Abstract
In a search for new anti-autoimmune agents that selectively suppress activation of autoreactive T cells, one such agent, 5-methyl-3-(1-methylethoxy)benzo[b]thiophene-2-carboxamide (CI-959-A), was found to be effective. This compound, which is known to suppress tumor necrosis factor alpha (TNF-α)-induced CD54 expression, inhibited the primary proliferative response of the T cell to antigen (Ag)-presenting cells (APCs) including allogenic dendritic cells (DCs), autologous Epstein-Barr virus-infected B cells, and human T lymphotropic virus type I (HTLV-I)-infected T cells. Autoreactive T cells from patients with HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) spontaneously proliferate in vitro, and their activation is reported to be associated with CD54 expression. The spontaneous proliferation of T cells from patients with HAM/TSP was entirely blocked by CI-959-A. However, in this study, the T-cell proliferation in 15 patients with HAM/TSP was found to depend more extensively on major histocompatibility complex (MHC) class II and CD86 than on CD54 Ags. Since most important APCs for the development of HAM/TSP are DCs and HTLV-I-infected T cells, the effect of CI-959-A on DC generation and on the expression of surface molecules on activated T cells is examined. CI-959-A suppressed recombinant granulocyte-macrophage colony stimulating factor (GM-CSF)- and recombinant interleukin-4-dependent differentiation of DCs from monocytes and inhibited the expression of CD54 and, more extensively, MHC class II and CD86 Ags. CI-959-A showed little toxicity toward lymphoma or HTLV-I-infected T-cell lines or toward monocytes and cultured DCs. These results suggest that CI-959-A might be a potent anti-HAM/TSP agent.
Human T lymphotropic virus type I (HTLV-I)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is thought to be an autoimmune disease induced by HTLV-I infection (8, 9, 24). The T lymphocytes obtained from patients with HAM/TSP patients produce interleukin-2 (IL-2) in vivo and proliferate spontaneously in vitro without any additional stimuli or cytokines (35). This spontaneous proliferation of T lymphocytes (SPL) depends on the interaction of T cells with antigen (Ag)-presenting cells (APCs) such as dendritic cells (DCs) (17, 25) and HTLV-I-infected CD4+ T cells (15, 32). The DCs localized in the blood and nonlymphoid organs are considered to be functionally immature, in that they are optimized for the uptake and processing of Ag but not for the initiation of primary T-cell responses. However, after the uptake of Ag and exposure to inflammatory agents including tumor necrosis factor alpha (TNF-α) and IL-1, the DCs undergo a process of maturation and gain the ability to present Ag to T cells for their priming (22, 26). In addition to DCs, HTLV-I-infected CD4+ T cells directly stimulate autologous CD4+ T cells in a major histocompatibility complex (MHC) class II- and CD86 molecule-dependent fashion (32). Among the T cells stimulated with these APCs, some might cross-react with self Ags and closely associate with the development of HAM/TSP.
We have been searching for compounds that inhibit the cellular interaction between APCs and T cells to suppress the activation of autoreactive and Ag-specific T cells. The molecules associated with the APC-T cell interaction may provide an effective target for therapy for autoimmune diseases. Binding of APCs and T cells is initiated by contact of adhesion molecules, such as CD54 and CD11a/CD18, expressed on both cells, and induction of sustained proliferation of T cells requires two independent signals provided by APCs: a T-cell receptor-mediated Ag-specific signal and a signal mediated by costimulatory molecules (CSMs) (10, 20) including CD86 and CD58 Ags (1, 11, 31). Blocking of their tight binding through adhesion molecules or interaction of the CSMs with CSM ligands effectively suppressed the abnormal expansion of disease-associated T cells in vivo and in vitro (19, 30, 32) and sometimes effectively induced a long-term unresponsiveness of T cells to recall stimuli.
5-Methyl-3-(1-methylethoxy)benzo[b]thiophene-2-carbox-amide (CI-959-A) is known to inhibit CD54 expression, and its derivative is reported to inhibit casein kinase II (4). In the present study, we found that CI-959-A markedly suppressed SPL in patients with HAM/TSP. Furthermore, the compound suppressed the primary T-cell proliferative response to stimuli provided by various APCs, the differentiation of immature DCs from monocytes and their subsequent maturation, and the induction of expression of MHC class II, CD54, and CD86 Ags on activated CD4+ T cells.
MATERIALS AND METHODS
Preparation of responder cells and stimulators.
Peripheral blood mononuclear cells (PBMCs) were donated, with informed consent, by 15 patients diagnosed with HAM/TSP at the Kagoshima University Hospital and by 8 healthy donors. The disease was diagnosed according to the World Health Organization criteria for HAM/TSP. The age range of the patients was 39 to 75 years, and the range of years with disease was 4 to 37. The patient population comprised 10 females and 5 males, and the severity of disease was scored as 0 to 7 by motor disability grading (22). No patient was coinfected with human immunodeficiency virus type 1. The PBMCs were isolated from heparinized blood by using Ficoll-Paque Plus (Pharmacia, Uppsala, Sweden) and were cryopreserved in liquid nitrogen as described previously (12). The HTLV-I-infected cell lines (HT-1, HT-2, and HT-3) were established by cocultivation of CD4+ T cells from healthy donors with a mitomycin C-treated HTLV-I-producing cell line, MT-2, which was a generous gift from I. Miyoshi, Kochi Medical School, for more than 3 months in the presence of 2 μg of phytohemagglutinin (PHA) (Difco Laboratories, Detroit, Mich.) per ml and 100 U of recombinant IL-2 (rIL-2; TGP-3; Takeda Chemical Industries, Osaka, Japan). B cells were infected with Epstein-Barr virus (EBV) by using the virus-producing cell line B95-8, which was kindly provided by Y. Eizuru, Kagoshima University.
The DCs were prepared from PBMCs as described previously (18). In brief, 106 PBMCs were plated in a 24-well flat-bottom tissue culture plate and were cultured for 7 to 10 days in the presence of 1,000 U of recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF; Kirin Brewery Co., Tokyo, Japan) and of 200 U of rIL-4 (Genzyme, Cambridge, Mass.) per ml. In some cases, various numbers of PBMCs were plated in a 96-well flat-bottom plate. CD83+ DCs were induced by the addition of 20 ng of TNF-α (Boehringer GmbH, Mannheim, Germany) per ml (29). The morphologically apparent DCs were counted under a microscope as described previously (26), and their rate of differentiation from peripheral monocytes was calculated as follows: 100 × (number of differentiated DCs/number of viable PBMCs plated). CI-959-A and its control analog, 5-methoxy-3-(1-methylethoxy)-N-(1H-tetrazol-5-yl)benzo[b]thiophene-2-carboxamide (CI-959), were kindly synthesized and provided by Daiichi Pharmaceutical Co. (Tokyo, Japan). The purities of both compounds were determined to be more than 99%.
Proliferation assay.
Allogeneic or autologous mixed lymphocyte reactions were conducted. Unseparated PBMCs obtained from healthy donors (5 × 104 per well) were stimulated with the following: allogeneic PBMCs (5 × 104 cells/well), allogeneic cultured DCs (5 × 103 cells/well), autologous EBV-infected B cells (3 × 104 cells/well), and autologous HTLV-I-infected CD4+ T cells (5 × 104 cells/well). The optimal concentrations of the mitomycin C-treated stimulators were determined in advance. An APC-dependent mitogen, PHA, was also used as a stimulator at a concentration of 2 μg per ml. The proliferation of responder cells during the last 16 h of the 6-day culture in the presence or absence of test compounds was quantified by incubating the cells with 1 μCi of [3H]thymidine. The results were expressed as the mean difference in counts per minute obtained from triplicate cultures. The proliferation of the three different HTLV-I-infected cells in the presence of PHA (2 μg/ml) and rIL-2 (100 U/ml) and that of the T-cell lymphoma lines Jurkat, CCRF-CEM, and MOLT-4 after 4-day cultures were also measured. The spontaneous proliferation of lymphocytes obtained from patients with HAM/TSP in the 6-day culture was determined in the absence of any additional stimulators or cytokines. The spontaneous proliferation assay was done by quantification of [3H]thymidine uptake, and in the proliferation assay, 10% heat-inactivated human pooled serum was used. The SPL was observed from 3 days after culture and reached a maximum at day 6. The uptake of [3H]thymidine by T cells from healthy uninfected donors cultured for 6 days was less than 1,500 cpm; therefore, uptake of more than 4,000 cpm was considered a positive SPL. The following monoclonal antibodies (MAbs) were used to suppress the SPL: W6/32 (anti-HLA-ABC), L243 (anti-HLA-DR), L307 (anti-CD80), IT2.1 (anti-CD86), HA58 (anti-CD54), TS2/9.1.4.3 (anti-CD58), and TRAP1 (anti-CD40L; Pharmingen, San Diego, Calif.). The MAbs were purified from culture supernatants or ascites fluid by using 40% saturated ammonium sulfate and caprylic acid (Sigma, St. Louis, Mo.). The purity of the MAbs was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the optimal concentration of them for suppression was determined in advance. The percent suppression was calculated as 100 × [1 − (mean counts per minute for cultures with MAb or compound/mean counts per minute for cultures without test materials)].
Analysis of cell surface Ag.
The expression of cell surface Ag on PHA-stimulated CD4+ T cells was determined by flow cytometry (FACScan; Becton Dickinson Immunocytometry Systems, San Jose, Calif.). Live CD4+ T cells (104) were gated by using fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-labeled anti-CD4 MAb (Leu-3a; Becton Dickinson) and were examined for Ag expression by using PE-labeled MAbs against CD54 (HA58), CD58 (L304.4) (Becton Dickinson), CD86 (IT2.2), and CD28 (CD28.2) (Pharmingen) and FITC-labeled anti-HLA-DR MAb (L243; Becton Dickinson). The surface Ag on cultured DCs was analyzed with FITC-labeled MAbs against HLA-DR, CD54, CD58, and CD40 (5C3; Pharmingen) and purified MAb to CD1a (NA1/34; Serotec Ltd., Oxford, England); this was followed by staining with FITC-labeled goat F(ab′)2 anti-mouse immunoglobulins (Igs; Tagoimmunologicals, Camarillo, Calif.) and with PE-labeled MAbs to CD83 (HB15a; Immunotech, Marseille, France) and CD86. The optimal concentrations of the MAbs were determined in advance. The toxicities of CI-959-A and CI-959 for nonproliferating monocytes and cultured DCs were determined by microscopically counting them while they were undergoing apoptosis. Furthermore, these cells, which were cultured for 3 to 5 days in the presence of compounds, were stained with Annexin-V (Genzyme) and propidium iodide (Sigma), and the number of cells undergoing apoptosis was determined by using FACScan. Monocytes were cultured with medium that included 20% human serum, and DCs were maintained in the medium containing rGM-CSF and rIL-4.
Statistical analysis.
Student’s t test was applied to reveal statistically significant differences.
RESULTS
Inhibition of T-cell proliferative response to APC-dependent antigenic stimuli by CI-959-A.
Since CI-959-A suppressed TNF-α-induced CD54 expression, the effects of CI-959-A and its control analog, CI-959, on the APC-T cell interaction were determined by examining the T-cell proliferative response to various APC-dependent stimuli (Table 1). In the 6-day primary culture, T-cell proliferation was induced by PHA, allogeneic PBMCs and cultured DCs, autologous EBV-infected B cells, and autologous HTLV-I-infected CD4+ T cells. The EBV- or HTLV-I-infected cells acted as APCs and stimulated autologous T cells in an Ag-specific and CD86-dependent fashion, as reported previously (32). While CI-959 did not interfere with T-cell proliferation, CI-959-A markedly suppressed the proliferation. The 50% effective concentration (EC50) of CI-959-A for each stimulator were from 0.51 μM (PHA) to 0.65 μM (allogeneic cultured DCs) (Table 1).
TABLE 1.
Inhibitory effects of CI-959 and CI-959-A on primary proliferative response of T cells
| Compound | EC50
(μM)a
|
||||
|---|---|---|---|---|---|
| PHA | Allogeneic PBMCs | Allogeneic cultured DCs | Autologous EBV-infected B cells | Autologous HTLV-I-infected T cells | |
| CI-959 | >4.0 | >4.0 | >4.0 | >4.0 | >4.0 |
| CI-959-A | 0.51 ± 0.24 | 0.52 ± 0.04 | 0.65 ± 0.10 | 0.52 ± 0.01 | 0.62 ± 0.05 |
The EC50s of CI-959 and CI-959-A were determined by quantification of the inhibition of the proliferative response of T cells to various stimulators. The proliferative response of unseparated PBMCs (5 × 104/well) in the absence of compounds was 156,274 ± 112,391 cpm for PHA (2 μg/ml), 11,937 ± 3,514 cpm for allogeneic PBMCs (5 × 104/well), 14,771 ± 10,026 cpm for allogeneic cultured DCs (5 × 103/well), 28,165 ± 11,205 cpm for autologous EBV-infected B cells (3 × 104/well), and 30,306 ± 19,772 cpm for autologous HTLV-I-infected T cells (5 × 104/well). Values are means ± standard deviations for three to six independent donors.
Cytotoxic effect of CI-959-A on lymphocytes.
The toxicities of the compounds for T cells were determined by culturing HTLV-I-immortalized T cells and T lymphoma cells in the presence of various concentrations of the compounds. Neither CI-959 nor CI-959-A inhibited the proliferation of these cells at 1 μM, and the 50% inhibitory concentrations of CI-959-A for proliferation of those cells were more than 8 μM (Table 2). The toxic effect of CI-959-A on nonproliferating cells was determined by culturing monocytes or cultured DCs in the presence of the compound at 1 μM. The live cell number was counted microscopically, and cultured cells were stained with Annexin-V and propidium iodide. However, neither monocytes nor DCs cultured in the presence of CI-959-A showed increasing cell death or apoptosis (data not shown).
TABLE 2.
Inhibitory effects of CI-959 and CI-959-A on proliferation of transformed T cells
| Compound | IC50
(μM)a
|
|||||
|---|---|---|---|---|---|---|
| HTLV-I-infected cell
line
|
T-cell lymphoma line
|
|||||
| HT-1 | HT-2 | HT-3 | Jurkat | CCRF-CEM | MOLT-4 | |
| CI-959 | >10.0 | >10.0 | >10.0 | >10.0 | >10.0 | >10.0 |
| CI-959-A | >10.0 | >10.0 | >10.0 | 9.9 ± 0.3 | 8.2 ± 0.5 | >10.0 |
The 50% inhibitory concentrations (IC50s) of CI-959 and CI-959-A were determined by quantification of the inhibition of proliferation of HTLV-I-infected CD4+ cell lines and T-cell lymphoma lines. The proliferation of cell lines cultured for 4 days in the absence of compounds was 6,728 ± 512 cpm for HT-1 (2 × 104/well), 1,304 ± 1,859 cpm for HT-2 (2 × 104/well), 7,135 ± 990 cpm for HT-3 (2 × 104/well), 14,541 ± 7,618 cpm for Jurkat (2 × 104/well), 10,990 ± 4,619 cpm for CCRF-CEM (2 × 104/well), and 19,185 ± 8,003 cpm for MOLT-4 (2 × 104/well). Values are means ± standard deviations for three separate experiments.
Suppression of SPL in patients with HAM/TSP by CI-959-A.
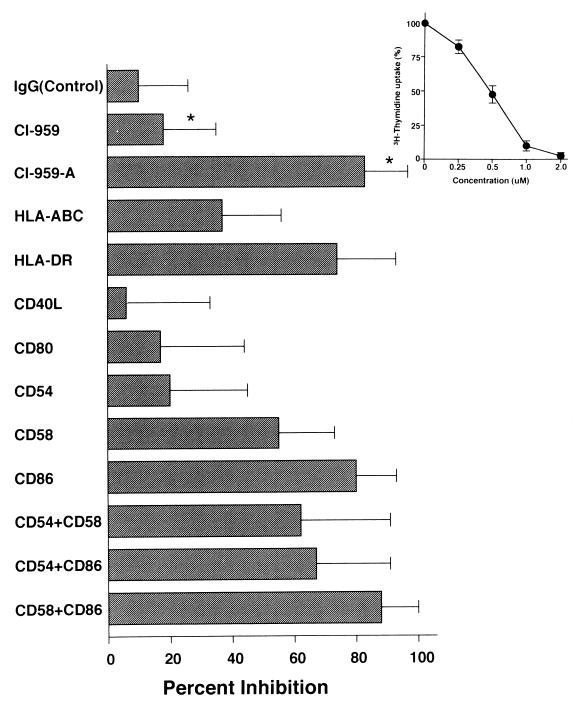
We obtained PBMCs from 15 patients with HAM/TSP and individually determined the therapeutic effect of CI-959-A on them (Fig. 1). To this end, we induced SPL in the presence of the compounds and found that 1 μM CI-959-A inhibited the T-cell proliferation by 83.1%, on average, in patients with HAM/TSP, whereas CI-959 suppressed T-cell proliferation by less than 18% (P < 0.001) (Fig. 1). SPL suppression by CI-959-A was constantly observed in most patients examined. In order to make sure that SPL is induced by contact of T cells with APCs mainly through CD54 Ag, we determined the molecules that are chiefly associated with SPL induction. The MAb to MHC class I Ags mildly affected the suppression of SPL (36.6% suppression), and the MHC class II MAb was markedly effective in the suppression of SPL (74.4%). Of the various MAbs to CSMs (CSMs CD40L, CD80, CD54, CD58, and CD86), the MAb to CD86 was the most effective (80.1%), and the MAb to CD58 showed moderate inhibition of SPL (55.4%). However, in contrast to previous reports (13, 36), the MAb to CD54 suppressed SPL by only 19.6%. These findings were further confirmed by combination treatment. All the combinations of MAbs (MAbs to CD54 plus CD58, CD54 plus CD86, and CD58 plus CD86) achieved more than 60% inhibition, and the combination of MAbs to CD58 and CD86 was the most effective (88.4%). However, no additive suppressive effects of the MAb to CD54 to the suppressive effect of the MAb to CD58 or CD86 were observed. The other two MAbs (MAbs to CD40L and CD80) had minimal effects. T cells have been reported to express the CTLA-4 Ag on the cell surface after activation. However, neither CD4+ T cells nor CD8+ T cells expressed the CTLA-4 Ag before or after in vitro induction of SPL. These results suggest that SPL is induced by the interaction with APCs and that it depends largely on the MHC class II, CD86, and CD58 molecules expressed on the APCs.
FIG. 1.
Inhibitory effects of CI-959-A and MAbs to CSMs on SPL for patients with HAM/TSP. PBMCs were donated by 15 patients with HAM/TSP, and 7.5 × 104 cells were cultured for 6 days in the presence of 10 μg of MAbs per ml or a 1-μM concentration of compound. The proliferation of T cells in the absence of test materials was 14,314 ± 7,255 cpm (mean ± standard deviation for 15 individuals; the individual titers were as follows: 27,368, 8,512, 8,585, 10,864, 4,154, 24,777, 11,593, 15,348, 10,636, 16,215, 10,723, 25,308, 5,256, 20,074, and 15,301 cpm). A mixture of normal mouse IgG subclasses (IgG1, Ig2a, Ig2b, and Ig3) was used as control antibody. ∗, P < 0.001. The inset shows a dose-response curve for CI-959-A on SPL for patients with HAM/TSP. Data for three representative patients were used, and [3H]thymidine uptake by T cells without the compound was determined to be 100%. The mean ± standard deviation for three patients is shown.
Suppression of DC generation and CSM expression on CD4+ T cells by CI-959-A.
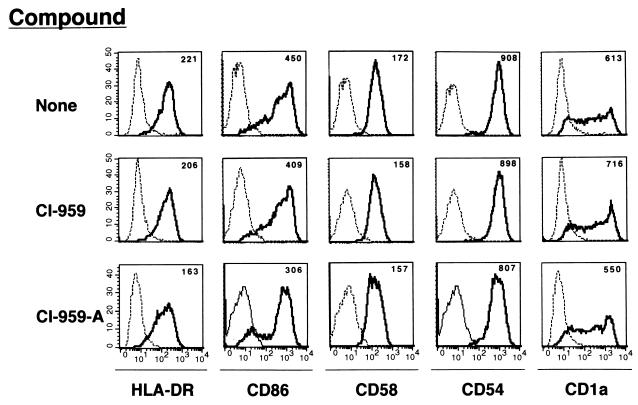
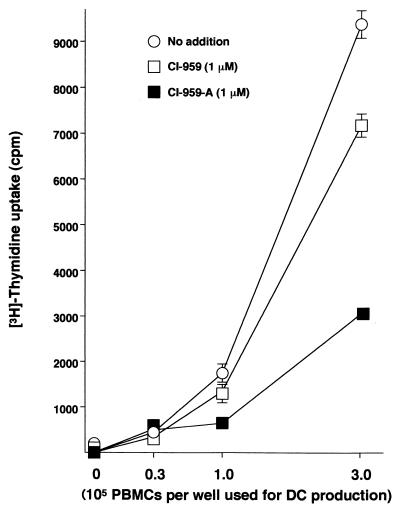
In order to clarify a mechanism of CI-959-A on SPL suppression in patients with HAM/TSP, we examined the effects of the compound on APCs, among which the most important are DCs and HTLV-I-infected CD4+ T cells (15, 17, 25, 32). CI-959-A suppressed the differentiation of DCs from normal monocytes (Table 3). The PBMCs donated by healthy individuals and plastic-adherent monocytes were cultured in the presence of rGM-CSF and rIL-4 for 7 to 10 days to induce DCs. The DCs that express CD83 at low levels and CD86 at moderate levels (termed CD83− DCs) differentiated in the presence of the cytokines, and DCs that were CD83 positive and that expressed the CD86 Ag at high levels (CD83+ DCs) differentiated following the addition of TNF-α. The rates of production of CD83− and CD83+ DCs were 7.3 and 2.7%, respectively. CI-959-A at 1 μM suppressed the differentiation of both types of DCs by more than 50%. There was a statistical difference in the DC differentiation rate between CI-959-A and CI-959. However, the DCs that differentiated in the presence of CI-959-A expressed MHC class II and CD54, CD58, CD1a, and CD86 molecules to the same extent as those that differentiated in the absence of the compound or in the presence of control compound, although their differentiation rates were reduced. The mean fluorescence intensity for those Ags on DCs generated with CI-959-A was minimally reduced compared to that for the other two DCs (Fig. 2). The inefficient generation of DCs was supported by the functional evidence (Fig. 3). CD83+ DCs differentiated from various numbers of PBMCs in the presence of CI-959-A at 1 μM in 96-well flat-bottom culture plates. After 10 days of culture, the APC activity of the DCs was assessed by allogeneic MLR. The DCs that had differentiated in the presence of 1 μM CI-959-A stimulated the allogeneic responder cells less efficiently than those which had differentiated normally, and the difference was statistically significant. These results suggested that CI-959-A suppressed the generation efficiency of functionally competent DCs.
TABLE 3.
Inhibitory effects of CI-959 and CI-959-A on differentiation of DCs from PBMCsa
| Compound | Concn (μM) | % Differentiation of
DCs
|
|
|---|---|---|---|
| CD83− DCs | CD83+ DCs | ||
| None | 0 | 7.30 ± 3.50 | 2.70 ± 1.11 |
| CI-959 | 1 | 6.41 ± 1.30 | 2.50 ± 0.40 |
| CI-959-A | 0.5 | 3.90 ± 1.31b | NDc |
| 1.0 | 2.25 ± 0.61de | 1.20 ± 0.65be | |
The effect of CI-959-A on differentiation of DCs from PBMCs was determined. CD83− DCs were differentiated by culturing PBMCs in the presence of GM-CSF and IL-4 for 7 days, and CD83+ DCs were differentiated by using GM-CSF, IL-4, and TNF-α. In each experiment, five independent cultures were used. The DC differentiation rate was calculated as follows: 100 × (number of DCs differentiated/number of PBMCs used). Means ± standard deviations are shown.
P < 0.05 compared with no compound.
ND, not determined.
P < 0.01 compared with no compound.
P < 0.05 compared with CI-959.
FIG. 2.
Expression of various molecules on DCs differentiated in the presence of CI-959-A. DCs were differentiated by using rGM-CSF (1000 U/μl) and rIL-4 (200 U/μl) for 5 days in the presence of CI-959-A (1 μM) or CI-959 (1 μM). The DCs were gated and examined for expression of HLA-DR, CD86, CD58, CD54, and CD1a. ----, control MAb; ———, the indicated MAb. The number above each histogram represents the mean fluorescence intensity.
FIG. 3.
Inhibition of functional DC production by CI-959-A. Various numbers of PBMCs were cultured in a 96-well plate in the presence of 1 μM CI-959-A or CI-959 to produce mature DCs. After 7 days, the DCs were treated with mitomycin C, washed, and used for T-cell stimulation. Allogeneic, unseparated PBMCs (5 × 104/well) were used as responders. Data represent those from three separate experiments.
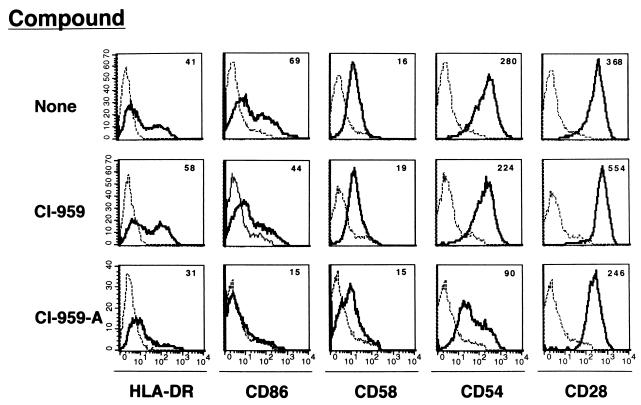
Furthermore, PBMCs donated by three healthy donors were stimulated with PHA (2 μg/ml) (Fig. 4). Seven days later, CD4+ T cells expressed HLA-DR, CD54, CD58, and CD86 Ags. However, the surface expression of these Ags except for that of the CD58 Ag on the CD4+ T cells activated in the presence of 1 μM CI-959-A was substantially suppressed. The production of cells expressing HLA-DR and CD86 Ags was most strikingly reduced, and expression of CD54 was moderately reduced by CI-959-A. In contrast to these Ags, the molecules constitutively expressed on CD4+ T cells, such as CD28 and CD2, were not affected by CI-959-A. A control compound, CI-959, caused no suppression of Ag expression.
FIG. 4.
Inhibition by CI-959-A of MHC, costimulatory molecule, and adhesion molecule expression on CD4+ T cells. PBMCs were stimulated with PHA (2 μg/ml) for 7 days in the presence of CI-959-A (1 μM) or CI-959 (1 μM). The activated CD4+ T cells were gated and examined for expression of CD54, CD58, CD86, HLA-DR, and CD28. ----, control MAb; ———, the indicated MAb. The number above each histogram represents the mean fluorescence intensity. Data represent those from more than three separate experiments.
DISCUSSION
Although HAM/TSP is induced by infection with HTLV-I, it is categorized as an autoimmune disease (8, 9, 24), in that disease development is closely associated with the activation of CD4+ and CD8+ T cells which recognize HTLV-I gene products (6) and which may also cross-recognize neural Ags (21). We have been searching for compounds capable of suppressing the APC-dependent activation of autoreactive T cells. In such processes, we focused on a benzothiophene derivative, CI-959-A, since the compound has been shown to suppress TNF-α-induced expression of CD54 and CD62E molecules on nonprofessional APCs such as human umbilical vein endothelial cells (3). Actually, CI-959-A entirely blocked the interaction of T cells with various APCs including DCs, EBV-infected B cells, and HTLV-I-infected CD4+ T cells (Table 1), probably through the suppression of expression of adhesion molecule CD54. In previous reports, CD54 and CD58 molecules were associated with the activation of T cells in patients with HAM/TSP (13, 36). This information prompted us to examine whether CI-959-A could inhibit the disease-associated activation of T cells in patients with HAM/TSP. The production of autoreactive T cells in patients with HAM/TSP is reflected in the extension of the in vitro SPL, and we found that the compound certainly suppressed the SPL in most of 15 patients with HAM/TSP. In contrast to previous reports, however, the SPL induction in the 15 patients examined in this study was found to be largely dependent on MHC class II, CD86, and CD58 molecules but not on CD54 molecules (Fig. 1). Thus, the suppression of SPL by CI-959-A seemed to be independent of its inhibition of CD54 expression. Then we determined the effect of the compound on the HAM/TSP-associated APCs, the most important of which are DCs (17, 35) and HTLV-I-immortalized CD4+ T cells (15, 32). While CI-959-A exhibited little toxicity against nonproliferating DCs and their precursor monocytes and proliferating lymphocytes such as HTLV-I-infected T cells and T-cell lymphoma lines (Table 2) at the dose that suppressed T-cell proliferative responses to antigenic stimuli, this compound inhibited the differentiation of DCs from peripheral monocytes and the induction of expression of MHC class II, adhesion, and costimulatory molecules on activated CD4+ T cells (Fig. 2 and 4). In addition to the fact that CI-959-A suppressed CD54 Ag expression on PHA-activated CD4+ T cells, it more strongly affected the expression of HLA-DR and CD86 Ags. Therefore, the suppression of SPL in patients with HAM/TSP by CI-959-A might be more closely associated with its pharmacological action on disease-associated APCs.
The MAbs to CD80 or CD86 are shown to be effective in the prevention of autoimmune diseases in vivo in animal models of disease such as experimental autoimmune encephalomyelitis (14) and nonobese diabetes observed in NOD mice (16). There are also several human autoimmune diseases including rheumatoid arthritis in which DCs, CSMs, and autoreactive T cells play major roles in the induction and progression of disease (5, 33, 34). The culture of peripheral monocytes in the presence of rGM-CSF and rIL-4 results in a directed differentiation into DCs (27, 29) that bear the phenotypic and functional characteristics of immature DCs (28, 29). Upon exposure to inflammatory cytokines, these cells undergo a maturation step and express CD83 Ag (2, 23, 26, 37). The fact that CI-959-A inhibited the differentiation of the immature type of DCs (CD83− DCs) as well as the mature type of DCs (CD83+ DCs) suggests that it suppresses both the uptake of Ag by CD83− DCs and the presentation of Ag to naive T cells by CD83+ DCs. Furthermore, while CI-959-A affected the induction of MHC class II and CD86 molecules, it had no effect on the constitutively expressed molecules including CD28 and CD2 (Fig. 4). This may suggest that this compound is most effective at the phase of induction and rapid progression of diseases.
A derivative of CI-959-A, termed PD144795, is reported to inhibit casein kinase II (4) and quite recently has been reported to inhibit calcineurin (7). Although these pharmacological effects are associated with the transcriptional inhibition of human immunodeficiency virus type 1 and the blocking of transactivation of the human immunodeficiency virus type 1 long-term repeat, the relationship between those effects and the suppression of CSM expression and DC generation by CI-959-A has not been uncovered. Furthermore, it is not yet clear whether CI-959-A could similarly suppress casein kinase II and calcineurin. In terms of the chemotherapy and prophylaxis of autoimmune diseases such as HAM/TSP, CI-959-A seems to be a potent drug candidate that should be further pursued.
ACKNOWLEDGMENTS
This work was supported in part by a Grant-in-Aid for a Second-Term Comprehensive 10-year Strategy for Cancer Control from the Ministry of Health and Welfare of Japan.
We acknowledge the contribution of N. Makino in the preparation of the manuscript. We thank M. L. Robbins for reviewing the manuscript.
REFERENCES
- 1.Azuma M, Ito D, Yagita H, Okumura K, Phillips J H, Lanier L L, Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature (London) 1993;366:76–79. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 2.Bender A, Sapp M, Schuler G, Steinman R M, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 3.Boschelli D H, Kramer J B, Connor D T, Lesch M E, Schrier D J, Ferin M A, Wright C D. 3-Alkoxybenzo[b]thiophene-2-carboxamides as inhibitors of neutrophil-endothelial cell adhesion. J Med Chem. 1994;37:717–718. doi: 10.1021/jm00032a001. [DOI] [PubMed] [Google Scholar]
- 4.Critchfield J W, Coligan J E, Folks T M, Butera S T. Casein kinase II is a selective target of HIV-1 transcriptional inhibitors. Proc Natl Acad Sci USA. 1997;94:6110–6115. doi: 10.1073/pnas.94.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis L S, Kavanaugh A F, Nichols L A, Lipsky P E. Induction of persistent T cell hyporesponsiveness in vivo by monoclonal antibody to ICAM-1 in patients with rheumatoid arthritis. J Immunol. 1995;154:3525–3537. [PubMed] [Google Scholar]
- 6.Elovaara I, Koenig S, Brewah A Y, Woods R M, Lehky T, Jacobson S. High human T cell lymphotropic virus type 1 (HTLV-1)-specific precursor cytotoxic T lymphocyte frequencies in patients with HTLV-1-associated neurological disease. J Exp Med. 1993;177:1567–1573. doi: 10.1084/jem.177.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gualberto A, Marquez G, Carballo M, Yungblood G L, Hunt III S W, Baldwin A S, Sobrino F. p53 transactivation of the HIV-1 long terminal repeat is blocked by PD 144795, a calcineurin-inhibitor with anti-HIV properties. J Biol Chem. 1998;273:7088–7093. doi: 10.1074/jbc.273.12.7088. [DOI] [PubMed] [Google Scholar]
- 8.Hollsberg P, Hafler D A. Pathogenesis of diseases induced by human lymphotropic virus type I infection. N Engl J Med. 1993;328:1173–1182. doi: 10.1056/NEJM199304223281608. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson S, Shida H, McFarlin D E, Fauci A S, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature (London) 1990;348:245–248. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins M K. The role of cell division in the induction of clonal anergy. Immunol Today. 1992;13:69–73. doi: 10.1016/0167-5699(92)90137-V. [DOI] [PubMed] [Google Scholar]
- 11.June C H, Bluestone A, Nadler L M, Thompson C B. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 12.Katahira Y, Yashiki S, Fujiyoshi T, Nomura K, Tara M, Tomita M, Setoyama M, Kanzaki T, Shida H, Sonoda S. In vitro induction of cytotoxic T lymphocytes against HTLV-I-infected T-cells from adult T-cell leukemia patients, asymptomatic HTLV-I carriers and seronegative healthy donors. Jpn J Cancer Res. 1995;86:21–27. doi: 10.1111/j.1349-7006.1995.tb02983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimata J T, Palker T J, Ratner L. The mitogenic activity of human T-cell leukemia virus type I is T-cell associated and requires the CD2/LFA-3 activation pathway. J Virol. 1993;67:3134–3141. doi: 10.1128/jvi.67.6.3134-3141.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuchroo V K, Dsa M P, Brown J A, Ranger A M, Zamvil S S, Sobel R A, Weiner H L, Nabavi N, Glimcher L H. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 15.Lal R B, Rudolph D L, Dezzutti C S, Linsley P S, Prince H E. Costimulatory effects of T cell proliferation during infection with human T lymphotropic virus type I and II are mediated through CD80 and CD86 ligands. J Immunol. 1996;157:1288–1296. [PubMed] [Google Scholar]
- 16.Lenschow D J, Ho S C, Sattar H, Rhee L, Gray G, Nabavi N, Herold K C, Bluestone J A. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macatonia S E, Cruickshank J K, Rudge P, Knight S C. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-I and stimulate autologous lymphocyte proliferation. AIDS Res Hum Retroviruses. 1992;8:1699–1706. doi: 10.1089/aid.1992.8.1699. [DOI] [PubMed] [Google Scholar]
- 18.Makino M, Baba M. Establishment of a cryopreservation method of human peripheral blood mononuclear cells for efficient production of dendritic cells. Scand J Immunol. 1997;45:618–622. doi: 10.1046/j.1365-3083.1997.d01-441.x. [DOI] [PubMed] [Google Scholar]
- 19.Makino M, Yoshimatsu K, Azuma M, Okada Y, Hitoshi Y, Yagita H, Takatsu K, Komuro K. Rapid development of murine AIDS is dependent on signals provided by CD54 and CD11a. J Immunol. 1995;155:974–981. [PubMed] [Google Scholar]
- 20.Mueller D L, Jenkins M K, Schwartz R H. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 21.Nagai M, Yashiki S, Fujuyoshi T, Fujiyama C, Kitze B, Izumo S, Osame M, Sonoda S. Characterization of a unique T-cell clone established from a patient with HAM/TSP which recognized HTLV-I-infected T-cell antigens as well as spinal cord tissue antigens. J Neuroimmunol. 1996;65:97–105. doi: 10.1016/0165-5728(96)00002-1. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa M, Izumo S, Ijichi S, Kubota H, Arimura K, Kawabata M, Osame M. HTLV-I-associated myelopathy: analysis of 213 patients based on clinical features and laboratory findings. J Neurovirol. 1995;1:50–61. doi: 10.3109/13550289509111010. [DOI] [PubMed] [Google Scholar]
- 23.O’Doherty U, Steinman R M, Peng M, Cameron P U, Gezelter S, Kopeloff I, Swiggard W J, Pope M, Bhardwaj N. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J Exp Med. 1993;178:1067–1078. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, Matsumoto M, Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;i:1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 25.Prince H E, York J, Golding J, Owen S M, Lal R B. Spontaneous lymphocyte proliferation in human T-cell lymphotropic virus type I (HTLV-I) and HTLV-II infection: T cell subset responses and their relationships to the presence of provirus and viral antigen production. Clin Diagn Lab Immunol. 1994;1:273–282. doi: 10.1128/cdli.1.3.273-282.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roake J A, Rao A S, Morris P J, Larsen C P, Hankins D F, Austyn J M. Dendritic cell loss from non-lymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J Exp Med. 1995;181:2237–2247. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch P O, Steinman R M, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor a. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartz R H. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz R H. Costimulation of T lymphocytes: the role of CD28, CTLA-4 and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 32.Takamoto T, Makino M, Azuma M, Kanzaki T, Baba M, Sonoda S. HTLV-I-infected T cells activate autologous CD4+ T cells susceptible to HTLV-I infection in a costimulatory molecule-dependent fashion. Eur J Immunol. 1997;27:1427–1432. doi: 10.1002/eji.1830270620. [DOI] [PubMed] [Google Scholar]
- 33.Thomas R, Davis L S, Lipsky P E. Rheumatoid synovium is enriched in mature antigen-presenting dendritic cells. J Immunol. 1994;152:2613–2623. [PubMed] [Google Scholar]
- 34.Thomas R, Lipsky P E. Could endogenous self-peptides presented by dendritic cells initiate rheumatoid arthritis? Immunol Today. 1996;17:559–564. doi: 10.1016/s0167-5699(96)20030-1. [DOI] [PubMed] [Google Scholar]
- 35.Usuku K, Sonoda S, Osame M, Yashiki S, Takahashi K, Matsumoto M, Sawada T, Tsuji K, Tara M, Igata A. HLA haplotype-linked high immune responsiveness against HTLV-I in HTLV-I-associated myelopathy: comparison with adult T-cell leukemia/lymphoma. Ann Neurol. 1988;23:S143–S150. doi: 10.1002/ana.410230733. [DOI] [PubMed] [Google Scholar]
- 36.Wucherpfennig K W, Hollsberg P, Richardson J H, Benjamin D, Hafler D A. T cell activation by autologous human T cell leukemia virus type I-infected T cell clones. Proc Natl Acad Sci USA. 1992;89:2110–2114. doi: 10.1073/pnas.89.6.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou L-J, Tedder T F. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–3835. [PubMed] [Google Scholar]