Highlights
-
•
UP treatment was effective in microbial decontamination of crayfish.
-
•
The applied treatments suppressed the protein oxidation during storage.
-
•
UP treatment retarded the flavor and textural deterioration of crayfish.
-
•
UP treatment reduced the water migration in crayfish during storage.
Keywords: Aquatic product, Decontamination, Preservation, Microbial quality, Physicochemical properties
Abstract
In this study, a decontamination technology combining ultrasound (US) and plasma-activated water (PAW) was developed to better preserve crayfish. First, the decontamination efficacy of US, PAW and their combinations (UP) on crayfish was quantified after 0, 20, 40, or 60 min of treatments. The total viable count (TVC) was reduced by 0.27–0.77 Log CFU/g after individual US or PAW treatments, while a TVC reduction of 1.17 Log CFU/g was achieved after 40 min of UP treatment. Besides, the changes in psychrotrophic bacteria, lactic acid bacteria, yeasts and molds followed a similar trend to TVC. UP treatments normally resulted in more significant reductions in the natural microbiota of crayfish than US or PAW treatments. Furthermore, the microbial quality, physicochemical properties and sensory properties of crayfish after different treatments were assessed during storage at 4 °C for 12 days. According to TVC and total volatile basic nitrogen (TVB-N) values, the control group became unacceptable from 4 days, US or PAW groups became unacceptable from 6 days, while UP group extended the storage time to 8–10 days. During storage, thiobarbituric acid reactive substances (TBARS) values of all the groups were maintained below 0.5 mg/kg, among which the control group exhibited the highest value (0.39 mg/kg). Moreover, UP treatment effectively retarded the deterioration in color and texture properties of crayfish. Fourier transform infrared (FTIR) spectroscopy analysis indicated that UP treatment decreased the α-helix contents and increased the β-sheet contents of crayfish proteins, while the structural changes were not evident at the end of storage. Low-field nuclear magnetic resonance (LF-NMR) analysis revealed that UP treatment reduced the water migration and enhanced the stability of bond water in crayfish. In addition, E-nose analysis revealed the protection of UP treatment on the sensory properties of crayfish during storage. This study demonstrated that the combinations of US and PAW treatments effectively accelerated the decontamination of crayfish and contributed to better storage quality.
1. Introduction
Crayfish (Procambarus clarkii) is the third largest farmed crustacean species in the world [1]. Owing to the high nutritional value and delicious taste, crayfish is gaining increasing popularity among consumers. In China, approximately 1.64 million tons of crayfish were consumed each year, while more than 2.64 million tons were annually outputted [2], [3]. Nevertheless, Crayfish is extremely perishable due to the abundant nitrogenous compounds and high levels of natural microorganisms. Before food processing, most crayfish have to undergo several days of storage or long logistics transportation. During this period, undesirable microbial growth potentially leads to the spoilage of crayfish and even causes food safety risks [4]. Therefore, reducing the initial microbial loads is an essential step to maintain the quality of crayfish during storage. However, conventional water washing exerts limited decontamination efficacy and might cause cross-contamination on crayfish. To improve decontamination efficiency, chemical sanitizers (e.g., chlorination) have been generally utilized over the past decades, potentially leaving chemical residues and associated with carcinogenic by-product formation [5]. Therefore, chemical sanitizers present a decreasing acceptance by consumers in the recent years, forcing manufacturers to seek alternative techniques to maintain the quality of crayfish in a safe, effective, and eco-friendly way.
Various novel techniques have been proposed for aquatic product decontamination, such as pulsed light, cold plasma, ultrasound (US), ozonation, gamma radiation, pulsed electric field (PEF) and ultraviolet. These decontamination technologies are generally effective and environmentally friendly, meanwhile minimizing negative effects on the food quality [6]. However, some of these methods are limited in practice due to the potential adverse effects. For instance, pulsed light has limited penetrating capacity and requires high capital cost [7]. Besides, long exposure time of pulsed light could raise product temperatures, resulting in quality degradation [8]. Cold plasma is ineffective for decontaminating inner layers of foodstuffs and not suitable for high-fat foods [9]. Moreover, more research is required to scale up the technology for industrial use [10]. For US treatment, excessive processing might result in degradation of food properties, including lipid oxidation, color and texture changes, and off-flavors. Besides, the decontamination effect of US treatment is insufficient towards high levels of microorganisms. Ozone and irradiation potentially lead to lipid oxidation, surface discoloration, degradation of vitamins and phenolic compounds, and formation of off-flavor in food products [11]. Ultraviolet is limited in application due to the low penetration depth, long processing time and public apprehensiveness for its safety [11]. For PEF, the decontamination efficiency is heavily dependent on the electrical conductivity of the treated products and requires high costs of set-up and maintenance [9]. Among the novel techniques, plasma-activated water (PAW) has been demonstrated as an effective antimicrobial disinfectant without changing the product characteristics [12]. PAW is a functionalized liquid generated by treating water with a plasma source, which triggers chemical reactions between gaseous plasma and liquid water, generating antimicrobial components, principally reactive oxygen species (ROS) and reactive nitrogen species (RNS) [13]. The antimicrobial efficiency of PAW has been demonstrated on a wide range of microorganisms, including spoilage microorganisms and foodborne pathogens [5], [14], [15]. Besides, PAW has been applied in the preservation of various food categories, such as fruits, vegetables, meats and bakery products [12], [13], [16], [17], [18], [19]. Currently, only limited studies focused on the effect of PAW on the preservation of aquatic products [13], [15], [16], [19], [20], while the antimicrobial efficiency of PAW can be heavily influenced by the treated food matrix [15]. The irregular surface of crayfish provides numerous niches for microbial growth, thus hampering the decontamination effect of PAW. To overcome this deficiency, the hurdle technology combining US with PAW can be a preeminent strategy. Generally, the combinations of hurdles are more energy-efficient and simultaneously diminish the deterioration of food qualities. US has attracted worldwide interest in the food processing field [21], [22], [23], [24], [25]. Although the antimicrobial effect of individual US treatment is limited [26], it could significantly benefit the decontamination effect of PAW, especially on the foodstuffs with rough surfaces [19]. On the one hand, the cavitation effect of US could generate a strong jet at the solid–liquid interface, therefore dislodging the microorganisms attached to the food surface [26], [27]. On the other hand, the propagation of sound waves generated by US could increase the exposure of hard-to-reach spots to PAW, therefore enabling maximal microbial reductions [28]. Moreover, the cavitation could facilitate the penetration of PAW into microbial cell membranes, thus accelerating microorganism disaggregation [29].
Nevertheless, limited studies are currently available regarding the combined effects of PAW and US on the preservation of aquatic products, while none of them is focused on crustaceans [13], [19]. Although combinations of US and PAW treatments could improve their decontamination efficiency, they potentially affect the quality properties of crayfish, such as protein and lipid oxidation, color and texture changes, and off-flavors. Moreover, the residual PAW on the crayfish after decontamination could further influence their qualities during storage [16]. However, quality changes of the treated samples during storage were still unclear. Therefore, the objective of this study was to quantify the individual and combined effects of US and PAW treatments on the preservation of crayfish. In addressing this objective, the decontamination effects of US, PAW and their combinations on the crayfish were quantified. Furthermore, the microbial quality, physicochemical properties and sensory properties of crayfish were assessed during refrigerated storage. This is one of the first studies which explored the combined effects of PAW and US on the decontamination of crustaceans, as well as their influence on the food qualities during storage.
2. Materials and methods
2.1. Sample collection and preparation
Fresh crayfish (Procambarus clarkia) were purchased from a local supermarket (Nanjing, China). The Crayfish with a similar body weight (25 ± 2 g), size (8 ± 1 cm) and apparent color (red shell) were selected as the experimental samples. The crayfish were kept in a sealed polyethylene box with a crayfish/ice ratio of 1:2 (w/w) and transported to the laboratory within 1 h. Upon arrival, the live crayfish were immediately killed by being immersed in ice water and then treated with the subsequent decontamination procedures.
2.2. Preparation and characterization of PAW
2.2.1. Generation of PAW
A non-thermal atmospheric pressure plasma system (PG-1000Z/D, Nanjing Suman Electronics Co., Ltd., Nan Jing, China) was used to generate PAW. As shown in Fig. 1, the system consisted of a high-voltage generator (800 W), plasma jet nozzle and gas control device. Compressed air at 0.18 MPa was used as working gas with a flow rate of 20–30 L/min. The device was set up to discharge PAW beneath the water surface at a distance of 0.5 cm between the end of the plasma jet and the water surface. Every 1000 mL of sterile distilled water (DW) was activated by cold plasma for 1, 3, 5, 7 and 10 min.
Fig. 1.
A schematic diagram of the plasma jet and the experimental arrangement.
2.2.2. US treatments
To investigate the influence of US treatments on the antimicrobial effect of PAW, the generated PAW was sonicated for different time periods. In detail, the US treatments were performed by a 4 L US tank (KQ-100DE, Ultrasonic Instrument Co., Ltd., Kun Shan, China) filled with 3 L distilled water. The US was operated at a frequency of 40 kHz with a power of 100 W. The effective power dissipated in the system was determined by the calorimetric method as 79.13 W, using the equation below:
| (1) |
where Pdiss is ultrasonic power dissipated into the water, Cp is the specific heat capacity per unit mass of water (4187 J/kg K), M is the mass of the water in the tank (L), and dT/dt is the slope of the temperature rise versus time (K/s).
The temperature during the process was controlled under 25 °C by a circulating water bath. After generation, 500 mL PAW was transferred into a 1000 mL glass beaker placed in the middle of the US tank, and sonicated for 0, 5 and 15 min.
2.2.3. Characterization of PAW
To quantified the basic physicochemical properties of PAW, the electrical conductivity (EC), pH and oxidation–reduction potential (ORP) of PAW were measured immediately by using a multimeter (DZS-708 T, Lei Ci Co., Ltd., Shang Hai, China). The concentrations of nitrate (NO3–) and nitrite (NO2–) in PAW were determined via spectrophotometry method as described previously (Shen et al., 2016). The O3 concentration was measured by a benchtop ozone meter (DOZ30, Chuangyue Environmental Protection Technology Co., Ltd., Guangzhou, China).
2.3. Decontamination treatment
For individual PAW treatment, the crayfish samples were immersed in a 1000 mL glass beaker with 500 mL PAW, and gentle agitations were performed to provide uniform decontamination. The total treatment time in PAW was 0, 20, 40 and 60 min. For individual US treatment, the crayfish samples were immersed in a 1000 mL glass beaker with 500 mL DW. Then the glass beaker with crayfish samples was placed in the middle of the US tank and treated with US for 10 min. For the combinations of US and PAW (UP) treatments, the crayfish samples were immersed in a 1000 mL glass beaker with 500 mL PAW and placed in the middle of the US tank. After 10 min of US treatment, the crayfish samples were further agitated in the PAW for 10, 30 and 50 min. Therefore, the total UP treatment time was 0, 20, 40 and 60 min. The crayfish samples without any treatment were used as the control. For all the treatments, five samples were treated each time. After decontamination, the crayfish samples were packaged with sterile polyamide/polyethylene bags (dimensions: 200 × 270 mm). Then all the packaged samples were stored at 4 °C for 12 days and analyzed every two days.
2.4. Microbiological analysis during storage
The microbiological quality of the crayfish samples was determined every two days according to the method of Sun et al. [30]. For each group, three crayfish samples were randomly selected on the day of analysis and blended using a stomacher (Nanjing Ningkai instrument Co. Ltd., XC07-II, China). Afterward, an aliquot (15 ± 1 g) of the crayfish mixtures was brought into a sterile stomacher bag aseptically in a laminar flow bench (SJ-CJ-1D, Suzhou purification equipment Co. Ltd., China), and homogenized with Peptone Physiological Solution (PPS) (1 g/L peptone (Sinopharm Group Co., Ltd., Shanghai, China) + 8.5 g/L NaCl (Sinopharm)). Subsequently, a decimal dilution series was prepared in PPS and the appropriate dilutions were plated on different media for the enumeration of different microbial populations. The total viable count (TVC, Hopebio, Qingdao, China) was determined by spread plating on plate count agar (PA, Hopebio) plates and incubated at 37 °C for 2 days, while the total psychrotrophic count (TPC, Hopebio) was determined on PA plates and incubated at 7 °C for 14 days. The cell counts of lactic acid bacteria (LAB) were determined on deMan, Rogosa Sharpe (MRS, Hopebio) plates and incubated at 30 °C for 5 days. Yeasts and molds (Y&M, Hopebio) were counted on Rose Bengal Chloramphenicol Agar (RBCA; Hopebio) and incubated at 22 °C for 5 days.
2.5. Physicochemical analysis during storage
2.5.1. Color analysis
The color of the crayfish samples was measured using a CM-700d spectrophotometer (Konica Minolta, Shanghai, China) with standard blackboard zeroing and calibrated with a standard white plate, according to the method in Wei et al [31]. The samples were selected from the 2nd to 3rd abdominal segments of crayfish tails for analysis. The working parameters of the CM-700d spectrophotometer were set to sci mode, 10° observation angle, and D65 illuminant. The CIE color coordinates of L*(lightness/darkness), a* (redness/greenness), and b* (yellowness/blueness) of each sample were recorded. The total color difference (ΔE) was calculated using the following equation:
| (2) |
where L0*, a0* and b0* were the initial values of L*, a* and b*.
2.5.2. Texture property analysis (TPA)
The texture properties (hardness, cohesiveness, adhesiveness, springiness and chewiness) of the crayfish were determined using a texture analyzer (TMS-Touch, FTC Co., Ltd., US), connected to a 100 N load-cell. The second and third segments of the crayfish were selected for TPA, and the analysis was conducted using a Cylinder probe (75 mm in diameter). TPA is constructed of 2 consecutive compression cycles, imitating the action of the masticate. The test speed, deformation ratio, and trigger force were set as 1 mm/s, 30%, and 0.15 N, respectively.
2.5.3. Thiobarbituric acid reactive substances (TBARS) analysis
The TBARS of the crayfish samples was measured according to the method described by Bian et al. [32] with minor modifications. In brief, 5 g of the crayfish samples were homogenized with 50 mL of 7.5% (w/v) trichloroacetic acid (TBA) and 0.1% (w/v) ethylenediaminetetraacetic acid (EDTA) for 1 min. The homogenate was centrifuged twice at 8000 × g at 4 °C for 10 min. Then, 5 mL of the supernatant was mixed with 5 mL of 0.02 mol/L TBA solution, followed by heating at 90 °C for 40 min in a hot water bath. Besides, 5 mL of distilled water instead of the supernatant was used as the blank. After cooling to room temperature, the absorbance was determined at 532 nm using a UV–VIS spectrophotometer (UV-6300; Mapada Instruments, Shanghai, China). The TBARS values of the samples were determined according to the standard curve of malondialdehyde (MDA).
2.5.4. Total volatile basic nitrogen (TVB-N) analysis
The TVB-N of the crayfish samples was measured according to the method described by Liao et al. [16]. In brief, about 5 ± 1 g sample was homogenized in 50 mL distilled water and stirred for 30 min. Then, the mixture was centrifuged at 8000 g for 1 min (4 °C) and 5 mL of the supernatant was mixed with 5 mL of 0.1% (w/v) MgO. Afterward, the mixture was mixed with 10 mL 2% (w/v) boric acid containing two or three drops of a mixed indicator, which consisted of 0.1% methyl red and 0.5% bromocresol green. Then, 0.01 M HCl was used for titration until a faint pink color appeared. The TVB-N values were calculated using the following equation:
| (3) |
where V and V0represent the volumes (mL) of HCl required for titration of the tested mixture and the blank, respectively, and m was the mass (g) of the crayfish sample.
2.6. Fourier transform infrared (FTIR) spectroscopy analysis
FTIR was measured as described by Tian et al. [33] with minor modifications. The crayfish samples were freeze-dried (Boyikang Experimental Instrument Co. LTD., Beijing, China) and ground to powder, which were then fully mixed with dried potassium bromide (1:100, w/w) for grinding. Afterward, the mixtures were pressed into a slice (0.3–0.5 mm thick) by a tablet-forming machine (Kimbell Technology Co. LTD, Tianjin, China). Infrared spectra were recorded using an ATR-FTIR spectrometer Nicolet iS50 FTIR (Thermo ScientificTM Co., Ltd., USA) with 32 scans in a range of 400–4000 cm−1 at a 4 cm−1 resolution. The recorded spectra were analyzed by OMNIC9.2 (Thermo Nicole Inc., Waltham, MA, USA), and the secondary structures of the crayfish proteins was determined by Peakfit 4.12 (SeaSolve Software, Inc., Framingham, MA, USA). The contents of α-helix, β-sheet, β-turn, and random coil structures were calculated in percentage.
2.7. Low-field nuclear magnetic resonance (LF-NMR) analysis
LF-NMR was measured by using MesoMR23-060H-I NMR imaging and analysis system (Suzhou Niumag Analytical Instrument Co., Ltd., Suzhou, China), according to the method of Han et al. [34]. The crayfish was placed in a glass tube (outer diameter: 60 mm) and inserted into the NMR probe. The spin–spin relaxation time of water was measured based on Carr-Purcell-Meiboom-Gill (CPMG) pulse sequences. The test settings were as follows: a 14 μs 90° pulse followed by a 23.04 μs 180° pulse; waiting time, 3000 ms; repeated scan number, 16; echo count, 15,000.
2.8. Electronic nose (E-nose) analysis
The aroma compounds of the crayfish samples were analyzed using a computerized E-nose PEN3 (Airsense Analytics Co., Ltd., Schwerin, Germany), according to the method described by Xu et al. [35]. Briefly, crayfish samples (4.0 g) were placed in a 20 mL headspace bottle closed hermetically by plastic film and equilibrated at 25 ± 1 °C for 1 h. Afterward, the volatile compounds were carried by ultrapure air at a 300 mL/min gas flow rate with a pre-sampling time of 10 s and a sampling time of 80 s. The analysis was based on the array system of ten sensors (Table 1), including W1C, W1S, W1W, W2S, W2W, W3C, W3S, W5C, W5S, and W6S. Before each measurement, the sensors were flushed with clean air for around 80 s until the sensor signals returned to baseline. The collected data, based on the sensor matrix, were processed and computerized once every second during the E-nose assessment.
Table 1.
Sensor sensitivities within the sensor array of the PEN3 E-nose.
| Order number | Sensor name | Sensitive compound |
|---|---|---|
| 1 | W1C | Aromatic components |
| 2 | W5S | Broad range, oxynitride |
| 3 | W3C | Ammonia, aromatic components |
| 4 | W6S | Hydrogen |
| 5 | W5C | Short chain alkanes, aromatic components |
| 6 | W1S | Methane |
| 7 | W1W | Sulphur-organic compound |
| 8 | W2S | Alcohol, aldehyde ketone |
| 9 | W2W | Organic sulfur compounds, aromatic components |
| 10 | W3S | Long chain alkanes |
2.9. Statistical analysis
All the experiments were conducted in triplicate and the results were expressed as the mean ± standard deviations. The data were assessed by one-way analysis of variance (ANOVA), and the significant difference between the mean values was determined by Duncan’s multiple range test (p < 0.05) using SPSS statistical software (version 19.0, SPSS, Inc., Chicago, IL, USA). Origin pro-2019 and excel 2016 software were used in the graphical report.
3. Results and discussion
3.1. Characterization of PAW
The basic physicochemical properties of PAW with different plasma activation and ultrasonic time were shown in Fig. 2. Overall, the values of all the measured parameters (ORP, EC, pH, O3, NO2– and NO3–) significantly depended on the plasma activation time, while slight increases in the EC values, O3 and NO2– concentrations were observed after the applied US treatments. Similarly, Royintarat et al. [36] observed that a US treatment (40 Hz) did not result in significant changes in the ORP and pH values of PAW. With the plasma activation time from 1 to 10 min, the ORP values of PAW raised from 394.49 to 435.16 mV (Fig. 2a), and the EC values raised from 132.20 to 900.33 μS/cm (Fig. 2b). In comparison, the ORP value and EC value of distilled water are 238.98 and 2.71 μS/cm, respectively. The results are in accordance with previous studies [19], [37], [38]. Guo et al. [37] observed that the ORP value of distilled water was 236.29 ± 2.95 and notably increased with activation time. It has been approved that PAW with high ORP and EC values could damage the membrane of microorganisms [5]. ORP indirectly indicated the levels of global ROS in PAW, while EC could determine the presence of active ions in PAW. The results of the current study indicated that abundant active ions were generated in PAW, including ROS and other reactive species, which contributed to its antimicrobial activity.
Fig. 2.
Physicochemical properties of PAW after different cold plasma activation time and ultrasonic time: (a) ORP values, (b) EC values, (c) Ozone concentrations, (d) Nitrite concentrations (e) Nitrate concentrations, and (f) pH values.
As shown in Fig. 2c, a nearly linear increase was observed in the O3 concentrations along with prolonged activation time, reaching a maximum value of 30.08 μmol/L. Besides, both NO2– (Fig. 2 d) and NO3– (Fig. 2e) concentrations exhibited an increasing trend with longer activation time and reached the maximum values of 120.00 mg/L and 138.85 mg/L, respectively. The reactions of H2O, N2 and O2 molecules during the plasma activation can generate abundant reactive species, principally ROS and RNS, playing a major role in the antimicrobial effect of PAW [39]. O3 is typically a long-lived species of ROS, while NO2– and NO3– are major species of RNS. These reactive species potentially result in oxidative and nitrosative damage to microorganisms by DNA destruction, lipid peroxidation, and inhibition of enzyme activity [14]. In addition, the applied US treatments further increased the O3 and NO2– concentrations of PAW to 35.58 μmol/L and 135.38 mg/L, implying an enhanced antibacterial ability.
Furthermore, the acidic species (e.g., nitrate and nitrite acid) generated during the plasma activation have been demonstrated to cause acidification of PAW. As shown in Fig. 2f, the pH values rapidly decreased from 6.6 to 3.6 during the first minute of plasma activation, then gradually decreased to 2.7 with the activation time increased to 7 min (P < 0.05), and then reached a steady state during 7–10 min. The faster decrease rate of pH values during the initial stage of plasma activation was observed previously [38], [40]. For instance, Xu et al. [38] observed that the pH values quickly dropped during the first 5 min of activation and then slowly dropped until achieving a steady state. pH is an important indicator of the antimicrobial ability of PAW. The low pH values and reactive species in PAW could generate a synergistically antimicrobial effect, as acid conditions would favor the penetration of reactive species into bacterial cell walls, making microorganisms more susceptible to ROS [38].
3.2. Microbial decontamination efficacy
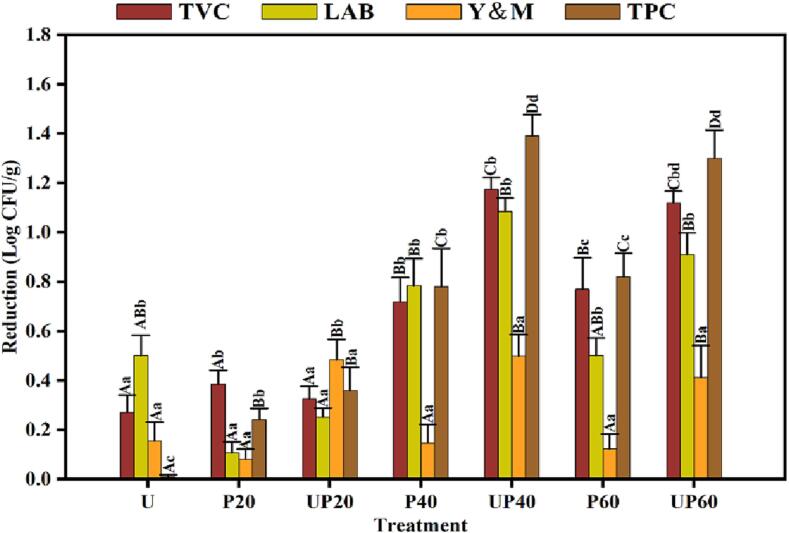
The microbial decontamination efficacy of different treatments (US, PAW and their combination) on the natural microbiota (TVC, LAB, Y&M and TPC) of crayfish is presented in Fig. 3. Fresh crayfish without any treatment was regarded as control, with a TVC around 5.65 log CFU/g, which is consistent with the reported values (5.00–6.44 log CFU/g) [2], [41]. For individual US treatment, a limited decontamination effect was observed towards the natural microbiota of crayfish, indicated by a TVC reduction of 0.27 log CFU/g. For individual PAW treatment, 0.39, 0.72 and 0.77 log CFU/g of TVC were decreased by 20, 40, and 60 min of treatments, respectively. After combining US with PAW, a TVC reduction of 1.17 was achieved after 40 min of UP treatment, revealing the synergistic decontamination effect of US and PAW treatments. Besides, the changes of LAB, Y&M, and TPC followed a similar trend to TVC, the combination of US and PAW normally resulted in more significant reductions than the individual treatments. Regarding LAB, 20, 40, and 60 min of PAW treatments resulted in a decrease of 0.11, 0.75, 0.56 log CFU/g, respectively. After combining US with PAW, a reduction of 1.09 log CFU/g was achieved after 40 min of UP treatment. In addition, no obvious reduction for TPC of crayfish was observed after individual US treatment, a reduction of 0.78 log CFU/g was observed after 40 min of individual PAW treatment, and a reduction of 1.39 log CFU/g was achieved after 40 min of UP treatment. The limited decontamination efficacy of individual US treatment was also observed towards the pathogens or natural microbiota on aquatic products [19], [42]. Besides, the decontamination efficacy of individual PAW treatments was less effective towards Y&M, compared with other investigated microbial groups. It was due to the fact that Y&M was more resistant to PAW [40]. When combing US with PAW, a more significant reduction was achieved in Y&M, highlighting the significance of their combinations on microbial decontamination. Currently, only a few studies focused on the combined decontamination effects of US and PAW on aquatic products. Zhao et al. [13] observed that 10 min of PAW treatment resulted in a reduction of 0.24 log CFU/g on the TPC of mackerel fillets, while a reduction of 0.38 log CFU/g was achieved by the combination of PAW with US treatment. Esua et al. [19] observed that the combinations of PAW and US were more effective in reducing the pathogens on grass carp, with significant reductions of 1.39 and 1.49 log CFU/g for Escherichia coli and Shewanella putrefaciens, respectively. It needs to be noted that the natural microbiota of aquatic products varied depending on the species and habitats, which could heavily influence the decontamination efficacy of applied treatments. Additionally, lengthening PAW or UP treatment time from 40 to 60 min failed to achieve more significant reductions in the microbial counts of crayfish, implying the limited contribution of treatment time after 40 min. Therefore, 40 min of PAW and UP treatments were chosen for further experiments.
Fig. 3.
The microbial reductions of the crayfish after different treatments. Error bars represent standard errors of measurements. Different lowercase letters indicate significant (P < 0.05) differences between microbial groups, while different uppercase letters indicate significant (P < 0.05) differences between treatments.
3.3. Microbial quality of the crayfish during storage
To evaluate the microbial quality of the crayfish during storage, microbial population changes (TVC, TPC, LAB and Y&M) of the crayfish after different treatments were monitored at 4 °C (Fig. 4). During the whole storage period, the crayfish after UP treatment generally exhibited the lowest microbial counts among all the treatments, irrespective of the investigated microbial populations. More specifically, TVC, TPC, LAB and Y&M of the crayfish after UP treatment at day 0 of storage were 4.48, 2.46, 2.30 and 2.87 log CFU/g, respectively. It revealed that the highest antimicrobial efficiency was achieved by UP treatment. Moreover, all the investigated microbial populations continuously increased with prolonged storage time.
Fig. 4.
Microbial changes of the crayfish after different treatments during storage at 4 °C: (a) TVC, (b) LAB, (c) Y&M, (d) TPC, (e) TVB-N and (f) TBARS.
Generally, a TVC below 6.00 log CFU/g represents an acceptable level for the microbial quality of aquatic products [16]. Accordingly, the TVC of the control group became unacceptable from the 4th day, US or PAW groups became unacceptable from the 6th day, while UP group exceeded the acceptable limit until the 10th day. Besides, TPC of the control, US and PAW groups exceeded 6.00 log CFU/g at day 8 of storage, while a delay of 2 days was observed in UP group. Psychotropic bacteria have been demonstrated as the major microbial group that was responsible for the spoilage of aquatic products stored in cold conditions [43]. It is not surprising that the growth of psychotropic bacteria was more rapid than other investigated microorganisms, as the storage temperature (4 °C) in the current study was more optimal for the growth of psychotropic bacteria than mesophilic bacteria. The result indicated that UP treatment achieved a significantly antimicrobial effect on the psychotropic bacteria of crayfish. Moreover, the growth of LAB in the crayfish samples was evaluated, which has been reported as a major group causing the spoilage of aquatic products [44]. As illustrated in Fig. 4b, the growth of LAB followed a similar trend as TVC, UP group always exhibited the lowest counts during storage, compared with other investigated groups. However, it needs to be noted that the LAB for all the groups kept below 6.00 log CFU/g during the whole storage period. It implied that LAB should not be the major spoilage bacteria that directly caused the decay of crayfish in the current study. As for Y&M, only UP treatment exerted a significant inhibition during the first 4 days of storage, while no obvious differences among the investigated groups were observed after 4 days. As the application of PAW to aquatic product preservation is still at the initial stages of development, limited data are currently available regarding the effect of PAW and its related technologies on the microbial qualities of aquatic products during storage. Liao et al. [16] observed that the TVC of the shrimps on TW ice exceeded 6.00 log CFU/g on day 6 of storage, while that on PAW ice was below the acceptable limit until day 8 of storage. In the current study, a shelf-life extension of 4 days was obtained after the applied UP treatment compared with the US or PAW treatments, demonstrating the enhanced antimicrobial effect after their combinations. This enhanced antimicrobial effect of UP treatment might result from the cavitation generated by the US treatment, which improved the contact area between PAW and the microorganisms [15], [41]. Besides, the cavitation was generally accompanied with localized pressure spots and high temperature, which could make the bacterial cytoplasmic membranes fragile and facilitate the penetration of reactive species into bacterial cell walls [26].
3.4. Physical quality of the crayfish during storage
3.4.1. Changes in TVB-N values
During storage, the protein degradation levels of the crayfish after different treatments were estimated using TVB-N values (Fig. 4e). The TVB-N values of the control, US, PAW and UP groups were 10.5 mg/100 g, 6.65 mg/100 g, 6.3 mg/100 g, and 5.25 mg /100 g, respectively. The TVB-N value of untreated crayfish was corresponding to the previously reported values, which ranged from 6.95 to 16.00 mg/100 g [34], [45]. TVB-N represents the undesirable nitrogen-containing compounds generated from the protein degradation of food products, such as ammonia, dimethylamine and trimethylamine. During the decay of aquatic products, TVB-N would be produced by the activities of bacteria and endogenous enzymes, generating unpleasant odors. Therefore, the TVB-N level can be used as an important indicator to estimate the freshness of crayfish. It has been recommended that the accepted limit of TVB-N value for aquatic products is 20 mg/100 g, exceeding which indicated inedible products [46]. Accordingly, the crayfish in the control group became unacceptable from the 4th day, individual US group remained acceptable until the 6th day, while PAW or UP groups exceeded the acceptable limit on the 8th day. The results indicated that PAW efficiently inhibited the production of TVB-N, which might result from suppressing the activities of spoilage bacteria and endogenous enzymes [47]. Besides, the lower increase of TVB-N in US group might be due to the potentially bacterial damage induced by US treatment, which delayed microbial multiplication (Fig. 4a), therefore suppressing the spoilage process. Various results have been observed regarding the influence of PAW treatments on the TVB-N values of aquatic products [16], [19], [20], [47].For instance, Liao et al. [16] observed that PAW ice significantly inhibited the increase in TVB-N values of shrimps during storage. Zhu et al. [20] observed that 20 min of PAW treatment reduced the TVB-N values of salmon fillets from 17.27 to 12.00 mg/100 g. On the contrary, Esua et al. [19] observed that PAW treatment resulted in an increase in the TVB-N values of grass carp, which were further increased when combined with US treatment. The different effects of PAW can be attributed to multiple factors, such as the plasma exposure dose, treatment time, food compositions, types of target microorganisms, and storage conditions [20], [40], [48].
3.4.2. Changes in TBARS values
TBARS values were used to assess the degree of lipid oxidation for crayfish during storage. As shown in Fig. 4f, the initial TBARS value of untreated crayfish was 0.14 mg/kg, which was comparable to the value reported previously [34]. At day 0 of storage, TBARS values of the crayfish from all the investigated groups did not differ statistically. After 12 days of storage, slight increases occurred in the TBARS values of US, PAW and UP groups, while a significant increase was observed in the TBARS value of the control group, indicating lipid oxidation occurred during storage. lipid oxidation is an intricate mechanism [49]. In the first step, unsaturated fatty acids could react with oxygen molecules to form initial oxidation products, such as peroxides and hydroperoxides. Then, these hydroperoxides and peroxides will continuously degrade to produce secondary oxidation products such as aldehydes and ketones. Malondialdehyde (MDA) is a widely used indicator of overall changes in secondary oxidation products. TBARS indicates the degree of lipid oxidation by measuring the level of MDA production. Lipid oxidation initiated by autoxidation and enzymatic reaction would generate off-flavor, playing an important role in the spoilage of aquatic products [47]. It is generally recognized that the acceptable limit of TBARS values is 1–2 mg/kg for aquatic products, while off-flavors were sometimes detected with a TBARS value above 0.5 mg/kg [13], [19]. At the end of storage, the TBARS values of all the groups maintained below 0.5 mg/kg, among which the control group exhibited the highest value of 0.39 mg/kg. The low TBARS values among all the groups should be due to the low lipid contents of crayfish [50]. Zhu et al. [20] observed that 20 min of PAW treatment efficiently decreased the TBARS values of the salmon fillet from 0.18 to 0.03 mmol/kg. Zhao et al. [13] observed that individual PAW treatment increased the TBARS values of mackerel fillets, while no significant change was observed after combining US with PAW treatments. On the one hand, the oxidant agents in PAW or formed during US treatment potentially led to lipid oxidation of food products, resulting in an increase in TBARS [16]. On the other hand, the applied treatments could inhibit the growth of spoilage bacteria, therefore suppressing lipid oxidation [51]. Moreover, the inhibitory effects of US and PAW treatments on lipid oxidation might be attributed to the destruction of prooxidative enzymes, which triggered an antioxidative mechanism [13]. The results of the current study indicated that the applied treatments did not result in an evident oxidation to the lipids of crayfish, and significantly suppressed the lipid oxidation during storage.
3.4.3. Changes in color properties
Color is an important factor in evaluating the quality of aquatic products. The CIE color coordinates of the crayfish after treatments were monitored during storage (Table 2). For the control group, the initial L*, a* and b* value of crayfish was 42.27, 5.81 and 2.23, respectively. Similar color coordinates of crayfish were reported previously [26].
Table 2.
The color properties of crayfish during storage at 4 °C.
| Treatments | Storage time(d) |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | |
| L* | |||||||
| CK | 42.27 ± 0.98Aa | 39.85 ± 0.59ABa | 41.62 ± 1.22Ab | 37.80 ± 1.41Ba | 34.68 ± 1.55Ca | 34.81 ± 1.62Ca | 29.63 ± 2.11Da |
| U | 44.93 ± 0.95Ab | 42.91 ± 0.97ABb | 40.55 ± 0.77Cab | 41.35 ± 1.05BCb | 34.65 ± 1.23DEa | 36.14 ± 1.24 Da | 32.78 ± 0.98Eb |
| P | 44.62 ± 0.81Ab | 41.16 ± 0.88Ba | 40.51 ± 0.65Bab | 38.28 ± 0.67Ca | 38.28 ± 0.67Cb | 34.68 ± 0.56 Da | 33.06 ± 0.99Db |
| UP | 41.34 ± 1.01Aa | 40.00 ± 1.11ABa | 39.12 ± 0.92BCa | 37.01 ± 0.68 Da | 37.38 ± 0.79CDb | 35.60 ± 0.88 Da | 36.12 ± 0.86Dc |
| a* | |||||||
| CK | 5.81 ± 0.31Aa | 7.06 ± 0.30Ba | 7.37 ± 0.66Ba | 7.55 ± 0.45Ba | 7.33 ± 0.74Ba | 8.59 ± 0.44Ca | 9.06 ± 0.35Ca |
| U | 6.27 ± 0.26Aab | 9.25 ± 0.46Bb | 9.25 ± 0.84Bbc | 10.39 ± 0.34BCc | 10.74 ± 0.88Cc | 11.09 ± 0.99Cb | 11.11 ± 0.63Cb |
| P | 7.69 ± 0.44Ac | 9.75 ± 0.28Bb | 8.83 ± 0.74Bab | 9.07 ± 0.64Bb | 8.94 ± 0.51Bb | 9.91 ± 0.65Bab | 9.38 ± 0.44Ba |
| UP | 6.50 ± 0.15Ab | 9.55 ± 0.51Bb | 10.51 ± 0.65BCc | 10.89 ± 0.88Cc | 11.69 ± 0.35CDc | 13.22 ± 0.83Ec | 12.86 ± 0.62DEc |
| b* | |||||||
| CK | 2.23 ± 0.08Ab | 2.08 ± 0.21Aa | 5.71 ± 0.05Bc | 4.75 ± 0.06Ca | 7.94 ± 0.44Dc | 7.23 ± 0.38Dc | 7.30 ± 0.44Dab |
| U | 2.41 ± 0.06Ac | 4.33 ± 0.32Bc | 4.86 ± 0.38Ba | 8.75 ± 0.04Cc | 6.13 ± 0.45Db | 5.76 ± 0.32Da | 7.45 ± 0.05Eb |
| P | 2.46 ± 0.10Ac | 3.81 ± 0.05Bb | 5.06 ± 0.09Cb | 6.38 ± 0.08Db | 5.02 ± 0.07Ca | 7.60 ± 0.06Ec | 7.13 ± 0.43Ea |
| UP | 2.03 ± 0.03Aa | 3.83 ± 0.06Bb | 6.73 ± 0.02Cd | 8.48 ± 0.10Dc | 7.52 ± 0.24Ec | 6.48 ± 0.31Cb | 7.38 ± 0.06Eb |
| Net colour difference | |||||||
| CK | 0.00 ± 0.00Aa | 2.73 ± 0.11Ba | 3.87 ± 0.06Ca | 5.42 ± 0.14 Da | 9.62 ± 0.21Ec | 9.40 ± 0.09Ea | 14.00 ± 0.51Fc |
| U | 0.00 ± 0.00Aa | 4.08 ± 0.12Bc | 5.83 ± 0.14Cb | 8.37 ± 0.16Dc | 11.81 ± 0.33Ed | 10.57 ± 0.15Fc | 14.02 ± 0.44Gc |
| P | 0.00 ± 0.00Aa | 4.25 ± 0.15Bc | 5.00 ± 0.18Cc | 7.58 ± 0.17Db | 6.95 ± 0.18Ea | 11.41 ± 0.21Fd | 12.58 ± 0.53Gb |
| UP |
0.00 ± 0.00Aa | 3.79 ± 0.09Bb | 6.56 ± 0.20Cd | 8.92 ± 0.11Dd | 8.52 ± 0.15Eb | 9.89 ± 0.11Fb | 9.81 ± 0.28Fa |
| Whiteness index | |||||||
| CK | 41.94 ± 0.94Aa | 39.40 ± 0.85Bab | 40.88 ± 1.12ABb | 37.17 ± 0.99Ca | 33.80 ± 1.11Dab | 33.85 ± 1.12 Da | 28.68 ± 1.46Ea |
| U | 44.52 ± 0.95Ab | 42.00 ± 2.11Bb | 39.65 ± 1.32Bab | 39.80 ± 1.22Bb | 33.50 ± 1.23CDa | 34.94 ± 1.43Ca | 31.46 ± 1.87Dab |
| P | 44.03 ± 1.11Ab | 40.24 ± 0.97Bab | 39.65 ± 1.51BCab | 37.29 ± 1.02Ca | 37.43 ± 0.98Cc | 33.50 ± 1.27 Da | 32.04 ± 1.96Dab |
| UP | 40.94 ± 1.31Aa | 39.12 ± 0.87Aa | 37.86 ± 0.98BCa | 35.52 ± 1.01CDa | 35.86 ± 0.77CDbc | 33.95 ± 1.54 Da | 34.43 ± 2.06Db |
The values are mean ± standard deviation (n = 3). Different uppercase letters in the same row and different lowercase letters in the same column are significantly different (p < 0.05).
As shown in Table 2, a* values of all the groups showed an increasing trend throughout the storage period, indicating an increased redness. The redness is closely related to the myoglobin content and interconversion of related proteins in food products. A similar trend was also observed in frozen rainbow trout fillets and refrigerated grass carp, of which the a* values increased during storage [52]. The increase of a* values might be composed of multiple factors, such as the changes in light absorption and scattering caused by substances formed during protein denaturation (Fig. 4e). Moreover, a* values of US and UP groups increased more rapidly than that of the control group, implying that US treatment accelerated the increase of redness. Consistent with the current study, Ling et al. [26] observed the acceleration effect of US treatments on the increase of redness towards crayfish. It might be attributed to the mechanical effects of US treatment on the crayfish samples, which resulted in cytochrome release.
For b* values, all the treated groups showed a gradually increasing trend throughout the storage period, while slight variations was observed among the different groups. It has been proved that the increase of b* values is frequently associated with lipid oxidations in food products [52], [53]. Although lipid oxidations in the crayfish samples slightly occurred during storage, the effect of each treatment on lipid oxidation was insufficient to reflect obvious changes in b* values. This should be due to the fact that the fat content of crayfish meat is quite low, which was consistent with the low TBARS values in our study (Fig. 4f).
Besides, the L* values for all the groups exhibited a decreased trend as the storage time increased, while UP group exhibited the slowest rate of decline, reaching an L* value of 36.13. The decrease in L* values of aquatic products are mainly associated with the oxidation of myofibrillar proteins and microbial spoilage during storage [19], [54]. This was supported by the current study, where UP group exhibited the lowest TVB-N and TVC values among all the groups at the end of storage. Liao et al. [16] observed a similar trend of L* values towards the shrimps stored with ice, while PAW ice efficiently alleviated the reduction in L* values compared with tap water ice. Moreover, UP group exhibited the minimum total color difference (△E = 9.81) among all the investigated groups at the end of storage, proving that UP treatment effectively retarded the color deterioration of crayfish.
3.4.4. Changes in textural properties
The textural properties (hardness, cohesiveness, adhesiveness, springiness and chewiness) of the crayfish after treatments were present in Table 3. During storage, all the groups followed a decreased trend in hardness, while UP group generally exhibited higher hardness values than the other groups. More specifically, the hardness value of crayfish in UP group was reduced to 1.40 N after 12 days of storage, while the hardness values of the control, US and PAW groups were reduced to 0.79, 1.19 and 1.23 N, respectively. A similar trend was observed in the springiness of the crayfish samples. UP treatment increased the springiness of crayfish, and UP group generally exhibited higher springiness values than the other groups during the whole storage period. For adhesiveness and cohesiveness, no obvious changes were observed during the whole storage period. The textural properties of food products depend on several factors, including muscle fiber densities, water contents and distributions, and protein and fat contents. Moreover, proteolysis, microbiological activities and storage conditions could significantly alter the textural properties of food products during storage [55]. Zhu et al. [20] found that the hardness and springiness of the salmon fillets treated with PAW were higher than the untreated ones. This might be due to the reaction substances in PAW, which could improve the meat hydrability of salmon fillets, thus maintaining their textural properties. In the current study, the higher hardness values of UP group than PAW group might be linked to the enhanced aggregation of crayfish myofibrillar proteins, which resulted from the compression and mechanical shockwaves generated by US treatment [19]. Nevertheless, PAW combined with US treatment did not result in significant changes in the hardness of chicken meat [12]. This might be due to the high content of connective tissues in chicken meat, enabling the muscle properties to remain stable during treatments. Normally, long-term storage would result in a soft texture of seafood, which might be due to the proteolysis process during storage [10]. The results of the current study indicated that UP treatment delayed the deteriorating changes in the hardness and springiness of crayfish.
Table 3.
The textural profiles of crayfish during storage at 4 °C.
| Treatments | Storage time(d) |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | |
| Hardness(N) | |||||||
| CK | 1.27 ± 0.00Aa | 1.19 ± 0.05Aa | 1.10 ± 0.06Ba | 0.93 ± 0.06Ca | 0.97 ± 0.02Ca | 0.83 ± 0.00 Da | 0.79 ± 0.04Da |
| U | 1.36 ± 0.00Ab | 1.31 ± 0.09ABa | 1.30 ± 0.09ABab | 1.29 ± 0.11ABb | 1.16 ± 0.09Bb | 1.17 ± 0.06Bb | 1.19 ± 0.00Bb |
| P | 1.32 ± 0.10Aab | 1.27 ± 0.05ABa | 1.30 ± 0.04Aab | 1.33 ± 0.11Ab | 1.19 ± 0.05ABb | 1.14 ± 0.08Bb | 1.23 ± 0.06ABb |
| UP | 1.56 ± 0.06Ac | 1.48 ± 0.10ABb | 1.40 ± 0.16ABCb | 1.36 ± 0.01BCb | 1.36 ± 0.00BCc | 1.29 ± 0.06Cc | 1.40 ± 0.08ABCc |
| Adhesiveness(N.mm) | |||||||
| CK | 0.06 ± 0.00Aa | 0.07 ± 0.01ABa | 0.07 ± 0.02ABa | 0.11 ± 0.03Ca | 0.10 ± 0.02BCa | 0.07 ± 0.02ABa | 0.05 ± 0.01Aa |
| U | 0.07 ± 0.02Aa | 0.08 ± 0.01Aa | 0.09 ± 0.04Aa | 0.11 ± 0.01Aa | 0.07 ± 0.02Aa | 0.09 ± 0.03Aa | 0.09 ± 0.01Ab |
| P | 0.06 ± 0.01Aa | 0.08 ± 0.01Aa | 0.09 ± 0.02Aa | 0.10 ± 0.02Aa | 0.09 ± 0.02Aa | 0.08 ± 0.03Aa | 0.08 ± 0.01Ab |
| UP | 0.09 ± 0.01Aa | 0.09 ± 0.01Aa | 0.11 ± 0.05Aa | 0.09 ± 0.02Aa | 0.08 ± 0.03Aa | 0.06 ± 0.02Aa | 0.08 ± 0.01Ab |
| Cohesiveness | |||||||
| CK | 0.29 ± 0.02ABa | 0.23 ± 0.06Ba | 0.25 ± 0.04ABa | 0.25 ± 0.08ABa | 0.22 ± 0.08Ba | 0.35 ± 0.04Aa | 0.29 ± 0.03ABa |
| U | 0.26 ± 0.03Aa | 0.28 ± 0.05Aa | 0.27 ± 0.04Aa | 0.30 ± 0.04Aa | 0.25 ± 0.04Aa | 0.26 ± 0.07Aa | 0.33 ± 0.07Aa |
| P | 0.27 ± 0.07Aa | 0.23 ± 0.07Aa | 0.22 ± 0.01Aa | 0.29 ± 0.03Aa | 0.30 ± 0.08Aa | 0.28 ± 0.05Aa | 0.33 ± 0.08Aa |
| UP | 0.26 ± 0.06Aa | 0.24 ± 0.07Aa | 0.29 ± 0.04Aa | 0.33 ± 0.03Aa | 0.25 ± 0.10Aa | 0.25 ± 0.10Aa | 0.36 ± 0.06Aa |
| Springiness | |||||||
| CK | 0.63 ± 0.12Aa | 0.59 ± 0.16Aa | 0.61 ± 0.07Aa | 0.64 ± 0.18Aa | 0.67 ± 0.18Aa | 0.70 ± 0.00Aa | 0.60 ± 0.09Aa |
| U | 0.82 ± 0.02Aab | 0.81 ± 0.14Aa | 0.76 ± 0.04Aa | 0.75 ± 0.24ABa | 0.66 ± 0.07Ba | 0.64 ± 0.08Ba | 0.60 ± 0.06Ba |
| P | 0.73 ± 0.01Aab | 0.68 ± 0.15Aa | 0.70 ± 0.08Aa | 0.73 ± 0.16Aa | 0.71 ± 0.17Aa | 0.67 ± 0.04Aa | 0.49 ± 0.12Ba |
| UP | 0.89 ± 0.15Ab | 0.93 ± 0.15Aa | 0.81 ± 0.17Aa | 0.84 ± 0.12Aa | 0.84 ± 0.03Aa | 0.83 ± 0.04Aa | 0.85 ± 0.12Ab |
| Gumminess | |||||||
| CK | 0.27 ± 0.02Aa | 0.25 ± 0.02Aa | 0.39 ± 0.02Bb | 0.34 ± 0.03ABa | 0.28 ± 0.03Aab | 0.61 ± 0.11Cb | 0.34 ± 0.04ABa |
| U | 0.30 ± 0.03ABa | 0.37 ± 0.03BCc | 0.36 ± 0.04BCb | 0.39 ± 0.04Cab | 0.24 ± 0.04Aa | 0.30 ± 0.02ABa | 0.48 ± 0.06Dab |
| P | 0.31 ± 0.02ABa | 0.31 ± 0.04ABb | 0.22 ± 0.03Aa | 0.46 ± 0.05Cb | 0.36 ± 0.05BCb | 0.38 ± 0.04BCa | 0.62 ± 0.13Db |
| UP | 0.38 ± 0.05ABb | 0.27 ± 0.01ACab | 0.40 ± 0.04ABb | 0.43 ± 0.05Bab | 0.21 ± 0.04Ca | 0.30 ± 0.03ABCa | 1.00 ± 0.22Dc |
The values are mean ± standard deviation (n = 3). Different uppercase letters in the same row and different lowercase letters in the same column are significantly different (p < 0.05).
3.5. Secondary structures of the crayfish proteins during storage
The FTIR spectra were detected on the 0, 6 and 12 days of storage, to evaluate the influence of different treatments on the proteins of crayfish meat. Overall, the spectra for all the tested samples exhibited similar trends. For each investigated group, the absorption peak of the spectrum slightly shifted to high wavenumber areas with the prolongation of storage time (Fig. 5). It implied that the protein structures of crayfish were destructed during refrigerated storage, which has been proved as the destruction of hydrogen bonds in the protein structures [56]. Moreover, it needs to be noted that the UP treatment accelerated this shift. More specifically, the amide I band of the control, US, PAW and UP group along 12 days of storage shifted from 1635.8 to 1640.2 cm−1, from 1636.3 to 1640.6 cm−1, from 1636.3 to 1640.6 cm−1, and from 1636.3 to 1644.0 cm−1, respectively. This might result from the denaturation and unfolding effects of PAW and US treatments on the secondary structures of proteins. Ekezie et al. [57] has pointed out that the oxidation effect of reactive species in PAW on amino acids residues and cavitation-induced shear actions of US treatment might result in the molecular unfolding of muscle proteins.
Fig. 5.
Fourier transform infrared spectra of the crayfish after different treatments during storage at 4 °C. (CK: the control group, U: the ultrasound treated group, P: the plasma-activated water treated group and UP: the US and PAW treated group).
The amide I band (1600–1700 cm−1) primarily represents the C O stretching vibrations of the peptide linkages, and is regarded as the fingerprint for the secondary structural components of proteins [31], [58]. Therefore, the characteristic peaks of the amide I band were used to quantitatively analyze the secondary structural elements of proteins, including α-helix, β-turn, β-sheet, and random coil (Table 4). On each day of analysis, spectral differences were observed among the samples, which might be due to conformation changes in the polypeptide chains. At day 0 of storage, the α-helix contents of the US, PAW and UP groups significantly decreased after treatments, while the β-sheet contents increased compared to the control. This was consistent with the previous findings that PAW and US treatments resulted in decreases in α-helix contents and increases in β-sheet contents for the proteins of aquatic products [58], [59]. The α-helix is a dense ordered structure, the decrease of which represents an unfolding of MP molecules. Li et al. [59] pointed out that PAW promoted the structure unfolding of α-helix and further modified the molecular interactions in the proteins of freshwater fish. Besides, the cavitation of ultrasound treatment could enhance the exposure of hydrophobic groups and result in the fracture of intramolecular hydrogen bonds, therefore causing the protein molecules to unfold, depolymerize and rearrange [58]. The structural changes induced by US and PAW treatments depend on the native protein structures and might enhance the biological properties of proteins [19], [59]. For instance, Li et al. [59] observed that PAW treatment on freshwater fish improved the water-holding capacity of its myofibrillar protein gels. Furthermore, the β-sheet contents for the control group exhibited a decreased trend, while the contents of β-turn and random coil generally followed an increased trend with prolonged storage time. These results implied that the secondary structure of crayfish proteins was destroyed along with the storage time, with the transformation from the ordered structure to the disordered structure [60]. In the current study, although the applied treatments induced slight changes in the protein structures of crayfish on day 0 of storage, no significant changes were observed at the end of storage.
Table 4.
The secondary structural changes of crayfish proteins during storage at 4 °C.
| Storage time | Group | β-sheet (%) | Random (%) | α-helix(%) | β-turn (%) |
|---|---|---|---|---|---|
| 0d | CK | 46.67 ± 0.80abc | 15.06 ± 0.06a | 18.35 ± 0.23a | 19.92 ± 0.52a |
| U | 46.93 ± 1.50abc | 16.49 ± 1.16c | 15.35 ± 0.76bcd | 21.24 ± 0.45abc | |
| P | 48.38 ± 0.32ab | 15.47 ± 0.22ab | 14.58 ± 0.11 cd | 21.57 ± 0.07abc | |
| UP | 49.12 ± 0.15a | 15.57 ± 0.16abc | 14.51 ± 0.05d | 20.80 ± 0.11ab | |
| 6d | CK | 47.78 ± 1.47ab | 15.02 ± 0.26a | 14.42 ± 0.18d | 22.78 ± 1.50abcd |
| U | 45.70 ± 1.47abc | 15.78 ± 0.26abc | 15.33 ± 0.18bcd | 23.19 ± 1.50abcd | |
| P | 44.45 ± 1.27abc | 16.12 ± 0.21bc | 15.69 ± 0.22bc | 23.75 ± 1.27bcd | |
| UP | 43.36 ± 1.02abc | 16.07 ± 0.25bc | 15.80 ± 0.14b | 24.77 ± 1.15cd | |
| 12d | CK | 43.91 ± 1.09abc | 16.26 ± 0.04bc | 15.83 ± 0.25b | 24.00 ± 0.85bcd |
| U | 43.66 ± 1.04abc | 16.41 ± 0.07c | 15.95 ± 0.24b | 23.98 ± 0.76bcd | |
| P | 42.27 ± 0.69bc | 16.16 ± 0.10bc | 15.98 ± 0.16b | 25.59 ± 0.61d | |
| UP | 41.27 ± 0.57c | 16.42 ± 0.05c | 16.34 ± 0.21b | 25.97 ± 0.38d |
The values are mean ± standard deviation (n = 3). Different letters in the same column indicate significant differences (p < 0.05).
3.6. LF-NMR analysis of the crayfish during storage
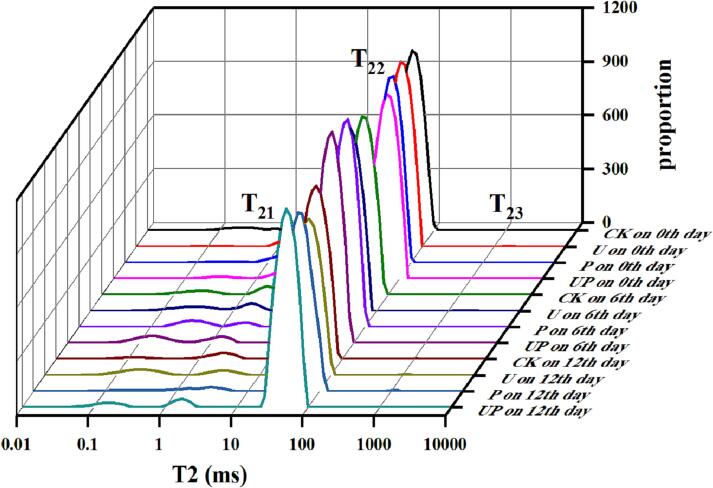
LF-NMR was used to analyze the changes in water distribution and dynamic water status of the crayfish during storage. The transverse relaxation time (T2) of the crayfish after different treatments were shown in Fig. 6. Three peaks were observed, T21 (0.1–10 ms), T22 (10–100 ms) and T23 (100–1000 ms). T21 stands for bound water in the crayfish. It was not surprising that no significant changes were observed in the T21 values for all the investigated groups during storage, as the water molecules tightly bonded with the macromolecules of crayfish proteins [17]. T22 stands for immobilized water, representing the water trapped by the dense myofibrillar network. After 12 days of storage, T22 amplitudes of crawfish samples in all the investigated group were obviously weaker than that of the fresh samples, while PAW and UP groups exhibited higher T22 amplitudes than control and US groups. The decreases in T22 values might be attributed to structural changes in the crayfish tissues during storage, which could result from the activities of microorganisms or enzymes during storage [51]. T23 stands for free water that is easily lost outside the cell [56]. The fluctuation of T23 was tiny, as free water plays a little part in the water contents of crayfish [2], [3]. After 12 days of storage, a slight increase in T23 amplitudes was observed for control and US groups compared with the fresh samples, implying that some trapped water was converted into free water during storage. PAW and UP treatments might retard the transition of water within myofibrillar macromolecules to free water. which may be due to the influence of muscle tissue structure on water-holding capacity. A similar result was observed by [61] that T22 amplitudes of crawfish samples diminished progressively, while T23 amplitudes observably increased during refrigerated storage. This might be due to the degradation of muscle fibers, leading to the decreased binding force of immobilized water. The freshness of aquatic products was tightly related to the changes in the water content and distribution during storage. LF-NMR is regarded as an effective method to assess the changes in the water distribution of aquatic products. The results revealed that PAW treatments reduced water migration and enhanced the stability of bond water in crayfish during storage, which potentially through enhancing the interaction of water and proteins macromolecules. The enhancement of PAW on the water-holding capacity was also demonstrated previously [17], benefiting the storage stability of food products.
Fig. 6.
Low-field nuclear magnetic resonance spectra of the crayfish after different treatments during storage at 4 °C. (CK: the control group, U: the ultrasound treated group, P: the plasma-activated water treated group and UP: the US and PAW treated group).
3.7. Sensory properties of the crayfish during storage
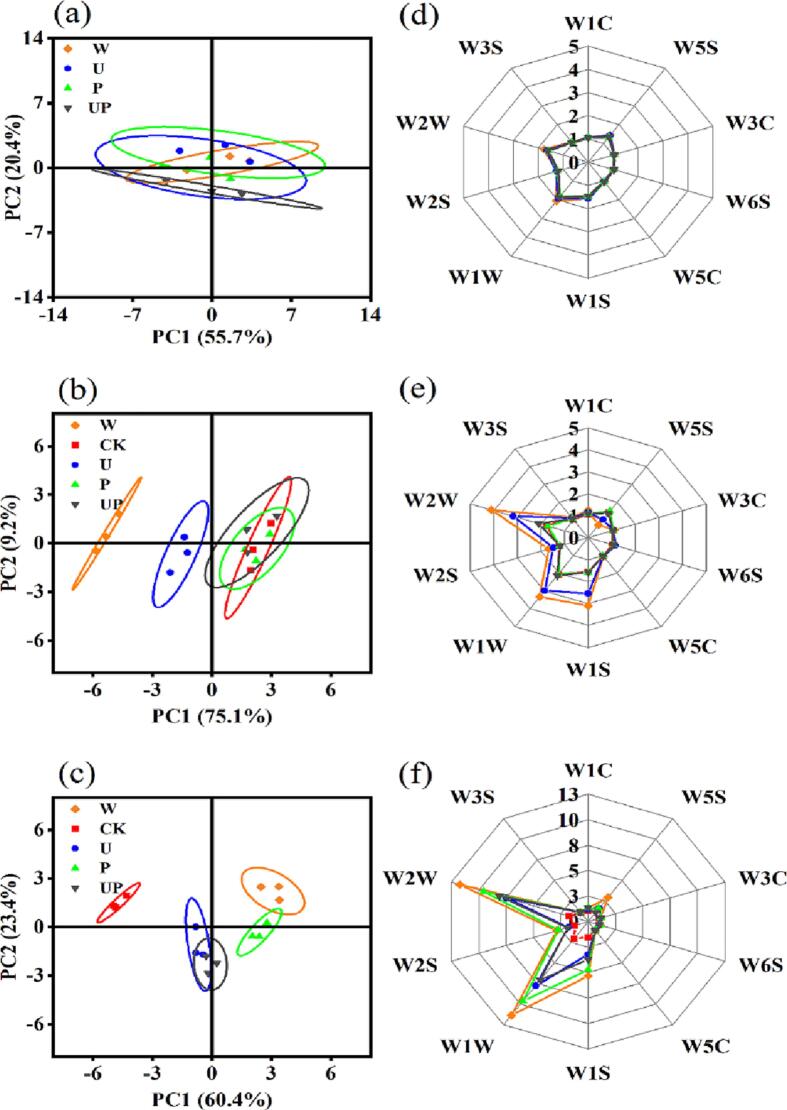
The volatile profiles of the crayfish samples after different decontamination treatments were characterized by E-nose. The volatile profile of fresh crayfish was used as CK. Principal component analysis (PCA) method was performed to analyze the E-nose data by reducing the variables to a small number of representatives (Fig. 7a-c). For each time point, the contribution variances of the first two principal components (PC1 and PC2) were over 75% of the total variance (76.1% of day 0, 84.3% of day 6 and 83.8% of day 12). This indicated that the first two PCs were sufficient to explain the total variances of the dataset [62], [63]. On day 0, the volatile profiles of crayfish samples were close to each other and even overlapped, indicating that the effects of different decontamination treatments were not obvious at the initial of storage [64]. On day 6, the volatile profiles of untreated and US groups were relatively far from the CK group, while the volatile profiles of PAW and UP groups were close to the CK group. It indicated that the volatile profiles of untreated and US groups were significantly different from the fresh crayfish with increased storage time, while P and UP groups still maintained the fresh smell. At the end of storage (D12), all the treated samples were far from CK, especially the untreated and PAW groups.
Fig. 7.
The principal component analyses (a-c) and radar charts (d-f) of the E-nose response data for crayfish samples after different treatments on 0 day (a, d), 6th day (b, e) and 12th day (c, f).
Furthermore, the radar charts of the E-nose responses to the crayfish samples after different storage times were presented in Fig. 7d-f. In consistent with the PCA results, all the samples have similar flavors on day 0, indicated by the similar responses of each sensor to the tested samples. After 6 days of storage, untreated and US groups had higher response values at sensors W1W, W2W, and W1S, indicating that untreated and US groups might have higher abundances of sulfides. At the end of storage (D12), all the samples had higher response values compared with CK, especially at sensors WIW and W2W [65]. The enhanced sensors conductance indicated that the concentrations of volatile compounds increased with storage time [66]. These variations in volatile compounds may be related to the deterioration of crayfish, revealing that sulfides played an important role in the spoilage odor of crayfish. sulfides have been detected as an important fraction of aroma in numerous foods and could be derived from sulfur amino acids [67]. Sulfides like carbon disulfide, hydrogen sulfide, and dimethyl disulfide have been considered as indicators of potential bacterial spoilage in packed aquatic products [65]. Overall, the results of the E-nose analysis revealed the protection of UP treatment on the sensory properties of crayfish during storage.
4. Conclusions
This study comprehensively evaluated the effects of US, PAW and their combinations on the preservation of crayfish. Compared to US or PAW treatments, UP treatment was more effective in microbial decontamination towards the natural microbiota (TVC, LAB, Y&M, and TPC) of crayfish. Moreover, UP treatment effectively inhibited microbial growth and suppressed the oxidations of proteins and lipids during storage, resulting in longer shelf life. Besides, UP treatment effectively retarded the deterioration in textual and sensory properties of crayfish. In addition, UP treatment accelerated the increase of redness while retarded the decrease of lightness for crayfish. Overall, UP treatments effectively accelerated the decontamination of crayfish and contributed to better qualities during refrigerated storage. The results demonstrated that UP treatment can be a promising method to improve the microbiological safety and quality attributes of aquatic crustaceans.
Funding
This research was funded by the Jiangsu Agricultural Science and Technology Innovation Fund [CX (21) 2030], the Basic Scientific Research Project of Jiangsu Academy of Agricultural Sciences [ZX (22) 5004] and Jiangsu modern agriculture (Procambarus clarkii) industrial technology system [JATS [2022] 444].
CRediT authorship contribution statement
Rongxue Sun: Conceptualization, Methodology, Writing – original draft. Weicheng Xu: Data curation, Formal analysis, Software, Investigation. Lingming Xiong: Resources, Supervision. Ning Jiang: Project administration, Funding acquisition. Jiangyue Xia: Writing – review & editing. Yongzhi Zhu: Validation. Cheng Wang: Methodology, Data curation. Qianyuan Liu: Visualization. Yanhong Ma: Validation. Haibo Luo: Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are thankful to the support from the Jiangsu Agricultural Science and Technology Innovation Fund [CX (21) 2030], the Basic Scientific Research Project of Jiangsu Academy of Agricultural Sciences [ZX (22) 5004] and Jiangsu modern agriculture (Procambarus clarkii) industrial technology system [JATS[2022]444].
Data availability
Data will be made available on request.
References
- 1.Xiao X., Liu X., Mei T., Xu M., Lu Z., Dai H., Pi F., Wang J. Estimation of contamination level in microplastic-exposed crayfish by laser confocal micro-Raman imaging. Food Chem. 2022;397 doi: 10.1016/j.foodchem.2022.133844. [DOI] [PubMed] [Google Scholar]
- 2.Huang J., Hu Z., Li G., Xiang Y., Chen J., Hu Y. Preservation mechanism of liquid nitrogen freezing on crayfish (Procambarus clarkia): Study on the modification effects in biochemical and structural properties. J. Food Process. Preserv. 2022;46:e17116. [Google Scholar]
- 3.Han J., Sun Y., Zhang T., Wang C., Xiong L., Ma Y., Zhu Y., Gao R., Wang L., Jiang N. The preservable effects of ultrasound-assisted alginate oligosaccharide soaking on cooked crayfish subjected to freeze-thaw cycles. Ultrason. Sonochem. 2023;92 doi: 10.1016/j.ultsonch.2022.106259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y., Wang C., Xia Z., Wang Q., Duan S. Monitoring freshness of crayfish (Prokaryophyllus clarkii) through the combination of near-infrared spectroscopy and chemometric method. J. Food Meas. Charact. 2022;16(5):3438–3450. [Google Scholar]
- 5.Ma R., Wang G., Tian Y., Wang K., Zhang J., Fang J. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 2015;300:643–651. doi: 10.1016/j.jhazmat.2015.07.061. [DOI] [PubMed] [Google Scholar]
- 6.Misra N.N., Tiwari B.K., Raghavarao K.S.M.S., Cullen P.J. Nonthermal plasma inactivation of foodborne pathogens. Food Eng. Rev. 2011;3(3-4):159–170. [Google Scholar]
- 7.Mir S.A., Dar B.N., Shah M.A., Sofi S.A., Hamdani A.M., Oliveira C.A.F., Hashemi Moosavi M., Mousavi Khaneghah A., Sant'Ana A.S. Application of new technologies in decontamination of mycotoxins in cereal grains: challenges, and perspectives. Food Chem. Toxicol. 2021;148:111976. doi: 10.1016/j.fct.2021.111976. [DOI] [PubMed] [Google Scholar]
- 8.Deng L.-Z., Mujumdar A.S., Pan Z., Vidyarthi S.K., Xu J., Zielinska M., Xiao H.-W. Emerging chemical and physical disinfection technologies of fruits and vegetables: a comprehensive review. Crit. Rev. Food Sci. Nutr. 2020;60(15):2481–2508. doi: 10.1080/10408398.2019.1649633. [DOI] [PubMed] [Google Scholar]
- 9.Bigi F., Maurizzi E., Quartieri A., De Leo R., Gullo M., Pulvirenti A. Non-thermal techniques and the “hurdle” approach: how is food technology evolving? Trends Food Sci. Technol. 2023;132:11–39. [Google Scholar]
- 10.Liu B., Li D.-Y., Wu Z.-X., Yang W.-J., Zhou D.-Y., Zhu B.-W. Combined effects of ultrasound and antioxidants on the quality maintenance of bay scallop (Argopecten irradians) adductor muscles during cold storage. Ultrason. Sonochem. 2022;82 doi: 10.1016/j.ultsonch.2021.105883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pankaj S.K., Shi H., Keener K.M. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci. Technol. 2018;71:73–83. [Google Scholar]
- 12.Royintarat T., Choi E.H., Boonyawan D., Seesuriyachan P., Wattanutchariya W. Chemical-free and synergistic interaction of ultrasound combined with plasma-activated water (PAW) to enhance microbial inactivation in chicken meat and skin. Sci Rep-uk. 2020;10:1559. doi: 10.1038/s41598-020-58199-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y.M., Oliveira M., Burgess C.M., Cropotova J., Rustad T., Sun D.W., Tiwari B.K. Combined effects of ultrasound, plasma-activated water, and peracetic acid on decontamination of mackerel fillets. LWT-Food Sci. Technol. 2021;150 [Google Scholar]
- 14.Choi E.J., Park H.W., Kim S.B., Ryu S., Lim J., Hong E.J., Byeon Y.S., Chun H.H. Sequential application of plasma-activated water and mild heating improves microbiological quality of ready-to-use shredded salted kimchi cabbage (Brassica pekinensis L.) Food Control. 2019;98:501–509. [Google Scholar]
- 15.Zhao Y.-M., Ojha S., Burgess C.M., Sun D.-W., Tiwari B.K. Influence of various fish constituents on inactivation efficacy of plasma-activated water. Int. J. Food Sci. Technol. 2020;55(6):2630–2641. [Google Scholar]
- 16.Liao X., Su Y., Liu D., Chen S., Hu Y., Ye X., Wang J., Ding T. Application of atmospheric cold plasma-activated water (PAW) ice for preservation of shrimps (Metapenaeus ensis) Food Control. 2018;94:307–314. [Google Scholar]
- 17.Wang Y., Liu Y., Li M., Ma M., Sun Q. Evaluation of the storage stability and quality properties of fresh noodles mixed with plasma-activated water. Foods. 2022;11:133. doi: 10.3390/foods11010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q., Salvi D. Recent progress in the application of plasma-activated water (PAW) for food decontamination. Curr. Opin. Food Sci. 2021;42:51–60. [Google Scholar]
- 19.Johnson Esua O., Cheng J.-H., Sun D.-W. Novel technique for treating grass carp (Ctenopharyngodon idella) by combining plasma functionalized liquids and ultrasound: Effects on bacterial inactivation and quality attributes. Ultrason. Sonochem. 2021;76:105660. doi: 10.1016/j.ultsonch.2021.105660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu W., Tan G., Han M., Bu Y., Li X., Li J. Evaluating the effects of plasma-activated slightly acidic electrolyzed water on bacterial inactivation and quality attributes of Atlantic salmon fillets. Innovative Food Sci. Emerging Technol. 2023;84 [Google Scholar]
- 21.Tao Y., Li D., Siong Chai W., Show P.L., Yang X., Manickam S., Xie G., Han Y. Comparison between airborne ultrasound and contact ultrasound to intensify air drying of blackberry: Heat and mass transfer simulation, energy consumption and quality evaluation. Ultrason. Sonochem. 2021;72 doi: 10.1016/j.ultsonch.2020.105410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu R., Shen J., Law C.L., Ma X., Li D., Han Y., Kiani H., Manickam S., Tao Y. Combined calcium pretreatment and ultrasonic/microwave drying to dehydrate black chokeberry: Novel mass transfer modeling and metabolic pathways of polyphenols. Innovative Food Sci. Emerging Technol. 2023;83 [Google Scholar]
- 23.Zhang X., Chen X., Gong Y., Li Z., Guo Y., Yu D., Pan M. Emulsion gels stabilized by soybean protein isolate and pectin: Effects of high intensity ultrasound on the gel properties, stability and β-carotene digestive characteristics. Ultrason. Sonochem. 2021;79 doi: 10.1016/j.ultsonch.2021.105756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu C., Zang M., Qiao X., Wang S., Zhao B., Shi Y., Bai J., Wu J. Effects of ultrasound-assisted thawing on lamb meat quality and oxidative stability during refrigerated storage using non-targeted metabolomics. Ultrason. Sonochem. 2022;90 doi: 10.1016/j.ultsonch.2022.106211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lan W., Sun Y., Liu S., Guan Y., Zhu S., Xie J. Effects of ultrasound-assisted chitosan grafted caffeic acid coating on the quality and microbial composition of pompano during ice storage. Ultrason. Sonochem. 2022;86 doi: 10.1016/j.ultsonch.2022.106032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling Y., Tan H., Shen L., Wei L., Xiong G., Wang L., Wu W., Qiao Y. Microbial evaluation of ozone water combined with ultrasound cleaning on crayfish (Procambarus clarkii) Foods. 2022;11:2314. doi: 10.3390/foods11152314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tao Y., Wu P., Dai Y., Luo X., Manickam S., Li D., Han Y., Loke Show P. Bridge between mass transfer behavior and properties of bubbles under two-stage ultrasound-assisted physisorption of polyphenols using macroporous resin. Chem. Eng. J. 2022;436 [Google Scholar]
- 28.Herianto S., Shih M.K., Lin C.M., Hung Y.C., Hsieh C.W., Wu J.S., Chen M.H., Chen H.L., Hou C.Y. The effects of glazing with plasma-activated water generated by a piezoelectric direct discharge plasma system on whiteleg shrimp (Litopenaeus vannamei) LWT-Food Sci. Technol. 2022;154 [Google Scholar]
- 29.do Rosário D.K.A., da Silva Mutz Y., Peixoto J.M.C., Oliveira S.B.S., de Carvalho R.V., Carneiro J.C.S., de São José J.F.B., Bernardes P.C. Ultrasound improves chemical reduction of natural contaminant microbiota and Salmonella enterica subsp. enterica on strawberries. Int. J. Food Microbiol. 2017;241:23–29. doi: 10.1016/j.ijfoodmicro.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Sun R., Vermeulen A., Devlieghere F. Extension of growth/no growth predictive models for the preservation of low-acid pasteurized sauces by incorporating water activity and model validation in sauces. Int. J. Food Microbiol. 2022;378 doi: 10.1016/j.ijfoodmicro.2022.109826. [DOI] [PubMed] [Google Scholar]
- 31.Luo L., Yang Z., Wang H., Ashokkumar M., Hemar Y. Impacts of sonication and high hydrostatic pressure on the structural and physicochemical properties of quinoa protein isolate dispersions at acidic, neutral and alkaline pHs. Ultrason. Sonochem. 2022;91 doi: 10.1016/j.ultsonch.2022.106232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bian C., Cheng H., Yu H., Mei J., Xie J. Effect of multi-frequency ultrasound assisted thawing on the quality of large yellow croaker (Larimichthys crocea) Ultrason. Sonochem. 2022;82 doi: 10.1016/j.ultsonch.2021.105907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian R., Feng J., Huang G., Tian B., Zhang Y., Jiang L., Sui X. Ultrasound driven conformational and physicochemical changes of soy protein hydrolysates. Ultrason. Sonochem. 2020;68 doi: 10.1016/j.ultsonch.2020.105202. [DOI] [PubMed] [Google Scholar]
- 34.Han J., Sun Y., Sun R., Zhang T., Wang C., Jiang N. Effects of freeze-thaw cycles on physicochemical properties and structure of cooked crayfish (Procambarus clarkii) Food Prod., Process. Nutr. 2022;4:25. [Google Scholar]
- 35.Xu Y., Lv Y., Zhao H., He X., Li X., Yi S., Li J. Diacylglycerol pre-emulsion prepared through ultrasound improves the gel properties of golden thread surimi. Ultrason. Sonochem. 2022;82 doi: 10.1016/j.ultsonch.2022.105915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.T. Royintarat, E.H. Choi, P. Seesuriyachan, W. Wattanutchariya, Ultrasound-assisted plasma-activated water for bacterial inactivation in poultry industry, in: 2019 IEEE Int. Conf. Ind. Technol, 2019, pp. 1028–1032.
- 37.Guo J., Qin D., Li W., Wu F., Li L., Liu X. Inactivation of Penicillium italicum on kumquat via plasma-activated water and its effects on quality attributes. Int. J. Food Microbiol. 2021;343 doi: 10.1016/j.ijfoodmicro.2021.109090. [DOI] [PubMed] [Google Scholar]
- 38.Xu Y., Tian Y., Ma R., Liu Q., Zhang J. Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem. 2016;197:436–444. doi: 10.1016/j.foodchem.2015.10.144. [DOI] [PubMed] [Google Scholar]
- 39.Pan Y.-W., Cheng J.-H., Sun D.-W. Inhibition of fruit softening by cold plasma treatments: affecting factors and applications. Crit. Rev. Food Sci. Nutr. 2021;61(12):1935–1946. doi: 10.1080/10408398.2020.1776210. [DOI] [PubMed] [Google Scholar]
- 40.Xiang Q., Fan L., Li Y., Dong S., Li K., Bai Y. A review on recent advances in plasma-activated water for food safety: current applications and future trends. Crit. Rev. Food Sci. Nutr. 2022;62(8):2250–2268. doi: 10.1080/10408398.2020.1852173. [DOI] [PubMed] [Google Scholar]
- 41.Yan S., Yu D., Tang C., Shen J., Xu Y., Xia W., Jiang Q., Yang F. Physicochemical and microbiological changes in postmortem crayfish (Procambarus clarkii) stored at 4 °C and 25 °C. INT. J. Food Sci. Technol. 2022;57(5):2992–3000. [Google Scholar]
- 42.Mikš-Krajnik M., James Feng L.X., Bang W.S., Yuk H.G. Inactivation of Listeria monocytogenes and natural microbiota on raw salmon fillets using acidic electrolyzed water, ultraviolet light or/and ultrasounds. Food Control. 2017;74:54–60. [Google Scholar]
- 43.Dong Z., Xu F., Ahmed I., Li Z., Lin H. Characterization and preservation performance of active polyethylene films containing rosemary and cinnamon essential oils for Pacific white shrimp packaging. Food Control. 2018;92:37–46. [Google Scholar]
- 44.Chaijan M., Chaijan S., Panya A., Nisoa M., Cheong L.-Z., Panpipat W. High hydrogen peroxide concentration-low exposure time of plasma-activated water (PAW): A novel approach for shelf-life extension of Asian sea bass (Lates calcarifer) steak. Innovative Food Sci. Emerging Technol. 2021;74 [Google Scholar]
- 45.Diler İ., Genç İ.Y., Diler A. Effects of different treatments on the quality and safety of crayfish (Astacus leptodactylus) J. Food Qual. 2017;2017:2904706. [Google Scholar]
- 46.Qin L., Wu Y., Chen J., Xia W., Liao E., Wang H. Effects of superchilling on quality of crayfish (Procambarus clarkii): water migration, biogenic amines accumulation, and nucleotides catabolism. Int. J. Food Sci. Technol. 2022;57:506–515. [Google Scholar]
- 47.Zouelm F., Abhari K., Hosseini H., Khani M. The Effects of cold plasma application on quality and chemical spoilage of Pacific white shrimp (Litopenaeus vannamei) during refrigerated storage. J. Aquat. Food Prod. Technol. 2019;28(6):624–636. [Google Scholar]
- 48.Wang H., Zhang Y., Jiang H., Cao J., Jiang W. A comprehensive review of effects of electrolyzed water and plasma-activated water on growth, chemical compositions, microbiological safety and postharvest quality of sprouts. Trends Food Sci. Technol. 2022;129:449–462. [Google Scholar]
- 49.Sun X., Guo X., Ji M., Wu J., Zhu W., Wang J., Cheng C., Chen L., Zhang Q. Preservative effects of fish gelatin coating enriched with CUR/βCD emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4 °C. Food Chem. 2019;272:643–652. doi: 10.1016/j.foodchem.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 50.Xu W.-N., Liu W.-B., Shen M.-F., Li G.-F., Wang Y., Zhang W.-W. Effect of different dietary protein and lipid levels on growth performance, body composition of juvenile red swamp crayfish (Procambarus clarkii) Aquacult. Int. 2013;21(3):687–697. [Google Scholar]
- 51.Chang H.-C., Wong R.-X. Textural and biochemical properties of cobia (Rachycentron canadum) sashimi tenderised with the ultrasonic water bath. Food Chem. 2012;132(3):1340–1345. doi: 10.1016/j.foodchem.2011.11.116. [DOI] [PubMed] [Google Scholar]
- 52.Sun L., Sun J., Thavaraj P., Yang X., Guo Y. Effects of thinned young apple polyphenols on the quality of grass carp (Ctenopharyngodon idellus) surimi during cold storage. Food Chem. 2017;224:372–381. doi: 10.1016/j.foodchem.2016.12.097. [DOI] [PubMed] [Google Scholar]
- 53.Guo Z., Ge X., Yang L., Ma G., Ma J., Yu Q.L., Han L. Ultrasound-assisted thawing of frozen white yak meat: Effects on thawing rate, meat quality, nutrients, and microstructure. Ultrason. Sonochem. 2021;70:105345. doi: 10.1016/j.ultsonch.2020.105345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiao L., Tu C., Mao J., Benjakul S., Zhang B. Impact of theaflavin soaking pretreatment on oxidative stabilities and physicochemical properties of semi-dried large yellow croaker (Pseudosciaena crocea) fillets during storage. Food Packag. Shelf Life. 2022;32 [Google Scholar]
- 55.Zhao Y., Yang X., Li L., Hao S., Wei Y.a., Cen J., Lin H. Chemical, microbiological, color and textural changes in Nile tilapia (Oreochromis niloticus) fillets sterilized by ozonated water pretreatment during frozen storage. J. Food Process. Preserv. 2017;41(1):e12746. [Google Scholar]
- 56.Zhang X., Huang W., Xie J. Effect of different packaging methods on protein oxidation and degradation of grouper (Epinephelus coioides) during refrigerated storage. Foods. 2019;8:325. doi: 10.3390/foods8080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ekezie F.G.C., Cheng J.H., Sun D.W. Effects of atmospheric pressure plasma jet on the conformation and physicochemical properties of myofibrillar proteins from king prawn (Litopenaeus vannamei) Food Chem. 2019;276:147–156. doi: 10.1016/j.foodchem.2018.09.113. [DOI] [PubMed] [Google Scholar]
- 58.Li H., Hu Y., Zhao X., Wan W., Du X., Kong B., Xia X. Effects of different ultrasound powers on the structure and stability of protein from sea cucumber gonad. LWT-Food Sci. Technol. 2021;137 [Google Scholar]
- 59.Li M., Shi T., Wang X., Bao Y., Xiong Z., Monto A.R., Jin W., Yuan L., Gao R. Plasma-activated water promoted the aggregation of Aristichthys nobilis myofibrillar protein and the effects on gelation properties. Curr. Res. Food Sci. 2022;5:1616–1624. doi: 10.1016/j.crfs.2022.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao Q., Lin J., Wang C., Yousaf L., Xue Y., Shen Q. Protein structural properties and proteomic analysis of rice during storage at different temperatures. Food Chem. 2021;361:130028. doi: 10.1016/j.foodchem.2021.130028. [DOI] [PubMed] [Google Scholar]
- 61.Liu W., Shen Y., Li N., Mei J., Xie J. Application of gelatin incorporated with red pitaya peel methanol extract as edible coating for quality enhancement of crayfish (Procambarus clarkii) during refrigerated storage. J. Food Qual. 2019;2019:1715946. [Google Scholar]
- 62.Liu X., Zhang C., Wang H., Wang Y., Zhu D., Liu H. Ultrasonic treatment maintains the flavor of the melon juice. Ultrason. Sonochem. 2023;92 doi: 10.1016/j.ultsonch.2022.106284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bao G., Niu J., Li S., Zhang L., Luo Y. Effects of ultrasound pretreatment on the quality, nutrients and volatile compounds of dry-cured yak meat. Ultrason. Sonochem. 2022;82 doi: 10.1016/j.ultsonch.2021.105864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H., Feng J., Shi S., Wang X., Xia X. Evaluation of effects of ultrasound-assisted saucing on the quality of chicken gizzards. Ultrason. Sonochem. 2022;86 doi: 10.1016/j.ultsonch.2022.106038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vajdi M., Varidi M.J., Varidi M., Mohebbi M. Using electronic nose to recognize fish spoilage with an optimum classifier. J. Food Meas. Charact. 2019;13(2):1205–1217. [Google Scholar]
- 66.Zhou X., Chong Y., Ding Y., Gu S., Liu L. Determination of the effects of different washing processes on aroma characteristics in silver carp mince by MMSE–GC–MS, e-nose and sensory evaluation. Food Chem. 2016;207:205–213. doi: 10.1016/j.foodchem.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 67.Noseda B., Islam M.T., Eriksson M., Heyndrickx M., De Reu K., Van Langenhove H., Devlieghere F. Microbiological spoilage of vacuum and modified atmosphere packaged Vietnamese Pangasius hypophthalmus fillets. Food Microbiol. 2012;30(2):408–419. doi: 10.1016/j.fm.2011.12.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.