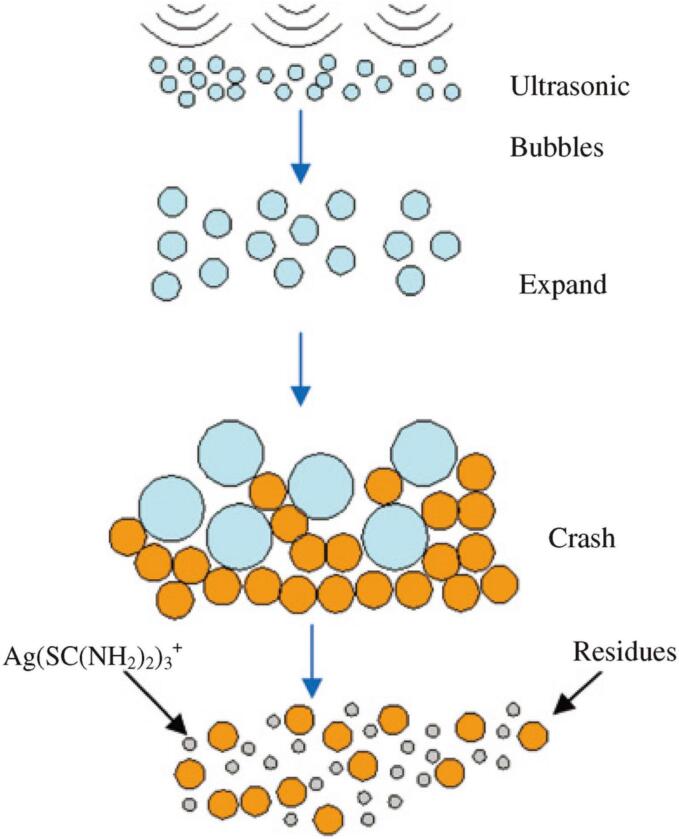
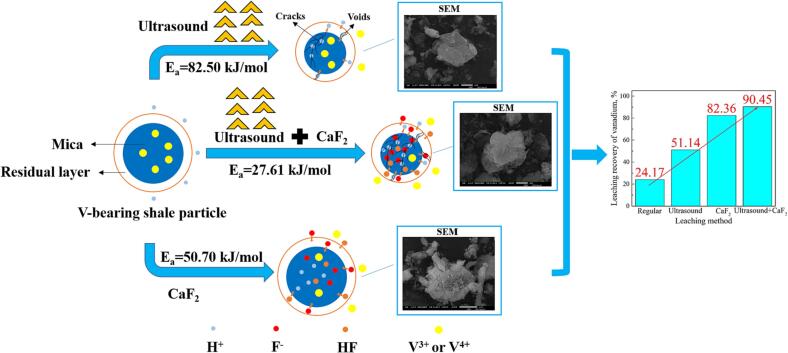
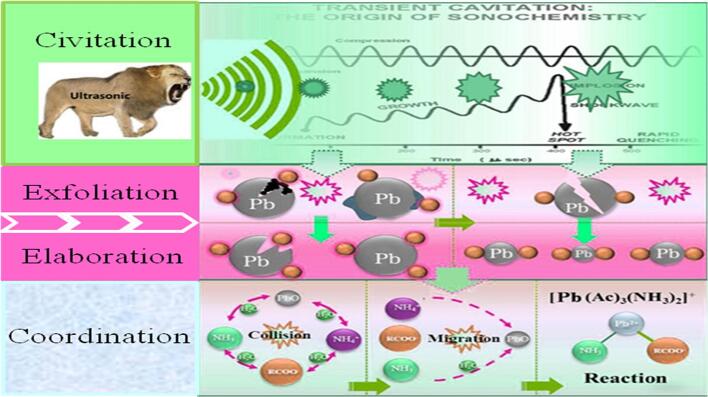
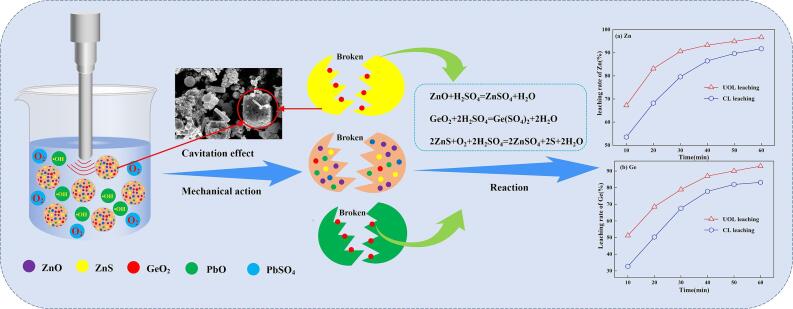
Graphical abstract
Keywords: Ultrasound-assisted leaching, Metal ores, Secondary resources, Leaching kinetics, Strengthened mechanisms
Highlights
-
•
Ultrasound-assisted leaching (UAL) of various metals is detailedly discussed.
-
•
Detailed discussions on the enhanced leaching mechanisms of ultrasound are proposed.
-
•
The development of the novel UAL techniques in the future are put forward.
-
•
Quantum chemical modeling point on the UAL process and its challenges are proposed.
-
•
The main obstacles for the industrial applications of UAL are proposed.
Abstract
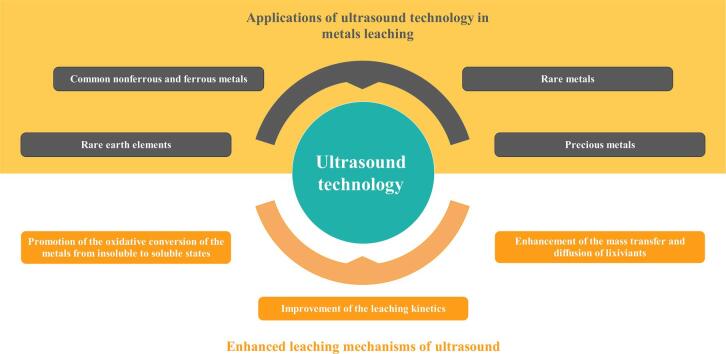
In recent two decades, ultrasound has been broadly applied to the hydrometallurgical leaching process to recover valuable metals within raw materials, aiming to solve the shortcomings of the conventional leaching process, including relatively low leaching recovery, long leaching duration, high reagent usage, high energy consumption and so on. The present work focuses on a comprehensive overview of the ultrasound-enhanced leaching of various metals, such as common nonferrous and ferrous metals, rare metals, rare earth elements, and precious metals, from raw metal ores and secondary resources. Moreover, the enhanced leaching mechanisms by ultrasound are discussed in detail and summarized based on the improvement of leaching kinetics, enhancement of the mass transfer and diffusion of lixiviants, and promotion of the oxidative conversion of metals from insoluble to soluble states. Lastly, the challenges and outlooks of future research on the leaching recovery for valuable metals with the assistance of ultrasound irradiation are proposed.
1. Introduction
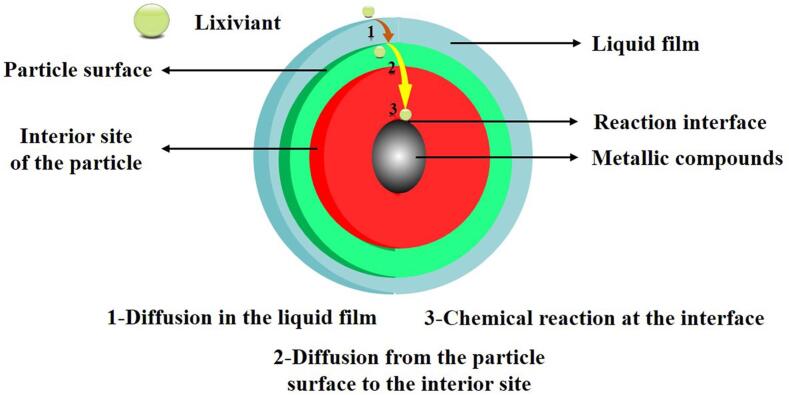
With the depletion and increasing complexity of metal ores in the world, hydrometallurgical technology has been extensively applied to extract valuable metals from low-grade metal ores and secondary resources, which is more economically, technically and environmentally attractive compared to pyrometallurgical process [1], [2], [3], [4]. At present, some metals, e. g., gold [5], [6], zinc [7], [8], aluminum [9], uranium [10], and rare earth elements [11], are produced mainly by hydrometallurgical techniques. Leaching is an essential operation during the hydrometallurgical process and aims to selectively dissolve the valuable metals contained in the metal-containing phases from solid to liquid [12], [13]. Generally, mechanical stirring is usually adopted as a conventional method to strengthen the mass transfer rate. However, the positive effects of mechanical stirring on mass transfer are very limited [14]. As a result, the traditional hydrometallurgical leaching process is generally considered relatively low leaching recovery, long leaching time, high reagent usage, and high energy consumption [15], [16], [17], [18]. Hence, more and more researchers have focused on finding alternative, economical and efficient techniques to solve the shortcomings associated with the conventional hydrometallurgical leaching.
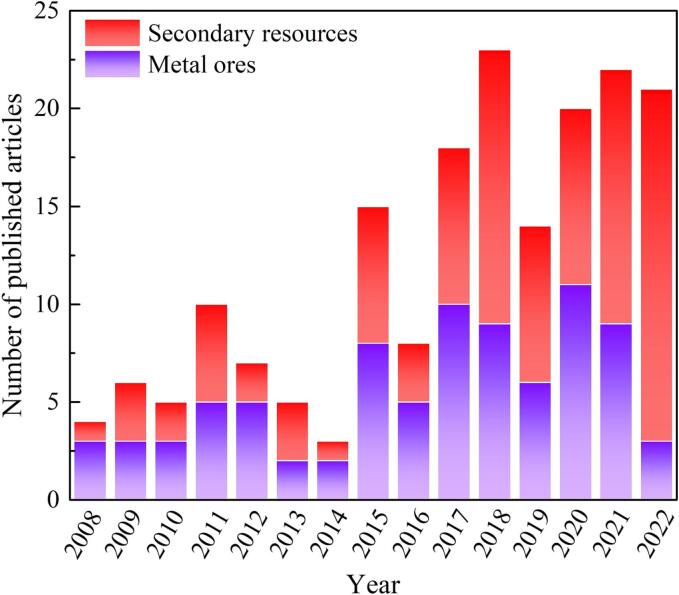
Ultrasound uses sound waves with frequencies higher than those to which human hearing can respond, i. e., above 20 kHz [19], [20], [21], [22]. It is widely accepted that ultrasound can be classified into two types: (i) low frequency ranging between 20 kHz and 2 MHz, where acoustic cavitation is generated and applied to induce and enhance chemical reactions and processes [23]; and (ii) high frequency sonic waves in the range of 2–10 MHz, which are principally adopted in the field of diagnostic [24]. In recent decades, the utilization of ultrasound for the leaching of metal ores [17], [19], [25], [26], [27], [28], [29], [30], [31], [32] and secondary resources [15], [33], [34], [35], [36], [37], [38], [39], [40], [41] has gained increasing attention in hydrometallurgical fields, due to the increased leaching recovery, shortened leaching time, and reduced reagent consumption, which are induced by the cavitation, mechanical, and thermal effect of ultrasound. As presented in Fig. 1, the interest in the promotion of metal leaching recovery by ultrasound has been increasing within the last fifteen years, according to the data collected from the web of science database. In particular, more and more attention has been given to the extraction of metals from secondary resources in the last five years. It has been well received that the cavitation effects induced play a vital role during the leaching process assisted by ultrasound waves [42], [43]. The collapse of cavitation bubbles generates an extremely high temperature and pressure environment [3], [16], producing cracks on the surface of particles and expediting the mass transfer and diffusion of lixiviants, and thus, increasing the leaching efficiency of valuable metals.
Fig. 1.
Number of articles published on “metal ores” and “secondary resources” leaching enhanced by ultrasound within the 2007–2022 periods.
This work aims to present a comprehensive overview of the state-of-the-art references and scientific knowledge in the field of ultrasound-assisted hydrometallurgical leaching. It reviews the fundamentals of ultrasonic cavitation theory and the applications of ultrasound towards valuable metals leaching, such as common nonferrous and ferrous metals, rare metals, rare earth elements, and precious metals. Furthermore, it provides a detailed discussion of the enhanced leaching mechanisms induced by ultrasound based on the analysis of the published references. Finally, the prospects for the research of ultrasound-assisted leaching, including deep insight into strengthened mechanisms and challenges in large-scale industrial applications, have also been proposed.
2. Ultrasonic cavitation theory
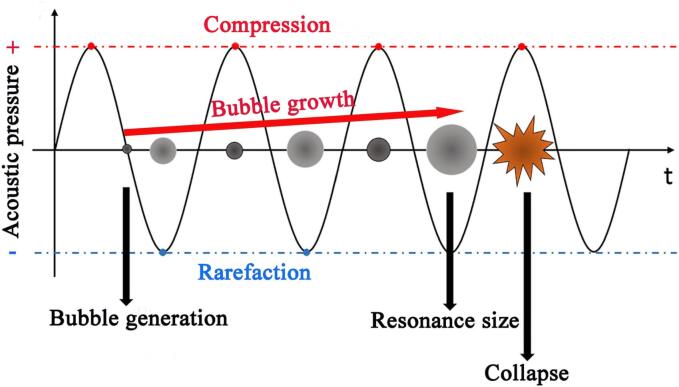
The most exciting phenomenon of ultrasound is acoustic cavitation, which includes the microbubbles’ generation, growth, and collapse (Fig. 2) [44]. It is well known that the microbubbles generally derive from the preexisting gas nuclei in solution, such as the dissolved O2, N2 or Ar. These gas nuclei oscillate and grow in the liquid under the function of alternating acoustic pressure (compression and rarefaction) until a resonance size (active size) is achieved, then they undergo an inertial collapse step. According to the thermodynamic stability of cavitation bubbles, cavitation bubbles are commonly divided into two categories: transient and stable cavitation bubbles. On one hand, transient bubbles are short lived or collapse speedily. On the other hand, stable cavitation bubbles would undergo hundreds of acoustic cycles. Generally, the transient cavitation bubbles are commonly generated at lower ultrasound frequencies, whereas the stable cavitation bubbles are usually produced at higher frequencies [44]. It is generally accepted that the active bubbles’ size decreases with the elevation of the ultrasound frequency, and hence the collapse intensity of the bubbles is much higher at lower frequencies than at higher frequencies.
Fig. 2.
Schematic diagram of the cavitation activity steps with respect to time: the generation, growth, and collapse of the microbubbles. Modified from [44]. Copyright (2019), with permission from Elsevier.
During the ultrasound irradiation treatment process, the significance of the cavitation effect to ultrasound is not how the cavitation bubbles are generated but rather what occurs when they implode. It is well known that the collapse of cavitation bubbles will produce a micro-environment termed local ‘hot spots’, where extremely high temperatures (up to 10000 K) and high pressures (up to 10000 atm) are generated [45]. The local ‘hot spots’ will induce the thermolysis of H2O to form •H and •OH radicals with high reactivity, as well as the thermolysis of the added surfactants, which may assist the chemical reaction by giving better catalyzing effect [46]. In heterogeneous systems (solid particles existing in liquids), both asymmetric and symmetric collapses can be generated, depending on the distance between the particles and the collapsing bubbles. Symmetric collapse commonly generates micro-scale turbulence and shock waves, whereas asymmetric collapse occurs where the particles are very close to the collapsing bubbles, which may form a powerful liquid jet [47]. The produced microjets or shockwaves will directly act on the solid particles’ surface, which further induces the fragmentation of particles or opens the inclusions on the particle’s surface [48].
The unique ultrasonic cavitation would cause specific impacts in the ultrasound-assisted leaching system such as thermal, mechanical and sonochemical effects. Mechanical effects would induce the formation of microcracks or the elimination of the passive layer on the particles’ surface, strengthening the mass transfer process of the lixiviant. Thermal effects could increase the temperature, which is responsible for the acceleration of the leaching reaction rate or the reaction extent. As for sonochemical effects, it could induce the generation of the free radicals, which intensifies the oxidation transformation process of low-valence metals to high and further promotes the dissolution of the targeted metals.
3. Applications of ultrasound technology in metal leaching processes
Ultrasound-assisted leaching technology has been extensively applied to extract valuable metals from metal ores, such as vanadium-bearing shale [16], [19], chalcopyrite [26], [49], scheelite [25], [50], [51], zinc oxide ore [30], [52], [53], nickel laterite ore [17], rare earth ore [29], refractory gold ore [27], [54], [55], [56], [57], [58], uranium ore [59], [60], K-feldspar [31], copper-bearing biotite [43], refractory silver ore [61], eudialyte [62], quartz sand [63], [64], [65], [66], [67], [68], poly-metallic sulfide ore [13], deep-sea nodules [69], phosphorus-potassium associated ore [21], sphalerite [32], and magmatic rocks [70]. In addition, ultrasound-assisted leaching has also been developed with successful enhancement in the leaching efficiency of metals from secondary resources, including metallurgical residues [3], [15], [35], [38], [39], [42], [48], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], spent catalysts [33], [37], [87], [88], [89], [90], [91], [92], [93], [94], waste electric and electronic equipment [23], [40], [95], [96], [97], [98], [99], [100], [101], spent batteries [34], [102], [103], [104], [105], [106], [107], [108], [109], [110], fly ash [36], [111], [112], [113], sludge [114], [115], [116], [117], cutting waste [118], [119], [120], fluorescent lamp waste [121], waste magnet [122], boron carbide waste-scrap [123], and fluoride matrix [124]. From recent literature, it can be found that ultrasound-assisted leaching has shown great potential in increasing leaching efficiency, shortening leaching duration, reducing chemical reagent consumption, etc., ascribing to the superiority of ultrasound irradiation compared to conventional mechanic stirring.
3.1. Common nonferrous and ferrous metals
In recent years, the utilization of ultrasound for the recovery of common nonferrous and ferrous metals has been pursued by many researchers worldwide, and many encouraging results have been obtained.
3.1.1. Common nonferrous metal leaching
3.1.1.1. Zinc leaching
As a significant base metal, zinc (Zn) has been broadly adopted in galvanizing, coating, manufacturing batteries, and alloying with other metals [52], [125]. The leaching characteristics of Zn from various raw materials with the assistance of ultrasound are summarized and compared in Table 1. Recently, ultrasound has been applied to recover Zn from zinc ores, such as zinc oxide ores and zinc sulphide ores, to elevate the leaching ratio of Zn. Li et al. [52] probed the influence of ultrasound wave on NH3–(NH4)2SO4 leaching of Zn from a low-grade zinc oxide ore. They found that ultrasound increased the leaching ratio of Zn mainly by accelerating the leaching rate and shortening the leaching duration at lower ammonium sulfate concentrations, with 83.33% of Zn being recovered under the optimized ultrasound leaching conditions. In order to further enhance the recovery of Zn, Zhang et al. [53] developed a new NH3-C6H5O7(NH4)3 leaching system intensified via ultrasound-assisted leaching combined with microwave pre-roasting. The results manifested that the optimal leaching recovery of Zn was 88.57% under ultrasound leaching at ambient temperature. Taking the synergistic effect between ultrasound and other auxiliary leaching technologies into consideration, He et al. [30] proposed a novel synergistic system with the combination of mechanical activation and ultrasound irradiation for the extraction of Zn from ZnO ore. The results indicated that a satisfactory improvement in Zn recovery (about 12%) was obtained with the simultaneous assistance of mechanical activation and ultrasound irradiation. Salmi et al. [32] established a modeling system for the leaching kinetics and mass transfer of sphalerite in Fe2(SO4)3-H2SO4 solutions with ultrasound assistance. The model can also be used to predict the reactivities of rough, porous or non-porous particles during solid–liquid leaching reactions.
Table 1.
Comparisons for the leaching characteristics of Zn from different raw materials assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | Zn Leaching Recovery | Reference |
|---|---|---|---|---|
| Zinc oxide ore | Ultrasound Lixiviant: NH3–(NH4)2SO4 | 600 W, 60 min, 30 °C | 83.33% | [52] |
| Zinc oxide ore | Microwave and ultrasound Lixiviant: NH3-C6H5O7(NH4)3 |
600 W, 120 min, 25 °C | 88.57% | [53] |
| Zinc oxide ore | Bead milling and ultrasound Lixiviant: H2SO4 |
150 W, 90 min, 60 °C | >75% | [30] |
| Electric arc furnace dust | Ultrasound Lixiviant: H2SO4 |
60 W/cm2, 30 min, 80 °C | about 90% | [3] |
| Zinc residue | Ultrasound Lixiviant: H2SO4 |
160 W, 180 min, 65 °C | about 80% | [74] |
| Corundum flue dust | Ultrasound Lixiviant: H2SO4 |
900 W, 50 min, 90 °C | 99.57% | [15] |
| Germanium-containing slag dust | Oxygen and ultrasound Lixiviant: H2SO4 |
300 W, 60 min, 90 °C | 96.66% | [126] |
In addition to natural zinc ores, researchers have engaged in extracting Zn from secondary resources [3], [15], [42], [74], [102], [116], [126]. Brunelli et al. [3] recovered Zn from electric arc furnace dust via ultrasound-augmented leaching. They stated that ultrasound waves were highly influential during the leaching process with 0.5 M sulfuric acid at 80 °C because they strengthened the dissolution process of franklinite, leading to a 55% increase of the Zn recovery in comparison to that obtained by conventional leaching. Wang et al. [74] leached Zn from zinc residue by comparing conventional and ultrasound-enhanced leaching. It was stated that the maximum leaching ratio of Zn was increased from 67% to 80%, and the activation energy was reduced from 13.07 kJ/mol to 6.57 kJ/mol with ultrasound assistance, making the leaching reaction easier to accomplish. A similar standpoint was also reported and proved in the work of Ding et al. and Xin et al. Ding et al. discovered that the Zn contained in a corundum flue dust was almost completely leached (leaching recovery of 99.57%) using ultrasound-assisted sulfuric acid leaching, whereas the leaching ratio achieved by conventional leaching was only 94.43% [15], attributing to the reduction of activation energy of Zn leaching reaction from 16.22 kJ/mol to 4.26 kJ/mol with the aid of ultrasound. Xin et al. [126] concluded that the combination of oxygen and ultrasound can noticeably decrease the activation energy, further bringing down the energy obstacle of Zn leaching. Hence, the leaching ratio of Zn was increased from 91.74% to 96.66% with the induction of ultrasound.
From the current research, the leaching efficiency of Zn is relatively high when ultrasound is coupled with aid-leaching reagents or other techniques. In comparison to conventional leaching method, the coupled leaching process not only obviously improve the leaching ratio of Zn, but also shorten the leaching time.
3.1.1.2. Nickel leaching
Nickel (Ni) is a significant alloying metal which has wide industrial applications ascribing to the specific properties of nickel alloy, e.g., high strength, ductility, and excellent heat resistance [17], [127]. The leaching characteristics of Ni from various raw materials with the assistance of ultrasound are summarized and compared in Table 2. The research on the extraction of Ni from nickel ores, such as nickel sulfide, nickel sulfate, and laterite ore, by ultrasound-assisted leaching have received extensive attention. Xue et al. [18] explored the oxidation leaching of Ni from nickel sulfide concentrate in Na2S2O8-AgNO3 solutions enhanced with ultrasound radiation. It was stated that the leaching ratio of Ni with the aid of ultrasound for 150 min is the same as that obtained by conventional leaching for 300 min. In particular, the oxidation leaching of nicopyrite could be directly improved using oxidants, as shown in Eq. (1)-(3), which could also be further enhanced with ultrasound induction. Li et al. recovered Ni from nickel block via an ultrasound-assisted oxidation leaching process. The results demonstrated that ultrasound leaching could increase the leaching ratio of Ni from 43.87% to 60.41% under the same conditions compared with conventional leaching, attributing to the peeling of the oxide film, shattering of the particles, and the reduction of activation energies of Ni leaching reaction with ultrasound assistance [128]. Furthermore, the phenomenon of activation energy reduction was also demonstrated in Cetintas’s work. Cetintas et al. [17] developed a novel and alternative process involving the combination of reagent-assisted mechanochemistry and ultrasound to recover Ni from a low-grade nickel laterite ore. The experimental outcomes indicated that the nickel leaching recovery obtained without ultrasound at 30 min was realized at only 10 min in the presence of ultrasound radiation, ascribing to the strengthened leaching reaction rate brought by ultrasound cavitation.
| (1) |
| (2) |
| (3) |
Table 2.
Comparisons for the leaching characteristics of Ni from different raw materials assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | Ni Leaching Recovery | Reference |
|---|---|---|---|---|
| Nickel sulfide ore | Ultrasound Lixiviant: Na2S2O8-AgNO3 | 220 W, 120 min, 70 °C | 82.95% | [18] |
| Nickel block | Ultrasound Lixiviant: H2SO4-H2O2 |
200 W, 240 min, 60 °C | 60.41% | [128] |
| Nickel laterite ore | Mechanochemical and ultrasound Lixiviant: H2SO4 |
720 W, 10 min, 95 °C | 96.18% | [17] |
| Spent catalysts | Ultrasound Lixiviant: HNO3 |
30 kHz, 50 min, 90 °C | 95% | [88] |
| Sapphire kerf waste | Ultrasound Lixiviant: H2SO4-HNO3 |
240 W, 30 min, 45 °C | 99.67% | [119] |
| Spent lithium-ion batteries (LIBs) | Ultrasound Lixiviant: lemon juice-H2O2 |
37 kHz, 35 min, 40 °C | 100% | [107] |
| Spent LIBs | Ultrasound Lixiviant: citric or acetic |
110 W, 1440 min, 50 °C | >99% | [109] |
| Spent hydroprocessing catalysts | Vacuum pyrolysis and ultrasound Lixiviant: H2SO4 |
60 W, 20 min, 45 °C | 98.99% | [33] |
In the meantime, ultrasonic leaching has also been used as a high-efficiency method for the recovery of Ni from electroplating sludge, spent catalysts, spent batteries, etc. [33], [81], [87], [88], [103], [108], [119]. Oza et al. [88] probed the effects of various leaching factors on the recovery of Ni from spent catalysts by HNO3 leaching assisted by ultrasound. They concluded that 95% of the Ni could be recovered using the ultrasonication technique at 50 min, while a maximum recovery of Ni (93%) was achieved at a longer time of 9 h by traditional leaching. Wang et al. [119] improved the leaching efficiency of Ni from sapphire kerf waste by a hybrid acid system (H2SO4 and HNO3) with ultrasound assistance. It was found that a high leaching recovery of Ni (99.67%) was achieved with ultrasound-assisted leaching for 30 min. To improve the leaching recovery of Ni, many researchers have combined ultrasound irradiation with other methods, such as additives, organic acid, and vacuum pyrolysis. Esmaeili et al. [107] recovered 100% Ni from spent lithium-ion batteries (LIBs) using organic acids in lemon juice together with H2O2 in the presence of ultrasound. Xiao et al. [109] also reported an ultrasound-assisted natural organic acids (citric or acetic) leaching technique to recover valuable metals within spent LIBs. The results showed that the highest Ni recovery over 99% was achieved, due to the improved mass transfer of metal ions in the residue layers of nickel-manganese-cobalt oxide. Feng et al. [33] proposed a sustainable technique involving vacuum pyrolysis and ultrasound leaching to recover Ni from uncrushed spent hydroprocessing catalysts. The leaching efficiency of Ni reached 98.99% with the combined approach.
It can be concluded from the research mentioned above that the promotion of Ni dissolution is more obvious when ultrasound is introduced into the leaching systems using oxidants or organic acids. The introduction of ultrasound makes it easier to achieve the highest leaching recovery of Ni in a shorter time compared to regular leaching process in the absence of ultrasound.
3.1.1.3. Copper leaching
Copper (Cu) is considered one of the most abundant and valuable metals, which has been broadly applied in the fields of electrodeposition, metal finishing, plastics, etc. [129], [130]. The leaching characteristics of Cu from various raw materials with the assistance of ultrasound are summarized and compared in Table 3. In recent, the recovery of copper from copper-containing ores, such as deep-sea manganese nodules, chalcopyrite, and biotite, via ultrasound-assisted leaching has been investigated by researchers. Knaislova et al. [69] explored the influence of ultrasound on Cu leaching from deep-sea manganese nodules in a reductive (NH4)2S2O3 leaching system. The results implied that the extraction efficiency of Cu was 83% by ultrasound leaching for 90 min, while the efficiency was just 67% by microwave leaching for 210 min. The oxidation leaching technique with ultrasound assistance has also been put forward to improve the leaching of Cu. Wang et al. [49] adopted ultrasound into the leaching process of chalcopyrite in Fe2(SO4)3-H2SO4 media. It was shown that ultrasonic waves efficiently improved the leaching rate, reduced the acid consumption, and shortened the reaction duration, hence the extraction ratio of copper from chalcopyrite was increased from 50.4% to 57.5% with the application of ultrasound. Turan et al. [26] recovered Cu from chalcopyrite by a novel leaching media with the usage of titriplex III-H2O2 assisted by ultrasound. The outcomes showed that the leaching ratio of Cu noticeably increased from 83% to 93% using ultrasound.
Table 3.
Comparisons for the leaching characteristics of Cu from different raw materials assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | Cu Leaching Recovery | Reference |
|---|---|---|---|---|
| Deep-sea manganese nodules | Ultrasound Lixiviant: (NH4)2S2O3 | 100 W, 90 min, 85 °C | 83% | [69] |
| Chalcopyrite | Ultrasound Lixiviant: Fe2(SO4)3-H2SO4 |
80 W, 300 min, 80 °C | 57.5% | [49] |
| Chalcopyrite | Ultrasound Lixiviant: titriplex III-H2O2 |
10 % ultrasound power, 90 min, 45 °C | 93% | [26] |
| Print circuit boards | Ultrasound Lixiviant: spent etching solution |
300 W, 30 min, 25 °C | 93.76% | [96] |
| Cu-Fe containing sludge | Ultrasound Lixiviant: H2SO4 |
35.58 W, 60 min, 25 °C | 97.47% | [115] |
| Copper anode slime | Ultrasound Lixiviant: H2SO4 |
800 W, 600 min | 85.18% | [83] |
| Blended copper slag | Ultrasound Lixiviant: H2O2-CH3COOH |
10 % ultrasound power, 60 min, 65 °C | 93% | [78] |
| Copper anode slime | Ultrasound Lixiviant: H2SO4-Na2S2O8 |
400 W, 50 min, 50 °C | 98.11% | [84] |
Ultrasound has also been applied to selectively recover Cu from print circuit boards waste sludge, metal-containing sludge, blend copper slag, and copper anode slime. Huang et al. [96] tried to recover Cu from print circuit boards waste sludge using copper-containing print circuit board spent etching solutions as a leaching media. They found that the leaching rate of Cu and Fe was 93.76% and 2.07%, respectively, indicating the high separation efficiency of Cu from Fe with ultrasound assistance. Zhang et al. [115] reported a method to efficiently dissolve and separate metals (Cu/Fe, Cu/Cr, and Cr/Fe) existing in metal-containing sludge, which relies on the assistance of ultrasound irradiation. Wang et al. [83] found that the residual Cu content in the ultrasound-leaching residue was 2.64%, which was apparently lower than that (10%) in the conventional leaching residue. Other elements, such as tellurium and selenium, remained in the leaching residues, implying the selective leaching of Cu with the use of ultrasound. Turan et al.[78] conducted the work of dissolving Cu from blended copper slag in H2O2-CH3COOH with the aid of ultrasound. The outcomes showed that the optimum leaching ratio of Cu (93%) and minimum of Fe (3%) were realized with ultrasound, indicating the selective leaching of Cu from Fe. Liu et al. [84] proposed an eco-friendly cooperative decopperization technique from copper anode slime via ultrasound-enhanced leaching with sodium persulfate. It was demonstrated that the combination of ultrasound and Na2S2O8 induced the transformation of insoluble copper into copper sulfate, which increased the total leaching recovery of Cu from 66.64% to 98.11%. Furthermore, the content of noble metals in the ultrasound-Na2S2O8 leaching residues was higher than that in the Na2S2O8 leaching residues and raw materials, making the hazardous waste turn into a valuable industrial material.
From the published literatures, the leaching systems with the combinations of oxidative reagents and ultrasound pose apparent intensification for the leaching of Cu. The ultrasound-assisted leaching process is not only characterized by higher leaching percentage and shorter leaching time, but also lower reagents consumption and higher selectivity compared with conventional leaching method.
3.1.1.4. Cobalt leaching
As a transition metal, cobalt (Co) has broad applications in magnets, high-strength materials, and rechargeable batteries ascribing to its unique physical properties [131]. The leaching characteristics of Co from various raw materials with the assistance of ultrasound are summarized and compared in Table 4. Up to now, ultrasound wave has been adopted to elevate the leaching ratio of Co during the hydrometallurgical process of deep-sea manganese nodules and spent LIBs [34], [69], [105], especially the reductive leaching process. Knaislova et al. [69] compared the leaching behavior of Co from deep-sea manganese nodules in (NH4)2S2O3 media by ultrasound waves and microwaves. It was found that the use of ultrasound could elevate the leaching ratio of Co from 8% to 32% and shorten the leaching duration from 210 min to 90 min, indicating the high efficiency of ultrasound towards the extraction of Co. Jiang et al. [103] found that both the leaching rate and efficiency of Co from spent LIBs in the H2SO4-H2O2 system were improved by ultrasound in comparison to conventional leaching. The report from Ning et al. has demonstrated the higher efficiency of the combination of malic acid (C4H6O5) and H2O2 for Co leaching from spent LIBs [106]. The main reductive leaching reactions in C4H6O5 using H2O2 as reductant are shown in Eq. (4)-(5), and the optimal leaching percentage of Co attained 97.6% at a leaching time of 30 min and leaching temperature of 80 °C. A novel acid-leaching process using organic acids in lemon juice (mainly citric, malic, and ascorbic acid) together with H2O2 by ultrasound radiation was proposed to recover Co within spent LIBs.[107]. It was obviously shown that the optimal leaching percentage of Co could reach 100% in 35 min.
| (4) |
| (5) |
Table 4.
Comparisons for the leaching characteristics of Co from different raw materials assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | Co Leaching Recovery | Reference |
|---|---|---|---|---|
| Deep-sea manganese nodules | Ultrasound Lixiviant: (NH4)2S2O3 | 100 W, 90 min, 85 °C | 32% | [69] |
| Spent LIBs | Ultrasound Lixiviant: H2SO4-H2O2 |
360 W, 30 min, 30 °C | 94.63% | [103] |
| Spent LIBs | Ultrasound Lixiviant: malic acid-H2O2 |
90 W, 30 min, 80 °C | 97.3% | [106] |
| Spent LIBs | Ultrasound Lixiviant: lemon juice-H2O2 |
37 kHz, 35 min, 40 °C | 100% | [107] |
| NMC type Li-ion battery | Ultrasound Lixiviant: H2SO4 |
400 W, 300 min, 65 °C | 73% | [105] |
| Spent LIBs | Ultrasound Lixiviant: citric or acetic |
110 W, 1440 min, 50 °C | >99% | [109] |
Meanwhile, the selective leaching of Co from other metals within the spent LIBs was also reported using mild organic acids with ultrasound assistance. Wang et al. [105] reported that the leaching ratio of Co could be augmented by about 40% with ultrasound enhancement. Interestingly, manganese was not determined in the leachate. Instead, it was precipitated as MnO2 with the induction of ultrasound. Xiao et al. [109] compared the leaching characteristics of Co in the leaching media of mild organic citric or acetic acids in the presence of ultrasound. It was found that the highest leaching ratio of Co reached more than 99%, and the leaching time was decreased by more than 50% with the application of ultrasound in these leaching media. Furthermore, it was also found that citric acid could suppress the dissolution of Cu compared to the use of acetic acid, ascribing to the inhibition effect of citric acid on the Cu surface.
From the presented researches, it is obvious that the combinations of ultrasound and organic acids or oxidative reagents give more enhancement towards Co leaching. In the meanwhile, the leaching duration is apparently reduced, and the dissolution of other impurity metals can be effectively restrained.
3.1.1.5. Lead leaching
Lead (Pb) is a significant industrial raw material, which has been extensively used in our daily life, for instance, batteries, cables, and chemical products [132], [133]. The leaching characteristics of Pb from various secondary resources with the assistance of ultrasound are summarized and compared in Table 5. At present, ultrasound has been introduced into the hydrometallurgical recovery of Pb from secondary resources, such as lead-rich oxidizing slag, fly ash, solid waste incineration fly ashes, waste printed circuit boards, landfilled metallurgical residues, electrolytic manganese anode mud and industrial waste sludges [39], [73], [75], [100], [111], [132], [134], [135] to remove the residual Pb and eliminate the potential environmental pollution. Zhang et al. [75] optimized the leaching conditions of Pb from antimony and lead-rich oxidizing slag in HCl-NaCl media with the assistance of ultrasound. They found that the leaching percentage of Pb by ultrasound leaching of 15 min resembled that obtained by conventional leaching at 45 min, which implies that ultrasound accelerated the dissolution of Pb. Similarly, Bisercic et al. [111] also drew the conclusion that ultrasound hardly increased the total amount of the extracted Pb, but the leaching efficiency of ultrasound treatment for 15 min was as effective as that of conventional shaking for 6 h. This phenomenon might be attributed to that ultrasound failed to generate new reaction pathways, and the maximal dissolution of Pb was determined by the composition of the Pb-containing phases.
Table 5.
Comparisons for the leaching characteristics of Pb from different secondary resources assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | Pb Leaching Recovery | Reference |
|---|---|---|---|---|
| Antimony and lead-rich oxidizing slag | Ultrasound Lixiviant: HCl-NaCl | 200 W, 90 min, 95 °C | about 75% | [74] |
| Electrolytic manganese anode mud | Ultrasound Lixiviant: CH3COONH4 |
450 W, 60 min, 70 °C | 93.09% | [132] |
| Lead-tin solders | Ultrasound Lixiviant: HBF4-H2O2 |
40 kHz 180 min, 5–20 °C | about 100% | [100] |
| Landfilled metallurgical residues | Ultrasound Lixiviant: NaCl |
20 W, 360 min, 25 °C, three leaching stages | 61% | [39] |
| Electrolytic manganese anode mud | Microwave and ultrasound Lixiviant: CH3COONH4 |
450 W, 60 min, 70 °C | 96.36% | [134] |
Xie et al. [132] extracted Pb from electrolytic manganese anode mud in CH3COONH4 solutions using roasting pretreatment and ultrasound-assisted leaching. It was discovered that the leaching rate of Pb was increased by about 7% with the assistance of ultrasound compared to conventional leaching, attributing to the reduced activation energy of the leaching reaction of Pb induced by ultrasound. Zhu et al. [100] compared the dissolution of Pb from lead-tin solders on waste printed circuit boards with and without the assistance of ultrasound wave. It indicated that the dissolution of the SnPb solder was 2 to 3 times faster with an ultrasonic field than without ultrasound, which is also ascribed to the decrease of the activation energy for the dissolution reactions of Pb. John et al. [39] explored the influence of ultrasound on the leaching of Pb from landfilled metallurgical residues. It was clearly found that the dissolution of Pb was improved by 19–26%, and ultrasound as a pre-treatment step in the first 5 min could be more sufficient compared to the entire ultrasound-assisted reaction. In order to further elevate the leaching ratio of Pb from electrolytic manganese anode mud, Xie et al. [134] attempted the technique of microwave roasting coupled with ultrasound leaching. It was reported that the leaching percentage of Pb was 96.36%, which is apparently higher than their previous work at the same leaching parameters.
It can be concluded from the current research that the leaching systems with the combination of new lixiviants or other techniques in high ultrasound power is more conducive for the removal of Pb from the solid wastes. The leaching rate of Pb is obviously elevated in the presence of ultrasound compared to that in the absence of ultrasound, which further improve the removal ratio of Pb.
3.1.1.6. Alumina leaching
Alumina (Al) and its alloys have broad applications in the aerospace, construction, and automobile industry due to their unique properties [136]. The leaching characteristics of Al from various raw materials with the assistance of ultrasound are summarized and compared in Table 6. The ultrasound-assisted leaching recovery of Al mainly focused on quartz sand, red mud, aluminum dross, and spent automobile catalysts. Lim et al. [72] recovered Al from red mud in H2SO4 media with the aid of ultrasound. It was apparent that the leaching efficiency of Al could be improved from 57.02% to 76.33% with the use of 150 W ultrasound. Li et al. [137] reported that the leaching ratio of Al from quartz sand was increased from 12.22% to 42.25% with Na2CO3 under ultrasound irradiation for only 25 min. Nguyen and Lee [79] also proposed the ultrasound-assisted NaOH leaching technique for the recovery of Al from aluminum dross, and it was obviously found that the leaching percentage of Al was improved from 35% to 60% with the use of ultrasound. In the meanwhile, researchers attempted to combine ultrasound with other processes to further improve the leaching recovery of Al. Hosseinzadeh et al. [37] leached Al from spent automotive catalysts using an ultrasound-assisted Fenton-like process. It was stated that the recovery of Al was 81.7% and 69.5% with and without the assistance of ultrasound, respectively, and 100% platinum and palladium within the residue could be recovered with further processing.
Table 6.
Comparisons for the leaching characteristics of Al from different raw materials assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | Al Leaching Recovery | Reference |
|---|---|---|---|---|
| Red mud | Ultrasound Lixiviant: H2SO4 | 150 W, 120 min, 70 °C | 76.33 | [72] |
| Quartz | Ultrasound Lixiviant: Na2CO3 |
150 W, 25 min, 80 °C | 42.3% | [137] |
| Aluminum dross | Ultrasound Lixiviant: NaOH |
100 W 240 min, 50 °C | 60% | [79] |
| Spent automotive catalysts | Ultrasound Lixiviant: FeSO4-H2O2 |
37 kHz, 40 min, 70 °C, two leaching stages | 81.7% | [37] |
From the current leaching researches, the leaching efficiency of Al is obviously enhanced with the assistance of ultrasound compared to conventional leaching. Especially, the leaching recovery of Al is higher in the oxidative leaching process assisted by ultrasound, meanwhile, other impurity metals remain in the leaching residues.
3.1.1.7. Other nonferrous metals leaching
Ultrasound has also been adopted to promote the recovery of other nonferrous metals, such as calcium (Ca), potassium (K), sodium (Na), magnesium (Mg), cadmium (Cd), and antimony (Sb). Ozkan et al. [70] dissolved Ca and Mg from magmatic rocks with the aid of ultrasound, and it was stated that the Ca and Mg encapsulated within the rocks could be easily and efficiently released with the assistance of ultrasound waves. The leaching of K from phosphorus-potassium associated ore in H2SO4-CaF2 system with the application of ultrasound was performed by Zhang et al. [21], and the results showed that the dissolution ratio of K could be obviously improved with ultrasound assistance, and the leaching efficiency of K attained as 94%. Ma et al. [31] studied the leaching kinetics of K-feldspar using H2SO4 media with the assistance of an ultrasound wave. It was found that the leaching rate constant was significantly increased with the assistance of ultrasound, ascribing to the reduced activation energy, which leads to the intensified dissolution of K. Srivalli et al. [138] investigated ultrasound-assisted leaching of K and Na from Indian coal under continuous and pulsed modes. It was discovered that the maximum leaching ratio of K and Na in the pulsed mode is 91.3% and 54.4%, respectively, while that in the continuous mode is only 62% and 24.5%. Kusaka et al. [82] studied the leaching of Ca from steelmaking slag in CH3COOH under ultrasound irradiation. They found that ultrasound could promote the mass transfer process and remarkably enhance the leaching rate of Ca in a short duration. Huang et al. [135] improved the removal efficiency of Cd from municipal solid waste incineration fly ashes by ultrasound. They discovered that the maximum leaching efficiency of Cd could reach 59.93% with the activation of ultrasound. Ultrasound has also been applied to enhance the recovery of Sb from the refractory gold ores [57]. It was reported that the leaching ratio of Sb could be significantly improved from 58.37% to 94.50% with the assistance of ultrasound compared to conventional leaching under the optimal leaching conditions.
3.1.2. Ferrous metal leaching
The enhancement of the leaching process towards ferrous metals such as iron, manganese, and chromium from natural ores and secondary resources is another significant application of ultrasound [77], [110], [115], [118], [120], [137], [139], [140].
3.1.2.1. Iron leaching
The leaching characteristics of Fe from various raw materials with the assistance of ultrasound are summarized and compared in Table 7. In recent years, ultrasound has been widely applied to remove iron (Fe) from raw materials, such as silica sand, quartz, kaolin, silicon diamond-wire saw cutting waste, and boron carbide waste-scrap [68], [120], [123], [140], [141] to improve the purity. In the early years, many studies were conducted in relatively single leaching systems, such as inorganic acid, alkali, and organic solvent. Du et al. [68] improved the recovery of Fe from silica sand using ultrasound-assisted H2C2O4 leaching method (Eq. (6)-(8)). The optimal leaching recovery of Fe was elevated from 65.8% to 75.4% in a lower acid concentration with ultrasound assistance, indicating that the ultrasound could promote the leaching efficiency of Fe and reduce the consumption of H2C2O4. The removal of Fe was carried out using CH4N2S with the aid of an ultrasound wave by Xia et al [140]. It was illustrated that ultrasound resulted in a significant acceleration of Fe leaching, an obvious reduction in the CH4N2S concentration, leaching duration and reaction temperature compared to conventional leaching. Zhang et al. [67] also found a similar phenomenon when they tried to remove Fe from quartz sand using H3PO4 by ultrasound-assisted leaching. The optimal removal efficiency of Fe reached 81%, which was 30 ∼ 40% higher than other methods. Wang et al. [66] concluded that ultrasound would induce a conspicuous acceleration of the dissolution and removal process of Fe from silica sand compared to the regular leaching method, attributing to the increase in the diffusion rate and the promotion of chemical reaction. In order to further promote the removal effect of Fe, many researchers have attempted to explore new techniques by the combination of ultrasound and other technologies. Yang et al. [65] removed Fe from quartz by calcination pretreatment and ultrasound-assisted leaching in hybrid acid systems (HCl and H2C2O4). It was discovered that the combined method could obviously promote the dissolution of Fe and elevate the removal ratio of Fe, leading to the increase of SiO2 content from 99.68% to 99.90%. Li et al. [141] put forward an environment-friendly process for removing Fe from quartz by a combination of microwave heating and ultrasound-assisted HNO3 leaching. It was reported that the maximum leaching efficiency of Fe was achieved as high as 99.94% under the optimum conditions, ascribing to the improved removal of Fe through the formed cracks.
| (6) |
| (7) |
| (8) |
Table 7.
Comparisons for the leaching characteristics of Fe from different raw materials assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | Fe Leaching Recovery | Reference |
|---|---|---|---|---|
| Silica sand | Ultrasound Lixiviant: H2C2O4 | 150 W, 30 min, 95 °C | 75.4% | [68] |
| Kaolin | Ultrasound Lixiviant: CH4N2S |
500 W, 20 min, 20 °C | Fe2O3 content was reduced from 0.85 to 0.7% | [140] |
| Quartz sand | Ultrasound Lixiviant: H3PO4 |
150 W 240 min, 80 °C | 81% | [67] |
| Silica sand | Ultrasound Lixiviant: FeSO4-H2O2 |
180 W, 40 min, 30 °C | 93.9% | [66] |
| Quartz | Ultrasound Lixiviant: HCl-H2C2O4 |
400 W, 30 min, 60 °C | 74% | [65] |
| Quartz sand | Microwave and ultrasound Lixiviant: HNO3 |
400 W, 30 min, 90 °C | 99.94% | [141] |
| Boron carbide waste-scrap | Ultrasound Lixiviant: H2SO4 |
210 W, 50 min, 50 °C | 94.5% | [123] |
| Silicon cutting slurry waste | Ultrasound Lixiviant: H2SO4 |
210 W, 45 min, 60 °C | 95.46% | [118] |
| Silicon diamond-wire saw cutting waste | Ultrasound Lixiviant: H2SO4 |
270 W, 50 min, 60 °C | 95.24% | [120] |
Besides natural ores, ultrasound was also found efficient in Fe leaching from secondary resources. Li et al. [123] investigated the ultrasound-assisted leaching process of Fe from boron carbide waste-scrap, and the outcomes showed that the leaching ratio of Fe was improved from 87.4% to 94.5%. Meantime, the leaching time was shortened from 80 min to 50 min with the induction of ultrasound. Liu et al.[118] also reported that the removal ratio of Fe from solar grade silicon cutting slurry waste could be improved by 7.84%, and the leaching time was reduced by 50% with ultrasound assistance. Kong et al. [120] found that the maximum Fe removal efficiency, 95.24%, could be obtained with ultrasound leaching for 50 min, which was achieved by conventional leaching for 80 min. It indicated that the aid of ultrasound could obviously accelerate the leaching reaction rate of Fe.
As for the secondary resources, the simple introduction of ultrasound can obviously boost the dissolution of Fe from secondary resources. However, ultrasound commonly combine with oxidative reagents or microwave so as to accelerate the removal rate of Fe from the non-metal ores, such as quartz and kaolin, meantime, the consumed lixiviant is clearly reduced.
3.1.2.2. Manganese and chromium leaching
The leaching characteristics of Mn or Cr from various raw materials with the assistance of ultrasound are summarized and compared in Table 8. Recently, ultrasound has been mainly used to recover manganese (Mn) and chromium (Cr) from secondary resources, such as waste sludge and spent LIBs [106], [109], [115]. Zhang et al. [115] explored the action of ultrasound on the selective leaching of Cr from Cr and Fe-bearing sludge. The results displayed that 97.38% of Cr was dissolved into the leaching solution, while 98.14% Fe remained in the leaching residue, indicating a more efficient segregation between Cr and Fe with the ultrasound assistance than conventional leaching process. Wen et al. [77] also discovered that ultrasound would make 96.67% of vanadium leached by H2SO4, while more than 99% Cr remained in the leaching residue of chromium-vanadium slag, realizing the effective separation between vanadium and chromium. Lots of work have been conducted in the organic acid media combined with H2O2 to recover Mn from spent LIBs with the aid of ultrasound. Ni et al. [106] probed the recovery of Mn from spent LIBs using DL-malic acid assisted by ultrasound. They found that the leaching efficiency of Mn could attain 97.3% in 30 min, indicating a higher leaching rate and shorter reaction time with the assistance of ultrasound. Xiao et al. [109] tried to recover Mn from spent LIBs using critic acid under ultrasound irradiation. The outcomes manifested that the ultrasound decreased the leaching duration by 50% and improved the leaching percentage of Mn to 96%, attributing to the local heat and enhanced mass transfer rate.
Table 8.
Comparisons for the leaching characteristics of Mn/Cr from different raw materials assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | Mn/Cr Leaching Recovery | Reference |
|---|---|---|---|---|
| Cr-Fe containing sludge | Ultrasound Lixiviant: H2SO4 |
35.58 W, 60 min, 25 °C | 97.38% | [115] |
| Chromium-vanadium slag | Ultrasound Lixiviant: H2SO4 |
800 W, 60 min, 60 °C | Less than 1% | [77] |
| Spent LIBs | Ultrasound Lixiviant: DL-malic acid-H2O2 |
90 W, 30 min, 80 °C | 97.3% | [106] |
| Spent LIBs | Ultrasound Lixiviant: critic acid-H2O2 |
110 W, 1440 min, 50 °C | 96% | [109] |
As is concluded from the current researches, the simple introduction of ultrasound in the conventional H2SO4 leaching can apparently increase the leaching selectivity of Cr with other metals. In particular, the leaching systems of organic acids and H2O2 assisted by ultrasound generally pose higher leaching recovery of Mn from the wastes obtained during the industrial production process.
3.2. Rare metals
The leaching of rare metals, including lithium [103], [104], [106], [107], [109], [110], vanadium [16], [19], [33], [36], [87], [113], molybdenum [33], [87], [89], [90], [91], tungsten [25], [50], [51], indium [38], [40], [97], [99], gallium [15], [117], uranium [59], [124], [142], germanium [35] and zirconium [62], is also a significant application of ultrasound. Recently, the research mainly focused on the leaching of rare metals from the metal ores and the secondary resources by ultrasound wave, especially for the latter.
3.2.1. Lithium leaching
Lithium (Li) has been broadly applied in the atomic reactor, light alloys, and batteries, attributing to its remarkable properties [143]. The leaching characteristics of Li from spent LIBs with the assistance of ultrasound are summarized and compared, which are displayed in Table 9. Recently, the leaching recovery of Li from spent LIBs assisted by ultrasound has been given more and more attention. Jiang et al. [103] recovered Li within spent LIBs in H2SO4-H2O2 media via ultrasound-assisted leaching. They discovered that the optimal leaching efficiency of Li attained 98.62% with the aid of ultrasound compared to 81.02% by conventional leaching at 30 min leaching duration, indicating a higher leaching efficiency induced by ultrasound irradiation. Apart from inorganic acids, researchers attempted to enhance the leaching efficiency of Li using organic acids as the leaching reagents. Shih et al. [104] found that ultrasound accelerated the dissolution process of Li from the spent LIBs at lower leaching temperature in H2SO4, citric acid, and succinic acid. Ning et al. [106] developed a novel process involving ultrasound-assisted malic acid and H2O2 leaching to recover Li from the spent LIBs. The outcomes manifested that the leaching ratio of Li achieved as 98% at 80 °C in 30 min. Esmaeili et al. [107] also proposed an ultrasound-assisted leaching method using organic acids in lemon juice and H2O2 to extract Li from the spent LIBs. The leaching recovery was gained as high as 100% under the conditions optimized by response surface methodology. In order to realize the selective leaching of Li, the combined technique was put forward to separate valuable metals from the spent LIBs. Makuza et al. [110] used a coupled technique (carbothermal reduction-dry grinding-carbonated ultrasound-assisted water leaching) to improve selective leaching of Li within spent LIBs. It was discovered that the leaching recovery of Li reached 92.25% with induction of ultrasonic cavitation effects, whereas over 99% of the other metals in the water-leaching residue, such as Ni, Mn, and Co, could be further leached with H2SO4.
Table 9.
Comparisons for the leaching characteristics of Li from spent LIBs assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | Li Leaching Recovery | Reference |
|---|---|---|---|---|
| Spent LIBs | Ultrasound Lixiviant: H2SO4-H2O2 |
360 W, 30 min, 30 °C | 98.62% | [103] |
| Spent LIBs | Ultrasound Lixiviant: DL-malic acid-H2O2 |
90 W, 30 min, 80 °C | 98% | [106] |
| Spent LIBs | Ultrasound Lixiviant: lemon juice-H2O2 |
37 kHz, 35 min, 40 °C | 100% | [107] |
| Spent LIBs | Dry grinding and ultrasound Lixiviant: H2O-CO2 |
40 kHz, 180 min, 50 °C | 92.25% | [110] |
From the presented leaching researches for spent LIBs, it can be concluded that both the oxidative leaching systems using inorganic acids or organic acids with the aid of ultrasound can boost the leaching recovery of Li. Especially, Li can be selectively dissolved in the leachate from the spent LIBs with other metals remained in the leaching residues when ultrasound is combined with other techniques.
3.2.2. Vanadium leaching
Vanadium (V) plays a critical role in catalysts, alloy steels, and vanadium redox flow battery, ascribed to its remarkable and unique performance [22], [144], [145], [146]. The leaching characteristics of V from various raw materials with the assistance of ultrasound are summarized and compared, which are displayed in Table 10. Chen et al [16]. first introduced the ultrasound technique into the leaching process of V from low-grade vanadium-bearing shale. It was showed that the leaching percentage of V could be increased from 87.86% to 92.93% in H2SO4-CaF2 media with ultrasound assistance, and the leaching time was decreased 240 min to 30 min. Meanwhile, it was apparently found that the combination of ultrasound and CaF2 exerts more positive effects than applying ultrasound or adding CaF2 alone given the enhancement in V recovery, indicating the existence of the synergetic action between CaF2 and ultrasound [19].
Table 10.
Comparisons for the leaching characteristics of V from different raw materials assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | V Leaching Recovery | Reference |
|---|---|---|---|---|
| Vanadium-bearing shale | Ultrasound Lixiviant: H2SO4-CaF2 |
900 W, 30 min, 95 °C | 92.93% | [16] |
| Spent hydroprocessing catalysts | Ultrasound Lixiviant: critic acid |
320 W, 360 min, 60 °C | >95% | [87] |
| Chromium-vanadium slag | Ultrasound Lixiviant: H2SO4 |
800 W, 60 min, 60 °C | 96.67% | [77] |
| Fuel-oil fly ash | Ultrasound Lixiviant: lemon juice-H2O2 |
159 W, 180 min, 35 °C | 100% | [113] |
| Coal fly ash | Ultrasound Lixiviant: H2SO4-H2O2 |
60 kHz, 60 min, 50 °C | 100% | [36] |
| Spent hydroprocessing catalysts | Vacuum pyrolysis and ultrasound Lixiviant: H2SO4 |
60 W, 20 min, 45 °C | 98.6% | [33] |
Ultrasound has also been found to be efficient in recovering V from secondary resources besides metal ores. Marafi et al. [87] compared the leaching behavior of V from spent hydroprocessing catalysts using sulfuric acid/citric acid with and without ultrasound assistance. The leaching of V was more effective in the leaching media of sulfuric acid with ultrasound irradiation than that in critic acid, and more than 95% of V that existed in the spent catalysts was extracted with the combination of critic acid and ultrasound at relatively low temperature in short time. Wen et al. [77] compared the effects of leaching conditions on the leaching behavior of V in ultrasound-assisted leaching and regular leaching in H2SO4 media. The outcomes manifested that the ultrasound significantly decreased the leaching duration and reaction temperature and increased V leaching efficiency from 90.89% to 96.67% under relatively mild conditions compared to regular leaching. The extraction process of V from fly ash was carried in inorganic and organic acids media with the assistance of ultrasound by Rohimi et al. [113] and Masoum et al. [36]. In both cases, the leaching recovery of V could attain as high as 100% under the conditions optimized via response surface methodology. In the meanwhile, ultrasound has been combined with vacuum pyrolysis to recover V from spent hydroprocessing catalysts [33]. It was found that the optimal leaching recovery of V reached 98.6%, with only 7.63% of Al being dissolved, indicating the selective leaching capability of the combined technique.
It can be concluded from the current researches that the leaching systems with aid-leaching reagents and oxidants assisted by ultrasound-assisted inorganic or organic acids can achieve the dissolution of most V within the raw materials. It is interesting that the combination of ultrasound and other methods not only strengthen the leaching recovery of V, but also improve the leaching selectivity of V over other metals.
3.2.3. Molybdenum leaching
The application of molybdenum (Mo) in steel manufacture, the chemical industry, and the energy industry has gained more and more attention [147], [148]. The leaching characteristics of Mo from various secondary resources with the assistance of ultrasound are summarized and compared, which are displayed in Table 11. In recent years, the recovery of Mo by ultrasound-assistance leaching was dominantly focused on the spent hydroprocessing catalysts. Marafi et al. [87] found that the combination of critic acid and ultrasound showed high efficiency in improving the Mo leaching ratio to 95.7%. Pinto and Soares [89] performed a leaching process of Mo from the spent hydrodesulphurization catalysts using ultrasound-assisted NaOH leaching. It was stated that ultrasound led to a faster dissolution of 66% Mo in a relatively short time (10 min).
Table 11.
Comparisons for the leaching characteristics of Mo from different secondary resources assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | Mo Leaching Recovery | Reference |
|---|---|---|---|---|
| Spent hydroprocessing catalysts | Ultrasound Lixiviant: Critic acid |
320 W, 360 min, 60 °C | 95.7% | [87] |
| Spent hydrodesulphurization catalysts | Ultrasound Lixiviant: NaOH |
200 W, 10 min, 80 °C | 66% | [89] |
| Spent hydrodesulphurization catalysts | Microwave and ultrasound Lixiviant: Na2CO3 |
600 W, 120 min, 55 °C | 94.3% | [90] |
| Spent hydrodesulphurization catalysts | Bioleaching and ultrasound Lixiviant: Escherichia coli and inoculum |
37 kHz and 80 kHz, 30 min, 35 °C, two-steps bioleaching | 54% | [91] |
| Spent hydroprocessing catalysts | Vacuum pyrolysis and ultrasound Lixiviant: H2SO4 |
60 W, 20 min, 45 °C | 94.96% | [33] |
Apart from a relatively single ultrasound-assisted leaching system, ultrasound has also been combined with other technologies to further enhance the leaching efficiency of Mo from spent hydroprocessing catalysts. Wang et al. [90] proposed a combined microwave roasting and ultrasound leaching method for oil removal and recovery of Mo from the spent hydrodesulphurization catalysts. The outcomes manifested that the leaching ratio of Mo could achieve as 94.3% under the optimal conditions, which indicated that the ultrasound-microwave method could obviously improve the leaching recovery of Mo and remarkably decrease the processing time. Vyas and Ting [91] combined the bioleaching process with ultrasound irradiation to recover Mo from spent hydrodesulphurization catalysts. They discovered that the application of sonication leaching led to an enhancement of Mo leaching recovery from 46% to 54%, and the pulse mode was more efficient than that of the continuous mode in strengthening the leaching efficiency of Mo. Feng et al. [33] recovered Mo from spent hydroprocessing catalysts by a sustainable process featuring vacuum pyrolysis and fast H2SO4 leaching assisted by ultrasound. The optimal leaching recovery of Mo was attained at 94.96% within a leaching of 20 min, whereas the leaching ratio of Al was maintained in a low level.
From the current researches, it is apparent that the combinations of ultrasound and organic acids or other techniques can largely promote the dissolution of Mo from various secondary resources in a relatively short time compared to conventional leaching method.
3.2.4. Indium leaching
Indium (In) is dominantly used in the production of alloys, semiconductors, and electric light sources [149]. The leaching characteristics of In from various secondary resources with the assistance of ultrasound are summarized and compared, which are displayed in Table 12. The application of ultrasound in the recovery of In was concentrated on secondary resources, such as waste liquid crystal display (LCD) panels and by-products of zinc metallurgy [38], [40], [97], [99]. Zhang et al. [97] explored the recovery of In within waste LCD by an innovative non-crushing leaching process with the assistance of ultrasound in HCl media. They stated that In could be efficiently leached even at a low concentration of HCl without extra heating, and the leaching efficiency of In achieved as 96.80% without Mo dissolution in 60 min. Souada et al. [99] also adopted ultrasonication to the H2SO4 leaching process of In from the end-of-life LCD. The results showed that the leaching efficiency was only 13% without ultrasound at 4 min, whereas the leaching efficiency was more than 82% with ultrasound assistance at the same conditions. The optimal leaching recovery of In was achieved as 92% with ultrasound assistance at 20 min, which is higher than that (70%) without ultrasound.
Table 12.
Comparisons for the leaching characteristics of In from different secondary resources assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | In Leaching Recovery | Reference |
|---|---|---|---|---|
| Waste liquid crystal display (LCD) | Ultrasound Lixiviant: HCl |
300 W, 60 min, room temperature | 96.8% | [97] |
| End-of-life LCD | Ultrasound Lixiviant: H2SO4 |
320 W, 20 min, 60 °C | 92% | [99] |
| Spent hydrodesulphurization catalysts | Ultrasound Lixiviant: Na2CO3 |
600 W, 120 min, 55 °C | 94.3% | [90] |
| By-products of zinc metallurgy | Ultrasound Lixiviant: HCl-CaCl2-Ca(ClO)2 |
700 W, 50 min, 70 °C | 96.42% | [38] |
| Waste LCD | Microwave and ultrasound Lixiviant: HCl |
650 W, 142 S, 45 °C | 100% | [40] |
Ultrasound has also been integrated with additives or other technologies to form novel processes to elevate the leaching ratio of In. Zou et al. [38] found that the optimal leaching rate of In from the by-products of zinc metallurgy could attain 96.42% using HCl-CaCl2 as leaching reagent and Ca(ClO)2 as oxidant with the assistance of ultrasound. The outcomes proved that ultrasound treatment could effectively elevate the leaching efficiency, reduce the leaching time and decrease the consumption of additives. Zhang et al. [40] recovered In from waste LCD using different inorganic acids (HCl, H3PO4, and HClO4) under a new microwave-ultrasound heating system. It was discovered that HCl was more effective than the other acids, and the leaching recovery of In was achieved at 100% under the optimal leaching conditions.
It is obvious that the introduction of ultrasound into the leaching process can not only elevate the leaching ratio of In and shorten the leaching duration, but also restrain the dissolution of other metals. Particularly, the leaching efficiency of In from the secondary resources is further improved at relatively mild conditions when ultrasound is coupled with microwave.
3.2.5. Uranium leaching
Uranium (U) is a significant fuel for the generation of nuclear energy. Hence, the exploitation of the high-efficient technique for the extraction of U from uranium ores and uranium-bearing waste has gained more and more attention [150], [151]. The leaching characteristics of U from various raw materials with the assistance of ultrasound are summarized and compared, which are displayed in Table 13. Avvaru et al. [59] explored the functions of ultrasound wave on the leaching of U from uranium ores in HNO3 and H2SO4 media. The outcomes manifested that the enhancement in leaching rate was high at a low concentration of HNO3 in the presence of ultrasound compared with traditional mechanical agitation due to the faster oxidative conversion of acid insoluble U(IV) to the soluble U(VI).
Table 13.
Comparisons for the leaching characteristics of U from different raw materials assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | U Leaching Recovery | Reference |
|---|---|---|---|---|
| Uranium ores | Ultrasound Lixiviant: HNO3 |
36 W, 360 min, room temperature | about 80% | [59] |
| Black shale | Ultrasound Lixiviant: Phoma tropica and molasses |
/ | 57.73% | [142] |
| Fluoride matrix | Air/O2 flow and utrasound Lixiviant: HNO3 |
150 W, 195 min, 65 °C | about 91% | [124] |
Other researchers have also attempted to incorporate ultrasound waves with other techniques, such as O2 flow and fungal leaching, to further intensify the leaching process of U. Anjum et al. [142] designed research to determine the sound effects of ultrasound and the biologically mediated extraction of U from low-grade black shale. They discovered that the leaching recoveries of U in various fungal strains were all improved with ultrasound assistance, but the enhancement varied in different fungi. It was also found that the highest leaching ratio of U (57.73%) was achieved by phoma tropica using molasses as substrate with the treatment of ultrasound compared to other fungi. Kalsi et al. [124] studied the recovery of U from the fluoride matrix by employing ultrasound-assisted HNO3 leaching in air/O2 flow. It was showed that the leaching percentage of U was improved by 76 ∼ 91% with the treatment of ultrasound. Meantime, the reduction of leaching time and acid consumption were also achieved.
From the presented leaching researches of U, it can be concluded that the oxidative leaching systems assisted by ultrasound can not only achieve the high leaching recovery of U, but also reduce the leaching time and reagents consumption.
3.2.6. Tungsten leaching
Tungsten (W) has been broadly utilized in the chemical industry, machine equipment manufacturing, and national defense industry [50]. The leaching characteristics of W from scheelite with the assistance of ultrasound are summarized and compared, which are displayed in Table 14. Many researches have focused on extracting W from scheelite by ultrasound in different alkaline leaching media, such as NaOH, Na2CO3, and Na3PO4. Zhao et al. [51] investigated the dissolution of W from scheelite with and without ultrasound in NaOH. The outcomes manifested that ultrasound had an apparent effect on the leaching rate of W, which was attributed to the increased collision between OH– and crystal lattice. Yang et al. [50] recovered W from scheelite in Na3CO3 with the aid of ultrasound. By comparison, it was found that ultrasound would induce the exfoliation and elimination of the product layer of Ca3(PO4)2, which strengthened the diffusion of the leaching media and further promoted the leaching reaction of W. They also concluded that the enhancement primarily lied in the improvement of the frequency factor for the scheelite with a loose and porous product layer. Johansson et al. [25] probed the effects of hydrodynamic and acoustic cavitation on the leaching efficiency of W from scheelite concentrate in NaOH. The results implied that ultrasound could elevate the leaching ratio of W from 36.7% to 71.5% at the same leaching conditions compared to conventional stirring leaching.
Table 14.
Comparisons for the leaching characteristics of W from scheelite assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | W Leaching Recovery | Reference |
|---|---|---|---|---|
| Scheelite | Ultrasound Lixiviant: NaOH |
176 W, about 146 min, 90 °C | about 90% | [51] |
| Scheelite | Ultrasound Lixiviant: Na3CO3 |
1000 W, about 450 min, 90 °C | about 22.5% | [50] |
| Scheelite | Ultrasound Lixiviant: HNO3 |
131 kWh/kg, 360 min, 80 °C | 71.5% | [25] |
It can be concluded that the introduction of ultrasound into the conventional leaching process promote the collision of the lixiviants with the solid particles, which is responsible for the improvement of the dissolution of W from scheelite.
3.2.7. Other rare metal leaching
The high efficiency of ultrasound towards rare metals extraction was not only reflected in the above-mentioned six rare metals but also showed significant advantages in the extraction of other rare metals, such as gallium (Ga), germanium (Ge), and zirconium (Zr). Chanturiya et al. [62] applied ultrasound to the leaching process of Zr from eudialyte concentrate in HNO3 media. It was found that the highest leaching recovery of Zr was 97.1%, while that in the conventional process was only 76.9%. Tonanzi et al. [117] put forward a pre-treatment process involving ultrasound and thermal hydrolysis to promote the release of Ga from municipal sludge and found that the pre-treatment indeed increased the removal efficiency of Ga by anaerobic bioleaching. Xin et al. [35] recovered Ge within zinc oxide dust by ultrasound-assisted H2SO4-H2O2 oxidation-leaching process, and the outcomes manifested that the leaching rate of Ge reached 88.29%, which is 5.65% higher than that obtained in the traditional leaching process. Ding et al. [15] studied the recovery of Ga from corundum flue dust using ultrasound-assisted H2SO4 leaching. The outcomes manifested that the leaching recovery of Ga was elevated from 62.78% to 82.56% with the aid of ultrasound compared to regular leaching, ascribing to the breakage of the agglomerated particles and the generation of cracks, which further improved the dissolution of the encapsulated Ga. Xin et al. [126] investigated the recovery of Ge from germanium-containing slag dust in H2SO4 media with the assistance of ultrasound combined with O2. They also concluded that ultrasound would break the minerals and open the mineral inclusions, further improving the release of Ge from the sulfide inclusions.
3.3. Rare earth elements
Rare earth elements (REEs) have been widely used in the fields of metallurgy, ceramics, electronics, vehicle, and permanent magnet motors [1]. In recent years, the recovery of REEs from the raw metal ores [29], [62] and secondary resources [36], [37], [92], [101], [121], [122], [152], [153] by ultrasound leaching technique has been given more and more attention, especially from secondary resources. The leaching characteristics of REEs from various raw materials with the assistance of ultrasound are summarized and compared, which are displayed in Table 15.
Table 15.
Comparisons for the leaching characteristics of REEs from different raw materials assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | REEs Leaching Recovery | Reference |
|---|---|---|---|---|
| Weathered crust elution-deposited ore | Ultrasound Lixiviant: MgSO4 |
700 W, 30 min, 25 °C, two-stage leaching | >99% | [29] |
| Eudialyte concentrate | Ultrasound Lixiviant: HNO3 |
22 kHz ± 1.65 kHz, 40 min (first and second stage) and 100 min (third stage), 80 °C, three-stage leaching | 94.5% | [62] |
| Fluorescent lamp waste | Ultrasound Lixiviant: HNO3 |
120 W, 1440 min, 20 °C | >95% | [121] |
| Phosphogypsum | Mechanical grinding and ultrasound Lixiviant: H2SO4 |
50 W, 120 min | >70% | [152] |
| Waste magnet | Ultrasound Lixiviant: CH3COOH |
90 W, 120 min, 30 °C | 99.99% | [122] |
| LCD screen wastes | Ultrasound Lixiviant: P2O74- |
120 W, 60 min, room temperature | >85% | [101] |
| Spent fluid cracking catalysts | Ultrasound Lixiviant: HCl |
200 W, 60 min, 60 °C | 97.1% | [92] |
| Coal fly ash | Ultrasound Lixiviant: H2SO4-H2O2 |
60 kHz, 60 min, 50 °C | 97% | [36] |
| Spent automotive catalysts | Ultrasound Lixiviant: FeSO4-H2O2 |
37 kHz, 40 min, 70 °C, two leaching stages | 47.2% | [37] |
| Graphite substrate | Ultrasound Lixiviant: HNO3-H2O2 |
8 W/cm−2, 45 min, 45 °C, | 7.7% | [153] |
Yin et al. [29] reported the recovery of REEs from weathered crust elution-deposited ore using MgSO4 as a leaching reagent by ultrasound-assisted leaching to eliminate the ammonia–nitrogen pollution issue and improve the leaching efficiency. The results manifested that the leaching ratio of REEs was elevated from 75.5% to 92.3% with the induction of ultrasound. In addition, the leaching recovery could be further elevated to more than 99% by a two-stage ultrasound-leaching process. Chanturiya et al. [62] found that three stages of HNO3 leaching of eudialyte concentrate by continuous ultrasound treatment in the first stage could increase the leaching recovery of REEs from 79.6% to 94.5%. The enhancement was ascribed to the dispersion of colloidal silicate gel, prevention of the generation of gel precipitation on the particles’ surface, and promotion of the formation of microcracks and defects induced by ultrasound irradiation.
As for secondary resources, the research mainly concentrated on the recovery of REEs from fluorescent lamp waste, phosphogypsum, waste magnet, LCD screen wastes, coal ash, spent fluid cracking catalysts (FCC), graphite substrate and spent automobile catalysts. The recovery flowsheet commonly includes the combination of ultrasound waves with inorganic acids, organic acids, additives, and other technologies. Tunsu et al. [121] discovered that efficient leaching of europium (Eu) and yttrium (Y) was achieved at more than 95% and 97%, respectively, using weak HNO3 solution as leachant with ultrasound assistance. Rychkov. et al. [152] recovered REEs from phosphogypsum by the combination of ultrasound treatment with mechanical grinding and resin-in-pulp process. They found that the combined process provided a significantly higher degree of REE leaching recovery from the raw materials (from 15 ∼ 17% to more than 70%). Furthermore, the by-products obtained after leaching could be recycled as raw materials for building. Behera et al. [122] applied an ultrasound-assisted technique to dissolve neodymium (Nd) within waste magnet by CH3COOH. The outcomes showed that almost all (99.99%) Nd could be leached from the raw materials under ultrasound-assisted leaching of 120 min, while only 65.03% of Nd was extracted by regular mechanical stirring for 2 h. Toache-perez et al. [101] put forward a effective method for the selective separation of gadolinium (Gd) and praseodymium (Pr) within LCD screen wastes in P2O74- leaching media assisted by ultrasound. It was stated that 85% Gd and 87% Pr could be selectively recovered from the original materials with ultrasound enhancement, whereas other REEs remained in the leaching residue. Sadeghi et al. [92] developed a simple and effective flowsheet using ultrasound-assisted HCl leaching for lanthanum (La) recovery within spent FCC. They discovered that the leaching recovery of La was obtained as 97.1%, an increase of 27.3% in comparison to that (69.8%) during the conventional leaching process. Masoum et al. [36] extracted Y from coal fly ash by a H2SO4 leaching process enhanced with ultrasound and H2O2. They discovered that the leaching recovery rate of Y could reach 97% under optimal conditions optimized by response surface methodology. Hosseinzadeh et al. [37] explored the recovery of cerium (Ce) from spent automobile catalysts by a Fenton-like leaching process assisted by ultrasound. The results manifested that the leaching percentage of Ce was significantly elevated from 13.4% to 47.2% with the enhancement of ultrasound. Lahiri et al. [153] found that ultrasound would bring about a threefold increase in the leaching recovery of Ce from graphite substrate compared to a silent process without ultrasound in HNO3-H2O2 media. Meanwhile, the obtained carbon residue after the stripping of graphite by ultrasound could be recycled as an absorbent for Ce in the leachate, hence realizing the decontamination and recyclability of the graphite.
From the current leaching researches of REEs from raw ores, it is obvious that the assistance of ultrasound can obviously boost the leaching recovery of REEs without ammonium addition, which eliminate the ammonia nitrogen pollution problems from the source. In the meanwhile, the oxidative leaching systems with the assistance of ultrasound is conducive to the enhancement of REEs leaching from secondary resources in a shorter time in comparison to conventional leaching method.
3.4. Precious metals
Ultrasound has wide applications in the recovery of precious metals, such as silver, gold, and platinum group metals from raw metal ores and secondary resources, ascribing to that any effective improvement for the recovery of precious metals can bring significant economic benefits. At present, the recovery of precious metals by ultrasound-assisted technology dominantly focused on refractory gold and silver ores [27], [54], [55], [56], gold concentrate [20], [154], polymetallic sulfide [13], [28], and spent automotive catalysts [93].
3.4.1. Gold leaching
Gold (Au) has been given more and more attention attributing to its broad application in industrial fields, including electronic materials, catalysts and so on [56]. Recently, the effective ultrasound-assisted leaching technique has been introduced to enhance the leaching efficiency of Au from refractory gold ores and gold concentrates to eliminate or mitigate cyanide pollution. The leaching characteristics of Au from various raw materials with the assistance of ultrasound are summarized and compared, which are displayed in Table 16.
Table 16.
Comparisons for the leaching characteristics of Au from different raw materials assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | Au Leaching Recovery | Reference |
|---|---|---|---|---|
| Refractory gold ores | Ultrasound Lixiviant: HCl-Cl2 |
120 min, 50 °C | about 50% | [54] |
| Refractory ores | Air flow and ultrasound Lixiviant: NaCN |
180 W, 300 min | 73.4% | [55] |
| Refractory gold ores | Chlorination-oxidation and utrasound Lixiviant: NaClO-NaOH |
200 W, 120 min, room temperature | 68.55% | [56] |
| Gold concentrate | Ultrasound Lixiviant: NaCN |
650 W, 60 min, 10 °C | >99% | [154] |
| Gold concentrate | Ultrasound Lixiviant: Co-NH3-S2O32- |
750 W, 480 min, 50 °C | about 80% | [20] |
| Refractory gold ore | Ozone and ultrasound Lixiviant: Na3(CN)3C3H3N6O3 |
480 W, 240 min, 80 °C | 93.52% | [27] |
Zhu et al. [54] investigated the action of ultrasound on the leaching of Au from refractory gold ores in the chloride (HCl-Cl2) system (Eq. (9)-(10)). They found that the use of ultrasound could improve the leaching ratio of Au by 13% in comparison to conventional stirring leaching. Zhang et al. [55] explored the function of ultrasound energy on the NaOH pretreatment and NaCN leaching of Au from the refractory ores. They found that the leaching ratio of Au was improved from 40.3% to 73.4% with ultrasound induction, indicating the high efficiency of the ultrasound technique in pretreatment and leaching of the raw refractory ores. Interestingly, it was proved that high ultrasonic power would lead to the decomposition of the CN– (Eq. (11)), which decreased the efficiency of cyanide.
| (9) |
| (10) |
| (11) |
Fu et al. [56] proposed a synergistic technique to recover Au from refractory gold ores by chlorination-oxidation and ultrasound in NaClO-NaOH system. The results manifested that the leaching ratio of Au was elevated from 45.8% to 68.55% with the aid of ultrasound. Meanwhile, the leaching duration was apparently reduced from 6 h to 2 h. They concluded that the enhancement of ultrasound was attributed to the peeling of the interface, the exposure of the new reaction surface, and the decrease of the reaction resistance. A similar conclusion was also drawn by other researchers. For instance, Yu et al. [154] studied the ultrasound-assisted cyanide extraction process of Au from gold concentrate. They found that the ultrasound extraction recovery of Au (99%) at 10 °C was almost equal to that under conventional leaching at 25 °C, meanwhile, the unit consumption of sodium cyanide was decreased by 16%. Gui et al. [20] recovered Au from gold concentrate in thiosulfate combined with cobalt ammonia with ultrasound assistance. It was discovered that the leaching rate of Au was eight folds faster, and the leaching ratio of Au was approximately 25% higher in the presence of ultrasound than under conventional conditions. Gui et al. [27] also utilized the synergistic pretreatment of ozone and ultrasound method to elevate the leaching efficiency of Au from refractory gold ore using the novel leaching media (Na3(CN)3C3H3N6O3). The results showed that the leaching percentage of Au was obviously elevated from 49.12% to 93.52% after ultrasound-assisted ozone pretreatment, ascribing to that ultrasound irradiation would destroy the oxidized pyrite enclosure and suppress the generation of the secondary enclosure in a short time.
It is obvious that the introduction of ultrasound into the cyanide leaching process can reduce the consumption of cyanide and elevate the leaching ratio of Au. In the meanwhile, the leaching systems of oxidants or new aid-leaching reagents combined with ultrasound can also realize the high leaching recovery of Au in a short time and low leaching temperature without the addition of cyanide.
3.4.2. Silver leaching
Silver (Ag) is widely adopted in various fields, such as chemical materials and photosensitive materials [61]. The leaching characteristics of Ag from various raw materials with the assistance of ultrasound are summarized and compared, which are displayed in Table 17. Ultrasound has been adopted for the recovery process of Ag from silver-containing metal ores to elevate the utilization efficiency of cyanide during the cyanide leaching process or promote Ag dissolution by no-cyanide leaching. Alarcon et al. [28] probed the applicability of ultrasound to recover Ag from polymetallic sulfide ores and stated that ultrasound is efficient in increasing the leaching ratio of Ag and the utilization efficiency of cyanide. Cilek et al. [61] investigated the ultrasound-assisted leaching recovery of Ag from refractory silver ores in a cyanide system. The outcomes showed that almost equal or better Ag recoveries (up to 15.5%) was obtained at 24 h ultrasound-assisted cyanide leaching than those of 48 h conventional cyanide leaching, indicating that leaching duration could be considerably shortened by 50% with ultrasound assistance. Larrabure et al. [13] found that the leaching recovery of Ag from polymetallic sulfide ores was enhanced from 25% to 60% with the application of ultrasound, attributing to exposing fresh sulfide surfaces and the decrease of the surface concentration of Mn. Gui et al. [27] introduced a NaOH pretreatment process, including ozone and ultrasound irradiation prior to Na3(CN)3C3H3N6O3 leaching to strengthen the leaching recovery of Ag from refractory gold ore. It was discovered that the leaching recovery of Ag was elevated from 4.01% to 61.25% with ultrasound and ozone pretreatment.
Table 17.
Comparisons for the leaching characteristics of Ag from different raw materials assisted by ultrasound.
| Raw materials | Leaching system | Characteristic leaching conditions | Ag Leaching Recovery | Reference |
|---|---|---|---|---|
| Refractory silver ores | Air flow and ultrasound Lixiviant: NaCN |
100 W, 2880 min, room temperature | about 90% | [61] |
| Polymetallic sulfide ores | Ultrasound Lixiviant: NaCN |
40 kHz, 650 min, 80 °C | 60% | [13] |
| Refractory gold ore | Ozone and ultrasound Lixiviant: Na3(CN)3C3H3N6O3 |
480 W, 240 min, 80 °C | 61.25% | [27] |
| Water-leaching residue of sintering dust | Ultrasound Lixiviant: CH4N2S |
200 W, 120 min, 45 °C | 94% | [85] |
| Sintering dust | Ultrasound Lixiviant: CH4N2S |
400 W, 120 min, 50 °C | about 95% | [86] |
| Spent symbiosis lead–zinc mine | Ultrasound Lixiviant: Na2S2O3 |
100 W, 120 min, 30 °C | 77.34% | [155] |
| Lead-zinc tailings | Ultrasound Lixiviant: Na2S2O3 |
200 W, 5 min, 30 °C | 71.5% | [41] |
In addition, ultrasound has also been applied to recover Ag from secondary resources, such as sintering dust, spent symbiosis lead–zinc mine, and lead–zinc tailings, by a cyanide-free leaching process. Chang et al. [85] attempted to use ultrasound to leach Ag from the water-leaching residue of sintering dust by acidic thiourea (Eq. (12)). They found that the leaching rate of Ag was up to 94% under the optimal conditions. Meanwhile, Chang et al. [86] also compared the leaching behavior of Ag from the sintering dust in traditional and ultrasound leaching systems. The outcomes manifested that the leaching recovery of Ag was increased by approximately 6% with ultrasound induction, which might be ascribed to the lower activation energy with ultrasound assistance. Li et al. [155] developed an efficient process to recover Ag from spent symbiosis lead–zinc mine via ultrasound-assisted Na2S2O3 leaching. It was discovered that the leaching rate of Ag after 5 min of ultrasound-assisted leaching was 73.88%. Nevertheless, the Ag leaching rate was only 72.51% after 2 h of reaction under regular conditions, indicating the high efficiency of ultrasound in increasing the leaching recovery of Ag and shortening the reaction period. In the meanwhile, the reaction activation energy was also found to be reduced with the aid of ultrasound, which was also reported in other literature. For example, Li et al. [41] also extracted Ag from lead-zinc tailings via Na2S2O3 leaching and discovered that the leaching ratio of Ag was improved from 61.7% to 71.5% with the aid of ultrasound irradiation. They concluded the enhanced leaching mechanisms were embodied in the destruction of the surface, increase of the active reaction area, acceleration of the mass transfer and diffusion, and reduction in the activation energy.
| (12) |
From the current researches, the combinations of ultrasound and other techniques can not only improve the leaching ratio of Ag, but also elevate the utilization efficiency of the cyanide. In the meantime, the reductive leaching systems with the assistance of ultrasound elevate the dissolution of Ag from the secondary resources in a short time without cyanide addition.
3.4.3. Platinum group metals
Apart from Au and Ag, the recovery of platinum (Pt) group metals within secondary resources with the use of ultrasound has also been studied by researchers. Recently, research has combined ultrasound pretreatment with bioleaching technology to accelerate the recovery of Pt group metals. Karim and Ting [93] tried to recover Pt group metals, Pt, rhodium (Rh), and palladium (Pd), within spent automotive catalysts via cyanogenic bacteria with ultrasound-assisted HNO3 pretreatment. A maximum leaching ratio of Pt (38%), Rh (91%), and Pd (44%) was achieved by mesophilic bacteria Pseudomonas fluorescens. The results demonstrated that ultrasound-assisted HNO3 pretreatment combined with bioleaching was a promising bio-recovery technology for the extraction of Pt group metals within spent automotive catalysts.
4. Enhanced leaching mechanisms of ultrasound
The enhanced leaching mechanisms of ultrasound mainly include the improvement of leaching kinetics, enhancement of the mass transfer and diffusion of lixiviants, and promotion of the oxidative conversion of the metals from insoluble to soluble state. The mechanisms are thoroughly discussed in the following sections.
4.1. Improvement of the leaching kinetics
The leaching process of metals from raw materials is a solid–liquid multi-phase reaction, which commonly undergoes three steps as follows [30]: (1) diffusion of the lixiviant through the fluid film formed on the particle surface, (2) diffusion of the lixiviant through the particle surface to the interior site of the particle, and (3) chemical reaction between the lixiviant and the metallic compound at the interface (Fig. 3). The slowest process is usually accepted as the rate-controlling step of the overall reaction. The leaching kinetics has been widely explored with and without ultrasound assistance to illustrate the enhanced leaching mechanism. The shrinking core model (SCM) is the most common solid–liquid reaction model, which has been broadly adopted to describe the leaching kinetics of metals during the ultrasound-assisted hydrometallurgical leaching process.
Fig. 3.
Schematic diagram of the leaching process of metals from solid particles.
The SCM is commonly used to depict the reaction surface that shrinks gradually from the surface to the solid particle’s center, the equation of which could be expressed as Eq. (13) to Eq. (16) [19]. Furthermore, the diffusion resistance through the fluid film is commonly negligible when the leaching reaction process is conducted under strong mechanical stirring and ultrasound irradiation [31].
When the liquid film diffusion controls:
| (13) |
When the product layer diffusion controls:
| (14) |
When the chemical reaction controls:
| (15) |
When mixed (layer diffusion and interfacial reaction) controls:
| (16) |
where X is the leaching percentage of metals (%), k is the reaction rate constant (min−1), t is the leaching time (min).
Additionally, other models were also adopted to depict the leaching kinetic process aided by ultrasound, such as the unreacted shrinking core model (USCM) [64], the logarithmic rate law [82], and the first-order kinetic model [85]. The USCM is basically utilized to describe the leaching process in which the particle size remains almost unchanged during the leaching process. The kinetics equation referred to as logarithmic rate law can be used when the leaching ratio of metals decreases dramatically with the prolonging of the reaction time.
The leaching temperature dependence of the reaction rate constant is generally depicted by the Arrhenius equation, the linear form of which is listed as Eq. (17). The activation energy and frequency factor can be computed according to the slope and intercept of the fitting line, respectively.
| (17) |
where Ea is the activation energy of the leaching reaction (kJ/mol), k is the reaction rate constant (min−1), k0 is the frequency factor, T is the leaching reaction (K), and R is the universal gas constant (8.314 J/K•mol−1).
As is displayed in Table 18, most of the relevant ultrasound-assisted leaching kinetics research have shown that the use of ultrasound would decrease the activation energy of the leaching reaction and hence improve the leaching efficiency of metals from the raw materials. Ma et al. [31] utilized the classic SCM to depict the leaching process of K-feldspar and found that the leaching of K with and without the assistance of ultrasound was both controlled by the diffusion in the product layer. In the meantime, the apparent activation energy of the leaching process was sharply decreased from 72.33 kJ/mol to 55.67 kJ/mol, leading to the increase of the rate constant and the dissolution of K from K-feldspar. The SCM model was also used to describe the leaching process of Zn from ZnO ores [30], which disclosed that ultrasound and bead milling (BM) combination could remarkably decrease the activation energy from 54.6 kJ/mol to 26.4 kJ/mol. In comparison, ultrasound and BM alone only decreased the activation energy to 44.9 kJ/mol and 41.5 kJ/mol, respectively. Gui et al. [20] used the USCM to describe the leaching of Au from gold concentrate and found that the increase in the leaching rate of Au was ascribed to the reduction of activation energy from 22.65 kJ/mol to 13.86 kJ/mol with the induction of ultrasound. The leaching kinetics of antimony (Sb) could be well fitted by the chemical reaction control model in SCM [58], and the reaction rate constant in the ultrasound system at different temperatures was higher than that in the traditional leaching system. In addition, the activation energy was reduced from 48.39 kJ/mol to 33.88 kJ/mol, while the frequency factor of the ultrasound-assisted method was 1.56 times higher than that of the traditional leaching method, increasing of the collision frequency between the reaction molecules. The research Chen et al. [19] manifested that the leaching process of V from vanadium-bearing shale in the CaF2 system in the presence and absence of ultrasound was controlled by mixed controls using SCM, that is the product layer diffusion and interfacial chemical reaction. At the same time, the activation energy could be decreased from 50.70 kJ/mol to 27.71 kJ/mol with the aid of ultrasound irradiation, and the kinetic rate constant at various reaction temperatures in the ultrasound system was consistently higher than that in the regular leaching system.
Table 18.
Comparisons of the leaching reaction activation energy and rate-controlling step in regular leaching (RL) and ultrasound-assisted leaching (UAL) systems.
| Raw materials | Leaching system | Dominant characteristics | References |
|---|---|---|---|
| Sintering dust | Metal: Ag Lixiviant: CS(NH2)2 |
Ea (RL) = 28.01 kJ/mol and Ea (UAL) = 18.19 kJ/mol; The rate-controlling step is diffusion through the product layer in both CL and UL system |
[86] |
| K-feldspar | Metal: K Lixiviant: H2SO4-CaF2 |
Ea (RL) = 72.33 kJ/mol and Ea (UAL) = 55.67 kJ/mol; The rate-controlling step is diffusion through the product layer in both CL and UL system |
[31] |
| Silica sand | Metal: Fe Lixiviant: HCl |
Ea (RL) = 67.5 kJ/mol and Ea (UAL) = 43.6 kJ/mol; The rate-controlling step is diffusion through the product layer in both CL and UAL system |
[66] |
| ZnO ore | Metal: Zn Lixiviant: H2SO4 |
Ea (RL) = 44.9 kJ/mol and Ea (UAL) = 26.4 kJ/mol; The rate-controlling step is diffusion through the product layer in both CL and UAL system |
[30] |
| Nickel sUALfate ore | Metal: Ni Lixiviant: H2SO4-H2O2 |
Ea (RL) = 16.2 kJ/mol and Ea (UAL) = 11.83 kJ/mol; The rate-controlling step is diffusion control in both CL and UAL system |
[128] |
| Electrolytic manganese anode mud |
Metal: Pb Lixiviant: CH3COONH4 |
Ea (RL) = 29.40 kJ/mol and Ea (UAL) = 26.95 kJ/mol; The rate-controlling step is diffusion control in both CL and UAL system |
[132] |
| Waste printed circuit boards | Metal: Pb and Sn Lixiviant: CH3COONH4 |
For Pb: Ea (RL) = 33.98 kJ/mol and Ea (UAL) = 17.22 kJ/mol; For Sn: Ea (RL) = 44.47 kJ/mol and Ea (UAL) = 11.87 kJ/mol; The rate-controlling step is surface chemical reaction in both CL and UAL system |
[100] |
| Lead-zinc tailings | Metal: Ag Lixiviant: Na₂S₂O3 |
Ea (RL) = 6.65 kJ/mol and Ea (UAL) = 5.6 kJ/mol; The rate-controlling step is diffusion control in both CL and UAL system |
[41] |
| Gold concentrate | Metal: Au Lixiviant: Na₂S₂O3-CoSO4-CH3COONH4 |
Ea (RL) = 22.65 kJ/mol and Ea (UAL) = 13.86 kJ/mol; The rate-controlling step is internal diffusion in both CL and UAL system |
[20] |
| Laterite | Metal: Ni Lixiviant: Na₂SO4/Na2CO3 |
For Na2SO4 system: Ea (RL) = 39.86 kJ/mol and Ea (UAL) = 13.25 kJ/mol; For Na2CO3 system: Ea (RL) = 30.43 kJ/mol and Ea (UAL) = 10.17 kJ/mol; The rate-controlling step is a combination of chemical and diffusion reaction in all leaching processes, except for RL in Na2SO4 system |
[17] |
| Refractory gold ore |
Metal: Sb Lixiviant: Na₂S |
Ea (RL) = 48.39 kJ/mol and Ea (UAL) = 33.88 kJ/mol; The rate-controlling step is surface chemical reaction in both CL and UAL system |
[58] |
| Copper anode slime | Metal: Cu Lixiviant: H2SO4-O3 |
Ea (RL) = 31.52 kJ/mol and Ea (UAL) = 10.37 kJ/mol; The rate-controlling step is chemical reaction and internal diffusion in CL and UAL system, respectively |
[48] |
| Nickel-containing residue | Metal: Ni Lixiviant: H2SO4-O3 |
Ea (RL) = 17.74 kJ/mol and Ea (UAL) = 5.04 kJ/mol; The rate-controlling step is diffusion control in both CL and UAL system |
[81] |
| Vanadium-bearing shale | Metal: V Lixiviant: H2SO4-CaF2 |
Ea (RL) = 62.03 kJ/mol and Ea (UAL) = 27.62 kJ/mol; The rate-controlling step is product layer diffusion and interfacial reaction in both CL and UAL system |
[19] |
| Graphite substrate | Metal: Ce Lixiviant: HNO3 |
Ea (RL) = 102 kJ/mol and Ea (UAL) = 80 kJ/mol; The rate-controlling step is surface chemical reaction in both CL and UAL system |
[153] |
| Germanium-containing slag dust |
Metal: Zn and Ge Lixiviant: H2SO4-O2 |
For Zn: Ea (RL) = 32.10 kJ/mol and Ea (UAL) = 23.13 kJ/mol; For Ge: Ea (RL) is unknown and Ea (UAL) = 14.99 kJ/mol; The rate-controlling step is diffusion and chemical reaction control, in both CL and UAL system |
[126] |
| Copper anode slime | Metal: Cu Lixiviant: H2SO4-Na₂S₂O8 |
Ea (RL) = 36.11 kJ/mol and Ea (UAL) = 10.37 kJ/mol; The rate-controlling step is internal diffusion in CL and UAL system, respectively |
[84] |
Note: Ea (RL) and Ea (UAL) is the leaching reaction activation energy in the RL and UAL system, respectively.
Wang et al. [74] adopted the SCM to analyze the leaching kinetics of Zn from zinc residue. They discovered that the diffusion through the product layer was identified as the rate-determining step for both the ultrasound-assisted and conventional leaching processes. The activation energy was 13.07 kJ/mol and 6.57 kJ/mol in the ultrasound-assisted and conventional leaching systems, respectively. The leaching process of Pb from electrolytic manganese anode mud was described by USCM [132]. The fitting results manifested that the diffusion model was the rate-controlling step during the traditional and ultrasound-assisted leaching processes, and the activation energy was reduced from 29.40 kJ/mol to 26.95 kJ/mol with the aid of ultrasound waves. The SCM was applied to describe the leaching process of Cu from copper anode slime [48], and it was found that the solid film diffusion controls the leaching process with ultrasound assistance. At the same time, the chemical reaction was the rate-controlling step for the conventional leaching. Moreover, the activation energy was sharply decreased from 31.52 kJ/mol to 10.37 kJ/mol. The diffusion through the product layer controlled the leaching process of Ni from nickel-containing residue in the conventional and ultrasound systems using the SCM [81], and the activation energy of the leaching reaction was 17.74 kJ/mol and 5.04 kJ/mol, respectively, with and without the assistance of ultrasound.
Nevertheless, some researchers reported that ultrasound cannot reduce the activation energy, but affects the collision frequency factor, as shown in Table 19. Yang et al. [50] found that the leaching of W from scheelite with loose and porous product layer using Na2CO3 in ultrasound-assisted and conventional leaching systems was controlled by chemical reaction, and the activation energy was 72 kJ/mol for the two leaching systems. However, the collision frequency factor was increased with the appliance of ultrasound, which indicated that the enhanced leaching of W by ultrasound is primarily due to the elevation of the collision frequency between reaction molecules. Ultrasound did not affect the activation energy of Cu leaching reaction from chalcopyrite [49], which instead increased the pre-exponential factor in the Arrhenius equation by 3 folds, resulting in the increase of the collision of H+ with SO42- in the crystal lattice of chalcopyrite. The leaching process of Ag from the spent lead–zinc mine was found to be controlled by the diffusion model in SCM [155]. The activation energy was almost the same (12.47 kJ/mol vs. 12.35 kJ/mol) under conventional and ultrasonic leaching conditions, which indicated that the introduction of ultrasound had no apparent effect on the activation energy. However, both the reaction equilibrium constant and the frequency factor were augmented with the assistance of ultrasound, implying the intensification of the ongoing leaching reaction. Yang et al. [64] discovered that the activation energy of the leaching reaction of Fe from quartz by ultrasound irradiation was 27.72 kJ/mol, which was 7.28 kJ/mol higher than that of the conventional leaching process. The frequency factor of the ultrasound-assisted leaching process was about eight times higher than that of the traditional leaching process, implying that the improvement of Fe leaching is mainly embodied in the elevation of the collision frequency between the lixiviant molecules and solid particles.
Table 19.
Comparisons of the leaching reaction activation energy and the frequency factor (K0) in RL and UAL systems.
| Raw materials | Leaching system | Dominant characteristics | References |
|---|---|---|---|
| Scheelite | Metal: W Lixiviant: NaOH |
Ea (RL) = 80 kJ/mol and Ea (UAL) = 80 kJ/mol; The frequency factor in the UAL system is 10.2 times than that in the CL system |
[51] |
| Scheelite | Metal: W Lixiviant: Na2CO3 |
Ea (RL) = 72 kJ/mol and Ea (UAL) = 72 kJ/mol; The frequency factor in the UAL system is 1.30 times than that in the CL system |
[50] |
| Spent lead–zinc mine | Metal: Ag Lixiviant: Na₂S₂O3 |
Ea (RL) = 12.47 kJ/mol and Ea (UAL) = 12.35 kJ/mol; The frequency factor in the UAL system is 1.18 times than that in the CL system |
[155] |
| Chalcopyrite | Metal: Cu Lixiviant: H2SO4-Fe2(SO4)3 |
Ea (RL) = 5.44 kJ/mol and Ea (UAL) = 5.75 kJ/mol; The frequency factor in the UAL system is 3 times than that in the CL system |
[49] |
| Quartz | Metal: Fe Lixiviant: H2C2O4-HCl |
Ea (RL) = 20.44 kJ/mol and Ea (UAL) = 27.72 kJ/mol; The frequency factor in the UAL system is 8 times than that in the CL system |
[64] |
4.2. Enhancement of the mass transfer and diffusion of lixiviants
The characteristics of the particles, such as grain size, microstructure, and morphology, generally pose significant effects on the leaching process of metals from raw materials [58], [124], [156]. In the presence of acoustic cavitation, cavitation bubbles commonly undergo formation, growth, and collapse, leading to the generation of strong shear stress in the local micro-environment [58], [86], [94]. The cavitation effect will alter the properties of the particles, for instance, the reduction of the particle size, formation of surface cracks or voids, exfoliation of the passivated layer, and opening of the mineral inclusions. Consequently, it is well accepted that the leaching ratio of the metals would be improved in the ultrasound-assisted system by enhancing the mass transfer and diffusion of lixiviants in the pores and cracks of the particles.
In Fig. 4(a) and (c), it is evident that the particles of the ultrasound-assisted leaching residue were finer and more uniform than the regular leaching residue. In the meanwhile, the regular leaching residue particles’ surface was characterized by dense structure, while the surface of the ultrasound-assisted leaching residue particle was loose (Fig. 4 (b) and (d)). Additionally, as is shown in Fig. 4(e), the difference between the particle size of the leaching residues obtained from ultrasound and regular leaching process also demonstrated the fragmentation effect of ultrasound. The results proved that the vanadium-bearing shale particles could be smashed with the induction of ultrasound energy, improving the leaching reaction between V and lixiviant [16]. Xia et al. [140] also found that some agglomerates formed by large kaolin flakes in the conventional leaching process were broken into small flakes under the function of ultrasound, which further opened the gaps between the kaolin particles and improved the dissolution of Fe.
Fig. 4.
The microstructure (a-d) and particle size distribution (e) of the leaching residues by regular (a-b) and ultrasound leaching (c-d). [16]. Copyright (2020), with permission from Elsevier.
Zhang et al. [21] discovered that the small globules particles deposited on the leaching residue particles’ surface might severely cover the pores of the product layer in the conventional leaching system (Fig. 5), which would hinder the diffusion of the leaching reagent into the unreacted interior surface of the phosphorus-potassium assisted ore. However, ultrasound would strip the deposited particles on the surface and induce the formation of a thin strip structure, further enhancing the dissolution of K. The thick and insoluble layer was also found on the surface of the conventional leaching residue, which was eliminated by the clean-up action of the ultrasonic radiation [74], thus the leaching of Zn from the zinc residue was improved with the assistance of ultrasound.
Fig. 5.
SEM images of the raw ore (a), conventional leaching residue (b) and ultrasound leaching residue (c). [21]. Copyright (2016), with permission from Elsevier.
Yu et al. [154] found that the leaching ratio of Au encapsulated within pyrite (FeS2) was improved by ultrasound, ascribing to the destruction and opening of the passive film of FeS2 (Fig. 6). It was found that ultrasound destroyed and peel the passive film formed on the refractory gold ore particles’ surface [56], which promoted the contact of the lixiviant with the gold phase. Hence the mass transfer on the solid–liquid interface and leaching reaction of Au would be further strengthened. Alarcon et al. [28] also demonstrated that using ultrasound could eliminate the formation of passivating layers on the surface of the ores during the leaching process.
Fig. 6.
SEM images of the FeS2 in the raw ore (a) and the conventional (b) and ultrasound-assisted leaching residues (c). [154]. Copyright (2020), with permission from Elsevier.
As displayed in Fig. 7, under the function of ultrasound cavitation and etching effect, the particle size decreased, and there were more holes on the particles’ surface [106]. Chang et al. [86] found that internal pores and micro cracks grew and extended on the surface of the sintering dust with prolonged ultrasound leaching time, leading to the elevation of the mass transfer rate. As presented in Fig. 8, Li et al. [155] observed numerous holes on the grain surface after ultrasound-assisted leaching, which would form a fresh reaction interface and further intensify the leaching of Ag from the spent lead–zinc mine. Our previous work also demonstrated that ultrasound wave induced the generation of holes and cracks on the surface of the leaching residues, which further promoted the diffusion of lixiviants and realized a significant enhancement of the V leaching efficiency from a vanadium-bearing shale [19].
Fig. 7.
Microstructure of the raw materials (a-b), conventional leaching residues (c-d) and ultrasound-assisted leaching residues (e-f). [106]. Copyright (2020), with permission from Elsevier.
Fig. 8.
SEM images of the leaching residue, (a-b) conventional leaching residue, (c-d) ultrasound leaching residue. [155]. Copyright (2018), with permission from Elsevier.
4.3. Promotion of the oxidative conversion of the metals from insoluble to soluble states
During the leaching process, the oxidation of low-valence metals to high is commonly responsible for the transition from insoluble to soluble states [16], [156], [157]. As is shown in Eq. (18), ultrasound irradiation produces hydroxyl radicals (•OH) and hydrogen atoms (•H) in the aqueous solution as a result of acoustic cavitation [90], [116], [158]. The produced •OH can combine and react with each other to form hydrogen peroxide (H2O2) according to Eq. (19). Both •OH and H2O2 are strong oxidizing reagents for the oxidation leaching process.
| (18) |
| (19) |
Avvaru et al. [59] stated that the enhancement of the leaching ratio of U was attributed to the oxidative conversion of insoluble U(IV) to soluble U(VI) in the sulfuric acid system with the action of •OH and H2O2 produced during the ultrasound cavitation process. Chang et al. [85] found that the leaching of Ag in ferric sulfate-thiourea system (Eq. (20)) was promoted, ascribing to the oxidation of ferrous to ferric ions (Eq. (21)) with the H2O2 produced in the ultrasound-assisted leaching process. Rahimi et al. [113] monitored the Eh variation during the ultrasound-assisted leaching process with H2O2 as an oxidant. They discovered that the initial Eh potential of the leaching system with ultrasound was obviously higher than that without ultrasound, demonstrating the production of •OH induced by ultrasound.
| (20) |
| (21) |
Toache-perez et al. [101] reported the formation of Fe-based alloys containing Gd and Pr under the function of H2O2 produced by ultrasound irradiation, which improved the recovery of Gd and Pr from spent hydrogenation catalysts. The ultrasound was also found to promote the generation of •OH during the Fenton-like oxidation leaching process, improving the regeneration of Fe2+, which in turn improved the leaching efficiency of Al and Ce [37]. Liu et al. [48] reported that the primary improvement mechanism for Cu leaching from copper anode slime using ozone (O3) with the assistance of ultrasound was the generation of •OH with the reaction of O3 and H2O2 (Eq. (22)-(24)). The sonolysis of the HNO3 aqueous solution in the presence of ultrasound occurred according to Eq. (25)-(26) [94]. In particular, the generation of HNO2 radicals induced by ultrasound facilitated the leaching of Ni and contributed to the higher leaching ratio of Ni from the spent hydrogenation catalysts in a shorter time.
| (22) |
| (23) |
| (24) |
| (25) |
| (26) |
4.4. Summary of the enhanced leaching mechanisms
In summary, the strengthening effect of ultrasound on metal leaching dominantly lies on the mechanical effect, sonochemical effect, and thermal effect brought by acoustic cavitation. According to the literature mentioned above, it can be concluded that the strengthening mechanisms of ultrasound irradiation during the leaching process are usually reflected in many aspects rather than a single aspect.
As depicted in Fig. 9, Chang et al. [85] concluded that the improvement of Ag liberation from the sintering dust was not only ascribed to the damage of the surface of the particles and the removal of the adsorbed impurities but also the improvement of the oxidation leaching process under the function of cavitation bubbles. Chen et al. [19] revealed that the enhancement mechanism of ultrasound mainly lies in two aspects (Fig. 10). On the one hand, ultrasound cavitation augmented the specific area of the particles and provided a more porous structure, which promoted the diffusion of lixiviants through the pores and cracks on the surface of the particles. On the other hand, the synergetic function of CaF2 and ultrasound decreased the activation energy of vanadium leaching reaction, which further strengthened the leaching reaction of V.
Fig. 9.
Schematic improvement mechanism of Ag leaching from Sintering dust with ultrasound. [85]. Open access.
Fig. 10.
Enhancement mechanism of V extraction from vanadium-bearing shale with the assistance of ultrasound and CaF2. [19]. Open access.
As is observed from Fig. 11, Xie et al. [134] revealed that ultrasound quickly stripped off the inclusions, fillers, and impurities attached to the surface of the mineral particles, which exposed more active surfaces engaged in the leaching reaction and promoted the contact between the lixiviant and particles. Additionally, the mechanical and thermal effects indirectly elevated the collision between the lixiviant molecules with the mineral particles, which contributed to the high leaching efficiency of Pb from electrolytic manganese anode mud in a short time. Xin et al. [126] concluded that the strengthening mechanisms of ultrasound for Zn and Ge leaching were reflected in three aspects (Fig. 12). Firstly, the cavitation effect of ultrasound broke the particles’ surface, opened the mineral inclusions and made the exfoliation of the attachments on the surface, which elevated the contact between lixiviants and the particles. Secondly, the oxidation synergy between ultrasound, H2SO4, and O2 generated more •OH and improved the oxidation leaching reaction of Zn and Ge. Lastly, the induction of ultrasound and O2 lowered the activation energy and increased the leaching kinetics of Zn and Ge.
Fig. 11.
Diagram of the ultrasound improved leaching of Pb from electrolytic manganese anode mud. [134]. Copyright (2021), with permission from Elsevier.
Fig. 12.
Mechanism of ultrasound towards Zn and Ge leaching from germanium-containing slag dust.[126]. Copyright (2022), with permission from Elsevier.
5. Conclusions and outlook
With the depletion of high-grade metal ores and the generation of vast metal-containing secondary resources, ultrasound-assisted hydrometallurgical leaching of valuable metals from raw materials has become an outstanding alternative technique for conventional leaching. Herein, in this review, we systematically summarized the recent developments and corresponding strengthened mechanisms of ultrasound-assisted leaching recovery of various metals from metal ores and secondary resources. Based on the literature review, the conclusions and outlooks are concluded as follows:
(1) In recent two decades, the technology of UAL has attracted extensive attention in the fields of hydrometallurgical leaching recovery of various valuable metals, including common nonferrous and ferrous metals, rare metals, rare earth elements, and precious metals, from low-grade metal ores and secondary resources. In the last five years, it is worth noting that the application trend of ultrasound has transferred from metal ores to metal-containing secondary resources, which can not only guarantee the sustainable recycling of valuable metal resources but also preserve the environment. Notably, the techniques of ultrasound combined with additives or other technologies have shown great potential in strengthening the leaching efficiency of metals, which will attract more and more attention in future research. The UAL technique is characterized by green, low carbon, and low energy consumption, which is conducive to realizing the objective of carbon neutrality.
(2) The strengthening mechanisms of ultrasound are mainly reflected in three aspects: (i) ultrasound improves the leaching kinetics of metals, such as reduction in the activation energy of leaching reactions, enhancement of the collision frequency between lixiviant molecules and metal-containing particles, and acceleration of leaching reaction rate; (ii) ultrasound cavitation effect alters the characteristics of the particles, such as reduction in the particle size, formation of surface cracks or voids, exfoliation of the passivated layer, and opening of the mineral inclusions, which benefit the mass transfer and diffusion of lixiviants in the pores and cracks on the particle surface and further promote the release of metals; (iii) the generation of strong oxidizing reagents, for instance, hydroxyl radicals or hydrogen peroxide, promotes the oxidative conversion of metals from insoluble to soluble states, which further facilitates the dissolution of targeted metals. In summary, the strengthening mechanisms of ultrasound irradiation during the leaching process are usually reflected in many aspects rather than a single aspect. In addition, the synergistic mechanisms of ultrasound combined with additives and other technologies should be further explored and revealed in the future.
(3) Ultrasound commonly affects the leaching process of metals by changing the liquid environment, such as agitation and heat transfer. Hence, the velocity and temperature distribution in various leaching media under CL and UAL should be comparatively simulated using software, such as COMSOL Multiphysics, to visualize the leaching environment. In addition, whilst the strengthening mechanisms of ultrasound towards metals leaching are revealed at a relative macro-scale, the lack of observation for ultrasound action at molecular or atomic scale impedes the profound interpretation of the mechanisms. Thus, it is proposed that future research should be focused on the quantum chemical modeling of the leaching process induced by ultrasound irradiation, the main challenges of which may be the loading of ultrasound boundary conditions during the modeling process.
(4) Although the technique of UAL has been extensively investigated and reported in the hydrometallurgical leaching recovery of metals from metal ores and secondary resources, there are only a few reports on the industrial pilot-scale applications of UAL technology [95], [114]. The main obstacles in industrial applications may be reflected in two aspects. On the one hand, the design and exploitation of high-efficient ultrasound reactors operated at various rigorous leaching conditions, such as high leaching temperature, highly acidic, and highly alkaline environments. The design of large-scale ultrasonic reactors may be based on multiple transducers in the presence/absence of multiple frequencies and on the mode of indirect or direct sonication. On the other hand, there is a lack of economic analysis evaluating the overall cost of metal recovery during the UAL process, including energy, chemicals, ultrasonic reactors, manpower consumption, and so on.
CRediT authorship contribution statement
Shenxu Bao: Conceptualization, Writing – review & editing, Funding acquisition, Supervision. Bo Chen: Conceptualization, Writing – review & editing, Supervision. Yimin Zhang: Writing – review & editing. Liuyi Ren: Writing – review & editing. Chunfu Xin: Writing – review & editing. Wei Ding: Writing – review & editing. Siyuan Yang: Writing – review & editing. Wencai Zhang: Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This research was supported by the National Natural Science Foundation of China (52074204) and Major Scientific and Technological Project of Zhejiang Province (2022C03061).
Contributor Information
Shenxu Bao, Email: sxbao@whut.edu.cn.
Bo Chen, Email: bochen2012@whut.edu.cn.
Data availability
Data will be made available on request.
References
- 1.Bao S.X., Tang Y.P., Zhang Y.M., Liang L. Recovery and separation of metal Ions from aqueous solutions by solvent-impregnated resins. Chem. Eng. Technol. 2016;39(8):1377–1392. [Google Scholar]
- 2.Abkhoshk E., Jorjani E., Al-Harahsheh M.S., Rashchi F., Naazeri M. Review of the hydrometallurgical processing of non-sulfide zinc ores. Hydrometallurgy. 2014;149:153–167. [Google Scholar]
- 3.Brunelli K., Dabala M. Ultrasound effects on zinc recovery from EAF dust by sulfuric acid leaching. Int. J. Min. Met. Mater. 2015;22(4):353–362. [Google Scholar]
- 4.Ding W., Bao S.X., Zhang Y.M., Xiao J.H. Efficient selective extraction of scandium from red mud. Min. Proc. Ext. Met. Rev. 2023;44(4):304–312. [Google Scholar]
- 5.Yin X.H., Opara A., Du H., Miller J.D. Molecular dynamics simulations of metal-cyanide complexes: fundamental considerations in gold hydrometallurgy. Hydrometallurgy. 2011;106(1–2):64–70. [Google Scholar]
- 6.Li J.S., Safarzadeh M.S., Moats M.S., Miller J.D., Levier K.M., Dietrich M., Wan R.Y. Thiocyanate hydrometallurgy for the recovery of gold. Part I: Chemical and thermodynamic considerations. Hydrometallurgy. 2012;113:1–9. [Google Scholar]
- 7.Xie Y.F., Xie S.W., Chen X.F., Gui W.H., Yang C.H., Caccetta L. An integrated predictive model with an on-line updating strategy for iron precipitation in zinc hydrometallurgy. Hydrometallurgy. 2015;151:62–72. [Google Scholar]
- 8.Park J., Jung Y., Kusumah P., Lee J., Kwon K., Lee C.K. Application of ionic liquids in hydrometallurgy. Int. J. Mol. Sci. 2014;15(9):15320–15343. doi: 10.3390/ijms150915320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David E., Kopac J. Aluminum recovery as a product with high added value using aluminum hazardous waste. J. Hazard. Mater. 2013;261:316–324. doi: 10.1016/j.jhazmat.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 10.Mahfouz M.G., Galhoum A.A., Gomaa N.A., Abdel-Rehem S.S., Atia A.A., Vincent T., Guibal E. Uranium extraction using magnetic nano-based particles of diethylenetriamine-functionalized chitosan: Equilibrium and kinetic studies. Chem. Eng. J. 2015;262:198–209. [Google Scholar]
- 11.Sapsford D.J., Bowell R.J., Geroni J.N., Penman K.M., Dey M. Factors influencing the release rate of uranium, thorium, yttrium and rare earth elements from a low grade ore. Miner. Eng. 2012;39:165–172. [Google Scholar]
- 12.Avvaru B., Roy S.B., Chowdhury S., Hareendran K.N., Pandit A.B. Enhancement of the leaching rate of uranium in the presence of ultrasound. Ind. Eng. Chem. Res. 2006;45(22):7639–7648. [Google Scholar]
- 13.Larrabure G., Chero-Osorio S., Silva-Quinones D., Benndorf C., Williams M., Gao F., Gamarra C., Alarcon A., Segura C., Teplyakov A., Rodriguez-Reyes J.C.F. Surface processes at a polymetallic (Mn-Fe-Pb) sulfide subject to cyanide leaching under sonication conditions and with an alkaline pretreatment: Understanding differences in silver extraction with X-ray photoelectron spectroscopy (XPS) Hydrometallurgy. 2021;200 [Google Scholar]
- 14.Ji J.B., Lu X.H., Cai M.Q., Xu Z.C. Improvement of leaching process of geniposide with ultrasound. Ultrason. Sonochem. 2006;13(5):455–462. doi: 10.1016/j.ultsonch.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Ding W., Bao S.X., Zhang Y.M., Xiao J.H. Mechanism and kinetics study on ultrasound assisted leaching of gallium and zinc from corundum flue dust. Miner. Eng. 2022;183 [Google Scholar]
- 16.Chen B., Bao S.X., Zhang Y.M., Li S. A high-efficiency and sustainable leaching process of vanadium from shale in sulfuric acid systems enhanced by ultrasound. Sep. Purif. Technol. 2020;240 [Google Scholar]
- 17.Cetintas S., Bingol D. Performance evaluation of leaching processes with and without ultrasound effect combined with reagent-assisted mechanochemical process for nickel recovery from Laterite: Process optimization and kinetic evaluation. Miner. Eng. 2020;157 [Google Scholar]
- 18.Xue J.Q., Lu X., Du Y.W., Mao W.B., Wang J.Y., Li J.X. Ultrasonic-assisted oxidation leaching of nickel sulfide concentrate. Chinese. J. Chem. Eng. 2010;18(6):948–953. [Google Scholar]
- 19.Chen B., Bao S.X., Zhang Y.M. Synergetic strengthening mechanism of ultrasound combined with calcium fluoride towards vanadium extraction from low-grade vanadium-bearing shale. Int. J. Min. Sci. Techno. 2021;31(6):1095–1106. [Google Scholar]
- 20.Gui Q.H., Khan M.I., Wang S.X., Zhang L.B. The ultrasound leaching kinetics of gold in the thiosulfate leaching process catalysed by cobalt ammonia. Hydrometallurgy. 2020;196 [Google Scholar]
- 21.Zhang Y.F., Ma J.Y., Qin Y.H., Zhou J.F., Yang L., Wu Z.K., Wang T.L., Wang W.G., Wang C.W. Ultrasound-assisted leaching of potassium from phosphorus-potassium associated ore. Hydrometallurgy. 2016;166:237–242. [Google Scholar]
- 22.Chen B., Bao S.X., Zhang Y.M., Ren L.Y. A novel and sustainable technique to precipitate vanadium from vanadium-rich solutions via efficient ultrasound irradiation. J. Clean. Prod. 2022;339 [Google Scholar]
- 23.Doche M., Mandroyan A., Mourad-Mahmoud M., Moutarlier V., Hihn J. An ultrasonic-assisted process for copper recovery in a des solvent: Leaching and re-deposition. Chem. Eng. Process. 2017;121:90–96. [Google Scholar]
- 24.Prasad R., Dalvi S.V. Sonocrystallization: Monitoring and controlling crystallization using ultrasound. Chem. Eng. Sci. 2020;226(6) [Google Scholar]
- 25.Johansson O., Pamidi T., Shankar V. Extraction of tungsten from scheelite using hydrodynamic and acoustic cavitation. Ultrason. Sonochem. 2021;71 doi: 10.1016/j.ultsonch.2020.105408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turan M.D., Silva J.P., Sari Z.A., Nadirov R., Toro N. Dissolution of chalcopyrite in presence of chelating agent and hydrogen peroxide. T. Indian. I. Metals. 2022;75(1):273–280. [Google Scholar]
- 27.Gui Q.H., Hu Y.T., Wang S.X., Zhang L.B. Mechanism of synergistic pretreatment with ultrasound and ozone to improve gold and silver leaching percentage. Appl. Surf. Sci. 2022;576 [Google Scholar]
- 28.Alarcon A., Segura C., Gamarra C., Rodriguez-Reyes J.C.F. Green chemistry in mineral processing: chemical and physical methods to enhance the leaching of silver and the efficiency in cyanide consumption. Pure. Appl. Chem. 2018;90(7):1109–1120. [Google Scholar]
- 29.Yin S.H., Pei J.N., Jiang F., Li S.W., Peng J.H., Zhang L.B., Ju S.H., Srinivasakannan C. Ultrasound-assisted leaching of rare earths from the weathered crust elution-deposited ore using magnesium sulfate without ammonia-nitrogen pollution. Ultrason. Sonochem. 2018;41:156–162. doi: 10.1016/j.ultsonch.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 30.He H.P., Cao J., Duan N. Synergistic effect between ultrasound and fierce mechanical activation towards mineral extraction: a case study of ZnO ore. Ultrason. Sonochem. 2018;48:163–170. doi: 10.1016/j.ultsonch.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Ma J.Y., Zhang Y.F., Qin Y.H., Wu Z.K., Wang T.L., Wang C.W. The leaching kinetics of K-feldspar in sulfuric acid with the aid of ultrasound. Ultrason. Sonochem. 2017;35:304–312. doi: 10.1016/j.ultsonch.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Salmi T., Grenman H., Bernas H., Warna J., Murzin D.Y. Mechanistic modelling of kinetics and mass transfer for a solid–liquid system: leaching of zinc with ferric iron. Chem. Eng. Sci. 2010;65(15):4460–4471. [Google Scholar]
- 33.Feng C.L., Zhang C., Yuan S.H., Liu M., Chen R.R., Hu H.P., Hu J.G. Sustainable recovery of surface-deposited oils and valuable metals from uncrushed spent hydroprocessing catalysts. J. Clean. Prod. 2022;338 [Google Scholar]
- 34.Xuan W., Chagnes A., Xiao X., Olsson R.T., Forsberg K. Antisolvent precipitation for metal recovery from citric acid solution in recycling of NMC cathode materials. Metals-Basel. 2022;12(4):607. [Google Scholar]
- 35.Xin C.F., Xia H.Y., Zhang Q., Zhang L.B., Zhang W. Recovery of Zn and Ge from zinc oxide dust by ultrasonic-H2O2 enhanced oxidation leaching. Rsc. Adv. 2021;11(53):33788–33797. doi: 10.1039/d1ra06510f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masoum H.G., Rastegar S.O., Khamforoush M. Ultrasound-assisted leaching of vanadium and yttrium from coal ash: optimization, kinetic and thermodynamic study. Chem. Eng. Technol. 2021;44(12):2249–2256. [Google Scholar]
- 37.Hosseinzadeh F., Rastegar S.O., Ashengroph M., Gu T. Ultrasound-assisted Fenton-like reagent to leach precious metals from spent automotive catalysts: process optimization and kinetic modeling. Int. J. Environ. Sci. Te. 2021;18(11):3449–3458. [Google Scholar]
- 38.Zou J.T., Luo Y.G., Yu X., Li J., Xi Y.H., Zhang L.B., Guo W.Q., Lin G. Extraction of Indium from by-products of zinc metallurgy by ultrasonic waves. Arab. J. Sci. Eng. 2020;45(9):7321–7328. [Google Scholar]
- 39.John J.J., De Houwer V., Van Mechelen D., Van Gerven T. Effect of ultrasound on leaching of lead from landfilled metallurgical residues. Ultrason. Sonochem. 2020;69 doi: 10.1016/j.ultsonch.2020.105239. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W., Lin Y., Chien S., Wu T., Chen S., Cheng P.C., Lai C.N. Efficient indium leaching and recovery from waste liquid crystal displays panels using microwave and ultrasound-assisted heating system. Sep. Purif. Technol. 2020;250 [Google Scholar]
- 41.Li H.Y., Zhang L.B., Xie H.M., Yin S.H., Peng J.H., Li S.W., Yang K., Zhu F. Ultrasound-assisted silver leaching process for cleaner production. JOM-US. 2020;72(2):766–773. [Google Scholar]
- 42.Wang X., Srinivasakannan C., Duan X.H., Peng J.H., Yang D.J., Ju S.H. Leaching kinetics of zinc residues augmented with ultrasound. Sep. Purif. Technol. 2013;115:66–72. [Google Scholar]
- 43.Yu B.Q., Kou J., Sun C.B., Xing Y. Extraction of copper from copper-bearing biotite by ultrasonic-assisted leaching. Int. J. Min. Met. Mater. 2022;29(2):212–217. [Google Scholar]
- 44.Nalesso S., Bussemaker M.J., Sear R.P., Hodnett M., Lee J. A review on possible mechanisms of sonocrystallisation in solution. Ultrason. Sonochem. 2019;57:125–138. doi: 10.1016/j.ultsonch.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 45.Cogne C., Labouret S., Peczalski R., Louisnard O., Baillon F., Espitalier F. Theoretical model of ice nucleation induced by acoustic cavitation. Part 1: Pressure and temperature profiles around a single bubble. Ultrason. Sonochem. 2016;29:447–454. doi: 10.1016/j.ultsonch.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 46.Muthoosamy K., Manickam S. State of the art and recent advances in the ultrasound-assisted synthesis, exfoliation and functionalization of graphene derivatives. Ultrason. Sonochem. 2017;39:478–493. doi: 10.1016/j.ultsonch.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 47.Nalajala V.S., Moholkar V.S. Investigations in the physical mechanism of sonocrystallization. Ultrason. Sonochem. 2011;18(1):345–355. doi: 10.1016/j.ultsonch.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 48.Liu J., Wang S.X., Liu C.H., Zhang L.B., Kong D.S. Mechanism and kinetics of synergistic decopperization from copper anode slime by ultrasound and ozone. J. Clean. Prod. 2021;322 [Google Scholar]
- 49.Wang J.X., Faraji F., Ghahreman A. Effect of ultrasound on the oxidative copper leaching from chalcopyrite in acidic ferric sulfate media. Minerals-Basel. 2020;10(7):633. [Google Scholar]
- 50.Yang J.H., He L.H., Liu X.H., Ding W.T., Song Y.F., Zhao Z.W. Comparative kinetic analysis of conventional and ultrasound-assisted leaching of scheelite by sodium carbonate. T. Nonferr. Metal. Soc. 2018;28(4):775–782. [Google Scholar]
- 51.Zhao Z.W., Ding W.T., Liu X.H., Liang Y. Effect of ultrasound on kinetics of scheelite leaching in sodium hydroxide. Can. Metall. Quart. 2013;52(2):138–145. [Google Scholar]
- 52.Li S.W., Chen W.H., Yin S.H., Ma A.Y., Yang K., Xie F., Zhang L.B., Peng J.H. Impacts of ultrasound on leaching recovery of zinc from low grade zinc oxide ore. Green. Process. Synth. 2015;4(4):323–328. [Google Scholar]
- 53.Zhang L.B., Li H.Y., Peng J.H., Srinivasakannan C., Li S.W., Yin S.H. Microwave and ultrasound augmented leaching of complicated zinc oxide ores in ammonia and ammonium citrate solutions. Metals-Basel. 2017;7(6):216. [Google Scholar]
- 54.Zhu P., Zhang X.J., Li K.F., Qian G.R., Zhou M. Kinetics of leaching refractory gold ores by ultrasonic-assisted electro-chlorination. Int. J. Min. Met. Mater. 2012;19(6):473–477. [Google Scholar]
- 55.Zhang G.W., Wang S.X., Zhang L.B., Peng J.H. Ultrasound-intensified leaching of gold from a refractory ore. ISIJ. Int. 2016;56(4):714–718. [Google Scholar]
- 56.Fu L.K., Zhang L.B., Wang S.X., Peng J.H. Synergistic extraction of gold from the refractory gold ore via ultrasound and chlorination-oxidation. Ultrason. Sonochem. 2017;37:471–477. doi: 10.1016/j.ultsonch.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 57.Guo P., Wang S.X., Zhang L.B. Selective removal of antimony from refractory gold ores by ultrasound. Hydrometallurgy. 2019;190 [Google Scholar]
- 58.Hu Y.T., Guo P., Wang S.X., Zhang L.B. Leaching kinetics of antimony from refractory gold ore in alkaline sodium sulfide under ultrasound. Chem. Eng. Res. Des. 2020;164:219–229. [Google Scholar]
- 59.Avvaru B., Roy S.B., Ladola Y., Chowdhury S., Hareendran K.N., Pandit A.B. Sono-chemical leaching of uranium. Chem. Eng. Process. 2008;47(12):2107–2113. [Google Scholar]
- 60.Ladola Y.S., Chowdhury S., Roy S.B., Pandit A.B. Application of cavitation in uranium leaching. Desalin. Water. Treat. 2014;52(1–3):407–414. [Google Scholar]
- 61.Cilek E.C., Ciftci H., Karagoz S.G., Tuzci G. Extraction of silver from a refractory silver ore by sono-cyanidation. Ultrason. Sonochem. 2020;63 doi: 10.1016/j.ultsonch.2020.104965. [DOI] [PubMed] [Google Scholar]
- 62.Chanturiya V.A., Minenko V.G., Samusev A.L., Chanturia E.L., Koporulina E.V., Bunin I.Z., Ryazantseva M.V. The effect of energy impacts on the acid leaching of eudialyte concentrate. Min. Proc. Ext. Met. Rev. 2021;42(7):484–495. [Google Scholar]
- 63.Arslan V. The modeling and optimization of iron removal from silica sand under ultrasound-assisted leaching by response surface methodology. Mining. Metall. Explor. 2021;38(5):2229–2237. [Google Scholar]
- 64.Yang C.Q., Li S.Q. Kinetics of iron removal from quartz under ultrasound-assisted leaching. High. Temp. Mat. Pr-Isr. 2020;39:395–404. [Google Scholar]
- 65.Yang C.Q., Li S.Q., Bai J.X., Han S.S. Advanced purification of industrial quartz using calcination pretreatment combined with ultrasound-assisted leaching. Acta. Geodyn. Geomater. 2018;15(2):187–195. [Google Scholar]
- 66.Wang J.Q., Xing P.F., Du X.H., Luo X.T., Zhuang Y.X., Lyu T., Dong X. Kinetics analysis and effects of various factors on removing iron from silica sand under ultrasound-assistance. Silicon-Neth. 2017;9(2):265–272. [Google Scholar]
- 67.Zhang Z.Z., Li J.S., Li X.X., Huang H.Q., Zhou L.F., Xiong T.T. High efficiency iron removal from quartz sand using phosphoric acid. Int. J. Miner. Process. 2012;114:30–34. [Google Scholar]
- 68.Du F.H., Li J.S., Li X.X., Zhang Z.Z. Improvement of iron removal from silica sand using ultrasound-assisted oxalic acid. Ultrason. Sonochem. 2011;18(1):389–393. doi: 10.1016/j.ultsonch.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 69.Knaislova A., Vu H.N., Dvorak P. Microwave and ultrasound effect on ammoniacal leaching of deep-sea nodules. Minerals-Basel. 2018;8(8):351. [Google Scholar]
- 70.Ozkan M.H., Gurkan R., Ozkan A., Akcay M. Dissolution kinetics of magmatic rocks via ultrasonic leaching for calcium, magnesium and aluminum. Gazi. U. J. Sci. 2008;21(4):117–122. [Google Scholar]
- 71.Bese A.V. Effect of ultrasound on the dissolution of copper from copper converter slag by acid leaching. Ultrason. Sonochem. 2007;14(6):790–796. doi: 10.1016/j.ultsonch.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 72.Lim K., Shon B. Metal components (Fe, Al, and Ti) recovery from red mud by sulfuric acid leaching assisted with ultrasonic waves. Int. J. Emerg. Technol. Adv. Eng. 2008;5(2):25–32. [Google Scholar]
- 73.Cheikh M., Magnin J.I., Gondrexon N., Willison J., Hassen A. Zinc and lead leaching from contaminated industrial waste sludges using coupled processes. Environ. Technol. 2010;31(14):1577–1585. doi: 10.1080/09593331003801548. [DOI] [PubMed] [Google Scholar]
- 74.Wang X., Yang D.J., Srinivasakannan C., Peng J.H., Duan X.H., Ju S.H. A comparison of the conventional and ultrasound-augmented leaching of zinc residue using sulphuric acid. Arab. J. Sci. Eng. 2014;39(1):163–173. [Google Scholar]
- 75.Zhang R.L., Zhang X.F., Tang S.Z., Huang A.D. Ultrasound-assisted HCl–NaCl leaching of lead-rich and antimony-rich oxidizing slag. Ultrason. Sonochem. 2015;27:187–191. doi: 10.1016/j.ultsonch.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 76.Liu F.P., Liu Z.H., Li Y.H., Wilson B.P., Lundstrom M. Recovery and separation of gallium(III) and germanium(IV) from zinc refinery residues: Part I: Leaching and iron(III) removal. Hydrometallurgy. 2017;169:564–570. [Google Scholar]
- 77.Wen J., Jiang T., Gao H.Y., Liu Y.J., Zheng X.L., Xue X.X. Comparison of ultrasound-assisted and regular leaching of vanadium and chromium from roasted high chromium vanadium slag. JOM-US. 2018;70(2):155–160. [Google Scholar]
- 78.Turan M.D., Sari Z.A., Demiraslan A. Ultrasound-assisted leaching and kinetic study of blended copper slag. Metall. Mater. Trans. B. 2019;50(4):1949–1956. [Google Scholar]
- 79.Nguyen T.T.N., Lee M.S. Improvement of alumina dissolution from the mechanically activated dross using ultrasound-assisted leaching. Korean. J. Met. Mater. 2019;57(3):154–161. [Google Scholar]
- 80.Sadeghi S.M., Ferreira C.M.H., Soares H.M.V.M. Evaluation of two-step processes for the selective recovery of Mn from a rich Mn residue. Miner. Eng. 2019;130:148–155. [Google Scholar]
- 81.Guo Z.Y., Guo P., Su G., Li F.C. Study on ultrasonically-enhanced sulfuric acid leaching of nickel from nickel-containing residue. Crystals. 2021;11(7):810. [Google Scholar]
- 82.Kusaka E., Suehiro R., Iwamizu Y. Kinetics of calcium leaching from particulate steelmaking slag in acetic acid solution. ISIJ. Int. 2016;62(1):263–274. [Google Scholar]
- 83.Wang S.X., Cui W., Zhang G.W., Zhang L.B., Peng J.H. Ultra fast ultrasound-assisted decopperization from copper anode slime. Ultrason. Sonochem. 2017;36:20–26. doi: 10.1016/j.ultsonch.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 84.Liu J., Wang S.X., Zhang Y.T., Zhang L.B., Kong D.S. Synergistic mechanism and decopperization kinetics for copper anode slime via an integrated ultrasound-sodium persulfate process. Appl. Surf. Sci. 2022;589 [Google Scholar]
- 85.Chang J., Zhang E.D., Yang C.J., Zhou J.W., Peng J.H., Zhang L.B., Srinivasakannan C. Kinetics of ultrasound-assisted silver leaching from sintering dust using thiourea. Green. Process. Synth. 2016;5(1):31–40. [Google Scholar]
- 86.Chang J., Zhang E.D., Zhang L.B., Peng J.H., Zhou J.W. A comparison of ultrasound-augmented and conventional leaching of silver from sintering dust using acidic thiourea. Ultrason. Sonochem. 2017;34:222–231. doi: 10.1016/j.ultsonch.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 87.Marafi M., Stanislaus A. Waste catalyst utilization: Extraction of valuable metals from spent hydroprocessing catalysts by ultrasonic-assisted leaching with acids. Ind. Eng. Chem. Res. 2011;50(16):9495–9501. [Google Scholar]
- 88.Oza R., Shah N., Patel S. Recovery of nickel from spent catalysts using ultrasonication-assisted leaching. J. Chem. Technol. Biot. 2011;86(10):1276–1281. [Google Scholar]
- 89.Pinto I.S.S., Soares H.M.V.M. Selective leaching of molybdenum from spent hydrodesulphurisation catalysts using ultrasound and microwave methods. Hydrometallurgy. 2012;129:19–25. [Google Scholar]
- 90.Wang L., Chao L., Qu W.W., Xu S.M., Zhang L.B., Peng J.H., Xia X.L. Ultrasound-assisted Oil removal of gamma-Al2O3-based spent hydrodesulfurization catalyst and Microwave roasting recovery of metal Mo. Ultrason. Sonochem. 2018;49:24–32. doi: 10.1016/j.ultsonch.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 91.Vyas S., Ting Y. Effect of ultrasound on bioleaching of hydrodesulphurization spent catalyst. Environ. Technol. Inno. 2019;14 [Google Scholar]
- 92.Maryam Sadeghi S., Jesus J., Pinto E., Almeida A.A., Soares H.M.V.M. A simple, efficient and selective process for recycling La (and Al) from fluid cracking catalysts using an environmentally friendly strategy. Miner. Eng. 2020;156 [Google Scholar]
- 93.Karim S., Ting Y. Ultrasound-assisted nitric acid pretreatment for enhanced biorecovery of platinum group metals from spent automotive catalyst. J. Clean. Prod. 2020;255 [Google Scholar]
- 94.Lim M.S.W., Yang T.C.K., Yap Y.H., Pan G., Chong S., Tiong T.J. Intensification and optimisation of nickel recovery from spent hydrogenation catalysts via ultrasound-augmented hydrometallurgy. J. Environ. Chem. Eng. 2021;9(4) [Google Scholar]
- 95.Xie F.C., Li H.Y., Ma Y., Li C.C., Cai T.T., Huang Z.Y., Yuan G.Q. The ultrasonically assisted metals recovery treatment of printed circuit board waste sludge by leaching separation. J. Hazard. Mater. 2009;170(1):430–435. doi: 10.1016/j.jhazmat.2009.04.077. [DOI] [PubMed] [Google Scholar]
- 96.Huang Z.Y., Xie F.C., Ma Y. Ultrasonic recovery of copper and iron through the simultaneous utilization of printed circuit boards (PCB) spent acid etching solution and PCB waste sludge. J. Hazard. Mater. 2011;185(1):155–161. doi: 10.1016/j.jhazmat.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 97.Zhang K.H., Li B., Wu Y.F., Wang W., Li R.B., Zhang Y.N., Zuo T.Y. Recycling of indium from waste LCD: A promising non-crushing leaching with the aid of ultrasonic wave, Waste. Manage. 2017;64:236–243. doi: 10.1016/j.wasman.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 98.Javed U., Farooq R., Shehzad F., Khan Z. Optimization of HNO3 leaching of copper from old AMD Athlon processors using response surface methodology. J. Environ. Manage. 2018;211:22–27. doi: 10.1016/j.jenvman.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 99.Souada M., Louage C., Doisy J., Meunier L., Benderrag A., Ouddane B., Bellayer S., Nuns N., Traisnel M., Maschke U. Extraction of indium-tin oxide from end-of-life LCD panels using ultrasound assisted acid leaching. Ultrason. Sonochem. 2018;40:929–936. doi: 10.1016/j.ultsonch.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 100.Zhu P., Tang J.K., Tao Q., Wang Y.J., Wang J.P., Li Z.L., Cao Z.B., Qian G.R., Theiss F., Frost R.L. The kinetics study of dissolving SnPb solder by hydrometallurgy. Environ. Eng. Sci. 2019;36(9):1236–1243. [Google Scholar]
- 101.Toache-Perez A.D., Bolarin-Miro A.M., Sanchez-De J.F., Lapidus G.T. Facile method for the selective recovery of Gd and Pr from LCD screen wastes using ultrasound-assisted leaching. Sustain. Environ. Res. 2020;30(1):20. [Google Scholar]
- 102.Sadeghi S.M., Vanpeteghem G., Neto I.F.F., Soares H.M.V.M. Selective leaching of Zn from spent alkaline batteries using environmentally friendly approaches, Waste. Manage. 2017;60:696–705. doi: 10.1016/j.wasman.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 103.Jiang F., Chen Y.Q., Ju S.H., Zhu Q.Y., Zhang L.B., Peng J.H., Wang X.M., Miller J.D. Ultrasound-assisted leaching of cobalt and lithium from spent lithium-ion batteries. Ultrason. Sonochem. 2018;48:88–95. doi: 10.1016/j.ultsonch.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 104.Shih Y., Chien S., Jhang S., Lin Y. Chemical leaching, precipitation and solvent extraction for sequential separation of valuable metals in cathode material of spent lithium ion batteries. J. Taiwan. Inst. Chem. E. 2019;100:151–159. [Google Scholar]
- 105.Wang W.Y., Yen C.H., Hsu J.K. Selective recovery of cobalt from the cathode materials of NMC type Li-ion battery by ultrasound-assisted acid leaching and microemulsion extraction. Sep. Sci. Technol. 2020;55(16):3028–3035. [Google Scholar]
- 106.Ning P.C., Meng Q., Dong P., Duan J.G., Zhang Y.J. Recycling of cathode material from spent lithium ion batteries using an ultrasound-assisted DL-malic acid leaching system, Waste. Manage. 2020;103:52–60. doi: 10.1016/j.wasman.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 107.Esmaeili M., Rastegar S.O., Beigzadeh R., Gu T. Ultrasound-assisted leaching of spent lithium ion batteries by natural organic acids and H2O2. Chemosphere. 2020;254 doi: 10.1016/j.chemosphere.2020.126670. [DOI] [PubMed] [Google Scholar]
- 108.Nivetha E.S., Saravanathamizhan R. Recovery of nickel from spent NiCd batteries by regular and ultrasonic leaching followed by electrodeposition. J. Electrochem. Sci. En. 2020;10(1):41–47. [Google Scholar]
- 109.Xiao X., Hoogendoorn B.W., Ma Y.Q., Sahadevan S.A., Gardner J.M., Forsberg K., Olsson R.T. Ultrasound-assisted extraction of metals from lithium-ion batteries using natural organic acids. Green. Chem. 2021;23(21):8519–8532. [Google Scholar]
- 110.Makuza B., Yu D.W., Huang Z., Tian Q.H., Guo X.Y. Dry grinding-carbonated ultrasound-assisted water leaching of carbothermally reduced lithium-ion battery black mass towards enhanced selective extraction of lithium and recovery of high-value metals. Resour. Conserv. Recy. 2021;174 [Google Scholar]
- 111.Bisercic M.S., Pezo L., Ignjatovic I.S., Ignjatovic L., Savic A., Jovanovic U., Andric V. Ultrasound and shacking-assisted water-leaching of anions and cations from fly ash. J. Serb. Chem. Soc. 2016;81(7):813–827. [Google Scholar]
- 112.Tasic A.M., Ignjatovic I.D.S., Ignjatovic L.M., Ilic M.A., Antic M.P. Comparison of sequential and single extraction in order to estimate environmental impact of metals from fly ash. J Serb. Chem. Soc. 2016;81(9):1081–1096. [Google Scholar]
- 113.Rahimi G., Rastegar S.O., Chianeh F.R., Gu T. Ultrasound-assisted leaching of vanadium from fly ash using lemon juice organic acids. Rsc. Adv. 2020;10(3):1685–1696. doi: 10.1039/c9ra09325g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li C.C., Xie F.C., Ma Y., Cai T.T., Li H.Y., Huang Z.Y., Yuan G.Q. Multiple heavy metals extraction and recovery from hazardous electroplating sludge waste via ultrasonically enhanced two-stage acid leaching. J. Hazard. Mater. 2010;178(1–3):823–833. doi: 10.1016/j.jhazmat.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 115.Zhang P., Ma Y., Xie F.C. Impacts of ultrasound on selective leaching recovery of heavy metals from metal-containing waste sludge. J. Mater. Cycles. Waste. 2013;15(4):530–538. [Google Scholar]
- 116.Mikhailov I., Komarov S., Levina V., Gusev A., Issi J., Kuznetsov D. Nanosized zero-valent iron as Fenton-like reagent for ultrasonic-assisted leaching of zinc from blast furnace sludge. J. Hazard. Mater. 2017;321:557–565. doi: 10.1016/j.jhazmat.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 117.Tonanzi B., Gallipoli A., Annesini M.C., La Penna C., Gianico A., Braguglia C.M. Pre-treatments and anaerobic hydrolysis as strategical key steps for resource recovery from sludge: the role of disintegration degree in metals leaching. J. Environ. Chem. Eng. 2021;9(1) [Google Scholar]
- 118.Liu Y., Xing P.F., Liu J., Kong J., Du X.H., Gao B., Luo X.T. Removal of iron from solar grade silicon (SoG-Si) cutting slurry waste by ultrasound-assisted leaching with dilute sulfuric acid. Silicon-Neth. 2019;11(1):301–311. [Google Scholar]
- 119.Wang S., Liu Y., Gao S.B., Xing P.F., He Y.L., Yan S., Yin H.Y. Purification and comprehensive utilization of sapphire kerf waste. J. Clean. Prod. 2019;214:248–258. [Google Scholar]
- 120.Kong J., Xing P.F., Wei D.H., Jin X., Zhuang Y.X. Ultrasound-assisted leaching of iron from silicon diamond-wire saw cutting waste. JOM-US. 2021;73(3):791–800. [Google Scholar]
- 121.Tunsu C., Ekberg C., Retegan T. Characterization and leaching of real fluorescent lamp waste for the recovery of rare earth metals and mercury. Hydrometallurgy. 2014;144:91–98. [Google Scholar]
- 122.Behera S.S., Panda S.K., Mandal D., Parhi P.K. Ultrasound and microwave assisted leaching of neodymium from waste magnet using organic solvent. Hydrometallurgy. 2019;185:61–70. [Google Scholar]
- 123.Li X., Xing P.F., Du X.H., Gao S.B., Chen C. Influencing factors and kinetics analysis on the leaching of iron from boron carbide waste-scrap with ultrasound-assisted method. Ultrason. Sonochem. 2017;38:84–91. doi: 10.1016/j.ultsonch.2017.02.037. [DOI] [PubMed] [Google Scholar]
- 124.Kalsi P.K., Tomar B.S., Ramakumar K.L., Venugopal V. Studies on recovery of uranium from fluoride matrix employing sonochemistry. J. Radioanal. Nucl. Ch. 29 2012,;3(3):863–867. [Google Scholar]
- 125.Xie X.F., Zhang Z.H., Chen Z.K., Wu J.Y., Li Z.L., Zhong S.P., Liu H., Xu Z.F., Z.L., Liu, In-situ preparation of zinc sulfide adsorbent using local materials for elemental mercury immobilization and recovery from zinc smelting flue gas. Chem. Eng. J. 2022;429 [Google Scholar]
- 126.Xin C.F., Xia H.Y., Jiang G.Y., Zhang Q., Zhang L.B., Xu Y.J., Cai W.C. Mechanism and kinetics study on ultrasonic combined with oxygen enhanced leaching of zinc and germanium from germanium-containing slag dust. Sep Purif Technol. 2022;302 [Google Scholar]
- 127.Rao M.J., Li G.H., Jiang T., Luo J., Zhang Y.B., Fan X.H. Carbothermic reduction of nickeliferous laterite ores for nickel pig iron production in China: a review. JOM-US. 2013;65(11):1573–1583. [Google Scholar]
- 128.Li H.Y., Li S.W., Peng J.H., Srinivasakannan C., Zhang L.B., Yin S.H. Ultrasound augmented leaching of nickel sulfate in sulfuric acid and hydrogen peroxide media. Ultrason. Sonochem. 2018;40:1021–1030. doi: 10.1016/j.ultsonch.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 129.Al-Saydeh S.A., El-Naas M.H., Zaidi S.J. Copper removal from industrial wastewater: a comprehensive review. J. Ind. Eng. Chem. 2017;56:35–44. [Google Scholar]
- 130.Zou Y.D., Wang X.X., Khan A., Wang P.Y., Liu Y.H., Alsaedi A., Hayat T., Wang X.K. Environmental remediation and application of nanoscale zero-valent iron and Its composites for the removal of heavy metal ions: a review. Environ. Sci. Technol. 2016;50(14):7290–7304. doi: 10.1021/acs.est.6b01897. [DOI] [PubMed] [Google Scholar]
- 131.Dehaine Q., Tijsseling L.T., Glass H.J., Trmnen T., Butcher A.R. Geometallurgy of cobalt ores: a review. Miner. Eng. 2021;160 [Google Scholar]
- 132.Xie H.M., Li S.W., Zhang L.B., Wang Y.M., Long H.L. Roasting pretreatment combined with ultrasonic enhanced leaching lead from electrolytic manganese anode mud. Metals-Basel. 2019;9(5):601. [Google Scholar]
- 133.Duan N., Dan Z.G., Wang F., Pan C.X., Zhou C.B., Jiang L.H. Electrolytic manganese metal industry experience based China's new model for cleaner production promotion. J. Clean. Prod. 2011;19(17–18):2082–2087. [Google Scholar]
- 134.Xie H.M., Li S.W., Guo Z.H., Xu Z. Extraction of lead from electrolytic manganese anode mud by microwave coupled ultrasound technology. J. Hazard. Mater. 2021;407 doi: 10.1016/j.jhazmat.2020.124622. [DOI] [PubMed] [Google Scholar]
- 135.Huang T., Zhou L.L., Liu L.F., Xia M. Ultrasound-enhanced electrokinetic remediation for removal of Zn, Pb, Cu and Cd in municipal solid waste incineration fly ashes. Waste Manage. 2018;75:226–235. doi: 10.1016/j.wasman.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 136.Mukiza E., Zhang L.L., Liu X.M., Zhang N. Utilization of red mud in road base and subgrade materials: a review. Resour. Conserv. Recy. 2019;141:187–199. [Google Scholar]
- 137.Li X.X., Li T.H., Gao J.X., Huang H.Q., Li L.B., Li J.S. A novel “green” solvent to deeply purify quartz sand with high yields: a case study. J. Ind. Eng. Chem. 2016;35:383–387. [Google Scholar]
- 138.Srivalli H., Nagarajan R. Mechanistic study of ultrasound-assisted solvent leaching of sodium and potassium from an Indian coal using continuous and pulsed modes of operation. Chem. Eng. Commun. 2019;206(2):207–226. [Google Scholar]
- 139.Nakamura T., Okawa H., Kawamura Y., Sugawara K. Sonication enables effective iron leaching from green tuff at low temperature. Jpn. J. Appl. Phys. 2011;50(7):07HE16. [Google Scholar]
- 140.Xia G.H., Lu M., Su X.L., Zhao X.D. Iron removal from kaolin using thiourea assisted by ultrasonic wave. Ultrason. Sonochem. 2012;19(1):38–42. doi: 10.1016/j.ultsonch.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 141.Li F.F., Jiang X.S., Zuo Q.X., Li J.W., Ban B.Y., Chen J. Purification mechanism of quartz sand by combination of microwave heating and ultrasound assisted acid leaching treatment. Silicon-Neth. 2021;13(2):531–541. [Google Scholar]
- 142.Anjum F., Bukhari S.A., Shahid M., Akcil A., Tahir A., Jaafar H.Z.E., Zia-Ul-Haq M., Samota I. Bio-mechanical leaching of uranium from low grade black shale. Environ. Eng. Manag. J. 2015;14(12):2939–2946. [Google Scholar]
- 143.Wang J., Zhang Y.Y., Yu L.H., Cui K.K., Fu T., Mao H.B. Effective separation and recovery of valuable metals from waste Ni-based batteries: a comprehensive review. Chem. Eng. J. 2022;439 [Google Scholar]
- 144.Zhang Y.M., Bao S.X., Liu T., Chen T.J., Huang J. The technology of extracting vanadium from stone coal in China: history, current status and future prospects. Hydrometallurgy. 2011;109(1–2):116–124. [Google Scholar]
- 145.Chen B., Bao S.X., Zhang Y.M., Li C. Reactive crystallization of ammonium polyvanadate from vanadium-bearing solution assisted by ultrasound irradiation: Crystallization characteristics and growth process. J. Mater. Res. Technol. 2023;25:667–680. [Google Scholar]
- 146.Bao S.X., Xin C.F., Zhang Y.M., Chen B., Ding W., Luo Y.P. Application of capacitive deionization in water treatment and energy recovery: A review. Energies. 2023;16(3):1136. [Google Scholar]
- 147.Wang J.L., Sui L.H., Huang J., Miao L., Nie Y.B., Wang K.S., Yang Z.C., Huang Q., Gong X., Nan Y.Y., Ai K.L. MoS2-based nanocomposites for cancer diagnosis and therapy. Bioact. Mater. 2021;6(11):4209–4242. doi: 10.1016/j.bioactmat.2021.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang X., Jia F.F., Song S.X. Recent advances in structural engineering of molybdenum disulfide for electrocatalytic hydrogen evolution reaction. Chem. Eng. J. 2021;405 [Google Scholar]
- 149.Woods-Robinson R., Han Y., Zhang H., Ablekim T., Khan I., Persson K.A., Zakutayev A. Wide band gap chalcogenide semiconductors. Chem. Rev. 2020;120(9):4007–4055. doi: 10.1021/acs.chemrev.9b00600. [DOI] [PubMed] [Google Scholar]
- 150.Dai Z.R., Zhen Y., Sun Y.S., Li L., Ding D.X. ZnFe2O4/g-C3N4 S-scheme photocatalyst with enhanced adsorption and photocatalytic activity for uranium(VI) removal. Chem. Eng. J. 2021;415 [Google Scholar]
- 151.Liao J., Xiong T., Ding L., Zhang Y., Zhu W.K. Effective separation of uranium(VI) from wastewater using a magnetic carbon as a recyclable adsorbent. Sep. Purif. Technol. 2022;282 [Google Scholar]
- 152.Rychkov V.N., Kirillov E.V., Kirillov S.V., Semenishchev V.S., Bunkov G.M., Botalov M.S., Smyshlyaev D.V., Malyshev A.S. Recovery of rare earth elements from phosphogypsum. J. Clean. Prod. 2018;196:674–681. [Google Scholar]
- 153.Lahiri S., Mandal D., Gogate P.R., Bhardwaj R.L. Intensified ceria recovery from graphite substrate and cleanup of leachant using sonication. Chem. Eng. Process. 2022;174 [Google Scholar]
- 154.Yu S.M., Yu T.T., Song W.P., Yu X.Y., Qiao J.X., Wang W.Y., Dong H.J., Wu Z.G., Dai L.Z., Li T.L. Ultrasound-assisted cyanide extraction of gold from gold concentrate at low temperature. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.105039. [DOI] [PubMed] [Google Scholar]
- 155.Li H.Y., Li S.W., Srinivasakannan C., Zhang L.B., Yin S.H., Yang K., Xie H.M. Efficient cleaning extraction of silver from spent symbiosis lead-zinc mine assisted by ultrasound in sodium thiosulfate system. Ultrason. Sonochem. 2018;49:118–127. doi: 10.1016/j.ultsonch.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 156.Li Y., Kawashima N., Li J., Chandra A.P., Gerson A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid. Interfac. 2013;197:1–32. doi: 10.1016/j.cis.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 157.Jha M.K., Lee J., Kim M., Jeong J., Kim B., Kumar V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review. Hydrometallurgy. 2013;133:23–32. [Google Scholar]
- 158.Bu X.N., Danstan J.K., Hassanzadeh A., Vakylabad A.B., Chelgani S.C. Metal extraction from ores and waste materials by ultrasound assisted leaching-an overview. Min. Proc. Ext. Met. Rev. 2022 doi: 10.1080/08827508.2022.2117173. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.