tendons to the middle and ring fingers are divided at the metacarpal head and delivered through a proximal incision. ( B ) One tendon is routed radially, and another is tunneled through the interosseous membrane. The tendons are then delivered into a distal incision on the dorsal wrist. ( C ) The tendons are wrapped once around the third metacarpal in opposing directions and sutured back onto itself using permanent suture and one weave. (Courtesy of Shriners Hospital for Children – Philadelphia.)
The FDS tendons are looped around the metacarpal base, one from radial to ulnar, and the other ulnar to radial. The FDS tendon is then woven back through itself. The transfer is tensioned to yield wrist extension tenodesis and is secured using nonabsorbable suture. Two tendons can create bulk at the tenorrhaphy site. Hence, we perform a single weave through each tendon as opposed to multiple Pulvertaft weaves. The remaining tendon is sutured back over itself in side-to-side fashion using a running locking suture. A short arm splint is applied.
Free Functional Muscle Transfer
For a variety of reasons, patients with BPBI can have residual deficits in elbow flexion/extension or finger flexion/extension. In the absence of options for tendon transfer, the only available treatment may be a free gracilis muscle transfer. The entire length of the gracilis is harvested as a myocutaneous flap and inset into the affected upper extremity based on the deficit to be addressed. For elbow flexion, the proximal end of the muscle is fixed to the coracoid, and the distal tendon secured to the proximal radius or coronoid. If finger flexion is also required, the distal muscle is tunneled underneath the lacertus and fixed to the FDP and flexor pollicis longus tendons en masse. For concomitant wrist extension, the distal muscle is tunneled through the proximal brachioradialis and woven into the extensor carpi radialis brevis. Our choice of recipient nerve depends on availability, but the spinal accessory nerve or intercostals are most commonly used. Patients are immobilized in a splint with the elbow flexed for 6 weeks, followed by therapy.
Conclusion
Our approach to managing brachial plexus injuries in infants continues to evolve at such a pace that the recommendations contained in this text will likely be out of date by the time they are published. As our experience grows, both clinically and in research, we are continually fine tuning our understanding and therefore our treatment strategies. We rely heavily on the collective and individual wisdom of the authors featured in this volume, without whom the practice of brachial plexus surgery would not have advanced to what it is today. As we have and continue to do, we encourage anyone in this field to visit and learn from innovators and thought leaders and to keep abreast of new developments and techniques. An open-minded and collaborative approach is the secret of our limited success.
Footnotes
Conflict of Interest None declared.
References
- 1.Lee B H, Park T C, Lee H J. Association between fetal abdominal circumference and birthweight in maternal hyperglycemia. Acta Obstet Gynecol Scand. 2014;93(08):786–793. doi: 10.1111/aogs.12420. [DOI] [PubMed] [Google Scholar]
- 2.Louden E, Marcotte M, Mehlman C, Lippert W, Huang B, Paulson A. Risk factors for brachial plexus birth injury. Children (Basel) 2018;5(04):46. doi: 10.3390/children5040046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abzug J M, Chafetz R S, Gaughan J P, Ashworth S, Kozin S H. Shoulder function after medial approach and derotational humeral osteotomy in patients with brachial plexus birth palsy. J Pediatr Orthop. 2010;30(05):469–474. doi: 10.1097/BPO.0b013e3181df8604. [DOI] [PubMed] [Google Scholar]
- 4.Richardson R T, Russo S A, Chafetz R S. Reachable workspace with real-time motion capture feedback to quantify upper extremity function: a study on children with brachial plexus birth injury. J Biomech. 2022;132:110939. doi: 10.1016/j.jbiomech.2021.110939. [DOI] [PubMed] [Google Scholar]
- 5.Russo S A, Chafetz R S, Rodriguez L M. Comparison of shoulder motion measurements by visual estimate, goniometer and motion capture. J Pediatr Orthop. 2022;42(08):443–450. doi: 10.1097/BPO.0000000000002212. [DOI] [PubMed] [Google Scholar]
- 6.Donohue K W, Little K J, Gaughan J P, Kozin S H, Norton B D, Zlotolow D A. Comparison of ultrasound and MRI for the diagnosis of glenohumeral dysplasia in brachial plexus birth palsy. J Bone Joint Surg Am. 2017;99(02):123–132. doi: 10.2106/JBJS.15.01116. [DOI] [PubMed] [Google Scholar]
- 7.van Dijk J G, Malessy M J, Stegeman D F. Why is the electromyogram in obstetric brachial plexus lesions overly optimistic? Muscle Nerve. 1998;21(02):260–261. doi: 10.1002/(sici)1097-4598(199802)21:2<260::aid-mus20>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Kozin S H, Zlotolow D A, Soldado F. Springer; 2021. Priorities of treatment and rationale (babies are not small adults) [Google Scholar]
- 9.Hale H B, Bae D S, Waters P M. Current concepts in the management of brachial plexus birth palsy. J Hand Surg Am. 2010;35(02):322–331. doi: 10.1016/j.jhsa.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 10.Garg R, Merrell G A, Hillstrom H J, Wolfe S W. Comparison of nerve transfers and nerve grafting for traumatic upper plexus palsy: a systematic review and analysis. J Bone Joint Surg Am. 2011;93(09):819–829. doi: 10.2106/JBJS.I.01602. [DOI] [PubMed] [Google Scholar]
- 11.Little K J, Zlotolow D A, Soldado F, Cornwall R, Kozin S H. Early functional recovery of elbow flexion and supination following median and/or ulnar nerve fascicle transfer in upper neonatal brachial plexus palsy. J Bone Joint Surg Am. 2014;96(03):215–221. doi: 10.2106/JBJS.L.01405. [DOI] [PubMed] [Google Scholar]
- 12.Zlotolow D A, Low S L, Lin I C, Williamson C, Tinsley B, Kozin S H. Suprascapular ligament release from an anterior approach: an anatomic feasibility study. J Hand Surg Am. 2019;44(10):9000–9.0E6. doi: 10.1016/j.jhsa.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Greenhill D A, Smith W R, Ramsey F V, Kozin S H, Zlotolow D A. Double versus single tendon transfers to improve shoulder function in brachial plexus birth palsy. J Pediatr Orthop. 2019;39(06):328–334. doi: 10.1097/BPO.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 14.Nikolaou S, Peterson E, Kim A, Wylie C, Cornwall R. Impaired growth of denervated muscle contributes to contracture formation following neonatal brachial plexus injury. J Bone Joint Surg Am. 2011;93(05):461–470. doi: 10.2106/JBJS.J.00943. [DOI] [PubMed] [Google Scholar]


